Summary
Background
External radiation therapy (RT) is often a primary treatment for inoperable meningiomas in the absence of established chemotherapy. Histone deacetylase 6 (HDAC6) overexpression, commonly found in cancer, is acknowledged as a driver of cellular growth, and inhibiting HDACs holds promise in improving radiotherapeutic efficacy. Downregulation of HDAC6 facilitates the degradation of β-catenin. This protein is a key element in the Wnt/β-catenin signalling pathway, contributing to the progression of meningiomas.
Methods
In order to elucidate the associations and therapeutic potential of HDAC6 inhibitors (HDAC6i) in conjunction with RT, we administered Cay10603, HDAC6i, to both immortalised and patient-derived meningioma cells prior to RT in this study.
Findings
Our findings reveal an increase in HDAC6 expression following exposure to RT, which is effectively mitigated with pre-treated Cay10603. The combination of Cay10603 with RT resulted in a synergistic augmentation of cytotoxic effects, as demonstrated through a range of functional assays conducted in both 2D as well as 3D settings; the latter containing syngeneic tumour microenvironment (TME). Radiation-induced DNA damage was augmented by pre-treatment with Cay10603, concomitant with the inhibition of β-catenin and minichromosome maintenance complex component 2 (MCM2) accumulation within the nucleus. This subsequently inhibited c-myc oncogene expression.
Interpretation
Our findings demonstrate the therapeutic potential of Cay10603 to improve the radiosensitisation and provide rationale for combining HDAC6i with RT for the treatment of meningioma.
Funding
This work was funded by Brain Tumour Research Centre of Excellence award to C Oliver Hanemann.
Keywords: HDAC6 inhibition, Cay10603, Meningioma, β-catenin nuclear translocation, Radiosensitiser
Research in context.
Evidence before this study
Meningiomas are the most common primary brain tumour and there is a need to improve treatment beyond surgery. Histone deacetylase inhibitors (HDACi) have been identified as potential radiosensitisers. However, their efficacy has been limited by the broad spectrum of targets affected by pan-HDACi, despite the distinct and unique functions attributed to each specific HDAC.
Abnormal regulation of β-catenin has been associated with the development of meningioma. The level of β-catenin deacetylation on K49 can be regulated by HDAC6, a cytoplasmic enzyme implicated in various cellular processes related to tumorigenesis.
Added value of this study
The present investigation elucidates that radiation-induced damage leads to an upregulation of HDAC6 expression. Pretreatment with the HDAC6 inhibitor, Cay10603, augments the efficacy of radiotherapy in meningioma. Furthermore, we demonstrate that the combination treatment of radiation and Cay10603 leads to a reduced β-catenin accumulation within the nucleus, consequently resulting in a decrease in the expression of the c-myc oncogene. This synergistic combination treatment ultimately leads to inhibited cellular growth and cell death in meningioma, demonstrated both in 2D and 3D experimental settings.
Implications of all the available evidence
Our findings indicate that inhibition of HDAC6, in combination with radiation therapy, represent a potential promising approach for improving the treatment outcomes of malignant meningioma.
Introduction
Meningiomas, the most prevalent primary tumours within the central nervous system, originate from the dura mater and the outer layer of the arachnoid.1 According to the WHO classification 2021, meningiomas are categorised into three grades; Benign meningiomas (grade 1, 80%) are typically associated with a favourable outcome.2 Atypical meningiomas (grade 2, 15–20%) have increased mitotic activity and malignant meningiomas (grade 3, 3–5%) are associated with the poorest prognosis.3 DNA-methylation-based classification for meningioma has been introduced, offering predictive insights into tumour recurrence.4 Although certain asymptomatic meningiomas can be managed by monitoring without active treatments, surgery remains the primary treatment modality for symptomatic or enlarging tumours. External radiation therapy (RT) often becomes a first-line treatment for inoperable meningiomas in surgical difficult locations due to the absence of widely accepted chemotherapeutic approach for meningioma care.5
Adjuvant radiotherapy, including stereotactic radiosurgery and fractionated stereotactic radiotherapy, could be considered for cases of incomplete resection in benign and atypical meningioma, and it is the standard of care for malignant meningiomas.6 Despite considerable research endeavours, recurrence rates for grade 2/3 meningiomas vary. The rate of recurrence range is from 9 to 50% after gross total resection and 36–83% after subtotal resection.7 Thus, there has been substantial interest in the development of radiosensitisers to optimise treatment plans while minimising radiation-induced complications. Histone deacetylase inhibitors (HDACis), identified as potential radiosensitiser candidates, have been studied over the past two decades.
The control of HDAC activity has been a hot topic in the development of anticancer strategies.8 HDACs restore a condensed chromatin structure through deacetylation, which maintains highly positive charges and reduces gene expression by restricting the binding of transcription factors.9 The radiosensitisation effect of HDACis on meningiomas remains unexplored. However, elevated HDAC activity has been observed to induce a radioresistant phenotype in the breast cancer cell model.10 Earlier research utilising AR-42, a pan-HDAC inhibitor, raised the prospect of its therapeutic application in NF2-associated meningiomas.11, 12, 13 AR-42, now known as REC-2282 and licensed by Recursion Pharma, is currently registered for clinical evaluation at clinicaltrials.gov (NCT05130866). Notably, several HDACis such as Vorinostat, Belinostat, and Romidepsin have received FDA approval for the treatment of haematological malignancies.14 However, despite the diverse cellular functions associated with each subtype of HDACs (encompassing 11 types of classical HDACs and 7 types of sirtuins across 4 different classes), the majority of investigations into HDACis, including all FDA-approved HDACis and REC-2282, have been conducted using pan-HDACis. Consequently, some studies employing pan-HDACis in conjunction with RT have yielded contentious outcomes regarding efficacy. Here, we focused on the efficacy of HDAC6, a functionally and structurally unique cytoplasmic enzyme that regulates biological processes involved in tumorigenesis. It is also related to promoting anchorage-independent proliferation, metastasis, migration, and contributes to resistance to the drug and/or radiation treatment in various cancer types.15,16 Previous research demonstrated that HDAC6 promotes tumour growth across numerous human cancers.17 Furthermore, HDAC6 plays a crucial role in facilitating the nuclear localisation of β-catenin, which is indispensable for the progression of cancers via transcriptional upregulation of downstream genes.18,19 Abnormal regulation of β-catenin has been identified as a contributory factor in the development of meningiomas.20 Once β-catenin translocates into the nucleus, it forms complexes with TCF/LEF1 (T cell factor/lymphoid enhancer factor) transcription factors targeting genes, primarily oncogenic transcription factors, associated with c-myc, N-myc, and cyclin D1.21
We show that HDAC6 expression is upregulated in meningiomas and is further increased by RT, prompting us further to control the radiation-induced HDAC6 expression using HDACi. Therefore, we applied Cay10603, a highly potent HDAC6i, to both immortalised cell lines and patient-derived meningiomas to assess the clinical feasibility of using a selective HDACi in conjunction with RT. Our study unveiled an enhancement in radiation-induced DNA double-strand breaks (DSBs), an increase in G2/M cell cycle arrest, and a promotion of apoptotic events. These effects were accompanied by a notable reduction in nuclear accumulation of β-catenin. We observed the role of HDAC6 and HDAC6 inhibition-induced radiosensitivity, not only in conventional 2D but also in more physiologically relevant 3D models of meningiomas, aiming to capture the complexities of the syngeneic tumour microenvironment (TME). Our data suggests that the targeting of HDAC6 as a radiosensitiser may represent a promising therapeutic strategy for the management of meningiomas.
Methods
Cell culture and ethics
Immortalised meningioma cell lines KT21-MG1,22 and IOMM-Lee were maintained in high glucose DMEM (Gibco-Thermo Fisher Scientific, MA) supplemented with 10% FBS (Thermo Fisher, Waltham, MA) without antibiotics. Cells were maintained at 37 °C and 5% CO2 in a humidified incubator. Isolation and culture were carried out as previously described.23 Penicillin-streptomycin (1%) has been added to the complete media for primary culture due to the high risk of contamination. IOMM-Lee and KT21-MG1 cell lines were received from Dr Randy Jensen (University of Utah) and Dr Long-Sheng Chang (Nationwide Children's Hospital), respectively.13,24 The validation information for BTB58 cell line is attached in reagent validation file.
Primary meningiomas were obtained from patients at the University Hospitals Plymouth NHS Trust and at Southmead Hospital Bristol. Written informed consent was obtained for all patients by the care team pre-operatively. The national Research Ethics Committee provided ethical approval for the biobank (UK registered) under the reference numbers REC 19/SC 0267, IRAS nr 246,667.
Western blotting and immunofluorescence
Western Blotting was carried out as previously described.23 Immunocytochemistry was conducted as previously described,25 except for some conditions indicated in this section. Briefly, paraformaldehyde 4% was used for fixation 30 min after RT (details of timeline in Fig. 3a [right bottom]). The information of antibodies is listed in Supplementary Table T1. The validation information for antibodies are attached in reagent validation file.
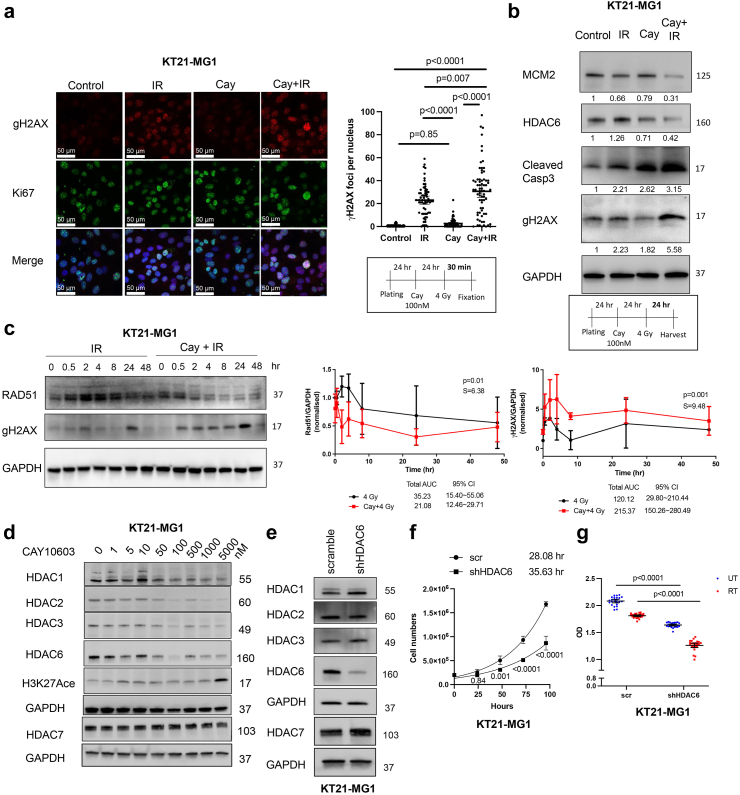
Fig. 3.
Cay10603 combination therapy with RT prolonged DNA DSBs whilst DNA repair protein was rapidly degraded over time. (a) γH2AX foci increased 30 min after RT and this enhanced with pre-treatment of Cay10603 although cell proliferation has not been detected within 30 min (n = 3, mean with 95% CI, one-way ANOVA, Dunn). (b) Combined therapy increased programmed cell death, DNA DSBs, and reduced replication. (c) Cay10603 prolongs DNA DSB while decreasing DNA damage repair protein expression (mean with SD, paired t-test, n = 3). (d) Cay10603, known as HDAC6 specific inhibitor, also regulate other HDACs. (e) Stable cell line establishment after shHDAC6 transfection after monoclononal selection (Details in Supplementary Figure S4a and b). (f) HDAC6 KD cell shows a slower doubling time than scramble (n = 3). (g) The effect of RT and/or HDAC6 KD on cell viability has been shown via MTT assay (n = 3, mean with 95% CI, two-way ANOVA, Šídák).
