Abstract
Electrocardiogram (ECG) changes after primary percutaneous coronary intervention (PCI) in ST-segment elevation myocardial infarction (STEMI) patients are associated with prognosis. This study investigated the feasibility of predicting left ventricular (LV) dysfunction in STEMI patients using an artificial intelligence (AI)-enabled ECG algorithm developed to diagnose STEMI. Serial ECGs from 637 STEMI patients were analyzed with the AI algorithm, which quantified the probability of STEMI at various time points. The time points included pre-PCI, immediately post-PCI, 6 h post-PCI, 24 h post-PCI, at discharge, and one-month post-PCI. The prevalence of LV dysfunction was significantly associated with the AI-derived probability index. A high probability index was an independent predictor of LV dysfunction, with higher cardiac death and heart failure hospitalization rates observed in patients with higher indices. The study demonstrates that the AI-enabled ECG index effectively quantifies ECG changes post-PCI and serves as a digital biomarker capable of predicting post-STEMI LV dysfunction, heart failure, and mortality. These findings suggest that AI-enabled ECG analysis can be a valuable tool in the early identification of high-risk patients, enabling timely and targeted interventions to improve clinical outcomes in STEMI patients.
Keyword: ST-segment elevation myocardial infarction, Heart failure, Artificial intelligence, Electrocardiogram
Subject terms: Cardiology, Machine learning
Introduction
Acute myocardial infarction (AMI) is a leading cause of mortality worldwide1. Prompt diagnosis and timely revascularization are crucial, especially ST-segment elevation myocardial infarction (STEMI)2,3. Even with proper revascularization through percutaneous coronary intervention (PCI), some patients may experience left ventricular (LV) dysfunction, affecting both short- and long-term outcomes4. LV dysfunction is caused not only by myocardial injury during coronary occlusion, but also by reperfusion injury or microvascular obstruction (MVO) after revascularization5–7. It is difficult to predict these events because they occur even if patency of the epicardial coronary artery is secured. Furthermore, no biomarkers are currently available to indicate the myocardial injury after revascularization.
Dynamic electrocardiogram (ECG) changes, such as the recovery of ST elevation after PCI, can reflect reperfusion at the myocardial level, not the vascular level, and have been linked with myocardial salvage and better outcomes8,9. However, these various changes in ECG parameters are difficult to quantify and are currently interpreted by clinicians based on their observations.
Digital biomarkers are biometric data obtained through various digital health devices, and in a broader sense, data obtained by analyzing such data. Recent advancements in artificial intelligence (AI)-enabled ECG have shown remarkable efficacy in diagnosing and predicting cardiovascular disease10,11 by generating probability indices that serve as novel digital biomarkers for decision-making12,13. This study aimed to evaluate the prognostic significance of the AI-based probability index derived from serial ECGs in patients who underwent primary PCI for STEMI.
Results
Serial ECG analysis in patients with STEMI following primary PCI
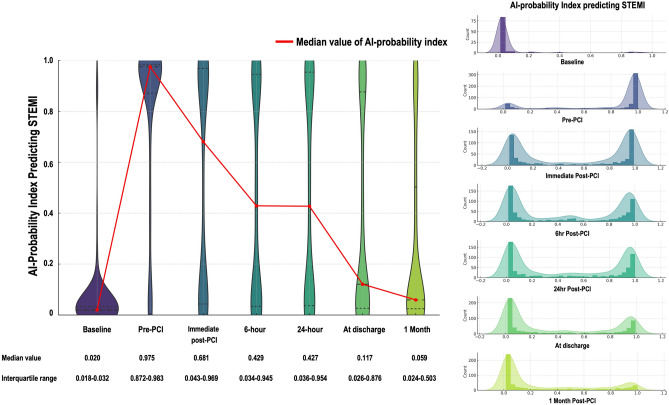
A total of 637 STEMI patients (mean age 64.7 ± 13.0 years, male 80.1%) were finally included in the analysis (Supplementary Fig. S1, online). The median probability index estimated by the AI-enabled ECG at various time points decreased over time after PCI and revealed significant differences (P < 0.001), with a value of 0.020 (interquartile range [IQR]: 0.018–0.032) at baseline, 0.975 (0.872–0.983) at Pre-PCI, 0.681 (0.043–0.969) at Immediate Post-PCI, 0.429 (0.034–0.945) at 6 h Post-PCI, 0.427 (0.036–0.954) at 24 h Post-PCI, 0.117 (0.026–0.876) at discharge, and 0.059 (0.024–0.503) at 1 M Post-PCI. Figures 1 shows the distribution of the probability index in predicting STEMI. Before primary PCI, most patients had values close to 1, whereas after PCI, this distribution is left-shifted towards 0.
Figure 1.
Distribution of the probability index predicting STEMI. STEMI, ST elevated myocardial infarction.
