Abstract
Several gammaherpesviruses contain open reading frames encoding proteins homologous to mammalian D-type cyclins. In this study, we analyzed the expression and function of the murine gammaherpesvirus 68 (γHV68) viral cyclin (v-cyclin). The γHV68 v-cyclin gene was expressed in lytically infected fibroblasts as a leaky-late mRNA of approximately 0.9 kb encoding a protein of approximately 25 kDa. To evaluate the effect of the γHV68 v-cyclin on cell cycle progression in primary lymphocytes and to determine if the γHV68 v-cyclin gene is an oncogene, we generated transgenic mice by using the lck proximal promoter to express the γHV68 v-cyclin in early T cells. Expression of the γHV68 v-cyclin significantly increased the number of thymocytes in cell culture, as determined by measuring both DNA content and incorporation of 5-bromo-2-deoxyuridine following in vivo pulse-labeling. Expression of the γHV68 v-cyclin interfered with normal thymocyte maturation, as shown by increased numbers of CD4+ CD8+ double-positive thymocytes and decreased numbers of CD4+ or CD8+ single-positive and T-cell-receptor-bright thymocytes and splenocytes in transgenic mice. Despite increased numbers of cycling thymocytes, γHV68–v-cyclin–transgenic mice did not have proportionately increased thymocyte numbers, and staining by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling demonstrated increased apoptosis in the thymi of v-cyclin-transgenic mice. Fifteen of 38 γHV68–v-cyclin–transgenic mice developed high-grade lymphoblastic lymphoma between 3 and 12 months of age. We conclude that (i) the γHV68 v-cyclin is expressed as a leaky-late gene in lytically infected cells, (ii) expression of the γHV68 v-cyclin in thymocytes promotes cell cycle progression and inhibits normal T-cell differentiation, and (iii) the γHV68 v-cyclin gene is an oncogene.
Gammaherpesviruses are characterized by their tropism for host B and/or T cells and an association with B- and T-cell malignancies. Their oncogenic potential may result, at least in part, from the ability of gammaherpesviruses to regulate cell cycle progression by means of the D-type cyclins. Host D-type cyclins (i) bind to cyclin-dependent kinases (cdk’s) 4 and 6 (42, 44), (ii) are required for the activation as well as the specificity of the kinase complex, and (iii) are essential for cell cycle progression through G1. D cyclins are not expressed in the G0 phase of the cell cycle but are constantly expressed in cycling cells (1, 43). Although Epstein-Barr virus (EBV) does not encode a cyclin homolog, EBV infection (or expression of EBV latent membrane protein 1 [LMP-1]) upregulates expression of cyclin D2 (3, 9, 63). In contrast, the gammaherpesviruses Kaposi’s sarcoma-associated herpesvirus (KSHV) (55), herpesvirus saimiri (HVS) (2, 49), and murine gammaherpesvirus 68 (γHV68) (70) all contain open reading frames (ORFs) predicted to encode proteins with high levels of homology to mammalian D-type cyclins. Studies of the KSHV and HVS v-cyclins have demonstrated alterations in sensitivity to cyclin-dependent kinase inhibitors and broadened substrate specificity of v-cyclin–cdk complexes (see Discussion). The murine γHV68 cyclin homolog is 25% identical to the KSHV cyclin homolog and 21% identical to the HVS cyclin homolog (70).
Transgenic-mouse models have been used to analyze the function of both mammalian cyclins and gammaherpesvirus oncogenes in vivo. Overexpression of mammalian D-type cyclins induces dysplasia of squamous epithelium (46, 48), abnormal development of other epithelia (53), multinucleation and abnormal DNA synthesis in cardiomyocytes (65), and mammary adenocarcinomas (72). Expression of mammalian D-type cyclins in B cells results in an increase in B-cell tumors when cyclin-transgenic mice are crossed to c-myc-transgenic mice (5, 39). Transgenic mice expressing the HVS STP protein develop tumors in multiple organs (47). Expression of the EBV nuclear antigen 2 (EBNA2) protein under the control of a simian virus 40 (SV40) promoter-enhancer induced renal adenocarcinomas (69). Expression of the EBV LMP-1 protein causes epithelial cell hyperplasia (78). Both EBV LMP-1 and EBNA1 can independently cause B-cell lymphomas when expressed from an immunoglobulin (Ig) heavy-chain promoter-enhancer (35, 77).
These studies suggested that analysis of the possible oncogenic properties of the γHV68 v-cyclin in a transgenic model would shed light on the function of this protein independent of other viral proteins. In this study, we demonstrated expression of the γHV68 v-cyclin in infected cells and used transgenic mice to assess the functional and transforming potential of the γHV68 v-cyclin in primary lymphocytes. Transgenic expression of the γHV68 v-cyclin promoted cell cycle progression in primary lymphocytes and led to the development of lymphoblastic lymphoma.
MATERIALS AND METHODS
Virus and viral DNA.
γHV68 clone WUMS, kindly provided by P. Doherty and A. Nash, was cloned, passaged, and grown as previously described (73, 74). Briefly, γHV68 was grown in NIH 3T12 cells in Dulbecco’s modified Eagle medium supplemented with 10% fetal calf serum (FCS), 100 U of penicillin/ml, 100 mg of streptomycin/ml, and 2 mM l-glutamine, and titers were determined by plaque assay. DNA was prepared from virus stocks grown (multiplicity of infection, 0.05) in NIH 3T12 cells, culture supernatants were collected, and DNA was isolated as previously described (70).
Isolation of RNA from lytically infected fibroblasts and Northern blotting.
3T12 fibroblasts (2 × 107 cells/T175 flask) were infected at a multiplicity of infection of 5 in a 10-ml volume of medium for 1 h, 15 ml of medium containing drugs was added, and the flasks were then incubated at 37°C in a 5% CO2 atmosphere for 12 or 24 h prior to harvesting of cells and preparation of RNA. The flask cultures were untreated or were treated with drugs as follows: (i) γHV68 DNA synthesis was inhibited by adding phosphonoacetic acid to a final concentration of 200 μg/ml, and (ii) protein synthesis was inhibited by adding a combination of cycloheximide (to a final concentration of 40 μM) and anisomycin (to a final concentration of 10 μM). Total cellular RNA was harvested by the single-step guanidinium thiocyanate-phenol method (51) and analyzed by Northern blot hybridization (57). Blots were probed for rat cyclophilin (16) to assess loading and RNA quality. All probes were radiolabeled by using the Megaprime DNA labeling system (Amersham, Arlington Heights, Ill.) per the manufacturer’s protocol. The cyclin probe was generated by PCR amplification of γHV68 DNA utilizing PCR primers containing 8-nucleotide (nt) overhangs and enzyme sites flanking the γHV68 genomic sequence. PCR primers utilized to amplify the region of the γHV68 genome from bp 102703 to 103116 were 5′-TACAGCTCCTCGAGTGAAGAAGACTGCAGACAGA TG-3′ and 5′-TACAGCTCGCGGCCGCATGATATGCAGGATGTAACA-3′. PCRs were carried out in a total volume of 20 μl of 1× Vent buffer-MgSO4 (New England Biolabs [NEB]) containing 0.5 U of Vent polymerase (NEB), 0.2 mM each deoxynucleoside triphosphate (dNTP), 0.15 μM each primer, and 1 μg of γHV68 genomic DNA. PCR conditions were as follows: a 5-min denaturation at 94°C; 12 cycles consisting of 30s at 94°C, 1 min at 55°C, and 30s at 72°C; a 5-min extension at 72°C; and a 4°C hold. The cyclophilin probe was generated from the cDNA (16). Additional probes were generated by PCR as described elsewhere (71).
Mice and tissue harvests.
Mice were housed and bred at Washington University School of Medicine, in accordance with all federal and university policies, or were purchased from Charles River Laboratories. Mice were sacrificed by cervical dislocation after metofane anesthetization. The thymus and spleen were removed and fixed for histological examination or dissociated for analysis. Tissues were disrupted in a TenBroek homogenizer, cells were centrifuged and washed, and erythrocytes were lysed with ammonium chloride. The remaining lymphocytes were centrifuged, filtered over 70-μm-pore-size Nitex mesh (Tetko), counted, and resuspended for staining. For in vivo labeling with 5-bromo-2-deoxyuridine (BrdU), 120 mg of BrdU per kg of body weight was injected intraperitoneally 2 h before sacrifice and tissues were harvested as previously described (62).
Pathology, TUNEL staining, and staining for BrdU.
For histological studies, tissues were fixed in phosphate-buffered formalin, paraffin embedded, and sectioned onto slides. Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) was performed as described elsewhere (54). Briefly, after removal of the paraffin with xylene, the slides were washed in decreasing concentrations of ethanol and incubated in a solution of proteinase K (Sigma). Biotinylated dUTP was added, with or without terminal deoxynucleotidyltransferase (Boehringer Mannheim), and incorporated biotin-dUTP was detected with streptavidin-horseradish peroxidase (SHRP) (Caltag) developed with aminoethyl carbazole substrate (Zymed). The slides were then counterstained with hematoxylin. The cortical and medullary areas of hematoxylin- and eosin-stained step sections through thymi of two sets of transgenic mice and matched littermates were measured by the use of Northern Eclipse (Empix Imaging, Missauga, Ontario, Canada) image analysis software. BrdU staining was performed as described previously (62). Briefly, after removal of paraffin from the slides with xylene, they were washed in decreasing concentrations of isopropanol, incubated in PBS phosphate-buffered saline (PBS)-blocking buffer for 20 min, and then incubated overnight at 4°C with goat anti-BrdU antibody (14) or normal goat antiserum (Vector Laboratories). Incorporated BrdU was detected by incubation with biotinylated anti-goat Ig (Jackson Immunoresearch) followed by incubation with SHRP, biotin-tyramide (NEB), and, finally, SHRP and then detection with diaminobenzidine substrate (Pierce) (5-min development).
