ABSTRACT
Recently, liquid-based cytology (LBC) has been widely applied to various samples in diagnostic cytology and its usefulness has been reported. In this study, we investigated thyroid cytology that applied LBC and immunocytochemistry to achieve more objective diagnosis and greater diagnostic accuracy. This study included 125 cases (57 papillary carcinomas (PCs), 22 follicular tumors, 43 adenomatous goiters and 3 with Basedow’s disease). After preparing the LBC slide, immunocytochemical staining was performed on each slide with six antibodies (HBME-1, cytokeratin 19 (CK19), high molecular weight cytokeratin (34βE12), galectin-3, CD15 and CA 19-9). All antibodies presented immunopositivity frequently in PCs, but only a few or some of them were positive in other cases. These antibodies were considered positive markers for PCs, and the most reliable marker was 34βE12; its sensitivity, specificity and diagnostic accuracy were 82.5%, 100% and 92.0%, respectively. Relations of immunocytochemical profiles against these markers were assessed using panel 34βE12, GAL-3 and CK19. More than or equal to two of these markers showed co-positive in 53 of 57 PCs, and negative for all markers was observed in only one case. In the other (non PC) cases, the former was 0 of 58 and the latter was 40 cases. In this panel, the sensitivity, specificity and diagnostic accuracy were 93.0%, 100% and 96.8%, respectively. All of these values were higher than or equal to single values of 34βE12. We concluded that the panel in this study is useful for more objective and accurate diagnosis of thyroid cytology.
Key Words: Liquid-based cytology, Diagnostic cytology, Thyroid, Immunocytochemistry
INTRODUCTION
Liquid-based cytology (LBC) techniques have been widely applied not only to gynecological cytology but also to non-gynecological cytology such as fine needle aspiration cytology.1-5) LBC systems have served not only to facilitate screening of cytological specimens, but also to preserve samples for some time, and to make residual samples available for further investigations such as immunocytochemistry or molecular analysis.6-8) These advantages may result in more objective diagnosis and greater diagnostic accuracy.
Although novel methods such as LBC have been developed and made available for diagnostic cytology, diagnostic cytology has remained a subjective diagnostic method because, as before, it is based on morphology. We considered that the immunocytochemical techniques will contribute to more objective diagnosis of cytology and improved diagnostic accuracy. Particularly, fine needle aspiration cytology including thyroid cytology requires higher diagnostic accuracy because the cytological diagnosis will be the final diagnosis. Therefore, we attempted to investigate objective cytological diagnosis of thyroid with immunocytochemistry for improvement of diagnostic accuracy.
Many immunohistochemical studies on thyroid lesions have suggested several antibodies as useful diagnostic markers, including, for example, galectin-3 (GAL-3),9-13) high molecular weight cytokeratin (34βE12),12,14,15) cytokeratin 19 (CK19),11-14,16-21) HBME-1,11-13,19,22,23) CD15,23-25) and CA 19-9.23,25) However, most of them were obtained from histological paraffin-embedded sections, and there were few studies using cytological specimens, because it is difficult to prepare many conventional (including fine needle aspiration cytology) cytological slides for immunocytochemistry with several antibodies.
We considered that the application of LBC to fine needle aspiration cytology allows easy preparation of several slides for immunocytochemistry unless the sample amount is very small. Therefore, we attempted to apply thyroid cytology to LBC for immunocytochemical analysis with several antibodies.
In this study, we used cell samples obtained by direct scratch from thyroid lesions which were removed surgically as the substitute for samples of fine needle aspiration cytology of thyroid, and then prepared LBC specimens. We made an immunocytochemical analysis of these specimens with six antibodies (GAL-3, 34βE12, CK19, HBME-1, CD15 and CA 19-9) which were reportedly useful for diagnosis of thyroid diseases.
