Abstract
Objectives
Knowledge, access, and use of testing and antiviral treatments is critical to managing and mitigating the continuing burden of the novel Corona Virus (COVID-19) in the United States. This study measured knowledge, attitude, behaviors, and self-reported barriers towards COVID-19 testing and outpatient anti-viral medications (OPA) treatments among Black and older individuals who face greater hospitalization and mortality from the disease.
Study design
Cross-sectional structured survey.
Methods
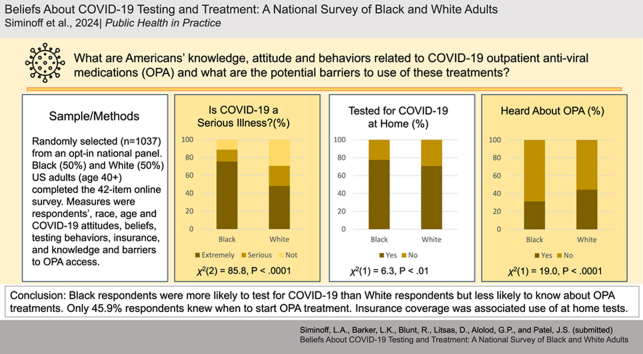
Respondents were randomly selected from an opt-in national panel in December 2022. Equal numbers of Black and White US adults over the age of 40 (n = 1037) completed the 42 item online survey. The main measures were key sociodemographic variables of respondents, race, age, political affiliation and COVID-19 attitudes, beliefs, testing behaviors, and knowledge and barriers to OPA access.
Results
Overall, awareness and knowledge of COVID-19 outpatient treatments was low. Black respondents were more likely to test for COVID-19 than White respondents but less likely to know about OPA treatments. Insurance coverage was a significant factor in use of home tests. Knowledge of OPA treatments was low across groups. White respondents were more likely than Black respondents to be aware of OPA treatments (1.75, 95 % CI [1.31–2.33]) as were higher income respondents (1.13, 95 % CI [1.08–1.17]) and self-identified Liberals (1.79, 95 % CI [1.29–2.49]).
Conclusions
Clinicians should know large numbers of patients may not be testing for COVID-19, nor are they aware of outpatient treatment options and may hold inaccurate beliefs about them. Developing culturally specific patient education materials are warranted to increase testing, utilization of vaccinations and OPAs.
Keywords: COVID-19 attitudes and knowledge, Infectious disease prevention, Antiviral treatment, Health disparities, Health communication
Graphical abstract
1. Introduction
Even though the novel Corona Virus (COVID-19) pandemic is over [1], excess morbidity and death from COVID-19 continues, making it the third leading cause of death in the US [2]. This burden is not equitably distributed in the US and disparities in COVID-19 infection and outcomes vary between older and younger people [3], individuals within different workplaces and occupations [4,5], and between people from different racial and ethnic groups [6,7]. Blacks are over twice as likely as Whites to be hospitalized due to COVID-19 8 and older individuals are at greatest risk for hospitalization and death. When adjusted for age differences [9], Blacks have a 1.6 times higher mortality rate than Whites [8]. It is likely that large swaths of the population will never be vaccinated [[10], [11], [12], [13], [14]]. As COVID-19 testing and vaccination requirements are de-emphasized [15], knowledge of and access to COVID-19 treatment, especially the newer outpatient anti-viral medications (OPA) become important to reduce mortality and morbidity. Nonetheless, utilization of OPAs is low [16] although factors inhibiting adoption are unclear.
Currently, there are two OPA drugs available to the public (Paxlovid and Lagevrio/Molnupiravir) [17] with more in development [18]. Both drugs are antiviral therapies but work differently. Paxlovid includes an antiviral booster and inhibits a key COVID enzyme [19]. Molnupiravir works by incorporating itself into viral RNA synthesis resulting in mutations that inhibit the COVID virus from functioning [20]. Both are taken orally in an outpatient setting within 5 days of onset of symptoms and can reduce rates of hospitalization and death by up to 89 % [21,22].
COVID-19 testing hesitancy or lack of access is a significant barrier to treatment. Although a "Test-to-Treat" COVID-19 protocol was introduced in Pharmacies in March 2022 [23,24] to facilitate rapid testing and treatment, access to OPAs continues to be limited [25]. Pharmacies have charged patients up to $100 for appointments to obtain a prescription [26] and the program has shown geographical inaccessibility for large portions of the population [27]. Further, patients’ beliefs about the seriousness of COVID-19, fear of loss of income or social stigma may influence whether or not they test [[28], [29], [30], [31], [32], [33], [34]]. However, the studies examining predictors of COVID-19 testing have had small sample sizes [30,35], or were conducted early in the pandemic, before the availability of OPAs [[31], [32], [33],[36], [37], [38]].
Another potential barrier to the administration of OPAs include misinformation about COVID-19 treatments [[39], [40], [41], [42]]. Patients may have apprehensions about symptom “rebound” [43,44], a concern shared by some physicians [45]. Only a single study has surveyed the American public's reasons for taking or not OPA treatment [16]. Its results suggest reluctance to take early treatment when initial symptoms are perceived as mild or when no recommendation from their healthcare provider is forthcoming [16].
