Abstract
Background
Zinc finger-containing transcription factor Osterix/Specificity protein-7 (Sp7) is an essential transcription factor for osteoblast differentiation. However, its functions in differentiated osteoblasts remain unclear and the effects of osteoblast-specific Sp7 deletion on osteocytes have not been sufficiently studied.
Methods
Sp7floxneo/floxneo mice, in which Sp7 expression was 30 % of that in wild-type mice because of disturbed splicing by neo gene insertion, and osteoblast-specific knockout (Sp7fl/fl;Col1a1−Cre) mice using 2.3-kb Col1a1 enhanced green fluorescent protein (EGFP)-Cre were examined by micro-computed tomography (micro-CT), bone histomorphometry, serum markers, and histological analyses. The expression of osteoblast and osteocyte marker genes was examined by real-time reverse transcription (RT)-PCR analysis. Osteoblastogenesis, osteoclastogenesis, and regulation of the expression of collagen type I alpha 1 chain (Col1a1) were examined in primary osteoblasts.
Results
Femoral trabecular bone volume was higher in female Sp7floxneo/floxneo and Sp7fl/fl;Col1a1−Cre mice than in the respective controls, but not in males. Bromodeoxyuridine (BrdU)-positive osteoblastic cells were increased in male Sp7fl/fl;Col1a1−Cre mice, and osteoblast number and the bone formation rate were increased in tibial trabecular bone in female Sp7fl/fl;Col1a1−Cre mice, although osteoblast maturation was inhibited in female Sp7fl/fl;Col1a1−Cre mice as shown by the increased expression of an immature osteoblast marker gene, secreted phosphoprotein 1 (Spp1), and reduced expression of a mature osteoblast marker gene, bone gamma-carboxyglutamate protein/bone gamma-carboxyglutamate protein 2 (Bglap/Bglap2). Furthermore, alkaline phosphatase activity was increased but mineralization was reduced in the culture of primary osteoblasts from Sp7fl/fl;Col1a1−Cre mice. Therefore, the accumulated immature osteoblasts in Sp7fl/fl;Col1a1−Cre mice was likely compensated for the inhibition of osteoblast maturation at different levels in males and females. Vertebral trabecular bone volume was lower in both male and female Sp7fl/fl;Col1a1−Cre mice than in the controls and the osteoblast parameters and bone formation rate in females were lower in Sp7fl/fl;Col1a1−Cre mice than in Sp7fl/fl mice, suggesting differential regulatory mechanisms in long bones and vertebrae. The femoral cortical bone was thin and porous in Sp7floxneo/floxneo and Sp7fl/fl;Col1a1−Cre mice of both sexes, the number of canaliculi was reduced, and terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL)-positive lacunae and the osteoclasts were increased, whereas the bone formation rate was similar in Sp7fl/fl;Col1a1−Cre and Sp7fl/fl mice. The serum levels of total procollagen type 1 N-terminal propeptide (P1NP), a marker for bone formation, were similar, while those of tartrate-resistant acid phosphatase 5b (TRAP5b), a marker for bone resorption, were higher in Sp7fl/fl;Col1a1−Cre mice. Osteoblasts were less cuboidal, the expression of Col1a1 and Col1a1-EGFP-Cre was lower in Sp7fl/fl;Col1a1−Cre mice, and overexpression of Sp7 induced Col1a1 expression.
Conclusions
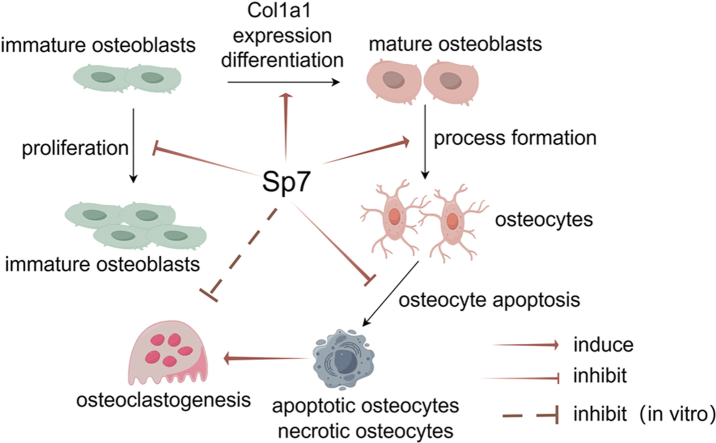
Our studies indicated that Sp7 inhibits the proliferation of immature osteoblasts, induces osteoblast maturation and Col1a1 expression, and is required for osteocytes to acquire a sufficient number of processes for their survival, which prevents cortical porosity.
The translational potential of this article
This study clarified the roles of Sp7 in differentiated osteoblasts in proliferarion, maturation, Col1a1 expression, and osteocyte process formation, which are required for targeting SP7 in the development of therapies for osteoporosis.
Keywords: Sp7, Osteoblast differentiation, Osteoblast proliferation, Osteocyte apoptosis, Osteocyte processes, Cortical porosity
Graphical abstract
1. Introduction
Runt related transcription factor 2 (Runx2), a member of the Runx family of transcription factors, induces the commitment of multipotent mesenchymal cells to osteoblast lineage cells and induces the proliferation of osteoblast progenitors. Sp7, which has three zinc finger motifs and belongs to the SP family of transcription factors, and Wnt signaling, together with Runx2 are required for osteoblast differentiation. After differentiation into immature osteoblasts, Runx2 regulates the expression of major bone matrix protein genes, including Col1a1, collagen type I alpha 2 chain (Col1a2), Spp1, integrin binding sialoprotein (Ibsp), Bglap/Bglap2 [1]. Although the SP family of transcription factors bind GC-rich target sequences through the zinc finger domain, Sp7 interacts with distal-less homeobox (Dlx) and binds to the AT-rich motifs of Dlx target genes [2].
The germline deletion of Sp7 (Sp7−/−) results in death just after birth, and Sp7−/− mice completely lack osteoblasts and bone [3]. Furthermore, Sp7fl/–;CAG−CreER mice treated with tamoxifen, which causes the ubiquitous deletion of Sp7 after birth, markedly reduced both bone formation and trabecular and cortical bone volumes [4]. Therefore, Sp7 is essential for osteoblast differentiation and bone formation during the embryonic stage and after birth. Moreover, SP7 is a locus associated with osteoporosis and a rare pathogenetic locus of osteogenesis imperfecta [5]. Sp7 is expressed in both osteoblasts and hypertrophic chondrocytes, and is required for matrix metallopeptidase 13 (Mmp13) expression in terminal hypertrophic chondrocytes [6].
To investigate the functions of Sp7 in differentiated osteoblasts, Baek et al. generated Sp7fl/–;Col1a1−Cre mice, in which one Sp7 allele was deleted in germline and the other Sp7 allele was conditionally deleted in osteoblasts using 2.3-kb Col1a1 Cre. In femurs, the trabecular bone volume increased, while cortical bone volume was normal. In vertebrae, the trabecular bone volume and the bone formation were reduced. The expression of Bglap/Bglap2, but not Col1a1, was reduced [7]. The same group also generated Sp7fl/–;Col1a1−CreERT2 mice and 4-hydroxytamoxifen (4-OHT) was injected after birth. The numbers of osteoblasts, osteoclasts, and BrdU-positive cells were similar in 4-OHT treated and control mice, while the expression of Col1a1 and Bglap/Bglap2 was lower in 4-OHT treated mice [8]. Osteocyte anomalies were not examined in either of the mouse models. Although the sexes of the mice examined were not specified, the two mouse models indicated that the deletion of Sp7 in osteoblasts in the Sp7 haplodeficient state decreased vertebral trabecular bone by inhibiting osteoblast maturation, increased femoral trabecular bone via unknown mechanisms, and did not affect cortical bone volume, osteoblast proliferation, and bone resorption. Another group generated Sp7OcyKO mice using Dmp1 Cre, which directs Cre expression in mature osteoblasts and osteocytes. Sp7OcyKO mice had porous cortical bone, fewer osteocyte processes and canaliculi, and more TUNEL-positive lacunae in the cortical bone, indicating the need for Sp7 to acquire a sufficient number of osteocyte processes [9].
Osteocytes form a dense network through their processes and canaliculi throughout bone. Reductions in the numbers of osteocyte processes and canaliculi induce osteocyte apoptosis through decreases in the supply of oxygen and nutrients to osteocytes via these processes and canaliculi [[9], [10], [11], [12]]. Apoptotic osteocytes have been shown to increase the expression of TNF superfamily member 11 (Tnfsf11, also known as Rankl), which encodes a ligand for TNF receptor superfamily member 11 (Tnfrsf11a/Rank), in neighboring osteocytes and osteoblasts through the release of adenosine triphosphate (ATP), thereby promoting osteoclastogenesis and bone resorption [[13], [14], [15], [16]]. Furthermore, secondary necrosis occurs in the majority of apoptotic osteocytes because scavengers cannot reach the apoptotic osteocytes imbedded in the bone matrix. Inflammatory molecules, including damage-associated molecular patterns, are released through the canaliculi to the bone surface and vascular canals in bone, leading to the production of proinflammatory cytokines that induce Tnfsf11 expression [[16], [17], [18]].
Although Sp7 is essential for osteoblast differentiation, the functions of Sp7 in bone formation, bone resorption, and bone matrix protein gene expression remain unclear, and the effects of the deletion of Sp7 on osteocytes have not been examined in previous osteoblast-specific Sp7 knockout mice using 2.3-kb Col1a1 Cre. Therefore, we examined Sp7floxneo/floxneo mice, in which Sp7 expression was reduced to 30 % of that in wild-type mice through disturbed splicing by neo gene insertion, and osteoblast-specific knockout (Sp7fl/fl;Col1a1−Cre) mice generated using 2.3-kb Col1a1 EGFP-Cre transgenic mice [19]. Although the knockdown or deletion of Sp7 in osteoblasts exerted differential effects in males and females, in long bones and vertebrae, and in trabecular and cortical bone, the results demonstrated that Sp7 inhibits the proliferation of immature osteoblasts and induces their differentiation, and that Sp7 is required for Col1a1 expression and for acquiring a sufficient number of osteocyte processes. The reduction in the number of osteocyte processes resulted in severe cortical porosity due to the osteocyte death, which enhanced osteoclastogenesis.
2. Results
2.1. Reduced Sp7 expression in neo-inserted Sp7 floxed mice
The Sp7 gene comprises two exons, and two major mRNAs (Types I and II) were transcribed from different transcription start sites (Supplementary Fig. 1A) [20]. This was confirmed by Cap Analysis of Gene Expression (CAGE) using RNA from wild-type calvariae (Supplementary Fig. 1B). The neo gene interposed between frts was inserted into the intron and loxp sequences in the 5’ of neo and the untranslated region of exon 2 (Supplementary Fig. 1A) [6]. We initially examined neo-inserted Sp7 floxed mice (Sp7floxneo/+ and Sp7floxneo/floxneo). Real-time RT-PCR analyses using the primer sets F1 and R1 in exon 1 of Type I mRNA or F2 and R2 in exon 1 of Type II mRNA showed no reduction in Sp7 mRNA in Sp7floxneo/+ and Sp7floxneo/floxneo mice compared to those in Sp7+/+ mice, indicating that the transcriptional activities for Type I and Type II mRNA were not affected by neo gene insertion. However, real-time RT-PCR analyses using the primer sets F3 (exon 1 in Type I mRNA) and R3 (exon 2) or F4 (exon 1 in Type II) and R3 (exon 2) revealed a decrease in Sp7 mRNA in Sp7floxneo/+ and Sp7floxneo/floxneo mice compared to Sp7+/+ mice (Supplementary Fig. 1C). Furthermore, real-time RT-PCR using the primers in exon 2 (F5 and R3) also showed a reduction in Sp7 mRNA in Sp7floxneo/floxneo mice compared to Sp7+/+ mice (Supplementary Fig. 1C). These results indicated that neo gene insertion in the intron disturbed splicing and reduced Type I and Type II Sp7 mRNAs. Type I and Type II Sp7 mRNAs in Sp7floxneo/+ mice and Sp7floxneo/floxneo mice were approximately 60 and 30 %, respectively, of those in Sp7+/+ mice. Similar results were obtained in western blot analyses of Sp7+/+, Sp7floxneo/+, and Sp7floxneo/floxneo newborn limbs using the Sp7 antibody (Supplementary Figs. 1D and E). Therefore, Sp7floxneo/floxneo mice, in which Sp7 mRNA is less than one-third of that in wild-type mice, are a useful model for examining the role of Sp7.