Flow cytometry
Cell cycle and apoptosis analysis were performed as previously described,25 except for some conditions elaborated in this section. The PI fluorescence signal was collected by a 585 nm filter using FACS Accuri C6 (BD Biosciences, New Jersey, NY, USA). For Annexin V apoptosis assay, stained cells were analysed with FACS Aria II (BD Biosciences, New Jersey, NY, USA) within 1 h of staining. Acquired data were analysed by FlowJo software (BD Biosciences, New Jersey, NY, USA).
Clonogenic assay
Cells were plated in 6-well plates containing 2 ml of fresh medium with appropriate numbers (1600 cells for KT21-MG1, 500 cells for IOMM-Lee for all 0–10 Gy) in triplicate. Irradiation was given 24 h after HDACi treatment and incubated for 2 weeks without removing the drug. Crystal violet staining was conducted as previously described.25
Drug treatment
Cay10603 (S7596), SB939 (S1515), TSA (S1045), AR42 (S2244), LAQ824 were purchased from Selleckchem. Hydroxyurea (HU; H8627, Merck) and vorambucil (Liaoning Kuke Biotechnology, China) were procured from their respective manufacturers as specified. Cells were plated 1 day before drug treatment and HDACis were treated 24 h before RT in all experiments for consistency.
Ionising radiation
Cells were treated with high-energy X-rays (0–10 Gy) using a linear accelerator (LINAC) (Varian Medical System, Palo Alto, CA). Radiation was given by qualified radiophysists, radiotherapy engineers, or clinical scientists in the radiotherapy department at Derriford Hospital, UK (see Acknowledgements).
MTT spectrophotometric assay
Cells were cultured in 96-well plates (3000 cells/well). MTT (2.5 mg/ml, M6494, Thermo) was added and incubated for 3 h before being dissolved in 100 μl of DMSO. Data was acquired using Fluostar Omega multi-mode microplate reader (BMG Labtech, Ortenberg, Germany).
shRNA transfection and establishing stably knocked-out cell lines
HDAC1, HDAC2, HDAC3, and HDAC6 shRNA lentiviral particles (sc-29343-V, sc-29345-V, sc-35538-V, sc-35544-V) and scramble shRNA lentiviral particles (SC-108080) were purchased from Santa Cruz Biotechnology. All infections were performed according to the manufacturer's instructions and cells were monoclonally selected on a 96-well plate following puromycin (2 μg/ml) treatment for 3 days.
Wound healing and boyden chamber migration assay
The cells were plated at a density of 2 × 105 cells/well in a 6-well plate, treated with Cay10603 the following day, and treated with radiation a day after. A scratch wound was generated 1 h after radiation using a sterile pipette tip. Millicell hanging cell culture insert (PTEP24H48, Millipore, Burlington, MA) was applied for the Boyden chamber migration assay following the manufacturer's instructions. Media with 10% FBS (700 μl) was filled each well as a chemoattractant and 30,000 cells in 300 μl of media without FBS filled chamber. Cells were stained using crystal violet solution at 20 h post-irradiation to minimise the confusion by proliferation.
Public datasets
The expression analysis data from patients with cancer were obtained from Gepia2 (http://gepia2.cancer-pku.cn/#survival), GEO2R (https://www.ncbi.nlm.nih.gov/geo/geo2r/), and UCSC Xena (https://xenabrowser.net/).
3D spheroid
The 3D spheroid culture method for meningioma followed the previous study.26 Live/dead cell viability assays (L3224, Thermo Fisher) were conducted as per the manufacturer's instructions.
Statistics
The surviving fraction was calculated based on the number of colonies on non-irradiated plates. Radiation survival curves were plotted in GraphPad Prism 10, using the linear-quadratic model with the equation SF = exp [– (αD + βD2)], where D is a dose of radiation. P values for ANOVA multiple comparisons (Dunn's, Tukey, or Šídák test) were calculated in GraphPad Prism 10. The levels of variables and correlation of two relative proteins were compared using a two-way ANOVA multiple comparison and Pearson correlation coefficient, respectively, in GraphPad Prism 10. We assumed Gaussian populations and employed either Bartlett's test (if every group has at least five values) or Brown–Forsythe test for ANOVA, while for the t-test, we utilised the F-test in GraphPad Prism 10. Each analysis method and sample size are explained in their respective legend. The results of the normality test are presented in Supplementary Figure S11. According to Wu et al.,27 a valid application of western blotting requires starting with three independent biological replicates in an accepted standard in this field, which we applied.
Role of funders
The funders (Brain Tumour Research) do not play any roles in study design, data collection, data analyses, interpretation, or writing of the report.
Results
Radiation induces HDAC6 expression in meningioma which positively correlates with cellular proliferation
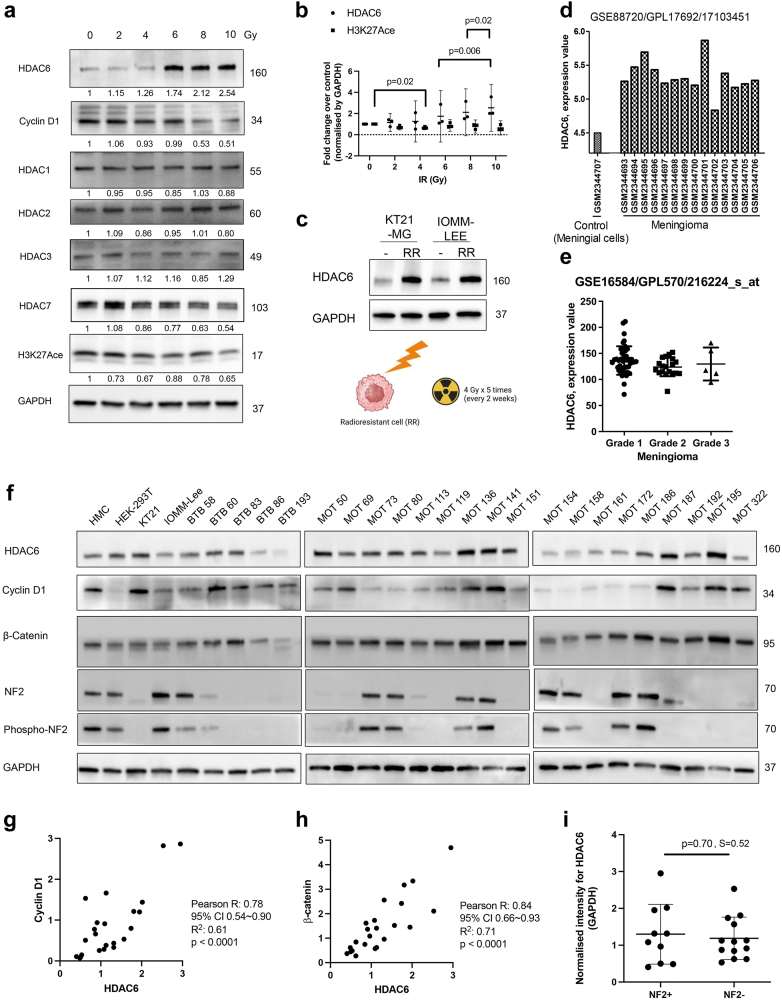
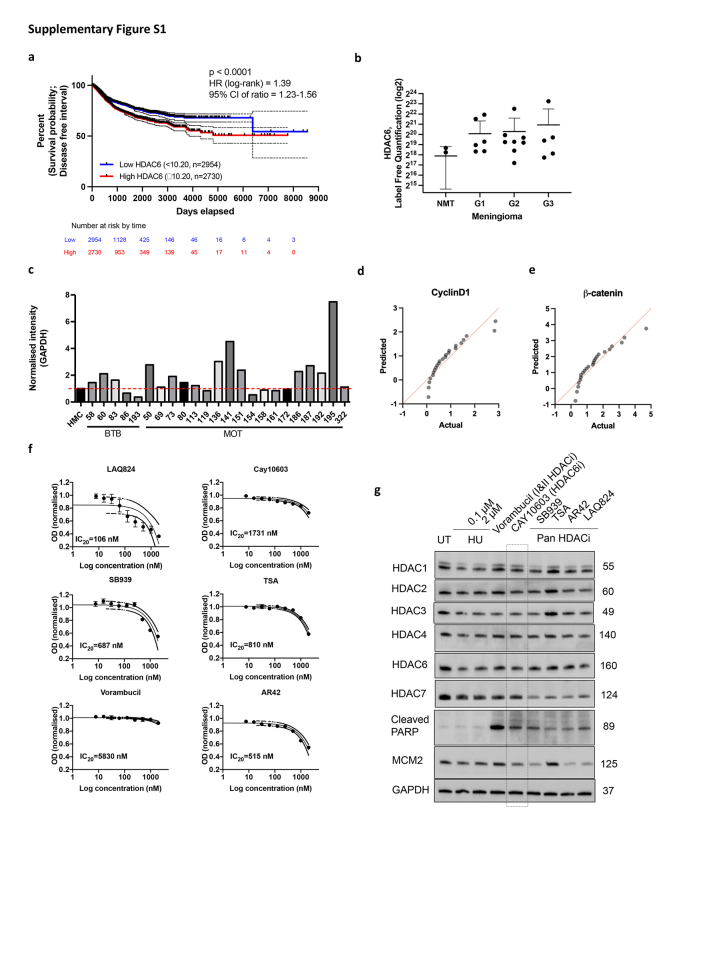
Initially, we examined the expression of HDACs following RT. The extent of damage inflicted by radiation was validated through the rapid degradation of cyclin D1, a mediator of G1 cell cycle arrest. Notably, HDAC6 expression increased dose-dependently, while the expression levels of HDAC1, HDAC2, HDAC3, and HDAC7 did not increase. The significant upregulation of HDAC6 expression following radiation led to reduction in H3K27 acetylation (Fig. 1a and b). Repeated exposure to radiation provided consistent evidence that radiation induces a stable upregulation of HDAC6 expression (Fig. 1c). To observe HDAC6 expression levels in patients included in meningioma and pan-cancer study, we utilised Gepia2, GEO2R database repository of high throughput gene expression data. Additionally, we referenced proteomic LC-MS/MS data from a previous study involving grade 1, 2, and 3 meningioma tissue.28 In TCGA database,29,30 elevated levels of HDAC6 expression are associated with poorer survival probability based on the disease-free interval. This is supported by a hazard ratio of 1.39 with a 95% confidence interval (CI) of 1.23–1.56, indicating a statistically significant and clinically meaningful decrease in survival probability for patients with pan-cancer with high HDAC6 expression (Supplementary Figure S1a). In meningioma, HDAC6 expression is notably high, and this is independent of the WHO grade classification (Fig. 1d and e; Supplementary Figure S1b). To further validate these findings derived from the database searches, we conducted a screening of 23 human primary meningioma cell lysates (Grade 1). In Fig. 1f and Supplementary Figure S1c, sixteen out of twenty threesamples of patient-derived meningiomas showed higher level of HDAC6 expression compared to human meningeal cells (HMC). Cyclin D1 significantly correlates with HDAC6 expression (Fig. 1f and g, Supplementary Figure S1d), suggesting that higher level of HDAC6 expression corresponds to increased cellular proliferation (Fig. 3f; Supplementary Figure S4c). Previous studies in colon and ovarian cancer cells have demonstrated that the downregulation of HDAC6 induces β-catenin degradation via acetylation.19 Intriguingly, we found that the expressions of HDAC6 and β-catenin positively correlate in meningioma (Fig. 1h, Supplementary Figure S1e). Also, we observed that the expression of HDAC6 is independent of NF2 loss (Fig. 1i) which is frequently found in meningiomas. Therefore, we hypothesised that HDAC6 inhibition could potentially control cell cycle progression and development of cancer by mediating transcriptional activity in both NF2 mutated and non-mutated meningioma.
Fig. 1.
