Abstract
Objective:
The pharyngeal swallow typically begins within 400 ms following the arrival of a liquid bolus in the pharynx. By contrast, processed food particles aggregate in the valleculae prior to swallow initiation. With solid foods, swallow reaction time (SRT), the interval between bolus passing the ramus of mandible and hyoid burst onset (HYB) can be subdivided into components of vallecular aggregation time (VAT) and the subsequent end of aggregation to hyoid burst interval (EOA-to-HYB). However, expected durations of these timing measures remain unclear. We aimed to study bolus aggregation in healthy swallowing for International Dysphagia Diet Standardisation Initiative Food Levels 5 (minced and moist [MM5]), 6 (soft and bite-sized [SB6]), and 7 (regular [RG7]). Understanding typical patterns and durations of vallecular aggregation with solids in healthy swallowing will inform the identification of impaired swallow timing in patient populations.
Design:
Twenty healthy adults (10 males, Mage = 28 years, range: 23–55 years) swallowed two boluses each of MM5, SB6, and RG7 foods in videofluoroscopy. Blinded duplicate ratings determined bolus location at swallow onset, SRT, VAT, and EOA-to-HYB. Texture-based differences were measured using Friedman's tests.
Bolus location was at/above the valleculae at swallow onset for 85% of boluses, with no differences by texture. SRT, VAT, and EOA-to-HYB did not vary by texture, with overall median values (interquartile range) of 99 ms (−66 to 743 ms) for SRT, 347 ms (66 to 891 ms) for VAT, and −132 ms (−231 to −83 ms) for EOA-to-HYB.
Conclusions:
These data corroborate prior evidence that it is not unusual for food particles to aggregate in the valleculae prior to swallow initiation in healthy swallowing. However, durations of vallecular aggregation are typically < 1 s in healthy adults.
Understanding normal physiology and pathophysiology is fundamental to evaluating and treating disorders of eating and swallowing (Matsuo & Palmer, 2008). When swallowing liquid boluses, it is widely accepted that onset of the pharyngeal phase of swallowing is marked by a rapid superior–anterior hyolaryngeal movement (“hyoid burst” [HYB]). The HYB facilitates airway protection by moving the larynx out of the path of the bolus and should be initiated promptly upon arrival of the bolus in the pharynx (Logemann, 2007). In early descriptions, movement of the leading edge of the bolus past the faucial pillars was used as an event marker, from which the timeliness of swallow initiation was measured. However, later work proposed moving this landmark to the point where the shadow of the ramus of mandible is seen to intersect the tongue base on lateral-view videofluoroscopy (Robbins et al., 1992). This location is thought to correspond to a population of sensory receptors for the internal branch of the superior laryngeal nerve, located in the region of the tongue base and along the laryngeal surface of the epiglottis (Steele & Miller, 2010). Although historic guidance suggested that pharyngeal swallow onset should occur promptly following bolus passing the mandible (BPM), more recent studies report variations in bolus location at swallow onset (BLSO; Bhutada et al., 2020; Martin-Harris et al., 2007; Stephen et al., 2005); healthy reference values show thin liquid bolus location at HYB to be distributed anywhere from above/at the ramus of mandible to as low as the pyriform sinuses (Steele et al., 2019). Swallow reaction time (SRT) is a measure of the timeliness of swallow onset, defined as the interval between BPM and HYB (Humbert et al., 2018; Steele et al., 2019). This measure has previously gone by different names, including “pharyngeal delay time” (Logemann et al., 2000, 2002), “duration of stage transition” (Robbins et al., 1992), and “swallow response time” (Power et al., 2007). SRT is typically < 400 ms for thin liquids, with longer time frames seen for thicker consistencies (Steele et al., 2019). The reference value for extremely thick liquids (i.e., Level 4 on the International Dysphagia Diet Standardisation Initiative [IDDSI] framework) typically falls < 800 ms (Steele et al., 2019, 2023).
Although timely initiation of a swallow is expected with discrete liquid boluses, the pattern seen with masticated boluses is different. In their Process Model of Feeding, Hiiemae and Palmer (Hiiemae & Palmer, 1999; Palmer et al., 1992) outline two stages of food processing: (a) in Stage 1 processing, the bolus is moved to the occlusal surface of the postcanine molar teeth for chewing and reduction into particles; and (b) in Stage 2 transport, triturated particles of food are collected from the molar teeth, gathered in midline, squeezed backward toward the pharynx by the tongue, and then carried on the dorsal surface of the tongue as it moves in a cyclical pattern through the faucial isthmus, to aggregate in the valleculae before initiation of the pharyngeal swallow. Several Stage 2 transport cycles may occur for a single bolus, with additional portions of food accumulating in the valleculae during continued chewing and oral processing of food in the oral cavity (Matsuo & Palmer, 2008).
