Abstract
The cellular and humoral immune responses to adenovirus (Ad) remain a major barrier to Ad-mediated gene therapy. We recently reported that mice deficient in tumor necrosis factor alpha (TNF-α) or Fas (APO-1, CD95) have prolonged expression of an Ad transgene expressing a foreign protein in the liver. To determine whether blockade of TNF-α or Fas would have the same effect in normal mice, we created transgenes that expressed soluble murine CD8 or CD8 fused to the extracellular regions of TNF receptor 1 (TNFR) or Fas and inserted into the left-end region of first-generation (E1/E3−) Ad vectors. Consistent with the results observed in TNF-deficient mice, expression of the TNFR-CD8 fusion protein was prolonged in vivo compared to that of control proteins. Not only did expression of TNFR-CD8 persist in the liver and the lung, but when coadministered with another first-generation vector, the protein provided “transprotection” for the companion vector and transgene. In addition, TNFR-CD8 attenuated the humoral immune response to the Ad. Together, these findings demonstrate that blockade of TNF-α is likely to be useful in extending the expression of an Ad-encoded transgene in a gene therapy application.
In view of their high level of transgene expression and ability to infect nondividing cells, first-generation adenovirus (Ad)-based vectors remain an important tool for gene therapy. The utility of these vectors is, however, limited by the immune response to Ad or its transgene. The cellular arm of the immune response eliminates transgene expression 2 to 3 weeks postinfection, and the humoral component prevents reinfection (reviewed in references 35 and 38).
Despite the fact that >90% of Ad is taken up by macrophages following intravenous (i.v.) administration (37), little attention has been paid to the earliest events whereby virus infection activates the innate immune response. Tumor necrosis factor alpha (TNF-α) is a major proinflammatory cytokine that is secreted by infected macrophages and is known to be released following wild-type Ad infection (15), as well as following infection with first-generation Ad vectors (21). Wild-type Ad synthesizes at least four proteins encoded in the E3 gene to counteract TNF-α action (36). In addition, since the E3 region has been removed from almost all Ad vectors to accommodate transgenes, Horwitz and colleagues (17) reinserted the E3 region into gene therapy vectors and demonstrated markedly prolonged persistence of expression. We have recently shown that TNF-α-deficient mice have a marked impairment in the ability to clear Ad compared to that of wild-type mice (10). Taken together, these findings suggest that TNF-α plays a critical role in the elimination of wild-type and Ad gene therapy vectors.
Although granzyme/perforin-mediated cytotoxicity is generally regarded as the most common effector pathway through which viruses are eliminated, perforin-deficient mice are able to mount efficient immune responses against viruses such as vesicular stomatitis virus, influenza virus, Semliki Forest virus, and rotavirus (13, 18). We recently observed that perforin-deficient mice were also able to efficiently clear Ad expressing the chloramphenicol acetyltransferase (CAT) transgene (AdCAT) (10). In contrast, Fas-deficient (lpr) mice had a modest prolongation in the expression of AdCAT compared to wild-type mice, suggesting that Fas-mediated apoptosis played a role in immune elimination of the virus. This suggestion is supported by recent studies demonstrating Fas receptor degradation as an antihost strategy employed by wild-type Ad (29, 31).
Both Fas (8)- and TNF-α (22, 27)-deficient mice develop immunological abnormalities caused by the deficiency of the gene product from birth. To determine whether blockade of TNF-α or Fas in an immunologically intact mouse would affect the duration of expression of Ad vectors, we constructed soluble inhibitors of TNF-α and Fas. As observed with TNF-α-deficient mice, the TNF-α inhibitor prolonged transgene expression whereas the Fas inhibitor was much less effective.
MATERIALS AND METHODS
Mice.
C57BL/6 (B6) (H-2b), BALB/c/J (H-2d), C3H (H-2k), and C57/BL-Faslpr mice were purchased from Jackson Laboratory, Bar Harbor, Maine. SCID mice were bred and maintained at HSS in a pathogen-free environment. TNF-α-deficient mice (22) were kindly provided by M. W. Marino.
RT-PCR.
Total RNA was extracted from lung tissue by RNAzol (TelTest) in accordance with the manufacturer’s protocol. cDNAs were synthesized with the Superscript Kit (Gibco). PCR amplification (30 cycles) was performed with primers specific for CD8 (5′) paired with either the TNF receptor (TNFR) or Fas (3′) using the primers described below. Reverse transcription (RT)-PCR for β-actin was used as a housekeeping gene control. For PCR amplification of CAT cDNA, the following primers (5′→3′) and conditions were used: CACTGGATATACCACCGT and CGCCCCGCCCTGCCACTC; 95°C for 30 s, 55°C for 30 s, and 72°C for 50 s for 40 cycles. The common primers CCCAGGTCCAACTGCAGCCC and GGTACTTGTGAGCCAAGGCAG were used for PCR of the Ad vector spanning the different transgenes.
Generation of replication-defective Ad expressing soluble murine proteins.
The extracellular domains of murine TNFR1, Fas, and CD8-α chain were amplified from cDNA by using the following primers (in the 5′→3′ orientation): Fas, ACACTCTGCGATGAAGAGCA; TNFR1, CCTGTAAGGAGACTCAGAAC; CD8, CGCTAAGCTTCCACCATGGCCTCACCGTTGACCCGC and GCTGCTCGAGCTATTAATCACAGGCGAAGTCCAATCC. Chimeric TNFR-CD8 and Fas-CD8 fusion proteins were created by overlapping PCR, taking care to avoid the introduction of foreign amino acids, by using the following primers: FasCD8, CGGAGTTCGGGTGCCTGTGGCTTAGCT CATTTCTGGGACTTTGTTTCCTGCAGTTTGT and ACAAACTGCAGGAAA CAAAGTCCCAGAAATGAAGCAAGCCACAGGCACCCGAACTCCG; TNFRCD8, CGGAGTTCGGGTGCCTGTGGCTTAGCTTCCGCAGTACCTGAGTCCTGGGGGTTTGTG and CACAAACCCCCAGGACTCAGGTACTGGGGAAGCTAAGCCACAGGCACCCGAACTCCG. All fragments were ligated between the HindIII and XhoI sites of pAd. The viruses expressing TNFR-CD8 and Fas-CD8 were produced as previously described (10). Briefly, the pAd vector containing a transgene was cotransfected with PJM17 into 293 cells. The cell lysate was used to infect 293 cells. Viral DNA was extracted by a modified Hirt assay, and recombination was verified by restriction enzyme digestion and PCR, and the protein product was verified by enzyme-linked immunosorbent assay (ELISA). The viruses were further plaque purified. Each clone was rescreened as described above. Finally, large-scale virus was purified by two-step CsCl concentration and stored in glycerol at −20°C or in sucrose at −70°C. Viral particles were quantitated by measurement of optical density (OD) at 260 nm.
ELISA.
The antibodies used for ELISA and their sources were as follows: anti-CD8-α chain, TIB 105 hybridoma (American Type Culture Collection); mouse YST 169 (Caltag); anti-Fas; Jo-2 (Pharmingen); R-anti-Fas, a rabbit polyclonal antibody (9); and anti-mTNFR1 clone55R-593 (Genzyme). ELISA plates were coated with antibody (5 μg/ml) and then incubated overnight at 4°C. The plates were blocked with phosphate-buffered saline (PBS)–3% bovine serum albumin (BSA) for 1 h at room temperature and then incubated with the test sample for 4 to 5 h at room temperature. The plates were washed and sequentially incubated with biotinylated secondary antibody, avidin-alkaline phosphatase, and substrate. The OD was read at 405 nm. Unless indicated otherwise, cell culture supernatants and BALs (broncheoalveolar lavage fluids) were tested without further dilution whereas serum was diluted 1/2.
