Abstract
p21(WAF1/SDII/CIP1) (p21) arrests cell growth by inhibiting cyclin-depend kinases. To explore the potential of using p21 for the gene therapy of cervical cancer, we infected human papillomavirus (HPV)-positive cervical cancer cells (HeLa, SiHa, and Z172) and HPV-negative cervical cancer cells (C33A) with recombinant adenovirus encoding p21 cDNA. The results revealed that effective inhibition of cell growth could be achieved by sense p21 adenovirus but not antisense p21 adenovirus infection and occurred through apoptosis as measured by DNA fragmentation and chromatin condensation. Apoptosis was also observed in xenografts of human cervical cancer cells infected with sense p21 adenovirus, as confirmed by in situ terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL). The apoptosis was not prevented by overexpression of the bcl-2 transgene. To sum up, the apoptotic effect suggests that p21 should be a tumoricidal agent instead of a tumoristatic agent in preventing cervical cancers. In addition, our report substantiates the combination of the high efficiency of adenovirus vector-mediated gene delivery and the apoptotic effect of p21.
The protein p21(Waf1/SdiI/CipI) (p21) is encoded by a recently cloned gene (Sdi), which is overexpressed in senescent fibroblasts (22, 28). The same p21 protein was found to associate with different cyclin-dependent kinase–cyclin complexes and to inhibit the kinase activity that is required for cell cycle progression (30, 31). Independent research had shown that p53 induces the p21 gene (10). Hence, p21 can mediate the cell proliferation arrest induced by p53 (10). The utility of p21 as an antitumor agent in a number of human cancer cell lines had been reported. Introduction of p21 into human prostate cancer cells (9), human brain tumor cells (1), and colon carcinoma cells (3) can significantly inhibit cell growth and tumorigenesis. To explore the potential of using p21 for the gene therapy of cervical cancers, we infected human papillomavirus (HPV)-positive cervical cells (HeLa, SiHa, and Z172) and HPV-negative cervical cancer cells (C33A) with recombinant adenovirus encoding p21 cDNA. HeLa, SiHa, and Z172 cells contain HPV-18, HPV-16, and HPV-16 genomes, respectively (25). These HPV-positive cervical cancer cell lines lack normal retinoblastoma (RB) and p53 functions, at least in part, since they express normal pRB and wild-type p53 proteins, which are presumed to be abrogated in function as a consequence of association with HPV E7 and E6 oncoproteins, respectively (25). In contrast, the HPV-negative C33A cells harbor the mutated p53 and RB genes (25).
The results of this study demonstrate that effective inhibition of cervical cancer cell growth could be achieved by sense p21-encoding adenovirus infection. However, instead of p21-induced growth arrest of infected cells, as we would expect, a massive detachment of infected cells from the culture surface was observed. Infection of cervical cancer cells with recombinant adenovirus encoding antisense p21, however, did not cause cell detachment. This prompted us to study the possible occurrence of apoptosis of p21-transduced cells. The obvious finding of DNA fragmentation and chromatin condensation in cervical cancer cells infected with p21-encoding recombinant adenovirus further confirmed apoptosis as the mechanism of cell death. Apoptosis was also shown to be the mechanism of cell death in human cervical xenografts infected with p21-encoding recombinant adenovirus. The mechanism of p21-induced apoptosis is not clear. Our results reveal that p21-induced apoptosis was not prevented by overexpression of the bcl-2 transgene and seemed to be p53 and RB independent.
MATERIALS AND METHODS
Virus generation.
To generate replication-deficient recombinant viruses carrying either sense or antisense p21 cDNA, we isolated a 2.1-kb NotI fragment from pCEP-WAF1-S and ligated it with pAdE1CMV/pA (15). After restriction enzyme mapping, a virus containing the sense orientation of p21 cDNA (pW8) and one containing the antisense orientation of p21 cDNA (pW5) were cotransfected with pJM17 into 293 cells to generate recombinant viruses (15). The genomic structures of both sense p21 (i.e., W8) and antisense p21 (i.e., W5) viruses were confirmed by genomic PCR (15). For large-scale virus production, the recombinant viruses were harvested from 20 plates of 293 cells grown on a P-150 dish after 36 h of infection and subjected to two cycles of CsCl gradient ultracentrifugation (16). After dialysis overnight, the stock of viruses was aliquoted and stored at −80°C until use. The average titers of viral stocks were determined by a plaque assay in triplicate.
Cells and transfection.
The HeLa, SiHa, and C33A cervical cancer cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum and antibiotics. Z172 cells were maintained in DMEM with 10% NuIV serum (Collaborative Research) and antibiotics.
The bcl-2 cDNA cloned in the pCEP4 vector (2) was introduced into HeLa, SiHa, Z172, and C33A cells separately, and then HeLa/bcl2, SiHa/bcl2, Z172/bcl2, and C33A/bcl2 cells were established from the pooled clones, which were selected by growth in hygromycin (200 μg/ml) for 3 or 4 weeks.
Growth rate assay.
A total of 105 cells were seeded on 10-cm plates in triplicate. After 24 h, the cells were infected with either sense or antisense p21-encoding adenovirus at a multiplicity of infection (MOI) of 25; the cells were harvested at 2, 4, 6, 8, and 10 days after virus infection and counted with a hemacytometer, and their viability was determined by trypan blue exclusion.