Several studies have explored durational aspects of Stage 1 processing. In one study in 14 healthy young adults, Mikushi et al. (2014) reported interquartile range (IQR) values for the duration of Stage 1 processing of ~1.2 to 1.4 s with banana and ~1.3 to 1.6 s with a shortbread cookie. Variations of bolus size from 2 to 12 g within food type did not affect Stage 1 duration. In a related study, Inokuchi et al. (2014) reported significantly more chewing cycles for the cookie stimulus than for the banana or a soft tofu stimulus. These results are consistent with the general premise that the function of chewing is to render foods of differing initial consistency to a uniform swallow-ready consistency prior to delivery to the pharynx. The food science literature contains numerous studies showing that variations in the initial properties of those foods will translate to different durations and degrees of chewing to reach that swallow-ready consistency (Bandini et al., 2022; Chen et al., 2013; Devezeaux de Lavergne et al., 2017; Fontijn-Tekamp et al., 2004; Hoebler et al., 2000; Jalabert-Malbos et al., 2007; Mishellany et al., 2006; Peyron et al., 2004, 2011).
Less information is available in prior studies regarding Stage 2 transport behaviors. The overall duration of oropharyngeal aggregation, including postfaucial aggregation and vallecular aggregation, is reported to last 8–10 s (Hiiemae & Palmer, 1999). Post hoc analysis of data reported by Hiraoka et al. (2017) reports median (IQR) values for overall processing duration, from food entering the mouth until onset of the pharyngeal swallow, as 7 s (5.5–10.2 s) for tofu, 8 s (6–9.3 s) for banana, and 12.4 s (11.5–13.7 s) for cookie. However, the expected duration of vallecular aggregation and whether this varies according to bolus consistency remains unclear. Given that clinical approaches to measuring the timeliness of swallow onset with liquids have adopted BPM as the initial event in these timing measures, the goal of the current study was to explore vallecular aggregation time (VAT) and swallow initiation patterns in healthy swallowing across foods of different consistencies as recently defined by IDDSI, specifically IDDSI Food Levels 5 (minced and moist [MM5]), 6 (soft and bite-sized [SB6]), and 7 (regular [RG7]). We aimed to measure the following parameters in healthy swallowing across food textures representing IDDSI Levels 5, 6, and 7:
BLSO (Humbert et al., 2018);
SRT, that is, the interval, in milliseconds, from BPM to HYB onset;
VAT, that is, the interval, in milliseconds, from BPM to end of aggregation (EOA), with EOA defined as the first frame showing the leading edge of the bolus moving below the level of the pit of the vallecular space; and
The EOA-to-HYB interval, in milliseconds.
Based on the assumption that oral processing serves to convert food boluses of varying initial texture into a uniform swallow-ready consistency, we adopted the null hypothesis, namely, that we would not see differences in timing measures across textures. As a final step, we performed exploratory post hoc investigations of within-participant variability in measures across task repetitions and across textures to determine whether any participants consistently produced values that were located in the upper or lower tails of the distribution.
Method
Data were collected as part of a larger study protocol exploring chewing, food oral processing, and swallowing behaviors in adults without dysphagia across a range of bolus consistencies. The study protocol received human subjects ethical approval from the local institutional review board. Community-dwelling adult volunteers were recruited to participate in the study at the Steele Swallowing Lab at the KITE Research Institute, Toronto Rehabilitation Institute – University Health Network. Telephone screening was completed and written informed consent was obtained from each participant who met the following inclusion criteria:
intact comprehension to follow two-step commands;
no history of previous medical conditions or interventions that could impact swallowing function, including (but not limited to) previous head and neck cancer, prior surgery or radiation to the speech or swallowing apparatus, motor speech diagnoses, respiratory disorders, gastroesophageal disorders, chronic sinusitis, or taste disturbances;
no known allergies to latex or any of the stimulus ingredients used in data collection; and
not pregnant.
Participants who met these criteria then attended a session with study staff where the study procedures were reviewed before signing informed consent and proceeding to a videofluoroscopic swallow study.
Videofluoroscopy
All participants underwent a videofluoroscopic swallowing study using continuous fluoroscopy on an Ultimax ADR-1000 (Toshiba America Medical Systems Inc.), and captured at 30 frames per second on a TIMS 2000 SP recording system (TIMS Medical). These examinations included two boluses each of foods prepared to represent MM5, SB6, and RG7 foods (i.e., IDDSI Levels 5, 6, and 7; www.iddsi.org). The barium stimuli were prepared using Bracco E-Z-PAQUE powdered barium in 20% w/v barium concentration and thickened with a gum-based thickening agent (Nestlé Resource ThickenUp Clear) according to previously reported standard recipes (please see the Appendix).