RT increases HDAC6 expression and primary lysate screening shows that HDAC6 correlates with cell cycle progression. (a) RT increases HDAC6 expression dose-dependently, although HDAC1, 2, and 3 expressions were consistent after RT. Cyclin D1 decreases after RT in the KT21-MG1 meningioma cell line. (b) HDAC6 and H3K27Ace expression depends on various RT doses (n = 3, mean with 95% CI, two-way ANOVA, Fisher's LSD). (c) The increased level of HDAC6 was maintained after repeated RT treatment and additional passages. (d–e) HDAC6 expression in patients with meningioma was analysed using Gene Expression Omnibus. (d) Demonstrates heightened HDAC6 expression in meningioma compared to meningeal cells. (e) Highlights the independence of HDAC6 expression from meningioma grades (mean with SD). (f) HDAC6, Acetyl H3, β-catenin, NF2, pNF2, and cyclin D1 expressions in human meningioma samples from patients (n = 23). (g) Correlation between HDAC6 and Cyclin D1. (h) Correlation between HDAC6 and β-catenin. (g–h) The Pearson correlation coefficient (R value) indicates a positive correlation. The normality assumption was confirmed to be met using the Kolmogorov–Smirnov test. (i) HDAC6 expression is independent of NF2 deletion (Mean with SD; unpaired t-test, two-tail).
Cay10603 synergistically reduces cell survival rate with radiation, accompanied by an increase in morphological signs of damage
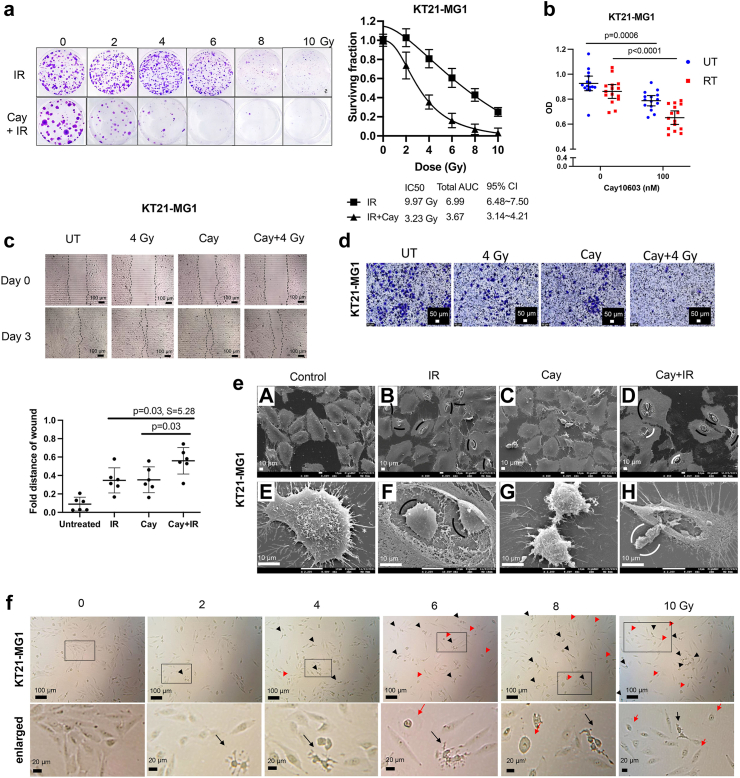
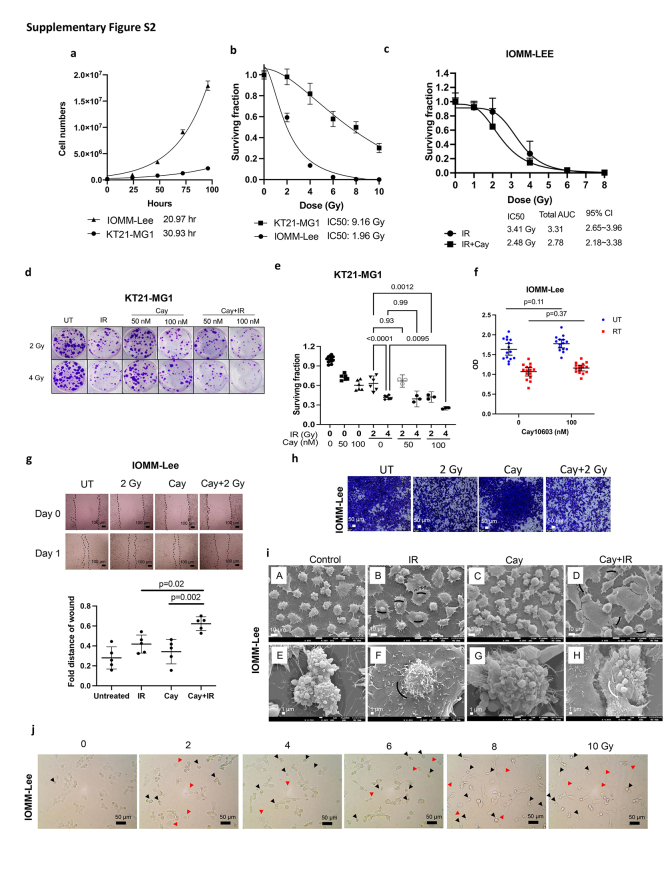
To determine the efficacy of pharmacological HDAC6 inhibition specifically, we determined the IC20 values for various HDACis and treated cells with each drug at their respective IC20 concentration (Supplementary Figure S1f–g). Vorambucil, Cay10603, SB939, and LAQ824 noticeably induced apoptosis. Among these, Vorambucil showed the most pronounced increase in apoptosis. However, it is important to note that Vorambucil did not decrease minichromosome maintenance complex component 2 (MCM2) expression (Supplementary Figure S1e), which is known as a marker for the high-grade meningioma molecular group (MG4) which is found to have shorter times to recurrence in meningioma according to Nassiri et al.31 HDACis reducing HDAC7 expression were excluded from consideration, as low HDAC7 expression may lead to increased levels of c-myc, indicating a potent anti-oncogenic effect of HDAC7.32 We opted for Cay10603 due to its demonstrated efficiency in inhibiting MCM2, and HDAC6, accompanying increased cleaved PARP, for further experiments. Due to the significantly faster cell doubling time of IOMM-Lee (20.97 h) compared to KT21-MG1 (30.93 h), a greater proportion of IOMM-Lee cells were found in the late G2 and mitosis phase, which represent the most radiosensitive phase of the cell cycle (Supplementary Figure S2a and b). Hence, the IC50 value for KT21-MG1 (9.2 Gy) was observed to be higher than that of IOMM-Lee (2.0 Gy). To mitigate the potential confounding effects of excessive dosages of singular Cay10603 treatment on combination therapy-induced damages, a cell survival assay was conducted to ascertain the optimal concentration for achieving synergistic effect. Notably, an encouraging outcome was observed at a concentration of 100 nM Cay10603 (Supplementary Figure S2d–f).
Various doses of radiation were applied with pre-treatment of Cay10603 to observe its cell survival rate and Cay10603 synergistically reduces cell survival rate dose-dependently (Fig. 2a and b; Supplementary Figure S2c). We show the effect of combined treatment on the cell viability, which resulted in significantly reduced viability in KT21-MG1, but a non-significant decrease in IOMM-Lee (Fig. 2b; Supplementary Figure S2f). This discrepancy is hypothesised to arise from a lack of CDKN2A on 9p21.3 in IOMM-Lee, thereby enhancing cellular proliferation,33 and potentially obscuring disparities in the number of viable cells. Next, we tested the effectiveness of the combined Cay10603 and RT in restraining migration, observing a synergistic delay in wound closure (Fig. 2c and d; Supplementary Figure S2g–h). Although cell viability did not significantly decrease in IOMM-Lee by Cay10603 + RT combination, the migration assay, which inherently encompasses aspects of cell viability and proliferation, indicated suppressed migratory capacity under the influence of Cay10603 + RT. We next investigated morphological attributes utilising scanning electron microscopy (SEM) and bright field imaging using a conventional microscope (Fig. 2e and f; Supplementary Figure S2i–j). Notably, a discernible proportion of nuclei showed shrinkage and detachment from the cellular body in cells subjected to Cay10603 + RT-treatment.
Fig. 2.
Cay10603 combination therapy with RT synergistically decreased cell survival and viability in addition to morphological modification. (a) Representative images for clonogenic assay and its calculation after various doses of RT ± Cay10603 (n = 3). (b) The effect of RT and/or Cay10603 on cell viability has been shown via MTT assay (n = 3, mean with 95% CI, two-way ANOVA, Šídák). (c–d) Cell migration was synergistically inhibited in combination treatment shown via both wound healing and Boyden assay (n = 2, mean with SD, one-way ANOVA, Šídák). (e) SEM imaging after Cay10603 and/or RT treatment. Pyknosis and karyorrhexis (completely shrunken nucleus, marked with black arcs and removed the nucleus from the cell body, marked with white arcs) were shown in damaged cells indicating cell death. (f) Cell morphology changes upon various doses of RT. Red arrow; cell swelling, black arrow; cell shrinkage.
Cay10603 synergistically increases DNA damage via sustained γ-H2AX and suppressed Rad51 over time
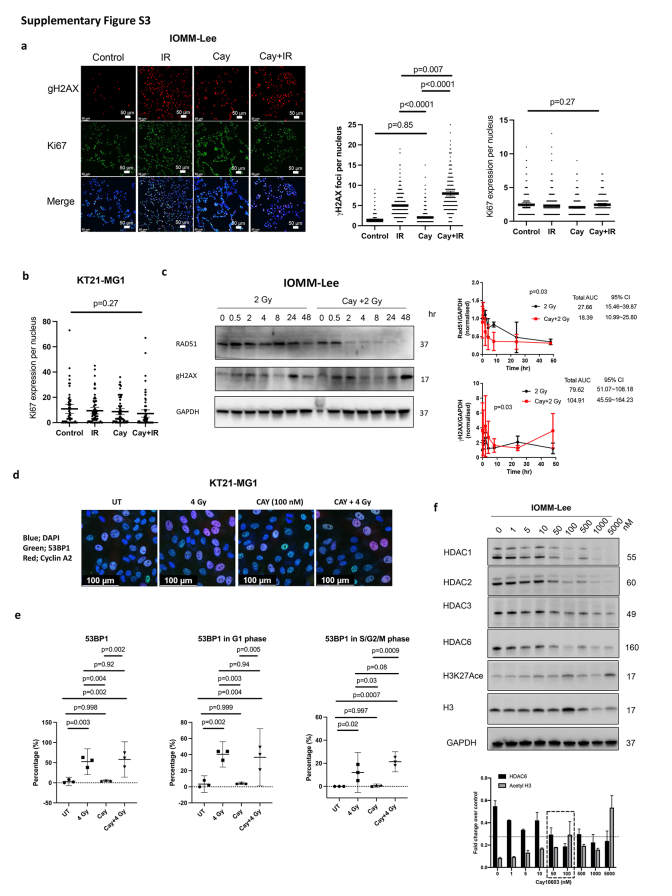
Next, we analysed DNA damage. The level of DNA DSBs marker, γH2AX foci, was unchanged after 100 nM of Cay10603 alone but the combined treatment with RT significantly increased the number of γH2AX foci compared to RT alone at 30 min post-irradiation (Fig. 3a; Supplementary Figure S3a). The proliferation marker, Ki67, did not show significant differences (Supplementary Figure S3a and b). It is conceivable that 30 min incubation period post-RT is conducive for a rapid reaction such as γH2AX, but perhaps it is too brief timeframe to observe proliferation considering DNA replication was impeded after 24 h (Fig. 3b). The cleaved form of caspase 3, an apoptosis marker, along with γH2AX, increased following combined treatment in comparison to RT or Cay10603 treatment alone at 24 h post-irradiation (Fig. 3b). Radiation increases HDAC6 expression (Fig. 1a), however, in the presence of Cay10603, radiation adversely reduced HDAC6 expression even further (Fig. 3b). To ascertain the concordance between DNA DSBs and DNA damage repair regulation, we measured the radiation-induced Rad51 and γH2AX expression levels over time (Fig. 3c; Supplementary Figure S3c). Radiation-induced upregulation of Rad51, a highly conserved protein that catalyses DNA repair via homologous recombination (HR), was attenuated by Cay10603, while increased γH2AX expression was prolonged by Cay10603. In order to scrutinise the preferences associated with non-homologous end joining (NHEJ), we quantified the expression of 53BP1 during the G1 phase as well as the S/G2/M phase considering the major NHEJ processes occurring in the G1 phase (Supplementary Figure S3d and e). Exposure to RT alone resulted in 52.3% of cells exhibiting positive 53BP1 expression, while the combined treatment with Cay10603 yielded a slightly increased proportion of 58%; however, this difference was not deemed statistically significant.
