Abstract
Background
Psychological stress has been widely implicated in asthma exacerbation. Evidence suggests that written emotional disclosure, an intervention that involves writing about traumatic or stressful experiences, helps to reduce stress and promote physical and psychological well‐being. Written emotional disclosure may have a role in the management of asthma.
Objectives
This review aims to determine the effectiveness of written emotional disclosure for people with asthma, specifically, to assess:
1. overall efficacy of emotional disclosure compared with emotionally neutral writing on self reported quality of life in people with asthma;
2. overall efficacy of emotional disclosure compared with emotionally neutral writing on objective measures of health outcome in people with asthma; and
3. comparative efficacy of different types of emotional disclosure for people with asthma.
Search methods
Trials were identified from the Cochrane Airways Group Specialised Register of trials, CENTRAL, MEDLINE, EMBASE, CINAHL, AMED and PsycINFO. The latest search was conducted in January 2014.
Selection criteria
Randomised controlled trials published in any language comparing written emotional disclosure intervention versus a control writing (emotionally neutral) intervention in participants with asthma were included in the review.
Data collection and analysis
Two review authors independently assessed studies against predetermined inclusion criteria and extracted the data. Corresponding authors were contacted when necessary to provide additional information.
Main results
Four studies, involving a total of 414 participants, met the inclusion criteria. Three studies were conducted in adult participants and one in adolescents. The average age of participants ranged from 14 to 43 years. The trials lasted between two months and 12 months. The interventions were based on Pennebaker's method. The risk of bias across most domains of the studies was generally considered to be low, however three of four studies were considered at high risk of bias due to lack of assessor blinding and one study was at high risk of bias for selective reporting. The interpretation of these studies was limited by diverse outcome measurements, measurement tools, control group techniques, and number and/or times of follow‐up. A pooled result from the four studies, including a total of 146 intervention and 135 control participants, indicated uncertain effect in forced expiratory volume in one second (FEV1) % predicted between the disclosure group and the control group (mean difference (MD) 3.43%, 95% confidence interval (CI) ‐0.61% to 7.47%; very low‐quality evidence) at ≤ three months' follow‐up. Similarly, evidence from two studies indicated that written emotional disclosure found uncertain effect on forced vital capacity (FVC) (standardised mean difference (SMD) ‐0.02, 95% CI ‐0.30 to 0.26; low‐quality evidence) and asthma symptoms (SMD ‐0.22, 95% CI ‐0.52 to 0.09; low‐quality evidence) but may result in improved asthma control at ≤ three months' follow‐up (SMD 0.29, 95% CI 0.01 to 0.58; low‐quality evidence). We were unable to pool the data for other outcomes. Results from individual trials did not reveal a significant benefit of written emotional disclosure for quality of life, medication use, healthcare utilisation or psychological well‐being. Evidence from one trial suggests a significant reduction in beta agonist use (MD ‐1.62, 95% CI ‐2.62 to ‐0.62; low‐quality evidence) at ≤ three months' follow‐up in the disclosure group compared with controls. The review did not address any adverse effects of emotional writing.
Authors' conclusions
Evidence was insufficient to show whether written emotional disclosure compared with writing about non‐emotional topics had an effect on the outcomes included in this review. Evidence is insufficient to allow any conclusions as to the role of disclosure in quality of life, psychological well‐being, medication use and healthcare utilisation. The evidence presented in this review is generally of low quality. Better designed studies with standardised reporting of outcome measurement instruments are required to determine the effectiveness of written emotional disclosure in the management of asthma.
Keywords: Adolescent; Adult; Humans; Disclosure; Writing; Asthma; Asthma/psychology; Asthma/therapy; Forced Expiratory Volume; Psychotherapy; Psychotherapy/methods; Randomized Controlled Trials as Topic; Stress, Psychological; Stress, Psychological/therapy
Plain language summary
Writing about emotional topics for asthma control and well‐being
Background
Stress may cause worsening of asthma. Previous studies showed that "written emotional disclosure," an activity that encourages people to write about stressful experiences, helps to reduce stress and improve well‐being. Therefore written emotional disclosure may have a role in the management of asthma by reducing stress.
Review question
We reviewed the medical literature to find out whether written emotional disclosure improves lung function and asthma symptoms in asthmatic patients. We looked at studies that compared the effectiveness of completing written emotional disclosure versus writing about topics unrelated to emotion.
Study characteristics
Four studies, involving 414 participants, were included in this review. The trials lasted between two months and 12 months. One study was conducted in the UK, the other three in the USA. All studies compared emotional disclosure writing versus non‐stressful writing. Three studies were conducted in adult participants and one in adolescents. The average age of participants ranged from 14 to 43 years. In all trials, most of the participants were female.
Key results
There is no evidence to support that written emotional disclosure is helpful in improving lung function or symptoms in patients with asthma. However, disclosure may be beneficial for patients' perceptions of their own asthma control. Based on evidence obtained from the studies, we are not able to draw conclusions about the role of written emotional disclosure in quality of life, psychological well‐being, asthma medication use or use of healthcare facilities for asthma‐related problems. Better designed studies are necessary to determine the effects of written emotional disclosure for patients with asthma.
Quality of the evidence
Our interpretation of the studies was limited by variation in study settings, topics of the non‐stressful writing exercise and study duration. The evidence presented in this review is generally of low quality. This summary was current to January 2014.
Summary of findings
for the main comparison.
| Written emotional disclosure compared with neutral writing for asthma | ||||||
|
Patient or population: adults and children with asthma Settings: home, healthcare, community and university settings Intervention: written emotional disclosure Comparison: neutral writing | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Neutral writing | Written emotional disclosure | |||||
|
Average FEV1 % predicted value Follow‐up: 2 to 3 months |
Mean FEV1 % predicted ranged across control groups from 65.8% to 94.76% | Mean FEV1 % predicted in the intervention groups was
3.43% higher (‐0.61% lower to 7.47% higher) |
286 (4 studies) |
⊕⊝⊝⊝ very low1,3,4 | Fixed effect I2 = 2% | |
|
Average FVC value Follow‐up: 2 to 3 months |
See comment | Mean FVC in the intervention groups was
‐0.02 standard deviations lower (‐0.30 lower to 0.26 higher ) |
SMD ‐0.02 (‐0.30 to 0.26) | 197 (2 studies) |
⊕⊕⊝⊝ low2,4 | Fixed effect I2 = 0% As studies reported FVC on different scales, we pooled using SMD. No significant group difference in FVC between the 2 groups |
|
Quality of life: Marks Asthma Quality of Life Questionnaire Follow‐up: 3 months (higher score indicated greater impact on quality of life; scales from 1 to 7) |
See comment | See comment | 108 (1 study) | ⊕⊕⊝⊝ low4,5 | Only 1 trial contributed to this outcome, so we were unable to pool data | |
|
Asthma symptoms Follow‐up: 2 to 3 months (different self report questionnaires, lower scores mean fewer symptoms) |
Mean symptom score for control group ranged from 11.05 to 18.25 | Mean asthma symptoms in the intervention groups were
‐0.22 standard deviations lower (‐0.52 lower to 0.09 higher ) |
SMD ‐0.22 (‐0.52 to 0.09) | 166 (2 studies) | ⊕⊕⊝⊝ low2,4 | As studies reported asthma symptoms on different scales, we pooled using SMD. No significant group difference in asthma symptoms between the 2 groups |
|
Asthma control Follow‐up: 2 to 3 months (different instruments) |
See comment | Mean asthma control in the intervention groups was
0.29 standard deviations higher (0.01 higher to 0.58 higher ) |
SMD 0.29 (95% CI 0.01 to 0.58) | 194 (2 studies) |
⊕⊕⊝⊝ low2,6 | Fixed effect I2 = 0% As studies reported asthma control on different scales, we pooled using SMD |
|
Beta agonist use; puffs/d Follow‐up: 3 months |
See comment | See comment | 117 (1study) |
⊕⊕⊝⊝ low5,6 | Only one trial contributed to this outcome, so we were unable to pool data | |
|
Asthma distress; Asthma Bother Profile Follow‐up: 3 months |
See comment | See comment | 101 (1 study) |
⊕⊕⊝⊝ low4,5 | Only one trial contributed to this outcome, so we were unable to pool data | |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; FEV1: Forced expiratory volume in one second; FVC: Forced vital capacity; SMD: Standardised mean difference. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Outcome assessors were not blinded in 3 of 4 studies.
2Outcome assessors were not blinded in 1 of 2 studies.
3One study was judged to be at high risk of bias for selective reporting.
4Confidence interval includes important benefit and no effect.
5Single study.
6Wide confidence interval.
Background
Description of the condition
Asthma is a chronic inflammatory disease that is associated with heightened irritability of the airways and reversible, episodic airway obstruction (Beasley 2004). The World Health Organization (WHO) estimates that 235 million people currently suffer from asthma (WHO 2013); furthermore, rates of asthma are likely to increase as more communities become urbanised (Beasley 2004). This continued growth will generate an additional treatment and diagnostic burden for healthcare systems, affecting both developed and developing countries. Despite the availability of a range of pharmaceutical treatments for people with asthma, many still experience residual and troublesome symptoms that impair their quality of life. Thus, there remains a need for complementary interventions that are effective, easily accessible and ideally low in cost.
Description of the intervention
The emotional disclosure intervention (also known as expressive writing) was originally developed by Pennebaker and Beall in 1986 (Pennebaker 1986)and was based on the idea that being unable to share experiences or inhibiting emotions following a stressful or traumatic event is associated with poorer psychological and physical health (Pennebaker 1986a). The emotional disclosure intervention asks people to disclose traumatic or stressful experiences through writing. Proponents suggest that emotional disclosure can have positive effects on both physical and psychological health, and several theories have been proposed to explain these benefits. Initial explanations stemmed from the Freudian theory of catharsis, whereby inhibiting stressful or traumatic events leads to impairment of physical and mental health, which can be alleviated by disclosure (Pennebaker 1986a; Pennebaker 1993). More recently, emerging theories regarding cognitive processing have suggested that enabling individuals to write about a stressful or traumatic experience allows creation of a coherent narrative, which, in turn, leads to insight into and an understanding of the experience (Pennebaker 1993). A further explanation is that benefit occurs via self regulation, as written disclosure of both real and imaginary trauma provides the individual with a mastery experience, allowing expression and control of emotions (Lepore 2002). Emotional disclosure can also be conducted within 'talking therapies'; however, this type of emotional disclosure may be influenced by different psychological processes and is excluded from this systematic review. If effective, written emotional disclosure would provide an inexpensive and safe adjunct to pharmacotherapy in the routine care of asthma.
