Abstract
Background:
Midlife residential exposure to greenspace may slow cognitive decline by increasing opportunities for physical activity and social connection, restoring attention, or reducing stress or adverse environmental exposures. However, prospective studies on the association between greenness and cognitive decline are sparse.
Objective:
We investigated the prospective association between greenness at midlife and cognitive decline later in life. We explored effect measure modification by apolipoprotein E (APOE)-ɛ4 carrier status, neighborhood socioeconomic status (NSES), and rural/urban regions.
Methods:
The Nurses’ Health Study () started in 1976 with married female nurses, 30–55 years of age, located across 11 US states. We examined 16,962 nurses who were enrolled in a substudy starting in 1995–2001 (mean ) through 2008. We assessed average summer residential greenness in a buffer using Landsat Normalized Difference Vegetation Index data from 1986–1994. Starting in 1995–2001, participants underwent up to four repeated measures of five cognitive tests. A global composite score was calculated as the average of all -scores for each task to evaluate overall cognition. We used linear mixed models to evaluate the association of average greenness exposure at midlife with cognitive decline in later life, adjusted for age, education, NSES, and depression.
Results:
In adjusted models, higher midlife greenness exposure [per interquartile range (IQR): 0.18] was associated with a 0.004-unit (95% CI: 0.001, 0.006) slower annual rate of cognitive decline. For comparison, we found that 1 year of age is related to a mean annual difference for global cognition in the full sample; thus, higher midlife greenness appeared equivalent to slowing cognitive decline by months. In analysis exploring gene–environment interactions, we found that among APOE-ɛ4 carriers, an IQR increase in greenness was associated with a rate of decline that was slower by 0.01 units of global composite score (95% CI: 0.0004, 0.02). This association was attenuated among APOE-ɛ4 noncarriers. We did not observe associations between greenness and baseline or annual rate of cognitive decline of verbal memory.
Discussion:
Higher midlife greenness exposure is associated with slower cognitive decline later in life. Future research is necessary to confirm these findings. https://doi.org/10.1289/EHP13588
Introduction
Literature has suggested that Alzheimer’s disease and related dementia (ADRD), the seventh leading cause of death in the United States,1 may begin before the onset of identifiable symptoms.2–4 Cognitive decline is a fundamental aspect of dementia5 and can be considered a spectrum from cognitive health to dementia; thus research that examines novel risk factors predicting cognitive decline is a powerful approach to understanding dementia prevention. An increasing number of studies have suggested inverse associations between exposure to residential greenness, or natural vegetation around the home, and cognitive function in adulthood.6,7 The hypothesized mechanisms through which greenness may be beneficial for cognition are attention restoration, reduction of stress8 and air pollution,9,10 and increased physical activity11 and social connection.12 However, the majority of studies are cross-sectional, and the evidence for a longitudinal association between residential greenness and cognition and cognitive decline remains scarce.6,13
Research has shown that reducing midlife (45–65 years of age) risk factors may help to delay or prevent ADRD.14 For example, midlife vascular risk factors are strongly associated with later-life cognitive decline15 and late-life dementia.16 A recent meta-analysis showed that midlife modifiable risk factors could significantly increase the dementia risk by 41%–78%, including psychological stress and physical exercise.17 A 2020 report on dementia prevention highlighted physical activity and social contact in midlife as potential preventive factors of dementia.14 Greenness exposure has been associated with reduction of vascular risk factors, psychological stress,18 and increase of physical exercise and social contact19 and, thus, represents a novel and modifiable exposure at midlife that can be critical to slow the development of ADRD.
In addition, there is interest in exploring gene–environment interactions, that is, situations in which genetic effects connected to a phenotype are dependent upon variability in the environment, or when genes modify sensitivity to particular environmental features.20 The ɛ4 allele is a polymorphism in the apolipoprotein E (APOE) gene, and a strong risk factor for dementia.21 There are no known ways for APOE-ɛ4 carriers to reduce dementia risk. Thus it is critical to explore effect modification by APOE-ɛ4 in prevention research, to help identify potential interventions that might be particularly important for carriers.22,23 Previous work has shown significant variation in the effect of the ɛ4 allele on cognition across neighborhoods with different levels of social disorder,24,25 greenness,26 and built environment.27 However, studies that evaluate variation in the association between greenness and cognitive decline by APOE ɛ4 allele are scarce.
In this study we use prospective data from a US-based nationwide cohort to assess the association between residential greenness at midlife with cognition at baseline and cognitive decline over time. We hypothesized that midlife greenness could restore attention, reduce stress and air pollution, promote physical activity or social engagement, thereby slowing down cognitive decline later in life. Epidemiological studies indicate that greenness may have health effects that are especially beneficial for socioeconomically disadvantaged groups, who usually have lower access to affordable alternative health-promoting resources.28 This potential of greenness exposure to mitigate health inequalities is known as the “equigenesis hypothesis.”29,30 With the aim to determine which groups would benefit most from greater midlife greenness exposure, we stratified the life course model by the presence of an APOE ɛ4 allele, neighborhood socioeconomic status (NSES), and urbanicity (urban vs. rural).
Methods
Population
We used data from the Nurses’ Health Study (NHS; ), which started in 1976 with female registered nurses, 30–55 years of age, located across 11 US states. All addresses have been geocoded since 1986, and participants now live in every state in the United States. Participants have completed questionnaires every 2 y on medical history and health-related behaviors, with a follow-up of .31 This study was approved by the institutional review board of Brigham and Women’s Hospital and Boston University (Boston, MA). Women gave informed consent to participate at the time of their cognitive assessment.
Study Participants
From 1995 to 2001, participants years of age with no history of stroke were invited to participate in a telephone-based study of cognitive function. Among eligible women (), 92% participated in the first cognitive interview. Three follow-up cognitive assessments were administered at intervals from 1995 to 2008 (median time between the first and fourth y; Figure S1), and participation rate remained high () over time.23 For the present analyses, we excluded participants with missing data on educational attainment, residential addresses, or baseline cognitive function, leading to a sample of 17,070 women. Because of differences in the availability of follow-up cognitive assessments, our analytical sample included 16,962 women with at least one cognitive assessment.
Exposure: Greenness at Midlife
We used the normalized difference vegetation index (NDVI), a satellite-based metric, to estimate the amount of vegetation surrounding participants’ residential addresses from 1986 [age: ] to 1994 (age: ). NDVI is the most widely used satellite-derived indicator of the quantity of green vegetation on the ground and has been used as a marker for exposure to greenness in many previous epidemiological studies.18,19,32 For this study, we used Land Remote-Sensing Satellite System (Landsat) 5 data at a resolution from July for each year because NDVI reaches its maximum and highest level of geographic variation during the height of the summer in most areas. In sensitivity analysis, we used the annual average NDVI to account for seasonal variation. Using all longitudinal greenness exposure data collected at midlife, from 1986 to the most updated available data before the first cognitive assessment, we estimated the cumulative average summer NDVI value at a buffer for the main analysis and at a buffer for the sensitivity analyses around each participant’s home based on prior work within the NHS cohorts.33 Greenness exposure incorporated changes in participants’ residential addresses throughout time. We examined NDVI in 270- and buffers to evaluate both the immediate area around residences and the walkable area as potential relevant geographic contexts.34 These buffers have also been used in previous epidemiological studies using NHS data, which allow for the comparison of results.31,33 NDVI ranges between and 1, with higher values indicating higher exposure to green vegetation at midlife. Greenness exposure was assessed as a continuous interquartile range (IQR).
