ABSTRACT
Osteogenic cells have been found within periosteal tissue. Periosteal cells will also form a membranous structure under the appropriate culture conditions. We have characterized the osteogenic potential of this membranous cultured periosteum (CP) and have demonstrated that CP can successfully regenerate alveolar bone defects in a canine periodontitis model. The aim of this study is to demonstrate periodontal tissue regeneration by using CP for patients with severe periodontitis. CP was applied in treatments for severe alveolar bone defects for a total of seven teeth among four periodontitis patients. Bone formation was evaluated by dental radiography 4 months after grafting, with a follow-up period of 12 to 15 months. CP was successfully generated and formed a membrane (approximately 4 cm in diameter) about 4 weeks after attachment to the dish. Vertical bone gain (3 to 8 mm) was observed in all grafted areas at 4 months post-surgery. The probing depth was also reduced to its normal depth and remained so beyond one year. Results from the present cases suggest that periodontitis patients with bone defects can benefit from CP treatment. Post-operative evaluation indicates periodontal tissue regeneration after CP treatment, suggesting a broad application for patients with periodontal disease.
Key Words: Key Words:Cultured periosteum, Guided tissue regeneration, Platelet-rich plasma, Periodontal tissue, Regeneration
ABSTRACT
Osteogenic cells have been found within periosteal tissue. Periosteal cells will also form a membranous structure under the appropriate culture conditions. We have characterized the osteogenic potential of this membranous cultured periosteum (CP) and have demonstrated that CP can successfully regenerate alveolar bone defects in a canine periodontitis model. The aim of this study is to demonstrate periodontal tissue regeneration by using CP for patients with severe periodontitis. CP was applied in treatments for severe alveolar bone defects for a total of seven teeth among four periodontitis patients. Bone formation was evaluated by dental radiography 4 months after grafting, with a follow-up period of 12 to 15 months. CP was successfully generated and formed a membrane (approximately 4 cm in diameter) about 4 weeks after attachment to the dish. Vertical bone gain (3 to 8 mm) was observed in all grafted areas at 4 months post-surgery. The probing depth was also reduced to its normal depth and remained so beyond one year. Results from the present cases suggest that periodontitis patients with bone defects can benefit from CP treatment. Post-operative evaluation indicates periodontal tissue regeneration after CP treatment, suggesting a broad application for patients with periodontal disease.
Key Words:Cultured periosteum, Guided tissue regeneration, Platelet-rich plasma, Periodontal tissue, Regeneration
INTRODUCTION
Periodontal disease is a leading cause of tooth loss among the elderly in Japan.1) As the disease progresses, periodontal tissues (including alveolar bone) are gradually lost and eventually reach a point where the tooth is no longer supported. Although the primary treatment for periodontal disease is a thorough removal of dental calculus, plaque and bacteria from tooth root surfaces, they have been shown insufficient to regenerate lost tissue. The ultimate goal of periodontal treatment is the complete reconstruction of lost periodontal tissues and alveolar bone.
Guided tissue regeneration (GTR) is a surgical treatment procedure for periodontal disease that utilizes a barrier membrane to induce the growth of bone and periodontal tissue at sites where the volumes or dimensions of the periodontal tissue are insufficient. GTR has been proven effective in both animal studies2-4) and clinical trials.5-7) More recently, a matrix derived from enamel has also been used for periodontal tissue regeneration.8) This matrix contains a mixture of proteins, mainly amelogenin, which has been suggested to promote cementum formation on the root surface.9) The above procedures are widely used alone or in combination with bone grafting. Although each of these techniques has gained wide acceptance, their efficacy involves limitations that are influenced by the type and level of the bone defect. Furthermore, complications such as exposure of the membrane, perforation of the gingival flap, abnormal pain and postoperative swelling have been reported, any one of which makes prognosis more unpredictable.10)
Tissue engineering is a novel paradigm for regenerating a tissue by using cells, bioactive molecules and a scaffold made of biodegradable material. Periosteal cells possess osteogenic and chondrogenic potential, and are a unique cell source for bone tissue engineering.11,12) Periosteum can easily be harvested from the oral cavity leaving no outwardly visible scar. The easy access and minimal invasiveness of harvesting periosteum make it a preferred donor site for osteogenic cells. Our laboratory has developed methods for culturing periosteal cells to form a membranous structure, so that the membrane derived from cultured periosteum (CP) can successfully regenerate critical sized bone defects in rats.13) Furthermore, when CP was grafted onto an artificially constructed canine tooth-bone defect, it promoted a regeneration of both the bone defect and the periodontal tissues.14) Qualities such as decreased immunogenicity and superior osteogenic potential make autologous CP a novel treatment adjunct for the regeneration of dysfunctional periodontal tissues.
In this study, CP was used to treat patients with severe periodontal disease with the aim of determining its ability to promote periodontal tissue regeneration.
