Abstract
The aim of this study was to determine the frequency of TP53 mutation among Pakistani head and neck cancer (HNC) patients who visited Nishtar Hospital Multan and Nishtar Institute of Dentistry (NID), Multan, Pakistan. While significant research has been conducted on the role of p53 in HNC throughout the world, this study is the first of its kind in Southern Punjab, Pakistan. A total of 242 samples (121 cases and 121 controls) were collected from Nishtar Hospital Multan and NID, Multan, Pakistan. After histopathological analysis to determine the stage type and grade of malignancy, DNA extraction and sequencing were carried out to assess any mutations in the TP53 region (exons 5–8). Genetic screening was performed by the polymerase chain reaction (PCR)-single strand conformation polymorphism (SSCP) technique and Chromas 2.6.6 was used to visualise the sequencing results. The mean age of cases was 50.73 ±16.41 years and controls were 37.55 ± 15.51 years. The frequency of HNC was higher in male patients (65.28%) than in female patients (34.71%). Overall, this cancer was found to be significantly more prevalent in the age group of >35–55 years (p < 0.001). Smoking (51% versus 14%), naswar usage (15.7% versus 6.6%), poor oral hygiene (52.9% versus 29.8%) and anemic status (57.0% versus 4.1%) were significantly associated with cases (p ≤ 0.05) compared to controls. Only 04 samples exon 5 (1 sample), exon 7 (2 samples) and exon 8 (1 sample) with changed mobility patterns were found on the SSCP gel. All mutations were missense and heterozygous. Out of four mutant samples, three mutations were in the hotspot regions (codon 175, 245 and 248) of p53. Based on this study, there may be a weak association between the TP53 exon 5–8 mutation and HNC patients in Southern Punjab, Pakistan.
Keywords: TP53, head and neck cancer, hotspot mutation, SSCP, missense mutation
Introduction
Head and neck cancer (HNC) accounts for 5% of all cancers globally and is the seventh most common cancer worldwide [1–5]; however, in Pakistan, HNC accounts for 32.6% of all cancers [65] and is considered as second most usual cancer [65, 66]. HNC prevalence is expected to increase by 30% yearly in 2030 in both advanced and poor countries [3, 4, 6]. Its prevalence is different because of anatomical sub sites and geographical differences. Smoking-linked HNC has been found to be decreased in developed countries and a rising trend has been found in Human papillomavirus (HPV) linked HNC but in developing countries, a continuous rise has been seen [67–70]. The highest ratio of HNC has been recorded in South East Asia and some countries of Central and South Europe such as France and Belgium. The frequency of different types of HNC is high in Pakistan, India, France, Brazil, Bangladesh, Afghanistan, Nepal, Sri Lanka, Iran, Maldives and Bhutan [71, 72].
Major causes of HNC include hereditary and natural harmful components such as tobacco, Epstein-Barr Virus, HPV, arecanut, liquor, eating less carbs and other ecological and occupational exposures [7, 8]. Other harmful factors are ecological exposures to wool dust, wood dust, mineral filaments and low consumption of vegetables and fruits [9, 10]. The most common modifications in HNSCC are the disturbance in the pathway of p53 [11, 12].
HNC is a complex illness that arises from changes in the tumour suppressor genes due to several gene-gene and gene-environment interactions. One of these genes is TP53, which is essential for controlling regular cell division and whose disturbance is the most frequent alteration in HNSCC (TP53; MIM#191170) [12]. In humans, the TP53 gene encodes the transcription factor tumour suppressor p53 protein. To maintain normal cellular functions and genome integrity, it is responsible for DNA repair, cellular homeostasis, metabolism, senescence and stimulation of non-apoptotic and apoptotic cell death. It is responsible for the activation of countless genes engaged in cell death metabolism and arrest of cell cycle in reaction to hypoxia, DNA damage, DNA mutations and oxidative stress, leading to stop the development of cancer [13]. So the loss of p53 activity leads to gene deletion or mutation, results in failure of normal cell activity to regulate and repair DNA death or damage, and finally causes cancer due to genomic variability [73].
TP53 mutations were confirmed in large-scale data in 2007 [14]. Almost 40%–60% of HNC patients have TP53 mutations leading to cancer progression from premalignant to invasive form and early recurrence of the disease is increased in these patients [15]. Point mutation makes the p53 protein non-functional or unstable, incapable of checking the proliferation of the cell. Non-functional p53 protein increases due to overexpression of the gene p53 and is reported in many studies [16]. Variations in p53 are common and appear early in HNC and both tobacco carcinogenic agents and HPV disease, two major risk factors, appear to target p53 [17]. The varying frequency (30% to 70%) of modifications in TP53 HNC patients in different studies is due to HNC heterogeneity (like cancer of various organs) and various methods used to detect TP53 mutational changes in HNC (such as mutational screening and immunohistochemistry). Many studies confirmed the role of TP53 mutations with a frequency range of 75% to 85% in non-HPV-associated HNSCC patients [18–21]. The majority of TP53 mutations shown by the International Agency for Research on Cancer affect exons 5–8, which make up the DNA-binding region specific to each site [22]. The goal of the current study was to determine if HNC patients from the Southern Punjab area had any mutations in the TP53 gene exon 5–8.
Materials and methods
In this study, we recruited a total of 242 participants, including 121 cases and 121 controls, from Nishtar Hospital Multan and Nishtar Institute of Dentistry, Multan, Pakistan, between 2017 and 2020. Informed consent was obtained from all the patients after informing them about the potential benefits of the study, in compliance with hospital rules. We collected Patient’s personal history, histopathological and other related information from the hospital and patients using a predesigned questionnaire. Ethical Approval for research work was obtained from the University Research Ethics Committee of Women University Multan (Reference No: WUM/UREC/00014).