Outcome: Cognitive Assessment in Late Life
Assessment of cognitive function via validated telephone interviews has been previously described in detail,35 and within this cohort.23,36 Initially, we administered the Telephone Interview of Cognitive Status (TICS),37 a telephone adaptation of the Mini-Mental State Exam (MMSE), and five more cognitive tests: a) delayed recall of the TICS 10-word list, East Boston Memory Test (EBMT)—b) immediate and c) delayed recalls, d) category fluency (animal naming test), and e) digit span backward.38,39 Because our cognitive tests are scaled differently from each other, we used -scores to create composite measures. A global composite score was calculated as the average of all -scores to evaluate overall cognitive functioning. A composite verbal memory score was also derived by averaging -scores of the immediate and delayed recalls of the EBMT and the TICS 10-word list component. Our primary results are based on the global composite score, but we also evaluated the verbal memory score as a secondary outcome, given that it is strongly associated with Alzheimer’s dementia in particular. Previous research has confirmed the validity and reliability of the telephone cognitive interview compared with in-person examinations.40 Previous work in the same cohort has established high validity ( comparing the global composite score from telephone interviews to in-person exams) and high reliability ( for two administrations of the TICS to our participants, 31 d apart) of our telephone method of assessing cognitive function in highly educated, high-functioning subjects.40–42 The average time in years of follow-up from baseline greenness exposure (i.e., 1986) to the last cognitive assessment was 22 y.
Covariates
Potential confounders were selected based on previous knowledge and on a directed acyclic graph (Figure S2).43,44 Demographic variables included age in years at first cognitive assessment (continuous), participant’s education level (bachelor’s degree, master’s or doctorate degree) and husband’s education level (some high school, high school graduate, college graduate, or graduate school graduate), and marital status (married, yes/no). NSES measures included the 1990 median income and median home value based on the US Census tract of the 1990 addresses. Mental health was measured in 1992 using the Short Form (SF-36) Mental Health Index (, poor mental health; , better mental health). Antidepressant use was assessed using the most updated available data before the first cognitive assessment. Further, health factors also measured in 1992 included physical activity (mean energy expended in vigorous, moderate, and light exercise) and body mass index (BMI). Air pollution was quantified in 1994 as residential address-level 12-month average particulate matter in aerodynamic diameter () predicted from a spatiotemporal generalized additive mixed model.45 Social engagement was assessed in 1992 with the Berkman-Syme Social Network Index (SNI),46 a multidimensional measure that has been widely used in prior research.47 This index assesses four distinct dimensions of social networks: a) marital status (married/partnered, separated/divorced, widowed); b) number of close relatives and close friends, separately (0, 1–2, 3–5, 6–9, ); c) frequency of religious activities (, 1/wk, 1/month–1/y, never); and d) frequency of activities with community organizations ( h/wk, 6–10 h/wk, 3–5 h/wk, 1–2 h/wk, no community activities).47 We imputed missing values to the modal category or the median value when they represented of the sample, otherwise a “missing” category was created.23
Statistical Analysis
We used linear mixed models to evaluate the association of average greenness exposure at midlife with cognitive decline in later life. The models included an intercept that represents the mean cognitive level at baseline and a slope parameter that represents the mean annual rate of cognitive decline over time. All models included a random intercept and random slope to account for interindividual variability. For each outcome of interest (global composite and verbal memory score), we ran several regression models where covariates were added in a hierarchical fashion, beginning with a minimally adjusted model and concluding in a fully adjusted model. Model A adjusted for age. Model B further included individual SES. Model C further added NSES. Last, model D included mental health and antidepressant use. To assess potential deviations from linearity for associations with all cognition measurements, we fit generalized additive models for continuous exposures and natural splines with 3-4 knots based on the Akaike information criterion.33 In supplementary analyses, we applied a more stringent definition of participants with the highest greenness exposure (90th percentile) vs. participants with the lowest greenness exposure (10th percentile) to assess potential differences in cognitive decline trajectories between these groups. Further, to examine shorter-term effects, we assessed the association between greenness exposure in the immediate year prior to the first cognitive assessment (vs. up to 9 y prior) and subsequent cognitive function. In sensitivity analyses, we further excluded participants in the lowest 10% of the distribution of cognitive performance at baseline (), participants who had just moved to the address for y (), and participants who had changed their address during the follow-up period ().
To examine factors that could modify the longitudinal association between midlife greenness exposure and subsequent cognitive function, we evaluated effect modification by stratifying models based on: a) NSES (tertiles: low, medium, high), b) US Census tract population density (number per square kilometers; tertiles: low, medium, high; ) as a measure of urban vs. rural, and c) APOE-ɛ4 status (carriers, noncarriers). We also included interaction terms to formally test the interaction between each of the hypothesized effect modifiers and greenness for each cognitive outcome. To obtain APOE-ɛ4 status (carrier/noncarrier) data, blood samples were collected from participants in 1989–1990 (, 59% of our sample). Participants who had not provided blood samples were invited to provide buccal cell samples in 2002–2004 (, 41% of our sample). Genomic DNA was extracted using the ReturPureGene DNA Isolation Kit from Qiagen #158489 (Gentra Systems), further information can be found in previous studies.23 The health status of included women was better than those without genotype data.23 Finally, we conducted a sensitivity analysis that restricted the sample to women without any APOE-ɛ2 allele (further excluding , 15% of participants with genotype data).
Greenness has been associated with decreased levels of air pollution,19,32 and increased levels of physical activity, social connection, and mental health.18 We used a causal mediation framework48–50 to determine whether the effect of greenness (at midlife) on cognition and cognitive decline over time was mediated through pathways represented by air pollution, physical activity, social connection, and mental health (all measured between 1992 and 1994, before the initial cognitive interview). We used the “mediation” package in R, which incorporates estimates from the mediator and outcome models to estimate natural direct and indirect effects.51 Mediation analysis requires the assumptions that there are no unmeasured exposure–outcome, mediator–outcome, or exposure–mediator confounders and no mediator–outcome confounders affected by exposure.52 Although these assumptions are not verifiable, we believe ours are reasonable because we have included major confounders in our mediation analyses. We used 100 Monte Carlo draws for nonparametric bootstrap confidence intervals (CIs). Data management was performed in SAS (version 9.4; SAS Institute, Inc.) and data analyses were conducted using R software (version 4.1.3; R Development Core Team). We used the hlme function of the lcmm R package (version 1.7.8) for linear mixed models.53
Results
Among the 16,962 women included in the study sample, the average age at the first cognitive interview was (Table 1). US Census tract median income was higher in the areas with highest greenness exposure () vs. the lowest greenness exposure (; Table 1), whereas areas with higher greenness had lower neighborhood home value (), compared with areas with less greenness (). The average global cognitive score over follow-up was higher for women who lived in areas with the highest greenness exposure across midlife than for women who lived in areas with the lowest greenness exposure (e.g., vs. ). Baseline characteristics were similar between participants recruited for the substudy of cognitive function and those in the analytic sample (Table S1).
