Abstract
Selenium, an essential micronutrient with potent anticancer and antioxidant properties, the inorganic form of selenium is highly toxic, while organic and elemental nanoforms are more bioavailable and less toxic and have gained attention owing to their dietary and clinical relevance. This study aims to optimize conditions for the biosynthesis and production of elemental selenium nanoparticles for selenium supplements using marine microalgae, Nannochloropsis oceanica CASA CC201. The 10 mM precursor solution treated with 1 % of the algal extract (10:1 ratio of precursor and algal extract, respectively) was shown to be the optimal concentration for synthesizing highly stable selenium nanoparticles with a size of 183 nm and a zeta potential of −38.5 mV. AFM and TEM analysis suggest that the spherical-shaped nanoparticles with smooth surfaces were polydispersely distributed. The nanoparticles are well characterized using various analytical and advanced techniques, including Raman spectroscopy and X-ray photoelectron spectroscopy. FT-IR analyses reveal the presence of microalgae proteins and peptides as stabilizing and fabricating agents of Se-NPs to further understand the mode of bioreduction. The synthesized elemental nanoform (Se0) has been validated for its biological functions, showing enhanced radical scavenging activity (74 % in a concentration-dependent manner). Subsequently, algal-mediated selenite reduction and nanoparticle synthesis is an eco-friendly, non-toxic, and sustainable method for the large-scale production of highly stable Se-NPs for niche applications as dietary and feed supplements.
Keywords: Selenium, Bio-transformed nanoparticles, Antioxidant's system, Microalgae, Human and feed supplements
Graphical abstract

Highlights
-
•
Biosynthesized an organic and elemental nanoform of Selenium Nanoparticles.
-
•
Biotransfomred selenium nanoparticles were characterized for shape, stability, porosity and surface topography.
-
•
Demonstrated microalga proteins and peptides as stabilizing agents.
-
•
A stable SeNPs with improved antioxidant properties proposed as an organic selenium dietary supplements.
1. Introduction
In recent years, selenium has gained great importance among the scientific community due to its potent anticancer and antioxidant properties [1,2], and it has been considered an essential micronutrient involved in various biological functions. The trace metal selenium is added to animal feed as selenium supplementation promoting animal growth and development [3]. Currently, selenium supplementation in animal feed is the mostly inorganic form (sodium selenite) of selenium; moreover, the inorganic form of selenium is highly toxic and has negative impacts. Whereas the organic form of selenium (selenocysteine, selenomethionine) is more bioavailable, less toxic, and thus appropriate for therapeutic and food/feed supplements, Selenium toxicity is dose-dependent, and it will be highly toxic at certain concentrations. At present, the application of selenium nanoparticles as selenium supplements in animal diets has been widely gaining attention because of their high bioavailability and low toxicity [4,5]. Recent studies suggest that Se–Np possesses less toxicity than other forms of selenium compounds; it improves the oxidative stress of animals, increases the level of antioxidant enzymes like GSH-Px, SOD, and AOC, and decreases the level of lipid peroxidation [[6], [7], [8]]. Presenting selenium nanoparticles as a selenium supplementation in a diet exhibiting it and there is need to metabolize them before being incorporated into selenoproteins; hence, the body can take up the selenium source much faster than inorganic selenium [[9], [10], [11]]. The nanoparticles are synthesized by three common methods. Physical [[12], [13], [14]], chemical [15], and biological [16] methods. Among these,the biological method is most preferred because of its properties, like being simple, non-toxic, cost-effective, and eco-friendly. The biological method emphasizes the use of microorganisms like bacteria [17], plants [16,21], microalgae [18,19], seaweeds [20], etc., as a green chemistry approach. The algae are photoautotrophs, the largest primary producers of the planet Earth, and have several potential resources within them. These organisms play a significant role in the nutrient cycle in aquatic ecosystems as food for invertebrates and fish.
Notably, humans do not have a metabolic system to synthesize Se, but it is essential; thus, it must be supplemented through diet. The biological functions of selenium are accomplished through selenioproteins. Se has an important clinical significance to human health, and it involves regulating the Se-dependent enzymes. Its deficiency leads to an imbalance in the antioxidant defense system. Thus, an adequate amount of Se is required to improve the antioxidant system and immunity. In addition, Se is also involved in the physiopathological conditions of many diseases, like anti-aging, cardiovascular, and cerebrovascular diseases [28]. Recently, our group decoded the selenium protein T and associated SECIS machinery from Microalgae [31]. We also developed a patented bioprocess to augment the bioconversion of organic selenium in an edible marine microalgae [29] and validated its applicability in the murine model [30]. However, organic selenium-enriched edible microalgae has wide applications in the therapeutic and feed industries. The nanoform of selenium is more bioavailable and less toxic. Thus, the synthesized elemental nanoform (Se0) is validated for its biological functions. Primarily, selenium and selenoproteins are involved in the perpetuation of cellular redox balance through their antioxidant properties. Also, it has an attractive commercial demand in the feed industry, particularly in poultry and aquaculture niche markets.
