Abstract
Introduction
Patients with non-ST segment elevation acute coronary syndrome (NSTE-ACS) and concomitant multivessel coronary artery disease (CAD) are considered patients with extremely high-risk atherosclerotic cardiovascular disease (ASCVD), and current guidelines specify a lower low-density lipoprotein cholesterol (LDL-C) target for this population. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors have been shown to effectively reduce LDL-C levels on a statin background. Additionally, several studies have confirmed the role of PCSK9 inhibitors in plaque regression and reducing residual cardiovascular risk in patients with ACS. However, those studies included coronary lesions with a degree of stenosis <50%. Whether the application of PCSK9 inhibitors in patients with NSTE-ACS with non-culprit artery critical lesions (stenosis degree between 50% and 75%) has a similar effect on plaque regression and improvement of cardiovascular outcomes remains unknown, with a lack of relevant research. This study aims to further investigate the safety and efficacy of evolocumab in patients with NSTE-ACS and concomitant multivessel CAD (non-culprit artery stenosis between 50% and 75%).
Methods and analysis
In this single-centre clinical randomised controlled trial, 122 patients with NSTE-ACS and concomitant multivessel CAD (non-culprit artery stenosis between 50% and 75%) will be randomly assigned to either the evolocumab treatment group or the standard treatment group after completing culprit vessel revascularisation. The evolocumab treatment group will receive evolocumab in addition to statin therapy, while the standard treatment group will receive standard statin therapy. At baseline and week 50, patients in the evolocumab treatment group will undergo coronary angiography and OCT imaging to visualise pre-existing non-lesional vessels. The primary end point is the absolute change in average minimum fibrous cap thickness (FCT) from baseline to week 50. Secondary end points include changes in plaque lipid arc, lipid length, macrophage grading, lipid levels and major adverse cardiovascular events during the 1-year follow-up period.
Ethics and dissemination
Ethics: this study will adhere to the principles outlined in the Helsinki Declaration and other applicable ethical guidelines. This study protocol has received approval from the Medical Research Ethics Committee of the First Affiliated Hospital of the University of Science and Technology of China (Anhui Provincial Hospital), with approval number 2022-ky214. Dissemination: we plan to disseminate the findings of this study through various channels. This includes publication in peer-reviewed academic journals, presentation at relevant academic conferences and communication to the public, policymakers and healthcare professionals. We will also share updates on the research progress through social media and other online platforms to facilitate the exchange and application of scientific knowledge. Efforts will be made to ensure widespread dissemination of the research results and to have a positive impact on society.
Trial registration number
ChiCTR2200066675.
Keywords: coronary intervention, myocardial infarction, cardiovascular disease, coronary heart disease
STRENGTHS AND LIMITATIONS OF THIS STUDY.
A strength of our study is that the SPECIAL study focuses on non-culprit vessels of patients with non-ST segment elevation acute coronary syndrome with a stenosis degree ranging from 50% to 75%, indicating more severe lesions compared with GLAGOV and HUYGENS.
A limitation of our study is its single-centre design, which could restrict the generalisability of findings due to potential variability in regional practices, population demographics and treatment facilities across centres, possibly leading to different outcomes.
Another limitation of this study is that each subject received a daily dose of 10 mg of rosuvastatin, which, while more reflective of real-world research settings, does not align with the guideline-recommended dosage for statins, potentially resulting in many patients not achieving their low-density lipoprotein cholesterol treatment goals.
Introduction
With the increasing ageing population and rising prevalence of chronic diseases such as diabetes, the incidence of acute coronary syndrome (ACS) is on the rise year by year.1 Non-ST segment elevation acute coronary syndrome (NSTE-ACS) accounts for approximately 70% of all ACS cases.2 Although the incidence of in-hospital complications (such as cardiogenic shock, heart failure and arrhythmias) in patients with NSTE-ACS is lower than in ST-segment elevation myocardial infarction (STEMI), the long-term mortality rate after discharge is higher than in STEMI.3 Among patients with NSTE-ACS, a considerable number have concomitant multivessel coronary artery disease, meaning that in addition to the culprit vessel, there are non-culprit vessels that may contribute to myocardial ischaemia.4 5 These non-culprit lesions may also undergo plaque rupture, leading to acute cardiovascular events. In fact, approximately half of cardiovascular events in patients with ACS postpercutaneous coronary intervention (PCI) are related to non-culprit lesions.6 7 As a result, there is a growing focus on the long-term management of patients diagnosed with NSTE-ACS and multivessel coronary artery disease following PCI.