METHODS
Subjects and preparation of membranous cultured periosteum
The protocol used in this study conformed to the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of the Nagoya University School of Medicine. A total of 4 patients were enrolled from the Nagoya University Hospital. The purpose and procedures were fully explained (including the use of animal derived materials), and written informed consent was obtained from the research subjects together with complete medical and dental histories. Smokers and subjects incurring systemic diseases within the previous 6 months were excluded. Each patient underwent a clinical examination including panoramic and dental radiographs. All patients presented chronic periodontitis with a probing depth of 6 mm or deeper at multiple sites and an attachment loss of 4 mm or greater at the same site. Pre-surgery clinical examination included probing depth, plaque index and teeth mobility. Two clinicians measured all parameters independently, and inter-examiner consistency was established.
A schematic drawing of the procedure used is shown in Fig. 1. Alveolar bone defects of the 4 periodontitis patients are summarized in Table 1. A total of seven teeth were treated with CP grafts. Prior to their participation, patients received brushing instructions and root scaling with planing, and their oral hygiene status was established before surgery. Prior to CP grafting, a square of periosteum (5 × 5 mm) was obtained under local anesthesia from the posterior mandibular body of each patient. Approximately 5 samples of periosteum can be obtained from a 20-mm mucosal incision at the first molar, as shown in Fig. 1. The mucosal layer was detached from the periosteum. As a source for CP, approximately 25-mm2 periosteum samples were peeled from the mandibular body using a scalpel. Periosteum specimens were placed directly in 100-mm culture dishes and left for about 30 min in an incubator at 37°C until the tissue was attached to the dish. Subsequently, culture medium was gently added to avoid the tissue detaching from the dish. The culture medium used was medium 199 (Gibco BRL, Grand Island, NY) supplemented with 10% FBS (Invitrogen, Carlsbad, CA, USA), which was certified to be free from known pathogens, 25 mg of ascorbic acid (Sigma Chemical Co., St. Louis, MO), penicillin (100 IU/ml), streptomycin (100 µg/ml), and amphotericin B (250 ng/ml). The culture medium was changed every 2–3 days. Periosteal cells spread out from the attached periosteum samples and the cell layer gradually increased in thickness. Dishes were maintained until the cells formed a relatively thick membrane that could be handled with forceps (approximately four weeks). At this point, the CP typically contained ~20 to 30 cell layers with extracellular molecules such as type I and II collagens, osteocalcin, osteonectin and osteopontin, with the final thickness of approximately 100–200 µm.14) Alkaline phosphatase activity increased after 2 weeks of culture and was maintained thereafter.24)
Fig. 1.
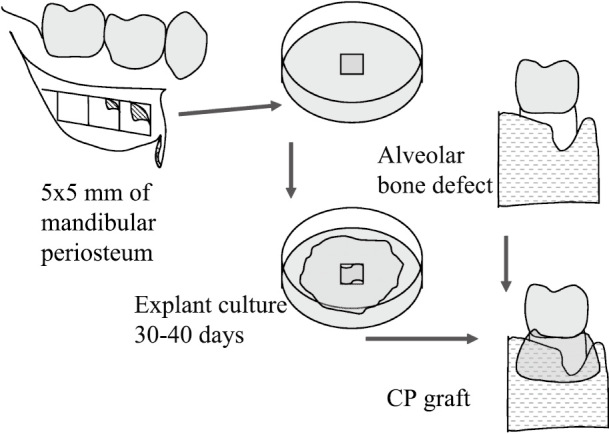
Schematic illustration of study protocol. As the cell source for CP, square-shaped periosteum (5 × 5 mm) was harvested from the posterior mandibular body of each patient under local anesthesia and placed directly on 100-mm culture dishes. Periosteum samples were incubated in culture medium until they formed multiple layers strong enough for grafting. CP membrane was detached from culture dish mechanically without enzymes. Membrane was carried by two forceps to graft site and placed around the exposed roots.
Table 1.
Pre-surgical conditions of each case for CP application
| Cases | Age | Sex | Tooth | Type of defect |
| 1 | 49 | male | left 2nd premolar | vertical |
| 2-1 | 41 | female | right 2nd incisor | horizontal |
| 2-2 | right 2nd molar | vertical | ||
| 3-1 | 54 | female | right 1st incisor | horizontal |
| 3-2 | right 2nd incisor | horizontal | ||
| 4-1 | 61 | female | right 2nd premolar | vertical |
| 4-2 | left 2nd premolar | vertical |
Donor screening
Patients’ serum (4 ml) was screened using the HBs antigen test (CLIA), HBs antibody test (CLIA), HBc antigen test (CLIA), HCV antibody test (RIA solid phase) test, HIV antigen/antibody test (ELISA), Syphilis serology test (RPR), Syphilis serology test (TPHA), HTLV-1 antibody test (CLIA), parvovirus B19 DNA (PCR), and Mycoplasma antibody test (ELISA). Patients showing positive results from any of these precautionary tests were excluded from this study, and then patients underwent a conventional flap procedure and routine periodontal treatment. Peripheral blood (3 ml) was also evaluated by hematological tests including leukocyte count, red blood count, hemoglobin content, hematocrit content, MCV, MCH, MCHC, and platelet count.