In this study, marine microalgae Nannochloropsisoceanica CASA CC201 was chosen as the organism of interest for the extracellular synthesis of selenium nanoparticles since it is an oleaginous microalga, a rich source of essential fatty acids. There are several reports on the biosynthesis of selenium nanoparticles from bacteria [17], blue-green algae [18,19], as well as plant extracts [16,21]. However, this is the first report on the green microalgae-mediated synthesis of selenium nanoparticles. Therefore, the present study aims to optimize conditions for the biosynthesis and production of selenium nanoparticles for selenium supplements. Further, the selenium nanoparticles were biosynthesized by using algal extract as a bioreductant and characterized by various analytical techniques like UV–visible spectroscopy (UV-VIS), dynamic light scattering (DLS), Zeta potential measurement (ZP), high-resolution transmission electron microscopy (HR-TEM), energy dispersive spectroscopy (EDX), atomic force microscope (AFM), Fourier transformed infrared spectroscopy (FT-IR), Raman spectroscopy, and X-ray photoelectron spectroscopy (XPS) for the size, shape, structural nature, stability, elemental composition, and surface chemistry of SeNPs. Thus, in the present study, we examined the antioxidant potential of the synthesized SeNPs. The results demonstrate that the SeNPs show radical scavenging activity (RSA) in a concentration-dependent manner. The synthesized SeNPs have applications as a regular health supplement and for specific therapeutic applications. the microalgae mediated biosynthesis of seNps is environmentally friendly process for producing extremely stable Se-NPs on a wide scale for specialized uses as feed and dietary supplements.
2. Materials and methods
2.1. Culture, growth conditions and maintenance
Nannochloropsisoceanica CASA CC201 was cultured on Walne's medium, and it was sub-cultured by using 10 % of the inoculum and maintained at 24 °C with 14 h of light period by fluorescent white light with an intensity of 50 μ mol/m2/s, alternating with 10 h of the dark period. The light and dark periods were regulated by an automatic timer fixed to the culture chamber in the algal culture room equipped with an air conditioner. The growth of the algae was estimated by measuring optical density at 680 nm at regular intervals. After the cells reached the exponential phase, the biomass was collected by centrifugation (8000 g for 15 min), and the cell pellets were washed three times with distilled water to remove the remaining media traces. The cell pellets were subjected to lyophilization and stored in a deep freezer for further use.
2.2. Preparation of algal extract
Aqueous extract (1 % solution) was prepared by incubating 500 mg of lyophilized algal biomass with 50 mL of Milli-Q-water for 1 h at 900C on the water bath and sonicated the sample, for a few minutes, and centrifuge the sample at 8000 g for 15 min to obtain the cell-free extract. The supernatant obtained after centrifugation contains cell-free extract that was filtered through a Whatman No.1 filter paper and stored at 40C until further use.
2.3. Synthesis of selenium nanoparticles and purification
For the synthesis of selenium nanoparticles, different concentrations (0.0 mM, 1 mM, 2.5 mM, 5 mM, and 10 mM) of sodium selenite (Na2SeO3) were added in a 1 %–5 % cell-free extract of Nannochloropsis oceanica CASA CC 201 for each flask containing 50 ml of Milli-Q water. The reaction mixture was incubated in dark conditions for 130 rpm at 37 °C on an orbital shaker. The pH of the reaction mixture was also recorded at regular intervals. After 72–96 h of incubation, the color of the reaction mixture changed from pale green to ruby red, then red, as shown in Fig. 1. The color change from green to red is a visible indication of selenite reduction into selenium and the formation of selenium NPs. After the color change, selenium NPs are purified by centrifugation and further characterized by various techniques.
Fig. 1.
Biosynthesis of Selenium Nanoparticles. Color change of reaction mixture from pale green into red varying concentrations (1–10 mM). A) 0 h (initial condition of reaction) B) condition of the reaction mixture after the incubation period. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2.4. Characterization of bio-transformed selenium nanostructures
2.4.1. UV–visible spectroscopy
After the visible confirmation of selenite reduction and the formation of selenium nanoparticles in the reaction mixture, it was further characterized in UV–visible spectroscopy by analyzing its corresponding surface plasmon resonance (SPR) peaks. The UV–visible spectrum was recorded in the 200–800 nm range by using the UV–Vis 1601Shimadzu spectrophotometer.
2.4.2. Dynamic light scattering and zeta-potential measurement
The dynamic light scattering analysis will reveal the size and distribution of the particles in the fluid suspension and show the average hydrodynamic diameter of the nanoparticles. The charges that are present on the surface of the nanoparticles were measured and expressed as a zeta potential. The zeta potential values will also reveal the stability of the nanoparticles, and they were estimated by how the charged particles respond in an electric field. They were measured using Zeta-sizer Nano ZS equipment. The size, distribution, and zeta potential of synthesized selenium nanoparticles were measured using a Zeta-sizer Nano ZS Particle Analyzer (Malvern Instruments, Malvern, UK), and the zeta potential was measured in the range of −200 to + 200 mV.