To obtain a standard for ELISA, Fas-CD8 was isolated from culture supernatants by step elution on a DEAE ion-exchange column. The protein was isolated to ∼70% purity as determined by silver staining and Western blot analysis.
Western blotting and gel filtration.
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis was performed on a 12% gel under reducing conditions. Western blotting was performed as previously described (5) by using rabbit anti-Fas or goat anti-TNFR antibodies (Santa Cruz). To evaluate the molecular masses of the fusion proteins under nondenaturing conditions, gel filtration on a Sephadex G-100 column (Pharmacia) equilibrated in PBS was performed following calibration with immunoglobulin G (IgG), BSA, and ovalbumin. Concentrated supernatant from virus-infected 293 cells was loaded onto the column, and 1-ml fractions were collected. Fas-CD8 or TNFR-CD8 was detected by ELISA as described above. The same supernatants were run on the same column under dissociating conditions (0.2% SDS).
T-cell cytotoxicity and proliferation assays.
To determine whether the Fas-CD8 fusion protein could inhibit apoptosis by authentic FasL, Fas-CD8 or CD8 supernatants were incubated with soluble functional human FasL (25) at 4°C for 6 h. The supernatants were then incubated with FasL-sensitive cell line A20 (24), and apoptosis was quantified by the alamar blue assay of viability as previously described (24) 12 to 16 h later. To evaluate TNFR-CD8 function, recombinant TNF-α (Genzyme) was incubated with serial dilutions of TNFR-CD8 supernatants or anti-mTNF-α clone MP6-XT22 (Pharmingen) at 4°C for 6 h. Cytotoxicity was then evaluated at 16 h by the alamar blue assay using the L929 cell line in the presence of cycloheximide (10 μg/ml). Fas-CD8 supernatant and Rat IgG were included as controls.
Allogeneic cytotoxic T-lymphocyte reactions were generated by injection of C3H B6/F1 spleen cells into C3H mice. Spleen cells were harvested at 7 days and tested for cytotoxicity against 51Cr-labeled EL4 cell (H-2b) targets. Percent lysis was calculated according to the formula [(cpm sample − cpm spontaneous)/(cpm maximum − cpm spontaneous)] × 100%.
To quantify T-cell sensitization to Ad, splenocytes were harvested at day 10 after i.v. infection. Cultures (2.5 × 105 cells/well) were incubated with various concentrations of virus in 96-well plates. At day 5, cells were pulsed with [3H]thymidine (1 μCi/well) and proliferation was quantified on a scintillation counter.
Jo-2- and LPS-induced hepatic damage.
To induce hepatic apoptosis through the Fas receptor (23), SCID mice were injected with 6 μg of Jo-2 antibody i.v. The mice were observed for 24 h, and any live mice were sacrificed. To induce TNF-α-mediated septic shock, C3HeSnJ mice were sensitized and then injected with lipopolysaccharide (LPS) as previously described (22). In brief, six C3H/HeSnJ mice per group were injected with 2 × 1010 particles of AdTNFR-CD8 or the AdCAT control. Five days later, they were sensitized with 25 mg of d-galactosamine, followed by 0.3 μg of LPS given intraperitoneally. The mice were observed for 3 days, after which surviving mice were sacrificed. At the time of death, the livers were harvested and fixed in formalin. Formalin-fixed tissue was embedded in paraffin, and sections were stained with hematoxylin and eosin for examination by light microscopy.
Anti-Ad antibody response.
Quantitation of anti-Ad IgG was performed as described previously (9). Briefly, plates were coated with purified Ad (108 particles/well) at 4°C overnight. Following blocking with 3% BSA, serum samples were diluted from 1/10 to 1/2,000 and incubated with antigen. The plates were sequentially blocked with 3% BSA, incubated with serum (diluted 1/2,000 for i.v. infected mice and 1/200 for intratracheally [i.t.] infected mice) and alkaline phosphatase-conjugated anti-mouse IgG. Neutralizing antibody titers were evaluated as previously reported (7). Briefly, twofold serial dilutions of sera were preincubated with Ad expressing LacZ (500 particles/cell) for 1 h at 37°C and added to A549 cells in 96-well plates (20,000 cells/well). Expression of LacZ was measured by 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining 16 h after infection.
Statistical analysis.
Test samples were analyzed for normal distribution and then compared by either Student’s t test (normal distribution) or the Mann-Whitney rank sum test (nonparametric data). Multiple comparisons were performed by analysis of variance with the Student-Newman-Keuls method for pairwise comparisons.
RESULTS
Construction and expression of CD8 fusion protein.
Trimerization has been shown to be important for TNF family ligand-receptor interaction (3). Given the property of spontaneous oligomerization of the CD8 α-chain extracellular (EC) domain and successful employment of a CD8-CD40L fusion protein (6), the CD8 EC domain was fused with the Fas or TNFR1 EC domain to generate a chimeric fusion protein. As a control, a virus expressing only the CD8 α-chain EC domain was also produced. To minimize any possible foreign elements in the transgene, no extra amino acid was introduced between these domains.
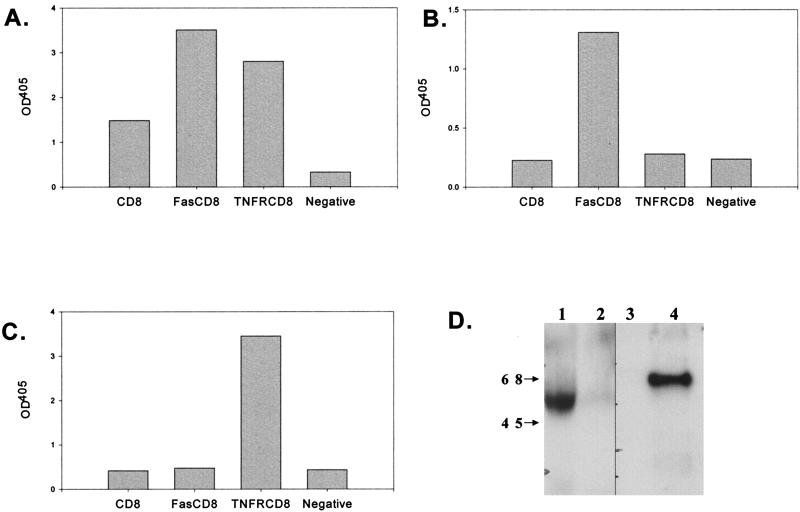
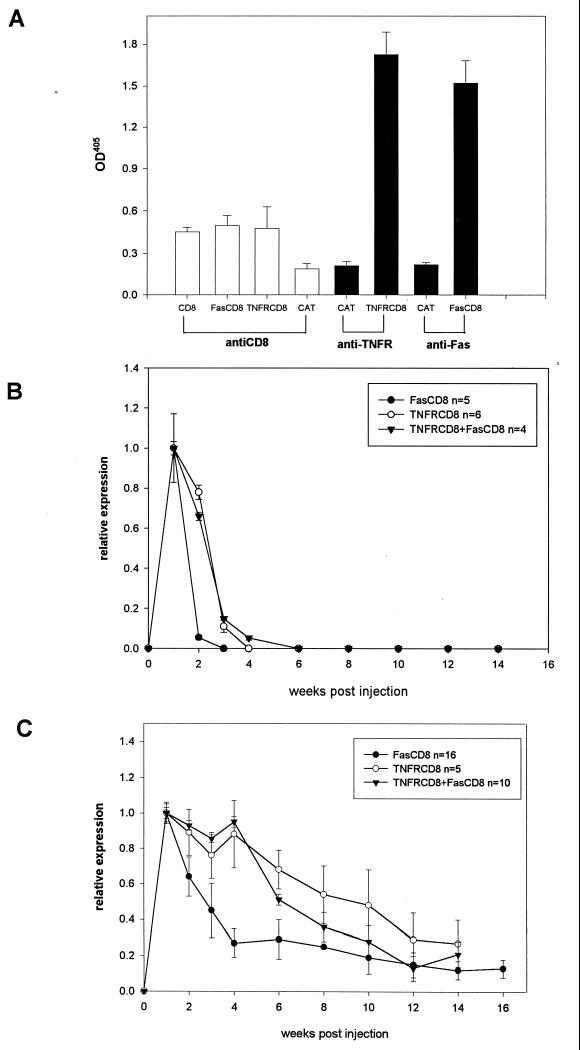
To determine whether full-length, functional proteins were produced, HeLa cells were transiently infected with the Ad vectors and the culture medium was tested for protein expression by ELISA and Western blot analysis and for function by inhibition of cell death. As shown in Fig. 1A, all three proteins could be detected by ELISA using two different monoclonal antibodies to CD8. These results were specific, since when the coating antibody was directed against either Fas (Fig. 1B) or TNF-R1 (Fig. 1C), only the appropriate transgene product was detected. Western blot analysis revealed that TNFR-CD8 and Fas-CD8 had molecular masses of ∼60 and 56 kDa, respectively (Fig. 1D), consistent with the sizes predicted from the DNA sequences and glycosylation (34). To examine the molecular weights of these fusion proteins under native (PBS) and dissociating (0.5% SDS) conditions, we applied culture supernatants to a Sephadex G-100 gel filtration column and detected the fusion proteins by ELISA. Under native conditions, TNFR-CD8 and Fas-CD8 eluted near the void volume of the column, whereas under dissociating conditions, the size of TNFR-CD8 was ∼45 kDa (data not shown).
