INTRODUCTION
Undifferentiated pleomorphic sarcoma (UPS) and myxofibrosarcoma (MFS) are the most common histologic subtypes of soft tissue sarcoma (STS) found in the trunk and extremities, together representing approximately 25% of STS diagnosed in these locations. The lesions are rarely identified in the retroperitoneum (Fig. 1). They are generally diagnosed during the sixth and seventh decades and present as a painless mass. Preoperative evaluation includes cross-sectional imaging of the tumor, tissue sampling to establish diagnosis and staging to rule out metastases, particularly pulmonary disease. Surgery forms the backbone of therapeutic algorithms for localized disease, although adjuvant radiation plays a role in preventing local recurrence in many patients and neoadjuvant chemotherapy can be considered in a subset of high-risk cases. Systemic therapies for advanced disease include cytotoxic regimens based on, among others, doxorubicin and novel therapeutics such as PD-L1 inhibitors.
Fig. 1.
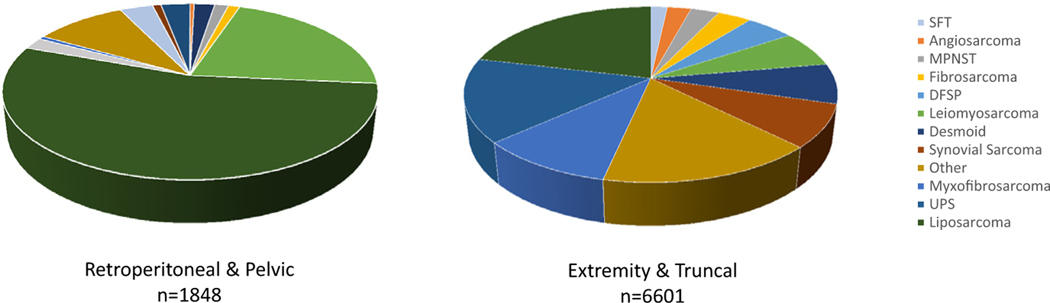
Histologic classification of STS tumors diagnosed in patients admitted to MSKCC between 1982 and 2021. Liposarcoma, leiomyosarcoma, solitary fibrous tumor, and malignant peripheral nerve sheath tumor represent common histologies in the retroperitoneum and pelvis. UPS/MFH, myxofibrosarcoma, synovial sarcoma, and dermatofibrosarcoma protuberans represent a greater proportion of those tumors diagnosed in the extremity and trunk.
PATIENT EVALUATION OVERVIEW
Histologic Diagnosis
Core biopsy and histologic evaluation by an expert STS pathologist establishes the diagnosis of UPS/MFS. UPS, which represents a diagnosis of exclusion, was previously termed malignant fibrous histiocytoma (MFH). The term historically encompassed a catch-all for many of the high-grade, poorly differentiated STS with large and irregular nuclei. Although UPS may still represent a heterogeneous group of lesions with variable outcomes, differing progenitor cells and complex karyotypes, modern genomic analysis and immunohistochemical staining techniques have allowed for the term to be more narrowly defined. Many MFH described in historic series were likely dedifferentiated liposarcomas, malignant peripheral nerve sheath tumors, poorly differentiated leiomyosarcomas, or malignant solitary fibrous tumors, each of which can seem similar to UPS microscopically. Clinical findings as well as directed staining for markers such as MDM2 and CDK4, loss of H2k27me3, smooth muscle markers such as SMA, desmin, and caldesmin, and nuclear STAT6 can rule out these respective histologies.1–4
Genomic analysis shows significant similarity between UPS and MFS, previously termed myxoid MFH. Both histologies are commonly characterized by complex genomic karyotypes. Loss of TP53, RB, and PTEN are consistently reported copy number alterations observed in at least 10% to 20% of UPS and MFS; mutations in these genes are also identified in both tumor types.5–8 Copy number alterations affect additional cell cycle regulators such as CCNE1 and mutations have been observed in ATRX, which control telomere length and cellular senescence.5 Despite these genetic similarities, on histologic evaluation, MFS differs from UPS in that it is associated with an infiltrative and multinodular growth pattern, myxoid stroma in portions of the tumor, spindled and vacuolated cells, and distinctive curvilinear vessels (Fig. 2). The tumors can be high grade or low grade, defined in large part by tumor cellularity, whereas UPS is almost uniformly high grade.9,10
Fig. 2.
