Abstract
INTRODUCTION
Commercially available plasma p‐tau217 biomarker tests are not well studied in ethnically diverse samples.
METHODS
We evaluated associations between ALZPath plasma p‐tau217 and amyloid‐beta positron emission tomography (Aβ‐PET) in Hispanic/Latino (88% of Cuban or South American ancestry) and non‐Hispanic/Latino older adults. One‐ and two‐cutoff ranges were derived and evaluated to assess agreement with Aβ‐PET.
RESULTS
A total of 239 participants underwent blood draw and Aβ‐PET (age 70.8 ± 7.8, 55.2% female, education 15.6 ± 3.4 years, 48.9% Hispanic/Latino, 94.9% white). Plasma p‐tau217 showed excellent discrimination of Aβ‐PET positive and negative participants (visual read: AUC = 0.91 [0.87–0.95], p < 0.001; Centiloids quantification: AUC = 0.90 [0.86–0.94]). There was a greater percent agreement between low p‐tau217 and negative Aβ‐PET (95.8%) than high p‐tau217 and positive Aβ‐PET (86.3%). Analyses within ethnicity‐specific subgroups suggested similar p‐tau217 performance.
DISCUSSION
Plasma p‐tau217 (ALZPath) relates to brain Aβ in Hispanic/Latino and non‐Hispanic/Latino older adults. Independent validation and replication are necessary to establish reference ranges and inform appropriate contexts of use across ethno‐racially diverse populations.
HIGHLIGHTS
Plasma p‐tau217 (ALZPath) and Aβ‐PET were measured in Hispanic/Latino and non‐Hispanic/Latino older adults.
Plasma p‐tau217 accurately discriminated Aβ‐PET positive and negative participants.
Applying a two‐cutoff “intermediate” plasma p‐tau217 approach could reduce need for more invasive and costly testing.
Plasma p‐tau217 associations with Aβ‐PET were strong within both Hispanic/Latino and non‐Hispanic/Latino groups.
Keywords: Alzheimer's, ALZPath, amyloid PET, biomarkers, dementia, ethnicity, Hispanic, Latino, plasma, p‐tau217
1. BACKGROUND
Alzheimer's disease (AD) defined by the presence of beta‐amyloid (Aβ) plaques and tau‐containing neurofibrillary tangles is the most common cause of dementia. 1 , 2 Accurately identifying AD pathology during life typically has relied on cerebrospinal fluid (CSF) or positron emission tomography (PET) measurement of Aβ and phosphorylated tau (p‐tau). The rapid development and validation of plasma AD biomarkers hold great promise for improving access to accurate diagnostics and clinical trials, reducing medical costs, and limiting the need for more invasive CSF or PET AD biomarker testing. 3 , 4 , 5 However, there are fewer data evaluating concordance between plasma‐ and PET‐based AD biomarkers among ethno‐racially diverse populations where individuals are at higher risk of cognitive impairment and dementia but are disproportionately excluded from AD‐related clinical trials. 1 , 6 , 7 , 8
There are several important contexts of use for plasma AD biomarkers including supplementing cognitive and behavioral assessments in geriatric primary care and specialty memory disorders clinics, eligibility screening for disease‐modifying AD therapies and clinical trials, and monitoring treatment response. 9 Plasma tau phosphorylated at residue threonine 217 (p‐tau217) has emerged as one of the top blood‐based AD biomarker candidates with an increasing number of studies showing high sensitivity and specificity to AD (Aβ‐PET, tau PET, post mortem neuropathology) 3 , 10 , 11 , 12 , 13 and prognostic ability 13 , 14 superior to other p‐tau epitopes (e.g., p‐tau181). 15 , 16 , 17
Strong associations between plasma p‐tau217 and PET or CSF markers of AD are documented across broad ethno‐racial groups, 5 , 18 , 19 though there is within‐group heterogeneity in country of origin and ancestral lines. Studies also vary in their recruitment sources (e.g., clinic versus community‐based), proportion of cognitively impaired versus unimpaired participants, and representation across the spectrum of AD pathology (e.g., Aβ burden on PET). Given that all these factors potentially impact strength of agreement between biomarkers and associated “cutoffs” for relevant plasma p‐tau217 concentrations, continued investigations are essential to inform clinical translation efforts.
We previously reported good agreement between plasma p‐tau181 and Aβ‐PET in Hispanic/Latino (H/L) and non‐Hispanic/Latino (non‐H/L) older adults in the 1Florida Alzheimer's Disease Research Center (ADRC). 20 Here, we build on this work by evaluating correspondence of p‐tau217 with Aβ‐PET using one of the few commercially available plasma p‐tau217 assays (ALZpath) in our ethnically diverse cohort. Given the predominant participant characteristics in this sample, the most relevant context of use for applying study results is a clinic‐based patient population where AD is on the etiological differential and plasma p‐tau217 testing may help rule in or rule out AD as a potential contributing neuropathology, thus also informing decisions about appropriateness for anti‐amyloid therapies. In addition to associations with positive versus negative Aβ‐PET, we evaluated how well ALZpath p‐tau217 differentiated varying severities of Aβ pathology (low, intermediate, high burden). We derive reference ranges classifying “intermediate” p‐tau217 concentrations as described previously 3 to inform the percentage of individuals who may require confirmatory PET or CSF AD biomarker testing. All results are reported for the combined sample and for H/L and non‐H/L cohorts.
2. METHODS
2.1. Participants
All study participants were enrolled in the 1Florida ADRC, which includes older adults spanning the continuum of normal cognition, mild cognitive impairment, and dementia. Over 50% of participants self‐identify as H/L, mostly of Cuban or South American origin. Participants in the current study were recruited primarily from outpatient memory disorders clinics (i.e., referred from professional/healthcare provider contact; 70%) with other recruitment sources including free memory screening programs and community outreach (i.e., non‐professional contact or self/relative/friend referral; 30%). Recruitment source did not differ between H/L and non‐H/L participants.
2.2. Plasma biomarkers
Venous blood was collected using 10 mL Purple Top blood tubes, mixed by inversion 10 times, centrifuged at room temperature for 12 min at 1200 rcf within 1 h of collection. Five hundred microliter aliquots of plasma were then placed in freezer boxes and stored at −80°C. Prior to analysis, samples were thawed (1 freeze‐thaw cycle) at room temperature, vortexed for 30 s, and placed on ice until centrifuging at 10,000 g for 5 min at 4°C.
