ABSTRACT
Although the SLC22A12 (uric acid transporter 1) 258X allele is known to cause hypouricemia, the genotype frequency according to the serum uric acid (SUA) level has not been reported. This study investigated the SLC22A12 258WX frequency according to SUA levels among Japanese health-checkup examinees. In addition, the changes were reported in SUA levels during five years for individuals with 258WX. Subjects were 746 Japanese aged 39–86 years in 2003. Their SUA records were linked during the five years from 2003 to 2007. SLC22A12 W258X was genotyped using a polymerase chain reaction with confronting two-pair primers. The 258X allele comprised 1.9% (95% CI, 1.3–2.8%) of all the subjects. Among those with SUA <3.0 mg/dL, 258WX was more common in males (66.7%, 95% CI, 22.2–95.7%) than in females (39.3%, 95% CI, 21.5–59.4%). Among subjects with a SUA of 3.0–4.9 mg/dL, those with 258WX totaled 10.7% (95% CI, 4.0–21.9%) and 2.6% (95% CI, 1.1–5.0%), respectively. There were no subjects with 258WX among those with a SUA of 5.0 mg/dL or more. During the five years from 2003 to 2007, the changes in SUA among 23 individuals with 258WX were found to be similar to those among 258WW subjects (n=536). This study indicated that SLC22A12 258WX was more common among those with a lower serum uric acid concentration. The observed SUA level changes in individuals with 258WX suggested that lifestyle factors could influence the levels of those with 258WX.
Key Words: Hypouricemia, Urate transporter, SLC22A12 W258X, Polymerase chain reaction with confronting two-pair primers
INTRODUCTION
Although idiopathic hypouricemia is clinically asymptomatic, it is associated with the risks of acute renal failure,1,2) urolithiasis,1,3,4) and hematuria.5) While there is no doubt that lifestyle affects serum uric acid (SUA) levels, genetic traits are the factors that determine SUA levels.
Uric acid transporter 1 (URAT1) encoded by SLC22A12 is a uric acid anion exchanger that reabsorbs uric acid in renal tubules.6,7) SLC22A12 genotypes with a reduced function have been reported to cause renal hypouricemia. To date, the 16 polymorphisms so far reported in SLC22A12 are: 11 missense polymorphisms (R90H, V138M, G164S, T127M, A226V, R228E, E298D, Q312L, Q382L, M430T, and R477H), two nonsense polymorphisms (W258X and Q297X), two short deletions (1639-1643delGTCCT, del313D-333P), and one splicing polymorphism (IVS2+1 G>A).8-11) The W258X polymorphism, which produces a non-functioning truncated protein that lacks half of the mature protein, is predominant among Japanese patients with hypouricemia; in one study, six (85.7%) out of seven patients had a 258X allele,8) while the allele was found in 29 (90.6%) out of 32 in another study.9) Among 30 Korean hypouricemic examinees, seven (23.3%) were found to have a 258X allele.12)
Although the SLC22A12 W258X frequencies among those with hypouremia were reported as above, the frequency according to SUA levels in a general population remains unknown. Furthermore, the changes in SUA among those with 258WX have yet to be reported. The present study investigated the frequency of SLC22A12 W258X polymorphism according to the SUA levels among Japanese health checkup examinees. In addition, the changes in SUA levels of individuals with 258WX were examined to document the possible effects of lifestyle on the SUA.
MATERIALS AND METHODS
Subjects
Subjects were 864 residents in a rural area of Hokkaido, Japan, who attended a health checkup in August, 2003. Among them, 803 subjects (280 males and 523 females) aged 39–86 years agreed to participate in this study along with written informed consent. After excluding 57 examinees who reported being under medication for gout, or with blood urea nitrogen greater than 23 mg/dL, or with creatinine greater than 1.1 mg/dL, the remaining 746 subjects (238 males and 508 females) were deemed eligible for the present analysis. This study was approved by the Ethics Committee of the Nagoya University School of Medicine (approval number 398).
Data collection
Using a self-administered questionnaire, their health and lifestyle including habits of smoking (never, former, or current) and drinking (never, former, or current) were obtained at the time of their health checkup. The resulting health checkup data, including blood tests, were used for this study. Peripheral blood was drawn in the morning from those fasting overnight, or in the afternoon from those without lunch. Biochemical analysis of the sampled sera was performed using an auto-analyzer (JCA-RX20, Nihon Denshi Co. Ltd.), and SUA levels ware measured by the uricase-POD method. Body height and weight were measured during the health checkup, with the body mass index (BMI) calculated from body weight (kg) divided by height (m) squared. The changes in SUA levels were followed for subjects who attended the annual health checkup during the five years from 2003 to 2007.