FIG. 1.
Detection of fusion proteins by ELISA and Western blotting. ELISA plates were coated with capture antibody (A, anti-CD8; B, anti-Fas; C, anti-TNFR1) and incubated with the culture supernatants from HeLa cells infected with Ad vectors expressing the proteins indicated (x axis). The proteins were detected by sequential incubation with biotinylated anti-CD8, streptavidin-alkaline phosphatase, and substrate. The results are expressed as OD at 405 nm. Panels A to C are representative of six experiments. The negative control was culture medium alone. (D) Concentrated supernatants obtained from AdFas-CD8 (lane 1), AdCD8 (lanes 2 and 3)-, and AdTNFR-CD8 (lane 4)-infected HeLa cells were analyzed by Western blotting using rabbit anti-Fas (lanes 1 and 2) or goat anti-TNFR1 antibodies (lanes 3 and 4) followed by the appropriate peroxidase-labeled secondary antibodies and substrate. The values to the left are molecular sizes in kilodaltons.
CD8 fusion proteins specifically inhibit their cognate ligands in vitro.
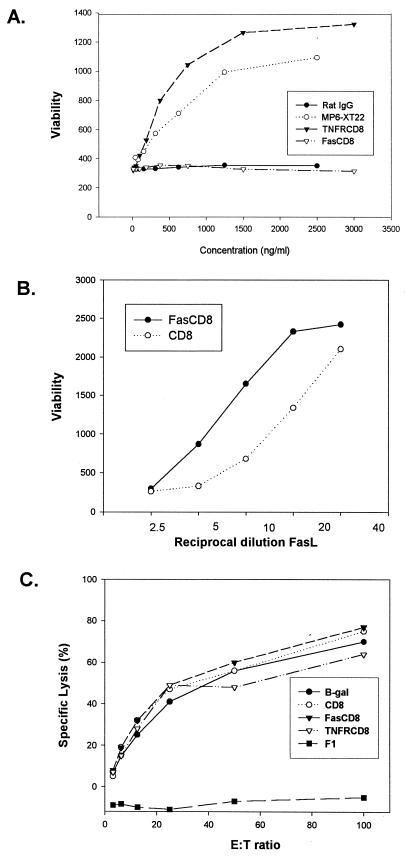
To ensure that the fusion proteins retained the function of binding authentic ligand, we tested the culture supernatants containing each protein in an in vitro functional assay. As shown in Fig. 2A, TNFR-CD8 efficiently inhibited TNF-α induced death of L929 cells. Similarly, Fas-CD8 attenuated FasL-mediated apoptosis of the B-cell lymphoma A20 (Fig. 2B). Since CD8 can bind to major histocompatibility complex class I via its CDR1 and CDR2 domains, we also evaluated the possible interference of soluble CD8 in a classical allogeneic cytotoxic T-lymphocyte reaction. As shown in Fig. 2C, addition of supernatants containing Fas-CD8 or TNFR-CD8 failed to inhibit this in vitro reaction. In addition, no evidence for binding of these fusion proteins to several major histocompatibility complex class I-positive cell lines (AE7 and EL4) was observed by flow cytometry analysis (data not shown).
FIG. 2.
CD8 fusion proteins specifically inhibit their cognate ligands in vitro. (A) Twofold dilutions of TNFR-CD8 viral supernatant or MP6-XT22 anti-TNFR-α monoclonal antibody were preincubated with 300 pg of TNF-α per ml for 4 h at 4°C and then incubated with L929 cells in the presence of cycloheximide (10 μg/ml) for 16 h. Cell viability was evaluated by using alamar blue. (B) A serial twofold dilution of soluble human Fas ligand (FasL) (25) was added to the B-cell lymphoma A20 in the presence of either Fas-CD8 or soluble CD8 alone. Cell viability was quantified by the alamar blue assay as for panel A. Results are presented as mean of duplicates and are representative of three experiments. (C) Alloreactive (anti-H-2b) or control (F1) T cells were generated in vivo as described in Materials and Methods and tested for cytotoxicity against 51Cr-labeled EL4 cells. Supernatants from HeLa cells infected with Ad vectors expressing β-galactosidase, CD8, Fas-CD8, or TNFR-CD8 were coincubated in the cytotoxicity assay, and the results are expressed as percent specific lysis. The results shown are means of triplicates and are representative of two experiments. E:T ratio, effector-to-target cell ratio.
The soluble Fas and TNFR fusion proteins are expressed and functional in vivo.
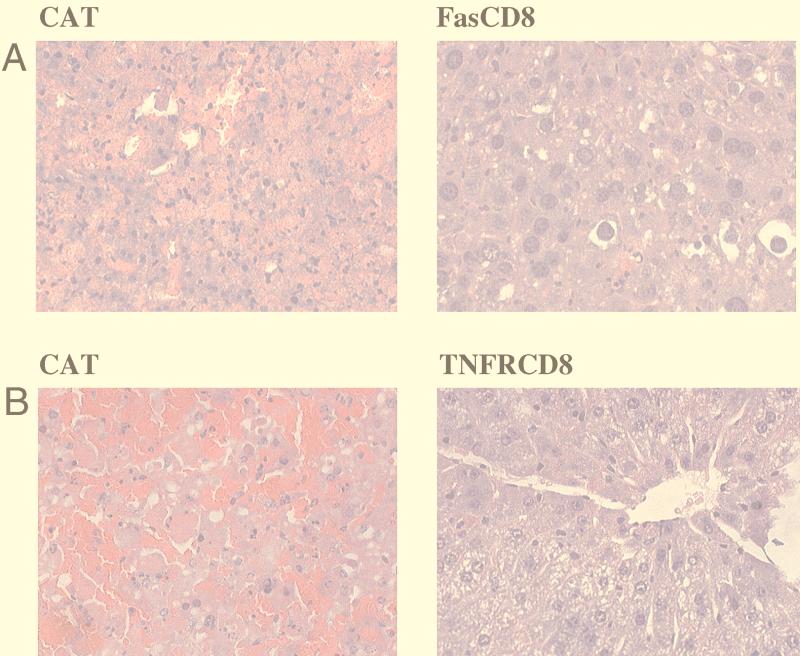
Purified viruses were injected into BALB/c mice i.v. and serum was assayed for transgene expression at 1 week postinjection. To determine whether the secreted soluble fusion proteins were functional, inducers of cell death were administered to Ad-infected SCID mice at 1 week postinfection. As shown in Fig. 3A, 6 μg of anti-Fas antibody Jo2 induced massive hepatic apoptosis in SCID mice infected with the control AdCAT virus (all six mice died by 24 h), whereas mice infected with AdFas-CD8 were substantially protected (none of six mice died at 24 h). To investigate the functional capacity of the TNFR-CD8 fusion protein in vivo, we exploited the high sensitivity of mice to lethal shock when exposed to bacterial LPS systemically. Following LPS injection, the mice were observed for 3 days, after which surviving mice were sacrificed. All of the six mice injected with AdCAT died within 6 h of injection of LPS. In striking contrast, all six mice that had been injected with AdTNFR-CD8 survived (similar to TNF-α deficient mice [21]). At autopsy (immediately after death), the AdCAT-injected mice showed extensive hemorrhagic liquifaction of the liver whereas mice receiving AdTNFR-CD8 were protected (Fig. 3B).