The aim of this study was to explore the attitudes, behaviors, and self-reported barriers towards COVID-19 testing and early treatment such as OPAs among older (over 40 years old) Black and White adults in the US. The significance of this study is that it would provide new knowledge about the barriers to COVID-19 testing and OPA treatment and inform development of culturally-specific COVID-19 education to increase testing and OPA treatment.
2. Methods
This anonymized survey study was deemed exempt by the Temple University Institutional Review Board after review, consistent with the updated Common Rule guidelines [46].
2.1. Survey design and measurement
The survey was designed to collect information about attitudes and past and projected future behaviors concerning COVID-19 testing and treatment. Best practices in survey design were adhered to by employing an approach that considered the language needs of nonwhite and lower literacy populations, as highlighted by Barreto et al. (2018) [47].
The survey was comprised of 42 items designed for ease of self-administration and comprehension by a heterogeneous group of respondents. Survey questions used standardized scales or previously piloted questions. Respondents were asked to complete 11 sociodemographic questions (See Table 1). Testing behaviors were assessed using 5 closed-ended questions including the number and type of tests taken and how they paid for them. Two 5-point Likert items measured COVID-19 testing self-efficacy. Reasons why respondents chose to test for COVID-19 and perceived barriers and access to COVID-19 testing were collected using single multi-select questions based on prior research and piloting [[48], [49], [50]]. Infection history, hospitalization, and long COVID-19 experience were assessed with yes/no questions. Early treatment knowledge was assessed with 5 true/false questions comprising a composite scale. Basic awareness of OPA treatments was assessed with 1 yes/no question and 6 true/false questions, which formed a summary scale of OPA knowledge with higher scores indicating more knowledge. Perceived access to OPA treatments was assessed with a yes/no question and source of prescription was measured with a 6 choice multi-select question. Attitudes towards the utility of testing and the perceived severity of COVID-19 were measured with two 5-point Likert items. (See survey included in appendix).
Table 1.
Sample demographic and outcome variables (n = 1037).
| All |
Self-Tested |
Aware of OPAs |
|||
|---|---|---|---|---|---|
|
N=1037 |
Yes (N=768) |
No (N=269) |
Yes (N=394) |
No (N=643) |
|
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |
| Race | |||||
| Black | 516 (49.8) | 400 (52.1) | 116 (43.1) | 162 (41.1) | 354 (55.1) |
| White | 521 (50.2) | 368 (47.9) | 153 (56.9) | 232 (58.9) | 289 (44.9) |
| Hispanic | |||||
| Yes | 27 (2.6) | 21 (2.7) | 6 (2.2) | 13 (3.3) | 14 (2.2) |
| Sex | |||||
| Female | 521 (50.2) | 391 (50.9) | 130 (48.3) | 200 (50.8) | 321 (49.9) |
| Age (Years), M(SD) | 58.4 (11.5) | 58.2 (11.5) | 58.9 (11.6) | 59.4 (11.3) | 57.8 (11.6) |
| Annual Income | |||||
| Less than $30,000 | 318 (30.7) | 224 (29.2) | 94 (34.9) | 96 (24.4) | 222 (34.5) |
| $30,000 - $59,999 | 316 (30.5) | 239 (31.1) | 77 (28.6) | 103 (26.1) | 213 (33.1) |
| More than $60,000 | 366 (35.3) | 284 (37) | 82 (30.5) | 184 (46.7) | 182 (28.3) |
| NR | 37 (3.6) | 21 (2.7) | 16 (5.9) | 11 (2.8) | 26 (4) |
| Education | |||||
| High School or Less | 274 (26.4) | 188 (24.5) | 86 (32) | 69 (17.5) | 205 (31.9) |
| Some College/Trade | 427 (41.2) | 318 (41.4) | 109 (40.5) | 142 (36) | 285 (44.3) |
| 4-year Degree or More | 336 (32.4) | 262 (34.1) | 74 (27.5) | 183 (46.4) | 153 (23.8) |
| Insurance | |||||
| Yes | 932 (89.9) | 713 (92.8) | 219 (81.4) | 364 (92.4) | 568 (88.3) |
| Insurance Type | |||||
| Public | 585 (56.4) | 437 (56.9) | 148 (55) | 227 (57.6) | 358 (55.7) |
| Private | 403 (38.9) | 322 (41.9) | 81 (30.1) | 169 (42.9) | 234 (36.4) |
| Political Affiliation | |||||
| Conservative | 327 (31.5) | 228 (29.7) | 99 (36.8) | 108 (27.4) | 219 (34.1) |
| Moderate | 457 (44.1) | 350 (45.6) | 107 (39.8) | 161 (40.9) | 296 (46) |
| Liberal | 252 (24.3) | 189 (24.6) | 63 (23.4) | 125 (31.7) | 127 (19.8) |
| Self-Tested (y/n) | |||||
| Yes | 768 (74.1) | 768 (100) | 0 (0) | 314 (79.7) | 454 (70.6) |
| No | 269 (25.9) | 0 (0) | 269 (100) | 80 (20.3) | 189 (29.4) |
| Aware of OPAs (y/n) | |||||
| Yes | 394 (38) | 314 (40.9) | 80 (29.7) | 394 (100) | 0 (0) |
| No | 643 (62) | 454 (59.1) | 189 (70.3) | 0 (0) | 643 (100) |
2.2. Study population and data collection
Data was collected in December 2022 via a nationally distributed, online survey hosted by Qualtrics Inc. utilizing a representative research panel to target nonhealth professional adults over the age of 40who self-identified as either Black/African American or White/Caucasian. Respondents also self-identified sex (male/female/nonbinary). Quota sampling ensured a balanced distribution of race and male/female. Respondents reporting another sex were not included in the analysis (n = 4). The approach yielded 1037 respondents with an average survey completion time of 7.0 min. Surveys completed under 2 min and surveys that were not submitted as directed were excluded. Respondents received compensation from Qualtrics for completion.
