Abstract
Cancer immunotherapy has rapidly become the fourth mainstream treatment alternative after surgery, radiotherapy, and chemotherapy, with some promising results. It aims to kill tumor cells by mobilizing or stimulating cytotoxic immune cells. However, the clinical applications of tumor immunotherapies are limited owing to a lack of adequate delivery pathways and high toxicity. Recently, nanomaterials and genetic engineering have shown great potential in overcoming these limitations by protecting the delivery of antigens, activating targeted T cells, modulating the immunosuppressive tumor microenvironment, and improving the treatment efficacy. Bacillus Calmette-Guérin (BCG) is a live attenuated Mycobacterium bovis vaccine used to prevent tuberculosis, which was first reported to have antitumor activity in 1927. BCG therapy can activate the immune system by inducing various cytokines and chemokines, and its specific immune and inflammatory responses exert antitumor effects. BCG was first used during the 1970s as an intravesical treatment agent for bladder cancer, which effectively improved immune antitumor activity and prevented tumor recurrence. More recently, nano-BCG and genetically engineered BCG have been proposed as treatment alternatives for bladder cancer due to their ability to induce stronger and more stable immune responses. In this study, we outline the development of nano-BCG and genetically engineered BCG for bladder cancer immunotherapy and review their potential and associated challenges.
Keywords: Bladder cancer, Bacillus Calmette-Guérin vaccine, Nanocarrier, Genetic engineering, Immunotherapy
Abstract
癌症免疫治疗已成为继手术、放疗和化疗之后的第四大主流治疗选择,并取得了令人鼓舞的成果。肿瘤免疫治疗通过调动或激发机体自身的免疫功能,从而抑制和杀伤肿瘤细胞。然而,肿瘤免疫治疗作为一种新兴的治疗手段,由于缺乏有效的免疫细胞传递途径以及具有较高的毒副作用,在临床上的应用受到限制。近年来,纳米材料和基因工程在保护抗原递送、激活靶向T细胞、调节免疫抑制的肿瘤微环境和提高治疗效果等方面显示出巨大的潜力。卡介苗是一种用于预防结核病的减毒牛分枝杆菌活疫苗,于1927年首次报道其抗肿瘤活性。卡介苗可通过诱导多种细胞因子和趋化因子激活免疫系统,其特异性免疫和炎症反应可发挥抗肿瘤作用。20世纪70年代,卡介苗首次作为治疗膀胱癌的膀胱灌注药物,有效地提高了免疫抗肿瘤活性,防止肿瘤复发。最近,纳米卡介苗和基因工程卡介苗因其能诱导更强且更稳定的免疫反应,被提出作为膀胱癌的治疗方案。在本研究中,我们概述了纳米卡介苗和基因工程卡介苗用于膀胱癌免疫治疗的发展,并回顾了它们的潜力和挑战。
Keywords: 膀胱癌, 卡介苗, 纳米载体, 基因工程, 免疫治疗
1. Introduction
Bladder cancer (BCa) is the tenth most common cancer in humans (Siegel et al., 2021). Its global age-standardized incidence (per 100 000 person-years) is 9.5, and it is more frequent in people aged 50–70 years (Siegel et al., 2021). BCa is 3–4 times more common in men than in women. A common subtype, non-muscular invasive BCa (NMIBC), is typically treated with transurethral resection of the bladder tumor (TURBT) and intravesical therapy (Babjuk et al., 2022).
Bacillus Calmette-Guérin (BCG) can be used for the prevention of pulmonary tuberculosis as a type of immunotherapy derived from bacteria, which can generate a specific immune response, guide inflammatory reactions in the body, and exert anti-tumor effects. In the 1970s, BCG was first used as an immunotherapeutic agent for BCa, and intravesical instillation with BCG has since become the most common treatment for NMIBC to prevent recurrence or progression of the disease. Clinical evidence indicates that BCG is more effective than TURBT alone or TURBT plus chemotherapeutic agents in reducing the risk of recurrence of moderate-to-high-risk NMIBC (Chou et al., 2017; Larsen et al., 2020; Álvarez-Maestro et al., 2021; Bhindi et al., 2021). The mechanism of action of BCG in BCa remains unclear, although it is generally believed that BCG acts as an immune adjuvant inducing the response of multiple immune cell types to activate a complex inflammatory cascade in urothelial cells, thereby causing a specific cytotoxic immune response (Brandau and Suttmann, 2007; Redelman-Sidi et al., 2014).
Despite its effectiveness in the treatment of NMIBC, BCG-induced immunotherapy can cause worse local and systemic side effects, such as systemic infections, sepsis, and even death, compared to intravesical instillation chemotherapy (Lamm et al., 1992; Kawai et al., 2013). To improve the antitumor effects of BCG therapy and mitigate its side effects, ameliorative methods include genetically engineered BCG and Mycobacterium cell wall (CW) as a substitute for BCG (Tham et al., 2020; Kim and Seo, 2021). In addition, nanomaterials can effectively deliver various antigens, with the advantages of targeting and controllable release. Thus, they have attracted increasing attention in the field of tumor immunotherapy (Szoka, 2008; Zhang et al., 2008; Park et al., 2009; Wang et al., 2009; Gadiot et al., 2011).
2. History and mechanisms of action of BCG vaccine in BCa
BCG is a live vaccine prepared from a suspension of attenuated bovine Mycobacterium tuberculosis, which was first used for tuberculosis prevention after observing the transmission of the strongly virulent bovine mycobacterium over 13 years and 230 generations. In the 1950s and 1960s, BCG application was demonstrated to have therapeutic effects in leukemia, colon, liver, and lung cancers, as well as melanoma. Morales (1978) further reported promising results for the treatment of BCa with BCG. Lamm et al. (1980) conducted a rigorously controlled study on the therapeutic use of BCG in BCa.
