Abstract
Surface Plasmon Resonance (SPR) based optical biosensors are recently the most attractive sensing devices that can detect minor changes in refractive index. Multiple methods have been developed to design SPR based biosensors with high-performance and ease of fabrication. This research is about a grating based biosensor that utilizes Silver (Ag) and Titanium (Ti) to produce the SP resonance state. The structure has a resonance wavelength, which displays sensitivity to changes in the surrounding medium of the refractive index. The study has been conducted using numerical simulations, utilizing the finite-difference-time-domain (FDTD) method.The simulation results shows a sharp resonance peaks in the wavelength range of 450–700 nm with a remarkable sensitivity of 172 nm/RIU (for mode 1 at SPR peak 465 nm) and 515 nm/RIU (for mode 2 at SPR peak 585 nm), which is superior to other on-chip device. The investigation involves a comparative analysis of sensing performance, focusing on parameters like transmission, reflection, FWHM and Quality factor to measure the detection accuracy of the proposed material combination. Later, we employed this miniature biosensor device to detect hemoglobin concentrations in the blood. Our findings indicate that this developed structure has great potential for detecting any biomolecule, such as proteins, glucose, fructose, nucleic acids, and cells.
Keywords: SPR biosensor, Silver (Ag), Titanium (Ti), Hemoglobin and sensitivity
1. Introduction
Researchers are currently exploring SPR based photonic structures as a possible replacement to conventional devices. This is due to their numerous advantages, including a compact sensing unit, rapid responsiveness, label-free detection, affordability, high sensitivity, real-time monitoring, and low sample consumption [1,2]. Additionally, photonic crystal structures can be one dimensional (1D), two dimensional (2D) or three dimensions (3D) [3].
When a polarized light falls on a photonic metal surface at the interface with different refractive indices, it excites collective oscillation of free electrons (plasmons) occurs. This excitation occurs when the momentum of the incident photons matches that of the surface plasmons leading to Surface Plasmon Resonance (SPR). Moreover, Localized Surface Plasmon Resonance (LSPR) refers to the localized oscillation of electrons in nanoparticles and Surface Enhanced Raman Scattering (SERS) utilizes the enhancement of Raman scattering signals by molecules in close to metal nanostructure undergoing LSPR. Each phenomenon has unique properties and uses, but they all result from light interacting with metal surfaces or nanoparticles.
SPR is highly sensitive to changes in the refractive index of the medium near the metal surface. Therefore, in recent years, it has received keen attention in biosensing applications since various biomaterials, liquid analytes, gasses, nucleic acids and proteins have specific RI [2]. This feature facilitates convenient sensing of their RI change, allowing for easy detection with high sensitivity [2]. As a result, biosensors play a crucial role in medical diagnostics, point of care testing, environmental testing, food safety, bioprocess monitoring, drug discovery and forensic analysis [[4], [5], [6], [7]]. For instance, they have been used for detecting several diseases, blood component detection, glucose detection in urine and blood, protein detection, cancerous cell, virus and bacteria.
Currently, lab-on-a- chip plasmonic biosensors are more popular as they are miniaturized and portable devices that integrate multiple components for biomolecular sensing and analysis at a good resolution on a single chip. These innovative on chip biosensors have many advantages over traditional laboratory setup. They enable quick and convenient analysis of biological samples, allowing for timely decision making in clinical settings, emergency response and resource limited environments [8]. To make our SPR-based biosensor smarter, we require sophisticated data processing algorithms and real-time monitoring automation. Furthermore, we need qualified and skilled staff to operate our photonic biosensor properly; otherwise, existing healthcare systems may face resistance owing to apathy. Clinical studies are necessary to demonstrate the effectiveness and dependability of photonic biosensors in real-world settings because they have not been conducted in real time. Biosensors can be categorized into various types based on their detection principles and components. The most common types include SPR based optical biosensors, electrochemical biosensors [9], piezoelectric biosensors [10], thermal biosensors [11], mass sensitive biosensors, magnetic biosensors [12], nanomaterial based biosensors and enzyme based biosensors.
Recent paper [13] which is a MIM based SPR biosensor has elliptical resonators on silver material. It is used to detect hb hemoglobin concentration in blood of sensitivity of 1100 nm/RIU. Although it has high sensitivity, its complex structure causes challenges in fabrication and its resonator's performance can be affected by temperature, noise or external interference. Additionally, the paper [14] MIM based multilayered bimetallic, semiconductor and 2D nanomaterials is used to detect glucose concentration in urine. And also the paper [15] metal-nitride layers on a bimetallic (Au–Ag) SPR biosensor is used to detect urine glucose. Although both have sensitivity of 440°/RIU and 411°/RIU compared to our proposed work it has very less sensitivity, complicated structure, hard to fabricate, not biocompatible enough and costly.
Another recent paper [16] which is multilayered grating based SPR biosensors of plasmonics Cytop Polymer grating structure with periodicity comparable to the incident wavelength are applied to evaluate the plasmonics Talbot biosensor. It operates with a sensitivity of 324 nm/RIU which is very less compared to our proposed sensor. Moreover, our proposed structure is biocompatible, easy to fabricate and cost effective. The mentioned paper has not examined any particular biomolecules numerically whereas our biosensor can detect Hb hemoglobin in blood and the results are mentioned.
In recent years, plasmonic biosensors have been the most researched sector, as many SPR based integrated biosensor devices have been launched to support the medical sector [17]. In this paper, we proposed a novel, easy to make cavity coupled plasmonic biosensor, which can be rapidly fabricated to get a label free, real time, sterile, reusable device with high sensitivity. This miniature structure is particularly promising for biomedical and biochemical applications. The microcavities of the plasmonic nanostructure significantly impact its performance by boosting the sensitivity and resolution while requiring minimum sample volume. Our sensor achieves sensitivity of 515 nm/RIU which is much better compared to other on chip devices.
2. Sensing mechanism
In this study, we have designed a cavity coupled waveguide biosensor using Silver (Ag) and Titanium (Ti) which will act as a well effective tool to detect Hb concentration in modern health care services. Several quantifiable techniques have been developed to determine the integral, local, or average RI of single living cells or multiple cells using digital holographic microscopy [1], optical trapping technique [18], integrated chip technique [19], Hilbert phase microscopy [20], tomographic phase microscopy [21], tomographic bright field microscopy [22] and several interferometry techniques [23].
