Abstract
In nature, aflatoxins, especially aflatoxin B1 (AFB1), are the common mycotoxins, which cause serious health problems for humans and animals. This paper aimed to study the effects of AFB1 on flesh flavor and muscle development of grass carp (Ctenopharyngodon idella) and its mechanism. There were 1440 individual fish in total, with 6 treatments and each treatment replicated 3 times. The 6 treatments were fed a control diet with different doses of AFB1 (0.04, 29.48, 58.66, 85.94, 110.43 and 146.92 μg/kg diet) for 60 d. AFB1 increased myofiber diameter, as well as decreased myofiber density of grass carp muscle (P < 0.05). The contents of free amino acid decreased gradually (P < 0.05) as dietary AFB1 increased in the muscle of grass carp. The levels of reactive oxygen species, malonaldehyde and protein carbonyl (PC) were increased (P < 0.05) with the dietary AFB1 increased. The levels of antioxidant enzyme (glutathione peroxidase, glutathione, glutathione reductase, total antioxidant capacity, anti-superoxide anion, and anti-hydroxyl radical) were decreased (P < 0.05) with the dietary AFB1 increased. In addition, dietary AFB1 decreased the content of collagen, and downregulated the mRNA and protein levels of transforming growth factor-β (TGF-β)/Smads signaling pathway in grass carp muscle (P < 0.05). The mRNA and protein levels of myogenic regulatory factors were downregulated in grass carp muscle (P < 0.05). Furthermore, the activities of matrix metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9) were increased (P < 0.05), and the protein levels of phosphorylate-38 mitogen-activated protein kinase (p-p38MAPK), phosphorylate-c-Jun N-terminal kinase, urokinase-type plasminogen activator (uPA), MMP-2 and MMP-9 were upregulated (P < 0.05), but collagen Ⅰ, laminin β1 and fibronectin were downregulated (P < 0.05) with the dietary AFB1 increased in the muscle of grass carp. Based on the results of this study, we can draw the following conclusion: dietary AFB1 might damage flesh flavor and inhibit the muscle development through MAPK/uPA/MMP/extracellular matrix (ECM) signaling pathway in grass carp. Moreover, the recommended safe limit of AFB1 in feed is no more than 26.77 μg/kg diet according to the PC levels in grass carp muscle.
Keywords: Aflatoxin B1, Flesh flavor, Muscle development, Grass carp
1. Introduction
The protein content in fish flesh ranges between 17% and 22%, and is easily digestible with all essential amino acids required for human nutrition (Wan et al., 2023). In recent years, the rise in consumption levels of aquatic products has led to the rapid development of aquaculture industries. However, this growth has also brought out concerns regarding the safety of aquatic products. Most fish feeds are susceptible to mycotoxin contamination, especially aflatoxin B1 (AFB1) (Marijani et al., 2019). However, the effect of AFB1 on the flesh quality and its possible mechanism of grass carp has not been reported.
Muscle quality is affected by the levels of free amino acids in muscle as well as muscle hardness. Free amino acids play an important role in the flavor of fish muscle (Jiang et al., 2016). AFB1 covalently binds nucleotides in human liver cell during metabolism and hence reduces nucleotide levels (Macé et al., 1997), while the deficiency of nucleotides reduces the levels of free amino acids in the muscle of grass carp (Tie et al., 2019). Thus, AFB1 might reduce the contents of free amino acids in the muscle of grass carp. Muscle stiffness increased with the increase of the collagen content in muscle, which in turn improved the texture of the grass carp muscle (Dong et al., 2022). Fish muscle is dominated by the collagen Ⅰ. Previous studies from our laboratory found that the content of collagen Ⅰ in fish was regulated by transforming growth factor-β (TGF-β)/Smads signaling pathway (Dong et al., 2022). It was reported that AFB1 downregulated TGF-β mRNA levels in skin of grass carp (He et al., 2022). Thus, AFB1 might reduce the levels of collagen through TGF-β/Smads signaling pathway. Nevertheless, the effects of AFB1 on the free amino acid contents, the contents of type Ⅰ collagen and the relative molecular mechanism in muscle of grass carp have not been reported.
The flesh quality is closely related to muscle development (Chen et al., 2020). The development of skeletal muscle is a highly complex and sophisticated biological process that is regulated by multiple factors, including myogenic regulatory factors (MRF) (Zhao et al., 2020), and the extracellular matrix (ECM) containing collagen, laminin, and fibronectin (Zhang et al., 2021). AFB1 damaged muscle structure of zebrafish (He et al., 2023a), but the related mechanism is unclear. Furthermore, the members of the matrix metalloproteinases (MMP) family are key regulators that degrade the ECM (Zhang et al., 2021). Urokinase-type plasminogen activator (uPA) is a serine protease (Alfano et al., 2009), which could activate MMP in myeloma cell (Fux et al., 2009). It was reported that the expression of uPA was regulated by mitogen-activated protein kinase (MAPK) signaling in human umbilical vein endothelial cell line (HUVEC) (Ye et al., 2016). Our previous study found that AFB1 activated the p38MAPK signaling in grass carp skin (Zeng et al., 2019). However, there have been no reports on whether AFB1 can activate uPA through MAPK signaling pathway to degrade ECM and affect muscle development, which deserves further exploration.
The grass carp is a freshwater economic fish with the highest output in China, even worldwide (Tang et al., 2019). As part of a major study, we aimed to explore the effects of dietary AFB1 on flesh flavor and muscle development of grass carp. It was reported that AFB1 should not exceed 20 μg/kg in fish feed, and in some countries, the content of AFB1 in fish feed is as high as 150 μg/kg (Marijani et al., 2019). Therefore, we designed an experiment where the content of AFB1 in grass carp diet varied between 30 and 150 μg/kg. We measured the histological structure of the muscle, the levels of free amino acids, antioxidant enzyme activities and its related mRNA expression, the mRNA and protein levels of MRF, and ECM components and related signal molecules. This study investigated the potential mechanism of dietary AFB1 damaging flesh flavor and inhibiting muscle development, and provided reference for healthy culture of grass carp.
2. Materials and methods
2.1. Animal ethics statement
All animal studies were approved by the Sichuan Agricultural University Animal Care Advisory Committee, in keeping with Chinese ethical Guidelines for Experimental Animals. The protocols were approved by the license HXN2020114011.
2.2. Animals
A total of 1440 healthy grass carp were kept in temporary cages (1.4 m × 1.4 m × 1.4 m, length × wide × height) for 4 weeks to acclimate the environment after purchase. Grass carp were fed 4 times a day during the temporary rearing period, and kept the same feeding conditions (water temperature 26.5 to 30.5 °C, oxygen concentration 6.0 mg/L, pH 7.5) throughout the experiment. Before the start of the experiment, we randomly assigned grass carp (12.96 ± 0.03 g) to 6 treatments, which had 3 replicates of 80 grass carp each. The growth trial involved feeding the fish 4 times a day for 60 d. There was no grass carp mortality during the 60-d experiment. The fish were kept in 1.4 m long × 1.4 m wide × 1.4 m high cages, the cage is installed in outdoor ponds. A feeding disc (100 cm diameter) was hung in each cage to estimate the remaining material. After 30 min of feeding, lifted the disc, collected the remaining feed, dried the feed and weighed it. The water temperature was maintained between 26.5 and 30.5 °C, oxygen concentration was 6.0 mg/L, the pH level was around 7.5.
2.3. Feeds and experimental design
The detailed feed formulation is listed in Table S1, which was consistent with our previous study (Zeng et al., 2019). Briefly, dietary proteins sources were primarily derived from fish meal, gelatin and casein, and dietary lipids originate primarily from soybean oil and fish oil. The experiment diets were designed to meet grass carp nutritional requirements. AFB1 (purity > 98%) was purchased from Pribolab Pte. Ltd. (Singapore). The diets contained 6 different concentrations of AFB1 (0, 30, 60, 90, 120, 150 μg/kg diet), and the actual concentration was 0.04, 29.48, 58.66, 85.94, 110.43 and 146.92 μg/kg diet, respectively, which was measured by high-performance liquid chromatographic (HPLC) according to the method of Zychowski et al. (2013). Furthermore, we chose corn starch as the substrate material. We first mixed AFB1 into corn starch to make a premix, and then mixed AFB1 premix with other feed ingredients to make a diet containing different concentrations of AFB1. The feeds were pelleted into uniform particles, which store at 4 °C after natural drying. According to AOAC (1995) methods, the dry matter was determined by the oven-drying method, and the crude protein and crude lipid contents of basal diet were determined by the Kjeldahl method and Soxhlet extraction techniques, respectively.