The inclusion criteria for cases were HNC patients of any age (7 to 90 years), those not undergoing any treatment for cancer, and those with a clinical diagnosis of HNC. Fresh tissues and blood samples from 121 HNC cases were collected at the time of surgery before therapy. Various factors were compared between cases and controls including non-demographic factors (such as oral hygiene, diet, gum bleeding and anemic condition) and demographic factors (such as age, naswar use and smoking habit). The biopsy-proven HNC fresh tissues of any stage (TNM staging system includes tumour size and primary site, absence and presence of a metastatic condition of disease and nodal disease). The TNM stages are used to determine the overall stage of cancer, which ranges from 0 to IV [74], and were used in this investigation for the molecular analysis of the TP53 gene. To preserve the tissue, it was immediately put in liquid nitrogen and stored at −80°C. Following the manufacturer’s instructions, genomic DNA was isolated from fresh, frozen tissues of HNC using Thermo Scientific DNA Purification (Cat # K0721). Sequencing was carried out following Single Strand Conformation Polymorphism analysis (SSCP). SSCP was used to find mutational alterations in the core DNA binding domain of the TP53 gene, which contains important hot spots. Exon 5–8 regions of P53 were selected that give amplicons of sizes ranging from 180–247 base pairs. A mutant forward primer with a single nucleotide alteration by replacing T with G was chosen to provide the mutant positive control of exons 5 and 8. A DNA sequence altered from A to G was chosen as a mutation-positive control for exon 6, and a DNA sequence changed from G to C was chosen as a mutation-positive control for exon 7. Sequencing was used for the confirmation of all these mutant controls.
PCR cycling conditions were set at 95°C for 5 minutes, followed by 30 cycles of 30 seconds at 95°C, 45 seconds at the annealing temperature of the primers used (Exon 5 = 57°C, Exon 6 = 54°C, Exon 7 = 59°C and Exon 8 = 56°C), and 30 seconds at 72°C using the thermal cycler G-Storm 4822. The gel electrophoresis was conducted in 1× TAE buffer for 30 to 45 minutes at 100V followed by visualisation and photography using an ultraviolet transilluminator.
When Exon 5–8 of the TP53 gene were amplified then SSCP analysis was carried out on the Decode™ Universal Mutation Detection System, Biorad (Cat. # 170-9092). For the optimisation of SSCP of all four exons and gels were run in varying conditions, i.e., a. Exon 5 and 6: 60 mL of 12% non-denaturing polyacrylamide gel solution was prepared by mixing 24 mL of 30% acrylamide mix (29:1 acrylamide/bisacrylamide), 6 mL of 10× Tris boric acid EDTA (TBE) (pH 8.3) and 30 mL of deionized water and stored at 4°C. b. Exon 7 and 8: 60 mL of 12% non-denaturing polyacylamide gel solution was prepared by mixing 24 mL of 30% acrylamide mix (29:1 acrylamide/bisacrylamide), 33 mL of 10× TBE (pH 8.3) and 30 mL of deionized water and stored at 4°C.
For SSCP analysis, 5 µL PCR product was mixed with 15 µL of loading buffer (0.5 M NaOH, 0.05% bromophenol blue, 0.05% xylene cyanol and 50% (w/v) Sucrose) denatured at 96°C for 8 minutes and immediately placed on ice. Twenty microliters of each denatured PCR product (containing 30–40 ng DNA) were loaded onto a 12% gel. Electrophoresis was conducted with TBE running buffer (1× for exon 5 and 6: 0.5× for exon 7 and 8) at 220V for 10–12 hours until xylene cyanol reached the bottom of the gel. The temperature for electrophoresis was 20°C for exon 5 but for exon 6, 7 and 8, the best results were found at 4°C. After electrophoresis, the gels were separated from the plates using a gel separator and transferred to a tank containing an ethidium bromide solution (250 mL of deionized water and 250 uL of 10 mg/mL ethidium bromide solution) for 5 to 10 minutes. The gel was visualised on a UV transilluminator and photographed.
For results interpretation, we compared the SSCP band patterns of normal control, mutant control DNA and cases. No mutations were observed in control samples. Confirmation of all mutation-positive cases was done at least three times by a separate SSCP run and PCR reaction. Control samples did not show any mutation. Only those samples that exhibited a changed pattern in the form of extra bands and mobility shifts with respect to normal samples have proceeded for sequencing. These PCR products were amplified under the appropriate conditions, run on a 1.5% agarose gel and the bands exhibiting a changed pattern were cut to isolate DNA for sequencing using a Thermo Scientific GeneJET Gel Extraction kit (Cat. # K0691). Sequencing analysis was performed using the Sanger sequencing method and chromatograms were used to record sequencing results. The chromatograms were used to visually analyse the data and identify changes in the human TP53 gene in HNC patients.
The SPSS software, (version 20) was used to conduct statistical analyses. The demographic and non-demographic characteristics of cases and controls were assessed by the χ2 test. Multiple logistic regression was applied for multivariate analyses. We considered p-values of ≤0.05 as statistically significant.
Results
Out of the total 242 study participants, there was no difference in gender between the cases and controls, with an equal number of males (n = 79) and females (n = 42) in both groups. The mean age of the study population, ranged from 7 to 90 years, with cases having a mean age of 50.73 ± 16.41 years and controls having a mean age of 37.55 ± 15.51 years. A statistically significant difference (p ≤ 0.05) was observed among the groups with reference to demographic factors. HNC patients were significantly more frequent in the age group of >35–55 years (p < 0.001). HNC patients showed a high frequency of smoking (51% versus14%; p < 0.001), smoking frequency >10 times/day (90% versus 55%; p < 0.001), smoking duration >15 years (74% versus 5%; p < 0.001), naswar usage (15% versus 6%; p = 0.029), naswar use frequency 6–10 times/day (68% versus 50%; p = 0.370) and naswar use duration >20 years (52% versus 12%; p = 0.069) as compared to controls (Table 1).