Blood, a vital bodily fluid, plays a critical role in oxygen and nutrient transport, as well as waste removal [24]. Blood optics, essential for biophotonic and clinical applications in therapy and diagnostics [25], includes diagnostic methods based on Mie theory, leveraging light scattering and absorption properties of human blood [26]. The light absorption and scattering characteristics of blood depend on erythrocyte refractive index, largely influenced by hemoglobin concentration [25]. Variations in Hb concentration can lead to conditions such as anemia, polycythemia, and other hematological disorders, underscoring the need for accurate detection methods.
Early detection of abnormal Hb levels can significantly impact patient outcomes, potentially preventing postoperative hemorrhages and enabling timely interventions [27]. Our biosensor aims to contribute to these efforts by offering sensitive and specific measurements of Hb concentration, thereby potentially saving lives through early intervention.
3. Theoretical analysis
When light passes over the photonic crystal from one end it will interact with the blood and get detected from another end. The propagation of light will vary in the photonic crystal depending on the refractive indices of the blood analyte [28]. When electromagnetic (EM) fields in the form of a polarized light pass across the interface between metal and dielectric they interact with the surface and cause the free electrons to oscillate. When the nanoparticles are much smaller than the wavelength of the incident light the EM Wave can induce resonance of free electrons. This resonance is known as surface plasmon resonance (SPR).
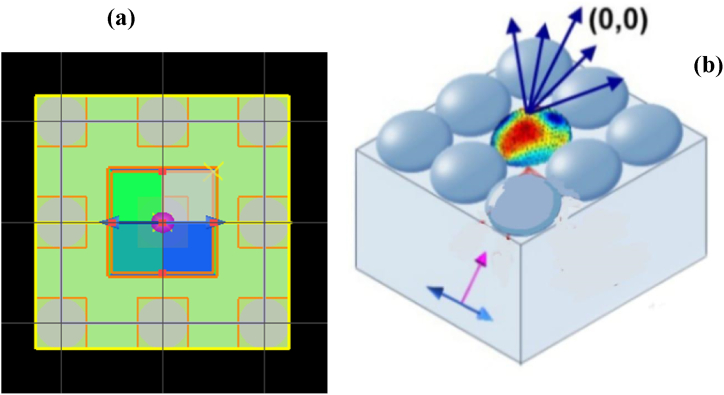
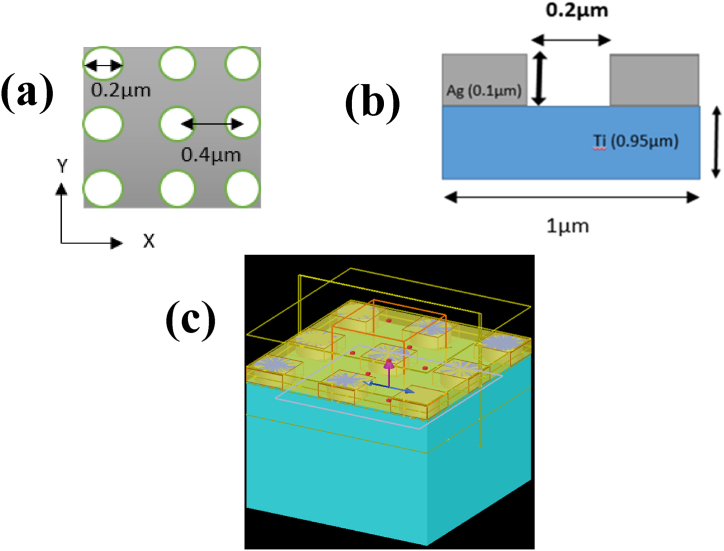
Resonance wavelength strongly depends on size, shape and particles of the structure [29]. As shown here, Fig. 1 the designed structure is a grating based structure which has nanohole cavities. Here the periodicity of the grating affects the coupling conditions for surface plasmons, influencing their resonance properties with their dispersion relations. When the incident light energy couples with the metal-dielectric layers, electrons of it result in changes in the reflectance or transmittance of the incident light.
Fig. 1.
(a)XY plane of simulation view, (b) Schematic representation of the biosensor when the light source is projected, SPR base transmission shifting is taking place and the monitor from above is capturing the spectrum (Inset blue arrow shows the direction of TFSF source and pink arrow indicates the direction of plane wave).
Free electrons at the silver nanohole boundaries must oscillate coherently for SPR to function. For this oscillation, the relationship between the wave vector (kSP) and frequency (ω) can be represented in equation (1) as follows.
| (1) |
Where, na is the refractive index of the medium inside the cavity, c is the speed of light and εm(ω) is the silver nanohole’ dielectric function. We can represent the complex dielectric function below using the Drude model using equation (2) as follows:
| (2) |
Here, γ0 is the damping constant of the silver layer and ΩP represents the plasma frequency. We use the values ΩP = 1.375 ⨯ 1016 rad/s and γ0 = 3.12 ⨯ 1013 rad/s from Ref. [30].
Stable oscillation now requires the wave vectors of the plasmon polaritons and the incident light to match. The wave in the grating surface can be represented by the wave vector shown by equation (3) as follows:
| (3) |
Where, is the light incidence angle, m is the diffraction order (0, ±1, ±2 …), c is the speed of light and P is the period of the grating.