Genotyping procedure
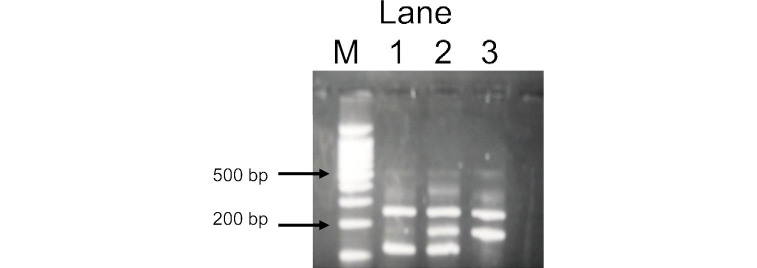
DNA was extracted from a buffy coat conserved at –80°C using a BioRobot® EZ1 (QIAGEN Group, Tokyo). The SLC22A12 W258X polymorphism was genotyped by a polymerase chain reaction with confronting two-pair primers (PCR-CTPP).13) Each 25 µl reaction tube contained 50–80 ng DNA, 0.12 mM dNTP, 12.5 pmol of each primer, 0.5 U AmpliTaq Gold (Perkin-Elmer, Foster City, CA) and 2.5 µl of 10x PCR buffer including 15 mM MgCl2. The PCR-CTPP was conducted with the initial denaturation at 95°C for 10 minutes, 35 cycles of denaturation at 95°C for 1 minute, annealing at 61.5°C for 1 minute, and extension at 72°C for 1 minute, and with a final extension at 72°C for 5 minutes. The W258X primers were F1: 5’-TCC ATG CAG GCT CCA GG-3’, R1: 5’-ACC ACC AGC TGC AGC AGT GTT-3’, F2: 5’-TAC GGT GTG CGG GAC TGG-3’, and R2: 5’-GGC AGG ATC TCC TCT GAG G-3’. The amplified DNA fragments were 117-base pairs (bp) for the G (258W) allele, 176-bp for the A (258X) allele, and 255-bp for a common band, as illustrated in Fig. 1.
Fig. 1.
Representative gel for SLC22A12 W258X polymorphism. Lane M, a 100-bp ladder; lane 1, a WW homozygote with fragments of 117 bp and 255 bp; lane 2, a WX heterozygote with fragments of 117 bp, 176 bp and 255 bp; lane 3, a XX homozygote with fragments of 176 bp and 255 bp.
Statistical analyses
Hyperuricemia was defined as SUA level ≥ 7.0 mg/dL, and hypouricemia as SUA level < 3.0 mg/dL. The Hardy-Weinberg equilibrium was examined with a chi-square test. Means between two groups were examined with a t-test. The 95% confidence interval (CI) of percentages was calculated based on binomial distribution. Age- and sex-adjusted odds ratios (ORs) and 95% CIs were estimated using an unconditional logistic regression model. Two-sided p-values less than 0.05 were considered statistically significant. All statistical analyses were performed using STATA software version 7.0 (STATA, College Station, TX).
RESULTS
Subject characteristics according to sex are summarized in Table 1. The mean and standard deviation (SD) of age was 62.3±10.5 years in males and 60.0±9.9 years in females. Hyperuricemia was found to be 16.4% (n=39) in males and 1.8% (n=9) in females, while hypouricemia was 2.5% (n=6) and 5.5% (n=28), respectively. The SUA mean was significantly higher in males than in females (5.7±1.3 mg/dL vs 4.6±1.1 mg/dL, p<0.001).
Table 1.
