Graphical abstract
Keywords: Sonochemical synthesis, Lithium (I) detection, Graphene oxide, Silvers oxide, Nanozymes, Biological fluids analysis
Highlights
-
•
Unleashing the potential of sono-fabrication of GO-Ag2O nanozyme was done.
-
•
Sono-fabrication of GO/Ag2O nanozyme was designed as an oxidize-like activity catalyst.
-
•
Detection of therapeutic and toxic levels of Li (I) in human blood serum was conducted.
-
•
LOD for Li (I) was determined at 0.01 µg/mL.
Abstract
Bipolar disorder is commonly treated with lithium carbonate. The concentration of lithium in the blood serum should be closely monitored in patients who require long-term lithium therapy. To date, no colorimetric method of detecting lithium ions has been reported using nanosensors. We have developed a novel chemosensor based on nanozyme (NZ) to address this clinical need. The GO-Ag2O NZs were synthesized by a sonochemical method and used as a colorimetric nanosensor to detect lithium ions in human blood serum (Li (I)). To characterize NZs, various techniques were employed, including XRD, FTIR, TEM, FESEM, EDX, Raman spectroscopy, BET, DLS, Zeta potential, and ICP-OES. According to TEM and FESEM images of GO-Ag2O, the nanoparticles (NPs) of Ag2O are uniformly distributed on the surface of 2D graphene oxide sheets. In addition, silver oxide nanoparticles exhibited a cubic morphology with an average size of 3.5 nm. We have examined the performance of the NZs in an aqueous medium and in human blood serum that contains Li (I). A colorimetric test revealed that NZs synthesized in the presence of ultrasound were more sensitive to Li (I). According to the linearity of the calibration curves’ ranges, Li (I) has a limit of detection (LOD) of 0.01 µg/mL. Furthermore, it displayed a linear range between 0 and 12 µg/mL. GO-Ag2O NZs showed noticeable color changes from green to orange after exposure to Li (I). An incubation time of two minutes was found to be the most effective for sensing. This innovative approach provides a reliable method for monitoring lithium levels and ensuring patient safety during long-term lithium therapy for bipolar disorder.
1. Introduction
The most commonly prescribed drug for bipolar disorder is lithium carbonate. The concentration of this drug in the blood serum must be monitored since it is usually used for a long period [1], [2]. As lithium's therapeutic and toxic limits are close together, Li (I) concentrations must remain within a narrow therapeutic range (0.4 to 1.2 mM) to maintain sufficient and stable efficacy. There is a risk of kidney failure, convulsions, comas, and even death when Li (I) concentrations exceed 1.5 mM [3], [4].
Lithium concentration in blood is currently measured by flame emission photometry [5], atomic absorption spectroscopy [6], ion selective electrodes [7], electrophoresis [8], optical spectrophotometric techniques [9], and spectrofluorimetry [10]. Even though these methods provide accuracy and sensitivity for measurement, complex laboratory methods cannot be used for personal monitoring, as they require specialized laboratories and highly trained personnel [11]. A simple and easy-to-use diagnostic method is believed to be beneficial for patients receiving lithium therapy. One of the most promising methods of determining toxic lithium levels in blood serum is colorimetric sensing, which is a sensitive, rapid, and eco-friendly technique [12]. Colorimetric nanosensors based on metal oxides have recently received considerable attention due to their cost-effectiveness, ease of use, and ability to detect objects with the naked eye [13], [14], [15], [16].
The use of nanomaterials as enzymes (i.e. nanozymes) has many advantages over natural enzymes [17]. Nanozymes (NZs) are inorganic nanomaterials capable of performing biological enzyme-like functions, including peroxidases, catalases, oxidases, phosphatases, and others [18], [19], [20]. Despite their superior sensitivity and convenience, these colorimetric methods require hydrogen peroxide (H2O2) for detection. The identification of NZs with stable and novel catalytic properties is essential for the preparation of a colorimetric detection system. The oxidase-mimicking activity is simpler and more stable than peroxidase activity since it does not require H2O2. Because of its excellent oxidase-mimicking activity, cube-shaped Ag2O was first reported to possess oxidase-mimicking activity in 2015 and was used to detect sulfites in foods [21].
One of the research studies involved the use of sodium alginate-modified Ag2O at room temperature in an aqueous solution for the colorimetric analysis of eight amino acids. L-cystine was detected selectively among the studied compounds by decolorizing the brown colloid that was visible to the naked eye [22]. Additionally, Ag2O NPs have been demonstrated to possess intrinsic oxidase-like activity and can act as catalysts for the oxidation of typical peroxidase substrates, including guaiacol, o-phenylenediamine, and tetramethylbenzidine, without requiring H2O2. However, Ag2O NZs have some disadvantages, including their instability and low reproducibility due to their aggregation [23].
To address these problems, it has been proposed to use support materials with a large specific surface area to distribute Ag2O on the surface of the support. As a result, there will be a reduction in accumulation, an increase in the number of surface-active sites, and a promotion of the nanosensor's activity and response [24]. Graphene and graphene oxide (GO) have been used in colorimetric nanosensor systems in recent years due to their excellent properties, including biocompatibility and a high surface-to-volume ratio [25]. In general, GO consists of two-dimensional sheets of carbon that contain the following oxygen-based functional groups: hydroxyl (–OH), alkoxy (C–O–C), carbonyl (C–O), and carboxylic acid (–COOH). As a result of the presence of these functional groups, GO has several desirable properties, including its solubility, reactive surface [26], high electrocatalytic activity [27], strength, and stability [28], [29].
In recent years, GO-based nanocomposites have been used for the detection of some hazardous compounds including chloramphenicol [27], methyl paraoxon [30], nitrite [31], roxarsone [32], methyl parathion [33] in food samples, and epinephrine hormone in blood serum [34].
Due to its oxygen-based functional groups, GO is also a water-soluble nanomaterial. Additionally, surface functionalization of GO has provided numerous opportunities for its use in the development of nanocomposite materials. GO exhibits high conductivity and is suitable for a variety of applications in the field, including sensors [35]. When compared to reduced GO (rGO), GO is highly hydrophilic and forms stable and homogeneous colloidal suspensions of negatively charged GO sheets in both aqueous and polar organic solvents. To deoxygenate GO, oxygen-containing groups are chemically reduced. Separating the graphene sheets individually is the most challenging aspect of the rGO production process. In the process of GO reduction by chemical methods, limited solubility or even irreversible agglomerates of rGO are formed during preparation in water and most organic solvents [36].
One of the most effective methods for preparing nanoparticles and nanostructures is sonochemical synthesis. In comparison to conventional methods, ultrasound irradiation offers several key advantages for the production of nanomaterials [26], [37]. As a result of this process, reaction times are significantly reduced, particle size can be controlled precisely, energy is conserved, and the process represents an environmentally friendly synthetic pathway [38], [39]. In contrast to traditional nanomaterial synthesis techniques, high-intensity ultrasound eliminates the need for harsh conditions. Unlike methods that require high temperatures, high pressures, or extended reaction times, ultrasound irradiation is an accessible way to fabricate nanostructures. Using ultrasonic waves as a source of energy, this sonochemical approach drives the rapid nucleation and growth of nanoscale materials. By intensifying mass transfer and causing localized heating effects, ultrasound facilitates the efficient production of nanomaterials with tuneable physical properties [40].
We have developed a colorimetric assay technique to detect lithium ions in an aqueous medium and blood serum using GO-Ag2O NZs. Ultrasonic irradiation was used to prepare the GO–Ag2O NZs with higher activity and more sensitivity to detecting Li (I). The GO–Ag2O NZs have relatively low oxidase-mimicking activity, but they can catalyze the reaction of 3,3′,5,5′-tetramethylbenzidine (TMB) to produce blue products (oxTMB). GO–Ag2O NZs displayed significantly enhanced oxidase-mimicking activity after Li (I) ions addition. Consequently, by increasing Li (I) concentration to 1 µg/mL, TMB molecules in the catalytic solution were oxidized to form dark-orange products within two minutes, resulting in an absorption peak at 460 nm. In the colorimetric method, the absorbance variation was positively correlated with the quantity of Li (I) ions, which could be used for the quantitative analysis of Li (I). The purpose of this study is to describe an innovative sonochemical method for the preparation of highly sensitive GO–Ag2O NZs with enhanced detection capability of Li (I) in human blood serum. It is expected that the constructed colorimetric method will facilitate the accurate and reliable detection of Li (I) ions without the use of H2O2.
2. Experimental
2.1. Materials
The analytical-grade reagents were used without further purification. 5,5,3,3-tetramethylbenzidine (TMB) (C16H20N2) with 98 % purity, ethanol (C2H5OH) with 99.9 % purity, hydrochloric acid (HCl) with 37 % purity, sodium hydroxide (NaOH), nitric acid (HNO3), lithium carbonate (Li2CO3), potassium chloride (KCl), copper(II) acetate (Cu(CH3COO)2), nickel(II) acetate tetrahydrate (Ni(OCOCH3)2 4H2O), chromium(III) acetate hydroxide ((CH3CO2)7Cr3(OH)2), magnesium nitrate hexahydrate (Mg(NO3)2·6H2O), glucose monohydrate (C6H12O6·H2O), sodium chloride (NaCl), iron (III) chloride (FeCl3), iron (II) sulfate (FeSO4), zinc sulfate heptahydrate (ZnSO4 7H2O), calcium chloride (CaCl2), vitamin D3 (C27H44O), folic acid (C19H19N7O6), ascorbic acid (C6H8O6), dopamine (C8H11NO2), L-valine (C5H11NO2), L-Methionine (C5H11NO2S), and trichloroacetic acid (C2HCl3O2) with 99 % purity were purchased from Sigma Aldrich. Graphene oxide (20 %) was purchased from Explorer Company of Iran. Silver nitrate (AgNO3) was obtained from Kimia Next Company (Iran). Table 1 describes the physicochemical characteristics of lithium carbonate. In all stages of the synthesis process, Milli-Q water was used with a minimum resistivity of 18.2 MΩ/cm.
Table 1.