Table 1.
Characteristics of women in the cognitive function substudy of the US-based nationwide Nurses’ Health Study by quintile of greenness [normalized difference vegetation index (NDVI), 1986–1994] at midlife ().
| Total sample characteristics | Total sample () | Quintile for greenness | ||||
|---|---|---|---|---|---|---|
| Q1 () IQR: | Q2 () IQR: 1.96–2.45 | Q3 () IQR: 2.46–2.81 | Q4 () IQR: 2.81–3.15 | Q5 () IQR: | ||
| Age, mean (y) | ||||||
| Participant’s education [ (%)] | ||||||
| Registered nursing degree | 13,166 (77.62) | 2,490 (73.49) | 2,713 (79.79) | 2,703 (79.81) | 2,661 (78.40) | 2,599 (76.60) |
| Bachelor’s degree | 2,802 (16.52) | 688 (20.15) | 507 (14.85) | 479 (14.03) | 525 (15.38) | 603 (17.66) |
| Master’s or doctoral degree | 994 (5.86) | 210 (6.15) | 180 (5.27) | 205 (6.00) | 208 (6.09) | 191 (5.59) |
| Husband’s education [ (%)] | ||||||
| Less than high school | 272 (1.60) | 46 (1.36) | 62 (1.82) | 31 (0.92) | 59 (1.73) | 74 (2.18) |
| Some high school | 871 (5.14) | 176 (5.16) | 186 (5.45) | 169 (4.95) | 162 (4.75) | 178 (5.21) |
| High school graduate | 5,840 (34.43) | 1,174 (34.39) | 1,211 (35.47) | 1,229 (36.00) | 1,112 (32.57) | 1,114 (32.63) |
| College graduate | 3,762 (22.18) | 723 (21.18) | 732 (21.44) | 747 (21.88) | 820 (24.02) | 740 (21.68) |
| Graduate school | 2,733 (16.11) | 549 (16.08) | 473 (13.85) | 522 (15.29) | 589 (17.25) | 600 (17.57) |
| Missing [ (%)] | 3,484 | 720 | 736 | 689 | 652 | 687 |
| Marital status, married [ (%)] | ||||||
| Yes | 12,072 (71.17) | 2,354 (68.95) | 2,345 (68.69) | 2,400 (70.30) | 2,518 (73.76) | 2,455 (71.91) |
| No | 4,890 (28.83) | 1,034 (30.51) | 1,055 (31.02) | 987 (29.14) | 876 (25.81) | 938 (27.64) |
| Low mental health score [ (%)] | ||||||
| Yes | 654 (3.86) | 132 (3.87) | 139 (4.07) | 131 (3.84) | 133 (3.90) | 119 (3.49) |
| No | 16,308 (96.14) | 3,256 (96.10) | 3,261 (95.91) | 3,256 (96.13) | 3,261 (96.08) | 3,274 (96.49) |
| Antidepressant use [ (%)] | ||||||
| Yes | 968 (5.71) | 201 (5.89) | 216 (6.33) | 175 (5.13) | 201 (5.89) | 175 (5.13) |
| No | 15,994 (94.29) | 3,187 (94.06) | 3,184 (93.64) | 3,212 (94.83) | 3,193 (94.07) | 3,218 (94.84) |
| Physical activity, mean (MET-h/wk) | ||||||
| BMI () [ (%)] | ||||||
| 3,217 (18.96) | 683 (20.17) | 608 (17.88) | 657 (19.39) | 620 (18.26) | 649 (19.13) | |
| 22–24.9 | 4,286 (25.27) | 885 (26.14) | 819 (24.09) | 833 (24.58) | 850 (25.04) | 899 (26.50) |
| 25–29.9 | 6,503 (38.34) | 1,274 (37.63) | 1,374 (40.41) | 1,308 (38.60) | 1,296 (38.17) | 1,251 (36.87) |
| 2,957 (17.43) | 544 (16.07) | 599 (17.62) | 591 (17.44) | 629 (18.53) | 594 (17.51) | |
| APOE-ɛ4 carriers (at least one allele) [ (%)] | ||||||
| Yes | 1,942 (11.45) | 327 (9.58) | 403 (11.80) | 394 (11.54) | 395 (11.57) | 423 (12.39) |
| No | 15,020 (88.55) | 3,061 (90.34) | 2,997 (88.14) | 2,993 (88.36) | 2,999 (88.36) | 2,970 (87.53) |
| US Census tract median income | ||||||
| US Census tract home value | ||||||
| Population density () | ||||||
| Average global cognition composite score over follow-up | ||||||
Note: Values are expressed as , unless otherwise specified. Quintiles of greenness are based on cumulative average summer NDVI value at a buffer. APOE, apolipoprotein E; BMI, body mass index; MET, metabolic equivalent of task; Q, quintile; SD, standard deviation.
Baseline Cognitive Function
Table 2 shows the estimates for the fixed effects for greenness (IQR: 0.18) in association with baseline cognitive function. Analyses showed a consistent relationship between higher midlife greenness exposure (per IQR) and higher baseline cognition that was robust to adjustment for individual- and neighborhood-level covariates. In midlife, participants with higher exposure to greenness had higher baseline cognitive function later in life than participants with lower greenness exposure at midlife (Table 2). In models adjusted for age, individual SES, and NSES, participants with higher greenness exposure (per IQR) in the area around their homes had a 0.03 higher standard units global composite cognitive score at baseline (95% CI: 0.01, 0.04; Table 2). In the fully adjusted model, further adjustment for depression and antidepressant use, slightly attenuated the association (0.02; 95% CI: 0.01, 0.04; Table 2), although the CIs were the same as in the estimate from the previous model. Results were consistent after adjustment for physical activity and BMI (Table S2), for the buffer (Table S3), and using annual average NDVI (Table S4). To help interpret this estimate, we find that 1 year of age is related to a -unit mean difference in cognition at baseline; thus, more greenness appeared equivalent to higher cognition at baseline corresponding to an age of 10 months younger. Estimates were similar for verbal memory at baseline, but the CIs included the null. Natural splines analysis suggested that the association between exposure to greenness and cognitive function did not deviate from linearity (Figure S3). Results were consistent for the association between greenness exposure in the immediate year prior to the first cognitive assessment and baseline cognitive function (Table S5), as well as excluding participants in the lowest 10% of the distribution of cognitive performance (Table S6). Further, results remained consistent and strengthened after excluding participants who had just moved to the address for y and those who had changed their address during the follow-up period (Table S7).
Table 2.
Association between midlife exposure to residential greenness [normalized difference vegetation index (NDVI), 1986–1994] and baseline cognitive function (1995–2001) among women in the Nurses’ Health Study ().
| Modelsa | Mean difference in baseline cognitive function per IQR increase in NDVI (95% CI) |
|---|---|
| Global cognition | |
| Age-adjusted | 0.02 (0.01, 0.04) |
| Plus socioeconomic indicators | 0.03 (0.01, 0.04) |
| Plus neighborhood socioeconomic indicators | 0.03 (0.01, 0.04) |
| Plus depression + antidepressant use | 0.02 (0.01, 0.04) |
| Verbal memory | |
| Age-adjusted | 0.01 (, 0.02) |
| Plus socioeconomic indicators | 0.01 (, 0.02) |
| Plus neighborhood socioeconomic indicators | 0.01 (, 0.02) |
| Plus depression + antidepressant use | 0.01 (, 0.02) |
Note: Participants average age ranged from 61 to 69 y at midlife exposure to residential greenness and cognitive function was assessed at age y. CI, confidence interval; IQR, interquartile range.
