Abstract
Background:
Robotic gastrectomy is a safe and feasible approach for gastric cancer (GC); however, its long-term oncological efficacy remains unclear. The authors evaluated the long-term survival outcomes and recurrence patterns of patients with locally advanced proximal GC who underwent robotic total gastrectomy (RTG).
Methods:
This prospective study (FUGES-014 study) enrolled 48 patients with locally advanced proximal GC who underwent RTG between March 2018 and February 2020 at a tertiary referral teaching hospital. Patients who underwent laparoscopic total gastrectomy (LTG) in the FUGES-002 study were enrolled in a 2:1 ratio to compare the survival outcomes between RTG and LTG. The primary endpoint of the FUGES-014 study was postoperative 30-day morbidity and has been previously reported. Here, the authors reported the results of 3-year disease-free survival (DFS), 3-year overall survival (OS), and recurrence patterns.
Results:
After propensity score matching, 48 patients in the RTG and 96 patients in the LTG groups were included. The 3-year DFS rates were 77.1% (95% CI: 66.1–89.9%) for the RTG and 68.8% (95% CI: 60.1–78.7%) for the LTG groups (P=0.261). The 3-year OS rates were not significantly different between the groups (85.4 vs. 74.0%, P=0.122). Recurrence occurred in nine patients (18.8%) in the RTG and 27 (28.1%) patients in the LTG groups (P=0.234). Recurrence patterns and causes of death were similar between the groups (P>0.05).
Conclusions:
The oncological outcome of RTG was noninferior to that of LTG. Thus, RTG might be an alternative surgical treatment for locally advanced proximal GC.
Keywords: disease-free survival, gastric cancer, laparoscopic total gastrectomy, overall survival, robotic total gastrectomy
Introduction
Highlights
This is the first prospective study to report survival of robotic total gastrectomy.
A prospective cohort with laparoscopic total gastrectomy was used to compare.
The oncological outcomes of robotic total gastrectomy were comparable to those of laparoscopic total gastrectomy.
Gastric cancer (GC) is the fifth most common malignancy and the fourth leading cause of cancer-related deaths worldwide1. Surgical resection is the cornerstone of treatment for locally advanced GC2. In recent years, minimally invasive surgery (MIS) for GC has gained popularity worldwide. Several large-scale phase 3 trials have demonstrated better postoperative and oncological outcomes with laparoscopic gastrectomy than with open gastrectomy3–6. However, technical difficulties (e.g. amplified physiological tremors, restricted range of motion, and ergonomic discomfort)7 and learning curve8 associated with laparoscopic gastrectomy restrict its wider application.
Hashizume et al.9 first reported the application of robotic gastrectomy in 2002, and since then, robotic surgery has been considered as an option for MIS10. Robotic surgery has several technical advantages over laparoscopic surgery, including high-definition three-dimensional surgical vision, increased degree of freedom, fewer physiological tremors, improved ergonomics, and decreased fatigue11. These technical advantages improve the perioperative outcome12. Several studies have collectively demonstrated the surgical safety of robotic gastrectomy10,13–15. However, robotic surgery was also associated with a longer operation time and a higher cost16. More importantly, the long-term outcomes of robotic gastrectomy have not been well established. Although several retrospective population-based studies and a single-arm prospective study have reported promising long-term results of robotic gastrectomy17, the oncological safety of the robotic approach in patients with locally advanced proximal GC remains controversial.
Laparoscopic total gastrectomy (LTG) is considered a more complicated procedure than distal gastrectomy because performing lymph node dissection around the splenic hilum can be challenging, potentially increasing the risk of postoperative complications18. Considering the technical advantages of robotic gastrectomy, we conducted the first prospective study (FUGES-014) to investigate the feasibility and safety of robotic total gastrectomy (RTG) in patients with locally advanced proximal GC. We had previously reported the short-term outcomes of RTG, which included less intraoperative blood loss, increased lymph node retrieval, decreased surgical errors, and reduced surgeon workload19. Here, we present the 3-year survival outcomes of RTG compared with LTG for locally advanced proximal GC.
Methods
Study design
The FUGES-014 was a prospective, single-arm, open-label study conducted at a tertiary referral teaching hospital in China between 5 March 2018, and 10 February 2020. The approved study protocol is available in Supplemental Digital Content 1, http://links.lww.com/JS9/C246. The study findings adhere to the strengthening the reporting of cohort, cross-sectional, and case–control studies in surgery (STROCSS, Supplemental Digital Content 6, http://links.lww.com/JS9/C251) reporting guidelines20. This study was registered with clinicaltrials.gov.
Study patients
The FUGES-014 study enrolled patients scheduled to undergo RTG. Eligible patients were aged between 18 and 75 years, and had histologically confirmed primary gastric adenocarcinoma located in the proximal stomach without greater curvature invasion (cT2 to cT4a, N0/+, and M0 tumors) during preoperative evaluation. Patients with other malignancies or a history of chemotherapy or radiotherapy were excluded. Written informed consent was obtained from all patients. Details of the eligibility criteria are shown in eTable 1 (Supplemental Digital Content 3, http://links.lww.com/JS9/C248).
Because of budget limitations, a randomized study was not feasible. Therefore, we selected patients who underwent LTG between January 2015 and December 2018 in the FUGES-002 study, a prospective randomized controlled trial (RCT), as the control group, as both the prospective trials had similar inclusion and exclusion criteria. The FUGES-002 study compared surgical outcomes for LTG with and without splenic hilar lymphadenectomy in locally advanced proximal GC (Supplemental Digital Content 2, http://links.lww.com/JS9/C247)19. For historical controls, 263 patients who underwent LTG with splenic hilar lymphadenectomy were included to ensure the patients included in both the laparoscopic and robotic surgery groups received the same interventions except the surgical approach. The Institutional Review Board approved the design and reporting procedures of the current study.
Propensity score matching was performed to compensate for differences in baseline characteristics between the RTG and LTG groups. A biostatistician who was blinded to the outcomes estimated the propensity score using a logistic regression model (eFigure 1, Supplemental Digital Content 3, http://links.lww.com/JS9/C248) by identifying 40 covariates before surgery (as previously reported)19. Greedy matching (1:2 ratio) was performed with a caliper width of 0.01 SD of the estimated logit. In addition to the propensity score, seven factors (cT and cN categories: age, sex, and BMI, cross-sectional area, and tumor location) were introduced to make additional adjustments6. Of the 536 patients in the FUGES-002 study, 96 were exactly matched using this method. The cut-off for the absolute standardized mean difference, which indicated a significant imbalance, was set at 0.10021.