2.3. Data analysis
Bivariate analyses between the independent and dependent variables were performed using chi-square for nominal data and t-test for continuous data. Results were deemed significant at α = 0.05. Two multiple logistic regression models were used to evaluate the relationship between demographic characteristics and the primary outcomes: (1) COVID-19 testing behaviors and (2) awareness of OPA treatment options (Paxlovid and Lagevrio/Molnupiravir). Model variables included age, race, sex, annual income, political self-characterization, and insurance status. Although interaction terms were incorporated to assess relationships among the independent variables, in both instances the simpler models outperformed across several measures (AIC, AUC, and the Hosmer-Lemeshow test) while maintaining interpretability. All data analyses were performed using SAS 9.4.
3. Results
3.1. Sample demographics
The sample was comprised of 49.8 % (n = 516) of Black and 50.2 % (n = 521) White respondents. Of the Black and White respondents, 2.6 % (n = 27) of the sample also identified as Hispanic ethnicity. Females represented 50.2 % (n = 521) of the sample. A majority of the sample (n = 763, 73.6 %) reported some college or technical education with the remaining (n = 274, 26.4 %) reporting a high school diploma equivalent or less (see Table 1).
Black and White respondents differed significantly on several sample characteristics. Blacks were significantly younger than Whites (55.5 years (SD 10.7) vs. 61.2 years (SD 11.7), P < 0.001) and Whites were more likely to report a bachelor or higher degree (n = 195, 37.4 %) as compared to Blacks (n = 141, 27.3 %; P = 0.004). Blacks leaned towards 'liberal' or 'moderate' political beliefs more than Whites (n = 153 (29.7 %) vs. n = 99 (19.0 %); n = 258 (50 %) vs. n = 199 (38.2 %)), while Whites were notably more inclined to identify as 'conservative' (n = 223 (42.8 %) vs. n = 104 (20.2 %)); all differences were statistically significant (P < 0.001).
3.2. COVID-19 experience
One-third of the sample reported having had COVID-19 (n = 325, 31.3 %), with no statistically significant differences between Whites and Blacks. Of these, 5.5 % (n = 18) were hospitalized, and 30.5 % (n = 99) reported long term health problems of at least 1–3 months or longer duration. Blacks were more likely to have tested for COVID-19 than Whites (n = 400 (77.5 %) vs n = 368 (70.6 %); P < 0.01). Overall, 74.1 % (n = 768) of the sample had taken a COVID-19 test, had not paid out-of-pocket and 92 % (n = 954) felt confident that they could obtain a COVID-19 test if needed.
3.3. COVID-19 severity beliefs
Over 20 % (n = 210) of respondents rated COVID-19 infection as “Not serious” with men more likely than women to hold this belief (n = 120 (23.4 %) vs n = 90 (17.3 %); P < 0.001). Likewise, politically conservative respondents regarded the disease as less serious (n = 109, 33.3 %) compared to moderate (n = 80, 17.5 %) or liberal respondents (n = 22, 8.7 %; P < 0.001) (Table 2). Blacks were more likely to characterize COVID-19 as a serious/extremely serious disease (n = 458 (88.8 %) vs. n = 368 (70.7 %); P < 0.001), and rate COVID-19 testing as very or moderately useful (n = 467 (90.5 %) vs. n = 419 (80.5 %; P < 0.001) compared to Whites.
Table 2.
Perceived severity of COVID-19.
| How serious a disease do you believe COVID-19 is? |
||||
|---|---|---|---|---|
|
Extremely Serious |
Serious |
Not Serious |
P Value | |
| No. (%) | No. (%) | No. (%) | ||
| Sex | ||||
| Female (n = 521) | 353 (67.8) | 78 (15) | 90 (17.3) | P < 0.001 |
| Male (n = 512) | 286 (55.9) | 106 (20.7) | 120 (23.4) | |
| Race | ||||
| Black/African American (n = 516) | 390 (75.6) | 68 (13.2) | 58 (11.2) | P < 0.001 |
| White/Caucasian (n = 521) | 251 (48.2) | 117 (22.5) | 153 (29.4) | |
| Education | ||||
| High School or Less | 170 (62) | 42 (15.3) | 62 (22.6) | P = 0.12 |
| Some College/Trade | 265 (62.1) | 70 (16.4) | 92 (21.6) | |
| 4-year Degree or More | 206 (61.3) | 73 (21.7) | 57 (17) | |
| Political Affiliation | ||||
| Conservative (n = 327) | 154 (47.1) | 64 (19.6) | 109 (33.3) | P < 0.001 |
| Moderate (n = 457) | 290 (63.5) | 87 (19) | 80 (17.5) | |
| Liberal (n = 252) | 196 (77.8) | 34 (13.5) | 22 (8.7) | |
Note: Boldface indicates statistical significance (p < .05).