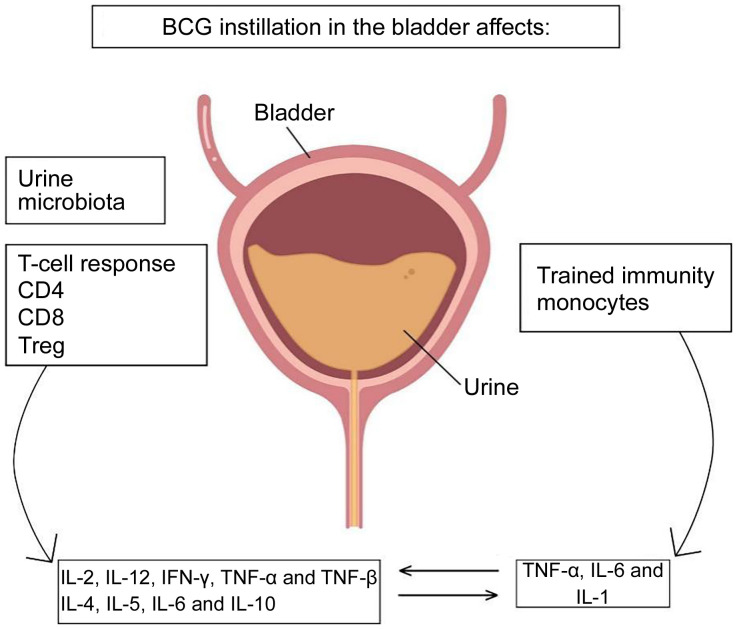
Following extensive experimentation and clinical evidence, it was indicated that intravesical infusion of BCG can prevent postoperative tumor recurrence and progression, treat carcinoma in situ, improve survival rates, and prolong survival time in tumors (Patard et al., 2002). However, the exact mechanism of action of BCG in the treatment of superficial BCa has yet to be investigated. The antitumor effects of BCG involve local immune mechanisms that depend on the integrity of the immune system. BCG is first engulfed by tumor cells, bladder mucosal epithelial cells, and macrophages, and then it induces many immune cells, such as cluster of differentiation 4 (CD4), CD8, and macrophages, to infiltrate tumor and bladder mucosal tissues with strong antigenic signals. The infiltrated immune cells mediate the release of a series of immune factors and prompt T cells and natural killer (NK) cells to kill tumor cells either directly or indirectly (Böhle and Brandau, 2003; Luo et al., 2003) (Fig. 1).
Fig. 1. Mechanism of action of Bacillus Calmette-Guérin (BCG) vaccine for bladder cancer. Reproduced from Larsen et al. (2020) by permission of John Wiley & Sons Ltd., Copyright 2019 APMIS. CD: cluster of differentiation; Treg: regulatory T cells; IL: interleukin; IFN: interferon; TNF: tumor necrosis factor.
3. Nanocarrier-mediated immunotherapy
Owing to the intermittent nature of urinary excretion, the short retention time of instillation drugs in the bladder can attenuate the therapeutic effects. The effect of intravesical instillation therapy is proportional to the drug concentration and independent of the drug dose (Tyagi et al., 2004). The exposure time of BCG in the urinary epithelium rarely lasts beyond the first urination after instillation; thus, the frequency and dose of instillation during treatment must be increased (Buss et al., 2018; Masuda et al., 2018). However, the frequency of bladder intravesical instillation is also positively correlated with certain adverse events, including tuberculous cystitis and hematuria, which can negatively affect patients and cause the termination of BCG treatment (Burger et al., 2013). Challenges facing BCG intravesical instillation therapy thus include how to effectively increase retention time in bladder tumor tissues and to reduce the frequency of instillation while alleviating adverse events associated with this technique.
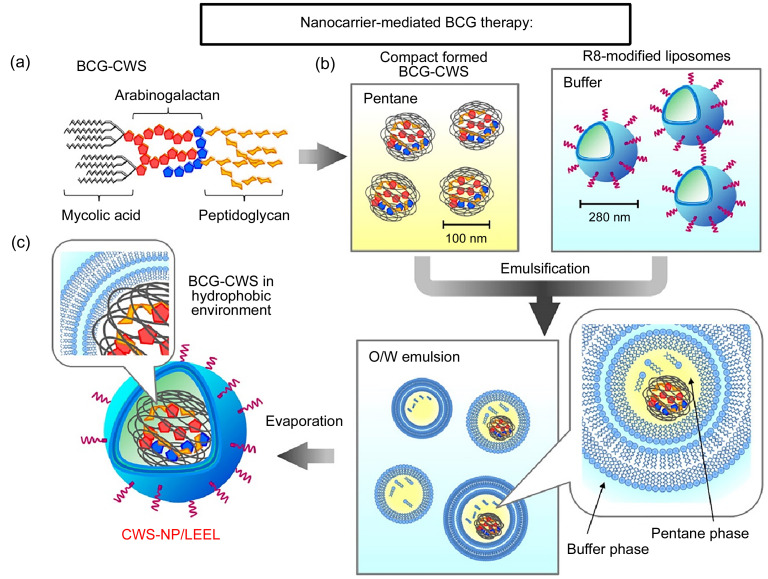
The use of nanotechnology in therapeutics is an emerging field. Nanomedicine carrier constructs currently include magnetic nanospheres, microemulsions, and polymeric nanoparticles. The current research results (Fig. 2) show that nanomedicine carriers can prolong drug release time, enable the simultaneous loading of multiple drugs for combination therapy, reduce the systemic side effects, and improve bioavailability. Due to the high mutation rate of BCa cells and the overexpression of tumor antigens on their surface, BCG therapy is a prime candidate to improve BCa-targeting treatments using nanotechnology delivery strategies.
Fig. 2. Nanocarrier-mediated immunotherapies. (a) Schematic illustration of the clinical application of Fe3O4-BCG-CS/GP delivery system. (b) Schematic illustration of the clinical application of CWS-NP/LEEL delivery system. (c) Schema of liposomes evaporated via the emulsified lipid method. Reprinted from Nakamura et al. (2014), Copyright 2013, with permission from Elsevier. BCG: Bacillus Calmette-Guérin; CS: chitosan; GP: glycerophosphate; CWS: cell wall skeleton; NP: nanoparticle; LEEL: liposome evaporated via emulsified lipid; R8: octaarginine; O/W: oil/water.
3.1. Application of BCG-chitosan (CS) in BCa treatment
Intravesical therapy is a form of mucosal drug delivery that is helpful in BCa treatment, since the bladder wall is covered by tightly connected uroepithelial cells, making it difficult for conventional drug molecules to penetrate the tumor tissue through the bladder wall. CS, an adherent polymer with a positive surface charge, has a powerful pro-permeation effect, which helps drugs to penetrate the urinary epithelium and increases the extent of drug diffusion into the bladder wall. In addition, CS can help build immunity when applied in intravesical instillation therapy (Zaharoff et al., 2009). Erdoğar et al. (2015) explored the antitumor effect of CS nanomaterials loaded with BCG in a rat bladder tumor model. The BCG-CS group of tumor-bearing rats had significantly longer survival than the BCG-alone group, and the histopathological results confirmed BCG antitumor activity in all treatment groups. Moreover, there was a significant accumulation of nanoparticles in the bladder tissue of rats in the BCG-CS group, which predicted that BCG would likely elicit a potent immune response. CS noticeably contributed to the internalization of BCG, and BCG-CS offered significant improvement in bladder tumor immunotherapy response (Erdoğar et al., 2015).
Temperature-sensitive hydrogels composed of CS and glycerophosphate (GP) possibly have great potential for drug delivery and cell encapsulation (Shi et al., 2011; Niranjan et al., 2013; Wang et al., 2013). Aqueous solutions of CS/GP form free-flowing media at room temperature and a viscous hydrogel at body temperature, which can act as a slow-release reservoir for drug agents in situ (Gong et al., 2013; Niranjan et al., 2013). The advantages of this kind of drug delivery system have been demonstrated using anti-inflammatory drugs in a rat model of interstitial cystitis (Tyagi et al., 2004). In another study, Leakakos et al. (2003) applied magnetic field outside the bladder to aid in loading a hydrogel of CS/GP with nontoxic ferromagnetic microparticles to be introduced into the bladder of pigs, in order to achieve long retention and targeting of adriamycin.