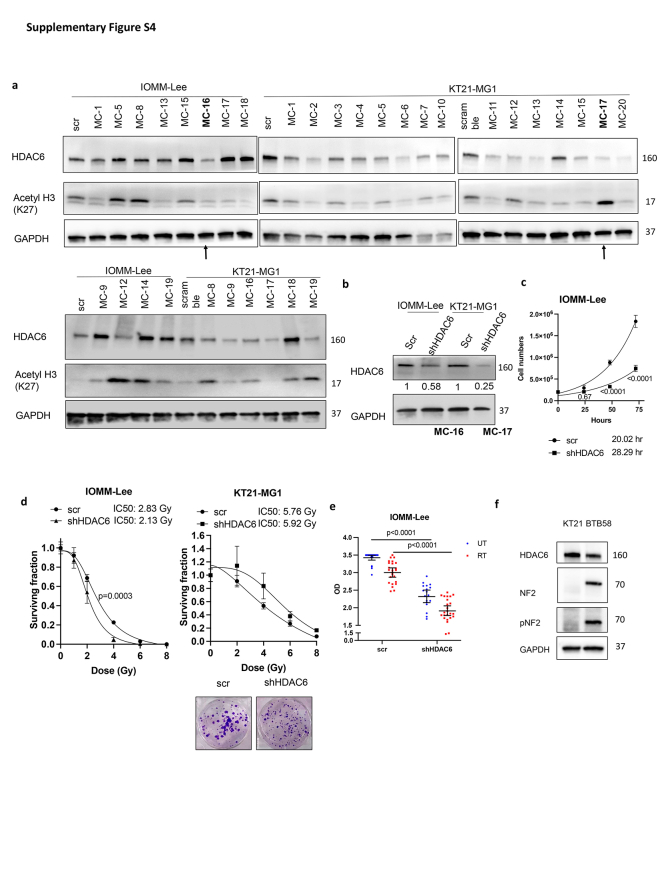
We found that Cay10603 inhibits not only HDAC6 but also Class I HDAC groups such as HDAC1, HDAC2, and HDAC3 in a dose-dependent manner, with HDAC7 remaining largely unaffected (0–5 μM) (Fig. 3d; Supplementary Figure S3f). Therefore, we established HDAC6 knockdown (KD) stable cell lines using shRNA for HDAC6 and selected single clones to investigate whether the radiosensitising effect was induced by HDAC6 inhibition (Fig. 3e, Supplementary Figure S4a and b). Given that there were no significant changes in HDAC1, 2, 3, and 7 after shRNA transfection for HDAC6, we confirmed that HDAC6 KD using a lentiviral shRNA system was HDAC6 selective (Fig. 3e). HDAC6 KD delayed cell doubling time and helped cells to be more radiosensitive in terms of cell survival rate and viability, although KT21-MG1 HDAC6 KD did not show significant difference in cell survivals (Fig. 3f and g; Supplementary Figure S4c and e). Altogether, this suggests that both pharmacological and genetic inhibition of HDAC6 can increase radiosensitivity, with one of the mechanisms involving augmented prolongation of DNA DSBs while attenuating DNA repair capability.
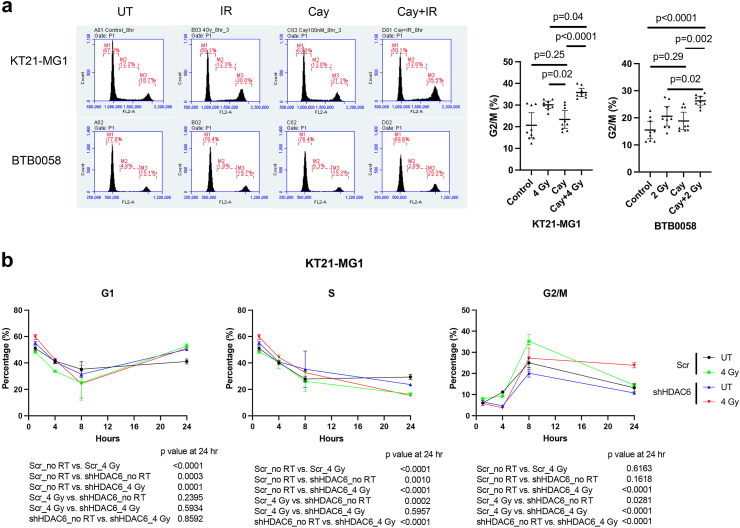
HDAC6 inhibition promoted radiation-induced G2/M cell cycle arrest and cell death in both immortalised and primary meningioma cells
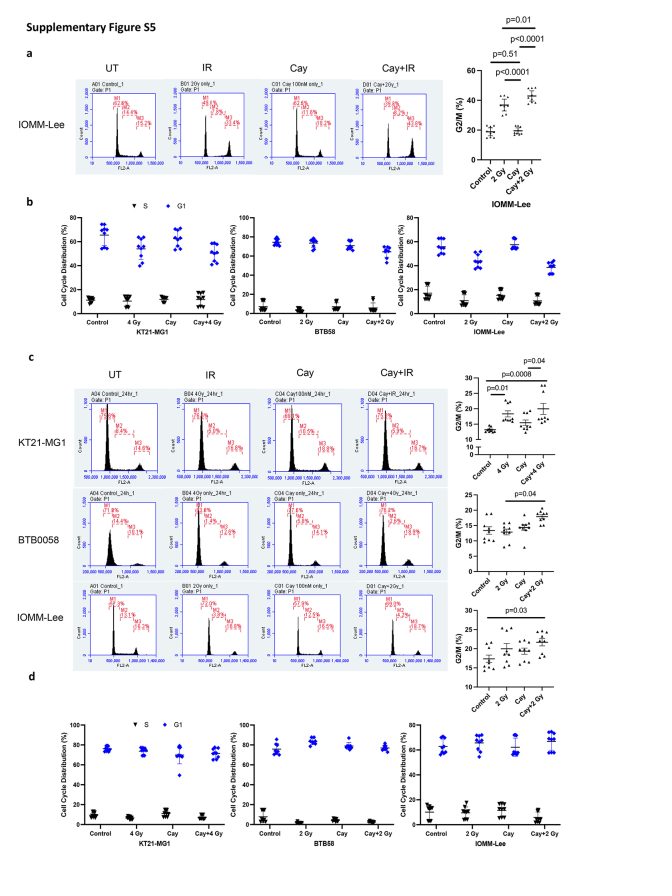
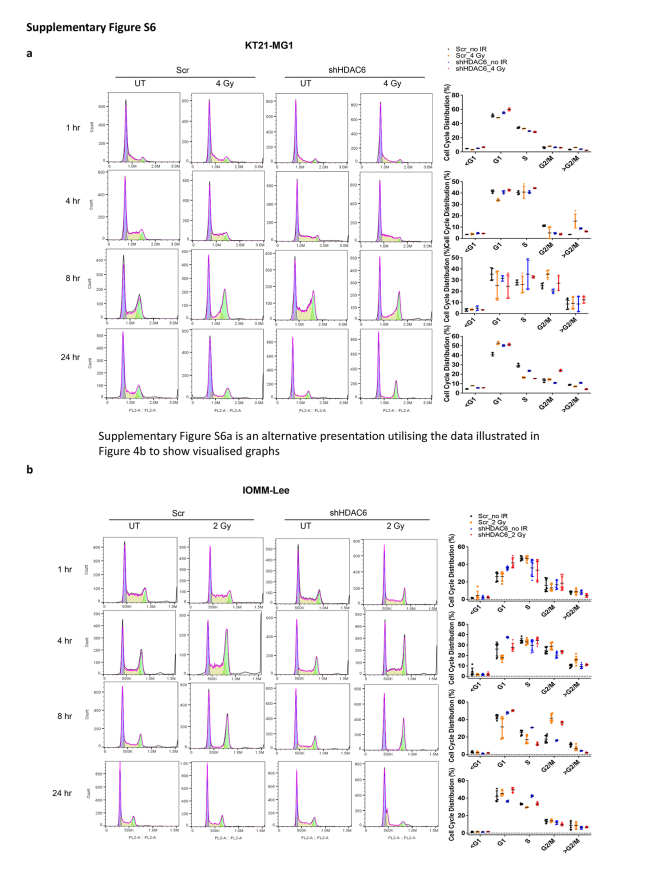
Next, we allowed varying intervals for DNA repair subsequent to RT, followed by a comprehensive analysis of cell cycle dynamics. G2/M arrest, an important mechanistic response of cells to RT, indicates that the damage of intracellular DNA is considerably challenging to be repaired and it is known that RT induces irreversible G2/M arrest in meningioma.34 We demonstrate that a synergistic escalation of G2/M arrest following the combined administration of Cay10603 and RT, observed at both 8 h and 24 h post-RT (Fig. 4a; Supplementary Figure S5a–d). Also, the pattern of cell cycle distribution over time shows that HDAC6 KD results in extended radiation-induced G2/M arrest while concurrently preserving the G1 and S phases (Fig. 4b; representative images shown in Supplementary Figure S6a). In HDAC6 KD IOMM-Lee cells, an observed increase in G2/M arrest was not evident. Instead, G1 arrest was prominent (Supplementary Figure S6b). This phenomenon can be attributed to the deletion of CDKN2A, the gene responsible for encoding cell-cycle inhibitor p16/INK4A, in IOMM-Lee and the downregulation of p16/INK4A induce G1 cell cycle arrest according to Zhang et al.35 To support the effect of combination therapy on the cell cycle, we investigated primary human meningioma cells; BTB0058 (WHO grade 1, meningothelial type from left temporal convexity, patients with non-NF2; Supplementary Figure S4f). G2/M arrest was induced in BTB0058, while a minimal proportion of cells were observed in the S phase, reflective of limited chromosomal replication (Fig. 4a; Supplementary Figure S5a).
Fig. 4.
Pharmacological inhibition and genetic knockdown of HDAC6 increases RT-induced G2/M arrest in both immortalised and primary meningioma cells. BTB0058; patient-derived primary meningioma cells. shHDAC6 and scramble were transfected in KT21-MG1 immortalised meningioma cell line. (a) Cell cycle analysis was performed after Cay10603 and/or RT treatment. Cells were harvested 8 h after RT. Combination treatment with Cay10603 and RT showed significantly increased G2/M arrest compared with that of RT alone samples (30.10% ± 2.18% vs 35.79% ± 2.51%, 20.59% ± 4.71% vs 26.29% ± 2.14%, in KT21-MG1, and BTB0058, respectively; n = 3, mean with 95% CI, one-way ANOVA, Tukey). (b) Cell cycle kinetics shows that HDAC6 KD delays the recovery from RT-induced G2/M arrest (n = 3, mean with SD, one-way ANOVA, Tukey).