Table 1. Demographic features among cases and controls.
| Variable | Cases (n = 121) n (%) |
Control (n = 121) n (%) |
OR | Cl (95%) | p- value |
|---|---|---|---|---|---|
| Age ≤ 35 >35–55 >55 |
22 (18.18) 52 (42.97) 40 (50.63) |
62 (51.3) 42 (34.7) 17 (14.0) |
Reference 3.49 7.79 |
1.851–6.577 3.726–16.294 |
<0.001*** <0.001*** |
| Smoking No Yes Smoking frequency 1–10 times/day >10 times/day Smoking duration (years) 1-15 >15 |
59 (48.7) 62 (51.2) 6 (9.7) 56 (90.3) 16 (25.8) 46 (74.2) |
103 (85.1) 18 (14.9) 17 (94.4) 1 (5.6) 17 (94.4) 1 (5.6) |
Reference 6.01 Reference 158.6 Reference 48.87 |
3.25–11.11 17.83–1411 6.01–397.3 |
<0.001*** <0.001*** <0.001*** |
| Naswar use No Yes Naswar use frequency 1–5 times/day 6–10 times/day Naswar use (years) 1–10 11–20 >20 |
102 (84.3) 19 (15.7) 6 (31.6) 13 (68.4) 4 (21.1) 5 (26.3) 10 (52.6) |
113 (93.4) 8 (6.6) 4 (50.0) 4 (50.0) 4 (50.0) 3 (37.5) 1 (12.5) |
Reference 2.63 Reference 2.16 Reference 1.66 0.06 |
1.10–6.27 0.40–11.74 0.22–12.2 0.83–119.3 |
0.029* 0.370 0.615 0.069 |
p ≤ 0.05 = significant;
p ≤ 0.001 = very highly significant; OR: odds ratio; Cl: Confidence Interval.
p-value = Chi-square test
Poor oral hygiene condition was significantly associated with cases (52% versus 29%; p = 0.026) as compared to controls but there was no significant association (57% versus 5%; p = 0.055), regarding gum bleeding factor between cases and controls. Anemic status was highly significantly associated with cases (57% versus 4%; p < 0.001) as compared to controls. With respect to diet, meat fonder was more frequent in cases as compared to controls (12% versus 3%; p = 0.055) (Table 2).
Table 2. Non-demographic factors among cases and controls.
| Variable | Cases (n = 121) n (%) |
Control (n = 121) n (%) |
OR | Cl (95%) | p-value |
|---|---|---|---|---|---|
| Oral hygiene Yes No Tooth brush/Miswak use >2 times/day 1 time/day |
57 (47.1) 64 (52.9) 19 (33.3) 38 (66.7) |
85 (70.2) 36 (29.8) 15 (17.6) 70 (82.4) |
Reference 2.82 Reference 0.429 |
1.64–4.78 0.19–0.938 |
0.026* 0.034* |
| Gum bleeding No Yes |
105 (86.8) 16 (57.0) |
114 (94.2) 7 (5.8) |
Reference 2.48 |
0.98–6.27 |
0.055 |
| Anemic status No Yes |
52 (43.0) 69 (57.0) |
116 (95.9) 5 (4.1) |
Reference 30.78 |
11.73–80.7 |
< 0.001** |
| Diet Vegetarian Mixed Meat fonder |
79 (65.3) 27 (22.3) 15 (12.4) |
65 (53.7) 52 (43.0) 4 (3.3) |
Reference 0.42 3.08 |
0.24–0.75 1.03–1.02 |
0.003* 0.055 |
p ≤ 0.05 = significant;
p ≤ 0.01 = highly significant; OR: odds ratio; Cl: Confidence Interval; p-value = Chi-square test
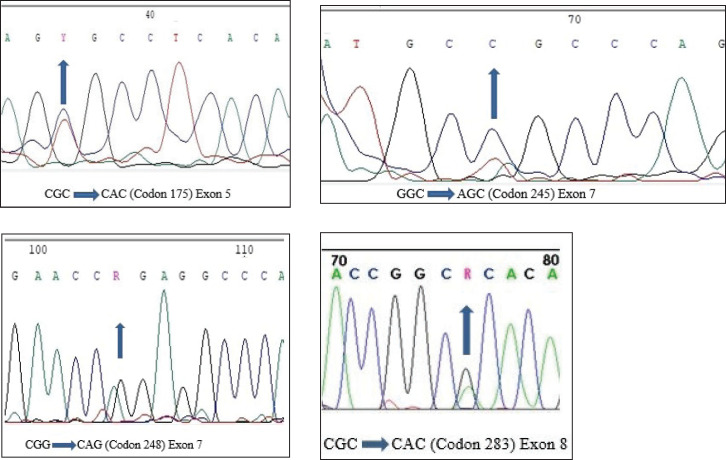
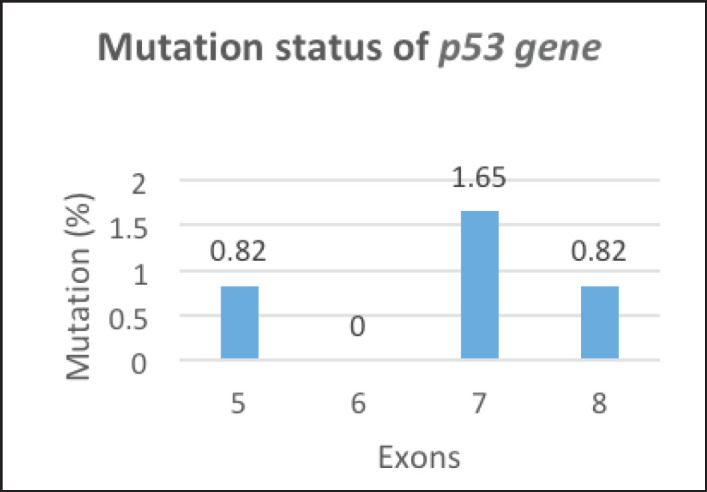