FIG. 3.
Fusion proteins Fas-CD8 and TNFR-CD8 protect mice from lethal anti-Fas hepatic apoptosis or LPS-induced shock. (A) SCID mice were injected i.v. with 2 × 1010 particles of AdCAT (n = 6) or AdFas-CD8 (n = 6). Six days later, the mice were injected with 6 μg of anti-Fas i.v. Livers of mice that died or were sacrificed at 24 h postinjection were analyzed by hematoxylin- and eosin staining. Livers from AdCAT-infected mice showed extensive hemorrhage with liquefaction “necrosis”, whereas mice infected with AdFas-CD8 were protected. Magnification, ×40. (B) C3H/HeSnJ mice were injected i.v. with 2 × 1010 particles of AdTNFR-CD8 (n = 6) or the AdCAT control (n = 6). Five days later, they were sensitized with 20 mg of d-galactosamine followed by 0.3 μg of LPS given intraperitoneally. The livers of mice that died or were sacrificed were sectioned and stained with hematoxylin and eosin. The AdCAT-injected mice showed extensive hemorrhagic liquefaction of the liver, whereas mice receiving AdTNFR-CD8 were protected. Magnification, ×40.
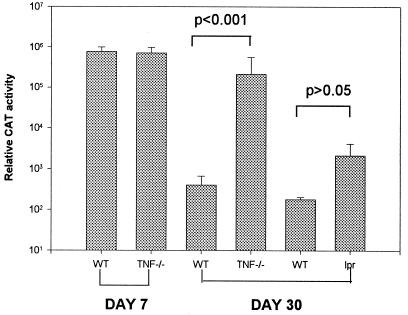
FIG. 6.
Transgene expression in the lung. (A) AdCAT (2.5 × 109 viral particles) was administered to TNF-α-deficient (TNF−/−) mice, Fas-deficient (lpr) mice, or paired wild-type (WT) controls (n = 5) by the i.t. route, and CAT expression was quantified at days 7 and 30 as previously described (10).
Duration of transgene expression in the liver.
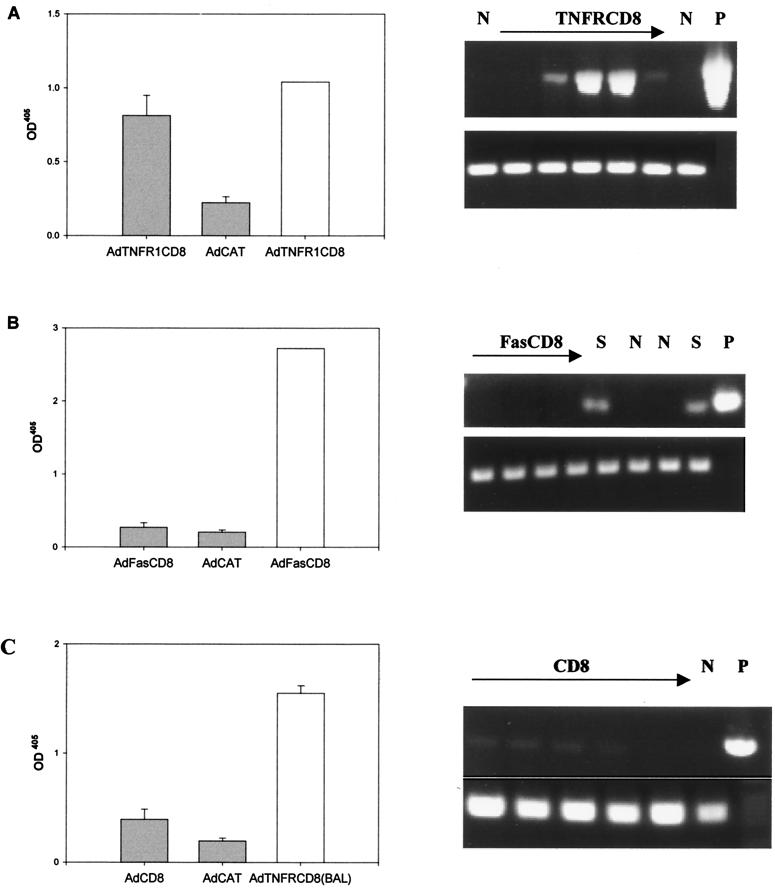
To compare the expression levels of the different transgenes in vivo, SCID mice were injected with 2 × 1010 particles of AdCD8, AdTNFR-CD8, or AdFas-CD8 and pooled serum from each group was tested for CD8 expression by ELISA. As shown in Fig. 4A, CD8, Fas-CD8, and TNFR-CD8 were produced at similar levels in vivo as determined by the CD8 sandwich ELISA. In contrast, when anti-Fas or anti-TNFR was used as the coating antibody, the ELISA sensitivities were severalfold higher for Fas-CD8 and TNFR-CD8, respectively. Because of the low level of CD8 detection, this transgene was not used for kinetic studies.
FIG. 4.
Detection and kinetics of transgene expression in vivo. (A) To compare the relative sensitivities of the ELISAs for the transgenes, SCID mice (four or five per group) were infected with 2 × 1010 particles of Ad vectors expressing CD8, FasCD8, or TNFR-CD8. The 7-day serum samples were pooled, diluted 1:2, and tested by ELISAs using the capture antibodies shown and anti-CD8 antibody YST 169 as the detecting antibody. AdFas-CD8, AdTNFR-CD8, or both viruses together were used to infect BALB/c (B) or B6 (C) mice. Serum was analyzed at weekly intervals for Fas-CD8 (closed symbols) or TNFR-CD8 (open symbols) transgene expression by ELISA as described in the legend to Fig. 1. The results are expressed relative to the OD at week 1. Expression of Fas-CD8 was significantly higher in AdFas-CD8-plus-AdTNFR-CD8-infected mice compared to AdFas-CD8-infected mice (BALB/c mice at week 2, P < 0.0001; B6 mice at week 6, P < 0.001).