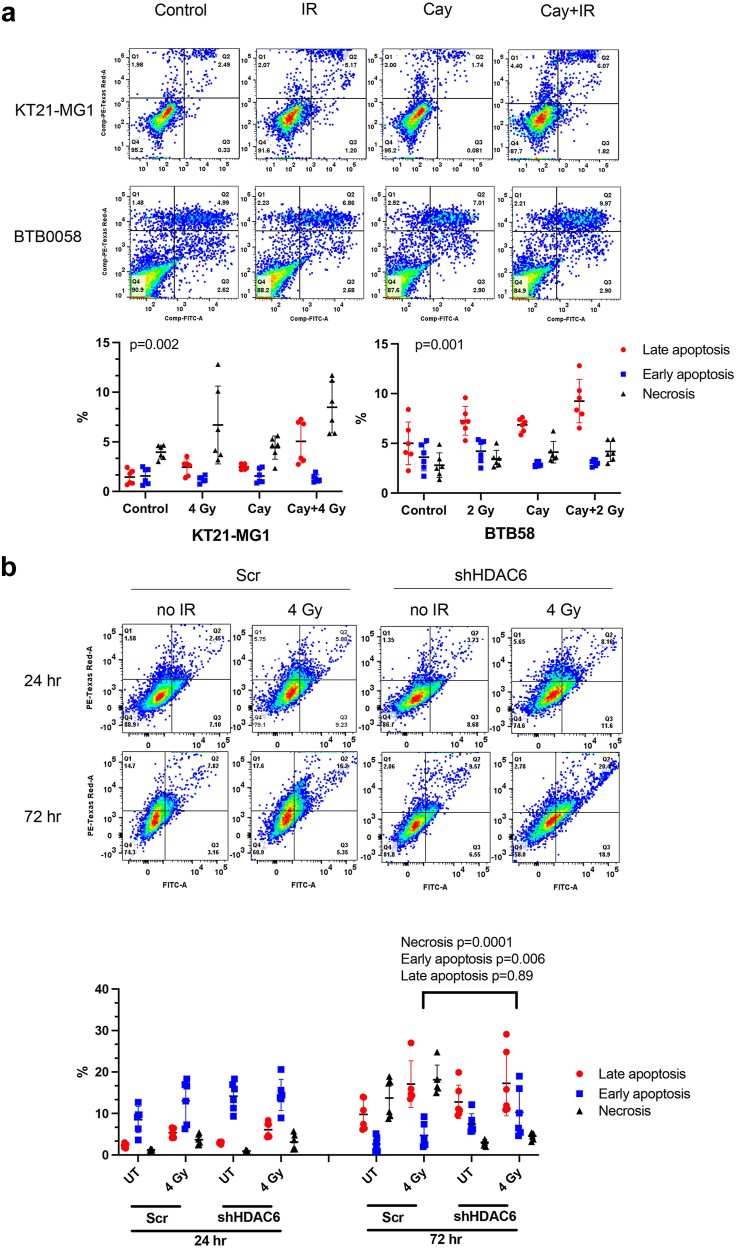
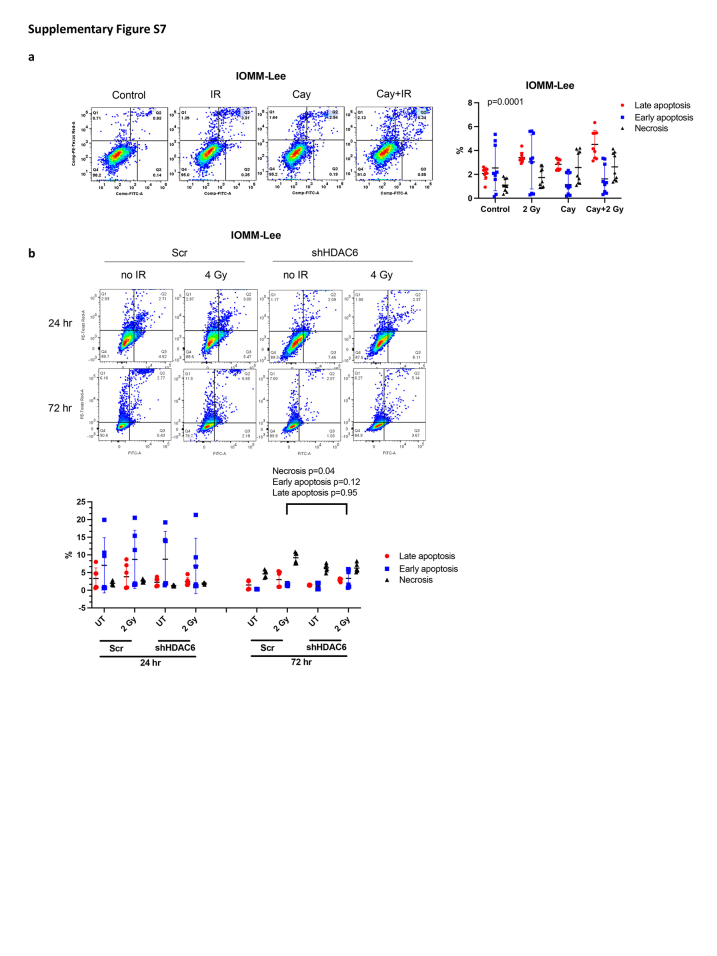
Next, we further assessed cell death levels and analysed early/late apoptotic and necrotic status. We noticed a significant augmentation in radiation-induced late apoptotic cells upon Cay10603 treatment; 2.1-fold in KT21-MG1, 1.3-fold in BTB0059 and IOMM-Lee to IR alone (Fig. 5a; Supplementary Figure S7a). The limited lifespan inherent to primary cells derived from patient with meningioma (BTB0058) held upregulated late apoptosis without any treatment, in contrast to immortalised cell lines. As shown in Fig. 5b and Supplementary Figure S7b, HDAC6 KD showed an increase in radiation-induced early apoptosis while concurrently suppressing radiation-induced necrosis at 72 h post-RT, albeit there was no significant difference made by HDAC6 KD at 24 h post-RT. These results collectively suggest that the synergistic interplay between HDAC6 inhibition and RT delays the cell cycle progression of meningioma cells by increasing G2/M phase arrest, ultimately contributing to increased apoptosis.
Fig. 5.
Pharmacological inhibition and genetic knockdown of HDAC6 increases RT-induced late apoptosis in both immortalised and primary meningioma cells. BTB0058; patient-derived primary meningioma cells. shHDAC6 and scramble were transfected in KT21-MG1 immortalised meningioma cell line. (a) Late apoptosis level increased significantly after Cay10603 and/or RT treatment (24 h post-irradiation, n = 3, mean with SD, two-way RM ANOVA with Geisser-greenhouse correction, alpha = 0.05). (b) HDAC6 KD and RT increased cell death at 72 h compared to 24 h. HDAC6 KD was prone to have increased apoptosis and decreased necrosis at 72 h post-irradiation (Bottom left: alive, Bottom right: early apoptosis, Top right: late apoptosis, Top left: necrosis, n = 3, mean with SD, two-way ANOVA, Fisher's LSD).
Combining HDAC6 inhibition with radiation treatment attenuates nuclear β-catenin accumulation
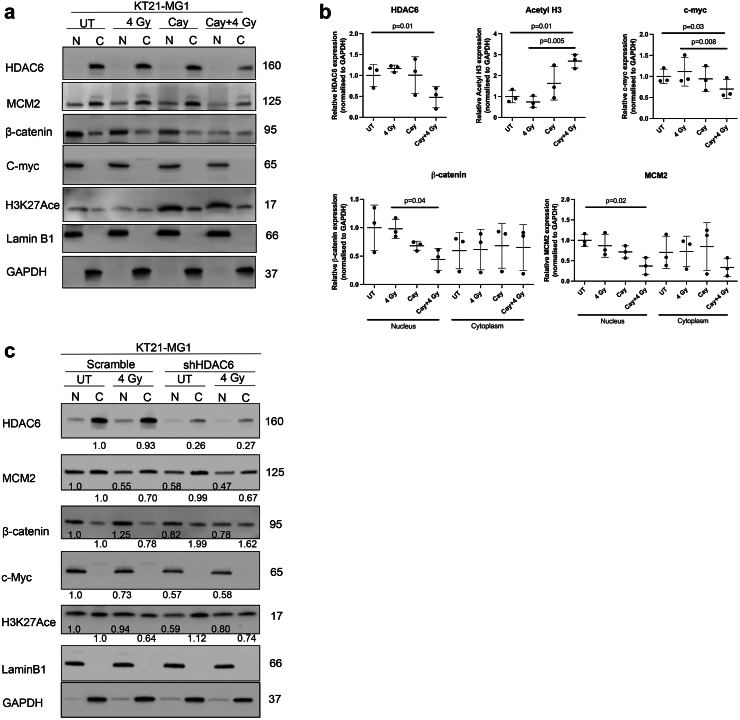
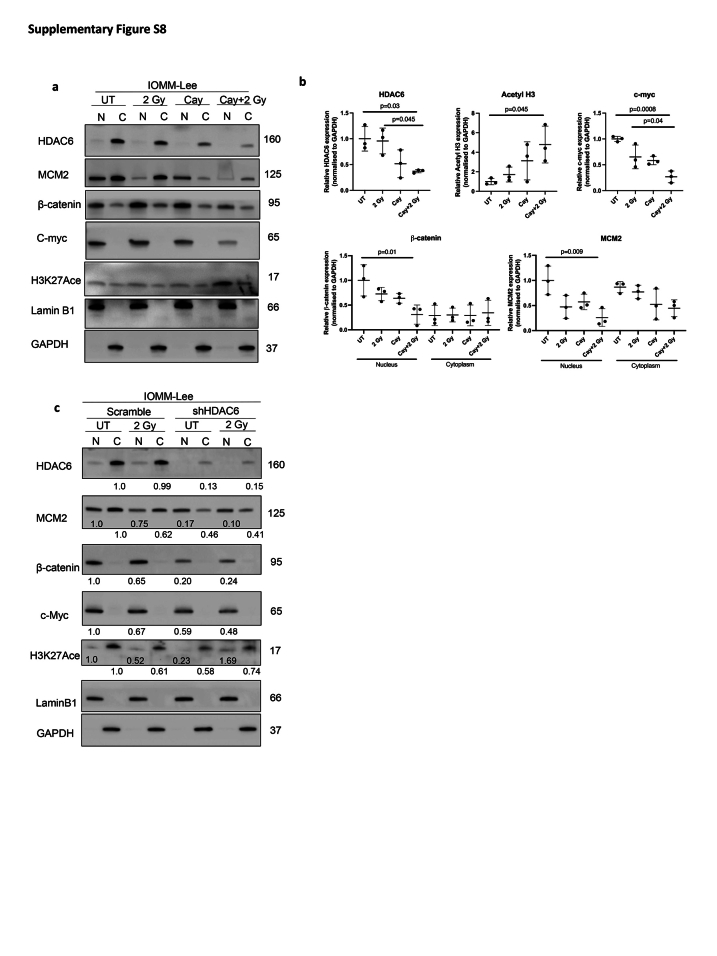
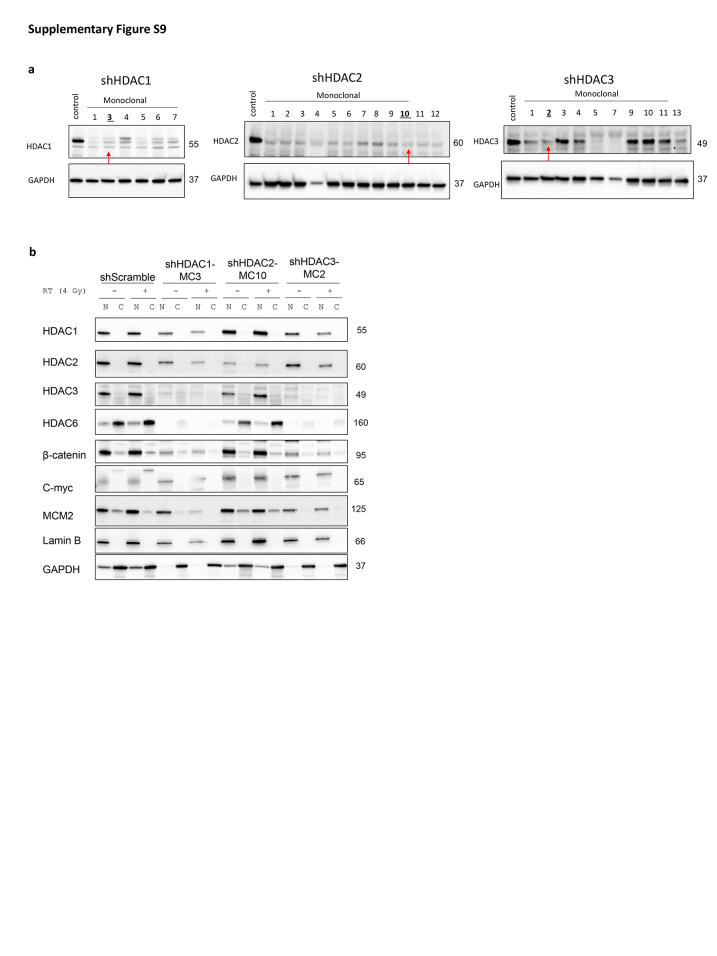
Given the correlation between HDAC6 expression and β-catenin levels (Fig. 1f and h), coupled with Mak et al.19 indicating the role of HDAC6 in stabilising β-catenin, we conducted subcellular nucleic/cytoplasmic fractionation to ascertain alterations in nuclear β-catenin accumulation upon Cay10603 and/or RT (Fig. 6; Supplementary Figure 8). Additionally, we included an assessment of MCM2 to evaluate our findings. MCM2 is known to be an interacting partner of Rad51,36 which showed a significant reduction in Fig. 3c and Supplementary Figure S3c. Cay10603 treatment with RT resulted in a significant reduction in both MCM2, and β-catenin protein levels in the nucleus along with suppressed expression of c-myc oncogene (Fig. 6a and b; Supplementary Figure S8a and b) which is known to result in reduced cell growth, survival, and cell cycle progression in various cancer types.37 However, we did not observe an additive or synergetic decrease in nuclear β-catenin and MCM2 levels after RT in HDAC6 KD cells, whilst clear KD of HDAC6 induced by shRNA (Fig. 6c; Supplementary Figure S8c). Given the study from Li et al.,18 demonstrating that HDAC6 inactivation impedes epidermal growth factor-induced β-catenin nuclear localisation in colon cancer cell models, we postulate that an excessive level of HDAC6 KD achieved through specific shRNA and subsequent monoclonal selection might have masked the anticipated radiation effect. This assumption is based on the idea that the complex interactions between HDAC6 and cellular signalling pathways, as evidenced in the literature,18,19 may contribute to nuanced responses to radiation therapy. The synergistic impact of RT with Cay10603 is likely dependent on a further decrease of HDAC6 induced by combined treatment. This rational is supported by Fig. 3b, which shows an additional decrease in HDAC6 expression following RT in the presence of Cay10603. This indicates that nuclear localisation of β-catenin is dependent on HDAC6, but independent of RT. Moreover, we investigated the influence of HDAC1, HDAC2, and HDAC3 on β-catenin nuclear localisation by individually knocking down each of these HDACs (Supplementary Figure S9). Our findings indicated that HDAC2 operated independently from β-catenin nuclear localisation. However, knockdown of HDAC1 and HDAC3, which influenced each other's expression significantly, led to a substantial decrease in both HDAC6 and β-catenin expression. In summary, these results revealed that RT in combination with HDAC6 inhibition via Cay10603 attenuates nuclear β-catenin accumulation, along with MCM2 as illustrated in Fig. 7. These concerted actions led to downregulation of c-myc oncogene, providing an explanation for the increased cell death along with decreased proliferation, survival, migration, and cell cycle progression in meningioma.