Why it was important to complete this review
Several research syntheses regarding written emotional disclosure have reported variable and conflicting results.
Smyth 1998 reviewed 13 studies conducted with healthy participants. Results suggested that written emotional disclosure significantly enhanced physical health, psychological well‐being, physiological functioning and general functioning.
Frisina 2004 carried out a meta‐analysis of nine studies conducted in people diagnosed with physical or psychiatric disorders; the analysis demonstrated a positive effect on physical health outcomes.
Meads 2005 carried out a meta‐analysis of 60 studies that included both healthy participants and those with preexisting morbidity. The analysis showed no significant difference in health centre visits between treatment groups. However, Meads 2005 excluded many positive studies from the analysis because they did not report mean differences, even though effect sizes could be calculated from other reported data. This biased the analysis towards a negative result.
Frattaroli 2006 carried out a meta‐analysis by using a comprehensive definition of a disclosure task that involved writing (or talking) about a real or imagined significant life event or personal topic. Included were 146 trials of healthy participants, trials including participants with a diverse range of health conditions and trials in participants who had experienced traumatic events such as sexual assault. In contrast to earlier meta‐analyses (Frisina 2004; Smyth 1998), Frattaroli 2006 calculated composite effect sizes drawn from data extracted from a number of different rating scales related to psychological and physical health. This analysis showed statistically significant differences favouring disclosure.
All previously published reviews have explored the effects of written emotional disclosure on broad populations that may not be suitable for meta‐analysis. Such an approach overlooks that differing illnesses can place different stressors on a person (e.g. through the invasiveness of needed medical treatment or the disease prognosis), and so the effects of written emotional disclosure may be disease specific. The specific effects of written emotional disclosure for patients with asthma are unclear.
In asthma, a well‐evidenced link has been identified between stress and exacerbation of asthma symptoms (Wright 1998). Therefore, in comparison with other illness, written emotional disclosure has a particular potential to affect health outcomes through reduction of stress caused by distressing or traumatic experiences. Two Cochrane systematic reviews (Yorke 2005; Yorke 2009) have focused on the use of psychological interventions for asthma (one focusing on adults, the other focusing on children) and were unable to draw conclusions. As written emotional disclosure is not delivered by a therapist, it did not fall within the scope of either of these reviews. The current review complements these two existing Cochrane reviews by exploring the effectiveness of expressive writing for people with asthma.
In summary, existing published reviews on written emotional disclosure have used small and heterogeneous samples with diverse outcome measures, thereby preventing firm conclusions. Existing reviews on psychological therapies for asthma have not included written emotional disclosure. This review addresses these problems by focusing specifically on the effects of emotional disclosure for patients with asthma.
Objectives
This review aims to determine the effectiveness of written emotional disclosure for people with asthma, specifically, to assess:
overall efficacy of emotional disclosure compared with emotionally neutral writing on self reported quality of life in people with asthma;
overall efficacy of emotional disclosure compared with emotionally neutral writing on objective measures of health outcome in people with asthma; and
comparative efficacy of different types of emotional disclosure for people with asthma.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) assessing the effectiveness of an emotional writing intervention for people with asthma. We excluded quasi‐randomised trials.
Types of participants
This review included people diagnosed with asthma by a general practitioner or consultant or according to standard guidelines for diagnosis (e.g. British Thoracic Society, American Thoracic Society). We included both men and women of any age.
Studies conducted in all settings (including hospital, family practice and the community) were considered, as no preexisting theoretical reason suggested that setting affected outcome, and this approach makes the findings more generalisable.
Types of interventions
Written emotional disclosure interventions based on the protocol developed by Pennebaker et al (Pennebaker 1986) or on the guided disclosure intervention developed by Gidron et al (Gidron 2002) were included.
Studies that included a control group given a non‐emotional writing intervention (such as writing about time management) were included in the review. Studies that included a non‐writing control group were excluded to control for the potential effect of the writing process itself and/or the allocation of time to reflect on outcomes. Only studies with a neutral writing control group were included to ensure that any observed treatment effects were due to the emotional component of the writing rather than to the writing itself.
Types of outcome measures
Primary outcomes
Physiological measure of lung function (forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC)).
Secondary outcomes
Self reported quality of life using a validated questionnaire.
Self reported symptom scores using a validated questionnaire.
Self reported medication use.
Scheduled or unscheduled healthcare utilisation.
Psychological well‐being based on a validated questionnaire.
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Group Specialised Register of trials (CAGR); the Cochrane Central Register of Controlled Trials (CENTRAL) 2014, Issue 12, part of The Cochrane Library; MEDLINE (Ovid) 1950 to January week 1, 2014; EMBASE (Ovid) 1974 to week 2, 2014; CINAHL (Ebsco) 1982 to January 2014; AMED (Ebsco) 1985 to January 2014; and PsycINFO (Ovid) 1806 to January, week 1, 2014. The search strategies used for each database are presented in Appendix 1. The latest searches were conducted in January 2014, with no restriction on language or type of publication. Handsearched conference abstracts and grey literature were searched through the CENTRAL database and the CAGR.
Searching other resources
We reviewed reference lists of all primary studies and review articles to look for additional references. Authors of identified trials were contacted and were asked to identify other published and unpublished studies. We contacted experts in the field, including Professor JW Pennebaker, who originated the written emotional disclosure protocol. The search attempted to identify all relevant studies, irrespective of language.
Data collection and analysis
Selection of studies
Two of three review authors (AT, PH and PP) independently assessed the relevance of abstracts identified by the search against the inclusion criteria. Full‐text articles were obtained for studies potentially fulfilling the inclusion criteria. The same review authors independently assessed each study against the inclusion criteria using a study selection form. Disagreements were resolved through discussion to reach a consensus decision with all review authors. Contact with study investigators was made when necessary to clarify eligibility. Reasons for inclusion or exclusion were recorded on the study selection form.
Data extraction and management
Data from each eligible study were extracted using a specifically designed form. Data extraction was completed independently by two review authors (PH and PP). The review authors were not blinded to the study authors nor to the publishing journal of each paper. On completion, results were compared and inconsistencies resolved by discussion. Aspects of study design, participant characteristics, interventions and outcomes were described and entered into RevMan 5 software.
We attempted to contact the authors of studies by using corresponding email addresses provided in the original articles or institutional Websites to ask for additional information required for the review that had not been included in the original article.
Assessment of risk of bias in included studies
Two review authors (PP and PH) independently performed the risk of bias assessment for each study. To facilitate assessment, information was collected by using The Cochrane Collaboration tool to assess risk of bias (Table 8.5.a in the Cochrane Handbook for Systematic Reviews of Interventions, version 5.0.0). For each domain, the procedures undertaken for each study were documented, including verbatim quotes. A judgement was made as to the possible risk of bias for each of the six domains, with the answer 'low' indicating low risk and the answer 'high' indicating high risk of bias. If insufficient detail was reported in the study, a judgement of 'unclear' was made, and the original study investigators were contacted to provide more information with the judgement adjusted accordingly. Graphic representations of potential bias within and across studies were computed using RevMan 5 software.
Measures of treatment effect
For continuous data, end point scores were expressed as mean differences (MDs) or standardised mean differences (SMDs) with associated 95% confidence intervals (CIs). For dichotomous data, the number of participants for each outcome event was entered into a 2 × 2 contingency table, and the fixed‐effect odds ratio (OR) with 95% CIs was reported.
Dealing with missing data
We contacted the authors of studies to ask for information not reported in the original article that was required for the review.
Assessment of heterogeneity
We assessed heterogeneity of the trials through visual inspection of forest plots and calculation of the I2 statistic in RevMan 5. We used a 50% limit to indicate substantial heterogeneity (Higgins 2011) and intended to explore the reasons for statistical variation if results exceeded this limit.
Assessment of reporting biases
We intended to use funnel plots to assess the possibility of publication bias if we found more than 10 studies; however, given the relatively small number of studies reporting each outcome, we did not undertake such assessment.
Data synthesis
Data extracted from each study were entered into a summary table to enable comparison of study characteristics, quality and results. As variation was noted in numbers and/or times of follow‐up measurement across studies, we have extracted and presented data for short‐term (≤ three months), medium‐term (> three months to six months) and long‐term (> six months) outcomes. If more than one measurement was taken during an outcome period, we used the longest follow‐up time measurement in our analysis.
We performed the analysis with RevMan 5 software, using fixed‐effect models.
Subgroup analysis and investigation of heterogeneity
We planned to evaluate the effectiveness of written emotional disclosure with regard to:
asthma severity (as defined by FEV1 baseline reading); and
age (< 18 years vs ≥ 18 years).
We planned to present and analyse self reported quality of life at one, three and six months' follow‐up rather than using only the longest follow‐up time.
None of these analyses were undertaken because of the small number of studies in the meta‐analysis.
Sensitivity analysis
Sensitivity analyses that were planned to recalculate the meta‐analysis by excluding studies of poorer quality were not undertaken because of the small number of studies.
Results
Description of studies
Study design
All four included studies were RCTs. Three studies were published between 1999 and 2006 (Harris 2005; Smyth 1999; Warner 2006). One study was unpublished, the draft of which was provided by the author (Smith 2013). Trial duration varied from two months to 12 months. All included studies were conducted in developed countries: One study was conducted in the UK (Smith 2013), the other three in the USA. Three studies had one intervention group and one control group (Smith 2013; Smyth 1999; Warner 2006). The fourth study (Harris 2005) consisted of two intervention groups and one control group, but for the purposes of this review, only the results obtained for the expressive writing intervention arm have been included. Only one study was described as double‐blind (Smith 2013).
Study participants
A total of 414 participants were included in this review. Three studies were conducted in adult participants (Harris 2005; Smith 2013; Smyth 1999) and one in adolescents (Warner 2006). All studies, except Warner 2006, reported a sample size calculation. The numbers of eligible and randomly assigned participants were detailed in all studies. Harris 2005 approached 168 participants; 163 were eligible and 137 were randomly assigned. Smith 2013 screened 267 eligible participants, and 146 were randomly assigned. Warner 2006 screened 222 participants, 70 of whom were eligible, and all 70 were randomly assigned. Smyth 1999 identified 210 potentially eligible participants; 180 were eligible, and only 61 were randomly assigned. Three studies provided a flow diagram of participants' adherence and dropout rates at various phases of the study and detailed the reasons for attrition (Harris 2005; Smith 2013; Smyth 1999). The fourth study reported the magnitude of attrition between pre/post assessments but did not report the reason for the withdrawal (Warner 2006). However, this study reported that the characteristics of non‐completers were similar to those of completers.