Duplicate blood samples were analyzed at the Quanterix Accelerator Lab (Quanterix, Billerica, MA) blinded to all clinical and demographic data, using single‐molecule array (Simoa) technology for P‐tau217 (ALZpath; Catalog# ACCALZ217‐D2, Lot# 48872). All samples had coefficients of variation <20% (mean ± SD 4.9 ± 3.8%) and good analytic performance (LOD = 0.001 pg/mL, LLOQ = 0.02 pg/mL, ULOQ = 10.0 pg/mL).
2.3. Amyloid‐PET imaging
Aβ‐PET was performed with either [18F] florbetaben (FBB; 90% of scans) or [18F] Florbetapir (FBP; 10% of scans). PET imaging protocols are described further in Supplementary methods. For quantification, we calculated a global composite standardized uptake value ratio (SUVR; cerebellar grey matter reference) and converted to a Centiloid (CL) scale. 21 Aβ‐PET scans were classified as either positive or negative by a trained reader following manufacturer interpretation protocols. Based on internal development and data published across other studies, binary quantification based on CL was defined as CL < 25 for negative Aβ‐PET and CL ≥ 25 for positive Aβ‐PET. We additionally classified each participant as Low Aβ‐PET (CL < 10), Intermediate Aβ‐PET (CL 10–49), or High Aβ‐PET (CL > 49). Cutoffs were based on PET‐to‐autopsy data showing CL < 10 reflected absence of any neuritic plaques at autopsy and CL > 49 best confirmed both neuropathological AD and clinicopathological diagnosis of AD‐related dementia. 22 In this cohort, there is ≥ 99% agreement between negative visual read and CL < 10 and between positive visual read and CL > 49, with greater ambiguity occurring in the CL 10–49 range (67% visual read negative). All Aβ‐PET scans corresponded to the same study visit as the blood draw.
RESEARCH IN CONTEXT
Systematic Review: Existing research on Alzheimer's biomarkers primarily includes white, non‐Hispanic/Latino older adults. We evaluated the commercially available ALZPath plasma p‐tau217 test and beta‐amyloid (Aβ) positron emission tomography (PET) Alzheimer's biomarkers in Hispanic/Latino and non‐Hispanic/Latino individuals from the 1Florida Alzheimer's Disease Research Center.
Interpretation: Elevated plasma p‐tau217 (ALZpath) has excellent discriminability of Aβ‐PET positive and negative individuals. There is relatively stronger agreement between low p‐tau217 and negative Aβ‐PET than between high p‐tau217 and positive Aβ‐PET. Analyses within Hispanic/Latino and non‐Hispanic/Latino participants suggested similar performance of plasma p‐tau217 for identifying brain Aβ with very modest differences in associated “cutoff” values. Plasma p‐tau217 may substantially reduce the need for more costly and invasive confirmatory CSF or PET testing in ethno‐racially diverse patients.
Future Directions: Plasma p‐tau217 may aid etiological diagnosis of cognitively impaired older adults from Hispanic/Latino and non‐Hispanic/Latino ethnic origins. Longitudinal follow‐up and replication in independent cohorts are critical. Additional work is needed to directly assess the role for plasma p‐tau217 as a potential screening or diagnostic tool in the clinical workup of ethnically diverse, cognitively impaired older adults and those who may be considering anti‐amyloid immunotherapies.
2.4. Clinical evaluation and medical history
The 1Florida ADRC participants completed comprehensive neurological and neuropsychological evaluations including elements from the National Alzheimer's Coordinating Center (NACC) Uniform Data Set (UDS) plus cognitive measures specific to the center. Overall functioning was assessed with the Clinical Dementia Rating (CDR) scale. H/L participants were tested in either Spanish or English according to participant preference by bilingual psychometricians. Data for each participant were reviewed during a multidisciplinary consensus conference. Clinical disease severity based on CDR and consensus clinical diagnosis are reported but did not factor into analyses, which focused on the correspondence between plasma p‐tau217 and Aβ‐PET across the cognitive and Aβ burden continuum. Given prior reports of different rates of medical history risk factors like heart disease(s) across ethno‐racial populations, we describe frequency of cardiovascular medical history factors from information collected through the UDS Health History. Indications of renal function or kidney disease were not available.
2.5. APOLIPOPROTEIN E genotyping
Apolipoprotein E (APOE) genotyping was performed in Dr. Nilüfer Ertekin‐Taner's laboratory (Mayo Clinic, Jacksonville, FL, USA); the APOE ɛ2, ɛ3, and ɛ4 alleles used predesigned TaqMan SNP Genotyping Assays for SNPs rs7412 and rs429358 (Thermo Fisher Scientific, MA, USA) on the QuantStudio 7 Flex Real‐Time PCR (polymerase chain reaction) system (Applied Biosystems, CA, USA) following the manufacturer's protocol.
2.6. Statistical analyses
Data were analyzed using R and SPSS v28. In the combined sample, we evaluated the correlation between plasma p‐tau217 and Aβ‐PET CLs using Spearman's rho and correspondence between p‐tau217 and Aβ‐PET results using area under the curve (AUC) analysis based on multiple Aβ‐PET classification approaches: visual read of positive or negative, CL quantification of positive or negative, High Aβ‐PET versus Low Aβ‐PET, and Intermediate Aβ‐PET versus Low Aβ‐PET. For each analysis, we derived p‐tau217 cutoffs that corresponded to an optimal balance of sensitivity and specificity to the “positive state” of the Aβ‐PET AUC analysis using Youden's Index. Like methods applied by Ashton et al., 3 we also used a two‐cutoff approach to establish an intermediate range of plasma p‐tau217 concentrations where the lower limit represents a fixed sensitivity of 95% with maximized specificity and the upper limit represents a fixed specificity of 95% with maximized sensitivity. Two intermediate ranges were calculated – one based on AUCs from correspondence of plasma p‐tau217 with Aβ‐PET visual read, and one based on correspondence with CL quantification of positive and negative Aβ‐PET. We report percent agreement and discordance between plasma‐ and PET‐based biomarker classification and examined potential predictors (demographics, disease severity, APOE status) of discordance using chi‐square and independent samples t‐tests. Analyses were repeated within ethnicity‐specific subgroups (non‐H/L white and H/L white) to examine whether there was evidence for different discriminability or derived cutoffs. Last, given that our study differs from prior work with its focus on predominantly cognitively impaired participants and clinic‐based recruitment, findings are also reported separately for cognitively impaired participants only (CDR > 0) in Supplementary results.
3. RESULTS
The sample included 239 participants (age 70.8 ± 7.8, 55.2% female, education 15.6 ± 3.4 years, 48.9% H/L, 94.9% white). Most participants identified as either non‐H/L white (46.9%) or H/L white (48.1%) and were cognitively impaired (73% CDR Global ≥ 0.5). The H/L participants had fewer years of education, were slightly younger, and had a higher proportion of females than non‐H/L (Table 1). Regarding cardiovascular risk factors, H/L were more likely to report a history of hypercholesterolemia. Trend‐level differences included H/L having a higher likelihood of diabetes and hypertension and a lower likelihood of atrial fibrillation compared to non‐H/L (Supplemental results – Table S1).