MATERIALS AND METHODS
Materials and preparations of cytological specimens
A total of 125 thyroid lesions were obtained for this study. All samples were from Nagoya Daini Red Cross Hospital and Kami-iida Daiichi General Hospital between Dec. 2006 and May 2010. The cases in this study included 57 papillary carcinomas (PCs), 22 follicular tumors, 43 adenomatous goiters and 3 with Basedow’s disease. The information on these cases was shown in Table 1. All cell specimens of LBC were obtained by direct scratch with scalpel or spatula from thyroid lesions that were removed surgically, and these cell samples were suspended in the preservation solution of a Liqui-PrepTM (LGM International Inc., Melbourne, FL)26) for at least 15 min and processed according to the manufacturer’s instructions. Briefly, the samples in the preservation solution were centrifuged at 1,000 G for 10 min, the supernatants were decanted, and cell pellets were mixed into a cellular base reagent for encapsulating and adhering to the slide glass. Aliquots of homogeneous mixtures were placed onto clean microscope slides by micropipette and dried at room temperature for at least 30 min. Then Papanicolaou stain and immunocytochemical staining were performed on these slides.
Table 1.
List of all cases
| Diagnosis | No. of cases (Male) | Age range (Average) |
| PC | 57 (13) | 25–83 (55.1) |
| FT | 22 (5) | 28–80 (56.6) |
| AG | 43 (9) | 22–73 (54.3) |
| BD | 3 (1) | 48–74 (58.3) |
| Total | 125 (28) | 22–83 (55.1) |
PC: papillary carcinoma. FT: follicular tumor.
AG: adenomatous goiter. BD: Basedow’s disease.
The study protocol was approved by the Board of Ethics of Nagoya University School of Health Sciences.
Immunocytochemistry
Immunocytochemical staining was performed on LBC cytologic slides with the antibodies listed in Table 2. The slides were rinsed in 0.3% hydrogen peroxide solution with absolute methanol for 20 min to remove endogenous peroxidase. After rehydration with tap and distilled water, these were immersed in 10 mM phosphate-buffered saline (PBS) pH 7.2. Antigen retrieval was done for anti-CK19 and 34βE12 antibodies by heating the slides with 10 mM citrate buffer (pH 6.0) for 20 min in autoclave at 121°C. Ten percent normal goat serum was applied for 20 min at room temperature, then primary antibody was replaced and incubated overnight at 4°C. Histofine® Simple Stain MAX PO (Nichrei Biosciences Inc., Tokyo, Japan) was used for the secondary peroxidase-labeled antibody and visualized by DAB substrate kit (Vector Laboratories, Inc., Burlingame, CA). Both procedures were performed according to the manufacturer’s instructions. The slides were counterstained with hematoxylin and finally coverslipped by cover glass after dehydration through ethanol and xylene.
Table 2.
Characteristics of antibodies
| Clone | Dilution | Pretreatment | Source | |
| HBME-1 | HBME-1 | 1:50 | - | Dako |
| Cytokeratin 19 (CK19) | RCK108 | 1:200 | AC | Dako |
| High molecular weight cytokeratin (34βE12) | 34βE12 | 1:50 | AC | Dako |
| Galectin-3 (GAL-3) | NCL-GAL3 | 1:200 | - | Novocastra |
| CD15 | MMA | 1:100 | - | BD |
| CA 19-9 | 116-NS19-9 | 1:50 | - | Dako |
Dako: Dako Japan, Inc., Tokyo, Japan. Novocastra: Novocastra Laboratories Ltd., Newcastle, UK. BD: BD Biosciences, Franklin Lake, NJ.
AC: Antigen retrieval by autoclave.
Evaluation of immunocytochemical results and statistical analyses
At first, we confirmed with Papanicolaou stain whether or not all specimens were suitable samples for evaluation of immunocytochemical analysis, and all assessable specimens were then investigated blindly. The immunocytochemical results were scored 0 to 3 as follows: (0): no staining; (1): less than 5% of cells stained, and no discernible specific staining; (2): 5% to 50% of cells stained; and (3): more than 50% of cells stained. Both (2) and (3) were regarded as positive. These data were summarized into each diagnostic category according to diagnosis by histopathology, and then aggregated. The sensitivity, specificity, diagnostic accuracy, positive predictive value (PPV) and negative predictive value (NPV) were calculated and aggregated, and each value was stated as a percentage. Relations of immunocytochemical profiles to these antibodies were also investigated. Then, an antibody panel useful for diagnosis of thyroid lesions was made and evaluated. We evaluated the panel similarly and attempted to establish more useful diagnostic criteria for differential diagnosis of thyroid lesions.