Pretest for transplantation
Cell culture supernatant was collected 5 days before the cell harvest and tested by two types of safety checks. First, to test for sterility, the supernatant was spread onto horse blood agar plates (Nissui, Tokyo, Japan) to check for the presence of bacteria or fungi colonies. Second, the supernatant was screened for mycoplasma contamination as follows. Phenol:chloroform:isoamyl alcohol (PCI) (Sigma, MO, USA) was used to extract any mycoplasma DNA. Equal volumes of PCI were added to each supernatant sample (600 µl) and centrifuged at 15,000 rpm for 5 min at room temperature. The upper aqueous layer was transferred to a new tube, mixed with 400 µl ice-cold 100% isopropanol (Wako, Osaka, Japan) and centrifuged at 15,000 rpm for 10 min. The pellet was then rinsed with 400 µl ice-cold 70% ethanol (Wako, Osaka, Japan) and centrifuged at 15,000 rpm for 5 min. The pellet was air dried for 3 min then dissolved in distilled water (20 µl) for subsequent PCR analysis. The PCR reaction (25 µl) consisted of 2.5 µl of 10× buffer, 2 µl of 10 mM dNTPs, 0.5 µl of each primer (10 mM, F1: ACACCATGGGAGYTGGTAAT, R1: CTTCWTCGACTTYCAGACCCAAGGCAT), and 0.1 µl of Taq-DNA polymerase (Takara Bio Inc., Otsu, Japan). Thirty-five cycles of the following conditions were run:
94°C for 1 min for denaturation, 55°C for 30 sec for annealing, and 72°C for 30 sec for polymerization. Amplified DNA was separated on a 1.5% agarose gel and soaked in TAE buffer containing 0.1 µg/ml ethidium bromide. PCR products were analyzed using the Chemi Doc system (BIO-RAD Laboratories, Hercules, CA, USA).
Before harvesting the CP, 5 µl of supernatant was diluted in normal saline solution (Otsuka Seiyaku, Tokyo, Japan), diluted 100-fold and checked for endotoxin using the Endosafe endotoxin testing kit (Charles River, MA, USA). Samples indicating over 1.0 EU were considered positive. Only CPs that passed all three tests were used in this study.
Surgical procedure
After soft tissue curettage, root scaling and planing, the surgical procedure was performed using the conventional flap operation technique. Immediately after the graft recipient site was prepared, the CP membrane was carried to the operating room in a culture dish with reduced medium, then mechanically detached from the base of the dish (i.e., without enzymes). The membrane was transferred with two forceps to the graft site and placed around the exposed roots. For cases 3 and 4, since the defect area was large, the defects were initially filled with autologous platelet-rich plasma to maintain a space for regeneration. The CP graft was then performed by placing the upper end of the membrane around the cervical portion of the tooth and sutured to the surrounding connective tissue using an absorbable thread. The gingival flap was repositioned and protected by a gingival bandage for two weeks.
Platelet-rich plasma (PRP) preparation
PRP used for cases 3 and 4 was prepared according to a previously reported protocol.15) Briefly, approximately 50 ml of peripheral blood was collected in a heparin-coated centrifuge tube and centrifuged at 1100 rpm for 5 min. Yellow plasma was subsequently transferred to a new tube and further centrifuged at 2500 rpm for 10 min to remove platelet-poor-plasma (PPP). The resulting pellet of platelets was resuspended in 5 ml of residual plasma and used to make PRP. Powdered thrombin (10,000 units, Mitsubishi Pharma Co., Tokyo, Japan) was dissolved in 10 ml of 10% calcium chloride. PRP (3.5 ml) and 500 µl of the thrombin/calcium chloride mixture were then loaded into separate syringes. When needed, the PRP and thrombin/calcium mixture were added to a disposable plate and mixed to produce an insoluble PRP gel.
Radiographic analysis of bone regeneration
To monitor bone regeneration, dental radiographs using a bisected-angle technique were taken at three time points: before the operation, three months after, and four months after. Radiographic images were captured with a digital camera at the same magnification (Olympus, Tokyo, Japan), transferred to a computer and manually assessed using Image J software (Scion Corporation, Frederick, MD, USA). The vertical length of the teeth on the three dental radiographs was measured to calibrate any vertical variations. In this study, the precision of tooth measurements on these radiographs was 5.8%. To determine the bone defect or gain, the distance from the cemento-enamel junction to the lowest edge of the alveolar bone was measured before and after the operation, using this vertical calibration.
Clinical observations
Probing depths were measured pre-operatively at six sites around the tooth, and again at 4 months and over one year after the operation. The deepest probing depth for each tooth was compared to evaluate the degree of periodontitis. The follow-up period was 12 to 14 months.