TABLE 1.
Descriptive characteristics of the combined study sample and stratified by non‐Hispanic/Latino and Hispanic/Latino white participants.
| Parameter | All a | Non‐Hispanic/Latino White | Hispanic/Latino White b | Sig. (p) |
|---|---|---|---|---|
| N | 239 | 112 | 115 | |
| Age, years | 70.8 ± 7.8 | 71.9 ± 8.0 | 69.7 ± 7.3 | 0.04 |
| Sex, %female | 55.2 | 47.3 | 64.3 | 0.01 |
| Education, years | 15.6 ± 3.4 | 16.5 ± 3.3 | 14.9 ± 3.3 | <0.001 |
| APOE e4, %carrier | 38.0 | 38.3 | 40.7 | 0.45 |
| CDR‐SOB | 2.1 ± 2.6 | 2.0 ± 2.1 | 2.2 ± 3.0 | 0.60 |
| CDR Global | ||||
| 0 | 26.7 | 21.4 | 28.7 | 0.25 |
| 0.5 | 56.9 | 63.3 | 53.0 | |
| 1.0+ | 16.3 | 15.2 | 18.2 | |
| WMH burden (log) | 7.8 ± 1.0 | 7.8 ± 1.0 | 7.8 ± 0.9 | 0.11 c |
| Aβ‐PET | ||||
| Centiloids (CL) | 29.0 ± 40.6 | 29.6 ± 41.8 | 28.9 ± 36.2 | 0.90 |
| Visual read, %positive | 37.7 | 38.3 | 40.0 | 0.80 |
| Quantification, %positive (CL ≥ 25) | 42.2 | 40.2 | 45.2 | 0.44 |
| 3‐Level Aβ‐PET | 0.63 | |||
| Low Aβ‐PET (CL < 10), % | 46.4 | 48.2 | 42.6 | |
| Intermediate Aβ‐PET (CL 10–49), % | 23.8 | 21.4 | 26.1 | |
| High Aβ‐PET (CL > 49), % | 29.7 | 30.3 | 31.3 | |
| Plasma p‐tau217, pg/mL | 0.67 ± 0.50 | 0.67 ± 0.53 | 0.67 ± 0.49 | >0.99 |
Abbreviations: Aβ‐PET, beta‐amyloid positron emission tomography; APOE, apolipoprotein E; CDR (SOB), Clinical Dementia Rating Scale (Sum of Boxes); CL, Centiloids; pg/mL, picograms per milliliter; WMH, white matter hyperintensity.
N = 10 Black/African American and N = 2 Black/Hispanic were included in the combined cohort analyses, but detailed breakdown and within‐group analyses not provided due to low N.
Hispanic/Latino white participants by self‐reported country/region of origin: Cuban (49.6%), South American (35.7%), Puerto Rican (8.7%), Central American (3.5%), Other (1.7%), Dominican (<1%).
Analysis of covariance comparing Hispanic/Latino White and non‐Hispanic/Latino White on log transformed white matter hyperintensity burden controlling for age and total intracranial volume.
3.1. Plasma p‐tau217 correspondence with Aβ‐PET results
Based on visual read, 91 (37.7%) participants had a positive Aβ‐PET and 148 had negative Aβ‐PET. Based on quantification (CL ≥ 25 vs. CL < 25), 104 (42.2%) participants had a positive Aβ‐PET and 135 had negative Aβ‐PET. Plasma p‐tau217 showed excellent discrimination of Aβ‐PET status (visual read: AUC = 0.91 [0.87–0.95], p < 0.001, Figure 1A–C; CL quantification: AUC = 0.90 [0.86–0.94]; Figure 1D–F).
FIGURE 1.
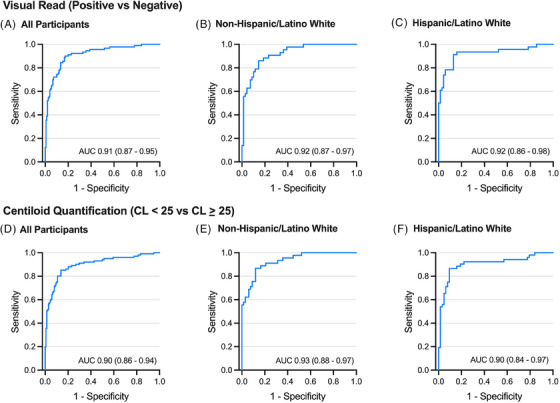
Receiver operating characteristic (ROC) curves showing plasma p‐tau217 discriminability of positive versus negative amyloid‐beta positron emission tomography (Aβ‐PET) results based on different interpretation methods and thresholds. (A–C) Visual read interpretation of Aβ‐PET results for the overall sample (panel A, All Participants) and separately within non‐Hispanic/Latino white (B) and Hispanic/Latino white (C) groups. (D–F) Centiloids‐based quantification of positive versus negative Aβ‐PET based on a threshold of 25 Centiloids for the overall sample (D), within non‐Hispanic/Latino white (E), and within Hispanic/Latino white (F). Area under the curve (AUC) estimate with 95% confidence interval is shown for each. See Tables 2, 3, 4 for corresponding single‐ and two‐cutoff ranges of p‐tau217 concentrations.
Overall, 72 participants had High Aβ‐PET (CL > 49), 59 had Intermediate Aβ‐PET (CL 10–49), and 108 had Low Aβ‐PET (CL < 10). Plasma p‐tau217 showed excellent discrimination of High versus Low Aβ‐PET (AUC = 0.94 [0.91–0.98], p < 0.001, Figure 2A–C) but weaker discrimination of Intermediate versus Low Aβ‐PET (AUC = 0.70 [0.62–0.78], p < 0.001; Figure 2D–F and Table 2, and Table S2).
FIGURE 2.