Fig. 6.
Subcellular protein analysis of Cay10603 in combination with radiation confirms inhibited nuclear accumulation of β-catenin. (a, b) Subcellular fractionation was performed to confirm the localisation of the protein of interest such as HDAC6, H3K27Ace, β-catenin, c-myc, and MCM2 (n = 3, mean with SD, one-way ANOVA, Tukey). (c) Subcellular fractionation was conducted in HDAC6 KD cell lines.
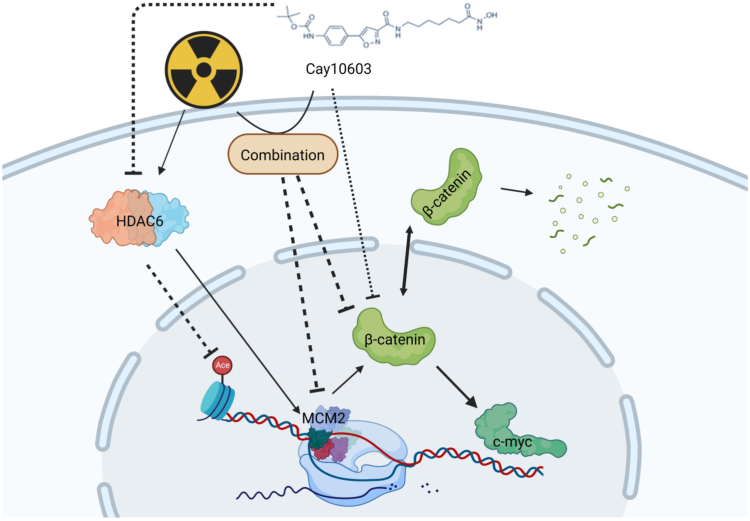
Fig. 7.
A schematic diagram depicting the mechanism of Cay10603-mediated controls towards β-catenin nuclear accumulation.
3D spheroid models of meningioma replicate the combined Cay10603 and RT responses observed from 2D functional assays
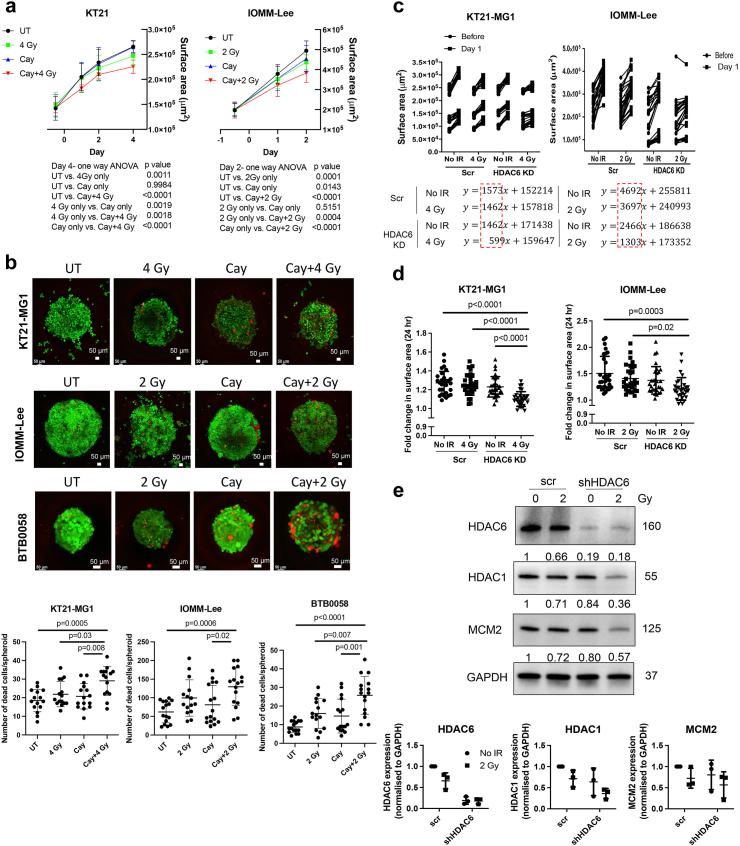
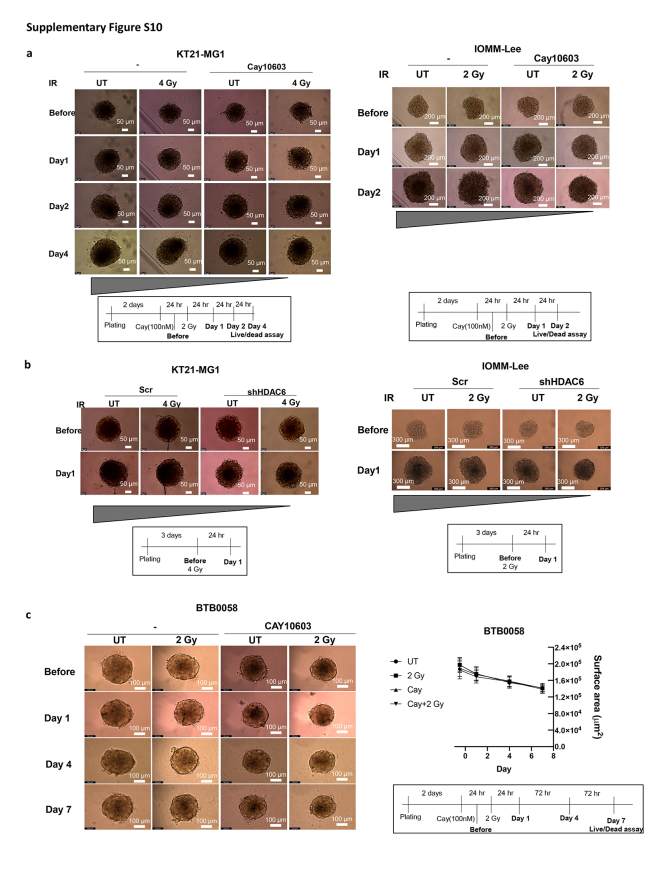
While the 2D cultures system is the predominant model in cancer research, 3D models for culturing both cell lines or patient-derived cells offer a more valid representation of crucial aspects akin to the natural tumour architecture such as cell–cell interaction including TME,26 an external proliferating zone with an internal quiescent zone with limited oxygen, nutrient and growth factor distribution which can reflect realistic drug response. Therefore, we conducted measurements of 3D spheroid growth and performed live/dead assay after treatments with Cay10603 and/or RT, using both immortalised meningioma cell lines and early passage (p-3) of patient-derived cells (Fig. 8; Supplementary Figure S10). The immortalised meningioma cell lines showed markedly reduced growth and an increase in dead cells following Cay10603 and RT treatment (Fig. 8a and b; Supplementary Figure S10a, c). In the case of HDAC6 KD cells, a shallower growth curve was observed, attributable to diminished DNA replication and reduced level HDAC1 after RT (Fig. 8c–e; Supplementary Figure S10b). The reduction in HDAC1 following HDAC6 KD and RT may result from the complex regulatory interplay between HDAC6 and HDAC1, given pivotal role of HDAC1 for cellular proliferation, analogous to the role of MCM2 in DNA replication. Patient-derived meningioma (BTB0058) did not show substantial growth in size-wise, consistent with their limited replicative capacity; however, a significant increase in the number of dead cells, along with swelling morphology, was observed after Cay10603 and RT (Fig. 8b, Supplementary Figure S10c). Overall, our findings indicate that the combined HDAC6 inhibition and RT leads to an augmentation in cell death and a deceleration in spheroid growth within a 3D context.
Fig. 8.
RT with HDAC6 inhibition synergistically increased cell death and attenuated the growth of 3D spheroid. (a) Pre-treatment of Cay10603 synergistically mitigated spheroid growth with RT (n = 3, one-way ANOVA, Tukey). (b) A combination treatment of Cay10603 and RT significantly increased the number of dead cells per spheroid in KT21-MG1, IOMM-Lee, and patient-derived primary meningioma cells, BTB0058 (n = 3, mean with SD, one-way ANOVA, Tukey). Red; dead cells (Ethidium homodimer-1), Green; live cells (Calcein AM). (c, d) The RT treatment applied to HDAC6 KD spheroids resulted in the mitigation of spheroid growth. Panel (c) illustrates the slope of the growth curve, while panel (d) provides statistical data on the increased surface area after RT (n = 3, mean with SD, one-way ANOVA, Tukey). (e) RT treatment to HDAC6 KD spheroid decreased HDAC1 expression and DNA replication licensing factor MCM2 (n = 3).
Discussion
HDAC6 is frequently overexpressed in various cancer types,17 and it regulates DNA damage response (DDR) genes. This has been associated with resistance to both drugs and radiation in certain cancer types.38 Also, the inhibition of HDAC6 is known to reduce the expression of DNA damage repair genes and induce DNA damage in glioblastoma.39 Here, we observed not only increased HDAC6 expression in meningioma but an increase in HDAC6 levels following exposure to RT accompanied by a reduction in histone H3 acetylation. Elevated HDAC6 expression in meningioma subsequent to RT suggests its involvement in a compensatory mechanism against radiation-induced damage, despite the effectiveness of RT in cancer treatment; notably, other types of HDACs such as HDAC1, 2, 3, and 7 remained unchanged (Fig. 1a). Although the expression of HDAC6 and Cyclin D1 showed a positive correlation in our patient-derived meningioma cell lysates (Fig. 1f and g), the expression of HDAC6 in omic datasets does not seem to show a significant correlation between HDAC expression and WHO stage of the meningioma (Fig. 1d and e; Supplementary Figure S1b). This may be attributed to factors associated with the prior treatment history of each patient in these datasets including radiation, which reduces cyclin D1 (Fig. 1a). We hypothesised that pre-treatment with an HDAC6i could prevent the radiation-induced upregulation of HDAC6, enhance the efficacy of RT and provide a therapeutic benefit in meningioma treatment. We have demonstrated that Cay10603 inhibited HDAC6 expression in paving the way for RT, resulting in enhanced therapeutic efficacy to meningioma 2D and 3D cell models (Figs. 2–3, 8; Supplementary Figures S2–S4, S8). Our results also revealed that pre-treatment with Cay10603 enhances radiation-induced G2/M cell cycle arrest and apoptosis (Fig. 4, Fig. 5; Supplementary Figures S5–S7). We show that these cellular responses can be attributed to the reduced accumulation of nuclear β-catenin, and MCM2 (Fig. 6, Fig. 7).