3.4. COVID-19 testing behaviors
A substantial majority (74.1 %, n = 768) of respondents reported having used a COVID19 home test while 25.9 % (n = 269) had not. A bare majority of respondents indicated that they were highly confident in their ability to use (n = 527, 50.8 %) and interpret (n = 531, 51.3 %) a COVID-19 home test with 15 % (n = 155) expressing no confidence and 13.1 % (n = 136) doubting their ability to interpret the results. Conservative respondents were more likely to express a lack of confidence in home testing (n = 66, 20.2 %) as compared to moderates (n = 64, 14 %) or liberals (n = 25, 9.9 %; P < 0.001). Conservatives also expressed greater doubt concerning test interpretation than liberals (n = 60 (18.4 %) vs. n = 19 (7.5 %); P < 0.001). No racial differences were found (P = .84), although Blacks (n = 373, 72.3 %) reported a greater belief that home testing was useful compared to Whites (n = 289, 55.5 %; P < 0.001).
Modeling use of COVID-19 home tests (y/n) found significant associations between the likelihood of testing and respondent race, income, and insurance status (Table 4). White participants were 29 % less likely to have tested themselves for COVID-19 (0.71, 95 % CI [0.52–0.97]) and although annual income showed a small effect (1.05, 95 % CI [1.004–1.11]) on the decision to self-test, the participant's insurance status showed a substantial impact. Insured individuals were 2.95 times more likely to test (2.95, 95 % CI [1.88–4.63]) than those without insurance, highlighting the importance of health coverage. The model was significant (P < 0.001) and results of the Hosmer-Lemeshow test indicated an adequate fit (P = 0.51).
Table 4.
Results from logistic regression analysis: Indicators of COVID-19 testing and awareness of OPAs.
| Used COVID-19 Home Test (y/n) |
Awareness of OPAs (y/n) |
|||
|---|---|---|---|---|
| Odds Ratio | 95 % CI | Odds Ratio | 95 % CI | |
| Age (Years) | 0.99 | 0.98–1.01 | 1.01 | 0.99–1.02 |
| Race (White v. Black) | 0.71 | 0.52–0.97 | 1.75 | 1.31–2.33 |
| Sex (Female v. Male) | 1.10 | 0.82–1.48 | 1.11 | 0.85–1.46 |
| Political Beliefs | ||||
| Conservative (y/n) | 0.72 | 0.51–1.02 | 0.74 | 0.53–1.03 |
| Liberal (y/n) | 0.88 | 0.61–1.29 | 1.79 | 1.29–2.49 |
| Annual Incomea | 1.05 | 1.00–1.11 | 1.13 | 1.08–1.17 |
| Insurance v. Uninsured | 2.95 | 1.88–4.63 | 1.15 | 0.71–1.88 |
Note.
$10k increments.
3.5. Modeling results: OPA treatment, knowledge and beliefs
General awareness of OPAs was low, with only 38 % (n = 394) of respondents indicating awareness of OPAs; a majority (n = 643, 62 %) had not heard of them. Specific knowledge of treatment options was also low with almost a quarter (n = 254, 24.6 %) of the sample reporting that Hydroxychloroquine and Ivermectin were safe and effective and 47.7 % (n = 493) stating uncertainty. While 79.5 % (n = 824) agreed that early medical treatments can be valuable, most people (n = 636, 61.4 %) reported that they did not know whether the treatments were for individuals who were in-hospital or were to be taken by patients at home and did not know when in the course of the illness they should be taken (n = 524, 50.6 %). Finally, although almost half the sample (n = 507, 48.9 %) did not know whether they could access these treatments, a majority reported their primary care physician as a source (n = 756, 72.9 %) (Table 3).
Table 3.
Knowledge about COVID-19 treatment and antivirals (n = 1037).