Physicochemical properties of lithium carbonate [41].
| Compound | Lithium carbonate |
|---|---|
| Chemical formula | Li2CO3 |
| pKa | 6.38 (the first ionization steps);10.25 (the second ionization steps) |
| Molar mass [g/mol] | 73.891 |
| Solubility | (Soluble in H2O) 1 g per 100 mL (37 °C) |
| Appearance | White, granular, odorless, light alkaline powder |
| Structure |  |
2.2. Sonochemical preparation of GO-Ag2O nanozyme (GO-Ag2O NZs)
The preparation of GO-Ag2O NZs was conducted in the presence of ultrasonic waves. High-power sonochemical synthesis of GO-Ag2O was carried out using an ultrasonic homogenizer (Branson Digital Sonifier, USA, W‐450 D, working frequency: 20 kHz). As a summary, 5 mg of GO in 50 mL of distilled water was dispersed under ultrasonic irradiation at 50 % amplitude (23.1 W/cm intensity, 22 W acoustic power determined by calorimetry [14]) for 30 min at 50 ± 1 °C. Then, under continuous sonication, 25 mL of 0.007 M AgNO3 was slowly added to the suspension. Following 30 min of sonochemical reaction, the suspension turned pale gray, confirming the successful synthesis of GO-Ag2O nanocomposite. In the final step, the resultant mixture was transferred to a dark container and kept at a temperature of 4 °C. The lithium ions concentration was detected using a colloidal solution of GO-Ag2O synthesized from GO. Several intensities (21, 23.1, and 26.9 W/cm) were used during the sonochemical synthesis process to study the effect of ultrasound power on synthesis conditions and determine optimal conditions.
As mentioned above, the preparation of the GO-Ag2O nanocomposite was also performed without ultrasonic irradiation to investigate the effect of ultrasonic irradiation.
2.3. Sonochemical preparation of Ag2O nanoparticles (NPs)
0.06 g of AgNO3 was dissolved in 50 mL of distilled water at room temperature in a 250 mL water jacket reactor. The solution was then irradiated with ultrasonics at 50 % amplitude and 23.1 W/cm intensity. A dropwise addition of 25 mL of 6.0 M NaOH was added to the reaction solution for one hour until the black color precipitated confirming Ag2O formation. Upon synthesis, Ag2O nanoparticles were washed with double distilled water and dried overnight at 60° C.
2.4. Oxidase-like catalytic activity of nanoprobes (GO-Ag2O NZ)
GO-Ag2O colloidal solution was used as a nanozyme for Li (I) colorimetric detection. Briefly, 300 μL of Li (I) aqueous solution with different concentrations (0–10 µg/mL) were mixed separately with 300 μL of GO-Ag2O suspension and 300 μL of TMB solution (0.3 mM in ethanol). By using UV–Vis spectroscopy and a digital camera, the mixture's color change after 2 min was evaluated. It was found that the mixture color changed from green to dark orange at Li (I) concentrations from 0.3 to 10 µg/mL. Additionally, transmission electron microscopy (TEM) was used to determine the size of GO-Ag2O before and after the addition of Li (I) to confirm the nanosensor's aggregation.
2.5. Oxidase-like catalytic activity of GO-Ag2O NZs for detection of Li (I) in human blood serum (real sample)
Samples of lithium-free human blood were obtained from a healthy volunteer (37-year-old woman, 51 kg weight, and 160 cm height). Samples were collected according to institutional guidelines and based on the “Medical Research Ethics, Good Practice Handbook”, published by the Research Center for Medical Ethics and Medical History, Tehran, Iran [42]. A blood serum sample was obtained and diluted. Using the standard additive method, certain levels of lithium ions were added to all real samples to determine the amount of Li (I) in human serum. The Ethical Committee of the Mashhad University of Medical Sciences approved this research (IRMUMS.REC.1402.304).
2.6. Characterization and apparatus
The X-ray diffraction (XRD) patterns of the as-synthesized samples were obtained by an XRD analyzer (XRD GNR Explorer) with Co Kα radiation (λ = 1.541874 Ao) at 2θ = 5–80 degrees. Also, the Fourier-transform infrared (FTIR) spectrum was recorded with a Thermo Nicolet AVATAR 370 spectrophotometer (Thermo Nicolet Corp., USA) by the standard KBr pellet reference. Field emission scanning electron microscopy (FESEM) and Energy-dispersive X-ray spectroscopy (EDS) imaging were performed on an MIRA3 device (TESCAN, Czech Republic). To evaluate the size and morphology of the samples, Transmission electron microscopy (TEM) images were obtained using LEO 912AB (LEO, Germany). A UV–Vis spectrophotometer (Evolution 201 Spectrophotometer) was used to measure the UV–Vis spectra of the samples (Thermo Fisher Scientific). Also, the BELSORP MINI II BET Analyzer was used to perform pore volume and specific surface area measurements using the Brunauer–Emmett–Teller (BET) and Barret–Joyner–Halenda (BJH) techniques.
The Zeta potential measurements were obtained using a Zetacompact Z8000 model (CAD Instrumentation). Also, Dynamic light scattering (DLS) was performed by a Vasco particle size analyzer (Cordouan Technologies, Pessac, France). The Raman spectrum of the samples was obtained with a UniDRON device manufactured by CL Technology Co., Taiwan with a 532 nm laser. Moreover, inductively coupled plasma-optical emission spectrometry (ICP-OES, Spectro Arcos, Model 76,004,555 Germany) was used to determine Lithium in the blood serum samples.
3. Results and discussion
A green synthesis of GO-Ag2O NZs was conducted using GO and AgNO3 precursors in conjunction with ultrasonic irradiation. An overview of the ultrasonic and stirring methods used in the preparation of GO-Ag2O NZs is shown in (Scheme 1). It has been shown that ultrasonic irradiation of GO results in the cavitation phenomenon, which is responsible for the separation of the GO layers from each other [43], [44], [45].
Scheme 1.
The synthetic strategy of GO-Ag2O NZs for (a) Ultrasonic method, (b) Stirring method.
Surfaces of GO contain a variety of functional groups, including epoxy, hydroxyl, carbonyl, and carboxyl groups. As a result of the ionization of functional groups on the surface of GO sheets, a negative charge is generated on the surface when they are dispersed in water. As a result, positively charged Ag+ ions are adsorbed onto negatively charged GO sheets through electrostatic interactions [46].
It is possible that Ag2O nanoparticles precipitate from a silver-containing solution on the graphene oxide surface under ultrasonic irradiation through the following mechanisms:
-
1.
Microbubbles are formed when water molecules are exposed to ultrasound waves. As the ultrasonic power increases, these bubbles grow in size [47]. The rapid collapse of these high-energy bubbles produces localized hotspots with temperatures exceeding 5000 K and pressures exceeding 1000 bar. Within these regions, there is an incredible rate of heating and cooling, which reaches 1010 ks−1. As a consequence, this phenomenon leads to the formation of highly reactive H• and OH• radicals (Eq. (1)). H2O2 is produced in the reaction medium by highly reactive hydroxyl radicals (Eq. (2)) [47]:
| (1) |
| (2) |
-
2.
Furthermore, in an air atmosphere, •OOH radicals can also be generated through the reaction between H• radicals and O2 molecules (Eq. (3)). In turn, the recombination of these hydroperoxide radicals leads to hydrogen peroxide (Eq. (4)).
| (3) |
| (4) |
-
3.
Lastly, silver oxide nanoparticles form on the surface of graphene oxide (GO) in the presence of an H2O2-oxidizing agent and functional groups containing oxygen. As shown in Eq. (5), this reaction can be described as follows [48]:
| (5) |
In turn, these processes promote the formation of Ag2O nanoparticles on GO surfaces with a high degree of dispersion.
3.1. Optimization of GO-Ag2O NZs synthesis conditions
After the successful synthesis of GO-Ag2O NZs, the effect of various factors including ultrasonic irradiation, acoustic intensity, temperature, and amount of AgNO3 was investigated in the preparation of GO-Ag2O NZs and its Li (I) colorimetric detection. Table 2 and Fig. 1 present the obtained results.
Table 2.
Synthesis conditions for the GO-Ag2O NZs, and test results in the detection of Li (I) (4 µg/mL).
| Sample No. | Acoustic intensity [W/cm2] |
GO [g] |
AgNO3 [M] |
T [°C] |
Absorbance after interaction of GO-Ag2O NZs with Li (I) (λmax, 460 nm) |
|---|---|---|---|---|---|
| S1 | 21 (40 %) | 5 × 10−3 | 7 × 10−3 | 50 | 0.877 |
| S2 | 26.9 (60 %) | 5 × 10−3 | 7 × 10−3 | 50 | 1.2 |
| S3 | 23.1 (50 %) | 5 × 10−3 | 7 × 10−3 | 50 | 1.611 |
| S4 | − | 5 × 10−3 | 7 × 10−3 | 50 | 0.493 |
| S5 | 23.1 (50 %) | 5 × 10−3 | 5 × 10−3 | 50 | 0.227 |
| S6 | 23.1 (50 %) | 5 × 10−3 | 3 × 10−3 | 50 | 0.227 |
| S7 | 23.1 (50 %) | 5 × 10−3 | 1 × 10−3 | 50 | 0.024 |
| S8 | 23.1 (50 %) | 5 × 10−3 | 7 × 10−3 | 60 | 0.989 |
| S9 | 23.1 (50 %) | 5 × 10−3 | 7 × 10−3 | 40 | 1.213 |
| S10 | 23.1 (50 %) | 5 × 10−3 | 7 × 10−3 | 30 | 0.445 |
Fig. 1.
Effect of different parameters on the UV‐Vis spectra of the GO-Ag2O NZs after the addition of Li (I) with a concentration of 4 µg/mL; effect of (a) ultrasonic irradiation, (b) acoustic power, (c) reaction temperature, and (d) Ag ion concentration.
