ABSTRACT
Phase III multi-country studies (ZOE-50/70) demonstrated that the adjuvanted recombinant zoster vaccine (RZV) was well tolerated and prevented herpes zoster (HZ) in healthy ≥ 50-year-olds, with a vaccine efficacy (VE) > 90% across age groups. These pivotal trials did not enroll participants from mainland China where RZV is licensed, therefore similar clinical data are missing for this population. In this phase IV observer-blind study (NCT04869982) conducted between 2021 and 2023 in China, immunocompetent and medically stable ≥ 50-year-olds were randomized 1:1 to receive two RZV or placebo doses, 2 months apart. This study assessed the VE (overall, as confirmatory objective, and descriptively by age category [50–69-year-olds/≥ 70-year-olds]), reactogenicity, and safety of RZV in this Chinese population. Of the 6138 enrolled participants, 99.2% completed the study. During a mean follow-up period of 15.2 (±1.1) months, 31 HZ episodes were confirmed (RZV = 0; placebo = 31) for an incidence rate of 0.0 vs 8.2 per 1000 person-years and an overall VE of 100% (89.82–100). The descriptive VE was 100% (85.29–100) for 50–69-year-olds and 100% (60.90–100) for ≥ 70-year-olds. Solicited adverse events (AEs) were more frequent in the RZV vs the placebo group (median duration: 1–3 days for both groups). Pain and fatigue were the most frequent local and general AEs (RZV: 72.1% and 43.4%; placebo: 9.2% and 5.3%). The frequencies of unsolicited AEs, serious AEs, potential immune-mediated diseases, and deaths were similar between both groups. RZV is well tolerated and efficacious in preventing HZ in Chinese ≥ 50-year-olds, consistent with efficacy studies including worldwide populations with similar age and medical characteristics.
KEYWORDS: Herpes zoster, adjuvanted recombinant zoster vaccine, vaccine efficacy, safety, reactogenicity
Plain Language Summary
What is the context?
Herpes zoster, commonly known as shingles, is a painful rash resulting from the reactivation of the dormant virus causing chickenpox.
Vaccines preventing shingles, such as Shingrix, were shown to be well tolerated and efficacious in healthy adults over 50 years of age from Europe, North and Latin America, Australia, and Asia (Taiwan, Hong Kong, Korea, Japan).
However, data on real-world protective effect of Shingrix are limited in some regions where the vaccine is licensed for use, such as mainland China.
What is new?
We analyzed data from Chinese adults aged 50 years or older to determine the efficacy and safety of Shingrix.
Around 6000 participants were divided in two equal groups to receive two doses of Shingrix or two doses of a placebo, given 2 months apart.
We found that, during the study period, the vaccine was 100% efficacious in preventing shingles.
We showed that the vaccine had an acceptable safety profile in this Chinese population.
What is the impact?
Shingrix is efficacious and well tolerated in Chinese adults over 50 years of age, as it is in similarly aged populations from other evaluated regions.
Introduction
Herpes zoster (HZ), also known as shingles, is a viral infection that is caused by the reactivation of latent varicella-zoster virus and is characterized by a painful, dermatomal rash.1 The risk of developing HZ and its complications, e.g., postherpetic neuralgia (PHN), increases with age.2 HZ and PHN can be prevented through vaccination with an adjuvanted recombinant zoster vaccine (RZV; Shingrix, GSK) that consists of lyophilized glycoprotein E antigen and the adjuvant system AS01B.3 RZV has been evaluated in several studies involving medically stable adults ≥ 50 years of age.4–9 The vaccine elicited strong cellular and humoral immune responses underlying its high efficacy at preventing HZ, and had a clinically acceptable safety and reactogenicity profile.3
Two randomized, multi-country phase III studies, one conducted with ≥ 50-year-old participants (ZOE-50, NCT01165177),7 and the other one conducted with ≥ 70-year-old participants (ZOE-70, NCT01165229),8 evaluated the vaccine efficacy (VE), immunogenicity, safety, and reactogenicity of RZV.7–9 A post-hoc subgroup analysis of the data from ZOE-50 and ZOE-70 studies involving all participants from Asian countries (China [Taiwan Province and Hong Kong Special Administrative Region], South Korea, and Japan) showed that the descriptive VE against HZ ranged between 94.7% and 95.6%,10 consistent with overall VE results reported in the ZOE-50 (97.2%) and ZOE-70 (89.8%) studies.7,8 Another post-hoc subgroup analysis of ZOE-50 and ZOE-70 data also showed that the VE of RZV in preventing HZ was not impacted by the geographic ancestry, region, or ethnicity.11 Furthermore, the reactogenicity and safety profile of RZV in this Asian subpopulation was similar to overall results from ZOE-50 and ZOE-70 studies.7–10
Studies evaluating the real-world effectiveness of RZV have shown that two doses of RZV were effective in 83.5–85.5% of individuals aged ≥ 50 years that were not immunocompromised12,13 and 70.1% of individuals aged ≥ 65 years,14 supporting and complementing clinical trial data.15 The real-world safety profile of RZV in ≥ 50-year-old individuals was also consistent with the one determined in clinical trials.15
Since 2017, RZV received marketing authorization in several countries for the prevention of HZ in ≥ 50-year-old adults worldwide.16 In 2018, RZV was included in the list of 48 “clinically urgently needed new medicines” for fast track licensure in China, and 1 year later the National Medical Products Administration (NMPA) approved the use of RZV in the country for preventing HZ in adults ≥ 50 years of age.17 The vaccine is expected to prevent many HZ cases and related complications among Chinese older adults.18,19
While efficacy, immunogenicity, and safety data for RZV are available for a variety of populations, including populations with Asian ancestry, no data is available for the population from mainland China. The aim of this post-marketing study was to evaluate the VE, reactogenicity, and safety of RZV in ≥ 50-year-old adults from this region.
Materials and methods
Study design and enrolled participants
This phase IV randomized (1:1), placebo-controlled, observer-blind study was conducted at six centers across mainland China, between May 2021 and April 2023, among ≥ 50-year-old participants.