Oxidised low-density lipoprotein (ox-LDL) is a harmful form of cholesterol, and its continuous accumulation is a crucial factor in the formation of atherosclerotic plaques.8 9 Lowering plasma low-density lipoprotein cholesterol (LDL-C) levels has been shown to significantly improve cardiovascular outcomes in patients.10,12 Patients who recently experienced ACS and have concomitant multivessel coronary artery disease are considered patients with extremely high-risk atherosclerotic cardiovascular disease (ASCVD), and current guidelines specify a lower LDL-C target for this population.13 14 Statins are the first-line treatment to lower LDL-C levels in patients with coronary artery disease; however, over 50% of patients with coronary artery disease treated with statins do not achieve target LDL-C levels (<70 mg/dL).15 Non-culprit coronary plaques continue to progress in patients even after 2 years of routine medication, including statins, following percutaneous coronary stent implantation (PCI).8 Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors have been demonstrated to significantly reduce LDL-C levels on a statin foundation.16,19 Furthermore, current knowledge on the pathophysiological action of PCSK9 suggests that the rapid pharmacological inhibition might provide an effective intervention in ACS to prevent further cardiovascular events. An effective and complete inhibition of PCSK9 with monoclonal antibodies, together with the use of statins, might reduce platelet activation and control the inflammatory response associated with ACS.20 Additionally, large clinical randomised controlled studies, including GLAGOV, HUYGENS and PACMAN-AMI, have consistently confirmed the ability of PCSK9 inhibitors to reverse plaque formation and reduce residual cardiovascular risk in patients with ASCVD.16 21 22 It is important to note, however, that the aforementioned studies included coronary lesions with a degree of stenosis <50%. Whether the application of PCSK9 inhibitors has a similar plaque-reversing effect in patients with NSTE-ACS and concomitant non-culprit artery critical lesions remains unknown due to lack of relevant research. Therefore, this study aims to investigate the safety and efficacy of PCSK9 inhibitor (evolocumab) in patients with NSTE-ACS with non-culprit artery critical lesions (stenosis degree between 50% and 75%).
Methods
Study design
The SPECIAL trial is a single-centre, randomised controlled study focusing on patients with NSTE-ACS and concomitant non-culprit artery critical lesions. Patients will be recruited from The First Affiliated Hospital of University of Science and Technology of China, Anhui Provincial Hospital. As outlined in box 1, within 24 hours of completing culprit vessel revascularisation, the principal investigator will confirm patient eligibility and obtain written informed consent. In summary, patients meeting the following criteria will be eligible:
Box 1. Eligible criteria.
Inclusion criteria
Age ≥18 years old.
NSTE-ACS with at least one coronary segment (culprit lesion) requiring PCI.
LDL-C ≥70 mg/dL assessed prior to or during PCI in patients receiving any stable statin regimen within ≥4 weeks prior to enrolment; OR LDL-C ≥130 mg/dL in statin-naïve patients or those not on a stable statin regimen for ≥4 weeks prior to enrolment.
At least one major native coronary arteries (‘target vessels’) each meeting the following criteria for intracoronary imaging immediately following the qualifying PCI procedure.
Angiographic evidence of 50%–75% reduction in lumen diameter by angiographic visual estimation.
Target vessel deemed to be accessible to imaging catheters and suitable for intracoronary imaging in the proximal (50 mm) segment (‘target segment’).
Target vessel may not be a bypass (saphenous vein or arterial) graft or a bypassed native vessel.
Target vessel must not have undergone previous PCI within the target segment.
Haemodynamic stability allowing the repetitive administration of nitroglycerine.
Ability to understand the requirements of the study and to provide informed consent.
Willingness of patient to undergo follow-up intracoronary imaging.
Exclusion criteria
Left main disease, defined as ≥50% reduction in lumen diameter of the left main coronary artery by angiographic visual estimation.
Three-vessel disease, defined as ≥75% reduction in lumen diameter of three major epicardial coronary arteries by angiographic visual estimation or in major branches of one or more of these arteries, irrespective of the localisation (proximal 50 mm or more distal localisation) of the obstructive lesions.
History of coronary artery bypass surgery.
Unstable clinical status (haemodynamic or electrical instability).
Severe renal dysfunction, defined by estimated glomerular filtration rate <30 mL/min/1.73 m2.
Active liver disease or hepatic dysfunction.
Known intolerance to rosuvastatin OR known statin intolerance.
Known allergy to contrast medium, heparin, aspirin, ticagrelor or prasugrel.
Patients who previously received PCSK9 inhibitor.
Patient who received cholesterol ester transfer protein inhibitors in the past 12 months prior to screening.
Treatment with systemic steroids or systemic ciclosporin in the past 3 months.
Known active infection or major haematological, metabolic or endocrine dysfunction in the judgement of the investigator.
Patients who will not be available for study required visits in the judgement of the investigator.
Current enrolment in another investigational device or drug study.
Estimated life expectancy <1 year.