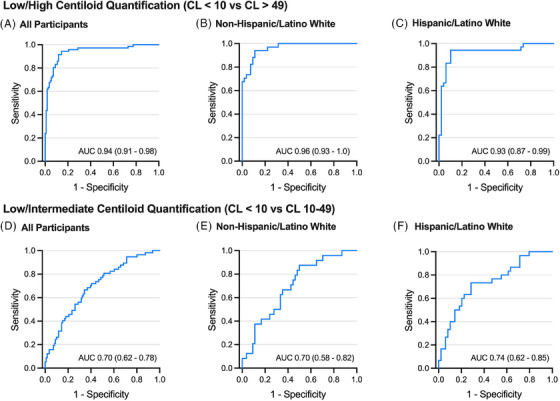
Receiver operating characteristic (ROC) curves showing plasma p‐tau217 discriminability of different levels of amyloid‐beta (Aβ) burden on Aβ‐positron emission tomography (PET) (“Low,” “Intermediate,” “High”). (A‐C) Plasma p‐tau217 discriminability of Low (Centiloids < 10) vs. High (Centiloids > 49) Aβ‐PET burden for the overall sample (A, All Participants) and separately within non‐Hispanic/Latino white (B) and Hispanic/Latino white (C) groups. (D–F) Plasma p‐tau217 discriminability of Low (Centiloids < 10) vs. Intermediate (Centiloids 10–49) Aβ‐PET burden for the overall sample (D), within non‐Hispanic/Latino white (E), and within Hispanic/Latino white (F). Area under the curve (AUC) estimate with 95% confidence interval is shown for each.
TABLE 2.
Area under the curve (AUC) and 95% confidence intervals for plasma p‐tau217 predicting Aβ‐PET results in the combined sample (“All”) and stratified by the two largest ethnicity‐specific subgroups (non‐Hispanic/Latino white, Hispanic/Latino white).
| Plasma p‐tau217 (ALZPath) | |||
|---|---|---|---|
| Aβ‐PET result | All | Non‐Hispanic/Latino White | Hispanic/Latino White |
| Visual read (positive vs. negative) | 0.91 (0.87–0.95) | 0.92 (0.87–0.97) | 0.92 (0.86–0.98) |
| CL quantification (CL < 25 vs. CL ≥ 25) | 0.90 (0.86–0.94) | 0.93 (0.88–0.97) | 0.90 (0.84–0.97) |
| High PET (CL > 49) vs. Low PET (CL < 10) | 0.94 (0.91–0.98) | 0.96 (0.93–1.0) | 0.93 (0.87–0.99) |
| Intermediate PET (CL 10–49) vs. Low PET (CL < 10) | 0.70 (0.62–0.78) | 0.70 (0.58–0.82) | 0.74 (0.62–0.85) |
Abbreviations: Aβ‐PET, beta‐amyloid positron emission tomography; CL, Centiloids; PET, positron emission tomography.
3.2. One‐ and two‐cutoff approach for plasma p‐tau217
We determined p‐tau217 cutoffs optimizing a balance of sensitivity and specificity to Aβ‐PET status (defined multiple ways) based on Youden's Index (Tables 3 and S3). The one‐cutoff approach yielded near identical values across both visual read and quantification‐based Aβ‐PET status (positive vs. negative) and for differentiating High versus Low Aβ‐PET (p‐tau217 > 0.55–0.56 pg/mL). As expected, the cutoff for differentiating Intermediate versus Low Aβ‐PET was lower (>0.39 pg/mL) and less accurate (sensitivity and specificity <70%).
TABLE 3.
Single cutoff values (bolded) for p‐tau217 (ALZPath) along with corresponding sensitivity and specificity to Aβ‐PET results for the overall combined sample (“All”) and reported separately for each ethnicity‐specific analysis.
| Plasma p‐tau217 (ALZPath) | ||||
|---|---|---|---|---|
| Aβ‐PET result | All | Non‐Hispanic/Latino White | Hispanic/Latino White | |
| Visual read (positive vs. negative) | Single cutoff, pg/mL | 0.55 | 0.55 | 0.60 |
| Sens/Spec (group‐specific) a | 89.0/82.4 | 86.0/85.1 | 91.1/86.8 | |
| PPV/NPV (group‐specific) | 75.7/93.2 | 78.7/90.8 | 82.4/93.8 | |
| Sens/Spec (universal) b | 89.0/82.4 | 86.0/85.1 | 93.3/83.8 | |
| PPV/NPV (universal) | 75.7/93.2 | 78.7/90.8 | 79.6/95.1 | |
| CL quantification (CL < 25 vs. CL ≥ 25) | Single cutoff, pg/mL | 0.56 | 0.55 | 0.60 |
| Sens/Spec (group‐specific) | 84.6/87.4 | 86.7/87.7 | 86.5/91.8 | |
| PPV/NPV (group‐specific) | 81.9/88.8 | 83.0/90.8 | 88.2/89.1 | |
| Sens/Spec (universal) | 84.6/87.4 | 86.7/87.7 | 86.5/88.5 | |
| PPV/NPV (universal) | 81.9/88.8 | 83.0/90.8 | 86.5/88.9 | |
| High PET (CL > 49) vs. Low PET (CL < 10) | Single cutoff, pg/mL | 0.56 | 0.56 | 0.61 |
| Sens/Spec (group‐specific) | 94.4/87.0 | 94.1/88.5 | 94.4/91.5 | |
| PPV/NPV (group‐specific) | 80.7/96.0 | 84.2/96.0 | 85.7/92.5 | |
| Sens/Spec (universal) | 94.4/87.0 | 94.1/88.5 | 94.4/89.4 | |
| PPV/NPV (universal) | 80.7/96.0 | 84.2/96.0 | 85.0/95.6 | |
| Intermediate PET (CL 10–49) vs. Low PET (CL < 10) | Single cutoff, pg/mL | 0.39 | 0.30 | 0.37 |
| Sens/Spec (group‐specific) | 66.1/66.6 | 87.5/48.1 | 73.3/72.3 | |
| PPV/NPV (group‐specific) | 48.6/77.7 | 43.8/90.0 | 60.0/79.5 | |
| Sens/Spec (universal) | 66.1/66.6 | 66.7/62.5 | 66.7/72.3 | |
| PPV/NPV (universal) | 48.6/77.7 | 44.1/79.5 | 58.8/77.8 | |
Abbreviations: Aβ‐PET, beta‐amyloid positron emission tomography; CL, Centiloids; PET, positron emission tomography.
aSensitivity (Sens) and Specificity (Spec) / positive predictive value (PPV) and negative predictive value (NPV) when applying the single cutoff (Youden's Index) derived from group‐specific area under the curve (AUC) curve.
Sensitivity (Sens) and Specificity (Spec) / positive predictive value (PPV) and negative predictive value (NPV) when universally applying the single cutoff (Youden's Index) derived from overall (“All”) sample area under the curve (AUC) curve to each subgroup. PPV and NPV refer only to this cohort and do not account for known or expected prevalence of disease (AD) in the population.