Surgical procedures, quality control, and follow-up
All patients underwent RTG or LTG under Enhanced Recovery After Surgery (ERAS) protocol (Supplemental Digital Content 4, http://links.lww.com/JS9/C249)22. Both RTG and LTG adhered to the principles of the surgical management of the extents of gastric resection and lymphadenectomy, based on the Japanese GC treatment guidelines 2014 (ver. 4)23 and as reported in previous research. The patients enrolled in the two studies underwent surgery by either of the two surgeons (C.-H.Z. or C.-M.H.) from the same team, both of whom had performed over 50 RTG and 50 LTG procedures before the trials. To reduce any bias caused by surgical variability, both the studies required that the lymphadenectomy and digestive reconstruction be performed independently by one of the two surgeons. The da Vinci Si system was used for RTG.
The main anastomosis was performed extracorporeally (Supplemental Digital Content 5, http://links.lww.com/JS9/C250). LTG and RTG were categorized into five major stages: removal of the greater omentum, and lymph node dissection of the infrapyloric, suprapancreatic, splenic hilar, and left cardiac areas. A FUGES devised a checklist (reported previously) used to assess the success of D2 lymphadenectomies19. Another group of surgeons reviewed the unedited videos of the participants once a week and referred them to a checklist for quality control. Lymph node-bearing soft tissues were separated from the surgical specimens and were divided into stations 1-7, 8a, 9, 10, 11p, 11d, and 12a according to the Japanese GC treatment guidelines 2014 (ver. 4)23. All specimens were evaluated pathologically in a standardized manner.
Patients in the RTG and LTG groups underwent similar perioperative management and follow-up protocols. Patients with pathological stage II or more advanced disease were recommended to receive adjuvant chemotherapy, which consisted of a 6-month fluorouracil-based regimen. The treating oncologist had the discretion in choosing the specific regimen and treatment duration.
The final follow-up date was 15 March 2023, with a minimum mandatory postoperative follow-up period of 36 months for each patient. Follow-up appointments were conducted every 3 months for the first 2 years and every 6 months for the subsequent 3 years. Routine follow-up procedures included (1) physical examination and blood tests every 3 months for the first 2 years and every 6 months thereafter; (2) chest radiography and abdominal computed tomography scans every 6 months for 3 years; and (3) upper gastrointestinal endoscopy annually for 3 years. Positron emission tomography/computed tomography was performed for patients with suspected recurrence. Recurrence was identified using a combination of medical history, physical examination, imaging, cytology, and tissue biopsy (preferred when feasible). Otherwise, patients attended follow-up visits at shorter intervals than the planned schedule. Those exhibiting symptoms such as abdominal mass, weight loss, or obstruction, which were indicative of recurrence, were evaluated regardless of their scheduled follow-up.
Outcomes and definitions
The primary endpoint of the FUGES-014 study was the overall postoperative morbidity rate, and the secondary endpoints were 3-year disease-free survival (DFS), 3-year overall survival (OS), and 3-year recurrence pattern. Notably, we assessed postoperative morbidity during the first 30 postoperative days because the majority of complications occur during this period24. Outcomes pertaining to safety and efficacy, such as perioperative morbidity and mortality rates (≤30 days) and postoperative recovery, have been previously reported19.
OS was defined as the time from surgery to death from any cause or the last follow-up. DFS was defined as the time from surgery to recurrence or death from any cause or the last follow-up. Postoperative complications were graded according to the Clavien–Dindo classification25. A delay in adjuvant chemotherapy beyond 8 weeks after surgery was defined as chemotherapy delay. Completion of at least six cycles of adjuvant chemotherapy was defined as chemotherapy completion.
Statistical analysis
The per-protocol analysis population was used for all analyses in this study. Data management and site-visit monitoring were performed by a data manager (M.L.). Continuous variables are expressed as means (SD) and categorical variables as numbers. Differences between the groups were assessed using the t-test, χ2 test, or Fisher exact test. All tests were two-sided, and a P<0.05 was considered statistically significant.
The 3-year DFS and OS rates were calculated using the Kaplan–Meier method, and the log-rank test was used to determine statistical significance. The hazard ratios (HRs) for the comparisons between RTG and LTG groups were estimated using Cox regression analysis after confirming the proportional hazards assumption. Multivariate Cox regression analyses were performed to evaluate the effect of operation type on survival after adjusting for clinicopathological covariates that were significantly associated with outcomes in univariate analyses. All-cause mortality was considered a competing event for recurrence. The cumulative incidence in the presence of competing risks was calculated, and competing-risk survival regression was used as an alternative to Cox regression analysis.
This was a noninferiority study. The primary hypothesis was noninferiority of RTG relative to LTG in terms of HR on DFS and OS, in which the noninferiority margin for the HR was set to be 1.33 according to the LOC-A study6. This margin was less than that of the CLASS-0126 and KLASS-025 studies (1.46 and 1.43, respectively). Noninferiority would be established if the upper bounds of the two-sided 95% CIs of HRs did not exceed this noninferiority margin.
All data were analyzed using SPSS statistical software version 25.0 (SPSS Inc), and R software version 4.2.0 (R Foundation for Statistical Computing). Statistical analyses were done from March to April 2023.
Results
Patient characteristics
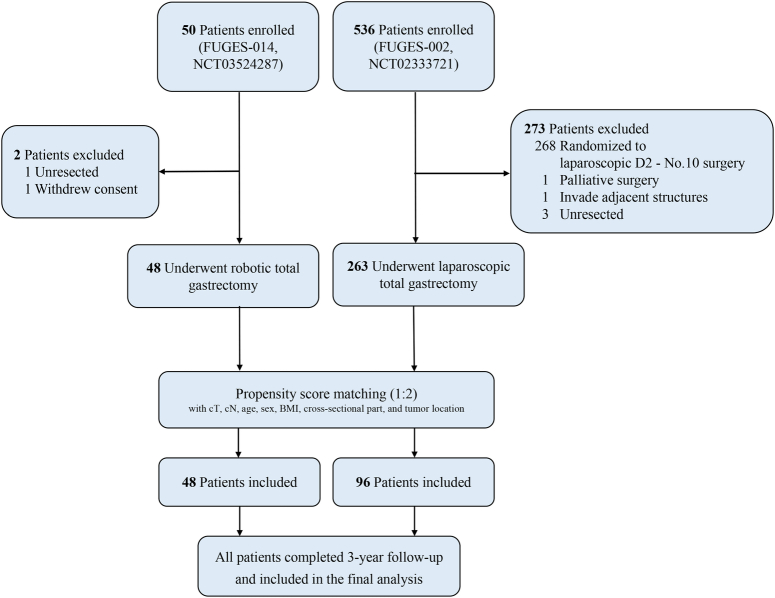
The FUGES-014 study enrolled 50 patients between 5 March 2018 and 10 February 2020. After excluding one patient with an unresectable tumor and one patient who withdrew from the study, a total of 48 patients who underwent RTG were included in the analysis. Of these, 38 (79.2%) patients were male and 10 (20.8%) were female, with a mean age of 61.3 years (SD=9.3).