RESULTS
Results of immunocytochemical analysis
Immunocytochemical staining with each antibody was shown in Fig. 1, and the results of the immunocytochemical analysis were summarized in Table 3. In general, the reactivities against all antibodies used in this study were higher in PC cases than others including follicular tumor, adenomatous goiter and Basedow’s disease. Both cases of follicular tumor and adenomatous goiter were shown to be slightly positive for some antibodies, but all of Basedow’s disease cases (3/3) were negative for all antibodies. In PC cases, especially high positivity was exhibited in HBME-1, CK19 and 34βE12. These positive rates were 93.0%, 89.5% and 82.5%, respectively. GAL-3 (78.9%), CD15 (75.4%) and CA 19-9 (68.4%) also showed relatively high positive rates. HBME-1 evidenced the highest reactivity (93.0%) in PC cases, but also showed some positive in both follicular tumor and adenomatous goiter, at 31.8% and 23.3%, respectively, the highest positive rates in these cases. As for the other antibodies, CK19 and CD15 reacted less than 20% in both follicular tumor and adenomatous goiter cases, and GAL-3 and CA 19-9 evidenced little positivity. Especially, 34βE12, like Basedow’s disease, did not show positivity in both cases of follicular tumor and adenomatous goiter.
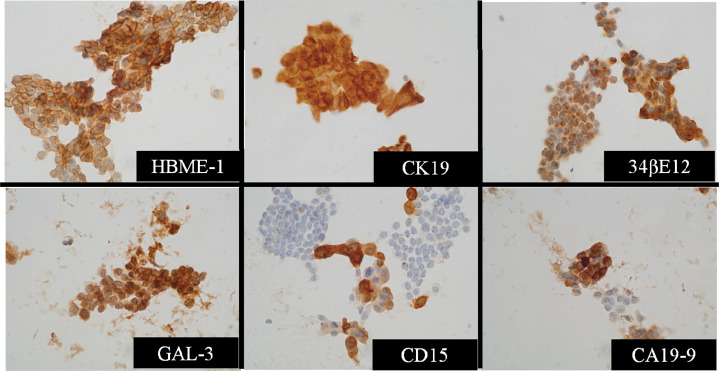
Fig. 1.
Immunocytochemical staining in papillary carcinoma with each antibody.
Positive staining of GAL-3 shows in both nucleus and cytoplasm, and the other antibodies are stained in cytoplasm or cellular membrane.
Table 3.
Results of immunocytochemistry
| Diagnosis | Qualitative evaluation | Semi-quantitative evaluation | |||||
| (n) | Nagative | Positive (%) | 0 | 1 | 2 | 3 | |
| Negative | Positive | ||||||
| 34βE12 | 10 | 47 (82.5) | 1 | 9 | 23 | 24 | |
| GAL-3 | 12 | 45 (78.9) | 4 | 8 | 7 | 38 | |
| PC | CK19 | 6 | 51 (89.5) | 1 | 5 | 15 | 36 |
| (57) | HBME-1 | 4 | 53 (93.0) | 0 | 4 | 5 | 48 |
| CD15 | 14 | 43 (75.4) | 0 | 14 | 20 | 23 | |
| CA 19-9 | 18 | 39 (68.4) | 6 | 12 | 17 | 22 | |
| 34βE12 | 22 | 0 ( 0.0) | 18 | 4 | 0 | 0 | |
| GAL-3 | 21 | 1 ( 4.5) | 20 | 1 | 1 | 0 | |
| FT | CK19 | 18 | 4 (18.2) | 9 | 9 | 3 | 1 |
| (22) | HBME-1 | 15 | 7 (31.8) | 14 | 1 | 2 | 5 |
| CD15 | 18 | 4 (18.2) | 15 | 3 | 4 | 0 | |
| CA 19-9 | 20 | 2 (13.6) | 19 | 1 | 2 | 0 | |
| 34βE12 | 43 | 0 ( 0.0) | 35 | 8 | 0 | 0 | |
| GAL-3 | 42 | 1 ( 2.3) | 34 | 8 | 1 | 0 | |
| AG | CK19 | 38 | 5 (11.6) | 22 | 16 | 5 | 0 |
| (43) | HBME-1 | 33 | 10 (23.3) | 29 | 4 | 3 | 7 |
| CD15 | 41 | 2 ( 4.7) | 32 | 9 | 2 | 0 | |
| CA 19-9 | 41 | 2 ( 4.7) | 29 | 12 | 2 | 0 | |
| 34βE12 | 3 | 0 ( 0.0) | 3 | 0 | 0 | 0 | |
| GAL-3 | 3 | 0 ( 0.0) | 3 | 0 | 0 | 0 | |
| BD | CK19 | 3 | 0 ( 0.0) | 3 | 0 | 0 | 0 |
| (3) | HBME-1 | 3 | 0 ( 0.0) | 2 | 1 | 0 | 0 |
| CD15 | 3 | 0 ( 0.0) | 3 | 0 | 0 | 0 | |
| CA 19-9 | 3 | 0 ( 0.0) | 3 | 0 | 0 | 0 | |
PC: papillary carcinoma. FT: follicular tumor. AG: adenomatous goiter.