Antisera and immunoblot analyses.
A polyclonal rabbit antiserum (Cocalico, Reamstown, Pa.) was generated from purified bacterially expressed v-cyclin protein. ORF72 was amplified from γHV68 genomic DNA, ligated into the NdeI-XhoI sites of pET30a(+) (Novagen), and sequenced in full. Primers contained 8-nt overhangs and enzyme sites flanking the v-cyclin ORF. The forward primer was also designed to incorporate a 6-histidine tag (6HN) at the amino terminus of the v-cyclin to aid in purification. PCR primers utilized to amplify the v-cyclin (γHV68 genome coordinates, bp 102423 to 103161) were 5′-TACAGC TCCATATGCACCATCATCATCACATGGCCAGTCAAGAATTCCAA-3′ and 5′-TACAGCTCGAGCTCCTAGGCATTTATTTTGAAATAGTT-3′. PCRs were carried out in total volumes of 20 μl of 1× Vent buffer-MgSO4 (NEB) containing 0.5 U of Vent polymerase (NEB), 0.2 mM each dNTP, 0.15 μM each primer, and 1 μg of γHV68 genomic DNA. PCR conditions were as follows: a 5-min denaturation at 94°C, 12 cycles of 30s at 94°C, 1 min at 48°C, and 30s at 72°C; a 5-min extension at 72°C; and a 4°C hold. BL21 cells (Novagen) were transformed and grown to log phase before being subjected to isopropyl-β-d-thiogalactopyranoside (IPTG) induction (0.4 mM final concentration) for 4 h at 37°C. The cells were pelleted and lysed, and 6HN–v-cyclin was purified according to the recommendations of the manufacturer (Qiagen). Briefly, cells were lysed in 6 M guanidine hydrochloride, sonicated, spun at 10,000 × g, and incubated with nickel-nitrilotriacetic acid (Ni-NTA) resin. 6HN–v-cyclin was eluted from the Ni-NTA in 150 mM imidazole. A polyclonal rabbit antiserum (Cocalico) was generated by an initial inoculation of 100 μg of 6HN–v-cyclin in complete Freunds adjuvant followed by boosts of 50 μg of 6HN–v-cyclin in incomplete Freunds adjuvant administered at days 14, 21, 49, and 77. Serum was collected 7 days following the final boost.
Whole-cell lysates for use in Western analyses were made from frozen cell pellets resuspended in 1× reducing sample loading buffer and boiled for 10 min (4). A total of 2.5 × 105 cell equivalents was loaded per lane on 15% polyacrylamide gels, and after electrophoresis the proteins were transferred to HybondN membranes (Amersham). Blots were blocked in PBS containing 5% nonfat milk and 0.05% Tween 20 for 45 min and then incubated with polyclonal rabbit anti-v-cyclin serum (1:2,000 dilution) overnight at room temperature. Blots were washed three times in PBS–0.05% Tween 20, incubated in horseradish peroxidase-conjugated donkey anti-rabbit antiserum (1:5,000; Jackson Immunoresearch), washed again three times, and developed with the ECL chemiluminescence reagent (Amersham).
Generation of γHV68–v-cyclin–transgenic (γHV68–v-cyclin–TG) mice.
The γHV68 v-cyclin transgene construct was generated by PCR amplification of γHV68 genomic DNA and ligation into the BamHI site of the p1017 vector (12). The p1017 vector contains the human growth hormone enhancer and the lck proximal promoter for tissue-specific expression in the thymus. PCR primers contained 8-nt overhangs and BamHI sites flanking the amplified γHV68 sequences. PCR primers utilized to amplify the γHV68 v-cyclin (γHV68 genome coordinates, bp 102422 to 103161) were 5′-TACAGCTCGGATCCATGGCC AGTCAAGAATTCCAA-3′ and 5′-TACAGCTCGGATCCCTAGGCATTTATTTTGAAATAGTT-3′. PCRs were carried out in total volumes of 20 μl of 1× Vent buffer-MgSO4 (NEB) containing 0.5 U of Vent polymerase (NEB), 0.2 mM each dNTP, 0.15 μM each primer, and 1 μg of γHV68 genomic DNA. PCR conditions were as follows: a 5-min denaturation at 94°C; 12 cycles of 30s at 94°C, 1 min at 50°C, and 30s at 72°C; a 5-min extension at 72°C; and a 4°C hold. The transgene insert and insertion junctions were sequenced in full. The transgene fragment used for microinjection was isolated by SpeI digestion and gel purified by standard methods. C57BL/6 embryo manipulations were performed as described in reference 18, using standard methods (28). Transgene-positive F1 mice were detected by PCR analysis, bred with C57BL/6 mice (Charles River), and carried as heterozygotes. The mice used for the experiments described in this paper were first-, second-, and third-generation BL/6 × γHV68 v-cyclin heterozygotes.
PCR analysis of transgenics.
Transgene-positive founder lines were identified by PCR and confirmed by Southern blotting. Tail biopsy specimens were obtained at 3 weeks and digested in 10 mM Tris plus 1 mM EDTA (pH 8.0) containing 1% Triton X-100 and 100 μg of proteinase K (Sigma)/ml overnight at 55°C. After digestion, the DNA was extracted, precipitated, and resuspended in Tris-EDTA. PCR analysis of tail DNA specimens was carried out on 2 μl of 50-ng/μl samples in 20-μl reaction volumes (5 ng/μl final concentration). PCR reactants included 1× Taq buffer with MgCl (Promega), 0.3 U of Taq polymerase, 0.2 mM dNTP, and 0.225 μM each primer. PCR conditions were as follows: a 5-min denaturation at 94°C; 45 cycles of 30s at 94°C, 1 min at 66°C, and 30s at 72°C; a 5-min extension at 72°C; and a 4°C hold. Primers utilized were specific for the human growth hormone enhancer within the transgenic expression construct, and the assay was sensitive to 103 copies of transgene plasmid DNA. The PCR primers employed were 5′-GGCCAAGCGCTTGGGCACTGTTCCCTCCCT-3′ and 5′-GCCCCCGGGCAGCACAGCCACTGCCGGTCC-3′.
Flow-cytometric analysis.
Flow cytometry was performed on either a Becton Dickinson FACScan or FACScaliber, and 50,000 events were collected per sample. Surface staining of thymocytes and splenocytes was performed as described elsewhere (15, 32). Briefly, 106 cells were incubated with directly conjugated monoclonal antibodies in Dulbecco’s modified Eagle medium supplemented with 4% FCS and 0.1% azide for 20 min on ice, washed three times, and fixed in 0.1% paraformaldehyde, pH 7.0. The following monoclonal antibodies were used: fluorescein isothiocyanate (FITC)-conjugated anti-CD8 (Caltag RM2201), phycoerythrin (PE)-conjugated anti-CD4 (Caltag RM2504), PE-conjugated anti-T-cell receptor β-chain (anti-TCRβ) (Pharmingen 01305A), the isotype control PE-conjugated anti-keyhole limpet hemocyanin (Pharmingen 11145A), FITC-conjugated anti-BrdU (Caltag MD5201), and the isotype control FITC-conjugated mouse IgG1 (Caltag MG101). BrdU staining and analysis were performed as described previously (38). Briefly, cells were acid ethanol fixed and permeabilized, denatured, neutralized, incubated with antibody, washed, and resuspended in RNase-free propidium iodide (PI). Cells were prepared for cell cycle analysis by fixation in 70% ethanol for 20 min at −20°C and resuspended in PBS containing 1% FCS, 1 μg of RNase A/ml, and 10 μg of PI/ml; this was followed by incubation at room temperature for >10 min.
Analysis of tumor development in transgenic and control mice.
Data reported in this paper were derived by monitoring a cohort of transgenic mice and matched littermate controls (a total of 72) for illness and tumor development for up to 1 year. Thirty-four mice were derived from founder 83, 16 of which were v-cyclin transgenics and 18 of which were littermate controls. Thirty-eight mice were derived from founder 100, 22 of which were v-cyclin transgenics and 16 of which were littermate controls. Mice were evaluated for tumors at the time of death, when seriously ill, or on reaching 1 year of age. Because the mice were born over a period of several months, not all mice were monitored for an entire year: the data presented here was as of 9 February 1999. Tumor occurrence was graphed and analyzed as a survival curve, using development of lymphoma as the primary variable (GraphPad Software, San Diego, Calif.). Tumors were examined by light microscopy by a hematopathologist (J.L.H.) and characterized using standard criteria for lymphoma. In some experiments, tumors were explanted and the expression of the γHV68 v-cyclin in resulting tumor lines was examined by Western blot analysis as described above.
Statistical methods.
Data are presented as average percentages of positive cells (fluorescence-activated cell sorter analysis [FACS] data) or total cell counts (cellularity data) ± standard errors of the means (SEMs). Tumor incidence was analyzed by the use of survival curves. All statistical analyses were performed with Graphpad Prism software (Graphpad Software).