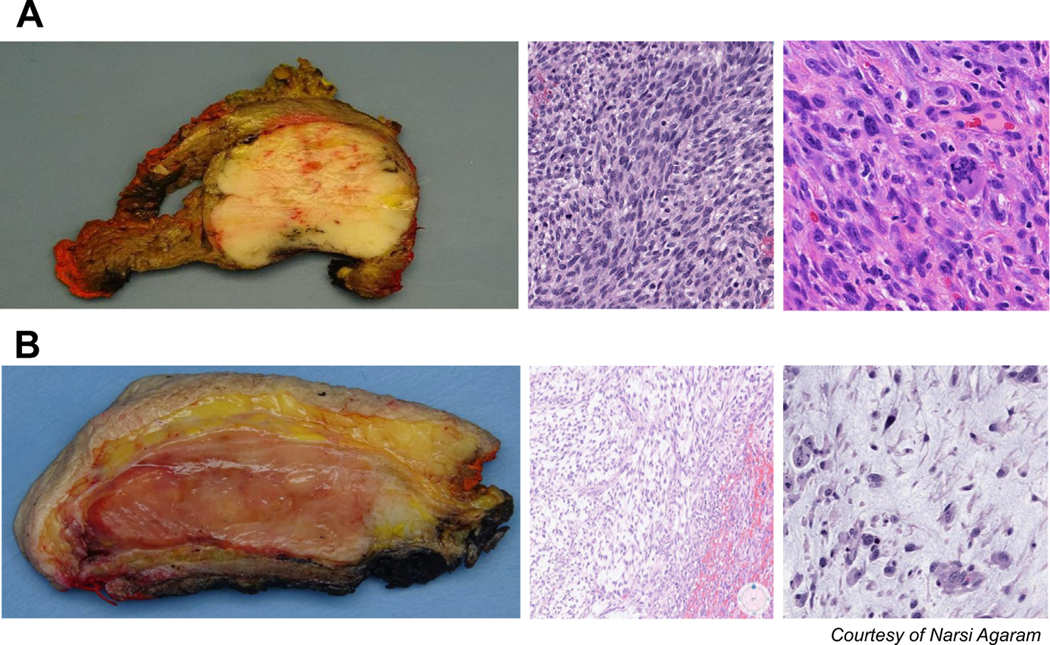
Gross and microscopic images depicting a representative (A) UPS and (B) MFS. The latter has a myxoid appearance on gross specimens, and thin, pale, myxoid stroma with curvilinear vessels. UPS is characterized by densely packed cells with large, pleomorphic nuclei, and numerous mitotic bodies.
Outcome is Determined by Histology
Early analysis of MFH outcomes showed poor prognosis with high rates of distal metastases. In a study of 100 patient diagnosed with MFH before 2001, 5-year distant recurrence free survival (DRFS) was only 64%.11 A similar study analyzing 239 patients undergoing complete surgical resection of MFH between 1982 and 1996 had a 5-year disease-specific survival (DSS) of 65%.12 Commonly described risk factors, such as tumor size more than 10 cm, were associated with poor outcomes in this study. Because more precise subclassification of pleomorphic sarcomas was described, risk was also shown to be closely associated with presumed histologic origin. For example, in a study by Fletcher and colleagues, 30 of the MFH were reclassified as having myogenic origin (eg, pleomorphic rhabdomyosarcoma, leiomyosarcoma), a finding associated with significantly worse outcomes as compared with the group as a whole.11
There is some debate in the literature regarding the percent of the tumor that is required to be myxoid to define a tumor as being an MFS as opposed to UPS and how this in turn may associate with prognosis. A modern series of UPS and MFS was examined in attempt to define the histologic characteristics of each tumor and describe outcomes more precisely after classification using updated ancillary pathology techniques. Tumors were resected between 1992 and 2013 and meticulous rereview of slides was performed to exclude subtypes of pleomorphic sarcoma other than UPS and MFS.10 The percent of each UPS or MFS that was composed of myxoid stroma was characterized and cut point analysis was used to determine thresholds defining subgroups of the cancers with significant differences in outcome. Tumors with less than 5% myxoid stromal were associated with a DSS of only 36% (vs 60% for those with ≥5% myxoid component). The authors suggested that such a cut point would be appropriate to differentiate UPS and MFS for clinical decision-making. A second subgroup of MFS, defined by those tumors with greater than 70% myxoid content, was associated with particularly good prognosis and a DSS of 66% 5 years after resection (compared with 52% for tumors with 5%–69% myxoid component).