Linear mixed models were used, including an intercept that represents the mean cognitive level at baseline. All models include the same sample size .
Cognitive Decline
We observed that exposure to greenness at midlife was associated with slower annual rates of cognitive decline based on the global composite in models adjusted for age and individual socioeconomic indicators (Table 3). In the fully adjusted model, we found that higher midlife greenness exposure (per IQR) was associated with an annual rate of decline that was slower by 0.004 units of the global composite score (95% CI: 0.001, 0.006; Table 3). We found that 1 year of age (in a model without controlling for greenness) was related to a -unit mean annual difference for global cognition; thus, more greenness appeared equivalent to slowing cognitive aging by months. Although participants in general experienced cognitive decline over time, those with higher midlife greenness exposure (90th percentile) had a slower decline than participants with lower midlife greenness exposure (10th percentile) (Figure S4, Excel Tables S2 and S3). Results were consistent for the buffer (Table S8) and for annual average NDVI (Table S9). We did not observe associations between average greenness and verbal memory decline (Table 3; Tables S8 and S9). Results were consistent for the association between greenness exposure in the immediate year prior to the first cognitive assessment and annual rate of decline of the global composite score (Table S10), as well as for excluding participants in the lowest 10% of the distribution of cognitive performance (Table S11). Of note, results of the association between greenness exposure in the immediate year prior to the first cognitive assessment and annual rate of decline of the verbal memory score were significant (Table S10). Results of the association between midlife exposure to greenness and cognitive decline slightly attenuated after excluding participants who had just moved to the address for y and those who had changed their address during the follow-up (Table S12).
Table 3.
Longitudinal association between midlife exposure to residential greenness [normalized difference vegetation index (NDVI), 1986–1994] and cognitive decline (1995–2008) among women in the Nurses’ Health Study ().
| Modelsa | Mean difference in slope of cognitive decline per IQR increase in NDVI (95% CI) |
|---|---|
| Global cognition | |
| Age-adjusted | 0.004 (0.001, 0.006) |
| Plus socioeconomic indicators | 0.004 (0.001, 0.006) |
| Plus neighborhood socioeconomic indicators | 0.004 (0.001, 0.006) |
| Plus depression + antidepressant use | 0.004 (0.001, 0.006) |
| Verbal memory | |
| Age-adjusted | 0.003 (, 0.006) |
| Plus socioeconomic indicators | 0.003 (, 0.006) |
| Plus neighborhood socioeconomic indicators | 0.003 (, 0.006) |
| Plus depression + antidepressant use | 0.003 (, 0.006) |
Note: Participants average age ranged from 61 to 69 y at midlife exposure to residential greenness and cognitive function was assessed at age y. CI, confidence interval; IQR, interquartile range.
Linear mixed models were used, including an intercept that represents the mean cognitive level at baseline and a slope parameter that represents the mean annual rate of cognitive decline over time. All models include the same sample size .
Stratified Analyses
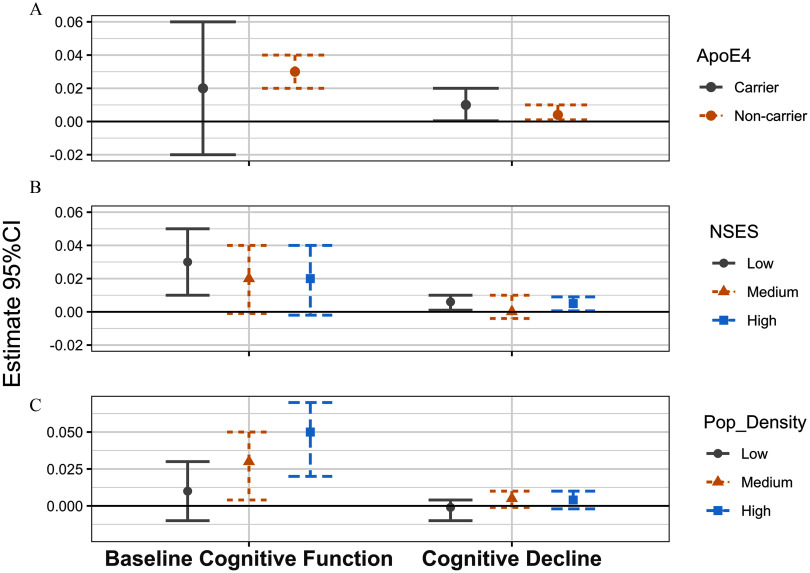
In low NSES, participants with higher greenness exposure (per IQR) had a 0.03 higher baseline global composite score (95% CI: 0.01, 0.05; Figure 1; Table S13) and an annual slope of decline that was slower by 0.006 units of the global composite score (95% CI: 0.001, 0.01; Figure 1; Table S13). We did not observe associations between greenness and cognitive function and cognitive decline in neighborhoods with medium SES. We did observe that in high NSES, participants with higher greenness exposure had an annual slope of decline that was slower by 0.005 units of the global composite score (95% CI: 0.001, 0.009; Figure 1; Table S13). In neighborhoods with medium and high population density, higher midlife greenness exposure was associated with a 0.03 and 0.05 higher baseline global composite scores, respectively (95% CI: 0.004, 0.05; 95% CI: 0.02, 0.07; Figure 1; Table S13). We did not observe associations between greenness and cognitive decline in any of the population density strata. Finally, among participants who did not carry the APOE-ɛ4 allele, higher midlife greenness exposure was associated with a 0.03 higher baseline global composite score (95% CI: 0.02, 0.04; Figure 1; Table S13) and an annual slope of decline that was slower by 0.004 units of the global composite score (95% CI: 0.001, 0.01; Figure 1; Table S13). Among participants who did carry the APOE ɛ4 allele, higher greenness exposure was associated with an annual slope of decline that was slower by 0.01 units of the global composite score (95% CI: 0.0004, 0.02; Figure 1; Table S13). Results from a sensitivity analysis that restricted the sample to women without any APOE-ɛ2 allele were attenuated, and overall results remained consistent except that the CI included the null for cognitive decline among APOE ɛ4 carriers (Table S14).
Figure 1.
Association of midlife exposure to residential greenness [normalized difference vegetation index (NDVI), 1986–1994] with baseline cognitive function and cognitive decline of global cognition (1995–2008), stratified by NSES, population density, and apolipoprotein E ɛ4 (apoE-ɛ4) carriers, the Nurses’ Health Study (). Linear mixed models were used, including an intercept that represents the mean cognitive level at baseline and a slope parameter that represents the mean annual rate of cognitive decline over time. Numeric data for effect estimates are shown in Table S13. Sample sizes were as follows: low NSES ; medium NSES ; high NSES ; low population density ; medium population density ; high population density ; ApoE-e4 noncarriers ; and ApoE-e4 carriers . Interaction -values for baseline cognitive function are as follows: ApoE4 , NSES , and population density . Interaction -values for cognitive decline are as follows: ApoE4 , NSES , and population density . Note: CI, confidence interval; NSES, neighborhood socioeconomic status; Pop_Density, population density.