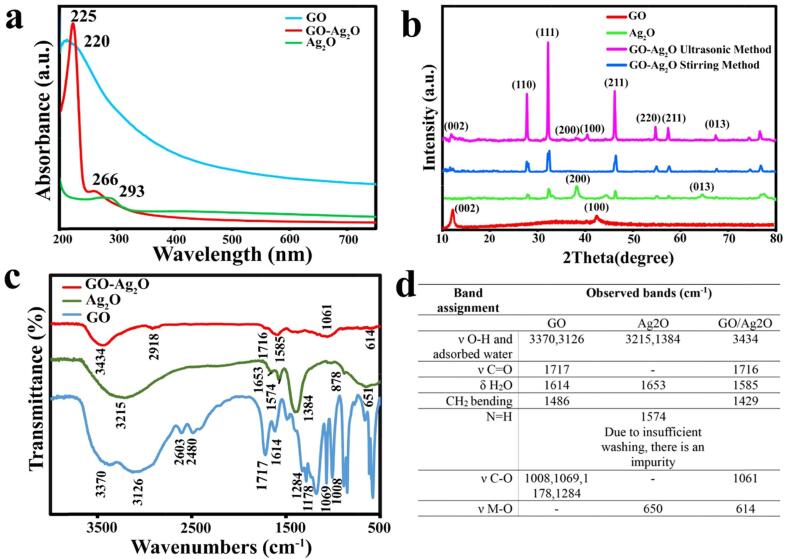
A characteristic peak has been reported in UV–Vis spectrums of suspensions containing Ag2O nanoparticles at 430 nm by other researchers [49]. UV–Vis spectra of Ag2O nanoparticles with an average particle size of less than 4 nm, however, do not show any peaks [50]. As GO-Ag2O nanoparticles in this research have an average particle size of 3.5 nm (see TEM results), UV–Vis spectra of the GO-Ag2O colloidal solution were used to study its performance after Li (I) addition. The appearance of a characteristic peak around 460 nm occurred upon the addition of Li (I) (4 µg/mL, 300 μL) at pH = 7.0 ± 0.2 after 2 min. The absorbance of the peak at 460 nm was considered a measure of the performance of the GO-Ag2O colloidal solution in Li (I) detection (4 µg/mL, 300 μL).
As shown in (Fig. 1a), significant changes in absorbance and color are observed with samples prepared with ultrasonic irradiation rather than the stirring method. This confirms that the GO-Ag2O synthesized by the ultrasonic method had better Li (I) detection performance.
However, the acoustic power effect shows that increasing ultrasonic power cannot improve the performance of the nanozyme, and the optimum amplitude was 50 % (Fig. 1b). It is assumed that high-intensity ultrasonic irradiation broke bonds between GO and Ag2O, which resulted in the nanozyme's descending performance. A study by another researcher reported similar results [51], [52]. Generally, ultrasonication time and intensity affect nanoparticle size and morphology [53], as well as bonding between nanocomposites. According to technical terms, high-intensity sonication can potentially break the bonds between Ag2O and GO. As a result of intense acoustic cavitation and shear forces during sonication, the bond between Ag2O and GO can be disrupted or even completely broken. Consequently, nanocomposites can lose structural integrity and properties. Nevertheless, the extent of bond breaking depends on several factors, such as the parameters of sonication, the duration, and the specific characteristics of the GO-Ag2O bond.
The effects of reaction temperature (30 °C–60 °C) on Li (I) detection performance of GO-Ag2O NZs have been investigated, and the results are shown in Fig. 1c. Using the nanozyme synthesized at room temperature (RT, 30 °C), Li (I) could be detected at a concentration of 4 µg/L. Additionally, increasing the temperature up to 50 °C improved the performance of the nanozyme, but increasing the temperature up to 60 °C resulted in a significant decrease in color change and Li (I) detection, possibly due to the breaking of bonds between Ag2O and GO due to increased heat agitation. According to Fig. 1d, when Ag ions concentration was increased up to 0.007 M, the prepared sample performed optimally for detecting lithium ions. According to Table 1 and Fig. 1, sample S3 is the most effective sample for detecting Li (I).
3.2. Characterization of the optimized GO-Ag2O NZs
Different techniques were used to characterize GO, Ag2O, and GO-Ag2O NZs (sample S3). Fig. 2a illustrates the UV–vis spectra of GO, Ag2O, and GO-Ag2O nanocomposite materials. The GO spectrum exhibits a significant peak at 220 nm, linked to the C=C aromatic bond and the π → π* transition [54], [55]. In the absorption spectrum of Ag2O, there is a peak at 293 nm, which is attributed to the quantum confinement of charge carriers within the nanoparticles [55]. GO-Ag2O nanocomposite spectrum shows a strong peak at 225 nm that is associated with GO [56]. The absorption peak around 266 nm is associated with Ag2O and exhibits a blue shift because Ag2O NPs are extremely small (see TEM results) [57], [58].
Fig. 2.
The results of (a) UV–Vis spectra, (b) XRD patterns, (c) FTIR spectra, and (d) Infrared transmission frequencies of GO, Ag2O, and GO-Ag2O nanocomposite.
Fig. 2b shows the results of X-ray diffraction analysis (XRD) carried out on the GO, Ag2O, and GO-Ag2O nanocomposite to determine its crystal structure. A sharp XRD peak can be observed at 12.23° in the GO XRD pattern, which is associated with the (0 0 2) plane and corresponds to oxygen-containing functionalized groups. The static disorder and defects in the GO structure are also reflected in the peak at 42.50° of the (1 0 0) plane [59].
The XRD pattern of Ag2O NPs displays the peaks at 28.13° (1 1 0), 32.5° (1 1 1) and 38.4° (2 0 0), 46.4° (2 1 1), 55.3° (2 2 0), 57.71° (2 2 1), and 64.78° (0 1 3) (JCPDS 1393-1393) [60]. The XRD patterns of the GO-Ag2O nanocomposite synthesized by ultrasonic and stirring method show that the peaks at 27.7°, 32.19°, 40.03°, 46.2°, 54.7°, 57.5°, and 67.4° correspond to the (1 1 0), (1 1 1), (2 0 0), (2 1 1), (2 2 0), (2 2 1) and (0 1 3) crystal planes of Ag2O by the standard pattern (JCPDS 1393-76) [61], [62]. Further, the intensity of the peak at 12.23° for GO-Ag2O nanocomposite synthesized by two ultrasonic and stirring methods decreased, which is related to the growth of Ag2O nanoparticles on the GO sheets [52]. The sample synthesized by ultrasonic irradiation also exhibits high crystallinity and sharp peaks.
To study the surface functionalized groups of fabricated samples and confirm the formation of Ag2O nanoparticles on GO, FTIR spectroscopy was performed. (Fig. 2c, and d).
In the case of GO, the observed peak at 3370, 3126 cm−1 is attributed to the stretching of the hydroxyl group (–OH) [63], [64]. Also, the observed peaks at 1717 cm−1 are responsible for ν C=O [65]. Furthermore, the FTIR spectrum of GO shows the peak at 1008 [66], 1069 [67], 1178 [68], 1284 cm−1, which corresponds to C–O stretching vibrations [69]. In the FTIR spectrum of Ag2O, the broad peak at 3215 [70] and 1384 [71] cm−1 refers to the hydroxyl group. The obtained characteristic absorption bands at 650 cm−1 are ascribed to vibration modes of Ag–O stretching and bending [72]. As silver oxide nanoparticles grow onto the surface of GO, the intensity of this peak has decreased in the nanocomposite. In the FTIR spectrum of GO-Ag2O nanocomposite, the broad peak at 3434 cm−1 refers to the hydroxyl group [63]. Also, the peaks at 2918 cm−1 are related to the stretching of C–H and 1061 cm−1 corresponds to C–O stretching vibrations [73]. Also, the observed peak at 614 cm−1 is attributed to Ag–O stretching.
It is obvious that the peak intensity of oxygen-containing functional groups has decreased in GO-Ag2O, which indicates that GO was reduced during the nanocomposite synthesis [63], [67], [73], [74], [75], [76], [77], [78].
As a rapid and non-destructive method of characterizing chemical bonds, Raman spectroscopy is a suitable tool for investigating carbon structures. (Fig. 3) shows Raman spectra of GO, Ag2O, and GO-Ag2O nanocomposites. GO exhibits Raman peaks at 1319 cm−1 (D band) and 1564 cm−1 (G band). The D band refers to sp3 carbon atom vibrations that are caused by defects and disorder, and the G band refers to sp2 carbon atoms bonded in hexagons. Raman spectra of GO-Ag2O nanocomposite show a D band at 1320 cm−1 and a G band at 1533 cm−1 [79], [80].
Fig.3.
The results of Raman spectroscopy of GO, Ag2O NPs, and GO-Ag2O nanocomposite.
As shown in Fig. 3, the Raman spectrum of Ag2O shows Raman peaks at about 159, 262, 358, and 463 cm−1 [81]. Raman spectra of nanocomposite show the characteristic bands of graphite, as well as peaks at 202, 393, 558, and 730 cm−1 corresponding to Ag2O, indicating that Ag2O is well composited with GO [81]. These bands are associated with the motion of oxygen anions and silver cations in the silver oxide lattice [82].
The morphology of GO-Ag2O nanocomposite was investigated using FESEM. The GO morphology comprises a porous network of small three-dimensional interconnected plates (Fig. 4a) [83], [84], [85]. The type and percentage of elements were determined using energy-dispersive X-ray spectroscopy (EDS). According to (Fig. 4b), graphene oxide contains 26.35 % carbon, 59.10 % oxygen, and 14.54 % sulfur by weight. Several studies have suggested that sulfur impurities in graphene oxide are caused by sulfur species hydrolyzing, resulting in stable sulfonic species in graphene oxide. Furthermore, FESEM analysis of graphene oxide nanosheets confirmed the random deposition of cube-shaped Ag2O NPs on the surfaces (Fig. 4c). FESEM results confirm the deposition of Ag2O NPs on the graphene oxide surface.
Fig. 4.
Low and high magnification FESEM images and EDX of (a), (b) GO and (c), (d) GO-Ag2O NZs.
Fig. 4d shows the elemental analysis of GO-Ag2O nanocomposite. This confirms the presence of carbon, oxygen, silver, and nitrogen elements with weight percentages of 12.51 %, 3.38 %, 9.14 %, and 74.98 %, respectively. As a result of these results, oxygen functional groups are removed from GO nanosheets during the formation of GO-Ag2O nanocomposite. In addition, the ratio of carbon, oxygen, and silver confirms the formation of silver oxide nanoparticles on graphene oxide, as previously observed by FESEM. Insufficient washing of the synthesized sample could lead to the presence of nitrogen in the structure of the GO-Ag2O nanocomposite due to the dissolution of AgNO3 salt into the reaction medium.