Medically stable male and female individuals, who were ≥ 50 years of age at the time of first vaccination, without any history of HZ, and who were not previously vaccinated against varicella or HZ were enrolled. Individuals with stable comorbidities (e.g., diabetes mellitus type 2, depression, asthma, hypertension, hyperlipidemia, gastritis) were eligible. Female participants of childbearing potential needed to have a negative pregnancy test on the day of vaccination and to practice adequate contraception 30 days prior to first vaccination and 2-months post-last vaccination. Those individuals planning to become pregnant or those who were immunosuppressed were excluded from the study. Urine samples were collected from all female participants of childbearing potential prior to any study vaccine administration (i.e., at Day 1 and Month 2) to determine any pregnancy. Full inclusion and exclusion criteria are listed in the supplementary material.
This study comprised two phases: a vaccination phase and a follow-up phase, each consisting of monthly visits. Participants were randomized 1:1 to receive either two RZV or placebo doses 2 months apart (at Day 1 and Month 2). All participants were followed up until last enrolled participant completed 12 months of follow-up post-last vaccination (i.e., 14 months from the enrollment of the last participant), and at least 27 confirmed HZ cases for the primary VE analysis were reported. When this trigger for primary VE analysis was reached, participants with an ongoing HZ episode were further followed until 29 days after onset (Figure 1).
Figure 1.
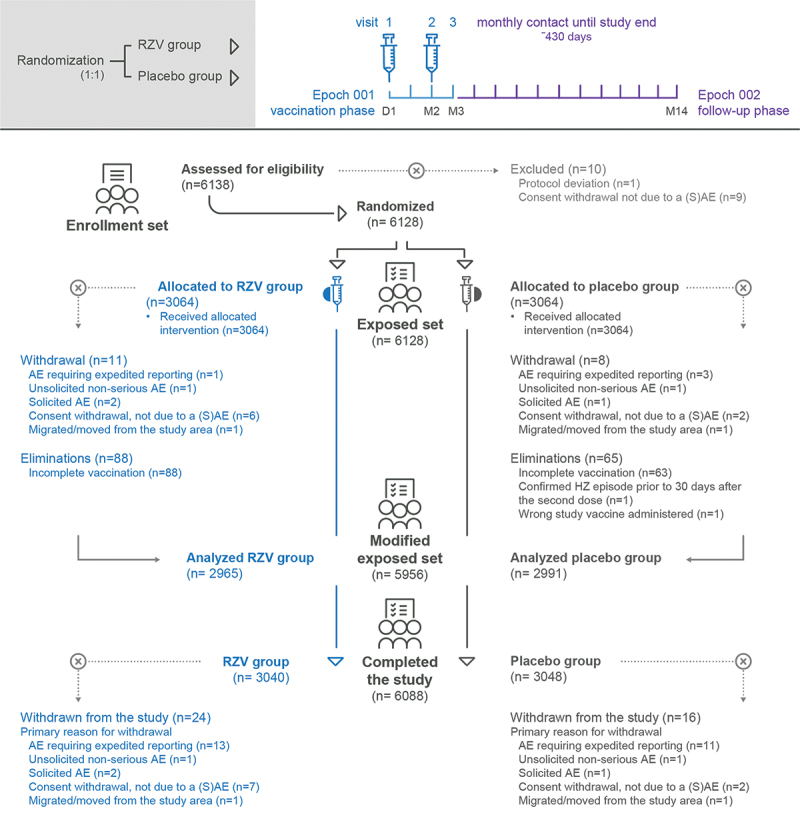
Study design and flow diagram of study participants.
RZV group, participants receiving the adjuvanted recombinant zoster vaccine; placebo group, participants receiving placebo; D, day; M, month; n, number of participants; (S)AE, (serious) adverse event; HZ, herpes zoster.
The study was conducted in an observer-blind manner, in which vaccine recipients and those responsible for the evaluation of study endpoints (e.g., safety, reactogenicity, and efficacy) were unaware of the study interventions. Each participant was assigned to an identification code that was not linked in any way to the attributed vaccine. Authorized medical personnel, who did not participate in any of the study outcomes, were responsible for the vaccine preparation and administration. The personnel in charge of the laboratory testing were also blinded to the study interventions.
All participants/legally authorized representatives provided written informed consent at enrollment. The study was conducted in accordance with the Declaration of Helsinki, the principles of Good Clinical Practice, and all applicable regulatory requirements. The study protocol and any subsequent amendments were approved by a national, regional, or investigational Independent Ethics Committee or Institutional Review Board. The trial is registered at ClinicalTrials.gov (NCT04869982).
Study vaccine and placebo composition, as well as administration mode were similar to what has been described previously.7,8 Placebo consisted of lyophilized sucrose (20 mg) reconstituted in 0.5 mL of saline (NaCl; 150 mM) solution.
Outcomes and assessments
The primary objective of this study was to evaluate the VE of RZV compared to placebo in ≥ 50-year-old adults from China, as measured by the reduction in HZ risk (Table S1).
Secondary objectives were to evaluate the VE of RZV compared to placebo in participants within each of the following age categories: 50–69 years and ≥ 70 years, descriptively, and to evaluate the reactogenicity and safety profile of the vaccine (Table S1).
The primary efficacy endpoint was assessed from 30 days post-second vaccination until study end.
The definition of suspected HZ was the same as in the ZOE-50 and ZOE-70 studies.7,8 In case of a suspected HZ episode, clinical specimens of HZ lesions and complete HZ-specific diary cards were collected from each participant; additional visits and contacts for the follow-up of HZ were conducted. All suspected HZ cases were referred to the HZ Ascertainment Committee (HZAC), located in China and consisting of 3–5 physicians with HZ expertise that were blinded to group assignments. The HZAC were to classify all referred cases as either “HZ”, “not HZ”, or “not able to decide”. The suspected HZ cases had to be confirmed by polymerase chain reaction (PCR),7,8 and if PCR results were unavailable or cases could not be confirmed or excluded by PCR, the HZAC classification served as the final case definition. Suspected cases were confirmed if HZAC members concurred unanimously.
