Abstract
Background
Gastric cancer (GC) is the fourth leading cause of cancer deaths globally. There is a paucity of real-life data on GC in Brazil. Our study aimed to evaluate survival trends in gastric adenocarcinoma (GA) in a large cancer center in Brazil during 2000–2017.
Methods
Based on our Hospital Cancer Registry Database, all individuals diagnosed with GA between 2000 and 2017, and treated at A.C. Camargo Cancer Center, were retrospectively included. The primary objectives were to describe the patient demographics, clinicopathological characteristics, treatment modalities and survival trends during four separate periods of diagnosis (2000–2004; 2005–2009; 2010–2014 and 2015–2017). χ2 test was performed between two specified periods (2000–2004 and 2015–2017) to compare categorical variables. Overall survival (OS) curves were stratified by four separate periods and compared with log-rank tests.
Results
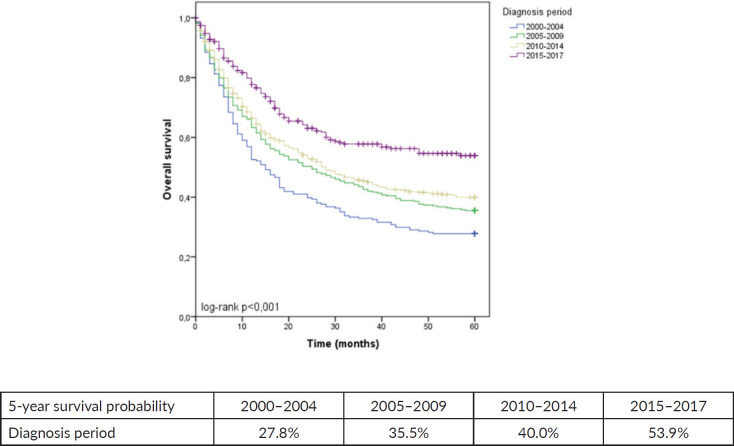
This analysis included 1,406 individuals. Across all periods, most patients were men aged 50–69 and presented with Lauren’s intestinal subtype. The frequency of stage IV disease significantly decreased between 2000–2004 and 2015–2017 (43.6% to 32.8%, p < 0.001). In contrast, we observed a rise in stage II (9.4% to 24.8%, p < 0.001) in the same comparison. We noticed an increased utilization of a combined approach involving chemotherapy and surgery (12% in 2000–2004 and 36.3% in 2015–2017, p < 0.001). The predicted 5-year OS of patients with GA in 2000–2004 was 27.8%, which increased to 53.9% in 2015–2017 (p < 0.001).
Conclusion
Our retrospective cohort showed an upward trend in survival rates during the period. We observed that 5-year OS almost doubled among men and women during 2000–2017.
Mini Abstract
The present retrospective cohort showed an upward trend in survival rates during the period from 2000 to 2017, in which the OS almost doubled among men and women.
Keywords: gastric cancer, prognosis, epidemiology
Introduction
Gastric cancer (GC) is the fifth most common cancer in Brazil [1] and worldwide [2], ranking fourth in global cancer-related mortality [3]. There has been a decrease in the global incidence and mortality of GC over the past decades [4, 5]. Nevertheless, the incidence has increased among young adults below the age of 50 [6].
Gastric adenocarcinoma (GA) constitutes approximately 90% of the total cases of GC [7]. Significant progress has been achieved in the management of GA over the past two decades [8, 9]. Curative treatment often involves a multimodal approach comprising R0 resection, D2 lymphadenectomy, radiotherapy and chemotherapy [10]. For advanced-stage patients, palliative systemic therapy is the standard of care, though it remains associated with an unfavourable prognosis [11].
Improvements range from adequate clinical staging with modern imaging techniques [12, 13] and diagnostic laparoscopy [14], to perioperative chemotherapy (PCT) for locally advanced disease [15, 16], and minimally invasive surgery [17]. Furthermore, systemic chemotherapy [18] and targeted agents [19] have increased survival in the advanced setting.