As-synthesized GO-Ag2O nanocomposites were examined under a transmission electron microscope (TEM). The TEM of GO-Ag2O nanocomposite before exposure to the drug shows Ag2O NPs are deposited on the surface of GO with high dispersion. The size of cube-shaped Ag2O NPs was determined to be about 3.5 nm based on the histogram obtained from measuring 108 nanoparticles (Fig. 5b). A TEM image of GO-Ag2O after exposure to the drug clearly shows size enhancement (Fig. 5c). By measuring 163 nanoparticles after GO-Ag2O reaction with Li (I), Ag2O NP dimensions increased to 20.34 nm (Fig. 5d). Therefore, it is clear that the addition of Li (I) to GO-Ag2O nanocomposite causes accumulation and change in Ag2O NP size.
Fig. 5.
TEM images and particle size histograms of GO-Ag2O NZs (a), (b) before, and (c), (d) after adding Li (I) with a concentration of 4 µg/mL to the reaction medium.
Furthermore, DLS analysis was conducted to determine the hydrodynamic size of nanocomposites before and after the addition of Li (I). Nanocomposite of hydrated GO- Ag2O was measured to have a particle size of 339.15 nm and a dispersion index of 0.2150. Also, the average particle size and dispersion index of GO-Ag2O in the presence of Li (I) with a concentration of 4 µg/mL were reported as 402.23 nm and 0.0970, respectively. Therefore, the nanoparticle accumulation after Li (I) addition has been confirmed, as previously shown by TEM [86].
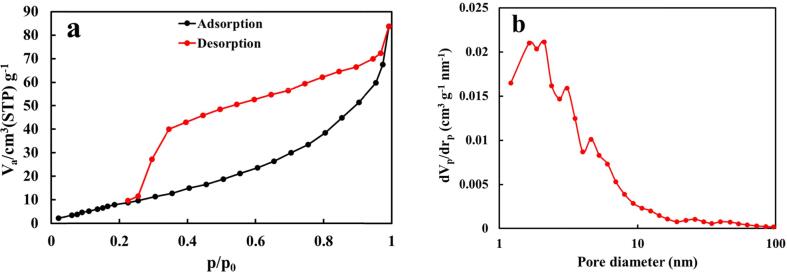
The GO-Ag2O nanozyme’s specific surface area, pore volume, and pore size distribution play crucial roles in its detection efficiency [87]. These parameters were investigated using BET-BJH analysis, and the results are depicted in Fig. 6. According to the Brunauer-Emmett-Teller (BET) method, the specific surface area of the GO-Ag2O nanozyme was determined to be 47.29 m2/g (Fig. 6a).
Fig. 6.
(a) Adsorption/desorption isotherm and (b) BJH pore size distribution of the GO-Ag2O nanozyme.
The observed type-V isotherm suggests an appropriate surface area with abundant active sites for lithium (Li) detection within the relative pressure range. Additionally, the presence of hysteresis in the isotherm confirms the existence of micro-mesopores in the structure of GO-Ag2O [88]. Furthermore, the BJH pore size distribution curve for the GO-Ag2O sample (Fig. 6b) indicates a pore volume of 0.1381 cm3/g and a mean pore size of 2.1 nm. This suggests that the GO-Ag2O nanozyme exhibits a broad pore range, spanning from 1 nm to over 100 nm, enabling it to adsorb and detect various compounds of different sizes.
Zeta potential analysis was used to determine the surface charge of nanoparticles in solution. Zeta potentials of GO-Ag2O nanocomposite were −23.49 and −28.97 mV before and after Li (I) addition. The change in zeta potential in the presence of Li (I) indicates an interaction between Li (I) and GO-Ag2O nanocomposite. The negative zeta potential is also caused by negatively charged functional groups such as carbonyls and hydroxyls [89]. Additionally, the presence of negative functional groups on the surface of GO causes electrostatic repulsion between nanomaterials, which prevents Ag2O nanoparticles from aggregating. Therefore, it increases nanoparticle dispersion and stability [90], [91].
Inductively coupled plasma (ICP) spectroscopic analysis was conducted to determine the amount of silver ions in the reactant solution and the supernatant after the nanocomposite separation. The amounts of Ag ions before and after synthesis were 11189 mg/L (0.007 M) and 218.987 mg/L (0.001 M), respectively. The formation of Ag2O NPs on the active sites of GO nanosheets is confirmed by the reduction of silver ions after the synthesis of GO-Ag2O nanocomposite.
3.3. Colorimetric assay of lithium ions (Li (I))
3.3.1. Linear range of calibration curves
A calibration curve was determined under optimum conditions (sample S3) to detect Li (I). The experimental conditions for sensing Li (I) are presented in Table 3.
Table 3.
The experimental conditions for sensing Li (I) using GO-Ag2O NZs.
| Sample | pH | Temp. | Incubation time | Color change from colorless |
λmax after the addition of Li (I) (nm) |
|---|---|---|---|---|---|
| S3 | 7.0 ± 0.2 | Room Temperature (RT) | 2 min | Blue-green (<1 μg/mL)Dark orange (>1 μg/mL) |
460 |
The calibration curves exhibited a linear range of 0–12 μg/mL with y = 0.2898x + 0.1316 (R2 = 0.9805), and a limit of detection (LOD) of 0.01 μg/mL (Fig. 7). The detection limit is calculated from the following equation:
| (6) |
In this equation, Sb is the standard deviation of the witness and m is the slope of the linear graph [92].
Fig. 7.
(a) UV‐Vis spectra of GO-Ag2O NZs solutions with different concentrations of Li (I), (b) calibration graphs for the quantification of Li (I) by using GO-Ag2O NZs as a colorimetric probe, and photographic images of GO-Ag2O NZs and Li (I) in the concentration range of 0–12 μg/mL.
A comparison between this method and other methods is presented in Table 4. As shown in Table 4, no colorimetric nanosensors have been developed for the detection of Li (I).
Table 4.
Comparison of detection method for Li (I) in blood serum.
| Method | Linear range | LOD | Reference |
|---|---|---|---|
| Potentiometric flow injection analysis system | 5 × 10−5–1 × 10−1 M | 3.4 × 10−5 M | [93] |
| Paper-Based Colorimetric | variance is below 6.1 % | 0.054 mM | [11] |
| Flame Atomic Emission Spectrometry | – | 1.17 pg dm−3 | [94] |
| Optical and impedance measurements | – | 0.1 mM | [5] |
| Electrophoresis | – | 0.1 mM | [95] |
| Electrophoresis | – | 0.15 mM | [8] |
| Chip-based capillary electrophoresis (CE) | – | less than 250 μM |
[96] |
| Electrophoresis | – | 0.4 mM | [97] |
| Graphite furnace atomic absorption spectrometry |
– | 0.8 pg and 0.01 μM | [98] |
| Argon plasma atomic emission spectrometry | – | 0.017 μM | [99] |
| Atomic absorption spectrometer | 0.05–2 mEq/L | limit of quantification 0.05 mEq/L | [100] |
| Paper-Based Colorimetric (detection window contains a concentrated detection reagent (F28 tetraphenylporphyrin, F28TPP) |
0–2 mM | 0.054 mM | [11] |
| Colorimetric (formation of LiKFe(IO6) complex) |
– | 0.14 mM | [101] |
| Colorimetric (23-membered macrocyclic mononuclear Sm(III) complex Sm-2f) |
0–1 mM | 1.147 mM | [102] |
| Colorimetric (GO-Ag2O NZs) |
0–12 μg/mL | 0.01 μg/mL | This work |
3.3.2. Kinetic studies of GO-Ag2O NZs
A steady-state kinetic assay was performed in the presence of various TMB concentrations to better understand GO-Ag2O NZs' kinetic properties. Experiments were conducted according to enzyme kinetic theory. To fit the Michaelis–Menten equation, kinetic data at varying substrates (TMB) were plotted. (Fig. 8) shows the typical Michaelis–Menten (Eq. (7)) and Lineweaver–Burk plots (Eq. (8)) for a given TMB concentration range.
| (7) |
| (8) |
where V0 is the initial velocity (initial rate), [S] is the concentration of the substrate (TMB), Km is the Michaelis–Menten constant and Vm is the maximal reaction velocity [103], [104].
Fig. 8.
The steady-state kinetic study of GO-Ag2O NZs; (a) TMB concentration varied between 0.01 and 0.5 mM (Li (I), 4 µg/mL), (b) the corresponding Lineweaver–Burk plots of the Michaelis–Menten equation.
The enzymatic kinetic parameters like Michaelis–Menten constant (Km) and the maximum reaction velocity (Vmax) were determined by fitting the Lineweaver–Burk equation. Km is a kinetic parameter that identifies the enzyme affinity towards the substrates. The low value of Km is related to the higher enzyme affinity to a substrate and vice versa.
The Km and Vm values of GO-Ag2O for TMB have obtained 0.03 mM, and 0.0002 mM s−1 which offer GO-Ag2O as a suitable artificial enzyme.
3.3.3. Interference effect
An accurate identification of Li (I) in biological samples requires the sensor to respond selectively to the analyte in the presence of interfering species. As our nanozyme detects Li (I) in blood serum, the specificity of the GO-Ag2O NZs was studied by comparing the color change of solution upon the addition of different substances to blood serum. During this test, Li (I) at a concentration of 4 (µg/mL) was used. The concentrations of the other substances were as follows: K+ (min 25 µg/mL, max 70 µg/mL), Mg2+ (min 17 µg/mL, max 90 µg/mL), Na+ (min 3105 µg/mL, max 3450 µg/mL), Zn2+ (min 0.7 µg/mL, max 1.8 µg/mL), Cu2+ (min 0.85 µg/mL, max 1.8 µg/mL), Fe2+ (min 3.5 µg/mL, max 5 µg/mL), Fe3+ (min 3.5 µg/mL, max 5 µg/mL), Ca2+ (min 0.233 µg/mL, max 0.288 µg/mL), Ni2+ (min 0.007 µg/mL, max 0.1 µg/mL), vitamin D3 (min 0.02 µg/mL, max0.15 µg/mL), folic acid (min 0.0027 µg/mL, max0.017 µg/mL), vitamin C (min 3 µg/mL, max6 µg/mL), glucose (min 700 µg/mL, max 1400 µg/mL), L-Methionine (Met) (min 1.9 µg/mL, max 6.7 µg/mL), L-valine (Val) (min 17 µg/mL, max 43.3 µg/mL), and dopamine (Dop) (3 × 10−5 µg/mL) (Fig. 9 and S1). Following the addition of Li (I) as shown in (Fig. 9 and S1), a significant color change occurred with maximum shifts toward 460 nm.