Fe3O4 magnetic nanoparticles, a kind of iron oxide with superparamagnetic properties, prevent the thermosensitive hydrogel from being washed away during urination and ensure attachment to the bladder wall (Liu et al., 2021). Based on these exciting observations, Zhang et al. (2013) proposed a temperature-sensitive hydrogel composed of CS and GP combined with Fe3O4 magnetic nanoparticles to form an in situ gel system (Fe3O4-CS/GP). Therein, the CS/GP ambient flow solution can rapidly agglomerate at body temperature, and the magnetic injectable hydrogel can significantly prolong the retention time of the loaded drug in the bladder when a magnetic field is applied. Later, Zhang et al. (2013) added BCG to this system (Fe3O4-BCG-CS/GP) to test its antitumor and local immunostimulatory activity in an in situ BCa rat model. They found that Fe3O4-BCG-CS/GP was superior to BCG alone in reducing tumor volume, and immunohistochemical staining showed that Fe3O4-BCG-CS/GP induced further CD4+ lymphocyte infiltration into the submucosal layer of the rat bladder. In addition, urine cytokine analysis was performed to show that both therapies caused the production of helper T cell 1 (Th1) cytokines (interleukin-2 (IL-2) and interferon-γ (IFN-γ)), while Fe3O4-BCG-CS/GP generated higher levels of these cytokines as compared with BCG alone. In this way, the hydrogel slow-release system allowed for the sustained release of BCG in the bladder, improving its antitumor effects and inducing higher levels of localized immune activity in the bladder (Zhang et al., 2013).
3.2. Application of BCG-cell wall skeleton (CWS) in BCa treatment
Although intravesical BCG infusion is an effective treatment option for NMIBC, it can cause serious side effects, such as systemic BCG infection, sepsis, and even death (Pérez-Jacoiste Asín et al., 2014). These side effects may lead to the termination of BCG therapy for approximately 20% of patients; therefore, it is essential to develop non-infectious, low-toxicity BCG immunotherapies. The BCG-CWS is the primary center of activity for BCG (Azuma and Seya, 2001) where cytotoxic or suppressive T cells are induced against tumors. However, it has not been widely utilized in clinics for the following reasons: (1) BCG-CWS easily aggregates, and thus, it is challenging to develop a suitable water-soluble drug; (2) the efficiency of BCG-CWS uptake by cancer cells is extremely low; and (3) when BCG-CWS is applied in animal and human studies, detergents containing oil/water (O/W) emulsions are typically used to forcibly disperse the BCG-CWS, while emulsified BCG-CWS can cause strong inflammatory reactions (Ochiai et al., 1983). Therefore, it is extremely difficult to apply BCG-CWS O/W emulsions in BCa treatment.
In order to overcome the challenges of BCG-CWS O/W therapies, Nakamura et al. (2014) prepared oil- and detergent-free nanoparticles (CWS-NP/LEEL) using the liposome evaporated via emulsified lipid (LEEL) method and observed that CWS-NP/LEEL was effectively taken up by mouse bladder tumor cells (MBT-2) in vitro. This effect effectively inhibited tumor growth in tumor-bearing mice, the median survival time of the mice was significantly improved, and adverse effects on the bladder environment were negligible (Nakamura et al., 2014; Masuda et al., 2018). Nakamura et al. (2014) further obtained naive CD4 T cells from four healthy human volunteers and verified that CWS-NP/LEEL could guide the Th1/Th2 balance toward Th1 immunity. These nanoparticles bear potential as a non-toxic therapeutic alternative to live BCG, that is, a non-infectious agent with no side effects. The clinical application of BCG-CWS may improve the quality of life of patients and benefit approximately 80% of those with BCa.
The phenomena illustrated above have been extensively studied (Matsumoto et al., 2001; Akazawa et al., 2004). The structure of liposomes is similar to that of cell membranes, with high biocompatibility, biodegradability, and modifiability. Structural modification with ligands or other functional groups can make liposomes tissue-specific, rendering them an ideal drug carrier. For example, octaarginine-modified cationized liposome nanoparticle containing BCG-CW (R8-liposome-BCG-CW) has been shown to improve the immunotherapeutic activity of BCG-CW against NMIBC through cellular internalization (Joraku et al., 2009). Using the n-butyl-N-(4-hydroxybutyl) nitrosamine (BBN)-induced BCa rat model, R8-liposome-BCG-CW showed significant cytotoxic effects against tumors, validating the inhibitory effects of R8-liposome-BCG-CW on BCa in vivo (Miyazaki et al., 2011b). Although the relevant mechanism of action is unclear, in a previous study, R2-liposome-BCG-CWS induced surface natural killer group 2, member D (NKG2D, a powerful activating receptor expressed by NK cells) ligands, making cancer cells more susceptible to lymphokine-activated killer cell lysis (Miyazaki et al., 2011a). R8 liposomes are similar to enveloped viruses, with their surface modified by anchored R8. R8-liposome-BCG-CW makes cancer cells more susceptible to lysis by lymphokine-activated killer cells (Miyazaki et al., 2011a). These findings demonstrate the potential advantages of nanotechnology in optimizing and developing novel BCG immunotherapy approaches.
Compared with conventional BCG to treat superficial bladder tumors, BCG loaded in nanocarrier systems can remain in the urinary epithelium for a longer period, overcoming the disadvantages of short retention time and low internalization of the drug in the bladder due to urination. Through the targeted transport of nanocarriers, BCG can be better internalized by bladder tumor cells, induce a stronger Th1 immune response, and exert high levels of antitumor activity. Indeed, nanotechnologies have demonstrated satisfactory results in BCG intravesical instillation therapy, and we believe that nano-BCG will provide significant advantages for improved BCa treatments.
4. Application of recombinant BCG (rBCG) in BCa
Recently, research on recombinant vaccines using BCG vaccine as a carrier has intensified, including that on BCG strains that express exogenous genes with immunomodulatory effects as well as BCG subgroups with the same immunogenic properties as live BCG strains. rBCG strains, which induce a more specific immune response by overexpressing immunogenic molecules or exogenous antigens, are a promising route to improving the effectiveness of BCa treatment. Evidence indicates that granting BCG the ability to secrete Th1-stimulating cytokines is a promising strategy (Kleinnijenhuis et al., 2014).