2.4.3. High resolution - transmission electron microscopy
The shape, morphology, size, and elemental composition of the synthesized nanoparticle were characterized by transmission electron microscopy coupled with energy-dispersive X-ray (EDX). Selected-Area Electron Diffraction (SAED) is also analyzed to identify the nature (crystalline or amorphous) of synthesized selenium nanoparticles. The purified Se-NPs were drop-casted on a carbon-coated copper grid and allowed to completely dry, then the TEM images and their corresponding selected area electron diffraction were taken in the JOEL JEM F200 with STEM EELS operating at 200 kV.
2.4.4. Atomic force microscopy
The synthesized Se-NPs were investigated by an atomic force microscope to obtain the height, three-dimensional morphological information, surface topography, and porosity of the nanoparticles. The purified Se-NPs were drop-casted on the cover glass and allowed to completely dry at room temperature, then AFM photomicrographs were procured from Multimode SPM (Bruker Nanoscope V) operating in tapping mode, and the resonant frequency of 300 kHz and spring constant of 40 Nm-1 were used.
2.4.5. Fourier transformed infrared spectroscopy
Fourier-transformed infrared spectroscopy is a very useful technique to investigate the surface chemistry of particles. It will reveal the surface residues, functional groups, and functional moieties that are present on the nanoparticles, as well as the biomolecules that are involved in the selenite reduction and stabilization of the nanoparticles. The lyophilized selenium nanoparticle, algal extract, and the precursor sodium selenite were analyzed using the Shimadzu IRTracer-100 (Shimadzu Corporation, Kyoto, Japan), and the spectrum was collected in a frequency range of 400–4000 cm− 1..
2.4.6. Raman spectroscopy
For Raman spectroscopic analysis, a small suspension of selenium nanoparticles was placed on a glass coverslip and allowed to completely dry at room temperature, then the Raman spectra were collected in the spectral range between 200 and 2400 cm1 by using WITec, Inc., Ulm, Germany: ALPHA 300R, with an excitation source of 633 nm.
2.4.7. X-ray photoelectron spectroscopy
The lyophilized Se-NPs were examined under X-ray photoelectron spectroscopy for characterizing surface chemistry, binding energy, and bonding pattern of biomolecules on the nanoparticles by XPS (PHI 5000 Versa Probe II, ULVAC-PHI Inc., USA) equipped with a micro-focused (200 μm, 15 kV) monochromatic Al-Kα X-ray source (h ν = 1486.6 eV). The overall survey spectra and narrow scans (high-resolution spectra) were recorded. Survey scans were recorded with an X-ray source power of 50W and a pass energy of 187.85 eV. High-resolution spectra of the major elements were recorded at 46.95 eV pass energy. The binding energy was referenced to the C1s peak at 284.8 eV [33].
2.5. Antioxidant activity of selenium nanoparticles
The free radical-scavenging processes are well-known mechanisms for determining the radical-scavenging activity and inhibition of lipid oxidation [32]. To replace the antioxidants derived from synthetic ones, the antioxidants from natural sources are seeking much attention, and efforts have been put into identifying more suitable antioxidant compounds. The radical scavenging activity of selenium nanoparticles was carried out by a 1-diphenyl-2-picrylhydrazyl (DPPH) assay. The DPPH assay is a very familiar assay for evaluating the antioxidant activity of many biological samples, including plant extract, microalgae, bacterial samples, etc. All samples were prepared at a 1 mg/mL concentration. Dried powdered samples of selenium nanoparticles were used for this assay in different concentrations like 50, 250, 500, 750, and 1000 μg/ml. The DPPH assay was referred to according to Refs. [25,26]. In brief, a DPPH solution of 0.4 mM was prepared, followed by a dilution with 99 % methanol. After that, different concentrations of selenium nanoparticles were added with methanol, and DPPH solutions were thoroughly mixed, and absorbance was recorded at 517 nm by a UV–visible spectrophotometer. The solutions of DPPH and methanol were used as controls and blanks, respectively, and ascorbic acid was used as a standard. By this assay, we observed 74 ± 2 percent of RSA activities in selenium nanoparticles. The scavenging activity was calculated as % inhibition of DPPH radical by the following method [24].
| Percentage of Scavenging activity = (A0 – A1)/A0 X 100 |
Where A0, the absorbance values of DPPH control OD, and A1, the absorbance values of test sample OD.
2.6. Selenium NPs stabilizing proteins
To analyze the associated proteins with the selenium NPs, the synthesized nanoparticles using aqueous extract of Nannochloropsis oceanica which contains proteins as well. The biosynthesized SeNPs were sonicated using a Vibracell™ Ultrasonicator (Model VCX130) for 10 min (2 s pulse on, 10 s pulse off at 30 % amplification) on ice. The resulting SeNPs associated proteins were collected through centrifugation at 10000 rpm for 10 min at 4 °C. The supernatant containing the SeNPs associated microalga proteins were denatured at 95 °C for 5 min in a Laemmle buffer. Subsequently the proteins were resolved through 12 % SDS-PAGE at a constant voltage of 100 V and silver stained.