2.2. Micro-CT and gene expression analyses in Sp7floxneo/floxneo and Sp7floxneo/floxneo;Col1a1−Cre mice at 9 weeks of age
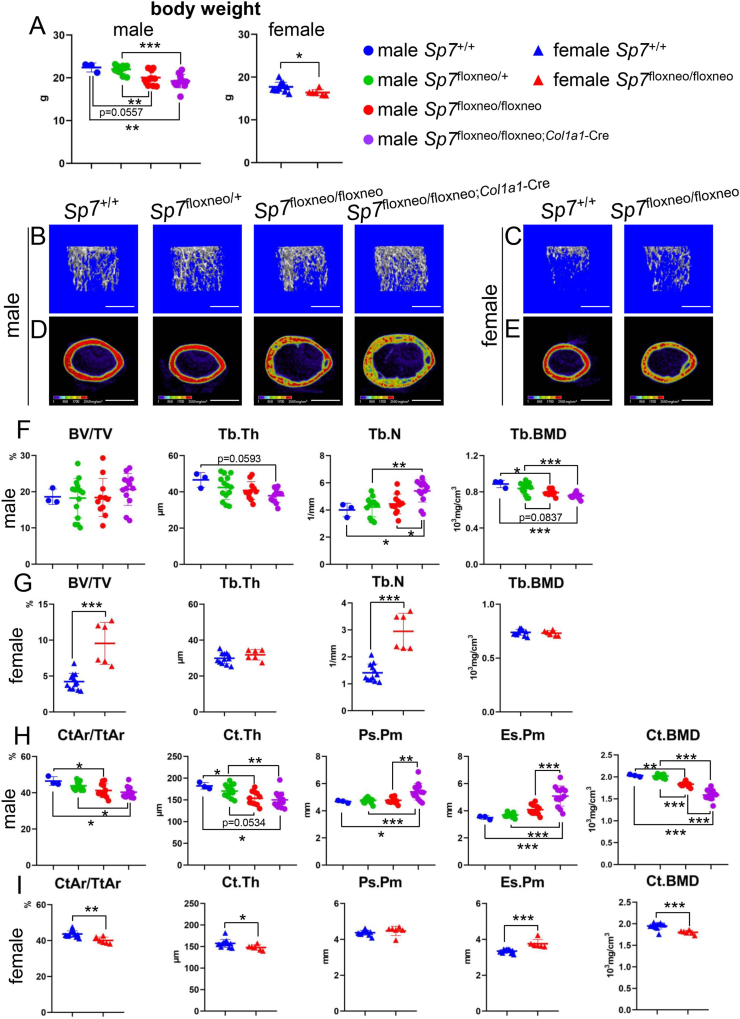
We generated Sp7floxneo/floxneo;Col1a1−Cre mice using 2.3-kb Col1a1 EGFP-Cre transgenic mice [19], and compared the body weights and trabecular and cortical bone of femurs among male Sp7+/+, Sp7floxneo/+, Sp7floxneo/floxneo, and Sp7floxneo/floxneo;Col1a1−Cre mice and between female Sp7+/+ and Sp7floxneo/floxneo mice. Body weights were lower in male Sp7floxneo/floxneo;Col1a1−Cre mice and female Sp7floxneo/floxneo mice than in the respective Sp7+/+ mice (Fig. 1A). In micro-CT analyses of males, no significant differences were observed in trabecular bone volume (BV/TV) or trabecular thickness (Tb.Th) among the four groups, while the trabecular number (Tb.N) was increased in Sp7floxneo/floxneo;Col1a1−Cre mice and trabecular bone mineral density (Tb.BMD) was reduced in Sp7floxneo/floxneo and Sp7floxneo/floxneo;Col1a1−Cre mice (Fig. 1B–F). In females, BV/TV and Tb.N were higher in Sp7floxneo/floxneo mice than in Sp7+/+ mice, while Tb.Th and Tb.BMD were similar (Fig. 1C–G). Cortical area (CtAr/TtAr), cortical thickness (Ct.Th), and cortical (Ct) BMD were lower in male Sp7floxneo/floxneo and Sp7floxneo/floxneo;Col1a1−Cre mice and female Sp7floxneo/floxneo mice than in the respective Sp7+/+ mice (Fig. 1D, E, H, I). The periosteal perimeter (Ps.Pm) of cortical bone in male Sp7floxneo/floxneo;Col1a1−Cre mice and the endosteal perimeter (Es.Pm) in male Sp7floxneo/floxneo;Col1a1−Cre mice and female Sp7floxneo/floxneo mice were higher than those in the respective Sp7+/+ mice (Fig. 1H and I). Body weights and all parameters in the micro-CT analysis were similar in Sp7floxneo/+ and Sp7+/+ male mice (Fig. 1).
Figure 1.
Micro-CT analyses of Sp7+/+, Sp7floxneo/+, Sp7floxneo/floxneo, and Sp7floxneo/floxneo;Col1a1−Cre mice at 9 weeks of age (A) Body weights of male Sp7+/+, Sp7floxneo/+, Sp7floxneo/floxneo, and Sp7floxneo/floxneo;Col1a1−Cre mice and female Sp7+/+ and Sp7floxneo/floxneo mice (B–E) Three-dimensional trabecular bone architecture of the distal femoral metaphysis (B, C) and images of cortical bone at the mid-diaphysis of the femur (D, E) in male (B, D) and female (C, E) mice. Scale bars: 1 mm. (F–I) Quantification of trabecular bone (F, G) and cortical bone (H, I) parameters in male Sp7+/+, Sp7floxneo/+, Sp7floxneo/floxneo, and Sp7floxneo/floxneo;Col1a1−Cre mice (F, H) and female Sp7+/+ and Sp7floxneo/floxneo mice (G, I). These parameters include trabecular bone volume (bone volume/tissue volume, BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), trabecular bone mineral density (Tb.BMD), cortical area (CtAr/TtAr), cortical thickness (Ct.Th), periosteal perimeter (Ps.Pm), endosteal perimeter (Es.Pm), and cortical bone mineral density (Ct.BMD). n = 3 (male Sp7+/+), n = 14 (male Sp7floxneo/+), n = 12 (male Sp7floxneo/floxneo), n = 13 (male Sp7floxneo/floxneo;Col1a1−Cre), n = 12 (female Sp7+/+), and n = 6 (female Sp7floxneo/floxneo).
The expression of the osteoblast marker genes, Tnfsf11, and TNF receptor superfamily member 11b (Tnfrsf11b, also known as Opg), which is a decoy receptor for Tnfsf11, was compared using RNA from the osteoblast-enriched fraction in male Sp7floxneo/+, Sp7floxneo/floxneo, and Sp7neo/neo;Col1a1−Cre mice (Supplementary Fig. 2A). The expression of Sp7 was lower in Sp7floxneo/floxneo mice and Sp7floxneo/floxneo;Col1a1−Cre mice, but the expression of Spp1 and Tnfsf11 was higher in Sp7floxneo/floxneo;Col1a1−Cre mice than in Sp7floxneo/+ mice, Tnfsf11 expression in Sp7floxneo/floxneo;Col1a1−Cre mice was higher than in Sp7floxneo/floxneo mice, and the expression of the other genes examined was similar among the three groups (Supplementary Fig. 2A). The expression of osteocyte marker genes, including dentin matrix acidic phosphoprotein 1 (Dmp1) and sclerostin (Sost), using the osteocyte-enriched fraction in male mice was markedly lower in Sp7floxneo/floxneo and Sp7floxneo/floxneo;Col1a1−Cre mice than in Sp7floxneo/+ mice (Supplementary Fig. 2B).
2.3. Micro-CT analysis and the serum markers for bone formation and resorption in Sp7fl/fl;Col1a1−Cre mice at 10 weeks of age
The neo gene was deleted by mating Sp7floxneo/+ mice with CAG-Flp transgenic mice to generate and Sp7fl/+ and Sp7fl/fl mice. The body weights and all trabecular and cortical bone parameters on micro-CT were similar between the Sp7fl/+ and Sp7fl/fl mice (Supplementary Figs. 3A–E). Furthermore, the expression of Sp7, the osteoblast marker genes, Tnfsf11, and Tnfrsf11b in the osteoblast-enriched fraction, and osteocyte marker genes in the osteocyte-enriched fraction were similar (Supplementary Figs. 3F and G).
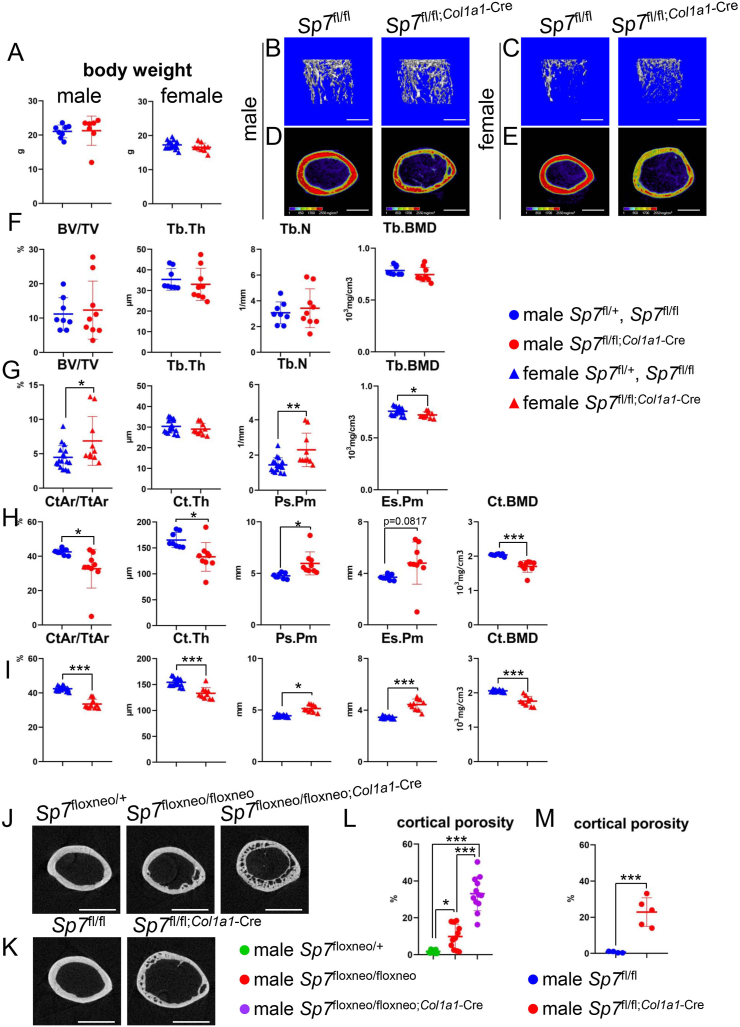
To examine Sp7 functions specifically in differentiated osteoblasts, Sp7fl/+ and Sp7fl/fl mice were mated with 2.3-kb Col1a1 EGFP-Cre transgenic mice to generate Sp7fl/fl;Col1a1−Cre mice. Sp7fl/fl;Col1a1−Cre mice were compared with the Sp7fl/+ and Sp7fl/fl littermates as the control, because there were no differences between the Sp7fl/+ and Sp7fl/fl littermates (Supplementary Fig. 3). Body weights were similar in Sp7fl/fl;Col1a1−Cre mice and the control in both sexes (Fig. 2A). In micro-CT analyses of femoral trabecular bone, BV/TV and Tb.N were higher and Tb.BMD was lower in female Sp7fl/fl;Col1a1−Cre mice than in the control, while these parameters in male Sp7fl/fl;Col1a1−Cre mice were similar to those in the control (Fig. 2B, C, F, G). CtAr/TtAr, Ct.Th, and Ct.BMD were lower and Ps.Pm was higher in both male and female Sp7fl/fl;Col1a1−Cre mice and Es.Pm was higher in female Sp7fl/fl;Col1a1−Cre mice than in the respective controls (Fig. 2D, E, H, I). As the cortical bone was porous in Sp7floxneo/floxneo, Sp7floxneo/flox neo;Col1a1−Cre, and Sp7fl/fl;Col1a1−Cre mice (Figure 1, Figure 2D, E), the level of the cortical pososity was compared at the mid-diaphysis of male femurs using high-resolution micro-CT. The cortical pososity in Sp7floxneo/floxneo mice was more severe than in Sp7floxneo/+ mice, that in Sp7floxneo/floxneo;Col1a1−Cre mice was more severe than in Sp7floxneo/floxneo mice, and that in Sp7fl/fl;Col1a1−Cre mice was more severe than in Sp7fl/fl mice (Fig. 2J–M).
