Abstract
Three different deletion mutants of simian immunodeficiency virus (SIV) that vary in their levels of attenuation were tested for the ability to protect against mucosal challenge with pathogenic SIV. Four female rhesus monkeys were vaccinated by intravenous inoculation with SIVmac239Δ3, four with SIVmac239Δ3X, and four with SIVmac239Δ4. These three vaccine strains exhibit increasing levels of attenuation: Δ3 < Δ3X <Δ4. The vaccinated monkeys were challenged by vaginal exposure to uncloned, pathogenic SIVmac251 at 61 weeks after the time of vaccination. On the basis of viral RNA loads in plasma, cell-associated virus loads in peripheral blood, and CD4 cell counts, strong protective effects were observed in all three groups of vaccinated monkeys. However, the degree of protection correlated inversely with the level of attenuation; the least-attenuated strain, SIVmac239Δ3, gave the greatest protection. One monkey in the Δ3X group and two in the Δ4 group clearly became superinfected by the challenge virus, but these animals had levels of SIV RNA in plasma that were considerably lower than those of naive animals that were challenged in parallel. Protection against vaginal challenge appears easier to achieve than protection against intravenous challenge, since four other SIVmac239Δ4-vaccinated monkeys showed no protection when challenged intravenously with a much lower inoculum of the same challenge virus stock. Protection against vaginal challenge in the Δ4-vaccinated group occurred in the absence of detectable serum neutralizing activities and appeared to be associated with the development of an early SIV-specific cytotoxic-T-lymphocyte response. Our results demonstrate that mucosal protection can be achieved by systemic immunization with the highly attenuated SIVmac239Δ4 more than 1 year prior to the time of challenge.
Live, attenuated simian immunodeficiency virus (SIV) deletion mutants have strongly protected rhesus monkeys against challenge by pathogenic strains of the virus (1, 4, 6, 34). Better knowledge of the features of this protection will be needed to move the live, attenuated vaccine approach for AIDS forward. At the very least, better understanding of the protection will aid in designing other vaccine approaches that can mimic it.
By analogy to other viral systems (11, 12), we might expect some viral strains to be lacking in safety because they are not attenuated enough and others to be lacking in protective efficacy because they are too attenuated. Thus, an important consideration for live, attenuated AIDS vaccines is the balance between safety and efficacy. A wide range of attenuation has been achieved in SIV by varying the number and location of deletion mutations (9). However, comparative analysis of the protective capacities of these different vaccine strains has not been undertaken. Such systematic comparisons may also provide clues to the immune responses associated with protection by live, attenuated SIV.
Although the majority of new human immunodeficiency virus type 1 (HIV-1) infections worldwide occur via mucosal transmission, most AIDS vaccine trials in monkeys have analyzed the abilities of different vaccines to protect against intravenous rather than mucosal challenge (30). Most studies of live, attenuated SIV deletion mutants have similarly examined the ability to protect against intravenous challenge with pathogenic SIV (1, 4, 6, 34), although at least one study has reported that systemic vaccination with a nef-defective strain of SIV protected against rectal exposure (5). There is a compelling need to provide more information on the ability of AIDS vaccines to provide protection against mucosal challenge.
Three different attenuated derivatives of SIVmac239 were compared for the ability to protect against vaginal challenge by an uncloned, pathogenic, slightly heterologous strain, SIVmac251. Protection appeared to vary inversely with the degree of attenuation. However, even a highly attenuated derivative lacking nef, vpr, vpx, and upstream sequences in U3 (SIVmac239Δ4) induced a reasonable level of protection against vaginal challenge.
MATERIALS AND METHODS
Virus stocks, immunization, and challenge.
The vaccine strains SIVmac239Δ3, -Δ3X, and -Δ4 were produced by transfection of cloned DNA into CEMx174 cells, and virus was harvested from the cell-free supernatant at or near the peak of virus-induced cytopathic effect 7 to 11 days later. The actual stocks used for these experiments have been described (9). SIVmac239Δ3 is missing unique nef, vpr, and nef sequences that overlap U3 (US); SIVmac239Δ3X is missing nef, vpx, and US sequences; SIVmac239Δ4 is missing nef, vpr, vpx, and US (9). Vaccine virus containing 100 ng of p27 was administered to each animal by intravenous inoculation. Intravenous challenge with uncloned, early-passage SIVmac251 was done with 10 rhesus monkey infectious doses (virus containing 0.032 ng of p27). The preparation, titration, and use of this challenge stock has been described previously (6, 18, 34). This same stock was used for vaginal challenge. Undiluted virus (0.5 ml) containing 48 ng of p27 was placed in the vaginal cavity atraumatically with the animal in a horizontal or slightly inverted position.
Virus load measurements.
The number of infectious cells in peripheral blood mononuclear cells (PBMC), i.e., the cell-associated virus load, was quantitated as previously described (9). Quantitation of viral RNA levels in plasma by real-time reverse transcriptase PCR has also been described (31).
Other measurements.
CD4 cell numbers were quantitated by flow cytometry with a whole-blood lysis technique that we have used previously (34). Procedures for amplifying across the nef gene by PCR for the analysis of wild-type (WT) versus vaccine sequences have been described (34). SIV was purified with the use of column chromatography and used to coat enzyme-linked immunosorbent assay (ELISA) plates as described previously (7). The presence of antibodies to SIV was detected with alkaline phosphatase-conjugated goat anti-human immunoglobulin G, which we have also used previously (9, 34). Procedures for the measurement of neutralization of SIV were performed as described previously (17, 34).
Measurement of viral envelope glycoprotein-specific antibody endpoint titer, conformational dependence, and avidity by ConA ELISA.
Serum samples from macaques infected with SIVmac239 deletion mutants (Δ3, Δ3X, and Δ4) were analyzed for their reactivity to SIVsmB7 (15) viral envelope glycoproteins in a concanavalin A (ConA) ELISA as previously described (3). Endpoint titers to viral envelope glycoproteins are reported as the last serial twofold dilution whose optical density was twice that of normal monkey serum or an optical density of 0.1, whichever value was greater, and all endpoint titer values represent at least two independent experiments. Measurements of conformational dependence were calculated from the ratios of serum antibody reactivities to native envelope glycoprotein substrates versus those to denatured substrates. Thus, the conformation ratio is a direct measure of the conformational dependence of a particular antibody sample (i.e., the larger the conformation ratio above 1.0, the greater the requirement for native envelope glycoprotein structure, while conformation ratios below 1.0 reflect predominant specificity for linear envelope determinants). Viral envelope glycoprotein-specific antibody avidities were determined by measuring the resistance of serum antibody-envelope glycoprotein complexes to treatment with 8 M urea in the ConA ELISA. The avidity index was then calculated from the ratio of the absorbance value obtained with urea treatment to that observed with phosphate-buffered saline treatment multiplied by 100%. All conformation ratios and avidity index values represent at least three independent experiments with several different serum dilutions within the linear range to ensure that the variation in actual values was within 10%.