Mediation Analyses
Estimates of the proportion of the association between greenness at midlife and cognition and cognitive decline that might be mediated by other factors (considering that the mediation analyses assumptions hold) were statistically significant for mental health (Table 4). Mental health was estimated to explain 18% (95% CI: 7%, 29%) of the association between greenness at midlife and cognitive function at baseline assessed by the global composite score (Table 4). Further, mental health was also estimated to explain 24% (95% CI: 9%, 50%) of the association between greenness at midlife and cognitive decline in the global composite score over time (Table 5). However, mental health did not mediate the association between greenness and verbal memory either at baseline or over time. The results did not suggest mediation of the association between greenness and cognitive function and cognitive decline by any other of the investigated mediators (Tables 4 and 5).
Table 4.
Estimated proportion of association between midlife exposure to residential greenness [normalized difference vegetation index (NDVI), 1986–1994] and baseline cognitive function (1995–2001) explained by physical activity, air pollution exposure, social engagement, and mental health ().
| Mediator | Proportion of association of cumulative average greenness and global cognition explained by mediator (95% CI) | Proportion of association of cumulative average greenness and verbal memory explained by mediator (95% CI) |
|---|---|---|
| Total physical activity | 0.02 (, 0.06) | 0.04 (, 1.36) |
| Air pollution | (, 0.08) | (, 0.50) |
| Social engagement | 0.00 (, 0.01) | 0.00 (, 0.04) |
| Mental health | 0.18 (0.07, 0.29) | 0.33 (, 1.16) |
Note: Participants average age ranged from 61 to 69 y at midlife exposure to residential greenness and cognitive function was assessed at age years. Mediation analyses assume that there is no unmeasured exposure–outcome confounding, no unmeasured mediator–outcome confounding, no unmeasured exposure–mediator confounding, and no mediator–outcome confounder affected by exposure. CI, confidence interval.
Table 5.
Estimated proportion of association between midlife exposure to residential greenness [normalized difference vegetation index (NDVI), 1986–1994] and cognitive decline (1995–2008) explained by physical activity, air pollution exposure, social engagement, and mental health.
| Mediator | Proportion of association of cumulative average greenness and global cognitive decline explained by mediator (95% CI) | Proportion of association of cumulative average greenness and verbal memory explained by mediator (95% CI) |
|---|---|---|
| Total physical activity | 0.02 (, 0.05) | 0.05 (, 0.46) |
| Air pollution | (, 0.15) | 1.10 (, 2.62) |
| Social engagement | 0.00 (, 0.01) | 0.01 (, 0.17) |
| Mental health | 0.24 (0.09, 0.50) | 0.46 (, 4.26) |
Note: Participants average age ranged from 61 to 69 y at midlife exposure to residential greenness and cognitive function was assessed at age y. Mediation analyses assume that there is no unmeasured exposure–outcome confounding, no unmeasured mediator–outcome confounding, no unmeasured exposure–mediator confounding, and no mediator–outcome confounder affected by exposure. CI, confidence interval.
Discussion
In this large prospective study of women, we assessed exposure to greenness up to 9 y prior to the first cognitive assessment, with cognitive function and cognitive decline based on a battery of five cognitive tests repeated up to four times over an average of 6 y. Results suggested that higher average exposure to greenness at midlife was associated with higher levels of baseline cognitive function and a slower annual rate of cognitive decline, based on global cognition scores, but not on verbal memory. This study indicates that greenness at midlife may offer cognitive benefits later in life to those with high residential exposure and that the association between greenness and cognitive function may differ by NSES, population density, and APOE-ɛ4 status.
Prior studies—characterized by smaller samples, shorter follow-up, and/or cross-sectional measures—have found modest associations between greenness and dementia outcomes, some similar to, as well as lower than, those in the present study.33,54–58 An analysis based on three waves of data from the Whitehall II cohort, providing a 10-y follow-up of 6,506 participants from the UK found that higher residential surrounding greenness was associated with slower cognitive decline over a 10-y follow-up among women, but not among men.58 A recent analysis of female nurses in the NHS II found that increasing greenspace was associated with higher scores of overall cognition and psychomotor speed/attention but not with learning/working memory.33 A review of 22 studies of outdoor greenness exposure and brain health measures (including cognitive function and clinical diagnosis of cognitive impairment/dementia) related to ADRD suggested positive greenness–brain health associations but stated that the methods and cohorts were limited and heterogeneous.59
Our study adds to the current literature by using a larger analytical sample, longer follow-up, and longitudinal measures for both greenness and cognitive decline. The association between greenness at midlife and cognitive function later in life was stronger among participants living in neighborhoods with low SES, high population density, and among participants with an APOE-ɛ4 allele. Our results for participants living in neighborhoods with low SES and APOE-ɛ4 status are similar to those of a study of the Lothian Birth Cohort that examined the association between lifetime availability of public parks and cognitive aging and found that the association was strongest for women and participants in the lowest socioeconomic groups, with the exception that the association was strongest for participants without an APOE-ɛ4 allele.26 A cross-sectional study of older adults in the United States did not observe an association between neighborhood built environment (such as walking destination density) and cognition among APOE-ɛ4 carriers.27 Several studies have shown that the best known genetic risk factor for Alzheimer’s dementia, the APOE-ɛ4 allele, may have a stronger association with ADRD in women than in men.4 Considering the latter, our results may suggest that among women with higher risk of ADRD, as indicated by the presence of the APOE-ɛ4 allele, greenness could potentially have a greater protective impact on cognitive decline. Our finding for participants living in neighborhoods with lower SES is consistent with the theory of equigenic environments, which suggests that greenness might be important to reduce socioeconomic health inequalities.30 The stronger associations between greenness and higher cognitive function among participants living in neighborhoods with high population density, compared with low population density, may indicate that policies to increase urban greenness may have sustainable cognitive health benefits. Our results show that the equigenesis hypothesis of greenness held for baseline cognitive function where we saw a beneficial association of greenness for participants living in areas of low SES but did not observe a beneficial association of greenness for participants living in areas of high SES. However, we did not see evidence supporting the equigenesis hypothesis for cognitive decline, where participants living in areas of low NSES had a similar beneficial association from greenness compared with participants living in areas of high NSES. We also observed evidence of the equigenesis hypothesis for cognitive decline among participants with a genetic predisposition, given that APOE-ɛ4 carriers had a 3-fold magnitude of slower cognitive decline by greenness exposure, compared with noncarriers.