Figure 2.
Micro-CT analyses of femurs in Sp7fl/fl;Col1a1−Cre mice without neo at 10 weeks of age (A) Body weight of male and female Sp7fl/fl;Col1a1−Cre and the control (Sp7fl/+ and Sp7fl/fl) mice. (B–E) Three-dimensional trabecular bone architecture of the distal femoral metaphysis (B, C) and images of the cortical bone at the mid-diaphysis in the femurs (D, E) of male (B, D) and female (C, E) Sp7fl/fl and Sp7fl/fl;Col1a1−Cre mice. Scale bars: 1 mm. (F–I) Quantification of trabecular bone (F, G) and cortical bone (H, I) parameters in male (F, H) and female (G, I) Sp7fl/fl;Col1a1−Cre and the control (Sp7fl/+ and Sp7fl/fl) mice. n = 8 (4 male Sp7fl/+ and 4 Sp7fl/fl), n = 7 (male Sp7fl/fl;Col1a1−Cre), n = 17 (7 female Sp7fl/+ and 10 Sp7fl/fl), and n = 10 (female Sp7fl/fl;Col1a1−Cre). (J–M) High resolution micro-CT images of cortical bone at the mid-diaphysis in male femurs (J, K) and the quantification of cortical porosity (L, M). The percentage of the pore area in the cortical area is shown. n = 14 (Sp7floxneo/+), n = 11 (Sp7floxneo/floxneo), n = 12 (Sp7floxneo/floxneo;Col1a1−Cre), n = 4 (Sp7fl/fl), and n = 5 (Sp7fl/fl;Col1a1−Cre).
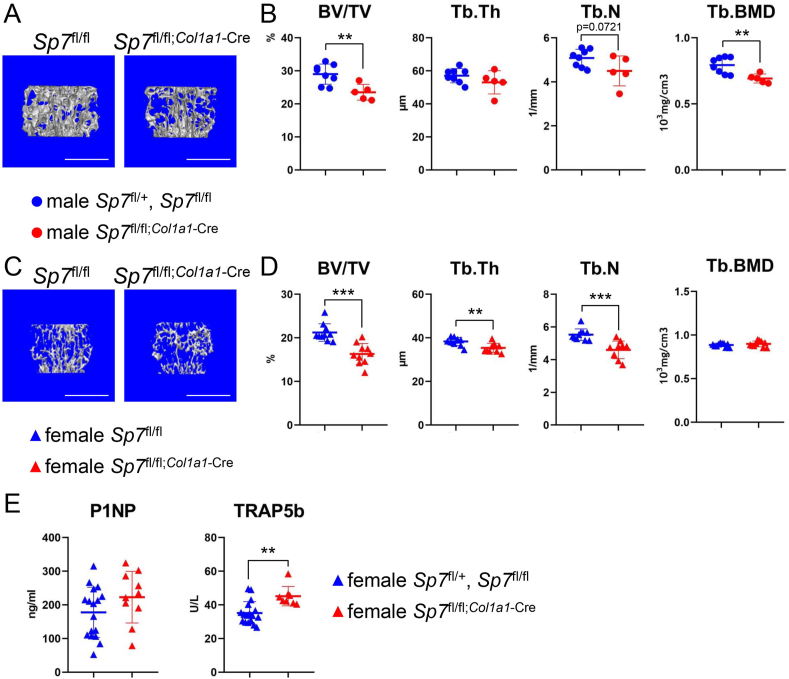
Because the male and female vertebrae were examined using different micro-CT systems (Skyscan1176 for males and R_mCT for females), the data could not be compared between males and females. In male lumbar vertebrae, BV/TV and Tb.BMD in Sp7fl/fl;Col1a1−Cre mice were lower than those in the control (Sp7fl/+ and Sp7fl/fl) (Fig. 3A and B). In females, BV/TV, Tb.Th, and Tb.N in lumbar vertebrae were lower in Sp7fl/fl;Col1a1−Cre mice than in the control (Sp7fl/fl), whereas Tb.BMD was similar (Fig. 3C and D).
Figure 3.
Micro-CT analysis of vertebrae and the serum markers for bone formation and resorption in Sp7fl/fl;Col1a1−Cre mice at 10 weeks of age (A–D) Three-dimensional trabecular bone architecture of the first lumbar vertebra (A, C) and the quantification of BV/TV, Tb.Th, Tb.N, and Tb.BMD (B, D) in male (A, B) and female (C, D) Sp7fl/fl;Col1a1−Cre and the control (male Sp7fl/+ and Sp7fl/fl and female Sp7fl/fl) mice. Scale bars: 1 mm. n = 8 (male 4 Sp7fl/+ and 4 Sp7fl/fl), n = 5 (male Sp7fl/fl;Col1a1−Cre), n = 10 (female Sp7fl/fl), and n = 10 (female Sp7fl/fl;Col1a1−Cre). Vertebrae from males and females were analyzed using different micro-CT systems. (E) Serum levels of P1NP and TRAP5b in female Sp7fl/fl;Col1a1−Cre and the control (Sp7fl/+ and Sp7fl/fl) mice. n = 17 (7 Sp7fl/+ and 10 Sp7fl/fl) and n = 8 (Sp7fl/fl;Col1a1−Cre).
The serum marker for bone formation, total procollagen type 1 N-terminal propeptide (P1NP), was similar in female Sp7fl/fl;Col1a1−Cre mice and controls, whereas that for bone resorption, tartrate-resistant acid phosphatase 5b (TRAP5b), was higher in the former (Fig. 3E).
2.4. Bone histomorphometric analyses in female Sp7fl/fl;Col1a1−Cre mice at 10 weeks of age
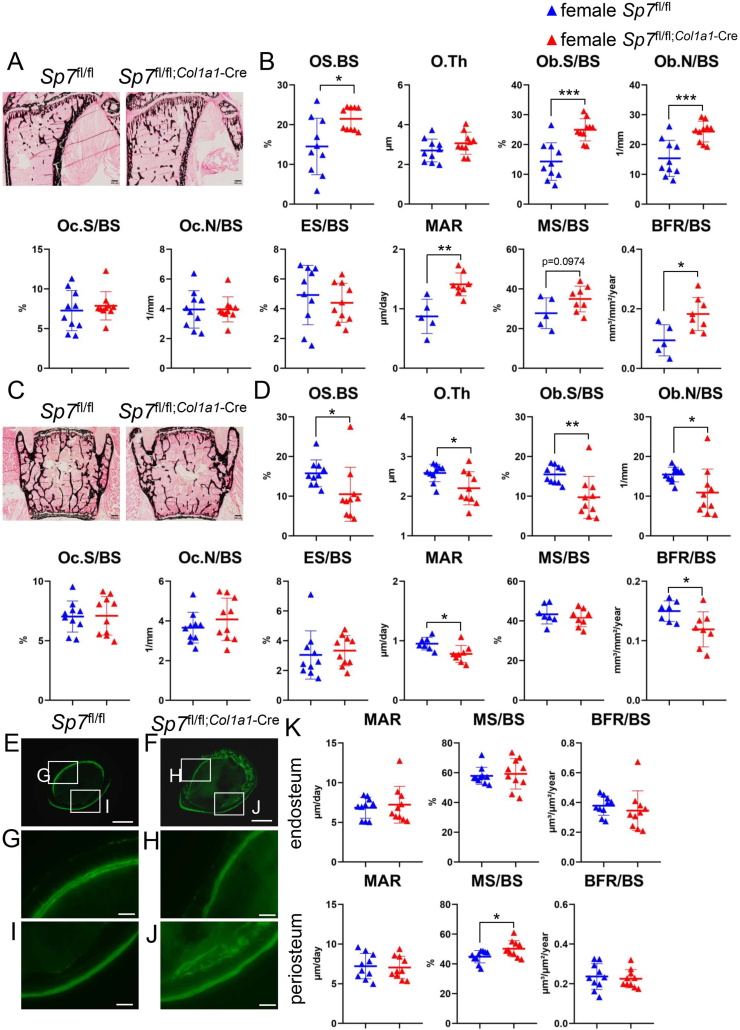
Bone histomorphometric analyses were performed using tibiae, vertebrae, and femoral cortical bone from female Sp7fl/fl and Sp7fl/fl;Col1a1−Cre mice at 10 weeks of age. In tibiae, osteoblast parameters, including the osteoid surface, osteoblast surface, and osteoblast number, and the mineral apposition and bone formation rate were higher in Sp7fl/fl;Col1a1−Cre mice than in Sp7fl/fl mice (Fig. 4B). In contrast, these parameters and osteoid thickness in vertebrae were lower in Sp7fl/fl;Col1a1−Cre mice than in Sp7fl/fl mice (Fig. 4D). Osteoclast parameters, including the osteoclast surface, osteoclast number, and eroded surface, in trabecular bone in tibiae and vertebrae were similar between Sp7fl/fl;Col1a1−Cre and Sp7fl/fl mice (Fig. 4B and D). In femoral cortical bone, the mineral apposition rate and bone formation rate in the periosteum and endosteum were similar between Sp7fl/fl;Col1a1−Cre mice and Sp7fl/fl mice, while the mineralizing surface in the periosteum, but not in the endosteum, was higher in Sp7fl/fl;Col1a1−Cre mice than in Sp7fl/fl mice (Fig. 4E–K).
Figure 4.