Fig. 9.
Absorbance plot of GO-Ag2O NZ s at 462 nm as a function of different substances present in blood serum within the maximum and minimum permissible range in blood serum, and related photographic images with a maximum concentration level.
Fig. 10 shows the absorbance at 462 nm of GO-Ag2O solutions after Li(I) is added in the presence of other components. In these experiments, the UV–Vis spectra of GO-Ag2O was measured when Li (I) (4 µg/mL) was added with other components at minimum and maximum levels. The photographs show that only Li (I) is detected selectively by the naked eye. The UV–Vis spectra confirmed that only Li (I) interacts specifically and selectively with the nanozyme, and that there are clear differences between all the combinations of drugs.
Fig. 10.
Absorbance plot of GO-Ag2O NZs at 462 nm as a function of different substances present in blood serum within the maximum and minimum permissible range in blood serum + Li (I), and related photographic images with maximum concentration level.
In the following section, we explain why lithium ions are selectively detected in comparison to other metals (sensing mechanism).
3.3.4. Sensing mechanism
3,3′,5,5′-tetramethylbenzidine (TMB) is commonly used as a chromogenic substrate, especially in enzyme-linked immunosorbent assay (ELISA). In the presence of H2O2, oxidation of TMB generates a blue-colored product with absorbance peaks at 370 and 652 nm. Using single electron transfer, TMB can be oxidized into its free cationic radical form. In addition, different TMB derivatives can be generated in the reaction medium, such as charge-transfer complexes and di-imines (di-cations because of double electron transfer). A blue charge-transfer complex with absorption peaks at 652 and 370 nm and a yellow di-imine derivative (460 nm) is formed by two-electron oxidation [17], [105].
In terms of reaction conditions, biological detection has some limitations. Further, enzymatic reactions require strict conditions, including pH control, catalyst type, and temperature control. It was found that the TMB could be oxidized without the addition of peroxides [21], [106]. This is a more important research topic that deserves further exploration. According to other reports, GO-Ag nanocomposites can oxidize TMB in the presence of hydrogen peroxide to produce blue products [107]. In this study, GO-Ag2O NZs catalyzed the oxidation of TMB without H2O2.
We conducted the following tests to investigate the role of GO-Ag2O NZs in the detection of Li (I). To purify and separate the nanozyme from the synthesis media, the final product was centrifuged and washed several times. After purification, GO-Ag2O NZs were dispersed in deionized water, then evaluated with a Li (I) solution and TMB (0.3 mL nanozyme solution combined with 0.3 mL Li (I) solution containing 4 µg/mL and 0.3 mL TMB solution containing 0.3 mM) (solution A, SA). As a result, the color of the final solution did not change and the UV–vis spectrum of the GO-Ag2O did not exhibit any peak at 460 nm. After that, 0.3 mL of a 1 mM Ag(I) solution was added to A (solution B, SB). With the addition of the Ag ions solution, both the color change and a peak at 460 nm in the UV–Vis spectrum of GO-Ag2O were observed (Fig. 11).
Fig. 11.
UV-vs spectra of GO-Ag2O NZs solutions in different conditions to determine the mechanism of Li detection.
In another experiment to investigate the role of Ag and Li ions in the oxidation of TMB in the absence of NZs, Ag (I) solution (1 mM, 0.3 mL) was added to 0.3 mL of Li (I) solution (0 and 4 µg/mL) and TMB solution (0.3 mM, 0.3 mL) (solution C, SC (0 µg/mL of Li (I)) and solution D, SD (4 µg/mL of Li (I))). After 2 min, the color of solutions C and D was changed to green and light orange, respectively. In addition, the UV–Vis spectra show new peaks at 370 and 652 nm for SC and a peak at 460 nm for SD.
The ability of Ag2O NPs to detect Li ions was evaluated through the synthesis and purification of Ag2O NPs. After purification, Ag2O NPs were dispersed in deionized water and evaluated in a Li (I) solution and a TMB solution (0.3 mL NP solution combined with 0.3 mL Li (I) solution containing 4 µg/mL and 0.3 mL TMB solution containing 0.3 mM) (solution E, SE). Thus, the color of the final solution did not change, and no peaks appeared in the UV–vis spectrum of GO-Ag2O.
During our research, we encountered several significant aspects, including the following. (1) Ag (I) ions present in the nanozyme solution (according to ICP analysis, 1 mM) cause TMB oxidation. This can be detected by absorption spectra (typical oxTMB peaks at 370 and 655 nm) and visually by blue color [17]. Hence, unreacted Ag+ ions in the GO-Ag2O colloidal solution may play a role in oxidizing TMB to oxTMB and producing Ag (0) [108].
(2) OxTMB can be oxidized into the di-imine derivative with Li (I) ions, which assist with electron withdrawal from the oxTMB structure, leading to double electron transfer and the production of the di-imine derivative. (3) The negative surface charge of NPs makes it easier to adsorb the positively charged TMB substrate on GO-Ag2O and increases TMB oxidation. Two amino groups in TMB bind to nanoparticle surfaces with negative charges [109]. (4) It is assumed that the increase in NP size after Li (I) and TMB addition (TEM images) is due to Ag (I) ions becoming Ag (0). (5) Two lithium ions are suggested to interact with Ag2O via adsorption on oxygen sites [110]. It begins by forming and stabilizing two Li–O contacts. The Li concentration on Ag2O is therefore inevitable to increase, which is evident in Li–O contacts [110]. The size of NPs may increase as a result of this phenomenon. Na and K ions, due to their larger ionic radius, cannot form such an adduct, so no change in color was observed. Consequently, Li was highly selectively detected in the presence of other mono-valence cations.
The following assumption could also explain the selective detection of Li (I) in comparison to the other metal ions. Lithium (520 kJ/mol) has lower ionization energy than Mg (II) (1440 kJ/mol), Zn (II) (1733 kJ/mol), Cu (II) (1958 kJ/mol), Ni (II) (1753 kJ/mol), Fe (II) (1651.9 kJ/mol), Fe (III) (2957 kJ/mol), and Ca (II) (1145.4 k) [111], [112]. Because of this, lithium ions have a higher oxidation potential than other metal ions and react more easily with oxygen atoms in Ag2O.
The ionization energies of potassium (495 kJ/mol) and sodium (419 kJ/mol) are less than those of lithium (520 kJ/mol). However, the hydration enthalpy of lithium is −515 kJ/mol while for sodium and potassium, it is −405 kJ/mol and −322 kJ/mol as lithium-ion has a higher charge density than sodium and its ions have a smaller ion size (thereby forming a stronger bond with the lone electrons in the oxygen atom of water molecules). Therefore, the hydration enthalpies of lithium and sodium differ much more than their ionization energies. Therefore, sodium and potassium atoms are easier to ionize than lithium atoms, but they are harder to hydrate. Because of this, aqueous/hydrated lithium ions have a lower reduction potential or a higher oxidation potential than aqueous/hydrated sodium and lithium ions [111], [112] (See Scheme 2).
Scheme 2.
Illustration of the detection mechanism of Li (I) using GO-Ag NZs.
3.3.5. Real sample
To determine whether the proposed method could be applied to actual samples, we measured the Li (I) content in spiked blood serum samples. The standard addition method was used to avoid matrix effects. Samples were prepared following the experimental method described in the Experimental section, and specific amounts of Li (I), which were checked in advance to ensure they were within the known linear range, were individually spiked into each sample. After 2 min of incubation, UV–Vis spectra of the solutions were measured.
Table 5 shows that reasonable recoveries (95–120 %) were obtained using the proposed method for the detection of Li (I). Each experiment was duplicated.
Table 5.
Analysis of Li (I) in spiked blood serum samples by the proposed method.
| Added [µg/ mL] |
Found [µg/ mL] |
Recovery [%] | Std.Dev(b) |
|---|---|---|---|
| 0 | ND(a) | – | – |
| 0.1 | 0.12 | 120 | 0.72 |
| 4 | 3.8 | 95 | 0.91 |
| 12 | 11.5 | 95.8 | 0.23 |
(a) ND: not detected.
(b) Std. Dev (standard deviation value for three measurements).
4. Conclusion
This research suggests a rapid, simple, low-cost, and highly specific nanosensor for the colorimetric detection of lithium in blood serum samples. The colorimetric nanoprobes are graphene oxide-silver oxide (GO-Ag2O) nanocomposites, which are synthesized using a green sonochemical process in the presence of ultrasonic irradiation. The obtained results of the colorimetric assay of lithium using the optimum sample showed a linear range of 0–12 μg/mL (R2 = 0.9805) with a limit of detection (LOD) of 0.100 μg/mL. Moreover, the fabricated GO-Ag2O nanocomposite exhibited a rapid response time (2 min) for the lithium ion at a concentration of 10 mg L−1. Generally, the results of this work show that the sonochemical-synthesized GO-Ag2O nanosensor has great potential for real-time naked-eye detection of lithium with high convenience, accuracy, and effectiveness, even in the presence of other pharmaceuticals. Also, it was clear that the ultrasonic treatment dramatically improved the sensitivity of lithium detection.
Funding sources
This research received no external funding.
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
CRediT authorship contribution statement
Maryam Entezari Khorasani: Methodology. Majid Darroudi: Funding acquisition. Tahereh Rohani Bastami: Supervision. Vahid Mahmoudi: Writing – original draft.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2024.106960.
Contributor Information
Tahereh Rohani Bastami, Email: t.rohani@qiet.ac.ir.
Vahid Mahmoudi, Email: v.mahmoudi@gonabad.ac.ir.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Singh N., Halliday A.C., Thomas J.M., Kuznetsova O.V., Baldwin R., Woon E.C., Aley P.K., Antoniadou I., Sharp T., Vasudevan S.R. A safe lithium mimetic for bipolar disorder. Nat. Commun. 2013;4:1332. doi: 10.1038/ncomms2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.R.S. El-Mallakh, R.H. Belmaker, Bipolar disorder, in: Tasman’s Psychiatry, Springer, 2023, pp. 1-54.