BD: Basedow’s disease.
Negative: 0 and 1 of semi-quantitative values.
Positive: 2 and 3 of semi-quantitative values.
We assumed that these antibodies might be diagnostic markers that could discriminate PC from the other thyroid lesions including follicular tumor, adenomatous goiter and Basedow’s disease. The sensitivity, specificity, diagnostic accuracy, PPV and PNV were then calculated and summarized in Table 4. The diagnostic accuracy of these six markers ranged 82.4% to 92.0%; the highest value was 34βE12 (92.0%), followed by GAL-3 (88.8%) and CK19 (88.0%), while the lowest was CA 19-9 (82.4%).
Table 4.
Statistical values of each antibody and in diagnosis of PC
| Statistical value | Antibodies | ||||||
| HBME-1 | CK19 | 34βE12 | GAL-3 | DC15 | CA 19-9 | Panel | |
| Sensitivity | 93.0 | 89.5 | 82.5 | 78.9 | 75.4 | 68.4 | 93.0 |
| Specificity | 75.0 | 86.8 | 100 | 97.1 | 91.2 | 94.1 | 100 |
| Diagnostic accuracy | 83.2 | 88.0 | 92.0 | 88.8 | 84.0 | 82.4 | 96.8 |
| PPV | 75.7 | 85.0 | 100 | 95.7 | 87.8 | 90.7 | 100 |
| NPV | 92.7 | 90.8 | 87.2 | 84.6 | 81.6 | 78.0 | 94.4 |
Panel is an antibody combination of 34βE12, GAL-3 and CK19.
Diagnostic criteria by means of panel in this study are defined by numbers positive for more than or equal to two panel antibodies.
Relations of immunocytochemical results
We investigated relations of immunocytochemical results of six antibodies to find the most useful antibody panel for differential diagnosis of PC. We then focused on three antibody markers that exhibited higher diagnostic accuracy in this study, including 34βE12, GAL-3 and CK19. The immunocytochemical results were integrated and shown in Fig. 2. Thus, these diagrams clearly revealed the patterns of presence and/or absence among the three markers. In addition, we limited ourselves to only three markers from a perspective of reduction in cost and handling.
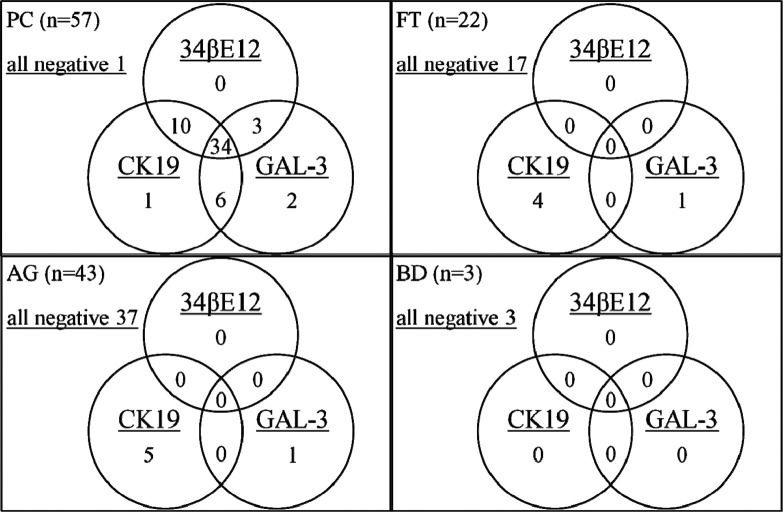
Fig. 2.