RESULTS
Expression of a D-type cyclin homolog in γHV68-infected cells.
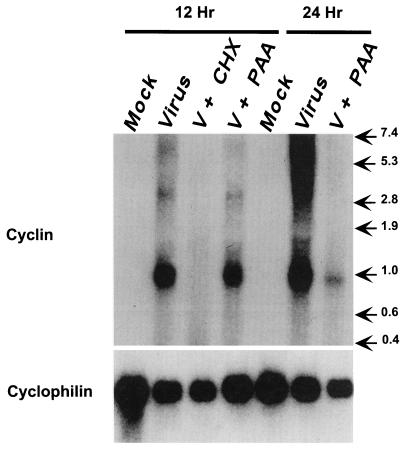
We previously demonstrated that murine γHV68 contains an ORF (gene 72) predicted to encode a protein homologous to both mammalian D-type cyclins and the v-cyclins of KSHV and HVS (70). To determine if this gene is transcribed and translated during viral infection, we examined γHV68-infected fibroblasts by Northern analysis and immunoblotting. Northern blot analysis demonstrated a single, predominant, ca. 900-nt transcript at 12 h after infection (Fig. 1). The larger transcripts, present at 24 h postinfection (Fig. 1), have not been characterized; however, gammaherpesviruses are known to produce long polycistronic transcripts late in infection. Blockade of protein synthesis, using cycloheximide and anisomycin, effectively inhibited expression of this transcript, demonstrating that gene 72 is not an immediate-early gene. Inhibition of viral DNA synthesis, using phosphonoacetic acid, did not ablate expression of the v-cyclin transcript at 12 h but did decrease its abundance at 24 h (Fig. 1), indicating that gene 72 is a leaky-late gene. Duplicate blots hybridized with probes specific for an early gene (single-stranded DNA binding protein [gene 6]) and two late genes (glycoprotein B [gene 8] and the major capsid protein [gene 25]) confirmed that phosphonoacetic acid treatment properly distinguished between early and late viral transcripts (data not shown). Stripping the v-cyclin Northern blot and reprobing with a probe specific for the cellular cyclophilin transcript demonstrated that loading did not influence assignment of kinetic class (Fig. 1).
FIG. 1.
Northern blot analysis of the expression of γHV68 gene 72 in infected fibroblasts. 3T12 cells either were mock infected or were infected with γHV68 in the absence (Virus) or presence of protein synthesis inhibitors (cycloheximide and anisomycin [V + CHX]) or a viral DNA replication inhibitor (phosphonoacetic acid [V + PAA]). RNA was collected at the times indicated and prepared as described in Materials and Methods. RNA (10 μg/lane) was fractionated on formaldehyde-agarose gels, transferred to a nylon membrane, and probed with DNA specific for the cyclin homolog (ORF72) and cellular cyclophilin as described in Materials and Methods. The numbers on the right indicate migration of RNA standards (in kilobases).
Immunoblot analysis with a polyclonal rabbit antibody raised against a His-tagged version of the γHV68 v-cyclin expressed in Escherichia coli (see Materials and Methods) demonstrated the presence of a protein with an apparent molecular mass of 25 kDa in infected fibroblasts but not in mock-infected fibroblasts (Fig. 2, lanes 1 and 2). The observed size of the viral protein expressed in infected cells is in good agreement with the predicted molecular mass of the γHV68 v-cyclin (29.0 kDa) and closely correlates with the observed molecular masses of the v-cyclins of KSHV (29 to 30 kDa with a flag tag [37] or 28 kDa with a myc tag [25]; 29 kDa [17]) and HVS (29 kDa [31].) These studies of RNA and protein expression identify the γHV68 v-cyclin ORF as a bona fide gene expressed in lytically infected cells as a leaky-late gene encoding a protein of ca. 25 kDa.
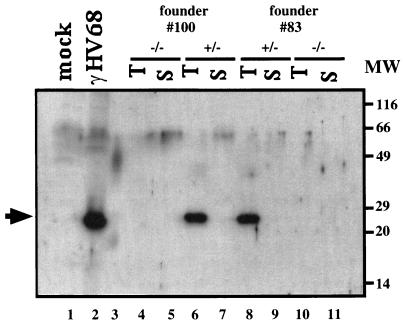
FIG. 2.
Western blot analysis of γHV68 v-cyclin protein expression in infected fibroblasts and γHV68–v-cyclin–TG mice. A total of 2.5 × 105 cell equivalents per sample was loaded on a 15% polyacrylamide gel, subjected to electrophoresis, transferred to nitrocellulose, and detected with a rabbit polyclonal antiserum to 6HN-tagged γHV68 v-cyclin. Lanes 1 and 2 contain mock- and γHV68-infected cell lysates, respectively, prepared at 24 h postinfection. Lanes 4 to 11 contain thymocytes (T) (lanes 4, 6, 8, and 10) or splenocytes (S) (lanes 5, 7, 9, and 11) from cyclin-transgenic mice (lanes 6 to 9) or wild-type littermate controls (lanes 4, 5, 10, and 11). Founder no. 100 is represented in lanes 4 to 7, and founder no. 83 is represented in lanes 8 to 11. Lane 3 contains molecular weight (MW) markers; sizes are indicated to the right of the gel. The solid arrow indicates the v-cyclin protein.
Generation of transgenic mice expressing the γHV68 v-cyclin under the control of the lck proximal promoter.
The gammaherpesviruses are lymphotrophic and are frequently associated with lymphoid malignancies. Therefore, we tested the hypothesis, based on in vitro studies of the HVS and KSHV v-cyclins, that the γHV68 v-cyclin is a pro-proliferative cyclin in primary lymphocytes. To this end, we generated transgenic mice expressing the γHV68 v-cyclin under the control of the lck proximal promoter (12). We selected this promoter since (i) it has been used in many studies and provides transgene expression at a well-defined early stage in thymocyte development (76); and (ii) thymic T-cell development has been extensively characterized, providing us with tools and a defined anatomic location for determining the effect of γHV68 v-cyclin expression on cell cycle and primary lymphocyte development.
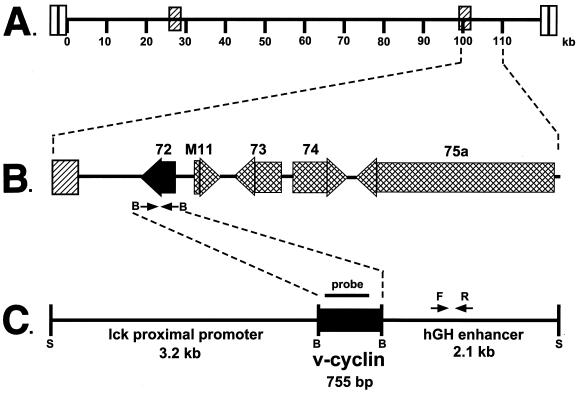
γHV68–v-cyclin–TG mice were derived by injecting C57BL/6 blastocysts with a construct containing the γHV68 v-cyclin ORF under the control of the lck proximal promoter (Fig. 3). The γHV68 v-cyclin ORF was derived by PCR, using the map coordinates for γHV68 gene 72 (70), and sequenced prior to construction of transgenic mice. PCR was used to screen for transgenic founders (see the legend to Fig. 3D for a description of the PCR assay used). Of a total of 26 potential founders, 4 contained the transgene. Western blot analysis showed that two transgenic mouse lines expressed levels of the γHV68 v-cyclin roughly equivalent to those seen in infected fibroblasts (Fig. 2). The v-cyclin protein expressed in lytically infected fibroblasts and in the γHV68–v-cyclin–TG cells showed no consistent differences in migration over the course of multiple experiments. As expected from the use of the lck proximal promoter, expression of the v-cyclin was detected in the thymi but not the spleens of the transgenic mice (Fig. 2). γHV68–v-cyclin–TG mice appeared normal and bred well. Southern analysis showed that the two lines, no. 83 and 100, contained approximately 11 and 23 copies of the transgene, respectively (data not shown). These two lines were further characterized. γHV68–v-cyclin–TG mice were compared to age- and sex-matched nontransgenic littermates in all experiments. Experiments included both males and females, and the ages of the mice examined ranged from 5 to 12 weeks, except for longitudinal studies of tumor development, which lasted for up to 1 year. In all experiments, data were collected on mice of both transgenic lines, 83 and 100, and pooled. No differences between these two lines were detected.
FIG. 3.