Diary cards were provided to each participant at every vaccination visit to record axillary body temperature, any solicited local (pain, redness, and swelling at injection site) and general (fatigue, fever, gastrointestinal symptoms [nausea, vomiting, diarrhea, abdominal pain], headache, myalgia, and shivering) adverse events (AEs) for 7 days after each vaccination as well as unsolicited AEs for 30 days post any vaccination. Solicited AEs were graded on a scale from 0 (absent) to 3 (preventing normal everyday activities), based on the US Food and Drug Administration (FDA) toxicity grading scale for vaccine clinical trials.20 Redness and swelling at the injection site were scored according to the diameter of the affected area; grade 3 was defined as having an affected area of > 100 mm in diameter.20 The grading of the intensity of solicited local and general AEs was also conducted using a grading scale used in China (NMPA Guidelines for Grading Criteria for Adverse Events in Clinical Trials of Preventive Vaccines) (Table S2). Unsolicited AEs were classified according to the Medical Dictionary for Regulatory Activities (MedDRA). Serious AEs (SAEs) and potential immune-mediated diseases (pIMDs) were evaluated within 30 days and 12 months post-last vaccination. All SAEs and pIMDs considered related to the study vaccine and participation as well as pregnancies were recorded throughout the entire study period.
Statistical analyses
Approximately 6138 eligible participants were planned to be enrolled in this study, 3069 in each study group. The sample size of the study was estimated to provide at least 90% power for the VE confirmatory objective on the overall population, aged 50 years or older. Sample size calculations were based on the assumption of a true conservative VE of 85% and targeted a minimum number of 27 HZ cases during the 12 months of follow-up post-last vaccination. Randomization of participants in each study group was done using a web-based randomization system that stratified participants by age (50–69 years of age and ≥ 70 years of age) and used a minimization procedure accounting for age (i.e., 50–59 and 60–69 years of age for the 50–69 years of age stratum, and 70–79 and ≥ 80 years of age for the ≥ 70 years of age stratum), center, gender, and preexisting medical conditions (including type 2 diabetes mellitus, asthma, chronic obstructive pulmonary disease, chronic kidney disease, and depression) to ensure recruitment of a broad age range of adults, representative of the population included in the RZV label in China.
The primary inferential and secondary analyses of efficacy were conducted on the modified exposed set (mES, including all participants who received two doses of study treatment as per protocol without developing any confirmed HZ prior to 30 days after the second dose) and complementary on the exposed set (ES, including all participants who received at least one dose of study treatment). The VE on the mES was calculated based on the confirmed HZ cases that have occurred anytime from 30 days after the administration of the second dose of the study vaccine up to study end, overall and within each of the age categories (50–69 years, ≥ 70 years). The primary efficacy objective for the overall VE was met if the lower limit of the two-sided 95% confidence interval (CI) of VE was > 25%. All p-values reported were related to the null hypothesis test VE = 0.
The primary analysis of efficacy considered the exact inference on the relative risk (RR) adjusted for age strata conditionally to the total number of confirmed HZ cases observed and time at risk. This method computed an exact CI around the rate ratio (i.e., ratio of the event rates in the RZV vs placebo group) and accounted for the sum of the time at risk of the participants within each group.
Incidence rate (IR) and VE with 95% CI were calculated using Poisson method. VE (Equation 1) and RR (Equation 2) are defined as:
| (1) |
| (2) |
For the secondary analysis of efficacy, the number of confirmed HZ cases, follow-up days, associated rate, and descriptive VE with 95% CIs were presented by age category.
Reactogenicity and safety data were evaluated using descriptive analyses, overall and by age category, in the ES.
Results
Study participants
Of the 6138 participants enrolled into the study, 6128 (99.8%) were included in the ES, of whom 5956 (97.2%) were included in the mES. In total, 6088 participants of the enrolled set (99.2%) completed the study. The main reasons for study discontinuation were AEs requiring expedited reporting (n = 24 participants, 0.4%) and consent withdrawal not due to a (S)AE (n = 9 participants, 0.1%) (Figure 1).
All participants were of Asian/East Asian heritage. The majority of participants in the mES were females (RZV: 60.8%; placebo: 61.2%), and more than one-fifth of the participants in the two groups were aged 70 years or older (RZV: 21.4%; placebo: 21.1%) (Table 1). Similar demographic characteristics were found in the ES (Table S3).
Table 1.
Characteristics of the participants at baseline (modified exposed set).
| Characteristic | RZV group N = 2965 |
Placebo group N = 2991 |
Total N = 5956 |
|---|---|---|---|
| Age (years) at first vaccination | |||
| Mean (±SD) | 62.4 (±7.8) | 62.4 (±7.8) | 62.4 (±7.8) |
| Sex, n (%) | |||
| Male | 1163 (39.2) | 1160 (38.8) | 2323 (39.0) |
| Female | 1802 (60.8) | 1831 (61.2) | 3633 (61.0) |
| Geographic ancestry, n (%) | |||
| Asian/East Asian heritage | 2965 (100) | 2991 (100) | 5956 (100) |
| Age strata, n (%) | |||
| 50–69 years of age | 2330 (78.6) | 2360 (78.9) | 4690 (78.7) |
| ≥ 70 years of age | 635 (21.4) | 631 (21.1) | 1266 (21.3) |
RZV group, participants receiving the adjuvanted recombinant zoster vaccine; placebo group, participants receiving placebo; N, total number of participants; SD, standard deviation; n (%), number (and percentage) of participants in each category.
Efficacy results
In the mES, 39 suspected HZ episodes (five in RZV group and 34 in placebo group) were reported from first vaccination up to study end. Of these, 31 HZ episodes were confirmed either by PCR or by HZAC after an overall mean follow-up period of 15.2 (±1.1) months: none in RZV recipients and 31 in placebo recipients. A PCR sample was not collected for one suspected HZ case in the placebo group of the mES. The case was not confirmed since the HZAC members did not reach a unanimous decision. The overall incidence of HZ per 1000 person-years was 0.0 in the RZV group and 8.2 in the placebo group, which resulted in an overall VE against HZ of 100% (95% CI: 89.82–100), p < .0001 (Table 2). The descriptive VE was 100% (95% CI: 85.29–100) and 100% (95% CI: 60.90–100) among participants 50–69 years of age and participants ≥ 70 years of age, respectively (Table 2).