3. Results
3.1. Synthesis of selenium nanoparticles and Purification
The selenium nanoparticles were successfully synthesized by using an aqueous extract of Nannochloropsis oceanica CASA CC201 as a bio-reductant to reduce sodium selenite (Fig. 1). The optimized concentration for synthesizing highly stable selenium nanoparticles was observed in a 10 mM precursor solution treated with 1 % algal extract (10:1 ratio of precursor and algal extract, respectively). It is believed that the protein, carbohydrate, fatty acids, and other biomolecules that are present in the aqueous extract are involved in the reduction of an inorganic form of sodium selenite (SeIV) to elemental selenium (Se0), and it is stabilizing the Se nanoparticles with various biomolecules by capping the nanoparticles. For characterizing the phyco-synthesized selenium nanoparticles, they were purified by centrifugation. (8000 g for 15 min) from the reaction mixture and washed three times with ethanol, followed by milli-q water.
3.2. Characterization
3.2.1. UV–visible spectroscopy
The phyco-synthesized selenium nanoparticles are extensively studied with various analytical techniques for characterization. Primarily, it starts with UV–visible spectroscopy. After the incubation period, the color of the reaction mixture turned red from pale green, indicating the selenite reduction and formation of selenium nanoparticles. This is further validated by UV–visible spectroscopy, with the samples showing the corresponding absorption maxima at the 260 nm range shown in Fig. 2A, indicating surface plasma resonance of the selenium nanoparticles [18,22]. Among all, the 10 mM concentration shows the highest absorption maxima; hence, it has been chosen as an optimal concentration in further experiments.
Fig. 2.
A) UV–vis spectrum of Se–Np after the incubation period.
3.2.2. Dynamic light scattering and zeta-potential measurement
The size and distribution of synthesized selenium nanoparticles were analyzed by the DLS method. From Fig. 2B, the size of the nanoparticles ranges from 78 to 396 nm, in which 122 nm particles are predominantly distributed among all. This was further interpreted and confirmed with TEM results, and the average size was measured at 183 nm. The results demonstrated that the nanoparticles are distributed in a polydisperse manner with a PDI of 0.075. The stability of the nanoparticles in a solution was estimated by their zeta potential, which is measured at 38.5 mV with the net negative charge shown in Fig. 2D. Generally, the zeta potential values fall between −25 and + 25 and are considered unstable and tend to aggregate in colloidal solutions. In our experiment, the zeta potential was measured at −38.5 mV, indicating a highly stable nanoparticle.
Fig. 2.
B) DLS analysis of Se–Np.
Fig. 2.
D) Zetapotential measurement.
3.2.3. Raman spectroscopy
The selenium nanoparticles are investigated by Raman spectroscopy for structural characterization. In Fig. 2C, the Raman spectra of purified selenium nanoparticles show a single, strong, narrow absorption peak at 252 cm1 in a low-frequency range. This corresponds to the A1 stretching of Se–Se mode.
Fig. 2.
C) Typical Raman spectrum of purified selenium nanoparticle.
3.2.4. High resolution - transmission electron microscopy and EDX
The transmission electron micrograph of purified Se-NPs revealed the shape, size, and organic shell layer wrapped around the particles. As it is very clear from the electron micrograph shown in Fig. 3A, the selenium nanoparticles have an electron-dense structure in a spherical shape with particle size in conformation with the DLS result and are distributed in a slightly polydisperse manner. It also shows the presence of the organic shell layer around the particle. Various biomolecules that are present in the extract are responsible for the selenite reduction, capping and stabilizing the nanostructure, and also preventing their aggregation. The SAED pattern suggests the amorphous nature of synthesized selenium nanoparticles. Additionally, the TEM-EDX elemental analysis of phyco-synthesized Se–Np exhibits the selenium signal along with carbon, oxygen, and copper; among these, the copper signal arises from the copper-coated grid. The EDX spectrum shown in Fig. 3B confirms the presence of an elemental form of selenium.
Fig. 3.
HR-TEM images of Se-NPs with SAED (A) and EDX Spectra of selenium nanoparticles (B).
3.2.5. Atomic force microscopy
Through atomic force microscopic analysis, the three-dimensional profile of the selenium nanoparticle revealed the shape, porosity, surface topography, and height of the Se-NPs. The (Fig. 4) atomic force photomicrograph depicts the spherical-shaped nanoparticles distributed in a polydisperse manner, which also correlates with TEM results. It is very clear from the 3D profile of Se-NPs that the surface of the nanoparticle was smooth, and the average height of the nanoparticles was measured at 98 nm.
Fig. 4.
Atomic force photomicrograph of selenium nanoparticle with Nanoscope analysis (a). Amplitude image showing the spherical nature, their corresponding height image (b & c) and their quantitative profile of the marked area (d & e).