Average age of participants in the trials ranged from 14 years (Warner 2006) to 43 years (Harris 2005). In all trials, most of the participants were female. Settings of studies varied and included home (Smith 2013), healthcare settings (Harris 2005; Smyth 1999; Warner 2006) and community and university settings (Harris 2005). Presentation with physician‐diagnosed asthma was an entry criterion in all studies. Asthma severity was measured in a variety of ways. Warner 2006 included participants with at least mild persistent asthma, Smith 2013 included participants who required regular inhaled medication (British Thoracic Society step two and above) and severity was not reported in other studies. Participants in the trials were excluded if they were unable to write for 20 minutes (Harris 2005; Smyth 1999), had received psychotherapy or had a defined psychiatric disorder (Harris 2005; Smith 2013; Smyth 1999; Warner 2006), had used a medication that could interfere with symptom reports (Smyth 1999; Warner 2006), had been diagnosed with chronic obstructive pulmonary disease (COPD) by a physician (Harris 2005) or had only seasonal or exercise‐induced asthma (Warner 2006). In an attempt to exclude patients who may have COPD, Smith 2013 excluded patients over 45 years of age. Smith 2013 also excluded patients who were unable to understand English or who had work or travel commitments during the trial period.
Outcome measures
A variety of outcomes were measured in the studies. Lung function was assessed in all studies: Harris 2005 measured FEV1 and FVC % predicted, Smith 2013 reported FEV1 % predicted and FVC (absolute value); other studies measured FEV1 % predicted only. Only one study measured quality of life (Smith 2013). Asthma symptoms were evaluated in two studies (Smith 2013 used a validated questionnaire; Warner 2006 used a scale that had been previously used but not clearly validated). Medication use was measured in one study (Smith 2013) and healthcare utilisation in two studies (Harris 2005; Smith 2013). In addition, some researchers measured psychological well‐being such as asthma distress (Smith 2013), stress (Harris 2005), positive affect and negative affect, with internalisation of behaviour problems (Warner 2006).
Intervention used
All studies adapted the intervention from the brief written emotional disclosure exercise developed by Pennebaker and Beall (Pennebaker 1986). Participants in the intervention group wrote on the most stressful experience that they had ever undergone. The written exercise for control groups varied across studies and included writing on neutral events of the previous day (Harris 2005), writing a factual account of a specific activity during the day (Smith 2013), writing plans for the day (Smyth 1999) and writing about time management (Warner 2006). In three studies, participants wrote for 20 minutes a day for three consecutive days (Smith 2013; Smyth 1999; Warner 2006); in one study, three sessions were spread over three consecutive weeks (Harris 2005). Warner 2006 encouraged participants to write on the same topic for three sessions, but participants in other studies were instructed to write about the same topic or to move from one topic to another. Location of the writing session varied across studies: Sessions occurred at home (Smith 2013; Warner 2006 ) or in the laboratory (Smyth 1999) or in both places (Harris 2005). In all studies, participants were asked to write continuously without worrying about spelling or writing style.
Results of the search
A total of 517 references were identified by electronic literature searches up to January 2014. Six additional references were identified through other sources. Of these 523, 17 studies were retrieved for further scrutiny. The article selection process is presented in a PRISMA diagram (Figure 1).
1.
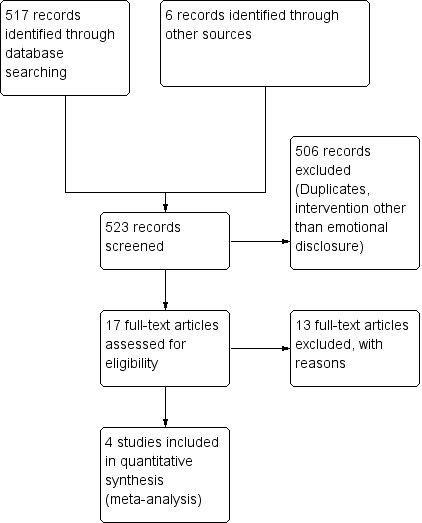
Flow chart of article selection process for the review.
Included studies
This review includes four randomised controlled trials that met the eligibility criteria. Summary details of the included studies are given below. Details of individual studies are summarised in the table Characteristics of included studies.
Excluded studies
In total, 13 studies failed to meet the eligibility criteria; reasons for exclusion have been provided in Characteristics of excluded studies. Seven studies (54%) did not include written emotional disclosure as an intervention; two (15%) were qualitative studies, one trial was non‐randomised (8%) and another was a narrative review of written emotional disclosure in different conditions (8%). Of the trials remaining, one (8%) consisted of multiple interventions, and it was impossible to tease out the effects of disclosure writing alone; the other trial (8%) also included participants with COPD and idiopathic pulmonary fibrosis. See Characteristics of excluded studies.
Risk of bias in included studies
Overall, the methodological quality of papers was good. Complete details on risk of bias judgements are described in Characteristics of included studies and in Figure 2 and Figure 3.
2.
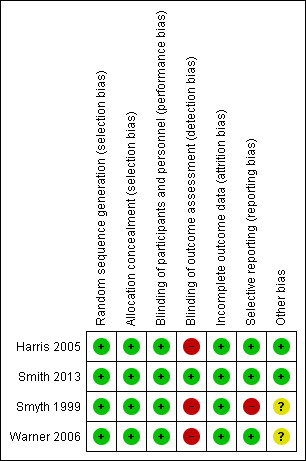
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
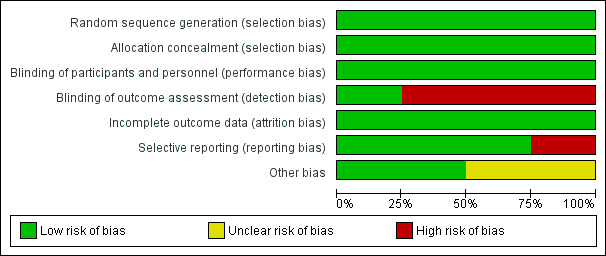
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Sequence generation was carried out adequately in all studies; computer‐generated programmes were used for randomisation. All studies used sealed envelopes for allocation and hence were judged to be at low risk of bias for allocation concealment (Harris 2005; Smith 2013; Smyth 1999; Warner 2006).
Blinding
Only one of the four included trials was double‐blind (Smith 2013). The other three studies (Harris 2005; Smyth 1999; Warner 2006) reported blinding of participants but did not describe blinding of outcome assessors; hence we judged these three studies to be at high risk of bias.
Incomplete outcome data
All studies had similar numbers of dropouts in the intervention and control groups and similar reasons for missing data and therefore were judged as having low risk of attrition bias. Only one study reported intervention‐related dropout (Harris 2005); two participants in the disclosure group discontinued, as they feared the writing task would be too upsetting
Selective reporting
Two studies reported measuring outcomes including lung function, asthma symptoms and psychological well‐being (Smith 2013; Warner 2006 ) and thus were judged to be at low risk of bias for selective reporting. Harris 2005 reported FEV1 % predicted and FVC % predicted, and Smyth 1999 reported FEV1 % predicted only. Corresponding authors of these two studies were contacted to provide further information on other outcomes. Harris 2005 provided data on additional outcomes (such as perceived stress, asthma control) and thus was judged to be at low risk of bias. Smyth 1999 confirmed the measurement of additional outcomes but provided no analyses of the data; hence, the study was judged to be at high risk of bias for selective reporting.
Effects of interventions
See: Table 1
Relevant data were entered and forest plots created. However, we were able to pool only the data for FEV1 % predicted, FVC, asthma symptoms and asthma control. We could not pool data for other outcomes because of divergent outcome measurements and measurement tools. Results are presented below for each outcome, starting with our primary outcome of lung function.
Lung function
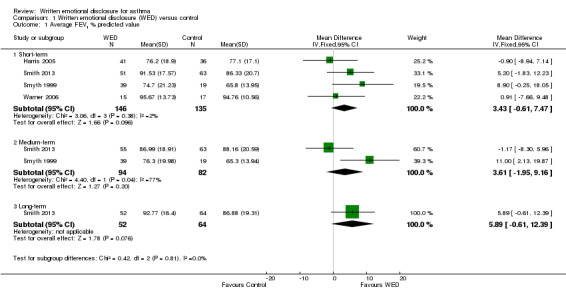
FEV1
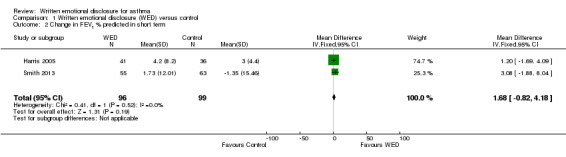
All four studies measured FEV1 % predicted (Harris 2005; Smith 2013; Smyth 1999; Warner 2006). A pooled analysis of the homogenous data (I2 = 2%; P value 0.38), including a total of 146 intervention and 135 control participants, indicated no statistically significant differences in FEV1 % predicted between the emotional disclosure group and the control group at short‐term follow‐up (MD 3.43%, 95% CI ‐0.61% to 7.47%). Evidence was rated as of very low quality after it had been downgraded for lack of assessor blinding, publication bias and imprecision because the confidence intervals included significant benefit and no effect.
A pooled result from two studies on 176 participants (Harris 2005; Smith 2013) showed that disclosure writing did not significantly improve FEV1 % predicted in participants with asthma at medium‐term follow‐up (MD 3.61%, 95% CI ‐1.95 to 9.16). Only one study measured FEV1 % predicted at long‐term follow‐up (Smith 2013) and reported no significant benefit of disclosure writing in participants with asthma (MD 5.89%, 95% CI ‐0.61 to 12.39) (Figure 4).
4.
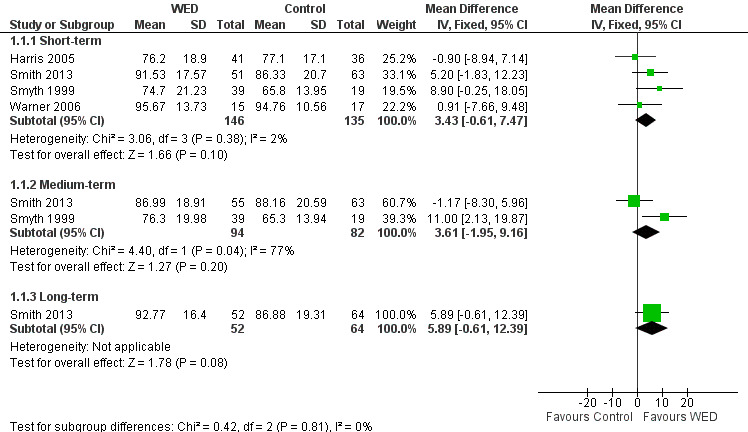
Forest plot of comparison: 1 Written emotional disclosure (WED) versus control, outcome: 1.1 Average FEV1 % predicted value.