Videofluoroscopy Postprocessing and Rating
The videofluoroscopy recordings were de-identified and segmented into video clips, each containing the swallowing events associated with a single bolus, including the initial swallow and any secondary swallows. Each clip was randomly assigned to two trained raters for rating according to the ASPEKT (Analysis of Swallowing Physiology: Events, Kinematics, and Timing) method (Steele et al., 2019) using ImageJ open-source software (National Institutes of Health). Blinded duplicate rating was completed to identify the key frames of BPM, EOA, and HYB for the initial swallow of each bolus to enable calculations of SRT, VAT, and the EOA-to-HYB interval. BLSO (i.e., the location of the leading edge of the bolus on the frame of HYB) was also coded according to the following six-zone method described by Humbert et al. (2018):
in the oral cavity or oropharynx, anterior/superior to the ramus of the mandible;
between the ramus of the mandible and valleculae;
within the valleculae, bounded by the leaf of the epiglottis;
between the valleculae and pyriform sinus, including the laryngeal surface of the epiglottis;
in the pyriform sinuses, inferior to the arytenoids;
within or inferior to the upper esophageal sphincter.
Interrater agreement was inspected for all measures. Any difference in frame identification > 3 frames across paired ratings, and all differences in BLSO cases were taken to a consensus meeting for review and resolution. For frame identification discrepancies ≤ 3 frames, the earlier of the two candidate frames was used as the frame of record. All analyses for timing measures were calculated in units of frames; results were subsequently converted to milliseconds in 33-ms increments, based on the frame rate of 30 frames per second.
Statistical Analyses
All statistical analyses were performed in SPSS (Version 29), with statistical significance defined as p < .05. Preconsensus reliability for event identification was calculated by calculating the absolute difference in frames between ratings, and determining the frequency of discrepancies greater than three frames. For BLSO, reliability was calculated similarly by measuring the absolute difference in scores across raters, and calculating frequencies for absolute differences by magnitude. Using participant mean values of confirmed BLSO scores after discrepancy resolution, BLSO frequencies were tabulated by anatomical zone and chi-square tests were performed to identify significant differences in BLSO by texture. With respect to timing measures, distributions were inspected for normality using Q-Q plots and Kolmogorov–Smirnov tests. One extremely high outlier value for VAT was identified and replaced by a missing value. Descriptive statistics were calculated for VAT, the EOA-to-HYB interval, and SRT by texture. Due to skewed distributions of residuals, it was not possible to perform general linear model analyses accounting for the within-participant repeated measures for each consistency. Instead, consistent with the methods used in our prior work on videofluoroscopy reference values for swallowing (Steele et al., 2023), we calculated participant mean values across the two repeated boluses of each texture and used these mean values in nonparametric Friedman's tests to identify significant differences in timing measures by texture. Post hoc Mann–Whitney U tests were performed to identify significant sex differences in timing measures within texture.
To explore within-participant variability in measures both within and across textures, we converted the individual bolus timing measures for consistency into z scores (i.e., two data points per consistency per participant). Each participant's z score range was then calculated per consistency as the difference between their highest and lowest z score. Graphs for each measure were then plotted showing z score range for each participant by texture.
Results
The study sample included 20 adults without dysphagia (10 males, 10 females), aged 23–55 years (M = 27.8, SD = 7.5). Interrater reliability for preconsensus event identification was excellent. Agreement within 3 frames across raters was achieved for 100% of the BPM and HYB measures. For EOA, agreement within 3 frames was seen in 96% of cases. For BLSO, 72% of preconsensus ratings were in perfect agreement, with 23% and 5% of ratings differing by 1 and 2 levels, respectively.
As shown in Table 1, BLSO was at or above the valleculae for 85% of the boluses in this data set, with 39% of swallows being initiated with the leading edge of the bolus still superior to the ramus of mandible, 17% between the ramus of mandible and the valleculae, and 29% located in the valleculae on the frame of HYB. Chi-square tests failed to find any significant differences in BLSO by texture (χ2 [df = 8] = 7.91, p = .44). Descriptive statistics for timing measures are listed by texture in Table 2. Friedman's tests showed no significant differences by texture for any timing measures: (a) SRT: χ2 (df = 2) = 1.6, p = .45; (b) VAT: χ2 (df = 2) = 2.78, p = .25; (c) xEOA-to-HYB: χ2 (df = 2) = 5.2, p = .07. Overall median times (IQR) were as follows: (a) SRT: 99 ms (−66 to 743 ms); (b) VAT: 347 ms (66 to 891 ms); and (c) EOA-to-HYB: −132 ms (−231 to −83 ms), with HYB typically occurring prior to EOA. No significant within-texture sex differences were identified for any of the timing measures studied.
Table 1.