Only f4 out of 121 HNC samples (Exon 5, 7 and Exon 8) showed changed mobility patterns on the SSCP gel (Figure 1). All four mutant samples were from males. The affected site in Exon 5 and 7 was the larynx in three males, while in one male, the affected site (Exon 8) was the oral cavity. The patients had different occupations, with three being farmers and one being a cook. Tumour grade 2 was common in all four patients and all patients with the mutation had tumour stage III, with the affected site being the larynx, except for one patient who had tumour stage II, with the affected site being the oral cavity. Three of the patients smoked at the frequency of 20 times per day. Among the patients showing mutation, one was 45 years old and three patients were 60 years old (Table 3). Figure 2 shows the DNA sequencing chromatogram of mutations in Exon 5, 7 and 8. All exons sequencing chromatograms showed heterozygous mutations. In all four mutant samples, a missense mutation was observed. In Exon 5, a CGC > CAC (Arg > His) mutation was observed, and in Exon 7, a CGG > CAG (Gly > Ser) and CGG > CAG (Arg > Gln) mutations were observed and in Exon 8 a CGC > CAC (Arg > His) mutation was found. All four mutant samples had transition mutations. Mutations were found in the following codons; codon 175 (Exon 5), 245, 248 (Exon 7) and 283 (Exon 8) of the p53 gene (Table 4). The percentage of p53 mutation in Exon 5 and 8 was 0.82% and in Exon 7, it was 1.65%, while in Exon 6, it was 0% (Figure 3).
Figure 1. SSCP analysis of normal and variant mobility pattern. CA represents HNC patient while N indicates normal control gene amplified for exons 5, 7, and 8 of p53 gene.
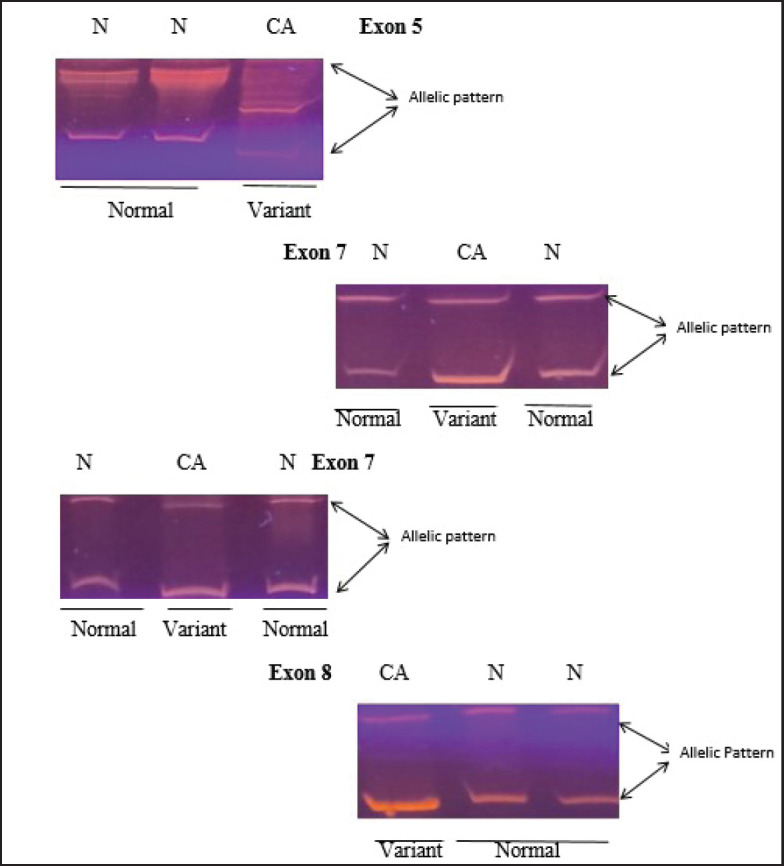
Table 3. Summary of mutant samples.
| Serial No. | Exon | Site | Gender | Age | Smoking frequency/day | Occupation | TNM classification | Tumour stage | Tumour grade |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Exon 5 | Larynx | Male | 60 years | 20/day | Farmer | T3N1M0 | III stage | Grade 2 |
| 2 | Exon 7 | Larynx | Male | 60 years | 20/day | Farmer | T3N1M0 | III stage | Grade 2 |
| 3 | Exon 7 | Larynx | Male | 45 years | 20/day | Shopkeeper | T3N0M0 | III stage | Grade 2 |
| 4 | Exon 8 | Oral cavity | Male | 60 years | No | Farmer | T2N0M0 | I stage | Grade 2 |
Figure 2. DNA sequencing chromatogram representing heterozygous mutations in each of exons 5, 7 and 8.
Table 4. Spectrum of TP53 mutations in HNC patients.
| Serial No. | Mutation name | Exon | Codon | Type | Event | Genetic alteration | Amino acid alteration |
|---|---|---|---|---|---|---|---|
| 1 | Arg175 His | 5 | 175 | Transition | G > A | CGC > CAC | Arg > His |
| 2 | Gly 245 Ser | 7 | 245 | Transition | G > A | GGC > AGC | Gly > Ser |
| 3 | Arg 248Gln | 7 | 248 | Transition | G> A | CGG > CAG | Arg > Gln |
| 4 | Arg 283 His | 8 | 283 | Transition | G > A | CGC > CAC | Arg > His |
Figure 3. Mutation status of p53 gene.