Assay of SIV-specific CTL activity.
PBMC were isolated from fresh heparinized blood by centrifugation over a Ficoll-sodium diatrizoate (Ficoll 1077; Sigma, St. Louis, Mo.) gradient and suspended at 2 × 106/ml in RPMI 1640 medium supplemented with 10% fetal bovine serum, 10 mM HEPES, 2 mM l-glutamine, 50 IU of penicillin/ml, and 50 μg of streptomycin/ml (R10). Antigen-specific stimulation of PBMC was carried out as described previously (14) with autologous B lymphoblastoid cell lines (B-LCL) infected with a recombinant vaccinia virus (vAbt388; provided by D. Panicali, Therion Biologics, Cambridge, Mass.) containing the SIVmac251 gag and pol genes and the SIVmac239 env gene. After an overnight incubation, vaccinia virus-infected B-LCL were inactivated with UV and psoralen and cultured with PBMC at a responder-to-stimulator ratio of 10:1 in R10 medium. Recombinant interleukin-2 (provided by Maurice Gately, Hoffman LaRoche) was added to a final concentration of 10 to 20 U/ml on day 4 of culture. Cytotoxic-T-lymphocyte (CTL) assays were performed 10 to 14 days after stimulation. The target cells consisted of autologous B-LCL infected with recombinant vaccinia viruses expressing SIV proteins. The recombinant vaccinia viruses used to infect the target cells included vAbt252 (encoding the SIVmac251 p55gag and protease proteins; Therion), rVV-239 (encoding the SIVmac239 envelope; provided by M. Mulligan [28]), and the control vaccinia virus NYCBH. B-LCL were infected overnight with a multiplicity of infection of 5 to 10 PFU/cell and then labeled with chromium 51 (DuPont NEN, Wilmington, Del.) at 100 μCi per 106 cells. Target and effector cells (104/well) were dispensed in duplicate for each effector/target (E/T) ratio into 96-well U-bottom plates (Costar). To decrease background lysis, cold targets consisting of unlabeled autologous B-LCL infected with the control vaccinia virus NYCBH were used at a cold target/hot target ratio of 15:1. Chromium release was assayed after a 5-h incubation at 37°C in a 5% CO2 incubator. The plates were spun at 1,000 rpm for 10 min at 4°C, after which 30 μl of supernatant was harvested from each well into wells of a LumaPlate-96 (Packard) and allowed to dry overnight. Emitted radioactivity was measured in a 1450 MicroBeta Plus liquid scintillation counter (Wallac, Turku, Finland). Spontaneous release was measured from wells containing only target cells and medium. Maximum release was measured from wells containing target cells and 0.1% Triton X-100 (Sigma). The percent cytotoxicity was calculated as follows: [(test release − spontaneous release)/(maximum release − spontaneous release)] × 100. SIV-specific cytotoxicity was calculated by subtracting the lysis of NYCBH-infected target cells from that obtained with targets expressing SIV proteins. Spontaneous release of target cells was <25% in all assays. E/T ratios for which background lysis of control targets exceeded 20% were excluded from analysis. Based on examination of 10 naive control animals not infected with SIV, SIV-specific lysis of greater than 5% seen at more than one E/T ratio was interpreted as significant.
Statistical analysis. Mann-Whitney tests and analysis of variance (ANOVA) were performed using StatView (Abacus Concepts, Berkeley, Calif.).
RESULTS
Vaccine phase.
The groups of four female rhesus monkeys were vaccinated by intravenous inoculation with normalized amounts of vaccine virus containing 100 ng of p27 antigen. One group received SIVmac239Δ3, one received SIVmac239Δ3x, and one received SIVmac239Δ4. These strains have been described (9). The cell-associated virus loads, plasma viral RNA loads, and antibody responses following vaccination of these twelve animals have recently been described (9).
Virus loads following vaginal challenge.
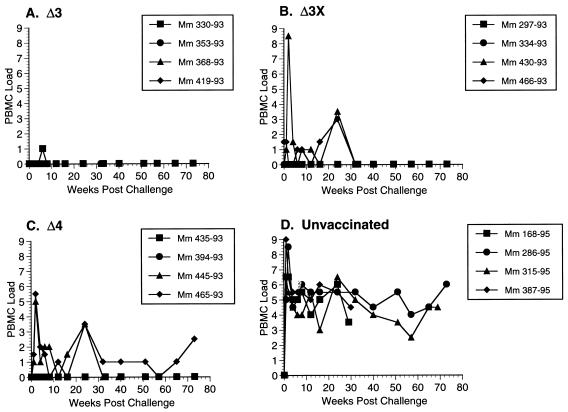
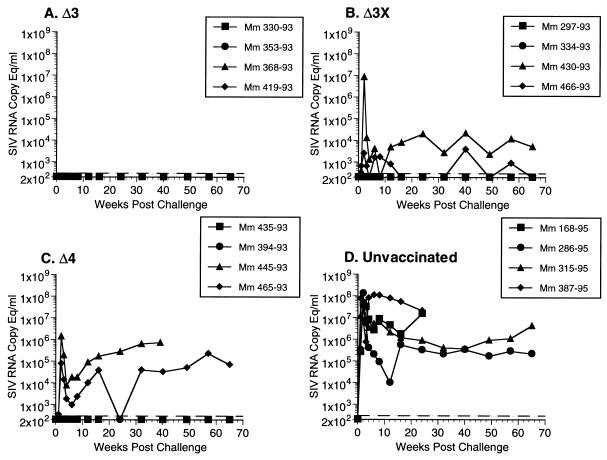
All 12 vaccinated monkeys and four unvaccinated controls were exposed to SIVmac251 challenge virus vaginally at 61 weeks after the time of vaccination. This is the same stock of virus that has been used for intravenous challenge in our previous studies (6, 8, 34). All four of the control animals became persistently infected on the basis of persistent virus recovery from PBMC (Fig. 1) and persisting high levels of viral RNA in plasma (Fig. 2). Both measures of virus load in these control animals were similar to what we have seen previously in animals infected with the same virus by the intravenous route (34). In addition to these four controls, two other rhesus monkeys that were passively administered antibody as part of other experiments also became infected when exposed vaginally at the same time with the same stock (data not shown). Two of two additional animals inoculated vaginally with a 1:5 dilution of SIV were also infected (data not shown). In all, we have exposed a total of eight unvaccinated female rhesus monkeys by the same route with the same stock used for these experiments, or a 1:5 dilution of it, and all eight have become infected.
FIG. 1.