3.2.6. FTIR analysis
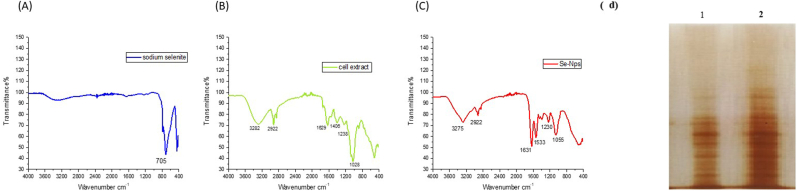
The purified selenium nanoparticles were examined under FTIR spectroscopy to determine the presence of biomolecules on the surface of the particle, which are involved in the fabrication and stabilization of the nanoparticle. The FTIR spectra of the control sample of sodium selenite (precursor) and algal cell extract, as well as purified selenium nanoparticles, were recorded and shown in Fig. 5. The spectrum of sodium selenite shows a strong absorption peak at 705 cm1, exhibiting the symmetric Se–O stretching vibration. This observation is similar to the recent report of Alipour 2021 synthesizing Se–Np from spirulina extract [19]. The FTIR spectrum of phyco-synthesized selenium nanoparticles indicates the presence of absorption peaks at 3275 cm1 and 2922 cm1, which correspond to the O–H stretching vibration of alcohol and the C–H stretching vibration of alkanes, respectively. Further, the presence of a strong characteristic peak at 1631 cm1 is mainly because CO–NH stretching vibration corresponds to amide-I (the characteristic region of proteins), and the peak at 1533 cm1 is N–H bending vibration, which corresponds to amide-II (the characteristic region of proteins). The absorption peaks at 1390 cm1, 1230 cm1, and 1055 cm1 correspond to symmetrical stretching of C–H, C–O stretching vibration of the carboxyl group, and C–O–C bending solid vibration in a characteristic region of polysaccharides, respectively. From Figs. (b and c), the characteristic absorption peaks in the infrared spectrum of Se–Np are similar to the spectrum of algal extract, indicating the presence of some algal compounds, including proteins (Fig. 5d), on the surface of nanoparticles, which are responsible for selenite reduction, fabrication, and stabilizing the nanoparticles. The presence of proteins coated over the SeNPs was resolved by SDS-PAGE (Fig. 5d); their identity and putative role are yet to be validated.
Fig. 5.
FTIR Spectrum A) sodium selenite. B) Algal cell extract, C) SeNPs D) SDS-PAGE protein profiling of SeNPs stabilizing agents. Lane 1 (1x) and 2 (2x) indicate the concentration of SeNPs extracts.
3.2.7. XPS analysis
The XPS analysis was performed to reveal surface chemistry, binding energy, and bonding patterns in nanoparticles. Fig. 6A XPS spectrum of the survey scan shows the presence of O 1S, N 1S, C 1S, and Se 3D signals under EDX results, except N. The N signals were detected in the XPS spectrum, while they were not detected in EDX. This is because of the overlapping of C and O signals on the N peak of the EDS spectrum. The overall composition of particles determined by the survey scan suggests the nanoparticles tend to be organic. The literature suggests the selenium 3D signal is composed of two sub-peaks, Se 3d3/2 and Se 3d5/2, and the metallic selenium (Se0) is expected to be at 55.1 eV in Se 3d5/2; Se II is approximately at 57.7 eV; Se IV is expected to appear at approximately 59.4 eV; and SeVI is expected to have a binding energy of 61 eV [23].
Fig. 6.
XPS spectra A) survey scan of purified Se–Np, B) High-resolution spectra of Se 3d
The high-resolution XPS spectrum of selenium nanoparticles is shown in Fig. 6, exhibiting the Se-3d peak signal typically at 56.75 eV binding energy, and the 3D selenium signal is very weak when compared with other signals in the spectrum. Moreover, the Se 3d, composed of two sub-peaks of binding energy at 55.18 and 57.65 eV, corresponds to Se 3d5/2 and Se 3d3/2, respectively. The results from XPS analysis reveal binding energy at 55.18 eV from the purified selenium nanoparticles conforming to the presence of elemental selenium (Se0). There is no signal detected corresponding to Se IV, which indicates the reduction of sodium selenite (Se IV) into elemental form (Se0).
3.3. Antioxidant activity of selenium nanoparticles
The results of the DPPH assay confirm the antioxidant activity of selenium nanoparticles synthesized from the aqueous extract of Nannochloropsis Oceanica CASA CC201. The SeNPs have shown radical scavenging activity (Fig. 7). Results show that antioxidant activity was directly proportional to the concentration of selenium nanoparticles. The Se-NPs have potent antioxidant activity compared to l-ascorbic acid. The synthesized selenium nanoparticles have 74 % of RSA activity at 1 mg/ml sample concentration (Fig. 7), and this percentage of inhibition gradually increases from 50, 250, 500, and 750 μg/ml concentrations to 1000 μg/ml concentrations. The results obtained in this study consistent investigation report the antioxidant activity of SeNPs [19,27].
Fig. 7.
Free Radical Scavenging Activity of biosynthesized Selenium nanoparticles (Unfilled vertical bar) and Ascorbic acid (Filled vertical bar). The average of three independent experiments was plotted, and the SD was expressed as an Error bar.
4. Discussion
In our present study, the highly stable selenium nanoparticle with antioxidant properties is bio-synthesized extracellularly in a simple and effective method by using an aqueous extract of marine microalgae Nannochloropsis oceanica CASA CC201 as a bio-reductant for reducing highly toxic sodium selenite into elemental selenium and characterized by various analytical methods. Although, several chemical and physical approaches are practiced to synthesis the bioactive Selenium nanoparticle [34], unsuitable for biocompatible applications due to aggregation and unstable nature. Whereas, biosynthesis of SeNPs using plant, algal and microbial resources are more stable due to the presence of biological active metabolites such as proteins, peptides, polyphenols and peptides as stabilizing and capping agents. Also the synthesis process is more sustainable and more reliable in nature [35].