So, the resonance condition is written by equation (4) as follows:
| (4) |
For metals like silver the real part of the metallic dielectric function is much greater than the imaginary part. So we can use the following by equation (5):
| (5) |
Linking the above equations we get the resonance equation:
| (6) |
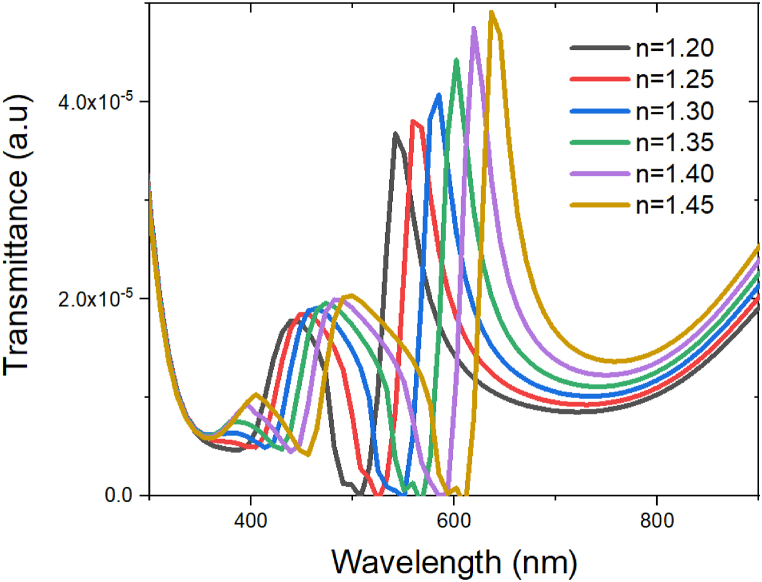
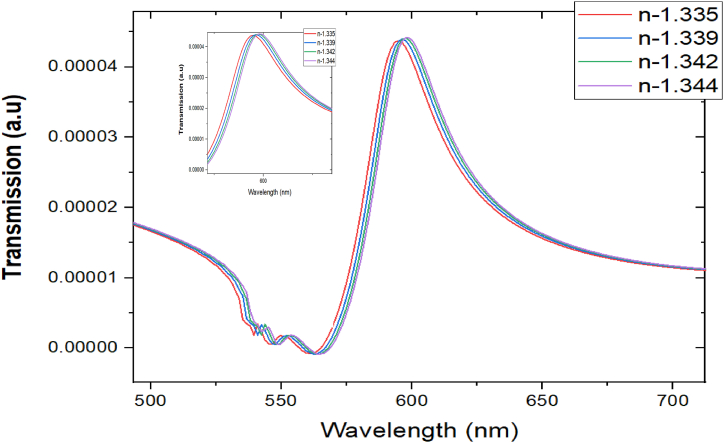
Equation (6) greatly depends on the refractive index inside the cavity (na) as expected. If the refractive index is changed inside the cavity, the resonance wavelength changes. Using the values of ΩP = 1.375 ⨯ 1016 rad/s, γ0 = 3.12 ⨯ 1013 rad/s, na = 1.3 we get, ⍵2 = 7.2511⨯1030 rad2/s2 using resonance wavelength we get 700.2 nm. From Fig. 5 we see that the resonance wavelength for refractive index 1.3 is 585 nm which is closest to our theoretical analysis. Therefore, the resonance wavelength increases if the refractive index is increased.
Fig. 5.
Transmittance vs wavelength graph.
The significant parameters used to measure the sensor's performance are Quality Factor (Q-factor), Wavelength sensitivity(S) and Full Width Half Maximum FWHM.
Sensitivity (S) is the ratio of obtained change in the resonant wavelength to the change in the refractive index. Sensitivity is measured in nm/RIU and it should be high which can be represented by equation (7) as follows:
| (7) |
where Δλ is change in resonant wavelength and Δn is change in refractive index.
3.1. Material selection
Although there are other different metals which have high sensitivity, they are very expensive in terms of mass production of Hb detection sensors. Also, some of these metals are not biocompatible enough, have lack of antimicrobial properties and are susceptible to corrosion, which can affect the environment and the stability & durability of the sensor.
Here, we investigate the optical properties of metal dielectric plasmonic substrates for refractive index sensing. Here we used silver (Ag) and Titanium (Ti) metals where nanohole arrays are etched in a silver metal layer (Fig. 2). Silver is a native metal which is cheap, highly reflective across a broad range of wavelengths particularly in the visible and near-infrared regions and has excellent conductivity properties. It exhibits antibacterial properties too. Moreover Titanium Ti is biocompatible, corrosion resistant and mechanically very strong. Combining these materials results in the design of a biosensor which enhances the performance, biocompatibility and durability of the sensor. The finite difference time-domain (FDTD) [31] approach is used to detect and study the optical spectra features and accompanying increased electric fields of silver (Ag) and titanium (Ti) nanohole arrays. The high sensitivity of resonant coupling mode between SPR from Silver Titanium (AgTi)-nanohole arrays and LSP [32] from Silver Ag nanoparticles to changes in the environment's refractive index variations is observed during the investigation.
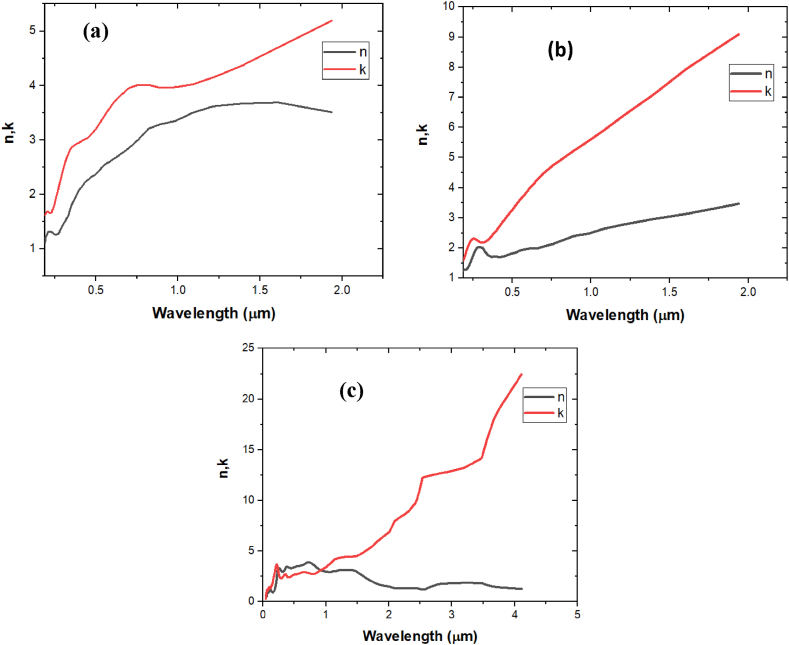
Fig. 2.
Real and imaginary part of refractive index a. Nickel b. Titanium c. Tungsten.