Association between the probability index and LV dysfunction
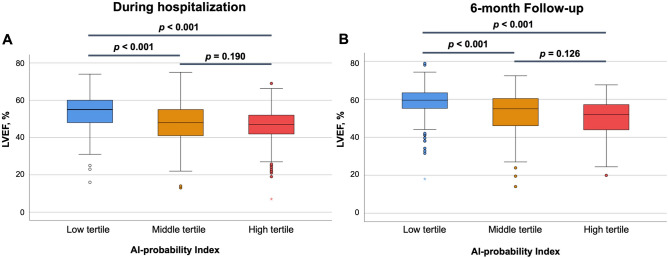
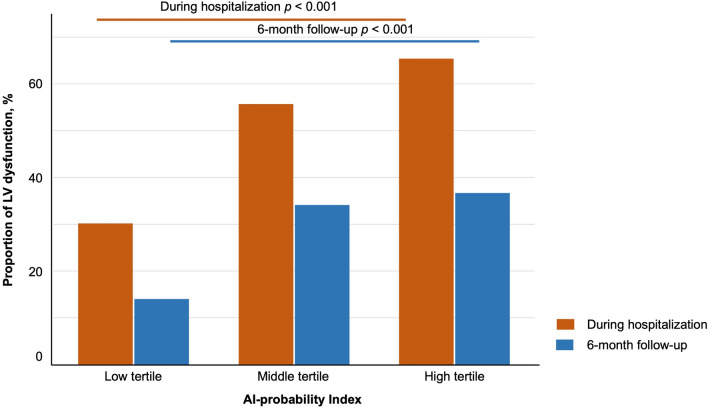
In terms of predicting LV dysfunction, the AUROC was found to be the highest for the 6 h Post-PCI time point (Supplementary Fig. S2, Table S1, online). Consequently, this specific index was chosen as the basis for further analysis. To assess the relationship between the 6 h Post-PCI index and LV dysfunction, patients were stratified into tertiles based on the index; the cutoff points for the tertiles were 0.058 and 0.873. Table 1 shows the baseline characteristics according to the probability index. The mean LVEF after primary PCI of the low tertile group was 53.3 ± 8.8%, 47.6 ± 10.4% for the middle tertile group, and 46.3 ± 9.5% for the high tertile group (ANOVA P < 0.001, Fig. 2A). The prevalence of LV dysfunction (LVEF < 50%) was 30.0%, 55.9%, and 65.4%, respectively (P < 0.001, Fig. 3, Supplementary Fig. S3, online). Follow-up echocardiography, conducted 3 to 6 months post-primary PCI, exhibited similar trends in LV dysfunction across the tertile groups (58.3 ± 8.6%, 52.4 ± 10.9%, and 50.9 ± 9.0%, respectively, ANOVA P < 0.001, Fig. 2B). In terms of LV remodeling, patients with increased LV chamber size were also most likely to be in the high tertile group (Supplementary Fig. S3, online).
Table 1.
Baseline characteristics according to the AI-probability index.
| AI-probability index | P value | |||
|---|---|---|---|---|
| Low tertile (n = 212) | Middle tertile (n = 212) | High tertile (n = 213) | ||
| Age, years | 64.9 ± 12.5 | 64.1 ± 12.6 | 65.2 ± 13.9 | 0.658 |
| Female, n (%) | 41 (19.8) | 44 (20.8) | 42 (19.7) | 0.931 |
| BMI, kg/cm2 | 24.9 ± 3.5 | 24.2 ± 3.6 | 24.3 ± 3.1 | 0.139 |
| Hypertension, n (%) | 117 (55.2) | 120 (56.6) | 114 (53.5) | 0.815 |
| Diabetes, n (%) | 66 (31.1) | 57 (26.9) | 75 (35.2) | 0.179 |
| ESRD, n (%) | 3 (1.4) | 3 (1.4) | 3 (1.4) | 0.999 |
| Prior PCI, n (%) | 9 (4.2) | 7 (3.3) | 8 (3.8) | 0.878 |
| Prior CVA, n (%) | 15 (7.1) | 16 (7.5) | 17 (8.0) | 0.939 |
| Systolic BP, mmHg | 131.1 ± 38.1 | 132.3 ± 35.2 | 133.4 ± 32.0 | 0.803 |
| Diastolic BP, mmHg | 77.2 ± 25.1 | 78.8 ± 24.0 | 78.8 ± 22.0 | 0.716 |
| Pulse rate, bpm | 72.4 ± 22.8 | 77.4 ± 20.7 | 81.4 ± 21.9 | < 0.001 |
| Killip Class, n (%) | 0.041 | |||
| I | 173 (81.6) | 174 (82.1) | 156 (73.2) | |
| II | 18 (8.5) | 17 (8.0) | 26 (12.2) | |
| III | 11 (5.2) | 10 (4.7) | 13 (6.1) | |
| IV | 10 (4.7) | 11 (5.2) | 18 (8.5) | |
| Disease severity, n (%) | 0.680 | |||
| 1 VD | 96 (45.3) | 105 (49.5) | 94 (44.1) | |
| 2 VD | 68 (32.1) | 67 (31.6) | 67 (31.5) | |
| 3 VD | 48 (22.6) | 40 (18.9) | 52 (24.4) | |
| Infarct-related artery, n (%) | < 0.001 | |||
| LM | 10 (4.7) | 6 (2.8) | 1 (0.5) | |
| LAD | 82 (38.7) | 135 (63.7) | 134 (62.9) | |
| LCx | 32 (15.1) | 16 (7.5) | 9 (4.2) | |
| RCA | 88 (41.5) | 55 (25.9) | 69 (32.4) | |
| Peak troponin I, ng/ml | 51.5 ± 43.8 | 74.9 ± 46.5 | 86.2 ± 42.5 | < 0.001 |
| NT-proBNP, pg/dl | 1682.2 ± 5631.8 | 1908.7 ± 5369.1 | 2543.5 ± 5668.7 | 0.259 |
| Hemglobin, g/dl | 14.1 ± 2.1 | 14.2 ± 2.0 | 14.1 ± 2.2 | 0.765 |
| Platelet, 103/μl | 236.4 ± 66.8 | 238.0 ± 70.1 | 241.9 ± 81.1 | 0.728 |
| eGFR, ml/min/1.73m2 | 69.6 ± 25.1 | 71.8 ± 24.3 | 69.0 ± 24.9 | 0.457 |
