Abstract
Objective:
Current meta-analysis was performed to systematically evaluate the potential prognostic factors for overall survival among resected cases with gallbladder carcinoma.
Methods:
PubMed, EMBASE, and the Cochrane Library were systematically retrieved and hazard ratio (HR) and its 95% confidence interval were directly extracted from the original study or roughly estimated via Tierney’s method. Standard Parmar modifications were used to determine pooled HRs.
Results:
A total of 36 studies with 11 502 cases were identified. Pooled results of univariate analyses indicated that advanced age (HR=1.02, P=0.00020), concurrent gallstone disease (HR=1.22, P=0.00200), elevated preoperative CA199 level (HR=1.93, P<0.00001), advanced T stage (HR=3.09, P<0.00001), lymph node metastasis (HR=2.78, P<0.00001), peri-neural invasion (HR=2.20, P<0.00001), lymph-vascular invasion (HR=2.37, P<0.00001), vascular invasion (HR=2.28, P<0.00001), poorly differentiated tumor (HR=3.22, P<0.00001), hepatic side tumor (HR=1.85, P<0.00001), proximal tumor (neck/cystic duct) (HR=1.78, P<0.00001), combined bile duct resection (HR=1.45, P<0.00001), and positive surgical margin (HR=2.90, P<0.00001) were well-established prognostic factors. Pathological subtypes (P=0.53000) and postoperative adjuvant chemotherapy (P=0.70000) were not prognostic factors. Pooled results of multivariate analyses indicated that age, gallstone disease, preoperative CA199, T stage, lymph node metastasis, peri-neural invasion, lymph-vascular invasion, tumor differentiation status, tumor location (peritoneal side vs hepatic side), surgical margin, combined bile duct resection, and postoperative adjuvant chemotherapy were independent prognostic factors.
Conclusion:
Various prognostic factors have been identified beyond the 8th AJCC staging system. By incorporating these factors into a prognostic model, a more individualized prognostication and treatment regime would be developed. Upcoming multinational studies are required for the further refine and validation.
Keywords: adenocarcinoma, surgery, cancer, tumor, carcinoma, cholecystectomy, gallbladder, malignant, neoplasm, surgical resection
Introduction
Highlights
Current study is the first to systematically evaluate the potential prognostic factors and independent prognostic factors among resected cases with gallbladder carcinoma.
To date, the sample size of current study is the largest.
Pooled results validated the prognostic value of conventional staging factors reported by 8th AJCC staging system (T, N).
Various unmasked potential prognostic factors, such as peri-neural invasion, tumor locations, lymph-vascular invasion, have also been explored and validated.
Gallbladder carcinoma (GBC) is a rare but highly lethal gallbladder epithelium-originated malignant disease with a reported poor 5-year survival rate less than 5%1. Curative-intent surgery has always been regarded the most effective intervention and a pure cholecystectomy has been demonstrated to be especially effective in patients with Tis or T1a disease with a reported 5-year survival rate reaching 100%2,3. Moreover, the radical cholecystectomy, including gallbladder removal, partial or more extended hepatectomy and a regional or more extensive lymphadenectomy has been widely applied in T1b or more advanced GBC1,4. Promising survival outcomes have been achieved and reported in those received curative surgery1,4.
According to the latest 8th American Joint Committee on Cancer (AJCC) staging system, the T stage (the depth of invasion), lymph node metastasis, and metastatic disease are the major staging factors and the most important prognostic factors for cases with GBC5. Apart from the three conventional prognostic factors mentioned above, many other potential prognostic factors with inconsistent validity of evidence have also been recommended for clinical care but have not been fully evaluated, including histological grade (differentiation status), pathological subtype, and lymph-vascular invasion6–8. Owing to the complex anatomic features that gallbladder is surrounded by various vital organs and structures, there might also be other hidden predictors for survival. For example, some researchers indicated that an elevated preoperative CA199 level indicated worse prognosis in postsurgical GBC patients9–11. Some easily-ignored predictors, such as age12,13 and the level of preoperative fibrinogen level14, have also been recommended but not fully evaluated. Just as our previous series indicated that the whole biliary tract was surrounded by dense neural network, it might serve as another potential metastatic route for GBC patients15 and prognostic significance of peri-neural invasion has also been validated in numerous previous publications16–18. Moreover, our recent research focusing on the significance of tumor locations (proximal tumor: neck/cystic duct tumors vs distal tumor: body/fundus tumors) in resected GBC indicated that proximal tumors shared much worse prognosis than distal tumors, especially cystic duct tumor19. However, the findings above have not been mentioned by 8th AJCC staging system. Additionally, the prognostic value of the extent of resection, especially the significance of extra-hepatic bile duct resection, has also been systematically evaluated in our previous series20 but has not been fully incorporated in the 8th AJCC staging system.
Therefore, current meta-analysis was performed to have a comprehensive evaluation and validation on the potential prognostic factors among surgically-treated GBC patients. Our findings might assist clinicians to stratify patients with different survival outcomes and treatment modalities.
Materials and methods
Search strategy
Eligible studies were retrieved and identified based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA, Supplemental Digital Content 1, http://links.lww.com/JS9/C299, Supplemental Digital Content 2, http://links.lww.com/JS9/C300)21 and Assessing the methodological quality of systematic reviews (AMSTAR, Supplemental Digital Content 3, http://links.lww.com/JS9/C301)22 guidelines. Comprehensive literature researching was performed in the following databases till 5 February 2024: PubMed, EMBASE, the Cochrane Library. Eligible studies were restricted to publications focusing on the prognostic factors for overall survival (OS) among resected GBC patients. The searching keywords were listed as follows: gallbladder, cancer, tumor, carcinoma, malignant, neoplasm, adenocarcinoma, surgery, surgical resection, and cholecystectomy. In order to broaden our research, we also reviewed any relevant studies listed in previous reviews or meta-analyses. The primary endpoint of current study is the potential prognostic factors for resected cases with GBC through the incorporation of results in univariate analyses. Moreover, all potential independent prognostic factors have also been furtherly evaluated via the incorporation of results reported in multivariate analyses. The current study was conducted based on published retrospective or prospective studies and was not registered. Additionally, we did not prepare a review protocol.
Inclusion criteria
Published English literature;
Only original researches reporting prognostic factors for OS among resected GBC were considered eligible.
Exclusion criteria
Abstracts, reviews, letters, meetings, and on-going clinical trials would be ruled out.
For certain studies, despite reporting prognostic factors, if those factors were rare and failed to be meta-analyzed or if the original data failed to be extracted, those studies would be also excluded.
For originated from the same center and a complete data duplication was detected, only the study with the largest sample size would be included.
Studies reported prognostic factors for disease-free survival (DFS) or cancer-specific survival but not for OS.
Studies with inadequate original data for further analysis would also be excluded.
Data extraction
The potential prognostic factors were confirmed via reviewing previous GBC-related publications as well as the latest 8th AJCC staging criteria by the first author (L.T.R) and second author (W.J.K). Based on the results of our own single-center experience3,15,23 and findings reported in previous publications, all authors discussed and finally identified sixteen relevant prognostic factors, including age (young vs old), preoperative obstructive jaundice (no vs yes), preoperative CA199 (low vs high), concurrent gallstone disease (no vs yes), T stage (T1-2 vs T3-4), lymph node metastasis (N- vs N+), peri-neural invasion (- vs +), lymph-vascular invasion (- vs +), vascular invasion (- vs +), pathology subtypes (AC vs others), differentiation status (well to moderate vs poor), tumor location (peritoneal side vs hepatic side), tumor location (distal vs proximal), surgical margin (- vs +), concurrent bile duct resection (not performed vs performed), and postoperative adjuvant chemotherapy (not performed vs performed).
Statistical analysis
Tierney’ method was the foundation of data extraction, transformation, and incorporation24. When hazard ratio (HR) and its 95% CI were directly provided, the HR and 95% CI were directed used and were transformed into the lnHR and selnHR for further analysis. However, when HR and 95% CI were not directly provided and only Kaplan–Meier curves were provided with the number of patients in both groups, a rough estimate of HR was performed via Tierney’s method using engauge software24. When HR and 95% CI as well as survival curves were both not provided, a rough calculation of HR and its 95% CI was conducted based on the number of cases in control group, the number of cases in experimental group, the total number of cases with final event (death), and the P-value from log rank test or univariable analysis, which was also based on Tierney’s method24. HRs larger than 1 indicated a negative survival impact on OS versus reference. Standard Parmar modifications was the major methodology of data extraction in our analysis25. Acquired lnHR and selnHR were incorporated via RevMan5.3 software. P-values lower than 0.05 indicated statistical difference.
Heterogeneity analyses were performed via removing every single study manually to find the major source of heterogeneity. A P-value lower than 0.1 and I2 greater than 50% indicated the existence of significant heterogeneity26. Any single study, which caused great heterogeneity would be excluded. Sensitivity analysis was also performed in a same manner. Publication bias was systematically evaluated via Stata14.0 software (Begg’s and Egger’s tests) for the comparison sharing the largest number of included studies. A P-value or corrected P-value (P*) lower than 0.05 indicated a significant publication bias27.
Results
Preliminary literature search identified a total of 57 097 publications. After removing duplicated articles, we were left with 45 957 unique papers. These papers underwent a screening process based on specific inclusion and exclusion criteria and 374 studies were considered potentially eligible. Full-text screening was furtherly conducted among these 374 articles and ultimately identified 36 studies3,9,10,16,17,19,23,28–56 for the final meta-analysis, which had sufficient clinical data (Fig. 1). For studies that performed based on public cancer databases like the Surveillance, Epidemiology, and End Results (SEER) or National Cancer Database (NCDB), we only included the study with the largest sample size. Specifically, we included a recently published study by Jiang et al.37 based on the SEER database with the largest sample size. In cases where multiple studies came from the same center, we applied a similar criterion of including only the study with the largest sample size. In our analysis, we included the study by Gani et al.36 and the study by Jiang et al.37, both of which were multicenter studies conducted in America. While there might be some overlap in patient sources between the two studies, their respective study periods differed, and the study by Jiang et al. had a significantly larger sample size (5451 vs 449) compared to the study by Gani et al. Similarly, multicenter studies by Vega et al., which included cases from Chile and America, were also included due to the similarity of their status. In the case of studies by Li et al., we considered several recent publications focusing on GBC. We included the study with the largest sample size (Lv TR et al., 2023, n=326) for further analysis23. However, two additional studies from the same center but with smaller sample sizes were also included. These two studies examined the prognostic value of tumor location (Lv TR et al., n=259)19 and the prognostic value of combined bile duct resection (Wang JK et al., n=213)3. The results of these two studies were included in different outcomes and did not generate any data overlap. The baseline characteristics of finally included studies were summarized in Table 1.