- 3.Sheikh M., Qassem M., Triantis I.F., Kyriacou P.A. Advances in therapeutic monitoring of lithium in the management of bipolar disorder. Sensors. 2022;22:736. doi: 10.3390/s22030736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altschul E., Grossman C., Dougherty R., Gaikwad R., Nguyen V., Schwimmer J., Merker E., Mandel S. Lithium toxicity: a review of pathophysiology, treatment, and prognosis. Pract. Neurol. 2016;12:42–45. [Google Scholar]
- 5.Qassem M., Constantinou L., Triantis I.F., Hickey M., Palazidou E., Kyriacou P.A. A method for rapid, reliable, and low-volume measurement of lithium in blood for use in bipolar disorder treatment management. I.E.E.E. Trans. Biomed. Eng. 2018;66:130–137. doi: 10.1109/TBME.2018.2836148. [DOI] [PubMed] [Google Scholar]
- 6.Rocks B.F., Sherwood R.A., Riley C. Direct determination of therapeutic concentrations of lithium in serum by flow-injection analysis with atomic absorption spectroscopic detection. Clin. Chem. 1982;28:440–443. [PubMed] [Google Scholar]
- 7.Novell M., Guinovart T., Blondeau P., Rius F.X., Andrade F.J. A paper-based potentiometric cell for decentralized monitoring of Li levels in whole blood. Lab Chip. 2014;14:1308–1314. doi: 10.1039/c3lc51098k. [DOI] [PubMed] [Google Scholar]
- 8.Vrouwe E.X., Luttge R., Vermes I., Van Den Berg A. Microchip capillary electrophoresis for point-of-care analysis of lithium. Clin. Chem. 2007;53:117–123. doi: 10.1373/clinchem.2007.073726. [DOI] [PubMed] [Google Scholar]
- 9.Gracia L.G., Rodríguez L.C., Ceba M.R. Spectrophotometric determination of lithium with Quinizarin in drugs and serum. Talanta. 1997;44:75–83. doi: 10.1016/s0039-9140(96)02018-8. [DOI] [PubMed] [Google Scholar]
- 10.Rodríguez L.C., Linares C.J., Ceba M.R. Selective spectrofluorometric determination of lithium (I) with quinizarin by extraction into tributyl phosphate. Fresenius J. Anal. Chem. 1996;356:320–325. doi: 10.1007/s0021663560320. [DOI] [PubMed] [Google Scholar]
- 11.Komatsu T., Maeki M., Ishida A., Tani H., Tokeshi M. Paper based device for the facile colorimetric determination of lithium ions in human whole blood. ACS Sensors. 2020;5:1287–1294. doi: 10.1021/acssensors.9b02218. [DOI] [PubMed] [Google Scholar]
- 12.Wu Y., Feng J., Hu G., Zhang E., Yu H.-H. Colorimetric sensors for chemical and biological sensing applications. Sensors. 2023;23:2749. doi: 10.3390/s23052749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rohani Bastami T., Bayat M., Paolesse R. Naked-eye detection of morphine by Au@ Ag nanoparticles-based colorimetric chemosensors. Sensors. 2022;22:2072. doi: 10.3390/s22052072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rohani Bastami T., Ghaedi A., Mitchell S.G., Javadian-Saraf A., Karimi M. Sonochemical synthesis of polyoxometalate-stabilized gold nanoparticles for point-of-care determination of acetaminophen levels: preclinical study in an animal model. RSC Adv. 2020;10:16805–16816. doi: 10.1039/d0ra00931h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rohani Bastami T., Ghamari Y., Khadempir S., Khorasani M.E., Paolesse R., Bayat M. Discriminative detection of morphine and methamphetamine-like street samples by label-free Cu doped-silver nanoparticles chemosensor. J. Ind. Eng. Chem. 2024;131:459–469. [Google Scholar]
- 16.Prasanna S.B., Sakthivel R., Lin L.-Y., Duann Y.-F., He J.-H., Liu T.-Y., Chung R.-J. MOF derived 2D-flake-like structured Mn3Co3O4 integrated acid functionalized MWCNT for electrochemical detection of antibiotic furazolidone in biological fluids. Appl. Surf. Sci. 2023;611 [Google Scholar]
- 17.Rohani Bastami T., Dabirifar Z. AuNPs@ PMo 12 nanozyme: Highly oxidase mimetic activity for sensitive and specific colorimetric detection of acetaminophen. RSC Adv. 2020;10:35949–35956. doi: 10.1039/d0ra06545e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei H., Wang E. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem. Soc. Rev. 2013;42:6060–6093. doi: 10.1039/c3cs35486e. [DOI] [PubMed] [Google Scholar]
- 19.Wu J., Wang X., Wang Q., Lou Z., Li S., Zhu Y., Qin L., Wei H. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II) Chem. Soc. Rev. 2019;48:1004–1076. doi: 10.1039/c8cs00457a. [DOI] [PubMed] [Google Scholar]
- 20.Tran H.V., Nguyen N.D., Tran C.T., Tran L.T., Le T.D., Tran H.T., Piro B., Huynh C.D., Nguyen T.N., Nguyen N.T. Silver nanoparticles-decorated reduced graphene oxide: A novel peroxidase-like activity nanomaterial for development of a colorimetric glucose biosensor. Arab. J. Chem. 2020;13:6084–6091. [Google Scholar]
- 21.Lu W., Shu J., Wang Z., Huang N., Song W. The intrinsic oxidase-like activity of Ag2O nanoparticles and its application for colorimetric detection of sulfite. Mater. Lett. 2015;154:33–36. [Google Scholar]
- 22.Andal V., Bhuvaneswari G. Silver oxide nano-colloid based selective colorimetric sensor for the recognition of cystine in aqueous solution. Mater. Res. Innov. 2020;24:202–209. [Google Scholar]
- 23.Zangeneh Kamali K., Pandikumar A., Jayabal S., Ramaraj R., Lim H.N., Ong B.H., Bien C.S.D., Kee Y.Y., Huang N.M. Amalgamation based optical and colorimetric sensing of mercury (II) ions with silver@ graphene oxide nanocomposite materials. Microchim. Acta. 2016;183:369–377. [Google Scholar]
- 24.Basiri S., Mehdinia A., Jabbari A. Green synthesis of reduced graphene oxide-Ag nanoparticles as a dual-responsive colorimetric platform for detection of dopamine and Cu2+ Sens. Actuators B: Chem. 2018;262:499–507. [Google Scholar]
- 25.Ji G., Tian J., Xing F., Feng Y. Optical biosensor based on graphene and its derivatives for detecting biomolecules. Int. J. Mol. Sci. 2022;23:10838. doi: 10.3390/ijms231810838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Govindasamy M., Rajaji U., Wang S.-F., Chang Y.-J., Ramalingam R.J., Chan C.-Y. Investigation of sonochemically synthesized sphere-like metal tungstate nanocrystals decorated activated carbon sheets network and its application towards highly sensitive detection of arsenic drug in biological samples. J. Taiwan Inst. Chem. Eng. 2020;114:211–219. [Google Scholar]
- 27.Rajaji U., Manavalan S., Chen S.-M., Govindasamy M., Chen T.-W., Maiyalagan T. Microwave-assisted synthesis of europium (III) oxide decorated reduced graphene oxide nanocomposite for detection of chloramphenicol in food samples. Compos. B Eng. 2019;161:29–36. [Google Scholar]
- 28.Pindiga N.Y., Musa B., Danbature W.L. Adv. Appl. Sci. Res. 2023 [Google Scholar]
- 29.Mohamed M.I., Eletr W., Awad E., Dahdouh S. Graphene oxide synthesis and characterizations as a carbonaceous nanoparticle by using modified Hummers’ method. Egypt. J. Soil Sci. 2023;63:537–552. [Google Scholar]
- 30.Rajaji U., Murugan K., Chen S.-M., Govindasamy M., Chen T.-W., Lin P.H. Graphene oxide encapsulated 3D porous chalcopyrite (CuFeS2) nanocomposite as an emerging electrocatalyst for agro-hazardous (methyl paraoxon) detection in vegetables. Compos. B Eng. 2019;160:268–276. [Google Scholar]
- 31.Govindasamy M., Wang S.-F., Huang C.-H., Alshgari R.A., Ouladsmane M. Colloidal synthesis of perovskite-type lanthanum aluminate incorporated graphene oxide composites: Electrochemical detection of nitrite in meat extract and drinking water. Microchim. Acta. 2022;189:210. doi: 10.1007/s00604-022-05296-4. [DOI] [PubMed] [Google Scholar]
- 32.Tamilalagan E., Akilarasan M., Chen S.-M., Govindasamy M., Lin K.-Y., Alzahrani F.M., Alsaiari N.S. Construction of perovskite structured ZnSnO3 embedded graphene oxide nanosheets for in-situ electrochemical quantification of organoarsenic roxarsone. Process Saf. Environ. Prot. 2023;171:705–716. [Google Scholar]
- 33.Govindasamy M., Umamaheswari R., Chen S.-M., Mani V., Su C. Graphene oxide nanoribbons film modified screen-printed carbon electrode for real-time detection of methyl parathion in food samples. J. Electrochem. Soc. 2017;164:B403. [Google Scholar]
- 34.Manavalan S., Rajaji U., Chen S.-M., Govindasamy M., Selvin S.S.P., Chen T.-W., Ali M.A., Al-Hemaid F.M., Elshikh M. Sonochemical synthesis of bismuth (III) oxide decorated reduced graphene oxide nanocomposite for detection of hormone (epinephrine) in human and rat serum. Ultrason. Sonochem. 2019;51:103–110. doi: 10.1016/j.ultsonch.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Meng N., Zhang S., Zhou Y., Nie W., Chen P. Novel synthesis of silver/reduced graphene oxide nanocomposite and its high catalytic activity towards hydrogenation of 4-nitrophenol. RSC Adv. 2015;5:70968–70971. [Google Scholar]
- 36.Gurunathan S., Han J.W., Park J.H., Kim E., Choi Y.-J., Kwon D.-N., Kim J.-H. Reduced graphene oxide–silver nanoparticle nanocomposite: a potential anticancer nanotherapy. Int. J. Nanomed. 2015:6257–6276. doi: 10.2147/IJN.S92449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen T.-W., Rajaji U., Chen S.-M., Govindasamy M., Selvin S.S.P., Manavalan S., Arumugam R. Sonochemical synthesis of graphene oxide sheets supported Cu2S nanodots for high sensitive electrochemical determination of caffeic acid in red wine and soft drinks. Compos. B Eng. 2019;158:419–427. [Google Scholar]
- 38.Rajaji U., Kumar K.Y., Arumugam R., Alothman A.A., Ouladsmane M., Chung R.-J., Liu T.-Y. Sonochemical construction of hierarchical strontium doped lanthanum trisulfide electrocatalyst: An efficient electrode for highly sensitive detection of ecological pollutant in food and water. Ultrason. Sonochem. 2023;92 doi: 10.1016/j.ultsonch.2022.106251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajaji U., Raghu M., Kumar K.Y., Almutairi T.M., Mohammed A.A., Juang R.-S., Liu T.-Y. A sonochemical synthesis of SrTiO3 supported N-doped graphene oxide as a highly efficient electrocatalyst for electrochemical reduction of a chemotherapeutic drug. Ultrason. Sonochem. 2023;93 doi: 10.1016/j.ultsonch.2023.106293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen P.-Y., Reddy T.K., Rajaji U., Alothman A.A., Govindasamy M. Optimization of electrochemical sensitivity in anticancer drug quantification through ZnS@ CNS nanosheets: synthesis via accelerated sonochemical methodology. Ultrason. Sonochem. 2024;105 doi: 10.1016/j.ultsonch.2024.106858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.H.C. Stober, Lithium Carbonate, in: Analytical Profiles of Drug Substances, Elsevier, 1986, pp. 367-391.