This analysis did not show a significant difference in local recurrence free survival (LRFS) between UPS and MFS but, historically, MFS has been thought to be associated with high rates of recurrent disease (greater than 25% local recurrence risk 5 years after surgery). This finding is thought to be related to its infiltrative nature and has been correlated in multiple studies with close or microscopically positive margins.13–15 Up to half of these recurrences can be observed outside the direct surgical or radiation field given the multinodular growth pattern of MFS.16 Second local recurrences can occur in more than half of those patients after the initial recurrence with multiple recurrences historically requiring amputation even at specialty centers.17 Of note, local recurrence is more commonly observed in high-grade tumors as opposed to low-grade tumors, although this may be a function of a more rapid rate of recurrence in high-grade tumors. Low-grade tumors often recur as high-grade disease associated with an increased risk of distal metastases.9
Imaging Work-Up
Distant metastases in high-grade UPS/MFS are most commonly identified in the lung; therefore, staging with chest X-ray or CT is generally sufficient before the treatment of localized disease. Cross-sectional MRI is ideal for defining the extent of local disease before planned resection (Fig. 3). UPS often presents as a heterogeneously enhancing mass in the soft tissues. Signals on T1 sequences are similar to those observed for muscle. Central regions of hyperintensity on T2 sequences can represent necrosis or hemorrhage. This can sometimes lead to misdiagnosis of benign hematoma. Although this may be a reasonable consideration in patients prescribed anticoagulants or with history of acute injury, even in these cases, careful evaluation to rule out peripheral nodularity (ie, with subtraction imaging and diffusion weighted imaging on MRI) and consideration of biopsy to rule out occult malignancy should be considered before any attempt at draining the lesion is performed.
Fig. 3.
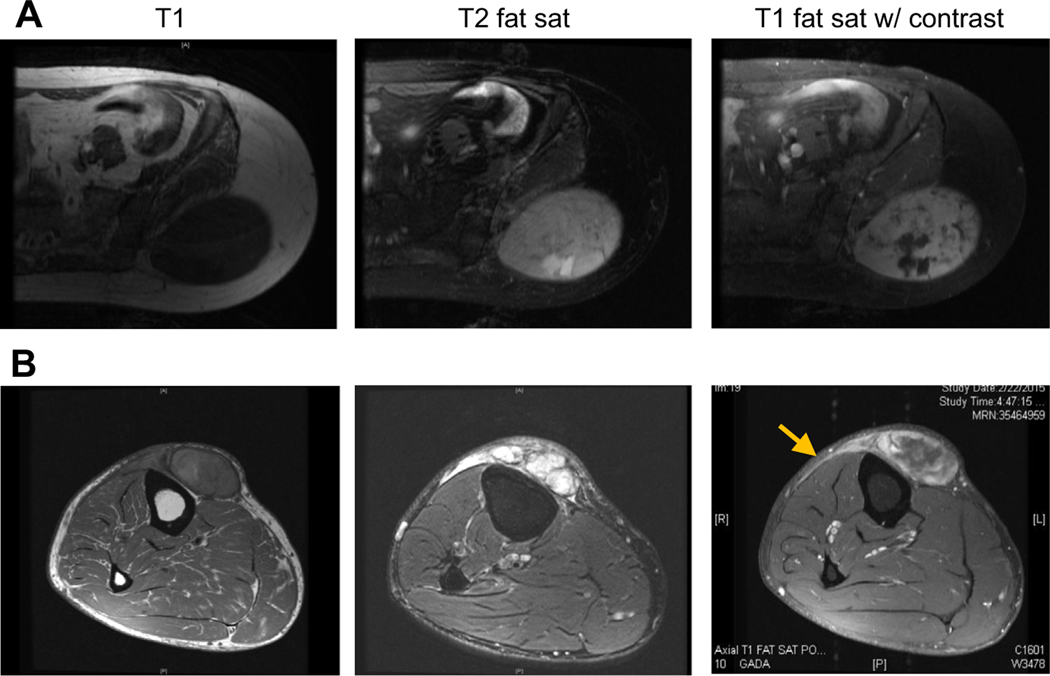
Although both (A) UPS and (B) MFS seem similar on T1 sequences, MFS is hyperintense on T2 sequences and can have infiltrative, enhancing tails that extend outward from gross disease (arrow).
Appearance of MFS on MRI imaging is similar to UPS on T1 sequences but on T2 sequences, the lesions are hyperintense, reflecting the myxoid component in the tumors. It is common, particularly in tumors located in the superficial tissues and along fascial planes between muscle compartments to observe multifocality of gross tumor nodules and infiltrative “tails,” which can represent microscopic extension of the tumor from areas of gross disease. These “tails” can be differentiated from edema, also hyperintense on T2 sequences, on postcontrast images. MFS-associated “tails” enhance but tumor-associated edema does not. Identification of these curvilinear enhancing “tails” can also be helpful in differentiating MFS from other subtypes of myxoid neoplasm (eg, intramuscular myxoma; see Fig. 3).18,19
SURGICAL TREATMENT OPTIONS
Retrospective clinical trials have shown a clear association between complete R0 resection and decreased rates of local recurrence in UPS and MFS. For example, in 425 patients treated by the French National Group, the 5-year local recurrence free rate after R1 resection was 51.6% versus 75.6% after R0 resection.13 Given such findings, the goal of surgery in UPS/MFS should generally be complete microscopic resection.