Figure 1.
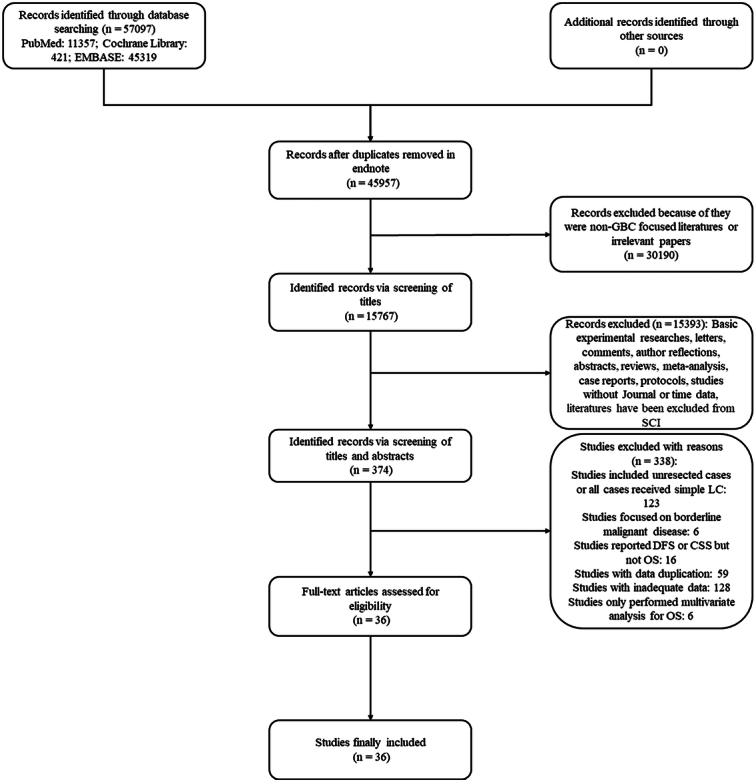
Specific process of literature research, selection, and identification. CSS, cancer-specific survival; DFS, disease-free survival; LC, laparoscopic cholecystectomy; OS, overall survival; SCI, scientific citation index.
Table 1.
Baseline characteristics of all studies included.
| Study author | Year | Study period | Cohort origin | Specific hospitals | No. cases included | Sex ratio (female/male) | Staging criteria | Follow-up time |
|---|---|---|---|---|---|---|---|---|
| Schauer et al.47 | 2001 | 1980–1997 | Germany | Ludwig-Maximilian University | 127 | NA | Nevin staging criteria | NA |
| Shimizu et al.48 | 2007 | 1980–2005 | Japan | Graduate School of Medicine, Chiba University | 79 | 50/29 | JSBS stage | Median 152, range, 6–288 months |
| Kohya and Miyazaki39 | 2008 | 1989–2007 | Japan | Saga University Hospital | 52 | 36/16 | AJCC staging criteria, 2th edition | NA |
| Murakami et al.44 | 2011 | 1990–2010 | Japan | Hiroshima University Hospital | 62 | 30/32 | UICC staging criteria, 2010 edition | Median 157, range 2– 255 months |
| Cziupka et al.34 | 2012 | 2001–2009 | Germany | Greifswald University | 33 | NA | UICC staging criteria, 2010 edition | NA |
| Choi et al.16 | 2013 | 2000–2010 | Korea | Korea University Medical Center | 71 | 39/32 | AJCC staging criteria, 7th edition | Till 31 November 2012 |
| Liu et al.43 | 2013 | 1995–2010 | China | Liaocheng People’s Hospital | 78 | 46/32 | AJCC staging criteria, 7th edition | Median 26.5, range: 2–132 months |
| Abe et al.9 | 2016 | 1996–2014 | Japan | Onomichi General Hospital | 54 | 33/21 | UICC staging criteria, 2010 edition | Median 3 years, range 0.04–13.8 years |
| Gani et al.36 | 2016 | 2000–2014 | USA | Ten medical centers: Johns Hopkins University; Emory University; Stanford University; University of Wisconsin; Ohio State University; Washington University; Vanderbilt University; New York University; University of Louisville; Wake Forest University | 449 | 292/157 | AJCC staging criteria, 7th edition | Median 37.6 months (IQR: 12.3–82.1). |
| Salman et al.46 | 2016 | 2000–2011 | Turkey | Atat€urk Training and Research Hospital | 47 | (35/12) | NA | Till July, 2013 |
| Kurahara et al.17 | 2017 | 2000–2015 | Japan | Kagoshima University | 80 | 44/36 | AJCC staging criteria, 7th edition | NA |
| Wang et al. | 2017 | 2008–2013 | China | Jinshan Hospital, Fudan University | 125 | 80/45 | AJCC staging criteria, 8th edition | Till December 2015 |
| Wen et al.52 | 2017 | 2003–2013 | China | Eastern Hepatobiliary Hospital | 390 | 240/150 | AJCC staging criteria, 7th edition | Till May 2014 |
| Vega et al.49 | 2019 | 2000–2017 | USA/Chile | The University of Texas MD Anderson Cancer Center/ Hospital Sotero del Rio | 255 | 195/60 | AJCC staging criteria, 7th edition | Median 70⋅8, 95% CI: 53⋅6–87⋅3 months |
| Wang et al. | 2019 | 2007–2016 | China | West China Hospital | 213 | 139/74 | AJCC staging criteria, 7th edition | Every 2–3 months in the first year and 3–6 months thereafter |
| Zheng et al.56 | 2019 | 2007–2016 | China | The Second Affiliated Hospital of Wenzhou Medical University | 83 | 60/23 | AJCC staging criteria, 7th edition | Till September 2018 |
| Bao et al.30 | 2020 | 2010–2017 | China | Third Affiliated Hospital of Soochow University | 144 | 107/37 | AJCC staging criteria, 8th edition | Till October 2020 |
| Lee et al.40 | 2020 | 2001–2013 | Korea | National Cancer Center | 158 | 85/73 | AJCC staging criteria, 8th edition | Every 3 or 6 months after discharge |
| Xu et al.53 | 2020 | 2005–2017 | China | Peking Union Medical College Hospital | 154 | 91/63 | AJCC staging criteria, 8th edition | Every 3 months during the first 2 years, every 6 months during the next 3 years, and annually after 5 years |
| Ando et al.28 | 2021 | 1982–2019 | Japan | Niigata University Medical and Dental Hospital and Niigata Cancer Center Hospital, Japan | 207 | 117/90 | AJCC staging criteria, 8th edition | Median 107, range: 0.5–424 months |
| Chaudhary et al.32 | 2021 | 1985–2016 | Japan | Tokyo Womens’ Medical University Hospital | 348 | 208/140 | AJCC staging criteria, 8th edition | NA |
| Chen et al.33 | 2021 | 2012–2020 | China | Affiliated Hospital of the Southwest Medical | 93 | 31/62 | AJCC staging criteria, 8th edition | Till July 2020 |
| Gupta et al.10 | 2021 | 2014–2018 | India | George’s Medical University | 115 | 91/24 | AJCC staging criteria, 7th edition | Median 30, range 8–62 months |
| Kim et al.38 | 2021 | 2002–2018 | Korea | Korea University Guro Hospital | 78 | 52/26 | AJCC staging criteria, 8th edition | Median 31, range: 0.5–147 months |
| Li et al.41 | 2021 | 2002–2019 | China | Chinese Research Group of gallbladder Cancer | 691 | 421/270 | AJCC staging criteria, 8th edition | Till June 2019 |
| Yao et al.54 | 2021 | 2000–2016 | China | Xinhua Hospital | 104 | 69/35 | AJCC staging criteria, 8th edition | Till June 2020 |
| Ashida et al.29 | 2022 | 2002–2014 | Japan | Shizuoka Cancer Center | 88 | 35/53 | AJCC staging criteria, 8th edition | Median 43.8 months |
| Wang et al.51 | 2022 | 2012–2020 | China | Shanghai Jiao Tong University Affiliated Sixth People’s Hospital and Renji Hospital | 55 | 35/27 | AJCC staging criteria, 7th edition | Till November 2020 |
| You et al.55 | 2022 | 2013–2018 | China | Eastern Hepatobiliary Surgery Hospital/Affiliated Hospital of North Sichuan Medical College | 102 | 59/43 | AJCC staging criteria, 8th edition | Till December 2019 |
| Cao et al.31 | 2023 | 2011–2018 | China | Sir Run-Run Shaw Hospital | 114 | 82/32 | AJCC staging criteria, 7th edition | Till January 2020 |
| Fan et al.35 | 2023 | 2014–2022 | China | 81st Group Army PLA/the First Affiliated Hospital of Hebei North University | 80 | 60/20 | AJCC staging criteria, 8th edition | NA |
| Jiang et al.37 | 2023 | 2004–2015 | USA | SEER | 5451 | 3825/1626 | AJCC staging criteria, 7th edition | NA |
| Li et al.42 | 2023 | 2011–2020 | China | The First Affiliated Hospital of Xi’an Jiaotong University | 540 | 367/173 | AJCC staging criteria, 8th edition | Till January 2022 |
| Lv et al. | 2023 | 2010–2020 | China | West China hospital | 259 | 178/81 | AJCC staging criteria, 8th edition | Till September 2022 |
| Lv et al. | 2023 | 2010–2021 | China | West China hospital | 326 | 221/105 | AJCC staging criteria, 8th edition | Till September 2022 |
| Park et al.45 | 2023 | 2010–2020 | Korea | Three tertiary referral hospitals | 197 | 100/97 | AJCC staging criteria, 8th edition | NA |
AJCC, American Joint Committee on Cancer; NA, not available; No, the number of; SEER, Surveillance, Epidemiology, and End Results; UICC, International Union Against Cancer; USA, The United States of America.
