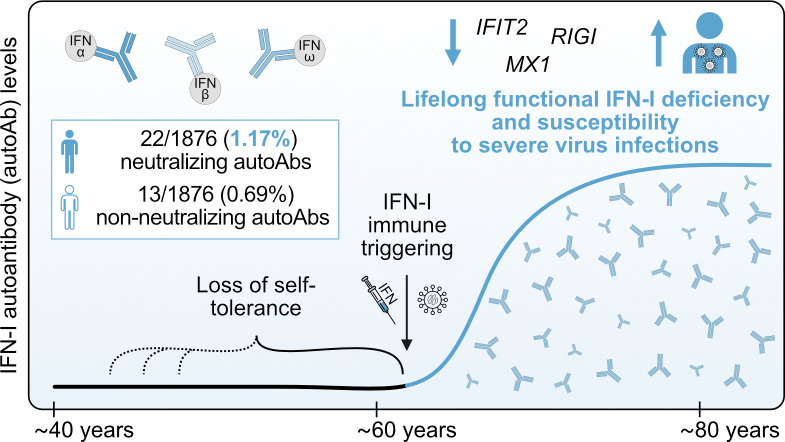
Rare autoantibodies neutralizing type I IFNs can underlie viral disease severity. Here, the authors dissect immunological and age-related factors associated with anti-IFN-I autoantibody development in individuals over a 35-year period and further reveal the lifelong infection susceptibility that results.
Abstract
Autoantibodies neutralizing type I interferons (IFN-Is) can underlie infection severity. Here, we trace the development of these autoantibodies at high-resolution using longitudinal samples from 1,876 well-treated individuals living with HIV over a 35-year period. Similar to general populations, ∼1.9% of individuals acquired anti-IFN-I autoantibodies as they aged (median onset ∼63 years). Once detected, anti-IFN-I autoantibodies persisted lifelong, and titers increased over decades. Individuals developed distinct neutralizing and non-neutralizing autoantibody repertoires at discrete times that selectively targeted combinations of IFNα, IFNβ, and IFNω. Emergence of neutralizing anti-IFNα autoantibodies correlated with reduced baseline IFN-stimulated gene levels and was associated with subsequent susceptibility to severe COVID-19 several years later. Retrospective measurements revealed enrichment of pre-existing autoreactivity against other autoantigens in individuals who later developed anti-IFN-I autoantibodies, and there was evidence for prior viral infections or increased IFN at the time of anti-IFN-I autoantibody triggering. These analyses suggest that age-related loss of self-tolerance prior to IFN-I immune-triggering poses a risk of developing lifelong functional IFN-I deficiency.
Graphical Abstract
Introduction
Deficiencies in the human type I interferon (IFN-I) system leave individuals susceptible to a range of severe viral diseases typically caused by pathogens to which they lack pre-existing humoral immunity (reviewed in Duncan et al. [2021]; Meyts and Casanova [2021]; Stertz and Hale [2021]). While life-threatening viral diseases linked directly to genetic defects in the IFN-I system are extremely rare (and mainly manifest themselves in the young) (Duncan et al., 2021), it has recently become apparent from cross-sectional studies that a functional defect caused by autoantibodies (autoAbs) targeting IFN-I cytokines is not rare in the elderly (reviewed in Bastard et al. [2024a]; Hale [2023]). Specifically, the prevalence of autoAbs neutralizing the IFN-I cytokines IFNα and/or IFNω increases sharply with age in apparently healthy individuals, such that a conservative estimate of prevalence in those >70 years old is about eight times higher (1.4%) than that in younger individuals (0.17%) (Bastard et al., 2021). Furthermore, the presence of neutralizing anti-IFN-I autoAbs in individuals has been associated with an increased susceptibility to severe infections caused by several viral pathogens, including SARS-CoV-2 (Akbil et al., 2022; Bastard et al., 2020, 2024b; Busnadiego et al., 2022; Chauvineau-Grenier et al., 2022; Credle et al., 2022; Eto et al., 2022; Frasca et al., 2022; Goncalves et al., 2021; Manry et al., 2022; Mathian et al., 2022; Scordio et al., 2022; Solanich et al., 2021; Troya et al., 2021; Wang et al., 2021), MERS-CoV (Alotaibi et al., 2023), influenza A virus (Zhang et al., 2022), West Nile virus (Gervais et al., 2023; Lin et al., 2023), and various herpesviruses (Bayat et al., 2015; Burbelo et al., 2010; Busnadiego et al., 2022; Hetemaki et al., 2021; Mathian et al., 2022; Mogensen et al., 1981; Pozzetto et al., 1984). Given that >100 million people worldwide have been estimated to harbor neutralizing anti-IFN-I autoAbs (Bastard et al., 2024a) and are therefore at increased risk of severe infectious disease outcomes, it is critical to understand factors associated with their development and pathogenic mechanisms to inform future mitigation strategies.
Multiple distinct host genetic defects converging on disruption of central T cell tolerance in the thymus have been shown to underlie the development of anti-IFN-I autoAbs in several patient cohorts (reviewed in Bastard et al. [2024a]; Hale [2023]). The best-characterized examples of these defects include mutations in the AIRE gene, which encodes an autoimmune regulator that normally ensures the negative selection of autoreactive T cells (Meager et al., 2006; Meyer et al., 2016), and mutations in the NFKB2, MAP3K14 (NIK), and RELB genes, which encode components of the alternative NF-κB pathway and regulate AIRE expression (Bodansky et al., 2022; Le Voyer et al., 2023; Ramakrishnan et al., 2018; Sjogren et al., 2022). Patients with these genetic defects exhibit thymic abnormalities and reduced self-tolerance and typically develop anti-IFN-I autoAbs in early childhood (Le Voyer et al., 2023; Meager et al., 2006), which has been speculated to occur following an infection event triggering “immunization” with endogenous IFN-I (Hale, 2023). However, the role of genetics and other predisposing factors in contributing to the increased prevalence of anti-IFN-I autoAbs in elderly populations is little understood, and it still remains to be resolved at the individual level at what point in life these autoAbs develop, their longevity, and their impact on an individual’s innate antiviral defenses. Furthermore, associations between potential “immunization” events, such as infections, and the development of anti-IFN-I autoAbs have yet to be investigated. These gaps in our knowledge mainly result from a lack of available longitudinal samples and a lack of systematic clinical histories taken from before anti-IFN-I autoAbs developed in individuals, as well as the limited time that has passed so far for patients recently identified to harbor anti-IFN-I autoAbs, which has prevented long-term follow-up studies.
Here, we sought to leverage the Swiss HIV Cohort Study (SHCS) as a large nationwide, systematic, longitudinal infectious disease cohort to study the development and consequences of anti-IFN-I autoAbs in elderly individuals. The SHCS was founded in 1988 and contains historic, semiannually biobanked plasma and cell samples, as well as clinical data from >21,000 people living with HIV (PLH) over the course of 35 years (Scherrer et al., 2022). With the improved success of long-term antiretroviral therapy, life expectancy in this cohort is now approaching that of the general population in Switzerland (Gueler et al., 2017). Thus, this well-treated cohort is aging, and individuals >50 years make up around 60% of the currently enrolled patients, with most having been followed up for almost half their adult lives. Furthermore, IFN-I therapy was widely used in this cohort for hepatitis C virus (HCV) treatment before directly acting antivirals became available (Baumann et al., 2024). Using this resource, we wanted to track the levels of neutralizing and non-neutralizing anti-IFN-I autoAb repertoires (targeting IFNα, IFNβ, and IFNω) in individuals over decades at high resolution, thus providing key information on the timing of autoAb induction and their longevity. Furthermore, we aimed to describe the impact of neutralizing anti-IFN-I autoAbs on innate antiviral defenses and to perform exploratory analyses of clinical record data to investigate whether predisposing factors or events influence the likelihood of anti-IFN-I autoAb development.
Results
Identification and characterization of anti-IFN-I autoAbs in a longitudinally sampled infectious disease patient cohort
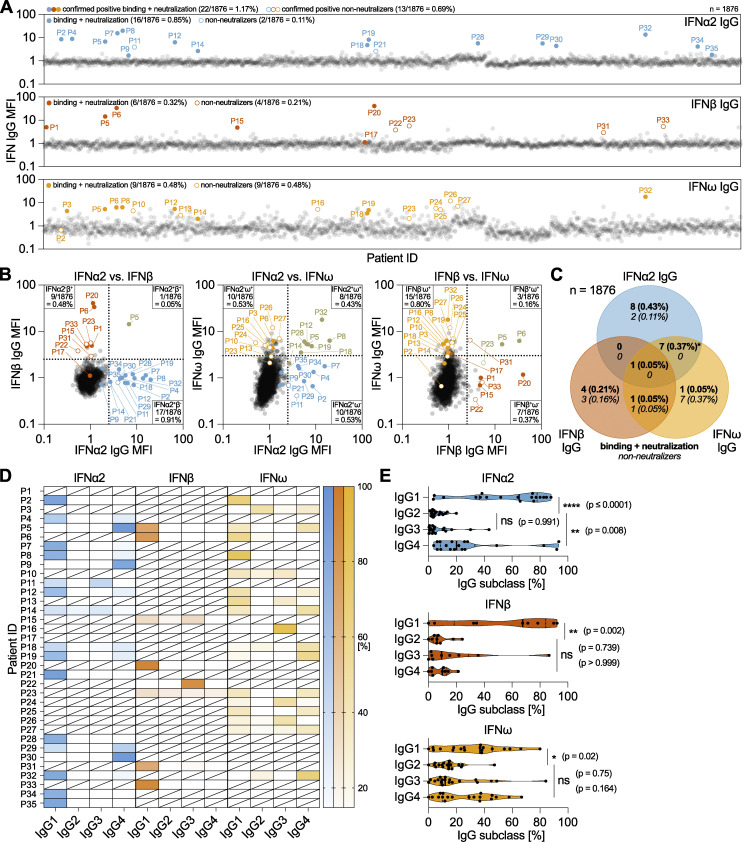
To systematically investigate the age-related development of anti-IFN-I autoAbs in the SHCS, we initially selected recent plasma samples taken from 1,876 well-treated individuals >65 years of age (82% male, median year of birth 1949, interquartile range 1943–1954; Table S1). Age and sample availability were the only criteria considered in this selection, and the resulting subcohort consisted of 857 65–69-year-olds (∼45.7%), 831 70–79-year-olds (∼44.3%), 183 80–89-year-olds (∼9.8%), and 5 90–94-year-olds (∼0.3%) at the time of sampling. We then applied a multiplexed bead-based assay to screen these samples in two rounds for IgG autoAbs binding to the representative type I IFNs: IFNα2, IFNβ, and IFNω. Samples identified to be positive were subsequently tested for neutralization capacity against the respective IFN-I at different doses (10, 1, or 0.2 ng/ml for IFNα2 and IFNω, and 1, 0.2, or 0.04 ng/ml for IFNβ). We observed that 0.85% of individuals (16/1,876) had neutralizing autoAbs against IFNα2, 0.32% of individuals (6/1,876) had neutralizing autoAbs against IFNβ, and 0.48% of individuals (9/1,876) had neutralizing autoAbs against IFNω (Fig. 1 A). Overall, 1.17% of the individuals in this subcohort (22/1,876) had neutralizing autoAbs against at least one IFN-I: 0.58% of 65–69-year-olds (5/857); 1.68% of 70–79-year-olds (14/831); and 1.64% of 80–89-year-olds (3/183). These age-related prevalences of neutralizing autoAbs are broadly similar to those previously reported in a very large, otherwise healthy, general population cohort: 0.27% and 0.87% of plasmas from 65- to 70-year-olds could neutralize IFNα2 and/or IFNω doses of 10 or 0.1 ng/ml, respectively, while 1.13% and 2.29% of plasmas from 70- to 80-year-olds could neutralize IFNα2 and/or IFNω doses of 10 or 0.1 ng/ml, respectively (Bastard et al., 2021). Our initial focus on screening for IFN-I binding IgG might nevertheless account for a small underestimation in the prevalence of neutralizing autoAbs in our cohort as neutralization assays are likely to be more sensitive than binding assays (Bastard et al., 2021). Indeed, in our own sensitivity analysis, we found that 0/320 and 1/320 of our “binding-negative” plasma samples could neutralize 10 or 0.2 ng/ml IFNα2, respectively, suggesting that only an additional ∼0.3% of our cohort may have very low titer neutralizing anti-IFN-I autoAbs that we did not detect. However, our screening strategy did allow us to identify an additional 0.69% of individuals in our >65 subcohort (13/1,876) who had detectable anti-IFN-I autoAbs that were non-neutralizing, even at the lowest 0.2 ng/ml dose of IFNα2/IFNω or 0.04 ng/ml dose of IFNβ tested (0.11% IFNα2, 0.21% IFNβ, and 0.48% IFNω).
Figure 1.