There is still a paucity of real-life data on GA survival analysis from underrepresented countries. Herein, we aim to describe the survival trends in patients with GA, regardless of their clinical staging, treated at a large cancer center in Brazil during a period of 17 years (2000–2017).
Methods
This is a retrospective hospital-based cohort of patients diagnosed with GA between 2000 and 2017 who were treated at A.C. Camargo Cancer Center. All patients were identified from the Hospital Cancer Registry Database using ICD C16; data was extracted on 10 August 2022.
Patients were considered eligible if they were 18 years old or above and had histological confirmation of GA independent of clinical staging. The date of diagnosis was determined as the date of histopathological analysis. We excluded individuals with esophageal squamous-cell carcinoma, gastrointestinal stromal tumours, gastric lymphomas and other rare histologies. The primary objectives were to describe the patient population demographics, clinicopathological characteristics, treatment modalities and survival trends during four distinguished periods of diagnosis (2000–2004; 2005–2009; 2010–2014 and 2015–2017).
Age group (<50, 50–69, 70+) and gender were analysed. We also collected the following information about the disease: histopathological type (according to Lauren’s classification), and clinical staging, according to the American Joint Committee on Cancer (AJCC) 7th Edition. Treatment modalities were grouped as follows: surgery alone, radiotherapy alone, chemotherapy alone or any combination of these approaches.
Descriptive statistics were used for demographics, clinicopathological characteristics and treatment modalities. χ2 tests were used to compare categorical variables between two specified periods (2000–2004 and 2015–2017).
Overall survival (OS) was calculated from diagnosis to date of death by any cause or last follow-up. Survival curves were stratified by four separate periods (2000–2004; 2005–2009; 2010–2014 and 2015–2017) and by sex. 5-year OS was estimated using Kaplan-Meier curves, and comparisons were performed with log-rank tests. All tests were considered statistically significant with a two-sided p-value of < 0.05.
Statistical analyses and 5-year survival probability curves were performed with IBM SPSS Statistics (version 23). The study protocol was approved by the institutional ethics committee (n. 2462/17) on 12 May 2017.
Results
The current analysis comprises a retrospective cohort of 1,406 patients (552 female and 854 male individuals). Demographics, clinicopathological characteristics and treatment modalities across the four periods of diagnosis are summarised in Table 1. The number of new cases per year was 46.8 during 2000–2004, and increased to 103.6 during 2015–2017. Across all periods, most patients were men aged 50–69 years and predominantly presented with Lauren’s intestinal subtype.
Table 1. Demographics, clinicopathological characteristics and treatment modalities during four separate periods of diagnosis.
| Variable | 2000–2004 N = 234 |
2005–2009 N = 324 |
2010–2014 N = 537 |
2015–2017 N = 311 |
*p-value |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 135 (57.7) | 199 (61.4) | 323 (60.1) | 197 (63.3) | 0.185 |
| Female | 99 (42.3) | 125 (38.6) | 214 (39.9) | 114 (36.7) | |
| Age | |||||