Table 2.
Vaccine efficacy against the first or only episode of herpes zoster infection, from 30 days post-second vaccination up to study end (modified exposed set).
| RZV group |
Placebo group |
VE |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type | N | n | T (years) | IR (per 1000 person-years) | N | n | T (years) | IR (per 1000 person-years) | % (95% CI) | p-value |
| Overall* | 2965 | 0 | 3752.3 | 0.0 | 2991 | 31 | 3769.0 | 8.2 | 100 (89.82–100) | <.0001 |
| 50–69 years | 2330 | 0 | 2957.0 | 0.0 | 2360 | 22 | 2981.8 | 7.4 | 100 (85.29–100) | |
| ≥ 70 years | 635 | 0 | 795.2 | 0.0 | 631 | 9 | 787.2 | 11.4 | 100 (60.90–100) | |
*VE adjusted by age strata.
Note: the follow-up for each participant started (start date) 30 days after the second dose was administered and ended (stop date) at the time a herpes zoster episode was confirmed for the participant. For those participants without a confirmed herpes zoster episode, the follow-up ended at the last visit; at the last contact date for participants who withdrew from the study and did not have a confirmed herpes zoster episode; or on the date of the herpes zoster and/or varicella zoster virus vaccination that occurred outside of the study, if not preceded by an event.
T (years) is the sum of follow-up time at risk expressed in years for confirmed herpes zoster of all participants in the modified exposed set. The follow-up time at risk for confirmed herpes zoster per participant, expressed in days, is computed using the following formula: .
RZV group, participants receiving the adjuvanted recombinant zoster vaccine; placebo group, participants receiving placebo; VE, vaccine efficacy; N, number of participants included in each group; n, number of participants having at least one confirmed herpes zoster episode; T, sum of follow-up period; IR, incidence rate; CI, confidence interval.
In the ES, 52 suspected HZ episodes (eight in RZV group and 44 in placebo group) were reported from first vaccination up to study end. Of these, 42 HZ episodes were confirmed either by PCR or by HZAC after an overall mean follow-up period of 18.0 (±1.6) months: two in RZV recipients and 40 in placebo recipients. The overall incidence of HZ per 1000 person-years was 0.4 in the RZV group and 8.7 in the placebo group, resulting in an overall VE of 95.03% (95% CI: 80.82–99.42) (Table S4). The descriptive VE was 93.13% (95% CI: 72.85–99.21) and 100% (95% CI: 68.97–100) among participants 50–69 years of age and participants ≥ 70 years of age, respectively (Table S4).
Safety and Reactogenicity
According to the US FDA grading scale, solicited AEs occurring within the 7-days post-vaccination period were more frequent in the RZV (76.3%) group than in the placebo (14.4%) group (Table 3). Incidence of any solicited AEs in the RZV group was slightly higher in 50–69 years of age category (79.7%) than in the ≥ 70 years of age category (63.4%) (Table 3). Median duration of solicited AEs (local and general) ranged between 1 and 3 days for the two groups. For grade 3 solicited AEs, median duration ranged between 1 and 2 days in the RZV group and between 1 and 3 days in the placebo group.
Table 3.
Safety and reactogenicity (exposed set).
| RZV group |
Placebo group |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall |
50–69 years |
≥70 years |
Overall |
50–69 years |
≥70 years |
|||||||
| n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | |
| Seven days post any vaccination | ||||||||||||
| N | 3054 | 2409 | 645 | 3058 | 2411 | 647 | ||||||
| Solicited AEs | 2329 | 76.3 (74.7–77.8) | 1920 | 79.7 (78.0–81.3) | 409 | 63.4 (59.6–67.1) | 439 | 14.4 (13.1–15.6) | 360 | 14.9 (13.5–16.4) | 79 | 12.2 (9.8–15.0) |
| Solicited local AEs | 2230 | 73.0 (71.4–74.6) | 1847 | 76.7 (74.9–78.3) | 383 | 59.4 (55.5–63.2) | 283 | 9.3 (8.3–10.3) | 236 | 9.8 (8.6–11.0) | 47 | 7.3 (5.4–9.5) |