Characteristics of participants according to sex
| Characteristics | Males (n=238) | Females (n=508) | ||||
| N | (%) | N | (%) | |||
| Age (years) | ||||||
| –49 | 31 | (13.0) | 76 | (15.0) | ||
| 50–59 | 56 | (23.5) | 169 | (33.3) | ||
| 60–69 | 89 | (37.5) | 175 | (34.4) | ||
| 70– | 62 | (26.1) | 88 | (17.3) | ||
| BMI (kg/m2) | ||||||
| –18.4 | 5 | (2.1) | 14 | (2.8) | ||
| 18.5–24.9 | 152 | (63.9) | 307 | (60.4) | ||
| 25.0– | 81 | (34.0) | 187 | (36.8) | ||
| SUA (mg/dL) | ||||||
| –0.9 | 1 | (0.4) | 0 | (0.0) | ||
| 1.0–1.9 | 0 | (0.0) | 3 | (0.6) | ||
| 2.0–2.9 | 5 | (2.1) | 25 | (4.9) | ||
| 3.0–3.9 | 13 | (5.5) | 113 | (22.2) | ||
| 4.0–4.9 | 43 | (18.1) | 200 | (39.4) | ||
| 5.0–6.9 | 137 | (57.6) | 158 | (31.1) | ||
| 7.0– | 39 | (16.4) | 9 | (1.8) | ||
| Creatinine (mg/dL) | ||||||
| –0.4 | 0 | (0.0) | 20 | (3.9) | ||
| 0.5–0.7 | 88 | (37.0) | 447 | (88.0) | ||
| 0.8–1.0 | 150 | (63.0) | 41 | (8.1) | ||
| BUN (mg/dL) | ||||||
| –7.9 | 1 | (0.4) | 11 | (2.2) | ||
| 8.0–19.9 | 210 | (88.2) | 477 | (93.9) | ||
| 20.0–22.9 | 27 | (11.3) | 20 | (3.9) | ||
| Smoking | ||||||
| Never | 47 | (19.7) | 407 | (80.1) | ||
| Former | 116 | (48.7) | 51 | (10.0) | ||
| Current | 75 | (31.5) | 47 | (9.3) | ||
| Unknown | 0 | (0.0) | 3 | (0.6) | ||
| Drinking | ||||||
| Never | 83 | (34.9) | 429 | (84.4) | ||
| Former | 17 | (7.1) | 11 | (2.2) | ||
| Current | 138 | (58.0) | 66 | (13.0) | ||
| Unknown | 0 | (0.0) | 2 | (0.4) | ||
BMI: body mass index; SUA: serum uric acid; BUN: blood urea nitrogen
Among the 746 examinees, 29 individuals (10 males and 19 females) were found with 258WX, and none with 258XX. The 258X allele was 1.9% (95% CI, 1.3–2.8). The genotype distribution was in Hardy-Weinberg equilibrium for males (p=0.74), for females (p=0.67), and for the combined (p=0.59).
Table 2 shows means and SD of age, BMI, blood tests, and blood pressure, according to the SLC22A12 W258X. A significant difference in SUA was observed between those with 258WW and 258WX ; 5.9±1.2 mg/dL and 3.1±1.2 mg/dL for males, and 4.6±1.0 mg/dL and 2.9±0.9 mg/dL for females, respectively. The age- and sex-adjusted OR of the 258WX genotype relative to the 258WW genotype was 45.6 (95% CI, 18.4–113.2) for hypouricemia.
Table 2.
Means and standard deviations (SD) of age, body mass index (BMI), blood tests, and blood pressure according to SLC22A12 W258X genotype
| Characteristics | Males | Females | |||||||||||||||
| WW (n=228) | WX (n=10) | p value | WW (n=489) | WX (n=19) | p value | ||||||||||||
| Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | ||||||
| Age (years) | 62.4 | ± | 10.6 | 59.9 | ± | 7.4 | 0.462 | 59.9 | ± | 9.9 | 63.4 | ± | 9.6 | 0.123 | |||
| BMI (kg/m2) | 24.1 | ± | 3.1 | 23.0 | ± | 1.9 | 0.293 | 24.0 | ± | 3.4 | 24.6 | ± | 2.9 | 0.431 | |||
| Uric acid (mg/dL) | 5.9 | ± | 1.2 | 3.1 | ± | 1.2 | <0.001 | 4.6 | ± | 1.0 | 2.9 | ± | 0.9 | <0.001 | |||
| Triglyceride (mg/dL) | 113.1 | ± | 76.4 | 80.9 | ± | 20.2 | 0.186 | 92.0 | ± | 47.4 | 115.3 | ± | 85.6 | <0.05 | |||