As for most high-grade sarcomas, R0 resection of UPS is generally attained by resecting a 1 cm margin of normal tissue around the lesion or removing adjacent fascial margins. In the extremity and trunk, obtaining adequate margins may be difficult due to proximity to neurovascular bundles. When major nerves or vessels are encased by high-grade tumors, these require en bloc resection with the tumor. Arteries can be reconstructed when distal ischemia is a concern. Morbidity related to resection of major nerves can be mitigated by bracing or, in the upper extremity, tendon transfer. Encasement of major neurovascular bundles is rare, however. In most cases, tumors instead displace the structures, and resection is performed by removing the perineurium and vascular sheath. Risk of local recurrence resulting from microscopically positive margins is accepted because local recurrences do not seem to negatively affect the survival and can be mitigated with adjuvant or neoadjuvant radiation.
The infiltrative nature of MFS requires modification of surgery with 2 cm margins resected en bloc with the tumor where feasible. The planned resection bed should include enhancing tails that are visualized during imaging work-up. As previously noted, these tails represent microscopic tumor extension and if not removed increase risk of microscopically positive margins and local recurrence. Such surgery may require the removal of significant portions of skin or create large areas of “dead space” that affect healing; hence, complex reconstruction with rotational or free flaps may be required to reconstruct the operative defect. This reconstruction may be performed in a delayed fashion to confirm by formal pathologic review that microscopically negative margins have been obtained. After initial resection, a vacuum-assisted closure is placed and reconstruction performed as a second procedure after permanent histologic sections have been reviewed. In a series of 53 MFS patients, delayed closured after resection and VAC placement was associated with a lower rate of local recurrence when compared with outcomes in patients treated with immediate reconstruction.20
Careful surgical planning and improved understanding of the biology of MFS has resulted in improved rates of local recurrence. In modern series of patients treated at major sarcoma centers are reported to range from 18% to 25%.10,13,15 These rates are not significantly different than those seen in modern series of the less infiltrative UPS histology.
ADJUVANT THERAPIES
Adjuvant and Neoadjuvant radiation
Decisions regarding the role of adjuvant radiation in treatment of UPS/MFS are generally based on the outcomes of a randomized phase III trial examining limb-sparing surgery after radiation. In the classic study, Yang and colleagues estimated risks of local recurrence after adjuvant radiation were reduced from 22% to 0% 10 years after STS resection. The study did not show that radiation affects overall survival (OS), so adjuvant treatment can be deferred in patients where morbidity associated with radiation may be high or in instances where risk of local recurrence may be low at baseline. For example, prospective data obtained after observation of small, high-grade tumors (T1, <5 cm) showed baseline rates of local recurrence were 7.6% 5 years after R0 resection, a rate that may not justify risk of radiation if recurrence could be managed with salvage surgery.21 Similarly, a range of retrospective studies has associated low-grade lesions with reduced risk of local recurrence, so observation may be appropriate after resection of these tumors. Baseline risk of local recurrence in STSs can be calculated using a nomogram recently published by Cahlon and colleagues.22
Detailed analysis of radiation use in MFS has been performed given its infiltrative nature and associated risk of local recurrence and close or microscopically positive margins after resection. A subset of these studies have shown radiation to be associated with improved rates of local recurrence in a subset (though not all) retrospective analyses.13 Selection bias may be the cause of variable results. Radiation would, therefore, generally be applied to patients with larger, high-grade tumors with microscopically positive margins as opposed to those with low-grade lesions resected with wide margins where salvage of local recurrence would not be morbid. Such an argument is strengthened by results presented in Mutter and colleagues. Although rates of microscopically positive margins were higher after resections for MFS than a control group of leiomyosarcomas, use of adjuvant radiation was more common in patients with MFS and 5-year LRFS rates were similar in both cohorts (14.6 vs 13.2%, respectively), suggesting the use of adjuvant radiation can optimize local control and compensate for the locally aggressive behavior of MFS.16
Adjuvant Chemotherapy
There is growing evidence of the efficacy of adjuvant, doxorubicin-based chemotherapy in a patient with high-risk STS including a subset of UPS/MFS. Retrospective analyses have shown associations between improved outcomes and the use of adjuvant anthracyclines and ifosfamide in large, high-grade sarcomas and in an older meta-analysis of 14 studies using perioperative chemotherapy, a modest benefit was observed in patients receiving adjuvant therapy (4% improvement in OS after 10 years). An update of this meta-analysis included 4 additional trials in which doxorubicin dosing was intensified and combinations with ifosfamide were used. This report showed an absolute risk reduction of death of 11% associated with adjuvant doxorubicin and ifosfamide (AI; 41% versus 30% in patients who did not receive adjuvant therapy.23