LDL-C, low-density lipoprotein cholesterol; NSTE-ACS, non-ST segment elevation acute coronary syndrome; PCI, percutaneous coronary intervention; PCSK9, proprotein convertase subtilisin/kexin type 9.
Patients with NSTE-ACS with concomitant multivessel coronary artery disease, having undergone culprit vessel revascularisation, with stenosis in non-culprit vessels, as assessed by coronary angiography, ranging from 50% to 75%, and no history of coronary stent implantation.
Patients who have received statin therapy for >4 weeks before enrolment with LDL-C levels ≥70 mg/dL, or patients who have not received statin therapy for >4 weeks before enrolment with LDL-C levels ≥130 mg/dL.
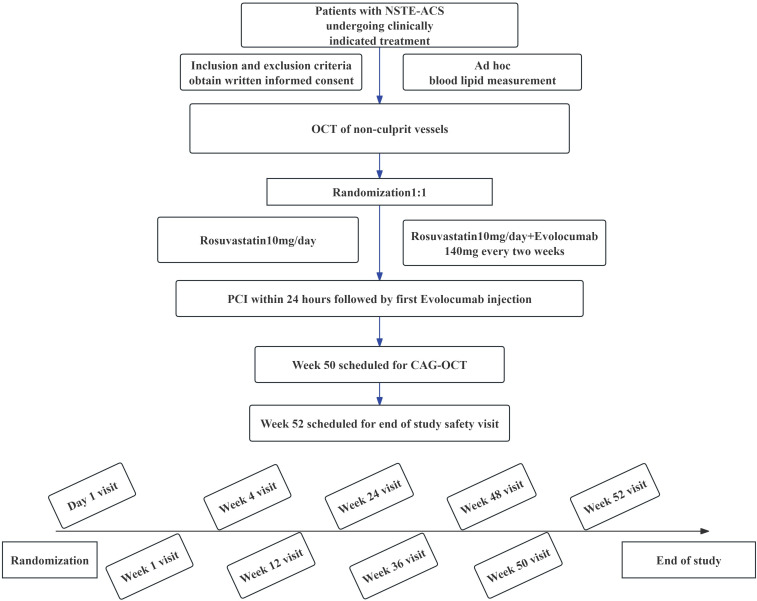
A total of 122 patients will be randomly allocated in a 1:1 ratio to either the evolocumab treatment group or the standard treatment group. All patients will receive statin therapy at the protocol-specified intensity, with rosuvastatin 10 mg/day throughout the study, and there will be no changes in the dose or type of statin during the entire study period. Concomitant medications may include aspirin, clopidogrel, ticagrelor, rosuvastatin, oral hypoglycaemic agents and insulin. The evolocumab treatment group will receive evolocumab (140 mg subcutaneous injection every 2 weeks) in addition to statin therapy. The first dose of evolocumab will be administered to patients within 24 hours post-PCI, followed by self-administration by patients at home. The study protocol flow chart is depicted in figure 1.
Figure 1. Study flow chart and schedule of study enrolment and interventions. CAG, coronary arteriography; NSTE-ACS, non-ST segment elevation acute coronary syndrome; OCT, optical CT; PCI, percutaneous coronary intervention.
After screening, enrolment and randomisation, each patient’s total study duration will be 52 weeks, comprising a treatment period of 50 weeks and a 2-week follow-up period after the end of the treatment. Baseline and follow-up coronary angiography and OCT assessments will be conducted on day 1 and week 50, respectively. OCT evaluations will assess plaque information in non-culprit vessels, including minimum fibrous cap thickness (FCT), lipid arc, lipid length and macrophage grading. Follow-up will occur at weeks 1, 4, 12, 24, 36, 48 and 52 through telephone or outpatient visits. Follow-up assessments will include recording changes in biomarkers such as total cholesterol, LDL-C, high-density lipoprotein cholesterol (HDL-C), triglycerides, non-HDL-C, lipoprotein (a) and monitoring for major adverse cardiovascular events, including death, non-fatal myocardial infarction, target vessel revascularisation, ischaemic stroke or transient ischaemic attack.
Randomisation
After signing the informed consent form, eligible patients will be randomly assigned to different lipid-lowering regimens in a 1:1 ratio for treatment (figure 1). The randomisation sequence will be generated by a computer program before the start of recruitment, and the results of the randomisation sequence will be maintained by designated personnel. Once patients meet the inclusion criteria, relevant staff will disclose the patient’s random sequence and will not be involved in subsequent data collection and patient follow-up. This randomisation method ensures both fairness and transparency in allocation, as well as the effectiveness and consistency of research data. Through this approach, our aim is to impartially assess the safety and efficacy of PCSK9 inhibitors (evolocumab) in patients with NSTE-ACS and multiple coronary artery lesions (with completed revascularisation of culprit vessels, and non-culprit vessels narrowed between 50% and 75%). This study seeks to provide an optimised treatment strategy for patients with NSTE-ACS.