| Solicited general AEs | 1675 | 54.8 (53.1–56.6) | 1440 | 59.8 (57.8–61.7) | 235 | 36.4 (32.7–40.3) | 261 | 8.5 (7.6–9.6) | 214 | 8.9 (7.8–10.1) | 47 | 7.3 (5.4–9.5) |
| 30 days post any vaccination | ||||||||||||
| N | 3064 | 2415 | 649 | 3064 | 2415 | 649 | ||||||
| Unsolicited AEs | 124 | 4.0 (3.4–4.8) | 96 | 4.0 (3.2–4.8) | 28 | 4.3 (2.9–6.2) | 97 | 3.2 (2.6–3.8) | 73 | 3.0 (2.4–3.8) | 24 | 3.7 (2.4–5.5) |
| Grade 3 | 8 | 0.3 (0.1–0.5) | 4 | 0.2 (0.0–0.4) | 4 | 0.6 (0.2–1.6) | 10 | 0.3 (0.2–0.6) | 7 | 0.3 (0.1–0.6) | 3 | 0.5 (0.1–1.3) |
| Medically attended | 64 | 2.1 (1.6–2.7) | 49 | 2.0 (1.5–2.7) | 15 | 2.3 (1.3–3.8) | 53 | 1.7 (1.3–2.3) | 37 | 1.5 (1.1–2.1) | 16 | 2.5 (1.4–4.0) |
| First vaccination up to 30 days post-last vaccination | ||||||||||||
| SAEs | 32 | 1.0 (0.7–1.5) | 20 | 0.8 (0.5–1.3) | 12 | 1.8 (1.0–3.2) | 36 | 1.2 (0.8–1.6) | 23 | 1.0 (0.6–1.4) | 13 | 2.0 (1.1–3.4) |
| Related SAEs | 0 | 0.0 (0.0–0.1) | 0 | 0.0 (0.0–0.2) | 0 | 0.0 (0.0–0.6) | 1 | 0.0 (0.0–0.2) | 1 | 0.0 (0.0–0.2) | 0 | 0.0 (0.0–0.6) |
| Deathsa | 3 | 0.1 (0.0–0.3) | 2 | 0.1 (0.0–0.3) | 1 | 0.2 (0.0–0.9) | 2 | 0.1 (0.0–0.2) | 1 | 0.0 (0.0–0.2) | 1 | 0.2 (0.0–0.9) |
| pIMDs | 2 | 0.1 (0.0–0.2) | 2 | 0.1 (0.0–0.3) | 0 | 0.0 (0.0–0.6) | 2 | 0.1 (0.0–0.2) | 2 | 0.1 (0.0–0.3) | 0 | 0.0 (0.0–0.6) |
| First vaccination up to 12 months post-last vaccination | ||||||||||||
| N | 3064 | 2415 | 649 | 3064 | 2415 | 649 | ||||||
| SAEs | 88 | 2.9 (2.3–3.5) | 55 | 2.3 (1.7–3.0) | 33 | 5.1 (3.5–7.1) | 93 | 3.0 (2.5–3.7) | 62 | 2.6 (2.0–3.3) | 31 | 4.8 (3.3–6.7) |
| Related SAEs | 0 | 0.0 (0.0–0.1) | 0 | 0.0 (0.0–0.2) | 0 | 0.0 (0.0–0.6) | 1 | 0.0 (0.0–0.2) | 1 | 0.0 (0.0–0.2) | 0 | 0.0 (0.0–0.6) |
| Deathsb | 11 | 0.4 (0.2–0.6) | 7 | 0.3 (0.1–0.6) | 4 | 0.6 (0.2–1.6) | 9 | 0.3 (0.1–0.6) | 5 | 0.2 (0.1–0.5) | 4 | 0.6 (0.2–1.6) |
| pIMDs | 2 | 0.1 (0.0–0.2) | 2 | 0.1 (0.0–0.3) | 0 | 0.0 (0.0–0.6) | 3 | 0.1 (0.0–0.3) | 3 | 0.1 (0.0–0.4) | 0 | 0.0 (0.0–0.6) |
aFatal AEs were as follows: gastrointestinal perforation (one in the placebo group); sudden death (one in RZV group); craniocerebral injury (one in RZV and one in placebo groups); hemorrhage intracranial (one in RZV group). No death was causally related to the vaccine, as assessed by the investigator and company.
bNo death was causally related to the vaccine, as assessed by the investigator and company.
Note: solicited AEs were ascertained only for participants who completed and returned the diary cards after any vaccination visit, whereas unsolicited AEs, medically attended AEs, SAEs, pIMDs, and deaths were assessed for all participants in the exposed set, independent of the status of the diary cards.
RZV group, participants receiving the adjuvanted recombinant zoster vaccine; placebo group, participants receiving placebo; n (%), number (percentage) of participants in each category; CI, confidence interval; N, total number of participants; AE, adverse event; SAE, serious adverse event; pIMD, potential immune-mediated disease.
Solicited local AEs occurred in 2230 (73.0%) of RZV recipients and 283 (9.3%) of placebo recipients. Pain was the most frequent solicited local AE, reported by 72.1% of participants in the RZV and 9.2% of participants in the placebo group. Grade 3 pain was reported by 3.4% RZV and 0.1% placebo recipients. Medically attended pain was reported by 0.9% of participants in the RZV and 0.0% of participants in the placebo group (Figure 2).
Figure 2.
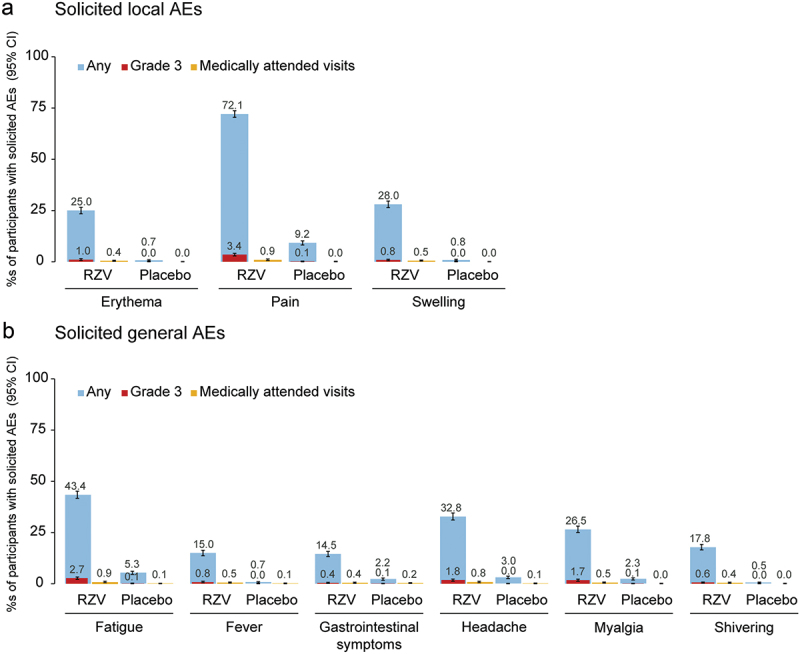
Frequency of solicited local (a) and general (b) adverse events occurring within 7 days after any vaccination (exposed set).
Fever is defined as axillary temperature ≥ 38.0°C.
Grade 3, diameter > 100 mm (erythema and swelling), temperature > 39.0°C (fever), prevents normal everyday activities (all the other events).
AE, adverse event; RZV group, participants receiving the adjuvanted recombinant zoster vaccine; placebo group, participants receiving placebo; CI, confidence interval.