To determine whether inhibition of Fas or TNF-α allowed prolonged expression of the respective fusion proteins in normal mice, BALB/c and B6 mice were infected by the i.v. route and tested for fusion protein expression by ELISA. In BALB/c mice, levels of Fas-CD8 in serum peaked at week 1 (corresponding to ∼4 μg/ml compared to a partially purified standard) and then approached background levels after 2 weeks, whereas TNFR-CD8 levels were sustained until week 2 and then declined in week 3 (Fig. 4B). When AdFas-CD8 and AdTNFR-CD8 were coinjected, Fas-CD8 persisted with kinetics similar to those of TNFR-CD8 (Fig. 4B). To examine strain differences, the experiments were repeated with B6 mice. In contrast to BALB/c mice, a high level of Fas-CD8 expression was detected in about a third of mice for 4 weeks postinjection, followed by a gradual decline. All of the B6 mice expressed high a level of TNFR-CD8 (n = 5) up to 6 weeks postinfection. As in BALB/c mice, TNFR-CD8 prolonged the expression of Fas-CD8.
Coinfection with AdCAT and AdTNFR-CD8 results in reduced mononuclear cell infiltration in the liver.
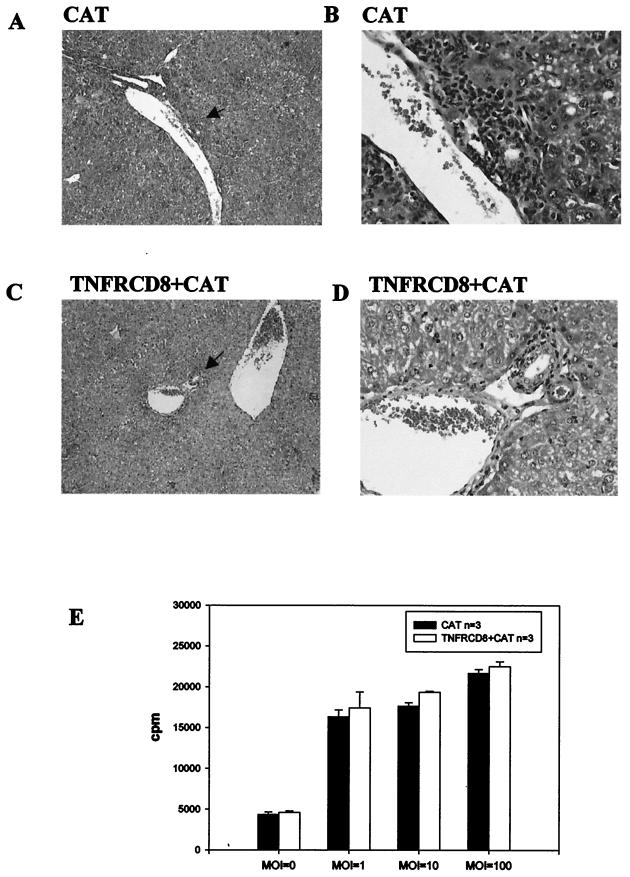
We previously reported that prolonged expression of AdCAT in the livers of TNF-α-deficient mice was explained, in part, by reduced hepatic infiltration of mononuclear cells. To determine whether a similar mechanism could explain prolonged TNFR-CD8 expression in B6 mice, we examined the livers of mice infected with AdTNFR-CD8 7 days postinfection. As shown in Fig. 5, AdCAT-infected mice showed extensive infiltration of mononuclear cells, especially around the portal vein, whereas coinjection of AdTNFR-CD8 with AdCAT greatly reduced the extent of inflammation. As shown in Fig. 5E, T cells from TNFR-CD8 mice were equivalently sensitized to virus, indicating that the absence of TNF-α results in a marked reduction in mononuclear cell recruitment but does not impair T-cell proliferation (this study) or T-cell cytotoxicity (9).
FIG. 5.
T cells in AdTNFR-CD8-infected mice proliferate in response to antigen but show reduced mononuclear cell infiltration in the liver. B6 mice were infected with 2 × 1010 particles of AdCAT (n = 4) or 2 × 1010 particles of AdTNFR-CD8 (n = 4) together with AdCAT and sacrificed at 7 days postinfection. Liver sections were stained with hematoxylin and eosin and examined by light microscopy (magnifications: A and C, ×10; B and D, ×40). Extensive perivascular infiltrates were observed in mice infected with AdCAT (A and B) but were much less prominent in mice receiving the coinjection (C and D). To determine whether T cells were sensitized to Ad, T-cell proliferative responses were compared at different multiplicities of infection (MOI) at day 5 by [3H]thymidine incorporation (E).
Absence or blockade of TNF-α prolongs transgene expression in the lung.
To determine whether mucosal immune responses in the airway were similar to the systemic immune response in the liver, we administered AdCAT to TNF-α- and Fas-deficient mice by the i.t. route and quantified CAT expression in the lungs. At 4 weeks postinjection, CAT activity was significantly higher in lung tissue obtained from TNF-α-deficient mice but only modestly higher in Fas-deficient mice compared to wild-type mice (Fig. 6).
We next examined whether the soluble fusion proteins would promote virus persistence in the lung. When B6 mice were infected with AdTNFR1-CD8, AdFas-CD8, or AdCD8 by the i.t. route, relatively high levels (compared to the SCID control) of the TNFR1-CD8 but not the Fas-CD8 fusion protein were detected in BALs 4 weeks postadministration (Fig. 7). In view of the differences in the sensitivities of the ELISAs, we evaluated mRNA transcription of the transgenes with the same primer pairs used to create the transgene. RT-PCR confirmed continued transcription of the TNFR1-CD8 transgene mRNA in four out of five mice and Fas-CD8 in none of four mice, and CD8 mRNA was barely detected in all five mice at this time (Fig. 7). Similar results were obtained when common vector primers were used for PCR. These findings indicate that blockade of TNF-α in the lung has a protective effect on immune elimination similar to that observed in the liver but that Fas plays a lesser role than that previously observed in the liver (10).
FIG. 7.
B6 mice (four or five per group) were infected with 2.5 × 109 viral particles of AdTNFR1, AdFas-CD8, AdCD8, or AdCAT (background control) by the i.t. route. BALs were obtained at day 28, and the levels of expression of TNFR-CD8 (A), Fas-CD8 (B) and CD8 (C) were quantified by ELISA as described in the legend to Fig. 1. The level of expression of the corresponding transgene in SCID serum (open bars in panels A and B) is shown for comparison. In panel C, BAL from AdTNFR-CD8-infected mice whose results are shown in panel A was used as a control (open bar). Expression of the mRNAs encoding these proteins was also evaluated by RT-PCR using the primers described in Materials and Methods. The lanes containing RNA from mice infected with the transgene are shown. N, mice injected with an irrelevant transgene; S, SCID mouse injected with the same transgene; P, plasmid positive control.
Transprotection mediated by TNFR-CD8.