Acridine orange staining.
Cells were fixed with ice-cold 75% ethanol and then washed with phosphate-buffered saline (PBS) three times. The cells were then permeabilized with PBS–0.1% Triton X-100 at room temperature for 30 min and were stained with 10 μg of acridine orange (Sigma, St. Louis, Mo.) per ml in PBS for 30 min. They were then washed with PBS and observed with a fluorescence microscope fitted with a blue filter.
Detection of DNA fragmentation in agarose gels.
DNA extraction was performed by lysing cells in a solution containing 0.5% sodium dodecyl sulfate (SDS), 100 mM EDTA, 10 mM Tris-HCl (pH 8.0), 20 μg of RNase A per ml, and 100 μg of proteinase K per ml and incubated at 37°C for 1 h with gentle shaking. The suspension of lysate was then extracted twice with phenol-chloroform, and the DNA was precipitated with ethanol. DNA was electrophoresed on a 1.4% agarose gel. The gel was stained with ethidium bromide and photographed under UV light.
Immunoblots.
Cellular proteins were extracted in SDS-polyacrylamide gel electrophoresis (PAGE) loading buffer. After a 10-min boiling step, about 100 μg of each crude protein lysate was separated by SDS-PAGE, transferred to nitrocellulose filters, reacted with specific monoclonal p21 antibody recognizing the full-length p21 protein (Pharmingen), and visualized by enhanced chemiluminescence (Amersham, Little Chalfont, United Kingdom) by procedures recommended by the manufacturer.
In vivo analysis for tumor suppression and apoptosis.
Experiments were performed as described previously (19). Briefly, 4- to 6-week-old nude female mice were anesthetized with ketamine (20 mg/kg) and subcutaneously injected with 2.5 × 106 tumor cells on their flanks. When the tumors grew to about 75 mm3, the animals were reanesthetized. The adenoviruses (2.5 × 107 PFU) in 100 μl of DMEM were injected in a volume of 30 to 35 μl into one tumor at three different positions for 1 min per position with a microliter syringe (Hamilton Co., Reno, Nev.) fitted with a 26-gauge needle, and the needle was left in the tissues for another 1 min and then withdrawn slowly. For tumor suppression analysis, the volume of the tumor [(length × width × height × 4/3 (πr3)] was measured weekly. For apoptosis analysis, the nude mice were sacrificed 72 h after virus infection and tumor tissue was isolated in situ for terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL).
In situ TUNEL.
The TUNEL procedure was performed as described by the manufacturer (Boehringer Mannheim). Briefly, paraffin-embedded tumor sections were dewaxed in xylene for 5 min three times each and were progressively hydrated by immersing the slides for 3 min each in 100, 90, 70, and 30% ethanol solutions. Endogenous peroxidase was inactivated by immersing the slides for 20 min in 0.75% (vol/vol) H2O2 in 100% methanol. The slides were washed in PBS, and the sections were digested with 0.1% (wt/vol) pepsin in 0.1 N HCl for 5 min at 37°C, extensively washed in PBS, and incubated in a moist chamber at 37°C for 1 h with an end-labeling cocktail that included 0.5 U of terminal deoxynucleotidyltransferase per μl, 0.06 mM fluorescein-dUTP, 10 μl of 5× TdT buffer (Boehringer Mannheim), and double-distilled water to 50 μl. The reaction was terminated by immersing the slides in a buffer containing 300 mM NaCl and 30 mM sodium citrate in double-distilled water. After the slides were washed in PBS, the sections were directly observed under a fluorescence microscope.
Immunohistochemistry.
Avidin-biotin immunohistochemistry was performed on 4-μm sections from routinely processed paraffin-embedded tissues. The sections were deparaffinized and cleared before being treated with a 3% solution of hydrogen peroxidase in methanol to block the endogenous peroxidase activity. Then the sections were processed as described previously (2) and incubated with an appropriate dilution of primary antibody against p21 protein (Pharmingen). The avidin-biotin experiment was performed as described by the manufacturer (DAKO). Hematoxylin was used for counterstaining.
RESULTS
Expression of p21 protein by the adenovirus-transduced p21 gene.
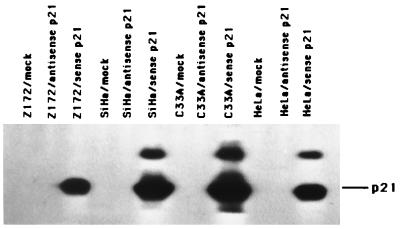
In this study, three HPV-positive cervical cancer cell lines (HeLa, SiHa, and Z172) and one HPV-negative cell line (C33A), all of which lack normal RB and p53 functions (as described in the introduction) (25), were used for investigating the mechanism of p21-mediated tumor suppression. Two replication-defective recombinant adenoviruses were generated as described in Materials and Methods. In sense p21 adenovirus, p21 gene expression was driven by a minimal human cytomegalovirus early promoter. A second adenovirus, antisense p21 adenovirus, had the same structure except that antisense p21 cDNA was encoded. To detect the expression of p21 protein, cells were infected at a MOI of 25 with either sense p21 or antisense p21 adenovirus. At 48 h after infection, total cellular proteins were extracted and Western blot analysis was performed. Figure 1 showed that only sense p21 adenovirus-infected cells could express the 21-kDa p21 protein. A 27-kDa protein band appeared only in the p21 virus-infected SiHa, C33A, and HeLa cells; we do not have enough evidence to speculate on the nature of this 27-kDa band. However, a similar band was observed when p21 protein was induced (17).