The prognostic value of age
A total of 31 studies9,10,16,17,23,28–33,35–38,40–44,46,48–56 with 10 818 cases were incorporated into this comparison. Pooled results indicated that old age was a risk factor for OS among resected cases with GBC (HR=1.02, 95% CI: 1.01–1.04, P=0.0002) (Figure S1A, Supplemental Digital Content 4, http://links.lww.com/JS9/C302) (Table 2). Consistently, age has also been demonstrated as an independent prognostic factor (HR=1.29, 95% CI: 1.17–1.43, P<0.00001) (Figure S2A, Supplemental Digital Content 5, http://links.lww.com/JS9/C303) (Table 2).
Table 2.
Pooled results of prognostic factors and independent prognostic factors for OS among resected patients with GBC.
| Heterogeneity | Begg’s test | Egger’s test | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcomes | No. studies | No. patients | Model (Fixed/random) | HR | 95% CI | P (overall test) | PC (overall test) | I2(%) | P | Pr>|z|a | Pr >|z|b | P>|t|a |
| Pooled results of univariate analyses | ||||||||||||
| Age (youngR vs old) | 31 | 10818 | Fixed | 1.02 | 1.01–1.04 | 0.00020 | 0.00020 | 49 | 0.00100 | 0.634 | 0.646 | <0.001 |
| Gallstone disease (noR vs yes) | 14 | 2201 | Fixed | 1.22 | 1.08–1.39 | 0.00200 | 0.00200 | 0 | 0.93000 | — | ||
| Preoperative jaundice (noR vs yes) | 11 | 3134 | Fixed | 1.93 | 1.72–2.18 | <0.00001 | <0.00001 | 30 | 0.16000 | |||
| Preoperative CA199 (lowR vs high) | 15 | 2813 | Fixed | 2.31 | 2.07–2.57 | <0.00001 | <0.00001 | 45 | 0.03000 | |||
| T stage (T1-2R vs T3-4) | 15 | 3180 | Random | 3.09 | 2.46–3.88 | <0.00001 | <0.00001 | 65 | 0.00030 | |||
| Node stage (N-R vs N+) | 26 | 4603 | Random | 2.78 | 2.35–3.28 | <0.00001 | <0.00001 | 60 | <0.0001 | |||
| Peri-neural invasion (-R vs +) | 15 | 3062 | Fixed | 2.20 | 1.95–2.49 | <0.00001 | <0.00001 | 3 | 0.42000 | |||
| Lymph-vascular invasion (-R vs +) | 13 | 2814 | Fixed | 2.37 | 2.02–2.77 | <0.00001 | <0.00001 | 38 | 0.08000 | |||
| Vascular invasion (-R vs +) | 8 | 1615 | Fixed | 2.28 | 1.87–2.79 | <0.00001 | <0.00001 | 25 | 0.23000 | |||
| Differentiation status (well to moderateR vs poor) | 27 | 10164 | Random | 3.22 | 1.65–2.87 | <0.00001 | <0.00001 | 76 | <0.00001 | |||
| Pathology subtype (OthersR vs AC) | 7 | 7683 | Random | 1.09 | 0.84–1.41 | 0.53000 | 0.53000 | 78 | 0.00010 | |||
| Tumor location (peritoneal sideR vs hepatic side) | 3 | 617 | Fixed | 1.85 | 1.51–2.28 | <0.00001 | <0.00001 | 10 | 0.33000 | |||
| Tumor location (distalR vs proximal) | 6 | 1244 | Fixed | 1.78 | 1.47–2.14 | <0.00001 | <0.00001 | 3 | 0.40000 | |||
| Surgical margin (negativeR vs positive) | 20 | 3846 | Random | 2.90 | 2.42–3.47 | <0.00001 | <0.00001 | 60 | 0.00030 | |||
| Bile duct resection (not performedR vs performed) | 10 | 1756 | Fixed | 1.28 | 1.10–1.48 | 0.00100 | <0.00001 | 50 | 0.04000 | |||
| Adjuvant chemotherapy (not performedR vs performed) | 14 | 7929 | Random | 0.96 | 0.80–1.16 | 0.70000 | 0.70000 | 57 | 0.00400 | |||
| Pooled results of multivariate analyses | ||||||||||||
| Age (youngR vs old) | 5 | 6138 | Fixed | 1.29 | 1.17–1.43 | <0.00001 | <0.00001 | 48 | 0.10000 | — | ||
| Gallstone disease (noR vs yes) | 1 | 540 | NA | 1.49 | 1.16–1.92 | 0.00200 | NA | NA | NA | |||
| Preoperative jaundice (noR vs yes) | 2 | 470 | Fixed | 1.05 | 0.75–1.47 | 0.79000 | 0.79000 | 0 | 0.33000 | |||
| Preoperative CA199 (lowR vs high) | 10 | 1896 | Fixed | 1.50 | 1.27–1.77 | <0.00001 | <0.00001 | 0 | 0.62000 | |||
| T stage (T1-2R vs T3-4) | 6 | 1431 | Random | 3.74 | 2.01–6.97 | <0.00010 | <0.00001 | 72 | 0.00300 | |||
| Node stage (N-R vs N+) | 14 | 2961 | Random | 2.20 | 1.74–2.78 | <0.00001 | <0.00001 | 56 | 0.00500 | |||
| Peri-neural invasion (-R vs +) | 7 | 1015 | Fixed | 1.34 | 1.04–1.73 | 0.02000 | 0.02000 | 3 | 0.40000 | |||
| Lymph-vascular invasion (-R vs +) | 5 | 791 | Fixed | 1.41 | 1.02–1.95 | 0.04000 | 0.04000 | 0 | 0.51000 | |||
| Vascular invasion (-R vs +) | 2 | 436 | Fixed | 1.29 | 0.80–2.10 | 0.30000 | 0.30000 | 0 | 0.86000 | |||
| Differentiation status (well to moderateR vs poor) | 16 | 8461 | Random | 2.02 | 1.59–2.56 | <0.00001 | <0.00001 | 72 | <0.00001 | |||
| Pathology subtype (OthersR vs AC) | 4 | 6255 | Fixed | 1.09 | 0.97–1.23 | 0.15000 | 0.15000 | 0 | 0.90000 | |||
| Tumor location (peritoneal sideR vs hepatic side) | 2 | 492 | Fixed | 1.52 | 1.19–1.95 | 0.00090 | 0.00090 | 0 | 0.78000 | |||
| Tumor location (distalR vs proximal) | 3 | 427 | Random | 1.78 | 0.84–3.78 | 0.13000 | 0.13000 | 67 | 0.05000 | |||
| Surgical margin (negativeR vs positive) | 11 | 2067 | Fixed | 2.28 | 1.91–2.73 | <0.00001 | <0.00001 | 0 | 0.60000 | |||
| Bile duct resection (not performedR vs performed) | 4 | 795 | Fixed | 1.73 | 1.55–1.93 | <0.00001 | <0.00001 | 0 | 0.64000 | |||
| Adjuvant chemotherapy (not performedR vs performed) | 4 | 6190 | Random | 0.68 | 0.48–0.96 | 0.03000 | 0.01000 | 77 | 0.00500 | |||
P-value.
P-value (continuity corrected).
GBC, gallbladder carcinoma; HR, hazard ratio; NA, not applicable; No, the number of; OR, odds ratio; OS, overall survival; PC, corrected P-value after the sensitivity analysis; R, reference.
The prognostic value of concurrent gallstone disease
A total of 14 studies3,9,10,16,28,31,35,42,43,46,51–53,56 with 2201 cases were incorporated into this comparison. Pooled results indicated that concurrent gallstone disease was a risk factor for OS among resected cases with GBC (HR=1.22, 95% CI: 1.08–1.39, P=0.002) (Figure S1B, Supplemental Digital Content 4, http://links.lww.com/JS9/C302) (Table 2). However, only one study incorporated gallstone disease into multivariate analysis and gallstone disease was also an independent prognostic factor (HR=1.49, 95% CI: 1.16–1.92, P=0.00200) (Figure S2B, Supplemental Digital Content 5, http://links.lww.com/JS9/C303) (Table 2).
The prognostic value of concurrent obstructive jaundice
A total of 11 studies23,28,29,32,35,41,42,49,51–53 with 3134 cases were incorporated into this comparison. Pooled results indicated that the concurrent of preoperative jaundice was a risk factor for OS among resected cases with GBC (HR=1.93, 95% CI: 1.72–2.18, P<0.00001) (Figure S1C, Supplemental Digital Content 4, http://links.lww.com/JS9/C302) (Table 2). However, in multivariate analysis, preoperative obstructive jaundice was not an independent prognostic factor (HR=1.05, 95% CI: 0.75–1.47, P=0.79000) (Figure S2C, Supplemental Digital Content 5, http://links.lww.com/JS9/C303) (Table 2).
The prognostic value of preoperative CA199 level
A total of 15 studies9,10,17,23,29–31,33,41,42,50,53–56 with 2813 cases were incorporated. Pooled results indicated that preoperative CA199 was an indicator of worse prognosis (HR=2.31, 95% CI: 2.07–2.57, P<0.00001) (Figure S1D, Supplemental Digital Content 4, http://links.lww.com/JS9/C302) (Table 2). The results of multivariate analyses indicated that preoperative CA199 level was also an independent prognostic factor (HR=1.50, 95% CI: 1.27–1.77, P<0.00001) (Figure S2D, Supplemental Digital Content 5, http://links.lww.com/JS9/C303) (Table 2).
The prognostic value of T category
A total of 15 studies9,16,23,28–30,34,36,38,41–44,49,54 with 3180 cases were incorporated. Pooled results indicated that T stage was a validating risk factor for OS (HR=3.09, 95% CI: 2.46–3.88, P<0.00001) (Figure S1E, Supplemental Digital Content 4, http://links.lww.com/JS9/C302). High heterogeneity was detected (I2=65%) and the results of heterogeneity analyses indicated that when the study by Lv TR et al.23 was excluded, a significantly lower heterogeneity value was furtherly acquired (HR=2.71, 95% CI: 2.38–3.07, P<0.00001, I2=3%) (Table 2). The results of multivariate analyses indicated that T stage was also an independent prognostic factor (HR=3.74, 95% CI: 2.01–6.97, P<0.00010) (Figure S2E, Supplemental Digital Content 5, http://links.lww.com/JS9/C303) (Table 2).