Discussion
In this study, we compared demographic factors and non-demographic factors between cases and controls. The mean age of cases was 50.73(±16.41) years and controls were 37.55(±15.51) years. Similar mean age ranges were observed in different studies [23, 24], while in contrast, a mean age of 63.8 ± 9.3 years was found in both cases and control in a 2019 study [25]. A significant difference in demographic features was observed between cases and controls, which is consistent with one Pakistani study [26], while a contrary result was found in another Pakistani study [24]. Regarding smoking, the number of smokers was higher in cases compared to controls, as found in different studies [24, 27], but contrary to other studies [26, 25] where the frequency of smokers was higher in controls. In the current study, smoking frequency (>10 times/day) was more in cases than in controls, as found in a study conducted in 2020 [27]. Additionally, we observed a significant association in the case of naswar usage between cases and controls. The frequency of naswar use was higher in cases than in controls in this study, similar to a Pakistani study [24]. Therefore, naswar may be one of the risk factors for HNC, which is in agreement with one Pakistani study [28].
Oral health is crucial for overall health and well-being. Poor oral health can lead to various oral diseases. In this study, poor oral health conditions were more prevalent in cases than controls, consistent with other studies [29, 24].
Due to low literacy rates and limited income, people in this area neglect their oral health. Gum bleeding was observed more frequently in cases in line with other studies [30, 31, 23]. Our study found a significant association between cases and controls regarding anemic status and diet. The percentage of anemic patients was higher in cases as reported in one study [32] and more vegetables were consumed by cases than controls consistent with other studies [33, 34]. However, unlike our study, some studies have reported an inverse relationship between vegetable consumption and HNC [35–37]. Additionally, our study found that cases consumed less meat, whereas studies conducted in Asia reported an inverse relationship between red meat consumption and HNC risk [38].
In this study, the frequency of TP53 mutations was low (3.3%) which is similar to the findings of many studies [39–43] where it was 3.2%, 3%, 2%, 3.4% and 0%, respectively. However other studies have reported a higher frequency of TP53 mutations [44–48], where it was 24%, 25%, 21.5%, 16% and 66.2%, respectively. The reasons for the variation in TP53 mutation frequency observed in different studies may be due to differences in detection techniques and the number of exons (2–11 exons) analysed. Therefore, it is more challenging to draw conclusions that can be applied across studies from a comprehensive evaluation of the literature [46, 49, 50]. Nonetheless, our study supports the notion that the incidence of TP53 gene mutations differs globally, even within the same country.
In the current study, three mutant samples had affected site larynx (Exon 5 and 7) similar to different studies [51, 8] and one had affected site oral cavity (Exon 8), consistent with the study of Peltonen et al [51]. 75% of mutations were found in hotspot codons of p53, i.e., 175, 245 and 248 codons, as found in different studies [45, 51, 8, 52, 53]. No mutation was found in Exon 6 contrary to the finding of Peltonen et al [51]. All mutations in the p53 gene found were missense mutations similar to different studies [51, 54, 8, 52, 55] that reported 90% of mutations in the p53 gene as missense mutations.
In the current study, all exon sequence chromatograms showed heterozygous mutations similar to the study of Leng et al [56] and contrary to the study of Baugh et al [52] which reported homosygous mutations in 50% to 60% of human cancer. Mutation in codon 245 was found in Exon 7, which matches with different studies [45, 58, 57, 8] that reported it as the most prevalent hotspot codon in HNC. The percentage of codon 245 mutation was 0.82%, compared to 0.4% in Baugh et al [52]. Mutation in codon 248 was found in Exon 7, similar to the study of Sisk et al [45], with a percentage of 0.82% and contrary to the study of Baugh et al [52], where codon 248% was 3.53%. In Exon 8, the mutation was found in codon 283 consistent with the study of Peltonen et al [51]. Mutation in codon 175 was found in Exon 5, similar to different studies [51, 52]. All four mutant samples showed only transition mutations contrary to the study of Schneider-Stock et al [58] where both transition and transversion mutations were observed. The age of patients was over 45 years old, consistent with the study of Poeta et al [14]. In this study with knowledge of the patients’ smoking habits and environmental exposure, we examined p53 aberrations in primary HNC patients. Three out of the four mutation-containing patients did smoking and were exposed to dust, insecticides and pesticides, similar to the factors observed in the study of Peltonen et al [51].
There is a well-established and significant link between smoking and HNC as found in several studies [59–63, 24, 25, 64] and it is known that p53 aberrations in general play a significant role in human cancers. Though the importance of p53 in the etiology of HNC has not yet been fully described in the literature, TP53 mutations in human malignancies have typically been the subject of substantial research. Mutant p53, the aberrant protein produced by TP53 alleles with missense mutations that frequently accumulate in cancer cells, has drawn a great deal of attention [51].
Conclusion
In the current study, we identified four mutations in exons 5–8 of the TP53 gene in HNC patients using SSCP. Our findings suggest that TP53 mutations may have a limited role in the development of HNC in the study population. However, further studies on a large scale are necessary to obtain more detailed results.
Conflicts of interest
The authors declare no conflict of interest.
Funding
No source of funding.
Author contributions
Summera Fatima: Data collection, data analysis, result compilation, writing – review, statistical analysis and editing and quality control.
Asia Bibi: Conceptualisation, methodology, writing – original draft, supervision.
Sara Samad Qureshi: Review, editing and data collection.
Suman Khan: Review, editing and data collection.
Acknowledgments
The authors acknowledge the participation of all HNC patients.