Cases
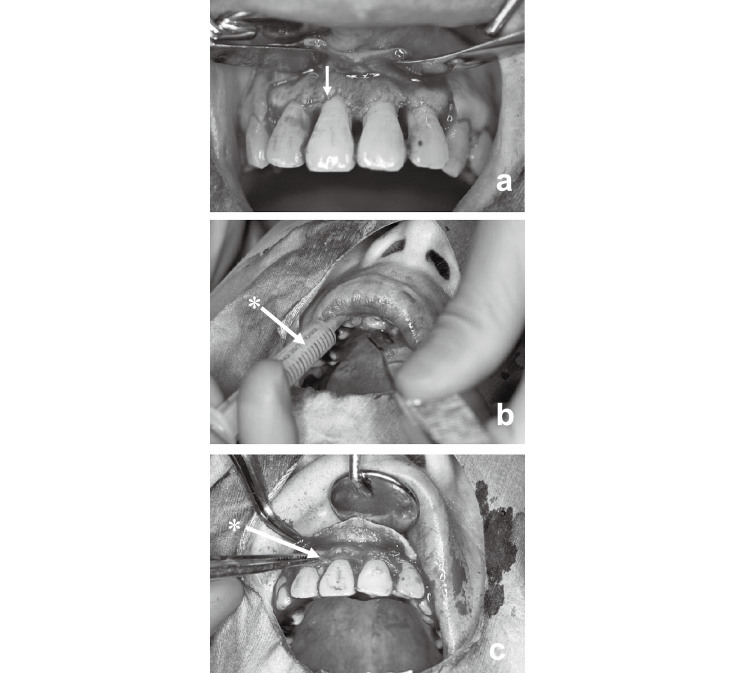
Case 1 was a 49-year-old male. After the initial periodontal treatment including brushing instructions as well as scaling and root planing for 6 months, most of the proving depth was restored (Fig. 2a). However, the proving depth on the distal side of the left 2nd pre-molar remained at 6 mm with a vertical bone defect (Fig. 2b). Autologous CP was prepared and grafted over the defect (Fig. 2c). The 2nd pre-molar was then fixed to the neighboring teeth on the medial and distal sides. The operated site was subsequently routinely checked (Fig. 2d).
Fig. 2.
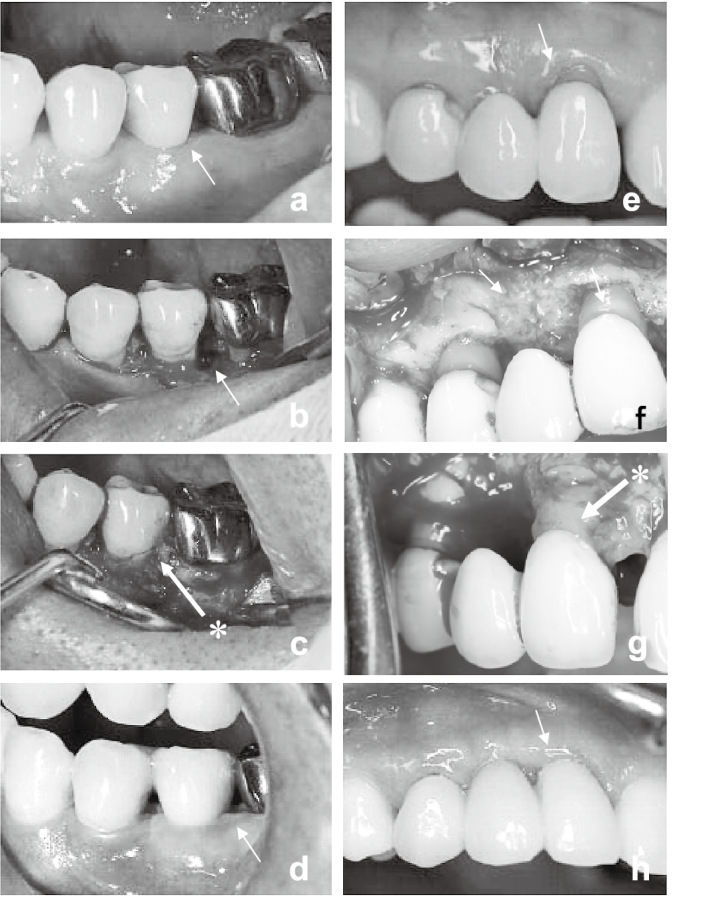
Representative cases: Case 1 (a, b, c, d) and case 2 (e, f, g, h). Pre-surgical findings (a, e). Arrows showing the sites of chronic periodontitis before operation. Preparation of graft sites (b, f). Arrows showing exposed bone defects. CP grafting to defects (c, g). Asterisk (*) showing the grafted CP. Macroscopic observations past one year after operation (d, h). Arrows indicate the treated sites.
Case 2 was a 41-year-old female. After conventional periodontal treatment including brushing instructions, together with scaling and root planing, a horizontal bone defect was observed at the right 2nd incisor with a 6-mm gingival pocket, and a vertical bone defect was also found on the distal side of the right 2nd molar with a 6-mm probing depth (Fig. 2e). After preparation of the graft site, CP was directly placed over the root surface of the 2nd incisor and 2nd molar tooth, and the flap was repositioned as shown (Fig. 2f and 2g). The operated site was also routinely checked afterwards (Fig. 2h).