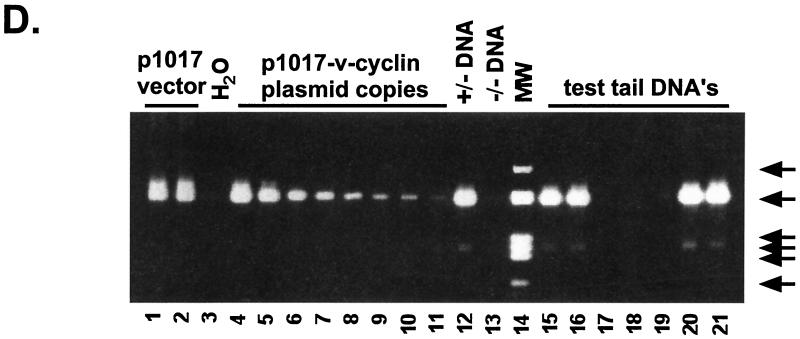
Construction of γHV68–v-cyclin–TG mice. (A) Schematic illustration of the 118,237-bp γHV68 genome. The terminal repeats are depicted as open rectangles at the ends of the genome, and the internal repeats are depicted as hatched rectangles. The numbers represent kilobases as described in reference 70. (B) The region from 100 to 110 kb in an expanded form. Large shaded arrows depict the positions and orientations of encoded ORFs, and the gene number is indicated above each ORF. Gene 72 (bp 103181 to 102426) was amplified from genomic γHV68 DNA as described in Materials and Methods. (C) Schematic illustration of the linearized transgene fragment. The 755-bp γHV68 v-cyclin was inserted under the control of the lck proximal promoter and the human growth hormone (hGH) enhancer. The transgene was linearized with SpeI and purified from the plasmid backbone. The probe used for Northern and Southern blots is indicated by the bar above the black box depicting the v-cyclin. The primers used for PCR detection of the transgene are indicated by arrows labeled F and R; S, SpeI sites. (D) A representative PCR analysis for the presence of the transgene. Input DNAs were as follows: lanes 1 and 2, 106 copies of p1017 vector in diluent DNA; lane 3, water; lanes 4 to 11, decreasing copy numbers of p1017-γHV68-cyclin plasmid in diluent DNA (lane 4, 107 copies; lane 5, 106 copies; lanes 6 and 7, 105 copies; lanes 8 and 9, 104 copies; lanes 10 and 11, 103 copies); lane 12, positive tail DNA; lane 13, negative tail DNA; lane 14, molecular weight markers; lanes 15 through 21, test tail samples. Molecular sizes of HaeII-cleaved Bluescript are indicated by arrows on the right side of the gel (602, 458/436, 290, 267, 242, and 172 bp).
Cell cycle profile of thymocytes from γHV68–v-cyclin–TG mice.
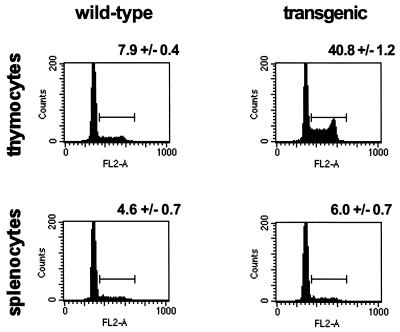
PI staining was used to compare the cell cycle profiles of thymocytes from 14 γHV68–v-cyclin–TG mice and 14 nontransgenic littermates. Notably, the proportion of thymic cells in S/G2/M of the cell cycle was increased from a mean ± SEM of 7.9% ± 0.4% in nontransgenic controls to 40.8% ± 1.2% in γHV68–v-cyclin–TG mice (Fig. 4). Consistent with the fact that we did not detect expression of v-cyclin in the spleen, we detected no significant alteration in the cell cycle profile when total splenocytes were evaluated (Fig. 4). However, the lack of a clear effect in whole splenocytes does not rule out the possibility that the γHV68 v-cyclin is expressed and functional in a subset of splenocytes.
FIG. 4.
Cell cycle analysis of lymphocytes from γHV68–v-cyclin–TG mice. Flow-cytometric profiles of thymocytes (top panels) and splenocytes (bottom panels) are displayed as histograms of PI fluorescence. Wild-type lymphocytes are shown in the histograms on the left, and γHV68–v-cyclin–TG lymphocytes are shown on the right. Cell cycle progression is indicated by cycling cells or cells in S/G2 and M phases (>2N DNA content). Numbers above each representative histogram are the mean percentages of S/G2/M cells ± the SEMs (n = 14).
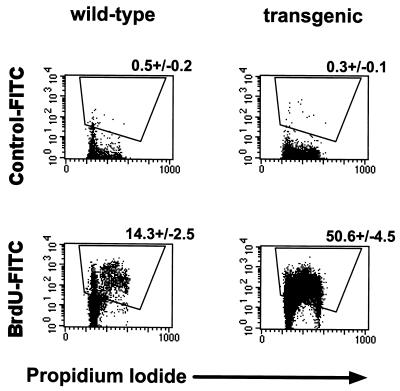
We considered the possibility that the increase in S/G2/M-phase thymocytes in v-cyclin-transgenic mice did not reflect increased numbers of cells passing through the cell cycle but instead represented a block in cell cycle progression. We therefore determined the proportion of thymocytes actively synthesizing DNA by measuring incorporation of BrdU (Fig. 5). Across four experiments, we found that 50.6% ± 4.5% of transgenic thymocytes incorporated BrdU during a 2-h in vivo pulse-labeling, compared with 14.3% ± 2.5% of normal thymocytes. Cells throughout S, G2, and M phases labeled with BrdU, consistent with increased numbers of cells transiting the cell cycle as opposed to a transgene-associated inhibition of cell cycle progression. Together, these data (Fig. 4 and 5) demonstrated that expression of the γHV68 v-cyclin promotes cell cycle progression in thymocytes.
FIG. 5.
BrdU pulse analysis of thymocyte cell cycle in γHV68–v-cyclin–TG mice. Two-color flow-cytometric analysis of in vivo-pulsed thymocytes at 2 h postinjection. FITC fluorescence (specific antibody staining) is indicated on the y axis, and PI fluorescence (cell cycle position) is indicated on the x axis. Dot plots of wild-type thymocytes are shown on the left, and γHV68–v-cyclin–TG thymocytes are shown on the right. Top panels are stained with an irrelevant isotype-matched antibody, and bottom panels are stained with a monoclonal antibody to BrdU. Numbers above representative dot plots are the mean percentages of BrdU-positive cells ± SEMs (n = 4).
Abnormalities of thymic T-cell development in γHV68–v-cyclin–TG mice.
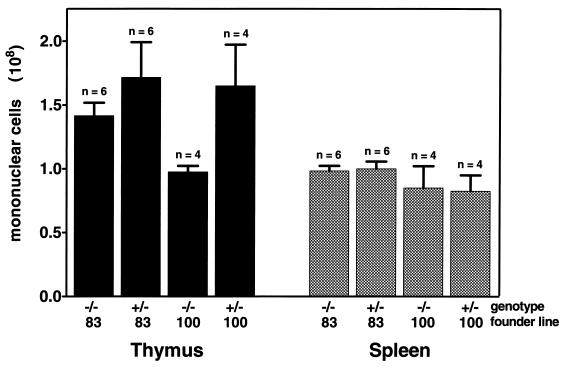
Consistent with our failure to detect v-cyclin expression in the spleen, cell counts revealed that the total numbers of mononuclear cells in the spleens of control and transgenic mice were similar (Fig. 6). However, despite v-cyclin expression (Fig. 2) and a four- to fivefold increase in the number of thymocytes in S/G2/M (Fig. 3 and 4), the total number of thymocytes was only somewhat higher (ca. 1.7-fold) in γHV68–v-cyclin–TG mice than in controls (Fig. 6).
FIG. 6.
Lymphocyte cellularity of thymus and spleen. The average total numbers of mononuclear cells ± the SEMs are shown as black bars (thymocytes) or as hatched bars (splenocytes). The total number of mice tested for each group is shown above the error bar; the average of six transgenic and six littermate controls for founder line no. 83 is shown, and the average of four transgenic and four littermate controls for founder line no. 100 is shown. Wild-type littermate controls and transgenics are indicated by −/− and +/− genotypes, respectively.
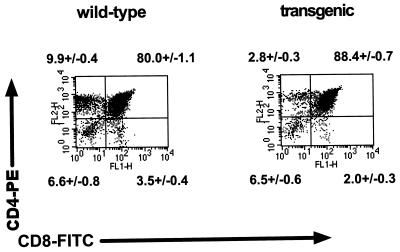
Because of the disparity between thymocyte numbers and thymocytes in S/G2/M of the cell cycle, we examined the differentiation state of thymocytes in v-cyclin-transgenic mice. Comparison of 13 transgenic mice with 13 littermate controls showed a transgene-associated increase in the mean number of CD4+ CD8+ (double-positive) thymocytes, from 80% ± 1.1% to 88.4% ± 0.7% (Fig. 7). This increase in double-positive cells was associated with a decrease in the mean number of single-positive CD4 T cells, from 9.9% ± 0.4% in controls to 2.8% ± 0.3% in transgenics, and a decrease in single-positive CD8 T cells, from 3.5% ± 0.4% in controls to 2.0% ± 0.3% in transgenics. There was no significant alteration in the proportion of double-negative thymocytes (Fig. 7). The transgene-associated increase in the proportion of double-positive cells was also apparent in absolute thymocyte numbers, which increased from 98 × 106 ± 7 × 106 to 150 × 106 ± 19 × 106. Likewise, the number of CD4 single-positive T cells in controls was 12.3 × 106 ± 1.0 × 106 and decreased to 4.3 × 106 ± 0.7 × 106 in transgenics. The number of single-positive CD8 thymocytes decreased very little, from 4.6 × 106 ± 0.7 × 106 in controls to 3.4 × 106 ± 0.3 × 106 in transgenics. The double-negative thymocyte numbers were relatively constant, with means of 9.3 × 106 ± 1.7 × 106 in controls and 10.6 × 106 ± 1.0 × 106 in transgenics.
FIG. 7.