Relations of each result for three markers.
Numbers within circles show number of positive cases for each marker. Numbers in overlaps of circles indicate numbers co-positive for each marker.
Among 57 cases of PC, 34 were positive for all three markers, whereas only one was negative for all of them. The number of positive cases for any two of these three markers, and its combination patterns of two markers, were 10 (34βE12 + CK19), 6 (CK19 + GAL-3) and 3 (34βE12 + GAL-3), respectively. On the other hand, neither of the follicular tumor and adenomatous goiter cases indicated positive for all of the three markers, whereas 17 of 22 follicular tumors and 37 of 43 adenomatous goiters were negative for all three. In addition, no case showed positive to more than or equal to two markers in follicular tumor and adenomatous goiter.
From the perspective of differential diagnosis of PC from the other thyroid lesions including follicular tumor, adenomatous goiter and Basedow’s disease, we considered that PC would express positive for any more than or equal to two of the three markers’ panel. Thus, these criteria were defined as the PC diagnostic criteria in the present study. We employed these diagnostic criteria and attempted to distinguish PC from the other lesions in all cases in the present study. These results were summarized and shown in Table 5, and the diagnostic statistical values were evaluated. The sensitivity, specificity, diagnostic accuracy, PPV and PNV of these criteria with the panel were 93.0%, 100%, 96.8%, 100% and 94.4%, respectively. Hence, all of the statistical values were higher than or equal to any single value of the highest among all six markers in this study (Table 3).
Table 5.
Results of antibody panel including 34βE12, GAL-3 and CK19 for diagnosis of PC
| PC | Others | Total | |
| Positive | 53 | 0 | 53 |
| Negative | 4 | 68 | 72 |
| Total | 57 | 68 | 125 |
Positive: Number positive for antibodies was more than or equal to two of the panel.
Negative: Number positive for antibodies was less than two of the panel.
PC: papillary carcinoma.
Other: follicular tumor, adenomatous goiter, and Basedow’s disease.
DISCUSSION
In the present study, we evaluated diagnostic cytology of thyroid lesions using LBC technique and immunocytochemistry with six antibodies.
Galectin-3 is a β-galactoside-binding protein that localizes in the cytoplasm and nucleus. A variety of physiological and pathological functions have been reported for this protein including cell adhesion,27) cell growth28,29) and apoptosis.29) GAL-3 is reportedly expressed preferentially in thyroid malignancies including papillary carcinoma and follicular carcinoma.9-13) In our study, 45 of 57 PCs indicated positive, but 12 cases were negative. Thus, although the positive rate (78.9%) was not good, the diagnostic accuracy was relatively high (88.8%) because there were only two false-positive cases among 68 non-PCs (Table 3, 4). Aratake et al.10) reported that 37 of 37 PCs, 7 of 20 follicular tumors and 0 of 16 adenomatous goiters were positive for GAL-3, respectively, in cytological specimens (not LBC). Follicular tumors included 6 cases of follicular carcinoma, and 5 cases were positive for GAL-3. There was only one follicular carcinoma in 22 follicular tumors in our study; it was negative not only for GAL-3, but also all other antibodies. Our cases included only one with follicular carcinoma, making further discussion impossible.
The antibody 34βE12, an anti-high molecular weight cytokeratin used in this study, recognizes cytokeratin 1, 5, 10 and 14.30-32) Raphael et al. reported that high molecular weight cytokeratin was a useful marker to distinguish papillary carcinoma from follicular neoplasms and nodular hyperplasias.14,15) This antibody was the most reliable marker for diagnosis of PC because its diagnostic accuracy (92.0%) was the highest among the six markers in our study. Though its sensitivity (82.5%) was lower than the optimum value of HBME-1 (93.0%), the specificity was 100% (Table 4). In their study of thyroid lesions using formalin-fixed, paraffin-embedded sections, Park et al.12) concluded that 34βE12 might limit utility as the result of low sensitivity because the sensitivity and specificity results were 59.7% and 100%, respectively. Their 34βE12 result was similar to ours in that the specificity was highest but the sensitivity was not so high. As for fine needle aspiration cytology including thyroid cytology, we considered that specificity is more important than sensitivity, because fine needle aspiration cytology is frequently adopted for the final diagnosis. On the other hand, most of the exfoliative cytology (including gynecological cytology, sputum and urine cytology, etc.) is employed for screening, so such cytology requires higher sensitivity.