2.4. Sample collection
After completion of the experiments, the grass carp were anaesthetized with benzocaine (50 mg/L). We used a scalpel to separate the muscle along the back and ventral lines of grass carp, removed the skin and red muscle, and collected the white muscle for experimental analysis (the diagram of muscle collection site of grass carp shown in Fig. S1). Each group had 3 grass carp randomly chosen to collect muscle which were subsequently fixed in 4% paraformaldehyde for histological analysis. For biochemical analysis, among the grass carp in each group, 6 carp (1 carp from each replicate) were randomly selected, and the muscle was collected and stored at −20 °C. For PCR and Western blot (WB) analysis, the muscle samples were collected by random sampling and stored at −80 °C.
2.5. Histological observation
For the hematoxylin-eosin (HE) staining, dimethylbenzene was used to deparaffinize the paraffin sections, followed by graded alcohol to rehydrate them. Then hematoxylin and eosin were used to counterstain the nuclei and cytoplasm. Finally, dehydration was accomplished using graded alcohol and sealed using neutral gum. The collagen fibers in the muscle tissue were stained with Sirius red. The method of dewaxing to water was the same as that of HE staining. The sections were stained with Sirius red after dewaxing and rehydration. Then hematoxylin was used to counterstain the nuclei. Finally, the sections were dehydrated and sealed. After drying overnight, microscopic images were then taken of the sections on an optical microscope (TS100, Nikon, Tokyo, Japan). The area of collagen fiber was counted by Image J, and the contents of collagen fiber were expressed by the relative expression quantity.
2.6. Biochemical analysis
All commercial kits related to antioxidant indicators and hydroxyproline (HYP) kits were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The contents of collagen were converted by measuring the content of HYP in muscle, which was calculated by multiplying HYP by 8. The antioxidant indicators included total superoxide dismutase (T-SOD), reactive oxygen species (ROS), protein carbonyl (PC), malondialdehyde (MDA), anti-superoxide anion (ASA), glutathione (GSH), glutathione reductase (GR), glutathione peroxidase (GPx), anti-hydroxyl radical (AHR), catalase (CAT) and total antioxidant capacity (T-AOC). We followed the instructions for the specific operation method. In brief, the muscle sample was weighed and placed in 2-mL centrifuge tube, and then 1.8 mL pre-cooled 0.9% saline was added. The supernatant was collected by centrifugation at 6000 × g for 20 min at 4 °C for further analysis. The methods and kits used are shown in Table S2.
2.7. Free amino acid analysis
A Hitachi L-8900 amino acid analyzer (Tokyo, Japan) was used for measuring amino acid contents. In brief, we added 0.1 g sample to 15-mL centrifuge tube and added 5 mL HCl (6 mol/L). The mixture was diluted with sodium citrate buffer (pH 2.2) after drying in a water bath. Then the samples were filtrated with 0.22-μm organic membrane.
2.8. Real-time PCR
A total RNA extract was prepared using Trizol (Takara, Dalian, Liaoning, China). The concentration of RNA was measured spectrophotometrically and the quality of RNA was determined through electrophoresis on a gel. The cDNA was synthesized using a cDNA synthesis kit (Takara, China). The primer sequences are listed in Table S3. qRT-PCR analysis was performed on the QuantStudio 5 Real-Time PCR System. The qRT-PCR cycling condition were as follows: 95 °C for 30 s (hold stage), followed by 95 °C for 15 s and 60 °C for 34 s (measure fluorescence), finally 60 °C for 1 min and 95 °C for 15 s for 40 cycles (melt curve stage). In the analysis of the expression data, we used 2−ΔΔCT method with β-actin as an internal reference gene.
2.9. Western blot
The muscle tissue was ground in a high-speed tissue grinder and then centrifuged to collect the supernatant. Assays for total proteins were conducted using the BCA Protein Assay Kits (Nanjing Jiancheng Bioengineering Institute, China). An electrophoretic separation of proteins was performed on sodium dodecyl sulfate polyacrylamide gels (24 μg/lane). Equal amounts of total proteins were separated by SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF) membranes. A primary antibody was then incubated overnight on the membranes (4 °C). Then secondary antibody was incubated at room temperature for 90 min. Finally, proteins were detected by enhanced chemiluminescence (ECL) system. The bands were quantified using Image J software. The antibodies information is shown in Table S4. Beta-actin, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and Lamin B1 were used as internal references.
2.10. Statistical analysis
One-way ANOVA was performed using SPSS18.0 and then mean values were compared using the Duncan's multiple range test. All data visualization was produced by Origin software and Hiplot website (https://hiplot.com.cn/home/index.html).
3. Results
3.1. AFB1 affected the muscle histological architecture of grass carp
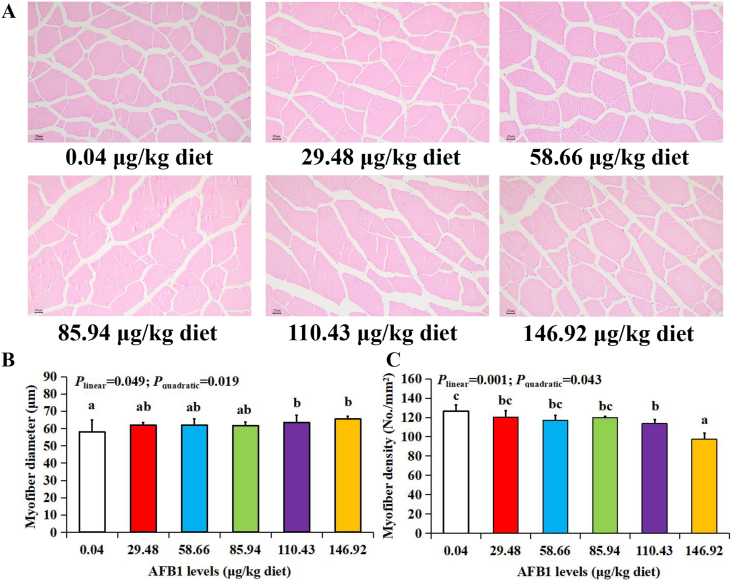
As shown in Table 1, AFB1 residue was detected in the muscle of grass carp in AFB1 groups. Histological observation of muscle can intuitively reflect the density and diameter of muscle fibers. As presented in Fig. 1A, with the increase of dietary AFB1 dose, the arrangement of muscle fibers was loose. The myofiber diameters significantly increased (P < 0.05) and the myofiber density significantly decreased (P < 0.05) with the dietary AFB1 level rising to 110.43 μg/kg diet, as shown in Fig. 1B and C. The data indicated that AFB1 destroyed the histological architecture of grass carp muscle.
Table 1.
The residues of aflatoxin B1 (AFB1) in grass carp muscle.
| Item | AFB1 levels, μg/kg diet |
Pquadratic | Plinear | |||||
|---|---|---|---|---|---|---|---|---|
| 0.04 | 29.48 | 58.66 | 85.94 | 110.43 | 146.92 | |||
| AFB1 residues | –1 | 1.43 ± 0.16a | 1.46 ± 0.17a | 1.32 ± 0.14a | 1.32 ± 0.16a | 1.42 ± 0.16a | 0.591 | 0.490 |
The data represent means ± SD. Different letters indicate significant differences (P < 0.05). The Pquadratic and Plinear indicate significant quadratic and linear dose response relationship, respectively (P < 0.05).
AFB1 residue in grass carp muscle was not detected.
Fig. 1.
The effects of aflatoxin B1 (AFB1) on muscle histology of grass carp (n = 6/group). (A) The histological characteristics of myofiber in grass carp flesh after feeding different doses of AFB1 (magnification, 100×). Scale bars = 20 μm. (B) The myofiber diameters in grass carp after feeding different doses of AFB1. (C) The myofiber density of grass carp fed different doses of AFB1. The data represent means ± SD. The Plinear and Pquadratic indicate significant linear and quadratic dose–response relationship, respectively (P < 0.05). a–cDifferent letters indicate significant differences (P < 0.05).