The FUGES-002 study enrolled 263 patients between 5 January 2015 and 10 December 2018, who underwent LTG. Of these, 189 (71.9%) patients were male and 74 (28.1%) were female, with a mean age of 59.8 years (SD=10.3). Before matching, patients in the LTG group had more advanced cT (P=0.044) and pT disease (P=0.006) than those in the RTG group (Table 1). Using 1:2 ratio propensity score matching, 96 patients who underwent LTG were included in the final analysis (Fig. 1). The clinicopathological characteristics of the two groups were similar (all P>0.05; Table 1).
Table 1.
Demographic and clinical characteristics of patients in the RTG and LTG groups before and after propensity score matching.
| Before matching, No. (%) | After matching, No. (%) | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | RTG (n=48) | LTG (n=263) | SMD | P | LTG (n=96) | SMD | P |
| Age, years | |||||||
| Mean±SD | 61.3±9.3 | 59.8±10.3 | 0.156 | 0.344 | 61.6±7.6 | 0.039 | 0.818 |
| BMI, kg/m2 | |||||||
| Mean±SD | 22.3±2.7 | 22.1±3.1 | 0.069 | 0.672 | 22.3±3.2 | 0.008 | 0.967 |
| Size, cm | |||||||
| Mean±SD | 4.4±2.6 | 5.0±2.5 | 0.188 | 0.132 | 4.5±2.1 | 0.027 | 0.872 |
| Sex | 0.170 | 0.295 | 0.079 | 0.651 | |||
| Male | 38 (79.2) | 189 (71.9) | 79 (82.3) | ||||
| Female | 10 (20.8) | 74 (28.1) | 17 (17.7) | ||||
| ECOG score | 0.011 | 0.945 | 0.021 | 0.903 | |||
| 0 | 30 (62.5) | 163 (62.0) | 59 (61.5) | ||||
| 1 | 18 (37.5) | 100 (38.0) | 37 (38.5) | ||||
| Tumor location | 0.065 | 0.674 | 0.088 | 0.615 | |||
| U/UM | 31 (64.6) | 178 (67.7) | 66 (68.8) | ||||
| M/MU | 17 (35.4) | 85 (32.3) | 30 (31.2) | ||||
| Histological type | 0.265 | 0.151 | 0.084 | 0.634 | |||
| Differentiated | 22 (45.8) | 92 (35.0) | 40 (41.7) | ||||
| Undifferentiated | 26 (54.2) | 171 (65.0) | 56 (58.3) | ||||
| Cross-sectional part | 0.156 | 0.820 | 0.083 | 0.974 | |||
| Lesser | 38 (79.2) | 197 (74.9) | 75 (78.1) | ||||
| Anterior | 2 (4.2) | 8 (3.0) | 3 (3.1) | ||||
| Posterior | 5 (10.4) | 40 (15.2) | 12 (12.5) | ||||
| Multiple | 3 (6.2) | 18 (6.8) | 6 (6.2) | ||||
| Lymphvascular invasion | 0.070 | 0.659 | 0.022 | 0.902 | |||
| Negative | 31 (64.6) | 161 (61.2) | 61 (63.5) | ||||
| Positive | 17 (35.4) | 102 (38.8) | 35 (36.5) | ||||
| Clinical T stage | 0.588 | 0.044 | 0.067 | 0.932 | |||
| cT2 | 17 (35.4) | 60 (22.8) | 32 (33.3) | ||||
| cT3 | 25 (52.1) | 131 (49.8) | 50 (52.1) | ||||
| cT4a | 6 (12.5) | 72 (27.4) | 14 (14.6) | ||||
| Clinical N stage | 0.111 | 0.053 | 0.064 | 0.718 | |||
| cN0 | 20 (41.7) | 73 (27.8) | 37 (38.5) | ||||
| cN+ | 28 (58.3) | 190 (72.2) | 59 (61.5) | ||||
| Pathologic T stage | 0.333 | 0.006 | 0.190 | 0.574 | |||
| ≤pT2 | 16 (33.3) | 54 (20.5) | 27 (28.1) | ||||
| pT3 | 24 (50.0) | 139 (52.9) | 46 (47.9) | ||||
| pT4a | 8 (16.7) | 70 (26.6) | 23 (24.0) | ||||
| Pathologic N stage | 0.333 | 0.219 | 0.112 | 0.943 | |||
| pN0 | 19 (39.6) | 73 (27.8) | 36 (37.5) | ||||
| pN1 | 10 (20.8) | 43 (16.3) | 19 (19.8) | ||||
| pN2 | 7 (14.6) | 53 (20.2) | 18 (18.8) | ||||
| pN3 | 12 (25.0) | 94 (35.7) | 23 (24.0) | ||||
| Pathologic TNM stage | 0.365 | 0.062 | 0.150 | 0.701 | |||
| I | 13 (27.1) | 41 (15.6) | 22 (22.9) | ||||
| II | 16 (33.3) | 74 (28.1) | 29 (30.2) | ||||
| III | 19 (39.6) | 148 (56.3) | 45 (46.9) | ||||
ECOG, Eastern Cooperative Oncology Group; LTG, Laparoscopic total gastrectomy; LVI, Lymphvascular invasion; M, Middle third of stomach; MU, Middle and Upper third of stomach; RTG, Robotic total gastrectomy; SMD, Standardized mean difference; U, Upper third of stomach; UM, Upper and Middle third of stomach.
Figure 1.
Patient enrollment.