| Percentage of Correct Responses (True/False) | No. (%) |
|---|---|
| Early Treatment Knowledge Items (M = 2.7, SD = 1.4) | |
| Currently, there is no effective cure for COVID-19. (T) | 586 (56.1) |
| Hydroxychloroquine or Ivermectin are safe and can treat COVID-19. (F) | 287 (27.7) |
| Some natural alternatives such as honey, ginger or eucalyptus can be used to treat the disease/fight the virus. (F) | 413 (39.8) |
| There are now anti-viral medications that can help patients recover from the infection. (T) | 661 (63.7) |
| Early medical treatment for symptoms can help most patients recover from the infection. (T) | 824 (79.5) |
| OPA Knowledge Items (M=2.3, SD=2.0) | |
| These treatments are for patients who are hospitalized with COVID-19. (F) | 177 (17.1) |
| You can take these treatments even if you have not taken a COVID-19 test but have been exposed to someone with COVID-19. (F) | 204 (19.7) |
| The treatment should be taken within 5 days of taking a test that shows you have COVID-19. (T) | 476 (45.9) |
| These treatments will definitely cure COVID-19. (F) | 409 (39.4) |
| These treatments will help you get better faster and reduce your risk of hospitalization. (T) | 565 (54.5) |
| These treatments are useless. (F) | 509 (49.1) |
Whites were more likely than Blacks to have heard of OPA treatments (n = 232 (44.5 %) vs. n = 162 (31.4 %); P < 0.001) and to correctly identify that these treatments could reduce the risk of hospitalization (n = 311 (59.8 %) vs. n = 254 (49.2 %); P < 0.001). Age also appears to be a factor: whereas 65.9 % (n = 108) of respondents between the ages of 70 and 79 understood OPAs to reduce the risk of hospitalization from COVID-19, only 47.7 % (n = 142) of respondents in their forties were aware (P = 0.004). This younger age group (41–49 years old) was also less aware of the 5-day period following a positive COVID test in which OPAs should be administered; 60.4 % (n = 180) believed this to be false or did not know the answer. Participants ages 70–79 were incorrect or unsure of the critical treatment period less than half the time (42.7 %, n = 70; P = 0.009)
Modeling awareness of OPAs (y/n) found significant associations between race, income, and political affiliation (Table 4). White respondents were 1.75 times more likely than Black respondents to be aware of OPA treatments (1.75, 95 % CI [1.31–2.33]). Each $10k increase in household income correlated with a 12.5 % awareness boost (1.13, 95 % CI [1.08–1.17]). Self-identified Liberals were 1.79 times more likely to know of OPA treatments (1.79, 95 % CI [1.29–2.49]). The model was significant (P < 0.001), and the Hosmer-Lemeshow test confirmed suitable fit (P = 0.23).
4. Discussion
The economic, socioemotional and clinical burden of COVID-19 is lower than at the height of the pandemic but remains outsized [51]. For patients, the burden of severe illness and death is unequally distributed, weighing more heavily on Black and older Americans. The rollout of new tools like OPAs have the capacity to reduce this burden, but require patients to know they have COVID-19 through prompt testing, be aware of OPAs, and have access to these treatments, including willingness of PCPs to prescribe. This study identified key groups of people who are less likely to test for COVID-19 and know about OPA treatments. Understanding who is at risk provides important knowledge to clinical providers, community health workers and policymakers as they work to mitigate the continuing impact of COVID-19.
The first step in taking an OPA is timely testing for COVID-19. Blacks were more likely than Whites (77.5 % vs. 70.6 %) to have tested for COVID and more likely to believe COVID-19 testing useful (72.3 % vs. 55.5 %). This may be because they were also more likely to assert that COVID-19 was an “extremely serious” disease compared to Whites (75.6 % vs. 48.2 %). This study was not designed to identify which messages and messengers are the most persuasive in specific populations.
Nonetheless, we found that this sample had significant gaps in their awareness and knowledge of OPAs that could keep them from seeking treatment. Only a minority (38 %) had heard of OPAs (31.4 % of Blacks and 44.5 % of Whites). The logistic regression analysis demonstrated that even with controlling for covariates Whites were 1.5 times more likely to have heard of OPA treatments than Blacks. Indeed, being Liberal, having higher income, and identifying as White were the shared characteristics of those most likely to be aware of OPA treatments.
Of concern is the finding that notable minorities of our samples held incorrect beliefs about outpatient COVID-19 treatment options, including 24.5 % who believed that Hydroxychloroquine or Ivermectin are safe and could treat COVID-19, and 21.5 % who thought OPAs were only available to hospitalized patients. While Blacks had significantly worse understanding than Whites in our sample on some key items, neither group showed high levels of knowledge. 57.7 % of Blacks and 50.3 % of Whites didn't know patients must begin treatment within the first five days of the disease. Half of Blacks (50.8 %) did not know OPAs can help speed recovery and reduce risk of hospitalization while 40.1 % of Whites were also unaware. Consistent with prior studies [52], this study's data indicate that younger respondents and politically conservative respondents were less likely to test for COVID-19 and have accurate information about OPAs.
These gaps point to a significant need for public education about OPAs. Social media campaigns may also be warranted. These results also suggest once diagnosed, patients need clear and concise messaging from the healthcare team that will counter misinformation and provide options for up-to-date treatment and care. A 2023 US survey reported low utilization of OPA treatments with only 20.5 % of patients aged 50–64 and 33.9 % those 65 and over taking an OPA when infected despite CDC guidance. In that same study, a common reason Black patients >65 did not take OPAs was because their healthcare provider didn't offer or recommend the treatment [16]. OPA treatments will continue to be underused if patients do not have an awareness of the treatment, a basic understanding of their benefits, and if physicians continue to be reluctant to prescribe them.