FIG. 1.
Expression of p21 protein detected by Western blot analysis. The cervical cancer cells, Z172, SiHa, C33A, and HeLa, were infected with sense p21 or antisense p21 adenovirus at a MOI of 25 or with PBS (mock); after 48 h, protein lysates were isolated and analyzed by immunoblotting with anti-p21 antibody (Pharmingen).
Effect of the p21 gene on cell growth.
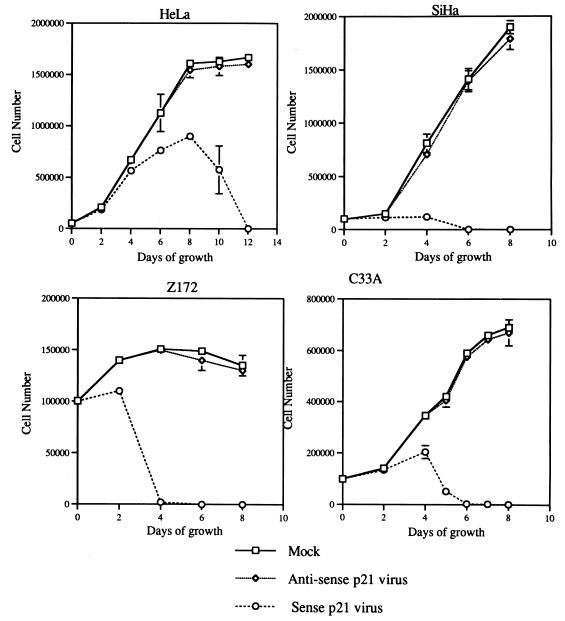
The effect of p21 on four different cervical cancer cells was determined by measuring the cell growth rate. Cells were infected with either sense p21 or antisense p21 adenovirus at a MOI of 25, and the number of viable cells which remained attachment to the culture surface was counted by measuring trypan blue dye exclusion with a hemacytometer every 2 days. As shown in Fig. 2, 2 days after sense p21 adenovirus infection, detachment of cells from the culture surface was observed in all four cell lines studied. The number of cells that remained attached to the culture surface was actually decreasing after 4 days for sense p21 adenovirus-infected SiHa, Z172, and C33A cells and after 8 days for HeLa cells. The majority of cells detached from the culture surface within 4 to 6 days in sense p21 adenovirus-infected SiHa, Z172, and C33A cells, and it took HeLa cells 12 days to completely detach from culture surface. This was unexpected since p21, as an inhibitor of cell cycle progression (26), was expected to arrest cell growth. The decrease in the cell number suggests that p21 has cytotoxic functions.
FIG. 2.
In vitro effect of p21 on cell growth. The growth of these four cervical cancer cell lines was determined by counting the cell number at different time points after infection of cells at a MOI of 25 with sense p21 or antisense p21 adenovirus. The results are the mean of three separate experiments.
In vitro analysis for apoptosis.
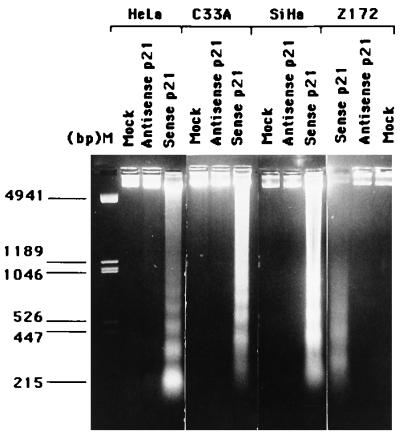
An unexpected observation during analysis of the growth rate was the massive detachment of cells from the culture surface 48 to 96 h after sense p21 adenovirus infection. Before the detachment, rounding up of cells and bleb formation in cells could be observed. This detachment from the culture surface and morphological changes of cells were not observed in cells infected with antisense p21 adenovirus at the same MOI. This indicates that p21 plays a role in inducing the morphological changes, cell detachment, etc. The detachment from the culture surface and blebbing of cytoplasm are typical finding of cells undergoing apoptosis (11). These observations prompted us to further analyze the biochemical changes of apoptosis in p21 adenovirus-infected cells. One of the characteristic markers of apoptosis is the biochemically observable appearance of the ladder of DNA fragments in electrophoresis of cellular DNA. Chromosomal DNA extracted from the adherent and detached cell after infection with sense or antisense p21 adenovirus for 72 h was subjected to agarose gel electrophoresis. As shown in Fig. 3, the appearance of DNA fragments equivalent to approximately 200 bp and multiples thereof was noticed in all tested cells infected with sense p21 adenovirus. No detectable fragmented DNA emerged from mock-infected or antisense p21 virus-infected cells.
FIG. 3.
DNA fragmentation assays. Low-molecular-weight DNA was harvested from attached and detached cells infected with sense p21 or antisense p21 adenovirus, and the DNA was analyzed by electrophoresis on a 1.4% agarose gel.