T-cell development in the thymus of γHV68–v-cyclin–TG mice. Two-color flow-cytometric analyses of one representative experiment are displayed as dot plots of CD4-CD8 staining of wild-type (left panel) and γHV68–v-cyclin–TG (right panel) thymocytes. PE-CD4 staining is indicated on the y axis, and FITC-CD8 staining is indicated on the x axis. Numbers indicate the mean percentages ± SEMs (n = 13), double negatives are below the corresponding lower left quadrant, CD4 single positives are above the corresponding upper left quadrant, double positives are above the corresponding upper right quadrant, and CD8 single positives are below the corresponding lower right quadrant.
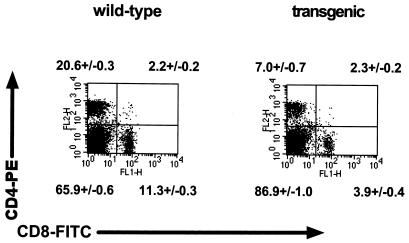
To determine whether changes in thymic T-cell development were reflected in peripheral lymphoid organs of γHV68–v-cyclin–TG mice, we examined T-cell populations in the spleen (Fig. 8). The mean percentage of single-positive CD4 splenocytes was decreased, from 20.6% ± 0.3% in controls to 7% ± 0.7% in transgenics. Similarly, the mean percentage of single-positive CD8 T cells in the spleen was decreased from 11.3% ± 0.3% in the control mice to 3.9% ± 0.4% in transgenics. The compensatory increase in the percentage of double-negative splenocytes was accounted for by an increase in the number of CD19-positive B cells (data not shown). These changes in percentages of single-positive CD4 and CD8 splenocytes were reflected in similarly decreased numbers of these cells, since the total numbers of splenocytes in control and transgenic mice did not differ (Fig. 6).
FIG. 8.
Cell subsets in the spleens of γHV68–v-cyclin–TG mice. Flow-cytometric dot plots from one representative experiment are shown for CD4-CD8 staining of wild-type (left panel) and γHV68–v-cyclin–TG (right panel) splenocytes. CD4-PE staining is indicated on the y axis, and CD8-FITC staining is indicated on the x axis. Representative dot plots are shown. Numbers indicate the mean percentages ± SEMs (n = 13); double negatives are below the corresponding lower left quadrant, CD4 single positives are above the corresponding upper left quadrant, double positives are above the corresponding upper right quadrant, and CD8 single positives are below the corresponding lower right quadrant.
TCRβ expression in γHV68–v-cyclin–TG mice.
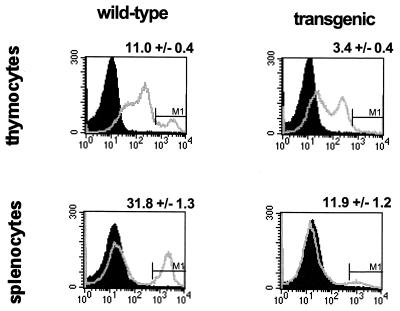
The above-described studies strongly suggested that thymocyte maturation was abnormal in γHV68–v-cyclin–TG mice. To confirm this, we examined levels of TCRβ expression in the thymi and spleens of control and v-cyclin-transgenic mice, using a monoclonal antibody that binds to all forms of the TCRβ (Fig. 9). Only mature T cells express a high level of TCRβ. The proportion of thymocytes expressing high levels of TCR was decreased from 11.0% ± 0.4% in control mice to 3.4% ± 0.4% in transgenic mice. The mean total number of TCRβhi thymocytes decreased from 13.4 × 106 in controls to 6.6 × 106 cells in transgenics, similar to the decrease in the mean total number of single-positive thymocytes (from 16.9 × 106 in controls to 7.7 × 106 cells in transgenics). Thus, the failure of thymocytes to differentiate normally was evidenced both by decreases in the numbers of single-positive CD4 and CD8 cells (Fig. 7 and 8) and by a similar decrease in the number of cells expressing high levels of the TCRβ (Fig. 9). This was paralleled by a decrease in the proportion of splenocytes expressing high levels of the TCRβ, from 31.8% ± 1.3% in control mice to 11.9% ± 1.2% in transgenic mice (Fig. 9). The possibility of an alteration in the γδ T-cell subset was not addressed.
FIG. 9.
TCRβ expression in spleens and thymi of γHV68–v-cyclin–TG mice. Flow-cytometric analyses of thymocytes (top panels) and splenocytes (bottom panels) are displayed as histograms of anti-TCRβ-PE fluorescence. Shaded peaks represent background fluorescence as measured with the irrelevant isotype matched control antibody; overlaid peaks represent TCRβ-specific fluorescence. Representative histograms are shown. The numbers above the FACS profiles represent the percentages of thymocytes or splenocytes expressing high levels of TCRβ ± SEMs (n = 12 for thymus; n = 12 for spleen). Wild-type lymphocytes are shown in the histograms on the left, and γHV68–v-cyclin–TG lymphocytes are shown on the right.
Thymic architecture in γHV68–v-cyclin–TG mice.
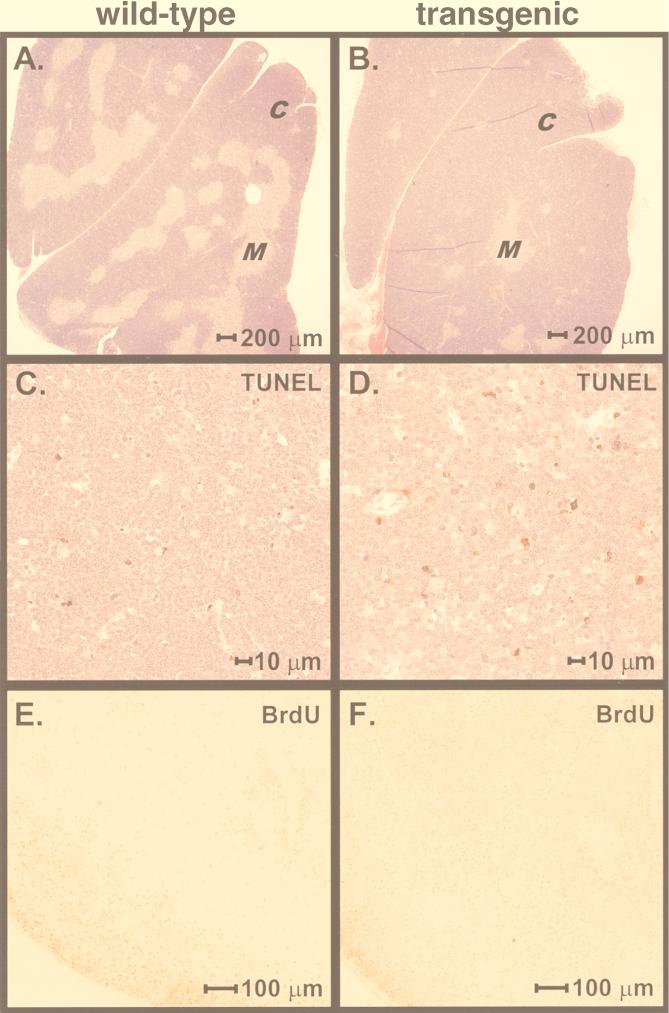
Abnormal thymic development was also evident upon examination of the thymic architecture. Littermate control thymi contained the expected ratio of cortex to medulla (Fig. 10A). In contrast, γHV68–v-cyclin–TG thymi showed an increase in the percentage of cortex and a greatly reduced medullary area (Fig. 10B). The proportions of thymic medulla and thymic cortex were quantitated by using image analysis software on random sections from the thymi of 10 γHV68–v-cyclin–TG mice and 10 control mice. In control mice, 78.4% ± 1.3% (mean ± SEM) of the thymus was cortex and 21.6% ± 2.6% was medulla. In contrast, γHV68–v-cyclin–TG mice had 93.4% ± 1.4% cortex and 6.6% ± 1.4% medulla. To ensure that the results were not biased by section selection, two control and two γHV68–v-cyclin–TG thymi were serially sectioned in their entirety. Quantitation of the cortical and medullary areas from these serial sections confirmed the data from random sections. Moreover, analysis of the total thymic area of these step sections showed no significant difference in the total thymic cross-sectional areas of control and transgenic mice, confirming the cell count data (Fig. 6) indicating that there was not significant thymic hyperplasia in γHV68–v-cyclin–TG mice. Staining with antibody to BrdU showed that cells synthesizing DNA were largely restricted to the peripheral cortex in normal mice but were distributed throughout the expanded cortex in γHV68–v-cyclin–TG mice (Fig. 10E and F). These data provided morphologic and histochemical confirmation of abnormal proliferative activity in γHV68–v-cyclin–TG mice and demonstrated an anatomic correlate of this increased proliferative activity.
FIG. 10.
Pathology, TUNEL, and BrdU staining of thymi from γHV68–v-cyclin–TG mice. (A and B) Representative hematoxylin- and eosin-stained sections of thymus. C, cortex; M, medulla. (A) Nontransgenic littermate control. (B) γHV68–v-cyclin–TG mouse. (C and D) Representative TUNEL-stained sections of thymus. TUNEL-positive cells are red, and background counterstaining is hemotoxylin. (C) Nontransgenic littermate control. (D) γHV68–v-cyclin–TG mouse. (E and F) Representative sections of thymus stained with antibody to BrdU. Mice were pulsed in vivo with BrdU for 2 h prior to sacrifice. The dark stain is the positive signal from the anti-BrdU antibody. (E) Nontransgenic littermate control. (F) γHV68–v-cyclin–TG mouse.