4.1. Limitations
This survey study has some limitations. It was conducted online, and although Qualtrics panels works to mitigate the inherent biases of online surveys, it should be noted that individuals with lower income and education and rural residence have lower participation rates. This study captured knowledge and attitudes about OPAs and COVID-19 testing at one point of time after OPAs became widely available. The landscape of COVID-19 information and misinformation in this country is constantly evolving and we recognize that the results may not be as robust over time. Another limitation of the study is that it was not sufficiently powered to review interactions between sociodemographic variables on the study outcomes. Future studies should consider and more fully explore the relationships between race, age and political affiliation. Finally, the generalizability of this study is limited since it surveyed a specific a cross-section of American adults and excluded individuals <40 and those who did not identify as either Black or White.
5. Conclusion
The COVID-19 pandemic demonstrated that worldwide pandemic preparedness is low, and the United States has significant work to do in order to be prepared for the next pandemic. Our results point to incomplete awareness and knowledge of critical OPA treatments in some of the most vulnerable populations and demonstrates how the health care system should continue to focus on educating patients about OPA treatments. The official end of the pandemic, inclusive of ending availability of free testing and limited insurance coverage of home tests and affordable access to OPAs, may make it more difficult to monitor and control COVID-19 in the future.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
[Name] University IRB reviewed this study (IRB Number: #30069) and deemed it exempt. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Declarations of interest: none.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.puhip.2024.100519.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Affairs (ASPA) AS for P Fact sheet: COVID-19 public health emergency transition roadmap. https://www.hhs.gov/about/news/2023/02/09/fact-sheet-covid-19-public-health-emergency-transition-roadmap.html HHS.gov. Published February 9, 2023.
- 2.Centers for Disease Control and Prevention . January 18, 2023. Leading Causes of Death.https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm [Google Scholar]
- 3.Centers for Disease Control and Prevention COVID-19 provisional counts - weekly updates by select demographic and geographic characteristics. January 11, 2023. https://www.cdc.gov/nchs/nvss/vsrr/covid_weekly/index.htm
- 4.Billock R., Steege A., Miniño A. National Center for Health Statistics (U.S.); 2020. COVID-19 Mortality by Usual Occupation and Industry: 46 States and New York City, United States. 2022. [DOI] [PubMed] [Google Scholar]
- 5.Lin S., Deng X., Ryan I., et al. COVID-19 symptoms and deaths among healthcare workers, United States. Emerg. Infect. Dis. 2022;28(8):1624–1632. doi: 10.3201/eid2808.212200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill L., Artiga S. KFF; August 22, 2022. COVID-19 Cases and Deaths by Race/Ethnicity: Current Data and Changes over Time.https://www.kff.org/coronavirus-covid-19/issue-brief/covid-19-cases-and-deaths-by-race-ethnicity-current-data-and-changes-over-time/ [Google Scholar]
- 7.Wong M.S., Haderlein T.P., Yuan A.H., Moy E., Jones K.T., Washington D.L. Time trends in racial/ethnic differences in COVID-19 infection and mortality. Int. J. Environ. Res. Publ. Health. 2021;18(9):4848. doi: 10.3390/ijerph18094848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention . Risk for COVID-19 Infection, Hospitalization, and Death by Race/Ethnicity. CDC; 2022. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html Published online December 28. [Google Scholar]
- 9.Centers for Disease Control and Prevention COVID-19 provisional counts - health disparities. 2023. https://www.cdc.gov/nchs/nvss/vsrr/covid19/health_disparities.htm Published.
- 10.Kates J., Tolbert J., Rouw A. The red/blue divide in COVID-19 vaccination rates continues: an update. 2022. https://www.kff.org/policy-watch/the-red-blue-divide-in-covid-19-vaccination-rates-continues-an-update/
- 11.Albrecht D. Vaccination, politics and COVID-19 impacts. BMC Publ. Health. 2022;22(1):96. doi: 10.1186/s12889-021-12432-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruiz J.B., Bell R.A. Predictors of intention to vaccinate against COVID-19: results of a nationwide survey. Vaccine. 2021;39(7):1080–1086. doi: 10.1016/J.VACCINE.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milligan M.A., Hoyt D.L., Gold A.K., Hiserodt M., Otto M.W. COVID-19 vaccine acceptance: influential roles of political party and religiosity. Psychol. Health Med. 2022;27(9):1907–1917. doi: 10.1080/13548506.2021.1969026. [DOI] [PubMed] [Google Scholar]
- 14.Zarębska-Michaluk D., Rzymski P., Moniuszko-Malinowska A., et al. Does hospitalization change the perception of COVID-19 vaccines among unvaccinated patients? Vaccines. 2022;10(3):476. doi: 10.3390/vaccines10030476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.House T.W. The biden-harris administration will end COVID-19 vaccination requirements for federal employees, contractors, international travelers, head start educators, and CMS-certified facilities. The white house. https://www.whitehouse.gov/briefing-room/statements-releases/2023/05/01/the-biden-administration-will-end-covid-19-vaccination-requirements-for-federal-employees-contractors-international-travelers-head-start-educators-and-cms-certified-facilities/ Published May 1, 2023.