3.2. AFB1 affected free amino acid enrichment of grass carp muscle
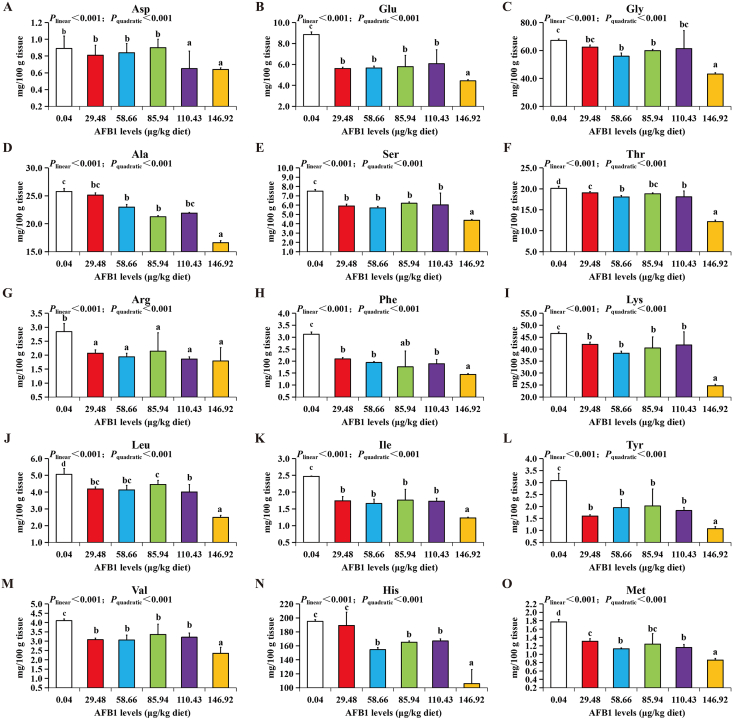
The levels of free amino acid in grass carp muscle are shown in Fig. 2. Compared with the control group, a high level of AFB1 (more than 85.94 μg/kg diet) significantly reduced the contents of umami taste amino acids, including aspartic acid (Asp) and glutamic acid (Glu) (P < 0.05) (Fig. 2A and B). Compared with the control group, the contents of glycine (Gly), alanine (Ala) and serine (Ser) were decreased significantly with the increase of dietary AFB1 levels (P < 0.05) (Fig. 2C–E). Threonine (Thr), arginine (Arg) and phenylalanine (Phe) levels were decreased significantly (P < 0.05) when the diet contained above 29.48 μg/kg AFB1 (Fig. 2F–H). Furthermore, the contents of lysine (Lys), leucine (Leu), isoleucine (Ile), tyrosine (Tyr), valine (Val) and methionine (Met) were decreased significantly (P < 0.05) when the levels of AFB1 reached 29.48 μg/kg diet (Fig. 2I–M, and O), while the content of histidine (His) was decreased significantly when the level of AFB1 reached above 29.48 μg/kg diet (Fig. 2N).
Fig. 2.
The effects of aflatoxin B1 (AFB1) on free amino acid levels in muscle of grass carp (n = 6/group). (A) Asp, (B) Glu, (C) Gly, (D) Ala, (E) Ser, (F) Thr, (G) Arg, (H) Phe, (I) Lys, (J) Leu, (K) Ile, (L) Tyr, (M) Val, (N) His, and (O) Met levels in muscle of grass carp. The data represent means ± SD. The Plinear and Pquadratic indicate significant linear and quadratic dose–response relationship, respectively (P < 0.05). a–dDifferent letters indicate significant differences (P < 0.05). Asp = aspartic acid; Glu = glutamic acid; Gly = glycine; Ala = alanine; Ser = serine; Thr = threonine; Arg = arginine; Phe = phenylalanine; Lys = lysine; Leu = leucine; Ile = isoleucine; Tyr = tyrosine; Val = valine; His = histidine; Met = methionine.
3.3. AFB1 affected antioxidant capacities and apoptosis of grass carp muscle
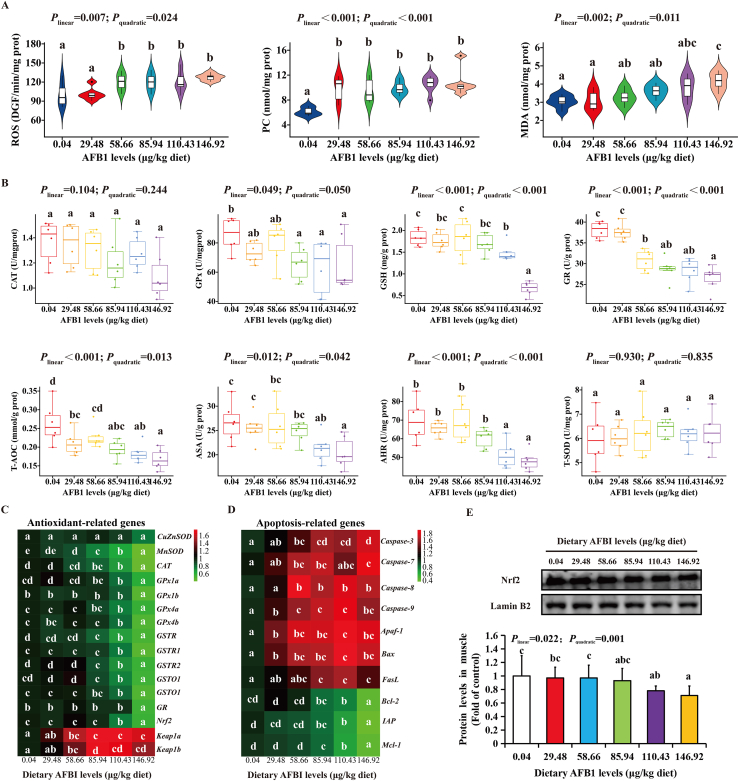
To probe the effects of dietary AFB1 on the antioxidant capacities of grass carp muscle, we detected the oxidative damage biomarkers and the activity of antioxidative enzymes, the related mRNA expression and protein levels in grass carp muscle. As shown in Fig. 3, compared with the control group, high levels of AFB1 significantly increased ROS, MDA and PC levels (P < 0.05) (Fig. 3A). Furthermore, GPx, GSH, GR, T-AOC, ASA and AHR levels were decreased with the increase of dietary AFB1 levels (P < 0.05). However, the levels of CAT and T-SOD have not been changed at any levels of AFB1 (Fig. 3B).
Fig. 3.
The effects of aflatoxin B1 (AFB1) on antioxidant and apoptosis related parameters in flesh of grass carp (n = 6/group). (A) ROS, PC, and MDA levels in grass carp muscle. (B) The antioxidant related enzyme activity in grass carp muscle. (C) The heat-map of AFB1 on the mRNA expression of antioxidant-related genes in grass carp muscle. (D) The heat-map of AFB1 on the mRNA expression of apoptosis-related genes in grass carp muscle. (E) The mRNA levels of Nrf2, Keap1a and Keap1b in grass carp muscle. (F) The protein level of Nrf2 in grass carp muscle after feeding different doses of AFB1. ROS = reactive oxygen species; PC = protein carbonyl; MDA = malondialdehyde; CAT = catalase; GPx = glutathione peroxidase; GSH = glutathione; GR = glutathione reductase; T-AOC = total antioxidant capacity; T-SOD = total superoxide dismutase; AHR = anti-hydroxyl radical; ASA = anti-superoxide anion; CuZnSOD = copper/zinc superoxide dismutase; MnSOD = manganese superoxide dismutase; GST = glutathione-S-transferase; Nrf2 = Nuclear factor-erythroid 2-related factor 2; Keap1 = Kelch-like ECH-associated protein 1; Caspase = cysteinyl aspartate specific proteinase; Apaf-1 = apoptotic protease activating factor-1; Bax = B-cell lymphoma 2-associated X protein; FasL = fatty acid synthetase ligand; Bcl-2 = B-cell lymphoma 2; IAP = inhibitor of apoptosis proteins; Mcl-1 = myeloid cell leukemia-1. The data represent means ± SD. The Plinear and Pquadratic indicate significant linear and quadratic dose–response relationship, respectively (P < 0.05). a–dDifferent letters indicate significant differences (P < 0.05).
In order to further detect whether AFB1 cause oxidative damage and apoptosis of grass carp muscle, we detected antioxidant and apoptosis relative mRNA expression. As shown in Fig. 3C, the mRNA expression related to muscle antioxidant in grass carp are exhibited. Compared with the control group, dietary AFB1 downregulated manganese superoxide dismutase (MnSOD), CAT, GPx1a, GPx1b, GPx4a, GPx4b, glutathione-S-transferase R (GSTR), GSTP1, GSTP2, GSTO1, GSTO2, and GR mRNA expression with the increase of dietary AFB1 levels (P < 0.05). Dietary AFB1 did not change copper/zinc superoxide dismutase (CuZnSOD) mRNA expression at any levels in grass carp muscle. As shown in Fig. 3D, the mRNA expression related to apoptosis in grass carp muscle are exhibited. Compared with the control group, dietary AFB1 significantly upregulated cysteinyl aspartate specific proteinase (caspase)-3, caspase-7, caspase-8, caspase-9, apoptotic protease activating factor-1 (Apaf-1), B-cell lymphoma 2-associated X protein (Bax) and fatty acid synthetase ligand (FasL) mRNA expression with the increase of dietary AFB1 levels (P < 0.05), and downregulated inhibitor of apoptosis proteins (IAP), myeloid cell leukemia-1 (Mcl-1) and B-cell lymphoma 2 (Bcl-2) mRNA levels with the increase of dietary AFB1 levels (P < 0.05).