We also derived two‐point cutoffs defining an “intermediate range” where the lower limit represents 95% sensitivity to positive Aβ‐PET (visual read and by quantification) and the upper limit represents 95% specificity to positive Aβ‐PET (Tables 4 and S4). In the overall sample, ∼40% of participants fell in the intermediate plasma p‐tau217 range when Aβ‐PET visual read was the predicted outcome and ∼20% fell within the intermediate range when quantification‐based Aβ‐PET was the predicted outcome.
TABLE 4.
Reference range for plasma p‐tau217 using a two‐cutoff approach where the lower limit represents sensitivity fixed at 95% with maximized specificity and the upper limit represents specificity fixed at 95% with maximized sensitivity.
| Parameter | Plasma p‐tau217 (ALZPath) | |||
|---|---|---|---|---|
| All | Non‐Hispanic/Latino White | Hispanic/Latino White | ||
| Visual read (positive vs. negative) | Two‐cutoff range, pg/mL | 0.31–0.80 | 0.41–0.89 | 0.30–0.80 |
| % Within group‐specific range a | 39.7 | 31.3 | 36.5 | |
| %Agreement (negative) | 95.8 | 93.6 | 91.7 | |
| %Agreement (positive) | 86.3 | 90.0 | 91.9 | |
| % Within universal range b | 39.7 | 42.9 | 35.7 | |
| %Agreement (negative) | 95.8 | 100.0 | 91.9 | |
| %Agreement (positive) | 86.3 | 84.8 | 91.9 | |
| CL quantification (CL < 25 vs. CL > 25) | Two‐cutoff range, pg/mL | 0.49–0.89 | 0.40–0.88 | 0.24–0.73 |
| % Within group‐specific range a | 20.9 | 33.0 | 51.3 | |
| %Agreement (negative) | 93.0 | 93.6 | 88.9 | |
| %Agreement (positive) | 89.0 | 93.3 | 91.9 | |
| % Within universal range b | 20.9 | 21.4 | 20.0 | |
| %Agreement (negative) | 93.0 | 100.0 | 89.2 | |
| %Agreement (positive) | 89.0 | 87.9 | 91.9 | |
Note: The percentage of participants falling within the intermediate two‐cutoff reference range is shown for when the range was based on group‐specific reference samples (i.e., within non‐Hispanic/white or within Hispanic/white) and for when the range derived from the overall sample was applied universally across the ethnicity subgroups. The percent agreement between “negative” tests (p‐tau217 below range plus negative Aβ‐PET) and between “positive” tests (p‐tau217 above range plus positive Aβ‐PET) is reported again using the group‐specific reference ranges and when applying the overall sample reference range universally across ethnicity subgroups).
Percent of participants within the intermediate range when using group (ethnicity) specific reference ranges.
Percent of participants within the intermediate range when applying the reference range derived from the overall combined sample (“universal”).
3.3. Plasma p‐tau217 and Aβ‐PET agreement and discordance
Plasma p‐tau217 correlated strongly with Aβ‐PET CLs (rho = 0.66 [0.58–0.73], p < 0.001; Figure 3). First, the single plasma p‐tau217 cutoff corresponding to optimal differentiation of Aβ‐PET status (>0.55 pg/mL) was applied to the entire sample to establish “positive” or “negative” p‐tau217 status and examine rates of discordance with visual read of Aβ‐PET. Overall, 35 (14.6%) participants had discordant plasma p‐tau217 and Aβ‐PET results. Discordance was more likely to be in the direction of p‐tau217+ / Aβ‐PET−. Of 107 p‐tau217+, 26 (24.3%) were Aβ‐PET−. Of 132 p‐tau217‐, 9 (6.8%) were Aβ‐PET+. Participants with discordant plasma‐PET results were older than those with concordant results (73.2 ± 4.8 vs. 70.4 ± 8.1, p = 0.04, small effect size) but did not differ in sex, ethnicity, CDR sum of boxes, or APOE e4 frequency.
FIGURE 3.
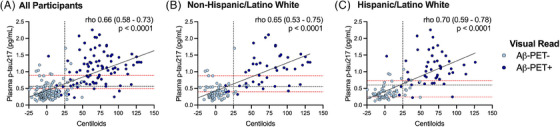
Scatterplots demonstrating a positive correlation between plasma p‐tau217 and amyloid‐beta (Aβ) burden measured in Centiloids for the overall sample (A) and separately within non‐Hispanic/Latino white (B), and Hispanic/Latino white (C) groups. Nonparametric linear association (Spearman's rho) is reported for each with a 95% confidence interval. The vertical dashed line represents the binary Centiloids‐based quantification threshold for a positive Aβ‐positron emission tomography (PET) scan (≥25). The horizontal gray dashed line represents the Youden's index single‐cutoff of plasma p‐tau217 that optimally balances sensitivity and specificity to discriminating a positive and negative Aβ‐PET scan (visual read). An intermediate plasma p‐tau217 range is shown as the lower and upper horizontal red dashed lines, which represent the p‐tau217 with 95% sensitivity (lower) and 95% specificity (upper) for discriminating a positive from negative Aβ‐PET scan (visual read). Participants falling between the red dashed lines represent a potential group that might be recommended to undergo confirmatory biomarker testing (CSF or PET) in a clinical setting. Youden's index and two‐point cutoffs (horizontal dashed lines) shown in panels (B) and (C) are derived from within‐group analyses (i.e., specific to race/ethnicity rather than universal cutoff applied from the overall sample). To limit axis distortion, three participants with Centiloids values were censored (outside axis range).
Using the two‐cutoff approach (excluding those in the intermediate range), we examined Aβ‐PET agreement among those with High (above range; N = 73) or Low (below range; N = 71) plasma p‐tau217 (Tables 4 and S4). There was greater percent agreement between Low p‐tau217 and negative Aβ‐PET (95.8%) than High p‐tau217 and positive Aβ‐PET (86.3%). In other words, like the binary single‐cutoff approach, discordance was more likely in the direction of elevated p‐tau217 with negative Aβ‐PET. A similar trend of older age associated with discordance was noted (73.1 ± 4.5 vs. 69.0 ± 8.2, p = 0.08, small effect size) and again no difference in sex, ethnicity, CDR Sum of Boxes, or APOE e4 frequency. Results were similar when using CL quantification for positive or negative Aβ‐PET status (Tables 4 and S4).