We evaluated several potential mediators on the association between greenness and cognitive function and cognitive decline. Our results on mental health as a potential mediator are in line with a previous study that found that greenness may be associated with cognitive function through depression.33 However, our results expand previous literature by showing that greenness may be associated with cognitive function, as well as cognitive decline, through mental health. Greenness has been consistently linked to lower levels of depression.60 In addition, depression has been documented to be an important modifiable risk factor of dementia.61,62 Our results strengthen the evidence of greenness as key environmental factor to reduce depressive symptoms and thus dementia risk. However, in our study we did not find evidence that the association between greenness and cognitive function and cognitive decline could be explained by physical activity, air pollution, or social engagement. These results align with a previous study that evaluated these as potential mediators between green space and cognitive function.33
Several limitations of this study should be noted. First, NDVI does not allow us to differentiate different types of vegetation that might be driving the beneficial aspects of greenness on cognitive health. Further, we did not have information on amenities, safety, aesthetics, access, or participant’s use of greenness. We measured greenness around participant’s residential addresses, and thus we do not have information on greenness exposure in other locations (e.g., work). We also have no information on their exposure to greenness earlier in life, which may have a larger impact on cognitive health than later life.26,63 Our study population was predominantly white and of high education level, and thus we acknowledge selection bias may exist and that this study may not be generalizable to populations with different demographic compositions, especially given the importance of education in dementia development.64 We acknowledge that it is potentially possible that reverse causation explains some of the effects observed. However, we think this would be attenuated owing to the nature of the longitudinal design of the NHS, and the clear temporal order of exposure at midlife, up to 9 y before the first cognitive assessment, and longitudinal change in the outcome later in life after this first cognitive assessment.65 Further, the richness of the NHS data allowed for a more detailed consideration of potential bias from a variety of health-related factors; for example, results were adjusted for numerous health indicators and health behaviors, including depression, BMI, and physical activity. In addition, we were able to assess potential reverse causation through sensitivity analyses, excluding participants in the lowest 10% of the distribution of cognitive performance at baseline, and our results were consistent. Finally, we did not measure dementia incidence in our cohort. However, cognitive decline assesses the continuum from healthy cognition through dementia, and it is an important and powerful phenotype.66
Our study has several strengths. Chief among them are the prospective findings relating greenness exposure up to 9 y prior to the first cognitive assessment in later life, and the assessment of cognitive decline based on a battery of cognitive tests was repeated up to four times. Second, this study applied objective satellite data to capture objective metrics of greenness exposure across time. Third, we have time-varying information on a number of important confounders, including socioeconomic indicators, NSES, depression, and antidepressant use. To our knowledge, no prior studies have examined effect modification by APOE-ɛ4 carrier status, and our findings suggest that the protective association between residential greenspace at midlife and cognitive decline later in life was observed among participants who carried the APOE-ɛ4 allele, who may be at higher risk of ADRD.
The latest report of the Lancet Commission on Dementia Prevention, Intervention, and Care observed that of worldwide dementias could be prevented by addressing modifiable risk factors.14 The effect estimates in our study are comparable to those from other modifiable risk factors examined in this cohort. For example, the size of the association between midlife greenness exposure and baseline global cognitive score (0.03; 95% CI: 0.01, 0.04) was half the size compared with previous studies that observed associations of physical activity and baseline cognitive function (0.06; 95% CI: 0.03, 0.09).67 When evaluating cognitive decline, the association between midlife greenness exposure and cognitive decline (0.004; 95% CI: 0.001, 0.006) was slightly less than half and in the opposite direction compared with previous studies on the association of cognitive decline with air pollution [2-y decline on global cognition score was 0.02 (95% CI: to )],10 and fasting glucose among nondiabetic nurses was (95% CI: to 0.01).68
Conclusion
This study’s findings may inform further longitudinal analyses on greenness in midlife as a potential sensitive period of exposure to slow the progression of cognitive decline later in life. Furthermore, this study suggested that high residential greenness at midlife may offer protective benefits for maintaining cognitive function and preventing cognitive decline later in life.
Supplementary Material
Acknowledgments
Funding sources for this work were R00AG066949 (M.P.J.); R01HL150119 (P.J.); and UM1 CA186107.
To request access to the Nurses’ Health Study (NHS) data for use by external investigators, a protocol is followed to preserve participant confidentiality with guidance and approval from the institutional review board. As an overview, external investigators can request access to the NHS cohort data in one of three ways: 1) through the NHS Data Repository, the external investigator is granted a login to our computer system, accesses and analyzes data, 2) the external investigator collaborates directly with an NHS investigator and programmer who conduct the analyses on the external investigator’s behalf, or 3) a specific, limited dataset is created to send to the external collaborator.
Conclusions and opinions are those of the individual authors and do not necessarily reflect the policies or views of EHP Publishing or the National Institute of Environmental Health Sciences.
References
- 1.National Institute on Aging. 2023. Alzheimer’s Disease Fact Sheet. https://www.nia.nih.gov/health/alzheimers-and-dementia/alzheimers-disease-fact-sheet [accessed 1 February 2024].
- 2.Wilson RS, Segawa E, Boyle PA, Anagnos SE, Hizel LP, Bennett DA. 2012. The natural history of cognitive decline in Alzheimer’s disease. Psychol Aging 27(4):1008–1017, PMID: 22946521, 10.1037/a0029857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grober E, Hall CB, Lipton RB, Zonderman AB, Resnick SM, Kawas C. 2008. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer’s disease. J Int Neuropsychol Soc 14(2):266–278, PMID: 18282324, 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alzheimer’s Association. 2019. 2019 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 15(3):321–387, 10.1016/j.jalz.2019.01.010. [DOI] [Google Scholar]
- 5.Hugo J, Ganguli M. 2014. Dementia and cognitive impairment: epidemiology, diagnosis, and treatment. Clin Geriatr Med 30(3):421–442, PMID: 25037289, 10.1016/j.cger.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Keijzer C, Gascon M, Nieuwenhuijsen MJ, Dadvand P. 2016. Long-term green space exposure and cognition across the life course: a systematic review. Curr Environ Health Rep 3(4):468–477, PMID: 27730509, 10.1007/s40572-016-0116-x. [DOI] [PubMed] [Google Scholar]