Bone histomorphometric analyses of trabecular bone in tibiae, vertebrae, and femoral cortical bone in female Sp7fl/fl and Sp7fl/fl;Col1a1−Cre mice at 10 weeks of age (A–D) von Kossa staining of tibiae (A) and lumber vertebrae (C), and trabecular bone parameters, including the osteoid surface (OS/BS), osteoid thickness (O.Th), osteoblast surface (Ob.S/BS), osteoblast number (Ob.N/BS), osteoclast surface (Oc.S/BS), osteoclast number (Oc.N/BS), eroded surface (ES/BS), mineral apposition rate (MAR), mineralizing surface (MS/BS), and bone formation rate (BFR/BS) in tibiae (B) and vertebrae (D). BS, bone surface. Scale bars: 0.2 mm. (E–K) Dynamic bone histomorphometric analyses of cortical bone. Cross-sections of the mid-diaphyses of femurs were analyzed. G and H show the endosteum and I and J show the periosteum. The boxed regions in E and F are magnified in G and I and in H and J, respectively. Scale bars: 0.5 mm (E, F) and 0.1 mm (G–J). MAR, MS/BS, and BFR/BS in the endosteum and periosteum are shown in K. n = 10 (Sp7fl/fl) and n = 10 (Sp7fl/fl;Col1a1−Cre).
2.5. Histological analyses of the femurs in male Sp7fl/fl;Col1a1−Cre mice by BrdU labeling and the staining with TUNEL, Safranin O, and TRAP
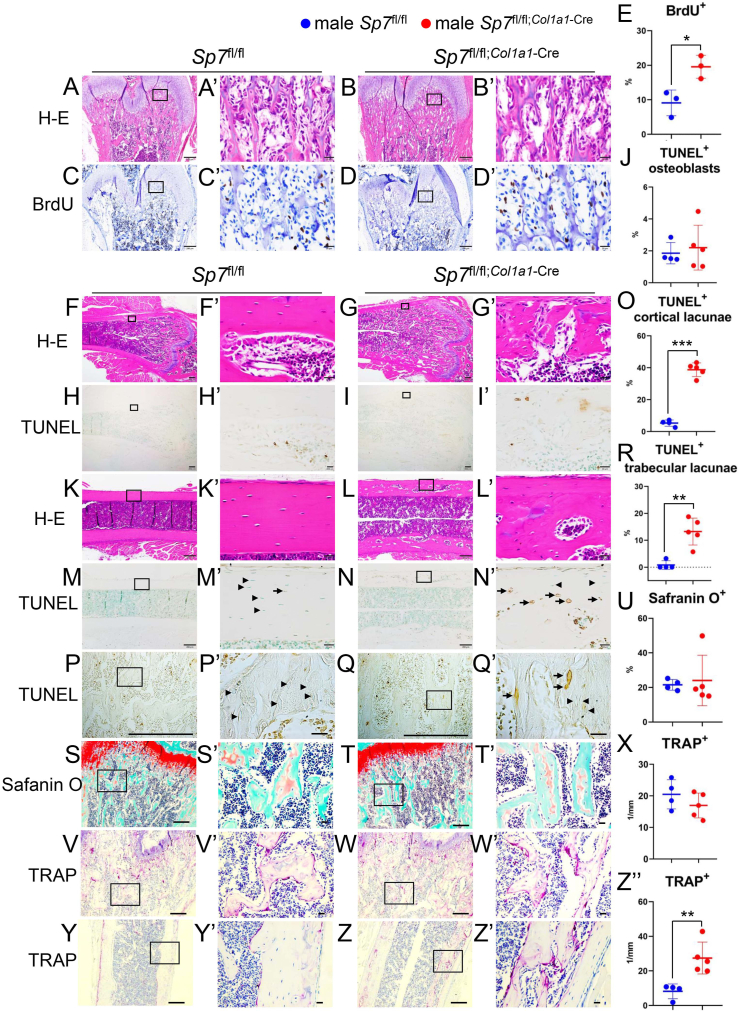
The proliferation of osteoblastic cells was examined by BrdU labeling at three weeks of age. The frequency of BrdU-positive osteoblastic cells was higher in Sp7fl/fl;Col1a1−Cre mice than in Sp7fl/fl mice (Fig. 5A–E). Furthermore, the frequencies of BrdU-positive osteoblastic cells in the two female Sp7fl/fl;Col1a1−Cre mice (19.7 % and 20.0 %) were similar to those in male Sp7fl/fl;Col1a1−Cre mice. The apoptosis of osteoblasts and osteocytes was examined using the TUNEL assay. The frequency of TUNEL-positive osteoblastic cells in the endosteum at the metaphysis was similar between Sp7fl/fl and Sp7fl/fl;Col1a1−Cre mice, whereas that of TUNEL-positive lacunae increased in both trabecular and cortical bones and was higher in cortical bone than in trabecular bone (Fig. 5F–R). Since calcified cartilage accumulated below the growth plate in Sp7fl/–;CAG−CreER mice treated with tamoxifen [4], the cartilage matrix was stained with safranin O. The safranin O-positive area below the growth plate was similar between Sp7fl/fl and Sp7fl/fl;Col1a1−Cre mice, indicating that the resorption of bone and cartilage similarly occurred in the primary spongiosa (Fig. 5S–U). The accumulation of calcified cartilage in Sp7fl/–;CAG−CreER mice treated with tamoxifen is likely due to the impaired degradation of the cartilage matrix by Mmp13 [4,6]. As osteocyte apoptosis has been shown to enhance bone resorption [16], TRAP-positive osteoclasts were counted. The number of TRAP-positive osteoclasts was similar between Sp7fl/fl and Sp7fl/fl;Col1a1−Cre mice in the trabecular bone, whereas it was markedly higher in Sp7fl/fl;Col1a1−Cre mice than in Sp7fl/fl mice in the cortical bone (Fig. 5V–Z″).
Figure 5.
Proliferation of osteoblastic cells, apoptosis of osteoblasts and osteocytes, and bone resorption in the femurs of male Sp7fl/fl and Sp7fl/fl;Col1a1−Cre mice (A–E) Proliferation of osteoblastic cells in trabecular bone of Sp7fl/fl and Sp7fl/fl;Col1a1−Cre mice at 3 weeks of age. A and B, H-E staining. C and D, BrdU staining, which was counterstained with hematoxylin. E, Frequency of BrdU-positive cells in the region shown in C′ and D’. n = 3 (Sp7fl/fl mice) and n = 3 (Sp7fl/fl;Col1a1−Cre mice). (F–R) Apoptosis of osteoblasts and osteocytes in Sp7fl/fl and Sp7fl/fl;Col1a1−Cre mice at 10 weeks of age. F–J, H-E (F, G), and TUNEL (H, I) staining of metaphyseal cortical bone and the frequency of TUNEL-positive osteoblasts in the endosteum (J). K–O, H-E (K, L), and TUNEL (M, N) staining of cortical bone at the mid-diaphysis and the frequency of TUNEL-positive lacunae in cortical bone (O). P–R, TUNEL staining of trabecular bone (P, Q) and the frequency of TUNEL-positive lacunae in trabecular bone (R). Arrows show TUNEL-positive lacunae and arrowheads show TUNEL-negative osteocytes in M′, N′, P′, and Q’. The number of TUNEL-positive lacunae and TUNEL-negative osteocytes were counted, and the frequency of TUNEL-positive lacunae was calculated. TUNEL stained sections were counterstained with methyl green. (S–Z) Safranin O staining of trabecular bone (S, T) and TRAP staining of trabecular bone (V, W) and cortical bone (Y, Z) in Sp7fl/fl and Sp7fl/fl;Col1a1−Cre mice at 10 weeks of age. The ratio of the safranin O-positive area to the total bone area is shown in U, and the number of TRAP-positive cells in the trabecular and cortical bones is shown in X and Z″, respectively. n = 4 (Sp7fl/fl) and n = 5 (Sp7fl/fl;Col1a1−Cre). Boxed regions in A–D, F, G, H, I, K, L, M, N, P, Q, S, T, V, W, Y and Z were magnified in A’ –D′, F′, G′, H′, I′, K′, L′, M′, N′, P′, Q′, S′, T′, V′, W′, Y'and Z′, respectively. Scale bars: 200 μm (A–D, F, G, H, I, K, L, M, N, P, Q, S, T, V, W, Y and Z), 20 μm (A′–D′, F′, G′, H′, I′, K′, L′, M′, N′, P′, Q′, S′, T′, V′, W′, Y'and Z′). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2.6. Histological analysis of canaliculi and Sost expression and gene expression analysis in Sp7fl/fl;Col1a1−Cre mice at 10 weeks of age
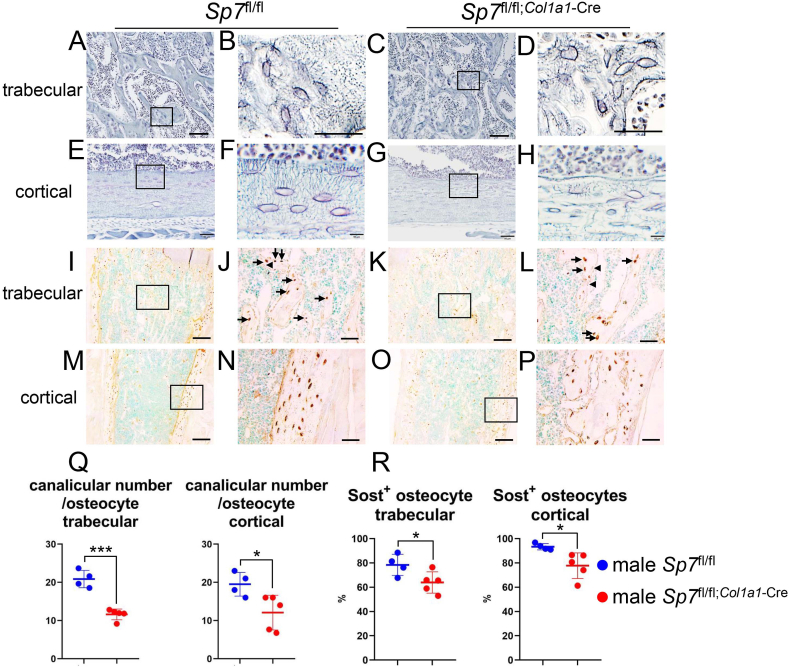
The number of canaliculi per osteocyte in both femoral trabecular and cortical bone was lower in male Sp7fl/fl;Col1a1−Cre mice than in Sp7fl/fl mice and the lacunocanalicular network was severely disorganized (Fig. 6A–H, Q). Sost is expressed in osteocytes and antagonizes canonical Wnt signaling through binding to Wnt co-receptor low density lipoprotein receptor-related ptotein (Lrp) 5 and Lrp6 [11,21,22]. The number of Sost-positive osteocytes in both femoral trabecular and cortical bone was lower in male Sp7fl/fl;Col1a1−Cre mice than in Sp7fl/fl mice (Fig. 6I–P, R).
Figure 6.