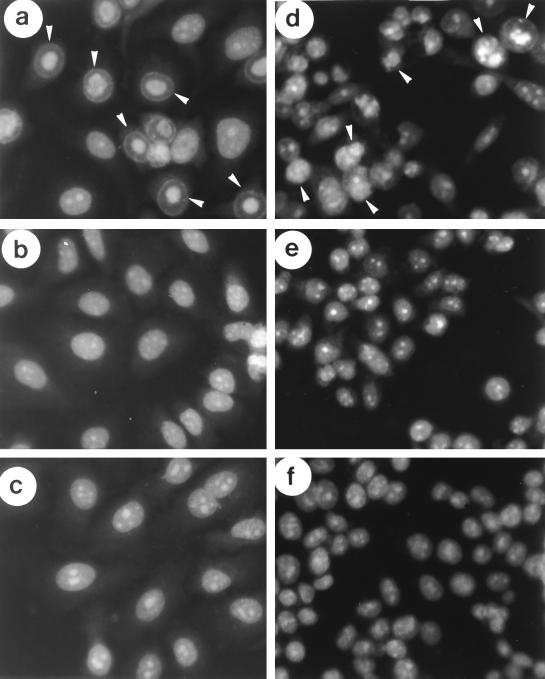
Another characteristic marker of apoptosis is the chromatin condensation and nuclear fragmentation which result from destruction of the structural organization of the nucleus. Hence, 2 days after virus infection, cells were stained with acridine orange and examined under fluorescence microscopy for apoptotic cells. Figure 4 shows the apoptotic features such as chromatin condensation, nuclear fragmentation, and formation of apoptotic bodies in HeLa and C33A cells after sense p21 adenovirus infection (Fig. 4a and d) but not with mock infection (Fig. 4c and f) or antisense p21 adenovirus infection (Fig. 4b and e).
FIG. 4.
In vitro analysis for apoptosis. Cells infected with sense p21 adenovirus, antisense p21 adenovirus, or PBS only (mock) for 2 days were stained with acridine orange and observed by fluorescence microscopy (a and d) HeLa and C33A cells infected with sense p21 adenovirus, respectively; (b and e) HeLa and C33A cells infected with antisense p21 adenovirus, respectively; (c and f) HeLa and C33A cells without virus infection (mock infected), respectively. The apoptotic bodies are indicated by arrowheads in panels a and d.
p21 induces tumor suppression and apoptosis in tumor xenografts.
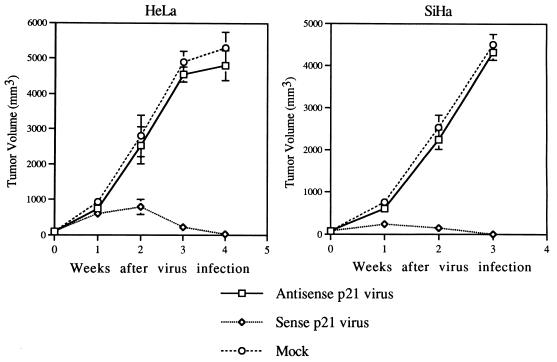
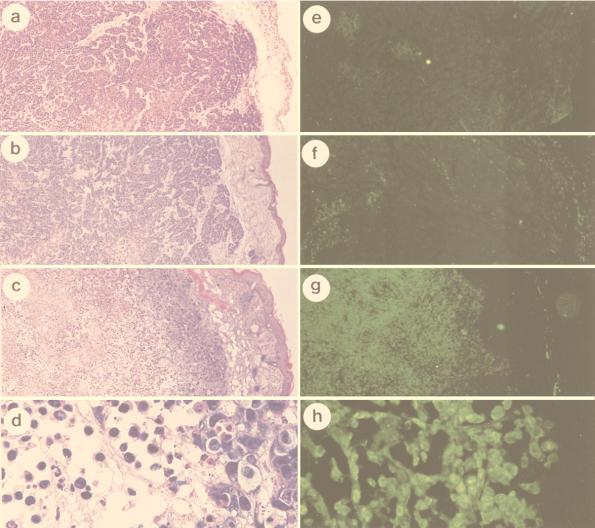
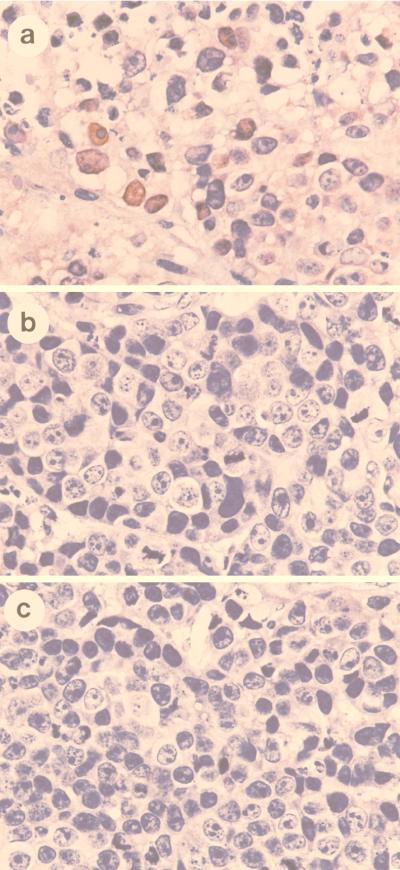
To further analyze the potential of sense p21 adenovirus in gene therapy of cervical cancers, the growth suppression effect of sense p21 adenovirus was tested in nude mice. First, we measured the transducible fraction of tumor cells by infecting tumor cells with recombinant adenovirus encoding Escherichia coli β-galactosidase combined with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining of tumor tissues. About 80% of tumor cells were shown to stain blue (data not shown), indicating highly efficient transduction of the gene into tumors by adenovirus. Experimental tumors were established with HeLa and SiHa cells in nude mice (15 nude mice for each cell line group). The cells were implanted subcutaneously, and the tumors grew to about 75 mm3. Sense p21 adenovirus was then injected into the tumor into five nude mice in each cell line group as described in Materials and Methods. Another five nude mice in each cell line group were injected into the tumor with antisense p21 adenovirus, and the remaining five mice were injected with the same volume of PBS as a mock control. The tumor size was monitored weekly, and the results show significant inhibition of tumor growth by sense p21 adenovirus injection compared with antisense p21 adenovirus or mock injection, indicating that tumor regression was due to the effect of p21 transduction (Fig. 5). To analyze the mechanism of tumor suppression, 3 days after virus injection the paraffin-embedded tumor tissues were sectioned and in situ TUNEL analysis was performed for every 10th section to detect apoptotic cells. The control experiment, in which the terminal transferase was left out, was performed to ensure the specificity of the TUNEL assay, and the result