- 42.Smith T. Cambridge University Press; 1999. Ethics in Medical Research: A Handbook of Good Practice. [Google Scholar]
- 43.Muthoosamy K., Manickam S. State of the art and recent advances in the ultrasound-assisted synthesis, exfoliation and functionalization of graphene derivatives. Ultrason. Sonochem. 2017;39:478–493. doi: 10.1016/j.ultsonch.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 44.Tyurnina A.V., Tzanakis I., Morton J., Mi J., Porfyrakis K., Maciejewska B.M., Grobert N., Eskin D.G. Ultrasonic exfoliation of graphene in water: A key parameter study. Carbon. 2020;168:737–747. [Google Scholar]
- 45.Mellado C., Figueroa T., Baez R., Meléndrez M., Fernández K. Effects of probe and bath ultrasonic treatments on graphene oxide structure. Mater. Today Chem. 2019;13:1–7. [Google Scholar]
- 46.Golsheikh A.M., Huang N., Lim H., Zakaria R. One-pot sonochemical synthesis of reduced graphene oxide uniformly decorated with ultrafine silver nanoparticles for non-enzymatic detection of H 2 O 2 and optical detection of mercury ions. RSC Adv. 2014;4:36401–36411. [Google Scholar]
- 47.Xu H., Zeiger B.W., Suslick K.S. Sonochemical synthesis of nanomaterials. Chem. Soc. Rev. 2013;42:2555–2567. doi: 10.1039/c2cs35282f. [DOI] [PubMed] [Google Scholar]
- 48.Rohani Bastami T., Entezari M.H. Synthesis of manganese oxide nanocrystal by ultrasonic bath: Effect of external magnetic field. Ultrason. Sonochem. 2012;19:830–840. doi: 10.1016/j.ultsonch.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 49.El-Ghmari B., Farah H., Ech-Chahad A. A new approach for the green biosynthesis of Silver Oxide nanoparticles Ag2O, characterization and catalytic application. Bull. Chem. React. Eng. Catal. 2021;16:651. [Google Scholar]
- 50.S. Laouini, A. Bouafia, M. Tedjani, Catalytic activity for dye degradation and characterization of silver/silver oxide nanoparticles green synthesized by aqueous leaves extract of Phoenix dactylifera L, (2021). [DOI] [PMC free article] [PubMed]
- 51.Ručigaj A., Connell J.G., Dular M., Genorio B. Influence of the ultrasound cavitation intensity on reduced graphene oxide functionalization. Ultrason. Sonochem. 2022;90 doi: 10.1016/j.ultsonch.2022.106212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bozkurt P.A. Sonochemical green synthesis of Ag/graphene nanocomposite. Ultrason. Sonochem. 2017;35:397–404. doi: 10.1016/j.ultsonch.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 53.Elsupikhe R.F., Shameli K., Ahmad M.B. Effect of ultrasonic radiation’s times to the control size of silver nanoparticles in κ-carrageenan. Res. Chem. Intermed. 2015;41:8829–8838. [Google Scholar]
- 54.Krishnamoorthy K., Veerapandian M., Kim G.-S., Jae Kim S. A one step hydrothermal approach for the improved synthesis of graphene nanosheets. Curr. Nanosci. 2012;8:934–938. [Google Scholar]
- 55.Zheng F., Xu W.-L., Jin H.-D., Hao X.-T., Ghiggino K.P. Charge transfer from poly (3-hexylthiophene) to graphene oxide and reduced graphene oxide. RSC Adv. 2015;5:89515–89520. [Google Scholar]
- 56.Fan B., Guo H., Shi J., Shi C., Jia Y., Wang H., Chen D., Yang Y., Lu H., Xu H. Facile one-pot preparation of silver/reduced graphene oxide nanocomposite for cancer photodynamic and photothermal therapy. J. Nanosci. Nanotechnol. 2016;16:7049–7054. [Google Scholar]
- 57.Munir I., Yesiloz G. Novel size-tunable and straightforward ultra-small nanoparticle synthesis in a varying concentration range of glycerol as a green reducing solvent. ACS Omega. 2023;8:28456–28466. doi: 10.1021/acsomega.3c02697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hebeish A., El-Rafie M., El-Sheikh M.A., El-Naggar M.E. Nanostructural features of silver nanoparticles powder synthesized through concurrent formation of the nanosized particles of both starch and silver. J. Nanotechnol. 2013;2013 [Google Scholar]
- 59.Gijare M., Chaudhari S., Ekar S., Garje A. A facile synthesis of GO/CuO-blended nanofiber sensor electrode for efficient enzyme-free amperometric determination of glucose. J. Anal. Sci. Technol. 2021;12:1–10. [Google Scholar]
- 60.B.H. Shinde, P.B. Shinde, A.K. Inamdar, S.N. Inamdar, S.B. Chaudhari, Green synthesis of silver/silver oxide composite nanoparticles using Cuscuta Reflexa plant for the insecticidal applications, Materials Today: Proceedings, 92 (2023) 549-553.
- 61.Patil M.P., Piad L.L.A., Bayaraa E., Subedi P., Tarte N.H., Kim G.-D. Doxycycline hyclate mediated silver–silver chloride nanoparticles and their antibacterial activity. J. Nanostruct. Chem. 2019;9:53–60. [Google Scholar]
- 62.Zhao X., Zhang J., Wang B., Zada A., Humayun M. Biochemical synthesis of Ag/AgCl nanoparticles for visible-light-driven photocatalytic removal of colored dyes. Materials. 2015;8:2043–2053. [Google Scholar]
- 63.Liu J., Liu L., Wu X., Zhang X., Li T. Environmentally friendly synthesis of graphene–silver composites with surface-enhanced Raman scattering and antibacterial activity via reduction with l-ascorbic acid/water vapor. New J. Chem. 2015;39:5272–5281. [Google Scholar]
- 64.Rohani Bastami T., Khaknahad S., Malekshahi M. Sonochemical versus reverse-precipitation synthesis of Cu x O/Fe 2 O 3/MoC nano-hybrid: removal of reactive dyes and evaluation of smartphone for colorimetric detection of organic dyes in water media. Environ. Sci. Pollut. Res. 2020;27:9364–9381. doi: 10.1007/s11356-019-07368-0. [DOI] [PubMed] [Google Scholar]
- 65.Gul W., Alrobei H. Effect of graphene oxide nanoparticles on the physical and mechanical properties of medium density fiberboard. Polymers. 2021;13:1818. doi: 10.3390/polym13111818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Farooq S., Aziz H., Ali S., Murtaza G., Rizwan M., Saleem M.H., Mahboob S., Al-Ghanim K.A., Riaz M.N., Murtaza B. Synthesis of functionalized carboxylated graphene oxide for the remediation of Pb and Cr contaminated water. Int. J. Environ. Res. Public Health. 2022;19:10610. doi: 10.3390/ijerph191710610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Y., Zhang P., Liu C.F., Huang C.Z. A facile and green method to fabricate graphene-based multifunctional hydrogels for miniature-scale water purification. RSC Adv. 2013;3:9240–9246. [Google Scholar]
- 68.Shaker F., Vardini M.T., Es' Haghi M., Kalhor E.G. Magnetic nanoparticles and ionic liquid effect on electrochemical sensors performance. Rudarsko-Geološko-Naftni Zbornik. 2022;37:109–120. [Google Scholar]
- 69.Surekha G., Krishnaiah K.V., Ravi N., Suvarna R.P. Journal of Physics: Conference Series. IOP Publishing; 2020. FTIR, Raman and XRD analysis of graphene oxide films prepared by modified Hummers method; p. 012012. [Google Scholar]
- 70.Olya M., Vafaee M., Jahangiri M. Modeling of acid dye decolorization by TiO2–Ag2O nano-photocatalytic process using response surface methodology. J. Saudi Chem. Soc. 2017;21:633–642. [Google Scholar]
- 71.Subalakshmi A., Kavitha B., Karthika A., Nikhil S., Srinivasan N., Rajarajan M., Suganthi A. Design of Mn and Zr incorporated Ag 2 O nanoparticles and their enhanced photocatalytic activity driven by visible light irradiation for degradation of rose bengal dye. New J. Chem. 2021;45:1876–1886. [Google Scholar]
- 72.Rita A., Sivakumar A., Dhas S.S.J., Dhas S.M.B. Structural, optical and magnetic properties of silver oxide (AgO) nanoparticles at shocked conditions. J. Nanostruct. Chem. 2020;10:309–316. [Google Scholar]
- 73.Eze F.N., Tola A.J., Nwabor O.F., Jayeoye T.J. Centella asiatica phenolic extract-mediated bio-fabrication of silver nanoparticles: characterization, reduction of industrially relevant dyes in water and antimicrobial activities against foodborne pathogens. RSC Adv. 2019;9:37957–37970. doi: 10.1039/c9ra08618h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wazalwar R., Tripathi N., Raichur A.M. Mechanical and curing behavior of epoxy composites reinforced with polystyrene-graphene oxide (PS-GO) core-shell particles. Composites Part C: Open Access. 2021;5 [Google Scholar]
- 75.Soroush A., Ma W., Silvino Y., Rahaman M.S. Surface modification of thin film composite forward osmosis membrane by silver-decorated graphene-oxide nanosheets. Environ. Sci. Nano. 2015;2:395–405. [Google Scholar]
- 76.D.L. Pavia, G.M. Lampman, G.S. Kriz, J.R. Vyvyan, Introduction to Spectroscopy, (2015).