The importance of HDAC roles in meningioma biology has been recently reported and their radiosensitising effects were explored based on their fundamental role in apoptosis induction, modulation of NHEJ and HR repair gene expression.40,41 One clinical trial study (phase 1) exploring the combination of pan-HDACi and RT for glioma and meningioma (NCT01324635; Panobinostat and RT) was discontinued. There are ongoing clinical trials evaluating the combination of HDACis with RT (eg. NCT02420613, NCT01236560; Vorinostat and RT for glioma, NCT02137759; Belinostat and RT for GBM). However, these HDACi studies are challenging due to following reasons; 1) Non-selective pan-HDACis are involved in a broad spectrum of adverse effects which complicate the interpretation of their mechanism of action. Thus, HDAC isoform-targeted inhibitors, known for their enhanced anticancer efficacy, can be promising over pan-HDACis.42 2) HDACis do not spare normal cells when administered, potentially leading to side effects. Therefore, our study has been focused on the role of specific isoform HDAC6 and using a low concentration of Cay10603, at a dose that does not escalate the occurrence of DNA DSBs, to mitigate potential adverse effects associated with HDAC6 inhibition. Cay10603 is not solely specific to HDAC6 because it unexpectedly affects other HDACs. However, despite this, Cay10603 has been described as an HDAC6-specific inhibitor in another study.43 Our findings also demonstrate that Cay10603 effectively targets HDAC6, with minimised effects on HDAC7 compared to other types of HDAC inhibitors (Fig. 3d, Supplementary Figures S1e, S3f).
We found that pre-treatment of Cay10603 leads to reduction in Rad51 levels and an extension of γH2AX persistence following RT indicating DNA damage. Rad51 plays a crucial role in HR, impeding the MRE11-mediated degradation and assists replication processing in response to DNA damage.44 Histone H2AX undergoes rapid phosphorylation during chromatin modification, facilitating the recruitment of other DDR protein components.25 Thus, the results above indicate that Cay10603 effectively mitigated DDR capability following RT. According to Gorgineni et al.,34 the induction of non-reversible G2/M arrest may hold therapeutic promise in the management of meningioma. It is attributed to the fact that the G2/M checkpoint plays a vital role in the survival of meningioma cells, rendering it an essential target for intervention. Although a single treatment of Cay10603(100 nM) alone sustained cell cycle without inducing a specific cell cycle arrest, along with a consistent level of DNA DSBs, combination treatment of Cay10603 with RT increased G2/M arrest and DNS DSBs level synergistically. Rad51 is recruited to the sites of DSBs, facilitating their repair in S/G2 phase cells.44 Therefore, its reduced expression of Rad51 shown in Fig. 3c; Supplementary Figure S3c supports synergistically increased G2/M arrest (Fig. 4; Supplementary Figure S5).
The majority of the functional assay results we conducted in IOMM-Lee cells were concordant with KT21-MG1 and primary cells BTB0058 in cell survival, migration, kinetics of γ-H2AX foci and Rad51, cell cycle arrest, cell death, β-catenin nuclear localisation, spheroid growth, spheroid cell death, and morphological changes by the combined treatment of HDAC6 inhibition and RT. However, the viability assay using Cay10603 and RT, as well as the G2/M arrest using HDAC6 shRNA in IOMM-Lee cells did not demonstrate a synergistic effect following RT as opposed to KT21-MG1. IOMM-Lee is a distinctive cell line, characterised by the loss of some critical genes (CDKN2A, CRIPAK), along with a significantly higher number of mutations.33 Loss of CDKN2A results in abnormal cell growth which was proven in cell doubling time assays in our study (20.97 h, shown in Supplementary Figure S2a) and this may disturb cell viability test and cell cycle regulation. Also, HDAC6 KD in KT21-MG1 did not show a synergistic survival rate after RT (Supplementary Figure S4d). We presume that the absence of a synergistic effect on survival rates following the knockdown of HDAC6 may be attributed to differences in read-out methodologies. Notably, the size of colonies was noticeably smaller in HDAC6 KD cells compared to scramble cells (Supplementary Figure S4d). Moreover, the potential presence of several confounding factors, such as off-target effects, heterogeneity contributing to variations in radiosensitivity, and compensatory mechanisms, could influence the differences observed in these results.
Our apoptosis assay demonstrated that cell death, especially late apoptosis, increases after Cay10603 + RT treatment (Fig. 5). KT21-MG1 cells have more necrotic cells than IOMM-Lee and BTB0058 cells, which is potentially related to the radioresistance of KT21-MG1 cells (Supplementary Figure S2b). Also, there was a higher fraction of necrosis shown in scramble than in HDAC6 KD (Fig. 5b, Supplementary Figure S7b). Consistent with our findings, Garcia-Segura et al.45 suggests that the combination of necrosis and brain invasion serves as a robust predictor of radioresistance in meningioma.
Dhandapani et al.46 have convincingly demonstrated that the 3D spheroid model effectively preserves the key characteristics of TME in breast cancer. To closely mimic the main features of solid tumours, including TME and heterogeneity, we used the early passage of primary human meningioma cells for the 3D spheroid model. In contrast, Zhang et al.47 faced challenges in successfully establishing the patient-derived orthotopic xenograft (PDOX) mouse model for meningioma. Their work revealed a success rate of only one out of nine primary tumours, suggesting that meningiomas may not be particularly amenable to PDOX mouse modelling. Moreover, it is important to note that the 3D spheroid model from primary cells is known to have high syngeneic immune cell infiltrations,26 which is less adequately found in PDOX models due to their compromised immune systems. In our patient-derived cell 3D model, we observed a significant increase in the number of dead cells after combination treatment and this increase in dead cells is separate from any contributions from a necrotic core (Fig. 8; Supplementary Figure S10).
Analysing the mechanism of action, we show that inhibition of HDAC6 promotes the therapeutic effect of RT through the attenuation of nuclear localisation of MCM2 and β-catenin. This leads to reduction of oncogenic c-myc expression, ultimately resulting in inhibited cell growth. While various signalling pathways such as Wnt, Rad, EGFR, and FGR are associated in malignancy in meningioma,48 our focus has been on nuclear accumulation of β-catenin within the Wnt signalling pathway. The lack of β-catenin is associated with inability to resolve DNA double-strand breaks after radiation,49 and β-catenin is widely recognised as a marker of cancer malignancy, driving cancer cell proliferation. Bukovac et al.50 also recently stated that nuclear translocation of β-catenin induces epithelial–mesenchymal transition and has a role in meningioma progression. Li et al.51 have previously reported that the knocked-down MCM2 leads to a reduction in β-catenin expression, along with its downstream factors such as ZEB1 and c-myc mRNA level in ovarian cancer cells. While it is widely established that nuclear localisation of β-catenin upregulates c-myc expression,52 the contribution of MCM2 has not received significant attention until now. In a related context, Sharma et al.48 conducted proteomics analysis in meningiomas and revealed the differentially expressed proteins in various signal transduction pathways in Wnt/β-catenin signalling cascade. Skiriute et al.53 also explained the malignancy of primary and recurrent meningioma depending on N-myc downstream-regulated gene 2 expression, which is engaged in Wnt signal by the modulation of β-catenin, suggesting the critical role of β-catenin in meningioma progression. The mechanism between HDAC6 and β-catenin remains unclear. However, it is known that HDAC6 deacetylates β-catenin at lysine 49, which frequently mutate in several cancer types resulting in major defects in embryonic stem cell differentiation.54 The work by Mak et al.19 demonstrated that the deacetylase activity of HDAC6 serves to stabilise β-catenin through its interaction with Prominin 1. Consequently, the downregulation of HDAC6 results in increased β-catenin degradation, ultimately contributing to decreased cell proliferation. Consistent with the findings of Mak et al.,19 here we show that the expression of HDAC6 in meningioma from human patients had a positive correlation with not only Cyclin D1 but also β-catenin expression (Fig. 1f–h).
In this study, the application of 3D model significantly strengthens the validity of our findings by replicating certain features of tumour microenvironment. We highlight the interplay between HDAC6, β-catenin, and cell cycle regulatory factors in the context of meningioma. Additionally, Cay10603, a potent hydroxamic acid-derived HDAC6i, has exhibited the potential to enhance the sensitivity of meningioma cells to RT, paving the way for more effective treatment strategies in the future. Nevertheless, a notable limitation lies in the translational gap from bench to bedside, which also restricts direct translatability of our results to the patient. Despite this limitation, this study contributes to the understanding of HDAC6 role in meningioma and offers a solid foundation for further investigation.
Contributors
Overall concept and coordination of the study: C.O.H., and J.N. Data acquisition and analysis: J.N., and S.S. Data interpretation: J.N. Drafting of the manuscript: C.O.H., and J.N. Financial support: C.O.H. All authors have read and approved the final manuscript.
Data sharing statement
The data for this study are available by contacting the corresponding author upon reasonable request.
Declaration of interests
COH received consultating fees from Recursion in 2022. COH is member of ICOM.
Acknowledgements
Radiation was delivered by the Radiotherapy Physics Department at University Hospitals Plymouth NHS Trust. We would like to thank Robin Laney, Dr. Scott Hanvey, Matthew Higman, Chris French, Jennifer Afonso, Mustafa Tumen, Martin Shingler, Donald Ndlovu, and Graham Arden for facilitating the delivery of megavoltage photon radiation to the samples in this study. We thank Dr Emanuela Ercolano for processing primary meningioma samples to produce cell-free extracts, and Dr Alexander Strachan in Plymouth Electron Microscopy Centre for the help getting SEM images. We also thank Dr Randy Jensen (University of Utah) for the IOMM-Lee cell line, and Dr Long-Sheng Chang (Nationwide Children's Hospital) for the KT21-MG1 cell line.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2024.105211.
Appendix A. Supplementary data
Supplementary Figure S1.
Supplementary Figure S2.
Supplementary Figure S3.
Supplementary Figure S4.
Supplementary Figure S5.
Supplementary Figure S6.
Supplementary Figure S7.
Supplementary Figure S8.
Supplementary Figure S9.
Supplementary Figure S10.
Supplementary Figure S11.
Supplementary Figure S12.