Recently, the results of the negative EORTC-STBSG 62931 trial (assessing the use of 5 cycles of AI vs no preoperative chemotherapy) were also reanalyzed to examine this question. Specifically, the subset of patients with predicted OS less than 60% 10 years after treatment (as calculated using the Sarculator nomogram that integrates risk based on characteristics such as age, histology, tumor size, and grade) were studied. The revision showed that in these patients, the use of preoperative chemotherapy was associated with significant increase in disease-free survival (DFS; HR 5 0.49) and OS (HR 5 0.50)24,25 In EORTC-STBSG 62931, patients with MFH/UPS accounted for 22% of the chemotherapy cohort and 33% of the control population. No benefit has been seen in the prescription of histology-specific regimens (eg, gemcitabine and docetaxel for UPS) as opposed to standard AI in a prospective trial (ISG-STS 1001) of neoadjuvant therapy and AI chemotherapy was related to improved OS and DFS.26 However, the use of preoperative AI in high-risk STS patients in ISG-STS 1001 was again associated with a better survival than predicted by Sarculator nomogram, which adds the additional evidence for the efficacy of neoadjuvant AI in this patient population.27
UPS/MFS greater than 10 cm have generally been associated with metastatic risk significant enough to consider systemic therapy, although risk stratification may be improved using nomograms that integrate multiple tumor and patient characteristics. The results presented in Lee and colleagues, also suggest that in UPS/MFS, patient selection may be further tailored by considering the percent myxoid component of the tumor specifically when considering UPS/MFS as UPS defined by a percent tumor myxoid component of less than 5%, risks of death from disease have been reported to be as high as 64%, so that adjuvant chemotherapy may be of benefit in select patients even with tumors 5 to 10 cm in diameter. For tumors with significant myxoid components (>70%), 5-year DRFS is 65% (vs 24% for tumors with <5% myxoid stroma), so that the treatment may not be associated with as significant a benefit and should be reserved for patients with larger tumors per standard protocols.
MEDICAL TREATMENT OPTIONS FOR ADVANCED DISEASE
Doxorubicin used alone or in combination with ifosfamide remains the standard first-line systemic treatment in patients with metastatic, locally advanced unresectable STS including UPS/MFS.28 In a randomized study by Judson and colleagues, doxorubicin in monotherapy was compared with a combination of AI in the first-line palliative treatment of patients with high-grade STS. There was no significant difference in OS between both groups (median OS 12.8 for doxorubicin vs 14.3 months in AI) but median progression-free survival (PFS) was significantly higher for the AI group than for the doxorubicin group (7.4 vs 4.6 months) and more patients in the combination arm had an overall response (26% vs 14%). Combination chemotherapy was associated with a significantly higher risk of grade 3/4 side effects of the treatment, however. Based on the results of this study, intensive combination chemotherapy ought to be prescribed when the aim for treatment is tumor shrinkage to provide relief from symptoms or before possible surgical excision of the metastases whereas single agent doxorubicin may be more appropriate for palliating asymptomatic patents.29
Alternative regimens include gemcitabine used alone or in combination, especially with docetaxel. In a study by Maki and colleagues of 19 patients diagnosed with UPS, 32% had documented responses and median PFS was 6.2 months in patients treated with gemcitabine–docetaxel.30 The GeDDis trial compared gemcitabine plus docetaxel with doxorubicin alone in the first-line treatment of palliative therapy in 257 patients diagnosed with STS, 12% to 13% of whom were diagnosed with UPS. PFS after 24 weeks (the primary endpoint) was 46% in both arms though OS was slightly better for doxorubicin. Gemcitabine plus docetaxel was more toxic and harder to administer than doxorubicin, so the combined regimen remains second line therapy in patients with metastatic UPS.31 Gemcitabine can also be combined with dacarbazine; in a Spanish study comparing gemcitabine plus dacarbazine versus dacarbazine alone, UPS patients were 19 of the 113 included patients. Median PFS was 4.2 months for combination versus 2 months for dacarbazine monotherapy (hazard ratio 0.58) and median OS was 16.8 months versus 8.2 months.30 Targeted therapy was considered in the PALETTE trial, a randomized, placebo-controlled trial assessing the efficacy of pazopanib, a multikinase inhibitor, in patients with previously treated advanced STS including UPS/MFS. The drug prolonged PFS by 3 months when compared with placebo, and thus it can be considered for patients with metastatic UPS/MFS.32