CK19 is an antibody that also reacts to cytokeratin, but it identifies specific cytokeratin 19, which is classified as a low molecular weight cytokeratin. Previous studies indicated that CK19 strongly reacted to PC, but also expressed positive in benign lesions including adenomatous goiter, follicular adenoma and normal follicular cells to some degree.11-14, 16-21) We observed 51 (89.5%) of 57 PCs positive for CK19, and 4 (18.2%) of 22 follicular tumors and 5 (11.6%) of 43 adenomatous goiters were also positive (Table 3). These results were similar to those of previous studies.
The results of the other markers including HBME-1,11-13,22,23) CD1523-25) and CA 19-923,25) were almost the same as those of previous studies. As for HBME-1, sensitivity was the highest (93.0%) but diagnostic accuracy was not so high (83.2%), because specificity was the lowest (75.0%) in six markers. The diagnostic accuracy of CD15 and CA 19-9 was also low as a result of their low sensitivity. We thus considered that these three markers were less likely to be useful among the six markers in our study. Therefore, we further evaluated the relations of 34βE12, GAL-3 and CK19.
The relations of the immunocytochemical results of the three markers (34βE12, GAL-3 and CK19) were presented in Fig. 2. These diagrams demonstrated that this marker panel is useful for diagnosis of PC. We hypothesized that PC would express positive for more than or equal to two markers of the three markers. As shown in Table 5, according to our diagnostic hypothesis, the sensitivity, specificity, diagnostic accuracy, PPV and PNV were 93.0%, 100%, 96.8%, 100% and 94.4%, respectively. All of these statistical values were higher than or equal to any single value of 34βE12, the most accurate marker in this study. Park et al.12) investigated a combination of markers including GAL-3, HBME-1 and CK19 for diagnosis of thyroid malignancies which included thyroid papillary carcinoma and follicular carcinoma. They evaluated the co-expression of either GAL-3 and HBME-1 or GAL-3 and CK19, and concluded that evaluation of co-expression of GAL-3 and HBME-1 or GAL-3 and CK19 improved diagnostic accuracy for thyroid malignancies, and their sensitivity, specificity and diagnostic accuracy were 93.2%, 100% and 95.3%, respectively. Nga et al.18) also reported the usefulness of PC diagnosis to evaluate the combination of HBME-1 and CK19; sensitivity, specificity and diagnostic accuracy were all 100%, in their small case study (all 22 cases of cytology specimens). Until now, there is no absolutely specific marker for carcinoma or malignancy. We therefore consider that the marker panel might be applied as a combination of several useful markers as the key to improved immunocytochemical diagnosis.
We investigated LBC cell specimens obtained by direct scratch from surgically-removed thyroid lesions. These specimens were considered to be almost similar in morphology to a specimen of fine needle aspiration cytology. But the sampling cell amount might be larger than for fine needle aspiration cytology (data not shown). Our immunocytochemistry results were similar to those of previous reported studies using formaldehyde-fixed paraffin sections and conventional cytological specimens. Therefore, we considered that the results of immunocytochemistry with LBC specimens in our study were available for diagnostic cytology. Moreover, in comparison with the conventional method, LBC is suitable for immunocytochemistry with the use of several markers because it enables easy processing of several slides if samples contain sufficient amounts of cells. We thus considered the LBC technique to be applicable to fine needle aspiration cytology of thyroid and that our immunocytochemical results are applicable.
We concluded that the antibody panel of 34βE12, GAL-3 and CK19 is useful for differential diagnosis of thyroid papillary carcinoma, and that the application of the LBC technique for immunocytochemistry with this panel will contribute to more objective and accurate diagnosis.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the support to K. Hashimoto by a Grant-in-Aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 20790280).