Solicited general AEs occurred in 1675 (54.8%) of RZV and 261 (8.5%) of placebo recipients. The most frequent solicited general AEs were fatigue (RZV: 43.4%; placebo: 5.3%) and headache (RZV: 32.8%; placebo: 3.0%). Grade 3 fatigue and headache were reported by 2.7% and 1.8% RZV, and 0.1% and 0.0% placebo recipients, respectively. Fatigue and headache requiring medical attendance were reported by 0.9% and 0.8% RZV, and 0.1% and 0.1% placebo recipients, respectively (Figure 2).
The incidence of solicited AEs (local and general) classified according to the NMPA Chinese grading scale is presented in Table S5. Pain was the most frequent solicited local AE (RZV: 72.1%; placebo: 9.2%), while fatigue (RZV: 43.4%; placebo: 5.3%) and fever (axillary temperature ≥ 37.3°C; RZV: 36.4%; placebo: 1.6%) were the most frequent solicited general AEs.
Within the 30 days post-vaccination period, at least one unsolicited AE was reported by 124 (4.0%) of RZV recipients and 97 (3.2%) of placebo recipients. Grade 3 unsolicited AEs were reported by eight (0.3%) of RZV recipients and 10 (0.3%) of placebo recipients (Table 3). The most frequently reported unsolicited AEs were dizziness (RZV: 0.8%; placebo: 0.3%) and nasopharyngitis (RZV: 0.3%; placebo: 0.2%).
From first vaccination up to 30 days post-last vaccination, at least one SAE occurred in 32 (1.0%) of participants in the RZV and 36 (1.2%) participants in the placebo group and at least one pIMD occurred in two (0.1%) participants in each group (Table 3). From first vaccination up to 12 months post-last vaccination, SAEs occurred in 88 (2.9%) of RZV recipients and 93 (3.0%) of placebo recipients, and pIMDs occurred in two (0.1%) participants in the RZV and three (0.1%) participants in the placebo group (Table 3). None were considered related to RZV vaccination. Overall, 11 participants in the RZV group and nine in the placebo group died during the follow-up period (Table 3). None of the reported deaths occurring at any time during the study were assessed by the investigator as causally related to vaccination. Finally, no pregnancy occurred during the study.
Discussion
This is the first randomized, placebo-controlled study that investigates the efficacy, reactogenicity, and safety of RZV in adults 50 years and older in China. Overall, the efficacy, reactogenicity, and safety results were similar to the ones from the pivotal multi-country phase III studies, ZOE-50 and ZOE-70, which supported RZV licensure in several countries worldwide, including China.7–9 These pivotal studies have demonstrated that RZV reduced the incidence of HZ by 97.2% in ≥ 50-year-old adults, and by 91.3% in ≥ 70-year-old adults.7,8
In the current study, point estimates for the descriptive VE against HZ were consistently high in both the mES and the ES among adults 50–69 years of age (mES: 100%; ES: 93.1%) and ≥ 70 years of age (mES: 100%; ES: 100%). Descriptive results showing 100% VE in the ≥ 70-year-olds match the conclusions of the ZOE-50 study,7 but neither the current study nor the ZOE-50 trial were powered for this age group. Analysis of pooled data among adults ≥ 70 years of age from ZOE-50 and ZOE-70 studies suggest that a VE slightly lower than 100% might be expected for this age group.8 Among those participants who completed the second vaccination visit of the current study, no HZ case was confirmed in any RZV recipients. Taken together, the findings from the current study and the ZOE-50/70 trials show that VE remained high regardless of age, thereby suggesting that RZV can provide protection against HZ to an immunosenescent population with greatest medical need.3,7,8
Several considerations should be taken into account before interpreting VE data with regard to the actual benefits provided to the intended population in China. First, the 100% VE observed in the mES does not imply that the vaccine is able to elicit a persisting and complete protection against HZ. The study met the primary objective as the lower limit of the two-sided 95% CI of VE was higher than 25%. The interim results of an extension study of ZOE-50 and ZOE-70 trials demonstrated that the VE of RZV against HZ remained > 84% up to 8-year post-vaccination, all age groups included.21 Despite minimal waning, consistently high levels of RZV-conferred protection against HZ could also be expected in the Chinese population up to several years after vaccination. Second, this event-driven study required less confirmed HZ cases to test the VE hypotheses compared the ZOE-50 study7 and explains the differences in follow-up time to achieve the required number of cases to trigger the final analysis (15.2 months vs 38.4 months).7 Third, since protection against PHN can be primarily driven by the vaccine-related reduction in HZ incidence, RZV could display high VE against both HZ and PHN in the Chinese population. Although the VE against PHN was not estimated in the current study, we can expect similar results to those from the ZOE-70 study, showing an 88.8% VE against PHN in adults aged 70 years and older.8
RZV did not raise any safety concerns after intramuscular administration on a two-dose schedule in ≥ 50-year-old adults from China. The frequency, severity, and duration of solicited local and general AEs were consistent with the known reactogenicity profile of the vaccine.7–9 The most common solicited AE was pain at the injection site, and the higher incidence of solicited AEs in the RZV group and among participants 50–69 years of age is in line with the known reactogenicity profile of the vaccine in adults worldwide.7–9 Analyses using the Chinese intensity measurement scale provided similar conclusions about incidence, grading, and duration of solicited local AEs, except for fever. Compared to the US FDA guidelines defining mild fever as 38.0–38.4°C,20 the NMPA Chinese grading scale uses a lower threshold to define fever, i.e., axillary temperature ≥ 37.3°C (Table S2), which might explain the higher frequency of this solicited general AE.
Overall, there were no apparent differences between the RZV and the placebo group for the frequency of unsolicited AEs during the 30-day post-vaccination period. Dizziness was the most frequently reported event irrespective of the study group. This unsolicited AE might be occurring secondary to reactogenicity symptoms. The frequency of SAEs and pIMDs was balanced between the RZV and the placebo group, also in line with the safety outcomes from ZOE-50 and ZOE-70 studies,7–9 and none were considered as causally related to RZV vaccination.