To probe whether AFB1 induced oxidative damage in grass carp flesh through nuclear factor-erythroid 2-related factor 2 (Nrf2) signaling pathway, we determined the expression of signal molecules related to Nrf2 signaling pathway. Compared with the control group, dietary AFB1 significantly downregulated the mRNA levels of Nrf2 and upregulated the mRNA levels of Kelch-like ECH-associated protein 1 (Keap1) a and Keap1b at 110.43, 58.66 and 58.66 μg/kg diet, respectively (Fig. 3C). The protein level of Nrf2 was significantly downregulated when dietary AFB1 level reached to 110.43 μg/kg diet (P < 0.05) (Fig. 3E).
3.4. AFB1 affected collagen enrichment and the relative gene expression of grass carp muscle
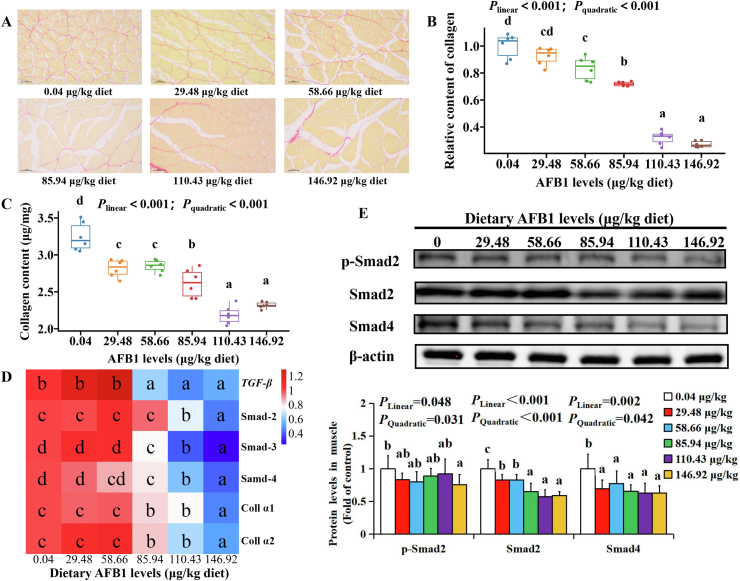
The contents of collagen and its regulated gene expression are shown in Fig. 4. Sirius red staining was used to determine collagen distribution (Fig. 4A), and the collagen distribution decreased significantly when the AFB1 level reached to 58.66 μg/kg diet (Fig. 4B). Compared with the control group, dietary AFB1 decreased collagen contents significantly (P < 0.001) (Fig. 4C). The mRNA levels of TGF-β, Smad-2, Smad-3 and Smad-4, the signaling molecules related to collagen synthesis, were significantly downregulated when AFB1 levels were above 85.94 μg AFB1/kg diet (P < 0.001) (Fig. 4D). AFB1 significantly downregulated coll α-1 and coll α-2 gene levels at 85.94 μg/kg diet (P < 0.001) (Fig. 4D). Furthermore, dietary AFB1 decreased p-Smad2, Smad2 and Smad4 proteins expression at 146.92, 29.48 and 29.48 μg/kg diet, respectively (P < 0.05) (Fig. 4E).
Fig. 4.
The effects of aflatoxin B1 (AFB1) on collagen in flesh of grass carp (n = 6/group). (A) The collagen distribution in grass carp flesh after feeding different doses of AFB1. (B) The quantification of collagen distribution in grass carp flesh after feeding different doses of AFB1. (C) The collagen contents in grass carp flesh after feeding different doses of AFB1. (D) The mRNA levels of TGF-β relative signaling pathway, coll α-1 and coll α-2 in grass carp flesh after feeding different doses of AFB1. (E) The protein levels of p-Smad2, Smad2 and Smad4 in grass carp flesh after feeding different doses of AFB1. TGF-β = transforming growth factor-β. The data represent means ± SD. The Plinear and Pquadratic indicate significant linear and quadratic dose–response relationship, respectively (P < 0.05). a–dDifferent letters indicate significant differences (P < 0.05).
3.5. AFB1 affected the expression of MRF of grass carp muscle
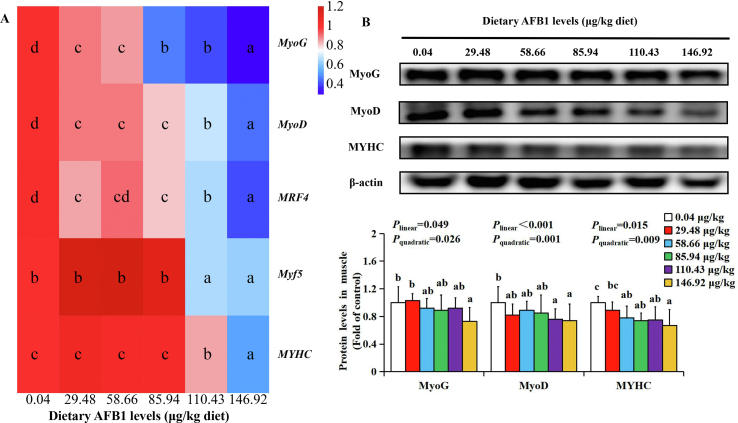
To probe whether AFB1 could inhibit muscle development of grass carp, we determined the gene expressions and protein levels of MRF. Compared with the control group, the mRNA levels of myogenin (MyoG), myogenic differentiation 1 (MyoD), and myogenic factor 6 (MRF4) were downregulated when the AFB1 levels more than 29.48 μg/kg diet, and myogenic factor 5 (Myf5) and myosin heavy chain (MYHC) mRNA levels were downregulated when the AFB1 levels more than 110.43 μg/kg diet (P < 0.001) (Fig. 5A). The protein levels of MyoG, MyoD and MYHC were downregulated when dietary AFB1 levels reached 146.92, 29.48 and 58.66 μg/kg diet, respectively (P < 0.05) (Fig. 5B).
Fig. 5.
The effects of aflatoxin B1 (AFB1) on (A) MyoG, MyoD, MRF4, Myf5, MYHC mRNA levels and (B) protein levels of MyoG, MyoD and MYHC in grass carp flesh after feeding different doses of AFB1. (n = 6/group). MyoG = myogenin; MyoD = myogenic differentiation 1; MRF4 = myogenic factor 6; Myf5 = myogenic factor 5; MYHC = myosin heavy chain. The data represent means ± SD. The Plinear and Pquadratic indicate significant linear and quadratic dose–response relationship, respectively (P < 0.05). a–dDifferent letters indicate significant differences (P < 0.05).
3.6. AFB1 affected the ECM main components, degradation related enzymes, and MAPK signaling of grass carp muscle
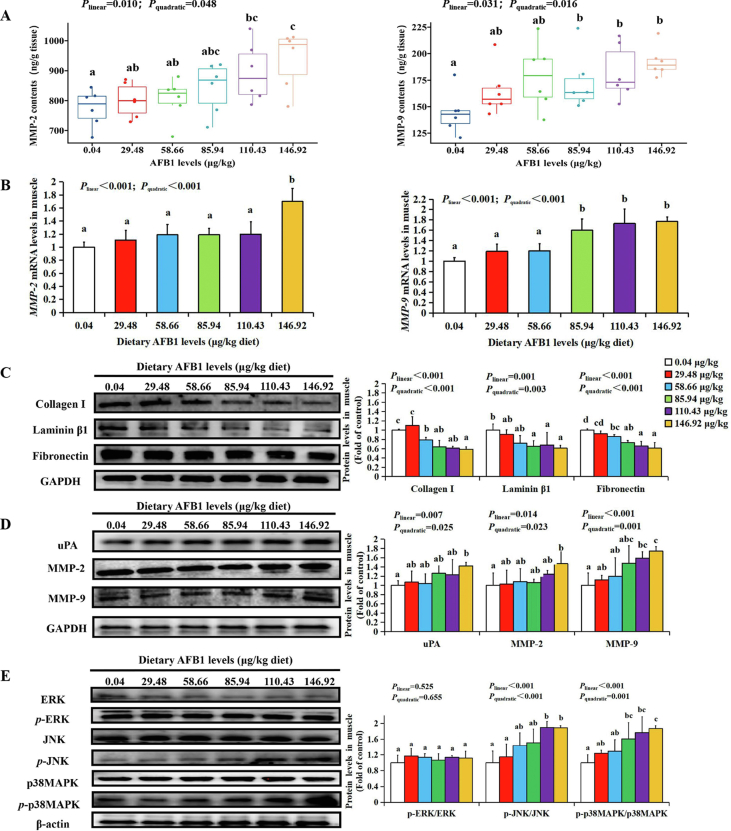
To probe the possible causes of the degradation of ECM components by AFB1, we first measured the enzyme contents and gene expression related to ECM degradation. Compared with the control group, dietary AFB1 increased the contents of MMP-2 and MMP-9 in grass carp muscle when dietary AFB1 levels reached 110.43 and 85.94 μg/kg diet, respectively (P < 0.05) (Fig. 6A). MMP-2 and MMP-9 mRNA levels in grass carp muscle were upregulated significantly at 146.92 and 85.94 μg AFB1/kg diet, respectively (P < 0.001) (Fig. 6B).