4.1. Application of rBCG-fibrin D-mannose specific adhesin (FimH) in BCa treatment
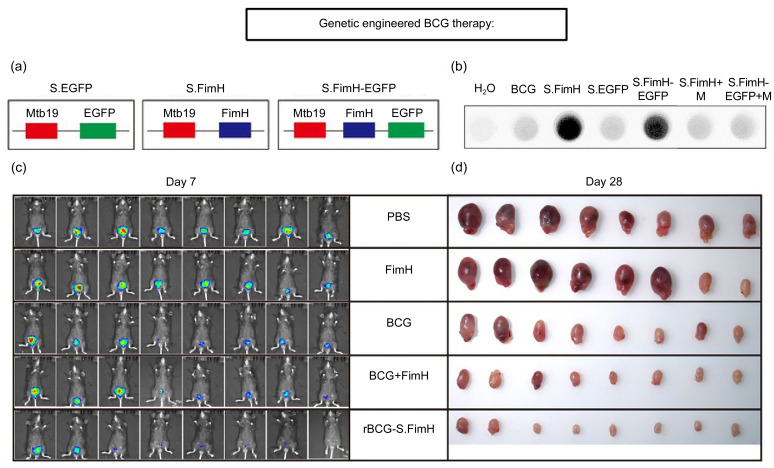
The attachment of BCG to the bladder uroepithelium is the initiating step of its antitumor effects, and low attachment may be a reason for treatment failure in some patients with BCa (Fleischmann et al., 1993; Bevers et al., 2004). Therefore, improving the attachment of BCG to the uroepithelium may boost its antitumor effects. Interestingly, urinary tract infections caused by Escherichia coli depend on the binding of bacterial adhesin protein FimH to mannose residues on the surface of urinary tract epithelial cells. The high binding affinity of FimH to mannose allows this pathogen to adhere to urinary tract epithelial cells (Yamamoto et al., 1997; Wurpel et al., 2013). FimH is a naturally occurring adhesion protein that can be used as an adjuvant for tumor immunotherapy (Zhang et al., 2020). This is possible via upregulating the expression of co-stimulatory molecules and major histocompatibility complex class II (MHC-II) to effectively promote dendritic cell (DC) activation and stimulating IL-12 production through Toll-like receptor 4 (TLR4)-dependent signaling pathways. FimH can also accelerate Th1-type immune activity by promoting DC antigen presentation and Th1 cell initiation (Lai et al., 2014). Zhang et al. (2022) successfully overexpressed this protein on the surface of BCG, resulting in rBCG-urinary epithelial cell adhesion, faster internalization of rBCG-surface FimH (rBCG-S.FimH) by uroepithelial cells, and longer retention time in the bladder uroepithelium (Fig. 3). Additionally, rBCG-S.FimH was shown to enhance the antigen-presenting ability of DCs via the TLR4-myeloid differentiation factor 88 (MyD88)-parallel Jun N-terminal kinase (pJNK)- nuclear factor-κB (NF-κB) axis in a mouse bladder tumor model and peripheral blood mononuclear cell (PBMC)-treated human BCa cell line, further confirming its adjuvant effect. Thus, BCG itself is considered as an immune adjuvant whose function is further enhanced by recombinant FimH.
Fig. 3. Construction, function, and enhanced antitumor effect of rBCG-S.FimH. (a) Schematic representation for the construction of plasmids expressing EGFP, FimH, or FimH-EGFP fused to the membrane protein Mbt19. (b) Only rBCGs expressing FimH can bind to mannose residues in the HRP protein. (c) IVIS images of mice from different treatment groups on Day 7. (d) Images of the bladders at the end of the experiment. Reprinted from Zhang et al. (2022). BCG: Bacillus Calmette-Guérin; rBCG: recombinant BCG; S: surface; FimH: fibrin D-mannose specific adhesion; EGFP: enhanced green fluorescent protein; HRP: horseradish peroxidase; IVIS: in-vehicle information system; M: 100 µmol/L D-mannose; PBS: phosphate-buffered saline.
4.2. Exploration of rBCG-streptococcal inhibitor of complement (SIC) treatment alternatives for BCa
Upon BCG acting on the urinary system to elicit a nonspecific immune response, uroepithelial cells secrete antimicrobial peptides (AMPs) to activate the host immune defense system to kill BCG. Other cell types avoid AMPs by secreting alternative specific proteins, such as SIC and D-alanyl carrier protein ligase (dltA) (Kovács et al., 2006; McBride and Sonenshein, 2011). Choi et al. (2015) previously speculated that the poor efficacy of BCG treatment in patients with BCa could be due to the killing of BCG by AMPs, resulting in reduced BCG internalization. Kim and Seo (2021) subsequently developed a BCG-expressing SIC and investigated its ability to effectively avoid AMPs and elicit specific immunity. They found that rBCG-SIC was more resistant to AMPs, while THP-1 cell migration was positively correlated with rBCG-SIC concentration. Furthermore, rBCG-SIC infection of BCa cells resulted in significantly higher IL-6 levels and significantly lower T24 cell survival compared with BCG infection. In an in situ BCa mouse model, the tumor volume in the rBCG-SIC group was further confirmed to be significantly smaller compared with that in the BCG group after 10 d of treatment (Kim et al., 2022).
rBCG-SIC may be an effective tool to overcome NMIBC after a lack of response to BCG therapy (Kim et al., 2022). Cho et al. (2019) cloned SIC and dltA into pMV306 and introduced them into BCG by electroporation to sensitize it to human α defensin-1 (HαD1), a histone protease inhibitor (cathelicidin), and cationic AMP. Compared with BCG, rBCG showed significantly enhanced inhibition of BCa cell growth and increased THP-1 migration. After 8 h of infection, BCa cells internalized higher levels of rBCG than BCG cells. Tumor cells infected with rBCG also had an increased ability to release antitumor cytokines, such as IL-6/12, tumor necrosis factor-α (TNF-α), and INF-γ, effectively evading bacterial destruction by AMP. Thus, with the addition of specific secreted proteins, rBCG could be an effective tool for the treatment of BCa via inhibiting AMPs, with a potent immunomodulatory effect (Cho et al., 2019).
4.3. Investigations on BCG and cytokine mixture for application in BCa treatment
Clinical experiments have previously demonstrated that mixtures of BCG and cytokines can be used to treat tumors with varying efficacy (Nave et al., 2019). Therefore, cytokine-secretable rBCG is expected to provide opportunities to improve the use and efficacy of immunotherapy in BCa. Yamada et al. (2000) ligated the gene encoding murine IL-2 with the gene encoding the M. tuberculosis antigen. The plasmid pS0246 was used as a vector to introduce BCG, and the results showed that IL-2 secreted by rBCG was biologically active and presented substantially more cytotoxicity against MBT-2 than normal BCG. Besides, although the addition of exogenous IL-2 alone could enhance BCG-mediated cytotoxicity, IL-2 expressed by rBCG was up to 100 times more potent. In another study, Luo et al. (2004) introduced the gene encoding mouse IL-18 into BCG and constructed an rBCG that automatically expressed IL-18. The results showed that rBCG significantly enhanced the immune activity of Th1 cells and the cytotoxicity of macrophages against MBT-2 BCa cells. These data suggest that using rBCG intravesical instillation could provide enhanced immunostimulatory effects and reduce the required dose, thereby improving efficacy and reducing the toxic side effects of BCG.