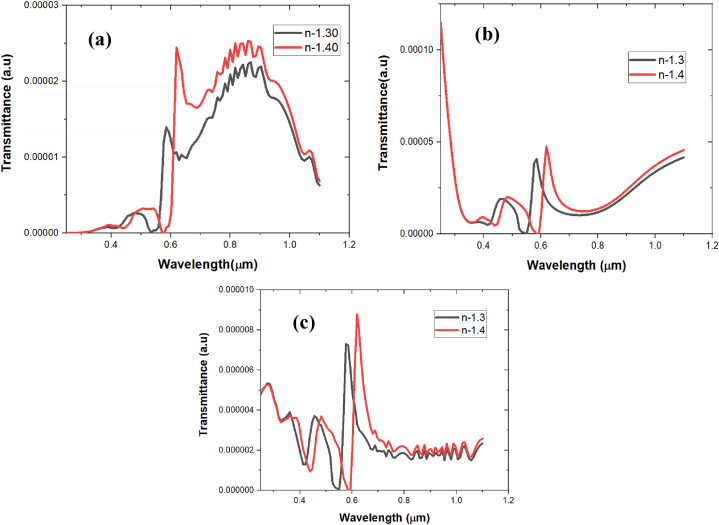
Other than Titanium we worked on Nickel (Ni) and Tungsten (W) also to check their sensitivity in-between refractive index 1.30 and 1.40 as shown in Table 1. We preferred Titanium over Nickel (Ni) and Tungsten (W) not only because of their sensitivity but due to its good material properties (Fig. 3). Nickel has limited transparency, low reflectivity and is susceptible to corrosion due to oxidizing effect. Moreover, Tungsten has high density, brittleness and has more oxidizing effect than all. Therefore, Titanium exhibits highest resistance to oxidizing among these three and has good biocompatibility, strength, durability for fabrication to biosensor devices which can be handled and operated in a clinical setting easily. The real and imaginary part of the refractive index of nickel, titanium and tungsten are shown in Fig. 2(a–c). Additionally sensitivity analysis is shown in Fig. 3(a–c).
Table 1.
Comparison of sensitivity when changing the bottom layer material while keeping the top layer material constant.
| Top layer | Bottom layer | Sensitivity |
|---|---|---|
| Silver | Tungsten | 343.44 nm |
| Silver | Titanium | 404.34 nm |
| Silver | Nickel | 386.36 nm |
Fig. 3.
Transmittance (sensitivity) analysis of (a) Tungsten, (b) Titanium, and (c) Nickel.
Moreover, ensuring the long-term stability and durability of SPR biosensors is a significant challenge. Degradation of materials or photonic structures over time can lead to reduced performance. Therefore, regular maintenance and recalibration may be necessary to maintain sensor accuracy, which can be time-consuming and costly. Additionally, the interaction between biological molecules like Hb hemoglobin and the sensor surface can lead to biofouling. To improve biocompatibility and minimize degradation of materials over time possible solutions could be to go for a coating method. There are several coating methods like Polyethylene glycol (PEG) coating, biomolecule coating, protein coating and carbon based coating [33]. Despite its benefits, these coatings can extant challenges. The stability of the coating layer's conditions can be variable, potentially leading to degradation over time. Additionally, achieving uniform and stable coatings can lead to complexity and increase the cost of sensor fabrication.
4. Device structure and simulation parameters
Our design embraces a simple manufacturing procedure with non-complicated sample-controlling techniques (Table 2). A fundamental issue in plasmonic resonance sensors is coupling the surface plasmon with an optical wave in free space, because the plasmonic wave vector is substantially bigger than the free-space wave vector, which causes the momentum mismatch between light and surface plasmon [34]. In this study we employed finite difference time domain simulation (FDTD) to simulate the metal-insulator-metal structure using transmission grating to cause surface plasmon resonance in the silver layer. Here fig-4 (a) and (b) demonstrate the design of the 1D grating structure which consists of nanoholes arrays of diameter 200 nm etched over silver (Ag) layer which is 100 nm thick. The Ag layer is placed on 950 nm of Titanium (Ti) substrate film. After a series of research and experiments these layers with specified dimensions have been determined to design a novel plasmonic structure (Fig. 4c). The plasmon coupling effect is demonstrated by the introduction of nanohole arrays in the silver layer. A stronger plasmonic near field coupling between two metallic nanoparticles results in a distance-dependent wavelength shift of the plasmon mode [29]. These resonance modes cause optical transmission at wavelengths between 450 nm −700nm.
Table 2.
Structure and dimension details.
| Layers dimension | Material | Purpose |
|---|---|---|
| Top layer: length = 1000 nm, width = 1000 nm, thickness = 100 nm | Silver | Lights interact with the nanoholes which are etched in silver and create resonance. |
| Nanohole's in a silver layer, Diameter = 200 nm, period = 400 nm | ||
| Bottom layer: length = 1000 nm, width = 1000 nm, thickness = 950 nm | Titanium | Works as a substrate. |
Fig. 4.
(a) 1D illustration for the simulation model in x-y plane (b) unit cell design in x-z plane and (c) Perspective view for simulation (Inset blue arrow shows the direction of TFSF source and pink arrow indicates the direction of plane wave).
Here, to simulate optical reflection and transmission data of the structure, an optical power monitor is placed in between the source and upper PML boundary [31].Here we studied transmission from plasmonic structure for a normal Electromagnetic incidence light in the wavelength range from 450 to 700 nm.
For the experiment, at first the refractive index was selected from 1.20 to 1.45 and then the above parameters were observed so that the sensing refractive index for hemoglobin concentration can give desired results.
In paper [35] we can see a 2 layer grating based sensor using silicon and silicon dioxide. Here, for three-waveguide thickness of silicon layer (220 nm, 150 nm, and 100 nm) the biosensing capability is evaluated. It is found that the sensor with a 100 nm thickness provides a higher sensitivity of 322.96 nm/RIU than all other designed structures mentioned in the paper. It has been also assessed that the higher gap width period 440 nm between the gratings has more sensitivity. Compared to the above paper our novel structure has a thickness of 100 nm of silver photonic layer and the gap between the periods is 400 nm which results in the highest sensitivity of 515 nm/RIU. This shows that our structure can exhibit better results flexibly than other parametric structures. Although we have examined the sensitivity by altering the thickness, period and diameter of nanoholes on our structure we obtained optimum parameters of the structure Table 2 which provides good fabrication feasibility and sensitivity. Compared to the mentioned paper our project has 37.28 % more improved sensitivity.
5. Result analysis and numerical discussion
As discussed, the simulation results of the nano hole cavity under z-polarized illumination across a wavelength range spanning from 450 nm to 700 nm is observed here Fig. 5. The analyte with varying refractive indices from 1.20 to 1.45 shows the transmission spectra of the silver titanium nanohole array. The sensitivity, FWHM, Detection accuracy and Quality factor has been calculated and shown in Table 3.
Table 3.