Cell-associated virus loads in monkeys (Mm) challenged with WT SIVmac251 by the vaginal route. The numbers of infectious cells in PBMC were quantitated as described in Materials and Methods. Code for PBMC load: 0, virus was not recovered even when 106 PBMC were used; 1, virus was recovered with an average of 106 but not fewer PBMC; 2, 333,333 PBMC; 3, 111,111 PBMC; 4, 37,037 PBMC; 5, 12,345 PBMC; 6, 4,115 PBMC; 7, 1,371 PBMC; 8, 457 PBMC.
FIG. 2.
Loads measured as amounts of viral RNA in plasma in monkeys (Mm) challenged with WT SIVmac251 by the vaginal route. The dashed lines indicate threefold sensitivity of the assay, i.e., 300 copy equivalents (Eq) per ml.
Vaccine virus was not recovered from PBMC of 11 of the 12 vaccinated animals on the day of challenge, or immediately prior to it, even when 106 PBMC were used (Fig. 1). Viral RNA in plasma was also below the threshold of detection (<300 copy equivalents per ml) in all 12 animals prior to challenge. This is consistent with the attenuated nature of these vaccine strains described previously (9).
In contrast to the control animals, all four of the Δ3-vaccinated animals exhibited little or no SIV recovery from PBMC for a prolonged period after challenge even when 106 PBMC were used (Fig. 1). Viral RNA in plasma also remained around or below the limit of detection in the four Δ3-vaccinated monkeys (Fig. 2). One of the four monkeys vaccinated with Δ3x (430-93) exhibited a sharp spike in cell-associated virus loads at 2 weeks postchallenge (Fig. 1). This corresponded to a similar spike in viral RNA in the plasma of the same animal (Fig. 2). Viral RNA in plasma in this animal persisted at measurable levels at all time points examined over the course of more than 60 weeks (Fig. 2). However, the persisting level of viral RNA was about 100-fold lower in this animal than in the controls in this experiment (Fig. 2) and in similar controls used previously (data not shown). One other animal in the Δ3x challenge group (466-93) exhibited measurable levels of viral RNA at some time points postchallenge, although genetic analysis of viral DNA in PBMC revealed only Δnef sequences (see below) (Fig. 2). The other two monkeys in the Δ3x challenge group were completely protected as assessed by viral cultures and viral RNA. Two of the four Δ4-vaccinated monkeys became virus recovery positive postchallenge (Fig. 1), and both of these monkeys developed measurable levels of persisting viral RNA postchallenge (Fig. 2). The levels of viral RNA in these animals 8 to 16 weeks postchallenge were about 100-fold lower than the levels observed in the controls for this experiment (Fig. 2) and in similar controls in other experiments (data not shown). The other two Δ4-vaccinated monkeys appeared to be solidly protected as measured by the virus load criteria.
To differentiate vaccine strain SIV from the challenge virus SIVmac251, DNA was prepared from PBMC of all challenged animals at weeks 2 and 24 postchallenge and used for genetic analysis by PCR. Primers that span the nef gene were used in such a way that DNA of WT challenge virus and DNA of vaccine strains from which nef was deleted yielded different-sized fragments following amplification (15, 34). The results of these genetic analyses agreed well with the results of all other analyses. PCR analysis of PBMC from animals 430-93 in the Δ3X group and 445-93 and 465-93 in the Δ4 group yielded a PCR product of WT size at both 2 and 24 weeks postchallenge (Table 1). All of the other animals were either negative for amplification of viral DNA or yielded PCR products consistent with the size of the product of the vaccine strain with the nef deletion at both time points (Table 1).
TABLE 1.
Genetic analysis of viral DNA in PBMC
| Virus strain | Animal no. | DNA at:
|
|
|---|---|---|---|
| Week 2 | Week 24 | ||
| Δ3 | 330-93 | Δnefa | Δnef |
| 353-93 | Δnef | Δnef | |
| 368-93 | Δnef | Δnef | |
| 419-93 | Δnef | Δnef | |
| Δ3X | 297-93 | Δnef | Δnef |
| 334-93 | Δnef | Δnef | |
| 430-93 | WTb | WT | |
| 466-93 | Δnef | Δnef | |
| Δ4 | 394-93 | Negative | Negative |
| 435-93 | Δnef | Δnef | |
| 445-93 | WT | WT | |
| 465-93 | WT | WT | |
Δnef, amplification of viral DNA with size of deleted nef.
WT, amplification of viral DNA with size of WT nef.
Intravenous challenge of animals vaccinated with SIVmac239Δ4.
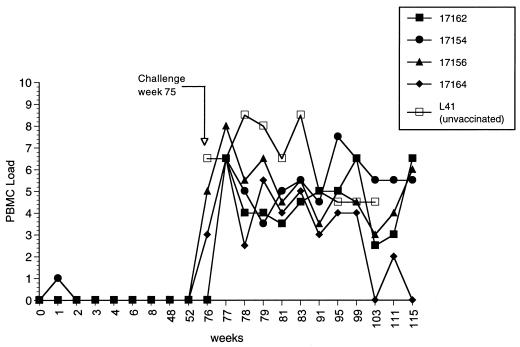
Four other monkeys that had been vaccinated with SIVmac239Δ4 were challenged in separate experiments by the intravenous route. The same stock of SIVmac251 that was used in the vaginal challenge experiments was also used for the intravenous challenges. The titers of this stock have been determined previously by the intravenous route in rhesus monkeys (18). Ten rhesus monkey infectious doses (intravenous) were used for the intravenous challenge. The challenge was performed 75 weeks after the time of vaccination. Virus load measurements postchallenge demonstrated no protective effects of the Δ4 vaccination (Fig. 3). A fifth monkey that had been vaccinated with SIVmac239Δ4 33 weeks previously was similarly challenged, and again there were no protective effects (data not shown).
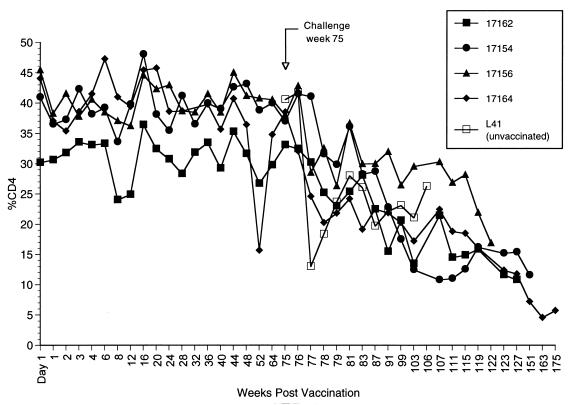
FIG. 3.
Cell-associated virus loads in Δ4-vaccinated monkeys challenged intravenously. Weeks indicate weeks postvaccination with SIVmac239Δ4. The animals were challenged at week 75 intravenously with SIVmac251. The numbers of infectious cells in PBMC are indicated on the y axis. The code for PBMC load is the same as that in the legend to Fig. 1. L41 is an unvaccinated control challenged in parallel.
CD4+-T-cell counts and anamnestic antibody responses.