Recently, the stimulator of interferon genes (STING) has received significant attention from researchers of cancer immunotherapy owing to its promotion of type I IFN and NF-κB-mediated antitumor responses (Medrano et al., 2017; Wu et al., 2020). STING has been found to play a pivotal role in the immune responses triggered by viral, bacterial, and parasitic infections, antitumor immune processes, and cellular autophagy through its phosphorylation and ubiquitination (Ma and Damania, 2016; Galon and Bruni, 2019). Singh et al. (2021) claimed that a BCG strain overexpressing the DNA integrity scanning protein A (disA) (BCG-disA-OE) showed excellent anticancer effects in a BCa model. In addition, BCG overexpression of the STING agonist induced a more robust pro-inflammatory cytokine response, greater myeloid polarization and M1 translocation, and enhanced phagocytosis in macrophages and BCa cells as compared with wild-type BCG. BCG-disA-OE was also less pathogenic than BCG in two BCa-loaded mouse models, suggesting its improved safety (Singh et al., 2021). These findings evidence the potential for BCG-disA-OE to induce local and remodel natural immune responses and ultimately enhance T cell immunity.
IL-15 is a pro-inflammatory cytokine with an important role in the treatment of tumors. Accordingly, it has undergone prior investigation as an anticancer agent (Tinhofer et al., 2000; Waldmann, 2015). IL-15 is involved not only in NK and memory CD8 T cell maintenance but also in neutrophil activation and migration (Waldmann et al., 2011; Perera et al., 2012). An rBCG expressing IL-15 and the Ag85B fusion protein (BCG-IL-15) was previously constructed by Takeuchi et al. (2016). BCG-IL-15 significantly prolonged the survival time of tumor-bearing mice, which exhibited a significant increase in neutrophil infiltration and in chemokine levels (macrophage inflammatory protein (MIP)-2 and MIP-1α) in their bladder tissues (Takeuchi et al., 2016). IL-15 has also been reported to play an essential role in neutrophil migration during inflammation, triggering a series of MIP-2, MIP-1α, and neutrophil migration signaling cascades (Verri et al., 2007). These observations may explain why BCG-IL-15 has a stronger anti-BCa effect as compared with BCG.
4.4. Preclinical studies on genetically modified BCG
The only BCG therapy currently used in clinical practice involves the live recombinant Mycobacterium bovis strain VPM1002BC. Specifically, the urease C gene is knocked out, and the hly gene of Listeria monocytogenes is introduced into VPM1002BC (DeltaureC hly+rBCG). DeltaureC hly+rBCG is then transferred into the hly gene of L. monocytogenes, which promotes antigen translocation into the cytoplasm and the apoptosis of infected macrophages. The urease C gene is deleted, making the pH value in the virus closer to the environment pH value of hly activity (Nieuwenhuizen et al., 2017). DeltaureC hly+rBCG can stimulate CD4+ and CD8+ T cell responses through DC nucleus macrophage cross-initiation antigen presentation, which provides greater immunogenicity and protection compared with parental BCG and is rapidly cleared from the host, supporting the potential for reduced side effects and lower systemic toxicity (Grode et al., 2005, 2013; Andersen et al., 2007; World Health Organization, 2018). Rentsch et al. (2020) first reported the application of VPM1002BC, its therapeutic effects, and the resulting immunological changes in six patients with NMIBC who failed BCG treatment. The patients’ urine and whole blood were collected and analyzed, and the plasma tumor necrosis factor and Th1 cytokine levels were found to be significantly elevated after treatment. In addition to being stimulated by M. tuberculosis (Mtb)-purified protein derivatives, intracellular granulocyte-macrophage colony-stimulating factor (GM-CSF) and IFN expression levels in blood-derived CD4+ T cells were significantly increased, and all patients successfully completed six intravesical instillation treatment sessions (Rentsch et al., 2020).
Several immunomodulatory genes have been introduced into BCG strains to enhance their ability to elicit immune responses and show their enhanced therapeutic efficacy in preclinical studies. Thus far, few gene-modified BCG strains have been used in clinical stage studies, yet they demonstrated promising value for treatments, with possible benefits to be elucidated in further studies.
5. Conclusions
BCG, the earliest biologic agent for the treatment of cancers, especially superficial BCa and postoperative cancer recurrences, has been remarkably successful; however, BCG administration triggers side effects. This has fueled research efforts toward exploring better BCa treatment methods. One such method involves the extraction of active components from BCG and the use of rBCG to improve its therapeutic effect and alleviate side effects. In this paper, we described the major advances in and applications of nanotechnology and genetic engineering to develop novel BCa therapies, which have improved the ability of BCG to induce immune response, enhanced the efficacy of BCa treatment, and provided new strategies for further clinical application.
Future challenges include the biosafety and biocompatibility concerns of the nanocarriers and their adaptation to their contained drugs. Another major concern is the selection of exogenous antigen genes for mating and introduction into BCG without mutual interference and in optimal combinations. For the delivery of exogenous antigens by BCG, it is uncertain whether optimal immunogenic activity and protection are possible. Nevertheless, nanotechnology and genetic engineering show great potential for the improvement of BCG treatments for BCa, offering hope for patients, such as those with high-risk NMIBC.
Acknowledgments
This work was supported by the Tianjin Natural Science Foundation (Nos. 21JCYBJC00220 and 22JCQNJC01640), the Tianjin Health Science and Technology Project (No. ZC20162), the Tianjin Key Medical Discipline (Specialty) Construction Project, and the National Natural Science Foundation of China (No. 82202323).
Author contributions
Sheng ZENG and Haifeng WANG wrote the manuscript. Shaoqiang XING and Yifei ZHANG collected references. Qian LIU supervised the whole work. All the authors have read and approved this manuscript.
Compliance with ethics guidelines
Sheng ZENG, Shaoqiang XING, Yifei ZhANG, Haifeng WANG, and Qian LIU declare that they have no conflict of interest.
This review does not contain any studies with human or animal subjects performed by any of the authors.