Analysis of proportion of thymocytes undergoing apoptosis in γHV68–v-cyclin–TG mice.
Our finding that the total number of thymocytes in γHV68–v-cyclin–TG mice did not increase proportionately to the increase in cycling thymocytes, combined with the decrease in mature T cells, raised the possibility that the increase in cycling cells observed in the thymus of γHV68–v-cyclin–TG mice was accompanied by an increased number of cells undergoing programmed cell death. We therefore evaluated the thymus by TUNEL to directly determine the number of thymocytes undergoing DNA fragmentation (Fig. 10C and D). In a blinded study, 10 individuals counted apoptotic cells in TUNEL-stained matched 40×-magnified microscopic fields of thymi from 10 wild-type littermates and 10 γHV68–v-cyclin–TG mice. We found that there was an increase (1.83-fold ± 0.10-fold) in the number of thymocytes undergoing apoptosis per microscopic field in the thymi of γHV68–v-cyclin–TG mice.
γHV68–v-cyclin–TG mice develop high-grade lymphoma.
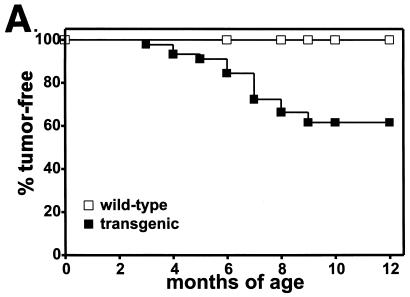
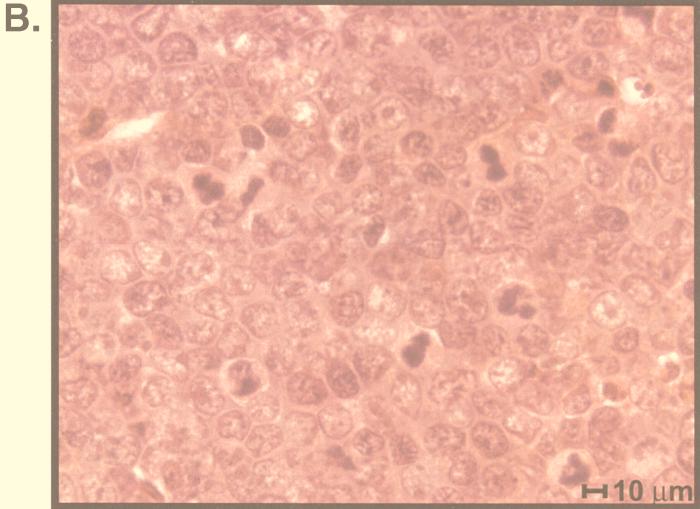
To determine whether expression of the pro-proliferative γHV68 v-cyclin can cause tumors, we followed a large cohort of transgenic mice and matched littermate controls over a period of up to 1 year. Mice that died, reached 12 months of age, or became ill were evaluated for tumors. Of a total of 38 transgenic mice, 17 developed tumors between 3 and 12 months of age (data for lymphoblastic lymphomas are shown in Fig. 11A). Fifteen of 17 tumors involved the thymus and were morphologically consistent with lymphoblastic lymphoma with a high mitotic index (Fig. 11B). Two of 17 tumors were plasmacytomas. None of the 34 matched littermate controls developed tumors. The difference between γHV68–v-cyclin–TG and control mice with regard to the development of lymphoblastic lymphoma was statistically significant (P < 0.0003). Tumor cell lines established from four mice with lymphoma were further characterized. All four grew well in culture and continued to express the γHV68 v-cyclin, as determined by Western blot analysis (data not shown). Two cell lines were characterized by FACS, and the immature phenotype of the tumor cells was confirmed by the expression of both CD4 and CD8 (data not shown). Two tumor lines derived independently from either the spleen or thymus of a mouse with lymphoma grew continuously for more than 2 months in culture. Cells from both the thymus- and spleen-derived lines were transferred into nine SCID mice. All mice developed tumors by 1 month, and these tumors were confirmed to be lymphoblastic lymphoma by histological analysis.
FIG. 11.
Incidence and pathology of lymphoblastic lymphoma in γHV68–v-cyclin–TG mice. (A) Thirty-eight γHV68–v-cyclin–TG mice from two different founders and 34 matched nontransgenic littermate controls were monitored for development of tumors for up to 1 year. Mice were evaluated at the time of death or sacrificed when ill, and tumor incidence was determined by autopsy and histologic analysis. Not all mice were followed for a full year. Statistical analysis took into account the fact that mice were monitored for different periods of time, and the difference between transgenic and nontransgenic mice was statistically significant (P < 0.0003). (B) Histopathology of tumors that arose in γHV68–v-cyclin–TG mice. A tumor from a mouse that died at 8 months of age is shown. Note the multiple mitotic figures.
DISCUSSION
The experiments reported here demonstrate that the γHV68 ORF72, identified by analysis of the γHV68 genomic sequence (70), is a bone fide gene encoding a protein of the predicted size which is expressed with leaky-late kinetics in lytically infected cells. The γHV68 v-cyclin promotes cell cycle progression in primary lymphocytes, as demonstrated by significant increases in the number of cycling thymocytes in γHV68–v-cyclin–TG mice. However, expression of the γHV68 v-cyclin does not result in increases in the number of thymocytes over the first few weeks of life, despite v-cyclin-induced cell cycle progression, but rather is associated with increased thymocyte apoptosis. One result of alterations in thymocyte cell cycle regulation is inhibition of normal T-cell differentiation. Importantly, expression of the γHV68 v-cyclin caused lymphoblastic lymphoma, identifying the v-cyclin gene as an oncogene.
The γHV68 v-cyclin promotes cell cycle progression in primary lymphocytes.
One of the primary goals of this study was to determine whether the γHV68 v-cyclin promotes cell cycle progression in primary lymphocytes. Expression of the γHV68 v-cyclin was sufficient to drive cell cycle progression in primary T lymphocytes, as evidenced by a four- to fivefold increase in cycling cells (assessed by PI incorporation and in vivo BrdU labeling). Notably, this effect on primary thymocytes was mediated by expression of the v-cyclin at a level comparable to that seen in lytically infected cells.
Our transgenic studies of the function of the γHV68 v-cyclin are consistent with cell culture and biochemical studies of related gammaherpesvirus v-cyclins. The KSHV and HVS v-cyclin–cdk complexes phosphorylate the retinoblastoma protein (pRb) and promote cell cycle progression in cultured cells (25, 31, 37). However, studies of the gammaherpesvirus v-cyclins have revealed several unusual properties. First, the proteins encoded by the v-cyclin ORFs lack the obvious LXCXE motif for pRb pocket binding that is found in mammalian D-type cyclins (21, 22, 70). However, with respect to this point, it should be noted that detailed transcript mapping of the v-cyclin mRNAs has not been carried out, and thus it is possible that the proteins encoded by the v-cyclin ORFs are extended by splicing and contain an LXCXE motif. Second, the HVS and KSHV v-cyclins interact (with various degrees of efficiency) with multiple cdk’s (25, 31, 37). Third, complexes of HVS and KSHV v-cyclins and cdk6 have a broader range of substrates than complexes of host D-type cyclins and cdk6, as demonstrated by the capacity of v-cyclin–cdk complexes to phosphorylate both pRb and histone H1 (25, 31, 37). Fourth, HVS and KSHV v-cyclin–cdk complexes are resistant to regulation by the cellular cyclin-cdk inhibitors p16Ink4a, p21Cip1, and p27Kip1 (25, 67). The broader substrate specificity, combined with resistance to inhibitors, raises the possibility that the gammaherpesvirus v-cyclins function at multiple stages in cell cycle progression and are themselves sufficient to drive lymphocytes to progress through the entire cell cycle, with consequent increases in lymphocyte numbers.
While the γHV68 v-cyclin increased the proportion of thymocytes in the cell cycle, there were not commensurate increases in the number of thymocytes. This argues that the γHV68 v-cyclin is not sufficient to drive cells through the entire cell cycle with a consequent increase in thymocytes. Consistent with this, we found that there was an increased rate of thymocyte apoptosis in γHV68–v-cyclin–TG mice, likely explaining why the significant increase in the proportion of cycling cells did not lead to significant thymic hyperplasia. One trivial explanation for this result is that during thymocyte maturation in the v-cyclin-transgenic mice, the lck proximal promoter is downregulated and, thus, v-cyclin expression drops, leading to thymocyte apoptosis. Alternatively, it is possible that expression of the v-cyclin is itself responsible for activating a cell death pathway via induction of premature S phase and E2F-dependent transcription (61, 79), as has been noted for overexpression of some mammalian cyclins (64). Finally, it is possible that the normal process of thymic selection and rapid apoptosis (66) of nonselected or negatively selected cells is responsible for eliminating cells driven into the cell cycle by the γHV68 v-cyclin.