3.4. Analyses within ethnicity‐specific subgroups
No clear differences between H/L and non‐H/L emerged in plasma p‐tau217 discriminability of PET‐defined Aβ pathology. Plasma p‐tau217 discriminability of positive versus negative PET and of High versus Low Aβ‐PET was similar in non‐H/L and H/L subgroups. Plasma p‐tau217 discriminability of Intermediate versus Low Aβ‐PET was somewhat greater in H/L (AUC = 0.74 [0.62–0.85], p < 0.001) than non‐H/L (AUC = 0.70 [0.58–0.82], p = 0.001), though the confidence intervals overlapped. To probe whether the modest AUC differences related to Aβ burden differences within the Intermediate Aβ‐PET groups, we compared Aβ burden (CLs) between H/L (N = 30) and non‐H/L (N = 24) classified as Intermediate Aβ‐PET. H/L with Intermediate Aβ‐PET had higher median CLs than non‐H/L (27.3 vs. 18.1) and were slightly more likely to be Aβ‐PET positive by quantification (53% vs. 46%), but neither difference was statistically significant (ps > 0.5).
Despite largely equivalent AUCs across analyses, cutoffs derived from within‐group, ethnicity‐specific analyses varied somewhat. Plasma p‐tau217 prediction of Aβ‐PET positivity (visual read) was identical in non‐H/L and H/L (AUC = 0.92) while the corresponding Youden's Index differed slightly (non‐H/L: 0.55 pg/mL; H/L: 0.60 pg/mL). We provide data showing discriminability and percent agreement when applying a) ethnicity‐specific cutoffs within non‐H/L and H/L, and b) universal cutoffs to each group (Tables 3, 4 and Tables S3, S4).
3.5. Diagnostic accuracy combining p‐tau217 with demographics and APOE
In the combined sample and when evaluating separately within ethnicity‐specific groups, there was minimal change in diagnostic accuracy beyond plasma p‐tau217 when considering demographics (age, sex) and APOE e4 carrier status. These results were consistent when the diagnostic reference was Aβ‐PET visual read or quantification based on positive or negative Aβ‐PET. Last, we evaluated whether demographics + APOE could improve upon the weak differentiation of Intermediate versus Low Aβ‐PET using plasma p‐tau217 alone, but results again suggested no added value of the combined model (Supplemental results – Table S5).
4. DISCUSSION
We evaluated correspondence between ALZpath plasma p‐tau217 and Aβ‐PET in a mixed cohort of H/L and non‐H/L older adults, most of whom were cognitively impaired and recruited from clinic‐based settings. Plasma p‐tau217 showed excellent discrimination of Aβ‐PET positive and negative participants and better discriminated high from low Aβ burden (AUC = 0.95) than intermediate from low Aβ burden (AUC = 0.70). Using a two‐cutoff approach to establish an intermediate plasma p‐tau217 range, we found stronger agreement between low plasma p‐tau217 (below range) and negative Aβ‐PET than between high plasma p‐tau217 (above range) and positive Aβ‐PET, suggesting a higher likelihood of discordance in the direction of elevated p‐tau217 with negative Aβ‐PET. A higher likelihood of plasma‐PET discordance was associated with being slightly older but not with sex, APOE status, or ethnicity. Assuming a clinical scenario where patients falling within the intermediate p‐tau217 range would undergo confirmatory PET or CSF testing, data from this cohort suggest that around 50%–80% of these more invasive and costly procedures could be eliminated. Discrimination (AUCs) was similar when restricting analyses to cognitively impaired participants only. As expected, given the higher level of Aβ burden in cognitively impaired individuals, single cutoffs corresponding to optimal differentiation of Aβ‐PET results and two‐cutoff intermediate ranges were higher when limiting to a cognitively impaired sample.
Plasma p‐tau217 discriminability of positive and negative Aβ‐PET did not clearly differ between H/L and non‐H/L participants, though corresponding cutoffs derived from ethnicity‐specific analyses suggested modestly different values for H/L participants (higher single‐cutoff Youden's Index, lower two‐cutoff intermediate range). While not statistically significantly different, H/L and non‐H/L participants differed slightly in proportion of Aβ‐PET positive participants and in Aβ burden. The distribution of Aβ burden in a reference group likely impacts associated p‐tau217 cutoff values. Regardless, p‐tau217 holds great promise as a screening tool for aiding in vivo identification of AD pathology and for informing eligibility for AD‐directed therapies and clinical trials among ethnically diverse older adults. 3 , 18 , 19 Plasma p‐tau217 concordance with Aβ‐PET in our mixed H/L and non‐H/L sample, based on Aβ‐PET as the reference standard, was similar to several other studies reporting AUCs ∼0.90 or higher. 3 , 4 , 17 , 23
With quantification based Aβ‐PET status (CL > 25) as the reference, plasma ALZpath p‐tau217 cutoffs using Youden's Index (>0.55pg/mL) and a two‐cutoff approach (0.49–0.89pg/mL) were somewhat higher than a recent study with the same p‐tau217 assay (Youden's Index cutoff >0.42pg/mL, intermediate range 0.40–0.63pg/mL). 3 Variability in cutoffs may reflect different distributions of Aβ burden between cohorts. Ashton et al. derived their cutoffs in a cohort where ∼4% of participants had cognitive impairment and ∼20% had a positive Aβ‐PET scan, 3 compared to ∼75% of our cohort having cognitive impairment and ∼40% having a positive Aβ‐PET scan. Individuals with cognitive impairment due to AD tend to have greater Aβ burden on average than individuals with preclinical AD. 24 , 25 We suspect the higher cutoffs in our sample relate to more individuals at the higher end of the Aβ burden spectrum leading to overall higher levels of plasma p‐tau217. This is further supported by observations of even higher corresponding cutoffs when we restricted analyses to our cognitively impaired participants only.
The methods for deriving cutoffs (e.g., one‐ vs. two‐cutoff) and the ideal reference sample from which cutoffs are determined depend on the proposed context of use (e.g., optimizing sensitivity versus specificity, early detection in asymptomatic older adults versus informing etiologic differential in cognitively impaired clinic patients). It is encouraging that there is consistently high concordance between plasma p‐tau217 and Aβ‐PET results with minor variability in cutoff ranges, which we now also demonstrate in both H/L and non‐H/L older adults in the context of ruling AD in or out for predominantly cognitively impaired individuals recruited mostly from memory clinics.