| Total cholesterol (mg/dL) | 207.4 | ± | 31.5 | 206.3 | ± | 31.9 | 0.911 | 217.6 | ± | 33.2 | 218.3 | ± | 34.2 | 0.925 | |||
| HDL (mg/dL) | 54.0 | ± | 12.7 | 55.6 | ± | 6.9 | 0.697 | 62.1 | ± | 13.7 | 57.9 | ± | 11.2 | 0.191 | |||
| Creatinine (mg/dL) | 0.79 | ± | 0.12 | 0.74 | ± | 0.11 | 0.189 | 0.61 | ± | 0.10 | 0.62 | ± | 0.12 | 0.635 | |||
| BUN (mg/dL) | 15.2 | ± | 3.2 | 16.2 | ± | 3.5 | 0.352 | 13.9 | ± | 3.2 | 14.4 | ± | 3.7 | 0.515 | |||
| Hematocrit (%) | 44.3 | ± | 3.4 | 43.8 | ± | 3.6 | 0.603 | 39.7 | ± | 3.1 | 39.1 | ± | 3.8 | 0.378 | |||
| ALP (IU/L) | 155.5 | ± | 43.8 | 160.1 | ± | 48.5 | 0.748 | 158.6 | ± | 48.8 | 171.2 | ± | 73.0 | 0.280 | |||
| Total protein (g/dL) | 7.5 | ± | 0.42 | 7.3 | ± | 0.46 | 0.236 | 7.6 | ± | 0.41 | 7.60 | ± | 0.29 | 0.716 | |||
| C reactive protein (mg/L) | 0.17 | ± | 0.46 | 0.05 | ± | 0.04 | 0.409 | 0.10 | ± | 0.29 | 0.14 | ± | 0.19 | 0.510 | |||
| Systolic blood pressure (mm Hg) | 138.3 | ± | 19.2 | 144.2 | ± | 24.0 | 0.349 | 135.0 | ± | 19.4 | 133.1 | ± | 16.9 | 0.660 | |||
| Diastolic blood pressure (mmHg) | 88.2 | ± | 10.7 | 92.4 | ± | 13.7 | 0.230 | 84.4 | ± | 10.5 | 82.9 | ± | 9.3 | 0.564 | |||
BMI: body mass index; HDL: high-density lipoprotein cholesterol; BUN: blood urea nitrogen; ALP: alkaline phosphatase
The frequency of the 258WX genotype according to the SUA is shown in Table 3. Among those with hypouricemia, 258WX was more common in males (66.7%, 95% CI, 22.2–95.7%) than in females (39.3%, 95% CI, 21.5–59.4%). Among subjects with an SUA of 3.0–4.9 mg/dL, those with 258WX were 10.7% (95% CI, 4.0–21.9%) and 2.6% (95% CI, 1.1–5.0%), respectively. There were no individuals with 258WX among those with a SUA equal to 5.0 mg/dL or more. The 258X allele was 1.9% (95% CI, 1.3–2.8%) of all the subjects.
Table 3.
SLC22A12 258WX genotype frequency (%) and 95% confidence interval (95% CI) according to serum uric acid (SUA)
| SUA (mg/dL) | |||||||
| 0.0–0.9 | 1.0–1.9 | 2.0–2.9 | 3.0–3.9 | 4.0–4.9 | 5.0– | Total | |
| Males | |||||||
| 258WX | 1 | 0 | 3 | 4 | 2 | 0 | 10 |
| % | 100.0 | − | 60.0 | 30.8 | 4.7 | 0.0 | 4.2 |
| (95%CI) | (2.5–100) | (−) | (14.7–94.7) | (9.1–61.4) | (0.6–15.8) | (0–2.1) | (2.0–7.6) |
| Females | |||||||
| 258WX | 0 | 2 | 9 | 4 | 4 | 0 | 19 |
| % | − | 66.7 | 36.0 | 3.5 | 2.0 | 0.0 | 3.7 |
| (95% CI) | (−) | (9.4–99.2) | (18.0–57.5) | (1.0–8.8) | (0.5–5.0) | (0–2.2) | (2.3–5.8) |
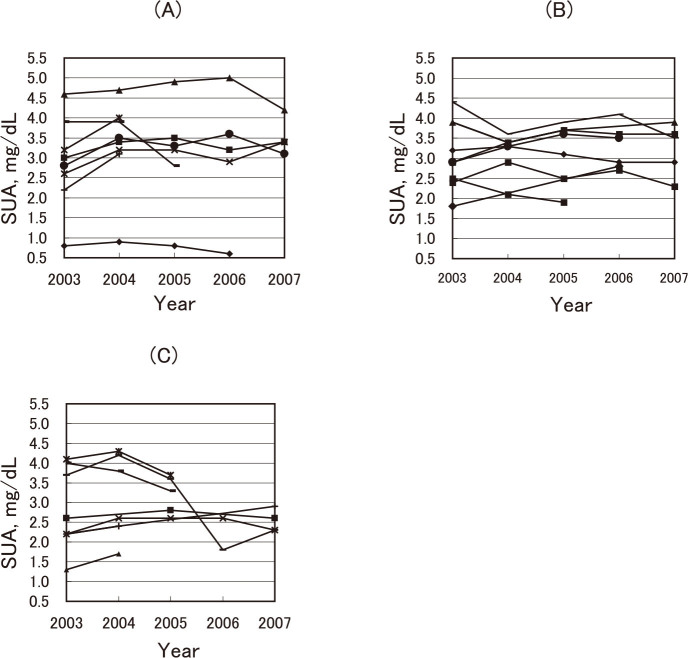
Among the subjects, 8 males and 15 females participated in a health checkup twice or more between 2003 and 2007, the period available for a record linkage. Fig. 2 demonstrates changes in the SUA of 23 participants; (A) for 8 males, (B) for 8 females with BMI<25, and (C) for 7 females with BMI>25; the respective differences from 2003 to 2007 were 0.3–1.1 mg/dL, 0.4–1.0 mg/dL, and 0.2–2.4 mg/dL. In one female depicted in Fig. 2 (C), a marked reduction in SUA was observed between 2005 and 2006. She was a 71-year-old participant who never smoked and had no drinking habit. The available data showed no obvious reasons; her BMI was 26.9 in 2005 and 25.7 in 2006, with a serum creatinine of 0.5 mg/dL in both years. The change in the percentage (difference between the maximum and minimum divided by the average of all obtained measurments ×100) ranged from 7.5% to 76.9% among 258WX subjects (n=23), whereas it ranged from 0.0% to 112.2% among 258WW subjects (n=536).