Fig. 6.
The effects of AFB1 on extracellular matrix (ECM) in grass carp muscle (n = 6/group). (A) The contents of MMP-2 and MMP-9 in grass carp muscle. (B) The mRNA expression of MMP-2 and MMP-9 in grass carp muscle. (C) The protein levels of collagen Ⅰ, laminin β1, and fibronectin in grass carp muscle. (D) The protein levels of uPA, MMP-2 and MMP-9 in grass carp muscle. (E) The protein levels of ERK, JNK and p38MAPK in grass carp muscle. MMP = matrix metalloproteinases; GAPDH = glyceraldehyde-3-phosphate dehydrogenase; uPA = urokinase-type plasminogen activator; ERK = extracellular signal-regulated kinase; JNK = c-Jun N-terminal kinase; p38MAPK = phosphorylate-38 mitogen-activated protein kinase. The data represent means ± SD. The Plinear and Pquadratic indicate significant linear and quadratic dose–response relationship, respectively (P < 0.05). a–dDifferent letters indicate significant differences (P < 0.05).
To probe whether AFB1 could destroy the main components of ECM and in grass carp muscle, we tested the proteins expression of collagen Ⅰ, laminin β1 and fibronectin. Compared with the control group, the protein expressions of collagen Ⅰ, laminin β1 and fibronectin were downregulated when dietary AFB1 level reached 58.66, 85.94 and 58.66 μg/kg diet, respectively (P < 0.05) (Fig. 6C). Furthermore, dietary AFB1 upregulated uPA, MMP-2 and MMP-9 protein levels in grass carp muscle at 146.92, 146.92 and 110.43 μg/kg diet, respectively (P < 0.05) (Fig. 6D).
To probe the possible pathway of ECM degradation by AFB1, we measured the protein expression of MAPK signaling pathway. Compared with the control group, dietary AFB1 upregulated phosphorylate-c-Jun N-terminal kinase (p-JNK) and p-p38MAPK proteins when dietary AFB1 levels reached 110.43 and 85.94 μg/kg diet, respectively (P < 0.05) (Fig. 6E), but it did not change the phosphorylate-extracellular-signaling-related kinase (p-ERK) protein level.
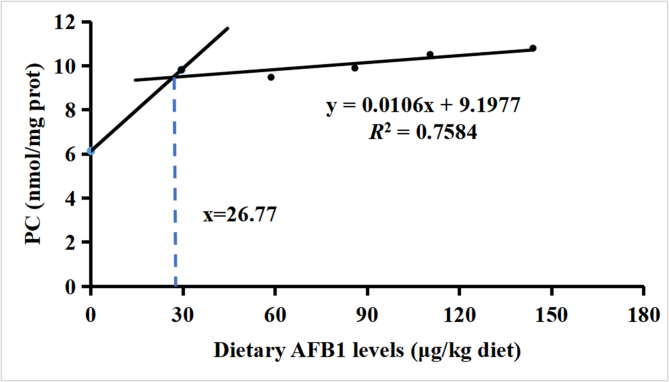
3.7. The recommended safe limit of AFB1 in feed
As shown in Fig. 7, according to the PC levels in grass carp muscle, the recommended safe limit of AFB1 in feed was determined by broken-line analysis, which was 26.77 μg/kg diet for grass carp.
Fig. 7.
The recommended safe limit of aflatoxin B1 (AFB1) in feed. PC = protein carbonyl.
4. Discussion
Our previous study found that AFB1 inhibited muscle development in zebrafish (He et al., 2023a), but its specific mechanism remains unclear. Furthermore, there was no information about the regulation of ECM on muscle development in fish. Therefore, for the first time, we explored whether AFB1 inhibited the muscle development in grass carp through regulating ECM and influenced flesh flavor. We found that AFB1 damaged the histological structure, induced oxidative damage and apoptosis in grass carp muscle. It also decreased the contents of free amino acids and inhibited the collagen synthesis of grass carp muscle. Furthermore, it inhibited muscle development and degraded ECM in grass carp muscle.
4.1. AFB1 damaged the histological structure and induced oxidative damage and apoptosis in grass carp muscle
AFB1 is a highly toxic mycotoxin that can damage animal tissue structure integrity (Huang et al., 2021). Our findings indicated that dietary AFB1 damaged the muscle histological structure, increased the myofiber diameters and decreased the myofiber density in grass carp. The results are consistent with the findings in zebrafish (He et al., 2023b) and in shrimp muscle (Huang et al., 2021). Cellular structural integrity influences tissue structural integrity in fish, which can be impaired by oxidative damage (Wei et al., 2018). Oxidative damage is characterized by ROS, MDA, and PC (He et al., 2023b). Our data showed that dietary AFB1 increased ROS, MDA and PC levels, which suggests that AFB1 could induce oxidative stress in grass carp muscle. The antioxidant defense system comprises enzymes and non-enzymatic antioxidants (Zhang et al., 2016). Antioxidant enzymes include CAT, SOD, GPx, etc. (García-Pérez et al., 2021). Our results showed that dietary AFB1 decreased the activities of GPx and GR, and the content of GSH in grass carp muscle. Our findings agree with previous studies that AFB1 decreased the activities of antioxidant enzymes (such as GPx) in zebrafish (He et al., 2023b). Thus, it appears that AFB1 might reduce antioxidant ability through decreasing the antioxidant enzyme activities in grass carp muscle.
Antioxidant enzyme activities are partly dependent on antioxidant enzyme gene transcription (Xu et al., 2022). RT-PCR showed that AFB1 inhibited transcription levels of genes associated with antioxidant enzymes (including CAT, MnSOD, GSTR, GSTP1, GSTP2, GSTO1, GSTO2, GPx1a, GPx1b, GPx4a, GPx4b, and GR) in grass carp muscle. A similar study found that AFB1 inhibited the transcription levels of antioxidant enzymes (including CAT and GPx) in zebrafish larvae (He et al., 2023b). Interestingly, unlike MnSOD, the mRNA level of CuZnSOD did not change at any AFB1 levels in our results. This may be due to the different localization of MnSOD and CuZnSOD in cells. In fact, MnSOD is localized in mitochondrial matrix, while CuZnSOD is localized in cytoplasm (Umasuthan et al., 2012). Our findings indicated that AFB1 increased the level of ROS. It is well known that ROS is mainly produced in the mitochondria (Jiang et al., 2022). Thus, AFB1 downregulated MnSOD mRNA level rather than CuZnSOD.
It has been demonstrated that Keap1 plays a crucial role in the regulation of Nrf2 by acting as a cytosolic repressor of the pathway (Malhotra et al., 2010). ROS overproduction causes Nrf2 to be activated and dissociated from Keap1 (Kudo et al., 2020). In our results, dietary AFB1 resulted in overproduction of ROS, and upregulated Keap1a and Keap1b mRNA levels, and downregulated Nrf2 mRNA level in grass carp muscle. The results indicated that AFB1 might induce oxidative damage in grass carp muscle through the Nrf2/Keap1 signaling pathway.