Canalicular staining and immunohistochemical analysis of Sost in femoral bone of male Sp7fl/fl and Sp7fl/fl;Col1a1-Cre mice at 10 weeks of age. (A–P) Canalicular staining (A–H) and immunostaining using an anti-Sost antibody (I–P) in trabecular bone (A–D, I–L) and cortical bone (E–H, M−P) of Sp7fl/fl (A, B, E, F, I, J, M, N) and Sp7fl/fl;Col1a1−Cre (C, D, G, H, K, L, O, P) mice. The boxed regions in A, C, E, G, I, K, M, and O are magnified in B, D, F, H, J, L, N, and P, respectively. The average number of canaliculi in one osteocyte was counted (Q), and the percentage of Sost-positive osteocytes was counted (R) in the trabecular and cortical bone.. n = 4 (Sp7fl/fl) and n = 5 (Sp7fl/fl;Col1a1−Cre). Arrows show Sost-positive osteocytes and arrowheads show Sost-negative osteocytes in J and L. The sections were then counterstained with methyl green. Scale bars: 200 μm (A, C, E, G, I, K, M, and O); 50 μm (B, D, F, H, J, L, N, and P). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
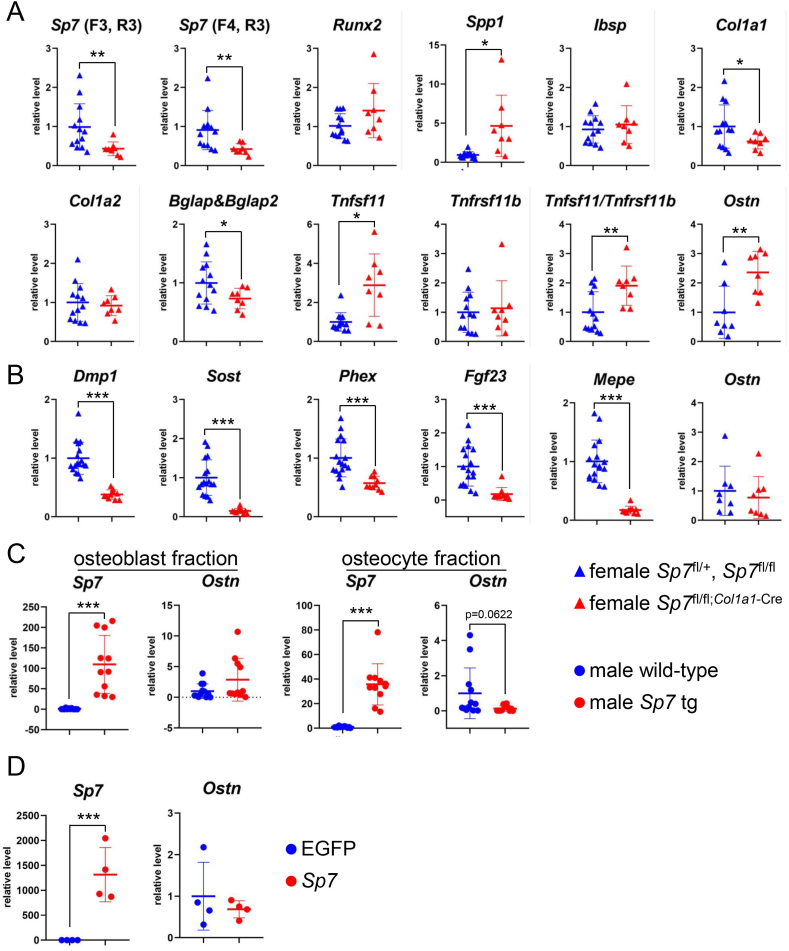
Since male mice were histologically analyzed by perfusion fixation, RNA samples of the osteoblast and osteocyte fractions were collected from female tibiae and the mRNA expression in Sp7fl/fl;Col1a1−Cre mice was compared with that in Sp7fl/+ and Sp7fl/fl mice. The expression of Sp7, Col1a1, and Bglap/Bglap2 was lower, whereas that of Spp1 and Tnfsf11 and the ratio of Tnfrsf11/Tnfrsf11b in the osteoblast fraction were higher in Sp7fl/fl;Col1a1−Cre mice than in the controls (Fig. 7A). The expression of all osteocyte marker genes in the osteocyte fraction, including Dmp1, Sost, phosphate regulating endopeptidase X-linked (Phex), fibroblast growth factor 23 (Fgf23), and matrix extracellular phosphoglycoprotein (Mepe), was markedly lower in Sp7fl/fl;Col1a1−Cre mice than in the controls (Fig. 7B). Although Ostn was previously reported to be responsible for the reduction in osteocyte processes [9], Ostn expression in the osteoblast fraction was higher in Sp7fl/fl;Col1a1−Cre mice than in the control (Fig. 7A). Ostn expression in the osteocyte fraction was similar in Sp7fl/fl;Col1a1−Cre mice and control mice (Fig. 7B). Ostn expression was also examined in osteoblast-specific Sp7 (Col1a1-Sp7) transgenic mice under the control of the 2.3-kb Col1a1 promoter. Ostn expression in both the osteoblast and osteocyte fractions was similar in male Col1a1-Sp7 transgenic mice and the male wild-type littermates (Fig. 7C). Moreover, Sp7 overexpression in osteoblastic MC3T3-E1 cells failed to induce Ostn (Fig. 7D).
Figure 7.
Real-time RT-PCR analyses of osteoblast and osteocyte marker genes, Tnfsf11, Tnfrsf11b, and Ostn (A, B) Real-time RT-PCR analysis of the osteoblast marker genes, Tnfsf11, Tnfrsf11b, and Ostn (A) and osteocyte marker genes and Ostn (B). RNA was extracted from the osteoblast fraction (A) and osteocyte fraction (B) of tibiae in female Sp7fl/fl;Col1a1-Cre and the control (Sp7fl/+ and Sp7fl/fl) mice at 10 weeks of age. The values of Sp7fl/+ and Sp7fl/fl mice were defined as 1 and relative levels are shown. n = 17 (7 Sp7fl/+ and 10 Sp7fl/fl) and n = 10 (Sp7fl/fl;Col1a1−Cre). (C) Sp7 and Ostn expression in osteoblast and osteocyte fractions from the tibiae in male wild-type and Col1a1-Sp7 transgenic (tg) mice at 14 weeks of age. The values of wild-type mice were defined as 1 and relative levels are shown. n = 11 (wild-type) and n = 11 (Sp7 tg). (D) MC3T3-E1 cells were transfected with either pME18-EGFP or pME18-Sp7. The values of pME18-EGFP were defined as 1 and relative levels are shown. n = 4 (pME18-EGFP) and n = 4 (pME18-Sp7). Similar results were obtained in three independent experiments, and representative data are shown.
2.7. Histological analysis of osteoblasts in the morphology and Col1a1 expression in Sp7fl/fl;Col1a1−Cre mice at 3 weeks of age
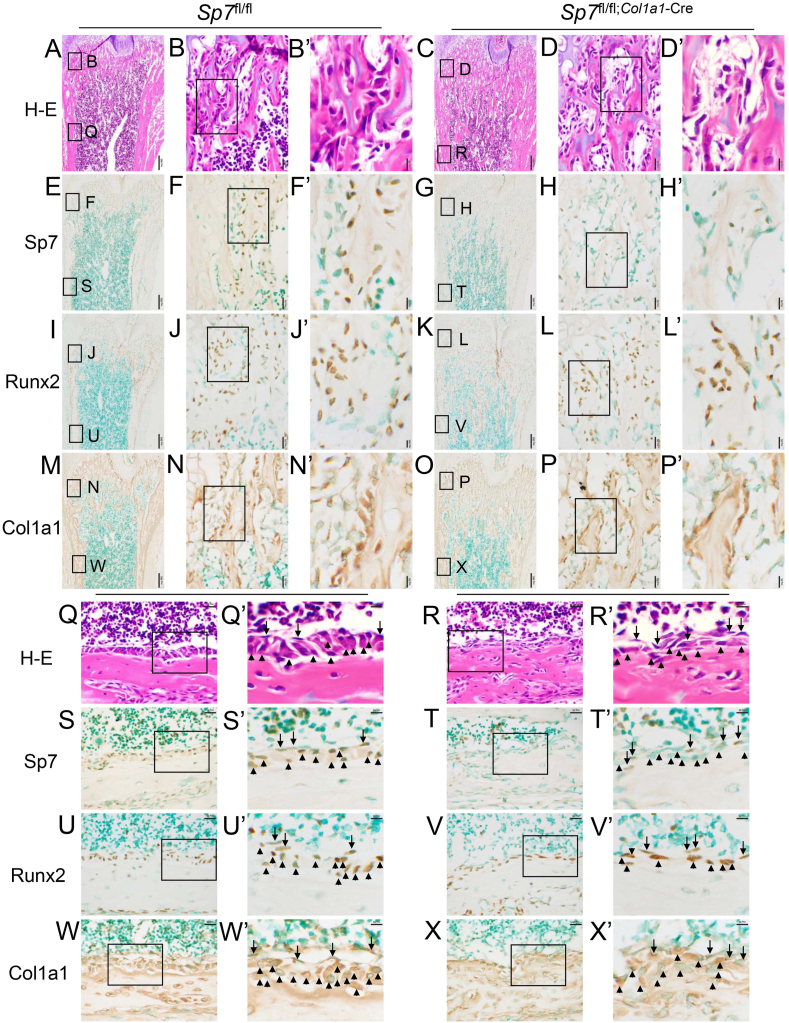
As Col1a1 expression was reduced in Sp7fl/fl;Col1a1−Cre mice (Fig. 7A), the protein expression of Sp7, Runx2, and Col1a1 and the morphology of osteoblasts were compared between Sp7fl/fl and Sp7fl/fl;Col1a1−Cre mice (Fig. 8). Two types of Sp7-positive cells were observed in Sp7fl/fl mice. Sp7-positive cuboidal osteoblasts with a large amount of cytoplasm, located on the surface of the bone, strongly reacted with the Col1a1 antibody in the trabecular bone (Fig. 8B’, F′, N′) and cortical bone (Fig. 8Q’, S′, W′). Sp7-positive flattened preosteoblasts with a small amount of cytoplasm, which were located on the bone marrow side of osteoblasts, were clealy discriminated from the osteoblasts on the cortical bone and weakly reacted with the Col1a1 antibody (Fig. 8Q’, S′, W′). Osteoblasts in Sp7fl/fl;Col1a1−Cre mice were negative for Sp7, less cuboidal than those in Sp7fl/fl mice, and strongly reacted with the Col1a1 antibody in the trabecular bone (Fig. 8D’, H′, P′) and cortical bone (Fig. 8R’, T′, X′). Flattened preosteoblasts in Sp7fl/fl and Sp7fl/fl;Col1a1−Cre mice reacted similarly to anti-Sp7 antibody (Fig. 8S’, T′). Since Runx2 is an upstream transcription factor of Sp7 [1], deletion of Sp7 in osteoblasts did not affect its expression in the trabecular bone (Fig. 8J’, L′) and cortical bone (Fig. 8U’, V’). Therefore, deletion of Sp7 in osteoblasts reduced the amount of Col1a1 in osteoblasts.
Figure 8.