OCT imaging acquisition and analysis
In patients with NSTE-ACS and multiple lesions after coronary intervention of the culprit vessel, operators perform coronary angiography and visual assessment of non-culprit vessels. If non-culprit vessels show non-invasive stenosis between 50% and 75%, OCT examination is conducted. After 50 weeks, patients undergo repeat coronary angiography and OCT imaging to visualise the original non-culprit vessels. The OCT imaging catheter is positioned as distally as possible and automatically retracted to the aorta at a speed of 36 mm/s to obtain OCT images. All imaging performed will be electronically transferred to the central core laboratory at the First Hospital of the University of Science and Technology of China, Anhui Provincial Hospital. The quality of all OCT images is then assessed, and on completion of the study, plaque phenotypes and burden will be measured in selected non-culprit vessels. For the acquired OCT images, cross-sectional images at intervals of 0.2 mm will be selected for analysis. Each frame will be classified based on a hierarchical approach as normal vessel, fibrous plaque, fibrocalcific plaque and fibroatheroma. A fibroatheroma is defined as a plaque with evidence of lipid pool >90°, characterised by a poorly defined or diffuse border between the signal-poor region and surrounding tissue, without lateral delineation. For each image depicting a plaque, measurements of minimum FCT and lipid arc will be conducted by analysts who are blinded to the treatment status of the patient and to imaging timepoint (baseline/follow-up). The lipid arc and lipid length (defined as the length of plaque with >90° of lipid measured on the longitudinal view) will be assessed. Grading will be performed based on the presence of macrophages and calcium deposits in each image.
Patient and public involvement
There was no patient or public involvement in the design and conduct of this study.
Trial stage
April 2023–December 2023: completion of subject recruitment.
January 2024–December 2024: completion of subject follow-up.
January 2025–March 2025: completion of research data analysis.
We successfully completed the recruitment of our first subject on 2 April 2023.
Results
As the primary focus of this study is to investigate whether the application of PCSK9 inhibitors has a reversing effect on plaques and improves cardiovascular outcomes in patients with NSTE-ACS and non-culprit vessel critical lesions, the primary end point is the absolute change in minimum FCT. Secondary end points include changes in plaque lipid arc, lipid length and macrophage grading. Additionally, assessments will be made for changes in lipid biomarkers and the evaluation of cardiovascular adverse events during the 1-year follow-up period (box 2).
Box 2. Summary of SPECIAL endpoints.
Primary end points
OCT
Absolute change in minimum FCT from baseline to week 50.
Secondary end points
OCT
Absolute change in the average of minimum FCT for all images.
Absolute change in maximum lipid arc throughout the segment.
Absolute change in maximum lipid length throughout the segment.
Macrophage grading.
Change in lipid levels (cholesterol, LDL-C, HDL-C, triglycerides, Lp(a))
MACE
All-cause death.
Cardiac death.
Non-fatal myocardial infarction.
Ischaemia-driven coronary revascularisation.
Ischaemic stroke/TIA.
FCT, fibrous cap thickness; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MACE, major adverse cardiovascular events; TIA, transient ischaemic attack.
Study plan and follow-up
The follow-up plan encompasses both baseline and long-term clinical outcomes (figure 1). Collected clinical data include patients’ preoperative baseline data, demographic information, coronary artery lesion details (including location, degree of stenosis, PCI treatment information, etc), laboratory tests (such as cholesterol, triglycerides, LDL-C), imaging examinations (ECGs, echocardiograms), medication history and primary and secondary end point data. The follow-up duration for all participants is 52 weeks, during which primary and secondary end points, as well as safety, will be assessed. The SPECIAL trial has been implemented in April 2023.
Sample size
The SPECIAL trial is an interventional clinical pilot study focusing on patients with NSTE-ACS and multiple coronary artery lesions (culprit vessel revascularisation completed, non-culprit vessel stenosis 50%–75%). The objective is to investigate whether evolocumab has a significant effect on reversing plaque thickening. Reviewing the literature, statin drugs have a thickening rate of 40% for FCT. Assuming that our drug’s effect on FCT will increase, with the thickening rate as an indicator, even if the rate reaches 55%, it represents a 15% increase over the baseline. With α=0.05, β=0.1, a dropout rate of 10%, according to the sample size calculation formula, the sample size for each group is n=(Uα+Uβ)22P(1−P)/(P1−P0)2, where P=(P1−P0)/2×100%. The calculated sample size for each group is 55 cases, with a total of 110 cases for both groups. Considering a 10% dropout rate, the estimated minimum sample size is 122 cases, with at least 61 cases per group.