shows no fluorescent signal (data not shown). As shown in Fig. 6, scattered clusters of fluorescent staining were observed in tissue sections isolated from SiHa cell tumors treated with mock infection (Fig. 6e) or antisense p21 adenovirus (Fig. 6f). Hematoxylin-and-eosin staining of the same section shows that tumors treated with mock infection (Fig. 6a) or antisense p21 adenovirus (Fig. 6b) had central necrosis and apoptosis surrounding the necrotic tissues. However, this apoptosis existed in only 5% of tumor tissue in the cross section. On the other hand, tissue sections isolated from SiHa tumors treated with sense p21 adenovirus stained brightly in the TUNEL assay, as shown in Fig. 6g (low magnification) and h (high magnification); and hematoxylin-and-eosin staining showed that apoptosis existed in 90% of the tumor tissue in a cross section and that only 10% of the tissue on the outskirts of tumor was spared from massive apoptosis (Fig. 6c [low magnification] and d [high magnification]), suggesting that apoptosis is the event involved in p21-induced suppression of tumor growth in vivo. To further confirm that apoptosis is indeed induced by overexpression of p21 protein, an immunohistochemistry assay was performed with tumor tissues. Figure 7 shows that after 36 h, the majority of tumors infected with sense p21 virus expressed p21 protein in the nucleus or cytoplasm (Fig. 7a) but that antisense p21 virus-treated (Fig. 7b) and or mock-treated (Fig. 7c) tumors did not.
FIG. 5.
p21-mediated tumor growth inhibition. HeLa and SiHa cells (2.5 × 106) were separately injected subcutaneously into the flanks of nude mice. When the tumors grew to about 75 mm3, the animals were reanesthetized and 2.5 × 107 PFU of adenovirus encoding sense p21 or antisense p21 or PBS only (mock infection) was injected into the tumor in five nude mice in each cell line group. The volumes of the tumors in five nude mice in each group were measured weekly. The error bars indicate the standard deviations of tumor volumes of five mice.
FIG. 6.
In vivo analysis of apoptosis by an in situ TUNEL assay. In situ end-labeling analysis was performed on paraffin-embedded sections obtained from tumor-bearing nude mice which were injected with sense p21 or antisense p21 adenovirus or PBS only (mock infected) 3 days earlier. SiHa cells were injected with PBS only (a and e), antisense p21 adenovirus (b and f), or sense p21 adenovirus (c, d, g, and h). (a to d) Hematoxylin and eosin stain; (e to h) fluorescent stain of apoptotic cells as shown by the in situ TUNEL assay. Magnifications, ×40 (a, b, c, e, f, and g) and ×300 (d and h); panels d and h are magnifications of panels c and g, respectively.
FIG. 7.
Expression of p21 in tumor tissues. At 36 h postinfection, the paraffin-embedded sections were obtained from sense p21 virus-treated (a), antisense p21 virus-treated (b) or mock-treated (c) tumors and stained with p21 antibody (Pharmingen) by the immunohistochemistry assay. Hematoxylin was used for counterstaining.
Bcl-2 cannot protect against p21 transgene-mediated apoptosis in cervical cancer cells.