References
- Akazawa T, Masuda H, Saeki Y, et al. , 2004. Adjuvant-mediated tumor regression and tumor-specific cytotoxic response are impaired in MyD88-deficient mice. Cancer Res, 64(2): 757-764. 10.1158/0008-5472.can-03-1518 [DOI] [PubMed] [Google Scholar]
- Álvarez-Maestro M, Guerrero-Ramos F, Rodríguez-Faba O, et al. , 2021. Current treatments for BCG failure in non-muscle invasive bladder cancer (NMIBC). Actas Urol Esp, 45(2): 93-102. 10.1016/j.acuro.2020.08.003 [DOI] [PubMed] [Google Scholar]
- Andersen CS, Dietrich J, Agger EM, et al. , 2007. The combined CTA1-DD/ISCOMs vector is an effective intranasal adjuvant for boosting prior Mycobacterium bovis BCG immunity to Mycobacterium tuberculosis . Infect Immun, 75(1): 408-416. 10.1128/iai.01290-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma I, Seya T, 2001. Development of immunoadjuvants for immunotherapy of cancer. Int Immunopharmacol, 1(7): 1249-1259. 10.1016/s1567-5769(01)00055-8 [DOI] [PubMed] [Google Scholar]
- Babjuk M, Burger M, Capoun O, et al. , 2022. European Association of Urology guidelines on non-muscle-invasive bladder cancer (Ta, T1, and carcinoma in situ). Eur Urol, 81(1): 75-94. 10.1016/j.eururo.2021.08.010 [DOI] [PubMed] [Google Scholar]
- Bevers RFM, Kurth KH, Schamhart DHJ, 2004. Role of urothelial cells in BCG immunotherapy for superficial bladder cancer. Br J Cancer, 91(4): 607-612. 10.1038/sj.bjc.6602026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhindi B, Kool R, Kulkarni GS, et al. , 2021. Canadian Urological Association guideline on the management of non-muscle-invasive bladder cancer – Abridged version. Can Urol Assoc J, 15(8): 230-239. 10.5489/cuaj.7487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhle A, Brandau S, 2003. Immune mechanisms in bacillus Calmette-Guerin immunotherapy for superficial bladder cancer. J Urol, 170(3): 964-969. 10.1097/01.ju.0000073852.24341.4a [DOI] [PubMed] [Google Scholar]
- Brandau S, Suttmann H, 2007. Thirty years of BCG immunotherapy for non-muscle invasive bladder cancer: a success story with room for improvement. Biomed Pharmacother, 61(6): 299-305. 10.1016/j.biopha.2007.05.004 [DOI] [PubMed] [Google Scholar]
- Burger M, Catto JWF, Dalbagni G, et al. , 2013. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol, 63(2): 234-241. 10.1016/j.eururo.2012.07.033 [DOI] [PubMed] [Google Scholar]
- Buss JH, Begnini KR, Bender CB, et al. , 2018. Nano-BCG: a promising delivery system for treatment of human bladder cancer. Front Pharmacol, 8: 977. 10.3389/fphar.2017.00977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho MJ, Kim MJ, Kim K, et al. , 2019. The immunotherapeutic effects of recombinant Bacillus Calmette-Guérin resistant to antimicrobial peptides on bladder cancer cells. Biochem Biophys Res Commun, 509(1): 167-174. 10.1016/j.bbrc.2018.12.097 [DOI] [PubMed] [Google Scholar]
- Choi SY, Kim SJ, Chi BH, et al. , 2015. Modulating the internalization of bacille Calmette-Guérin by cathelicidin in bladder cancer cells. Urology, 85(4): 964.e7-964.e12. 10.1016/j.urology.2014.12.028 [DOI] [PubMed] [Google Scholar]
- Chou R, Selph S, Buckley DI, et al. , 2017. Intravesical therapy for the treatment of nonmuscle invasive bladder cancer: a systematic review and meta-analysis. J Urol, 197(5): 1189-1199. 10.1016/j.juro.2016.12.090 [DOI] [PubMed] [Google Scholar]
- Erdoğar N, Iskit AB, Eroğlu H, et al. , 2015. Antitumor efficacy of bacillus Calmette-Guerin loaded cationic nanoparticles for intravesical immunotherapy of bladder tumor induced rat model. J Nanosci Nanotechnol, 15(12): 10156-10164. 10.1166/jnn.2015.11690 [DOI] [PubMed] [Google Scholar]
- Fleischmann JD, Park MC, Hassan MO, 1993. Fibronectin expression on surgical specimens correlated with the response to intravesical bacillus Calmette-Guerin therapy. J Urol, 149(2): 268-271. 10.1016/s0022-5347(17)36052-4 [DOI] [PubMed] [Google Scholar]
- Gadiot J, Hooijkaas AI, Kaiser ADM, et al. , 2011. Overall survival and PD-L1 expression in metastasized malignant melanoma. Cancer, 117(10): 2192-2201. 10.1002/cncr.25747 [DOI] [PubMed] [Google Scholar]
- Galon J, Bruni D, 2019. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov, 18(3): 197-218. 10.1038/s41573-018-0007-y [DOI] [PubMed] [Google Scholar]
- Gong C, Qi T, Wei X, et al. , 2013. Thermosensitive polymeric hydrogels as drug delivery systems. Curr Med Chem, 20(1): 79-94. 10.2174/0929867311302010009 [DOI] [PubMed] [Google Scholar]
- Grode L, Seiler P, Baumann S, et al. , 2005. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guérin mutants that secrete listeriolysin. J Clin Invest, 115(9): 2472-2479. 10.1172/jci24617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grode L, Ganoza CA, Brohm C, et al. , 2013. Safety and immunogenicity of the recombinant BCG vaccine VPM1002 in a phase 1 open-label randomized clinical trial. Vaccine, 31(9): 1340-1348. 10.1016/j.vaccine.2012.12.053 [DOI] [PubMed] [Google Scholar]
- Joraku A, Homhuan A, Kawai K, et al. , 2009. Immunoprotection against murine bladder carcinoma by octaarginine-modified liposomes incorporating cell wall of Mycobacterium bovis bacillus Calmette-Guérin. BJU Int, 103(5): 686-693. 10.1111/j.1464-410X.2008.08235.x [DOI] [PubMed] [Google Scholar]
- Kawai K, Miyazaki J, Joraku A, et al. , 2013. Bacillus Calmette-Guerin (BCG) immunotherapy for bladder cancer: current understanding and perspectives on engineered BCG vaccine. Cancer Sci, 104(1): 22-27. 10.1111/cas.12075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Seo HK, 2021. Emerging treatments for bacillus Calmette-Guérin-unresponsive non-muscle-invasive bladder cancer. Investig Clin Urol, 62(4): 361-377. 10.4111/icu.20200602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Choi J, Kim M, et al. , 2022. Immunotherapeutic effects of recombinant Bacillus Calmette-Guérin containing sic gene in ex vivo and in vivo bladder cancer models. Investig Clin Urol, 63(2): 228-237. 10.4111/icu.20210425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinnijenhuis J, Quintin J, Preijers F, et al. , 2014. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J Innate Immun, 6(2): 152-158. 10.1159/000355628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács M, Halfmann A, Fedtke I, et al. , 2006. A functional dlt operon, encoding proteins required for incorporation of D-alanine in teichoic acids in gram-positive bacteria, confers resistance to cationic antimicrobial peptides in Streptococcus pneumoniae . J Bacteriol, 188(16): 5797-5805. 10.1128/jb.00336-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai R, Jeyanathan M, Shaler CR, et al. , 2014. Restoration of innate immune activation accelerates Th1-cell priming and protection following pulmonary mycobacterial infection. Eur J Immunol, 44(5): 1375-1386. 10.1002/eji.201344300 [DOI] [PubMed] [Google Scholar]