References
- 1.Cogliano VJ, Baan R, Straif K, et al. Preventable exposures associated with human cancers. J Nat Cancer Inst. 2011;103(24):1827–1839. doi: 10.1093/jnci/djr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhull AK, Atri R, Dhankhar R, et al. Major risk factors in head and neck cancer: a retrospective analysis of 12-year experiences. World J Oncol. 2018;9(3):80–84. doi: 10.14740/wjon1104w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson DE, Burtness B, Leemans CR, et al. Head and neck squamous cell carcinoma. Nat Rev Dis Prim. 2020;6(1):92. doi: 10.1038/s41572-020-00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2020;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 5.Mody MD, Rocco JW, Yom SS, et al. Head and neck cancer. Lancet. 2021;398(10318):2289–2299. doi: 10.1016/S0140-6736(21)01550-6. [DOI] [PubMed] [Google Scholar]
- 6.Bravi F, Lee YA, Hashibe M, et al. Lessons learned from the INHANCE consortium: an overview of recent results on head and neck cancer. Oral Dis. 2021;27(1):73–93. doi: 10.1111/odi.13502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tumino R, Vicario G. Head and neck cancers: oral cavity, pharynx, and larynx. Epidemiol Prev. 2004;28(2 Suppl):28–33. [PubMed] [Google Scholar]
- 8.Zhou G, Liu Z, Myers JN. TP53 mutations in head and neck squamous cell carcinoma and their impact on disease progression and treatment response. J Cellular Biochem. 2016;9999:1–11. doi: 10.1002/jcb.25592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mork J, Lie AK, Glattre E, et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344:1125–1131. doi: 10.1056/NEJM200104123441503. [DOI] [PubMed] [Google Scholar]
- 10.Boccia S, Cadoni G, Sayed-Tabatabaei FA, et al. CYP1A1, CYP2E1, GSTM1, GSTT1, EPHX1 exons 3 and 4, and NAT2 polymorphisms, smoking, consumption of alcohol and fruit and vegetables and risk of head and neck cancer. J Cancer Res Clin Oncol. 2008;134(1):93–100. doi: 10.1007/s00432-007-0254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boutelle AM, Attardi LD. p53 and tumor suppression: it takes a network. Tren Cell Biol. 2021;31(4):298–310. doi: 10.1016/j.tcb.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine AJ. Targeting the P53 protein for cancer therapies: the translational impact of P53 research. Cancer Res. 2022;82(3):362–364. doi: 10.1158/0008-5472.CAN-21-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vousden KH, Prives C. P53 and prognosis: new insights and further complexity. Cell. 2005;120(1):7–10. doi: 10.1016/j.cell.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 14.Poeta ML, Manola J, Goldwasser MA, et al. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N Engl J Med. 2007;357(25):2552–2561. doi: 10.1056/NEJMoa073770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brennan JA, Sidransky D. Molecular staging of head and neck squamous carcinoma. Cancer Metast Rev. 1996;15(1):3–10. doi: 10.1007/BF00049484. [DOI] [PubMed] [Google Scholar]
- 16.Homann N, Nees M, Conradt C, et al. Overexpression of p53 in tumor-distant epithelia of head and neck cancer patients is associated with an increased incidence of second primary carcinoma. Clin Cancer Res. 2001;7(2):290–296. [PubMed] [Google Scholar]
- 17.Toyooka S, Tsuda T, Gazdar AF. The TP53 gene, tobacco exposure and lung cancer. Hum Mutat. 2003;21:229–239. doi: 10.1002/humu.10177. [DOI] [PubMed] [Google Scholar]
- 18.Agrawal N, Frederick MJ, Pickering CR, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333:1154–1157. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stransky N, Egloff AM, Tward AD, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pickering CR, Zhang J, Yoo SY, et al. Integrative genomic characterization of oral squamous cell carcinoma identifies frequent somatic drivers. Cancer Discov. 2013;3:770–781. doi: 10.1158/2159-8290.CD-12-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hainaut P, Hollstein M. P53 and human cancer: the first ten thousand mutations. Adv Cancer Res. 2000;77:81–137. doi: 10.1016/S0065-230X(08)60785-X. [DOI] [PubMed] [Google Scholar]
- 23.Chang JS, Lo HI, Wong TY, et al. Investigating the association between oral hygiene and head and neck cancer. Oral Oncol. 2013;49(10):1010–1017. doi: 10.1016/j.oraloncology.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed R, Malik S, Khan MF, et al. Epidemiological and clinical correlates of oral squamous cell carcinoma in patients from north-west Pakistan. J Pak Med Assoc. 2019;69(8):1074–1078. [PubMed] [Google Scholar]
- 25.Vučičević Boras V, Fučić A, Baranović S, et al. Environmental and behavioural head and neck cancer risk factors. Cent Eur J Public Health. 2019;27(2):106–109. doi: 10.21101/cejph.a5565. [DOI] [PubMed] [Google Scholar]
- 26.Akhtar A, Hussain I, Talha M, et al. Prevalence and diagnostic of head and neck cancer in Pakistan. Pak J Pharm Sci. 2016;29(5 Suppl):1839–1846. [PubMed] [Google Scholar]
- 27.Chang CP, Siwakoti B, Sapkota A, et al. Tobacco smoking, chewing habits, alcohol drinking and the risk of head and neck cancer in Nepal. Inter J Cancer. 2020;147(3):866–875. doi: 10.1002/ijc.32823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manzoor H, Ahmad N, Shuja J, et al. Epidemiology characteristic of head & neck cancers (HNCs) in Southwestern Pakistan: 21 years experience. Children. 2020;402:1–70. [Google Scholar]