We consider it likely that additional γHV68 gene products will regulate the cell cycle and/or apoptosis in the course of viral infection. In this scenario, the v-cyclin would promote cell cycle progression, but an additional gene product(s) would be required to protect cells from apoptotic death. This would be analogous to systems for regulating the cell cycle, and cell survival, utilized by other DNA viruses, such as adenovirus and human papillomavirus (HPV). For example, the adenovirus E1A protein induces host cell DNA synthesis as well as apoptosis, while the E1B gene-encoded 55- and 19-kDa proteins interfere with cell death (26, 75). Similarly, the HPV E7 protein binds pRb, induces host cell DNA synthesis, and results in apoptosis in the absence of the HPV E6 protein (68). In the γHV68 genome, one obvious candidate for protecting against apoptosis is the M11 gene, which, being similar to the gene encoding E1B 19K, is predicted to encode a protein homologous to members of the bcl-2 family (70). Studies to determine whether the γHV68 v-cyclin and v-bcl-2 can act together to promote lymphocyte proliferation and accelerate tumor development are under way.
Expression of v-cyclin during lytic γHV68 infection of fibroblasts.
The pattern of expression of gammaherpesvirus v-cyclins may provide clues to their functional roles. The HVS v-cyclin protein is expressed in transformed T cells (31), and the KSHV v-cyclin transcript is expressed during latent infection and in tumor cells (11, 13, 17, 19). Notably, in KSHV the adjacent gene, gene 73, encodes a protein that is expressed in spindle cells of Kaposi’s sarcoma (latency-associated nuclear antigen [LANA] [11, 34, 52]). KSHV gene 73 and its v-cyclin gene, along with gene 71 (encoding v-FLIP), form a transcriptional unit, driven by a single promoter, which gives rise to at least three distinct alternatively spliced transcripts, all of which are 3′ coterminal (19). Thus, transcriptional mapping studies show coregulation of the transcription of the KSHV v-cyclin gene and LANA-encoding gene 73, suggesting that the KSHV v-cyclin plays an important role during latent infection. While there may be some minimal upregulation of the KSHV v-cyclin-specific mRNA by phorbol esters (inducers of lytic gene expression), this is not impressive (19, 24, 58), and thus there is little evidence to support an important role for the KSHV v-cyclin in lytic infection.
In contrast to the KSHV-encoded v-cyclin, lytically infected NIH 3T12 fibroblasts transcribed γHV68 gene 72 and expressed a v-cyclin of ca. 25 kDa as a leaky-late protein, arguing that the γHV68 v-cyclin may be important in the lytic cycle. Studies of the role of the γHV68 v-cyclin in latent infection have not been performed. While we have detected γHV68 v-cyclin gene expression during lytic infection, we have found very little expression of gene 73 in lytically infected fibroblasts (71). Thus, in contrast to the situation with KSHV, in which both gene 73 and the v-cyclin gene appear to be transcribed during latent infection, gene 73 and the v-cyclin gene are independently regulated during lytic γHV68 infection. The genome structure of γHV68, which has the M11 gene (encoding a putative v-bcl-2) interposed in the opposite orientation between gene 73 and the v-cyclin gene, provides a further basis for believing that the γHV68 v-cyclin gene and gene 73 are independently regulated (70). It is tempting to speculate that the γHV68 v-cyclin will play an important role in reactivation from latency if some populations of latent cells are not in cell cycle. Studies of γHV68 v-cyclin mutants during acute infection, latent infection, and reactivation will be needed to determine the role of this protein in different phases of γHV68 pathogenesis.
Cell cycle regulation by herpesviruses.
What is the functional importance of expressing a cyclin that promotes cell cycle progression during γHV68 lytic infection? Substantial data from other herpesvirus systems provides a possible rationale for expression of a cyclin that promotes cell cycle progression. For EBV, transformation is associated with upregulation of D-type cyclins (3, 9, 29), and the latent membrane protein LMP-1 is thought to be a key regulator of D-type cyclins (3). Less is known about the regulation of the cell cycle during lytic infection by EBV. However, the Zta protein, which contributes to the switch from latent to lytic gene expression, inhibits cell cycle progression prior to S phase (10). For the alphaherpesvirus herpes simplex virus type 1, the important immediate-early protein ICP0 has been shown to interact with and stabilize the G1 regulatory cyclin D3 (33). ICP0 mutants are complemented by cellular factors that are active in the G1/S phase of the cell cycle (8), and viral replication is decreased by drugs that inhibit cdk’s involved in cell cycle progression through G1/S (60). For the alphaherpesvirus bovine herpesvirus 1 it has been argued that the latency-related gene (lrg) inhibits cell cycle progression through S phase (59). Infection with the betaherpesvirus human cytomegalovirus causes cell cycle arrest in G1/S (and perhaps G2/M) (7, 30, 40) (for reviews, see references 20 and 56). In addition, drugs that inhibit cdk’s inhibit human cytomegalovirus replication (6). Thus, for alpha-, beta-, and gammaherpesviruses, induction of the cell cycle (or at least induction of cellular responses associated with cell cycle progression) up to S phase and arrest of the cell cycle at G1/S appear to be important during lytic infection. One rationale for this is that viral DNA synthesis is optimal when the infected cell has made all the necessary preparations for replication of the host cell genome in S phase. It is tempting to speculate that the γHV68 v-cyclin gene is involved in inducing cell cycle progression from G1 to S and that additional γHV68 genes are responsible for preventing host cell DNA synthesis during lytic infection. Studies with γHV68 mutants that fail to express the v-cyclin will be needed to address this hypothesis and directly test the role of the γHV68 v-cyclin during lytic infection.
Developmental impact of v-cyclin-driven cell cycle progression on T-cell development.
While this study was designed to address the oncogenic potential of the γHV68 v-cyclin and to determine whether this protein is pro-proliferative in primary cells, an unexpected finding was that expression of the γHV68 v-cyclin interfered with normal T-cell development. Normal thymocyte development involves extensive cell proliferation, and cell cycle regulation is tied to T-cell development (27, 50) (for a review, see reference 23). Early TCRβ-positive thymocytes express on their surfaces the pre-TCR α-chain paired with the β-chain, and successful assembly of this complex is associated with significant proliferation. Subsequent steps in differentiation, including TCR α-chain rearrangement as well as positive and negative selection, result in single positive (CD4+ or CD8+) mature T cells (for reviews, see references 23, 41, 45 and 80). Expression of the γHV68 v-cyclin gene under the control of the lck proximal promoter resulted in an increase in double positive thymocytes and a decrease in single positive thymocytes. Consistent with the presence of double positive thymocytes, rearrangement of TCR β-chains appeared to be intact, as demonstrated by the presence of cells expressing low or intermediate levels of TCRβ. However, we observed a significant deficit in the number of thymocytes and splenocytes expressing high levels of the TCRβ and decreased numbers of singly positive thymocytes and splenocytes. This suggests a role for cell cycle control in the latter stages of thymocyte differentiation. Further studies will be required to define the mechanism underlying this defect in T-cell differentiation.
The γHV68 v-cyclin gene is an oncogene.
Transgenic studies have demonstrated the oncogenic potential of several gammaherpesvirus genes, including the HVS STP gene and the EBV genes EBNA1, EBNA2, and LMP-1 (35, 47, 69, 77, 78). This study adds the v-cyclin gene of the gammaherpesviruses to the list of genes that are oncogenic in the absence of additional viral gene products. It is interesting that EBV does not encode a D-type cyclin homolog but does upregulate cyclin D expression (9, 63) and that the EBV LMP-1 protein can independently upregulate cyclin D expression (3). Given the oncogenic potential of both EBV LMP-1 and the γHV68 v-cyclin, it is tempting to speculate that upregulation of an endogenous cyclin may contribute to oncogenesis by LMP-1. In this scenario, the related herpesviruses γHV68 and EBV may exploit the same pathway for tumor induction, with the mechanistic difference being that one upregulates an endogenous cyclin while the other encodes a homolog of the endogenous cyclin. Notably, it has also been recently shown that the walleye retroviruses (walleye dermal sarcoma virus and walleye epidermal hyperplasia viruses 1 and 2) all encode homologs of cellular D-type cyclins (36). Thus, two distinct families of viruses have independently usurped a key component of the cell cycle regulatory program, presumably to allow virus regulation of cell cycle progression.
ACKNOWLEDGMENTS
H.W.V. was supported by grant RPG-97-134-01-MBC from the American Cancer Society and by NIH grant RO1 CA74730. L.F.V. was supported by grant PF-4379 from the American Cancer Society. S.H.S. was supported by grants from the National Institutes of Health (R01 CA43143, R01 CA52004, R01 CA58524, and R01 CA74730).
We thank the members of the laboratories of H.W.V., S.H.S., David Leib, and Lynda Morrison for continued commentary on this project. We thank Darren Kreamalmeyer, Mike White, and Robert Schreiber for expert assistance in generation of transgenic mice. We thank Robin Lorenz for expertise in and suggestions with regard to histological staining. We thank Barry Sleckman and Beth Levine for critical reading of the manuscript.