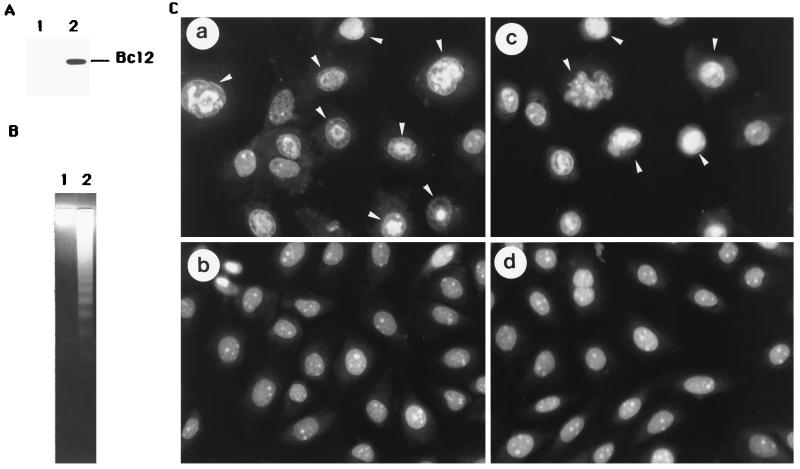
Bcl-2 is an integral intracellular membrane protein that inhibits programmed cell death induced by multiple insults in a wide variety of cell types (23, 24). To understand the ability of Bcl-2 protein to protect these cervical cancer cells from apoptosis, we introduced the bcl-2 gene into these four cervical cancer cell lines by a Lipofectin method. After 3 or 4 weeks, hygromycin B-resistant cell clones were pooled and established, and they all expressed high levels of Bcl-2 protein. When these Bcl-2-overexpressing cells and their respective parental control cells were infected with sense p21 adenovirus, similar degrees of chromatin condensation and DNA degradation were found. Figure 8 gives the results only for SiHa cells. Figure 8A shows Bcl-2 protein expression in SiHa/bcl2 cells (lane 2); Fig. 8B shows DNA fragmentation in SiHa/bcl2 cells infected with sense p21 virus (lane 2); and Fig. 8C shows chromatin condensation of SiHa cells with the bcl-2 transgene infected with sense p21 adenovirus (panel c). Taken together, these results indicate that Bcl-2 protein cannot protect cells from p21-induced apoptosis.
FIG. 8.
Bcl-2 cannot protect against p21-mediated apoptosis in cervical cancer cells. (A) Expression of Bcl-2 protein. Cellular proteins were extracted, then separated by a SDS-PAGE, and subjected to immunoblot analysis with antibody recognizing Bcl-2 (Santa Cruz). Lanes 1 and 2 represent SiHa cells and SiHa/bcl2 cells (which contain the bcl-2 gene). (B) Low-molecular-weight DNA was harvested from attached and detached SiHa/bcl2 cells which were infected with sense p21 (lane 2) or antisense p21 (lane 1) adenovirus and then analyzed by electrophoresis on a 1.4% agarose gel. (C) SiHa cells with or without Bcl-2 protein infected with sense p21 or antisense p21 adenovirus for 2 days were stained with acridine orange and observed with a fluorescence microscope. Panels a and b represent SiHa cells infected with sense p21 and antisense p21 adenovirus, respectively. Panels c and d represent SiHa/bcl2 cells infected with sense p21 and antisense p21 adenovirus, respectively. The apoptotic cells were observed in panels a and c (arrowheads).
DISCUSSION
A replication-defective adenovirus system was used to show that ectopically overexpressing p21 inhibited tumor growth due to human cervical cancer cell lines. These cervical cancer cell lines infected with sense p21 adenovirus clearly manifested apoptosis, as shown by nucleosomal DNA fragmentation and chromatin condensation in vitro. Moreover, in situ TUNEL analysis showed that apoptosis was also involved in suppression of cervical tumor growth in vivo. However, antisense p21 adenovirus produced no effect, suggesting that the process is not a function of transduced viral gene products.
p21 inhibits the growth of human brain tumor cells, colon carcinoma cells, and virally transformed chicken embryo fibroblasts by promoting cell cycle arrest (1, 3, 9, 12). However, recent reports also suggest that p21 has an apoptosis-inducing function. For example, repression of p21 gene expression by antisense p21 delays apoptosis induced by serum deprivation of mouse fibroblasts (8). Apoptosis is induced by p21 transfection in vascular smooth muscle cells and RB cells (17, 20). These findings call for a reinvestigation of the effect of p21 on more common human cancers. In the present study, using p21 genes delivered by recombinant adenovirus vectors, we demonstrated that p21 could cause apoptosis instead of cell cycle arrest of cervical cancer cells. The apoptotic effect suggests that p21 should be a tumoricidal instead of tumoristatic agent, and this may explain the effectiveness of p21 in preventing tumor growth. The massive apoptosis existed in sense p21 virus-treated tumors seems to result from p21 transgene overexpression, since p21 could be identified in the majority of cells soon (36 h) after infection (Fig. 7). The chance of apoptosis being caused by an adenovirus-induced immune system response is slim, since we did not observed massive lymphocyte infiltration into tumor tissues and since nude mice, which were used in this study, are defective in their cellular immune response. Taken together, our results indicate that the high efficiency of adenovirus vector-mediated gene delivery and the effect of p21 can be combined for the gene therapy of cervical cancer.
Our results reveal that Bcl-2 protein could not protect cells from p21-induced apoptosis. First identified for its role in B-cell malignancies, Bcl-2 inhibits cell death due to a variety of apoptotic stimuli and operates in numerous cell types (24). Bcl-2 can inhibit the activation of nematode death effector ced3 and mammalian protease interleukin-1β-converting enzyme, both of which are essential components of cell death pathways (24). However, a recent report demonstrated that Bcl-2 fails to inhibit Fas/Apo-1-induced apoptosis, suggesting the existence of distinct apoptosis signaling pathways which display differential sensitivity to Bcl-2 (5). Moreover, Bcl-2 is also reported to be a downstream death substrate of caspases, which can be induced to cause cell death. It has been proposed that a separate group of caspases may cleave Bcl-2 and abolish its antiapoptotic function (4). Why Bcl-2 cannot protect cervical cancer cells from p21-induced apoptosis is unclear and worthy of further investigation. However, Bcl-2 was expressed from an integrated low-copy-number plasmid and p21 was expressed from adenovirus at high MOI of 25 (Fig. 8). We cannot rule out the possibility that the lack of protection by Bcl-2 against p21-induced apoptosis is due to the low level of Bcl-2 expression.