Identification and characterization of anti-IFN-I autoAbs in a longitudinally sampled infectious disease patient cohort. (A–C) Validated screening results for the presence of anti-IFNα2, anti-IFNβ, and anti-IFNω IgG in plasma samples derived from unique patients enrolled in the SHCS and aged >65 years at the time of sampling (n = 1,876, representative of two independent screenings). MFI fold change (FC) of IgG values obtained from IFN-I–coated beads relative to the MFI of IgG values obtained from empty beads is shown, normalized to the cohort means for each IFN-I. All individual patient sample results from the initial screening are shown (circles), but only patients considered positive after secondary analysis of longitudinal samples are colored (see Materials and methods for thresholds). Solid-colored circles represent patients who also neutralized the respective IFN-I in subsequent assays. Numbers and percentages of positive patients (neutralizing and non-neutralizing IgG) are indicated for each anti-IFN-I IgG. (B) Pairwise representation of the data shown in A comparing the indicated combinations of anti-IFNα2, anti-IFNβ, and anti-IFNω IgG found in each patient. (C) Venn diagram analysis of the 35 anti-IFN-I autoAb-positive patients highlighting the anti-IFN-I autoAb specificities observed for neutralizing and non-neutralizing IgG. Percentages refer to the entire subcohort (n = 1,876). The asterisk denotes the inclusion of a single patient found to possess binding and neutralizing anti-IFNα2 IgG, as well as binding and non-neutralizing anti-IFNω IgG. (D and E) Plasma samples from anti-IFN-I autoAb-positive patients were analyzed to determine relative levels (%) of each of the four IgG subclasses targeting IFN-Is (n = 33 patients, with at least two independent samples tested per patient). (D) Patient-level analysis is shown as a heat map, where white blocks indicate no anti-IFN-I IgG subclass was detected, and slashed blocks indicate IgG subclass was not determined. (E) IgG subclass analysis in all patients for each IFN-I. Statistical analysis was performed using a one-way ANOVA with Tukey’s multiple comparison (single pooled variance). Exact P values are stated in the panel (* = significant; ns = non-significant).
With regard to patient ethnicity, the most likely source of HIV-1 infection, and baseline levels of HIV-1 RNA, there were no clear differences between those with detectable levels of anti-IFN-I autoAbs and those without (Table S1). However, males made up 94.3% (33/35) of those identified to have anti-IFN-I autoAbs as compared to 81.9% (1,507/1,841) of males in the population without anti-IFN-I autoAbs, suggesting a trend toward increased prevalence in males (Table S1). Notably, one individual (P5) had IgG autoAbs binding and neutralizing all three type I IFNs, while most had binding autoAbs specific for IFNα2 (0.53%, 10/1,876), IFNω (0.43%, 8/1,876), or both IFNα2 and IFNω (0.37%, 7/1,876) (Fig. 1, B and C). Seven individuals (0.37%) only had IgG autoAbs specific to IFNβ, while two individuals (0.11%) had IgG autoAbs against IFNβ and IFNω (Fig. 1, B and C). While most anti-IFN-I autoAb-positive plasmas neutralized the respective IFN-I, it was striking that the majority of plasmas positive for anti-IFNω autoAbs alone appeared to be non-neutralizing, even against low 0.2 ng/ml doses of IFNω, at the plasma dilution tested (Fig. 1 C). Further characterization of each individual’s anti-IFN-I IgG autoAb subclasses revealed a binding antibody response dominated by IgG1 autoAbs in most individuals (Fig. 1, D and E). However, it was interesting to observe that IgG4 was also a commonly identified subclass of anti-IFNα2 and anti-IFNω IgG autoAbs (statistically significant for IFNα2, but not for IFNω) and that IgG4 even dominated in a few individuals with these types of autoAbs. In contrast, it was notable that anti-IFNβ IgG4 did not dominate in any individual (Fig. 1 E). Anti-IFN-I IgG autoAbs of the IgG3 subclass were also sporadically detected and represented the main IgG autoAb in some individual patients, while anti-IFN-I IgG2 autoAbs were very rarely detected or of low titer (Fig. 1, D and E). Thus, the anti-IFN-I IgG autoAb landscape in our subcohort is mostly comprised of the IgG1 subclass. Overall, our screening data reveal the diversity of anti-IFN-I autoAbs in a subcohort of PLH >65 years of age, and indicated that the prevalence of these autoAbs in this subcohort (1.17% for neutralizing autoAbs only) is highly comparable with that previously reported in a similarly aged general population cohort (Bastard et al., 2021).
High-resolution longitudinal analysis of anti-IFN-I autoAb levels reveals their acute age-associated development and persistence over decades
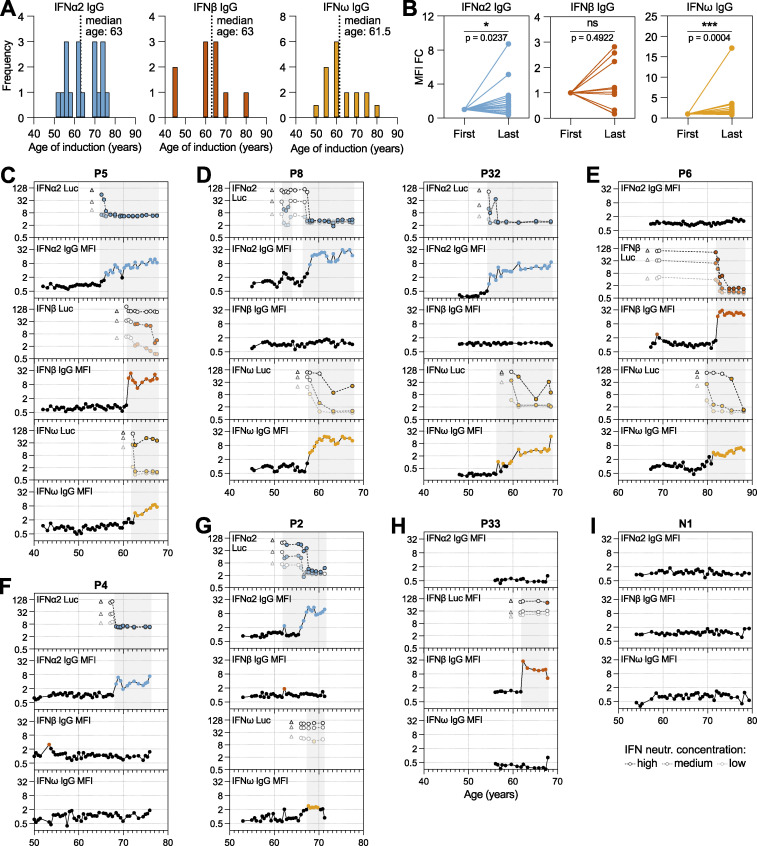
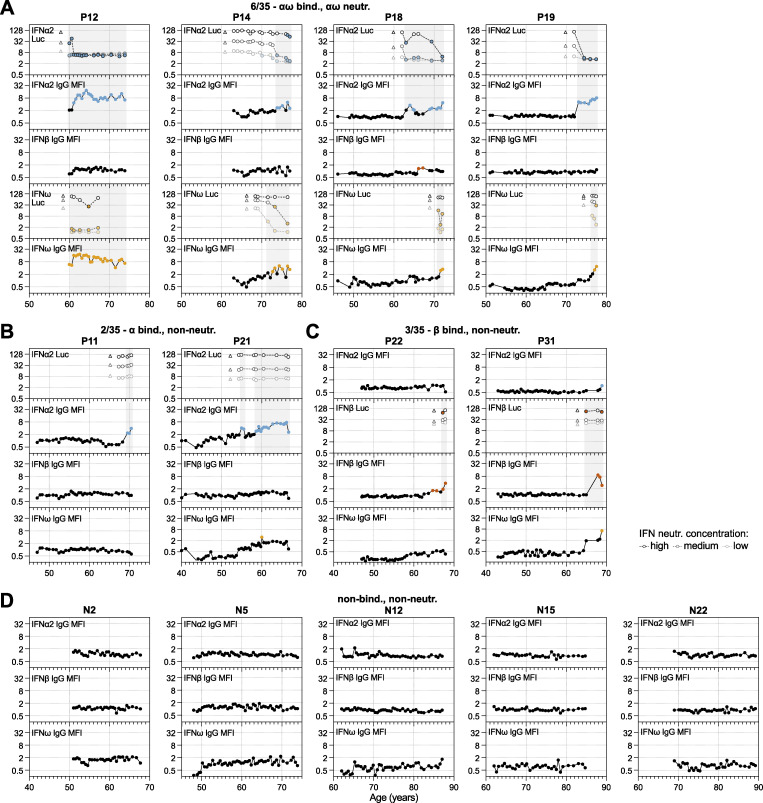
For all 35 anti-IFN-I autoAb positive individuals, as well as for 35 anti-IFN-I autoAb negative individuals (matched for sex and year of birth), we obtained all available biobanked plasma samples. Samples were typically available from two time points (∼6-monthly) per year and spanned an average of 20.2 years (range 9–27) for each anti-IFN-I autoAb positive individual. As an example, the anti-IFN-I autoAb positive individual with the most samples available had 52 plasma samples that were taken between the ages of 43 and 70 (i.e., over a 27-year period). For all longitudinal plasma samples, we assessed levels of IgG autoAbs binding to each type I IFN and subsequently tested most samples for IFN-I neutralization capacity at different doses (10, 1, or 0.2 ng/ml for IFNα2 and IFNω, and 1, 0.2, or 0.04 ng/ml for IFNβ), resulting in a temporal overview of anti-IFN-I autoAb binding and neutralization development for each individual (Fig. 2; and Figs. S1 and S2). Despite individual heterogeneity with regard to anti-IFN-I autoAb reactivity, several common features were apparent. Firstly, for 34/35 anti-IFN-I autoAb positive individuals, anti-IFN-I autoAbs were undetectable for many years in the available samples prior to the occurrence of a positive sample, indicating that the autoAbs did not exist in these individuals for most of their lives. This is in stark contrast with patients harboring mutations in the AIRE gene or in genes of the alternative NF-κB pathway, where anti-IFN-I autoAbs are detectable very early in childhood (Le Voyer et al., 2023; Meager et al., 2006). Indeed, confirming the age-related onset of anti-IFN-I autoAbs in our study population, the median ages of new-onset autoAb detection were remarkably similar at 63, 63, and 61.5 years for anti-IFNα2, anti-IFNβ, and anti-IFNω, respectively, with a range of ages spanning years 45–80 (Fig. 2 A). Second, anti-IFN-I autoAbs first developed at a specific, discrete time point in each individual, indicating that their induction was likely triggered by a certain acute event. Third, anti-IFN-I autoAbs generally increased in binding titers over time for anti-IFNα2 and anti-IFNω, but not for anti-IFNβ (Fig. 2 B), which might reflect increases in autoAb abundance or increases in avidity toward the IFNα2 and anti-IFNω antigens. However, it is unclear why this appears to be antigen specific. Finally, with the exception of rare transient “blips” at early time points (e.g., P8), it was apparent that the presence of anti-IFN-I autoAbs never resolved, and once developed they persisted continuously in subsequent plasma samples (Fig. 2, C–H; and Figs. S1 and S2). For example, the longest duration of neutralizing anti-IFN-I autoAbs detected in our study subcohort was ∼15 years (P12 and P32; Fig. 2 D and Fig. S2 A), and 11 individuals maintained anti-IFN-I autoAbs for at least 10 years each. On the contrary, anti-IFN-I binding autoAbs were never detected at any time point in the 35 negative individuals tested longitudinally (see examples in Fig. 2 I and Fig. S2 D). Overall, our data reveal acute induction of anti-IFN-I autoAbs that occurs in some individuals around the age of 60–65 years, which is followed by subsequent lifelong maintenance of these anti-IFN-I autoAbs.
Figure 2.
High-resolution longitudinal analysis of anti-IFN-I autoAb development over decades. Semiannually biobanked plasma samples available for all 35 anti-IFN-I autoAb-positive patients (and several negative patients) were analyzed for anti-IFNα2, anti-IFNβ, and anti-IFNω IgG levels, as well as for IFNα2, IFNβ, or IFNω neutralization capacity. (A) Frequency of ages (years) where each anti-IFN-I autoAb was first detected. The median age of first detection (induction) is noted (n = 35). (B) MFI FC values for each anti-IFNα2, anti-IFNβ, and anti-IFNω IgG in each patient comparing relative levels between the first time point where anti-IFN-I autoAbs were detected and the last available time point sampled (n = 35). Statistical analysis was performed using a Wilcoxon matched-pairs signed rank test. Exact P values are stated in the panel (* = significant; ns = non-significant). (C–I) Patient-level representation of anti-IFNα2, anti-IFNβ, and anti-IFNω IgG levels (MFI FC), as well as IFNα2, IFNβ, or IFNω neutralization (inhibition of IFN-induced luciferase [Luc] activity) at three different doses (see Materials and methods), for all available longitudinal samples plotted as a function of patient age (years). Each sample was tested in duplicate, and selected samples were retested for independent experimental validation. Colored circles represent samples considered positive for either binding IgG or neutralization (see Materials and methods for thresholds). Triangles in neutralization plots represent negative controls. The patient in I is a negative patient who never developed anti-IFN-I autoAbs. See also Figs. S1 and S2.
Figure S1.