Frequencies of bolus location at swallow onset (BLSO) across minced and moist (MM5), soft and bite-sized food (SB6), and regular (RG7) foods.
| BLSO | MM5 |
SB6 |
RG7 |
Total |
||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| 1 | 16 | 40 | 14 | 35 | 17 | 44 | 57 | 39 |
| 2 | 6 | 15 | 7 | 18 | 7 | 18 | 20 | 17 |
| 3 | 13 | 33 | 15 | 37 | 6 | 15 | 34 | 29 |
| 4 | 5 | 12 | 4 | 10 | 8 | 20 | 17 | 14 |
| 5 | 0 | 0 | 0 | 0 | 1 | 3 | 1 | 1 |
Note. 1: In the oral cavity or oropharynx, anterior/superior to the ramus of the mandible. 2: Between the ramus of the mandible and valleculae. 3: Within the valleculae, bounded by the leaf of the epiglottis. 4: Between the valleculae and pyriform sinus, including the laryngeal surface of the epiglottis. 5: In the pyriform sinuses, inferior to the arytenoids.
Table 2.
Differences in timing parameters across minced and moist (MM5), soft and bite-sized (SB6), and regular (RG7) foods.
| Parameter | Consistency | P25 | Median | P75 |
|---|---|---|---|---|
| Swallow reaction time (ms) | MM5 | −45 | 305 | 887 |
| SB6 | −33 | 66 | 743 | |
| RG7 | −111 | 66 | 1060 | |
| Overall | −66 | 99 | 743 | |
| Vallecular aggregation time (ms) | MM5 | 66 | 413 | 1015 |
| SB6 | 66 | 231 | 825 | |
| RG7 | 103 | 322 | 1044 | |
| Overall | 66 | 347 | 891 | |
| End of aggregation to hyoid burst onset (ms) | MM5 | −149 | −116 | −87 |
| SB6 | −198 | −132 | −83 | |
| RG7 | −248 | −231 | −74 | |
| Overall | −231 | −132 | −83 |
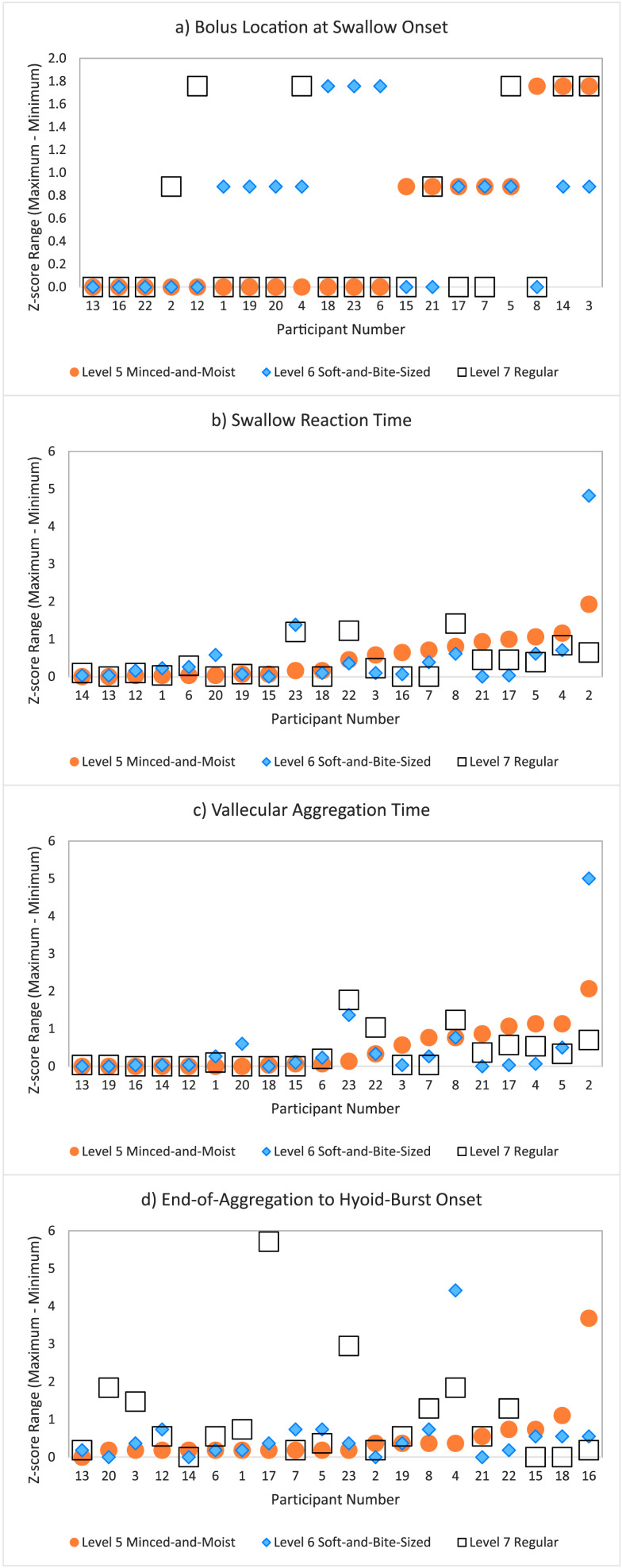
The results of the post hoc exploration of within-participant variability within and across textures can be seen in Figures 1a–1d. These graphs show z score range on the y-axis, with values closer to zero representing minimal variability across task repetitions within a participant. Values for the three textures are shown by different colors and symbols as indicated in the legend. Participant identity is shown along the x-axis in order of ascending z score range, prioritized by texture level (i.e., rank for Level 5 < rank for Level 6 < rank for Level 7). The order of participants along the x-axis is, therefore, not consistent across the four plots. Although it can be appreciated that Participant 13 shows low variability on all four measures, the same cannot be said for other participants, either with respect to a trend of low variability or a trend of high variability. Certainly, it does not appear to be the case that specific participants trended to having consistently more variable scores. It can also be appreciated that greater within-participant variability for the EOA-to-HYB measure is seen for the Level 7 regular food stimulus.