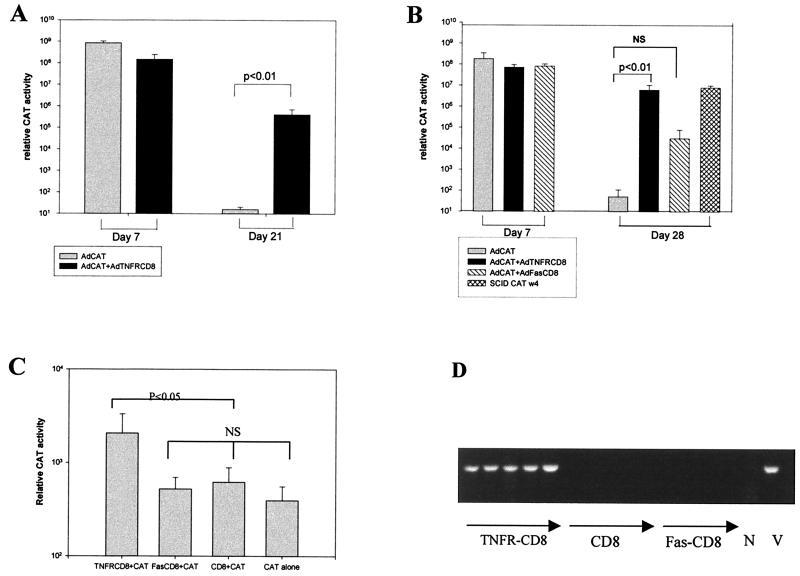
To approximate a gene therapy application more closely, we examined whether TNFR-CD8 would provide transprotection for a second gene product, in this case, the bacterial enzyme CAT (Fig. 8). AdTNFR-CD8 and AdCAT were coinjected i.v., and CAT expression was evaluated at day 21 in BALB/c mice (Fig. 8A) or at day 28 in B6 mice (Fig. 8B). As shown, coexpression of AdTNFR-CD8 with AdCAT markedly enhanced CAT expression in both strains of mice compared to AdCAT alone. To determine whether this effect could also be seen in the lung, the three transgenes were coadministered with AdCAT by the i.t. route and expression of CAT was quantified at day 28. As shown in Fig. 8C, TNFR-CD8 conferred significantly greater protection for CAT expression compared to the other transgenes. When analyzed at the level of transcription, CAT mRNA was only detected in AdTNFR-CD8-infected lungs (Fig. 8D). CAT mRNA expression was not detected in the lungs of mice infected with AdCAT alone (data not shown). Together, these findings indicate that TNFR-CD8 can extend the expression of a foreign transgene product and that this expression is due to continued transcription of the transgene.
FIG. 8.
Transprotection of CAT by TNFR-CD8. For liver tests, BALB/c (A) or B6 (B) mice (four or five per group) were either infected with 2 × 1010 particles of AdCAT alone or coinfected with 2 × 1010 particles of AdCAT together with 2 × 1010 particles of AdTNFR-CD8 i.v. The mice were sacrificed at the time points shown, and the livers were harvested for measurement of CAT activity. NS, not significant. For lung tests, B6 mice were infected with 2.5 × 109 particles of AdTNFR1 (n = 5), AdFas-CD8 (n = 4), or AdCD8 (n = 5) together with 2.5 × 109 particles of AdCAT by the i.t. route. At day 28, the mice were sacrificed and the lungs were harvested for measurement of CAT enzyme activity (C) or CAT mRNA expression by RT-PCR (D). V, CAT viral DNA; N, negative control.
TNFR-CD8 attenuates the humoral immune response to Ad.
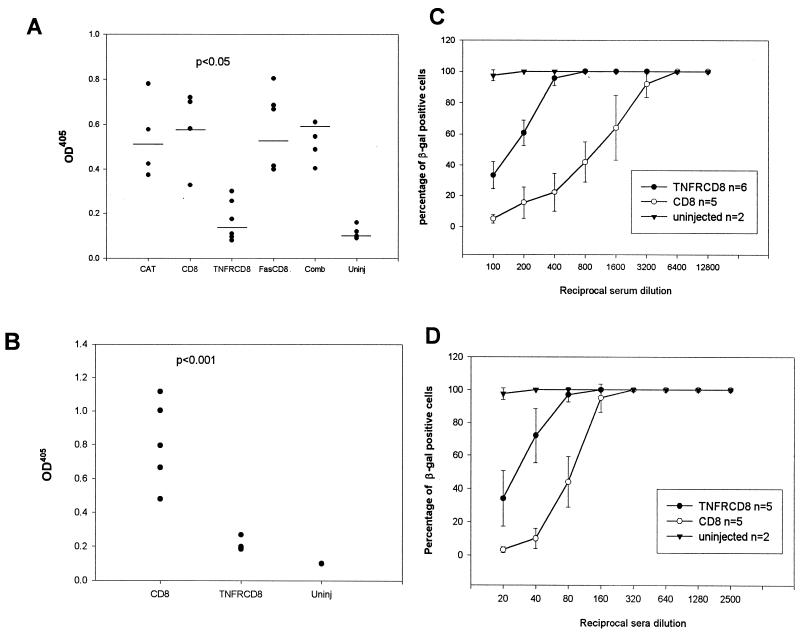
We previously showed that TNF-α-deficient mice had an impaired humoral immune response to AdCAT (10). This finding is of considerable importance, since the humoral immune response prevents successful readministration of the virus. T-cell-dependent B-cell responses in TNF-α-deficient mice are compromised due to a defect in germinal-center formation (22, 27). We therefore examined the effect of TNF-α blockade on humoral responses to Ad in the liver and lung 4 weeks postinfection. Following i.v. administration, IgG responses in AdTNFR-CD8 mice were substantially reduced compared to those in controls (CAT and CD8) (Fig. 9A). In contrast, mice infected with AdFas-CD8 alone or with both vectors simultaneously had antibody responses similar to those of controls. When mice were infected with AdTNFR-CD8 by the i.t. route, there was a statistically significant reduction in anti-Ad antibodies compared to controls (Fig. 9B). Neutralizing assays confirmed that the reduction in total antibody levels was associated with reduced neutralizing activity. About 10- and 4-fold less neutralizing antibody was produced in mice infected with AdTNFR-CD8 than in those infected with AdCD8 by the i.v. (Fig. 9C) and i.t. (Fig. 9D) routes, respectively.
FIG. 9.
TNFR-CD8, but not Fas-CD8, reduces the humoral immune response to Ad. Mice were infected with Ad vectors expressing the vectors shown (x axis) by either the i.v. (A and C) or the i.t. (B and D) route. Sera were tested for total IgG anti-Ad antibodies (A and B) or neutralizing antibodies (C and D) at day 28 by ELISA. In panel A, the TNFR-CD8 and uninjected (Uninj) levels, but not the Fas-CD8 and combined (TNFR-CD8 together with Fas-CD8 [Comb]) levels, were significantly lower than the control (CAT and CD8) levels (analysis of variance, P < 0.05). In panel B, antibody levels were significantly lower in the TNFR-CD8 group than in the CD8 group (P < 0.001).
DISCUSSION
TNF and Fas belong to the nerve growth factor-TNF (NGF-TNF) receptor NGF-TNF superfamily of proteins that now comprise almost 20 members (1, 33). TNF-α has dual antiviral potential based on the ability to directly induce cytotoxicity as well as to promote a potent proinflammatory immune response. This proinflammatory response is mediated, in part, by NF-κB transcription of numerous cytokine and adhesion molecule gene products (reviewed in reference 2). FasL is a cytotoxic effector that induces apoptosis in activated cells that bear the Fas receptor (reviewed in reference 26). FasL has been implicated in the host defense against several virus infections. CD4+ T cells can transfer lethal lymphocytic choriomeningitis virus disease to β2-microglobulin-deficient mice by a Fas-dependent pathway (39), and evidence for FasL-mediated tissue injury has been observed both in the hepatitis transgene model and in Fas null mice (20), as well as in human livers infected with hepatitis B virus (14, 16).
We previously reported a marked or moderate persistence of expression of a foreign transgene, that for CAT, in mice that were deficient in TNF-α or Fas, respectively (10). In the present study, we demonstrated the persistence of transgenes in normal animals by expressing soluble receptors that inhibited their respective ligands. To design Ad vectors expressing soluble TNFR1 and Fas, the extracellular domains of these proteins were ligated to the EC domain of CD8. We developed ELISAs to quantify recombinant protein levels and demonstrated that soluble CD8 and the chimeric fusion proteins TNFR-CD8 and Fas-CD8 were produced by virus-infected cells in vitro. It has previously been shown that TNFR and Fas function as trimmers (3, 19). Western blot analysis and gel filtration confirmed the correct size of the fusion proteins and, most important for ligand binding, confirmed that the fusion protein multimerized under nondissociating conditions. Both TNFR-CD8 and Fas-CD8 were found to block their respective ligands in vitro, as well as to prevent fatal LPS-induced shock mediated through TNF (22) or anti-Fas-mediated hepatic apoptosis (23) in vivo.