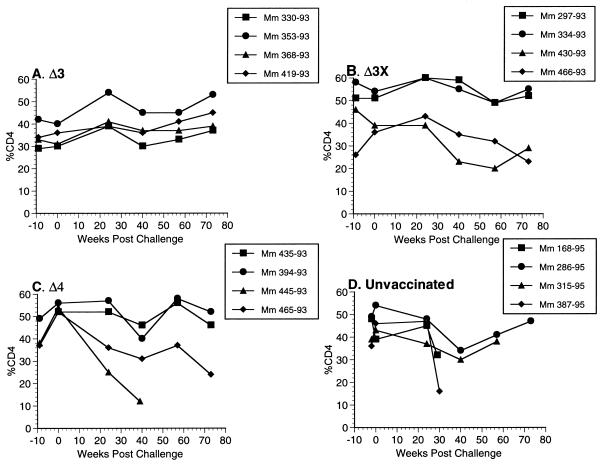
Changes in the percentage and absolute number of CD4+ T cells were quite consistent with the virus load measurements. All 12 of the vaccinated monkeys in the vaginal-challenge group exhibited percentages of CD4+ T cells in the normal range throughout the vaccine phase and at the time of challenge (Fig. 4). All four of the Δ3-vaccinated animals maintained stable percentages of CD4+ T cells for greater than 1 year of follow-up postchallenge (Fig. 4). However, 430-93 in the Δ3X group and 445-93 and 465-93 in the Δ4 group exhibited significant declines in the CD4+-cell population following vaginal challenge (Fig. 4). These same animals also developed consistently measurable viral loads postchallenge (Fig. 1 and 2). An additional Δ3x-vaccinated animal, 466-93, showed a rising and then falling percentage of CD4+ T cells (Fig. 4). Two of the unvaccinated control monkeys that were challenged (168-95 and 387-95) exhibited declining percentages of CD4+ T cells up until the time of their deaths from AIDS at 29 and 30 weeks postchallenge (Fig. 4). 315-95, an unvaccinated monkey, and 445-93 and 465-93, unprotected monkeys in the Δ4 challenge group, also died from AIDS at 69, 39, and 82 weeks postchallenge, respectively. The other 10 vaccinated monkeys in this study were still alive at 86 weeks postchallenge. The four Δ4-vaccinated monkeys that were challenged intravenously (Fig. 3) all exhibited declines in CD4+-cell counts (Fig. 5), and three of the four died or had to be euthanized due to AIDS. For all animals, analysis of the absolute number of CD4+ T cells demonstrated changes similar to those seen with analysis of the percentage of CD4+ T cells (data not shown).
FIG. 4.
Percentages of CD4+ T lymphocytes in monkeys (Mm) challenged with SIVmac251 by the vaginal route. The results are expressed as the percentage of cells in PBMC that were CD4+ T lymphocytes.
FIG. 5.
Percentages of CD4+ T lymphocytes in Δ4-vaccinated monkeys challenged intravenously. The animals were challenged intravenously with SIVmac251 at 75 weeks. L41 is the unvaccinated control challenged in parallel. The results are expressed as the percentage of cells in PBMC that were CD4+ T lymphocytes.
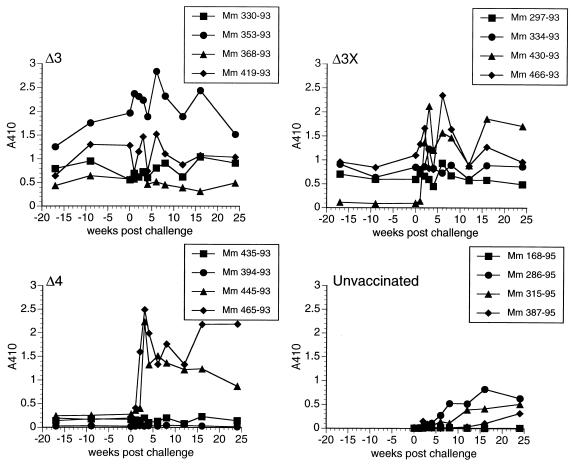
We also used anti-SIV antibody levels to look for anamnestic antibody responses. Animals 445-93 and 465-93 in the Δ4 group and 430-93 in the Δ3X group had clear, strong anamnestic antibody responses postchallenge (Fig. 6), consistent with the other measurements described above. None of the other vaccinated animals that were challenged vaginally showed any clear evidence of anamnestic responses (Fig. 6). All four of the Δ4-vaccinated monkeys that were challenged intravenously also exhibited anamnestic responses (data not shown).
FIG. 6.
Anamnestic antibody responses in monkeys (Mm) challenged vaginally. Plasma from monkeys obtained at the indicated weeks were reacted with purified, lysed SIV, using 1:200 dilutions of plasma and 1:100 dilutions of conjugate. Week 0 is the time of challenge.
Analysis of SIV-specific antibody responses.
In an effort to better understand which immune responses might be involved in mediating protection induced by these attenuated SIV strains, we attempted to correlate several measurements of SIV-specific antibodies and neutralizing antibody titers on the day of challenge with protection. SIV-specific antibody responses were evaluated with respect to endpoint dilution titer, conformational ratio, and avidity index. Previous studies of these parameters in vaccinated animals have shown that monkeys protected by live, attenuated SIV vaccines often had antibody responses characterized by a relatively high avidity and lower conformation dependence, characteristics that have been associated with maturation of the humoral immune response to SIV (3).
Plasma taken on the day of challenge, just prior to inoculation of the challenge virus, was used to measure anti-SIV antibody levels by using ELISA plates coated with antigens from lysed, purified virions. Using a similar assay, we previously showed that these same Δ3-, Δ3X-, and Δ4-vaccinated animals exhibited antibody responses in the initial weeks and months after vaccination that varied according to the vaccine strain: Δ3 > Δ3X > Δ4 (9). Plasma taken on the day of challenge 61 weeks after the time of vaccination exhibited this same trend. Among the Δ4-vaccinated animals, the two animals that had no detectable replication of challenge virus (394-93 and 435-93) had the lowest levels of SIV-specific antibodies (Fig. 7). In fact, monkey 394-93 in the Δ4 group, which originally showed marginal antibody levels around the borderline of detection (9), also showed questionable reactivity with plasma taken on the day of challenge (Fig. 7).
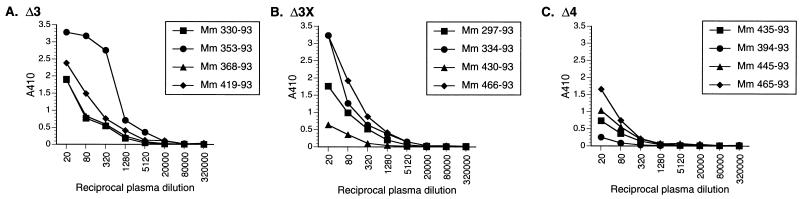
FIG. 7.