It was reported that cellular structural integrity influences tissue structural integrity in fish, which can also be impaired by apoptosis (Wei et al., 2018). Caspases are major effectors of apoptosis, and the activation of caspase-3 signifies irreversible apoptosis (He et al., 2018). Death receptor and mitochondrial pathways are the two main pathways that underpin apoptosis (Liu et al., 2011). The death receptor pathway is controlled by the apoptosis regulators FasL and caspase-8 (Patel et al., 2015), while the mitochondria-mediated apoptosis pathway is controlled by the apoptosis regulators Bax, Bcl-2 and caspase-3 (Du et al., 2019). Our data showed that dietary AFB1 upregulated FasL, Bax, Apaf-1, caspase-3, caspase-7, caspase-8, and caspase-9, downregulated IAP, Bcl-2, and Mcl-1 mRNA levels in grass carp muscle. Similarly, our previous studies indicated that AFB1 induced apoptosis by upregulating caspase-3 and Bax, and downregulating Bcl-2 mRNA levels in zebrafish larvae (He et al., 2023b). These studies suggest that AFB1 might induce apoptosis by extrinsic death receptor pathway and the intrinsic mitochondrial pathway in grass carp muscle.
4.2. AFB1 decreased the contents of free amino acid in grass carp muscle
AFB1 induced oxidative damage in grass carp muscle according to our previous study. Wu et al. (2022) found that oxidative damage could disrupt the flesh quality in grass carp, which, in turn, could decrease the contents of free amino acids in muscle. Free amino acids are the primary flavor components of aquaculture products, in which Glu and Asp are umami taste amino acids, and Gly, Ala and Ser are sweet taste amino acids (Jiang et al., 2016). Thr, Arg and Lys and other amino acids as well can produce aroma by the Maillard reaction (Yokoyama et al., 2020). Furthermore, Leu, Ile, Tyr, Val, His, and Met, as the essential amino acids (EAA), are the important nutritional value index in flesh quality (Jiang et al., 2016). Results showed that dietary AFB1 decreased the umami taste amino acids, sweet taste amino acids, EAA, and the Maillard reaction related amino acids in grass carp muscle. Similarly, AFB1 decreased Leu, Ile, Val and other amino acid level in the serum of dairy goats (Cheng et al., 2017). Our results indicated that AFB1 damaged the flesh quality through decreasing the contents of free amino acids of grass carp.
4.3. AFB1 inhibited collagen synthesis in grass carp muscle
As one of the important indicators of flesh quality, muscle hardness is affected by collagen contents (Dong et al., 2022). Collagen content is dependent on the balance between collagen metabolism and synthesis (Dong et al., 2022). The main collagen in grass carp muscle is type I collagen, which consists of two polypeptide chains, the α1 (col1 α1) chain and the α2 (col1 α2) chain, and the expression of col1α1 and col1 α2 is regulated by TGF-β/Smads signaling pathway (Dong et al., 2022). Our study found that dietary AFB1 decreased the contents of collagen I, downregulated the mRNA expressions of col1 α1, col1 α2, TGF-β, Smad-2, Smad-3, and Smad-4, and also decreased the proteins level of Smad-2 and Smad-4 in grass carp muscle. Our results indicated that AFB1 decreased the level of Thr. A previous study in our laboratory found that low dietary Thr decreased TGF-β mRNA and protein expression in muscle of grass carp (Wen et al., 2023). Therefore, we speculated that AFB1 might downregulated TGF-β gene expression by decreasing the level of Thr in grass carp muscle. Our data suggested that AFB1 might inhibit collagen synthesis through TGF-β/Smads signaling pathway in grass carp muscle.
4.4. AFB1 inhibited muscle development in grass carp
Collagen not only affects muscle quality, but also muscle cell development (Liu et al., 2020). Initial muscle development is controlled by the MRF (Zhao et al., 2020). Among MRF, muscle cell proliferation is mainly controlled by Myf5 and MyoD, while differentiation is primarily controlled by MRF4 and MyoG in muscle development (Song et al., 2015). There is a high level of expression of MYHC in differentiated myoblasts, which promotes cell fusion and leads to the formation of myotubes (Zi et al., 2022). Our findings demonstrated that AFB1 downregulated MyoG, MyoD, MRF4, Myf5, and MYHC mRNA expression in grass carp muscle. Dietary AFB1 also decreased MyoG, MyoD, and MYHC protein level in grass carp muscle. The results are consistent with our previous findings in zebrafish larvae (He et al., 2023b). These data indicate that dietary AFB1 inhibits muscle development through suppressing the expression of MRF genes in grass carp.
As a part of connective tissue, ECM not only provides structural support to cells, but also has a significant role in muscle cell development, of which the main components include collagen Ⅰ, laminin and fibronectin (Zhang et al., 2021). Our findings demonstrated that dietary AFB1 reduced collagen Ⅰ, laminin and fibronectin protein levels in grass carp muscle. Both MMP-2 and MMP-9, both zinc-dependent proteases that break down ECM, are directly activated by uPA (Gramling et al., 2010). We found that dietary AFB1 increased MMP-2 and MMP-9 contents, and upregulated the expression of their genes and proteins in grass carp muscle. AFB1 also upregulated the expression of uPA protein in grass carp muscle. Excessive ROS production could increase uPA expression and activation in MDA-MB-231 cell (Flores-López et al., 2016). Thus, the possible reason why AFB1 inhibits muscle development of grass carp is that ROS activates uPA, which, in turn, activates MMP and degrades ECM.
It was reported that the expression of uPA was regulated via the MAPK signaling pathway in HUVEC line (Ye et al., 2016). Furthermore, the MAPK pathway is frequently deregulated, leading to the overexpression of MMP-2, MMP-9, and uPA genes in glioblastoma (Qu et al., 2017). A MAPK family consists of three major members, ERK, p38, and JNK, the activation and function of which are controlled by upstream kinases and stress-related inducers (Beck et al., 2009). Our data showed that dietary AFB1 upregulated p-JNK and p-p38MAPK protein level in grass carp muscle. The p38MAPK signaling molecule could be strongly activated by oxidative stress. Our previous work showed that AFB1 caused oxidative damage in zebrafish muscle (He et al., 2023b). Moreover, AFB1 induced inflammatory response through upregulating gene expression related to proinflammatory cytokines in grass carp head kidney (He et al., 2022), and proinflammatory cytokines could activate JNK signaling molecule (López-Bergami et al., 2005). Thus, we hypothesized that AFB1 might activate uPA and MMP through p38MAPK and p-JNK signaling pathways, degrade ECM, and thus inhibit muscle development of grass carp.
As a highly toxic mycotoxin, the effects of AFB1 on fish flesh quality and muscle development have not been systematically studied. In aquatic animals, flesh quality depends on muscle development (Chen et al., 2020). At present, AFB1 has been demonstrated to damage the flesh quality of aquatic and terrestrial animals, but its mechanism has not been reported. The current study suggests that AFB1 might affect the flesh flavor of grass carp by inhibiting muscle development.
4.5. The recommended safe limit of AFB1 in feed
According to the PC levels in grass carp muscle, the recommended safe limit of AFB1 in feed is no more than 26.77 μg/kg diet (ymin = 9.4815, y = 0.0106x + 9.1977, R2 = 0.7584). Our previous research found that the recommended safe limits of AFB1 in head kidney, spleen and gill were 30, 29 and 31.10 μg/kg diet (He et al., 2023a; Zeng et al., 2019), respectively, which were higher than recommended for muscle.
5. Conclusion
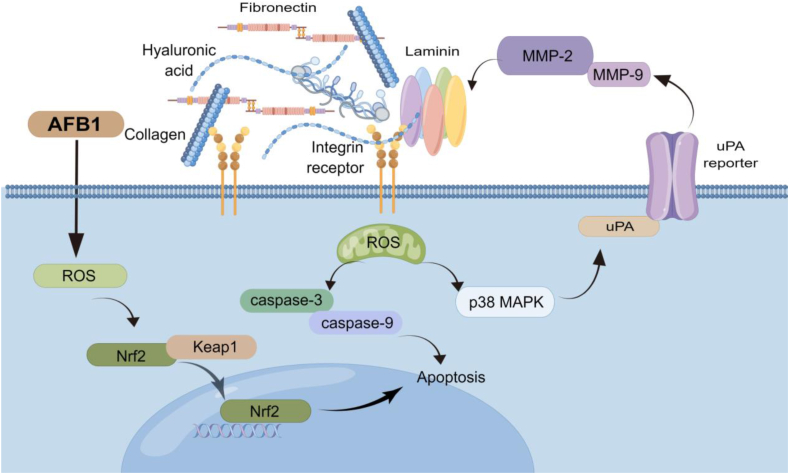
Dietary AFB1 damaged the histological structure and decreased the flesh quality through decreasing the contents of free amino acids in grass carp muscle. As shown in Fig. 8, AFB1 induces oxidative damage likely through the Nrf2/Keap1 signaling pathway, and induces apoptosis by extrinsic death receptor pathway and the intrinsic mitochondrial pathway in grass carp muscle. The inhibition of collagen synthesis might act through the TGF-β/Smads signaling pathway in grass carp muscle. AFB1 inhibits muscle development in grass carp possibly by inhibiting MRF gene expression and promoting ECM degradation through p38MAPK and p-JNK signaling pathways. The recommended safe limit of AFB1 in feed is 26.77 μg/kg of diet for grass carp. Our findings demonstrate that AFB1 might inhibit muscle development of grass carp through the MAPK/uPA/MMP/ECM signaling pathway, thus reducing its flavor quality.