Figure 1.
Plots illustrating within-participant variability for the parameters of interest, across task repetitions, within consistency: (a) bolus location at swallow onset, (b) swallow reaction time, (c) vallecular aggregation time, (d) end of aggregation to hyoid burst onset interval. Each plot shows the range (maximum–minimum) for z scores, ranking the participant's task-specific values along the standardized distribution of all values seen for that task. A low z score range represents minimal participant variability across task repetitions. The order of participants along the x-axis represents the order of ascending variability, beginning with IDDSI Level 5 minced and moist foods (orange circles) and progressing through Level 6 soft and bite-sized (blue diamonds) and Level 7 regular foods (white squares).
Discussion
This preliminary study provides new information regarding patterns of bolus transfer and swallow initiation for foods in the Level 5 to Level 7 range of the IDDSI framework, with implications for the identification of atypical behaviors in clinical assessment. The current data suggest that the leading edge of solid food boluses is located below the ramus of mandible in 61% of cases, but rarely lower than the valleculae. This is an interesting observation given recent evidence that BLSO with liquid boluses may be distal to the vallecular pit in as many as 50% of cases (Steele et al., 2019). In this study, particles of food were noted to aggregate in the region beyond the ramus of mandible and in the valleculae prior to swallow initiation 46% of the time, with the period of aggregation extending beyond the frame of HYB. Interestingly, the timing of HYB relative to BPM with foods in this study is similar to SRT values previously reported for IDDSI Level 4 stimuli (i.e., extremely thick liquids or pureed foods; Steele et al., 2019). However, the observation that HYB frequently occurs before the end of vallecular aggregation with food boluses, such that negative values for EOA-to-HYB were not unusual, challenges the convention of using the HYB to demarcate onset of the pharyngeal phase of swallowing with solid foods. Clinically, the data suggest that aggregation of solid food particles for ≤ 1 s should not be considered to indicate delayed swallow initiation; aggregation > 1 s was, however, unusual in these healthy young adult study participants. It should be noted that the definitions used for BPM and EOA identified the first frame showing the leading edge of the bolus moving past the anatomical landmark of interest, and did not capture the extent to which portions of the bolus were distributed across regions superior to those landmarks.
In our study, VAT did not differ significantly across the food textures that were tested. Hiiemae and Palmer noted there to be “considerable variation for the actual time the valleculae were filled.” The current study concurs with this observation: The IQRs seen for SRT and VAT were fairly wide, in the order of 800 to 900 ms (i.e., 24–27 frames). This degree of variation means that the magnitude of a minimally interesting texture-based difference in timing measures will be quite large. Additionally, the collection of larger data corpuses from larger samples will be needed to confirm the presence of statistically robust differences across textures.
Limitations
There are a number of limitations to note regarding this preliminary study. First, it is important to acknowledge that IDDSI Level RG7 is effectively an unlimited category that contains a wide variety of regular textured foods, defined simply by the fact that they do not display the properties of softness, particle size, and adhesiveness that are specified as required characteristics of Levels 6, 5, 4, or 3 on the IDDSI framework. Whether a single Level RG7 stimulus, in this case a Carr's Table Water Cracker, elicits swallow timing measures that are representative of those that may be expected across different RG7 foods, or compared to the Level 7 subcategory of easy-to-chew foods, is unknown. Notably, however, our study did not identify significant timing differences in measures of aggregation or swallow response time across the stimuli studied. Therefore, it seems reasonable to use the overall median and IQRs for these measures as the basis for judging a greater variety of Level 7 foods.