- 29.Hashim D, Sartori S, Brennan P, et al. The role of oral hygiene in head and neck cancer: results from International head and neck cancer epidemiology (INHANCE) consortium. Ann Oncol. 2016;27(8):1619–1625. doi: 10.1093/annonc/mdw224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreno-Lopez LA, Esparza-Gomez GC, Gonzalez-Navarro A, et al. Risk of oral cancer associated with tobacco smoking, alcohol consumption and oral hygiene: a case-control study in Madrid, Spain. Oral Oncol. 2000;36(2):170–174. doi: 10.1016/S1368-8375(99)00084-6. [DOI] [PubMed] [Google Scholar]
- 31.Lissowska J, Pilarska A, Pilarski P, et al. Smoking, alcohol, diet, dentition and sexual practices in the epidemiology of oral cancer in Poland. Eur J Cancer Prev. 2003;12(1):25–33. doi: 10.1097/00008469-200302000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Azria D, Zouhair A, Serre A, et al. Anémie et cancers des voies aérodigestives supérieures. Bull Cancer. 2005;92(5):445–451. [PubMed] [Google Scholar]
- 33.Zhou BF, Stamler J, Dennis B, et al. Nutrient intakes of middle-aged men and women in China, Japan, United Kingdom, and United States in the late 1990s: the INTERMAP study. J Hum Hypertens. 2003;17(9):623–630. doi: 10.1038/sj.jhh.1001605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.AICR W. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. American Institute for Cancer Research; 2007. [Google Scholar]
- 35.Lagiou P, Talamini R, Samoli E, et al. Diet and upper-aerodigestive tract cancer in Europe: the ARCAGE study. Inter J Cancer. 2009;124(11):2671–2676. doi: 10.1002/ijc.24246. [DOI] [PubMed] [Google Scholar]
- 36.Lucenteforte E, Garavello W, Bosetti C, et al. Dietary factors and oral and pharyngeal cancer risk. Oral Oncol. 2009;45(6):461–467. doi: 10.1016/j.oraloncology.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Chuang SC, Jenab M, Heck JE, et al. Diet and the risk of head and neck cancer: a pooled analysis in the INHANCE consortium. Cancer Causes Control: CCC. 2012;23(1):69–88. doi: 10.1007/s10552-011-9857-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tzoulaki I, Brown IJ, Chan Q, et al. Relation of iron and red meat intake to blood pressure: cross sectional epidemiological study. BMJ (Clinical research ed.) 2008;337:a258. doi: 10.1136/bmj.a258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinheiro NA, Villa LL. Low frequency of p53 mutations in cervical carcinomas among Brazilian women. Braz J Med Biol Res. 2001;34(6):727–733. doi: 10.1590/S0100-879X2001000600005. [DOI] [PubMed] [Google Scholar]
- 40.Hedau S, Jain N, Husain SA, et al. Novel germline mutations in breast cancer susceptibility genes BRCA1, BRCA2 and p53 gene in breast cancer patients from India. Breast Cancer Res Treat. 2004;88(2):177–186. doi: 10.1007/s10549-004-0593-8. [DOI] [PubMed] [Google Scholar]
- 41.Aziz I. Spectrum of Tp53 Tumor Suppressor Gene Mutations and Codon 72 Polymorphism In Pakistani Female Breast Cancer Patients. Lahore: University of the Punjab; 2011. [Doctoral Dissertation] [Google Scholar]
- 42.Yan B, Chen Q, Xu J, et al. Low-frequency TP53 hotspot mutation contributes to chemoresistance through clonal expansion in acute myeloid leukemia. Leukemia. 2020;34(7):1816–1827. doi: 10.1038/s41375-020-0710-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sadia H, Ullah M, Irshad A, et al. Mutational analysis of exons 5–9 of TP53 gene in breast cancer patients of Punjabi ethnicity. Adv Life Sci. 2022;9(1):18–23. [Google Scholar]
- 44.Haraf DJ, Nodzenski E, Brachman D, et al. Human papilloma virus and p53 in head and neck cancer: clinical correlates and survival. J Am Assoc Cancer Res. 1996;2(4):755–762. [PubMed] [Google Scholar]
- 45.Sisk EA, Soltys SG, Zhu S, et al. Human papillomavirus and p53 mutational status as prognostic factors in head and neck carcinoma. Head Neck: J Sci Spec Head Neck. 2002;24(9):841–849. doi: 10.1002/hed.10146. [DOI] [PubMed] [Google Scholar]
- 46.Bosch FX, Ritter D, Enders C, et al. Head and neck tumor sites differ in prevalence and spectrum of p53 alterations but these have limited prognostic value. Int J Cancer. 2004;111(4):530–538. doi: 10.1002/ijc.11698. [DOI] [PubMed] [Google Scholar]
- 47.Golusinski P, Lamperska K, Pazdrowski J, et al. Analiza wystepowania mutacji w obrebie genu TP53 u chorych na raka płaskonabłonkowego głowy i szyi [Analysis of mutations within the TP53 gene in patients with squamous cell carcinoma of the head and neck] Otolaryngol Pol. 2011;65(2):114–121. doi: 10.1016/S0030-6657(11)70640-0. [DOI] [PubMed] [Google Scholar]
- 48.Hashmi AA, Bukhari U, Aslam M, et al. Clinicopathological parameters and biomarker profile in a cohort of patients with head and neck squamous cell carcinoma (HNSCC) Cureus. 2023;15(7):e41941. doi: 10.7759/cureus.41941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Komarova EA, Gudkov AV. Chemoprotection from p53-dependent apoptosis: potential clinical applications of the p53 inhibitors. Biochem Pharmacol. 2001;62(6):657–667. doi: 10.1016/S0006-2952(01)00733-X. [DOI] [PubMed] [Google Scholar]