Fig. 2.
SUA levels of individuals with SLC22A12 258WX who participated in the health checkup twice or more during 2003 to 2007. (A) 8 males; 5 times for 4 males, 4 times for 1 male, 3 times for 1 male, and twice for 2 males, (B) 8 females with body mass index (BMI)<25; 5 times for 4 females, 4 times for 2 females, 3 times for 1 female, and twice for 1 female. (C) 7 females with BMI≥25; 5 times for 2 females, 3 times for 4 females, and twice for 2 females.
DISCUSSION
This study demonstrated that the mean SUA was significantly lower for those with SLC22A12 258WX than for those with 258WW. The 258X allele frequency was 1.9% (95% CI, 1.3–2.8) when 57 subjects were excluded due to gout, high blood urea nitrogen or high creatinine. Those with at least one 258X allele were 66.7% of 6 males and 39.3% of 28 females with SUA < 3.0 mg/dL, and 10.7% of 56 males and 2.6% of 313 females with SUA 3.0–4.9 mg/dL. This was the first study reporting the allele frequecy according to sex and SUA levels among Japanese. It was also the first that the SUA of those with a 258WX allele changed similarly to those with the 258WW genotype during the entire 5-year observation period.
The frequency of the 258X allele in a Japanese population was estimated to be 2.4% among 1,875 participants (83 heterozygotes and 3 homozygotes) in a cohort study,10) and 2.3% among 980 controls in a case-control study,14) both of which were slightly higher than the estimate in this study. When the 57 examinees under medication for gout, or with high serum levels of urea nitrogen or creatinine were included (n=803), the frequency in the present study was 1.8% (95% CI, 1.2–2.6). While the 258X allele was found in 3 of 5 hypouricemia patients in Korea,15) the frequency in a general Korean population was 1.1%.12) Since this allele was not reported among other ethnic groups, its origin was thought to be in East Asia.16)
Interestingly, the 258X allele was found in those with SUA 4.0–4.9 mg/dL in the present study. Such a finding indicates that there may be other genetic traits and/or lifestyle factors compensating for the effect of a 258X allele on SUA levels. It is well known that SUA is lower in females than males. In this study, the SUA mean was 5.7±1.3 mg/dL in males and 4.6±1.1 mg/dL in females (p<0.001). Age, menopause, meat consumption, alcohol intake, obesity, sedentary lifestyle, dyslipidemia, insulin resistance, blood pressure, renal function, and drugs for hypertension were the factors associated with SUA levels.17-22) Since there were only 29 participants with 258WX, associations with the above factors among them could not be examined; even between males and females, the difference was small (3.1 mg/dL in males and 2.9 mg/dL in females).