Surgical outcomes
A conversion to open or laparoscopic surgery was not reported for any patient in both groups. Although the total operation time was longer in the RTG group than in the LTG group (217.1 vs. 186.1 min, P<0.001), there was no significant difference in laparoscopic/robotic operation time between the two groups (97.5 vs. 92.5 min, P=0.054). Intraoperative blood loss was significantly lower in the RTG group than in the LTG group (38.7 vs. 66.4 ml, P=0.042). Moreover, the mean number of lymph nodes retrieved from the extraperigastric regions was higher in the RTG group than in the LTG group (20.2 vs. 17.5, P=0.039; eTable 2, Supplemental Digital Content 3, http://links.lww.com/JS9/C248). Seven patients (14.6%) in the RTG group and 16 (16.7%) in the LTG group had postoperative complications within 30 days after surgery, with no statistically significant differences (P=0.859; eTable 3, Supplemental Digital Content 3, http://links.lww.com/JS9/C248) between the groups. There was no significant difference in the severity of complications (P=0.912) between the groups. In terms of postoperative recovery, the RTG group had significantly shorter time to first flatus, ambulation, first liquid intake, and postoperative hospital stay than the LTG group (all P<0.05; eTable 3, Supplemental Digital Content 3, http://links.lww.com/JS9/C248).
Between 30 and 90 days after surgery, one patient in the RTG group (unknown reason) and two patients in the LTG group (one with ileus and one with pneumonia) experienced complications. The 90-day morbidity rates of the RTG and LTG groups were 16.7 and 18.8%, respectively (P=0.759). The 90-day mortality rates of the RTG and LTG groups were 2.1 and 0.0%, respectively (P=0.333).
Among patients with pathological stage II–III disease, 33 (94.3%) patients in the RTG group and 59 (79.7%) in the LTG group received adjuvant chemotherapy (P=0.051). There were no significant differences in the time to starting adjuvant chemotherapy from surgery or in the chemotherapy delay rate between the two groups (P>0.05). However, chemotherapy completion rate was significantly higher in the RTG group than in the LTG group (60.6 vs. 45.8%, P=0.049; eTable 4, Supplemental Digital Content 3, http://links.lww.com/JS9/C248).
Disease-free survival
The median follow-up period was 44 (range, 36–60) months in the RTG group and 62 (range, 43–87) months in the LTG group. The 3-year DFS rates were 77.1% (95% CI: 66.1–89.9%) in the RTG group and 68.8% (95% CI: 60.1–78.7%) in the LTG group, with no statistically significant differences between the two groups (log-rank P=0.261; HR 0.689, 95% CI: 0.357–1.329, P=0.266, Fig. 2A). The upper bound for the 95% CI was lower than 1.33, and thus the noninferiority of RTG relative to LTG was demonstrated. Multivariate analysis revealed that HR for DFS in the RTG group was not significantly different from that in the LTG group (HR 0.592, 95% CI: 0.306–1.148, P=0.121; eTable 5, Supplemental Digital Content 3, http://links.lww.com/JS9/C248).
Figure 2.

Kaplan–Meier curves comparing disease-free survival (A) and overall survival (B) between patients in the robotic and laparoscopic groups.
Overall survival
Seven patients (14.6%) in the RTG group died within 3 years of surgery compared to 25 patients (26.0%) in the LTG group. The 3-year OS rates were 85.4% (95% CI: 76.0–96.0%) in the RTG group and 74.0% (95% CI: 65.7–83.3%) in the LTG group, and the upper bound of the 95% CI was below the noninferiority margin of 1.33 (log-rank P=0.122; HR 0.563, 95% CI: 0.269–1.179, P=0.128, Fig. 2B). The 3-year OS was not significantly different after controlling for tumor size and pN stage (HR 0.504, 95% CI: 0.240–1.057, P=0.070; Supplemental Digital Content 3, http://links.lww.com/JS9/C248). The 3-year actual OS rates were 83.3% (95% CI: 73.4–94.6%) in the RTG group and 71.9% (95% CI: 63.4–81.5%) in the LTG group, with an absolute difference of 11.5%. Furthermore, the 3-year actual DFS rates were 77.1% (95% CI: 66.1–89.9%) in the RTG group and 67.7% (95% CI: 59.0–77.7%) in the LTG group, with an absolute difference of 10.4%. Both the actual OS and DFS between the two groups were comparable (log-rank P>0.05; eFigure 2, Supplemental Digital Content 3, http://links.lww.com/JS9/C248).
Recurrence
Within the first 3 years of follow-up, recurrence was recorded in 9 out of 48 patients in the RTG group (18.8%) and 27 out of 96 patients in the LTG group (28.1%); however, the differences were not statistically significant (log-rank P=0.234; HR 1.491, 95% CI: 0.732–3.036, P=0.271; Table 2 and eFigure 3A, Supplemental Digital Content 3, http://links.lww.com/JS9/C248). The recurrence sites were similar between the two groups (P>0.05). The most common site of recurrence was the peritoneum (including the patients with mixed recurrence), and was reported in four patients in the RTG group and in nine patients in the LTG group. The 3-year cumulative GC-specific survival rates were 14.6% in the RTG group and 26.0% in the LTG group, with no significant differences between the groups (log-rank P=0.131; HR 1.776, 95% CI 0.848–3.721, P=0.128, Table 2 and eFigure 3B, Supplemental Digital Content 3, http://links.lww.com/JS9/C248). Similarly, RTG was not independently associated with recurrence (HR 0.686, 95% CI: 0.334–1.409, P=0.305; eTable 7, Supplemental Digital Content 3, http://links.lww.com/JS9/C248).
Table 2.
Frequencies of causes of death and first recurrence within 3 years after surgery of patients in the RTG and LTG groups.
| No.(%) | |||||
|---|---|---|---|---|---|
| Events | RTG (n=48) | LTG (n=96) | RD | HR (95% CI)a | P |
| All-cause death | 7 (14.6) | 25 (26.0) | −0.21 | 0.563 (0.269–1.179) | 0.128 |
| Gastric cancer | 5 (10.4) | 22 (22.9) | −0.14 | 0.523 (0.228–1.200) | 0.126 |
| Other causesb | 2 (4.2) | 3 (3.1) | 0.01 | 0.709 (0.151–3.331) | 0.627 |
| Total recurrence | 9 (18.8) | 27 (28.1) | −0.11 | 0.671 (0.329–1.367) | 0.271 |
| Local | 1 (2.1) | 5 (5.2) | −0.04 | 0.392 (0.047–3.250) | 0.388 |
| Peritoneum | 3 (6.3) | 8 (8.3) | −0.02 | 0.942 (0.291–3.046) | 0.920 |
| Liver | 2 (4.2) | 4 (4.2) | 0.00 | 1.016 (0.187–5.362) | 0.985 |
| Multiple sitesc | 1 (2.1) | 6 (6.3) | −0.05 | 0.324 (0.040–2.631) | 0.290 |
| Other or uncertain sitesd | 2 (4.2) | 4 (4.2) | 0.02 | 1.210 (0.306–4.787) | 0.786 |
Cox proportional hazards model was used for all-cause death, while competing risk regression model was used for other events. For gastric cancer-specific death, other causes of death were the competing events, and vice versa. For total recurrence, all-cause death was the competing event. For the specific sites of recurrence, other sites of recurrence and other causes of death were the competing events.