A promising treatment option for advanced UPS/MFS is immunotherapy; it is one of the few STS subtypes with noted responses to treatment and prolonged survival. A large study of immunotherapy in sarcomas was published in 2017 (SARC028 Trial). This two-cohort, single-arm, open-label, phase 2 study enrolled 86 patients with STS or bone sarcoma. Ten patients with UPS were included. Seven (18%) of 40 patients with STS had an objective response, including 4 (40%) of 10 patients with UPS. Responses were reasonably durable, with a median duration of response being 33 weeks (median PFS for UPS cohort 30 weeks). The median OS for patients with STS was 49 weeks (95% CI 34–73). The median OS for patients with UPS had not been reached at the time of analysis.33 An expansion cohort increasing the number of UPS patients examined to 40 was reported at the American Society of Clinical Oncology conference in 2019. Overall response rate (by RECIST v1.1) in the UPS cohort was 23% (9/40), median PFS was 3 months, 12-week PFS rate was 50%, and median OS of 12 months.34
Combination of nivolumab and ipilimumab was also studied sarcoma patients. An open-label, unblinded, noncomparative, multicenter randomized phase II study enrolled 96 patients, who received either nivolumab or nivolumab and ipilimumab. Patients were heavily pretreated, with 61% having received at least 3 prior chemotherapy lines. Median overall response rates were 5% in the nivolumab monotherapy group and 16% in the combination arm with responses observed in UPS/MFS patients treated with a combination of nivolumab and ipilimumab.35
FUTURE DIRECTIONS
Recent trials have established a role for immunotherapy in the third-line setting for patients with advanced disease and ongoing studies are examining potential predictive markers and neoadjuvant approaches such as prescription in combination with radiation. For example, results of immunotherapy in a preoperative setting were presented on ASCO 2020. A randomized, phase II noncomparative trial was presented evaluating the efficacy of 3 to 4 cycles of neoadjuvant nivolumab or a combination of ipilimumab/nivolumab in patients with resectable retroperitoneal dedifferentiated liposarcoma or extremity/truncal UPS. Median pathologic response in 9 UPS patients (all of whom received concurrent neoadjuvant radiation) was 95%. Given association of pathologic response and ultimate outcome in STS, this is a promising finding.36
It is clear that rates and durability of response to immunotherapy may be less impressive than in other cancer types, so parallel genomic and translational studies continue to examine the role of targeted therapy in these diseases. The TCGA analysis confirmed not only mutations and copy number alterations in TP53, RB1, and PTEN affecting UPS and MFS but noted common copy number alterations affecting Hippo pathways components VGLL3 and YAP1.5 A second study examining MFS independently identified a high-risk subset of lesions characterized by increased expression of ITGA10. The gene product integrin α−10 was shown to interact with Rictor and TRIO, both encoded on a chromosome 5 amplicon to activate the oncogenic RAC/PAK and AKT/mTOR pathways.37 This suggests a basis for clinical trials to examine inhibitors of these pathways in patients with advanced disease.
KEY POINTS.
Undifferentiated pleomorphic sarcoma (UPS) and myxofibrosarcoma (MFS) are genomically complex tumors commonly diagnosed in the extremity and trunk of older patients.
Although genomically similar, the presence of myxoid stroma is associated with the diagnosis of MFS as opposed to UPS; tumors with highest proportion of myxoid stroma are associated with the lowest rates of metastasis in MFS/UPS.
MFS has a locally aggressive phenotype. Preoperative assessment with MRI allows for the identification of infiltrative tumor “tails” that are resected en bloc with dominant tumor nodules to minimize local recurrence.
Adjuvant radiation mitigates risks of local recurrence in UPS/MFS, and there is growing evidence of survival benefit associated with the use of perioperative chemotherapy in patients with high-risk of metastatic disease including those with larger UPS/MFS.
Treatments for advanced disease include anthracycline-based therapies in the first-line treatment and gemcitabine–docetaxel combination; recent research demonstrates the efficacy of immunotherapy in a proportion of patients
CLINICS CARE POINTS.
Core biopsy should be performed to establish the diagnosis of undifferentiated pleomorphic sarcoma (UPS)/myxofibrosarcoma (MFS).
Myxoid stroma, fibrous septa, and curvilinear vessels suggest a diagnosis of MFS; low-grade and high-grade forms are observed. UPS is an intermediate-grade or high-grade lesions composed of irregular, pleomorphic cells seen in several sarcomas, so ancillary techniques should be used to rule out histologies such as liposarcoma, leiomyosarcoma, malignant peripheral nerve sheath tumor, and solitary fibrous tumor.
Cross-sectional MRI of the primary lesion is performed in parallel with pulmonary staging and can be useful particularly in MFS to delineating nonpalpable tumor “tails” that extend outward from the gross nodules.
Surgical resection of UPS should be performed with 1 cm margins or with resection of adjacent fascia. Adjacent, but not encased, neurovascular bundles can be preserved by resection of the perineurium and vascular sheath.
The locally aggressive nature of MFS means that 2 cm margins should be planned when morbidity is not prohibitive and tumor “tails” identified on MRI should be resected en bloc with the primary lesion.
If R1 resection of high-grade tumors is planned to reduce minimize surgical morbidity, adjuvant or neoadjuvant radiation can be used to mitigate risk of local recurrence.
Neo-/adjuvant chemotherapy should be considered in patients with tumors at high-risk for metastasizing (high-grade MFS more than 10 cm in size and UPS at least 5 cm in greatest diameter).