Histological analyses of femurs in Sp7fl/fl and Sp7fl/fl;Col1a1−Cre mice at three weeks of age H-E staining (A–D, Q, R) and immunohistochemical analyses using anti-Sp7 (E–H, S, T), anti-Runx2 (I–L, U, V), and anti-Col1a1 (M−P, W, X) antibodies in the femoral trabecular (A–P) and cortical bone (Q–X) of Sp7fl/fl (A, B, E, F, I, J, M, N, Q, S, U, and W) and Sp7fl/fl;Col1a1−Cre (C, D, G, H, K, L, O, P, R, T, V, and X) mice. Sections were counterstained with methyl green. The boxed regions in A, C, E, G, I, K, M, and O are magnified in B and Q, D and R, F and S, H and T, J and U, L and V, N and W, and P and X, respectively. B, D, F, H, J, L, N, and P–X are magnified in B′, D′, F′, H′, J′, L′, N′, and P′–X′, respectively. The pictures in Q–X were rotated 90°. Arrows show preosteoblasts, and arrowheads show osteoblasts (Q′–X′). Six male Sp7fl/fl mice and three male and two female Sp7fl/fl;Col1a1−Cre mice were analyzed and similar results were obtained. Representative data are presented here. Scale bars: 200 μm (A, C, E, G, I, K, M, and O), 20 μm (B, D, F, H, J, L, N, and P–X), and 5 μm (B′, D′, F′, H′, J′, L′, N′, and P′–X′). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2.8. Regulation of Col1a1 expression, osteoblastogenesis, and osteoclastogenesis by Sp7
Because EGFP expression in 2.3-kb Col1a1 promoter EGFP-Cre transgenic mice reflects endogenous Col1a1 expression [19], it was compared between Sp7fl/+;Col1a1−Cre and Sp7fl/fl;Col1a1−Cre newborn femurs. EGFP intensity was weaker in Sp7fl/fl;Col1a1−Cre mice than in Sp7fl/+;Col1a1−Cre mice (Supplementary Figs. 4A–D). Real-time RT-PCR analysis showed that the expression of Sp7 and EGFP in newborn limbs was lower in Sp7fl/fl;Col1a1−Cre mice than in Sp7fl/+;Col1a1−Cre mice (Supplementary Fig. 4E). Furthermore, overexpression of Sp7 in primary osteoblasts induced Col1a1 expression (Supplementary Fig. 4F). These results suggest that Sp7 controls Col1a1 expression, at least in part, through regulation of the 2.3-kb Col1a1 promoter. Alkaline phosphatase (ALP) and von Kossa staining represent osteoblast differentiation at the early and late stages, respectively. ALP staining was stronger and von Kossa staining was weaker in Sp7fl/fl;Col1a1−Cre mice than in Sp7fl/fl mice, indicating that the number of immature osteoblasts increased among Sp7fl/fl;Col1a1−Cre primary osteoblasts and osteoblast maturation was inhibited (Supplementary Figs. 4G–J). In a co-culture of bone marrow-derived monocyte/macrophage lineage cells (BMMs) from wild-type mice with primary osteoblasts from Sp7fl/fl or Sp7fl/fl;Col1a1−Cre mice, the number of multinucleated osteoclasts was higher and the bone resorption area (pit area) was larger in Sp7fl/fl;Col1a1−Cre mice than in Sp7fl/fl mice (Supplementary Fig. 4K–N). These results suggest that deletion of Sp7 in osteoblasts increases the number of immature osteoblasts, inhibits osteoblast maturation, and promotes osteoclastogenesis in vitro.
3. Discussion
Knockdown of Sp7 (Sp7floxneo/floxneo) in germline or osteoblast-specific Sp7 deletion (Sp7fl/fl;Col1a1−Cre) affected the trabecular bone volume differentially in males and females and in long bones and vertebrae, probably through differential levels in the accumulation of immature osteoblasts. In contrast, the femoral cortical bone was thin and porous in Sp7floxneo/floxneo and Sp7fl/fl;Col1a1−Cre mice of both sexes compared with the respective controls, which was caused by the increased bone resorption due to osteocyte apoptosis through the reduction in the number of osteocyte processes and canaliculi. These findings demonstrated that Sp7 inhibits the proliferation of immature osteoblasts and induces their differentiation, and that Sp7 regulates the process formation of osteoblasts, which is finally essential for the survival of embedded osteocytes and the development and maintenance of cortical bone.
Female Sp7floxneo/floxneo and Sp7fl/fl;Col1a1−Cre mice showed similar phenotypes and the femoral trabecular bone volume was increased, while femoral trabecular bone volume in male Sp7floxneo/floxneo, Sp7floxneo/floxneo;Col1a1−Cre, and Sp7fl/fl;Col1a1−Cre mice was similar to the respective controls. Although we could not perform all experiments in both sexes, BrdU-positive osteoblastic cells were increased in the trabecular bone of male Sp7fl/fl;Col1a1−Cre mice, and osteoblast parameters and bone formation rate increased in the trabecular bone of female Sp7fl/fl;Col1a1−Cre mice. These findings suggest that immature osteoblasts accumulated in the trabecular bone in both male and female Sp7fl/fl;Col1a1−Cre mice, but at different levels, compensating for the inhibited osteoblast maturation, as shown by the increased expression of the immature osteoblast marker gene Spp1 and the reduced expression of the mature osteoblast marker gene Bglap/Bglap2. The increase in an early osteoblast differention marker, ALP, and the reduction of a late marker, mineralization, in the culture of primary osteoblasts from Sp7fl/fl;Col1a1−Cre mice were also compatible with the phenotypes. Thus, Sp7 was considered to inhibit the proliferation of osteoblast lineage cells and induces their differentiation. Estrogen and progesterone may be positively involved in the accumulation of immature osteoblasts in female Sp7fl/fl;Col1a1−Cre mice because they were previously shown to induce osteoblast proliferation and differentiation [23,24]. In contrast, the proliferation of osteoblastic cells was reduced in Runx2fl/fl;Col1a1−Cre mice, which were generated using the same 2.3-kb Col1a1 promoter EGFP-Cre transgenic mice. Bone volume, osteoblast parameters, and bone formation in the femoral trabecular bone decreased in both males and females, clarifying the opposite functions of Sp7 and Runx2 in the proliferation of immature osteoblasts [19,25,26]. The decrease in the number of Sost-expressing osteocytes appeared to have contributed to the accumulation of immature osteoblasts in Sp7fl/fl;Col1a1−Cre mice by enhancing canonical Wnt signaling pathway [11].
Differential changes in femoral and vertebral trabecular bone volumes were observed in both male and female Sp7fl/fl;Col1a1−Cre mice. The differences in the femoral and vertebral trabecular bone were due to the number of osteoblasts and the bone formation rate (Fig. 4A–D). Recently, skeletal stem cells in vertebrae were shown to be different from those in limb bones, with a different gene expression profile [27]. Thus, the proliferation of osteoblast lineage cells and their differentiation in vertebrae and limb bones are likely to be regulated by different transcription factors and signaling pathways.
Bone formation in the femoral cortical bone of Sp7fl/fl;Col1a1−Cre mice was similar to that in Sp7fl/fl mice, but the number of osteoclasts in the cortical bone increased. Thus, the thin and porous cortical bone was attributed to enhanced bone resorption, likely owing to osteocyte apoptosis and necrosis. Among SP7 mutations in humans, a homozygous mutation (R316C) and a heterozygous mutation in zinc finger 2 (E340A) cause osteogenesis imperfecta with high bone turnover and low bone turnover, respectively, and both patients have porous cortical bone [28,29]. Furthermover, the number and length of canaliculi are reduced in patients with the R316C mutation [9]. Although the lacunocanalicular structure was not examined in patients with the E340A mutation, the porous cortical bone in patients with the R316C or E340A mutation may have been caused by osteocyte death due to reductions in osteocyte processes and canaliculi.
The number of TRAP-positive cells increased in the cortical bone but not in the trabecular bone of Sp7fl/fl;Col1a1−Cre mice, probably because the frequency of TUNEL-positive lacunae in the cortical bone was much higher than that in the trabecular bone in Sp7fl/fl;Col1a1−Cre mice. This could be explained by two factors. Most osteocytes in the trabecular bone are close to the bone surface and easily obtain oxygen and nutrients for survival, even with a reduced number of processes. Furthermore, trabecular bone is remodeled faster than cortical bone, and apoptotic/necrotic osteocytes in trabecular bone are removed faster than those in cortical bone. The former reduces the number of apoptotic/necrotic osteocytes, while the latter reduces osteoclastogenesis induced by apoptotic/necrotic osteocytes. As osteoclastogenesis was enhanced in the co-culture of Sp7fl/fl;Col1a1−Cre primary osteoblasts and wild-type BMMs, however, the bone quality of Sp7fl/fl;Col1a1−Cre mice may have negatively affected osteoclastogenesis.
The frequency of Sost-positive osteocytes was lower in Sp7fl/fl;Col1a1−Cre mice than that in Sp7fl/fl mice (Fig. 6R). Direct regulation of the Sost gene by Sp7 was previously reported [4]. However, the frequency of Sost-positive osteocytes in the femur and the serum level of Sost in Col1a1-Sp7 transgenic mice, which have fewer osteocyte processes and canaliculi, were also lower than those in wild-type mice [12]. Therefore, the impaired lacunocanalicular system in Sp7fl/fl;Col1a1−Cre mice and Col1a1-Sp7 transgenic mice may also be responsible for the reduction in the frequency of Sost-positive osteocytes because the lacunocanalicular system is responsible for mechano-sensing and mechano-transduction, and Sost expression changes by mechanical loading [11,12,[30], [31], [32]].
Previous studies demonstrated that Sp7fl/–;CAG−CreER mice treated with tamoxifen and Sp7 deletion by Dmp1 Cre (Sp7OcyKO) reduced the number of osteocyte processes and canaliculi [4,9]. Canalicular number was also reduced in Sp7fl/fl;Col1a1−Cre mice (Fig. 6Q). However, the number of osteocyte processes and canaliculi was also decreased in Col1a1-Sp7 transgenic mice, and these reductions were larger than those in Sp7fl/fl;Col1a1−Cre mice (Fig. 6A–H, Q) [12,33]. As it is difficult for osteocytes to form canaliculi after mineralization, the number of canaliculi will be determined by the number of cell processes at the transitional stage from osteoblasts to osteocytes. Based on these findings, therefore, an appropriate Sp7 level appears to be required for the final stage of osteoblasts to acquire a sufficient number of processes. It has been shown that the knockdown of Sp7 reduces the number of processes in MC3T3-E1 cells, infection with an Ostn-expressing lentivirus restores this decrease, and AAV8-Ostn infection in Sp7OcyKO mice increases the number of canaliculi [9]. However, the expression of Ostn was not reduced in Sp7OcyKO femurs [9]; it was increased in the osteoblast-enriched fraction of Sp7fl/fl;Col1a1−Cre mice, and its expression in the osteocyte fraction of Sp7fl/fl;Col1a1−Cre mice and in the osteoblast and osteocyte fractions of Col1a1-Sp7 transgenic mice were similar to those in the respective controls. Moreover, Sp7 overexpression in MC3T3-E1 cells failed to induce Ostn (Fig. 7B–D). Therefore, the mechanisms underlying the regulation of osteoblast and osteocyte process formation warrant further investigation.
In conclusion, Sp7 is involved in osteoblast proliferation, osteoblast maturation, Col1a1 expression, and process formation in committed osteoblasts. The compensatory mechanisms for impaired osteoblast maturation differed between femurs and vertebrae and between males and females and need to be clarified in the future. To maintain bone volume and quality, it is important to elucidate the mechanisms by which mature osteoblasts/osteocytes acquire a sufficient number of processes for osteocyte survival as well as mechano-sensing and mechano-transduction.
4. Materials and methods
4.1. Mice
Generation of Sp7floxneo/floxneo mice, 2.3-kb Col1a1 EGFP-Cre transgenic mice, CAG-Flp transgenic mice, and osteoblast specific Sp7 transgenic mice under the control of 2.3-kb Col1a1 promoter has been previously described [6,19,33,34]. Sp7floxneo/floxneo mice contain a neomycin resistance gene (neo) in the Sp7 intron. Sp7floxneo/floxneo mice were crossed with 2.3-kb Col1a1 EGFP-Cre transgenic mice to generate Sp7floxneo/floxneo;Col1a1−Cre mice. Sp7floxneo/floxneo mice were crossed with CAG-Flp transgenic mice to remove neo, and Sp7fl/fl mice were generated. Sp7fl/fl mice were crossed with 2.3-kb Col1a1 EGFP-Cre transgenic mice to generate Sp7fl/fl;Col1a1−Cre mice. The backgrounds of Sp7floxneo/floxneo, Sp7fl/fl, and CAG-Flp transgenic mice were C57BL/6. 2.3-kb Col1a1 EGFP-Cre transgenic mice were generated in the B6C3H F1 background and backcrossed with C57BL/6 mice more than 14 times. Prior to the initiation of the study, all experimental protocols were reviewed and approved by the Animal Care and Use Committee of Nagasaki University Graduate School of Biomedical Sciences (No. 1903131520–9). Animals were housed three per cage in a pathogen-free environment on a 12-h light cycle at 22 °C ± 2 °C, with standard chow (CLEA Japan, Tokyo, Japan) and free access to tap water. All relevant guidelines for working with animals were adhered to in this study.