It has been shown in several reports that E2 of bovine papillomavirus type 1, HPV-16, and HPV-18 can induce growth arrest and apoptosis of cervical carcinoma cell lines (6, 7, 14). The apoptosis induced by E2 seems to be p53 dependent, since E2 stimulates the expression of the p53 gene while inducing apoptosis but fails to induce apoptosis in p53-negative cell lines (6). However, expression of the dominant negative p53 mutant abolishing the transcriptional activity of p53 can prevent only E2-induced growth arrest but not E2-induced apoptosis (6), indicating that E2-induced apoptosis may also involve a p53-independent pathway. On the other hand, p21 expression is induced by a p53-independent mechanism (10); thus, p21 may play a role in E2-induced apoptosis. No matter what the mechanism of E2-induced apoptosis turns out to be, our finding that overexpression of p21 can induce apoptosis in both p53-positive and p53-negative cells indicates that p21 is superior to E2 when used for gene therapy of cervical cancer.
In this study, the p21 gene was delivered via a recombinant adenovirus. Three adenovirus early genes (E1A, E3, and E4) have been reported to have an apoptosis potential function (18, 21, 27). Adenovirus E1A is known to induce apoptosis through a p53-dependent pathway (21), and E3 has been shown to induce cell lysis (27). In the recombinant adenovirus that we used in this study, both E1A and E3 genes were deleted (15). It has been reported that upon conditional induction, adenovirus E4 can induce apoptosis in rodent cells regardless of the p53 status and this apoptosis is significantly overcome by coexpression with either Bcl-2 and Bcl-XL (18). However, in this study, our results reveal that the failure of Bcl-2 to prevent p21-induced apoptosis and the lack of apoptosis induction by adenovirus delivering antisense p21 gene preclude the direct effect of adenovirus E4. Whether the apoptosis that we observed is the result of cooperation between p21 and E4 remains to be determined.
To sum up, this is the first demonstration that p21 plays an important role in the induction of apoptosis in human cervical cancer cells. In addition, our report substantiates the combination of a high efficiency of adenovirus vector-mediated gene delivery and the apoptotic effect of p21.
ACKNOWLEDGMENT
This work was supported by National Science Council grant NSC 87-2312-B106-003.
REFERENCES
- 1.Chen J, Willingham T, Shuford M, Bruce D, Rushing E, Smith Y, Nisen P D. Effects of ectopic overexpression of p21WAF1/CIP1 on aneuploidy and the malignant phenotype of human brain tumor cells. Oncogene. 1996;13:1395–1403. [PubMed] [Google Scholar]
- 2.Chen S L, Tsao Y P, Chen Y L, Huang S J, Chang J L, Wu S F. The induction of apoptosis by SV40 T antigen correlates with c-jun overexpression. Virology. 1998;244:521–529. doi: 10.1006/viro.1998.9109. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y Q, Cipriano S C, Arenkiel J M, Miller F R. Tumor suppression by p21WAF1. Cancer Res. 1995;55:4536–4539. [PubMed] [Google Scholar]
- 4.Cheng E H Y, Kirsch D G, Clem R J C, Ravi R, Kastan M B, Bedi A, Ueno K, Hardwick J M. Conversion of bcl-2 to a bax-like death effector by caspases. Science. 1997;278:1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 5.Chinnaiyan A M, Orth K, O’Rourke L, Duan H, Poirier G G, Disit V M. Molecular ordering of the cell death pathway: Bcl-2 and Bcl-xl function upstream of the ced-3-like apoptotic proteases. J Biol Chem. 1996;271:4573–4576. doi: 10.1074/jbc.271.9.4573. [DOI] [PubMed] [Google Scholar]
- 6.Desaintes C, Demeret C, Goyat S, Yaniv M, Thierry F. Expression of the papillomavirus E2 protein in HeLa cells leads to apoptosis. EMBO J. 1997;16:504–514. doi: 10.1093/emboj/16.3.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowhanick J J, McBride A A, Howley P M. Suppression of cellular proliferation by the papillomavirus E2 protein. J Virol. 1995;69:7791–7799. doi: 10.1128/jvi.69.12.7791-7799.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duttaroy A, Qian J F, Smith J S, Wang E. Up-regulated p21cip1 expression is part of the regulation quantitatively controlling serum deprivation-induced apoptosis. J Cell Biochem. 1997;64:434–446. [PubMed] [Google Scholar]
- 9.Eastham J A, Hall S J, Sehgal I, Wang J, Timme T L, Yang G, Connell-Crowley L, Elledge S J, Zhang W W, Harper J W, Thompson T C. In vivo gene therapy with p53 or p21 adenovirus for prostate cancer. Cancer Res. 1995;55:5151–5155. [PubMed] [Google Scholar]