(a) sensitivity at mode 1 and mode 2 (b)parameters for mode −1 (c)parameters for mode 2.
| Refractive Index | Mode 1 (nm) | Sensitivity nm/RIU | Mode 2 (nm) | Sensitivity nm/RIU |
|---|---|---|---|---|
| 1.25 | 456.06 | 305 | 559.09 | 343.4 |
| 1.30 | 464.65 | 171.8 | 584.85 | 515.2 |
| 1.35 | 473.23 | 171.6 | 602.02 | 343.4 |
| 1.40 | 481.82 | 171.8 | 619.19 | 343.4 |
| 1.45 | 498.99 | 343.6 | 636.36 | 343.4 |
(a)
| Refractive index | Sensitivity (nm/RIU) | FWHM (nm) | Accuracy (1/FWHM) | Quality Factor (Sensitivity/FWHM) |
|---|---|---|---|---|
| 1.25 | 305 | 76.95 | 0.012995 | 3.9636 |
| 1.30 | 171.8 | 83.55 | 0.011969 | 2.0562 |
| 1.35 | 171.6 | 91.37 | 0.010944 | 1.8780 |
| 1.40 | 171.8 | 96.97 | 0.010312 | 1.7716 |
| 1.45 | 343.6 | 106.95 | 0.009350 | 3.2127 |
(b)
| Refractive Index | Sensitivity (nm/RIU) | FWHM(nm) | Accuracy (1/FWHM) | Quality Factor (Sensitivity/FWHM) |
|---|---|---|---|---|
| 1.25 | 343.4 | 51.87 | 0.019278 | 6.62039 |
| 1.30 | 515.2 | 48.15 | 0.020768 | 10.69989 |
| 1.35 | 343.4 | 45.81 | 0.021829 | 7.496179 |
| 1.40 | 343.4 | 42.99 | 0.023261 | 7.987904 |
| 1.45 | 343.4 | 44.77 | 0.022336 | 7.670314 |
(c)
From the results, it is seen that as the refractive index of the surrounding medium increases, the biosensor's sensitivity also escalates, indicating that it becomes more responsive to changes in the refractive index. This heightened sensitivity is beneficial for detecting small variations in analyte concentrations or bio molecular interactions. In our designed structure we can see that there are a number of resonances with the strongest SPR at mode1 and mode2. Moreover, in the transmission spectra of mode1 the SPR is between 430 nm −490nm and for mode 2 the SPR wavelength is between 450 and 700 nm. Particularly, when the refractive index of the analyte is between 1.30 and 1.35 the SPR is approximately 584–602 nm the sensitivity is highest, which results 515.2nm/RIU at mode 2 in where the quality factor is also very high of 10.69989.
Grating-based surface plasmon resonance (SPR) biosensors, provides several advantages in terms of simplicity and miniaturization potential, however have a number of limits and obstacles when compared to other SPR biosensors.Grating-based biosensors are often less sensitive, more prone to noise, and have less effective plasmon excitation, resulting in a lower detection limit. On the other hand, fabrication complexity and reproducibility poses considerable challenges because creating high quality grating with precise periodicity and uniformity requires advanced lithography techniques which can be costly and difficult to scale.
Additionally, the angular and wavelength range over which gratings can efficiently couple light to plasmons is narrower, limiting the operational flexibility of grating-based sensors for the detection of a wide range of analytes.
Multiplexing with dual mode detection could be an intriguing area to focus on in the future. The ability to multiplex the biosensor boosts its efficiency and utility in clinical diagnosis. Implementing similar strategies in a photonic cavity-based biosensor could make it more versatile and practical for biomolecule analysis.
6. Application of biosensor in HB detection
Normal Hemoglobin concentration in blood for females is 12–16 g/dl and 14–18 g/dl for males but above or below this concentration is very abnormal, it may cause anemia or polycythemia, cancer, heart disease, lung disease, kidney or liver disease [36]. Moreover, postoperative hemorrhages and autologous transfusions can be observed from the HB level [37]. Hence a suitable amount of Hb must be maintained to ensure adequate oxygenation of the tissue. Generally, the volume of hemoglobin in blood is measured in grams per deciliter (g/dl) or grams per liter (g/l) [37]. In this investigation, the measurement of RIs of the oxygenated Hb concentration could be useful to determine the sensitivity of the designed structure (Fig. 6). As there is a slight change in RI due to the concentration of Hb the sensitivity of the transmission vs SPR wavelength can be detected. Particularly, for every incremental change of 0.001 in the refractive index, there is an associated increase of 6.1025 g per liter (g/L) in the hemoglobin level. This relationship underscores the significance of monitoring blood refractive index as a potential indicator of hemoglobin concentration [37]. This relationship can be denoted as
| (8) |
where Hr is the resultant HB level, H0 is the present HB level, B is the constant value of 5766.5, and the refractive index change is Δn [25].
Fig. 6.
Transmittance spectrum with respect to the wavelength for varying Hb concentrations.
Performance analysis: The research investigates a range of hemoglobin (Hb) concentrations from 0 to 60 g/dl, simulating diverse blood conditions (Fig. 6). The refractive index at the resonant wavelength varies between 1.335 and 1.344, depending on the specific Hb concentration. Since the surface plasmon resonance occurs at a wavelength of 589.3 nm, the investigation focuses on this wavelength.
According to the simulation results, when the Hb concentration is very low (0 g/dl) under unhealthy conditions, the outcome is 365 nm/RIU (Refractive Index Unit). Under healthy conditions with an Hb concentration of 20 g/dl, the sensitivity is 355 nm/RIU, and with a high Hb concentration of 40 g/dl, the sensitivity increases to 473 nm/RIU, as indicated in Table 4. This demonstrates how this structure can detect Hb concentrations with a resolution of 20 g/dl increments, which is notably higher than that achieved by many other papers.
Table 4.
Summary of sensitivity, FWHM, detective accuracy, quality factor and healthy/unhealthy status at varying blood hemoglobin concentration.
| sample with varied Hb level (g/dl) | Refractive index | Resonant wavelength (nm) mode 2 | Sensitivity nm/RIU | FWHM (nm) | Accuracy (1/FWHM) | Quality Factor | Status |
|---|---|---|---|---|---|---|---|
| 0 | 1.335 | 594.825 | 364.91 | 45.38 | 0.02203 | 8.04 | unhealthy |
| 20 | 1.339 | 596.244 | 354.75 | 45.18 | 0.02213 | 7.85 | healthy |
| 40 | 1.342 | 597.663 | 472.67 | 45.10 | 0.02217 | 10.48 | unhealthy |
| 60 | 1.344 | 598.373 | 355.00 | 45.05 | 0.02219 | 7.88 | unhealthy |
7. Comparison with literature
Table 5 represents the comparison of the obtained results of 2D designed photonic nanoholes cavity structure with other research papers which were also used to detect Hb concentration. Here, we can see that, comparatively, our proposed structure has the best sensitivity over all.