4.2. Real-time RT-PCR and western blot analysis
The osteoblast fraction was collected using a micro-intertooth brush (Kobayashi Pharmaceutical Co., Ltd., Osaka, Japan) from the endosteum of tibiae after bone marrow was flushed out by PBS, and the remaining bone was used for the osteocyte fraction, as previously reported [10]. Total RNA was extracted using ISOGEN (Wako, Osaka, Japan). Real-time RT-PCR was performed using THUNDERBIRD SYBR quantitative PCR (qPCR) Mix (Toyobo, Osaka, Japan) and Light Cycler 480 Real-Time PCR system (Roche Diagnostics, Tokyo, Japan). Primer sequences are shown in Supplemental Table 1. The primer set for Bglap/Bglap2 detected both Bglap and Bglap2. The values obtained were normalized to those of actin beta (Actb) using the 2(−delta delta C(t)) method. Western blotting was performed using rabbit polyclonal anti-Sp7 (Abcam, Cambridge, UK) and anti-β-actin (Santa Cruz Biotechnology, Dallas, TX, USA) antibodies.
4.3. Micro-CT analysis
Micro-CT analysis was performed using a μCT system (R_mCT; Rigaku Corporation, Tokyo, Japan), except for the analysis of the first lumbar vertebra in male mice. Data from scanned slices were used in a three-dimensional analysis to calculate femoral morphometric parameters. Trabecular bone parameters were measured on the distal femoral metaphysis. Approximately 2 mm (0.2 mm from the growth plate) was cranio-caudally scanned and 200 slices at 10-μm increments were taken. In femoral cortical bone, 20 slices in 10-μm increments were taken. To analyze the first lumbar vertebra in female mice, 100 slices were obtained. The threshold of mineral density was 500 mg/cm3. The first lumbar vertebra in male mice were scanned using a micro-CT system (Skyscan1176, Bruker, Aartselaar, Belgium), and 150 slices in 9-μm increments were obtained. The pore area in femoral cortical bone at the mid-diaphysis was measured by Skyscan 1272 (Bruker) at a resolution of 10 μm/voxel.
4.4. Bone histomorphometric analysis
Mice were intraperitoneally injected with calcein 7 and 2 days before sacrifice at a dose of 20 mg/kg body weight, and were examined at 10 weeks of age. Mice were euthanized and their tibiae, femurs, and lumbar vertebrae (L3–L5) were harvested and fixed in 70 % ethanol for three days. The fixed bones were dehydrated with graded ethanol, infiltrated and embedded in a mixture of methyl methacrylate and 2-hydroxyethyl methacrylate (Fujifilm Wako Pure Chemical, Osaka, Japan). Bone histomorphometric analysis of the proximal tibiae and lumbar vertebrae was performed using undecalcified 4-μm-thick sections, as previously described [35]. Bone histomorphometric analysis of cortical bone was conducted using approximately 50-μm-thick cross-sections from the mid-diaphyses of femurs. Structural, dynamic, and cellular parameters were calculated and expressed according to the standard nomenclature [36].
4.5. Serum testing
Blood was collected from the heart and left to stand at room temperature for at least 30 min. Serum was collected after centrifugation at 3000 rpm/min at room temperature for 10 min. Serum levels of P1NP and TRAP5b were measured using a Rat/Mouse P1NP enzyme-linked immunosorbent assay (Immunodiagnostic Systems, Boldon Business Park, UK) and Mouse TRAP Assay (Immunodiagnostic Systems), respectively.
4.6. Histological analysis
Mice were anesthetized and perfusion fixed in 4 % paraformaldehyde/0.1 M phosphate buffer (PFA), and femurs were separated and subjected to further oscillatory fixation with 4 % PFA at 4 °C overnight. After decalcification in 10 % EDTA, the bone samples were embedded in paraffin. Four-micrometer-thick sections were stained with hematoxylin and eosin (H-E), safranin O (Solarbio, Beijing, China), or fast red violet LB salt for TRAP (Absin, Shanghai, China). TUNEL staining was performed using an ApopTag Peroxidase In Situ Apoptosis Detection Kit (Sigma Aldrich, St. Louis, MO, USA) and counterstained with methyl green. To analyze BrdU incorporation, we injected BrdU intraperitoneally into 3-week-old mice at 100 μg/g body weight 1 h before sacrifice, and BrdU incorporation was detected using a BrdU staining kit (Invitrogen, Carlsbad, CA, USA). The sections were counterstained with hematoxylin. Bone canalicular staining (silver impregnation staining) was performed using Silver Protein (198–18101: FUJIFILM-Wako) according to a previously reported method [37]. BrdU-positive osteoblastic cells, TUNEL-positive lacunae, Sost-positive osteocytes, canalicular number, and Safranin O-positive area in trabecular bone were counted or measured in three enlarged trabecular areas and then averaged. TUNEL-positive lacunae, Sost-positive osteocytes, and the canalicular number in cortical bone were counted in four areas: anterior metaphyseal, posterior metaphyseal, anterior diaphyseal, and posterior diaphyseal cortical bones. TUNEL-positive osteoblasts were counted in three areas of the endosteum at the metaphysis. In the analysis of the canalicular number, at least 10 lacunae with live osteocytes were randomly selected, the number of canaliculi in each osteocyte was counted, and counts were averaged. In each experiment, one section was analyzed for each mouse. Immunohistochemistry was performed using polyclonal goat anti-Sost (R&D, Minneapolis, MN, USA), polyclonal rabbit anti-Sp7 (Abcam, Cambridge, UK), monoclonal rabbit anti-Runx2 (Cell Signaling, Danvers, MA, USA), and polyclonal rabbit anti-Col1a1 (Rockland, Limerick, PA, USA) antibodies. Secondary antibodies were Histofine Stain MAXPO (R) (Nichirei, Tokyo, Japan) for anti-Sp7, anti-Runx2, and anti-Col1a1 antibodies, and a goat two-step test kit (ZSGB-BIO, Beijing, China) for the anti-Sost antibody. Immunohistochemistry without the first antibodies resulted in no significant signals. To obtain frozen sections, newborn mice were euthanized and fixed in PFA at 4 °C for 2 h, washed with PBS at 4 °C for 1 h, immersed in 20 % sucrose at 4 °C overnight, embedded in optimum cutting temperature (O.C.T.) compound (Sakura Finetek, Tokyo, Japan), and sectioned at a thickness of 7 μm using a Leica CM3050S research cryostat (Leica Biosystems, Wetzlar, Germany).
4.7. Cell culture and Sp7 overexpression
Primary osteoblasts and osteoblast progenitors were isolated from the wild-type calvariae of newborn mice. The calvariae were cut into small pieces and cultured for 10–14 days in a three-dimensional collagen gel (Cell matrix, Nitta Gelatin, Co., Osaka, Japan) with α-modified Minimum Essential Medium (α-MEM) containing 10 % fetal bovine serum (FBS). Cells outgrowing from explants were retrieved by an incubation at 37 °C for 30 min with 0.2 % collagenase (Wako Pure Chemical Industries, Osaka, Japan) in PBS (−). In this method, the main cell types isolated were osteoblast progenitors and osteoblasts at an early differentiation stage with low ALP activity and virtually no Bglap/Bglap2 production [38]. Cells were plated in 24-well plates at a density of 1.9 × 104 cells/cm2 in αMEM supplemented with 10 % FBS. Cells were transfected with EGFP-expressing (pME18-EGFP) or type I Sp7-expressing (pME18-Sp7) vector using X-tremeGENE9 (Roche Diagnostics). Transfected cells were cultured for 48 h before RNA extraction.
4.8. In vitro osteoblastogenesis
Primary osteoblasts were isolated from the calvariae of Sp7fl/fl and Sp7fl/fl;Col1a1−Cre newborns by sequential digestion with 0.1 % collagenase A and 0.2 % dispase. Osteoblastic cells from the third to fifth fractions were pooled and used for osteoblast differentiation. Cells were seeded on 48-well plates at a density of 1.3 × 105 cells/cm2, and medium was changed to osteogenic medium containing 50 μg/ml of ascorbic acid and 10 mM β-glycerophosphate at confluence. Staining for ALP and mineralization (von Kossa) was performed 5 and 13 days after confluence, respectively, as previously described [19].
4.9. In vitro osteoclastogenesis
BMMs were isolated by density gradient centrifugation using Ficoll–Paque™ (GE Healthcare, Tokyo, Japan) from the bone marrow of 10-week-old wild-type mice. BMMs at 2.5 × 105 cells/cm2 were co-cultured with primary osteoblasts, which were prepared from the calvariae of Sp7fl/fl and Sp7fl/fl;Col1a1−Cre newborn mice using a three-dimensional collagen gel, at 1.3 × 104 cells/cm2 in α-MEM containing 10 % FBS in the presence of 50 μg/ml of ascorbic acid and 10−8 M 1α,25 (OH)2D3 in 24-well plates for 8 days. TRAP staining was performed as previously described [39]. To assess bone resorption, BMMs at 5 × 104 cells/cm2 were co-cultured with primary osteoblasts at 2.6 × 104 cells/cm2 in α-MEM containing 10 % FBS in the presence of 50 μg/ml of ascorbic acid and 10−8 M 1α,25 (OH)2D3 on dentin slices (Wako) in 96-well plates for 13 days, and the resorbed area was measured by scanning electron microscopy (H-7100; Hitachi, Tokyo, Japan).
4.10. Graphical abstract
Gaphical abstract was drawed by Figdraw.
4.11. Statistical analysis
Values are shown as the mean ± SD. Statistical analyses were performed using the Student's t-test, and those of more than three groups were conducted by ANOVA and the Tukey–Kramer post-hoc test.
5. Category 3
Approval of the version of the manuscript to be published (the names of all authors must be listed):
Qing Jiang, Kenichi Nagano, Takeshi Moriishi, Hisato Komori, Chiharu Sakane, Yuki Matsuo, Zhiguo Zhang, Riko Nishimura, Kosei Ito, Xin Qin, Toshihisa Komori.
6. Article Processing Charge
The corresponding author agrees to pay the Journal of Orthopaedic Translation Article Processing Charge upon acceptance of the work for publication in Journal of Orthopaedic Translation, unless prior arrangements have been made to waive the Article Processing Charge.
Author names and details of the conflict(s) of interest
This Authorship & Conflicts of Interest Statement is signed by all the authors listed in the manuscript to indicate agreement that the above information is true and correct (a photocopy of this form may be used if there are more than 10 authors).
Disclosures
The authors declare that they have no conflicts of interest.