Fig. 8.
The mechanism of toxic effects of aflatoxin B1 (AFB1) on muscle development of grass carp. MMP = matrix metalloproteinases; uPA = urokinase-type plasminogen activator; ROS = reactive oxygen species; caspase = cysteinyl aspartic acid-protease; MAPK = mitogen-activated protein kinase; Keap1 = Kelch-like ECH-associated protein 1; Nrf2 = nuclear factor-erythroid 2-related factor 2.
Author contributions
Xiang-Ning He: Writing-original draft preparation, Conceptualization, Methodology, Software. Zhen-Zhen Zeng: Data curation. Wei-Dan Jiang, Pei Wu: Methodology. Yang Liu: Validation, Investigation. Sheng-Yao Kuang, Ling Tang, Shu-Wei Li: Supervision. Lin Feng, Xiao-Qiu Zhou: Conceptualization, Writing-reviewing and editing, Project administration.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This research was financially supported by National Natural Science Foundation of China for Outstanding Youth Science Foundation (31922086), the Earmarked Found for China Agriculture Research System (CARS-45), National Key Research and Development Program of China (2019YFD0900200, 2018YFD0900400), and the Young Top-Notch Talent Support Program. The authors would like to express their sincere thanks to the personnel of these teams for their kind assistance. We would like to express our sincere thanks for the assistance from the personnel of teams. We also thank the Home for Researchers (www.home-for-researchers.com).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aninu.2024.03.012.
Contributor Information
Lin Feng, Email: fenglin@sicau.edu.cn.
Xiao-Qiu Zhou, Email: zhouxq@sicau.edu.cn, fishnutrition@126.com.
Appendix supplementary data
The following is the Supplementary data to this article:
References
- Alfano M., Mariani S.A., Elia C., Pardi R., Blasi F., Poli G. Ligand-engaged urokinase-type plasminogen activator receptor and activation of the CD11b/CD18 integrin inhibit late events of HIV expression in monocytic cells. Blood. 2009;113(8):1699–1709. doi: 10.1182/blood-2008-02-138412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC . 16th ed. AOAC International; Gaithersburg, MD: 1995. Official methods of analysis. [Google Scholar]
- Beck I.M., Vanden Berghe W., Vermeulen L., Yamamoto K.R., Haegeman G., De Bosscher K. Crosstalk in inflammation: the interplay of glucocorticoid receptor-based mechanisms and kinases and phosphatases. Endocr Rev. 2009;30(7):830–882. doi: 10.1210/er.2009-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.J., Gan L., Guo Y.C., Tian L.X., Liu Y.J. Changes in growth performance, aflatoxin B1 residues, immune response and antioxidant status of Litopenaeus vannamei fed with AFB1-contaminated diets and the regulating effect of dietary myo-inositol supplementation. Food Chem. 2020;324 doi: 10.1016/j.foodchem.2020.126888. [DOI] [PubMed] [Google Scholar]
- Cheng J., Huang S., Fan C., Zheng N., Zhang Y., Li S., Wang J. Metabolomic analysis of alterations in lipid oxidation, carbohydrate and amino acid metabolism in dairy goats caused by exposure to Aflotoxin B1. J Dairy Res. 2017;84(4):401–406. doi: 10.1017/S0022029917000590. [DOI] [PubMed] [Google Scholar]
- Dong M., Zhang L., Wu P., Feng L., Jiang W., Liu Y., Kuang S., Li S., Mi H., Tang L. Dietary protein levels changed the hardness of muscle by acting on muscle fiber growth and the metabolism of collagen in sub-adult grass carp (Ctenopharyngodon idella) J Anim Sci Biotechnol. 2022;13(1):109. doi: 10.1186/s40104-022-00747-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z., Chen S., Cui G., Yang Y., Zhang E., Wang Q., Lavin M.F., Yeo A.J., Bo C., Zhang Y. Silica nanoparticles induce cardiomyocyte apoptosis via the mitochondrial pathway in rats following intratracheal instillation. Int J Mol Med. 2019;43(3):1229–1240. doi: 10.3892/ijmm.2018.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-López L.A., Martínez-Hernández M.G., Viedma-Rodríguez R., Díaz-Flores M., Baiza-Gutman L.A. High glucose and insulin enhance uPA expression, ROS formation and invasiveness in breast cancer-derived cells. Cell Oncol. 2016;39:365–378. doi: 10.1007/s13402-016-0282-8. [DOI] [PubMed] [Google Scholar]
- Fux L., Ilan N., Sanderson R.D., Vlodavsky I. Heparanase: busy at the cell surface. Trends Biochem Sci. 2009;34(10):511–519. doi: 10.1016/j.tibs.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Pérez E., Ryu D., Lee C., Lee H.J. Ochratoxin A induces oxidative stress in HepG2 Cells by impairing the gene expression of antioxidant enzymes. Toxins. 2021;13(4):271. doi: 10.3390/toxins13040271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramling M.W., Beaulieu L.M., Church F.C. Activated protein C enhances cell motility of endothelial cells and MDA-MB-231 breast cancer cells by intracellular signal transduction. Exp Cell Res. 2010;316(3):314–328. doi: 10.1016/j.yexcr.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X.-N., Zeng Z.-Z., Feng L., Wu P., Jiang W.-D., Liu Y., Zhang L., Mi H.-F., Kuang S.-Y., Tang L. Aflatoxin B1 damaged structural barrier through Keap1a/Nrf2/MLCK signaling pathways and immune barrier through NF-κB/TOR signaling pathways in gill of grass carp (Ctenopharyngodon idella) Aquat Toxicol. 2023 doi: 10.1016/j.aquatox.2023.106424. [DOI] [PubMed] [Google Scholar]
- He X., Sun J., Huang X. Expression of caspase-3, Bax and Bcl-2 in hippocampus of rats with diabetes and subarachnoid hemorrhage. Exp Ther Med. 2018;15(1):873–877. doi: 10.3892/etm.2017.5438. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- He X.N., Wu P., Jiang W.D., Liu Y., Kuang S.Y., Tang L., Ren H.M., Li H., Feng L., Zhou X.Q. Aflatoxin B1 exposure induced developmental toxicity and inhibited muscle development in zebrafish embryos and larvae. Sci Total Environ. 2023;878 doi: 10.1016/j.scitotenv.2023.163170. [DOI] [PubMed] [Google Scholar]
- He X.N., Zeng Z.Z., Wu P., Jiang W.D., Liu Y., Jiang J., Kuang S.Y., Tang L., Feng L., Zhou X.Q. Dietary Aflatoxin B1 attenuates immune function of immune organs in grass carp (Ctenopharyngodon idella) by modulating NF-κB and the TOR signaling pathway. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.1027064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Sun L., Wang Y., Deng Q., Fang Z., Zhao L., Zhao J. Protective mechanism of tea polyphenols against muscle quality deterioration of shrimp (Penaeus vannamei) induced by aflatoxin B1. Aquaculture. 2021;532 [Google Scholar]
- Jiang H., Chen F., Song D., Zhou X., Ren L., Zeng M. Dynamin-related protein 1 is involved in mitochondrial damage, defective mitophagy, and NLRP3 inflammasome activation induced by MSU crystals. Oxid Med Cell Longev. 2022;2022 doi: 10.1155/2022/5064494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Wu P., Tang R.J., Liu Y., Kuang S.Y., Jiang J., Tang L., Tang W.N., Zhang Y.A., Zhou X.Q. Nutritive values, flavor amino acids, healthcare fatty acids and flesh quality improved by manganese referring to up-regulating the antioxidant capacity and signaling molecules TOR and Nrf2 in the muscle of fish. Food Res Int. 2016;89:670–678. doi: 10.1016/j.foodres.2016.09.020. [DOI] [PubMed] [Google Scholar]