Antibody titers to SIV on the day of vaginal challenge. Plasma obtained from monkeys on the day of, and just prior to, vaginal challenge were reacted with purified, lysed SIV, using serial fourfold dilutions starting at 1:20.
We also examined SIV-specific envelope responses by using a heterologous envelope from the SIV/B7 molecular clone, which was derived from SIVsmmH3 (Fig. 8). Again, the same trend was observed: SIV envelope-specific titers decreased with increasing attenuation of the vaccine strain (Fig. 8). Among the Δ4-vaccinated group, the protected animals had the lowest endpoint titers.
FIG. 8.
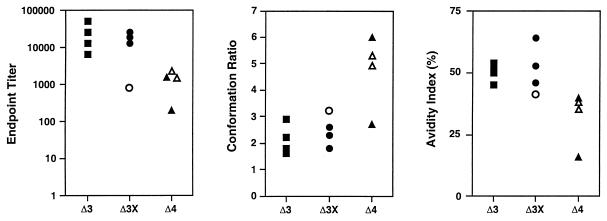
Analysis of envelope-specific anti-SIV antibody responses on the day of vaginal challenge. Serum samples were analyzed for reactivity to SIVsmB7 viral envelope glycoproteins in a ConA ELISA as previously described (3). Measurements of conformational dependence were calculated from the ratios of serum antibody reactivities to native versus denatured envelope glycoprotein substrates. Measurements of viral envelope glycoprotein-specific antibody avidity were determined by measuring the resistance of serum antibody-envelope glycoprotein complexes to mild treatment with 8 M urea in the ConA ELISA. Unprotected animals (430-93, 445-93, and 465-93) are represented by open symbols; protected animals are represented by solid symbols.
SIV-specific antibodies were also characterized with respect to their conformation ratios and avidity indexes (3). The conformation ratio was determined by measuring the serum reactivity to native viral glycoproteins compared to that with denatured viral glycoproteins by ELISA. The avidity index was determined by measuring the resistance of serum antibody envelope glycoprotein immune complexes to disruption by treatment with 8 M urea. By ANOVA, Δ4-vaccinated animals had significantly lower avidity indices (P = 0.02) and a trend toward lower endpoint titers and higher conformational ratios. In general, animals that were protected exhibited higher antibody avidity indices and lower conformational ratios whereas unprotected animals tended to have lower avidity indices and higher conformational ratios (Fig. 8). Using a Mann-Whitney test, correlation of protection with avidity index and with conformational ratio reached statistical significance (P = 0.03 for each). However, the trend of increased protection with higher avidity index and lower conformational ratio appeared to be largely related to the degree of attenuation of the vaccine strain rather than protection per se, since there was no clear distinction among the Δ4-vaccinated animals in either conformation ratio or avidity index between protected and unprotected animals.
We also measured the ability to neutralize viral infectivity by using a laboratory-passaged SIVmac251 (251L) and a primary stock of SIVmac251 (251p). As has been noted previously, (17, 22), the laboratory-passaged stock was more easily neutralized (Table 2). Plasma taken on the day of challenge was used for these measurements. Six of the eight monkeys vaccinated with SIVΔ3 and -Δ3X showed activity in plasma capable of neutralizing the laboratory-passaged stock. None of the plasma from the Δ4-vaccinated animals, including the two solidly protected animals, had neutralizing activity to the laboratory-passaged stock (Table 2). Plasma with the highest levels of neutralizing activity against the laboratory-passaged virus had low levels of neutralizing activity against the primary stock, with titers in the range of 1:10 to 1:24.
TABLE 2.
Summary of Δ3, Δ3X, and Δ4 trial
| Virus strain | Animal | Protectiona | SIVAbb | Nabc
|
CTLd
|
||
|---|---|---|---|---|---|---|---|
| 251L | 251P | Early | Late | ||||
| Δ3 | 330-93 | ++++ | +++ | 170 | <4 | + | +++ |
| 353-93 | ++++ | ++++ | 426 | 23 | + | + | |
| 368-93 | ++++ | +++ | <30 | <4 | + | + | |
| 419-93 | ++++ | +++ | 253 | 10 | ++++ | + | |
| Δ3x | 297-93 | ++++ | +++ | 369 | 24 | + | +++ |
| 334-93 | ++++ | +++ | 377 | 24 | − | +++ | |
| 430-93 | ++ | + | <30 | <4 | − | − | |
| 466-93 | +++ | +++ | 283 | <4 | + | ++ | |
| Δ4 | 394-93 | ++++ | +/− | <30 | <4 | ++++ | ++++ |
| 435-93 | ++++ | + | <30 | <4 | ++ | + | |
| 445-93 | + | + | <30 | <4 | +/− | + | |
| 465-93 | + | + | <30 | 8 | − | + | |
Degree of protection against vaginal challenge (see text).
Strength of antibodies at the time of challenge (see Fig. 7).
Neutralizing antibody titer against laboratory-passaged SIVmac251 (251L) or primary SIVmac251 (251p).
SIV-specific CTL activity against either Gag or envelope at 8 weeks (early) or 61 weeks (late) after infection. −, <5%; +/−, 5–10%; +, 11–20%; ++, 21–30%; +++, 32–40%; ++++, >41%.
Analysis of SIV-specific CTL responses prechallenge.
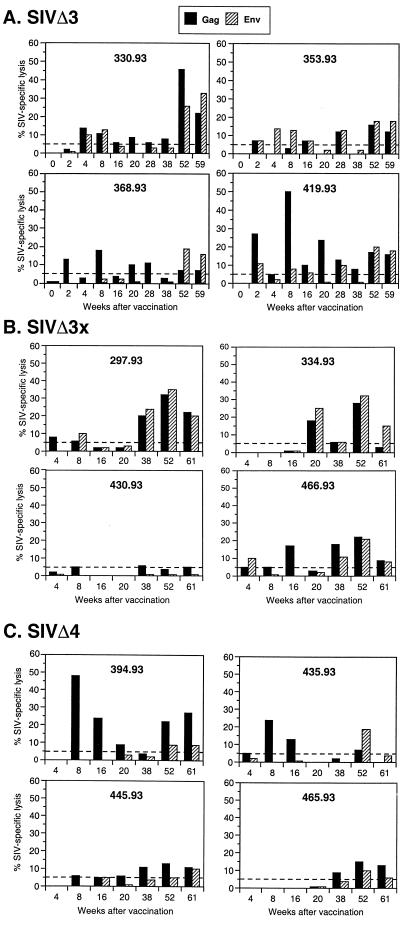
We also attempted to correlate SIV-specific CTL responses with the outcome of challenge. CTL responses against SIV Gag and envelope were prospectively evaluated during the vaccine phase with fresh PBMC stimulated with autologous B-LCL infected with recombinant vaccinia viruses expressing SIV proteins, as described previously (14). CTL activity was then assessed at multiple E/T ratios against autologous B-LCL expressing either SIV Gag or SIV envelope. As previously reported, all four animals vaccinated with SIVmac239Δ3 developed SIV-specific CTL activity within 4 weeks after infection (14). Serial evaluation of SIV-specific CTL activity in these animals at later time points revealed several different patterns (Fig. 9A). For two of the animals (353-93 and 368-93), relatively stable levels of SIV-specific CTL activity were observed to 61 weeks of infection. In one animal (330-93), a late increase in SIV-specific CTL activity was observed at 52 weeks and confirmed at 59 weeks, whereas in the other animal (419-93) CTL activity to the target antigens peaked at 8 weeks and decreased thereafter. The levels of SIV-specific CTL activity in these SIVΔ3-vaccinated animals were generally similar to those reported in our earlier publication (14).