The prior studies by Hiiemae and Palmer (1999) included a Scottish butter shortbread finger cookie smeared with a light coating of barium paste; this particular stimulus may not be comparable to the Carr's Table Water Cracker with barium that was used in this study, both in terms of consistency and/or in terms of the breakdown patterns that occur during mastication and oral processing. In addition, Hiiemae and Palmer studied chicken paste, banana, peanuts, and a high-concentration barium liquid. Their study predated release both of IDDSI's definitions and testing methods, and the mapping of their stimuli to IDDSI levels cannot be presumed because food texture can vary based on factors such as ripeness, temperature, and preparation (e.g., chopping).
It should also be noted that there are limitations to using videofluoroscopy to study VAT across different food stimuli; these include the potential impact of barium powder or paste on food texture and the radiation exposure involved. Currently, there are no FDA-approved standard barium stimuli available for IDDSI Levels 5, 6, and 7. The current data suggest that it may be reasonable to evaluate swallow initiation patterns for foods with a single Level 7 food stimulus such as a cracker, as proposed in several current standard videofluoroscopy protocols such as the MBSImP and DIGEST protocols (Hutcheson et al., 2022; Martin-Harris et al., 2008). In cases where clinicians seek to explore variations in swallow initiation patterns across a wider variety of food stimuli, endoscopy may prove to be a useful tool. However, caution is warranted in using videofluoroscopy timing measures as guidance for interpreting endoscopic exams; the correspondence between endoscopic and radiographic timing measures is unlikely to be exact, given that the structures used for videofluoroscopy measures include the faucial pillars, shadow of the ramus of mandible, and the hyoid, which are not visible on endoscopy.
Conclusion
These data corroborate prior evidence that it is not unusual for food particles to aggregate in the valleculae prior to swallow initiation in healthy swallowing. However, BLSO is rarely lower than the valleculae. The timing of HYB relative to the BPM is similar for foods to that reported for extremely thick liquids. However, HYB typically occurs prior to the EOA. Based on the results of this study, it is unusual for the duration of vallecular aggregation of food particles to exceed 1 s in healthy young adults.
Data Availability Statement
The data sets generated and/or analyzed during the current study are not publicly available due to ethical/legal restrictions. Inquiries regarding access to the data should be directed to the final author (C.M.S.).
Acknowledgments
The authors acknowledge salary support and grant funding for this project from the KITE Research Institute, Toronto Rehabilitation Institute – University Health Network, Toronto, Ontario, Canada; the Canada Research Chairs Secretariat, Ottawa, Ontario, Canada; and National Institutes of Health Grants R01DC011020 and R01AG077481 (Catriona M. Steele is the recipient of both grants listed).
Appendix
Recipes for Radio-Opaque Food Stimuli Used During Videofluoroscopic Swallowing Studies
Radio-opaque food stimuli for IDDSI Food Levels 5 (minced and moist), 6 (soft and bite-sized), and 7 (regular) were prepared according to the following recipes:
IDDSI Level 5 Minced and Moist Food:
Create 125 ml of a 30% w/v liquid barium solution using 39 g of Bracco E-Z-PAQUE powder and 116 g of bottled water. Mix using a kitchen stand mixer for 2 min 30 s.
In a separate container, combine 15.92 of breadcrumbs (NuCibo Texture Modified Bread & Bakery Mix) and half a tablespoon (7.5 ml) of cocoa powder (Fry's Premium Cocoa).
Measure 3.7 ml of canola oil (Longo's brand) using a syringe and add to the breadcrumb and cocoa powder mixture.
Add 60 ml of the 30% w/v liquid barium solution to the breadcrumb, cocoa powder, and oil mixture. Stir manually until evenly blended.
IDDSI Level 6 Soft and Bite-Sized Food:
Create 125 ml of a 40% w/v liquid barium solution using 52 g of Bracco E-Z-PAQUE powder and 113 g of bottled water. Mix using a kitchen stand mixer for 2 min 30 s.
In a separate container, combine 15.92 of breadcrumbs (NuCibo Texture Modified Bread & Bakery Mix) and half a tablespoon (7.5 ml) of cocoa powder (Fry's Premium Cocoa).
Measure 3.7 ml of canola oil (Longo's brand) using a syringe and add to the breadcrumb and cocoa powder mixture.
Add 30 ml of the 30% w/v liquid barium solution to the breadcrumb, cocoa powder, and oil mixture. Stir manually until evenly blended.
Allow the mixture to sit for 10 min to allow it to solidify.
Using gloves, remove half-teaspoon portions from the mixture and shape into cubes of 1.5 cm3. Serve by teaspoon.
IDDSI Level 7 Regular Food:
This consistency is prepared by smearing a barium paste onto a Carr's Table Water Cracker.
To create the barium paste, follow these instructions:
Create one cup of paste using 108 g of Bracco E-Z-PAQUE powder and a full 108-g container of Kraft Jell-O chocolate pudding. Stir manually until well blended.
Smear the chocolate barium paste onto the cracker just prior to use.