| Cholesterol, mg/dl | 151.9 ± 46.1 | 155.4 ± 39.8 | 163.0 ± 48.6 | 0.034 |
| Triglyceride, mg/dl | 166.0 ± 150.6 | 168.1 ± 144.5 | 158.2 ± 126.8 | 0.751 |
| HDL, mg/dl | 45.2 ± 11.2 | 45.0 ± 11.7 | 45.8 ± 10.8 | 0.783 |
| LDL, mg/dl | 108.9 ± 37.7 | 110.5 ± 37.3 | 120.0 ± 39.2 | 0.005 |
| CRP, mg/dl | 0.8 ± 2.5 | 0.9 ± 2.6 | 1.4 ± 3.0 | 0.066 |
AI, artificial intelligence; BMI, body mass index; BP, blood pressure; CRP, C-reactive protein; CVA, cerebrovascular accident; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HDL, high density lipoprotein; LAD, left anterior descending artery ; LCx, left circumflex artery; LDL, low density lipoprotein; LM, left main; NT-proBNP, N-terminal pro b-type natriuretic peptide; PCI, percutaneous coronary intervention; RCA, right coronary artery; VD, vessel disease.
Figure 2.
The mean LVEF after primary PCI according to the AI-probability index. AI, artificial intelligence; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention.
Figure 3.
Proportion of LV dysfunction according to the AI-probability index. AI, artificial intelligence; LV, left ventricular.
The multivariate logistic regression analysis showed that a high probability index was an independent predictive factor for LV dysfunction after primary PCI and the odds ratio of the high tertile of the probability index was 2.285 (95% CI: 1.428–3.656, P = 0.001; Table 2). Furthermore, similar results were seen when LV dysfunction was defined as LVEF < 40% (Supplementary Table S2, online).
Table 2.
Independent risk factors for post-STEMI LV dysfunction.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value | |
| AI-probability index low tertile | Reference | Reference | ||||
| AI-probability index middle tertile | 1.367 | 0.981–1.905 | 0.064 | 1.869 | 1.166–2.998 | 0.009 |
| AI-probability index high tertile | 2.549 | 1.807–3.594 | < 0.001 | 2.009 | 1.228–3.287 | 0.005 |
| Age, years | 1.006 | 0.994–1.018 | 0.344 | |||
| Body mass index, kg/cm2 | 0.904 | 0.861–0.949 | < 0.001 | 0.902 | 0.849–0.959 | 0.001 |
| Female | 1.301 | 0.768–2.206 | 0.203 | |||
| Systolic blood pressure, mmHg | 0.992 | 0.987–0.997 | < 0.001 | 0.986 | 0.975–0.997 | 0.015 |
| Diastolic blood pressure, mmHg | 0.992 | 0.986–0.999 | 0.023 | |||
| Pulse rate, bpm | 1.019 | 1.011–1.026 | < 0.001 | 1.019 | 1.009–1.030 | < 0.001 |
| Killip ≥ Class 3 | 2.852 | 1.659–4.902 | < 0.001 | 2.674 | 1.356–5.273 | 0.005 |
| Hypertension | 0.855 | 0.625–1.169 | 0.326 | |||
| Diabetes | 1.128 | 0.805–1.580 | 0.484 | |||
| End-stage renal disease | 1.981 | 0.491–7.991 | 0.337 | |||
| PCI to LM and/or LAD | 2.564 | 1.854–3.546 | < 0.001 | 2.903 | 1.926–4.377 | < 0.001 |
| eGFR, ml/min/1.73m2 | 0.989 | 0.983–0.996 | 0.001 | |||
| Peak troponin I | 1.016 | 1.012–1.020 | < 0.001 | 1.018 | 1.013–1.022 | < 0.001 |
| C-reactive protein, mg/dl | 1.250 | 1.107–1.412 | < 0.001 | 1.211 | 1.056–1.389 | 0.006 |
| Cholesterol, mg/dl | 1.003 | 0.999–1.006 | 0.131 | 1.006 | 1.000–1.012 | 0.043 |
| LDL cholesterol, mg/dl | 1.001 | 0.997–1.006 | 0.485 | |||
AI, artificial intelligence; CI, confidence interval; PCI, percutaneous coronary intervention; LAD, left anterior descending artery; LDL, Low density lipoprotein; LM, left main coronary artery.