- 50.Tandon S, Tudur-Smith C, Riley RD, et al. A systematic review of p53 as a prognostic factor of survival in squamous cell carcinoma of the four main anatomical subsites of the head and neck. Cancer Epidemiol Biomark Prev. 2010;19(2):574–587. doi: 10.1158/1055-9965.EPI-09-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peltonen JK, Helppi HM, Pääkkö P, et al. p53 in head and neck cancer: functional consequences and environmental implications of TP53 mutations. Head Neck Oncol. 2010;2(1):1–10. doi: 10.1186/1758-3284-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baugh EH, Ke H, Ke H, et al. Why are there hotspot mutations in the TP53 gene in human cancers? Cell Death Differ. 2018;25(1):154–160. doi: 10.1038/cdd.2017.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Srividya, Giridhar BH, Vishwanath S, et al. Profiling of germline mutations in major hotspot codons of TP53 using PCR-RFLP. Pathol Oncol Res POR. 2019;25(4):1373–1377. doi: 10.1007/s12253-018-0394-8. [DOI] [PubMed] [Google Scholar]
- 54.Perri F, Pisconti S, Scarpati GDV. P53 mutations and cancer: a tight linkage. Ann Transl Med. 2016;4:522. doi: 10.21037/atm.2016.12.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huszno J, Grzybowska EWA. TP53 mutations and SNPs as prognostic and predictive factors in patients with breast cancer (Review) Oncol Lett. 2018;16:34–40. doi: 10.3892/ol.2018.8627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leng K, Schlien S, Bosch FX. Refined characterization of head and neck squamous cell carcinomas expressing a seemingly wild-type p53 protein. J Oral Pathol Med. 2006;35(1):19–24. doi: 10.1111/j.1600-0714.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 57.Thomas JOMD. TP53 as a Biomarker in Head and Neck Squamous Cell Carcinoma The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences. Houston: The Texas Medical Center Library; 2011. p. 217. [Dissertations and Theses (Open Access)] [Google Scholar]
- 58.Schneider-Stock R, Mawrin C, Motsch C, et al. Retention of the arginine allele in codon 72 of the p53 gene correlates with poor apoptosis in head and neck cancer. Am J Pathol. 2004;164(4):1233–1241. doi: 10.1016/S0002-9440(10)63211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hashibe M, Brennan P, Benhamou S, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International head and neck cancer epidemiology consortium. J Natl Cancer Inst. 2007;99(10):777–789. doi: 10.1093/jnci/djk179. [DOI] [PubMed] [Google Scholar]
- 60.Hashibe M, Brennan P, Chuang SC, et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International head and neck cancer epidemiology consortium. Cancer Epidemiol Biomarkers Prev. 2009;18(2):541–550. doi: 10.1158/1055-9965.EPI-08-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koyanagi YN, Matsuo K, Ito H, et al. Cigarette smoking and the risk of head and neck cancer in the Japanese population: a systematic review and meta-analysis. Jpn J Clin Oncol. 2016;46(6):580–595. doi: 10.1093/jjco/hyw027. [DOI] [PubMed] [Google Scholar]
- 62.Lu Y, Sobue T, Kitamura T, et al. Cigarette smoking, alcohol drinking, and oral cavity and pharyngeal cancer in the Japanese: a population-based cohort study in Japan. Eur J Cancer Prev. 2018;27(2):171–179. doi: 10.1097/CEJ.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 63.Kanwal M, Haider G, Zareef U, et al. Addiction of tobacco chewing and smoking in the patients of head and neck squamous cell carcinoma: a descriptive epidemiological study in Pakistan. Pak J Med Sci. 2019;35(6):1712. doi: 10.12669/pjms.35.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rupe C, Basco A, Schiavelli A, et al. Oral health status in patients with head and neck cancer before radiotherapy: baseline description of an observational prospective study. Cancers. 2022;14(6):1411. doi: 10.3390/cancers14061411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hanif M, Zaidi P, Kamal S, et al. Institution-based cancer incidence in a local population in Pakistan: nine year data analysis. Asian Pac J Cancer Prev. 2009;10:227–230. [PubMed] [Google Scholar]
- 66.Tariq A, Mehmood Y, Jamshaid M, et al. Head and neck cancers: incidence, epidemiological risk, and treatment options. Int J Pharm Res Allied Sci. 2015;4(3):21–34. [Google Scholar]
- 67.Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers. Cancer. 2007;110(7):1429–1435. doi: 10.1002/cncr.22963. [DOI] [PubMed] [Google Scholar]
- 68.Westra WH. The changing face of head and neck cancer in the 21st century: the impact of HPV on the epidemiology and pathology of oral cancer. Head Neck Pathol. 2009;3(1):78–81. doi: 10.1007/s12105-009-0100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45(4–5):309–316. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 70.Vigneswaran N, Williams MD. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac Surg Clin North Am. 2014;26(2):123–141. doi: 10.1016/j.coms.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Camargo Cancela M, Voti L, Guerra-Yi M, et al. Oral cavity cancer in developed and in developing countries: population-based incidence. Head Neck. 2010;32(3):357–367. doi: 10.1002/hed.21193. [DOI] [PubMed] [Google Scholar]
- 72.Khan Z, Tönnies J, Müller S. Smokeless tobacco and oral cancer in South Asia: a systematic review with meta-analysis. J Cancer Epidemiol. 2014;2014:394696. doi: 10.1155/2014/394696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Muller PA, Vousden KH. P53 mutations in cancer. Nat Cell Biol. 2013;15:2–8. doi: 10.1038/ncb2641. [DOI] [PubMed] [Google Scholar]
- 74.Deschler DG, Day T. TNM staging of head and neck cancer and neck dissection classification. Am Acad Otolaryn Head Neck Surg Found. 2008. pp. 10–23.