- Lamm DL, Thor DE, Harris SC, et al. , 1980. Bacillus Calmette-Guerin immunotherapy of superficial bladder cancer. J Urol, 124(1): 38-42. 10.1016/S0022-5347(17)55282-9 [DOI] [PubMed] [Google Scholar]
- Lamm DL, van der Meijden APM, Morales A, et al. , 1992. Incidence and treatment of complications of bacillus Calmette-Guerin intravesical therapy in superficial bladder cancer. J Urol, 147(3): 596-600. 10.1016/s0022-5347(17)37316-0 [DOI] [PubMed] [Google Scholar]
- Larsen ES, Joensen UN, Poulsen AM, et al. , 2020. Bacillus Calmette-Guérin immunotherapy for bladder cancer: a review of immunological aspects, clinical effects and BCG infections. Apmis, 128(2): 92-103. 10.1111/apm.13011 [DOI] [PubMed] [Google Scholar]
- Leakakos T, Ji C, Lawson G, et al. , 2003. Intravesical administration of doxorubicin to swine bladder using magnetically targeted carriers. Cancer Chemother Pharmacol, 51(6): 445-450. 10.1007/s00280-003-0597-9 [DOI] [PubMed] [Google Scholar]
- Liu L, Shen TT, Liu HF, et al. , 2021. Toxicity effects of anthracycline-converted herceptin and azo-functionalized Fe3O4 nanoparticles on the heart of patients with breast cancer based on echocardiography. J Nanosci Nanotechnol, 21(2): 878-885. 10.1166/jnn.2021.18658 [DOI] [PubMed] [Google Scholar]
- Luo Y, Chen XH, O'Donnell MA, 2003. Role of Th1 and Th2 cytokines in BCG-induced IFN-γ production: cytokine promotion and simulation of BCG effect. Cytokine, 21(1): 17-26. 10.1016/s1043-4666(02)00490-8 [DOI] [PubMed] [Google Scholar]
- Luo Y, Yamada H, Chen X, et al. , 2004. Recombinant Mycobacterium bovis bacillus Calmette-Guérin (BCG) expressing mouse IL-18 augments Th1 immunity and macrophage cytotoxicity. Clin Exp Immunol, 137(1): 24-34. 10.1111/j.1365-2249.2004.02522.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Damania B, 2016. The cGAS-STING defense pathway and its counteraction by viruses. Cell Host Microbe, 19(2): 150-158. 10.1016/j.chom.2016.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H, Nakamura T, Noma Y, et al. , 2018. Application of BCG-CWS as a systemic adjuvant by using nanoparticulation technology. Mol Pharm, 15(12): 5762-5771. 10.1021/acs.molpharmaceut.8b00919 [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Seya T, Kikkawa S, et al. , 2001. Interferon gamma-producing ability in blood lymphocytes of patients with lung cancer through activation of the innate immune system by BCG cell wall skeleton. Int Immunopharmacol, 1(8): 1559-1569. 10.1016/s1567-5769(01)00071-6 [DOI] [PubMed] [Google Scholar]
- McBride SM, Sonenshein AL, 2011. The dlt operon confers resistance to cationic antimicrobial peptides in Clostridium difficile . Microbiology (Reading), 157(5): 1457-1465. 10.1099/mic.0.045997-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrano RFV, Hunger A, Mendonça SA, et al. , 2017. Immunomodulatory and antitumor effects of type I interferons and their application in cancer therapy. Oncotarget, 8(41): 71249-71284. 10.18632/oncotarget.19531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki J, Kawai K, Kojima T, et al. , 2011a. The liposome-incorporating cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guéin can directly enhance the susceptibility of cancer cells to lymphokine-activated killer cells through up-regulation of natural-killer group 2, member D ligands. BJU Int, 108(9): 1520-1526. 10.1111/j.1464-410X.2010.10056.x [DOI] [PubMed] [Google Scholar]
- Miyazaki J, Nishiyama H, Yano I, et al. , 2011b. The therapeutic effects of R8-liposome-BCG-CWS on BBN-induced rat urinary bladder carcinoma. Anticancer Res, 31(6): 2065-2071. [PubMed] [Google Scholar]
- Morales A, 1978. Adjuvant immunotherapy in superficial bladder cancer. Natl Cancer Inst Monogr, 49: 315-319. [PubMed] [Google Scholar]
- Nakamura T, Fukiage M, Higuchi M, et al. , 2014. Nanoparticulation of BCG-CWS for application to bladder cancer therapy. J Control Release, 176: 44-53. 10.1016/j.jconrel.2013.12.027 [DOI] [PubMed] [Google Scholar]
- Nave O, Hareli S, Elbaz M, et al. , 2019. BCG and IL-2 model for bladder cancer treatment with fast and slow dynamics based on SPVF method—stability analysis. Math Biosci Eng, 16(5): 5346-5379. 10.3934/mbe.2019267 [DOI] [PubMed] [Google Scholar]
- Nieuwenhuizen NE, Kulkarni PS, Shaligram U, et al. , 2017. The recombinant Bacille Calmette-Guérin vaccine VPM1002: ready for clinical efficacy testing. Front Immunol, 8: 1147. 10.3389/fimmu.2017.01147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niranjan R, Koushik C, Saravanan S, et al. , 2013. A novel injectable temperature-sensitive zinc doped chitosan/β-glycerophosphate hydrogel for bone tissue engineering. Int J Biol Macromol, 54: 24-29. 10.1016/j.ijbiomac.2012.11.026 [DOI] [PubMed] [Google Scholar]
- Ochiai T, Sato H, Hayashi R, et al. , 1983. Postoperative adjuvant immunotherapy of gastric cancer with BCG-cell wall skeleton. 3- to 6-year follow up of a randomized clinical trial. Cancer Immunol Immunother, 14(3): 167-171. 10.1007/bf00205355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Fong PM, Lu J, et al. , 2009. PEGylated PLGA nanoparticles for the improved delivery of doxorubicin. Nanomedicine, 5(4): 410-418. 10.1016/j.nano.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patard JJ, Rodriguez A, Leray E, et al. , 2002. Intravesical bacillus Calmette-Guerin treatment improves patient survival in T1G3 bladder tumours. Eur Urol, 41(6): 635-642. 10.1016/s0302-2838(02)00173-2 [DOI] [PubMed] [Google Scholar]