FIG. 9.
Prospective analysis of SIV-specific CTL responses in vaccinated monkeys prior to vaginal challenge. CTL activity following antigen-specific stimulation was examined at multiple E/T ratios, and representative data are shown for an E/T of 40:1 for animals vaccinated with SIVmac239Δ3 (A), SIVmac239Δ3X (B), and SIVmac239Δ4 (C).
Among the macaques vaccinated with SIVmac239Δ3x, three of four animals had developed SIV-specific CTL responses at some time point by 20 weeks after infection, although SIV-specific CTL activity in these animals was more consistently observed after 38 weeks of infection (Fig. 9B). One animal (430-93), the only unprotected Δ3x-infected animal, never developed significant SIV-specific CTL activity at any time point examined prior to challenge.
Two of the 4 animals vaccinated with SIVmac239Δ4 developed SIV-specific CTL responses against Gag or envelope in the first 8 weeks of infection (Fig. 9C). One of these animals (394-93) had one of the highest observed CTL responses of any animal studied in this vaccine trial. This same animal did not have detectable viral RNA at any time and had only marginally detectable levels of virus binding antibodies (9) (Fig. 7). Both of these early Δ4-vaccinated CTL responders were solidly protected against vaginal challenge (Table 2). The other two SIVmac239Δ4-vaccinated animals, which were not completely protected, developed SIV-specific CTL responses of greater than 10% specific lysis only after 38 to 52 weeks of infection.
DISCUSSION
Our results clearly demonstrate that the majority of vaccinated monkeys were solidly protected against vaginal SIVmac251 challenge on the basis of virus load measurements, stable antibody levels, lack of any declines in CD4+-T-cell numbers, and genetic analysis of viral sequences in PBMC. We cannot be certain that there was indeed “sterilizing immunity” in each of these cases or whether some animals might develop increasing virus loads and disease progression at a later date. However, our prior experience with monkeys vaccinated with live, attenuated SIV with this degree of protection is that such monkeys have remained solidly protected for as long as we have followed them, as long as 6 3/4 years (references 6 and 34 and results not shown). In contrast, 430-93 in the Δ3X group and 445-93 and 465-93 in the Δ4 group clearly became superinfected following vaginal SIV251 challenge and developed virus loads in the readily measurable range. While the persisting virus loads in these three animals that were not solidly protected were on average considerably lower than those in the control, unvaccinated group, our previous experience with such animals is that they will progress toward AIDS, but at a slower rate (reference 34 and results not shown).
Our data indicate that it is easier for live, attenuated SIV to protect against vaginal mucosal exposure than against intravenous challenge. None of the four Δ4-vaccinated monkeys that were challenged intravenously following a similar period of vaccination showed any level of protection at all, while protective effects were observed in all four of the Δ4-vaccinated monkeys that were challenged vaginally: two with solid protection and two with lowering of viral loads. It is important to note that the same stock of SIVmac251 was used for both the intravenous and vaginal challenges. Thus, differences in outcome cannot be ascribed to differences in the stocks used. It also appears unlikely that differences in the outcome of challenge reflect differences in the infectious dose of virus used for each of these routes. The vaginal-challenge dose used for these experiments contained 1,500 times more virus than was used for the intravenous challenge (48 ng of p27 antigen vaginally compared with 32 pg intravenously). In vivo titrations have demonstrated that the 32-pg intravenous-challenge dose contains 10 monkey infectious doses by this route (18). Although the vaginal dose used for these experiments has not been formally titrated, the fact that two of two animals challenged with a 1:5 dilution of this stock became infected suggests that this stock contains at least 5 monkey infectious doses. Thus, the dose of SIVmac251 used for vaginal challenge was likely to contain a level of animal infectious units of SIV at least comparable to that present in the intravenous challenge. Using recombinant poxvirus vaccines, Benson et al. have also observed better protection against mucosal challenge exposure than against intravenous challenge (2).
These results provide clear evidence for the ability of systemic immunization with a live, attenuated SIV to provide protection against mucosal challenge. Systemic immunization with nonreplicating antigens is relatively inefficient at inducing local immune responses at mucosal sites, and effective induction of mucosal immune responses generally requires administration of antigens at the mucosal surface (23). This feature has led to the suggestion that effective protection against mucosal exposure to SIV and HIV may require mucosal immunization (21, 25). Several previous attempts to induce protection against vaginal infection with pathogenic SIV have been unsuccessful, including immunization regimens employing live, attenuated SIV (20) or targeted lymph node immunization (21). Our present results, along with the previous demonstration that systemic immunization with a nef-defective SIV strain can protect against rectal challenge (5), provide clear documentation for protection against mucosal challenge following a simple systemic immunization. The immunologic mechanisms responsible for mediating this protection are not understood and may involve immunity at multiple sites, including the mucosal surface, submucosa, or regional or draining lymph nodes. The efficacy of live, attenuated SIV strains in this regard may in part be due to their ability to replicate significantly at mucosal sites during the early weeks after vaccination (33). Thus, highly attenuated SIV vaccine strains may create sufficient immunity to limit challenge virus replication at the mucosal site or limit its subsequent spread to systemic sites. Evidence that protection is indeed immune mediated has been summarized recently (13).
Our results also illustrate the relationship of the level of attenuation of the vaccine strain with the degree of protection. We previously reported solid, long-term protection of four monkeys that had previously been vaccinated with SIV239Δnef and subsequently challenged intravenously (6). Recently, Connor et al. (4) reported strong protection against intravenous challenge by SIVmac251 in eight monkeys that had been vaccinated with SIV239Δnef 15 or more weeks previously. Similarly, Wyand et al. (34) obtained solid protection in four of four monkeys that had been persistently infected with SIV239Δ3 for 79 weeks when they were challenged intravenously with SIV251. In contrast, there were no protective effects in the present study when four monkeys that were vaccinated with SIV239Δ4 were challenged intravenously with SIVmac251 79 weeks after vaccination. It is important to note that the same challenge stock of SIVmac251 at the same dose was used for these various studies, so differences in outcome cannot be ascribed to differences in the challenge stock used. Similarly, in the vaginal challenges described in this report, decreasing protection was observed with increasing levels of attenuation in the vaccine strain, going from Δ3 to Δ3X to Δ4. Thus, the degree of protection appears to correlate inversely with the level of attenuation of the vaccine strain. Lohman et al. have also noted an inverse relation of protection with level of attenuation for the highly attenuated cloned virus SIVmac1A11 and less attenuated derivatives obtained by recombination with SIVmac239 (19). In considering the live, attenuated vaccine approach for use in people, there will clearly be a need to balance the desire to attenuate the virus as much as possible to help ensure safety with the desire to make the vaccine as effective as possible.