- 16.Benchimol-Elkaim B., Dryden-Peterson S., Miller D.R., Koh H.K., Geller A.C. Oral antiviral therapy utilization among adults with recent COVID-19 in the United States. J. Gen. Intern. Med. 2023 doi: 10.1007/s11606-023-08106-6. Published online February 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raglow Z, Malani PN, Petty LA. Outpatient treatment for COVID-19. JAMA. Published online August 16, 2023. doi:10.1001/jama.2023.16666. [DOI] [PubMed]
- 18.Verderosa MAB and AD. Use of Antiviral Agents and other Therapies for COVID-19 | EndNote Click. Accessed May 20, 2023. https://click.endnote.com/viewer?doi=10.1055%2Fs-0042-1758837&token=WzE4MDQzMjEsIjEwLjEwNTUvcy0wMDQyLTE3NTg4MzciXQ.RTL1Z0QYOqz4L9hMcyErLWasZZM. [DOI] [PubMed]
- 19.Katella Kathy. The Latest COVID-19 Pill. Yale Medicine; March 25, 2024. 13 things to know about paxlovid.https://www.yalemedicine.org/news/13-things-to-know-paxlovid-covid-19 Published. [Google Scholar]
- 20.Katella Kathy. Yale Medicine; January 5, 2024. 9 Things You Need to Know about Molnupiravir, a New COVID-19 Pill.https://www.yalemedicine.org/news/9-things-to-know-about-covid-pill Published. [Google Scholar]
- 21.Dryden-Peterson S., Kim A., Kim A.Y., et al. Nirmatrelvir plus ritonavir for early COVID-19 in a large U.S. Health system. Ann. Intern. Med. 2023;176(1):77–84. doi: 10.7326/M22-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.US Department of Health and Human Services . 2023. Information Sheet: Paxlovid Eligibility and Effectiveness. Published online. [Google Scholar]
- 23.Administration for Strategic Preparedness & Response. Test to Treat | HHS/ASPR. Accessed October 21, 2022. https://aspr.hhs.gov:443/TestToTreat/Pages/default.aspx.
- 24.NIH launches Home Test to Treat, a pilot COVID-19 telehealth program. National Institute of Biomedical Imaging and Bioengineering. Accessed September 14, 2023. https://www.nibib.nih.gov/news-events/newsroom/nih-launches-home-test-treat-pilot-covid-19-telehealth-program.
- 25.How test to treat works for individuals and families | HHS/ASPR. https://aspr.hhs.gov:443/TestToTreat/Pages/process.aspx
- 26.Recht H. Kaiser Health News; April 15, 2022. How the Test-To-Treat Pillar of the US Covid Strategy Is Failing Patients.https://khn.org/news/article/test-to-treat-biden-covid-failing-patients-pharmacies-cvs/ Published. [Google Scholar]
- 27.Oakley ERS and EM. Geospatial Disparities in Federal COVID-19 Test-to-Treat Program | EndNote Click. Accessed May 20, 2023. https://click.endnote.com/viewer?doi=10.1016%2Fj.amepre.2023.01.022&token=WzE4MDQzMjEsIjEwLjEwMTYvai5hbWVwcmUuMjAyMy4wMS4wMjIiXQ.QSko-08e3BmZ9LFqFMxIvHHTBpQ. [DOI] [PMC free article] [PubMed]
- 28.Asabor E.N., Warren J.L., Cohen T. Racial/ethnic segregation and access to COVID-19 testing: spatial distribution of COVID-19 testing sites in the four largest highly segregated cities in the United States. Am. J. Publ. Health. 2022;112(3):518–526. doi: 10.2105/AJPH.2021.306558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bilal U., Tabb L.P., Barber S., Diez Roux A.V. Spatial inequities in COVID-19 testing, positivity, confirmed cases, and mortality in 3 U.S. Cities : an ecological study. Ann. Intern. Med. 2021;174(7):936–944. doi: 10.7326/M20-3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perry B.L. Contact tracing could exacerbate COVID-19 health disparities: the role of economic precarity and stigma. Am. J. Publ. Health. 2021;111(5):778–781. doi: 10.2105/AJPH.2021.306244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adepoju O.E., Ojinnaka C.O. County-level determinants of COVID-19 testing and cases: are there racial/ethnic disparities in Texas? 2021;24(5):589–594. doi: 10.1089/POP.2020.0300. home.liebertpub.com/pop. [DOI] [PubMed] [Google Scholar]
- 32.Ali S.H., Tozan Y., Jones A.M., Foreman J., Capasso A., DiClemente R.J. Regional and socioeconomic predictors of perceived ability to access coronavirus testing in the United States: results from a nationwide online COVID-19 survey. Ann. Epidemiol. 2021;58:7–14. doi: 10.1016/J.ANNEPIDEM.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perry B.L., Aronson B., Railey A.F., Ludema C. If you build it, will they come? Social, economic, and psychological determinants of COVID-19 testing decisions. PLoS One. 2021;16(7) doi: 10.1371/JOURNAL.PONE.0252658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levy A.G., Thorpe A., Scherer L.D., et al. Misrepresentation and nonadherence regarding COVID-19 public health measures. JAMA Netw. Open. 2022;5(10) doi: 10.1001/jamanetworkopen.2022.35837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valera E., Jankelow A., Lim J., et al. COVID-19 point-of-care diagnostics: present and future. ACS Nano. 2021;15(5):7899–7906. doi: 10.1021/ACSNANO.1C02981/ASSET/IMAGES/MEDIUM/NN1C02981_0003.GIF. [DOI] [PubMed] [Google Scholar]