The prognostic value of lymph node metastasis
A total of 26 studies9,10,16,17,23,28–30,32,33,36,38,39,41–45,47–51,54–56 with 4603 cases were incorporated. Pooled results indicated that lymph node metastasis was a validating risk factor for OS (HR=2.78, 95% CI: 2.35–3.28, P<0.00001) (Figure S1F, Supplemental Digital Content 4, http://links.lww.com/JS9/C302) (Table 2). The high heterogeneity was detected and the results of heterogeneity analyses failed to reveal any major source of heterogeneity. The results of multivariate analyses indicated that lymph node metastasis was also an independent prognostic factor (HR=2.20, 95% CI: 1.74–2.78, P<0.00001) (Figure S2F, Supplemental Digital Content 5, http://links.lww.com/JS9/C303) (Table 2).
The prognostic value of peri-neural invasion
A total of 15 studies16,17,23,28–30,38,41–43,45,46,49,55,57 with 3062 cases were incorporated. Pooled results indicated that peri-neural invasion was a validating risk factor for OS (HR=2.20, 95% CI: 1.95–2.49, P<0.00001) (Figure S1G, Supplemental Digital Content 4, http://links.lww.com/JS9/C302) (Table 2). The results of multivariate analyses indicated that peri-neural invasion was also an independent prognostic factor (HR=1.34, 95% CI: 1.04–1.73, P=0.02000) (Figure S2G, Supplemental Digital Content 5, http://links.lww.com/JS9/C303) (Table 2).
The prognostic value of lymph-vascular invasion
A total of 13 studies9,16,23,28,29,38,41,42,45,46,49,55,57 with 2814 cases were incorporated. Pooled results indicated that lymph-vascular invasion was a risk factor for OS among resected cases with GBC (HR=2.37, 95% CI: 2.07–2.77, P<0.00001) (Figure S1H, Supplemental Digital Content 4, http://links.lww.com/JS9/C302) (Table 2). The results of multivariate analyses indicated that lymph-vascular invasion was also an independent prognostic factor (HR=1.41, 95% CI: 1.02–1.95, P=0.04000) (Figure S2H, Supplemental Digital Content 5, http://links.lww.com/JS9/C303) (Table 2).
The prognostic value of vascular invasion
A total of 8 studies28,29,32,38,42,43,45,48 with 1615 cases were incorporated. Pooled results indicated that vascular invasion was a risk factor for OS among resected cases with GBC (HR=2.28, 95% CI: 1.87–2.79, P<0.00001) (Figure S1I, Supplemental Digital Content 4, http://links.lww.com/JS9/C302) (Table 2). However, in multivariate analysis, vascular invasion was not an independent prognostic factor (HR=1.29, 95% CI: 0.80–2.10, P=0.30000) (Figure S2I, Supplemental Digital Content 5, http://links.lww.com/JS9/C303) (Table 2).
The prognostic value of tumor differentiation status
A total of 27 studies9,10,17,23,28–33,35,37,38,40–44,46,49,50,52–57 with 10 164 cases were incorporated. Pooled results indicated that poorly differentiated tumor was a risk factor for OS among resected cases with GBC (HR=3.22, 95% CI: 1.65–2.87, P<0.00001) (Figure S1J, Supplemental Digital Content 4, http://links.lww.com/JS9/C302) (Table 2). The high heterogeneity (I2=76%) was detected and the results of heterogeneity analyses failed to reveal any major source of heterogeneity. The results of multivariate analyses indicated that tumor differentiation status was also an independent prognostic factor (HR=2.02, 95% CI: 1.59–2.56, P<0.00001) (Figure S2J, Supplemental Digital Content 5, http://links.lww.com/JS9/C303) (Table 2).
The prognostic value of tumor pathological subtypes
A total of 7 studies23,28,37,41–43,52 with 7683 cases were incorporated. Pooled results indicated that pathological subtypes were not a risk factor for OS among resected cases with GBC (HR=1.09, 95% CI: 0.84–1.41, P=0.53) (Figure S1K, Supplemental Digital Content 4, http://links.lww.com/JS9/C302) (Table 2). The high heterogeneity (I2=78%) was detected and the results of heterogeneity analyses failed to reveal any major source of heterogeneity. In multivariate analysis, pathological subtypes were not an independent prognostic factor (HR=1.09, 95% CI: 0.97–1.23, P=0.15000) (Figure S2K, Supplemental Digital Content 5, http://links.lww.com/JS9/C303) (Table 2).
The prognostic value of tumor location (peritoneal side vs hepatic side)
A total of 3 studies50,52,55 with 617 cases were incorporated. Pooled results indicated that hepatic tumor shared more worse prognosis than peritoneal tumor and tumor location (peritoneal side vs hepatic side) was a prognostic factor for resected GBC (HR=1.85, 95% CI: 1.51–2.28, P<0.00001) (Figure S1L, Supplemental Digital Content 4, http://links.lww.com/JS9/C302) (Table 2). The results of multivariate analyses indicated that hepatic tumor was also an independent prognostic factor (HR=1.52, 95% CI: 1.19–1.95, P=00090) (Figure S2L, Supplemental Digital Content 5, http://links.lww.com/JS9/C303) (Table 2).
The prognostic value of tumor location (distal vs proximal)
A total of 6 studies17,23,29,35,40,42 with 1244 resected cases were incorporated. Pooled results indicated that proximal tumors (neck/cystic duct) shared more worse prognosis than distal tumors (body/fundus) and tumor location (distal vs proximal) was a prognostic factor for resected GBC (HR=1.78, 95% CI: 1.47–2.14, P<0.00001) (Figure S1M, Supplemental Digital Content 4, http://links.lww.com/JS9/C302) (Table 2). However, in multivariate analysis, proximal tumor was not an independent prognostic factor (HR=1.78, 95% CI: 0.84–3.78, P=0.13000) (Figure S2M, Supplemental Digital Content 5, http://links.lww.com/JS9/C303) (Table 2).
The prognostic value of surgical margin
A total of 20 studies9,17,23,28,32,35,36,38,41,44,46–49,51–55,57 with 3864 cases were incorporated. Pooled results indicated that positive margin was a risk factor for OS among resected GBC (HR=2.90, 95% CI: 2.42–3.47, P<0.00001) (Figure S1N, Supplemental Digital Content 4, http://links.lww.com/JS9/C302) (Table 2). High heterogeneity (I2=60%) was detected and the results of heterogeneity analyses failed to reveal any major source of heterogeneity. The results of multivariate analyses indicated that surgical margin was also an independent prognostic factor (HR=2.28, 95% CI: 1.91–2.73, P<0.00001) (Figure S2N, Supplemental Digital Content 5, http://links.lww.com/JS9/C303) (Table 2).
The prognostic value of combined extra-hepatic bile duct resection
A total of 10 studies3,9,16,17,29,36,39,49,52,54 with 1756 cases were incorporated. Pooled results indicated that the combined bile duct resection was a risk factor for OS among resected GBC (HR=1.28, 95% CI: 1.10–1.48, P=0.001) (Figure S1O, Supplemental Digital Content 4, http://links.lww.com/JS9/C302) (Table 2). Moderate heterogeneity (I2=50%) was detected and the results of heterogeneity analyses indicated that when the study by Wang et al.3 was excluded, a more significant P-value with lower heterogeneity was acquired (HR=1.45, 95% CI: 1.23–1.71, P<0.00001, I2=0%). The results of multivariate analyses indicated that combined bile duct resection was also an independent prognostic factor (HR=1.73, 95% CI: 1.55–1.93, P<0.00001) (Figure S2O, Supplemental Digital Content 5, http://links.lww.com/JS9/C303) (Table 2).
The prognostic value of postoperative adjuvant chemotherapy
A total of 14 studies9,16,17,23,28,32,37,42,44,45,49–51,57 with 7929 cases were incorporated. Pooled results indicated that postoperative adjuvant chemotherapy was a protective factor but was not a prognostic factor for OS among resected GBC (HR=0.96, 95% CI: 0.80–1.16, P=0.70000) (Figure S1P, Supplemental Digital Content 4, http://links.lww.com/JS9/C302) (Table 2). High heterogeneity (I2=57%) was detected and the results of heterogeneity analyses failed to reveal any major source of heterogeneity. However, in multivariate analysis, postoperative adjuvant chemotherapy was proved to be a protective factor as well as an independent prognostic factor (HR=0.68, 95% CI: 0.48–0.96, P=0.03000) (Figure S2P, Supplemental Digital Content 5, http://links.lww.com/JS9/C303) (Table 2).
Publication bias, heterogeneity analysis, and sensitivity analysis
The potential publication bias in the outcome with the highest number of studies included was assessed through several analyses. Initially, Begg’s test and Egger’s test were conducted to evaluate the presence of publication bias in the age (young vs old) comparison. As summarized in Table 2, publication bias was detected in Egger’s test. To further assess the potential publication bias, Begg’s filled funnel plot and Egger’s linear regression (meta-trim command) were utilized (Figure S3B, Supplemental Digital Content 6, http://links.lww.com/JS9/C304). The results were consistent before and after trimming, with both P-values being lower than 0.05. This suggests that the identified publication bias could be disregarded. Heterogeneity analyses and sensitivity analyses were performed via removing every single study manually and their results were documented in each relevant outcome. Additionally, the sensitivity analysis for the outcome with the highest number of studies included was furtherly validated via using the db metaninf command (Figure S3A, Supplemental Digital Content 6, http://links.lww.com/JS9/C304). The stepwise exclusion of included studies did not result in any significant differences.
Discussion
This meta-analysis represents the first systematic evaluation of potential prognostic factors for OS among cases with resected GBC. Our findings not only reinforce the significance of prognostic factors outlined in the latest 8th edition of the AJCC staging system, such as the T category, lymph node metastasis, lymph-vascular invasion, differentiation status, and surgical margin, but also uncover other potentially useful predictors. These additional predictors include peri-neural invasion, preoperative CA199 level, preoperative jaundice, concurrent gallstone disease, tumor locations, and age. Notably, postoperative adjuvant chemotherapy was shown to be a protective factor for OS (HR=0.96000) but can provide limited survival benefit (P=0.70000). However, the pooled results of multivariate analyses indicated that postoperative adjuvant chemotherapy was an independent protective factor (HR=0.68, 95% CI: 0.48–0.96, P=0.03000). Additionally, the inconsistency in pathological subtype was also not found to be a prognostic factor for OS among resected GBC cases. The findings of this study could contribute to a more in-depth understanding of the prognostic factors as well as independent prognostic factors for OS among surgically-treated cases with GBC and provide insights into potentially valuable predictors beyond those included in the current AJCC staging system.