The morphological and structural characterization of synthesized selenium nanoparticles, like microscopic analysis (TEM, AFM, EDX) and spectroscopic analysis (FTIR, XPS), conforms the shape, elemental composition, and stability of nanoparticles and is fabricated with biomolecules (proteins and polysaccharides), which stabilize the nanoparticles. Similar, Selenium nanoparticle was characterized for its morphological and structural aspects, also stability and biofunonalised with bioactive metabolites are, comprehensively described in the to the current study the recent review article [36].
The present study, investigated the antioxidant properties suggested that the phyco-synthesized selenium nanoparticle has potential antioxidant activity and has 74 % RSA activity at a 1 mg/mL concentration. Therefore, this algal-mediated selenite reduction and nanoparticle synthesis is an eco-friendly, non-toxic, and sustainable method for the large-scale production of highly stable Se-Nps feed supplements.
Similar to the present study, very recently Zn and Se dual Nannoparticles were described by Somaghian et al. [37] using Rosmarinus officinalis leaf extract with 90.6 % antioxidant activity. An eco-friendly approach was adapted to systhesis the SeNPs using the aqueous extract of Ulva fasciata macromolecules as a reducing and capping agent. These SeNPs were evaluated for their anti-cancer activity by Shahzamani et al. [38], through inhibiting matrix metalloproteinase-2 and 9 (MMP-2 and 9) and also an enhanced lactate dehydrogenase leakage was observed. In addition, SeNPs exhibited synergistic effect with a bacteriostatic antibiotics chloramphenicol at 10 μg/ml concentration against S. aureus and P. aeruginosa [38].
A bimetallic silver-selenide chalcogenide nanostructure (Ag–Se NPs) were fabricated using Melilotus officinalis aqueous extract and characterised for its material and biological properties. It is noteworthy to mention that it is showing DPPH scavenging potential of 58.52 % [39] which is a relatively lower when compared to the present finding. ZnSe NPs were synthesized using the aqueous extract of seaweed, Gracilaria corticata were and well characterised. ZnSe NPs exhibited about 67 % antioxidant activity, biofilm inhibition and an antibacterial activity of multiple bacterial strains [40].
5. Conclusion
This study focuses on optimizing conditions for the biosynthesis and production of elemental selenium nanoparticles for selenium supplements using marine microalgae, Nannochloropsis oceanica CASA CC201. The optimal concentration for synthesizing stable nanoparticles was found to be 10 mM with 1 % algal extract. Further, the biosynthesized SeNPs were characterized for its morphological and structural features by appropriate advanced analytical methods. AFM and TEM analysis suggest that the spherical-shaped nanoparticles with smooth surfaces were polydispersely distributed. Further, the synthesized selenium NPs showed enhanced radical scavenging activity, making algal-mediated selenite reduction and nanoparticle synthesis an eco-friendly, non-toxic, and sustainable method for large-scale production of highly stable Se-NPs for niche applications.
CRediT authorship contribution statement
S. Vasanthakumar: Writing – original draft, Software, Methodology, Formal analysis, Data curation. M. Manikandan: Formal analysis. Muthu Arumugam: Writing – review & editing, Resources, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
On behalf of all the authors, the corresponding author declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The work was supported by a grant from the Department of Science and Technology-SERB, Government of India, through the Core Research Grant Project “CRG/2019/001913: Investigation on identification and biochemical validation of selenoproteins from Nannochloropsis oceanica CASA CC201 as functional food/feed supplements” to MA.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2024.101760.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- 1.Spyridopoulou K., Tryfonopoulou E., Aindelis G., et al. Biogenic selenium nanoparticles produced by Lactobacillus casei ATCC 393 inhibit colon cancer cell growth in vitro and in vivo. Nanoscale Adv. 2021;3:2516–2528. doi: 10.1039/D0NA00984A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rayman M.P. Selenium and human health. Lancet. 2012;379:1256–1268. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad W., Shams S., Ahmad A., et al. Synthesis of Selenium− silver nanostructures with enhanced antibacterial, photocatalytic and antioxidant activities. Appl. Nanosci. 2020;1:1191–1204. doi: 10.1007/s13204-019-01213-z. [DOI] [Google Scholar]