Includes other cancers, diseases other than cancer, unintentional injuries, and unknown causes.
Multiple sites recurrence was defined as the presence of recurrent disease simultaneously in 2 or more sites, including peritoneum, liver, lung, bone, brain, distant lymph node, or other hematogenous metastatic sites.
Includes hematogenous recurrence at sites other than liver (i.e. lung, bone, and brain), recurrence at distant lymph node, and recurrence at uncertain sites.
HR, hazard ratio; LTG, laparoscopic total gastrectomy; RD, risk difference; RTG, robotic total gastrectomy.
Subgroup analysis
No significant differences in DFS, OS, or recurrence were observed between the RTG and LTG groups based on the different pathological stages (log-rank P>0.05, Fig. 3). After excluding 27 patients with early GC (seven in the RTG group and 20 in the LTG group), the 3-year DFS rate (75.6 vs. 63.2%), 3-year OS rate (85.4 vs. 69.7%), and 3-year recurrence rate (22.0 vs. 35.5%) were similar between the two groups (log-rank P>0.05, eFigure 4, Supplemental Digital Content 3, http://links.lww.com/JS9/C248). However, RTG showed a trend for a lower recurrence rate in patients with pT4a (P for interaction=0.017) or pN+ disease (P for interaction=0.028); (eFigure 5, Supplemental Digital Content 3, http://links.lww.com/JS9/C248). No significant interaction was observed between patients in the RTG and LTG groups among other baseline characteristics including age, BMI, and tumor location (P for interaction>0.05, eFigure 6–8, Supplemental Digital Content 3, http://links.lww.com/JS9/C248). Moreover, the OS, DFS, and cumulative recurrence rates of patients undergoing robot-assisted surgery for stage II–III were comparable to those undergoing laparoscopic surgery irrespective of adjuvant chemotherapy (all P>0.05, eFigure 9, Supplemental Digital Content 3, http://links.lww.com/JS9/C248).
Figure 3.
Kaplan–Meier curves comparing disease-free survival, overall survival, and cumulative recurrence between patients with stage I (A), stage II (B), or stage III (C) tumors in the robotic and laparoscopic groups.
Discussion
To the best of our knowledge, this is the first prospective study to compare the long-term outcomes of RTG and LTG in patients with GC. As previously reported, RTG has been shown to result in less intraoperative blood loss, faster postoperative recovery, a higher number of retrieved lymph nodes, and lower rates of major lymph node noncompliance19. In the present study, we further confirmed the oncological safety of robotic surgery compared to that of laparoscopic surgery in complex total gastrectomy.
The purpose of MIS is to reduce postoperative pain, achieve a faster return to normal activities, and shorten the length of hospital stay10. Laparoscopic surgery, a cornerstone of MIS, has been proven to be a safe and efficacious approach for patients with locally advanced GC3,5,27. Although the short-term safety of robotic surgery is well established13,14,28,29, there is limited evidence on the oncological safety of robotic gastrectomy with D2 lymphadenectomy. A meta-analysis indicated statistically nonsignificant differences in OS and DFS between robotic and laparoscopic surgeries for GC12. Two real-world studies from China and Korea showed that long-term outcomes of robotic gastrectomy were comparable to those of laparoscopic gastrectomy30,31. However, it is difficult to assess the benefits of robotic complex total gastrectomy in a relatively high proportion of subtotal gastrectomies and early-stage tumors. In this study, after rigorous adjustment using propensity score matching, we confirmed that 3-year DFS, 3-year OS, 3-year cumulative recurrence, and 3-year recurrence patterns were comparable between the RTG and LTG groups. These findings indicate that there are no significant differences in tumor prognosis between RTG and LTG surgical approaches, and the findings are consistent with previous studies31,32. Thus, robotic surgery can be a reliable option for the treatment of MIS in patients with locally advanced proximal GC. Additionally, the multivariate Cox analysis showed that pN staging was an important factor affecting DFS, OS, and recurrence in patients with GC. Robot-assisted surgery is advantageous in intraoperative lymph node dissection as it can significantly increase the number of lymph nodes retrieved and reduce postoperative staging bias. Our findings will contribute to the design of future large-scale prospective RCTs.
Robotic surgery offers several technological advantages over traditional laparoscopic surgery, including three-dimensional vision, seven degrees of freedom, a stable optical platform, and tremor reduction technology, which enables surgeons to effectively conduct complex procedures, such as suprapancreatic and splenic hilum lymph node dissection33,34. Compared to laparoscopic surgery using unwristed instruments, robotic surgery allows the surgeon to approach the deep and narrow supra-pancreatic and splenic hilar areas easily, which enables adequate lymph node dissection within a restricted space30,35. These advantages facilitate a more complete lymph node dissection and minimize injury to the pancreas, spleen, and splenic vessels. Previous studies have shown that using RTG allows for a higher number of lymph nodes to be retrieved from the superior margin of the pancreas and splenic hilum compared with the LTG. Furthermore, RTG is associated with lesser intraoperative blood loss and fewer intraoperative errors than LTG19. However, these advantages of RTG have not been shown to improve survival rates. These findings could be explained by the notion that performing surgeries with similar scopes and under similar surgical conditions between the two approaches can yield comparable long-term results. It is possible that high-quality surgery may have already been achieved using the laparoscopic approach, leaving little room for improvement in survival outcomes using the robotic approach. Furthermore, the purpose of this study was not to investigate whether robotic surgery can improve survival, but rather to confirm the short-term advantages of robotic surgery and assess its feasibility as an alternative surgical approach to laparoscopic surgery in terms of long-term outcomes19,32–34,36. Nevertheless, RTG showed a trend toward survival benefits compared with LTG in patients with tumors invading the serosa or with lymph node metastasis. Robotic surgical systems can provide a technically superior surgical environments for MIS, which can have oncological advantages in cancer treatment, particularly in the treatment of advanced GC. The surgical complexity significantly increases as the tumor stage advances and the number of enlarged lymph nodes increase. Robotic systems can be advantageous to surgeons in overcoming intraoperative challenges, reduce intraoperative bleeding, lymphatic leakage, and cancer cell dissemination associated with surgical bleeding or lymphatic leakage37,38. Additionally, robotic surgery can increase the number of lymph node dissections, particularly for extraperigastric lymph nodes. Cumulative evidence has demonstrated that dissecting a sufficient number of LNs in the standard lymphadenectomy area is necessary for accurate disease staging and avoiding missed dissection of metastatic lymph nodes, thus having a positive impact on the prognosis of patients39,40. Moreover, robotic surgery has been reported to reduce staging bias in various malignancies41–43. Therefore, patients with advanced GC may potentially benefit in terms of survival from robotic surgery. However, further large-scale prospective studies are needed to identify the subgroups of patients who could particularly benefit more from a robotic approach.