Table 5.
The comparison of proposed design with recent reported results.
| References | Year | Structure | Sensitivity | FWHM |
|---|---|---|---|---|
| [38] | 2019 | 1 D structure of Air/(SnS/Nanocomposite/SiO2)/Graphene/D/Graphene/(SnS/Nanocomposite/SiO2)N/Air. Photonic crystal based with a single defective layer. | 51.49nm/RIU | NA |
| [39] | 2020 | 1D-Photonic crystal cavity based structure is designed by silicon (Si) material. | 323 nm/RIU | 0.6 nm |
| [40] | 2020 | A graphene layer of Metal Dielectric Metal configuration nano-scale ring structure. | 333 nm/RIU | – |
| [41] | 2019 | Sphere, cubic and cylinder shaped silver plasmonic nanoparticles are used to detect Hb concentration blood. | 235.7nm/RIU | 94 nm |
| [42] | 2022 | 1D photonic crystal-based sensor using an asymmetric periodic double layers structure of Titanium dioxide TiO2 and silicon dioxide SiO2 with a single defect layer. | 144.50nm/RIU | |
| Proposed work | 2024 | 2 D grating structure of nanoholes cavity silver titanium structure (dual mode) | 172 nm/RIU and 515 nm/RIU | 85 nm and 48.15 nm |
The research [42] focuses on a one-dimensional photonic crystal-based sensor using an asymmetric periodic double layers structure of Titanium dioxide TiO2 and silicon dioxide SiO2 with a single defect layer, showcasing sensitivity of 144.50 nm/RIU. And also in paper [38] one dimensional ternary photonic crystal with a defective layer is designed for bio sensing. Materials like Graphene, silicon dioxide SiO2/Ag and Tin sulfide SnS provide sensitivity 51.49nm/RIU. The research paper [39] focuses on designing a cavity based one-dimensional Photonic Crystal sensor. The sensor design includes alternate silicon layers with introduced porosity to achieve the desired index contrast, enhancing sensing capabilities. The structure demonstrates an average sensitivity of approximately 323 nm/RIU or 0.05 nm/(g/L). Compared to all other biosensors mentioned above where all are mainly detecting Hb in blood, our proposed work has the easiest structure containing nanomaterials silver and titanium only. It is the most available photoactivated nanomaterials found and the very cheapest among all the above materials mentioned in other papers. It has a nice analyte chamber which is a nanocavity based hole/grating structure, which helps to interact the analyte with the photonic nanomaterials efficiently. It enhances the electric field's intensity as light travels through the analyte and thin film of titanium and silver photonic metals. As a result, we have the highest sensitivity of 515 nm/RIU for which we can detect not only Hb in blood but also other biomolecules.
To conclude, the proposed grating-based Surface Plasmon Resonance (SPR) biosensor offers a novel approach for detecting hemoglobin (Hb) with high sensitivity and specificity. This biosensor leverages the unique properties of surface plasmons—oscillations of free electrons at the interface between a metal and a dielectric—to monitor changes in refractive index upon Hb binding. The grating structure, which is composed of periodic metallic or dielectric materials, enhances the SPR signal by diffracting incident light to excite surface plasmons more efficiently. This enhancement allows for real-time, label-free detection of Hb at low concentrations, making it suitable for clinical diagnostics and biomedical research. In future, the incorporation of specific antibodies or aptamers on the grating surface will ensure selective binding of Hb, reducing interference from other proteins. Moreover, the grating-based SPR biosensor offers advantages such as rapid detection times, minimal sample preparation, and the potential for miniaturization and integration into portable devices. This technology represents a significant advancement over traditional Hb detection methods, providing a powerful tool for early diagnosis and monitoring of conditions like anemia and hemoglobinopathies. As research progresses, further optimization of the grating design and functionalization strategies will likely enhance the sensitivity and robustness of this promising biosensing platform.
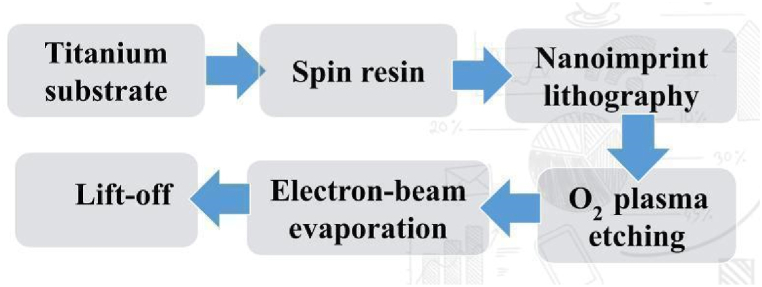
8. Fabrication realization
NIL (Nanoimprint Lithography) fabrication is a technique used for creating nanoscale patterns on various substrates (Fig. 7). It involves the transfer of a pattern from a mold onto a substrate using a combination of heat and pressure. NIL offers high resolution and high throughput, making it suitable for applications in nanoelectronics, photonics, and biotechnology. The process begins with the fabrication of a mold, which can be made using techniques such as electron beam lithography or nanoimprint lithography itself.
Fig. 7.
Proposed fabrication procedure of the proposed structure.
Here the spin based mold is then pressed onto the substrate titanium, causing the pattern to be transferred. After the pattern transfer, the mold is removed, leaving behind the desired pattern on the substrate. This biosensor device is made by replicating a pattern using NIL on a resin coated Titanium substrate. Due to its capacity to generate high-resolution patterns, conventional NIL generally uses solid-phase thermoplastic polymers such as polymethylmethacrylate (PMMA) as imprint resins. A high imprint temperature of more than 170 °C and pressure of up to 50 bar are required for PMMA resin in order to successfully transfer nanoscale patterns from a stamp mold to the PMMA layer [43]. Using an O2 plasma etching technique, any excess resin is removed after a 100 nm thick layer of silver is deposited via electron beam evaporation [44]. Here the cavities are 200 nm in diameter and 40 nm in period. The distance between the cavities is maintained at 200 nm in both the vertical and horizontal directions. Cavities are made using the lift off technique in order to remove the resin imprint [43].
NIL can be used to create various types of patterns, including lines, dots, and complex structures. The technique offers advantages such as high resolution, low cost, and compatibility with a wide range of materials. However, challenges such as mold fabrication, alignment, and defect control need to be addressed for widespread adoption of NIL.