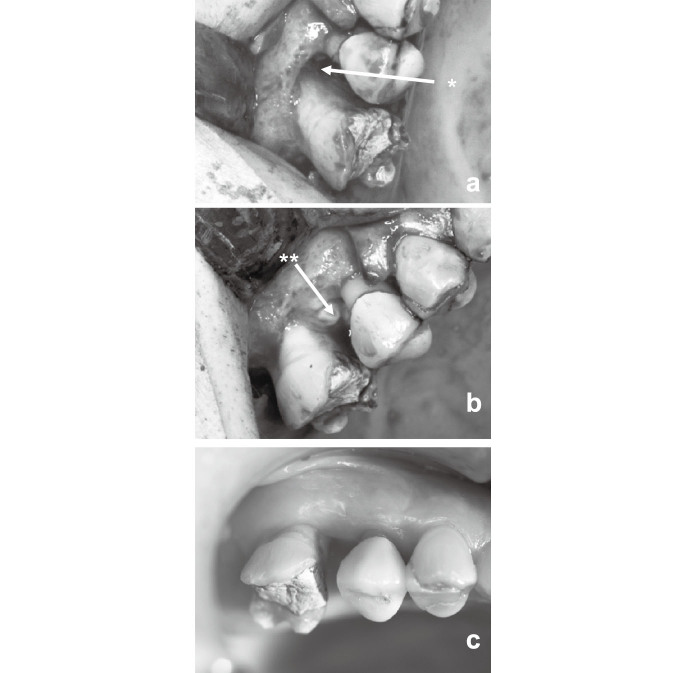
Case 3 was a 54-year-old female. A bone defect presented at the right 1st and 2nd incisors, and the palatal side of both teeth showed a 10- and 9-mm probing depth, respectively (Fig. 3a, Table 2). CP was placed on the bone defect after an autologous platelet-rich plasma (PRP) gel was injected prior to the application of CP to maintain the space and prevent gingival recession after surgery (Fig. 3b and 3c). After placing the PRP gel and CP graft, the flap was repositioned. A detailed protocol for the PRP gel was described previously.
Fig. 3.
Operative procedure of case 3. Panel a showing the grafted site after removal of granulation tissue and root scaling/planing. Panel b showing the placement of PRP gel (*) onto the bone defect. Panel c showing the grafted CP (*) on the PRP gel.
Table 2.
Evaluation of clinical results by pocket depth and radiographical new bone formation
| Cases | Probing depth (mm) |
Baseline bone defect (mm) |
New bone (mm) |
||||
| Before | 4 months | 1 year | Before – 4 months | ||||
| 1 | 6 | 2 | 2 | 5.3–3.8 | +1.5 | ||
| 2-1 | 6 | 3 | 1 | 6.0–3.0 | +3.0 | ||
| 2-2 | 6 | 3 | 1 | 5.0–1.5 | +3.5 | ||
| 3-1 | 10 | 2 | 2 | 2.5–1.0 | +1.5 | ||
| 3-2 | 9 | 3 | 2 | 7.0–3.3 | +3.7 | ||
| 4-1 | 5 | 3 | 2 | 6.5–3.7 | +2.8 | ||
| 4-2 | 9 | 3 | 3 | 5.1–0 | +5.1 | ||
Case 4 was a 61-year-old female. A bone defect presented at the right 2nd and left 2nd premolars. The distal side of the right 2nd molar and the medial side of the left 2nd molar showed 5- and 9-mm probing depths, respectively (Table 2). For the same reason as that in case 3, PRP gel was also used. CP was placed over PRP gel onto the bone defect, and the flap was repositioned.
RESULTS
Generation of cultured periosteum from patients
Human CP was successfully generated and appeared similar to CP made from bovine and canine periosteal cells. On day three, cells started to migrate from the tissue. On or around day 7, the cells began to exhibit signs of extracellular matrix, and some calcified nodules could be observed under a microscope inside the colonies. The number of cells increased rapidly, and together with the extracellular matrix, they formed a membranous structure approximately 4 cm in diameter about 4 weeks after attachment to the culture dish.
Regeneration of defect by new bone formation
In all four patients, no complications related to the CP grafting procedure were observed. Vertical bone gain was noted in all grafted areas 4 months after the operation. Proving depths were also reduced to within normal limits and remained so after at least one year. In all cases of this study, tooth mobility did not change throughout the survey period. These results are summarized in Table 2.
Macroscopic findings
In the 2nd premolar of case 1, a slight inflammation was observed around the gingival flap when the surgical pack was removed, but was shortly resolved. No inflammation was subsequently noted around the tooth (Fig. 2d). The probing depth prior to CP grafting was reduced from 6 mm to 2 mm at its deepest point. The superior probing depth, free of periodontitis, has been retained for one year.
In case 2, the probing depth of the 2nd incisor was reduced from 6 to 3 mm. The final probing depth decreased to 1 mm and was maintained for over one year (Fig. 2h). The probing depth of right second molar was also reduced from 6 to 3 mm.
In case 3, there was a severe bone defect on the palatal side (data not shown) as well as on labial side, exposing the upper root surface (Fig. 3a). After the removal of granulation tissue and root scaling/planing, PRP gel was placed onto the bone defect (Fig. 3b). CP was then grafted on the PRP gel (Fig. 3c). After autologous CP grafting, the probing depth was reduced from 10 to 2 mm, and the root surface was mostly covered by attached gingiva. The 2nd incisor showed a reduction in the pocket from 9 to 3 mm and a new bone height of 3.7 mm.