- 4.Zhang J., Wang X., Xu T. Elemental selenium at nano size (nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: comparison with Se-methylselenocysteine in mice. Toxicol. Sci. 2008;101:22–31. doi: 10.1093/toxsci/kfm221. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J., Wang H., et al. Comparison of short term toxicity between Nano-Se and selenite in mice. Life Sci. 2005;76:1099–1109. doi: 10.1016/j.lfs.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Wang H., Zhang J., Yu H. Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: comparison with selenomethionine in mice. Free Radical Biol. Med. 2007;42:1524–1533. doi: 10.1016/j.freeradbiomed.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Shoeibi S., Mozdziak P., Golkar-Narenji A. Biogenesis of selenium nanoparticles using green chemistry. Top. Curr. Chem. 2017;375:88. doi: 10.1007/s41061-017-0176-x. [DOI] [PubMed] [Google Scholar]
- 8.Wu Z., Ren Y., Liang Y., et al. Synthesis, characterization, immune regulation, and antioxidative assessment of yeast-derived selenium nanoparticles in cyclophosphamide-inducedrats. ACS Omega. 2021;6(38):24585–24594. doi: 10.1021/acsomega.1c03205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gangadoo S., Stanley D., Hughes R.J., et al. Nanoparticles in feed: progress and prospects in poultry research. Trends Food Sci. Technol. 2016;58:115–126. doi: 10.1016/j.tifs.2016.10.013. [DOI] [Google Scholar]
- 10.Suzuki K.T. Metabolomics of selenium: se metabolites based on speciation studies. J. Health Sci. 2005;51:107–114. doi: 10.1248/jhs.51.107. [DOI] [Google Scholar]
- 11.Suzuki K., Ogra Y. Metabolic pathway for selenium in the body: speciation by HPLC-ICP MS with enriched se. Food AdditContam. 2002;19:974–983. doi: 10.1080/02652030210153578. [DOI] [PubMed] [Google Scholar]
- 12.Guleria A., Chakraborty S., Neogy S., et al. Controlling the phase and morphology of amorphous Se nanoparticles: their prolonged stabilization and anticancer efficacy. Chem. Commun. 2018;54:8753–8756. doi: 10.1039/C8CC05375H. [DOI] [PubMed] [Google Scholar]
- 13.Guleria A., Neogy S., Raorane B.S., et al. Room temperature ionic liquid assisted rapid synthesis of amorphous Se nanoparticles: their prolonged stabilization and antioxidant studies. Mater. Chem. Phys. 2020;253 doi: 10.1016/j.matchemphys.2020.123369. [DOI] [Google Scholar]
- 14.Guleria A., Maurya D.K., Neogy S., et al. A 10minute approach for the phase-specific synthesis of Se nanoparticles with tunable morphology: their anticancer efficacy and the role of an ionic liquid. New J. Chem. 2020;44:4578–4589. doi: 10.1039/C9NJ06088J. [DOI] [Google Scholar]
- 15.Vahdati M., Tohidi Moghadam T. Synthesis and characterization of selenium nanoparticles-lysozyme nanohybrid system with synergistic antibacterial properties. Sci. Rep. 2020;10(1):1–10. doi: 10.1038/s41598-019-57333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gunti L., Dass R.S., Kalagatur N.K. Phytofabrication of selenium nanoparticles from emblicaofficinalis fruit extract and exploring its biopotential applications: antioxidant, antimicrobial, and biocompatibility. Front. Microbiol. 2019;10:931. doi: 10.3389/fmicb.2019.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J., Zhang D., Lv X., et al. Green synthesis of robust selenium nanoparticles via polysaccharide–polyphenol interaction: design principles and structure–bioactivity relationship. ACS Sustainable Chem. Eng. 2022;10(6):2052–2062. doi: 10.1021/acssuschemeng.1c06048. [DOI] [Google Scholar]
- 18.ElSaied B.E., Diab A.M., Tayel A.A., et al. Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract. Green Process. Synth. 2021;10(1):49–60. doi: 10.1515/gps-2021-0005. [DOI] [Google Scholar]
- 19.Alipour S., Kalari S., Morowvat M.H., et al. Green synthesis of selenium nanoparticles by cyanobacterium Spirulina platensis (abdf2224): cultivation condition quality controls. BioMed Res. Int. 2021:1–11. doi: 10.1155/2021/6635297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirzaei S.Z., Somaghian S.A., et al. Phyco-fabrication of bimetallic nanoparticles (zinc–selenium) using aqueous extract of Gracilariacorticata and its biological activity potentials. Ceram. Int. 2021;47(4):5580–5586. doi: 10.1016/j.ceramint.2020.10.142. [DOI] [Google Scholar]
- 21.Alagesan V., Venugopal S. Green synthesis of selenium nanoparticle using leaves extract of withaniasomnifera and its biological applications and photocatalytic activities. Bionanoscience. 2019;9(1):105–116. doi: 10.1007/s12668-018-0566-8. [DOI] [Google Scholar]
- 22.Cittrarasu V., Kaliannan D., Dharman K., et al. Green synthesis of selenium nanoparticles mediated from CeropegiabulbosaRoxb extract and its cytotoxicity, antimicrobial, mosquitocidal and photocatalytic activities. Sci. Rep. 2021;11(1):1–15. doi: 10.1038/s41598-020-80327-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castle J.E. In: Practical Surface Analysis by Auger and X‐ray Photoelectron Spectroscopy. Briggs D., Seah M.P., editors. John Wiley and Sons Ltd; Chichester: 1983. p. 533. , £ (1984) 44.50: 302-302. [DOI] [Google Scholar]