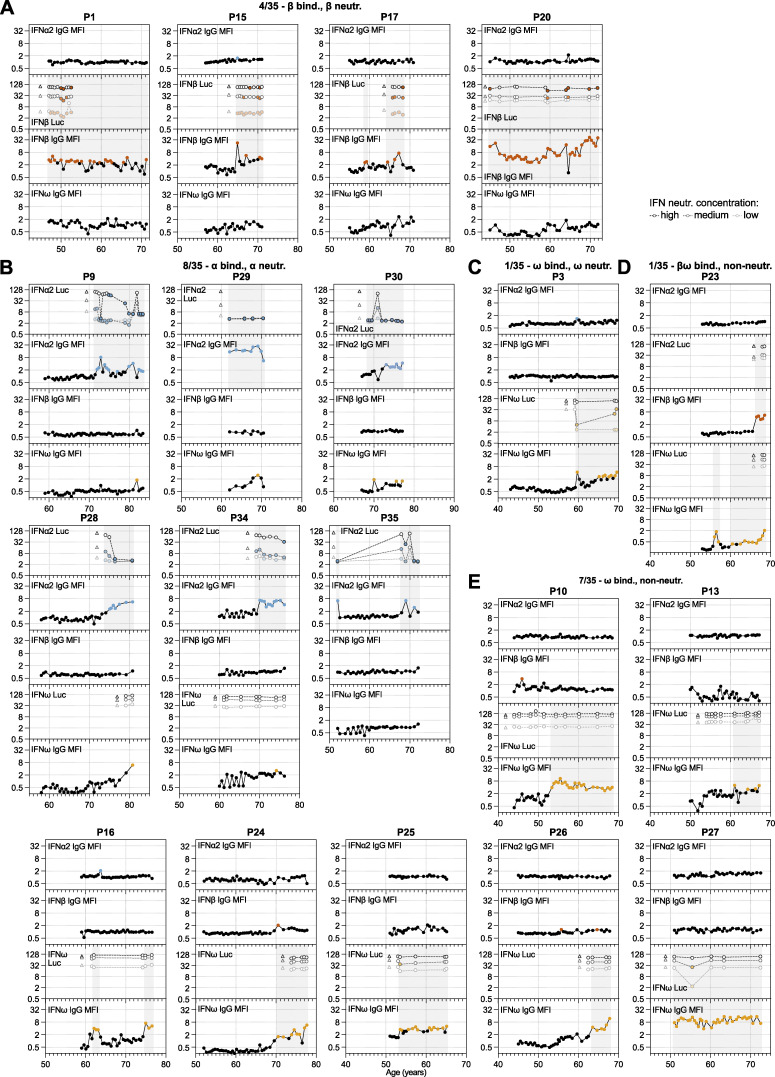
High-resolution longitudinal analysis of anti-IFN-I autoAb development over decades. Continued from Fig. 2. (A–E) Patient-level representation of anti-IFNα2, anti-IFNβ, and anti-IFNω IgG levels (MFI FC), as well as IFNα2, IFNβ, or IFNω neutralization (inhibition of IFN-induced luciferase [Luc] activity) at three different doses (see Materials and methods) for all available longitudinal samples plotted as a function of patient age (years). Each sample was tested in duplicate, and selected samples were retested for independent experimental validation. Colored circles represent samples considered positive for either binding IgG or neutralization (see Materials and methods for thresholds). Triangles in neutralization plots represent negative controls. In all panels, individual patients are grouped according to the types of IFN-I to which they possess binding and neutralizing IgG (indicated at the top of each panel, together with the n/35 patients who have a similar phenotype).
Figure S2.
High-resolution longitudinal analysis of anti-IFN-I autoAb development over decades, together with negative patients. Continued from Fig. 2. (A–C) Patient-level representation of anti-IFNα2, anti-IFNβ, and anti-IFNω IgG levels (MFI FC), as well as IFNα2, IFNβ, or IFNω neutralization (inhibition of IFN-induced luciferase [Luc] activity) at three different doses (see Materials and methods) for all available longitudinal samples plotted as a function of patient age (years). Each sample was tested in duplicate, and selected samples were retested for independent experimental validation. Colored circles represent samples considered positive for either binding IgG or neutralization (see Materials and methods for thresholds). Triangles in neutralization plots represent negative controls. In all panels, individual patients are grouped according to the types of IFN-I to which they possess binding and neutralizing IgG (indicated at the top of each panel, together with the n/35 patients who have a similar phenotype). (D) Semiannually biobanked plasma samples available for 5 patients who were negative for anti-IFN-I autoAbs in initial screenings were analyzed for anti-IFNα2, anti-IFNβ, and anti-IFNω IgG (n = 5). Each panel is a patient-level representation of anti-IFNα2, anti-IFNβ, and anti-IFNω IgG levels (MFI FC) plotted as a function of patient age (years). Each sample was tested in duplicate, and selected samples were retested for independent experimental validation. All samples were considered negative for binding IgG based on established thresholds (see Materials and methods for details).
Distinct individual variation in the timing and specificity of anti-IFN-I autoAb development
We observed various phenotypes relating to anti-IFN-I autoAbs that were particular to certain individuals or to groups of individuals. For example, it was striking in the single individual with autoAbs binding and neutralizing all three type I IFNs (P5) (Fig. 2 C) that each anti-IFN-I autoAb developed at different times over 7 years, with anti-IFNα2 autoAbs developing after age 55, anti-IFNβ autoAbs developing after age 61, and anti-IFNω autoAbs developing after age 62. This suggests that different events might have triggered the development of these different neutralizing autoAb reactivities at different times or that processes such as epitope spreading or molecular mimicry between the IFN-Is might have occurred. A similar pattern was observed in individual P32, who developed neutralizing anti-IFNα2 autoAbs after age 55, followed by neutralizing anti-IFNω autoAbs around age 60 (Fig. 2 D). However, anti-IFNβ autoAbs never developed in P32, highlighting inter-individual variability in the types of IFN-I antigens to which anti-IFN-I autoAbs can be raised. While reactivity to both IFNα2 and IFNω was not uncommon to observe (7/35 individuals; Fig. 2, D and G; and Fig. S2 A), there were many examples where reactivity to either only IFNα2 (10/35 individuals; e.g., Fig. 2 F, Fig. S1 B, and Fig. S2 B) or IFNω (8/35 individuals; Fig. S1, C and E) occurred, indicating that specificity of reactivity at the individual level, even between these two closely related type I IFNs, is possible despite the known potential for IFNα/IFNω crossreactive autoAbs to exist (Meyer et al., 2016). Furthermore, such lack of reactivity to both IFNα2 and IFNω was not simply a consequence of short time periods preventing the broadening of reactivity from one IFN to the other, as some individuals had specific anti-IFNα2 or anti-IFNω autoAbs for 8 years (P4, P21; Fig. 2 F and Fig. S2 B), 11 years (P7), or even 15 years (P10; Fig. S1 E) without developing autoAbs against the other IFN. In a similar specificity example, individual P6 developed anti-IFNω autoAbs after the age of 80 and then developed anti-IFNβ autoAbs after the age of 82, but never developed anti-IFNα2 autoAbs (Fig. 2 E). Thus, even within 35 individuals, almost all combinations of anti-IFN-I autoAb reactivities could be observed, which also extended to variations of whether the autoAbs were able to neutralize the action of IFN-I or not at the doses tested. Overall, our longitudinal analyses reveal that neither time of induction, binding specificity, nor neutralization capacity of anti-IFN-I autoAb repertoires can be generalized, but probably reflect distinct host or environmental factors specific to each individual.
Development of neutralizing anti-IFNα2 autoAbs is associated with subsequent COVID-19 hospitalization and with compromised baseline IFN-stimulated gene (ISG) levels
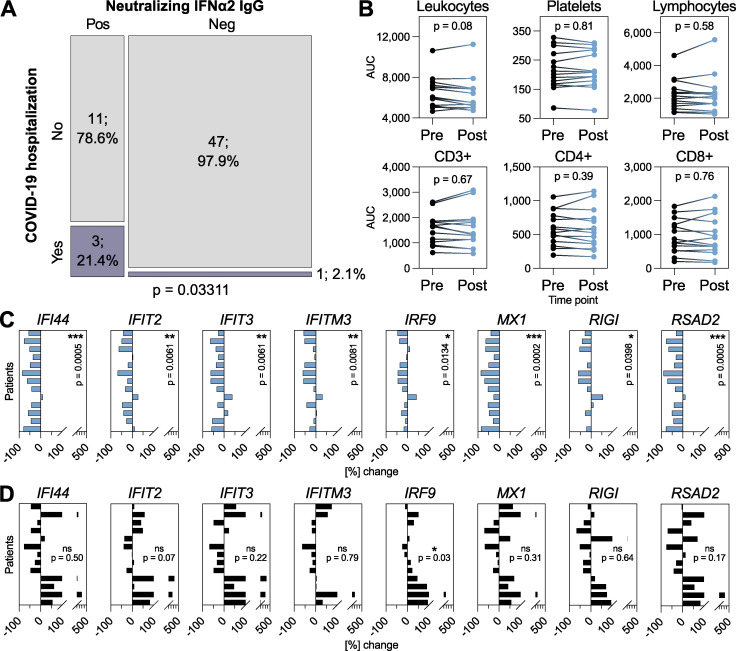
Our high-resolution data spanning multiple decades gave us the possibility to dissect the long-term consequences of anti-IFN-I autoAb development in individual patients. Given the heterogeneity of types of anti-IFN-I autoAbs observed in our subcohort, we focused studies on individuals who developed neutralizing anti-IFNα2 autoAbs, with the rationale that this was the largest “homogeneous” subgroup (n = 16), and that neutralization capacity was most likely to have explainable functional consequences. We began by looking at the consequences of anti-IFN-I autoAb development on well-documented database-recorded outcomes related to HIV-1 viral loads, incidences of other viral infections (e.g., herpes zoster, HSV-1, and severe COVID-19), opportunistic bacterial and fungal infections, and outcomes such as diabetes or cancers. For each individual, we analyzed these outcomes for their whole lifetime following the first detection of neutralizing anti-IFNα2 autoAbs and compared the results to anti-IFN-I autoAb negative individuals within the SHCS database who were matched for sex, registration date, study center, and year of birth (n = 62). We only observed a significant effect of neutralizing anti-IFNα2 autoAbs on the subsequent development of severe COVID-19 leading to hospitalization (P = 0.03311; Fig. 3 A and Table S2), and not on any of the other recorded outcomes which, for HIV-1 at least, might reflect the high effectiveness of long-term antiretroviral therapy. It is worth noting that the three autoAb-positive patients hospitalized with COVID-19 in 2020 (P18, P28, and P32; Fig. 2 D, Fig. S1 B, and Fig. S2 A) first developed neutralizing anti-IFNα2 autoAbs in 2008, 2013, and 2014 (i.e., 6–12 years prior to the start of the pandemic). At the cellular level, we did not observe that the development of neutralizing anti-IFNα2 autoAbs correlated with any changes to specific blood cell compositions, including leukocytes, platelets, or various lymphocyte subpopulations (Fig. 3 B). However, to investigate directly whether the development of neutralizing anti-IFNα2 autoAbs had consequences for ISG expression, we analyzed baseline ISG levels in multiple frozen biobanked peripheral blood mononuclear cell (PBMC) samples that were available from 13 individuals who developed neutralizing anti-IFNα2 autoAbs, as well as from 13 age-matched negative-control individuals who never developed anti-IFN-I autoAbs. For each autoAb-positive individual, we obtained two to three PBMC samples from time points before and after autoAb development. In the case of negative-control individuals, we obtained a similar number of samples from age-matched time points. Without culturing, total RNA was extracted directly from frozen PBMCs and subjected to quantitative reverse transcription PCR (RT-qPCR) analysis for a panel of eight ISG mRNAs: MX1, RIGI, IRF9, RSAD2, IFITM3, IFIT2, IFIT3, and IFI44. As shown in Fig. 3 C, mRNA levels of all ISGs were significantly reduced in autoAb-positive individuals subsequent to their development of neutralizing anti-IFNα2 autoAbs. In contrast, baseline ISG mRNA levels were not reduced in autoAb-negative individuals at age-matched time points, and more than half of these individuals actually exhibited increased levels of several ISGs over time (Fig. 3 D). These results build on previous cross-sectional descriptions of a correlation between neutralizing anti-IFNα autoAbs and low ISG levels (Abers et al., 2021; Bastard et al., 2020; Kisand et al., 2008; Koning et al., 2021; Lopez et al., 2021; van der Wijst et al., 2021; Wang et al., 2021), and reveal that age-associated development of neutralizing anti-IFNα autoAbs can have clear temporal functional consequences. In particular, our data indicate that neutralizing anti-IFNα autoAbs compromise baseline ISG levels, likely by impairing the low levels of constitutively secreted tonic IFN-I that prime innate antiviral defenses (Flagg et al., 2023; Gough et al., 2012). Importantly, the development of neutralizing anti-IFNα autoAbs can have long-term consequences, as demonstrated by the observation of severe COVID-19 susceptibility in several individuals over a decade after the first detection of IFNα neutralization.
Figure 3.
Neutralizing anti-IFNα2 autoAbs are associated with subsequent COVID-19 hospitalization and with compromised baseline ISG levels. (A) Mosaic plot comparing the SHCS recorded incidence of COVID-19 hospitalization between patients who developed neutralizing anti-IFNα2 autoAbs (n = 14) and matched control patients who did not (n = 48). Only patients who were still actively enrolled in the SHCS in 2020 were included (therefore n differs from that in Table S2). Statistical analysis was performed using Fisher’s exact test for count data, and the exact P value is indicated in the panel. (B) Area under the curve (AUC) values for clinically determined cell compositions in whole blood (as indicated) in patients who developed neutralizing anti-IFNα2 autoAbs. Existing clinical cell titers were obtained for each patient from the SHCS, and AUC values were determined from all available data up to 1 year before (pre) or 1 year after (post) the time point where anti-IFNα2 autoAbs were first detected (n = 16). Statistical analysis was performed using a paired Wilcoxon signed rank test. Exact P values are indicated in the panel. (C and D) RT-qPCR analysis of the indicated ISGs in PBMCs from patients who developed neutralizing anti-IFNα autoAbs (n = 13, two to three independent samples per time point) (C) or age-matched control patients who never developed anti-IFN-I autoAbs (n = 13, two to three independent samples per time point) (D). Data shown for each patient represent mean percentage changes in expression of the indicated ISG relative to the first time point (i.e., samples taken before the development of anti-IFNα autoAbs for C, or to the equivalent time point for D). The statistical significance of changes across all patients was determined based on the original ΔCt values (normalized to GAPDH) using a Mann–Whitney U test. Exact P values are indicated in the panels (* = significant; ns = non-significant).