The large, enrolled cohort and the high retention rate of the participants during the entire study period are considered strengths of this study. Even though the study was conducted over two years, during the coronavirus disease 2019 (COVID-19) pandemic, few people (2.8%) missed their second vaccination visit and even fewer dropped out during the study; 99.2% of the enrolled participants completed the study. Another study strength was the HZ case ascertainment methodology, based on the protocols from ZOE-50 and ZOE-70 trials. In the current study, all but one suspected HZ cases had available PCR samples and they were therefore reliably identified upon PCR diagnosis, ensuring no episodes were potentially missed throughout the study period.
The study has several limitations. Individuals with significant underlying illness and immunosuppressive conditions were excluded from the study, limiting the generalizability of our findings. Furthermore, excluding participants with medical conditions that in the opinion of the investigator might have interfered with the study evaluations could be considered a limitation, as it might contain subjective elements. However, the same exclusion criterion was used in other studies as well, such as the pivotal ZOE-50/70 trials,7,8 therefore, we expect no influence on the results of the current trial. Also, the current study was partly limited by the COVID-19 pandemic. The allowed interval between visits was extended during the COVID-19 pandemic and the associated lockdown periods, permitting participants with out-of-window visits to be included in the analyses. One monthly phone contact was missed or delayed due to the COVID-19 pandemic and the associated lockdown policies for 101 participants. However, this protocol deviation likely had no bearing on VE primary analysis performed on the mES, which included all participants receiving two doses of study treatment, regardless of vaccination window. In addition, extension of vaccination visits within schedule is included in the RZV label. Finally, extensive contact was maintained with participants despite the COVID-19 pandemic, and there were no missed HZ cases identified since all but one had available PCR results.
In conclusion, this phase IV study has demonstrated that RZV is highly efficacious in preventing HZ in Chinese adults 50 years and older, regardless of age, while having a clinically acceptable reactogenicity and safety profile. These findings are consistent with results in other geographical populations with similar age and medical characteristics. Prevention of HZ is a health priority in China as part of the “Healthy China 2030” initiative, which ensures improved access to healthcare for the Chinese population.22 Vaccine hesitancy continues to impede the efficacy of adult vaccination programs, which was seen most recently in China with the introduction of COVID-19 vaccines.23 The results of this study might support the evaluation of the benefit-risk profile of vaccine administration, help alleviate the vaccine hesitancy among the Chinese population, and be informative for Chinese healthcare professionals when recommending vaccination.
Supplementary Material
Acknowledgments
Authors thank Dan Lin, Valerie Massem, Celine Boutry, Valerie Sengers, Vidya Mallalli, Sonakshi Shankar, Phelix Ojwang, Madhura Saralaya, and Andrew Hastie for their support. Authors also thank Akkodis Belgium for editorial assistance and manuscript coordination, on behalf of GSK; Sara-Teodora Vulcu provided medical writing support.
Funding Statement
This work was supported by GlaxoSmithKline Biologicals SA. GlaxoSmithKline Biologicals SA covered all costs associated with the development and publishing of the present manuscript.
Contributor Information
Collaborators: Huang Zhuoying, Guo Xiang, Li Zhi, Jiang Yonggen, Lu Hongmei, Zhu Qi, He Lu, Du Jiaxi, Li Jia, Gao Qiang, Xu Zhenhui, Wang Jing, Cai Enmao, Pang Hong, Chen Jinyan, Jin Pengfei, Liu Sheng, Zhong Shan, Zhu Jiahong, and Song Zhizhou
Disclosure statement
J.Z., J.S., N.P., and S.O.A. are/were employees of GSK at the time the study was designed, initiated, conducted, and/or completed. J.Z., J.S., N.P. and S.O.A. received stock options from GSK as part of their employee remuneration. D.A.E.P. was contracted by GSK from Aixial-Alten company to serve as a clinical lead for the study. X.S. and F.Z. have nothing to declare. All authors have no other financial and non-financial interests to declare.
Author contributions
D.A.E.P. was involved in the study concept or design. F.Z. and X.S. participated in the data acquisition. D.A.E.P., J.Z., J.S., N.P., and S.O.A. performed the data analysis. D.A.E.P., F.Z., X.S., J.Z., J.S., N.P., and S.O.A. interpretated the data.
Data availability
The data that support the findings of this study are available from the corresponding author, S.O.A, upon reasonable request.
Trademarks
Shingrix and AS01 are trademarks owned by or licensed to the GSK group of companies.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2351584
References
- 1.Centers for Disease Control and Prevention . Shingles (herpes zoster): clinical overview. 2023. [accessed 2023 Aug 18]. https://www.cdc.gov/shingles/hcp/clinical-overview.html.