The prognostic value of age (young vs old)
Theoretically, older patients often have more severe preoperative comorbidities, especially respiratory and cardiovascular conditions, and their immune system may be weakened. As a result, their postoperative recovery is typically slower compared to younger patients. Additionally, surgery itself is considered a form of trauma to the body, and older patients generally share lower tolerance for surgical procedures compared to younger individuals. Consequently, it is expected that older patients would have a less favorable prognosis, and our findings supported this hypothesis by showing a slight yet significant difference in survival between younger and older patients. Our analysis revealed a HR of 1.02 in the univariate analyses, indicating that older patients had a 1.02 times higher risk of death compared to their younger counterparts. Moreover, in multivariate analyses, advanced age was furtherly demonstrated to be an independent prognostic factor. However, due to the relatively small impact on survival compared to other tumor-related factors, limited attention has been given to this issue, and therefore, we will not delve further into this aspect.
The prognostic value of concurrent gallstone disease
Accumulating evidence has indicated that the presence of concurrent gallstone disease is a risk factor for GBC, particularly in cases where there has been a prolonged tolerance of chronic inflammation, hyperplasia, and atypical differentiation resulting from gallstone-related factors3,42,43,46,51,56. The latest 8th AJCC staging system has also indicated that cholelithiasis is associated with carcinoma of the gallbladder in most cases. Recently, according to the analysis based on Chilean and European Genotype Data, gallstone disease was the major risk factor in the prevention of GBC58. Additionally, Ryu et al. analyzed 396 720 cases from south Korea between 2002 and 2012 and demonstrated that gallstone disease was associated with an elevated risk of mortality in hepatobiliary cancer, particularly liver and intrahepatic cancer, as well as mortality specifically related to gallbladder cancer. These associations remained regardless of other potential confounding factors59. Further epigenome-wide analysis focusing on methylation status revealed that gallstone disease was often accompanied by low-grade dysplasia60. Consequently, the evidence above collectively validated the fact the gallstone disease is a key factor in the development and prognosis of GBC.
The prognostic value of concurrent preoperative jaundice
Our previous meta-analysis has systematically evaluated the prognostic value of preoperative jaundice in radically-resected cases with GBC, that is, jaundice was associated with a lower resectability rate, a lower R0 rate, more postoperative complications and mortalities61. In consistent with our previous findings, current meta-analysis demonstrated that preoperative jaundice was a risk factor for OS among resected GBC cases (HR=1.93, P<0.00001). However, when results in multivariate were pooled together, preoperative jaundice failed to be an independent prognostic factor (HR=1.05, P=0.79000). We think this might be accounted for the limited number of studies (n=2) and patients included (n=470). Preoperative jaundice was previously regarded as a contraindication of curative surgery for advanced lesions62. Hyper bilirubin level in the blood could lead to liver dysfunction, which often greatly increase the risk of surgery and anesthesia, especially for surgical candidates with GBC who are considering receiving the combined partial or extended hepatectomy. Undoubtedly, preoperative percutaneous or naso-biliary drainage is extremely necessary for jaundiced cases.
The prognostic value of preoperative CA199
CA199 is a common and classical serum tumor biomarker of biliary malignancies and its obvious elevation can often be regarded as an indicator of early diagnosis and worse prognosis in various biliary malignancies63–65. Wen et al. reported an extremely satisfactory area under curve (AUC) value of 0.77 regarding the predictive value of preoperative CA199 level in 5-year survival rate in resected GBC patients52. In line with previous publications3,9,10,14,17,63–66, our pooled results furtherly validated the prognostic value of CA199 that patients with an elevated preoperative CA199 level shared a 2.31 times risk of death than those with lower level of CA199. Moreover, the pooled result of multivariate analyses also demonstrated it to be an independent prognostic factor. However, the prognostic value of preoperative CA199 was not fully-reflected in the 8th AJCC staging system, not even to be mentioned. Currently, the optimal cut-off value of survival-related CA199 level remains debating and a cut-off value of around 37 mmol/l has been widely applied in various studies3,9,10,17,66. Others have also put forward inconsistent cut-off values and they were also demonstrated to be effective to stratify patients with different survival outcomes14,67. The prognostic value of preoperative CA199 is expected to be officially-recognized in the upcoming AJCC staging system and more powerful well-designed controlled studies are required for further validation.
The prognostic value of peri-neural invasion
Anatomically, just as our previous series has indicated, the whole biliary tract was regulated by the sympathetic as well as parasympathetic nerves, which were primarily distributed around the portal hepatis, along with hepatic artery and extra-hepatic bile duct15. Due to the extensive attachment neural network to biliary system, the pathological or physiological functions of biliary epithelium are inevitably influenced. The interaction between the neural network and tumors originating from organs set up an anatomic foundation of peri-neural invasion-mediated metastasis. Previous multicenter studies have evaluated the significance of peri-neural invasion in patients with resected intrahepatic cholangiocarcinoma68. Peri-neural invasion was demonstrated to be an independent prognostic factor on OS or DFS in resected patients with intrahepatic cholangiocarcinoma68. Similarly, based on the evidence regarding GBC patients, peri-neural invasion also indicated a worse prognosis13,16–18,23,28–30,38,41–43,45,46,49,55,57. Subsequently, when their results were pooled together, we were surprisingly to find that patients with peri-neural invasion shared a two times risk of death than those without peri-neural invasion (HR=2.20, P<0.00010) and it also served as an independent prognostic factor (HR=1.34, P=0.02000)
The prognostic value of lymph-vascular invasion
Lymph-vascular invasion is defined as the micro invasion of tumor cells in endothelial-lined spaces, such as lymphatic or blood vessels69. The prognostic significance of lymph-vascular invasion has been demonstrated in oral cancer70, melanoma and breast cancer71, and bladder cancer72. According to the 8th AJCC staging system, lymph-vascular invasion was regarded as an additional factor requiring clinical care (1366 cases, AJCC level of evidence II) in cases with GBC8. Our analysis, however, with a much larger sample size (n=2814), furtherly validated its prognostic value in resected GBC patients (HR=2.37, P<0.00001). Lymph-vascular invasion also served as an independent prognostic factor (HR=1.41, P=0.04000). Our findings would provide a much higher level of evidence regarding the significance of lymph-vascular invasion in GBC patients.
The prognostic value of tumor differentiation status
Accumulating evidence has consistently indicated that a poor or moderate to poor differentiation status indicated a worse OS or DFS than those with well differentiation disease9,10,13,14,17,18,23,28–33,35,37,38,40–44,46,49,50,52–57,66,73. When their results were pooled together, a significant risk factor was furtherly validated (HR=3.22, P<0.00001). Tumor differentiation status (well to moderate vs poor) has also been suggested as an independent prognostic factor on OS or DFS in the 8th AJCC staging system (level of evidence II)7. Our study with 10 164 resected patients included furtherly validated its prognostic significance.
The prognostic value of pathological subtypes
Current meta-analysis revealed that tumor pathological subtypes were not a prognostic factor for resected GBC cases. The inconsistency of pathological subtypes in the comparison with adenocarcinoma might account for this phenomenon. Accumulating evidence have suggested that gallbladder adeno-squamous/squamous carcinoma shared a worse prognosis than those with adenocarcinoma74–79. Gallbladder mucinous adenocarcinoma80 and sarcomatoid carcinoma81 have also been demonstrated to share a worse prognosis than adenocarcinoma. However, there are also pathological subtypes that show a comparable or even more favorable prognosis compared to gallbladder adenocarcinoma. A study conducted by Do et al.82 revealed that gallbladder neuroendocrine carcinoma had a comparable prognosis to gallbladder adenocarcinoma after matching. Our previous research focusing on extra-hepatic cholangiocarcinoma also acquired the similar phenomenon83. Consequently, the inconsistency regarding pathological subtypes might serve as a confounding factor, and the prognostic value of pathological subtypes needs to be furtherly investigated more specifically, especially when focused on different subtypes.
The prognostic value of tumor locations
Compared with the 7th AJCC staging system for GBC, the latest 8th AJCC staging system furtherly classified T2 diseases into T2a (peritoneal side) and T2b (hepatic side) disease. Our research incorporated 3 studies with 617 cases included furtherly validated the reasonability of this novel classification criteria in terms of univariate analysis (HR=1.85, P<0.00001) and multivariate analysis (HR=1.52, P=0.00090). However, the 8th AJCC staging system does not mention the prognostic value brought by another type of classification criteria of tumor locations, which includes tumor located in the body, fundus, neck, and cystic duct of the gallbladder. Anatomically, tumors located in the neck or the cystic duct of the gallbladder are closer to the porta hepatis, adjacent to numerous major blood vessels and organs. Consequently, proximal tumors (neck/cystic duct tumor) have a high tendency to develop multiorgan infiltrations and metastasis versus distal tumors (body/fundus tumor). Therefore, proximal tumors of the gallbladder often require a more extended range of surgical resection, share a more advanced stage, and a worse prognosis. The prognostic value of tumor locations (distal vs proximal) has been validated in our previous research (HR=2.5, P=0.003)19, which has also been validated in the current meta-analysis (HR=1.85, P<0.00001). However, in multivariate analysis, proximal tumor was shown to be a risk factor as well but not an independent prognostic factor ((HR=1.78, P=0.13000). Further analysis on the potential reasons, we found that only two studies were incorporated into the analysis of multivariate analysis. Consequently, the prognostic value of tumor location (distal vs proximal) still requires to be furtherly validated in large multicenter studies.
The prognostic value of surgical margin and the combined EHBDR
A radical resection with a clear margin has always been linked with improved survival84,85. A lymphadenectomy along with the hepatoduodenal ligament combined with partial hepatectomy has been strongly recommended as the standard surgical procedures for patients with progressive GBC disease. A clear margin without residual disease microscopically or macroscopically has been proved to be closely-associated with improved survival than those with positive margin3,9,12–14,17,36,44,46,48,49,52,73, which was also validated in our synthetic results with an extremely significant P-value combined with a high level of risk of death in terms of univariate analysis (HR=2.90, P<0.00001) and multivariate analysis (HR=2.28, P<0.00001).