Most foreign transgenes expressed by Ad are eliminated between 2 and 3 weeks postinfection (35, 38). Few studies, however, have evaluated the duration of expression of self transgenes. Tripathy et al. (32) compared the duration of expression of transgenes encoding a foreign (human) soluble protein with that of a self (mouse) soluble protein, erythropoietin, following intramuscular injection into normal mice. Whereas the human-encoded protein was rapidly eliminated, mouse erythropoietin persisted for periods exceeding 4 months as determined by its biological effect on erythrocyte production. The authors concluded that limited transgene expression reported by other investigators could be explained by an immune attack on the foreign protein. Other investigators have shown a shorter duration of expression of self proteins. Although a biological effect of thrombopoietin was evident for 2 months, mRNA expression of the transgene was not detected beyond 2 weeks postinfection, regardless of the route of administration or the mouse strain infected (30).
To determine the duration of expression of CD8 self transgenes following i.v. administration in normal mice, we quantified the levels of soluble CD8 and the CD8 fusion proteins in serum at weekly intervals postinfection. Failure to detect CD8 in serum beyond 1 week was, at least in part, explained by the low sensitivity of the CD8 ELISA, whereas when serum was tested neat or diluted 1/2, the ELISAs for Fas and TNFR were approximately equivalent in sensitivity. The significant increase in the levels of TNFR-CD8 compared to Fas-CD8 therefore suggests that the TNFR fusion protein prolonged its own expression in the liver. This was particularly striking in the B6 strain, where the fusion protein was readily detectable in serum 2 to 3 months postinfection.
We previously reported that persistence of a foreign transgene, that for CAT, in TNF-α-deficient mice was explained by reduced recruitment of T cells to the liver rather than a failure of T-cell cytotoxicity (10). Since reduced mononuclear cells were also observed in the livers of mice infected with AdTNFR-CD8 yet T-cell proliferation in response to the virus was normal, we conclude that persistence of the virus in these mice is also due to impaired recruitment to the site of infection.
To further examine the effects of the soluble TNFR and Fas fusion proteins, similar experiments were performed in the lung. Following i.t. administration, TNFR-CD8, but not Fas-CD8 or CD8 alone, was detected at relatively high levels in the BAL at day 28. Detection of the protein was due to persistent expression, since mRNA was readily demonstrable in most of these mice at the time of sacrifice. Together, these findings indicate that infection of normal mice with AdTNFR-CD8 results in prolonged expression of the transgene and is consistent with the results obtained with TNF-α-deficient animals (10). Fas-CD8 had a much more modest effect on Ad persistence and, for reasons that are unexplained, was more variable from mouse to mouse. This result is also consistent with the lower expression of CAT in Fas-deficient mice compared to TNF-α-deficient mice (10) and suggests that redundant pathways are able to compensate for Fas-mediated cytotoxicity in this model. Although detection of AdTNFR-CD8 mRNA in the lungs of infected mice at 4 weeks indicated persistent expression of the transgene, the sensitivity of the ELISAs could be a limiting factor for accurate assessment of transgene persistence at the protein level. To simulate a potential gene therapy application, we examined the ability of the fusion proteins to protect against an immune response against a foreign transgene, that for CAT. Coadministration of AdTNFR-CD8 and AdCAT i.v. caused a striking increase in CAT expression in both BALB/c and B6 mice. Similarly, TNFR-CD8, but not the other two transgenes, extended CAT expression in the lungs. Ad-mediated transgene expression may vary between different mouse strains (4). Although our studies are not exhaustive, persistent expression of TNFR-CD8 in both BALB/c and B6 mice suggests that these results are likely to be generalizable. Together, these findings suggest that transprotection by TNFR-containing fusion proteins might be useful in extending the expression of a transgene product in human diseases, including cystic fibrosis.
Many strategies have been proposed to reduce the immune response against Ad-encoded transgenes. However, depletion of lymphocyte subpopulations or immunosuppressive drugs such as cyclosporine would be expected to predispose to secondary infection. TNF-α-deficient mice do not spontaneously develop infections, although they have higher mortality when infected with Listeria monocytogenes or Mycobacterium tuberculosis (22, 27). We did not observe any overt infection in mice receiving AdTNFR-CD8, suggesting that this may be a relatively safe form of immunosuppression, at least in normal individuals. Further studies are required to determine whether temporary blockade of TNF-α increases the risk of infection in hosts who have a pre-existing infection or are immunocompromised.
Humoral immune responses pose another hurdle for gene therapy by eliminating soluble foreign transgenes and by preventing readministration of the Ad vectors. While the striking reduction in the IgG antibody response to Ad in TNF-α-deficient mice (10) might be expected in view of the key role of TNF in the formation of germinal centers (22, 27), the significant decrease in the total and neutralizing anti-Ad IgG response observed following expression of a soluble inhibitor of TNF-α indicates that a similar effect can be obtained in mice that have normal maturation of lymph nodes. Blockade of TNF-α in normal mice may reduce humoral responses by also inhibiting T-B interaction in germinal centers or by one of the other numerous effects that TNF-α has on the immune system. These effects range from promoting maturation of dendritic cells that are important in antigen presentation (28), recruitment of effector cells to the site of infection, and regulation of T- and B-cell function (12). Despite reduction of the humoral immune response to Ad in AdTNFR-CD8-infected mice, preliminary attempts to reinfect the mice have not yielded a high level of expression of a second transgene 1 to 2 months later (unpublished observations).
The reduced antibody response was specific for TNF blockade, since mice injected with Fas-CD8 or CD8 had similar levels of anti-Ad IgG. Interestingly, coadministration of Fas-CD8 and TNFR-CD8 abrogated the humoral effect of TNFR, suggesting that blocking Fas most likely protects B cells from apoptosis mediated through Fas ligand expressed on Th1 CD4+ T cells (see reference 11). This finding emphasizes that whereas blockade of TNFR1 or Fas (to a much lower extent) independently prolongs the expression of transgenes, the combination of transgenes abrogates the effect of TNF inhibition on the humoral immune response. These findings caution against the combined use of immunomodulators without careful evaluation of their interactions.
ACKNOWLEDGMENTS
This work was supported by grants NIH AR45482 (to K.B.E.) and PO1 HL51746 (for E.F.-P. and R.G.C.) and Cystic Fibrosis Foundation grant Z990 (to E.F.-P.). E.F.P. and R.G.C. receive sponsored research support from GenVec.
We thank Stephan Worgall and Peter Bullough for help with these studies.