CRediT authorship contribution statement
Qing Jiang: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Funding acquisition. Kenichi Nagano: Investigation, Formal analysis, Methodology, Software, Funding acquisition. Takeshi Moriishi: Investigation, Methodology, Funding acquisition. Hisato Komori: Investigation, Funding acquisition. Chiharu Sakane: Investigation, Funding acquisition. Yuki Matsuo: Project administration, Investigation, Funding acquisition. Zhiguo Zhang: Methodology. Riko Nishimura: Methodology. Kosei Ito: Methodology. Xin Qin: Investigation, Data curation, Validation, Supervision, Writing – original draft, Funding acquisition. Toshihisa Komori: Data curation, Validation, Supervision, Writing – original draft, Writing – review & editing, Funding acquisition.
Declaration of competing interest
A conflict of interest occurs when an individual's objectivity is potentially compromised by a desire for financial gain, prominence, professional advancement or a successful outcome. The Editors of the Journal of Orthopaedic Translation strive to ensure that what is published in the Journal is as balanced, objective and evidence-based as possible. Since it can be difficult to distinguish between an actual conflict of interest and a perceived conflict of interest, the Journal requires authors to disclose all and any potential conflicts of interest.
The authors whose names are listed immediately below certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Acknowledgments
This work was supported by grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology, Japan to Toshihisa Komori (23H00440), Kenichi Nagano (19K10056), Takeshi Moriishi (23K09120), Hisato Komori (23K08591), Chiharu Sakane (22K11805), and Yuki Matsuo (24K12356), Key Laboratory of Orthopaedics of Suzhou (SZS2022017) to Qing Jiang and Xin Qin, and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), China to Qing Jiang and Xin Qin. We thank B. De Crombrugghe for the 2.3-kb Col1a1 promoter, H. Kaneko for technical and secretarial assistances.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2024.06.005.
Contributor Information
Xin Qin, Email: xqin@suda.edu.cn.
Toshihisa Komori, Email: komorit@nagasaki-u.ac.jp.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Komori T. Whole aspect of Runx2 functions in skeletal development. Int J Mol Sci. 2022;23:5776. doi: 10.3390/ijms23105776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hojo H., Ohba S., He X., Lai L.P., McMahon A.P. Sp7/Osterix is restricted to bone-forming vertebrates where it acts as a Dlx co-factor in osteoblast specification. Dev Cell. 2016;37:238–253. doi: 10.1016/j.devcel.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakashima K., Zhou X., Kunkel G., Zhang Z., Deng J.M., Behringer R.R., et al. The novel zinc finger-containing transcription factor Osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 4.Zhou X., Zhang Z., Feng J.Q., Dusevich V.M., Sinha K., Zhang H., et al. Multiple functions of Osterix are required for bone growth and homeostasis in postnatal mice. Proc Natl Acad Sci USA. 2010;107:12919–12924. doi: 10.1073/pnas.0912855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J.S., Tokavanich N., Wein M.N. SP7: from bone development to skeletal disease. Curr Osteoporos Rep. 2023;21:241–252. doi: 10.1007/s11914-023-00778-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishimura R., Wakabayashi M., Hata K., Matsubara T., Honma S., Wakisaka S., et al. Osterix regulates calcification and degradation of chondrogenic matrices through matrix metalloproteinase 13 (MMP13) expression in association with transcription factor Runx2 during endochondral ossification. J Biol Chem. 2012;287:33179–33190. doi: 10.1074/jbc.M111.337063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baek W.Y., Lee M.A., Jung J.W., Kim S.Y., Akiyama H., de Crombrugghe B., et al. Positive regulation of adult bone formation by osteoblast-specific transcription factor Osterix. J Bone Miner Res. 2009;24:1055–1065. doi: 10.1359/jbmr.081248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baek W.Y., Crombrugghe B., Kim J.E. Postnatally induced inactivation of Osterix in osteoblasts results in the reduction of bone formation and maintenance. Bone. 2010;46:920–928. doi: 10.1016/j.bone.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J.S., Kamath T., Mazur C.M., Mirzamohammadi F., Rotter D., Hojo H., et al. Control of osteocyte dendrite formation by Sp7 and its target gene osteocrin. Nat Commun. 2021;12:6271. doi: 10.1038/s41467-021-26571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moriishi T., Maruyama Z., Fukuyama R., Ito M., Miyazaki T., Kitaura H., et al. Overexpression of Bcl2 in osteoblasts inhibits osteoblast differentiation and induces osteocyte apoptosis. PLoS One. 2011;6 doi: 10.1371/journal.pone.0027487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moriishi T., Fukuyama R., Ito M., Miyazaki T., Maeno T., Kawai Y., et al. Osteocyte network; a negative regulatory system for bone mass augmented by the induction of Rankl in osteoblasts and Sost in osteocytes at unloading. PLoS One. 2012;7 doi: 10.1371/journal.pone.0040143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moriishi T., Ito T., Fukuyama R., Qin X., Komori H., Kaneko H., et al. Sp7 transgenic mice with a markedly impaired lacunocanalicular network induced Sost and reduced bone mass by unloading. Int J Mol Sci. 2022;23:3173. doi: 10.3390/ijms23063173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kennedy O.D., Laudier D.M., Majeska R.J., Sun H.B., Schaffler M.B. Osteocyte apoptosis is required for production of osteoclastogenic signals following bone fatigue in vivo. Bone. 2014;64:132–137. doi: 10.1016/j.bone.2014.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckley K.A., Hipskind R.A., Gartland A., Bowler W.B., Gallagher J.A. Adenosine triphosphate stimulates human osteoclast activity via upregulation of osteoblast-expressed receptor activator of nuclear factor-kappa B ligand. Bone. 2002;31:582–590. doi: 10.1016/s8756-3282(02)00877-3. [DOI] [PubMed] [Google Scholar]
- 15.Cheung W.Y., Fritton J.C., Morgan S.A., Seref-Ferlengez Z., Basta-Pljakic J., Thi M.M., et al. Pannexin-1 and P2X7-receptor are required for apoptotic osteocytes in fatigued bone to trigger RANKL production in neighboring bystander osteocytes. J Bone Miner Res. 2016;31:890–899. doi: 10.1002/jbmr.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komori T. Cell death in chondrocytes, osteoblasts, and osteocytes. Int J Mol Sci. 2016;17:2045. doi: 10.3390/ijms17122045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zong W.X., Thompson C.B. Necrotic death as a cell fate. Genes Dev. 2006;20:1–15. doi: 10.1101/gad.1376506. [DOI] [PubMed] [Google Scholar]
- 18.Lotze M.T., Tracey K.J. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 19.Qin X., Jiang Q., Komori H., Sakane C., Fukuyama R., Matsuo Y., et al. Runt-related transcription factor-2 (Runx2) is required for bone matrix protein gene expression in committed osteoblasts in mice. J Bone Miner Res. 2021;36:2081–2095. doi: 10.1002/jbmr.4386. [DOI] [PubMed] [Google Scholar]
- 20.Nishio Y., Dong Y., Paris M., O'Keefe R.J., Schwarz E.M., Drissi H. Runx2-mediated regulation of the zinc finger Osterix/Sp7 gene. Gene. 2006;372:62–70. doi: 10.1016/j.gene.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 21.Li X., Zhang Y., Kang H., Liu W., Liu P., Zhang J., et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280:19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- 22.Semenov M., Tamai K., He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem. 2005;280:26770–26775. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- 23.Ernst M., Heath J.K., Schmid C., Froesch R.E., Rodan G.A. Evidence for a direct effect of estrogen on bone cells in vitro. J Steroid Biochem. 1989;34:279–284. doi: 10.1016/0022-4731(89)90092-7. [DOI] [PubMed] [Google Scholar]
- 24.Scheven B.A., Damen C.A., Hamilton N.J., Verhaar H.J., Duursma S.A. Stimulatory effects of estrogen and progesterone on proliferation and differentiation of normal human osteoblast-like cells in vitro. Biochem Biophys Res Commun. 1992;186:54–60. doi: 10.1016/s0006-291x(05)80774-0. [DOI] [PubMed] [Google Scholar]
- 25.Kawane T., Qin X., Jiang Q., Miyazaki T., Komori H., Yoshida C.A., et al. Runx2 is required for the proliferation of osteoblast progenitors and induces proliferation by regulating Fgfr2 and Fgfr3. Sci Rep. 2018;8 doi: 10.1038/s41598-018-31853-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin X., Jiang Q., Miyazaki T., Komori T. Runx2 regulates cranial suture closure by inducing hedgehog, Fgf, Wnt and Pthlh signaling pathway gene expressions in suture mesenchymal cells. Hum Mol Genet. 2019;28:896–911. doi: 10.1093/hmg/ddy386. [DOI] [PubMed] [Google Scholar]
- 27.Sun J., Hu L., Bok S., Yallowitz A.R., Cung M., McCormick J., et al. A vertebral skeletal stem cell lineage driving metastasis. Nature. 2023;621:602–609. doi: 10.1038/s41586-023-06519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fiscaletti M., Biggin A., Bennetts B., Wong K., Briody J., Pacey V., et al. Novel variant in Sp7/Osx associated with recessive osteogenesis imperfecta with bone fragility and hearing impairment. Bone. 2018;110:66–75. doi: 10.1016/j.bone.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 29.Ludwig K., Ward L.M., Khan N., Robinson M.E., Miranda V., Bardai G., et al. Dominant osteogenesis imperfecta with low bone turnover caused by a heterozygous SP7 variant. Bone. 2022;160 doi: 10.1016/j.bone.2022.116400. [DOI] [PubMed] [Google Scholar]
- 30.Robling A.G., Niziolek P.J., Baldridge L.A., Condon K.W., Allen M.R., Alam I., et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 31.Lin C., Jiang X., Dai Z., Guo X., Weng T., Wang J., et al. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res. 2009;24:1651–1661. doi: 10.1359/jbmr.090411. [DOI] [PubMed] [Google Scholar]
- 32.Moustafa A., Sugiyama T., Prasad J., Zaman G., Gross T.S., Lanyon L.E., et al. Mechanical loading-related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered. Osteoporos Int. 2012;23:1225–1234. doi: 10.1007/s00198-011-1656-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshida C.A., Komori H., Maruyama Z., Miyazaki T., Kawasaki K., Furuichi T., et al. SP7 inhibits osteoblast differentiation at a late stage in mice. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otani S., Date Y., Ueno T., Ito T., Kajikawa S., Omori K., et al. Runx3 is required for oncogenic Myc upregulation in p53-deficient osteosarcoma. Oncogene. 2022;41:683–691. doi: 10.1038/s41388-021-02120-w. [DOI] [PubMed] [Google Scholar]
- 35.Qin X., Jiang Q., Nagano K., Moriishi T., Miyazaki T., Komori H., et al. Runx2 is essential for the transdifferentiation of chondrocytes into osteoblasts. PLoS Genet. 2020;16 doi: 10.1371/journal.pgen.1009169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dempster D.W., Compston J.E., Drezner M.K., Glorieux F.H., Kanis J.A., Malluche H., et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28:2–17. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kusuzaki K., Kageyama N., Shinjo H., Takeshita H., Murata H., Hashiguchi S., et al. Development of bone canaliculi during bone repair. Bone. 2000;27:655–659. doi: 10.1016/s8756-3282(00)00383-5. [DOI] [PubMed] [Google Scholar]
- 38.Komori T., Yagi H., Nomura S., Yamaguchi A., Sasaki K., Deguchi K., et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 39.Maruyama Z., Yoshida C.A., Furuichi T., Amizuka N., Ito M., Fukuyama R., et al. Runx2 determines bone maturity and turnover rate in postnatal bone development and is involved in bone loss in estrogen deficiency. Dev Dynam. 2007;236:1876–1890. doi: 10.1002/dvdy.21187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.