- Kudo Y., Sugimoto M., Arias E., Kasashima H., Cordes T., Linares J.F., Duran A., Nakanishi Y., Nakanishi N., L'Hermitte A. PKCλ/ι loss induces autophagy, oxidative phosphorylation, and NRF2 to promote liver cancer progression. Cancer Cell. 2020;38(2):247–262. e211. doi: 10.1016/j.ccell.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Uematsu H., Tsuchida N., Ikeda M.-A. Essential role of caspase-8 in p53/p73-dependent apoptosis induced by etoposide in head and neck carcinoma cells. Mol Cancer. 2011;10(1):1–13. doi: 10.1186/1476-4598-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Gao Y., Long X., Hayashi T., Mizuno K., Hattori S., Fujisaki H., Ogura T., Wang D.O., Ikejima T. Type I collagen promotes the migration and myogenic differentiation of C2C12 myoblasts via the release of interleukin-6 mediated by FAK/NF-κB p65 activation. Food Funct. 2020;11(1):328–338. doi: 10.1039/c9fo01346f. [DOI] [PubMed] [Google Scholar]
- López-Bergami P., Habelhah H., Bhoumik A., Zhang W., Wang L.-H., Ronai Ze. Receptor for RACK1 mediates activation of JNK by protein kinase C. Mol Cell. 2005;19(3):309–320. doi: 10.1016/j.molcel.2005.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macé K., Aguilar F., Wang J., Vautravers P., Gómez-Lechón M., Gonzalez F., Groopman J., Harris C., Pfeifer A. Aflatoxin B1-induced DNA adduct formation and p53 mutations in CYP450-expressing human liver cell lines. Carcinogenesis. 1997;18(7):1291–1297. doi: 10.1093/carcin/18.7.1291. [DOI] [PubMed] [Google Scholar]
- Malhotra D., Portales-Casamar E., Singh A., Srivastava S., Arenillas D., Happel C., Shyr C., Wakabayashi N., Kensler T.W., Wasserman W.W. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res. 2010;38(17):5718–5734. doi: 10.1093/nar/gkq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marijani E., Kigadye E., Okoth S. Occurrence of fungi and mycotoxins in fish feeds and their impact on fish health. Int J Microbiol. 2019;2019 doi: 10.1155/2019/6743065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V., Balakrishnan K., Keating M.J., Wierda W.G., Gandhi V. Expression of executioner procaspases and their activation by a procaspase-activating compound in chronic lymphocytic leukemia cells. Blood. 2015;125(7):1126–1136. doi: 10.1182/blood-2014-01-546796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu M., Yu J., Liu H., Ren Y., Ma C., Bu X., Lan Q. The candidate tumor suppressor gene SLC8A2 inhibits invasion, angiogenesis and growth of glioblastoma. Mol Cells. 2017;40(10):761. doi: 10.14348/molcells.2017.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y.J., Choi J.H., Lee H. Setdb1 is required for myogenic differentiation of C2C12 myoblast cells via maintenance of MyoD expression. Mol Cells. 2015;38(4):362. doi: 10.14348/molcells.2015.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M., Lu Y., Xiong Z., Chen M., Qin Y. The Grass Carp Genomic Visualization Database (GCGVD): an informational platform for genome biology of grass carp. Int J Biol Sci. 2019;15(10):2119. doi: 10.7150/ijbs.32860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tie H.M., Wu P., Jiang W.D., Liu Y., Kuang S.Y., Zeng Y.Y., Jiang J., Tang L., Zhou X.Q., Feng L. Dietary nucleotides supplementation affect the physicochemical properties, amino acid and fatty acid constituents, apoptosis and antioxidant mechanisms in grass carp (Ctenopharyngodon idellus) muscle. Aquaculture. 2019;502:312–325. [Google Scholar]
- Umasuthan N., Bathige S., Revathy K.S., Lee Y., Whang I., Choi C.Y., Park H.-C., Lee J. A manganese superoxide dismutase (MnSOD) from Ruditapes philippinarum: comparative structural-and expressional-analysis with copper/zinc superoxide dismutase (Cu/ZnSOD) and biochemical analysis of its antioxidant activities. Fish Shellfish Immunol. 2012;33(4):753–765. doi: 10.1016/j.fsi.2012.06.024. [DOI] [PubMed] [Google Scholar]
- Wan P., Cai B., Chen H., Chen D., Zhao X., Yuan H., Huang J., Chen X., Luo L., Pan J. Antidiabetic effects of protein hydrolysates from Trachinotus ovatus and identification and screening of peptides with α-amylase and DPP-IV inhibitory activities. Curr Res Food Sci. 2023 doi: 10.1016/j.crfs.2023.100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S.P., Jiang W.D., Wu P., Liu Y., Zeng Y.Y., Jiang J., Kuang S.Y., Tang L., Zhang Y.A., Zhou X.Q. Dietary magnesium deficiency impaired intestinal structural integrity in grass carp (Ctenopharyngodon idella) Sci Rep. 2018;8(1) doi: 10.1038/s41598-018-30485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen M.-L., Wu P., Jiang W.-D., Liu Y., Wu C.-M., Zhong C.-B., Li S.-W., Tang L., Feng L., Zhou X.-Q. Dietary threonine improves muscle nutritional value and muscle hardness associated with collagen synthesis in grass carp (Ctenopharyngodon idella) Food Chem. 2023;422 doi: 10.1016/j.foodchem.2023.136223. [DOI] [PubMed] [Google Scholar]
- Wu P., Zhang L., Jiang W., Liu Y., Jiang J., Kuang S., Li S., Tang L., Tang W., Zhou X. Dietary vitamin A improved the flesh quality of grass carp (Ctenopharyngodon idella) in relation to the enhanced antioxidant capacity through Nrf2/Keap 1a signaling pathway. Antioxidants. 2022;11(1):148. doi: 10.3390/antiox11010148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Zhang X., Song Y., Zheng B., Wen Z., Gong M., Meng L. Heat-killed Lacticaseibacillus paracasei ameliorated UVB-induced oxidative damage and photoaging and its underlying mechanisms. Antioxidants. 2022;11(10):1875. doi: 10.3390/antiox11101875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S., Mao B., Yang L., Fu W., Hou J. Thrombosis recanalization by paeoniflorin through the upregulation of urokinase-type plasminogen activator via the MAPK signaling pathway. Mol Med Rep. 2016;13(6):4593–4598. doi: 10.3892/mmr.2016.5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama I., Ohata M., Komiya Y., Nagasao J., Arihara K. Inhalation of odors containing DMHF generated by the Maillard reaction affects physiological parameters in rats. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-70843-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z.Z., Jiang W.D., Wu P., Liu Y., Zeng Y.Y., Jiang J., Kuang S.Y., Tang L., Zhou X.Q., Feng L. Dietary aflatoxin B1 decreases growth performance and damages the structural integrity of immune organs in juvenile grass carp (Ctenopharyngodon idella) Aquaculture. 2019;500:1–17. [Google Scholar]
- Zhang W., Liu Y., Zhang H. Extracellular matrix: an important regulator of cell functions and skeletal muscle development. Cell Biosci. 2021;11:1–13. doi: 10.1186/s13578-021-00579-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.J., Zhou T., Wang F., Zhou Y., Li Y., Zhang J.J., Zheng J., Xu D.P., Li H.B. The effects of Syzygium samarangense, Passiflora edulis and Solanum muricatum on alcohol-induced liver injury. Int J Mol Sci. 2016;17(10):1616. doi: 10.3390/ijms17101616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Li J.Y., Jiang Q., Zhou X.Q., Feng L., Liu Y., Jiang W.D., Wu P., Zhou J., Zhao J. Leucine improved growth performance, muscle growth, and muscle protein deposition through AKT/TOR and AKT/FOXO3a signaling pathways in hybrid catfish Pelteobagrus vachelli× Leiocassis longirostris. Cells. 2020;9(2):327. doi: 10.3390/cells9020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zi J., Xu J., Luo J., Yang X., Zhen Z., Li X., Hu D., Guo Y., Guo H., Ding X. PFN1 inhibits myogenesis of bovine myoblast cells via Cdc42-PAK/JNK. Cells. 2022;11:3188. doi: 10.3390/cells11203188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zychowski K.E., Pohlenz C., Mays T., Romoser A., Hume M., Buentello A., Gatlin I.I.I.D.M., Phillips T.D. The effect of NovaSil dietary supplementation on the growth and health performance of Nile tilapia (Oreochromis niloticus) fed aflatoxin-B1 contaminated feed. Aquaculture. 2013;376:117–123. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.