The prognostic significance of concurrent extra-hepatic bile duct resection in cases with GBC has always been discussed and remains debating. According to findings reported in previous in previous publications, EHBDR was not correlated with an improved survival and was even harmful in cases with early-staged disease15,20,86–88. However, some Asian surgeons, especially Japanese researches, have always insisted on performing EHBDR aggressively due to its potential contribution in tumor clearance as well as a more thorough lymph yield36,89–91. In current analysis, bile duct resection was a predictor of worse prognosis (HR=1.28, P<0.00001) and also served as an independent prognostic factor (HR=1.73, P<0.00001). According to the National Comprehensive Cancer Network (NCCN) (Version: 1.2022 to 29 March 2022, www.nccn.org/patients) guidelines, EHBDR should be only performed when there is an obvious positive cystic duct margin or extra-hepatic bile duct infiltration. Clinically, patients who received RHBDR were generally in a more advanced stage and therefore shared a worse prognosis20. What truly determines the prognosis is the tumor staging rather than the combined extra-hepatic bile duct resection. Bile duct resection is primarily performed to achieve curative resection.
The prognostic value of postoperative adjuvant chemotherapy
According to a previous NCDB-based retrospective study, adjuvant therapy was associated with an improved 3-year survival rate in resected GBC patients but only less than one thirds GBC patients were eligible for receiving adjuvant therapies92. Currently, according to the latest NCCN guidelines, postoperative adjuvant chemotherapy or chemo-radiotherapy were only recommended in resected GBC patients with positive margins or those with positive lymph node status93. Horgan et al. performed a meta-analysis with 6712 patients with biliary malignancies included focusing on the effect of postoperative adjuvant chemotherapies and found a moderate but not significant survival benefit in patients with positive surgical margin or node status93. Ma et al.94 also indicated that postoperative adjuvant therapies could bring survival benefit in patients with positive margin, positive node status or patients with II/III disease. Wang et al.95 also indicated adjuvant chemo-radiotherapy was beneficial in T3 or N1 GBC patients. Mantripragada et al.96 performed a propensity score matching analysis to evaluate the effect of adjuvant therapies in resected GBC patients and reported a moderate survival benefit in T3 or N1 GBC patients but was not correlated with survival benefit in the entire cohort, which was in consistent with our results in univariate analysis (HR=0.96, P=0.70000). The results of multivariate analysis indicated that postoperative adjuvant chemotherapy was an independent risk factor as well as a protective factor (HR=0.68, P=0.01000), which was consistent with the findings reported by Jiang et al. based on a SEER cohort (n=6712). However, inconsistent with our research, in order to avoid the survival impact brought by various confounding factors, Jiang et al. performed further investigation via propensity score matching and revealed that postoperative adjuvant chemotherapy was a prognostic factor as well as a protective factor (P=0.002). The propensity score matching analyses and subgroup analyses focusing on R1 or N1 patients were not feasible in current meta-analysis due to the inadequate original data.
Currently, there is a growing trend of strongly recommending a multidisciplinary approach for cases with biliary tract cancers, particularly for those with advanced GBC. Novel and emerging adjuvant strategies, including immunotherapy, targeted therapy, and combination strategies, are being investigated for GBC and the results of these studies are eagerly awaited97–99. In order to enhance the effectiveness of antitumor treatment, the combination of immune checkpoint inhibitors with chemotherapy has been investigated. It was found that chemotherapeutic agents could upregulate the expression of checkpoint proteins100–102. A current phase III, double-blind clinical trial called TOPAZ-1 is evaluating the role of chemotherapy agents in combination with the immunotherapy agent durvalumab compared to chemotherapy agents with a placebo. Undoubtedly, considering the evidence presented above, postoperative adjuvant chemotherapy should be considered a crucial prognostic factor for OS among resected GBC. To further evaluate its effectiveness, it is imperative to conduct more robust and well-designed studies. Randomized controlled trials, in particularly, would be invaluable in this regard.
Other potential prognostic factors
The significance of preoperative fibrinogen in resected GBC patients have also been discussed and validated14,103–105. However, the majority publications were based on Asian populations and therefore its prognostic value was less powerful and applicable14,103–105. Other nonconventional prognostic factors based on a small cohort, such as the level of survivin expression46, platelet to lymphocyte ratio66, or preoperative systemic inflammation status9, have also been reported. Moreover, the prognostic value of nutrition-related indicators has also been demonstrated in patients with GBC, such as sarcopenia57. Additionally, in the era of minimally surgery, numerous studies have focused on the surgery approaches, including laparotomy, laparoscopic surgery, or robotic surgery. Laparoscopic radical cholecystectomy has been demonstrated to be effective in T1/2 GBC patients that equivalent oncological adequacy and long-term survival has been achieved via laparoscopic approach versus conventional laparotomy approach106. However, for patients with more advanced disease, laparoscopic surgery seems to be less applicable106.
Limitations
Current study has to be interpretated with several limitations. Firstly, a rough estimate of HR and its 95% CI via Tierney’s method would introduce bias to some extent. Secondly, the inconsistency on the predictors selected for model establishment would also introduce bias. Some studies might have selectively avoided reporting negative results, which can have a certain impact on our research outcomes. Thirdly, our study not only assessed all potential prognostic factors but also evaluated all potential independent prognostic factors at the same time. However, independent prognostic factors in current study were evaluated via incorporating HRs and 95% CIs in multivariate analyses, which might introduce certain statistical limitations. The appropriate approach should involve collecting clinical information from all patients included in all included studies, merging the clinical information of all cases, and then conducting univariate and multivariate Cox regression analysis. This issue is expected to be addressed in future large-sample multicenter studies, which will provide more conclusive results. Fourth, in order to maximize the sample size and gather more compelling evidence, we included some studies where there might have been some overlapping data after evaluation across studies. While this approach did increase the overall sample size to some extent and provide more significant evidence, it is important to acknowledge that the presence of data overlap could somewhat weaken the robustness of our study. Fifth, there are also many other potential prognostic factors to be explored, such as the evolvement in the surgical techniques, peri-operative management, and adjuvant therapies in GBC in the past few decades. However, the inadequate original data has hindered us from further exploration.
Conclusion
Current systematic review and meta-analysis firstly evaluated and validated all potential prognostic factors as well as independent prognostic factors for OS among resected GBC patients. Our findings not only validated previously-approved prognostic factors such as lymph node metastasis, but also revealed other nonconventional and rarely-reported prognostic factors, such as peri-neural invasion, tumor locations, and lymph-vascular invasion. Due to the multicollinearity and interdependence between prognostic factors, the inconsistency regarding the factors selected for modeling were unavoidable. Future multinational studies are required for a better model establishment and evaluation.
Ethical approval
Current study was performed based on previously-published studies and the ethical approval was not required.
Consent
Current study was performed based on previously-published studies and the informed consent was not required.
Funding
This study was supported by Sichuan Natural Science Foundation Youth Foundation Project (2024NSFSC1949); 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC21046); 1.3.5 project for disciplines of excellence clinical Research Incubation Project, West China Hospital, Sichuan University (2021HXFH001); National Natural Science Foundation of China for Young Scientists Fund (82203782); Sichuan University-Sui Ning School-local Cooperation project (2022CDSN-18); China Telecom Sichuan Company Biliary Tract Tumor Big Data Platform and Application Phase I R&D Project (312230752)
Role of the funding source
Funding source has no role in design and preparation of the manuscript.
Author contribution
T.-R.L. and J.-K.W.: contributed equally to the study; T.-R.L.: contributed to data acquisition and drafted the manuscript; J.-K.W.: contributed to the literature review, manuscript editing, and subsequent minor revision; H.-J.H.: was involved in editing the manuscript; F.-Y.L.: contributed to the study design and revision of the manuscript.
Conflicts of interest disclosure
The authors declare that they have no conflicts of interest.
Research registration unique identifying number (UIN)
Current study was performed based on previously-published studies and the registered number is not available and required.
Guarantor
Tian-Run Lv and Fu-Yu Li.
Data availability statement
All data generated or analyzed during this study is included in the published article.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Supplementary Material
Footnotes
Tian-Run Lv and Jun-Ke Wang authors contribute equally to this work.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.lww.com/international-journal-of-surgery.
Published online 27 March 2024
Contributor Information
Tian-Run Lv, Email: 849211303@qq.com.
Jun-Ke Wang, Email: 1353781922@qq.com.
Fu-Yu Li, Email: lfy_74@hotmail.com.
Hai-Jie Hu, Email: hhj1063557621@163.com.