REFERENCES
- 1).Hutchinson ML, Cassin CM, Ball HG, 3rd. The efficacy of an automated preparation device for cervical cytology. Am J Clin Pathol, 1991; 96: 300–315. [DOI] [PubMed]
- 2).Perez-Reyes N, Mulford DK, Rutkowski MA, Logan-Young W, Dawson AE. Breast fine-needle aspiration. A comparison of thin-layer and conventional preparation. Am J Clin Pathol, 1994; 102: 349–353. [DOI] [PubMed]
- 3).Hees K, Lebeau PB. Comparison of conventional and ThinPrep preparations of mucoid cytology samples. Diagn Cytopathol, 1995; 12: 181–185. [DOI] [PubMed]
- 4).Biscotti CV, Hollow JA, Toddy SM, Easley KA. ThinPrep versus conventional smear cytologic preparations in the analysis of thyroid fine-needle aspiration specimens. Am J Clin Pathol, 1995; 104: 150–153. [DOI] [PubMed]
- 5).Leung CS, Chiu B, Bell V. Comparison of ThinPrep and conventional preparations: nongynecologic cytology evaluation. Diagn Cytopathol, 1997; 16: 368–371. [DOI] [PubMed]
- 6).Leung SW, Bedard YC. Immunocytochemical staining on ThinPrep processed smears. Mod Pathol, 1996; 9: 304–306. [PubMed]
- 7).Dabbs DJ, Abendroth CS, Grenko RT, Wang X, Radcliffe GE. Immunocytochemistry on the ThinPrep processor. Diagn Cytopathol, 1997; 17: 388–392. [DOI] [PubMed]
- 8).Cheung CC, Carydis B, Ezzat S, Bedard YC, Asa SL. Analysis of ret/PTC gene rearrangements refines the fine needle aspiration diagnosis of thyroid cancer. J Clin Endocrinol Metab, 2001; 86: 2187–2190. [DOI] [PubMed]
- 9).Xu XC, el-Naggar AK, Lotan R. Differential expression of galectin-1 and galectin-3 in thyroid tumors. Potential diagnostic implications. Am J Pathol, 1995; 147: 815–822. [PMC free article] [PubMed]
- 10).Aratake Y, Umeki K, Kiyoyama K, Hinoura Y, Sato S, Ohno A, Kuribayashi T, Hirai K, Nabeshima K, Kotani T. Diagnostic utility of galectin-3 and CD26/DPPIV as preoperative diagnostic markers for thyroid nodules. Diagn Cytopathol, 2002; 26: 366–372. [DOI] [PubMed]
- 11).Prasad ML, Pellegata NS, Huang Y, Nagaraja HN, de la Chapelle A, Kloos RT. Galectin-3, fibronectin-1, CITED-1, HBME1 and cytokeratin-19 immunohistochemistry is useful for the differential diagnosis of thyroid tumors. Mod Pathol, 2005; 18: 48–57. [DOI] [PubMed]
- 12).Park YJ, Kwak SH, Kim DC, et al. Diagnostic value of galectin-3, HBME-1, cytokeratin 19, high molecular weight cytokeratin, cyclin D1 and p27(kip1) in the differential diagnosis of thyroid nodules. J Korean Med Sci, 2007; 22: 621–628. [DOI] [PMC free article] [PubMed]
- 13).Saleh HA, Feng J, Tabassum F, Al-Zohaili O, Husain M, Giorgadze T. Differential expression of galectin-3, CK19, HBME1, and Ret oncoprotein in the diagnosis of thyroid neoplasms by fine needle aspiration biopsy. Cytojournal, 2009; 6: 18. [DOI] [PMC free article] [PubMed]
- 14).Raphael SJ, McKeown-Eyssen G, Asa SL. High-molecular-weight cytokeratin and cytokeratin-19 in the diagnosis of thyroid tumors. Mod Pathol, 1994; 7: 295–300. [PubMed]
- 15).Raphael SJ, Apel RL, Asa SL. Brief report: detection of high-molecular-weight cytokeratins in neoplastic and non-neoplastic thyroid tumors using microwave antigen retrieval. Mod Pathol, 1995; 8: 870–872. [PubMed]
- 16).Schelfhout LJ, Van Muijen GN, Fleuren GJ. Expression of keratin 19 distinguishes papillary thyroid carcinoma from follicular carcinomas and follicular thyroid adenoma. Am J Clin Pathol, 1989; 92: 654–658. [DOI] [PubMed]