| <50 | 52 (22.2) | 61 (18.8) | 100 (18.6) | 45 (14.5) | 0.064 |
| 50–69 | 109 (46.6) | 141 (43.5) | 274 (51.0) | 160 (51.4) | |
| 70+ | 73 (31.2) | 122 (37.7) | 163 (30.4) | 106 (34.1) | |
| Histologic type | |||||
| Intestinal | 98 (41.9) | 133 (41.0) | 210 (39.1) | 118 (37.9) | 0.093 |
| Diffuse | 75 (32.1) | 98 (30.2) | 202 (37.6) | 102 (32.8) | |
| Undetermined | 60 (25.6) | 88 (27.2) | 114 (21.2) | 80 (25.7) | |
| Other | 1 (0.4) | 5 (1.5) | 11 (2.0) | 11 (3.5) | |
| Clinical staging | |||||
| I | 33 (14.1) | 63 (19.4) | 85 (15.8) | 55 (17.7) | <0.001 |
| II | 22 (9.4) | 34 (10.5) | 106 (19.7) | 77 (24.8) | |
| III | 45 (19.2) | 64 (19.8) | 93 (17.3) | 53 (17.0) | |
| IV | 102 (43.6) | 125 (38.6) | 218 (40.6) | 102 (32.8) | |
| No data | 32 (13.7) | 38 (11.7) | 35 (6.5) | 24 (7.7) | |
| Treatment | |||||
| Surgery | 96 (41.0) | 91 (28.1) | 105 (19.6) | 56 (18.0) | <0.001 |
| Radiotherapy | 14 (6.0) | 8 (2.5) | 3 (0.6) | 3 (1.0) | |
| Chemotherapy | 31 (13.2) | 58 (17.9) | 133 (24.8) | 73 (23.5) | |
| Surgery + Radiotherapy | 5 (2.1) | 2 (0.6) | - | 2 (0.6) | |
| Surgery + Chemotherapy | 28 (12.0) | 69 (21.3) | 190 (35.4) | 113 (36.3) | |
| Radiotherapy + Chemotherapy | 5 (2.1) | 15 (4.6) | 28 (5.2) | 26 (8.4) | |
| Surgery + Radiotherapy + Chemotherapy | 29 (12.4) | 54 (16.7) | 40 (7.4) | 14 (4.5) | |
| Other treatment combination | - | - | - | 10 (3.2) | |
| No treatment performed | 26 (11.1) | 27 (8.3) | 38 (7.1) | 14 (4.5) |
X2 test between 2000 and 2004 and 2015-2017. p < 0.05
There were significant differences between staging groups during the study. Specifically, between 2000 and 2004, stage IV varied from 43.6% to 32.8% in 2015–2017 (p < 0.001). The most substantial variation in nonmetastatic cases occurred in stage II, comprising 9.4% of cases from 2000 to 2004 and rising to 24.8% in 2015–2017 (p < 0.001).
Concerning treatment modalities, we observed an increased utilisation of a combined approach involving chemotherapy and surgery throughout the investigation. Specifically, from 2000 to 2004, only 12% of patients received a multimodal treatment regimen consisting of chemotherapy and surgery, whereas, between 2015 and 2017, this proportion increased to 36.3% (p < 0.001). Also, patients receiving surgery + radiotherapy + chemotherapy varied from 12.4% of patients in 2000–2004 to 4.5% in 2015–2017 (p < 0.001).
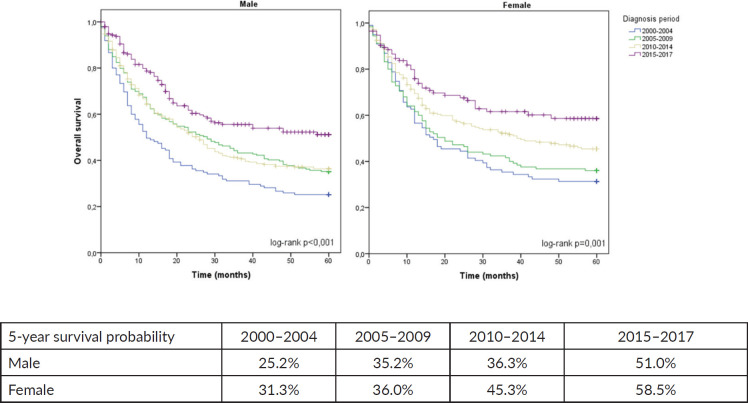
When the entire cohort was analysed, the predicted 5-year OS of patients with GA between 2000 and 2004 was 27.8%, which increased to 53.9% in the period from 2015 to 2017 (p < 0.001), as shown in Figure 1. Among females, 5-year OS increased from 31.3% between 2000 and 2004 to 58.5% between 2015 and 2017 (p = 0.001). Among males, 5-year OS increased from 25.2% to 51.0% in the same comparison (p < 0.001), as shown in Figure 2.
Figure 1. 5-year OS for GA stratified by diagnosis period.
Figure 2. 5-year OS for GA stratified by sex and diagnosis period.
Discussion
This study represents a pioneering investigation of GA utilising a hospital-based cancer registry data in the Latin American context. Our findings describe the OS trends over 17 years in a tertiary cancer center. This milestone research contributes to understanding GA outcomes in our region, shedding light on critical aspects of patient prognosis and healthcare management.