References
- 1.Kalamarides M., Stemmer-Rachamimov A.O., Niwa-Kawakita M., et al. Identification of a progenitor cell of origin capable of generating diverse meningioma histological subtypes. Oncogene. 2011;30(20):2333–2344. doi: 10.1038/onc.2010.609. [DOI] [PubMed] [Google Scholar]
- 2.Louis D.N., Perry A., Wesseling P., et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Champeaux C., Jecko V., Houston D., et al. Malignant meningioma: an international multicentre retrospective study. Neurosurgery. 2019;85(3):E461–E469. doi: 10.1093/neuros/nyy610. [DOI] [PubMed] [Google Scholar]
- 4.Sahm F., Schrimpf D., Stichel D., et al. DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol. 2017;18(5):682–694. doi: 10.1016/S1470-2045(17)30155-9. [DOI] [PubMed] [Google Scholar]
- 5.Zhao L., Zhao W., Hou Y., et al. An overview of managements in meningiomas. Front Oncol. 2020;10:1523. doi: 10.3389/fonc.2020.01523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maggio I., Franceschi E., Tosoni A., et al. Meningioma: not always a benign tumor. A review of advances in the treatment of meningiomas. CNS Oncol. 2021;10(2):CNS72. doi: 10.2217/cns-2021-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chohan M.O., Ryan C.T., Singh R., et al. Predictors of treatment response and survival outcomes in meningioma recurrence with atypical or anaplastic histology. Neurosurgery. 2018;82(6):824–832. doi: 10.1093/neuros/nyx312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eom G.H., Kook H. Role of histone deacetylase 2 and its posttranslational modifications in cardiac hypertrophy. BMB Rep. 2015;48(3):131–138. doi: 10.5483/BMBRep.2015.48.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park S.Y., Kim J.S. A short guide to histone deacetylases including recent progress on class II enzymes. Exp Mol Med. 2020;52(2):204–212. doi: 10.1038/s12276-020-0382-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharda A., Rashid M., Shah S.G., et al. Elevated HDAC activity and altered histone phospho-acetylation confer acquired radio-resistant phenotype to breast cancer cells. Clin Epigenetics. 2020;12(1):4. doi: 10.1186/s13148-019-0800-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bush M.L., Oblinger J., Brendel V., et al. AR42, a novel histone deacetylase inhibitor, as a potential therapy for vestibular schwannomas and meningiomas. Neuro Oncol. 2011;13(9):983–999. doi: 10.1093/neuonc/nor072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collier K.A., Valencia H., Newton H., et al. A phase 1 trial of the histone deacetylase inhibitor AR-42 in patients with neurofibromatosis type 2-associated tumors and advanced solid malignancies. Cancer Chemother Pharmacol. 2021;87(5):599–611. doi: 10.1007/s00280-020-04229-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welling D.B., Collier K.A., Burns S.S., et al. Early phase clinical studies of AR-42, a histone deacetylase inhibitor, for neurofibromatosis type 2-associated vestibular schwannomas and meningiomas. Laryngoscope Investig Otolaryngol. 2021;6(5):1008–1019. doi: 10.1002/lio2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu Z., Wei F., Su Y., et al. Histone deacetylase inhibitors promote breast cancer metastasis by elevating NEDD9 expression. Signal Transduct Target Ther. 2023;8(1):11. doi: 10.1038/s41392-022-01221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pham T.Q., Robinson K., Xu L., Pavlova M.N., Skapek S.X., Chen E.Y. HDAC6 promotes growth, migration/invasion, and self-renewal of rhabdomyosarcoma. Oncogene. 2021;40(3):578–591. doi: 10.1038/s41388-020-01550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aldana-Masangkay G.I., Sakamoto K.M. The role of HDAC6 in cancer. J Biomed Biotechnol. 2011;2011 doi: 10.1155/2011/875824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang S.L., Zhu H.Y., Zhou B.Y., et al. Histone deacetylase 6 is overexpressed and promotes tumor growth of colon cancer through regulation of the MAPK/ERK signal pathway. OncoTargets Ther. 2019;12:2409–2419. doi: 10.2147/OTT.S194986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y., Zhang X., Polakiewicz R.D., Yao T.P., Comb M.J. HDAC6 is required for epidermal growth factor-induced beta-catenin nuclear localization. J Biol Chem. 2008;283(19):12686–12690. doi: 10.1074/jbc.C700185200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mak A.B., Nixon A.M., Kittanakom S., et al. Regulation of CD133 by HDAC6 promotes beta-catenin signaling to suppress cancer cell differentiation. Cell Rep. 2012;2(4):951–963. doi: 10.1016/j.celrep.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pecina-Slaus N., Kafka A., Lechpammer M. Molecular genetics of intracranial meningiomas with emphasis on canonical Wnt signalling. Cancers. 2016;8(7) doi: 10.3390/cancers8070067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pai S.G., Carneiro B.A., Mota J.M., et al. Wnt/beta-catenin pathway: modulating anticancer immune response. J Hematol Oncol. 2017;10(1):101. doi: 10.1186/s13045-017-0471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka K., Sato C., Maeda Y., et al. Establishment of a human malignant meningioma cell line with amplified c-myc oncogene. Cancer. 1989;64(11):2243–2249. doi: 10.1002/1097-0142(19891201)64:11<2243::aid-cncr2820641110>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 23.Lyons Rimmer J., Ercolano E., Baiz D., et al. The potential of MLN3651 in combination with selumetinib as a treatment for merlin-deficient meningioma. Cancers. 2020;12(7) doi: 10.3390/cancers12071744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ragel B.T., Couldwell W.T., Gillespie D.L., Wendland M.M., Whang K., Jensen R.L. A comparison of the cell lines used in meningioma research. Surg Neurol. 2008;70(3):295–307. doi: 10.1016/j.surneu.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 25.Na J., Newman J.A., Then C.K., et al. SPRTN protease-cleaved MRE11 decreases DNA repair and radiosensitises cancer cells. Cell Death Dis. 2021;12(2):165. doi: 10.1038/s41419-021-03437-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van de Weijer L.L., Ercolano E., Zhang T., et al. A novel patient-derived meningioma spheroid model as a tool to study and treat epithelial-to-mesenchymal transition (EMT) in meningiomas. Acta Neuropathol Commun. 2023;11(1):198. doi: 10.1186/s40478-023-01677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu L., Hu X., Tang H., Han Z., Chen Y. Valid application of western blotting. Mol Biol Rep. 2014;41(5):3517–3520. doi: 10.1007/s11033-014-3215-5. [DOI] [PubMed] [Google Scholar]
- 28.Dunn J., Ferluga S., Sharma V., et al. Proteomic analysis discovers the differential expression of novel proteins and phosphoproteins in meningioma including NEK9, HK2 and SET and deregulation of RNA metabolism. eBioMedicine. 2019;40:77–91. doi: 10.1016/j.ebiom.2018.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldman M.J., Craft B., Hastie M., et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat Biotechnol. 2020;38(6):675–678. doi: 10.1038/s41587-020-0546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J., Lichtenberg T., Hoadley K.A., et al. An integrated TCGA pan-cancer clinical data resource to drive high-quality survival outcome analytics. Cell. 2018;173(2):400–416 e11. doi: 10.1016/j.cell.2018.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nassiri F., Liu J., Patil V., et al. A clinically applicable integrative molecular classification of meningiomas. Nature. 2021;597(7874):119–125. doi: 10.1038/s41586-021-03850-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barneda-Zahonero B., Collazo O., Azagra A., et al. The transcriptional repressor HDAC7 promotes apoptosis and c-Myc downregulation in particular types of leukemia and lymphoma. Cell Death Dis. 2015;6 doi: 10.1038/cddis.2014.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mei Y., Bi W.L., Greenwald N.F., et al. Genomic profile of human meningioma cell lines. PLoS One. 2017;12(5) doi: 10.1371/journal.pone.0178322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gogineni V.R., Nalla A.K., Gupta R., Dinh D.H., Klopfenstein J.D., Rao J.S. Chk2-mediated G2/M cell cycle arrest maintains radiation resistance in malignant meningioma cells. Cancer Lett. 2011;313(1):64–75. doi: 10.1016/j.canlet.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang C.Y., Bao W., Wang L.H. Downregulation of p16(ink4a) inhibits cell proliferation and induces G1 cell cycle arrest in cervical cancer cells. Int J Mol Med. 2014;33(6):1577–1585. doi: 10.3892/ijmm.2014.1731. [DOI] [PubMed] [Google Scholar]
- 36.Huang J., Luo H.L., Pan H., Qiu C., Hao T.F., Zhu Z.M. Interaction between RAD51 and MCM complex is essential for RAD51 foci forming in colon cancer HCT116 cells. Biochemistry (Mosc) 2018;83(1):69–75. doi: 10.1134/S0006297918010091. [DOI] [PubMed] [Google Scholar]
- 37.Niu Z., Liu H., Zhou M., et al. Knockdown of c-Myc inhibits cell proliferation by negatively regulating the Cdk/Rb/E2F pathway in nasopharyngeal carcinoma cells. Acta Biochim Biophys Sin. 2015;47(3):183–191. doi: 10.1093/abbs/gmu129. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z., Hu P., Tang F., et al. HDAC6 promotes cell proliferation and confers resistance to temozolomide in glioblastoma. Cancer Lett. 2016;379(1):134–142. doi: 10.1016/j.canlet.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Yang W.B., Wu A.C., Hsu T.I., et al. Histone deacetylase 6 acts upstream of DNA damage response activation to support the survival of glioblastoma cells. Cell Death Dis. 2021;12(10):884. doi: 10.1038/s41419-021-04182-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tatman P.D., Wroblewski T.H., Fringuello A.R., et al. High-throughput mechanistic screening of epigenetic compounds for the potential treatment of meningiomas. J Clin Med. 2021;10(14) doi: 10.3390/jcm10143150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Groselj B., Sharma N.L., Hamdy F.C., Kerr M., Kiltie A.E. Histone deacetylase inhibitors as radiosensitisers: effects on DNA damage signalling and repair. Br J Cancer. 2013;108(4):748–754. doi: 10.1038/bjc.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang T., Wang F., Elhassan R.M., et al. Targeting histone deacetylases for cancer therapy: trends and challenges. Acta Pharm Sin B. 2023;13(6):2425–2463. doi: 10.1016/j.apsb.2023.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hou Q., Kan S., Wang Z., et al. Inhibition of HDAC6 with CAY10603 ameliorates diabetic kidney disease by suppressing NLRP3 inflammasome. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.938391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhattacharya S., Srinivasan K., Abdisalaam S., et al. RAD51 interconnects between DNA replication, DNA repair and immunity. Nucleic Acids Res. 2017;45(8):4590–4605. doi: 10.1093/nar/gkx126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barisano G., Bergamaschi S., Acharya J., et al. Complications of radiotherapy and radiosurgery in the brain and spine. Neurographics. 2018;8(3):167–187. doi: 10.3174/ng.1700066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dhandapani H., Siddiqui A., Karadkar S., Tayalia P. In vitro 3D spheroid model preserves tumor microenvironment of hot and cold breast cancer subtypes. Adv Healthc Mater. 2023;12(21) doi: 10.1002/adhm.202300164. [DOI] [PubMed] [Google Scholar]
- 47.Zhang H., Qi L., Du Y., et al. Patient-derived orthotopic xenograft (PDOX) mouse models of primary and recurrent meningioma. Cancers. 2020;12(6) doi: 10.3390/cancers12061478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma S., Ray S., Mukherjee S., Moiyadi A., Sridhar E., Srivastava S. Multipronged quantitative proteomic analyses indicate modulation of various signal transduction pathways in human meningiomas. Proteomics. 2015;15(2–3):394–407. doi: 10.1002/pmic.201400328. [DOI] [PubMed] [Google Scholar]
- 49.Lento W., Ito T., Zhao C., et al. Loss of beta-catenin triggers oxidative stress and impairs hematopoietic regeneration. Genes Dev. 2014;28(9):995–1004. doi: 10.1101/gad.231944.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bukovac A., Kafka A., Raguz M., et al. Are we benign? What can Wnt signaling pathway and epithelial to mesenchymal transition tell us about intracranial meningioma progression. Cancers. 2021;13(7) doi: 10.3390/cancers13071633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li H.M., Lin Z.K., Bai Y.X., et al. Sinomenine inhibits ovarian cancer cell growth and metastasis by mediating the Wnt/beta-catenin pathway via targeting MCM2. Rsc Adv. 2017;7(79):50017–50026. [Google Scholar]
- 52.Rennoll S., Yochum G. Regulation of MYC gene expression by aberrant Wnt/beta-catenin signaling in colorectal cancer. World J Biol Chem. 2015;6(4):290–300. doi: 10.4331/wjbc.v6.i4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Skiriute D., Tamasauskas S., Asmoniene V., et al. Tumor grade-related NDRG2 gene expression in primary and recurrent intracranial meningiomas. J Neuro Oncol. 2011;102(1):89–94. doi: 10.1007/s11060-010-0291-9. [DOI] [PubMed] [Google Scholar]
- 54.Hoffmeyer K., Junghans D., Kanzler B., Kemler R. Trimethylation and acetylation of beta-catenin at lysine 49 represent key elements in ESC pluripotency. Cell Rep. 2017;18(12):2815–2824. doi: 10.1016/j.celrep.2017.02.076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.