- 77.Chettri P., Vendamani V., Tripathi A., Singh M.K., Pathak A.P., Tiwari A. Green synthesis of silver nanoparticle-reduced graphene oxide using Psidium guajava and its application in SERS for the detection of methylene blue. Appl. Surf. Sci. 2017;406:312–318. [Google Scholar]
- 78.Song T., Tian W., Qiao K., Zhao J., Chu M., Du Z., Wang L., Xie W. Adsorption behaviors of polycyclic aromatic hydrocarbons and oxygen derivatives in wastewater on N-doped reduced graphene oxide. Sep. Purif. Technol. 2021;254 [Google Scholar]
- 79.Mohammadi Z., Entezari M.H. Sono-synthesis approach in uniform loading of ultrafine Ag nanoparticles on reduced graphene oxide nanosheets: An efficient catalyst for the reduction of 4-Nitrophenol. Ultrason. Sonochem. 2018;44:1–13. doi: 10.1016/j.ultsonch.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 80.Kumari S., Sharma P., Yadav S., Kumar J., Vij A., Rawat P., Kumar S., Sinha C., Bhattacharya J., Srivastava C.M. A novel synthesis of the graphene oxide-silver (GO-Ag) nanocomposite for unique physiochemical applications. ACS Omega. 2020;5:5041–5047. doi: 10.1021/acsomega.9b03976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ahmad J., Majid K. In-situ synthesis of visible-light responsive Ag2O/graphene oxide nanocomposites and effect of graphene oxide content on its photocatalytic activity. Adv. Compos. Hybrid Mater. 2018;1:374–388. [Google Scholar]
- 82.Martina I., Wiesinger R., Jembrih-Simbürger D., Schreiner M. Micro-Raman characterisation of silver corrosion products: instrumental set up and reference database. E-Preserv. Sci. 2012;9:1–8. [Google Scholar]
- 83.Paulchamy B., Arthi G., Lignesh B. A simple approach to stepwise synthesis of graphene oxide nanomaterial. J. Nanomed. Nanotechnol. 2015;6:1. [Google Scholar]
- 84.Singh S., Verma P., Ghosh S.K. Numerical and experimental analysis of performance in a compact plate heat exchanger using graphene oxide/water nanofluid. Int. J. Numer. Meth. Heat Fluid Flow. 2021;31:3356–3372. [Google Scholar]
- 85.Lakshmi V.R., Balavijayalakshmi J. Silver nanocomposites decorated reduced graphene oxide nanosheets for electrochemical sensor applications. Orient. J. Chem. 2018;34:2872. [Google Scholar]
- 86.Raval N., Maheshwari R., Kalyane D., Youngren-Ortiz S.R., Chougule M.B., Tekade R.K. Basic Fundamentals of Drug Delivery. Elsevier; 2019. Importance of physicochemical characterization of nanoparticles in pharmaceutical product development; pp. 369–400. [Google Scholar]
- 87.Guan H., Xing K., Liu S. Green synthesis of Au magnetic nanocomposites using waste chestnut skins and their application as a peroxidase mimic nanozyme electrochemical sensing platform for sodium nitrite. Foods. 2023;12:3665. doi: 10.3390/foods12193665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thommes M., Kaneko K., Neimark A.V., Olivier J.P., Rodriguez-Reinoso F., Rouquerol J., Sing K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report) Pure Appl. Chem. 2015;87:1051–1069. [Google Scholar]
- 89.Niksirat M., Sadeghi R., Esmaili J. Removal of Mn from aqueous solutions, by activated carbon obtained from tire residuals. SN Appl. Sci. 2019;1:782. [Google Scholar]
- 90.Zhu J., Ni H., Hu C., Zhu Y., Cai J., Liu S., Gao J., Yang H., Liu H. Rapid synthesis and characterization of silver-loaded graphene oxide nanomaterials and their antibacterial applications. R. Soc. Open Sci. 2021;8 doi: 10.1098/rsos.201744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bao Y., Tian C., Yu H., He J., Song K., Guo J., Zhou X., Zhuo O., Liu S. In situ green synthesis of graphene oxide-silver nanoparticles composite with using gallic acid. Front. Chem. 2022;10 doi: 10.3389/fchem.2022.905781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shrivastava A., Gupta V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011;2:21–25. [Google Scholar]
- 93.Coldur F., Andac M. A flow-injection potentiometric system for selective and sensitive determination of serum lithium level. Electroanalysis. 2013;25:732–740. [Google Scholar]
- 94.Dol I., Knochen M., Vieras E. Determination of lithium at ultratrace levels in biological fluids by flame atomic emission spectrometry. Use of first-derivative spectrometry. Analyst. 1992;117:1373–1376. doi: 10.1039/an9921701373. [DOI] [PubMed] [Google Scholar]
- 95.Vrouwe E.X., Luttge R., Olthuis W., van den Berg A. Microchip analysis of lithium in blood using moving boundary electrophoresis and zone electrophoresis. Electrophoresis. 2005;26:3032–3042. doi: 10.1002/elps.200500012. [DOI] [PubMed] [Google Scholar]
- 96.Sewart R., Gärtner C., Klemm R., Schattschneider S., Becker H. Microfluidic device for fast on-site biomedical diagnostic on the example of lithium analysis in blood. Biomed. Eng./Biomedizinische Technik. 2012;57:729–732. [Google Scholar]
- 97.Vrouwe E.X., Luttge R., van den Berg A. Direct measurement of lithium in whole blood using microchip capillary electrophoresis with integrated conductivity detection. Electrophoresis. 2004;25:1660–1667. doi: 10.1002/elps.200405885. [DOI] [PubMed] [Google Scholar]
- 98.Zhao J., Gao P., Wu S., Zhu D. Superiority of nitric acid for deproteinization in the determination of trace lithium in serum by graphite furnace atomic absorption spectrometry. J. Pharm. Biomed. Anal. 2009;50:1075–1079. doi: 10.1016/j.jpba.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 99.Leflon P., Plaquet R., Rose F., Hennon G., Ledeme N. Rapid determination of lithium in human serum and urine, at physiological concentrations, by inductively coupled argon plasma atomic emission spectrometry. Anal. Chim. Acta. 1996;327:301–306. [Google Scholar]
- 100.Santo C.E.d.E., Carvalho T.M.d.J.P. Determination of serum lithium: comparison between atomic emission and absorption spectrometry methods. Jornal Brasileiro De Patologia e Medicina Laboratorial. 2014;50:12–19. [Google Scholar]
- 101.Quartarolli L.F., Silveira A.T., Toma H.E. Overcoming lithium analysis difficulties with a simple colorimetric/spectrophotometric method. Anal. Methods. 2021;13:3627–3631. doi: 10.1039/d1ay00937k. [DOI] [PubMed] [Google Scholar]
- 102.Zhang K., Chen T.-T., Zhang L.-F., Ma S., Shen Y.-J., Feng C.-C., Nie P.-P., Yang Z.-R., Zhu C. The selective colorimetric probe based on a macrocyclic Sm (III) complex for detecting lithium ion and its performance in the psychiatric drug. Dyes Pigm. 2020;174 [Google Scholar]
- 103.Wang Y., Li T., Wei H. Determination of the Maximum Velocity of a Peroxidase-like Nanozyme. Anal. Chem. 2023;95:10105–10109. doi: 10.1021/acs.analchem.3c01830. [DOI] [PubMed] [Google Scholar]
- 104.Singh S., Tripathi P., Kumar N., Nara S. Colorimetric sensing of malathion using palladium-gold bimetallic nanozyme. Biosens. Bioelectron. 2017;92:280–286. doi: 10.1016/j.bios.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 105.Busa L.S.A., Komatsu T., Mohammadi S., Maeki M., Ishida A., Tani H., Tokeshi M. 3, 3′, 5, 5′-Tetramethylbenzidine oxidation on paper devices for horseradish peroxidase-based assays. Anal. Sci. 2016;32:815–818. doi: 10.2116/analsci.32.815. [DOI] [PubMed] [Google Scholar]
- 106.Zhan X., Tang Y., Liu Y., Tao H., Wu Y. A novel colorimetric strategy for rapid detection of dimethoate residue in vegetables based on enhancing oxidase-mimicking catalytic activity of cube-shape Ag2O particles. Sensors Actuators B: Chem. 2022;361 [Google Scholar]
- 107.Zhao Q., Gou W., Zhang X., Zhang M., Bu Y., Wang L., Hu L., Yao W., Yan Z. Hg2+-activated oxidase-like activity of Ag2S@ graphene oxide nanozyme and its naked-eye monitoring Hg2+ application with obvious hyperchromic effect. Appl. Surf. Sci. 2021;545 [Google Scholar]
- 108.Zhang F., Wang X., Jie X., Wei W. Test paper for colorimetric inspection of fatty acids and edible oils. Sensors. 2018;18 doi: 10.3390/s18103252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yu F., Huang Y., Cole A.J., Yang V.C. The artificial peroxidase activity of magnetic iron oxide nanoparticles and its application to glucose detection. Biomaterials. 2009;30:4716–4722. doi: 10.1016/j.biomaterials.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Priyadarshini C.H., Sudha V., Harinipriya S. Computational mechanistic insights on Ag 2 O as a host for Li in lithium-ion batteries. Phys. Chem. Chem. Phys. 2022;24:16112–16124. doi: 10.1039/d2cp01674e. [DOI] [PubMed] [Google Scholar]
- 111.B. Fricke, Superheavy elements a prediction of their chemical and physical properties, in: Recent impact of physics on inorganic chemistry, Springer, 2007, pp. 89-144.
- 112.Mattolat C., Gottwald T., Raeder S., Rothe S., Schwellnus F., Wendt K., Thörle-Pospiech P., Trautmann N. Determination of the first ionization potential of technetium. Phys. Rev. A. 2010;81 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.