However, the advantages of robotic surgery over laparoscopic surgery in terms of postoperative complications remains controversial. A phase 3 RCT showed that the incidence of postoperative complications of grade II or higher (8.8 vs. 19.7%) and grade IIIa or higher (5.3 vs. 16.2%) was significantly lower in the robotic group than in the laparoscopic group29. A population-based study of 3552 patients revealed that robotic surgery significantly reduced the incidence of overall complications compared to laparoscopic surgery (12.6 vs. 15.2%), but did not reduce the incidence of serious complications (2.5 vs. 2.9%)30. However, a meta-analysis of prospective observational studies showed no statistically significant differences in the postoperative complications between robotic and laparoscopic approaches16. The present study also found that postoperative complication rates were similar between the RTG and LTG groups. Robotic gastrectomy is beneficial for reducing pancreatic fistula by avoiding pancreatic damage, which is associated with stable and constant pancreatic compression during surgery14,34,44. In our study patients, postoperative pancreatic fistula was not observed in both surgical groups because it is possible that skilled coordination between the surgeons and assistants could have resulted in similar postoperative complication rates for the surgeries14,34,44. Robotic surgery has a shorter learning curve45, which we believe can help beginners quickly master minimally invasive surgical techniques while effectively reducing the risk of complications during the learning phase.
The present study also demonstrated that RTG was superior to LTG in terms of intraoperative blood loss, postoperative recovery, and postoperative hospital stay, which is consistent with the findings of most previous studies28,29. These findings suggest that as robotic surgery is associated with stable and flexible movements of the robotic forceps, it can avoid excessive traction on the tissue and accidental injury to the blood vessels and decrease surgical trauma to the patients. Furthermore, our study showed that the rate of adjuvant chemotherapy completion was significantly higher in the RTG group than in the LTG group, which could be due to robotic surgery being a less invasive surgery. A faster recovery with RTG might enhance the patients’ confidence in the cure of GC and make them more willing to accept subsequent treatment arrangements. However, due to a small sample size and nonrandomized design, further studies are needed to confirm this association.
Although robotic surgery has several technical advantages over traditional laparoscopic surgery, it is associated with a longer operative time16. Multiple reasons can be attributed to this phenomenon, and one of them is additional robot-specific procedures, such as preparation and docking time. After excluding this, the actual operative time of robotic surgery was comparable to that of laparoscopic surgery28. In addition, the surgeon needs to independently adjust the positions of all robotic arms when switching between different dissection planes in robotic surgery31. In the newly developed robots, it is worth looking forward to not only an easier option for switching arms but also to a simpler system setup. A high cost is another major factor that impedes the wide adoption of robotic surgery46. To facilitate the broader application of the robotic system in gastrectomy, we have devised standardized operating procedures and conducted a systematic education program, which might reduce direct costs by minimizing operation time and unnecessary complications. Moreover, newly developed robots that will reduce both the cost and operation time, along with extension coverage of the National Health Insurance System are expected to further reduce indirect costs associated with robotic surgery.
Limitations
The present study has several limitations. First, as a single-center study, all surgical procedures were performed by senior general surgeons from East Asia, and all patients underwent total gastrectomy combined with spleen-preserving splenic hilar lymphadenectomy. Thus, our findings are not generalizable to other centers and regions. Second, previous studies have reported that a loss of normal tissue plane from chemotherapy-induced profibrotic reactions and cytotoxicity can increase the surgical difficulty and lead to poor quality lymphadenectomy27,47–49, thereby affecting postoperative short-term and long-term outcomes50. To avoid this confounding effect, we excluded patients who had received neoadjuvant treatment (NAT) before surgery. We aim to conduct an exclusive NAT-associated prospective clinical trial to investigate the feasibility and safety of robotic gastrectomy following NAT. Third, as this and previous studies did not specifically focus on total RTG or LTG3–6, we could not isolate the impact of extracorporeal anastomosis on surgical outcomes. Finally, this was not a RCT and the efficacy of the RTG arm was compared with that of a historical cohort rather than with the control arm. Strict quality control was ensured to compare the two prospective studies using similar patient inclusion and exclusion criteria to ensure the reliability of results. Nevertheless, the inclusion of a historical cohort could not completely replace a concurrent control group, which inevitably resulted in bias and reduced the overall validity. Further validation with the results of RCTs is warranted.
Conclusions
In conclusion, the long-term oncological outcomes after RTG with D2 lymphadenectomy for locally advanced proximal GC were noninferior to those after LTG. Although there were no statistically significant between-group differences for the postoperative complications, RTG was significantly associated with better outcomes than LTG in terms of intraoperative blood loss, lymphadenectomy quality, postoperative recovery, and completion of adjuvant chemotherapy. These findings support the use of RTG in this patient population if the surgery is to performed by qualified surgeons. In the future, we expect to validate our findings through an ongoing multicenter, phase 3 RCT conducted at our institution.
Ethical approval
The Institutional Review Board of the Fujian Medical University Union Hospital approved the design and reporting procedures of the current study (IRB number: 2020KY0113).
Consent
Written informed consent was obtained from the patient for publication and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Sources of funding
This study was supported by the Construction Funds for “High-level Hospitals and Clinical Specialties” of Fujian Province (No. [2021]76); Fujian provincial health technology project (No. 2023QNA019); and Fujian Medical University Union Hospital Talent Launch Fund Project (No. 2024XH007).
Author contribution
We thank who have devoted a lot to this study, including nurses, pathologists, further-study doctors, statisticians, reviewers and editors. Thanks for Dr. Zhi-Hong Huang, Public Technology Service Center, Fujian Medical University. Feng-Qiong Liu, Experimental Center of School of Public Health, Fujian Medical University.
Conflicts of interest disclosure
None reported.
Research registration unique identifying number (UIN)
ClinicalTrials.gov: NCT03524287 and NCT02333721.
Guarantor
HCM had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Data availability statement
Huang CM had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Deidentified data about the individual participants will be shared with researchers of further studies on reasonable request. Request for data sharing will be handled in line with the data access and sharing policy of Fujian Medical University Union Hospital.