FVC
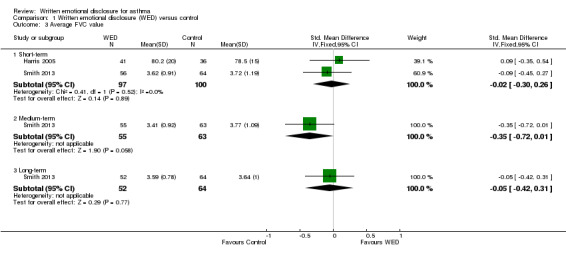
Two studies on 197 participants measured FVC (Harris 2005; Smith 2013). Harris 2005 reported FVC % predicted values, and Smith 2013 reported absolute FVC values. A pooled result from these two studies indicated that disclosure writing did not result in significant improvement in FVC in participants with asthma at short‐term follow‐up (SMD ‐0.02, 95% CI ‐0.30 to 0.26). The outcome was rated as of low quality after it had been downgraded for lack of assessor blinding and imprecision because the confidence intervals included significant benefit and no effect.
One study measured FVC (absolute value) at medium‐ and long‐term follow‐up (Smith 2013). This study found no significant differences in FVC values between the disclosure writing group and the control group at both follow‐up periods (Figure 5).
5.
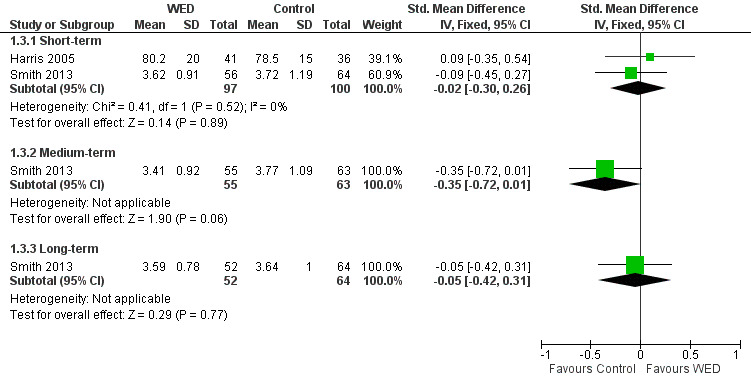
Forest plot of comparison: 1 Written emotional disclosure (WED) versus control, outcome: 1.3 Average FVC value.
Asthma quality of life
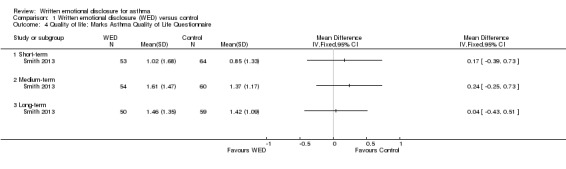
The impact of written emotional disclosure interventions on disease‐specific quality of life was assessed in one trial (Smith 2013). This study used the Marks Asthma Quality of Life Questionnaire (Marks 1992), a validated tool in which a higher score indicates greater impairment in quality of life. No significant difference in quality of life was found between the emotional disclosure group and the control group at any point of follow‐up (Figure 6). Evidence was rated as of low quality and was downgraded for imprecision because the confidence intervals show significant benefit and no effect, and because evidence was based on a single trial.
6.
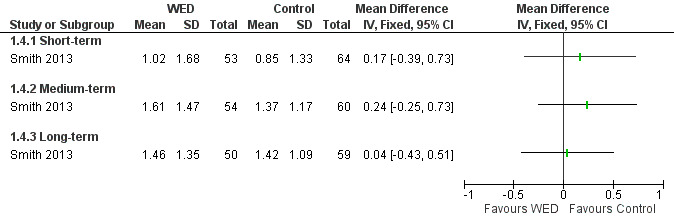
Forest plot of comparison: 1 Written emotional disclosure versus control, outcome: 1.3 Quality of life; Marks Asthma Quality of Life Questionnaire.
Asthma symptoms
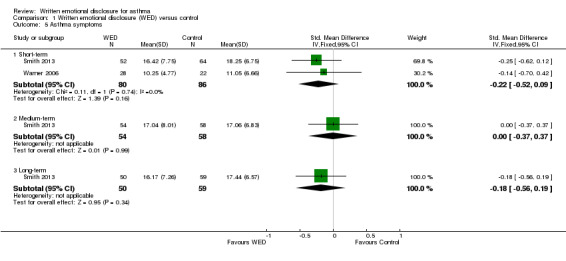
Two studies including 166 participants reported symptoms as an outcome measure (Smith 2013; Warner 2006). In Smith 2013, asthma symptoms were measured using the Symptom Score Questionnaire (SSQ) (Wasserfellan 1997), a 23‐item validated scale in which a lower score indicates fewer symptoms. Warner 2006 used the nine‐item Asthma Sum Scale (Wahlgren 1997) to measure symptoms. A pooled result from these two studies indicated that disclosure writing does not improve asthma symptoms at short‐term follow‐up (SMD ‐0.22, 95% CI ‐0.52 to 0.09). The outcome was rated as of low quality after it had been downgraded for lack of assessor blinding and imprecision because the confidence intervals included significant benefit and no effect.
One study measured asthma symptoms at medium‐ and long‐term follow‐up (Smith 2013); this study reported no significant benefit of disclosure writing for asthma symptoms at either follow‐up (Figure 7).
7.
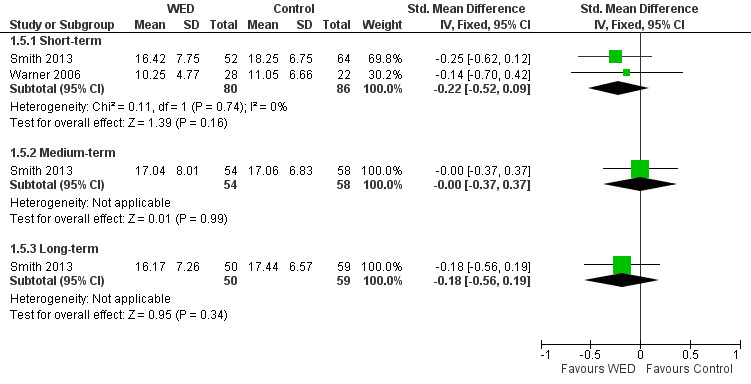
Forest plot of comparison: 1 Written emotional disclosure (WED) versus control, outcome: 1.5 Asthma symptoms.
Asthma control
Two studies on 194 participants measured asthma control (Harris 2005; Smith 2013) but used different instruments. Harris 2005 used the Asthma Control Questionnaire (Juniper 1999) to measure the adequacy of asthma control and changes in asthma control as a result of emotional disclosure writing. Smith 2013 used the Asthma Control Test, a validated tool in which higher scores reflect greater asthma control (Nathan 2004). A pooled result from these two studies indicated that disclosure writing results in short‐term improvement in asthma control (SMD 0.29, 95% CI 0.01 to 0.58). Evidence was rated as of low quality and had been downgraded for imprecision (wide confidence intervals) and lack of assessor blinding.
Only one study measured asthma control at medium‐term and long‐term follow‐up (Smith 2013). The beneficial effect of disclosure writing for asthma control was not statistically significant at later follow‐up (Figure 8).
8.
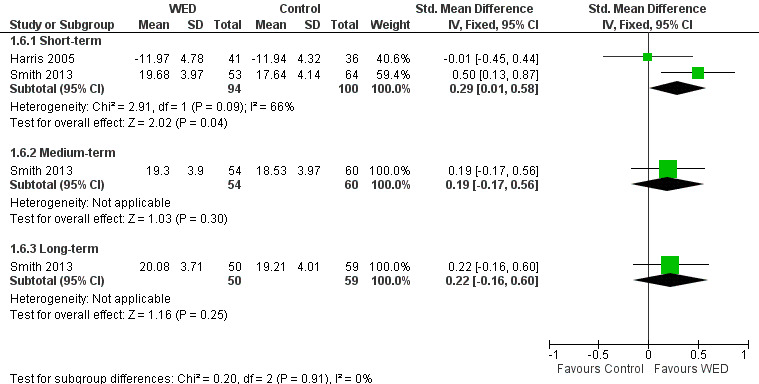
Forest plot of comparison: 1 Written emotional disclosure (WED) versus control, outcome: 1.6 Asthma control.
Medication use (puffs/d)
Smith 2013 examined the effects of written emotional disclosure for medication use (inhaled corticosteroids and beta agonists (MD ‐1.62, 95% CI ‐2.62 to ‐0.62)). This study found that the disclosure group used significantly less beta agonist compared with the control group at short‐term follow‐up. However, no significant differences in the use of these medications were found between groups at later follow‐up (Figure 9; Figure 10). The outcome was rated as of low quality after it had been downgraded for imprecision and for evidence based on a single trial.
9.
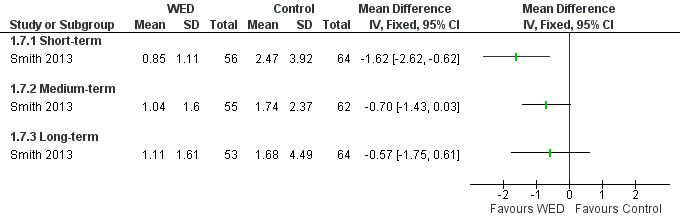
Forest plot of comparison: 1 Written emotional disclosure (WED) versus control, outcome: 1.7 Beta agonist use; puffs/d.
10.
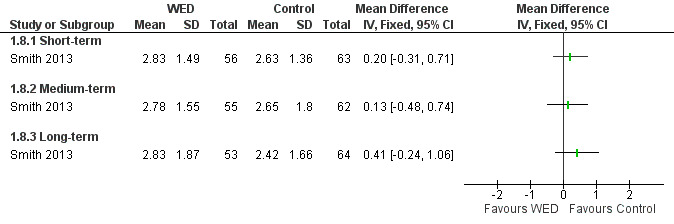
Forest plot of comparison: 1 Written emotional disclosure (WED) versus control, outcome: 1.8 Inhaled corticosteroid use; puffs/d.
Healthcare utilisation
Healthcare utilisation was measured in two studies; Harris 2005 measured utilisation over a two‐month period, whereas Smith 2013 recorded measurements over a 12=month period. Harris 2005 found no significant differences between groups on change in healthcare use (data to support this were not reported in the study, apart from data for healthcare use, which were available for 13 of 114 participants). Smith 2013 also revealed no significant differences in the odds of healthcare utilisation in the intervention groups when compared with the control group: OR 0.99 (95% CI 0.37 to 2.66) (Figure 11).
11.

Forest plot of comparison: 1 Written emotional disclosure (WED) versus control, outcome: 1.9 Healthcare utilisation.