The findings from our study reveal a consistent and notable increase in OS during the analysed period. This improvement may be attributed to several contributing factors, including a shift to earlier diagnoses, enhanced staging accuracy, more effective chemotherapy protocols, and improvements in surgical interventions. These factors collectively may elucidate the observed positive trends in survival outcomes.
Diagnosis of GA at earlier stages is related to higher OS [20, 21]. Our analysis revealed a significant trend towards more initial tumours, especially within AJCC 7th ed. clinical stage II. Although current guidelines do not recommend screening in the ‘average-risk’ population of Western countries [22], the more widespread availability of upper endoscopy [23] was an important factor leading to this transition of earlier staging GA cases.
Patients diagnosed with GA need a comprehensive staging evaluation to inform treatment decisions and enhance prognostication. In patients with locally advanced disease, the systematic use of staging laparoscopy detects occult peritoneal dissemination in 31% of patients [24]. A previous publication from our group highlighted that 57.7% of all GA patients underwent staging laparoscopy at our center [25].
International guidelines in Western countries recommend, as a preference, performing PCT as a multimodal approach [10, 26]. The pivotal Magic trial [16], published in 2006, compared perioperative Epirrubicin, Cisplatin and 5-Fluorouracil (ECF) versus surgery alone in 503 locally advanced gastroesophageal cancer patients in the United Kingdom. PCT significantly improved OS (HR, 0.75; 95%CI, 0.60–0.93; p = 0.009) and progression-free survival (HR, 0.66; 95CI, 0.53–0.81; p < 0.001).
Another randomised trial from France [27] compared doublet PCT with Cisplatin and 5-Fluorouracil, versus surgery in 224 locally advanced gastroesophageal cancer patients. PCT also demonstrated improved OS (5-year rate 38% versus 24%; HR, 0.69; 95%CI, 0.50–0.95; p = 0.02). At our institution, patients with locally advanced disease (mainly >cT3 and cN+) have been systematically referred to PCT, since 2006. Our previously published data revealed that 65.5% of nonmetastatic patients underwent PCT [28].
More recently, perioperative 5-Fluorouracil, Oxaliplatin and Docetaxel (FLOT) were compared to ECF in a phase 2/3, randomised trial [29, 30] in Germany. Patients with locally advanced gastroesophageal cancer (>cT2 or cN+) were included. Perioperative FLOT was significantly superior to ECF in OS (HR, 0.77; 95%CI, 0.63–0.94 p = 0.012). The period of this publication aside, FLOT has become our standard protocol since 2017.
Surgery remains the cornerstone of curative-intended treatment. The main goals of surgery are achieving an R0 resection [31] and performing a D2 lymphadenectomy [32]. In recent years, minimally invasive techniques, such as laparoscopic and robotic procedures, have become available. Surgical teams’ expertise and the perioperative care protocols are also highly relevant. Previously published data from patients treated at our cancer center demonstrated a low surgical mortality rate of 4.4% [25]. Also, the frequency of adjuvant chemoradiation, evaluated in the Intergroup US 0116 Trial [33], decreased during the period. It is known that this adjuvant strategy is less common in the present day due to standard D2 lymphadenectomy, which was performed in less than 10% of pivotal trials. Previously published from our cancer center demonstrated that D2-lymphadenectomy was performed in 90.8% of the patients [34].
To the best of our knowledge, this analysis represents the largest real-life GA database in our country. There might be some imprecision in collected information because of the extensive period covered in our research and changes made in the AJCC staging system over that period. It is also important to highlight that most of our patients were treated in the context of a private hospital. Hence, our results cannot be extrapolated to patients in the public health system. Moreover, interesting findings related to differences in OS, such as better survival in females, have not been fully clarified thus far and warrant further investigation.
Conclusion
Our hospital-based data shows an upward trend in survival rates across patients with GA. We observed that 5-year OS almost doubled among men and women during the analysed period. Recent improvements in staging methods and treatment have increased cure rates. Nevertheless, further investigation is essential to determine significant differences in OS between males and females.
Conflicts of interest
The authors declare no conflicts of interest.
Funding
None.