Similar to Ashton et al., 3 we found stronger agreement between low ALZpath p‐tau217 and negative Aβ‐PET than between high ALZpath p‐tau217 and positive Aβ‐PET, thus showing a higher likelihood of elevated p‐tau217 with a negative Aβ‐PET than vice versa. Aβ‐PET and more recently tau PET have been a standard of truth for validating plasma AD biomarkers, though a growing understanding of the temporal dynamics of these AD‐related biomarkers suggests both plasma and CSF‐based biomarkers may begin elevating prior to PET evidence of Aβ or tau accumulation. 16
Longitudinal plasma p‐tau217 data and investigations of other AD‐related plasma biomarkers support the interpretation that elevated plasma markers without PET evidence of AD reflect emerging pathology rather than a “false positive” plasma result. Elevated plasma p‐tau217 with negative tau PET at baseline is associated with faster rates of tau deposition longitudinally in early AD brain regions. 12 Our group recently reported that elevated plasma Aβ42/40 (measured via LC‐MS; Quest AD‐Detect) with negative Aβ‐PET was associated with greater microstructural brain changes compared to individuals with both normal plasma Aβ42/40 and Aβ‐PET. 26 Fluid and PET biomarkers offer complementary but distinct insights into the onset and progression of AD pathology. Determining an in vivo “gold standard” is context dependent and blood‐based markers may be more susceptible to measurement variability from non‐AD sources (e.g., kidney disease, body mass index). 15 , 27 , 28 Continued study of factors influencing blood‐based AD biomarker measurement and their longitudinal stability is essential for clinical translation.
Despite small differences in the derived reference ranges and acknowledging the importance of replication and validation of our findings in independent cohorts, our results provide preliminary support for using similar ALZpath p‐tau217 reference ranges for both H/L and non‐H/L individuals. While there are several compelling reasons that the risk for dementia, broadly, and AD‐related dementia, specifically, may differ by ethno‐racial group, 1 , 29 , 30 it is less clear that self‐identified race or ethnicity per se should be expected to directly impact the agreement between blood‐based and PET− or neuropathology‐based indicators of AD pathology. Any apparent differences in biomarker performance between ethno‐racial groups requires careful interpretation and evaluation of possible explanations (e.g., disproportionate prevalence of comorbidities, recruitment sources, frequencies of AD pathology, social determinants of health) rather than an assumption of inherent biological differences influencing results. 31 , 32 , 33 As this area of research advances, considering more conservative (i.e., wider) “intermediate” reference ranges would still offer a substantial reduction in the need for confirmatory PET or CSF testing while minimizing false negative or false positive risks.
4.1. Strengths and limitations
Major strengths of our study included the balanced representation of H/L and non‐H/L older adults, the focus on a commercially available assay (ALZpath) for plasma p‐tau217 quantification, good representation across the Aβ burden spectrum based on Aβ‐PET (low, intermediate, and high levels), and predominantly memory clinic‐based participant recruitment with a high proportion of participants having cognitive impairment (typical clinic context).
There were also several limitations. We lacked longitudinal data and independent cohort validation of derived AUCs and p‐tau217 reference ranges. While we infer that 50%–80% of confirmatory CSF or PET procedures might be eliminated by utilizing a two‐cutoff approach, ultimately longitudinal follow‐up is required to determine feasibility, cost savings, and accuracy of the p‐tau217 ranges above and below the intermediate range. Our “standard of truth” was Aβ‐PET and future work integrating tau PET in ethno‐racially diverse samples is critical for understanding plasma p‐tau217 as a proxy along the temporal and severity spectrum of AD neuropathology. Our quantification‐based definitions for positive or negative Aβ‐PET and for defining low, intermediate, and high ranges were data‐driven but are not widely established and some studies propose different thresholds than we used, 3 , 34 which may impact resulting AUCs and p‐tau217 reference ranges. We did not have detailed characterization of renal function (e.g., estimated glomerular filtration rate [eGFR]), which might influence plasma p‐tau217 measurement. 15 , 27 We previously noted slightly higher frequency of reported angiotensin II inhibitors use in H/L participants in our cohort, which can be used in treating chronic kidney disease, but we do not know reasons for self‐reported prescribed medications in the sample. While our ethnic diversity improves on prior work, our H/L population is predominantly of Cuban or South American descent and results may not generalize to other regions of H/L origin that may have different genetic admixture or social determinant of health considerations. Our sample also did not have racial diversity (95% white). We hope to integrate future efforts with other ethno‐racially diverse cohorts 18 , 19 , 35 , 36 to contribute to advancing AD‐related research in underserved populations.
5. CONCLUSIONS
Plasma p‐tau217 (ALZpath) strongly predicts elevated brain Aβ on Aβ‐PET in Hispanic/Latino and non‐Hispanic/Latino older adults. Implementing a two‐cutoff “intermediate range” approach could substantially reduce the need for more costly and invasive confirmatory CSF or PET testing. Findings add to the growing body of evidence supporting an important role for plasma p‐tau217 as a potential screening or diagnostic tool in the clinical workup of ethnically diverse, cognitively impaired older adults and those who may be considering anti‐amyloid immunotherapies. 3 , 7
CONFLICT OF INTEREST STATEMENT
The authors report no disclosures relevant to the content of this work. STDeK reports being a consultant with Biogen, Prevail, Vaccinex, and Acumen Dementia. DEV reports consulting for Neuroimaging Solutions. All other authors report no disclosures. Author disclosures are available in the Supporting information.
CONSENT STATEMENT
All study participants provided informed consent prior to undergoing study procedures.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
We are incredibly grateful to all 1Florida ADRC research participants and their families for their invaluable contributions to our research program. This research and several affiliated investigators are supported by the National Institute of Aging (P30AG066506‐01; MPI: Smith, Duara, Loewenstein). Funding sources did not play a role in the conduct of the research or manuscript preparation.