Among those with 258WX, the changes in SUA ranged from 7.5% to 76.9% of the average. Those changes were similar to those of the examinees with 258WW, indicating that lifestyle factors were similarly influential on SUA. Since hypouricemia increases the risk of acute renal failure, urolithiasis, and hematuria, the elevation of SUA to an appropriate level through lifestyle changes might benefit those with hypouricemia.
The present observational study has several limitations. The first was the limited number of subjects. Because of this limitation, the factors possibly associated with the SUA among those with 258WX could not be examined. Second, the follow-up period for the changes in SUA levels was only five years, so that lifestyle changes potentially affecting the SUA could not be examined for the long-term changes in the SUA. Lastly, genotypes other than SLC22A12 W258X were not examined.
In conclusion, 746 Japanese health checkup examinees were genotyped for SLC22A12 W258X. Although a strong association was found between the SLC22A12 258X allele and low SUA, some participants with 258X allele showed a normal SUA. The allele among those with hypouricemia was 66.7% in 6 males and 39.3% in 28 females. The SUA levels changed among those with 258WX, as they also did among those with 258WW, indicating that lifestyles could similarly influence SUA for those with 258WX.
ACKNOWLEDGMENTS
This study was supported in part by a Grant-in-Aid for Scientific Research on Special Priority Areas of Cancer from the Japanese Ministry of Education, Culture, Sports, Science and Technology. We are grateful to Ms. Yoko Mitsuda for her valuable technical assistance. Our data were derived from the Yakumo Study established by Emeritus Professor Kunio Aoki of the Nagoya University Graduate School of Medicine.
REFERENCES
- 1).Yeun JY, Hasbargen JA. Renal hypouricemia: prevention of exercise-induced acute renal failure and a review of the literature. Am J Kidney Dis, 1995; 25: 937–946. [DOI] [PubMed]
- 2).Ohta T, Sakano T, Ogawa T, Kato J, Awaya Y, Kihara H, Kinoshita Y. Exercise-induced acute renal failure with renal hypouricemia: a case report and a review of the literature. Clin Nephrol, 2002; 58: 313–316. [DOI] [PubMed]
- 3).Hirasaki S, Koide N, Fujita K, Ogawa H, Tsuji T. Two cases of renal hypouricemia with nephrolithiasis. Intern Med, 1997; 36: 201–205. [DOI] [PubMed]
- 4).Hisatome I, Tanaka Y, Kotake H, Kosaka H, Hirata N, Fujimoto Y, Yoshida A, Shigemasa C, Mashiba H, Sato R, et al. Renal hypouricemia due to enhanced tubular secretion of urate associated with urolithiasis: successful treatment of urolithiasis by alkalization of urin K+, Na(+)-citrate. Nephron, 1993; 65: 578–582. [DOI] [PubMed]
- 5).Hisatome I, Tanaka Y, Ogino K, Shimoyama M, Hiroe K, Tsuboi M, Yamamoto Y, Hamada N, Kato T, Manabe I, Kinugawa T, Ohtahara A, Yoshida A, Shigemasa C, Takeda A, Sato R. Hematuria in patients with renal hypouricemia. Intern Med, 1998; 37: 40–46. [DOI] [PubMed]
- 6).Taniguchi A, Kamatani N. Control of renal uric acid excretion and gout. Curr Opin Rheumatol, 2008; 20: 192–197. [DOI] [PubMed]
- 7).Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, Hosoyamada M, Takeda M, Sekine T, Igarashi T, Matsuo H, Kikuchi Y, Oda T, Ichida K, Hosoya T, Shimokata K, Niwa T, Kanai Y, Endou H. Molecular identification of a renal urate-anion exchanger that regulates blood urate levels. Nature, 2002; 417: 447–452. [DOI] [PubMed]