References
- 1. Rakić M, Patrlj L, Kopljar M, et al. Gallbladder cancer. HBSN 2014;3:221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ishihara S, Miyakawa S, Takada T, et al. Status of surgical treatment of biliary tract cancer. Dig Surg 2007;24:131–136. [DOI] [PubMed] [Google Scholar]
- 3. Wang JK, Ma WJ, Wu ZR, et al. Is combined extra-hepatic bile-duct resection justified for advanced gallbladder carcinoma? Gastroenterol Rep 2019;7:426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Krell RW, Wei AC. Gallbladder cancer: surgical management. Clin Oncol 2019;8:36. [DOI] [PubMed] [Google Scholar]
- 5. Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA: a cancer journal for clinicians 2017;67:93–99. [DOI] [PubMed] [Google Scholar]
- 6. Kanthan R, Senger JL, Ahmed S, et al. Gallbladder cancer in the 21st century. J Oncol 2015;2015:967472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ito H, Ito K, D’Angelica M, et al. Accurate staging for gallbladder cancer: implications for surgical therapy and pathological assessment. Ann Surg 2011;254:320–325. [DOI] [PubMed] [Google Scholar]
- 8. Roa I, Ibacache G, Muñoz S, et al. Gallbladder cancer in Chile: pathologic characteristics of survival and prognostic factors: analysis of 1,366 cases. Am J Clin Pathol 2014;141:675–682. [DOI] [PubMed] [Google Scholar]
- 9. Abe T, Amano H, Hanada K, et al. Preoperative systemic inflammation and complications affect long-term gallbladder carcinoma outcomes following surgery with curative intent. Anticancer Res 2016;36:4887–4894. [DOI] [PubMed] [Google Scholar]
- 10. Gupta S, Prakash P, Kumar V, et al. Radical surgery for de novo gallbladder carcinoma-Single-center analysis of prognostic factors and survival outcomes from an endemic region. Clin Oncol 2022;125:631–641. [DOI] [PubMed] [Google Scholar]
- 11. Maruyama S, Kawaida H, Hosomura N, et al. Indications for extrahepatic bile duct resection due to perineural invasion in patients with gallbladder cancer. World J Surg Oncol 2019;17:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lv TR, Wang JM, Ma WJ, et al. The consistencies and inconsistencies between distal cholangiocarcinoma and pancreatic ductal adenocarcinoma: a systematic review and meta-analysis. Front oncol 2022;12:1042493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shirai Y, Sakata J, Wakai T, et al. Extended” radical cholecystectomy for gallbladder cancer: long-term outcomes, indications and limitations. WJG 2012;18:4736–4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu WY, Zhang HH, Yang XB, et al. Prognostic significance of combined preoperative fibrinogen and CA199 in gallbladder cancer patients. WJG 2018;24:1451–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lv TR, Hu HJ, Liu F, et al. The role of extra-hepatic bile duct resection in patients with gallbladder carcinoma with peri-neural invasion: a ten-year experience in China. EJSO: BASO 2023;49:1009–1015. [DOI] [PubMed] [Google Scholar]
- 16. Choi SB, Han HJ, Kim WB, et al. Surgical strategy for T2 and T3 gallbladder cancer: is extrahepatic bile duct resection always necessary? Langenbeck’s. Arch Surg 2013;398:1137–1144. [DOI] [PubMed] [Google Scholar]
- 17. Kurahara H, Maemura K, Mataki Y, et al. Indication of extrahepatic bile duct resection for gallbladder cancer. Langenbecks Arch Surg 2018;403:45–51. [DOI] [PubMed] [Google Scholar]
- 18. Lim JH, Chong JU, Kim SH, et al. Role of common bile duct resection in T2 and T3 gallbladder cancer patients. Ann Hepatobiliary Pancreat Surg, AHBPS 2018;22:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lv T-R, Wang J-K, Hu H-J, et al. The significance of tumor locations in patients with gallbladder carcinoma after curative-intent resection. JOGS 2023;27:1387–1399. [DOI] [PubMed] [Google Scholar]
- 20. Lv TR, Liu F, Hu HJ, et al. The role of extra-hepatic bile duct resection in the surgical management of gallbladder carcinoma. A first meta-analysis EJSO EJSO 2022;48:482–491. [DOI] [PubMed] [Google Scholar]
- 21. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg (London, England) 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 22. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ (Clinical research ed) 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lv TR, Liu F, Ma WJ, et al. The significance of countable and treatable metastatic liver disease in patients with gallbladder carcinoma after curative‐intent surgery: a 10‐year experience in China. Cancer Med 2023;12:18503–18515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ (Clinical research ed) 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 26. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in medicine 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 27. Egger M, Davey, Smith G, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed) 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ando T, Sakata J, Nomura T, et al. Anatomic location of residual disease after initial cholecystectomy independently determines outcomes after re-resection for incidental gallbladder cancer. Langenbeck’s Arch Surg 2021;406:1521–1532. [DOI] [PubMed] [Google Scholar]
- 29. Ashida R, Yamamoto Y, Aramaki T, et al. Preoperative skeletal muscle fat infiltration is a strong predictor of poorer survival in gallbladder cancer underwent surgery. Clin Nutr ESPEN 2022;52:60–67. [DOI] [PubMed] [Google Scholar]
- 30. Bao Y, Yang J, Duan Y, et al. The C-reactive protein to albumin ratio is an excellent prognostic predictor for gallbladder cancer. Biosci Trends 2021;14:428–435. [DOI] [PubMed] [Google Scholar]
- 31. Cao J, Yan J, Hu J, et al. Estimating the influencing factors for T1b/T2 gallbladder cancer on survival and surgical approaches selection. Cancer Med 2023;12:16744–16755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chaudhary RK, Higuchi R, Yazawa T, et al. Resectional surgery in gallbladder cancer with jaundice—how to improve the outcome?. Langenbeck’s Arch Surg 2021;406:791–800. [DOI] [PubMed] [Google Scholar]
- 33. Chen H, Huang Z, Sun B, et al. The predictive value of systemic immune inflammation index for postoperative survival of gallbladder carcinoma patients. J Surg Oncol 2021;124:59–66. [DOI] [PubMed] [Google Scholar]
- 34. Cziupka K, Partecke LI, Mirow L, et al. Outcomes and prognostic factors in gallbladder cancer: a single-centre experience. Langenbeck’s Arch Surg 2012;397:899–907. [DOI] [PubMed] [Google Scholar]
- 35. Fan Z, Liu B, Shang P. Development and validation of a nomogram prediction model based on albumin-to-alkaline phosphatase ratio for predicting the prognosis of gallbladder carcinoma. POR 2023;28(no pagination):1610818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gani F, Buettner S, Margonis GA, et al. Assessing the impact of common bile duct resection in the surgical management of gallbladder cancer. J Surg Oncol 2016;114:176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jiang Y, Jiang L, Li H, et al. Adjuvant chemoradiotherapy in resected gallbladder cancer: a SEER-based study. Heliyon 2023;9:e14574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim HN, Lee SY, Kim BH, et al. Prognostic value of tumor budding in gallbladder cancer: application of the International Tumor Budding Consensus Conference scoring system. Virchows Archiv: an IJPA 2021;478:1071–1078. [DOI] [PubMed] [Google Scholar]
- 39. Kohya N, Miyazaki K. Hepatectomy of segment 4a and 5 combined with extra‐hepatic bile duct resection for T2 and T3 gallbladder carcinoma. J Surg Oncol 2008;97:498–502. [DOI] [PubMed] [Google Scholar]
- 40. Lee EC, Park SJ, Lee SD, et al. Effects of sarcopenia on prognosis after resection of gallbladder cancer. JOGS: SSAT 2020;24:1082–1091. [DOI] [PubMed] [Google Scholar]
- 41. Li L, Ren T, Liu K, et al. Development and validation of a prognostic nomogram based on the systemic immune-inflammation index for resectable gallbladder cancer to predict survival and chemotherapy benefit. Front oncol 2021;11:692647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li Q, Liu H, Gao Q, et al. Textbook outcome in gallbladder carcinoma after curative-intent resection: a 10-year retrospective single-center study. Chin Med J 2023;136:1680–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu GJ, Li XH, Chen YX, et al. Radical lymph node dissection and assessment: Impact on gallbladder cancer prognosis. WJG 2013;19:5150–5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Murakami Y, Uemura K, Sudo T, et al. Prognostic factors of patients with advanced gallbladder carcinoma following aggressive surgical resection. JOGS 2011;15:1007–1016. [DOI] [PubMed] [Google Scholar]
- 45. Park Y, Lee JS, Lee B, et al. Prognostic effect of liver resection in extended cholecystectomy for T2 gallbladder cancer revisited: a retrospective cohort study with propensity score-matched analysis. Ann Surg 2023;278:985–993. [DOI] [PubMed] [Google Scholar]
- 46. Salman T, Argon A, Kebat T, et al. The prognostic significance of survivin expression in gallbladder carcinoma. Apmis 2016;124:633–638. [DOI] [PubMed] [Google Scholar]
- 47. Schauer RJ, Meyer G, Baretton G, et al. Prognostic factors and long-term results after surgery for gallbladder carcinoma: a retrospective study of 127 patients. Langenbeck’s. Arch Surg 2001;386:110–117. [DOI] [PubMed] [Google Scholar]
- 48. Shimizu H, Kimura F, Yoshidome H, et al. Aggressive surgical approach for stage IV gallbladder carcinoma based on Japanese Society of Biliary Surgery classification. JHBPS 2007;14:358–365. [DOI] [PubMed] [Google Scholar]
- 49. Vega EA, De Aretxabala X, Qiao W, et al. Comparison of oncological outcomes after open and laparoscopic re-resection of incidental gallbladder cancer. BJS 2020;107:289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang J, Liu J, Chang Q, et al. The association between preoperative serum interleukin‐6 levels and postoperative prognosis in patients with T2 gallbladder cancer. J Surg Oncol 2018;117:1672–1678. [DOI] [PubMed] [Google Scholar]
- 51. Wang W, Yang CX, Yu XZ, et al. Clinicopathological characteristics and prognostic factors of patients with primary gallbladder neuroendocrine carcinomas. J Dig Dis 2022;23:166–173. [DOI] [PubMed] [Google Scholar]
- 52. Wen Z, Si A, Yang J, et al. Elevation of CA19-9 and CEA is associated with a poor prognosis in patients with resectable gallbladder carcinoma. HPB 2017;19:951–956. [DOI] [PubMed] [Google Scholar]
- 53. Xu W, Wu X, Wang X, et al. Prognostic significance of the preoperative lymphocyte to monocyte ratio in patients with gallbladder carcinoma. Cancer Manag Res 2020;12:3271–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yao WY, Wu XS, Liu SL, et al. Preoperative lymphocyte to C-reactive protein ratio as a new prognostic indicator in patients with resectable gallbladder cancer. HBPD INT 2022;21:267–272. [DOI] [PubMed] [Google Scholar]