Prior virus infections may influence anti-IFN-I autoAb development
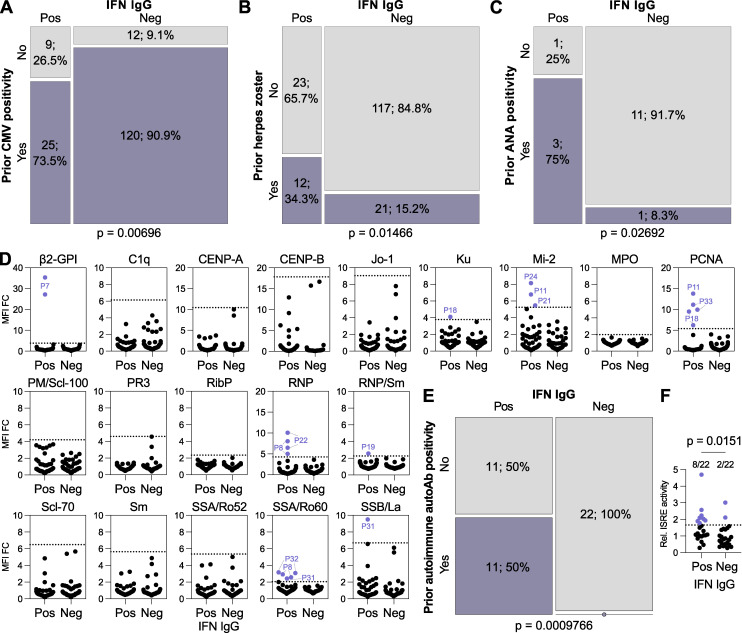
We probed the clinical records of each patient to understand factors potentially influencing the development of anti-IFN-I autoAbs. To do this, we initially analyzed differences between our 35 anti-IFN-I autoAb-positive patients and 138 autoAb-negative patients (matched for sex, registration date, years since HIV diagnosis, study center, and year of birth) at all time points that preceded the first onset of anti-IFN-I autoAbs in positive individuals. Given the matching for registration date and year of birth, the same time points could be considered for the negative individuals. Out of over 30 parameters investigated in an exploratory study, which were either chosen due to their routine recording in the SHCS database or their infection-relatedness, only prior CMV seropositivity and prior diagnosis of herpes zoster showed significant differences between the two groups (Table S3 and Fig. 4, A and B). There were no significant differences observed for prior CD4+ or CD8+ cell counts, HIV-1 RNA levels, or any of the common opportunistic bacterial or fungal infections investigated (Table S3). Prior CMV seropositivity had a small negative association with anti-IFN-I autoAb development (P = 0.00696; Fig. 4 A). In contrast, prior herpes zoster events had a small positive association with anti-IFN-I autoAb development (P = 0.01466; Fig. 4 B). This was particularly surprising, as further temporal analysis revealed that the prior recorded herpes zoster diagnoses did not always immediately precede the onset of anti-IFN-I autoAb development, as might be expected if the severe acute disease itself triggered autoAb production. Specifically, out of the 12 individuals who developed anti-IFN-I autoAbs and had documented prior herpes zoster, only two individuals (P10 and P24) had herpes zoster in the 6 mo prior to the first detection of anti-IFN-I autoAbs. Of the remaining 10 individuals, the herpes zoster events were recorded variously between 3 and 14 years prior to anti-IFN-I autoAb onset. However, it is possible that the recorded herpes zoster events might act as a well-documented indicator of other disparate types of severe infections that may also have occurred in these patients, perhaps closer to the time of anti-IFN-I autoAb development. In this regard, it is notable that a previous study postulated that a range of recurrent severe or chronic infections may be factors in promoting the development of autoAbs, including against IFN-Is, in certain infection-susceptible immunodeficient individuals (Walter et al., 2015). To explore this further on a case-by-case basis in our subcohort, we looked for additional recorded infection-related clinical events that occurred up to 1 year prior to the first detection of anti-IFN-I autoAbs. One individual (P4) was reported to have recently suffered from severe pneumonia with sepsis that led to hospitalization. Respiratory samples taken from P4 at this time were found to be PCR-positive for influenza A virus and bocavirus, the former agent being paradigmatic for inducing significant IFN-I responses (Dunning et al., 2018). Another individual (P7) was treated for HCV infection with ribavirin and pegylated IFNα, but the normal course of treatment was cut short for an unknown reason, and P7 developed anti-IFNα2 autoAbs during this period. Thus, our finding of a small, yet statistically significant, association between prior herpes zoster diagnoses and anti-IFN-I autoAb development, together with additional case-by-case analyses of individual infection-related events, may support the concept that previous infections might influence autoAb induction (Walter et al., 2015). Mechanistically, as discussed below for the IFNα-treated individual (P7), this could be due to events that increase levels of the IFN-I “immunogen” for subsequent autoAb development, such as severe virus infections.
Figure 4.
Prior infections and immune factors influence the development of anti-IFN-I autoAbs. (A–C) Mosaic plots comparing the SHCS recorded incidence of prior CMV positivity (A), prior herpes zoster diagnosis (B), or prior ANA test positivity (C) between patients who developed anti-IFN-I autoAbs and matched control patients who did not. In A and B, only patients with complete data for the indicated parameter were included (therefore n differs slightly from that in Table S3). In C, only patients who were tested are included. For all panels, n and % are shown. (D) Screening results for the presence of 19 different anti-autoantigen IgGs in plasma samples derived from anti-IFN-I autoAb positive (Pos) patients (n = 22 patients, with two independent samples tested per patient) and age-matched negative control (Neg) patients (n = 22 patients, with two independent samples tested per patient) who were confirmed to have never developed anti-IFN-I autoAbs. The two samples tested per patient were the two samples immediately preceding the first detection of anti-IFN-I autoAbs (for the positive patients; typically 6 and 12 mo before) or age-matched time points for the negative patients. MFI FC IgG values obtained from the indicated autoantigen-coated beads are shown relative to the MFI of IgG values obtained from empty beads normalized to the negative patient mean for each autoantigen. All patient samples are shown (circles). Patient plasmas exhibiting normalized MFI values >5 SDs above the mean MFIs obtained from the negative patient samples (dotted lines) were considered positive for the specific anti-autoantigen IgG and were colored and labeled. (E) Mosaic plot analysis of the data in D. (F) Average relative ISRE-driven luciferase (Luc) activity induced by the two plasma samples from each patient described in D (Pos, n = 22; Neg, n = 22). For A and B, statistical analyses were performed using conditional-logistic regression (taking into account the matched nature of the data) and likelihood-ratio tests; for C, statistical analysis was performed using Fisher’s exact test for count data (as the routine ANA data were not available for matched pairs of cases and controls); and for E, statistical analysis was performed using the exact McNemar test (as this takes into account both the paired nature of the data and the absence of events in one group). For F, statistical analysis was performed using a Mann–Whitney U test. Exact P values are indicated in the appropriate panel.
Pre-existing autoimmune reactivity predicts subsequent anti-IFN-I autoAb development
While exposure to virus-induced IFN-I may act as the “immunogen” for triggering anti-IFN-I autoAb production, a compromised ability to tolerate self-antigens, including IFN-Is, is likely an essential additional requirement for autoAb development. For example, defects in genes relating to correct thymus function, key to self-tolerance mechanisms, have been linked to the early lifetime development of anti-IFN-I autoAbs (Bodansky et al., 2022; Le Voyer et al., 2023; Meager et al., 2006; Ramakrishnan et al., 2018). Furthermore, in the elderly, it may be that common age-related thymic decline plays a key role in reducing self-tolerance and promoting autoAb development in some individuals (Liang et al., 2022). We therefore searched the available clinical records of our patients to identify instances where their prior autoimmune status had been assessed by antinuclear antigen (ANA) test. While the number of patients tested due to clinical need was small (n = 4 for those with anti-IFN-I autoAbs, n = 12 for matched controls), there was nevertheless a statistically significant association between prior ANA test positivity and subsequent development of anti-IFN-I autoAbs in our subcohort (P = 0.02692; Fig. 4 C). To test the association between loss of self-tolerance (e.g., ANA positivity) and subsequent anti-IFN-I autoAb induction more formally, and with a greater number of individuals, we selected 22 anti-IFN-I autoAb positive patients for whom we had at least 5 years of validated anti-IFN-I autoAb negativity prior to anti-IFN-I autoAb development. For each patient, we obtained the two plasma samples taken immediately before the first confirmed detection of anti-IFN-I autoAbs (this was typically 6 mo and 1 year prior) and used a multiplexed bead-based assay to screen these samples for IgG autoAbs binding to 19 different human autoantigens associated with autoimmune disease, including those likely detected by the clinical ANA test. In parallel, age-matched samples from 22 (negative) patients who never developed anti-IFN-I autoAbs were also tested. Using standard deviation (SD) thresholds based on these negative patient samples, we identified 11 patients with IgG autoAbs targeting diverse ANA-related and non-related autoantigens, and it was striking that all of these 11 patients (100%) were positive individuals who subsequently went on to develop anti-IFN-I autoAbs (Fig. 4 D). None of the 22 anti-IFN-I autoAb negative patients exhibited autoreactivity to these other autoantigens. There was no apparent antigen specificity to the observed prior autoreactivity in those who went on to develop anti-IFN-I autoAbs, with different patients harboring IgG targeting distinct autoantigens (including β2-GPI, Ku, Mi-2, PCNA, RNP, RNP/Sm, SSA/Ro60, and SSB/La). However, it is interesting to note that anti-SSA and anti-SSB autoAbs are associated with primary Sjögren’s syndrome where frequent development of anti-IFN-I autoAbs has been reported (Burbelo et al., 2019; Gupta et al., 2016). These data amounted to a statistically highly significant association between prior autoimmune autoAb positivity and subsequent development of anti-IFN-I autoAbs (P = 0.0009766; Fig. 4 E). Overall, the statistically significant association between prior ANA test positivity and subsequent development of anti-IFN-I autoAbs, together with our experimental identification of autoreactivity in 50% of those who went on to produce anti-IFN-I autoAbs, indicate that diminished self-tolerance can precede anti-IFN-I autoAb development. Furthermore, these data suggest that those with certain autoimmune diseases, which might be detectable by routine assays such as ANA screening, are at higher risk of subsequently developing anti-IFN-I autoAbs.
Development and lifelong persistence of neutralizing anti-IFNα autoAbs potentially triggered by therapeutic IFNα in an individual with pre-existing autoimmunity
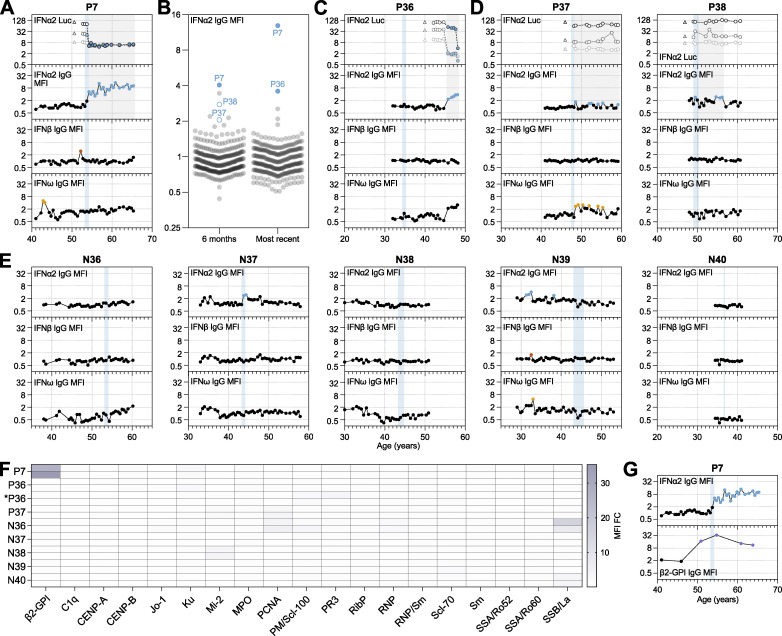
Based on previous hypotheses (Hale, 2023), and the data presented so far, we postulate that anti-IFN-I autoAbs might develop following exposure to unusually high levels of IFN-I (possibly driven by acute severe infections or by chronically elevated levels) in the context of diminished self-tolerance. Indeed, functional analysis of samples taken around 6 mo and 1 year prior to first confirmed detection of anti-IFN-I autoAbs in 22 patients suggested that many of these individuals may have had slightly elevated (chronic) levels of IFN in their plasmas as compared with 22 age-matched patients who never developed anti-IFN-I autoAbs (P = 0.0151; Fig. 4 F). Toward further validating this concept with data closer to the time of first anti-IFN-I autoAb detection, we followed-up on the specific case of P7, who developed neutralizing anti-IFNα autoAbs around the age of 54 years and maintained them lifelong ever since (>12 years) (Fig. 5 A). P7 was initially diagnosed with HCV around the age of 46 and was treated with the standard of care at the time, ribavirin and pegylated IFNα, 8 years later. Treatment with pegylated IFNα was started only 5 wk before neutralizing anti-IFNα autoAbs were first detected, and all previous samples tested (>12 years) were negative for anti-IFNα autoAbs (Fig. 5 A). This observation is highly suggestive that the treatment of P7 with pegylated IFNα may have stimulated the production of long-lasting anti-IFNα autoAbs in a manner similar to that previously reported (Jorns et al., 2006). However, such occurrences would seem to be atypical, as although production of anti-IFNα autoAbs has been described to occur in response to recombinant IFNα treatment, production appears to be transient and usually self-resolving, at least in the small numbers of individuals (20–40) tested previously (Bell et al., 1994; Jorns et al., 2006; Ronnblom et al., 1992). To formally address this on a larger scale, we took advantage of our access to plasma samples taken from 300 individuals in the SHCS (including P7; Table S1) who had been treated with pegylated IFNα and assessed whether they had detectable levels of anti-IFN-I IgG autoAbs. Two samples per patient were tested: the first sample available after IFNα treatment began (typically around 6 mo); and the last sample available (typically 10–20 years later). Only 3/300 individuals tested around 6 mo after starting IFNα treatment were confirmed to possess anti-IFNα IgG autoAbs in their plasmas (P7, P37, and P38), and only the plasma of P7 neutralized the activity of IFNα, even at high 10 ng/ml doses (Fig. 5 B). For P37 and P38, the presence of anti-IFNα autoAbs was transient, as these autoAbs were not readily detected in the last available plasma samples from these patients, or in any other longitudinal samples available (spanning up to 20 years) (Fig. 5, B–D). Analysis of the last available plasma samples from our IFNα-treated cohort revealed that only 2/300 individuals possessed anti-IFNα autoAbs in their plasmas at this time point, and both neutralized high 10 ng/ml doses of IFNα (P7 and P36) (Fig. 5 B). Notably, longitudinal analysis of biobanked plasma samples from P36 (spanning around 15 years) revealed that his induction of anti-IFNα IgG autoAbs occurred about 10 years after IFNα treatment stopped, suggesting that the IFNα treatment was not the acute trigger for anti-IFNα autoAbs in this individual. We also tested all available longitudinal plasma samples from five negative individuals treated with IFNα in case we had missed important time points. However, despite some low-level transient anti-IFNα IgG autoAb positive “blips,” all these IFNα-treated individuals remained negative for long-lasting neutralizing anti-IFNα autoAbs (Fig. 5 E). Thus, we conclude that the development of long-lasting neutralizing anti-IFNα autoAbs in response to IFNα treatment (as observed for P7) is a rare event.