- 24.Adebiyi O.E., Olayemi F.O., Ning-Hua T., et al. In vitro antioxidant activity, total phenolic and flavonoid contents of ethanol extract of stem and leaf of Grewiacarpinifolia. Beni-Suef University Journal of Basic and Applied Sciences. 2017;6(1):10–14. doi: 10.1016/j.bjbas.2016.12.003. [DOI] [Google Scholar]
- 25.Blois M. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- 26.Shanab S.M., Mostafa S.S., et al. Aqueous extracts of microalgae exhibit antioxidant and anticancer activities. Asian Pac. J. Trop. Biomed. 2012;2(8):608–615. doi: 10.1016/S2221-1691(12)60106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menon S., Ks S.D., Agarwa H., Shanmugam V.K. Efficacy of biogenic selenium nanoparticles from an extract of ginger towards evaluation on anti-microbial and anti-oxidant activities. Colloid and Interface Science Communications. 2019;29:1–8. doi: 10.1016/j.colcom.2018.12.004. [DOI] [Google Scholar]
- 28.Ye Q., Wang H., Li H. Arbuscular mycorrhizal fungi enhance drought stress tolerance by regulating osmotic balance, the antioxidant system, and the expression of drought-responsive genes in Vitis vinifera L. Aust. J. Grape Wine Res. 2023 doi: 10.1155/2023/7208341. [DOI] [Google Scholar]
- 29.Reshma R., Arumugam M. Organic selenium fortification in edible marine microalga Nannochloropsisoceanica CASA CC201 for food and feed applications. Algal Res. 2022;66 doi: 10.1016/j.algal.2022.102787. [DOI] [Google Scholar]
- 30.Ragini R., Arumugam M. In vivo studies on bioavailability, toxicity, and antioxidant defense of organic selenium-enriched microalga biomass in Wistar rats. J. Appl. Phycol. 2023:1–15. doi: 10.1007/s10811-023-03007-x. [DOI] [Google Scholar]
- 31.Reshma R., Kumari S., Arumugam M. Structural elucidation of selenocysteine insertion machinery of microalgalselenoprotein T and its transcriptional analysis. Biotechnol. Appl. Biochem. 2021;68(3):636–647. doi: 10.1002/bab.1974. [DOI] [PubMed] [Google Scholar]
- 32.Wong S.P., Leong L.P., Koh J.H. Antioxidant activities of aqueous extracts of selected plants. Food Chem. 2006;99(4):775–783. doi: 10.1016/j.foodchem.2005.07.058. [DOI] [Google Scholar]
- 33.Vazhayil A., Thomas J., Thomas N. Cobalt doping in LaMnO3 perovskite catalysts–B site optimization by solution combustion for oxygen evolution reaction. J. Electroanal. Chem. 2022;918 [Google Scholar]
- 34.Gilavand F., Saki R., Mirzaei S.Z., Lashgarian H.E., Karkhane M., Marzban A. Green synthesis of zinc nanoparticles using aqueous extract of Magnoliae officinalis and assessment of its bioactivity potentials. Biointerface Res Appl Chem. 2021;11(1):7765–7774. doi: 10.33263/BRIAC111.77657774. [DOI] [Google Scholar]
- 35.Mirzaei S.Z., Somaghian S.A., Lashgarian H.E., Karkhane M., Cheraghipour K., Marzban A. Phyco-fabrication of bimetallic nanoparticles (zinc–selenium) using aqueous extract of Gracilaria corticata and its biological activity potentials. Ceram. Int. 2022;47(4):5580–5586. doi: 10.1016/j.ceramint.2020.10.142. [DOI] [Google Scholar]
- 36.Karthik K.K., Cheriyan B.V., Rajeshkumar S., Gopalakrishnan M. A review on selenium nanoparticles and their biomedical applications. Biomedical Technology. 2024;(6):61–74. doi: 10.1039/D1MA00639H. 2024. [DOI] [Google Scholar]
- 37.Somaghian S.A., Mirzaei S.Z., Shakib M.E.K. Biogenic zinc selenide nanoparticles fabricated using Rosmarinus officinalis leaf extract with potential biological activity. BMC Complement Med Ther. 2024;24:20. doi: 10.1186/s12906-023-04329-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shahzamani K., Lashgarian H.E., Karkhane M., Ghaffarizadeh A., Ghotekar S., Marzban A. Bioactivity assessments of phyco-assisted synthesized selenium nanoparticles by aqueous extract of green seaweed, Ulva fasciata. Emergent Materials. 2022;5(6):1689–1698. doi: 10.1007/s42247-022-00415-6. [DOI] [Google Scholar]
- 39.Mirzaei S.Z., Lashgarian H.E., Karkhane M., Shahzamani K., Alhameedawi A.K., Marzban A. Bio-inspired silver selenide nano-chalcogens using aqueous extract of Melilotus officinalis with biological activities. Bioresources and Bioprocessing. 2021;(8):1–11. doi: 10.1186/s40643-021-00412-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mirzaei S.Z., Somaghian S.A., Lashgarian H.E., Karkhane M., Cheraghipour K., Marzban A. Phyco-fabrication of bimetallic nanoparticles (zinc–selenium) using aqueous extract of Gracilaria corticata and its biological activity potentials. Ceram. Int. 2021;47(4):5580–5586. doi: 10.1016/j.ceramint.2020.10.142. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.