- 10.El-Deiry W S, Tokino S T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 11.Fisher D E. Apoptosis in cancer therapy: crossing the threshold. Cell. 1994;78:539–542. doi: 10.1016/0092-8674(94)90518-5. [DOI] [PubMed] [Google Scholar]
- 12.Givol I, Givol D, Rulong S, Resau J, Tsarfaty I, Hughes S H. Overexpression of human p21 WAF1/CIP1 arrests the growth of chicken embryo fibroblasts transformed by individual oncogenes. Oncogene. 1995;11:2609–2618. [PubMed] [Google Scholar]
- 13.Harper J W, Adami G R, Wei N, Keyomarsi K, Elledge S J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 14.Hwang E S, RieseII D J, Settleman J, Nilson L A, Hong J, Flynn S, DiMaio D. Inhibition of cervical carcinoma cell line proliferation by the introduction of a bovine papillomavirus regulatory gene. J Virol. 1993;67:3720–3729. doi: 10.1128/jvi.67.7.3720-3729.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleinerman D I, Zhang W W, Lin S H, Van N T, von Eschenbach A C, Hsieh J T. Application of a tumor suppressor (C-CAM1)-expressing recombinant adenovirus in androgen-independent human prostate cancer therapy: a preclinical study. Cancer Res. 1995;55:2831–2836. [PubMed] [Google Scholar]
- 16.Kleinerman D I, Dinney C P N, Zhang W W, Lin S H, Van N T, Hsieh J T. Suppression of human bladder cancer growth by increased expression of C-CAM-1 gene in an orthotopic model. Cancer Res. 1996;56:3431–3435. [PubMed] [Google Scholar]
- 17.Kondo Y, Kondo S, Liu J, Haqqi T, Barnett G H, Barna B P. Involvement of p53 and WAF1/CIP1 in γ-irradiation-induced apoptosis of retinoblastoma cells. Exp Cell Res. 1997;236:51–56. doi: 10.1006/excr.1997.3693. [DOI] [PubMed] [Google Scholar]
- 18.Lavoie J N, Nguyen M, Marcellus R C, Branton P E, Shore G C. E4orf4, a novel adenovirus death factor that induces p53-independent apoptosis by a pathway that is not inhibited by zVAD-fmk. J Cell Biol. 1998;140:637–645. doi: 10.1083/jcb.140.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu T J, El-Naggar A K, McDonnell T J, Steck K D, Wang M, Taylor D L, Clayman G L. Apoptosis induction mediated by wild-type p53 adenoviral gene transfer in squamous cell carcinoma of the head and neck. Cancer Res. 1995;55:3417–3422. [PubMed] [Google Scholar]
- 20.Matsushita H, Morishita R, Kida I, Aoki M, Hayashi S I, Tomita N, Yamamoto K, Moriguchi A, Noda A, Kaneda Y, Higaki J, Ogihara T. Inhibition of growth of human vascular smooth muscle cells by overexpression of p21 gene through induction of apoptosis. Hypertension. 1998;31:493–398. doi: 10.1161/01.hyp.31.1.493. [DOI] [PubMed] [Google Scholar]
- 21.Mymryk J S. Tumour suppressive properties of the adenovirus 5 E1A oncogene. Oncogene. 1996;13:1581–1589. [PubMed] [Google Scholar]
- 22.Noda A, Ning Y, Venable S F, Pereira S O, Smith J R. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res. 1994;211:90–98. doi: 10.1006/excr.1994.1063. [DOI] [PubMed] [Google Scholar]
- 23.Oliver F J, Marvel J, Collins M K, Lopez-Rivas A. Bcl-2 oncogene protects a bone marrow-derived pre-B-cell line from 5′-fluor,2′-deoxyuridine-induced apoptosis. Biochem Biophys Res Commun. 1993;194:126–132. doi: 10.1006/bbrc.1993.1794. [DOI] [PubMed] [Google Scholar]
- 24.Reed J C. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994;124:1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheffner M, Münger K, Byrne J C, Howley P M. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc Natl Acad Sci USA. 1991;88:5523–5527. doi: 10.1073/pnas.88.13.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sherr C. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 27.Tollefson A E, Ryerse J S, Scaria A, Hermiston T W, Wold W S M. The E3-11.6-kDa adenovirus death protein (ADP) is required for efficient cell death: characterization of cells infected with adp mutants. Virology. 1996;220:152–162. doi: 10.1006/viro.1996.0295. [DOI] [PubMed] [Google Scholar]
- 28.Tsao Y P, Kuo S W, Li S F, Liu J C, Lin S Z, Chen K Y, Chen S L. Differential regulation of cyclin A, cyclin B, and p21 concentrations in a growth-restricted human fibroblasts cell line. Biochem J. 1995;312:693–698. doi: 10.1042/bj3120693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waga S, Hannon G J, Beach D, Stillman B. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature (London) 1994;369:574–578. doi: 10.1038/369574a0. [DOI] [PubMed] [Google Scholar]
- 30.Xiong Y, Hannon G J, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature (London) 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 31.Xiong Y, Zhang H, Beach D. Subunit rearrangement of the cyclin-dependent kinases is associated with cellular transformation. Genes Dev. 1993;7:1572–1583. doi: 10.1101/gad.7.8.1572. [DOI] [PubMed] [Google Scholar]