Asken BM, DeSimone JC, Wang W‐E, et al. Plasma p‐tau217 concordance with amyloid PET among ethnically diverse older adults. Alzheimer's Dement. 2024;16:e12617. 10.1002/dad2.12617
REFERENCES
- 1. 2022 Alzheimer's disease facts and figures. Alzheimers Dement. 2022;18(4):700‐789. [DOI] [PubMed] [Google Scholar]
- 2. 2023 Alzheimer's disease facts and figures. Alzheimers Dement. 2023;19(4):1598‐1695. [DOI] [PubMed] [Google Scholar]
- 3. Ashton NJ, Brum WS, Di Molfetta G, et al. Diagnostic accuracy of a plasma phosphorylated tau 217 immunoassay for Alzheimer disease pathology. JAMA Neurol. 2024;81(3):255‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Janelidze S, Bali D, Ashton NJ, et al. Head‐to‐head comparison of 10 plasma phospho‐tau assays in prodromal Alzheimer's disease. Brain. 2023;146(4):1592‐1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mendes AJ, Ribaldi F, Lathuiliere A, et al. Head‐to‐head study of diagnostic accuracy of plasma and cerebrospinal fluid p‐tau217 versus p‐tau181 and p‐tau231 in a memory clinic cohort. J Neurol. 2024;271(4):2053‐2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Manly JJ, Jones RN, Langa KM, et al. Estimating the prevalence of dementia and mild cognitive impairment in the US: the 2016 Health and Retirement Study Harmonized Cognitive Assessment Protocol Project. JAMA Neurol. 2022;79(12):1242‐1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weiner MW, Veitch DP, Miller MJ, et al. Increasing participant diversity in AD research: plans for digital screening, blood testing, and a community‐engaged approach in the Alzheimer's Disease Neuroimaging Initiative 4. Alzheimers Dement. 2023;19(1):307‐317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Franzen S, Smith JE, Van Den Berg E, et al. Diversity in Alzheimer's disease drug trials: the importance of eligibility criteria. Alzheimers Dement. 2022;18(4):810‐823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hansson O, Blennow K, Zetterberg H, Dage J. Blood biomarkers for Alzheimer's disease in clinical practice and trials. Nat Aging. 2023;3(5):506‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vandevrede L, La Joie R, Thijssen EH, et al. Evaluation of plasma phosphorylated tau217 for differentiation between Alzheimer disease and frontotemporal lobar degeneration subtypes among patients with corticobasal syndrome. JAMA Neurol. 2023;80(5):495‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Milà‐Alomà M, Ashton NJ, Shekari M, et al. Plasma p‐tau231 and p‐tau217 as state markers of amyloid‐β pathology in preclinical Alzheimer's disease. Nat Med. 2022;28(9):1797‐1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Janelidze S, Berron D, Smith R, et al. Associations of plasma phospho‐tau217 levels with tau positron emission tomography in early Alzheimer disease. JAMA Neurol. 2021;78(2):149‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mattsson‐Carlgren N, Salvadó G, Ashton NJ, et al. Prediction of longitudinal cognitive decline in preclinical Alzheimer disease using plasma biomarkers. JAMA Neurol. 2023;80(4):360‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Palmqvist S, Tideman P, Cullen N, et al. Prediction of future Alzheimer's disease dementia using plasma phospho‐tau combined with other accessible measures. Nat Med. 2021;27(6):1034‐1042. [DOI] [PubMed] [Google Scholar]
- 15. Mielke MM, Dage JL, Frank RD, et al. Performance of plasma phosphorylated tau 181 and 217 in the community. Nat Med. 2022;28(7):1398‐1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cogswell PM, Lundt ES, Therneau TM, et al. Modeling the temporal evolution of plasma p‐tau in relation to amyloid beta and tau PET. Alzheimers Dement. 2024;20(2):1225‐1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thijssen EH, La Joie R, Strom A, et al. Plasma phosphorylated tau 217 and phosphorylated tau 181 as biomarkers in Alzheimer's disease and frontotemporal lobar degeneration: a retrospective diagnostic performance study. Lancet Neurol. 2021;20(9):739‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brickman AM, Manly JJ, Honig LS, et al. Plasma p‐tau181, p‐tau217, and other blood‐based Alzheimer's disease biomarkers in a multi‐ethnic, community study. Alzheimers Dement. 2021;17(8):1353‐1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mohs RC, Beauregard D, Dwyer J, et al. The Bio‐Hermes Study: biomarker database developed to investigate blood‐based and digital biomarkers in community‐based, diverse populations clinically screened for Alzheimer's disease. Alzheimers Dement. 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Asken BM, Wang WE, McFarland K, et al. Plasma Alzheimer's biomarkers and brain amyloid in Hispanic and non‐Hispanic older adults. Alzheimers Dement. 2024;20(1):437‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klunk WE, Koeppe RA, Price JC, et al. The Centiloid Project: standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement. 2015;11(1):1‐15.e1‐e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Amadoru S, Doré V, Mclean CA, et al. Comparison of amyloid PET measured in Centiloid units with neuropathological findings in Alzheimer's disease. Alzheimers Res Ther. 2020;12(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Palmqvist S, Janelidze S, Quiroz YT, et al. Discriminative accuracy of plasma phospho‐tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2020;324(8):772‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hönig M, Altomare D, Caprioglio C, et al. Association between years of education and amyloid burden in patients with subjective cognitive decline, MCI, and Alzheimer disease. Neurology. 2024;102(6):e208053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Asken BM, Elahi FM, La Joie R, et al. Plasma glial fibrillary acidic protein levels differ along the spectra of amyloid burden and clinical disease stage. J Alzheimers Dis. 2020;78(1):265‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Desimone JC, Wang W‐E, Loewenstein DA, et al. Diffusion MRI relates to plasma Aβ42/40 in PET negative participants without dementia. Alzheimers Dement. 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Berry K, Asken BM, Grab JD, et al. Hepatic and renal function impact concentrations of plasma biomarkers of neuropathology. Alzheimers Dement (Amst). 2022;14(1):e12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ramanan VK, Graff‐Radford J, Syrjanen J, et al. Association of plasma biomarkers of alzheimer disease with cognition and medical comorbidities in a biracial cohort. Neurology. 2023;101(14):e1402‐e1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Balls‐Berry JJE, Babulal GM. Health disparities in dementia. Continuum (Minneap Minn). 2022;28(3):872‐884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fortinsky RH, Robison J, Steffens DC, Grady J, Migneault D, Wakefield D. Association of race, ethnicity, education, and neighborhood context with dementia prevalence and cognitive impairment severity among older adults receiving medicaid‐funded home and community‐based services. Am J Geriatr Psychiatry. 2023;31(4):241‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ramanan S, Bertoux M, Flanagan E, et al. Longitudinal executive function and episodic memory profiles in behavioral‐variant frontotemporal dementia and Alzheimer's disease. J Int Neuropsychol Soc. 2017;23(1):34‐43. [DOI] [PubMed] [Google Scholar]
- 32. Schindler SE, Karikari TK, Ashton NJ, et al. Effect of Race on prediction of brain amyloidosis by plasma Aβ42/Aβ40, phosphorylated tau, and neurofilament light. Neurology. 2022;99(3):e245‐e257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Turney IC, Lao PJ, Rentería MA, et al. Brain aging among racially and ethnically diverse middle‐aged and older adults. JAMA Neurol. 2023;80(1):73‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. La Joie R, Ayakta N, Seeley WW, et al. Multisite study of the relationships between antemortem [(11)C]PIB‐PET Centiloid values and postmortem measures of Alzheimer's disease neuropathology. Alzheimers Dement. 2019;15(2):205‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. O'Bryant SE, Zhang F, Petersen M, et al. Neurodegeneration from the AT(N) framework is different among Mexican Americans compared to non‐Hispanic Whites: a Health & Aging Brain among Latino Elders (HABLE) Study. Alzheimers Dement (Amst). 2022;14(1):e12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hall JR, Petersen M, Johnson L, O'bryant SE. Characterizing plasma biomarkers of alzheimer's in a diverse community‐based cohort: a cross‐sectional study of the HAB‐HD cohort. Front Neurol. 2022;13:871947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