Statistical analysis
All data will be statistically analysed using SPSS V.22.0 software. Continuous data will be presented as mean±SD (x±s), and intergroup comparisons will be conducted using analysis of covariance. This allows us to control for the effects of one or more covariates while comparing the main effects. Categorical data will be expressed as frequencies (percentages) (n(%)), and comparisons between groups will be made using the χ2 test. Logistic regression analysis will be employed to determine the correlation between evolocumab and plaque FCT. A significance level of p<0.05 will be considered statistically significant. All statistical tests will be two-tailed.
Discussion
The SPECIAL trial investigated the effects of the PCSK9 inhibitor evolocumab on non-culprit vessel plaques in patients with NSTE-ACS and multivessel coronary disease (completed culprit vessel revascularisation, non-culprit vessel stenosis between 50% and 75%). The study assessed changes in non-culprit vessel plaques, including alterations in minimum FCT, absolute changes in maximum lipid arc and macrophage grading, as well as the impact on residual cardiovascular risk after PCI.
Previous studies have consistently demonstrated that postoperative residual cardiovascular risk remains elevated in patients with ACS and multivessel coronary disease, with over half of cardiovascular events being attributed to non-culprit vessels.6 7 Despite the rapid advancements in PCI technology, this intervention fails to alter the unstable plaque status and the risk of thrombotic recurrence in patients with ACS.23,25 After 2 years post-PCI, even with standard medication, including statins, patients still experience continuous progression of non-culprit coronary plaques.7 This phenomenon may be linked to the dissatisfaction with lipid control rates among patients with ACS in the real world. Cannon et al26 discovered that within a 2-year timeframe, only 17.1% of patients with ASCVD received intensified lipid-lowering therapy, and two-thirds of them maintained LDL-C levels >70 mg/dL. These findings highlight the challenges in achieving optimal lipid control in the real-world scenario, potentially contributing to the persistent progression of non-culprit coronary plaques despite conventional treatments, including statins.
In pathological research, the morphological and compositional features of plaques have long been associated with plaque vulnerability and rupture, which can lead to significant cardiovascular events.9 27 Clinically, besides altering patients’ lipid metabolism, reducing LDL-C is crucial for modifying the vulnerability of plaques, ultimately aiming to lower the occurrence of cardiovascular events. Statins are the frontline treatment for lowering LDL-C levels in patients with coronary heart disease. Numerous intravascular ultrasound (IVUS) studies have also demonstrated that intensified statin therapy promotes the regression of coronary atherosclerosis, with the degree of benefit proportional to the reduction in lipid levels.920,31 Furthermore, statins have been shown to increase FCT measured by OCT32,35 and reduce lipid content derived from near-infrared spectroscopy.36
PCSK9 inhibitors have proven to significantly lower LDL-C levels on top of statin therapy.16 Large clinical randomised controlled studies, including GLAGOV, HUYGENS and PACMAN-AMI, have indicated that PCSK9 inhibitors have the ability to reverse plaques and reduce cardiovascular residual risk in patients with ACS.16 21 22 However, it is noteworthy that in the aforementioned studies, the observed narrowing of coronary arteries ranged between 20% and 50%. Whether the application of PCSK9 inhibitors has similar effects on plaque reversal and reduction of cardiovascular residual risk in patients with NSTE-ACS combined with non-culprit coronary critical lesions remains unclear and lacks relevant research.
The SPECIAL trial aims to provide further mechanistic insights into the impact of effective LDL-C reduction on patients with NSTE-ACS combined with non-culprit coronary critical lesions. This includes exploring composition, and microstructural characteristics, aiming to offer additional evidence for the efficacy of PCSK9 inhibitors in lowering LDL-C levels and improving clinical outcomes.
In comparison with prior intravascular imaging studies assessing the impact of lipid-lowering therapy on atherosclerosis, the SPECIAL trial will offer further insights into non-culprit coronary critical lesions.
Comparison with GLAGOV and HUYGENS trials
The GLAGOV trial primarily recruited patients with stable coronary heart disease, while the HUYGENS study focused on non-culprit vessels of patients with NSTEMI. Although both studies demonstrated the effects of evolocumab on plaque stability and reversal, they specifically targeted vessels with a 20%–50% degree of stenosis. In contrast, the SPECIAL study focuses on non-culprit vessels of patients with NSTE-ACS with a stenosis degree ranging from 50% to 75%, indicating more severe lesions compared with GLAGOV and HUYGENS. While the primary end points of interest remain plaque reversal or stability, considering lesions of varying severity implies different disease stages for patients. More severe stenosis indicates differences in haemodynamics, impacting patient prognosis. Therefore, the SPECIAL study aims to enrich plaque data for non-culprit coronary critical lesions and provide additional research evidence on the safety and efficacy of PCSK9 inhibitors in such patients.