- 7.Cerin E, Barnett A, Shaw JE, Martino E, Knibbs LD, Tham R, et al. 2021. From urban neighbourhood environments to cognitive health: a cross-sectional analysis of the role of physical activity and sedentary behaviours. BMC Public Health 21(1):2320, PMID: 34949175, 10.1186/s12889-021-12375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van den Berg AE, Maas J, Verheij RA, Groenewegen PP. 2010. Green space as a buffer between stressful life events and health. Soc Sci Med 1982 70(8):1203–1210, PMID: 20163905, 10.1016/j.socscimed.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Maas J, Verheij RA, de Vries S, Spreeuwenberg P, Schellevis FG, Groenewegen PP. 2009. Morbidity is related to a green living environment. J Epidemiol Community Health 63(12):967–973, PMID: 19833605, 10.1136/jech.2008.079038. [DOI] [PubMed] [Google Scholar]
- 10.Weuve J, Puett RC, Schwartz J, Yanosky JD, Laden F, Grodstein F. 2012. Exposure to particulate air pollution and cognitive decline in older women. Arch Intern Med 172(3):219–227, PMID: 22332151, 10.1001/archinternmed.2011.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardson EA, Pearce J, Mitchell R, Kingham S. 2013. Role of physical activity in the relationship between urban green space and health. Public Health 127(4):318–324, PMID: 23587672, 10.1016/j.puhe.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Jennings V, Bamkole O. 2019. The relationship between social cohesion and urban green space: an avenue for health promotion. Int J Environ Res Public Health 16(3):452, PMID: 30720732, 10.3390/ijerph16030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Besser LM, McDonald NC, Song Y, Kukull WA, Rodriguez DA. 2017. Neighborhood environment and cognition in older adults: a systematic review. Am J Prev Med 53(2):241–251, PMID: 28455123, 10.1016/j.amepre.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. 2020. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396(10248):413–446, PMID: 32738937, 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottesman RF, Albert MS, Alonso A, Coker LH, Coresh J, Davis SM, et al. 2017. Associations between midlife vascular risk factors and 25-year incident dementia in the Atherosclerosis Risk in Communities (ARIC) cohort. JAMA Neurol 74(10):1246–1254, PMID: 28783817, 10.1001/jamaneurol.2017.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. 2005. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 64(2):277–281, PMID: 15668425, 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 17.Li XY, Zhang M, Xu W, Li JQ, Cao XP, Yu JT, et al. 2019. Midlife modifiable risk factors for dementia: a systematic review and meta-analysis of 34 prospective cohort studies. Curr Alzheimer Res 16(14):1254–1268, PMID: 31902364, 10.2174/1567205017666200103111253. [DOI] [PubMed] [Google Scholar]
- 18.Jimenez MP, DeVille NV, Elliott EG, Schiff JE, Wilt GE, Hart JE, et al. 2021. Associations between nature exposure and health: a review of the evidence. Int J Environ Res Public Health 18(9):4790, PMID: 33946197, 10.3390/ijerph18094790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James P, Banay RF, Hart JE, Laden F. 2015. A review of the health benefits of greenness. Curr Epidemiol Rep 2(2):131–142, PMID: 26185745, 10.1007/s40471-015-0043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seabrook JA, Avison WR. 2010. Genotype–environment interaction and sociology: contributions and complexities. Soc Sci Med 70(9):1277–1284, PMID: 20181419, 10.1016/j.socscimed.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. 1993. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261(5123):921–923, PMID: 8346443, 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 22.Bos MM, Noordam R, Blauw GJ, Slagboom PE, Rensen PCN, van Heemst D. 2019. The ApoE ε4 isoform: can the risk of diseases be reduced by environmental factors? J Gerontol A Biol Sci Med Sci 74(1):99–107, PMID: 30321297, 10.1093/gerona/gly226. [DOI] [PubMed] [Google Scholar]
- 23.Fassier P, Kang JH, Lee IM, Grodstein F, Vercambre MN. 2022. Vigorous physical activity and cognitive trajectory later in life: prospective association and interaction by apolipoprotein E e4 in the Nurses’ Health Study. J Gerontol A Biol Sci Med Sci 77(4):817–825, PMID: 34125204, 10.1093/gerona/glab169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee BK, Glass TA, James BD, Bandeen-Roche K, Schwartz BS. 2011. Neighborhood psychosocial environment, apolipoprotein E genotype, and cognitive function in older adults. Arch Gen Psychiatry 68(3):314–321, PMID: 21383266, 10.1001/archgenpsychiatry.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boardman JD, Barnes LL, Wilson RS, Evans DA, Mendes de Leon CF. 2012. Social disorder, APOE-E4 genotype, and change in cognitive function among older adults living in Chicago. Soc Sci Med 74(10):1584–1590, PMID: 22465377, 10.1016/j.socscimed.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cherrie MPC, Shortt NK, Mitchell RJ, Taylor AM, Redmond P, Thompson CW, et al. 2018. Green space and cognitive ageing: a retrospective life course analysis in the Lothian Birth Cohort 1936. Soc Sci Med 196:56–65, PMID: 29128786, 10.1016/j.socscimed.2017.10.038. [DOI] [PubMed] [Google Scholar]
- 27.Besser L, Galvin JE, Rodriguez D, Seeman T, Kukull W, Rapp SR, et al. 2019. Associations between neighborhood built environment and cognition vary by apolipoprotein E genotype: Multi-Ethnic Study of Atherosclerosis. Health Place 60:102188, PMID: 31797769, 10.1016/j.healthplace.2019.102188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moran MR, Bilal U, Dronova I, Ju Y, Gouveia N, Caiaffa WT, et al. 2021. The equigenic effect of greenness on the association between education with life expectancy and mortality in 28 large Latin American cities. Health Place 72:102703, PMID: 34753000, 10.1016/j.healthplace.2021.102703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell R. 2013. What is equigenesis and how might it help narrow health inequalities? CRESH Blog Series. http://cresh.org.uk/2013/11/08/what-is-equigenesis-and-how-might-it-help-narrow-health-inequalities [accessed 1 February 2024].
- 30.Mitchell R, Popham F. 2008. Effect of exposure to natural environment on health inequalities: an observational population study. Lancet 372(9650):1655–1660, PMID: 18994663, 10.1016/S0140-6736(08)61689-X. [DOI] [PubMed] [Google Scholar]
- 31.James P, Hart JE, Banay RF, Laden F. 2016. Exposure to greenness and mortality in a nationwide prospective cohort study of women. Environ Health Perspect 124(9):1344–1352, PMID: 27074702, 10.1289/ehp.1510363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fong KC, Hart JE, James P. 2018. A review of epidemiologic studies on greenness and health: updated literature through 2017. Curr Environ Health Rep 5(1):77–87, PMID: 29392643, 10.1007/s40572-018-0179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jimenez MP, Elliott EG, DeVille NV, Laden F, Hart JE, Weuve J, et al. 2022. Residential green space and cognitive function in a large cohort of middle-aged women. JAMA Netw Open 5(4):e229306, PMID: 35476063, 10.1001/jamanetworkopen.2022.9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwan MP. 2012. The uncertain geographic context problem. Ann Assoc Am Geogr 102(5):958–968, 10.1080/00045608.2012.687349. [DOI] [Google Scholar]
- 35.Devore EE, Grodstein F, van Rooij FJA, Hofman A, Stampfer MJ, Witteman JCM, et al. 2010. Dietary antioxidants and long-term risk of dementia. Arch Neurol 67(7):819–825, PMID: 20625087, 10.1001/archneurol.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devore EE, Grodstein F, Duffy JF, Stampfer MJ, Czeisler CA, Schernhammer ES. 2014. Sleep duration in midlife and later life in relation to cognition. J Am Geriatr Soc 62(6):1073–1081, PMID: 24786726, 10.1111/jgs.12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Folstein MF, Folstein SE, McHugh PR. 1975. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3):189–198, PMID: 1202204, 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 38.Baddeley AD, Bressi S, Della Sala S, Logie R, Spinnler H. 1991. The decline of working memory in Alzheimer’s disease. A longitudinal study. Brain 114(pt 6):2521–2542, PMID: 1782529, 10.1093/brain/114.6.2521. [DOI] [PubMed] [Google Scholar]