Clinical outcomes according to the probability index of STEMI
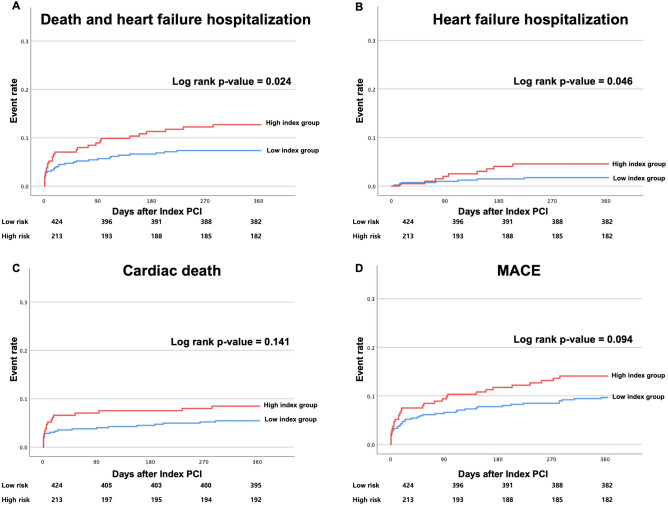
There was a total of 58 cases (9.1%) of cardiac death and heart failure hospitalization, with a higher frequency observed in the highest probability index group (7.5% in the low tertile group, 7.1% in the middle tertile group, and 12.7% in high tertile group, P = 0.066). According to the Kaplan–Meier survival curve, when divided into a low index group (low and middle tertile group) and a high index group (high tertile group), the rate of cardiac death and heart failure hospitalization were significantly higher in the high index group (Log-rank P = 0.024, unadjusted HR = 1.766, 95% CI: 1.054–2.958; Fig. 4).
Figure 4.
Clinical outcomes according to the AI-probability index. AI, artificial intelligence.
Discussion
The main finding of our study is that it is possible to quantify dynamic ECG changes in patients with STEMI after primary PCI using an AI-enabled ECG for STEMI detection. Furthermore, we could predict LV dysfunction and mortality in these patients by using these indices as a digital biomarker. To our knowledge, this is the first study to utilize a diagnostic AI-enabled ECG algorithm as a biomarker to predict prognosis.
During the thrombolysis era, it is impossible to confirm the patency of the coronary artery visually; thus, changes in the ECG were considered to be vital indicators of reperfusion, and several studies have focused on their prognostic implications8,14. The dynamics of ECG changes have often provided insights into the adequate reperfusion to the myocardial level following thrombolytic therapy15. In addition, Dizon et al. showed that the ST segment resolution after primary PCI was significantly correlated with infarct size and MVO16.
While primary PCI effectively ensures patency of the epicardial artery, it does not necessarily guarantee complete perfusion at the myocardial level. The procedure can be associated with reperfusion injury, characterized by oxidative stress and inflammatory responses, leading to further myocardial damage17. On a cellular level, prolonged ischemia–reperfusion injury may lead to cell death, including apoptosis, autophagy, necrosis, and necroptosis18,19. Microvascular obstruction (MVO) can also occur after PCI, preventing adequate myocardial level perfusion despite the opening of epicardial arteries. MVO can result from mechanical obstruction by a thrombus, embolus, or edema, and is associated with an adverse prognostic impact20,21. Restoring blood flow at the macrovascular level through successful PCI achieving a thrombolysis in myocardial infarction flow score of 3 may not always translate to effective reperfusion at the myocardial level due to reperfusion injury and MVO22. They can compromise myocardial salvage despite the restoration of epicardial blood flow. ECG changes can confirm reperfusion at the myocardial level, but ECG changes are challenging to quantify, so they cannot be widely utilized as a prognostic factor.