Choice of OCT imaging
Different intravascular imaging modalities yield distinct characteristics in imaging data. Reports suggest that features of vulnerable plaques on OCT are associated with larger cardiovascular risks. The SPECIAL study opts for OCT as the intravascular imaging tool to acquire plaque information. Compared with IVUS, OCT boasts higher imaging resolution, enabling insights into various aspects of PCSK9 antibody efficacy against atherosclerosis, including plaque composition, and microstructure. This choice enhances the specificity and granularity of the obtained data, contributing to a more comprehensive understanding of the impact of PCSK9 inhibitors on plaque characteristics in patients with NSTE-ACS with non-culprit coronary critical lesions.
Limitations
Our study has several limitations. First, it was conducted at a single medical centre, which may limit the generalisability of the results. Regional differences, population characteristics and treatment facilities across different centres could yield varying outcomes. Second, despite our efforts to maintain consistency in the treatment process, some variations were present during implementation. Although guidelines suggest escalating statin dosages to attain desired LDL-C levels, a significant proportion of the Chinese population fails to meet these therapeutic targets, primarily due to the administration of routine or low statin doses—a fact supported by existing literature. Compared with their European and American counterparts, Asian populations, which are generally smaller in stature, exhibit a higher incidence of muscle soreness and liver damage when subjected to high-dose statin therapy, thereby constraining the application of higher doses. Reflecting the realities of clinical practice in China, we opted for a standard dosage of rosuvastatin at 10 mg/day, a decision we believe mirrors the actual treatment landscape more accurately. But it cannot be denied that this is indeed a deficiency in our research as patients who did not achieve the guideline-recommended LDL-C levels with statin monotherapy at a fixed dose may impact cardiovascular prognosis. Finally, for non-culprit vessels with stenosis between 50% and 75%, although experienced clinicians assessed the presence of ischaemic evidence based on clinical symptoms and ECG results, functional tests such as fractional flow reserve measurements were not performed, representing a certain limitation. Recently, the FITTER study,37 currently underway, seeks to explore the impact of the cholesterol-lowering medication evolocumab when administered alongside standard statin therapy. The study focuses on assessing its effects on blood flow in non-infarct-related artery lesions among patients diagnosed with ACS and multivessel disease. This research endeavour holds promise for bridging existing gaps in understanding within this domain.
Ethics and dissemination
Ethics
This study will adhere to the principles outlined in the Helsinki Declaration and other applicable ethical guidelines. This study protocol has received approval from the Medical Research Ethics Committee of the First Affiliated Hospital of the University of Science and Technology of China (Anhui Provincial Hospital), with approval number 2022-ky214.
Dissemination
We plan to disseminate the findings of this study through various channels. This includes publication in peer-reviewed academic journals, presentation at relevant academic conferences and communication to the public, policymakers and healthcare professionals. We will also share updates on the research progress through social media and other online platforms to facilitate the exchange and application of scientific knowledge. Efforts will be made to ensure widespread dissemination of the research results and to have a positive impact on society.
Acknowledgements
The authors thank all the clinical staff for their assistance with the execution of the study.
Footnotes
Funding: This study was supported by the National Key Research and Development Programme of China (2021YFA0804904), the Anhui Department of Education Research Programme (grant no. 2022AH040193) and the Clinical Medical Research Transformation Project of Anhui Province (grant no. 202204295107020021, 202204295107020026).
Prepublication history for this paper is available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2023-083730).
Patient consent for publication: Consent obtained directly from patient(s).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Contributor Information
Yu-Wei Wang, Email: 1165004630@qq.com.
Jie Xu, Email: xujievera@gmail.com.
Likun Ma, Email: lkma@ustc.edu.cn.
Hao Hu, Email: huhao1977@ustc.edu.cn.
Hong-Wu Chen, Email: chenhongwu1975@163.com.
Jing-Sheng Hua, Email: doctorhuajs@sina.com.
Xiang-Yong Kong, Email: kxyong1234@163.com.
Dan Li, Email: 10157463@qq.com.
Long-Wei Li, Email: llw3831@163.com.
Jian-Yuan Pan, Email: eypanjianyuan@sina.com.
Jiawei Wu, Email: wjwei626@163.com.