This two-layered cavity-based system demands high precision to maintain consistent functioning. Acquiring accurate dimensions is technically difficult and necessitates advanced lithography techniques (such as electron-beam lithography). These procedures are time-consuming and costly. It also requires sophisticated clean rooms and skilled workers to properly handle and manufacture biosensors. Scaling up from laboratory-scale manufacturing to large production while keeping good performance and cheap costs is similarly difficult. While the manufacturing of this is outside the purview of this work, the mentioned fabrication methods may prove helpful for the device's real-time implementation and monitoring, which incorporates mobility.
Overall, NIL fabrication is a promising technique for creating nanoscale patterns with high resolution and high throughput, and it has the potential to revolutionize various fields of science and technology.
9. Conclusion
In conclusion, the development of SPR based nanohole on chip biosensors represents a significant advancement in biomolecular sensing and analysis. We demonstrated dual mode plasmonic biosensor for SPR mode 1 at 465 nm, sensitivity 172 nm/RIU and SPR mode 2 at 585 nm sensitivity 515 nm/RIU respectively. With its remarkable sensitivity this technology offers a promising solution for detecting hemoglobin (Hb) concentration, which is crucial for various medical applications including disease diagnosis and monitoring. Our design can be easily fabricated compared to other complex structured configuration models. The biosensor's high accuracy and portability further enhance its utility, enabling rapid and reliable Hb measurements at the point of care. By harnessing the unique properties of nanohole structures this biosensor demonstrates great potential to revolutionize healthcare by providing clinicians with a versatile tool for timely and precise diagnostics. Continued research and innovation in this field hold promise for further improving sensitivity, specificity, and usability, ultimately leading to enhanced medical outcomes and patient care.
Methods
We used only FDTD simulation and the hemoglobin refractive index to get the reported sensitivity with our device. In this study, we have not obtained blood from any living animals as we performed numerical simulation only.
Data and code availability
Data included in article/supp. material/referenced in article.
CRediT authorship contribution statement
Nishat Tasnim: Writing – original draft, Visualization, Validation, Methodology, Investigation, Formal analysis, Data curation. Abu S.M. Mohsin: Writing – review & editing, Validation, Supervision, Investigation, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
Authors would like to thank Brac University, Bangladesh for the research support.
References
- 1.Rappaz B., Marquet P., Cuche E., Emery Y., Depeursinge C., Magistretti P.J. J.O.e. Measurement of the integral refractive index and dynamic cell morphometry of living cells with digital holographic microscopy. 2005;13(23):9361–9373. doi: 10.1364/opex.13.009361. [DOI] [PubMed] [Google Scholar]
- 2.Al Mahmud R., Sagor R., Khan M.J.O., Technology L. Surface plasmon refractive index biosensors: a review of optical fiber, multilayer 2D material and gratings, and MIM configurations. 2023;159 [Google Scholar]
- 3.Gowdhami D., Balaji V., Murugan M., Robinson S., Hegde G.J.I., Systems S. Photonic crystal based biosensors: an overview. 2022;11(1):147–167. [Google Scholar]
- 4.Li J., et al. Based biosensors based on multiple recognition modes for visual detection of microbially contaminated food. 2024;4(1):61–70. [Google Scholar]
- 5.Shrikrishna N.S., Sharma R., Gandhi S.J.P. 2023. Based Biosensors: Overview from Past to Future. 1-1-1-26. [Google Scholar]
- 6.Mahmoodpour M., et al. Vol. 13. 2023. (Based Biosensors as Point-Of-Care Diagnostic Devices for the Detection of Cancers: a Review of Innovative Techniques and Clinical Applications). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y., Lu S., Zhang Z., Yang Z., Cui X., Liu G.J.L. 2023. Printable Biosensors towards Next-Generation Point-Of-Care Testing: Paper Substrate as an Example. [DOI] [PubMed] [Google Scholar]
- 8.Park M., Kang B.-H., Jeong K.-H.J.B.J. Vol. 12. 2018. pp. 1–10. (Based Biochip Assays and Recent Developments: A Review). [Google Scholar]
- 9.Yoo H., Shin J., Sim J., Cho H., Hong S.J.B., Bioelectronics Reusable surface plasmon resonance biosensor chip for the detection of H1N1 influenza virus. 2020;168 doi: 10.1016/j.bios.2020.112561. [DOI] [PubMed] [Google Scholar]
- 10.Tombelli S. Piezoelectric biosensors for medical applications. Biosensors for medical applications: Elsevier. 2012:41–64. [Google Scholar]
- 11.Ramanathan K., Danielsson B.J.B., Bioelectronics Principles and applications of thermal biosensors. 2001;16(6):417–423. doi: 10.1016/s0956-5663(01)00124-5. [DOI] [PubMed] [Google Scholar]
- 12.Hahm J.-i. J.S. Vol. 11. 2011. pp. 3327–3355. (Functional Polymers in Protein Detection Platforms: Optical, Electrochemical, Electrical, Mass-Sensitive, and Magnetic Biosensors). 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zafar S.N.R., Singh G., d'Alessandro A., Salim M. Plasmonics-based refractive index sensor for detection of hemoglobin concentration. IEEE Sensor. J. 2018;18 [Google Scholar]
- 14.Kumar S., Yadav A., Malomed B.A. Bimetal thin film, semiconductors, and 2D nanomaterials in SPR biosensors: an approach to enhanced urine glucose sensing. IEEE Trans. NanoBioscience. 2024;23(2):336–343. doi: 10.1109/TNB.2024.3354571. [DOI] [PubMed] [Google Scholar]
- 15.Yadav A., Kumar A., Sharan P., Mishra M. Highly sensitive bimetallic-metal nitride SPR biosensor for urine glucose detection. IEEE Trans. NanoBioscience. October 2023;22(4):897–903. doi: 10.1109/TNB.2023.3246535. 2023 Feb 20. [DOI] [PubMed] [Google Scholar]