Psychological symptoms
Distress
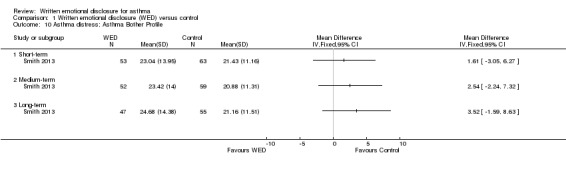
Smith 2013 used the Asthma Bother Profile (Hyland 1995), a 15‐item validated scale, to measure distress caused by asthma. This study found no significant differences in asthma distress between the disclosure writing group and the control group at any follow‐up period (Figure 12). The remaining trials (Harris 2005; Smyth 1999; Warner 2006) did not provide data on distress. The outcome was rated as of very low quality after it had been downgraded for imprecision because the confidence intervals included significant benefit and no effect, and because evidence was based on a single trial .
12.
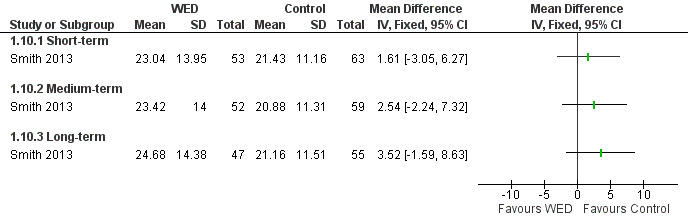
Forest plot of comparison: 1 Written emotional disclosure (WED) versus control, outcome: 1.10 Asthma distress; Asthma Bother Profile.
Perceived stress
Harris 2005 measured stress caused by asthma using the 14‐item Perceived Stress Scale (Cohen 1983). This study found no significant differences between intervention and control groups in measurements of stress (data not reported). The remaining studies (Smith 2013; Smyth 1999; Warner 2006 ) did not include data on stress.
Discussion
Summary of main results
This review identified four trials measuring the effectiveness of written emotional disclosure in the treatment of participants with asthma. Overall, this review failed to find evidence that emotional disclosure writing improves pulmonary function and symptoms in participants with asthma. These studies differed in reported outcomes, outcome measurement tools and frequency and/or time of follow‐up measurements, which, in turn, limited our ability to summarise the pool effect for other outcomes except FEV1 % predicted, FVC, asthma symptoms and asthma control. Although one study (Smyth 1999) reported significantly greater FEV1 % predicted at all follow‐up measurements, this study recruited participants with more severe conditions (FEV1 % predicted at baseline was 64%), which meant that there was more room for improvement in lung function compared with participants with less severe conditions who had been recruited in other trials (baseline FEV1 % predicted value: 73% in Harris 2005, 87% in Smith 2013 and 94% in Warner 2006).
Quality of life was measured in one study (Smith 2013), but no beneficial effect of disclosure writing for quality of life of participants with asthma was found. Asthma symptoms were assessed in two studies (Smith 2013; Warner 2006); however, the symptom measurement tools in these studies varied. For example, Smith 2013 used the Symptom Score Questionnaire (Wasserfellan 1997), and Warner 2006 used the Asthma Sum Scale (Wahlgren 1997), thus making the rigour of this assessment difficult to ascertain. Two studies measured asthma control (Harris 2005; Smith 2013); a pooled result from these studies indicated that disclosure writing results in improvement in asthma control at short‐term follow‐up.
In terms of medication use, Smith 2013 reported reduced use of a bronchodilator at three months but found no significant difference in the use of steroid inhalers. This may be explained by the fact that for many patients, steroid dosing is regular, whereas a bronchodilator medication is prescribed for use ‘as required’ to control breakthrough symptoms. Hence, improved symptom control and reduced necessity to intervene with rescue medication are consistent observations.
Of the four studies included in the review, only two measured healthcare utilisation (Harris 2005; Smith 2013). Both studies found no significant differences between groups in healthcare use. Both studies used self report measures and hence may not reflect accurately true actual healthcare utilisation.
Psychological well‐being was measured in three studies (Harris 2005; Smith 2013; Warner 2006); however, the outcomes examined were diverse. There seems to be no consensus as to which psychological outcomes are conceptually linked to asthma or to the written emotional disclosure intervention being studied. For example, Harris 2005 measured perceived stress, and Smith 2013 measured asthma distress. Warner 2006 measured positive and negative affect, along with internalising behaviour, in adolescents. For the purpose of this review, we reported only distress and stress, as the other outcomes were not prespecified in our protocol and were more child specific. One study (Smith 2013) reported greater distress in the disclosure group compared with the control group at one month of follow‐up.
RCTs evaluating written emotional disclosure for asthma are rare. The few studies identified were relatively homogeneous in terms of the intervention provided to experimental groups; all studies adapted a brief written emotional expression exercise developed by Pennebaker and Beall (Pennebaker 1986). However, many variations existed, especially in terms of the intervention provided to control groups, location (home and/or laboratory) and timing (daily sessions or weekly sessions) of the written exercise session, as well as instruction on flexibility of the topic (same topic for all sessions or moved from one topic to another). These procedural differences may have resulted in some diversity, which was further complicated by the multiplicity of outcomes. Although the diversity of outcomes measured in the studies reflects the potential breadth of the impact of written emotional disclosure, no consensus has yet been reached on which outcomes a written emotional disclosure might influence and the conceptual link between them.
Various moderating factors such as sample characteristics, outcome type (self report vs objective data), dose of the intervention and focus of the writing task (past traumatic event, ongoing traumatic event or both) may attenuate or enhance the effects of written emotional disclosure (Smyth 1998). However, in the current context of uncertainty around the effectiveness of disclosure writing for patients with asthma, it is not appropriate to look for mediators of this effect.
Overall completeness and applicability of evidence
We conducted a thorough systematic search for published and unpublished trials and could extract data from only four trials. At present, there is no evidence to support that written emotional disclosure provides significant benefit in improving the lung function and symptoms of patients with asthma in general.
Quality of the evidence
This review included four studies with 414 participants. Individual studies were small, ranging from 61 to 146 participants. Key methodological limitations included lack of assessor blinding in three of the four studies, and outcomes other than lung function were limited to a subset of studies; asthma symptoms and asthma control were measured in two, and all other outcomes were measured in only one trial. Thus, the quality of evidence for many outcomes was rated as low or very low.
Potential biases in the review process
We corresponded with the authors of all included trials to identify other published and non‐published studies in the area. We followed a prespecified protocol for trial selection and data extraction, and two review authors independently conducted the process. Nevertheless, we acknowledge that we might have missed unpublished trials, which might change our confidence in the conclusions. Some of the review authors were involved in a trial of written emotional disclosure for asthma. We have declared our interest in the Declarations of interest section.
Agreements and disagreements with other studies or reviews
This is the first review to focus on the effects of written emotional disclosure in the treatment of patients with asthma. It summarises the best evidence available to January 2014. This review is unable to find any evidence to support that written emotional disclosure improves lung function and asthma symptoms in asthmatic patients, but written emotional disclosure may have some beneficial effect on asthma control. Cochrane reviews on psychological interventions for asthma in children (Yorke 2005) and adults (Yorke 2009) were unable to draw firm conclusions because of the absence of an adequate evidence base. Previous reviews exploring the effects of written emotional disclosure for healthy and clinical participants have produced different results; three reviews (Frattaroli 2006; Frisina 2004; Smyth 1998) produced positive results, but a review by Meads 2005 produced a neutral result. This review, by focusing on a single disease, allows the evidence to be explored in greater depth.
Authors' conclusions
Implications for practice.
Based on the results from current RCTs, not enough evidence is available to show whether written emotional disclosure had an effect on the outcomes included in this review compared with writing about non‐emotional topics. Divergent outcomes and variation in measurement tools and follow‐up times do not allow us to address adequately any of the other outcomes. Further RCTs are needed to support or refute the effectiveness of written emotional disclosure for quality of life, medication use, healthcare utilisation and psychological well‐being. In the meanwhile, written emotional disclosure should not be used in routine clinical practice as a means of improving pulmonary function and symptoms in patients with asthma.
Implications for research.
The positive impact of expressive writing in reducing the use of asthma rescue medication and improving feelings of asthma control for short‐term outcomes should be explored in future trials. The study that showed the impact of written emotional disclosure had a population with more severe asthma. Future trials would benefit from stratifying participants by asthma severity, confirming airways reversibility, including an objective measure of asthma medication use and looking at whether the impact of the intervention can be sustained by repeating the intervention.
Well‐designed rigorous RCTs conducted under routine conditions are needed to determine the effectiveness of written emotional disclosure for patients with asthma. There is a need to standardise reporting of outcomes measurement instruments (e.g. using the same validated questionnaire for measuring symptoms and quality of life) and duration of follow‐up.
As the control writing exercise may reduce the ability of the study to show a true difference, three‐armed RCTs comparing the effectiveness of written emotional disclosure versus neutral writing versus usual care (no writing exercise) are needed.
Acknowledgements
No source of funding was available for this review. The review authors wish to acknowledge the contribution of Robert Horne, Richard Bowskill and Anthony Frew to the development of the review protocol. We thank the Cochrane Airways Review Group, particularly Emma Welsh, for providing ongoing support. We appreciate the trials authors, namely, Joshua M Smyth, Mark A Lumley and Alex HS Harris, who responded enthusiastically to our requests for further information.
Anne Chang was the Editor for this review and commented critically on the review.