References
- 1.Ruff CT, Braunwald E. The evolving epidemiology of acute coronary syndromes. Nat Rev Cardiol. 2011;8:140–7. doi: 10.1038/nrcardio.2010.199. [DOI] [PubMed] [Google Scholar]
- 2.Steg PG, Goldberg RJ, Gore JM, et al. Baseline characteristics, management practices, and in-hospital outcomes of patients hospitalized with acute coronary syndromes in the global Registry of acute coronary events (GRACE) Am J Cardiol. 2002;90:358–63. doi: 10.1016/s0002-9149(02)02489-x. [DOI] [PubMed] [Google Scholar]
- 3.Chan MY, Sun JL, Newby LK, et al. Long-term mortality of patients undergoing cardiac Catheterization for ST-elevation and non-ST-elevation myocardial infarction [published correction appears in circulation. Circulation. 2009;119:3110–7. doi: 10.1161/CIRCULATIONAHA.108.799981. [DOI] [PubMed] [Google Scholar]
- 4.Sato A, Ohigashi H, Nozato T, et al. Coronary artery spatial distribution, morphology, and composition of Nonculprit coronary plaques by 64-slice computed Tomographic angiography in patients with acute myocardial infarction. Am J Cardiol. 2010;105:930–5. doi: 10.1016/j.amjcard.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 5.Rioufol G, Finet G, Ginon I, et al. Multiple Atherosclerotic plaque rupture in acute coronary syndrome: a three-vessel Intravascular ultrasound study. Circulation. 2002;106:804–8. doi: 10.1161/01.cir.0000025609.13806.31. [DOI] [PubMed] [Google Scholar]
- 6.Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary Atherosclerosis. N Engl J Med . 2011;364:226–35. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 7.Kang J, Park KW, Lee MS, et al. The natural course of Nonculprit coronary artery lesions; analysis by serial quantitative coronary angiography. BMC Cardiovasc Disord. 2018;18:130. doi: 10.1186/s12872-018-0870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinberg D, Glass CK, Witztum JL. Evidence mandating earlier and more aggressive treatment of hypercholesterolemia. Circulation. 2008;118:672–7. doi: 10.1161/CIRCULATIONAHA.107.753152. [DOI] [PubMed] [Google Scholar]
- 9.Bentzon JF, Otsuka F, Virmani R, et al. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852–66. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after Statin therapy. N Engl J Med. 2005;352:20–8. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 11.Schubert J, Lindahl B, Melhus H, et al. Low-density lipoprotein cholesterol reduction and Statin intensity in myocardial infarction patients and major adverse outcomes: a Swedish nationwide cohort study. Eur Heart J. 2021;42:243–52. doi: 10.1093/eurheartj/ehaa1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boekholdt SM, Hovingh GK, Mora S, et al. Very low levels of Atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of Statin trials. J Am Coll Cardiol. 2014;64:485–94. doi: 10.1016/j.jacc.2014.02.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/Apha/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: A report of the American college of cardiology/American heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:3168–209. doi: 10.1016/j.jacc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Visseren FLJ, Mach F, Smulders YM, et al. ESC guidelines on cardiovascular disease prevention in clinical practice [published correction appears in Eur heart J. 2022 Nov 7;43(42):4468] Eur Heart J. 2021;42:3227–337. doi: 10.1093/eurheartj/ehab484. [DOI] [PubMed] [Google Scholar]
- 15.Baum SJ, Rane PB, Nunna S, et al. Geographic variations in lipid-lowering therapy utilization, LDL-C levels, and proportion retrospectively meeting the ACC/AHA very high-risk criteria in a real-world population of patients with major Atherosclerotic cardiovascular disease events in the United States. Am J Prev Cardiol . 2021;6:100177. doi: 10.1016/j.ajpc.2021.100177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicholls SJ, Puri R, Anderson T, et al. Effect of Evolocumab on progression of coronary disease in Statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:2373–84. doi: 10.1001/jama.2016.16951. [DOI] [PubMed] [Google Scholar]
- 17.Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–22. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 18.Leucker TM, Blaha MJ, Jones SR, et al. Effect of Evolocumab on Atherogenic lipoproteins during the Peri- and early postinfarction period: A placebo-controlled, randomized trial. Circulation. 2020;142:419–21. doi: 10.1161/CIRCULATIONAHA.120.046320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lou B, Liu H, Luo Y, et al. In-hospital initiation of Pcsk9 inhibitor and short-term lipid control in patients with acute myocardial infarction. Lipids Health Dis. 2022;21:105. doi: 10.1186/s12944-022-01724-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferri N, Ruscica M, Lupo MG, et al. Pharmacological rationale for the very early treatment of acute coronary syndrome with Monoclonal antibodies anti-Pcsk9. Pharmacol Res. 2022;184:S1043-6618(22)00384-X. doi: 10.1016/j.phrs.2022.106439. [DOI] [PubMed] [Google Scholar]