Figure 5.
Therapeutic IFNα likely triggered the development and lifelong persistence of neutralizing anti-IFNα autoAbs in an individual with pre-existing autoimmunity. (A) Representation of anti-IFNα2, anti-IFNβ, and anti-IFNω IgG levels (MFI FC), as well as IFNα2 neutralization (inhibition of IFN-induced luciferase [Luc] activity) at three different doses (see Materials and methods), for all available longitudinal samples from patient P7 (who was treated therapeutically with IFNα2) plotted as a function of patient age (years). Each sample was tested in duplicate, and selected samples were retested for independent experimental validation. (B) Validated screening results for the presence of anti-IFNα2 IgG in plasma samples derived from unique patients enrolled in the SHCS who were treated with IFNα2 (n = 300). Two independent samples per patient were assayed in duplicate: the first sample available after IFNα treatment (typically around 6 mo), and the last sample available (most recent: typically 10–20 years later). MFI FC values obtained from IFNα2-coated beads relative to the MFI of values obtained from empty beads are shown, normalized to the cohort mean. All individual patient samples are shown (circles), with samples considered positive after subsequent independent analysis of longitudinal samples colored (see Materials and methods for thresholds). Solid colored circles represent plasma samples that also neutralized IFNα when assayed. Positive patient samples are labeled. (C–E) Representation of data similar to A, but for all available longitudinal samples from the indicated patients who had also been treated therapeutically with IFNα2. Each sample was tested in duplicate, and selected samples were retested for independent experimental validation. (F) Heatmap representation of screening results for the presence of 19 different anti-autoantigen IgGs in plasma samples derived from several patients who had been treated therapeutically with IFNα2 (n = 8). Two independent samples per patient were tested, which were the two samples immediately preceding the start of IFNα2 treatment (typically 6 and 12 mo before), as well as immediately preceding first detection of anti-IFN-I autoAbs (for newly identified patient *P36). MFI FC IgG values obtained from the indicated autoantigen-coated beads are shown relative to the MFI of IgG values obtained from empty beads normalized to the means of controls shown in Fig. 4. Patient plasmas exhibiting normalized MFI values >5 SDs above the mean MFIs obtained from the controls shown in Fig. 4 were considered positive for the specific anti-autoantigen IgG and are colored. Note that the samples for P7 are the same as shown in Fig. 4, as the start of IFNα2 treatment coincided with the first detection of anti-IFN-I autoAbs. (G) Representation of anti-IFNα2 (from A) and anti-β2-GPI IgG levels (MFI FC), in selected longitudinal samples from patient P7 plotted as a function of patient age (years). Each sample was tested in duplicate. In A–E and G, colored circles represent samples considered positive for either binding IgG or neutralization (see Materials and methods for thresholds). Triangles in neutralization plots represent negative controls. Blue shading indicates the period of time when each patient underwent IFNα2 treatment.
Toward understanding why P7 alone developed a long-lasting neutralizing anti-IFNα IgG autoAb response during treatment, we obtained the two plasma samples taken immediately prior to IFNα treatment (typically 6 mo and 1 year prior) for seven IFNα-treated individuals in addition to P7. We then used the multiplexed bead-based assay to screen these samples for IgG autoAbs binding to different autoimmune disease–associated autoantigens as a means to identify those with a potential breakdown of self-tolerance. Notably, P7 had strong unambiguous evidence for autoimmune reactivity (anti-β2-GPI IgG autoAbs) in his plasma samples prior to developing anti-IFNα autoAbs, while autoreactivity was generally lacking in samples taken from all other patients who did not develop anti-IFNα autoAbs following IFNα treatment (except N36 who had weak reactivity to SSB/La) (Fig. 5 F). Furthermore, the longitudinal analysis revealed that P7 possessed anti-β2-GPI IgG autoAbs at least 3 years prior to receiving IFNα treatment and developing long-lasting neutralizing anti-IFNα IgG (Fig. 5 G). Taken together with our previous observation of a statistically significant association between prior autoreactivity and subsequent anti-IFN-I autoAb development, the results of this case study suggest that a pre-existing breakdown of self-tolerance in P7 was likely to have been decisive in him mounting a long-lasting anti-IFNα IgG response when treated with pegylated IFNα.
Discussion
Herein, we systematically investigated the age-related development and long-term dynamics of anti-IFN-I autoAbs in well-treated individuals living with HIV. The unique aspect of our work is the longitudinal analysis of historic biobanked patient samples and clinical records that span the decades before and after each patient developed anti-IFN-I autoAbs. Together with retrospective assays, this allowed us to dissect factors associated with anti-IFN-I autoAb induction and their consequences. Our observation that ∼1.9% of individuals ultimately developed anti-IFN-I autoAbs (1.17% for neutralizing autoAbs only, not including a possible false-negative rate of 0.3% for low titer autoAbs), with a median onset age of ∼63 years (range 45–80), aligns well with findings from a recent cross-sectional study that noted a sharp increase in neutralizing anti-IFN-I autoAb prevalence (to at least 1.4%) in generally healthy individuals over the age of 70 years (Bastard et al., 2021). This suggests that the underlying HIV-positive status of individuals within the subcohort we studied, the majority of whom were well-treated with long-term antiretroviral therapy, likely has a limited impact on anti-IFN-I autoAb development. Our results could therefore be broadly applicable to the general population, and our data now provide a high-resolution long-term perspective on this form of IFN system deficiency and its contribution to severe viral disease.
The triggering of anti-IFN-I autoAbs in some individuals around ages 60–65 is enigmatic. Such late-onset development is suggestive that, at least in this population, germline host genetic variants compromising thymic function may have only a limited contribution as compared with the situation in younger patient populations harboring mutations in AIRE or alternative NF-κB pathway genes (Le Voyer et al., 2023; Meager et al., 2006). Nevertheless, our finding that there is a significant temporal association between prior autoimmune reactivity and subsequent development of anti-IFN-I autoAbs is still indicative that these patients have diminished self-tolerance. While we cannot rule out an impact of the HIV-1 infection on patient autoimmune status (Roszkiewicz and Smolewska, 2016; Zandman-Goddard and Shoenfeld, 2002), we note that the observed anti-IFN-I autoAb prevalence in our cohort is very similar to that in the general population (Bastard et al., 2021). Given the genetic link between central T-cell tolerance in the thymus and anti-IFN-I autoAb development (Le Voyer et al., 2023; Meager et al., 2006), the most likely explanation in our aged patients is that the natural process of thymic involution has led to reduced thymus activity and an increased likelihood of developing autoimmunity (Liang et al., 2022), including against IFN-Is. Further studies will have to address this hypothesis directly, as well as understand why only a subset of aged individuals go on to develop such autoimmune reactions. In this regard, the availability of longitudinal biobanked plasma and PBMC samples, together with high-resolution tracking of anti-IFN-I autoAb induction (such as presented here), could prove invaluable to retrospectively assess thymus-related immunosenescence in specific individuals by assaying historic thymic output (e.g., by quantifying T cell receptor excision circles, or TRECs [Mitchell et al., 2010]) and correlating this directly with the subsequent likelihood of anti-IFN-I autoAb induction. Given the apparent severe consequences of anti-IFN-I autoAbs for infection susceptibility (reviewed in Bastard et al. [2024a]; Hale [2023]), in the future there may be a clear clinical role for performing diagnostic autoimmune (e.g., ANA test and others) or TREC quantifications to help predict an individual’s risk of developing this form of IFN system deficiency.
While aging and prior autoimmune reactivity appear to predispose an individual to develop anti-IFN-I autoAbs, the “two-hit” hypothesis suggests that (auto)immunization with IFN-I is a subsequently required trigger in this process (Hale, 2023). Our finding of a statistical association (albeit weak) between prior recorded virus infections such as reactivated herpes zoster, which activates a clear endogenous IFN-I response in patients (Vandoren et al., 2024), and the development of anti-IFN-I autoAbs provides some support to the idea that autoimmunization may occur. Similar support comes from our anecdotal finding that anti-IFN-I autoAbs developed in a patient immediately following hospitalization with severe pneumonia possibly caused by influenza A virus, again a scenario where endogenous IFN-I and inflammatory responses will be activated (Dunning et al., 2018). We also cannot rule out that a subset of our patients had specific subclinical undiagnosed systemic autoimmune diseases, such as primary Sjögren’s syndrome or systemic lupus erythematosus, which provided an environment where both loss of tolerance and atypically increased levels of IFN-I triggered anti-IFN-I autoAb development (Burbelo et al., 2019; Gupta et al., 2016; Mathian et al., 2022; Trutschel et al., 2022). However, additional evidence supporting the two-hit hypothesis comes from our analysis of 300 individuals who were treated (or “immunized”) with pegylated IFNα, where we identified that a single individual with unambiguous evidence for loss of self-tolerance prior to therapy was the only individual to generate long-lasting neutralizing anti-IFNα IgG in response to the exogenous IFNα. Overall, this could suggest that some previous acute infections in tolerance-compromised individuals might lead to the development of anti-IFN-I autoAbs and could thus have long-term consequences for enhancing susceptibility to future severe infections. This concept may also apply to some individuals who are still treated therapeutically with IFN-Is (European Association for the Study of the Liver, 2023), and given the two-hit hypothesis, it may be worth considering future implementation of diagnostic autoimmune prescreening and risk–benefit evaluations to assess the likely short- and long- term outcomes associated with IFN-I treatment specifically in those with poor self-tolerance.
In contrast to previous cross-sectional studies (Abers et al., 2021; Bastard et al., 2020; Kisand et al., 2008; Koning et al., 2021; Lopez et al., 2021; van der Wijst et al., 2021; Wang et al., 2021), our access to high-resolution longitudinal samples gave us the unique opportunity to study the within-individual consequences of neutralizing anti-IFN-I autoAb development. Compared with samples taken before anti-IFN-I autoAbs were detectable, we observed a clear temporal correlation between induction of neutralizing anti-IFNα2 autoAbs and a reduction of baseline ISG levels in patient PBMCs, which is likely due to neutralization of the low levels of constitutively secreted tonic IFN-I that prime innate antiviral defenses (Flagg et al., 2023; Gough et al., 2012). It is logical to assume that the appearance and maintenance of these neutralizing autoAbs led to this functional impairment of innate antiviral immunity, thus contributing to an increased susceptibility to severe viral infections as previously reported (reviewed in Bastard et al. [2024a]; Hale [2023]). The consequences of harboring other neutralizing anti-IFN-I autoAbs (e.g., against IFNβ or IFNω) could not be readily discerned in our subcohort due to the relatively low numbers of positive individuals identified, although a recent report suggests that neutralization of IFNα may have greater pathogenic consequences than neutralization of IFNω (Bastard et al., 2024b). Nevertheless, a key finding from our work was the observation that following induction, neutralizing anti-IFN-I autoAbs can be effectively maintained in circulation lifelong (over at least 15 years in one individual), meaning that the consequences of anti-IFN-I development could result in extremely long-lasting IFN-I functional deficiency and increased susceptibility to infection. Indeed, we noted severe COVID-19 susceptibility in several individuals at least 6–12 years after the first detection of neutralizing anti-IFN-I autoAbs in their plasmas. Transient induction of anti-IFN-I autoAbs has been described in some infection scenarios (Chang et al., 2021; Steels et al., 2022), as well as in this study, and could potentially have important physiological regulatory functions such as dampening immunopathologies (Babcock et al., 2024, Preprint). However, a distinction should be made with the class of long-lasting, lifelong neutralizing anti-IFN-I autoAbs that we mainly describe here, which functionally impair innate antiviral immunity and increase severe infection susceptibility.