Funding Statement
The authors acknowledge salary support and grant funding for this project from the KITE Research Institute, Toronto Rehabilitation Institute – University Health Network, Toronto, Ontario, Canada; the Canada Research Chairs Secretariat, Ottawa, Ontario, Canada; and National Institutes of Health Grants R01DC011020 and R01AG077481 (Catriona M. Steele is the recipient of both grants listed).
References
- Bandini, A., Gandhi, P., Sutton, D., & Steele, C. M. (2022). Bolus texture testing as a clinical method for evaluating food oral processing and choking risk: A pilot study. American Journal of Speech-Language Pathology, 31(6), 2806–2816. 10.1044/2022_AJSLP-22-00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutada, A. M., Dey, R., Martin-Harris, B., & Focht Garand, K. L. (2020). Factors influencing initiation of pharyngeal swallow in healthy adults. American Journal of Speech-Language Pathology, 29(4), 1956–1964. 10.1044/2020_AJSLP-20-00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J., Khandelwal, N., Liu, Z., & Funami, T. (2013). Influences of food hardness on the particle size distribution of food boluses. Archives of Oral Biology, 58(3), 293–298. 10.1016/j.archoralbio.2012.10.014 [DOI] [PubMed] [Google Scholar]
- Devezeaux de Lavergne, M., van de Velde, F., & Stieger, M. (2017). Bolus matters: The influence of food oral breakdown on dynamic texture perception. Food & Function, 8(2), 464–480. 10.1039/c6fo01005a [DOI] [PubMed] [Google Scholar]
- Fontijn-Tekamp, F. A., Slagter, A. P., Van der Bilt, A., Van't Hof, M. A., Kalk, W., & Jansen, J. A. (2004). Swallowing thresholds of mandibular implant-retained overdentures with variable portion sizes. Clinical Oral Implants Research, 15(3), 375–380. 10.1111/j.1600-0501.2004.01006.x [DOI] [PubMed] [Google Scholar]
- Hiiemae, K. M., & Palmer, J. B. (1999). Food transport and bolus formation during complete feeding sequences on foods of different initial consistency. Dysphagia, 14(1), 31–42. 10.1007/PL00009582 [DOI] [PubMed] [Google Scholar]
- Hiraoka, T., Palmer, J. B., Brodsky, M. B., Yoda, M., Inokuchi, H., & Tsubahara, A. (2017). Food transit duration is associated with the number of Stage II transport cycles when eating solid food. Archives of Oral Biology, 81, 186–191. 10.1016/j.archoralbio.2017.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebler, C., Devaux, M. F., Karinthi, A., Belleville, C., & Barry, J. L. (2000). Particle size of solid food after human mastication andin vitrosimulation of oral breakdown. International Journal of Food Science and Nutrition, 51(5), 353–366. 10.1080/096374800426948 [DOI] [PubMed] [Google Scholar]
- Humbert, I. A., Sunday, K. L., Karagiorgos, E., Vose, A. K., Gould, F., Greene, L., Azola, A., Tolar, A., & Rivet, A. (2018). Swallowing kinematic differences across frozen, mixed, and ultrathin liquid boluses in healthy adults: Age, sex, and normal variability. Journal of Speech, Language, and Hearing Research, 61(7), 1544–1559. 10.1044/2018_JSLHR-S-17-0417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson, K., Barbon, C. E. A., Alvarez, C. P., & Warneke, C. L. (2022). Refining measurement of swallowing safety in the Dynamic Imaging Grade of Swallowing Toxicity (DIGEST) criteria: Validation of DIGEST Version 2. Cancer, 128(7), 1458–1466. 10.1002/cncr.34079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuchi, H., Brodsky, M. B., Gonzalez-Fernandez, M., Yoda, M., Hiraoka, T., Matsuo, K., & Palmer, J. B. (2014). Frequency of Stage II oral transport cycles in healthy human. Dysphagia, 29(6), 685–691. 10.1007/s00455-014-9562-5 [DOI] [PubMed] [Google Scholar]
- Jalabert-Malbos, M. L., Mishellany-Dutour, A., Woda, A., & Peyron, M. A. (2007). Particle size distribution in the food bolus after mastication of natural foods. Food Quality and Preference, 18(5), 803–812. 10.1016/j.foodqual.2007.01.010 [DOI] [Google Scholar]
- Logemann, J. A. (2007). Swallowing disorders. Gastroenterology, 21(4), 563–573. 10.1016/j.bpg.2007.03.006 [DOI] [PubMed] [Google Scholar]
- Logemann, J. A., Pauloski, B. R., Rademaker, A. W., Colangelo, L. A., Kahrilas, P. J., & Smith, C. H. (2000). Temporal and biomechanical characteristics of oropharyngeal swallow in younger and older men. Journal of Speech, Language, and Hearing Research, 43(5), 1264–1274. 10.1044/jslhr.4305.1264 [DOI] [PubMed] [Google Scholar]