In case 4, there was a severe bone defect on the distal side of the right 2nd premolar (Fig. 4a), PRP gel was placed on the bone defect (Fig. 4b). After CP grafting, the probing depth was reduced from 5 to 3 mm (Fig. 4c). The probing depth on the medial side of the left 2nd premolar had been reduced from 9 to 3 mm after the operation, though the level of attached gingiva was not fully recovered.
Fig. 4.
Operative procedure of case 4. Panel a showing the site(*) where the vertical bone defect present. Panel b showing the placement of CP on PRP gel (**) over the bone defect. Panel c showing the grafted site after 4 months
Radiographic findings
In case 1, dental X-ray analysis revealed newly formed bone, while the distal alveolar bone height recovered to a level identical to the 1st and 2nd premolars (Fig. 5a and 5b). In case 2, the new bone height of the 2nd incisor had reached 3 mm at 4 months (Fig. 5c and 5d). The distal bone defect of the left 2nd molar was completely filled with newly formed bone (Fig. 5e and 5f). In case 3, dental X-ray analysis was performed 4 months after the operation, revealing alveolar bone regeneration (Fig. 5g and 5h). The new bone height of the right 1st incisor was 9 mm, and the alveolar ridge had been restored. In case 4, dental X-ray analysis that was performed 4 months after the operation and revealed alveolar bone regeneration (Fig. 5i, 5j, 5k and 5l). The new bone heights of the right 2nd and left 2nd premolars were 6 and 8 mm, respectively.
Fig. 5.
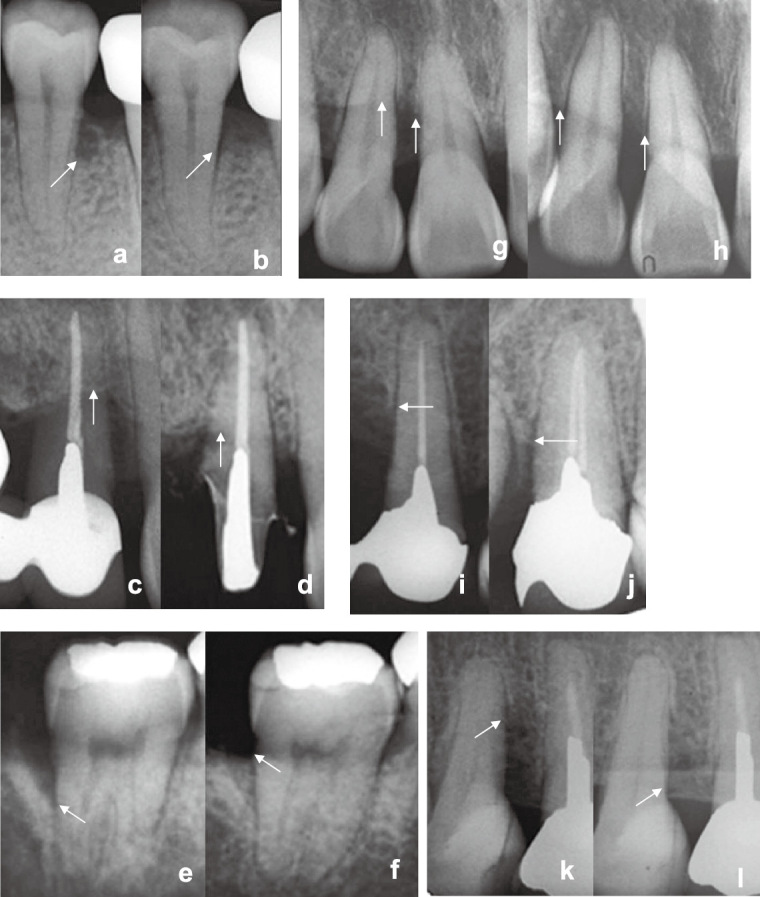
Dental X-ray radiographs of case 1 (a, b), case 2 (c, d, e, f), case 3 (g, h) and case 4 (i, j, k, l) at pre-surgery (a, c, g, i, k) and 4 months after CP grafting (b, d, f, h, j, l). Arrows indicate the treated defects.
DISCUSSION
Despite the limited number of patients treated, results from this study have demonstrated proof-of-concept for periodontal tissue regeneration using CP. CP might be especially effective for horizontal bone defects, which are difficult to treat using conventional methods.16) No adverse events or complications were observed in any patient during a postoperative period lasting over one year. These results exemplified the potential of CP and cultured periosteal cells for the treatment of periodontal disease.
One unique feature of this therapy is the absence of an exogenous scaffold. The current paradigm of tissue engineering is to generate new tissue with cultured or isolated cells seeded onto a designed scaffold. Although artificial scaffolds such as polyglycolic acid and poly lactic acid are useful in forming a three-dimensional structure, host reaction against their degraded products has been known to induce inflammation, leading to rejection of the graft.17-20) The extracellular matrix secreted by periosteal cells within CP acted as a natural scaffold, with a thickness and strength similar to those of a native periosteum.