Our study has some limitations, including the retrospective nature and potential biases inherent in long-term cohort studies. For example, as this cohort was not specifically established to study clinical aspects of autoAbs, autoimmune results (e.g., ANA tests) were only available for some patients that were performed based on clinical need. However, we were able to address this specific deficiency through systematic measurements made retrospectively from biobanked samples. Furthermore, not all (viral) infections can be recorded in an observational cohort study, and the low prevalence of neutralizing anti-IFN-I autoAbs limits the power to assess their impact on subsequent infections, perhaps with the exception of severe COVID-19. While we believe that the results from our work should also be broadly applicable to otherwise healthy general populations, we also acknowledge that all of our study patients are PLH, and we thus cannot rule out potential effects specifically related to the infection, unknown opportunistic co-infections, or antiretroviral therapy. In this regard, a major limitation to studying the functional consequences of anti-IFN-I autoAbs was actually that we could not effectively assess their impact on HIV-1 replication due to the majority of patients being on long-term antiretroviral therapy and therefore exhibiting virological suppression at the time of anti-IFN-I autoAb detection. Our initial sampling strategy (>65 years of age) may also have biased our assessment of anti-IFN-I autoAb lifelong persistence, and we may not yet have a true appreciation of transient anti-IFN-I autoAb induction. While future investigations with larger and more diverse cohorts are therefore clearly essential, we also highlight the unique resource that the SHCS represents for studies such as this given the rarity of decade-spanning longitudinally sampled cohorts focused on infectious diseases with the numbers of patients required to reveal uncommon anti-IFN-I autoAbs.
In summary, our study provides a comprehensive longitudinal exploration of the age-related development and specificities of anti-IFN-I autoAbs. Our work identifies prior loss of tolerance and infection-related factors as influencers of anti-IFN-I autoAb induction and thereby supports the hypothesis that at least two-hits probably underlie this process. These findings have implications for diagnosing those who may be predisposed to developing anti-IFN-I autoAbs and the lifelong IFN-I functional deficiency and severe infection susceptibility that results. Broader knowledge in this area should contribute to the development of targeted strategies to mitigate severe viral disease susceptibility.
Materials and methods
Patient samples, data, and ethics
The 1,876 plasma samples from patients >65 years at the time of initial testing (Table S1), 300 plasma samples from patients treated with pegylated IFNα (Table S1), and all retrospective follow-up longitudinal samples (including plasma and/or PBMCs) analyzed in this study were derived from samples stored in the biobanks of the SHCS. The SHCS is a prospective, nationwide, longitudinal, noninterventional, observational, clinic-based cohort with semiannual visits and blood collections, enrolling all PLH in Switzerland since 1988 (Scherrer et al., 2022). The SHCS maintains comprehensive, longitudinal, anonymous data collection for all participants, including extensive clinical and demographic data. The data are collected by the five Swiss university hospitals, two cantonal hospitals, 15 affiliated hospitals, and 36 private physicians (listed in http://shcs.ch/180-health-care-providers). Detailed information on the study is available at http://shcs.ch. The SHCS has been approved by the ethics committees of all participating institutions (Kantonale Ethikkommission Bern, Ethikkommission des Kantons St. Gallen, Comite Departemental d’Ethique des Specialites Medicales et de Medicine Communataire et de Premier Recours, Kantonale Ethikkommission Zürich, Repubblica et Cantone Ticino–Comitato Ethico Cantonale, Commission Cantonale d’Étique de la Recherche sur l’Être Humain, Ethikkommission beider Basel), and written informed consent has been obtained from all participants. The personnel who conducted the work with patient samples had no information on patient demographics at the time of analysis, and all data have subsequently been analyzed anonymously.
Analysis of autoreactive IgG in plasma samples
A previously described high-throughput multiplexed bead-based assay was implemented to assay patient plasma samples for anti-IFN-I IgG (Achleitner et al., 2023; Busnadiego et al., 2022; Liechti et al., 2018). Briefly, magnetic beads (MagPlex-C Microspheres, Luminex) were coupled to recombinant carrier-free human IFN-Is (IFNα2, Novus Biologicals; IFNβ, PeproTech; or IFNω, Novus Biologicals) at a concentration of 10 µg protein per million beads or left empty. Bead coating efficiency and bead specificity were assessed using mouse monoclonal antibodies as described previously (Busnadiego et al., 2022). For serological testing, obtained patient plasma samples were heat-inactivated at 56°C for 1 h before being diluted 1:50 in PBS supplemented with 1% BSA (PBS/BSA) and incubated in 96-well plates with 1:1:1:1 mixtures of IFNα2:IFNβ:IFNω:empty beads (2 × 103 beads per region per well; 1:100 final concentration of plasma). On all experimental plates, a human polyclonal anti-IFNα2b antiserum (BEI Resources) was used as a positive control and an in-house healthy donor pool of human plasmas was used both as a negative control and for batch normalization. Following incubation of the bead:plasma mixes for 1 h at room temperature, beads were washed twice with PBS/BSA before phycoerythrin (PE)-labeled secondary antibodies were added separately in PBS/BSA (pan-IgG, 1:500 dilution; Southern Biotech; IgG1, 1:500 dilution; Southern Biotech; IgG2, 1:100 dilution; Southern Biotech; IgG3, 1:100 dilution; Southern Biotech; IgG4, 1:100 dilution; Southern Biotech). After a further 1 h incubation at room temperature, bead mixtures were washed twice in PBS/BSA and samples were analyzed on a FlexMap 3D instrument (Luminex). A minimum of 50 beads per antigen were acquired. For each plasma sample, median fluorescence intensity (MFI) values from the IFN-I–coated beads were obtained and made relative to the corresponding MFI value obtained from the empty beads (fold over empty, FOE). Values were then normalized between plates and cohorts using results from the in-house healthy donor pool. In the primary screen, patient plasmas exhibiting normalized FOE values >2 MFI were considered preliminarily positive for the specific anti-IFN-I IgG. Secondary testing was then performed on all available longitudinal plasma samples from both these patients and selected negatives. When assaying longitudinal samples, patient plasmas exhibiting normalized FOE values >10 SDs above the mean MFIs of the first five available samples from each individual patient were considered positive for the specific anti-IFN-I IgG. Patients were overall confirmed as positive for anti-IFN-I IgG if at least four of their total longitudinal samples (or three consecutive samples) met these criteria.
To assay patient plasma samples for IgG targeting various human autoantigens associated with autoimmune diseases, a customized 19 autoantigen-containing MILLIPLEX Human Autoimmune Autoantibody Panel (Merck Millipore) was used. The assay was performed according to the manufacturer’s protocol using a 1:300 final dilution of each heat-inactivated patient plasma, and samples were analyzed on a FlexMap 3D instrument (Luminex). For each plasma sample, FOE MFI values for IgG targeting each autoantigen were calculated as described above. Within the dataset for each autoantigen, FOE values were then normalized to the mean MFI values obtained from the anti-IFN-I autoAb-negative patient samples. Patient plasmas exhibiting normalized FOE values >5 SDs above the mean MFIs obtained from the anti-IFN-I autoAb-negative patient samples were considered positive for the specific anti-autoantigen IgG.
Analysis of IFN-I neutralization in plasma samples
IFN-I neutralization was assessed as described previously using a 293T transfection-based dual-luciferase reporter assay in 96-well plates that is based around cotransfection of a plasmid containing the firefly luciferase (FF-Luc) gene under control of the IFN-inducible mouse Mx1 promoter (an IFN-stimulated response element, ISRE; pGL3-Mx1P-FFluc) (kindly provided by Georg Kochs, University of Freiburg, Freiburg, Germany) with a plasmid constitutively expressing the Renilla luciferase (Ren-Luc) gene (pRL-TK-Renilla) (Busnadiego et al., 2022). 24 h after transfection of both plasmids, heat-inactivated patient plasma samples (or multiple negative controls) were diluted 1:50 in DMEM supplemented with 10% fetal calf serum (FCS), 100 U/ml penicillin, and 100 mg/ml streptomycin, and incubated for 1 h at room temperature with 10, 1, or 0.2 ng/ml of IFNα2 or IFNω, or with 1, 0.2, or 0.04 ng/ml of IFNβ prior to their addition to the transfected cells. After 24 h, cells were lysed for 15 min at room temperature and FF-Luc and Ren-Luc activity levels were determined using the Dual-Luciferase Reporter Assay System (Promega) and a PerkinElmer EnVision plate reader (EV2104) according to the manufacturer’s instructions. FF-Luc values were normalized to Ren-Luc values and then to the median luminescence intensity of control wells that had not been stimulated with any IFN-I. Patient plasmas that reduced IFN-I–stimulated FF-Luc/Ren-Luc values by >2 SDs below the mean values of the negative control samples were considered to be neutralizing. As required, a similar ISRE-based luciferase assay was performed in the absence of exogenous IFN-I to determine the functional IFN content of patient plasmas diluted 1:10. The 293T cell stocks were originally from ATCC but were not authenticated following acquisition. The 293T cells used in assays were routinely tested for mycoplasma contamination and tested negative at all times.
Analysis of ISG expression in PBMC samples
13 patients who developed persistent neutralizing anti-IFNα autoAbs (positive patients) were selected, together with 13 age-matched control patients who were confirmed longitudinally to have never developed anti-IFN-I autoAbs (negative patients). For each positive patient, we obtained biobanked frozen PBMC samples corresponding to two to three annual donations given before the development of anti-IFNα autoAbs and two to three annual donations given after the development of autoAbs. For each negative patient, we obtained similar biobanked frozen PBMC samples corresponding to equivalent time points. Total RNA from freshly thawed PBMCs was extracted using the ReliaPrep RNA Cell Miniprep System (Promega), and mRNA was subsequently reverse transcribed into cDNA with an oligo(dT) primer using SuperScript III Reverse Transcriptase (Thermo Fisher Scientific) according to the manufacturer’s instructions. Real-time PCR was then performed with PowerTrack SYBR Green Master Mix (Thermo Fisher Scientific) using gene-specific forward and reverse primers (sequences available upon request) in a 7300 Real-Time PCR System (Applied Biosystems). Relative gene expression was calculated with the ΔΔCt method. Data were normalized to GAPDH levels, averaged between the two to three annual donations per patient and per time point, and were expressed as percent change relative to the first time point (i.e., before the development of anti-IFNα autoAbs for the positive patients, or to the equivalent time point for the age-matched negative patients). Statistical significance was determined based on the ΔCt values (normalized to GAPDH) using a Mann–Whitney U test.
Statistical analyses
Patient clinical and demographic data derived from the SHCS relating to the contents of Tables S1, S2, and S3, COVID-19 hospitalizations, blood cell compositions, prior CMV positivity, prior herpes zoster diagnoses, and prior ANA test positivity were examined using the Wilcoxon rank-sum test (for continuous variables), or Fisher’s exact test (for categorical variables). In addition, conditional logistic regression models were performed to take into account the effect of matching cases and controls. Analyses were performed using R version 4.2.2, with statistical tests noted in the appropriate figure legends. All other statistical analyses presented were performed in GraphPad Prism 9 using the statistical tests noted in the appropriate figure legends.
Online supplemental material
Fig. S1 and Fig. S2 contain high-resolution longitudinal data on anti-IFN-I autoAb development over decades for several additional individual patients, as well as negative individuals. Table S1 describes the baseline patient characteristics of the study subcohorts. Table S2 shows the analysis of neutralizing anti-IFNα autoAbs on patient outcomes, including severe COVID-19. Table S3 shows the analysis of prior infection-related events on the development of anti-IFN-I autoAbs.
Supplementary Material
shows baseline patient characteristics of the study sub-cohorts.
shows the impact of neutralizing anti-IFNα autoAbs on recorded outcomes.
shows the impact of prior events on development of anti-IFN-I autoAbs.
Acknowledgments
We thank the patients participating in the SHCS and their physicians and study nurses for patient care. We are grateful to Nadine Rist, Stefan Schmutz, and Kevin Steiner (University of Zurich, Switzerland) for their technical support and sample management. We are also grateful to Alexandra Calmy (University of Geneva, Switzerland) and Matthias Cavassini (University of Lausanne, Switzerland) for their support as representatives of SHCS centers.
Financial support for this study was provided by the Swiss National Science Foundation (SNF; grants 31003A_182464 and 310030_214957 to B.G. Hale), the Novartis Foundation for Medical-Biological Research (grant 23A069 to B.G. Hale), and the Promedica Foundation (to I.A. Abela). The study was also cofinanced within the framework of the SHCS, supported by the SNF (33CS30_201369 to H.F. Günthard), by the small nested SHCS project 898 (to B.G. Hale and I.A. Abela). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions: S. Fernbach: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing, N.K. Mair: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing, I.A. Abela: Investigation, Writing - review & editing, K. Groen: Investigation, Project administration, Validation, Visualization, R. Kuratli: Investigation, Methodology, Validation, Visualization, M. Lork: Formal analysis, Investigation, Methodology, Validation, Visualization, C.W. Thorball: Investigation, Validation, Writing - review & editing, E. Bernasconi: Data curation, Investigation, Resources, Supervision, Writing - review & editing, P. Filippidis: Writing - review & editing, K. Leuzinger: Investigation, Project administration, Resources, Writing - review & editing, J. Notter: Resources, Writing - review & editing, A. Rauch: Funding acquisition, Resources, Writing - review & editing, H.H. Hirsch: Data curation, Investigation, Resources, Writing - original draft, M. Huber: Methodology, Writing - review & editing, H.F. Guenthard: Conceptualization, Data curation, Funding acquisition, Resources, Writing - review & editing, J. Fellay: Conceptualization, Supervision, Writing - review & editing, R.D. Kouyos: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Visualization, Writing - review & editing, B.G. Hale: Conceptualization, Funding acquisition, Project administration, Supervision, Writing - original draft, Writing - review & editing.