- 36.Bruine de Bruin W., Bennett D. Relationships between initial COVID-19 risk perceptions and protective health behaviors: a national survey. Am. J. Prev. Med. 2020;59(2):157. doi: 10.1016/J.AMEPRE.2020.05.001. 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Facente S.N., De Zuzuarregui M., Frank D., et al. Risky business: a mixed methods study of decision-making regarding COVID-19 risk at a public university in the United States. Front. Psychol. 2022;13 doi: 10.3389/fpsyg.2022.926664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jimenez M.E., Rivera-Núñez Z., Crabtree B.F., et al. Black and latinx community perspectives on COVID-19 mitigation behaviors, testing, and vaccines. JAMA Netw. Open. 2021;4(7) doi: 10.1001/jamanetworkopen.2021.17074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loomba S., de Figueiredo A., Piatek S.J., de Graaf K., Larson H.J. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat. Human Behav. 2021;5(3):337–348. doi: 10.1038/s41562-021-01056-1. [DOI] [PubMed] [Google Scholar]
- 40.Mackey T.K., Purushothaman V., Haupt M., Nali M.C., Li J. Application of unsupervised machine learning to identify and characterise hydroxychloroquine misinformation on Twitter. Lancet Digit Health. 2021;3(2):e72–e75. doi: 10.1016/S2589-7500(20)30318-6. [DOI] [PubMed] [Google Scholar]
- 41.Erku D.A., Belachew S.A., Abrha S., et al. When fear and misinformation go viral: pharmacists' role in deterring medication misinformation during the “infodemic” surrounding COVID-19. Res. Soc. Adm. Pharm. 2021;17(1):1954–1963. doi: 10.1016/j.sapharm.2020.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sule S., DaCosta M.C., DeCou E., Gilson C., Wallace K., Goff S.L. Communication of COVID-19 misinformation on social media by physicians in the US. JAMA Netw. Open. 2023;6(8) doi: 10.1001/jamanetworkopen.2023.28928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leonhardt D. The Power of Paxlovid. The New York Times. https://www.nytimes.com/2022/10/07/briefing/covid-treatment-paxlovid.html. Published October 7, 2022. Accessed November 8, 2022.
- 44.Parums D.V. Editorial: rebound COVID-19 and cessation of antiviral treatment for SARS-CoV-2 with paxlovid and Molnupiravir. Med Sci Monit Int Med J Exp Clin Res. 2022;28 doi: 10.12659/MSM.938532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Medscape Medscape survey finds reasons paxlovid IS UNDERPRESCRIBED for those 65 and older, despite its effectiveness against COVID complications. https://www.prnewswire.com/news-releases/medscape-survey-finds-reasons-paxlovid-is-underprescribed-for-those-65-and-older-despite-its-effectiveness-against-covid-complications-301720681.html
- 46.Menikoff J., Kaneshiro J., Pritchard I. The common Rule, updated. N. Engl. J. Med. 2017;376(7):613–615. doi: 10.1056/NEJMp1700736. [DOI] [PubMed] [Google Scholar]
- 47.Barreto M.A., Frasure-Yokley L., Vargas E.D., Wong J. Best practices in collecting online data with asian, black, latino, and white respondents: evidence from the 2016 collaborative multiracial post-election survey. Polit Groups Identities. 2018;6(1):171–180. doi: 10.1080/21565503.2017.1419433. [DOI] [Google Scholar]
- 48.Sharmin R, Rayna SE, Khalequzzaman M, Rahman KMT, Islam SS. COVID-19 Testing: Perceived Barriers Among the Urban Slum Dwellers of Dhaka, Bangladesh. doi:10.31219/OSF.IO/W3QPB.
- 49.Sun N., Hua C.L., Qiu X., Brown J.S. Determinants of COVID-19 testing among late middle-aged and older adults: applying the health belief model. Aging Health Res. 2022;2(2):100066. doi: 10.1016/J.AHR.2022.100066. 100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McElfish P.A., Purvis R., James L.P., Willis D.E., Andersen J.A. Perceived barriers to COVID-19 testing. Int. J. Environ. Res. Publ. Health. 2021;18(5):1–8. doi: 10.3390/IJERPH18052278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Centers for Disease Control and Prevention Estimated COVID-19 burden. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/burden.html Published August 12, 2022.
- 52.Dayton L., Song W., Kaloustian I., Eschliman E., Strickland J., Latkin C. A longitudinal study of COVID-19 disclosure stigma and COVID-19 testing hesitancy in the United States | Elsevier Enhanced Reader. Publ. Health. 2022;212 doi: 10.1016/j.puhe.2022.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.