- Logemann, J. A., Pauloski, B. R., Rademaker, A. W., & Kahrilas, P. J. (2002). Oropharyngeal swallow in younger and older women: Videofluoroscopic analysis. Journal of Speech, Language, and Hearing Research, 45(3), 434–445. 10.1044/1092-4388(2002/034) [DOI] [PubMed] [Google Scholar]
- Martin-Harris, B., Brodsky, M. B., Michel, Y., Castell, D. O., Schleicher, M., Sandage, J., Maxwell, R., & Blair, J. (2008). MBS measurement tool for swallow impairment—MBSImp: Establishing a standard. Dysphagia, 23(4), 392–405. 10.1007/s00455-008-9185-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Harris, B., Brodsky, M. B., Michel, Y., Lee, F. S., & Walters, B. (2007). Delayed initiation of the pharyngeal swallow: Normal variability in adult swallows. Journal of Speech, Language, and Hearing Research, 50(3), 585–594. 10.1044/1092-4388(2007/041) [DOI] [PubMed] [Google Scholar]
- Matsuo, K., & Palmer, J. B. (2008). Anatomy and physiology of feeding and swallowing: Normal and abnormal. Physical Medicine and Rehabilitation Clinics of North America, 19(4), 691–707. https://doi.org/S1047-9651(08)00044-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikushi, S., Seki, S., Brodsky, M. B., Matsuo, K., & Palmer, J. B. (2014). Stage I intraoral food transport: Effects of food consistency and initial bolus size. Archives of Oral Biology, 59(4), 379–385. 10.1016/j.archoralbio.2014.01.002 [DOI] [PubMed] [Google Scholar]
- Mishellany, A., Woda, A., Labas, R., & Peyron, M. A. (2006). The challenge of mastication: Preparing a bolus suitable for deglutition. Dysphagia, 21(2), 87–94. 10.1007/s00455-006-9014-y [DOI] [PubMed] [Google Scholar]
- Palmer, J. B., Rudin, N. J., Lara, J., & Crompton, A. W. (1992). Coordination of mastication and swallowing. Dysphagia, 7(4), 187–200. 10.1007/BF02493469 [DOI] [PubMed] [Google Scholar]
- Peyron, M. A., Gierczynski, I., Hartmann, C., Loret, C., Dardevet, D., Martin, N., & Woda, A. (2011). Role of physical bolus properties as sensory inputs in the trigger of swallowing. PLOS ONE, 6(6), Article e21167. 10.1371/journal.pone.0021167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron, M. A., Mishellany, A., & Woda, A. (2004). Particle size distribution of food boluses after mastication of six natural foods. Journal of Dental Research, 83(7), 578–582. 10.1177/154405910408300713 [DOI] [PubMed] [Google Scholar]
- Power, M. L., Hamdy, S., Singh, S., Tyrrell, P. J., Turnbull, I., & Thompson, D. G. (2007). Deglutitive laryngeal closure in stroke patients. Journal of Neurology, Neurosurgery, & Psychiatry, 78(2), 141–146. 10.1136/jnnp.2006.101857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins, J., Hamilton, J. W., Lof, G. L., & Kempster, G. B. (1992). Oropharyngeal swallowing in normal adults of different ages. Gastroenterology, 103(3), 823–829. 10.1016/0016-5085(92)90013-O [DOI] [PubMed] [Google Scholar]
- Steele, C. M., Bayley, M. T., Bohn, M. K., Higgins, V., Peladeau-Pigeon, M., & Kulasingam, V. (2023). Reference values for videofluoroscopic measures of swallowing: An update. Journal of Speech, Language, and Hearing Research, 66(10), 3804–3824. 10.1044/2023_JSLHR-23-00246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele, C. M., & Miller, A. J. (2010). Sensory input pathways and mechanisms in swallowing: A review. Dysphagia, 25(4), 323–33. 10.1007/s00455-010-9301-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele, C. M., Peladeau-Pigeon, M., Barbon, C. A. E., Guida, B. T., Namasivayam-MacDonald, A. M., Nascimento, W. V., Smaoui, S., Tapson, M. S., Valenzano, T. J., Waito, A. A., & Wolkin, T. S. (2019). Reference values for healthy swallowing across the range from thin to extremely thick liquids. Journal of Speech, Language, and Hearing Research, 62(5), 1338–1363. 10.1044/2019_JSLHR-S-18-0448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen, J. R., Taves, D. H., Smith, R. C., & Martin, R. E. (2005). Bolus location at the initiation of the pharyngeal stage of swallowing in healthy older adults. Dysphagia, 20(4), 266–272. 10.1007/s00455-005-0023-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated and/or analyzed during the current study are not publicly available due to ethical/legal restrictions. Inquiries regarding access to the data should be directed to the final author (C.M.S.).