For the regeneration of periodontal tissue by GTR method, a barrier membrane is used to prevent soft tissue invasion and also to guide tissue regeneration. In our novel method of periodontal regeneration therapy, CP served as a barrier membrane similar to that in the GTR method. However, a major difference should be noted between the GTR method and the current CP-mediated regeneration. In contrast to the conventional GTR membrane, CP had an osteogenic characteristic. Having a membrane structure up to 300 µm in thickness and its osteogenic potential allow CP was able to serve two functions during periodontal tissue regeneration, which may eventually synergize to improve the efficacy of this therapy. The gingival flap also facilitated early neovascularization of the regenerating tissue because of its close contact with the grafted CP. The biological nature of the CP together with the gingival flap constituted another advantage over GTR.21)
Dispersion cell cultures from periosteum present differential phenotypic characteristics and require growth factors to obtain robust osteogenic capacity.22) CP does not require exogenous factors to sustain osteogenic cells and may have an autonomous regulation mechanism.14)
Several craniofacial structures have been engineered using mesenchymal stem cells from various tissues including dental pulp, deciduous tooth, and periodontium.23) Moreover, periosteum is also a source of somatic stem cells. The mechanistic role of periosteum-derived cells for periodontal tissue regeneration is not clear from this study. The regeneration of periodontal tissue requires not only alveolar bone but also periodontal ligament and cementum formation. Theoretically, periosteum contains some type of mesenchymal stem cell(s) that can differentiate into various cell types. Further investigations will be needed to discern the specific role of periosteum-derived cells for periodontal tissue regeneration.
The vertical bone defects of cases 1 and 2 were completely filled with new bone. The horizontal defect of case 2, however, exhibited exposure of the upper root surface after grafting, since there was no matrix to retain the original position of the flap. As in case 3, the use of PRP gel may have been effective in supporting the flap and maintaining the space needed for regeneration when the area of the defect is large. PRP contains various growth factors suggested to enhance bone regeneration.15) More recent reports have diminished the role PRP may play in influencing bone regeneration.24) Although animal experiments using a canine model have shown the efficacy of CP and PRP compared with PRP alone,14) they do not allow exact evaluation of the efficacy of CP in this study. A future study using an artificial scaffold might be able to clarify the influence of PRP on periodontal tissue regeneration with CP.
Animal experiments have shown that CP has osteogenic potential in vivo.13,14) It is unclear whether transplanted CP directly contributes to tissue regeneration in the way cementum and collagen fibers do, by anchoring the root surface to the surrounding bone. From experiments using a canine model, the regeneration of alveolar bone and periodontal tissues has been observed after CP grafting.14) In the present study, the periodontal space was maintained in clinical patients by CP grafting as determined by X-ray analysis, with no sign of root absorption being observed one year after the operation. These findings suggest a regeneration of periodontal tissue by periosteal cells aiding in the reconstruction of periodontal ligament. Previous studies have shown that a complete regeneration of periodontal tissue requires periodontal ligament.25,26) Considering the unique characteristics of periodontal ligament, it is conceivable that CP may have contributed to the regeneration of periodontal ligament and cementum in addition to regenerating alveolar bone.
Despite the growing enthusiasm for the potential of cell-based regenerative medicine, current limitations include the practicality of cell harvest, complexity of the procedure, and the fact that it requires two surgical procedures. Given that the preparation of CP is both costly and labor-intensive, a cost-benefit analysis should be considered. Before the usefulness of this novel treatment can be firmly established, the ethics and economics of these issues should be addressed. However, an important benefit of this procedure is the use of “autologous” cells without an exogenous scaffold. Currently, autologous serum can be used in cell culture intended for therapeutic purposes. If this system can be readily applied to the culture of CP, the clinical merit of CP therapy should be considerably enhanced.
This study has demonstrated the efficacy of CP grafting for patients with periodontal disease. Although the number of patients was limited to only four cases, the promising results achieved should encourage further evaluations of this novel therapy. It is important to expand the number of study subjects and to investigate the procedure’s long-term stability and its efficacy on various types of bone defects.
ACKNOWLEDGEMENTS
This study was partly supported by a grant for “Research on Human Genome and Tissue Engineering” from the Ministry of Health, Labor and Welfare of Japan, and a “Grant-in-Aid for Young Scientists (B)” from the Japanese Government.
REFERENCES
- 1).Ministry of Health, Labor and Welfare. White Paper on Health and Welfare 2000. 2000, Gyosei, Tokyo. (in Japanese).