Footnotes
Swiss HIV Cohort Study members: Irene A. Abela, Karoline Aebi-Popp, Alexia Anagnostopoulos, Manuel Battegay, Enos Bernasconi, Dominique Laurent Braun, Heiner Bucher, Alexandra Calmy, Matthias Cavassini, Angela Ciuffi, Günter Dollenmaier, Mattias Egger, Luisa Elzi, Jan Fehr, Jacques Fellay, Hansjakob Furrer, Christoph Fux, Huldrych Fritz Günthard (President of the SHCS), Anna Hachfeld, David Haerry (Deputy of “Positive Council”), Barbara Hasse, Hans Hirsch, Matthias Hoffmann, Irene Hösli, Michael Huber, David Jackson-Perry (patient representative), Christian Kahlert (Chairman of the Mother & Child Substudy), Laurent Kaiser, Olivia Keiser, Thomas Klimkait, Roger Dimitri Kouyos, Helen Kovari, Katharina Kusejko (Head of Data Centre), Niklaus Labhardt, Karoline Leuzinger, Begogna Martinez de Tejada, Catja Marzolini, Karin Jutta Metzner, Nicolas Müller, Johannes Nemeth, Dunja Nicca, Julia Notter, Paolo Paioni, Giuseppe Pantaleo, Matthieu Perreau, Andri Rauch (Chairman of the Scientific Board), Luisa Salazar-Vizcaya, Patrick Schmid, Roberto Speck, Marcel Stöckle (Chairman of the Clinical and Laboratory Committee), Philip Tarr, Alexandra Trkola, Gilles Wandeler, Maja Weisser, and Sabine Yerly.
Contributor Information
The Swiss HIV Cohort Study:
Irene A. Abela, Karoline Aebi-Popp, Alexia Anagnostopoulos, Manuel Battegay, Enos Bernasconi, Dominique Laurent Braun, Heiner Bucher, Alexandra Calmy, Matthias Cavassini, Angela Ciuffi, Günter Dollenmaier, Matthias Egger, Luisa Elzi, Jan Fehr, Jacques Fellay, Hansjakob Furrer, Christoph Fux, Huldrych Fritz Günthard, Anna Hachfeld, David Haerry, Barbara Hasse, Hans Hirsch, Matthias Hoffmann, Irene Hösli, Michael Huber, David Jackson-Perry, Christian Kahlert, Laurent Kaiser, Olivia Keiser, Thomas Klimkait, Roger Dimitri Kouyos, Helen Kovari, Katharina Kusejko, Niklaus Labhardt, Karoline Leuzinger, Begogna Martinez de Tejada, Catja Marzolini, Karin Jutta Metzner, Nicolas Müller, Johannes Nemeth, Dunja Nicca, Julia Notter, Paolo Paioni, Giuseppe Pantaleo, Matthieu Perreau, Andri Rauch, Luisa Salazar-Vizcaya, Patrick Schmid, Roberto Speck, Marcel Stöckle, Philip Tarr, Alexandra Trkola, Gilles Wandeler, Maja Weisser, and Sabine Yerly
Data availability
Data acquired specifically for this study are available within the article itself and its supplementary materials. Patient data from the SHCS are not publicly available due to privacy reasons and the sensitivities associated with HIV infection. However, upon reasonable request, selected data can be made available to interested researchers after submission of a proposal to the respective SHCS address (http://shcs.ch/contact). The provision of data will be considered by the Scientific Board of the SHCS and the study team and is subject to Swiss legal and ethical regulations as well as a material and data transfer agreement.
References
- Abers, M.S., Rosen L.B., Delmonte O.M., Shaw E., Bastard P., Imberti L., Quaresima V., Biondi A., Bonfanti P., Castagnoli R., et al. 2021. Neutralizing type-I interferon autoantibodies are associated with delayed viral clearance and intensive care unit admission in patients with COVID-19. Immunol. Cell Biol. 99:917–921. 10.1111/imcb.12495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achleitner, M., Mair N.K., Dänhardt J., Kardashi R., Puhan M.A., Abela I.A., Toepfner N., de With K., Kanczkowski W., Jarzebska N., et al. 2023. Absence of type I interferon autoantibodies or significant interferon signature alterations in adults with post-COVID-19 syndrome. Open Forum Infect. Dis. 11:ofad641. 10.1093/ofid/ofad641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbil, B., Meyer T., Stubbemann P., Thibeault C., Staudacher O., Niemeyer D., Jansen J., Mühlemann B., Doehn J., Tabeling C., et al. 2022. Early and rapid identification of COVID-19 patients with neutralizing type I interferon auto-antibodies. J. Clin. Immunol. 42:1111–1129. 10.1007/s10875-022-01252-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alotaibi, F., Alharbi N.K., Rosen L.B., Asiri A.Y., Assiri A.M., Balkhy H.H., Al Jeraisy M., Mandourah Y., AlJohani S., Al Harbi S., et al. 2023. Type I interferon autoantibodies in hospitalized patients with Middle East respiratory syndrome and association with outcomes and treatment effect of interferon beta-1b in MIRACLE clinical trial. Influenza Other Respir. Viruses. 17:e13116. 10.1111/irv.13116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock, B.R., Kosters A., Eddins D.J., Donaire M.S.B., Sarvadhavabhatla S., Pae V., Beltran F., Murray V.W., Gill G., Xie G., et al. 2024. Transient anti-interferon autoantibodies in the airways are associated with efficient recovery from COVID-19. medRxiv. 10.1101/2024.01.11.24301000 (Preprint posted January 11, 2024). [DOI] [Google Scholar]
- Bastard, P., Gervais A., Le Voyer T., Philippot Q., Cobat A., Rosain J., Jouanguy E., Abel L., Zhang S.Y., Zhang Q., et al. 2024a. Human autoantibodies neutralizing type I IFNs: From 1981 to 2023. Immunol. Rev. 322:98–112. 10.1111/imr.13304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard, P., Gervais A., Le Voyer T., Rosain J., Philippot Q., Manry J., Michailidis E., Hoffmann H.H., Eto S., Garcia-Prat M., et al. 2021. Autoantibodies neutralizing type I IFNs are present in ∼4% of uninfected individuals over 70 years old and account for ∼20% of COVID-19 deaths. Sci. Immunol. 6:eabl4340. 10.1126/sciimmunol.abl4340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard, P., Gervais A., Taniguchi M., Saare L., Sarekannu K., Le Voyer T., Philippot Q., Rosain J., Bizien L., Asano T., et al. 2024b. Higher COVID-19 pneumonia risk associated with anti-IFN-alpha than with anti-IFN-omega auto-Abs in children. J. Exp. Med. 221:e20231353. 10.1084/jem.20231353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard, P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.H., Zhang Y., Dorgham K., Philippot Q., Rosain J., Beziat V., et al. 2020. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 370:eabd4585. 10.1126/science.abd4585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann, L., Braun D.L., Cavassini M., Stoeckle M., Bernasconi E., Schmid P., Calmy A., Haerry D., Beguelin C., Fux C.A., et al. 2024. Long-term trends in hepatitis C prevalence, treatment uptake and liver-related events in the Swiss HIV Cohort Study. Liver Int. 44:169–179. 10.1111/liv.15754 [DOI] [PubMed] [Google Scholar]
- Bayat, A., Burbelo P.D., Browne S.K., Quinlivan M., Martinez B., Holland S.M., Buvanendran A., Kroin J.S., Mannes A.J., Breuer J., et al. 2015. Anti-cytokine autoantibodies in postherpetic neuralgia. J. Transl. Med. 13:333. 10.1186/s12967-015-0695-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, J.B., Barfoot R., Iveson T., Powles R.L., and Millar B.C.. 1994. Neutralising antibodies in patients with multiple myeloma receiving maintenance therapy with interferon alpha 2b. Br. J. Cancer. 70:646–651. 10.1038/bjc.1994.365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodansky, A., Vazquez S.E., Chou J., Novak T., Al-Musa A., Young C., Newhams M., Kucukak S., Zambrano L.D., Mitchell A., et al. 2022. NFKB2 haploinsufficiency identified via screening for IFNalpha2 autoantibodies in children and adolescents hospitalized with SARS-CoV-2-related complications. J Allergy Clin Immunol. 151:926–930.e2. 10.1016/j.jaci.2022.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbelo, P.D., Browne S., Holland S.M., Iadarola M.J., and Alevizos I.. 2019. Clinical features of Sjögren’s syndrome patients with autoantibodies against interferons. Clin. Transl. Med. 8:1. 10.1186/s40169-018-0218-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbelo, P.D., Browne S.K., Sampaio E.P., Giaccone G., Zaman R., Kristosturyan E., Rajan A., Ding L., Ching K.H., Berman A., et al. 2010. Anti-cytokine autoantibodies are associated with opportunistic infection in patients with thymic neoplasia. Blood. 116:4848–4858. 10.1182/blood-2010-05-286161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busnadiego, I., Abela I.A., Frey P.M., Hofmaenner D.A., Scheier T.C., Schuepbach R.A., Buehler P.K., Brugger S.D., and Hale B.G.. 2022. Critically ill COVID-19 patients with neutralizing autoantibodies against type I interferons have increased risk of herpesvirus disease. PLoS Biol. 20:e3001709. 10.1371/journal.pbio.3001709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, S.E., Feng A., Meng W., Apostolidis S.A., Mack E., Artandi M., Barman L., Bennett K., Chakraborty S., Chang I., et al. 2021. New-onset IgG autoantibodies in hospitalized patients with COVID-19. Nat. Commun. 12:5417. 10.1038/s41467-021-25509-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvineau-Grenier, A., Bastard P., Servajean A., Gervais A., Rosain J., Jouanguy E., Cobat A., Casanova J.L., and Rossi B.. 2022. Autoantibodies neutralizing type I interferons in 20% of COVID-19 deaths in a French hospital. J. Clin. Immunol. 42:459–470. 10.1007/s10875-021-01203-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Credle, J.J., Gunn J., Sangkhapreecha P., Monaco D.R., Zheng X.A., Tsai H.J., Wilbon A., Morgenlander W.R., Rastegar A., Dong Y., et al. 2022. Unbiased discovery of autoantibodies associated with severe COVID-19 via genome-scale self-assembled DNA-barcoded protein libraries. Nat. Biomed. Eng. 6:992–1003. 10.1038/s41551-022-00925-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan, C.J.A., Randall R.E., and Hambleton S.. 2021. Genetic lesions of type I interferon signalling in human antiviral immunity. Trends Genet. 37:46–58. 10.1016/j.tig.2020.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning, J., Blankley S., Hoang L.T., Cox M., Graham C.M., James P.L., Bloom C.I., Chaussabel D., Banchereau J., Brett S.J., et al. 2018. Progression of whole-blood transcriptional signatures from interferon-induced to neutrophil-associated patterns in severe influenza. Nat. Immunol. 19:625–635. 10.1038/s41590-018-0111-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto, S., Nukui Y., Tsumura M., Nakagama Y., Kashimada K., Mizoguchi Y., Utsumi T., Taniguchi M., Sakura F., Noma K., et al. 2022. Neutralizing type I interferon autoantibodies in Japanese patients with severe COVID-19. J. Clin. Immunol. 42:1360–1370. 10.1007/s10875-022-01308-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Association for the Study of the Liver . 2023. EASL Clinical Practice Guidelines on hepatitis delta virus. J. Hepatol. 79:433–460. 10.1016/j.jhep.2023.05.001 [DOI] [PubMed] [Google Scholar]
- Flagg, M., Goldin K., Pérez-Pérez L., Singh M., Williamson B.N., Pruett N., Hoang C.D., and de Wit E.. 2023. Low level of tonic interferon signalling is associated with enhanced susceptibility to SARS-CoV-2 variants of concern in human lung organoids. Emerg. Microbes Infect. 12:2276338. 10.1080/22221751.2023.2276338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca, F., Scordio M., Santinelli L., Gabriele L., Gandini O., Criniti A., Pierangeli A., Angeloni A., Mastroianni C.M., d’Ettorre G., et al. 2022. Anti-IFN-α/-ω neutralizing antibodies from COVID-19 patients correlate with downregulation of IFN response and laboratory biomarkers of disease severity. Eur. J. Immunol. 52:1120–1128. 10.1002/eji.202249824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais, A., Rovida F., Avanzini M.A., Croce S., Marchal A., Lin S.C., Ferrari A., Thorball C.W., Constant O., Le Voyer T., et al. 2023. Autoantibodies neutralizing type I IFNs underlie West Nile virus encephalitis in approximately 40% of patients. J. Exp. Med. 220:e20230661. 10.1084/jem.20230661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves, D., Mezidi M., Bastard P., Perret M., Saker K., Fabien N., Pescarmona R., Lombard C., Walzer T., Casanova J.L., et al. 2021. Antibodies against type I interferon: Detection and association with severe clinical outcome in COVID-19 patients. Clin. Transl. Immunol. 10:e1327. 10.1002/cti2.1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough, D.J., Messina N.L., Clarke C.J., Johnstone R.W., and Levy D.E.. 2012. Constitutive type I interferon modulates homeostatic balance through tonic signaling. Immunity. 36:166–174. 10.1016/j.immuni.2012.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueler, A., Moser A., Calmy A., Günthard H.F., Bernasconi E., Furrer H., Fux C.A., Battegay M., Cavassini M., Vernazza P., et al. 2017. Life expectancy in HIV-positive persons in Switzerland: Matched comparison with general population. AIDS. 31:427–436. 10.1097/QAD.0000000000001335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, S., Tatouli I.P., Rosen L.B., Hasni S., Alevizos I., Manna Z.G., Rivera J., Jiang C., Siegel R.M., Holland S.M., et al. 2016. Distinct functions of autoantibodies against interferon in systemic lupus erythematosus: A comprehensive analysis of anticytokine autoantibodies in common rheumatic diseases. Arthritis Rheumatol. 68:1677–1687. 10.1002/art.39607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale, B.G. 2023. Autoantibodies targeting type I interferons: Prevalence, mechanisms of induction, and association with viral disease susceptibility. Eur. J. Immunol. 53:e2250164. 10.1002/eji.202250164 [DOI] [PubMed] [Google Scholar]