Appendices
Appendix 1. Database search strategies
Cochrane Airways Group Register (CAGR)
((express* or emotion* or self* or truth* or guid* or experiment*) and (disclosure* or writ*))
[Limit to 'asthma' records]
CENTRAL (The Cochrane Library)
#1 MeSH descriptor Asthma explode all trees
#2 asthma*
#3 antiasthma* or anti‐asthma*
#4 MeSH descriptor Respiratory Sounds explode all trees
#5 wheez*
#6 MeSH descriptor Bronchial Spasm, this term only
#7 bronchospas*
#8 bronch* near/3 spasm*
#9 bronchoconstrict*
#10 MeSH descriptor Bronchoconstriction explode all trees
#11 bronch* near/3 constrict*
#12 MeSH descriptor Bronchial Hyperreactivity, this term only
#13 MeSH descriptor Respiratory Hypersensitivity, this term only
#14 (bronchial* or respiratory* or airway* or lung*) near/3 (hypersensitiv* or hyperreactiv* or allerg* or insufficiency*)
#15 atopic* or atopy
#16 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15)
#17 (express* or emotion*) near/3 writ*
#18 MeSH descriptor Writing, this term only
#19 MeSH descriptor Emotions, this term only
#20 MeSH descriptor Self Disclosure, this term only
#21 MeSH descriptor Truth Disclosure, this term only
#22 (guid* or experiment* or emotion*) near/3 disclos*
#23 (#17 OR #18 OR #19 OR #20 OR #21 OR #22)
#24 (#16 AND #23)
MEDLINE (Ovid)
1. exp Asthma/ 2. asthma$.mp. 3. (antiasthma$ or anti‐asthma$).mp. 4. Respiratory Sounds/ 5. wheez$.mp. 6. Bronchial Spasm/ 7. bronchospas$.mp. 8. (bronch$ adj3 spasm$).mp. 9. bronchoconstrict$.mp. 10. exp Bronchoconstriction/ 11. (bronch$ adj3 constrict$).mp. 12. Bronchial Hyperreactivity/ 13. Respiratory Hypersensitivity/ 14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp. 15. (atopic$ or atopy).mp. 16. or/1‐15 17. ((express$ or emotion$ ) and writ$).mp. 18. Writing/ 19. Emotions/ 20. Self Disclosure/ 21. Truth Disclosure/ 22. ((guid$ or experiment$) adj3 disclos$).mp. 23. or/17‐22 24. 16 and 23
RCT filter
1. (clinical trial or controlled clinical trial or randomised controlled trial).pt. 2. (randomised or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. Animals/ 10. Humans/ 11. 9 not (9 and 10) 12. 8 not 11
EMBASE (Ovid)
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Abnormal Respiratory Sound/
5. Wheezing/
6. wheez$.mp.
7. Bronchospasm/
8. bronchospas$.mp.
9. (bronch$ adj3 spasm$).mp.
10. bronchoconstrict$.mp.
11. Bronchus Hyperreactivity/
12. Respiratory Tract Allergy/
13. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
14. or/1‐13
15. writing/
16. emotion/
17. self disclosure/
18. ((express$ or emotion$) adj3 writ$).mp.
19. ((guid$ or experiment$ or emotion$) adj3 disclos$).mp.
20. or/15‐19
21. 20 and 14
RCT filter
1. Randomized Controlled Trial/
2. Controlled Study/
3. randomisation/
4. Double Blind Procedure/
5. Single Blind Procedure/
6. Clinical Trial/
7. Crossover Procedure/
8. follow up/
9. exp prospective study/
10. or/1‐9
11. (clinica$ adj3 trial$).mp.
12. ((singl$ or doubl$ or trebl$ or tripl$) adj5 (mask$ or blind$ or method$)).mp.
13. exp Placebo/
14. placebo$.mp.
15. random$.mp.
16. (latin adj3 square$).mp.
17. exp Comparative Study/
18. ((control$ or prospectiv$ or volunteer$) adj3 (trial$ or method$ or stud$)).mp.
19. (crossover$ or cross‐over$).mp.
20. or/11‐19
21. 10 or 20
22. exp ANIMAL/
23. Nonhuman/
24. Human/
25. 22 or 23
26. 25 not 24
27. 21 not 26
PSYCInfo (Ovid)
1. exp asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. wheez$.mp.
5. bronchospas$.mp.
6. (bronch$ adj3 spasm$).mp.
7. bronchoconstrict$.mp.
8. (bronch$ adj3 constrict$).mp.
9. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
10. or/1‐9
11. exp Self Disclosure/
12. self expression/
13. exp emotions/
14. expressed emotion/
15. creative writing/
16. ((express$ or emotion$) adj3 writ$).mp.
17. ((guid$ or experiment$ or emotion$) adj3 disclos$).mp.
18. or/11‐17
19. 18 and 10
RCT filter
1. random$.mp.
2. (clinical adj5 trial$).mp.
3. (control$ adj5 trial$).mp.
4. ((clinical or control$ or comparativ$) adj5 (study or studies)).mp.
5. placebo$.mp.
6. (single blind$ or single‐blind$).mp.
7. (double blind$ or double‐blind$).mp.
8. (triple blind$ or triple‐blind$).mp.
9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
CINAHL (EBSCO)
S22 S21 and S11
S21 S20 or S19 or S18 or S17 or S16 or S15 or S14 or S13 or S12
S20 emotion* N3 disclos*
S19 experiment* N3 disclos*
S18 guid* N3 disclos*
S17 emotion* N3 writ*
S16 express* N3 writ*
S15 (MH "Truth Disclosure")
S14 (MH "Self Disclosure")
S13 (MM "Emotions")
S12 (MM "Writing")
S11 S10 or S9 or S8 or S7 or S6 or S5 or S4 or S3 or S2 or S1
S10 (DE "RESPIRATORY HYPERSENSITIVITY")
S9 bronch* N3 constrict*
S8 bronchoconstrict*
S7 bronch* N3 spasm*
S6 bronchospas*
S5 wheez*
S4 (DE "RESPIRATORY SOUNDS")
S3 antiasthma* or anti‐asthma*
S2 asthma*
S1 (DE "asthma")
AMED (EBSCO)
S20 S19 and S10
S19 S18 or S17 or S16 or S15 or S14 or S13 or S12 or S11
S18 emotion* N3 disclos*
S17 experiment* N3 disclos*
S16 guid* N3 disclos*
S15 emotion* N3 writ*
S14 express* N3 writ*
S13 (DE "TRUTH DISCLOSURE")
S12 (DE "EMOTIONS")
S11 (DE "WRITING")
S10 S9 or S8 or S7 or S6 or S5 or S4 or S3 or S2 or S1
S9 (DE "RESPIRATORY HYPERSENSITIVITY")
S8 (DE "RESPIRATORY SOUNDS")
S7 (DE "ASTHMA")
S6 bronch* N3 constrict*
S5 bronchoconstrict*
S4 bronch* N3 spas*
S3 bronchospas*
S2 wheez*
S1 asthma*
Data and analyses
Comparison 1. Written emotional disclosure (WED) versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Average FEV1 % predicted value | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Short‐term | 4 | 281 | Mean Difference (IV, Fixed, 95% CI) | 3.43 [‐0.61, 7.47] |
| 1.2 Medium‐term | 2 | 176 | Mean Difference (IV, Fixed, 95% CI) | 3.61 [‐1.95, 9.16] |
| 1.3 Long‐term | 1 | 116 | Mean Difference (IV, Fixed, 95% CI) | 5.89 [‐0.61, 12.39] |
| 2 Change in FEV1 % predicted in short term | 2 | 195 | Mean Difference (IV, Fixed, 95% CI) | 1.68 [‐0.82, 4.18] |
| 3 Average FVC value | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Short‐term | 2 | 197 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.30, 0.26] |
| 3.2 Medium‐term | 1 | 118 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.72, 0.01] |
| 3.3 Long‐term | 1 | 116 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.42, 0.31] |
| 4 Quality of life; Marks Asthma Quality of Life Questionnaire | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Short‐term | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Medium‐term | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Long‐term | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Asthma symptoms | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Short‐term | 2 | 166 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.52, 0.09] |
| 5.2 Medium‐term | 1 | 112 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.37, 0.37] |
| 5.3 Long‐term | 1 | 109 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.56, 0.19] |
| 6 Asthma control | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Short‐term | 2 | 194 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.29 [0.01, 0.58] |
| 6.2 Medium‐term | 1 | 114 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐0.17, 0.56] |
| 6.3 Long‐term | 1 | 109 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.16, 0.60] |
| 7 Beta agonist use; puffs/d | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Short‐term | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Medium‐term | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Long‐term | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Inhaled corticosteroid use; puffs/d | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Short‐term | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Medium‐term | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Long‐term | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Healthcare utilisation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10 Asthma distress; Asthma Bother Profile | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 Short‐term | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Medium‐term | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 Long‐term | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
Comparison 1 Written emotional disclosure (WED) versus control, Outcome 1 Average FEV1 % predicted value.
1.2. Analysis.
Comparison 1 Written emotional disclosure (WED) versus control, Outcome 2 Change in FEV1 % predicted in short term.
1.3. Analysis.
Comparison 1 Written emotional disclosure (WED) versus control, Outcome 3 Average FVC value.
1.4. Analysis.
Comparison 1 Written emotional disclosure (WED) versus control, Outcome 4 Quality of life; Marks Asthma Quality of Life Questionnaire.
1.5. Analysis.
Comparison 1 Written emotional disclosure (WED) versus control, Outcome 5 Asthma symptoms.
1.6. Analysis.
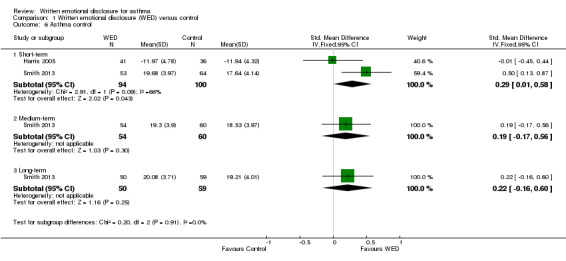
Comparison 1 Written emotional disclosure (WED) versus control, Outcome 6 Asthma control.
1.7. Analysis.
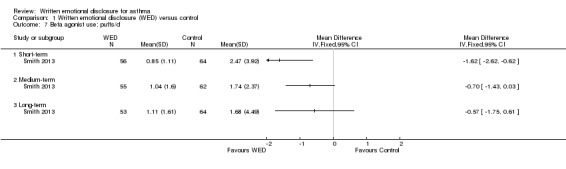
Comparison 1 Written emotional disclosure (WED) versus control, Outcome 7 Beta agonist use; puffs/d.
1.8. Analysis.
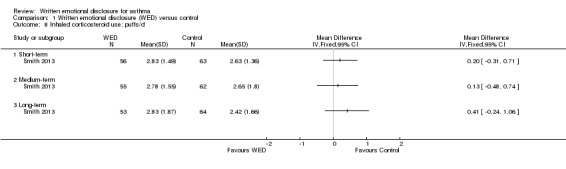
Comparison 1 Written emotional disclosure (WED) versus control, Outcome 8 Inhaled corticosteroid use; puffs/d.
1.9. Analysis.
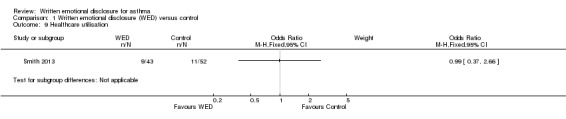
Comparison 1 Written emotional disclosure (WED) versus control, Outcome 9 Healthcare utilisation.