- 2).Aukhil I, Simpson DM, Schaberg TV. An experimental study of new attachment procedure in beagle dogs. J Periodontal Res, 1983; 18: 643–654. [DOI] [PubMed]
- 3).Caffesse RG, Smith BA, Castelli WA. New attachment achieved by guided tissue regeneration in beagle dogs. J Periodontol, 1988; 59: 589–594. [DOI] [PubMed]
- 4).Niederman R, Savitt ED, Heeley JD, Duckworth JE. Regeneration of furca bone using Gore-Tex periodontal material. Int J Periodontics Restorative Dent, 1989; 9: 469–480. [PubMed]
- 5).Becker W, Becker BE, Berg L, Prichard J, Caffesse R, Rosenberg E. New attachment after treatment with root isolation procedures: report for treated class III and class II furcations and vertical osseous defects. Int J Periodontics Restorative Dent, 1988; 8: 9–23. [PubMed]
- 6).Pontoriero R, Lindhe J, Nyman S, Karring T, Rosenberg E, Sanavi F. Guided tissue regeneration in the treatment of furcation defects in mandibular molars: a clinical study in degree III involvements. J Clin Periodontol, 1989; 16: 170–174. [DOI] [PubMed]
- 7).Dahlin C, Linde A, Gottlow J, Nyman S. Healing of bone defects by guided tissue regeneration. Plast Reconstr Surg, 1988; 81: 672–676. [DOI] [PubMed]
- 8).Craig RG, Kallur SP, Inoue M, Rosenberg PA, LeGeros RZ. Effect of enamel matrix proteins on the periodontal connective tissue-material interface after wound healing. J Biomed Mater Res A, 2004; 69: 180–187. [DOI] [PubMed]
- 9).Hammarstrom L. Enamel matrix, cementum development and regeneration. J Clin Periodontol, 1997; 24: 658–668. [DOI] [PubMed]
- 10).O’Neal R, Wang HL, MacNeil RL, Somerman MJ. Cells and materials involved in guided tissue regeneration. Curr Opin Periodontol, 1994; 2: 141–156. [PubMed]
- 11).Schantz JT, Hutmacher DW, Chim H, Ng KW, Lim TC, Teoh SH. Induction of ectopic bone formation by using human periosteal cells in combination with a novel scaffold technology. Cell Transplant, 2002; 11: 125–138. [PubMed]
- 12).Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum, 2005; 52: 2521–2529. [DOI] [PubMed]
- 13).Mase J, Mizuno H, Okada K, Sakai K, Mizuno D, Usami K, Kagami H, Ueda M. Cryopreservation of cultured periosteum: effect of different cryoprotectants and pre-incubation protocols on cell viability and osteogenic potential. Cryobiology, 2006; 52: 182–192. [DOI] [PubMed]
- 14).Mizuno H, Hata K, Kojima K, Bonassar LJ, Vacanti CA, Ueda M. A novel approach to regenerating periodontal tissue by grafting autologous cultured periosteum. Tissue Eng, 2006; 12: 1227–1335. [DOI] [PubMed]
- 15).Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 1998; 85: 638–646. [DOI] [PubMed]
- 16).Kotschv P, Munzker R. New dimensions in GTR treatment modalities for profound marginal periodontitis. Int J Periodontics Restorative Dent, 1995; 15: 284–297. [PubMed]
- 17).Laurencin CT, Attawia MA, Elgendy HE, Herbert KM. Tissue engineered bone-regeneration using degradable polymers: the formation of mineralized matrices. Bone, 1996; 19: 93S–99S. [DOI] [PubMed]
- 18).Bonassar LJ, Vacanti CA. Tissue Engineering: The first decade and beyond. J Cell Biochem Suppl, 1998; 30/31: 297–303. [PubMed]
- 19).Oreffo RO, Triffitt JT. Future potential for using osteogenic stem cells and biomaterials in orthopedics. Bone, 1999; 25: 5S–9S. [DOI] [PubMed]
- 20).Orban JM, Marra KG, Hollinger JO. Composition options for tissue-engineered bone. Tissue Eng, 2002; 8: 529–539. [DOI] [PubMed]
- 21).Selliseth NJ, Selvig KA. The vasculature of the periodontal ligament: a scanning electron microscopic study using corrosion casts in the rat. J Periodontol, 1994; 65: 1079–1087. [DOI] [PubMed]
- 22).Agata H, Asahina I, Yamazaki Y, Uchida M, Shinohara Y, Honda MJ, Kagami H, Ueda M. Effective bone engineering with periosteum-derived cells. J Dent Res, 2007; 86: 79–83. [DOI] [PubMed]
- 23).Mao JJ, Giannobile WV, Helms JA, Hollister SJ, Krebsbach PH, Longaker MT, Shi S. Craniofacial tissue engineering by stem cells. J Dent Res, 2006; 85: 966–979. [DOI] [PMC free article] [PubMed]
- 24).Casati MZ, de Vasconcelos Gurgel BC, Gencalves PF, Pimentel SP, da Rocha Nogueira Filho G, Nociti FH Jr, Sallum EA. Platelet-rich plasma does not improve bone regeneration around peri-implant bone defects – a pilot study in dogs. Int J Oral Maxillofac Surg, 2007; 36: 132–136. [DOI] [PubMed]
- 25).Boyko GA, Melcher AH, Brunette DM. Formation of new periodontal ligament by periodontal ligament cells implanted in vivo after culture in vitro. J Periodontal Res, 1981; 16: 73–88. [DOI] [PubMed]
- 26).Nyman S, Karring T, Lindhe J, Planten S. Healing following implantation of periodontitis-affected roots into gingival connective tissue. J Clin Periodontol, 1980; 7: 394–401. [DOI] [PubMed]