- Hetemäki, I., Laakso S., Välimaa H., Kleino I., Kekäläinen E., Mäkitie O., and Arstila T.P.. 2021. Patients with autoimmune polyendocrine syndrome type 1 have an increased susceptibility to severe herpesvirus infections. Clin. Immunol. 231:108851. 10.1016/j.clim.2021.108851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorns, C., Holzinger D., Thimme R., Spangenberg H.C., Weidmann M., Rasenack J., Blum H.E., Haller O., and Kochs G.. 2006. Rapid and simple detection of IFN-neutralizing antibodies in chronic hepatitis C non-responsive to IFN-alpha. J. Med. Virol. 78:74–82. 10.1002/jmv.20506 [DOI] [PubMed] [Google Scholar]
- Kisand, K., Link M., Wolff A.S., Meager A., Tserel L., Org T., Murumägi A., Uibo R., Willcox N., Trebusak Podkrajsek K., et al. 2008. Interferon autoantibodies associated with AIRE deficiency decrease the expression of IFN-stimulated genes. Blood. 112:2657–2666. 10.1182/blood-2008-03-144634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koning, R., Bastard P., Casanova J.L., Brouwer M.C., van de Beek D., van Agtmael M., Algera A.G., Appelman B., van Baarle F., Bax D., et al. 2021. Autoantibodies against type I interferons are associated with multi-organ failure in COVID-19 patients. Intensive Care Med. 47:704–706. 10.1007/s00134-021-06392-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Voyer, T., Parent A.V., Liu X., Cederholm A., Gervais A., Rosain J., Nguyen T., Perez Lorenzo M., Rackaityte E., Rinchai D., et al. 2023. Autoantibodies against type I IFNs in humans with alternative NF-κB pathway deficiency. Nature. 623:803–813. 10.1038/s41586-023-06717-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, Z., Dong X., Zhang Z., Zhang Q., and Zhao Y.. 2022. Age-related thymic involution: Mechanisms and functional impact. Aging Cell. 21:e13671. 10.1111/acel.13671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti, T., Kadelka C., Ebner H., Friedrich N., Kouyos R.D., Günthard H.F., and Trkola A.. 2018. Development of a high-throughput bead based assay system to measure HIV-1 specific immune signatures in clinical samples. J. Immunol. Methods. 454:48–58. 10.1016/j.jim.2017.12.003 [DOI] [PubMed] [Google Scholar]
- Lin, S.C., Zhao F.R., Janova H., Gervais A., Rucknagel S., Murray K.O., Casanova J.L., and Diamond M.S.. 2023. Blockade of interferon signaling decreases gut barrier integrity and promotes severe West Nile virus disease. Nat. Commun. 14:5973. 10.1038/s41467-023-41600-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez, J., Mommert M., Mouton W., Pizzorno A., Brengel-Pesce K., Mezidi M., Villard M., Lina B., Richard J.C., Fassier J.B., et al. 2021. Early nasal type I IFN immunity against SARS-CoV-2 is compromised in patients with autoantibodies against type I IFNs. J. Exp. Med. 218:e20211211. 10.1084/jem.20211211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manry, J., Bastard P., Gervais A., Le Voyer T., Rosain J., Philippot Q., Michailidis E., Hoffmann H.H., Eto S., Garcia-Prat M., et al. 2022. The risk of COVID-19 death is much greater and age dependent with type I IFN autoantibodies. Proc. Natl. Acad. Sci. USA. 119:e2200413119. 10.1073/pnas.2200413119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathian, A., Breillat P., Dorgham K., Bastard P., Charre C., Lhote R., Quentric P., Moyon Q., Mariaggi A.A., Mouries-Martin S., et al. 2022. Lower disease activity but higher risk of severe COVID-19 and herpes zoster in patients with systemic lupus erythematosus with pre-existing autoantibodies neutralising IFN-α. Ann. Rheum. Dis. 81:1695–1703. 10.1136/ard-2022-222549 [DOI] [PubMed] [Google Scholar]
- Meager, A., Visvalingam K., Peterson P., Möll K., Murumägi A., Krohn K., Eskelin P., Perheentupa J., Husebye E., Kadota Y., and Willcox N.. 2006. Anti-interferon autoantibodies in autoimmune polyendocrinopathy syndrome type 1. PLoS Med. 3:e289. 10.1371/journal.pmed.0030289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, S., Woodward M., Hertel C., Vlaicu P., Haque Y., Kärner J., Macagno A., Onuoha S.C., Fishman D., Peterson H., et al. 2016. AIRE-deficient patients harbor unique high-affinity disease-ameliorating autoantibodies. Cell. 166:582–595. 10.1016/j.cell.2016.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyts, I., and Casanova J.L.. 2021. Viral infections in humans and mice with genetic deficiencies of the type I IFN response pathway. Eur. J. Immunol. 51:1039–1061. 10.1002/eji.202048793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, W.A., Lang P.O., and Aspinall R.. 2010. Tracing thymic output in older individuals. Clin. Exp. Immunol. 161:497–503. 10.1111/j.1365-2249.2010.04209.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen, K.E., Daubas P., Gresser I., Sereni D., and Varet B.. 1981. Patient with circulating antibodies to alpha-interferon. Lancet. 2:1227–1228. 10.1016/S0140-6736(81)91460-4 [DOI] [PubMed] [Google Scholar]
- Pozzetto, B., Mogensen K.E., Tovey M.G., and Gresser I.. 1984. Characteristics of autoantibodies to human interferon in a patient with varicella-zoster disease. J. Infect. Dis. 150:707–713. 10.1093/infdis/150.5.707 [DOI] [PubMed] [Google Scholar]
- Ramakrishnan, K.A., Rae W., Barcenas-Morales G., Gao Y., Pengelly R.J., Patel S.V., Kumararatne D.S., Ennis S., Döffinger R., Faust S.N., and Williams A.P.. 2018. Anticytokine autoantibodies in a patient with a heterozygous NFKB2 mutation. J. Allergy Clin. Immunol. 141:1479–1482.e6. 10.1016/j.jaci.2017.11.014 [DOI] [PubMed] [Google Scholar]
- Rönnblom, L.E., Janson E.T., Perers A., Oberg K.E., and Alm G.V.. 1992. Characterization of anti-interferon-alpha antibodies appearing during recombinant interferon-alpha 2a treatment. Clin. Exp. Immunol. 89:330–335. 10.1111/j.1365-2249.1992.tb06957.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszkiewicz, J., and Smolewska E.. 2016. Kaleidoscope of autoimmune diseases in HIV infection. Rheumatol. Int. 36:1481–1491. 10.1007/s00296-016-3555-7 [DOI] [PubMed] [Google Scholar]
- Scherrer, A.U., Traytel A., Braun D.L., Calmy A., Battegay M., Cavassini M., Furrer H., Schmid P., Bernasconi E., Stoeckle M., et al. 2022. Cohort profile update: The Swiss HIV cohort study (SHCS). Int. J. Epidemiol. 51:33–34j. 10.1093/ije/dyab141 [DOI] [PubMed] [Google Scholar]
- Scordio, M., Frasca F., Santinelli L., Sorrentino L., Pierangeli A., Turriziani O., Mastroianni C.M., Antonelli G., Viscidi R.P., d’Ettorre G., and Scagnolari C.. 2022. High frequency of neutralizing antibodies to type I Interferon in HIV-1 patients hospitalized for COVID-19. Clin. Immunol. 241:109068. 10.1016/j.clim.2022.109068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjøgren, T., Bratland E., Røyrvik E.C., Grytaas M.A., Benneche A., Knappskog P.M., Kämpe O., Oftedal B.E., Husebye E.S., and Wolff A.S.B.. 2022. Screening patients with autoimmune endocrine disorders for cytokine autoantibodies reveals monogenic immune deficiencies. J. Autoimmun. 133:102917. 10.1016/j.jaut.2022.102917 [DOI] [PubMed] [Google Scholar]
- Solanich, X., Rigo-Bonnin R., Gumucio V.D., Bastard P., Rosain J., Philippot Q., Perez-Fernandez X.L., Fuset-Cabanes M.P., Gordillo-Benitez M.A., Suarez-Cuartin G., et al. 2021. Pre-existing autoantibodies neutralizing high concentrations of type I interferons in almost 10% of COVID-19 patients admitted to intensive care in barcelona. J. Clin. Immunol. 41:1733–1744. 10.1007/s10875-021-01136-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steels, S., Van Elslande J., Leuven COVID-Study Group, De Munter P., and Bossuyt X.. 2022. Transient increase of pre-existing anti-IFN-α2 antibodies induced by SARS-CoV-2 infection. J. Clin. Immunol. 42:742–745. 10.1007/s10875-022-01235-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stertz, S., and Hale B.G.. 2021. Interferon system deficiencies exacerbating severe pandemic virus infections. Trends Microbiol. 29:973–982. 10.1016/j.tim.2021.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troya, J., Bastard P., Planas-Serra L., Ryan P., Ruiz M., de Carranza M., Torres J., Martínez A., Abel L., Casanova J.L., and Pujol A.. 2021. Neutralizing autoantibodies to type I IFNs in >10% of patients with severe COVID-19 pneumonia hospitalized in Madrid, Spain. J. Clin. Immunol. 41:914–922. 10.1007/s10875-021-01036-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trutschel, D., Bost P., Mariette X., Bondet V., Llibre A., Posseme C., Charbit B., Thorball C.W., Jonsson R., Lessard C.J., et al. 2022. Variability of primary sjögren’s syndrome is driven by interferon-α and interferon-α blood levels are associated with the class II HLA-DQ locus. Arthritis Rheumatol. 74:1991–2002. 10.1002/art.42265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wijst, M.G.P., Vazquez S.E., Hartoularos G.C., Bastard P., Grant T., Bueno R., Lee D.S., Greenland J.R., Sun Y., Perez R., et al. 2021. Type I interferon autoantibodies are associated with systemic immune alterations in patients with COVID-19. Sci. Transl. Med. 13:eabh2624. 10.1126/scitranslmed.abh2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandoren, R., Boeren M., Schippers J., Bartholomeus E., Mullan K., Michels N., Aerts O., Leysen J., Bervoets A., Lambert J., et al. 2024. Unravelling the immune signature of herpes zoster: Insights into pathophysiology and the HLA risk profile. J. Infect. Dis. jiad609. 10.1093/infdis/jiad609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter, J.E., Rosen L.B., Csomos K., Rosenberg J.M., Mathew D., Keszei M., Ujhazi B., Chen K., Lee Y.N., Tirosh I., et al. 2015. Broad-spectrum antibodies against self-antigens and cytokines in RAG deficiency. J. Clin. Invest. 125:4135–4148. 10.1172/JCI80477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, E.Y., Mao T., Klein J., Dai Y., Huck J.D., Jaycox J.R., Liu F., Zhou T., Israelow B., Wong P., et al. 2021. Diverse functional autoantibodies in patients with COVID-19. Nature. 595:283–288. 10.1038/s41586-021-03631-y [DOI] [PubMed] [Google Scholar]
- Zandman-Goddard, G., and Shoenfeld Y.. 2002. HIV and autoimmunity. Autoimmun. Rev. 1:329–337. 10.1016/S1568-9972(02)00086-1 [DOI] [PubMed] [Google Scholar]
- Zhang, Q., Pizzorno A., Miorin L., Bastard P., Gervais A., Le Voyer T., Bizien L., Manry J., Rosain J., Philippot Q., et al. 2022. Autoantibodies against type I IFNs in patients with critical influenza pneumonia. J. Exp. Med. 219:e20220514. 10.1084/jem.20220514 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
shows baseline patient characteristics of the study sub-cohorts.
shows the impact of neutralizing anti-IFNα autoAbs on recorded outcomes.
shows the impact of prior events on development of anti-IFN-I autoAbs.
Data Availability Statement
Data acquired specifically for this study are available within the article itself and its supplementary materials. Patient data from the SHCS are not publicly available due to privacy reasons and the sensitivities associated with HIV infection. However, upon reasonable request, selected data can be made available to interested researchers after submission of a proposal to the respective SHCS address (http://shcs.ch/contact). The provision of data will be considered by the Scientific Board of the SHCS and the study team and is subject to Swiss legal and ethical regulations as well as a material and data transfer agreement.