1.10. Analysis.
Comparison 1 Written emotional disclosure (WED) versus control, Outcome 10 Asthma distress; Asthma Bother Profile.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Harris 2005.
| Methods | RCT (parallel design) Study duration: 3 months (3 week intervention period, 2 months postintervention follow‐up) Setting: VA Palo Alto Health Care System, Stanford University and the local community, California, USA |
|
| Participants | 163 eligible, 137 randomly assigned, 114 completed Intervention (stress‐writing group): 41; intervention (positive writing group): 37; and control (neutral‐writing group): 36 Age: mean age 43 (SD ± 17.7) years, range not reported Sex: male 49, female 65 Physician diagnosed asthma by history Inclusion criteria: adult patients with physician‐diagnosed asthma and evidence of reduced pulmonary function at baseline Exclusion criteria: younger than 18 years of age, not diagnosed with asthma by a physician, diagnosed with COPD by a physician, post‐traumatic stress disorder and unable to write for 20 minutes and comply with other expectations of study participation, such as getting to weekly writing sessions and assessment meetings |
|
| Interventions | Intervention 1: write on a traumatic and upsetting event or loss experienced in life Intervention 2: write on positive experiences such as events that stimulated feelings of happiness or joy Control: write on neutral topics focused on the events of the previous day Participants in all three groups wrote for 20 minutes, once a week, for 3 consecutive weeks. They could write about the same experience at all three sessions or about different experiences. Writing occurred alone in a private room: the first and third writing sessions in the laboratory and the second session in the participant's home |
|
| Outcomes | FEV1 and FVC % predicted measured by spirometry according to ATS guidelines, control of asthma (Asthma Control Questionnaire), perceived control of asthma (Perceived Asthma Control Questionnaire), healthcare utilisation and perceived stress (The Perceived Stress Scale) Outcomes measured at baseline, immediately post intervention and at 2 months after writing |
|
| Notes | Sample size calculation done The study reports only the measurement of FEV1 and FVC % predicted. On request, the study author supplied information on other outcome measures. Experimental and control groups did not differ at baseline in most demographic characteristics, health behaviours, psychological variables and disease severity; however, group differences did exist in terms of age, education and smoking status. Consideration of smoking status did not change the results for any of the outcome variables Study funded by fellowships from the Department of Veterans Affairs Office of Academic Affiliations to the first study author, and from the Fetzer Institute, Kalamazoo, Michigan, USA, to Dr. Thoresen |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomised to a writing group using computer‐generated, equal‐probability allocation" |
| Allocation concealment (selection bias) | Low risk | "Assignments were kept in sealed envelopes until immediately before the first scheduled writing session" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Participants were not informed about the specific nature of the other writing groups and were not aware whether they were in the control or experimental conditions" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Staff performing the pulmonary function assessments were not blind to the experimental condition" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout balanced in numbers across the three groups (8 in intervention group and 6 in control group) with similar reasons for missing data |
| Selective reporting (reporting bias) | Low risk | Only FEV1 and FVC % predicted reported in the paper; however, information on other outcome measures was supplied by the study author on request (reported in PhD dissertation) |
| Other bias | Low risk | No evidence of further systematic bias |
Smith 2013.
| Methods | RCT (parallel design) Study duration: 12 months Setting: 29 general practices in south east of England, UK |
|
| Participants | 3968 eligible, 146 randomly assigned, 120 completed the study Intervention (expressive writing): 55 Control (writing on time management): 65 Age: mean 36 (SD 6.99) years Sex: male 34, female 112 Physician‐diagnosed asthma Inclusion criteria: adults (18 to 45 years old) with a diagnosis of asthma and requiring regular inhaled medication (British Thoracic Society step two and above) Exclusion criteria: receipt of psychotherapy, diagnosis of psychotic disorder in the past, unable to understand English, having work or travel commitments during the trial period |
|
| Interventions | Intervention: write about very deepest thoughts and feelings about a stressful experience. Participants could write about the same topic for three sessions or move from one topic to another Control: write on a factual account of activity over the day (day 1), food and drink consumed (day 2) and leisure time activity (day 3) Participants in both groups wrote in their own home for 20 minutes daily for three consecutive days. |
|
| Outcomes | FEV1 % predicted and FVC (absolute values) using spirometer (vitalograph), quality of life (Mark’s Asthma Quality of Life Questionnaire), asthma symptoms (Symptom Score Questionnaire), distress caused by asthma (Asthma Bother Profile), medication use (corticosteroid and beta agonist use measured as puffs per day) and asthma‐related healthcare utilisation (measured as asthma‐related GP or hospital visits) Outcomes measured at baseline and at 1, 3, 6 and 12 months post intervention |
|
| Notes | Abstract only, full results extracted from draft manuscript (unpublished) Sample size calculation reported Intervention and control groups similar in terms of demographic characteristics, smoking history and asthma‐related outcome measures Study funded by Asthma UK |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were allocated to one of the two study groups, using computer generated randomised blocks of 12, changing to blocks of six as recruitment slowed" |
| Allocation concealment (selection bias) | Low risk | Study author confirmed the use of sealed opaque envelopes for assignment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The writing tasks were supplied in sealed envelopes to ensure researcher blinding. Participants were informed that the trial was examining the effect of two writing tasks to ensure that participants remained blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study author confirmed the blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout balanced in numbers across the two groups (13 in intervention and 11 in control) with similar reasons for missing data |
| Selective reporting (reporting bias) | Low risk | All possible outcomes stated in the methods section reported in the results section |
| Other bias | Low risk | No evidence of further systematic bias |
Smyth 1999.
| Methods | RCT (unbalanced design; 2 of every 3 participants allocated to the experimental condition) Study duration: 4 months Setting: outpatient community residents drawn from private and institutional practice, North Dakota, USA |
|
| Participants | 70 eligible, 70 randomly assigned, 61 received the intervention and 58 completed the study Intervention (writing on most stressful experience): 39 Control (writing about plan for the day): 19 Age: mean age 41 (SD ± 17.4) years, range not reported Sex: male 15, female 43 Physician‐diagnosed asthma based on history Inclusion criteria: patients with physician‐diagnosed asthma required to provide a documented reduction in expiratory function (in physician records or when evaluated by study staff) Exclusion criteria: ongoing psychotherapy or having a defined psychiatric disorder, using a medication that could interfere with symptom report, being deemed unable to comply with the protocol, being unable to write for a duration of 20 minutes |
|
| Interventions | Intervention: write on most stressful experience ever undergone Control: write about plans for the day Participants in both groups wrote for 20 minutes on 3 consecutive days a week. They could write about the same topic for three sessions or move from one topic to another. Writing took place in private rooms located in study laboratory |
|
| Outcomes | FEV1 % predicted measured by spirometry in accordance with ATS guidelines Outcomes measured at baseline and at 2 weeks, 2 months and 4 months post intervention |
|
| Notes | Trial included asthma and RA participants. Only the data on participants with asthma were extracted and included in the review Sample size calculation done No other data reported except FEV1 % predicted On email correspondence, study author informed that other outcomes were measured, but data analysis was carried out for FEV1 % predicted only Control and experimental groups similar at baseline in terms of demographic measures, health behaviours including smoking or psychological measures and asthma outcome Study funded by the Fetzer Institute, Kalamazoo, Michigan, USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomised into the control or experimental group using a computer‐generated random assignment scheme" |
| Allocation concealment (selection bias) | Low risk | "Assignments were kept in sealed opaque envelopes until participants were scheduled to complete the writing intervention" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Neither patients nor physicians were informed of the assignment" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Statistical analyses were conducted primarily by the first author, who was aware of group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Nine participants dropped out of the study before receiving the intervention; however, only two of 22 participants in the intervention arm left the study after randomisation |
| Selective reporting (reporting bias) | High risk | Only FEV1 reported as a study outcome, no other study outcomes reported |
| Other bias | Unclear risk | Insufficient information |
Warner 2006.
| Methods | RCT (parallel design) Study duration: 2 months Setting: six asthma/allergy clinics, Detroit, Michigan, USA |
|
| Participants | 180 eligible, 61 randomly assigned, 50 completed the study Intervention (writing on trauma or problem ever experienced): 28 Control (writing on time management): 22 Age: mean 14 years Sex: male 21, female 29 Physician‐diagnosed asthma Severity: 40% had mild persistent asthma, 52% had moderate persistent and 8% had severe persistent Inclusion criteria: adolescent participants, aged 12 to 17, with at least mild persistent asthma Exclusion criteria: having only seasonal or exercise‐induced asthma, the presence of a serious medical condition other than asthma and current use of psychotropic medication or participation in counselling or psychotherapy and known to have cognitive impairment |
|
| Interventions | Intervention: write about a trauma or problem ever experienced. Participants encouraged to write about the same event for 15 to 20 minutes daily for three consecutive days Control: write about time management. Written exercise varied across three days; activity over the past week (day 1), activity over the past 24 hours (day 2) and plan for next 24 hours (day 3) Both groups wrote in a private place at home or elsewhere |
|
| Outcomes | FEV1 % predicted measured by spirometry in accordance with ATS guidelines, asthma symptoms (9‐item Asthma Sum Scale), positive affect and negative affect (30‐item Positive and Negative Affect Schedule for Children), internalisation of behaviour problems (Youth Self Report), functional disability (15‐item Functional Disability Inventory) Outcome measured at baseline and at 1 and 2 months post intervention |
|
| Notes | No sample size calculation reported Spirometry data available for 32 participants only Two groups similar in terms of demographics and asthma history variables at baseline Study funded by dissertation grants from the Blue Cross/Blue Shield of Michigan Foundation, Wayne State University and the Ashok and Ingrid Sarniak Endowment through Children’s Hospital of Michigan, USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A random numbers table was used to randomise participants to groups separately for each gender" |
| Allocation concealment (selection bias) | Low risk | Study author confirmed the use of sealed envelopes for assignment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study authors contacted to provide more information on blinding; these authors confirmed the blinding of study participants and researcher during recruitment and baseline assignment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Information on blinding of outcome assessors not reported in the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data balanced in number across the intervention group and the control group (6 and 5 respectively) |
| Selective reporting (reporting bias) | Low risk | All possible outcomes stated in the methods section reported in the results section |
| Other bias | Unclear risk | Inability to assess lung function for all participants may have introduced a selection bias. Insufficient information on whether smoking was taken into account |
Abbreviations: ATS: American Thoracic Society; COPD: chronic obstructive pulmonary disease; GP: general practitioner; RA: rheumatoid arthritis; RCT: randomised controlled trial; FEV1: forced expiratory volume in one second; FVC: forced vital capacity.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bruzzese 2011 | Intervention consists of education only programme, no written emotional disclosure (WED) component |
| Hockemeyer 2002 | Multiple interventions consisting of tape‐recorded deep‐breathing relaxation exercise and WED, difficult to tease out the effects of WED alone |
| Horowitz 2008 | Narrative review of WED in different conditions |
| Hyland 1993 | Diary keeping refers to peak flow diaries—not relevant |
| Magar 2005 | Does not include WED |
| McGhan 2010 | Intervention other than WED |
| Okelo 2004 | Not an RCT |
| Shah 2001 | No apparent WED component |
| Sharifabad 2010 | Diagnosis other than asthma not excluded. Patients with COPD and idiopathic pulmonary fibrosis included in the study |
| Smyth 2002 | Substudy of Smyth 1999—analysis mostly qualitative |
| Theadom 2010 | Qualitative study using framework analysis |
| Tousman 2011 | No WED component |
| Urek 2005 | Intervention does not include WED component |
COPD: chronic obstructive pulmonary disease; RCT: randomised controlled trial; WED: written emotional disclosure.