REFERENCES
- 1.Ashkenazi A, Dixit V M. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin A S., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 3.Banner D, D’Arcy A, Janes W, Gentz R, Schoenfeld H, Broger C, Loetscher H, Lesslauer W. Crystal structure of the soluble human 55 kDa TNF receptor-human TNF beta complex: implications for TNF receptor activation. Cell. 1993;73:431–445. doi: 10.1016/0092-8674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- 4.Barr D, Tubb J, Ferguson D, Scaria A, Lieber A, Wilson C, Perkins J, Kay M A. Strain related variations in adenovirally mediated transgene expression from mouse hepatocytes in vivo: comparisons between immunocompetent and immunodeficient inbred strains. Gene Ther. 1995;2:151–155. [PubMed] [Google Scholar]
- 5.Bullard D C, King P D, Hicks M J, Dupont B, Beaudet A L, Elkon K B. ICAM-1 deficiency protects MRL/MpJ-Faslpr mice from early lethality. J Immunol. 1997;159:2058–2067. [PubMed] [Google Scholar]
- 6.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on human dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chirmule N, Hughes J V, Gao G-P, Raper S E, Wilson J M. Role of E4 in eliciting CD4 T-cell and B-cell responses to adenovirus vectors delivered to murine and nonhuman primate lungs. J Virol. 1998;72:6138–6145. doi: 10.1128/jvi.72.7.6138-6145.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen P L, Eisenberg R A. lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- 9.Drappa J, Brot N, Elkon K B. The Fas protein is expressed at high levels on double positive thymocytes and activated mature T cells in normal but not MRL/lpr mice. Proc Natl Acad Sci USA. 1993;90:10340–10344. doi: 10.1073/pnas.90.21.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elkon K B, Liu C C, Gall J G, Trevejo J, Marino M W, Abrahamsen K A, Old L J, Crystal R G, Song X, Zhou J L, Falck-Pedersen E. TNF-α plays a central role in immune mediated clearance of adenoviral vectors. Proc Natl Acad Sci USA. 1997;94:9814–9819. doi: 10.1073/pnas.94.18.9814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elkon K B, Marshak-Rothstein A. B cells in systemic autoimmune disease: recent insights from Fas-deficient mice and men. Curr Opin Immunol. 1996;8:852–859. doi: 10.1016/s0952-7915(96)80015-x. [DOI] [PubMed] [Google Scholar]
- 12.Feldmann M, Brennan F M, Maini R N. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1995;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 13.Franco M A, Tin C, Rott L S, VanCott J L, McGhee J R, Greenberg H B. Evidence for CD8+ T-cell immunity to murine rotavirus in the absence of perforin, Fas, and gamma interferon. J Virol. 1997;71:479–486. doi: 10.1128/jvi.71.1.479-486.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galle P R, Hofmann W J, Walczak H, Schaller H, Otto G, Stremmel W, Krammer P H, Runkel L. Involvement of the CD95 (APO-1/Fas) receptor and ligand in liver damage. J Exp Med. 1995;182:1223–1230. doi: 10.1084/jem.182.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ginsberg H S, Moldawer L L, Sehgal P B, Redington M, Kilian P L, Chanock R M, Prince G A. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci USA. 1991;88:1651–1655. doi: 10.1073/pnas.88.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiramatsu N, Hayashi N, Katayama K, Mochizuki K, Kawanishi Y, Kasahara A, Fusamoto H, Kamada T. Immunohistochemical detection of Fas antigen in liver tissue of patients with chronic hepatitis C. Hepatology. 1994;19:1354–1359. [PubMed] [Google Scholar]
- 17.Ilan Y, Droguett G, Chowdhury N R, Li Y, Sengupta K, Thummala N R, Davidson A, Chowdhury J R, Horwitz M S. Insertion of the adenoviral E3 region into a recombinant viral vector prevents antiviral humoral and cellular immune responses and permits long-term gene expression. Proc Natl Acad Sci USA. 1997;94:2587–2592. doi: 10.1073/pnas.94.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kagi D, Seiler P, Pavlovic J, Ledermann B, Burki K, Zinkernagel R M, Hengartner H. The roles of perforin- and Fas-dependent cytotoxicity in protection against cytopathic and noncytopathic viruses. Eur J Immunol. 1995;25:3256–3262. doi: 10.1002/eji.1830251209. [DOI] [PubMed] [Google Scholar]
- 19.Kischkel F C, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer P H, Peter M E. Cytotoxicity dependent APO-1 (Fas/CD95) associated proteins from a death inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kondo T, Suda T, Fukuyama H, Adachi M, Nagata S. Essential roles of the Fas ligand in the development of hepatitis. Nat Med. 1997;3:409–413. doi: 10.1038/nm0497-409. [DOI] [PubMed] [Google Scholar]
- 21.Lieber A, He C Y, Meuse L, Schowalter D, Kirillova I, Winther B, Kay M A. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J Virol. 1997;71:8798–8807. doi: 10.1128/jvi.71.11.8798-8807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marino M W, Dunn A, Grail D, Inglese M, Noguchi Y, Richards E, Jungbluth A, Wada H, Moore M, Williamson B, Basu S, Old L J. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci USA. 1997;94:8093–8098. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 24.Onel K, Ashany D, Nikolic-Zugic J, Lacy E, Elkon K B. Expression and function of the murine CD95 Fas/APO-1 receptor in relation to B cell tolerance. Eur J Immunol. 1995;25:2940–2947. doi: 10.1002/eji.1830251034. [DOI] [PubMed] [Google Scholar]
- 25.Orlinick J R, Elkon K B, Chao M V. Separate domains of the human Fas ligand dictate self-association and receptor binding. J Biol Chem. 1997;272:32221–32229. doi: 10.1074/jbc.272.51.32221. [DOI] [PubMed] [Google Scholar]
- 26.Orlinick, J. R., A. K. Vaishnaw, and K. B. Elkon. Structure and function of Fas/Fas ligand. Int. Rev. Immunol., in press. [DOI] [PubMed]
- 27.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNF-α-deficient mice: a critical requirement for TNF-α in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sallusto F, Lanzavecchia A. Efficient preparation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shisler J, Yang C, Walter B, Ware C F, Gooding L R. The adenovirus E3-10.4K/14.5K complex mediates loss of cell surface Fas (CD95) and resistance to Fas-induced apoptosis. J Virol. 1997;71:8299–8306. doi: 10.1128/jvi.71.11.8299-8306.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki M, Singh R, Moore M A, Song W R, Crystal R G. Similarity of strain- and route-dependent murine responses to an adenovirus vector using the homologous thrombopoietin cDNA as the reporter genes. Hum Gene Ther. 1998;20:1223–1231. doi: 10.1089/hum.1998.9.8-1223. [DOI] [PubMed] [Google Scholar]
- 31.Tollefson A E, Hermiston T W, Lichtenstein D L, Colle C F, Tripp R A, Dimitrov T, Toth K, Wells C E, Doherty P C, Wold W S M. Forced degradation of Fas inhibits apoptosis in adenovirus-infected cells. Nature. 1998;392:726–730. doi: 10.1038/33712. [DOI] [PubMed] [Google Scholar]
- 32.Tripathy S K, Black H B, Goldwasser E, Leiden J M. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat Med. 1996;2:545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- 33.Wallach D, Boldin M, Varfolomeev E, Beyaert R, Vandenabeele P, Fiers W. Cell death induction by receptors of the TNF family: towards a molecular understanding. FEBS Lett. 1997;410:96–106. doi: 10.1016/s0014-5793(97)00553-x. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe-Fukunaga R, Brannan C I, Copeland N G, Jenkins N A, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 35.Wilson C, Kay M A. Immunomodulation to enhance gene therapy. Nat Med. 1995;1:887–893. doi: 10.1038/nm0995-887. [DOI] [PubMed] [Google Scholar]
- 36.Wold W S M, Hermiston T W, Tollefson A E. Adenovirus proteins that subvert host defenses. Trends Microbiol. 1994;2:437–443. doi: 10.1016/0966-842x(94)90801-x. [DOI] [PubMed] [Google Scholar]
- 37.Worgall S, Leopold P, Wolff G, Ferris B, van Roijen N, Crystal R G. Role of alveolar macrophages in rapid elimination of adenovirus vectors administered by the epithelial surface of the respiratory tract. Human Gene Ther. 1997;8:1687–1696. doi: 10.1089/hum.1997.8.14-1675. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y, Li Q, Ertl H C, Wilson J M. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zajac A J, Quinn D G, Cohen P L, Frelinger J A. Fas-dependent CD4+ cytotoxic T cell-mediated pathogenesis during virus infection. Proc Natl Acad Sci USA. 1996;93:14730–14735. doi: 10.1073/pnas.93.25.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]