Provenance and peer review
This paper was not invited.
Supplementary Material
Footnotes
Zhong Q, Tang YH, and Liu ZY contributed equally to this work and should be considered co-first authors.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.lww.com/international-journal-of-surgery.
Published online 27 March 2024
Contributor Information
Qing Zhong, Email: 845733977@qq.com.
Yi-Hui Tang, Email: 269051109@qq.com.
Zhi-Yu Liu, Email: 751478940@qq.com.
Zhi-Quan Zhang, Email: zhangzhiquan997@163.com.
Qi-Chen He, Email: 1092550131@qq.com.
Ping Li, Email: pingli811002@163.com.
Jian-Wei Xie, Email: xjwhw2019@163.com.
Jia-Bin Wang, Email: 847044493@qq.com.
Jian-Xian Lin, Email: 158816524@qq.com.
Jun Lu, Email: 78379048@qq.com.
Qi-Yue Chen, Email: 690934662@qq.com.
Chao-Hui Zheng, Email: wwkzch@163.com.
Chang-Ming Huang, Email: hcmlr2002@163.com.
References
- 1. Sung H, Ferlay J, Siegel R, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 2. Songun I, Putter H, Kranenbarg E, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439–449. [DOI] [PubMed] [Google Scholar]
- 3. Huang C, Liu H, Hu Y, et al. Laparoscopic vs open distal gastrectomy for locally advanced gastric cancer: five-year outcomes from the CLASS-01 randomized clinical trial. JAMA Surg 2022;157:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim H, Han S, Kim M, et al. Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with stage i gastric cancer: the KLASS-01 randomized clinical trial. JAMA Oncol 2019;5:506–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Son S, Hur H, Hyung W, et al. Laparoscopic vs open distal gastrectomy for locally advanced gastric cancer: 5-year outcomes of the KLASS-02 randomized clinical trial. JAMA Surg 2022;157:879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kinoshita T, Uyama I, Terashima M, et al. Long-term outcomes of laparoscopic versus open surgery for clinical stage II/III gastric cancer: a multicenter cohort study in Japan (LOC-A Study). Ann Surg 2019;269:887–894. [DOI] [PubMed] [Google Scholar]
- 7. Sasako M. Is there role for laparoscopic gastrectomy for advanced gastric cancer. Eur J Surg Oncol 2017;43:965–967. [DOI] [PubMed] [Google Scholar]
- 8. Jin S, Kim D, Kim H, et al. Multidimensional learning curve in laparoscopy-assisted gastrectomy for early gastric cancer. Surg Endosc 2007;21:28–33. [DOI] [PubMed] [Google Scholar]
- 9. Hashizume M, Shimada M, Tomikawa M, et al. Early experiences of endoscopic procedures in general surgery assisted by a computer-enhanced surgical system. Surg Endosc 2002;16:1187–1191. [DOI] [PubMed] [Google Scholar]
- 10. Parisi A, Reim D, Borghi F, et al. Minimally invasive surgery for gastric cancer: A comparison between robotic, laparoscopic and open surgery. World J Gastroenterol 2017;23:2376–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marohn M, Hanly E. Twenty-first century surgery using twenty-first century technology: surgical robotics. Curr Surg 2004;61:466–473. [DOI] [PubMed] [Google Scholar]
- 12. Feng Q, Ma H, Qiu J, et al. Comparison of long-term and perioperative outcomes of robotic versus conventional laparoscopic gastrectomy for gastric cancer: a systematic review and meta-analysis of PSM and RCT studies. Front Oncol 2021;11:759509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim H, Han S, Yang H, et al. Multicenter prospective comparative study of robotic versus laparoscopic gastrectomy for gastric adenocarcinoma. Ann Surg 2016;263:103–109. [DOI] [PubMed] [Google Scholar]
- 14. Uyama I, Suda K, Nakauchi M, et al. Clinical advantages of robotic gastrectomy for clinical stage I/II gastric cancer: a multi-institutional prospective single-arm study. Gastric Cancer 2019;22:377–385. [DOI] [PubMed] [Google Scholar]
- 15. Choi S, Song J, Lee S, et al. Surgical merits of open, laparoscopic, and robotic gastrectomy techniques with D2 lymphadenectomy in obese patients with gastric cancer. Ann Surg Oncol 2021;28:7051–7060. [DOI] [PubMed] [Google Scholar]
- 16. Bobo Z, Xin W, Jiang L, et al. Robotic gastrectomy versus laparoscopic gastrectomy for gastric cancer: meta-analysis and trial sequential analysis of prospective observational studies. Surg Endosc 2019;33:1033–1048. [DOI] [PubMed] [Google Scholar]
- 17. Hikage M, Tokunaga M, Furukawa K, et al. Long-term outcomes of robotic gastrectomy for clinical stage I gastric cancer: a single-center prospective phase II study. Surg Endosc 2021;35:4160–4166. [DOI] [PubMed] [Google Scholar]
- 18. Kawaguchi Y, Shiraishi K, Akaike H, et al. Current status of laparoscopic total gastrectomy. Ann Gastroenterol Surg 2019;3:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen Q, Zhong Q, Liu Z, et al. Surgical outcomes, technical performance, and surgery burden of robotic total gastrectomy for locally advanced gastric cancer: a prospective study. Ann Surg 2022;276:e434–e443. [DOI] [PubMed] [Google Scholar]
- 20. Mathew G, Agha R, Albrecht J, et al. STROCSS 2021: strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg 2021;96:106165. [DOI] [PubMed] [Google Scholar]
- 21. Austin P. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biometrical J Biometrische Zeitschrift 2009;51:171–184. [DOI] [PubMed] [Google Scholar]
- 22. Mortensen K, Nilsson M, Slim K, et al. Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Br J Surg 2014;101:1209–1229. [DOI] [PubMed] [Google Scholar]
- 23. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017;20:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Putila E, Helminen O, Helmiö M, et al. Population-based nationwide incidence of complications after gastrectomy for gastric adenocarcinoma in Finland. BJS Open 2023;7:zrad101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu J, Huang C, Sun Y, et al. Effect of laparoscopic vs open distal gastrectomy on 3-year disease-free survival in patients with locally advanced gastric cancer: the CLASS-01 randomized clinical trial. JAMA 2019;321:1983–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li Z, Shan F, Ying X, et al. Assessment of laparoscopic distal gastrectomy after neoadjuvant chemotherapy for locally advanced gastric cancer: a randomized clinical trial. JAMA Surg 2019;154:1093–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lu J, Zheng C, Xu B, et al. Assessment of robotic versus laparoscopic distal gastrectomy for gastric cancer: a randomized controlled trial. Ann Surg 2021;273:858–867. [DOI] [PubMed] [Google Scholar]