Contributions of authors
HS had the idea of carrying out the original review. AT wrote the protocol in conjunction with HS, CA, CJ, JY and MH. Studies for inclusion were assessed by AT, PH and PP. Data extraction and risk of bias assessment were carried out by PP and PH. PP wrote the review with constructive feedback from all review authors. HS is the guarantor of the review.
Declarations of interest
HS, CJ, AT and MH were involved in a trial of written emotional disclosure for asthma.
New
References
References to studies included in this review
Harris 2005 {published data only}
- Harris AH, Thoresen CE, Humphreys K, Faul J. Does writing affect asthma? A randomised trial. Psychosomatic Medicine 2006;67(1):130‐6. [DOI] [PubMed] [Google Scholar]
Smith 2013 {unpublished data only}
Smyth 1999 {published data only}
- Smyth JM, Stone AA, Hurewitz A, Kaell A. Effects of writing about stressful experiences on symptom reduction in patients with asthma or rheumatoid arthritis: a randomised trial. JAMA 1999;281(14):1304‐9. [DOI] [PubMed] [Google Scholar]
Warner 2006 {published data only}
- Warner LJ, Lumley MA, Casey RJ, Pierantoni W, Salazar R, Zoratti EM, et al. Health effects of written emotional disclosure in adolescents with asthma: a randomised, controlled trial. Journal of Padeatric Psychology 2006;31(6):557‐68. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bruzzese 2011 {published data only}
- Bruzzese JM, Sheares BJ, Vincent EJ, Du Y, Sadeghi H, Levison MJ, et al. Effects of a school‐based intervention for urban adolescents with asthma: a controlled trial. American Journal of Respiratory and Critical Care Medicine 2011;183(8):998–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hockemeyer 2002 {published data only}
- Hockemeyer J, Smyth J. Evaluating the feasibility and efficacy of a self‐administered manual‐based stress management intervention for individuals with asthma: results from a controlled study. Behavioural Medicine 2002;27(4):161‐72. [DOI] [PubMed] [Google Scholar]
Horowitz 2008 {published data only}
- Horowitz S. Evidence‐based health outcomes of expressive writing. Alternative and Complementary Therapies 2008;14(4):194‐8. [Google Scholar]
Hyland 1993 {published data only}
- Hyland ME, Kenyon CA, Allen R, Howarth P. Diary keeping in asthma: comparison of written and electronic methods. British Medical Journal 1993;306(6876):487‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Magar 2005 {published data only}
- Magar Y, Vervloet D, Steenhouwer F, Smaga S, Mechin H, Rocca Serra JP, et al. Assessment of a therapeutic education programme for asthma patients: "un souffle nouveau". Patient Education and Counseling 2005;58(1):41‐6. [DOI] [PubMed] [Google Scholar]
McGhan 2010 {published data only}
- McGhan SL, Wong E, Sharpe HM, Hessel PA, Mandhane P, Boechler VL, et al. A children's asthma education program: Roaring Adventures of Puff (RAP), improves quality of life. Canadian Respiratory Journal 2010;17(2):67‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Okelo 2004 {published data only}
- Okelo SO, Wu AW, Krishnan JA, Rand CS, Skinner EA, Diette GB. Emotional quality‐of‐life and outcomes in adolescents with asthma. Journal of Paediatrics 2004;145:523‐9. [DOI] [PubMed] [Google Scholar]
Shah 2001 {published data only}
- Shah S, Peat JK, Mazurski EJ, Wang H, Sindhusake D, Bruce C, et al. Effect of peer led programme for asthma education in adolescents: cluster randomised controlled trial. BMJ 2001;322(7286):583‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharifabad 2010 {published data only}
- Sharifabad MA, Hurewitz A, Spiegler P, Bernstein M, Feuerman M, Smyth JM. Written disclosure therapy for patients with chronic lung disease undergoing pulmonary rehabilitation. Journal of Cardiopulmonary Rehabilitation and Prevention 2010;30(5):340‐5. [DOI] [PubMed] [Google Scholar]
Smyth 2002 {published data only}
- Smyth J, Anderson C, Hockemeyer J, Stone A. Does emotional non‐expressiveness or avoidance interfere with writing about stressful life events? An analysis in patients with chronic illness. Psychology and Health 2002;17(5):561‐9. [Google Scholar]
Theadom 2010 {published data only}
- Theadom A, Smith H, Horne R, Bowskill R, Apfelbacher CJ, Frew A. Participant experiences of a written emotional disclosure intervention in asthma. Stress and Health 2010;26(1):45‐50. [Google Scholar]
Tousman 2011 {published data only}
- Tousman SA, Zeitz H, Bond D, Stewart D, Rackow R, Greer R, et al. A randomized controlled behavioral trial of a new adult asthma self‐management program. Journal of Asthma and Allergy Educators 2011;2(2):91‐6. [Google Scholar]
Urek 2005 {published data only}
- Urek MC, Tudorić N, Plavec D, Urek R, Koprivc‐Milenović T, Stojić M. Effect of educational programs on asthma control and quality of life in adult asthma patients. Patient Education and Counseling 2005;58(1):47‐74. [DOI] [PubMed] [Google Scholar]
Additional references
Beasley 2004
- Beasley R. The Global Burden of Asthma Report, Global Initiative for Asthma (GINA). http://www.ginasthma.org/Global‐Burden‐of‐Asthma (accessed 6 January 2014).
Cohen 1983
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behaviour 1983;24(4):385‐96. [PubMed] [Google Scholar]
Frattaroli 2006
- Frattaroli J. Experimental disclosure and its moderators: a meta‐analysis. Psychological Bulletin 2006;132(6):823‐65. [DOI] [PubMed] [Google Scholar]
Frisina 2004
- Frisina PG, Borod JC, Lepore SJ. A meta‐analysis of the effects of written emotional disclosure on the health outcomes of clinical populations. Journal of Nervous and Mental Disease 2004;192(9):629‐34. [DOI] [PubMed] [Google Scholar]
Gidron 2002
- Gidron Y, Duncan E, Lazar A, Biderman A, Tandeter H, Shvartzman P. Effects of guided written disclosure of stressful experiences on clinic visits and symptoms in frequent clinic attenders. Family Practice 2002;19(2):161‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, www.cochrane‐handbook.org, 2011. [Google Scholar]
Hyland 1995
- Hyland ME, Ley A, Fisher DW, Woodward V. Measurement of psychological distress in asthma and asthma management programmes. British Journal of Clinical Psychology 1995;34(4):601‐11. [DOI] [PubMed] [Google Scholar]
Juniper 1999
- Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. European Respiratory Journal 1999;14(4):902‐7. [DOI] [PubMed] [Google Scholar]
Lepore 2002
- Lepore SJ, Greenberg MA, Bruno M, Smyth JM. Expressive writing and health: self‐regulation of emotion‐related experience, physiology, and behavior. In: Lepore SJ, Smyth JM editor(s). The Writing Cure: How Expressive Writing Promotoes Health and Emotional Well‐Being. Washington, DC: American Psychological Association, 2002:99‐117. [Google Scholar]
Marks 1992
- Marks GB, Dunn SM, Woolcock AJ. A scale for the measurement of quality of life in adults with asthma. Journal of Clinical Epidemiology 1992;45:461‐72. [DOI] [PubMed] [Google Scholar]
Meads 2005
- Meads C, Nouwen A. Does emotional disclosure have any effects? A systematic review of the literature with meta‐analyses. International Journal of Technology Assessment in Health Care 2005;21(2):153‐64. [PubMed] [Google Scholar]
Nathan 2004
- Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: a survey for assessing asthma control. Journal of Allergy and Clinical Immunology 2004;113(1):59‐65. [DOI] [PubMed] [Google Scholar]
Pennebaker 1986
- Pennebaker JW, Beall SK. Confronting a traumatic event: toward an understanding of inhibition and disease. Journal of Abnormal Psychology 1986;95(3):274‐81. [DOI] [PubMed] [Google Scholar]
Pennebaker 1986a
- Pennebaker JW, Hoover C. Inhibition and cognition: toward an understanding of trauma and disease. In: Davidson RJ, Schwartz DW, Shapiro D editor(s). Consciousness and Self Regulation. New York: Plenum, 1986:107‐36. [Google Scholar]
Pennebaker 1993
- Pennebaker JW. Putting stress into words: health, linguistic, and therapeutic implications. Behaviour Research Therapy 1993;31(6):539‐48. [DOI] [PubMed] [Google Scholar]
RevMan 5 [Computer program]
- Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration. Review Manager (RevMan) Version 5.2. Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration, 2012.
Smyth 1998
- Smyth JM. Written emotional expression: effect sizes, outcome types, and moderating variables. Journal of Consulting and Clinical Psychology 1998;66(1):174‐84. [DOI] [PubMed] [Google Scholar]
Wahlgren 1997
- Wahlgren DR, Hovell MF, Matt GE, Meltzer SB, Zakarian JM, Meltzer EO. Toward a simplified measure of asthma severity for applied research. Journal of Asthma 1997;34(4):291‐303. [DOI] [PubMed] [Google Scholar]
Wasserfellan 1997
- Wasserfallen JB, Gold K, Schulman KA, Baraniuk JN. Development and validation of a rhinoconjunctivitis and asthma symptom score for use as an outcome measure in clinical trials. Journal of Allergy and Clinical Immunology 1997;100(1):16‐22. [DOI] [PubMed] [Google Scholar]
WHO 2013
- WHO. Fact about asthma. http://www.who.int/mediacentre/factsheets/fs307/en/ (accessed 6 January 2014).
Wright 1998
- Wright RJ, Rodriguez M, Cohen S. Review of psychosocial stress and asthma: an integrated biopsychosocial approach. Thorax 1998;53(12):1066‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yorke 2005
- Yorke J, Fleming SL, Shuldham C. Psychological interventions for children with asthma. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD003272.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yorke 2009
- Yorke J, Fleming SL, Shuldham C. Psychological interventions for adults with asthma. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD002982.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