Although infection with increasingly attenuated SIV strains clearly decreased the strength of the SIV-specific humoral response (9) (Fig. 7 and Table 2), we did not observe such a clear relationship for the strength of the CTL response. Although early SIV-specific CTL responses (by 8 weeks of vaccination) were observed less frequently in animals immunized with SIVmac239Δ4 than in those immunized with SIVmac239Δ3, the SIV-specific CTL responses in those Δ4 animals that developed early CTL activity were as strong or stronger than those in animals immunized with Δ3 or Δ3X. The effect of attenuation on the magnitude of immune responses induced by live, attenuated SIV strains may therefore differ between humoral and cellular immune responses.
We are encouraged that a strain as attenuated as SIV239Δ4 can still give reasonable levels of protection against natural exposure to a virulent SIV more than 1 year after the time of vaccination. Although only two of four animals were solidly protected, the other two animals exhibited virus load reductions compared to unvaccinated controls. It may be possible in future experiments to get protection with even more attenuated strains by the inclusion of specific types of booster immunizations.
Our studies do not allow unambiguous assignment of immune parameters that are associated with protection. Factors that may contribute to this inability include the relatively small number of unprotected animals, the limited quantitative capabilities of bulk CTL assays, failure to measure immune responses that are the relevant ones, or failure to measure them in an appropriate fashion. Alternatively, protective immunity against primate lentiviruses may result from the sum effect of multiple different immune responses, each of which may play some additive role in resistance to infection. Analysis of the protected Δ4-vaccinated animals does suggest that protection against mucosal infection can be achieved in the absence of detectable neutralizing antibodies in plasma and, in one case, even with ELISA-binding antibodies at the borderline of detection. Similar conclusions have been reached by other investigators examining immunization or challenge with chimeric SIV strains that express the HIV-1 envelope (5, 24). Further analysis of larger cohorts of animals vaccinated with highly attenuated SIV strains, coupled with more quantitative assessments of cellular immune responses, such as those derived from the use of major histocompatibility complex tetramers (10, 32) should allow more detailed evidence for immune correlates of protection by live, attenuated SIV.
ACKNOWLEDGMENTS
We thank María García-Moll of Bio-Molecular Technology, Inc., for the genetic analyses. We also thank Dong-Ling Xia, Allan McPhee, and Theresa Wiltrout for technical assistance, MaryAnn DeMaria and Michael Rosenzweig for flow cytometric analysis, Dennis Panicali and Mark Mulligan for providing recombinant vaccinia viruses, Maurice Gately for providing recombinant IL-2, and Joanne Newton and Jane FitzPatrick for manuscript preparation. We also thank K. Mansfield, P. Sehgal, E. Roberts, and the Division of Primate Resources for animal care, blood sampling, and clinical care.
This work has been supported by PHS grants AI35365, AI43044 (R.C.D. and R.P.J.) AI43075 (R.M.), AI07487 (K.S.C.), and RR 00168 and with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-56000.
REFERENCES
- 1.Almond N, Kent K, Cranage M, Rud E, Clarke B, Stott E J. Protection by attenuated simian immunodeficiency virus in macaques against challenge with virus-infected cells. Lancet. 1995;345:1342–1344. doi: 10.1016/s0140-6736(95)92540-6. [DOI] [PubMed] [Google Scholar]
- 2.Benson J, Chougnet C, Robert-Guroff M, Montefiori D, Markham P, Shearer G, Gallo R C, Cranage M, Paoletti E, Limbach K, Venzon D, Tartaglia J, Franchini G. Recombinant vaccine-induced protection against the highly pathogenic simian immunodeficiency virus SIV(mac251): dependence on route of challenge exposure. J Virol. 1998;72:4170–4182. doi: 10.1128/jvi.72.5.4170-4182.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cole K S, Rowles J L, Jagerski B A, Murphy-Corb M, Unangst T, Clements J E, Robinson J, Wyand M S, Desrosiers R C, Montelaro R C. Evolution of envelope-specific antibody responses in monkeys experimentally infected or immunized with simian immunodeficiency virus and its association with the development of protective immunity. J Virol. 1997;71:5069–5079. doi: 10.1128/jvi.71.7.5069-5079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connor R I, Montefiori D C, Binley J M, Moore J P, Bonhoeffer S, Gettie A, Fenamore E A, Sheridan K E, Ho D D, Dailey P J, Marx P A. Temporal analyses of virus replication, immune responses, and efficacy in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine. J Virol. 1998;72:7501–7509. doi: 10.1128/jvi.72.9.7501-7509.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cranage M P, Whatmore A M, Sharpe S A, Cook N, Polyanskaya N, Leech S, Smith J D, Rud E W, Dennis M J, Hall G A. Macaques infected with live attenuated SIVmac are protected against super-infection via the rectal mucosa. Virology. 1997;229:143–154. doi: 10.1006/viro.1996.8419. [DOI] [PubMed] [Google Scholar]
- 6.Daniel M D, Kirchhoff F, Czajak S C, Sehgal P K, Desrosiers R C. Protective effects of a live-attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 7.Daniel M D, Letvin N L, Sehgal P K, Hunsmann G, Schmidt D K, King N W, Desrosiers R C. Long-term persistent infection of macaque monkeys with the simian immunodeficiency virus. J Gen Virol. 1987;68:3183–3189. doi: 10.1099/0022-1317-68-12-3183. [DOI] [PubMed] [Google Scholar]
- 8.Daniel M D, Mazzara G P, Simon M A, Sehgal P K, Kodama T, Panicali D L, Desrosiers R C. High titer immune responses elicited by recombinant vaccinia virus priming and particle boosting are ineffective in preventing virulent SIV infection. AIDS Res Hum Retroviruses. 1994;10:839–851. doi: 10.1089/aid.1994.10.839. [DOI] [PubMed] [Google Scholar]
- 9.Desrosiers R C, Lifson J D, Gibbs J S, Czajak S C, Howe A Y M, Arthur L O, Johnson R P. Identification of highly attenuated mutants of simian immunodeficiency virus. J Virol. 1998;72:1431–1437. doi: 10.1128/jvi.72.2.1431-1437.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doherty P C. The numbers game for virus-specific CD8+ T cells. Science. 1998;280:227. doi: 10.1126/science.280.5361.227. [DOI] [PubMed] [Google Scholar]