- 55. You C, Xie M, Ling M, et al. Residual cancer is a strong predictor of survival in T3 incidental gallbladder cancer. BMC surgery 2022;22:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zheng P, Wang X, Hong Z, et al. Preoperative fasting hyperglycemia is an independent prognostic factor for postoperative survival after gallbladder carcinoma radical surgery. Cancer Manag Res 2019;11:1425–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee EC, Park S-J, Lee SD, et al. Effects of sarcopenia on prognosis after resection of gallbladder cancer. JOGS 2019;24:1082–1091. [DOI] [PubMed] [Google Scholar]
- 58. Barahona Ponce C, Scherer D, Brinster R, et al. Gallstones, body mass index, C-reactive protein, and gallbladder cancer: Mendelian randomization analysis of chilean and European genotype data. Hepatology (Baltimore, Md) 2021;73:1783–1796. [DOI] [PubMed] [Google Scholar]
- 59. Ryu S, Chang Y, Yun KE, et al. Gallstones and the risk of gallbladder cancer mortality: a cohort study. Am J Gastroenterol 2016;111:1476–1487. [DOI] [PubMed] [Google Scholar]
- 60. Brägelmann J, Barahona Ponce C, Marcelain K, et al. Epigenome-wide analysis of methylation changes in the sequence of gallstone disease, dysplasia, and gallbladder cancer. Hepatology (Baltimore, Md) 2021;73:2293–2310. [DOI] [PubMed] [Google Scholar]
- 61. Lv TR, Hu HJ, Regmi P, et al. The effect of preoperative jaundice in the surgical management of gallbladder carcinoma: an updated meta-analysis. ANZ J Surg 2021;91:E455–e64. [DOI] [PubMed] [Google Scholar]
- 62. Lee H, Kwon W, Han Y, et al. Optimal extent of surgery for early gallbladder cancer with regard to long-term survival: a meta-analysis. J Hepatobiliary Pancreat Sci 2018;25:131–141. [DOI] [PubMed] [Google Scholar]
- 63. Strom BL, Maislin G, West SL, et al. Serum CEA and CA 19-9: potential future diagnostic or screening tests for gallbladder cancer? Int J Cancer Res 1990;45:821–824. [DOI] [PubMed] [Google Scholar]
- 64. Wang YF, Feng FL, Zhao XH, et al. Combined detection tumor markers for diagnosis and prognosis of gallbladder cancer. WJG 2014;20:4085–4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yu T, Yu H, Cai X. Preoperative prediction of survival in resectable gallbladder cancer by a combined utilization of CA 19-9 and carcinoembryonic antigen. Chin Med J 2014;127:2299–2303. [PubMed] [Google Scholar]
- 66. Pang Q, Zhang LQ, Wang RT, et al. Platelet to lymphocyte ratio as a novel prognostic tool for gallbladder carcinoma. WJG 2015;21:6675–6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Liu F, Hu HJ, Ma WJ, et al. Prognostic significance of neutrophil-lymphocyte ratio and carbohydrate antigen 19-9 in patients with gallbladder carcinoma. Medicine 2019;98:e14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wei T, Zhang XF, He J, et al. Prognostic impact of perineural invasion in intrahepatic cholangiocarcinoma: multicentre study. BJS 2022;109:610–616. [DOI] [PubMed] [Google Scholar]
- 69. Larson AR, Kemmer J, Formeister E, et al. Beyond depth of invasion: adverse pathologic tumor features in early oral tongue squamous cell carcinoma. The Laryngoscope 2020;130:1715–1720. [DOI] [PubMed] [Google Scholar]
- 70. Mascitti M, Togni L, Caponio V, et al. Lymphovascular invasion as a prognostic tool for oral squamous cell carcinoma: a comprehensive review. Int J Oral Maxillofac Surg 2022;51:1–9. [DOI] [PubMed] [Google Scholar]
- 71. Thompson N, Storr S, Zhang S, et al. Lymphovascular invasion: assessment and prognostic impact in melanoma and breast cancer. Histol Histopathol 2015;30:1001–1009. [DOI] [PubMed] [Google Scholar]
- 72. Algaba F. Lymphovascular invasion as a prognostic tool for advanced bladder cancer. Current opinion in urology 2006;16:367–371. [DOI] [PubMed] [Google Scholar]
- 73. Murimwa G, Hester C, Mansour JC, et al. Comparative outcomes of adenosquamous carcinoma of the gallbladder: an analysis of the National Cancer Database. JOGS: SSAT 2021;25:1815–1827. [DOI] [PubMed] [Google Scholar]
- 74. Ayabe RI, Wach MM, Ruff SM, et al. Gallbladder squamous cell carcinoma: an analysis of 1084 cases from the National Cancer Database. J Surg Oncol 2020;122:716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kim WS, Jang KT, Choi DW, et al. Clinicopathologic analysis of adenosquamous/squamous cell carcinoma of the gallbladder. J Surg Oncol 2011;103:239–242. [DOI] [PubMed] [Google Scholar]
- 76. Leigh N, Solomon D, Pletcher E, et al. Adeno-squamous and squamous cell carcinoma of the gallbladder: the importance of histology in surgical management. Am J Surg 2020;220:1242–1248. [DOI] [PubMed] [Google Scholar]
- 77. Roa JC, Tapia O, Cakir A, et al. Squamous cell and adenosquamous carcinomas of the gallbladder: clinicopathological analysis of 34 cases identified in 606 carcinomas. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 2011;24:1069–1078. [DOI] [PubMed] [Google Scholar]
- 78. Samuel S, Mukherjee S, Ammannagari N, et al. Clinicopathological characteristics and outcomes of rare histologic subtypes of gallbladder cancer over two decades: a population-based study. PLoS One 2018;13:e0198809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Song HW, Chen C, Shen HX, et al. Squamous/adenosquamous carcinoma of the gallbladder: Analysis of 34 cases and comparison of clinicopathologic features and surgical outcomes with adenocarcinoma. J Surg Oncol 2015;112:677–680. [DOI] [PubMed] [Google Scholar]
- 80. Zou RQ, Hu HJ, Lv TR, et al. Clinicopathological characteristics and outcome of primary sarcomatoid carcinoma of the gallbladder. Front oncol 2022;12:1009673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zou RQ, Hu HJ, Liu F, et al. Comparison of clinicopathological characteristics of mucinous adenocarcinoma and conventional adenocarcinoma of gallbladder. Asian J Surg 2023;46:283–290. [DOI] [PubMed] [Google Scholar]
- 82. Do MY, Jang SI, Kang HP, et al. Comparison of the clinical features and outcomes of gallbladder neuroendocrine carcinoma with those of adenocarcinoma: a propensity score-matched analysis. Cancers 2021;13:4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lv TR, Liu F, Jin YW, et al. Neuroendocrine component in extrahepatic cholangiocarcinoma is associated with better survival: data from the SEER study. Adv Ther 2023;40:4032–4041. [DOI] [PubMed] [Google Scholar]
- 84. Dixon E, Vollmer CM, Jr, Sahajpal A, et al. An aggressive surgical approach leads to improved survival in patients with gallbladder cancer: a 12-year study at a North American Center. Ann Surg 2005;241:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hari DM, Howard JH, Leung AM, et al. A 21-year analysis of stage I gallbladder carcinoma: is cholecystectomy alone adequate? HPB 2013;15:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Donohue JH. Present status of the diagnosis and treatment of gallbladder carcinoma. J Hepatobiliary Pancreat Surg 2001;8:530–534. [DOI] [PubMed] [Google Scholar]
- 87. Kondo S, Nimura Y, Hayakawa N, et al. Extensive surgery for carcinoma of the gallbladder. Br J Surg 2002;89:179–184. [DOI] [PubMed] [Google Scholar]
- 88. Suzuki S, Yokoi Y, Kurachi K, et al. Appraisal of surgical treatment for pT2 gallbladder carcinomas. World J Surg 2004;28:160–165. [DOI] [PubMed] [Google Scholar]
- 89. Kai M, Chijiiwa K, Ohuchida J, et al. A curative resection improves the postoperative survival rate even in patients with advanced gallbladder carcinoma. J Gastrointest Surg 2007;11:1025–1032. [DOI] [PubMed] [Google Scholar]
- 90. Sakamoto Y, Kosuge T, Shimada K, et al. Clinical significance of extrahepatic bile duct resection for advanced gallbladder cancer. J Surg Oncol 2006;94:298–306. [DOI] [PubMed] [Google Scholar]
- 91. Shimizu Y, Ohtsuka M, Ito H, et al. Should the extrahepatic bile duct be resected for locally advanced gallbladder cancer? Surgery 2004;136:1012–1017; discussion 8. [DOI] [PubMed] [Google Scholar]
- 92. Mitin T, Enestvedt CK, Jemal A, et al. Limited use of adjuvant therapy in patients with resected gallbladder cancer despite a strong association with survival. JNCI 2017;109. doi: 10.1093/jnci/djw324 [DOI] [PubMed] [Google Scholar]
- 93. Horgan AM, Amir E, Walter T, et al. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. Clin Oncol: Am J Clin Oncol 2012;30:1934–1940. [DOI] [PubMed] [Google Scholar]
- 94. Ma N, Cheng H, Qin B, et al. Adjuvant therapy in the treatment of gallbladder cancer: a meta-analysis. BMC cancer 2015;15:615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wang SJ, Lemieux A, Kalpathy-Cramer J, et al. Nomogram for predicting the benefit of adjuvant chemoradiotherapy for resected gallbladder cancer. Clin Oncol: Am J Clin Oncol 2011;29:4627–4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mantripragada KC, Hamid F, Shafqat H, et al. Adjuvant therapy for resected gallbladder cancer: analysis of the National Cancer Data Base. JNCI 2017;109:djw202. [DOI] [PubMed] [Google Scholar]
- 97. Rizzo A, Brandi G. First-line chemotherapy in advanced biliary tract cancer ten years after the ABC-02 trial: “And Yet It Moves!. CTARC 2021;27:100335. [DOI] [PubMed] [Google Scholar]
- 98. Rizzo A, Ricci AD, Tober N, et al. Second-line treatment in advanced biliary tract cancer: today and tomorrow. Anticancer Res 2020;40:3013–3030. [DOI] [PubMed] [Google Scholar]
- 99. Ricci AD, Rizzo A, Brandi G. The DNA damage repair (DDR) pathway in biliary tract cancer (BTC): a new Pandora’s box? ESMO open 2020;5:e001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ueno M, Ikeda M, Morizane C, et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: a non-randomised, multicentre, open-label, phase 1 study. The lancet Gastroenterology & hepatology 2019;4:611–621. [DOI] [PubMed] [Google Scholar]
- 101. Rizvi S, Gores GJ. Emerging molecular therapeutic targets for cholangiocarcinoma. J Hepatol 2017;67:632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rizzo A, Ricci AD, Brandi G. Durvalumab: an investigational anti-PD-L1 antibody for the treatment of biliary tract cancer. Expert opinion on investigational drugs 2021;30:343–350. [DOI] [PubMed] [Google Scholar]
- 103. Shu YJ, Weng H, Bao RF, et al. Clinical and prognostic significance of preoperative plasma hyperfibrinogenemia in gallbladder cancer patients following surgical resection: a retrospective and in vitro study. BMC Cancer 2014;14:566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Cao P, Jiang L, Zhou LY, et al. The clinical significance of preoperative serum fibrinogen levels and platelet counts in patients with gallbladder carcinoma. BMC Gastroenterol 2021;21:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Yang Z, Ren T, Liu S, et al. Preoperative serum fibrinogen as a valuable predictor in the nomogram predicting overall survival of postoperative patients with gallbladder cancer. J Gastrointest Oncol 2021;12:1661–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lv TR, Yang C, Regmi P, et al. The role of laparoscopic surgery in the surgical management of gallbladder carcinoma: A systematic review and meta-analysis. Asian J Surg 2021;44:1493–1502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study is included in the published article.