- 17).Nasser SM, Pitman MB, Pilch BZ, Faquin WC. Fine-needle aspiration biopsy of papillary thyroid carcinoma: diagnostic utility of cytokeratin 19 immunostaining. Cancer, 2000; 90: 307–11. [PubMed]
- 18).Nga ME, Lim GS, Soh CH, Kumarasinghe MP. HBME-1 and CK19 are highly discriminatory in the cytological diagnosis of papillary thyroid carcinoma. Diagn Cytopathol, 2008; 36: 550–556. [DOI] [PubMed]
- 19).Cheung CC, Ezzat S, Freeman JL, Rosen IB, Asa SL. Immunohistochemical diagnosis of papillary thyroid carcinoma. Mod Pathol, 2001; 14: 338–342. [DOI] [PubMed]
- 20).Fonseca E, Nesland JM, Hoie J, Sobrinho-Simoes M. Pattern of expression of intermediate cytokeratin filaments in the thyroid gland: an immunohistochemical study of simple and stratified epithelial-type cytokeratins. Virchows Arch, 1997; 430: 239–245. [DOI] [PubMed]
- 21).Miettinen M, Kovatich AJ, Karkkainen P. Keratin subsets in papillary and follicular thyroid lesions. A paraffin section analysis with diagnostic implications. Virchows Arch, 1997; 431: 407–413. [DOI] [PubMed]
- 22).Miettinen M, Karkkainen P. Differential reactivity of HBME-1 and CD15 antibodies in benign and malignant thyroid tumours. Preferential reactivity with malignant tumours. Virchows Arch, 1996; 429: 213–219. [DOI] [PubMed]
- 23).van Hoeven KH, Kovatich AJ, Miettinen M. Immunocytochemical evaluation of HBME-1, CA 19-9, and CD-15 (Leu-M1) in fine-needle aspirates of thyroid nodules. Diagn Cytopathol, 1998; 18: 93–97. [DOI] [PubMed]
- 24).Schroder S, Schwarz W, Rehpenning W, Loning T, Bocker W. Prognostic significance of Leu-M1 immunostaining in papillary carcinomas of the thyroid gland. Virchows Arch A Pathol Anat Histopathol, 1987; 411: 435–439. [DOI] [PubMed]
- 25).Vierbuchen M, Schroder S, Uhlenbruck G, Ortmann M, Fischer R. CA 50 and CA 19-9 antigen expression in normal, hyperplastic, and neoplastic thyroid tissue. Lab Invest, 1989; 60: 726–732. [PubMed]
- 26).Park J, Jung EH, Kim C, Choi YH. Direct-to-vial comparison of a new liquid-based cytology system, liqui-PREP versus the conventional pap smear. Diagn Cytopathol, 2007; 35: 488–492. [DOI] [PubMed]
- 27).Moutsatsos IK, Wade M, Schindler M, Wang JL. Endogenous lectins from cultured cells: nuclear localization of carbohydrate-binding protein 35 in proliferating 3T3 fibroblasts. Proc Natl Acad Sci U S A, 1987; 84: 6452–6456. [DOI] [PMC free article] [PubMed]
- 28).Inohara H, Akahani S, Koths K, Raz A. Interactions between galectin-3 and Mac-2-binding protein mediate cell-cell adhesion. Cancer Res, 1996; 56: 4530–4534. [PubMed]
- 29).Yang RY, Hsu DK, Liu FT. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci U S A, 1996; 93: 6737–6742. [DOI] [PMC free article] [PubMed]
- 30).Gown AM, Vogel AM. Monoclonal antibodies to intermediate filament proteins of human cells: unique and cross-reacting antibodies. J Cell Biol, 1982; 95: 414–424. [DOI] [PMC free article] [PubMed]
- 31).Gown AM, Vogel AM. Monoclonal antibodies to human intermediate filament proteins. II. Distribution of filament proteins in normal human tissues. Am J Pathol, 1984; 114: 309–321. [PMC free article] [PubMed]
- 32).Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell, 1982; 31: 11–24. [DOI] [PubMed]