- 21.Nicholls SJ, Kataoka Y, Nissen SE, et al. Effect of Evolocumab on coronary plaque phenotype and burden in Statin-treated patients following myocardial infarction. JACC Cardiovasc Imaging. 2022;15:1308–21. doi: 10.1016/j.jcmg.2022.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Zanchin C, Koskinas KC, Ueki Y, et al. Effects of the Pcsk9 antibody Alirocumab on coronary Atherosclerosis in patients with acute myocardial infarction: a serial, Multivessel, Intravascular ultrasound, near-infrared spectroscopy and optical coherence tomography imaging study-rationale and design of the PACMAN-AMI trial. Am Heart J. 2021;238:33–44. doi: 10.1016/j.ahj.2021.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Higo T, Ueda Y, Oyabu J, et al. Atherosclerotic and Thrombogenic Neointima formed over sirolimus drug-Eluting Stent: an Angioscopic study. JACC Cardiovasc Imaging. 2009;2:616–24. doi: 10.1016/j.jcmg.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 24.Nakazawa G, Vorpahl M, Finn AV, et al. One step forward and two steps back with drug-Eluting-Stents: from preventing Restenosis to causing late thrombosis and Nouveau Atherosclerosis. JACC Cardiovasc Imaging. 2009;2:625–8. doi: 10.1016/j.jcmg.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Ohtani T, Ueda Y, Mizote I, et al. Number of yellow plaques detected in a coronary artery is associated with future risk of acute coronary syndrome: detection of vulnerable patients by Angioscopy. J Am Coll Cardiol. 2006;47:2194–200. doi: 10.1016/j.jacc.2006.01.064. [DOI] [PubMed] [Google Scholar]
- 26.Cannon CP, de Lemos JA, Rosenson RS, et al. Use of lipid-lowering therapies over 2 years in GOULD, a Registry of patients with Atherosclerotic cardiovascular disease in the US. JAMA Cardiol . 2021;6:1–9. doi: 10.1001/jamacardio.2021.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borén J, Chapman MJ, Krauss RM, et al. Low-density lipoproteins cause Atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis society consensus panel. Eur Heart J. 2020;41:2313–30. doi: 10.1093/eurheartj/ehz962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gili S, Iannaccone M, Colombo F, et al. Effects of Statins on plaque rupture assessed by optical coherence tomography in patients presenting with acute coronary syndromes: insights from the optical coherence tomography (OCT)-FORMIDABLE Registry. Eur Heart J Cardiovasc Imaging. 2018;19:524–31. doi: 10.1093/ehjci/jex102. [DOI] [PubMed] [Google Scholar]
- 29.Nissen SE, Tuzcu EM, Schoenhagen P, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary Atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071–80. doi: 10.1001/jama.291.9.1071. [DOI] [PubMed] [Google Scholar]
- 30.Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity Statin therapy on regression of coronary Atherosclerosis: the ASTEROID trial. JAMA. 2006;295:1556–65. doi: 10.1001/jama.295.13.jpc60002. [DOI] [PubMed] [Google Scholar]
- 31.Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive Statin regimens on progression of coronary disease. N Engl J Med. 2011;365:2078–87. doi: 10.1056/NEJMoa1110874. [DOI] [PubMed] [Google Scholar]
- 32.Kataoka Y, Puri R, Hammadah M, et al. Frequency-domain optical coherence Tomographic analysis of plaque Microstructures at Nonculprit Narrowings in patients receiving potent Statin therapy. Am J Cardiol. 2014;114:549–54. doi: 10.1016/j.amjcard.2014.05.035. [DOI] [PubMed] [Google Scholar]
- 33.Dai J, Hou J, Xing L, et al. Is age an important factor for vascular response to Statin therapy? A serial optical coherence tomography and Intravascular ultrasound study. Coron Artery Dis. 2017;28:209–17. doi: 10.1097/MCA.0000000000000465. [DOI] [PubMed] [Google Scholar]
- 34.Hou J, Xing L, Jia H, et al. Comparison of intensive versus moderate lipid-lowering therapy on fibrous cap and Atheroma volume of coronary lipid-rich plaque using serial optical coherence tomography and Intravascular ultrasound imaging. Am J Cardiol. 2016;117:800–6. doi: 10.1016/j.amjcard.2015.11.062. [DOI] [PubMed] [Google Scholar]
- 35.Komukai K, Kubo T, Kitabata H, et al. Effect of atorvastatin therapy on fibrous cap thickness in coronary Atherosclerotic plaque as assessed by optical coherence tomography: the EASY-FIT study. J Am Coll Cardiol. 2014;64:2207–17. doi: 10.1016/j.jacc.2014.08.045. [DOI] [PubMed] [Google Scholar]
- 36.Kini AS, Baber U, Kovacic JC, et al. Changes in plaque lipid content after short-term intensive versus standard Statin therapy: the YELLOW trial (reduction in yellow plaque by aggressive lipid-lowering therapy) J Am Coll Cardiol. 2013;62:21–9. doi: 10.1016/j.jacc.2013.03.058. [DOI] [PubMed] [Google Scholar]
- 37.Smith J, Johnson A. FITTER study Registry entry. 2019. https://clinicaltrials.gov/ct2/show/NCT04141579 Available.