- 8).Komoda F, Sekine T, Inatomi J, Enomoto A, Endou H, Ota T, Matsuyama T, Ogata T, Ikeda M, Awazu M, Muroya K, Kamimaki I, Igarashi T. The W258X mutation in SLC22A12 is the predominant cause of Japanese renal hypouricemia. Pediatr Nephrol, 2004; 19: 728–733. [DOI] [PubMed]
- 9).Ichida K, Hosoyamada M, Hisatome I, Enomoto A, Hikita M, Endou H, Hosoya T. Clinical and moleculer analysis of patients with renal hypouricemia in Japan - influence of URAT1 gene on urinary urate excretion. J Am Soc Nephrol, 2004; 15: 164–173. [DOI] [PubMed]
- 10).Iwai N, Mino Y, Hosoyamada M, Tago N, Kokubo Y, Endou H. A high prevalence of renal hypouricemia caused by inactive SLC22A12 in Japanese. Kidney Int, 2004; 66: 935–944. [DOI] [PubMed]
- 11).Tanaka M, Itoh K, Matsushita K, Matsushita K, Wakita N, Adachi M, Nonoguchi H, Kitamura K, Hosoyamada M, Endou H, Tomita K. Two male siblings with hereditary renal hypouricemia and exercise-induced ARF. Am J Kidney Dis, 2003; 42: 1287–1292. [DOI] [PubMed]
- 12).Lee JH, Choi HJ, Lee BH, Kang HK, Chin HJ, Yoon HJ, Ha IS, Kim S, Choi Y, Cheong HI. Prevalence of hypouricaemia and SLC22A12 mutations in healthy Korean subjects. Nephrology, 2008; 13: 661–666. [DOI] [PubMed]
- 13).Hamajima N, Saito T, Matsuo K, Kozaki K, Takahashi T, Tajima K. Polymerase chain reaction with confronting two-pair primers for polymorphism genotyping. Jpn J Cancer Res, 2000; 91: 865–868. [DOI] [PMC free article] [PubMed]
- 14).Taniguchi A, Urano W, Yamanaka M, Yamanaka H, Hosoyamada M, Endou H, Kamatani N. A common mutation in an organic anion transporter gene, SLC22A12, is a suppressing factor for the development of gout. Arthritis Rheum, 2005; 52: 2576–2577. [DOI] [PubMed]
- 15).Cheong HI, Kang JH, Lee JH, Ha IS, Kim S, Komoda F, Sekine T, Igarashi T, Choi Y. Mutational analysis of idiopathic renal hypouricemia in Korea. Pediatr Nephrol, 2005; 20: 886–890. [DOI] [PubMed]
- 16).Ichida K, Hosoyamada M, Kamatani N, Kamitsuji S, Hisatome I, Shibasaki T, Hosoya T. Age and origin of the G774A mutation in SLC22A12 causing renal hypouricemia in Japanese. Clin Genet, 2008; 74: 243–251. [DOI] [PubMed]
- 17).Burack RC, Keller JB, Higgins MW. Cardiovascular risk factors and obesity: are baseline levels of blood pressure, glucose, cholesterol and uric acid elevated prior to weight gain? J Chronic Dis, 1985; 38: 865–872. [DOI] [PubMed]
- 18).Benedek TG. Correlations of serum uric acid and lipid concentrations in normal, gouty, and atherosclerotic men. Ann Intern Med, 1967; 66: 851–861. [DOI] [PubMed]
- 19).Alderman M. Uric acid in hypertension and cardiovascular disease. Can J Cardiol, 1999; 15(Suppl F): 20F-22F. [PubMed]
- 20).Bengtsson C, Tibblin E. Serum uric acid levels in women: an epidemiological survey with special reference to women with high serum uric acid values. Acta Med Scand, 1974; 196: 93–102. [PubMed]
- 21).Rathmann W, Funkhouser E, Dyer AR, Roseman JM. Relations of hyperuricemia with the various components of the insulin resistance syndrome in young black and white adults: the CARDIA study. Coronary Artery Risk Development in Young Adults. Ann Epidemiol, 1998; 8: 250–261. [DOI] [PubMed]
- 22).Nakashima M, Uematsu T, Kosuge Kanamaru M. Pilot study of the uricosuric effect of DuP-753, a new angiotensin II receptor antagonist, in healthy subjects. Eur J Clin Pharmacol, 1992; 42: 333–335. [DOI] [PubMed]