The recent advancements in the AI-enhanced ECG algorithm allows the prediction of several diseases or medical conditions, such as LV dysfunction, valvular heart disease, anemia, and electrolyte abnormalities or renal impairment23–26. The AI algorithm can extract subtle patterns and changes from ECG signals and provide quantified probability values between 0 and 1 for target diseases or conditions. These probability values can be utilized as digital biomarkers, allowing for a more precise and timely diagnosis, prognosis, and risk stratification. The AI algorithm used in this study is a deep-learning algorithm that diagnoses STEMI using a 12-lead ECG. The accuracy of the diagnosis was confirmed to be 95.7% in the external validation10,27. We obtained a STEMI probability index from the serial ECGs over time after primary PCI using this algorithm. The algorithm can provide information about the ischemic burden at the myocardial level in STEMI patients after primary PCI, and this study was conducted to extend the prognostic predictive ability of this algorithm. We used it as a digital biomarker, which showed to be a good predictor of LV dysfunction and mortality after STEMI. The ECG at 6 h after primary PCI showed the highest predictive power. This result can be reasonable when considering the fact that revascularization should be performed within 12 h of symptom onset to salvage the myocardium.
Although it is possible to develop another algorithm to predicts LV dysfunction, in this study we applied the STEMI probability index to post-PCI ECGs to find that AI-enabled ECG algorithms can serve not only for binary decisions but also as biomarkers for diagnosis and predicting prognosis.
Several limitations of the present study are noteworthy. First, due to the retrospective nature of the study design, the timing of the ECG checks was not strictly standardized. We collected ECG data based on a window of approximately ± 3 h for our analysis. Second, our sample size was relatively small to substantiate differences in clinical outcomes in patients with STEMI. To generalize the results of this study, we believe that validation should be performed on a larger number of patients from different countries and ethnicities.
For this reason, we conducted our analysis with LV dysfunction as the primary endpoint. Third, as this is a study of reperfusion at the myocardial level in patients with STEMI, it would have been better to check infarct size measurements or MVO by cardiac magnetic resonance imaging; however, there are no data on this. Based on the findings of this study, we plan to conduct subsequent prospective research to validate the impact on clinical outcomes and correlation with cardiac image data.
In conclusion, this study shows that the ECG digital biomarker from an AI-enabled ECG algorithm is an effective tool for quantifying ECG changes after primary PCI in patients with STEMI. The digital biomarker can predict post-AMI heart failure and is associated with clinical outcomes, indicating its potential as a prognostic marker. Further studies are needed to validate our results and explore the use of the digital biomarker in assessing myocardial reperfusion after primary PCI.
Methods
Study design and population
This retrospective study was designed to validate the prognostic value of an AI-enabled ECG derived probability index as a digital biomarker for predicting the prognosis of patients with STEMI after primary PCI. We retrospectively included consecutive patients who underwent primary PCI for STEMI between January 2019 and June 2022 in our institution. STEMI was diagnosed by a new ST segment elevation in ≥ 2 contiguous leads, measuring > 0.2 mV in leads V1-3 or 0.1 mV in all other leads on a 12-lead ECG in a patient with the acute onset of chest pain or dyspnea. Patients who died within 24 h, those who underwent coronary artery bypass grafting, and patients for whom adequate serial ECGs could not be obtained were excluded from the study. This study was approved by the Institutional Review Board (IRB) of Biomedical Research Institute in Seoul National University.
Bundang Hospital (B-2201–733-104) and all methods were performed in accordance with the relevant guideline and regulation. The requirement for informed consent was waived by the IRB because of the retrospective study design using fully anonymized ECG and health data, with minimal potential harm.
Development of an AI-enhanced ECG prediction model for STEMI
In previous research, we developed a deep-learning algorithm for the detection of AMI and STEMI using a 12-lead ECG10,27. The performance of this algorithm was validated, substantiating its efficacy. The output values, situated between 0 and 1, denote that an elevated value corresponds to an increased probability of the presence of AMI or STEMI. Data were sourced from two different hospitals for internal and external validation. A total of 22,259 ECGs (13,916 ECGs of non-AMI and 8,343 ECGs of AMI) from 15,113 patients were used to develop and validate the model. The area under the receiver operating characteristics curve (AUROC) was 0.927 (95% confidence interval [CI]: 0.908–0.945) for AMI and 0.983 (95% CI: 0.976–0.989) for STEMI.