References
- 1.INSTITUTO NACIONAL DE CÂNCER JOSÉ ALENCAR GOMES DA SILVA. Estimativa 2023: incidência do Câncer no Brasil. Rio de Janeiro: INCA Dehwgb; [Google Scholar]
- 2.Morgan E, Arnold M, Camargo MC, et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020–40: a population-based modelling study. EClinicalMedicine. 2022;47:101404. doi: 10.1016/j.eclinm.2022.101404. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. (In eng) [DOI] [PubMed] [Google Scholar]
- 4.Bray F, Ferlay J, Laversanne M, et al. Cancer incidence in five continents: inclusion criteria, highlights from volume X and the global status of cancer registration. Int J Cancer. 2015;137(9):2060–2071. doi: 10.1002/ijc.29670. (In eng) [DOI] [PubMed] [Google Scholar]
- 5.Bertuccio P, Chatenoud L, Levi F, et al. Recent patterns in gastric cancer: a global overview. Int J Cancer. 2009;125(3):666–673. doi: 10.1002/ijc.24290. (In eng) [DOI] [PubMed] [Google Scholar]
- 6.Sung H, Siegel RL, Rosenberg PS, et al. Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. Lancet Public Health. 2019;4(3):e137–e147. doi: 10.1016/S2468-2667(18)30267-6. (In eng) [DOI] [PubMed] [Google Scholar]
- 7.Karimi P, Islami F, Anandasabapathy S, et al. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23(5):700–713. doi: 10.1158/1055-9965.EPI-13-1057. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin. 2021;71(3):264–279. doi: 10.3322/caac.21657. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alsina M, Arrazubi V, Diez M, et al. Current developments in gastric cancer: from molecular profiling to treatment strategy. Nat Rev Gastroenterol Hepatol. 2023;20(3):155–170. doi: 10.1038/s41575-022-00703-w. [DOI] [PubMed] [Google Scholar]
- 10.Lordick F, Carneiro F, Cascinu S, et al. Gastric cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(10):1005–1020. doi: 10.1016/j.annonc.2022.07.004. (In eng) [DOI] [PubMed] [Google Scholar]
- 11.Hu HM, Tsai HJ, Ku HY, et al. Survival outcomes of management in metastatic gastric adenocarcinoma patients. Sci Rep. 2021;11(1):23142. doi: 10.1038/s41598-021-02391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdalla EK, Pisters PW. Staging and preoperative evaluation of upper gastrointestinal malignancies. Semin Oncol. 2004;31(4):513–529. doi: 10.1053/j.seminoncol.2004.04.014. (In eng) [DOI] [PubMed] [Google Scholar]
- 13.Mocellin S, Pasquali S. Diagnostic accuracy of endoscopic ultrasonography (EUS) for the preoperative locoregional staging of primary gastric cancer. Cochrane Database Syst Rev. 2015;2015(2):Cd009944. doi: 10.1002/14651858.CD009944.pub2. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowy AM, Mansfield PF, Leach SD, et al. Laparoscopic staging for gastric cancer. Surgery. 1996;119(6):611–614. doi: 10.1016/S0039-6060(96)80184-X. (In eng) [DOI] [PubMed] [Google Scholar]
- 15.Xiong BH, Cheng Y, Ma L, et al. An updated meta-analysis of randomized controlled trial assessing the effect of neoadjuvant chemotherapy in advanced gastric cancer. Cancer Invest. 2014;32(6):272–284. doi: 10.3109/07357907.2014.911877. (In eng) [DOI] [PubMed] [Google Scholar]
- 16.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. doi: 10.1056/NEJMoa055531. (In eng) [DOI] [PubMed] [Google Scholar]
- 17.Viñuela EF, Gonen M, Brennan MF, et al. Laparoscopic versus open distal gastrectomy for gastric cancer: a meta-analysis of randomized controlled trials and high-quality nonrandomized studies. Ann Surg. 2012;255(3):446–456. doi: 10.1097/SLA.0b013e31824682f4. (In eng) [DOI] [PubMed] [Google Scholar]