- 11.Freestone D S. Yellow fever vaccine. In: Plotkin S A, Mortimer E A J, editors. Vaccines. W. B. Philadelphia, Pa: Saunders Company; 1988. pp. 387–419. [Google Scholar]
- 12.Henderson D A. Smallpox and vaccinia. In: Plotkin S A, Mortimer E A J, editors. Vaccines. W. B. Philadelphia, Pa: Saunders Company; 1988. pp. 8–30. [Google Scholar]
- 13.Johnson R P, Desrosiers R C. Protective immunity induced by live attenuated simian immunodeficiency virus. Curr Biol. 1998;10:436–443. doi: 10.1016/s0952-7915(98)80118-0. [DOI] [PubMed] [Google Scholar]
- 14.Johnson R P, Glickman R L, Yang J Q, Kaur A, Dion J T, Mulligan M J, Desrosiers R C. Induction of vigorous cytotoxic T-lymphocyte responses by live attenuated simian immunodeficiency virus. J Virol. 1997;71:7711–7718. doi: 10.1128/jvi.71.10.7711-7718.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirchhoff F, Kestler III H W, Desrosiers R C. Upstream U3 sequences in simian immunodeficiency virus are selectively deleted in vivo in the absence of an intact nef gene. J Virol. 1994;68:2031–2037. doi: 10.1128/jvi.68.3.2031-2037.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kraiselburd E N, Torres J V. Properties of virus-like particles produced by SIV-chronically infected human cell clones. Cell Mol Biol. 1995;41 (Suppl. 1):S41–S52. [PubMed] [Google Scholar]
- 17.Langlois A J, Desrosiers R C, Lewis M G, Kewalramani V N, Littman D R, Zhou J Y, Manson K, Wyand M S, Bolognesi D P, Montefiori D C. Neutralizing antibodies in sera from macaques immunized with attenuated simian immunodeficiency virus. J Virol. 1998;72:6950–6955. doi: 10.1128/jvi.72.8.6950-6955.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis M G, Bellah S, McKinnon K, Yalley-Ogunro J, Zack P M, Elkins W R, Desrosiers R C, Eddy G E. Titration and characterization of two rhesus derived SIVmac challenge stocks. AIDS Res Hum Retroviruses. 1994;10:213–220. doi: 10.1089/aid.1994.10.213. [DOI] [PubMed] [Google Scholar]
- 19.Lohman B L, McChesney M B, Miller C J, McGowan E, Joye S M, VanRompay K A, Reay E, Antipa L, Pedersen N C, Marthas M L. A partially attenuated simian immunodeficiency virus induces host immunity that correlates with resistance to pathogenic virus challenge. J Virol. 1994;68:7021–7029. doi: 10.1128/jvi.68.11.7021-7029.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marthas M, Sutjipto S, Miller C, Higgins J, Torten J, Unger R, Kiyono H, McGhee J, Marx P, Pedersen N. Efficacy of live-attenuated and whole-inactivated simian immunodeficiency virus vaccines against intravenous and intravaginal challenge. Vaccines. 1992;1992:117–122. [PubMed] [Google Scholar]
- 21.McGhee J, Lehner T, Miller C. Targeted lymph-node immunization with whole inactivated simian immunodeficiency virus (SIV) or envelope and core subunit antigen vaccines does not reliably protect rhesus macaques from vaginal challenge with SIVmac251. AIDS. 1998;12:1–10. doi: 10.1097/00002030-199801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Means R E, Greenough T, Desrosiers R C. Neutralization sensitivity of cell culture-passaged simian immunodeficiency virus. J Virol. 1997;71:7895–7902. doi: 10.1128/jvi.71.10.7895-7902.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987;7:265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- 24.Miller C J, McChesney M B, Lu X, Dailey P J, Chutkowski C, Lu D, Brosio P, Roberts B, Lu Y. Rhesus macaques previously infected with simian/human immunodeficiency virus are protected from vaginal challenge with pathogenic SIVmac239. J Virol. 1997;71:1911–1921. doi: 10.1128/jvi.71.3.1911-1921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller C J, McGhee J R, Gardner M B. Biology of disease: mucosal immunity, HIV transmission, and AIDS. Lab Investig. 1992;68:129–145. [PubMed] [Google Scholar]
- 26.Putkonen P, Makitalo B, Bottiger D, Biberfeld G, Thorstensson R. Protection of human immunodeficiency virus type 2-exposed seronegative macaques from mucosal simian immunodeficiency virus transmission. J Virol. 1997;71:4981–4984. doi: 10.1128/jvi.71.7.4981-4984.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quesada-Rolander M, Makitalo B, Thorstensson R, Zhang Y-J, Castanos-Velez E, Biberfeld G, Putkonen P. Protection against mucosal SIVsm challenge in macaques infected with a chimeric SIV that expresses HIV type 1 envelope. AIDS Res Hum Retroviruses. 1996;12:993. doi: 10.1089/aid.1996.12.993. [DOI] [PubMed] [Google Scholar]
- 28.Ritter G D J, Mulligan M J, Lydy S L, Compans R W. Cell fusion activity of the simian immunodeficiency virus envelope protein is modulated by the intracytoplasmic domain. Virology. 1993;197:255–264. doi: 10.1006/viro.1993.1586. [DOI] [PubMed] [Google Scholar]
- 29.Spira A I, Marx P A, Patterson B K, Mahoney J, Koup R A, Wolinsky S M, Ho D D. Cellular targets of infection and route of viral dissemination following an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med. 1996;183:215–225. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stott J, Hu S-L, Almond N. Candidate vaccines protect macaques against primate immunodeficiency viruses. AIDS Res Hum Retroviruses. 1998;14:S265–S270. [PubMed] [Google Scholar]
- 31.Suryanarayana K, Wiltrout T A, Vasquez G M, Hirsch V M, Lifson J D. Plasma SIV RNA viral load by determination by real-time quantification of product generation in reverse transcriptase-polymerase chain reaction. AIDS Res Hum Retroviruses. 1998;14:183–189. doi: 10.1089/aid.1998.14.183. [DOI] [PubMed] [Google Scholar]
- 32.Tough D F, Sprent J. Anti-viral immunity: spotting virus-specific T cells. Curr Biol. 1998;8:R498–R501. doi: 10.1016/s0960-9822(98)70317-3. [DOI] [PubMed] [Google Scholar]
- 33.Veazey R S, DeMaria M, Chalifoux L V, Shvetz D E, Pauley D R, Knight H L, Rosenzweig M, Johnson R P, Desrosiers R C, Lackner A A. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 34.Wyand M S, Manson K H, Garcia-Moll M, Montefiori D, Desrosiers R C. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J Virol. 1996;70:3724–3733. doi: 10.1128/jvi.70.6.3724-3733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]