We developed a ResNet-based model using PyTorch and Python, which is typically applied in image recognition, to classify ECG data by recognizing complex patterns. ECG classification is challenging because it involves deciphering rhythm and morphological features, both in image and time series analysis. Figure 5 shows the architecture of our deep-learning model, which includes a stem block, followed by six residual blocks, and ends with a fully connected layer to discern these patterns. Features from the ECG are progressively extracted through each block. We grouped every two residual blocks into what we call a "stage"; our model comprises three such stages. Within each residual block, we arranged a sequence of layer: a one dimensional (1D) convolutional layer, batch normalization, ReLU activation, another 1D convolutional layer, additional batch normalization, a dropout layer, and a skip connection. The stem block has a single layer plus a skip connection and max pooling. For training, we chose the Adam optimizer paired with a cosine warm-up optimization scheduler to adjust the weights and focal loss function for learning. We ran this scheduler for 150 epochs to test different hyperparameters and determine the best model structure, focusing on the highest area under the receiver operating characteristics (AUROC).
Figure 5.
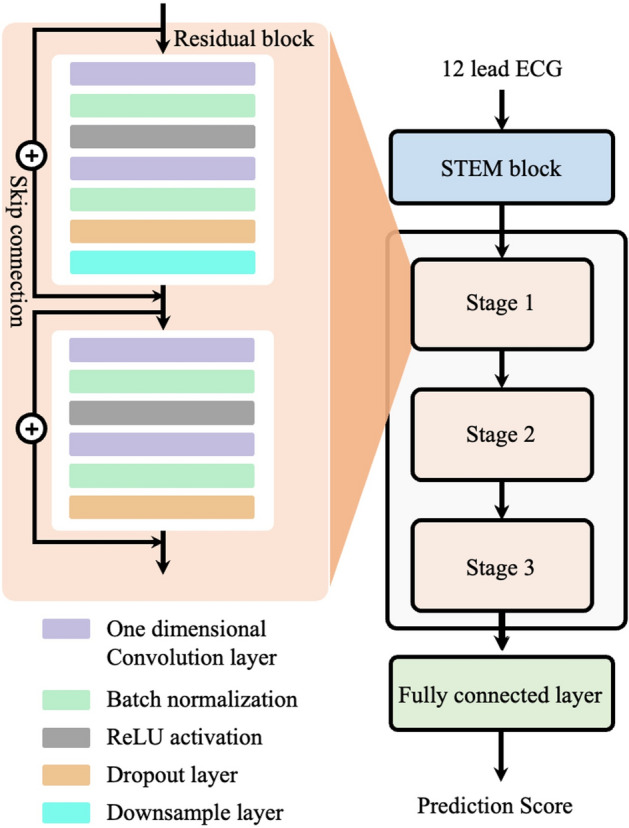
Architecture of the deep learning model. We utilized a ResNet-based model to predict AMI and STEMI. It consists of three parts: a stem block, stage block, and a full connected layer. AMI, acute myocardial infarction; STEMI, ST elevated myocardial infarction.
Data collection and analysis of serial ECGs
Serial ECGs were analyzed at multiple time points to quantify the dynamic ECG change and to evaluate myocardial reperfusion following primary PCI in patients with STEMI. The AI-enabled ECG quantified the probability of STEMI at each time points as a continuous variable between 0 and 1, i.e., as a digital biomarker. The time points at which the ECG was taken were as follows (Supplementary Fig. S4):
More than 1 month prior to the STEMI event (Baseline)
Time of arrival to the emergency department prior to PCI (Pre-PCI)
Immediately after primary PCI (Immediate Post-PCI)
Six hours after PCI (6 h Post-PCI)
Twenty-four hours after PCI (24 h Post-PCI)
Time of discharge (At discharge)
One month following the PCI (1 M Post-PCI)
Echocardiographic assessments were usually performed the day after primary PCI and follow-up echocardiography was performed around 6 months after the onset of STEMI. LV end systolic volume, LV end systolic volume, and LV ejection fraction (LVEF) were estimated using the bi-planar modified Simpson’s rule from apical two and four chamber views.
Study endpoint
The primary endpoint was post-STEMI LV dysfunction, defined as a LVEF < 50% during hospitalization and at the 6-month follow-up. The secondary endpoint was cardiac death or heart failure hospitalization within 1 year of the primary PCI. Cardiac death was defined as a mortality with a defined cardiovascular cause and heart failure hospitalization was defined as admission for ≥ 24 h with a primary diagnosis of heart failure, with ≥ 1 symptom and ≥ 2 physical examination, laboratory, or invasive findings of heart failure, and receives a heart failure-specific treatment28.