Advanced disease is generally treated with anthracycline-based regimens (the first-line therapy) or gemcitabine/docetaxel combinations. Pazopanib can be considered in progressive cases, anti-PD1 demonstrates promising activity in proportion of patients.
DISCLOSURE
A.M. Crago: Advisory Board, Springworks Therapeutics. K. Cardona: none to report. H. Kosela-Paterczyk: none to report. P. Rutkowski: honoraria for lectures and Advisory Boards from BMS < MSD, Novartis, Pierre Fabre, Sanofi, Merck, and Blueprint Medicines outside of the scope of this study. A.Crago - NIH/NCI P50-CA217694, P30-CA008748.
REFERENCES
- 1.Prieto-Granada CN, Wiesner T, Messina JL, Jungbluth AA, Chi P, Antonescu CR. Loss of H3K27me3 Expression Is a Highly Sensitive Marker for Sporadic and Radiation-induced MPNST. Am J Surg Pathol 2016;40(4):479–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheah AL, Billings SD, Goldblum JR, Carver P, Tanas MZ, Rubin BP. STAT6 rabbit monoclonal antibody is a robust diagnostic tool for the distinction of solitary fibrous tumour from its mimics. Pathology 2014;46(5):389–95. [DOI] [PubMed] [Google Scholar]
- 3.Doyle LA, Vivero M, Fletcher CD, Mertens F, Hornick JL. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol 2014;27(3):390–5. [DOI] [PubMed] [Google Scholar]
- 4.Dry SMaF, Leiomyosarcoma S. WHO classification of Tumours of soft tissue and bone. 5th edition. IARC; 2020. p. 195–7. [Google Scholar]
- 5.Cancer Genome Atlas Research Network. Electronic address edsc, Cancer Genome Atlas Research N. Comprehensive and Integrated Genomic Characterization of Adult Soft Tissue Sarcomas. Cell 2017;171(4):950–965 e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barretina J, Taylor BS, Banerji S, et al. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat Genet 2010;42(8):715–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chibon F, Mairal A, Freneaux P, et al. The RB1 gene is the target of chromosome 13 deletions in malignant fibrous histiocytoma. Cancer Res 2000;60(22):6339–45. [PubMed] [Google Scholar]
- 8.Perot G, Chibon F, Montero A, et al. Constant p53 pathway inactivation in a large series of soft tissue sarcomas with complex genetics. Am J Pathol 2010;177(4): 2080–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mentzel T, Calonje E, Wadden C, et al. Myxofibrosarcoma. Clinicopathologic analysis of 75 cases with emphasis on the low-grade variant. Am J Surg Pathol 1996;20(4):391–405. [DOI] [PubMed] [Google Scholar]
- 10.Lee AY, Agaram NP, Qin LX, et al. Optimal Percent Myxoid Component to Predict Outcome in High-Grade Myxofibrosarcoma and Undifferentiated Pleomorphic Sarcoma. Ann Surg Oncol 2016;23(3):818–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fletcher CD, Gustafson P, Rydholm A, Willen H, Akerman M. Clinicopathologic reevaluation of 100 malignant fibrous histiocytomas: prognostic relevance of subclassification. J Clin Oncol 2001;19(12):3045–50. [DOI] [PubMed] [Google Scholar]
- 12.Salo JC, Lewis JJ, Woodruff JM, Leung DH, Brennan MF. Malignant fibrous histiocytoma of the extremity. Cancer 1999;85(8):1765–72. [PubMed] [Google Scholar]
- 13.Boughzala-Bennadji R, Stoeckle E, Le Pechoux C, et al. Localized Myxofibrosarcomas: Roles of Surgical Margins and Adjuvant Radiation Therapy. Int J Radiat Oncol Biol Phys 2018;102(2):399–406. [DOI] [PubMed] [Google Scholar]
- 14.Look Hong NJ, Hornicek FJ, Raskin KA, et al. Prognostic factors and outcomes of patients with myxofibrosarcoma. Ann Surg Oncol 2013;20(1):80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanfilippo R, Miceli R, Grosso F, et al. Myxofibrosarcoma: prognostic factors and survival in a series of patients treated at a single institution. Ann Surg Oncol 2011; 18(3):720–5. [DOI] [PubMed] [Google Scholar]
- 16.Mutter RW, Singer S, Zhang Z, Brennan MF, Alektiar KM. The enigma of myxofibrosarcoma of the extremity. Cancer 2012;118(2):518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haglund KE, Raut CP, Nascimento AF, Wang Q, George S, Baldini EH. Recurrence patterns and survival for patients with intermediate- and high-grade myxofibrosarcoma. Int J Radiat Oncol Biol Phys 2012;82(1):361–7. [DOI] [PubMed] [Google Scholar]