- 29. Ojima T, Nakamura M, Hayata K, et al. Short-term outcomes of robotic gastrectomy vs laparoscopic gastrectomy for patients with gastric cancer: a randomized clinical trial. JAMA Surg 2021;156:954–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li Z, Zhou Y, Li T, et al. Robotic gastrectomy versus laparoscopic gastrectomy for gastric cancer: a multicenter cohort study of 5402 patients in China. Ann Surg 2023;277:e87–e95. [DOI] [PubMed] [Google Scholar]
- 31. Shin H, Son S, Wang B, et al. Long-term comparison of robotic and laparoscopic gastrectomy for gastric cancer: a propensity score-weighted analysis of 2084 consecutive patients. Ann Surg 2021;274:128–137. [DOI] [PubMed] [Google Scholar]
- 32. Obama K, Kim Y, Kang D, et al. Long-term oncologic outcomes of robotic gastrectomy for gastric cancer compared with laparoscopic gastrectomy. Gastric Cancer 2018;21:285–295. [DOI] [PubMed] [Google Scholar]
- 33. Son T, Lee J, Kim Y, et al. Robotic spleen-preserving total gastrectomy for gastric cancer: comparison with conventional laparoscopic procedure. Surg Endosc 2014;28:2606–2615. [DOI] [PubMed] [Google Scholar]
- 34. Suda K, Man-I M, Ishida Y, et al. Potential advantages of robotic radical gastrectomy for gastric adenocarcinoma in comparison with conventional laparoscopic approach: a single institutional retrospective comparative cohort study. Surg Endosc 2015;29:673–685. [DOI] [PubMed] [Google Scholar]
- 35. Yang K, Cho M, Roh C, et al. Robotic spleen-preserving splenic hilar lymph node dissection during total gastrectomy for gastric cancer. Surg Endosc 2019;33:2357–2363. [DOI] [PubMed] [Google Scholar]
- 36. Park S, Kim M, Park M, et al. Rapid adaptation of robotic gastrectomy for gastric cancer by experienced laparoscopic surgeons. Surg Endosc 2012;26:60–67. [DOI] [PubMed] [Google Scholar]
- 37. Han T, Kong S, Lee H, et al. Dissemination of free cancer cells from the gastric lumen and from perigastric lymphovascular pedicles during radical gastric cancer surgery. Ann Surg Oncol 2011;18:2818–2825. [DOI] [PubMed] [Google Scholar]
- 38. Marutsuka T, Shimada S, Shiomori K, et al. Mechanisms of peritoneal metastasis after operation for non-serosa-invasive gastric carcinoma: an ultrarapid detection system for intraperitoneal free cancer cells and a prophylactic strategy for peritoneal metastasis.. Clin Cancer Res 2003;9:678–685. [PubMed] [Google Scholar]
- 39. Son T, Hyung W, Lee J, et al. Clinical implication of an insufficient number of examined lymph nodes after curative resection for gastric cancer. Cancer 2012;118:4687–4693. [DOI] [PubMed] [Google Scholar]
- 40. Huang L, Zhang X, Wei Z, et al. Importance of examined lymph node number in accurate staging and enhanced survival in resected gastric adenocarcinoma-the more, the better? A cohort study of 8,696 cases from the US and China, 2010-2016. Front Oncol 2020;10:539030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Briganti A, Bianchi M, Sun M, et al. Impact of the introduction of a robotic training programme on prostate cancer stage migration at a single tertiary referral centre. BJU Int 2013;111:1222–1230. [DOI] [PubMed] [Google Scholar]
- 42. Espinoza-Mercado F, Imai T, Borgella J, et al. Does the approach matter? Comparing survival in robotic, minimally invasive, and open esophagectomies. Ann Thorac Surg 2019;107:378–385. [DOI] [PubMed] [Google Scholar]
- 43. Pan H, Tian Y, Wang H, et al. Perioperative and oncological outcomes of robotic-assisted, video-assisted thoracoscopic and open lobectomy for patients with n1-metastatic non-small cell lung cancer: a propensity score-matched study. Cancers 2022;14:5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nakauchi M, Suda K, Susumu S, et al. Comparison of the long-term outcomes of robotic radical gastrectomy for gastric cancer and conventional laparoscopic approach: a single institutional retrospective cohort study. Surg Endosc 2016;30:5444–5452. [DOI] [PubMed] [Google Scholar]
- 45. Kim M, Kim W, Hyung W, et al. Comprehensive learning curve of robotic surgery: discovery from a multicenter prospective trial of robotic gastrectomy. Ann Surg 2021;273:949–956. [DOI] [PubMed] [Google Scholar]
- 46. Lu J, Wu D, Huang J, et al. Comparison of robotic versus laparoscopic versus open distal gastrectomy for locally advanced gastric cancer: a prospective trial-based economic evaluation. Surg Endosc 2023;37:7472–7485. [DOI] [PubMed] [Google Scholar]
- 47. Fujisaki M, Mitsumori N, Shinohara T, et al. Short-term and long-term outcomes of laparoscopic versus open gastrectomy for locally advanced gastric cancer following neoadjuvant chemotherapy. Surg Endosc 2021;35:1682–1690. [DOI] [PubMed] [Google Scholar]
- 48. Zheng H, Shen L, Xu B, et al. Oncological outcomes of laparoscopic versus open radical total gastrectomy for upper-middle gastric cancer after neoadjuvant chemotherapy: a study of real-world data. Surg Endosc 2023;37:6288–6297. [DOI] [PubMed] [Google Scholar]
- 49. Tian Y, Guo H, Hu Y, et al. Safety and efficacy of robotic-assisted versus laparoscopic distal gastrectomy after neoadjuvant chemotherapy for advanced gastric cancer. Surg Endosc 2023;37:6761–6770. [DOI] [PubMed] [Google Scholar]
- 50. Fiflis S, Papakonstantinou M, Giakoustidis A, et al. Comparison between upfront surgery and neoadjuvant chemotherapy in patients with locally advanced gastric cancer: a systematic review. World J Gastrointest Surg 2023;15:1808–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Huang CM had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Deidentified data about the individual participants will be shared with researchers of further studies on reasonable request. Request for data sharing will be handled in line with the data access and sharing policy of Fujian Medical University Union Hospital.