- Perera PY, Lichy JH, Waldmann TA, et al. , 2012. The role of interleukin-15 in inflammation and immune responses to infection: implications for its therapeutic use. Microbes Infect, 14(3): 247-261. 10.1016/j.micinf.2011.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Jacoiste Asín MA, Fernández-Ruiz M, López-Medrano F, et al. , 2014. Bacillus Calmette-Guérin (BCG) infection following intravesical BCG administration as adjunctive therapy for bladder cancer: incidence, risk factors, and outcome in a single-institution series and review of the literature. Medicine (Baltimore), 93(17): 236-254. 10.1097/md.0000000000000119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redelman-Sidi G, Glickman MS, Bochner BH, 2014. The mechanism of action of BCG therapy for bladder cancer—a current perspective. Nat Rev Urol, 11(3): 153-162. 10.1038/nrurol.2014.15 [DOI] [PubMed] [Google Scholar]
- Rentsch CA, Bosshard P, Mayor G, et al. , 2020. Results of the phase I open label clinical trial SAKK 06/14 assessing safety of intravesical instillation of VPM1002BC, a recombinant mycobacterium Bacillus Calmette Guérin (BCG), in patients with non-muscle invasive bladder cancer and previous failure of conventional BCG therapy. Oncoimmunology, 9(1): 1748981. 10.1080/2162402X.2020.1748981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi WP, Ji YW, Zhang XG, et al. , 2011. Characterization of pH- and thermosensitive hydrogel as a vehicle for controlled protein delivery. J Pharm Sci, 100(3): 886-895. 10.1002/jps.22328 [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Fuchs HE, et al. , 2021. Cancer statistics, 2021. CA Cancer J Clin, 71(1): 7-33. 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- Singh AK, Srikrishna G, Bivalacqua TJ, et al. , 2021. Recombinant BCGs for tuberculosis and bladder cancer. Vaccine, 39(50): 7321-7331. 10.1016/j.vaccine.2021.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szoka F, 2008. The art of assembly. Science, 319(5863): 578-579. 10.1126/science.1154253 [DOI] [PubMed] [Google Scholar]
- Takeuchi A, Eto M, Tatsugami K, et al. , 2016. Antitumor activity of recombinant Bacille Calmette-Guérin secreting interleukin-15-Ag85B fusion protein against bladder cancer. Int Immunopharmacol, 35: 327-331. 10.1016/j.intimp.2016.03.007 [DOI] [PubMed] [Google Scholar]
- Tham SM, Mahendran R, Chiong E, et al. , 2020. Gmcsf and Ifnα gene therapy improves the response to BCG immunotherapy in a murine model of bladder cancer. Future Oncol, 16(17): 1179-1188. 10.2217/fon-2020-0137 [DOI] [PubMed] [Google Scholar]
- Tinhofer I, Marschitz I, Henn T, et al. , 2000. Expression of functional interleukin-15 receptor and autocrine production of interleukin-15 as mechanisms of tumor propagation in multiple myeloma. Blood, 95(2): 610-618. 10.1182/blood.V95.2.610 [DOI] [PubMed] [Google Scholar]
- Tyagi P, Li ZH, Chancellor M, et al. , 2004. Sustained intravesical drug delivery using thermosensitive hydrogel. Pharm Res, 21(5): 832-837. 10.1023/b:pham.0000026436.62869.9c [DOI] [PubMed] [Google Scholar]
- Verri WA, Cunha TM, Ferreira SH, et al. , 2007. IL-15 mediates antigen-induced neutrophil migration by triggering IL-18 production. Eur J Immunol, 37(12): 3373-3380. 10.1002/eji.200737488 [DOI] [PubMed] [Google Scholar]
- Waldmann TA, 2015. The shared and contrasting roles of IL2 and IL15 in the life and death of normal and neoplastic lymphocytes: implications for cancer therapy. Cancer Immunol Res, 3(3): 219-227. 10.1158/2326-6066.Cir-15-0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann TA, Lugli E, Roederer M, et al. , 2011. Safety (toxicity), pharmacokinetics, immunogenicity, and impact on elements of the normal immune system of recombinant human IL-15 in rhesus macaques. Blood, 117(18): 4787-4795. 10.1182/blood-2010-10-311456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WX, Zhang P, Shan WG, et al. , 2013. A novel chitosan-based thermosensitive hydrogel containing doxorubicin liposomes for topical cancer therapy. J Biomater Sci Polym Ed, 24(14): 1649-1659. 10.1080/09205063.2013.789357 [DOI] [PubMed] [Google Scholar]
- Wang X, Li J, Wang YQ, et al. , 2009. HFT-T, a targeting nanoparticle, enhances specific delivery of paclitaxel to folate receptor-positive tumors. ACS Nano, 3(10): 3165-3174. 10.1021/nn900649v [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization , 2018. BCG vaccine: WHO position paper, February 2018 – Recommendations. Vaccine, 36(24): 3408-3410. 10.1016/j.vaccine.2018.03.009 [DOI] [PubMed] [Google Scholar]
- Wu JJ, Zhao L, Hu HG, et al. , 2020. Agonists and inhibitors of the STING pathway: potential agents for immunotherapy. Med Res Rev, 40(3): 1117-1141. 10.1002/med.21649 [DOI] [PubMed] [Google Scholar]
- Wurpel DJ, Beatson SA, Totsika M, et al. , 2013. Chaperone-usher fimbriae of Escherichia coli . PLoS ONE, 8(1): e52835. 10.1371/journal.pone.0052835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Matsumoto S, Matsumoto T, et al. , 2000. Murine IL-2 secreting recombinant Bacillus Calmette-Guerin augments macrophage-mediated cytotoxicity against murine bladder cancer MBT-2. J Urol, 164(2): 526-531. 10.1016/S0022-5347(05)67417-4 [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Tsukamoto T, Terai A, et al. , 1997. Genetic evidence supporting the fecal-perineal-urethral hypothesis in cystitis caused by Escherichia coli . J Urol, 157(3): 1127-1129. 10.1016/S0022-5347(01)65154-1 [DOI] [PubMed] [Google Scholar]
- Zaharoff DA, Hoffman BS, Hooper HB, et al. , 2009. Intravesical immunotherapy of superficial bladder cancer with chitosan/interleukin-12. Cancer Res, 69(15): 6192-6199. 10.1158/0008-5472.Can-09-1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Sun P, Li P, et al. , 2013. A magnetic chitosan hydrogel for sustained and prolonged delivery of Bacillus Calmette-Guérin in the treatment of bladder cancer. Biomaterials, 34(38): 10258-10266. 10.1016/j.biomaterials.2013.09.027 [DOI] [PubMed] [Google Scholar]
- Zhang L, Gu FX, Chan JM, et al. , 2008. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther, 83(5): 761-769. 10.1038/sj.clpt.6100400 [DOI] [PubMed] [Google Scholar]
- Zhang W, Xu L, Park HB, et al. , 2020. Escherichia coli adhesion portion FimH functions as an adjuvant for cancer immunotherapy. Nat Commun, 11: 1187. 10.1038/s41467-020-15030-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Huo F, Cao Q, et al. , 2022. FimH confers mannose-targeting ability to Bacillus Calmette-Guerin for improved immunotherapy in bladder cancer. J Immunother Cancer, 10(3): e003939. 10.1136/jitc-2021-003939 [DOI] [PMC free article] [PubMed] [Google Scholar]