- 2.Kawai K, Gebremeskel BG, Acosta CJ.. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4(6):e004833. doi: 10.1136/bmjopen-2014-004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lecrenier N, Beukelaers P, Colindres R, Curran D, De Kesel C, De Saegher JP, Didierlaurent AM, Ledent EY, Mols JF, Mrkvan T, et al. Development of adjuvanted recombinant zoster vaccine and its implications for shingles prevention. Expert Rev Vaccines. 2018;17(7):619–10. doi: 10.1080/14760584.2018.1495565. [DOI] [PubMed] [Google Scholar]
- 4.Leroux-Roels I, Leroux-Roels G, Clement F, Vandepapeliere P, Vassilev V, Ledent E, Heineman TC.. A phase 1/2 clinical trial evaluating safety and immunogenicity of a varicella zoster glycoprotein E subunit vaccine candidate in young and older adults. J Infect Dis. 2012;206(8):1280–90. doi: 10.1093/infdis/jis497. [DOI] [PubMed] [Google Scholar]
- 5.Chlibek R, Bayas JM, Collins H, de la Pinta ML, Ledent E, Mols JF, Heineman TC. Safety and immunogenicity of an AS01-adjuvanted varicella-zoster virus subunit candidate vaccine against herpes zoster in adults ≥ 50 years of age. J Infect Dis. 2013;208(12):1953–61. doi: 10.1093/infdis/jit365. [DOI] [PubMed] [Google Scholar]
- 6.Chlibek R, Smetana J, Pauksens K, Rombo L, Van den Hoek JA, Richardus JH, Plassmann G, Schwarz TF, Ledent E, Heineman TC. Safety and immunogenicity of three different formulations of an adjuvanted varicella-zoster virus subunit candidate vaccine in older adults: a phase II, randomized, controlled study. Vaccine. 2014;32(15):1745–53. doi: 10.1016/j.vaccine.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 7.Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ, Levin MJ, McElhaney JE, Poder A, Puig-Barberà J, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087–96. doi: 10.1056/NEJMoa1501184. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang SJ, Díez-Domingo J, Godeaux O, Levin MJ, McElhaney JE, Puig-Barberà J, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375(11):1019–32. doi: 10.1056/NEJMoa1603800. [DOI] [PubMed] [Google Scholar]
- 9.López-Fauqued M, Campora L, Delannois F, El Idrissi M, Oostvogels L, De Looze FJ, Diez-Domingo J, Heineman TC, Lal H, McElhaney JE, et al. Safety profile of the adjuvanted recombinant zoster vaccine: pooled analysis of two large randomised phase 3 trials. Vaccine. 2019;37(18):2482–93. doi: 10.1016/j.vaccine.2019.03.043. [DOI] [PubMed] [Google Scholar]
- 10.Kim JH, Diaz-Decaro J, Jiang N, Hwang SJ, Choo EJ, Co M, Hastie A, Hui DSC, Irimajiri J, Lee J, et al. The adjuvanted recombinant zoster vaccine is efficacious and safe in Asian adults ≥ 50 years of age: a sub-cohort analysis of the ZOE-50 and ZOE-70 randomized trials. Hum Vaccin Immunother. 2021;17(7):2050–7. doi: 10.1080/21645515.2020.1859321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willer DO, Oostvogels L, Cunningham AL, Gervais P, Gorfinkel I, Hyung Kim J, Talarico C, Wascotte V, Zahaf T, Colindres R, et al. Efficacy of the adjuvanted recombinant zoster vaccine (RZV) by sex, geographic region, and geographic ancestry/ethnicity: a post-hoc analysis of the ZOE-50 and ZOE-70 randomized trials. Vaccine. 2019;37(43):6262–7. doi: 10.1016/j.vaccine.2019.09.028. [DOI] [PubMed] [Google Scholar]
- 12.Sun Y, Jackson K, Dalmon CA, Shapiro BL, Nie S, Wong C, Arnold BF, Porco TC, Acharya NR. Effectiveness of the recombinant zoster vaccine among Kaiser permanente Hawaii enrollees aged 50 and older: a retrospective cohort study. Vaccine. 2021;39(29):3974–82. doi: 10.1016/j.vaccine.2021.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Y, Kim E, Kong CL, Arnold BF, Porco TC, Acharya NR. Effectiveness of the recombinant zoster vaccine in adults aged 50 and older in the United States: a claims-based cohort study. Clin Infect Dis. 2021;73(6):949–56. doi: 10.1093/cid/ciab121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izurieta HS, Wu X, Forshee R, Lu Y, Sung H-M, Agger PE, Chillarige Y, Link-Gelles R, Lufkin B, Wernecke M, et al. Recombinant zoster vaccine (shingrix): real-world effectiveness in the first 2 years post-licensure. Clin Infect Dis. 2021;73(6):941–8. doi: 10.1093/cid/ciab125. [DOI] [PubMed] [Google Scholar]
- 15.Parikh R, Singer D, Chmielewski-Yee E, Dessart C. Effectiveness and safety of recombinant zoster vaccine: a review of real-world evidence. Hum Vaccin Immunother. 2023;19(3):2263979. doi: 10.1080/21645515.2023.2263979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parikh R, Widenmaier R, Lecrenier N. A practitioner’s guide to the recombinant zoster vaccine: review of national vaccination recommendations. Expert Rev Vaccines. 2021;20(9):1065–75. doi: 10.1080/14760584.2021.1956906. [DOI] [PubMed] [Google Scholar]
- 17.GSK . GSK announces approval of shingrix in China for prevention of shingles in adults aged 50 and over. 2019. [accessed 2023 Aug 17]. https://www.gsk.com/en-gb/media/press-releases/gsk-announces-approval-of-shingrix-in-china-for-prevention-of-shingles-in-adults-aged-50-and-over/.
- 18.Lee C, Jiang N, Tang H, Ye C, Yuan Y, Curran D. Potential public health impact of the adjuvanted recombinant zoster vaccine among people aged 50 years and older in Beijing. Hum Vaccin Immunother. 2021;17(10):3735–46. doi: 10.1080/21645515.2021.1932216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin D, Van Oorschot D, Jiang N, Marijam A, Saha D, Wu Z, Tang H, Diaz-Decaro J, Watson P, Xie X, et al. A systematic literature review to assess the burden of herpes zoster disease in China. Expert Rev Anti Infect Ther. 2021;19(2):165–79. doi: 10.1080/14787210.2020.1792290. [DOI] [PubMed] [Google Scholar]
- 20.U.S. Food and Drug Administration, U.S. Department of Health and Human Services, Center for Biologics Evaluation and Research . Guidance for industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. 2007. [accessed 2023 Aug 17]. https://www.fda.gov/media/73679/download.
- 21.Boutry C, Hastie A, Diez-Domingo J, Tinoco JC, Yu CJ, Andrews C, Beytout J, Caso C, Cheng HS, Cheong HJ, et al. The adjuvanted recombinant zoster vaccine confers long-term protection against herpes zoster: interim results of an extension study of the pivotal phase 3 clinical trials ZOE-50 and ZOE-70. Clin Infect Dis. 2022;74(8):1459–67. doi: 10.1093/cid/ciab629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan X, Zhang Y, Shao H. Healthy China 2030, a breakthrough for improving health. Glob Health Promot. 2019;26(4):96–9. doi: 10.1177/1757975917743533. [DOI] [PubMed] [Google Scholar]
- 23.Wang G, Yao Y, Wang Y, Gong J, Meng Q, Wang H, Wang W, Chen X, Zhao Y. Determinants of COVID-19 vaccination status and hesitancy among older adults in China. Nat Med. 2023;29(3):623–31. doi: 10.1038/s41591-023-02241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, S.O.A, upon reasonable request.