Statistical analysis
Continuous variables are presented as mean (± standard deviation) and were compared using the unpaired and paired Student’s t-test or Mann–Whitney U test. Means of tertile groups were compared by analysis of variance (ANOVA). Categorical variables are expressed as frequencies and percentages and were compared using the Pearson χ2 test or Fisher’s exact test when the Cochran rule was not met. For the analysis of probability index values obtained at different time points, we used the median value and the statistical significance of differences in these median values across various time points was evaluated using the non-parametric Kruskal–Wallis H-test. Univariate and multivariate logistic regression analyses were performed to identify the proportional hazard risk for LV dysfunction in patients with STEMI after primary PCI, which were adjusted for known potential confounders (age, sex, body mass index, systolic blood pressure, diastolic blood pressure, pulse rate, Killip class ≥ 3, hypertension, diabetes mellitus, end stage renal disease, multiple vessel coronary artery disease, and infarct-related artery). Statistical significance was set at P-value < 0.05. All statistical analyses were performed using IBM/SPSSv24.0 (IBM/SPSS, Chicago, IL, USA), RStudio (Integrated Development Environment for R. RStudio, PBC, Boston, MA, USA), and Python (Python Software Foundation, Wilmington, DE, USA).
Supplementary Information
Abbreviations
- AMI
Acute myocardial infarction
- AI
Artificial intelligence
- AUROC
Area under the receiver operating characteristics curve
- ECG
Electrocardiogram
- IRB
Institutional Review Board
- LV
Left ventricle
- LVEF
Left ventricular ejection fraction
- MVO
Microvascular obstruction
- PCI
Percutaneous coronary intervention
- STEMI
ST-segment elevation myocardial infarction
Author contributions
HSL contributed to the conceptualization, methodology and wrote the first draft. SK and JHJ were involved methodology, data analysis and AI model development. YYJ, JMS, and MSL contributed to AI model development. JSK, HWC, SHK, WL, CHY, and JWS contributed methodology and clinical data collection and verified the clinical coding. TJY and IHC contributed conceptualization and supervised the project. KHJ and JMK were the principal investigators, contributed to the study idea and design, prepared and verified the clinical coding, performed data analysis, wrote the first draft, and contributed to the subsequent drafts.
Data availability
The datasets used and/or analyzed in this study are available from the corresponding author upon reasonable request and to the extent permitted by the Personal Information Protection Act of Korea.
Competing interests
Medical AI Inc. provided support in the form of salaries for authors (Hak Seung Lee, Sora Kang, Jong-Hwan Jang, Yong-Yeon Jo, Jeong Min Son, Min Sung Lee, Joon-myoung Kwon). Joon-myoung Kwon is the founder and stakeholder in Medical AI Inc., a medical artificial intelligence company. Dr. Jeon have received research grant support from Medical AI Inc. Other authors have nothing to declare. There are no patents, products in development of marketed products to declare.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ki-Hyun Jeon and Hak Seung Lee.
Contributor Information
Ki-Hyun Jeon, Email: imcardio@gmail.com.
Hak Seung Lee, Email: cardiolee@gmail.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-67532-6.
References
- 1.Salari N, et al. The global prevalence of myocardial infarction: a systematic review and meta-analysis. BMC Cardiovasc. Disord. 2023;23:206. doi: 10.1186/s12872-023-03231-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee SH, Hong YJ, Ahn Y, Jeong MH. Past, present, and future of management of acute myocardial infarction. J. Cardiovasc. Interv. 2023;2:51–65. doi: 10.54912/jci.2022.0023. [DOI] [Google Scholar]
- 3.De Luca G, Suryapranata H, Ottervanger JP, Antman EM. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: Every minute of delay counts. Circulation. 2004;109:1223–1225. doi: 10.1161/01.CIR.0000121424.76486.20. [DOI] [PubMed] [Google Scholar]
- 4.Frantz S, Hundertmark MJ, Schulz-Menger J, Bengel FM, Bauersachs J. Left ventricular remodelling post-myocardial infarction: Pathophysiology, imaging, and novel therapies. Eur. Heart J. 2022;43:2549–2561. doi: 10.1093/eurheartj/ehac223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ibanez B, Heusch G, Ovize M, Van de Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. J. Am. Coll. Cardiol. 2015;65:1454–1471. doi: 10.1016/j.jacc.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 6.Schroder R. Prognostic impact of early ST-segment resolution in acute ST-elevation myocardial infarction. Circulation. 2004;110:e506–510. doi: 10.1161/01.CIR.0000147778.05979.E6. [DOI] [PubMed] [Google Scholar]
- 7.Heusch G. Coronary microvascular obstruction: the new frontier in cardioprotection. Basic Res. Cardiol. 2019;114:45. doi: 10.1007/s00395-019-0756-8. [DOI] [PubMed] [Google Scholar]