- 18.Lefkowitz RA, Landa J, Hwang S, et al. Myxofibrosarcoma: prevalence and diagnostic value of the “tail sign” on magnetic resonance imaging. Skeletal Radiol 2013;42(6):809–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waters B, Panicek DM, Lefkowitz RA, et al. Low-grade myxofibrosarcoma: CTand MRI patterns in recurrent disease. AJR Am J roentgenology 2007;188(2):W193–8. [DOI] [PubMed] [Google Scholar]
- 20.Fourman MS, Ramsey DC, Kleiner J, et al. Temporizing Wound VAC Dressing Until Final Negative Margins are Achieved Reduces Myxofibrosarcoma Local Recurrence. Ann Surg Oncol 2021. 10.1245/s10434-021-10242-4. [DOI] [PubMed] [Google Scholar]
- 21.Pisters PW, Pollock RE, Lewis VO, et al. Long-term results of prospective trial of surgery alone with selective use of radiation for patients with T1 extremity and trunk soft tissue sarcomas. Ann Surg 2007;246(4):675–81. [DOI] [PubMed] [Google Scholar]
- 22.Cahlon O, Brennan MF, Jia X, Qin LX, Singer S, Alektiar KM. A postoperative nomogram for local recurrence risk in extremity soft tissue sarcomas after limbsparing surgery without adjuvant radiation. Ann Surg 2012;255(2):343–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 2008;113(3): 573–81. [DOI] [PubMed] [Google Scholar]
- 24.Pasquali S, Pizzamiglio S, Touati N, et al. The impact of chemotherapy on survival of patients with extremity and trunk wall soft tissue sarcoma: revisiting the results of the EORTC-STBSG 62931 randomised trial. Eur J Cancer 2019;109:51–60. [DOI] [PubMed] [Google Scholar]
- 25.Callegaro D, Miceli R, Bonvalot S, et al. Development and external validation of two nomograms to predict overall survival and occurrence of distant metastases in adults after surgical resection of localised soft-tissue sarcomas of the extremities: a retrospective analysis. Lancet Oncol 2016;17(5):671–80. [DOI] [PubMed] [Google Scholar]
- 26.Gronchi A, Ferrari S, Quagliuolo V, et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol 2017;18(6):812–22. [DOI] [PubMed] [Google Scholar]
- 27.Pasquali S, Palmerini E, Quagliuolo V, et al. Neoadjuvant chemotherapy in highrisk soft tissue sarcomas: A Sarculator-based risk stratification analysis of the ISG-STS 1001 randomized trial. Cancer 2022;128(1):85–93. [DOI] [PubMed] [Google Scholar]
- 28.Casali PG, Abecassis N, Aro HT, et al. Soft tissue and visceral sarcomas: ESMOEURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29(Suppl 4):iv51–67. [DOI] [PubMed] [Google Scholar]
- 29.Judson I, Verweij J, Gelderblom H, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol 2014; 15(4):415–23. [DOI] [PubMed] [Google Scholar]
- 30.Maki RG, Wathen JK, Patel SR, et al. Randomized Phase II Study of Gemcitabine and Docetaxel Compared With Gemcitabine Alone in Patients With Metastatic Soft Tissue Sarcomas: Results of Sarcoma Alliance for Research Through Collaboration Study 002. J Clin Oncol 2007;25(19):2755–63. [DOI] [PubMed] [Google Scholar]
- 31.Seddon B, Strauss SJ, Whelan J, et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): a randomised controlled phase 3 trial. Lancet Oncol 2017;18(10):1397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379(9829):1879–86. [DOI] [PubMed] [Google Scholar]
- 33.Tawbi HA, Burgess M, Bolejack V, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol 2017;18(11):1493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burgess MA, Bolejack V, Schuetze S, et al. Clinical activity of pembrolizumab (P) in undifferentiated pleomorphic sarcoma (UPS) and dedifferentiated/pleomorphic liposarcoma (LPS): Final results of SARC028 expansion cohorts. J Clin Oncol 2019;37(15_suppl):11015. [Google Scholar]
- 35.D’Angelo SP, Mahoney MR, Van Tine BA, et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, noncomparative, randomised, phase 2 trials. Lancet Oncol 2018;19(3):416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roland CL, Keung EZ-Y, Lazar AJ, et al. Preliminary results of a phase II study of neoadjuvant checkpoint blockade for surgically resectable undifferentiated pleomorphic sarcoma (UPS) and dedifferentiated liposarcoma (DDLPS). J Clin Oncol 2020;38(15_suppl):11505. [Google Scholar]
- 37.Okada T, Lee AY, Qin LX, et al. Integrin-alpha10 Dependency Identifies RAC and RICTOR as Therapeutic Targets in High-Grade Myxofibrosarcoma. Cancer Discov 2016;6(10):1148–65. [DOI] [PMC free article] [PubMed] [Google Scholar]