- 18.Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358(1):36–46. doi: 10.1056/NEJMoa073149. (In eng) [DOI] [PubMed] [Google Scholar]
- 19.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–697. doi: 10.1016/S0140-6736(10)61121-X. (In eng) [DOI] [PubMed] [Google Scholar]
- 20.Onodera H, Tokunaga A, Yoshiyuki T, et al. Surgical outcome of 483 patients with early gastric cancer: prognosis, postoperative morbidity and mortality, and gastric remnant cancer. Hepatogastroenterology. 2004;51(55):82–85. (In eng) [PubMed] [Google Scholar]
- 21.Maharjan U, Kauppila JH. Survival trends in gastric cancer patients between 1987 and 2016: a population-based cohort study in Finland. Gastric Cancer. 2022;25(6):989–1001. doi: 10.1007/s10120-022-01326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lansdorp-Vogelaar I, Meester RGS, Laszkowska M, et al. Cost-effectiveness of prevention and early detection of gastric cancer in Western countries. Best Pract Res Clin Gastroenterol. 2021;50–51:101735. doi: 10.1016/j.bpg.2021.101735. (In eng) [DOI] [PubMed] [Google Scholar]
- 23.Januszewicz W, Kaminski MF. Quality indicators in diagnostic upper gastrointestinal endoscopy. Therap Adv Gastroenterol. 2020;13:1756284820916693. doi: 10.1177/1756284820916693. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarela AI, Lefkowitz R, Brennan MF, et al. Selection of patients with gastric adenocarcinoma for laparoscopic staging. Am J Surg. 2006;191(1):134–138. doi: 10.1016/j.amjsurg.2005.10.015. (In eng) [DOI] [PubMed] [Google Scholar]
- 25.Coimbra FJF, Jesus VHF, Ribeiro HSC, et al. Impact of ypT, ypN, and adjuvant therapy on survival in gastric cancer patients treated with perioperative chemotherapy and radical surgery. Ann Surg Oncol. 2019;26(11):3618–3626. doi: 10.1245/s10434-019-07454-0. (In eng) [DOI] [PubMed] [Google Scholar]
- 26.Ajani JA, D'Amico TA, Bentrem DJ, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(2):167–192. doi: 10.6004/jnccn.2022.0008. (In eng) [DOI] [PubMed] [Google Scholar]
- 27.Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29(13):1715–1721. doi: 10.1200/JCO.2010.33.0597. (In eng) [DOI] [PubMed] [Google Scholar]
- 28.Diniz TP, Costa WL, Gomes CC, et al. Symptomatic recurrence and survival outcomes after curative treatment of gastric cancer: does intensive follow-up evaluation improve survival? Ann Surg Oncol. 2022;29(1):274–284. doi: 10.1245/s10434-021-10724-5. [DOI] [PubMed] [Google Scholar]
- 29.Al-Batran SE, Hofheinz RD, Pauligk C, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016;17(12):1697–1708. doi: 10.1016/S1470-2045(16)30531-9. (In eng) [DOI] [PubMed] [Google Scholar]
- 30.Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393(10184):1948–1957. doi: 10.1016/S0140-6736(18)32557-1. (In eng) [DOI] [PubMed] [Google Scholar]
- 31.Roder JD, Böttcher K, Siewert JR, et al. Prognostic factors in gastric carcinoma. Results of the German gastric carcinoma study 1992. Cancer. 1993;72(7):2089–2097. doi: 10.1002/1097-0142(19931001)72:7<2089::AID-CNCR2820720706>3.0.CO;2-H. (In eng) [DOI] [PubMed] [Google Scholar]
- 32.Wu CW, Hsiung CA, Lo SS, et al. Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol. 2006;7(4):309–315. doi: 10.1016/S1470-2045(06)70623-4. (In eng) [DOI] [PubMed] [Google Scholar]
- 33.MacDonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 34.Costa Jr WL, Coimbra FJ, Fogaroli RC, et al. Adjuvant chemoradiotherapy after d2-lymphadenectomy for gastric cancer: the role of n-ratio in patient selection. Results of a single cancer center. Radiat Oncol. 2012;7:169. doi: 10.1186/1748-717X-7-169. [DOI] [PMC free article] [PubMed] [Google Scholar]