- 8.de Lemos JA, Braunwald E. ST segment resolution as a tool for assessing the efficacy of reperfusion therapy. J. Am. Coll. Cardiol. 2001;38:1283–1294. doi: 10.1016/s0735-1097(01)01550-9. [DOI] [PubMed] [Google Scholar]
- 9.Dong Q, et al. ST-segment resolution as a marker for severe myocardial fibrosis in ST-segment elevation myocardial infarction. BMC Cardiovasc. Disord. 2021;21:455. doi: 10.1186/s12872-021-02269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee BT, et al. Usefulness of deep-learning algorithm for detecting acute myocardial infarction using electrocardiogram alone in patients with chest pain at emergency department: DAMI-ECG study. J. Cardiovasc. Interv. 2023;2:100–112. doi: 10.54912/jci.2022.0028. [DOI] [Google Scholar]
- 11.Attia ZI, Harmon DM, Behr ER, Friedman PA. Application of artificial intelligence to the electrocardiogram. Eur. Heart J. 2021;42:4717–4730. doi: 10.1093/eurheartj/ehab649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito S, et al. Correlation between artificial intelligence-enabled electrocardiogram and echocardiographic features in aortic stenosis. Eur. Heart J. Digit. Health. 2023;4:196–206. doi: 10.1093/ehjdh/ztad009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeon K-H, et al. Identifying atrial fibrillation with sinus rhythm electrocardiogram in embolic stroke of undetermined source: A validation study with insertable cardiac monitors. Korean Circ. J. 2023;53:758–771. doi: 10.4070/kcj.2023.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Essen R, et al. Myocardial infarction and thrombolysis Electrocardiographic short term and long term results using precordial mapping. Br. Heart J. 1985;54:6–10. doi: 10.1136/hrt.54.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaturi M, Birnbaum Y. The use of the electrocardiogram to identify epicardial coronary and tissue reperfusion in acute myocardial infarction. J. Thromb. Thrombol. 2000;10:137–147. doi: 10.1023/a:1018762509887. [DOI] [PubMed] [Google Scholar]
- 16.Dizon JM, et al. Relationship between ST-segment resolution and anterior infarct size after primary percutaneous coronary intervention: Analysis from the INFUSE-AMI trial. Eur. Heart J. Acute Cardiovasc. Care. 2014;3:78–83. doi: 10.1177/2048872613508658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frohlich GM, Meier P, White SK, Yellon DM, Hausenloy DJ. Myocardial reperfusion injury: Looking beyond primary PCI. Eur. Heart J. 2013;34:1714–1722. doi: 10.1093/eurheartj/eht090. [DOI] [PubMed] [Google Scholar]
- 18.Wu MY, et al. Current mechanistic concepts in ischemia and reperfusion injury. Cell Physiol. Biochem. 2018;46:1650–1667. doi: 10.1159/000489241. [DOI] [PubMed] [Google Scholar]
- 19.Heusch G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat. Rev. Cardiol. 2020;17:773–789. doi: 10.1038/s41569-020-0403-y. [DOI] [PubMed] [Google Scholar]
- 20.Bonfig NL, et al. Increasing myocardial edema is associated with greater microvascular obstruction in ST-segment elevation myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2022;323:H818–H824. doi: 10.1152/ajpheart.00347.2022. [DOI] [PubMed] [Google Scholar]
- 21.Abbas A, et al. Cardiac MR assessment of microvascular obstruction. Br. J. Radiol. 2015;88:20140470. doi: 10.1259/bjr.20140470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gassler JP, Topol EJ. Reperfusion revisited: beyond TIMI 3 flow. Clin. Cardiol. 1999;22:20–29. doi: 10.1002/clc.4960221605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon JM, et al. Development and validation of deep-learning algorithm for electrocardiography-based heart failure identification. Korean Circ. J. 2019;49:629–639. doi: 10.4070/kcj.2018.0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon JM, et al. Deep learning-based algorithm for detecting aortic stenosis using electrocardiography. J. Am. Heart Assoc. 2020;9:e014717. doi: 10.1161/JAHA.119.014717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwon JM, et al. A deep learning algorithm to detect anaemia with ECGs: A retrospective, multicentre study. Lancet Digit. Health. 2020;2:e358–e367. doi: 10.1016/S2589-7500(20)30108-4. [DOI] [PubMed] [Google Scholar]
- 26.Kwon JM, et al. Artificial intelligence assessment for early detection and prediction of renal impairment using electrocardiography. Int. Urol. Nephrol. 2022;54:2733–2744. doi: 10.1007/s11255-022-03165-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho Y, et al. Artificial intelligence algorithm for detecting myocardial infarction using six-lead electrocardiography. Sci. Rep. 2020;10:20495. doi: 10.1038/s41598-020-77599-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abraham WT, et al. Standardized definitions for evaluation of heart failure therapies: Scientific expert panel from the heart failure collaboratory and academic research consortium. JACC Heart Fail. 2020;8:961–972. doi: 10.1016/j.jchf.2020.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed in this study are available from the corresponding author upon reasonable request and to the extent permitted by the Personal Information Protection Act of Korea.