- 39.Albert M, Smith LA, Scherr PA, Taylor JO, Evans DA, Funkenstein HH. 1991. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer’s disease. Int J Neurosci 57(3–4):167–178, PMID: 1938160, 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- 40.Kang JH, Grodstein F. 2003. Regular use of nonsteroidal anti-inflammatory drugs and cognitive function in aging women. Neurology 60(10):1591–1597, PMID: 12771247, 10.1212/01.WNL.0000065980.33594.B7. [DOI] [PubMed] [Google Scholar]
- 41.Kang JH, Logroscino G, De Vivo I, Hunter D, Grodstein F. 2005. Apolipoprotein E, cardiovascular disease and cognitive function in aging women. Neurobiol Aging 26(4):475–484, PMID: 15653176, 10.1016/j.neurobiolaging.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Kim IY, Grodstein F, Kraft P, Curhan GC, Hughes KC, Huang H, et al. 2019. Interaction between apolipoprotein E genotype and hypertension on cognitive function in older women in the Nurses’ Health Study. PLoS One 14(11):e0224975, PMID: 31697783, 10.1371/journal.pone.0224975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hernán MA, Hernández-Díaz S, Werler MM, Mitchell AA. 2002. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol 155(2):176–184, PMID: 11790682, 10.1093/aje/155.2.176. [DOI] [PubMed] [Google Scholar]
- 44.Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M, Ellison GT. 2016. Robust causal inference using directed acyclic graphs: the R package ‘dagitty.’ Int J Epidemiol 45(6):1887–1894, PMID: 28089956, 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
- 45.Yanosky JD, Paciorek CJ, Laden F, Hart JE, Puett RC, Liao D, et al. 2014. Spatio-temporal modeling of particulate air pollution in the conterminous United States using geographic and meteorological predictors. Environ Health 13(1):63, PMID: 25097007, 10.1186/1476-069X-13-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berkman LF, Syme SL. 1979. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am J Epidemiol 109(2):186–204, PMID: 425958, 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- 47.Trudel-Fitzgerald C, Poole EM, Sood AK, Okereke OI, Kawachi I, Kubzansky LD, et al. 2019. Social integration, marital status, and ovarian cancer risk: a 20-year prospective cohort study. Psychosom Med 81(9):833–840, PMID: 31592935, 10.1097/PSY.0000000000000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pearl J. 1995. Causal diagrams for empirical research. Biometrika 82(4):669–688, 10.1093/biomet/82.4.669. [DOI] [Google Scholar]
- 49.Pearl J. 2001. Direct and indirect effects. In: UAI’01: Proceedings of the Seventeenth Conference on Uncertainy in Artificial Intelligence. Breese J, Koller D, eds. 2–5 August 2001. Seattle, WA: Morgan Kaufmann Publishers, 411–420. [Google Scholar]
- 50.Robins JM, Greenland S. 1992. Identifiability and exchangeability for direct and indirect effects. Epidemiology 3(2):143–155, PMID: 1576220, 10.1097/00001648-199203000-00013. [DOI] [PubMed] [Google Scholar]
- 51.Tingley D, Teppei Y, Hirose K, Keele L, Imai K, Trinh M. 2019. Mediation: R package for causal mediation analysis. https://cran.r-project.org/web/packages/mediation/mediation.pdf [accessed 1 March 2020].
- 52.VanderWeele TJ. 2015. Explanation in Causal Inference: Methods for Mediation and Interaction. New York, NY: Oxford University Press. [Google Scholar]
- 53.Proust-Lima C, Philipps V, Liquet B. 2017. Estimation of extended mixed models using latent classes and latent processes: the R package lcmm. J Stat Soft 78(2):1–56, 10.18637/jss.v078.i02. [DOI] [Google Scholar]
- 54.Paul LA, Hystad P, Burnett RT, Kwong JC, Crouse DL, van Donkelaar A, et al. 2020. Urban green space and the risks of dementia and stroke. Environ Res 186:109520, PMID: 32344208, 10.1016/j.envres.2020.109520. [DOI] [PubMed] [Google Scholar]
- 55.Astell-Burt T, Navakatikyan MA, Feng X. 2020. Urban green space, tree canopy and 11-year risk of dementia in a cohort of 109,688 Australians. Environ Int 145:106102, PMID: 32979811, 10.1016/j.envint.2020.106102. [DOI] [PubMed] [Google Scholar]
- 56.Besser LM, Lovasi GS, Michael YL, Garg P, Hirsch JA, Siscovick D, et al. 2021. Associations between neighborhood greenspace and brain imaging measures in non-demented older adults: the Cardiovascular Health Study. Soc Psychiatry Psychiatr Epidemiol 56(9):1575–1585, PMID: 33388800, 10.1007/s00127-020-02000-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Slawsky ED, Hajat A, Rhew IC, Russette H, Semmens EO, Kaufman JD, et al. 2022. Neighborhood greenspace exposure as a protective factor in dementia risk among U.S. adults 75 years or older: a cohort study. Environ Health 21(1):14, PMID: 35033073, 10.1186/s12940-022-00830-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Keijzer C, Tonne C, Basagaña X, Valentín A, Singh-Manoux A, Alonso J, et al. 2018. Residential surrounding greenness and cognitive decline: a 10-year follow-up of the Whitehall II cohort. Environ Health Perspect 126(7):077003, PMID: 30028296, 10.1289/EHP2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Besser L. 2021. Outdoor green space exposure and brain health measures related to Alzheimer’s disease: a rapid review. BMJ Open 11(5):e043456, PMID: 33941628, 10.1136/bmjopen-2020-043456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gascon M, Sánchez-Benavides G, Dadvand P, Martínez D, Gramunt N, Gotsens X, et al. 2018. Long-term exposure to residential green and blue spaces and anxiety and depression in adults: a cross-sectional study. Environ Res 162:231–239, PMID: 29358115, 10.1016/j.envres.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 61.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. 2014. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol 13(8):788–794, PMID: 25030513, 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- 62.Jimenez MP, Gause EL, Hayes-Larson E, Morris EP, Fletcher E, Manly J, et al. 2023. Racial and ethnic differences in the association between depressive symptoms and cognitive outcomes in older adults: findings from KHANDLE and STAR. MedRxiv. Preprint posted online 7 September 2023, PMID: 37732261, 10.1101/2023.09.07.23295205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kabisch N, van den Bosch M, Lafortezza R. 2017. The health benefits of nature-based solutions to urbanization challenges for children and the elderly—a systematic review. Environ Res 159:362–373, PMID: 28843167, 10.1016/j.envres.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 64.Sharp ES, Gatz M. 2011. Relationship between education and dementia: an updated systematic review. Alzheimer Dis Assoc Disord 25(4):289–304, PMID: 21750453, 10.1097/WAD.0b013e318211c83c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Besser LM, Brenowitz WD, Meyer OL, Hoermann S, Renne J. 2021. Methods to address self-selection and reverse causation in studies of neighborhood environments and brain health. Int J Environ Res Public Health 18(12):6484, PMID: 34208454, 10.3390/ijerph18126484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kawas CH, Corrada MM, Brookmeyer R, Morrison A, Resnick SM, Zonderman AB, et al. 2003. Visual memory predicts Alzheimer’s disease more than a decade before diagnosis. Neurology 60(7):1089–1093, PMID: 12682311, 10.1212/01.WNL.0000055813.36504.BF. [DOI] [PubMed] [Google Scholar]
- 67.Weuve J, Kang JH, Manson JE, Breteler MMB, Ware JH, Grodstein F. 2004. Physical activity, including walking, and cognitive function in older women. JAMA 292(12):1454–1461, PMID: 15383516, 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 68.van Oijen M, Okereke OI, Kang JH, Pollak MN, Hu FB, Hankinson SE, et al. 2008. Fasting insulin levels and cognitive decline in older women without diabetes. Neuroepidemiology 30(3):174–179, PMID: 18421217, 10.1159/000126909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.