- 16.Farhadi S., Farmani A., Hamidi A. Figure of merit enhancement of surface plasmon resonance biosensor based on Talbot effect. Opt Quant Electron. 2021;53:518. doi: 10.1007/s11082-021-03168-4. [DOI] [Google Scholar]
- 17.Lopez G.A., Estevez M.-C., Soler M., Lechuga L.M. Recent advances in nanoplasmonic biosensors: applications and lab-on-a-chip integration. 2017;6(1):123–136. [Google Scholar]
- 18.Ghosh N., Buddhiwant P., Uppal A., Majumder S., Patel H., Gupta P.J. A.p. l. Simultaneous determination of size and refractive index of red blood cells by light scattering measurements. 2006;88(8) [Google Scholar]
- 19.Liang X., Liu A., Lim C., Ayi T., Yap P.J.S., Physical A.A. Determining refractive index of single living cell using an integrated microchip. 2007;133(2):349–354. [Google Scholar]
- 20.Lue N., et al. Live cell refractometry using Hilbert phase microscopy and confocal reflectance microscopy. 2009;113(47):13327–13330. doi: 10.1021/jp904746r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi W., Fang-Yen C., Badizadegan K., Oh S., Lue N., Dasari R.R., Feld M.S. Tomographic phase microscopy. Nat. Methods. 2007 Sep;4(9):717–719. doi: 10.1038/nmeth1078. [DOI] [PubMed] [Google Scholar]
- 22.Phillips K.G., Jacques S.L., McCarty O.J.J.P. Measurement of single cell refractive index, dry mass, volume, and density using a transillumination microscope. 2012;109(11) doi: 10.1103/PhysRevLett.109.118105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohsin ASM, Salim MB. Probing the intracellular refractive index and molecular interaction of gold nanoparticles in HeLa cells using single particle spectroscopy. Int J Nanomedicine. 2018 Oct 4;13:6019–6028. doi: 10.2147/IJN.S175523. PMID: 30323589; PMCID: PMC6177377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma P., Roy S.K., Sharan P. 2014 IEEE Region 10 Symposium. IEEE; 2014. Design and simulation of photonic crystal based biosensor for detection of different blood components; pp. 171–176. [Google Scholar]
- 25.Zhernovaya O., Sydoruk O., Tuchin V., Douplik A.J.P., Biology The refractive index of human hemoglobin in the visible range. 2011;56:4013. doi: 10.1088/0031-9155/56/13/017. 13. [DOI] [PubMed] [Google Scholar]
- 26.Friebel M., Meinke M.J.J. Determination of the complex refractive index of highly concentrated hemoglobin solutions using transmittance and reflectance measurements. 2005;10(6) doi: 10.1117/1.2138027. 064019-064019-5. [DOI] [PubMed] [Google Scholar]
- 27.Ajad A.K., Islam M.J., Kaysir M.R., Atai J.J.O. Vol. 226. 2021. (Highly Sensitive Biosensor Based on WGM Ring Resonator for Hemoglobin Detection in Blood Samples). [Google Scholar]
- 28.Sharma S.K.R. 2014. Design and Simulation of Photonic Crystal Based Biosensor for Detection of Different Blood Components. [Google Scholar]
- 29.Mohsin A.S.M., Salim M.B. Probing the plasmon coupling, quantum yield, and effects of tip geometry of gold nanoparticles using analytical models and FDTD simulation. IEEE Photon. J. 2018;10(3):1–10. [Google Scholar]
- 30.Mendoza Herrera L.J., Arboleda D.M., Schinca D.C., Scaffardi L.B. Determination of plasma frequency, damping constant, and size distribution from the complex dielectric function of noble metal nanoparticles. J. Appl. Phys. 2014;116(23) [Google Scholar]
- 31.Chamoli S.K., Singh S.C., Guo C. Design of extremely sensitive refractive index sensors in infrared for blood glucose detection. IEEE Sensor. J. 2020;20(9):4628–4634. [Google Scholar]
- 32.G. Y. L. Cheng Hung Chu, P. C. Wu, W. Y. Chou, S. H. Chen, H. C Liang, H. P. Chiang, "Enhanced optical sensing with metal-insulator-metal nanohole arrays integrated with silver nanoparticles.".
- 33.Naresh V., Lee N.J.S. A review on biosensors and recent development of nanostructured materials-enabled biosensors. 2021;21(4):1109. doi: 10.3390/s21041109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faramarzi V., Ahmadi V., Ghane Golmohamadi F., Fotouhi B.J.S.I. A biosensor based on plasmonic wave excitation with diffractive grating structure. 2017;24(6):3441–3447. [Google Scholar]
- 35.Sahu S.A., Jalil & Yupapin Preecha, Singh Ghanshyam. 2018. Optical Biosensor Based on a Cladding Modulated Grating Waveguide. [Google Scholar]
- 36.Kim MY, Jee SH, Yun JE, Baek SJ, Lee DC. Hemoglobin concentration and risk of cardiovascular disease in Korean men and women - the Korean heart study. J Korean Med Sci. 2013 Sep;28(9):1316–1322. doi: 10.3346/jkms.2013.28.9.1316. Epub 2013 Aug 28. PMID: 24015036; PMCID: PMC3763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ajad A.K., Islam J., Kaysir R., Atai J. 2021. Highly Sensitive Biosensor Based on WGM Ring Resonator for Hemoglobin Detection in Blood Samples. [Google Scholar]
- 38.El-Khozondar H.J., et al. Vol. 111. 2019. pp. 29–36. (Design of One Dimensional Refractive Index Sensor Using Ternary Photonic Crystal Waveguide for Plasma Blood Samples Applications). [Google Scholar]
- 39.Goyal A.J.P.R. Vol. 97. 2020. pp. 77–86. (Design Analysis of One-Dimensional Photonic Crystal Based Structure for Hemoglobin Concentration Measurement). [Google Scholar]
- 40.Farmani H., Farmani A., Biglari Z.J.P., Nanostructures A label-free graphene-based nanosensor using surface plasmon resonance for biomaterials detection. 2020;116 [Google Scholar]
- 41.Heidarzadeh H.J.O.C. Analysis and simulation of a plasmonic biosensor for hemoglobin concentration detection using noble metal nano-particles resonances. 2020;459 [Google Scholar]
- 42.Edappadikkunnummal S., Chembra Vasudevan R., Dinesh S., Thomas S., Desai N.R., Kaniyarakkal S. Detection of hemoglobin concentration based on defective one-dimensional photonic crystals. 2022;9(9):660. [Google Scholar]
- 43.Boltasseva Alexandra. Plasmonic components fabrication via nanoimprint. J. Opt. Pure Appl. Opt. 2009;11(11) [Google Scholar]
- 44.Jung Woo Kyung, Byun Kyung Min. Fabrication of nanoscale plasmonic structures and their applications to sphotonic devices and biosensors. Biomedical Engineering Letters. 2011;1:153–162. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.