REFERENCES
- 1.Ajchenbaum F, Ando K, DeCaprio J A, Griffin J D. Independent regulation of human D-type cyclin gene expression during G1 phase in primary human T lymphocytes. J Biol Chem. 1993;268:4113–4119. [PubMed] [Google Scholar]
- 2.Albrecht J-C, Nicholas J, Biller D, Cameron K R, Biesinger B, Newman C, Wittmann S, Craxton M A, Coleman H, Fleckenstein B, Honess R W. Primary structure of the herpesvirus saimiri genome. J Virol. 1992;66:5047–5058. doi: 10.1128/jvi.66.8.5047-5058.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arvanitakis L, Yaseen N, Sharma S. Latent membrane protein-1 induces cyclin D2 expression, pRb hyperphosphorylation, and loss of TGF-β-1-mediated growth inhibition on EBV-positive B cells. J Immunol. 1995;155:1047–1056. [PubMed] [Google Scholar]
- 4.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1987. p. 10.2.30. [Google Scholar]
- 5.Bodrug S E, Warner B J, Bath M L, Lindeman G J, Harris A W, Adams J M. Cyclin D1 transgene impedes lymphocyte maturation and collaborates in lymphomagenesis with the myc gene. EMBO J. 1994;13:2124–2130. doi: 10.1002/j.1460-2075.1994.tb06488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bresnahan W A, Boldogh I, Chi P, Thompson E A, Albrecht T. Inhibition of cellular Cdk2 activity blocks human cytomegalovirus replication. Virology. 1997;231:239–247. doi: 10.1006/viro.1997.8489. [DOI] [PubMed] [Google Scholar]
- 7.Bresnahan W A, Boldogh I, Thompson E A, Albrecht T. Human cytomegalovirus inhibits cellular DNA synthesis and arrests productively infected cells in late G1. Virology. 1996;224:150–160. doi: 10.1006/viro.1996.0516. [DOI] [PubMed] [Google Scholar]
- 8.Cai W, Schaffer P A. A cellular function can enhance gene expression and plating efficiency of a mutant defective in the gene for ICP0, a transactivating protein of herpes simplex virus type 1. J Virol. 1991;65:4078–4090. doi: 10.1128/jvi.65.8.4078-4090.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannell E J, Farrell P J, Sinclair A J. Epstein-Barr virus exploits the normal cell pathway to regulate Rb activity during the immortalisation of primary B-cells. Oncogene. 1996;13:1413–1421. [PubMed] [Google Scholar]
- 10.Cayrol C, Flemington E K. The Epstein-Barr virus bZIP transcription factor Zta causes G0/G1 cell cycle arrest through induction of cyclin-dependent kinase inhibitors. EMBO J. 1996;15:2748–2759. [PMC free article] [PubMed] [Google Scholar]
- 11.Cesarman E, Nador R G, Bai F, Bohenzky R A, Russo J J, Moore P S, Chang Y, Knowles D M. Kaposi’s sarcoma-associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi’s sarcoma and malignant lymphoma. J Virol. 1996;70:8218–8223. doi: 10.1128/jvi.70.11.8218-8223.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaffin K E, Beals C R, Wilkie T M, Forbush K A, Simon M I, Perlmutter R M. Dissection of thymocyte signaling pathways by in vivo expression of pertussis toxin ADP-ribosyltransferase. EMBO J. 1990;9:3821–3829. doi: 10.1002/j.1460-2075.1990.tb07600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang Y, Moore P S, Talbot S J, Boshoff C H, Zarkowska T, Godden-Kent D, Paterson H, Weiss R A, Mittnacht S. Cyclin encoded by KS herpesvirus. Nature. 1996;382:410. doi: 10.1038/382410a0. [DOI] [PubMed] [Google Scholar]
- 14.Cohn S M, Lieberman M W. The use of antibodies to 5-bromo-2′-deoxyuridine for the isolation of DNA sequences containing excision-repair sites. J Biol Chem. 1984;259:12456–12462. [PubMed] [Google Scholar]
- 15.Cosgrove D, Gray D, Dierich A, Kaufman J, Lemeur M, Benoist C, Mathis D. Mice lacking MHC class II molecules. Cell. 1991;66:1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- 16.Danielson P E, Forss-Petter S, Brow M A, Calavetta L, Douglass J, Milner R J, Sutcliffe J G. p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA. 1988;7:261–267. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- 17.Davis M A, Sturzl M A, Blasig C, Schreier A, Guo H G, Reitz M, Opalenik S R, Browning P J. Expression of human herpesvirus 8-encoded cyclin D in Kaposi’s sarcoma spindle cells. J Natl Cancer Inst. 1997;89:1868–1874. doi: 10.1093/jnci/89.24.1868. [DOI] [PubMed] [Google Scholar]
- 18.Dighe A S, Campbell D, Hsieh C-S, Clarke S, Greaves D R, Gordon S, Murphy K M, Schreiber R D. Tissue specific targeting of cytokine unresponsiveness in transgenic mice. Immunity. 1995;3:657–666. doi: 10.1016/1074-7613(95)90136-1. [DOI] [PubMed] [Google Scholar]
- 19.Dittmer D, Lagunoff M, Renne R, Staskus K, Haase A, Ganem D. A cluster of latently expressed genes in Kaposi’s sarcoma-associated herpesvirus. J Virol. 1998;72:8309–8315. doi: 10.1128/jvi.72.10.8309-8315.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dittmer D, Mocarski E S. Human cytomegalovirus infection inhibits G1/S transition. J Virol. 1997;71:1629–1634. doi: 10.1128/jvi.71.2.1629-1634.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dowdy S F, Hinds P W, Louie K, Reed S I, Arnold A, Weinberg R A. Physical interaction of the retinoblastoma protein with human D cyclins. Cell. 1993;73:499–511. doi: 10.1016/0092-8674(93)90137-f. [DOI] [PubMed] [Google Scholar]
- 22.Ewen M E, Sluss H K, Sherr C J, Matsushime H, Kato J-Y, Livingston D M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993;73:487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- 23.Fehling H J, von Boehmer H. Early αβ T cell development in the thymus of normal and genetically altered mice. Curr Opin Immunol. 1997;9:263–275. doi: 10.1016/s0952-7915(97)80146-x. [DOI] [PubMed] [Google Scholar]
- 24.Flore O, Gao S J. Effect of DNA synthesis inhibitors on Kaposi’s sarcoma-associated herpesvirus cyclin and major capsid protein gene expression. AIDS Res Hum Retroviruses. 1997;13:1229–1233. doi: 10.1089/aid.1997.13.1229. [DOI] [PubMed] [Google Scholar]
- 25.Godden-Kent D, Talbot S J, Boshoff C, Chang Y, Moore P, Weiss R A, Mittnacht S. The cyclin encoded by Kaposi’s sarcoma-associated herpesvirus stimulates cdk6 to phosphorylate the retinoblastoma protein and histone H1. J Virol. 1997;71:4193–4198. doi: 10.1128/jvi.71.6.4193-4198.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardwick J M. Virus-induced apoptosis. Adv Pharmacol. 1997;41:295–336. doi: 10.1016/s1054-3589(08)61063-7. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman E S, Passoni L, Crompton T, Leu T M, Schatz D G, Koff A, Owen M J, Hayday A C. Productive T-cell receptor beta-chain gene rearrangement: coincident regulation of cell cycle and clonality during development in vivo. Genes Dev. 1996;10:948–962. doi: 10.1101/gad.10.8.948. [DOI] [PubMed] [Google Scholar]
- 28.Hogan B, Costatini F, Lacy E. Manipulating the mouse embryo. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1998. [Google Scholar]
- 29.Hollyoake M, Stuhler A, Farrell P, Gordon J, Sinclair A. The normal cell cycle activation program is exploited during the infection of quiescent B lymphocytes by Epstein-Barr virus. Cancer Res. 1995;55:4784–4787. [PubMed] [Google Scholar]
- 30.Jault F M, Jault J-M, Ruchti F, Fortunato E A, Clark C, Corbeil J, Richman D D, Spector D H. Cytomegalovirus infection induces high levels of cyclins, phosphorylated Rb, and p53, leading to cell cycle arrest. J Virol. 1995;69:6697–6704. doi: 10.1128/jvi.69.11.6697-6704.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung J U, Stäger M, Desrosiers R C. Virus-encoded cyclin. Mol Cell Biol. 1994;14:7235–7244. doi: 10.1128/mcb.14.11.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katz J D, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74:1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 33.Kawaguchi Y, Van Sant C, Roizman B. Herpes simplex virus 1 α regulatory protein ICP0 interacts with and stabilizes the cell cycle regulator cyclin D3. J Virol. 1997;71:7328–7336. doi: 10.1128/jvi.71.10.7328-7336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kedes D H, Lagunoff M, Renne R, Ganem D. Identification of the gene encoding the major latency-associated nuclear antigen of the Kaposi’s sarcoma-associated herpesvirus. J Clin Invest. 1997;100:2606–2610. doi: 10.1172/JCI119804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kulwichit W, Edwards R H, Davenport E M, Baskar J F, Godfrey V, Raab-Traub N. Expression of the Epstein-Barr virus latent membrane protein 1 induces B cell lymphoma in transgenic mice. Proc Natl Acad Sci USA. 1998;95:11963–11968. doi: 10.1073/pnas.95.20.11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LaPierre L A, Casey J W, Holzschu D L. Walleye retroviruses associated with skin tumors and hyperplasias encode cyclin D homologs. J Virol. 1998;72:8765–8771. doi: 10.1128/jvi.72.11.8765-8771.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li M, Lee H, Yoon D-W, Albrecht J-C, Fleckenstein B, Neipel F, Jung J U. Kaposi’s sarcoma-associated herpesvirus encodes a functional cyclin. J Virol. 1997;71:1984–1991. doi: 10.1128/jvi.71.3.1984-1991.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lissy N A, Van Dyk L F, Becker-Hapak M, Vocero-Akbani A, Mendler J H, Dowdy S F. TCR antigen-induced cell death occurs from a late G1 phase cell cycle check point. Immunity. 1998;8:57–65. doi: 10.1016/s1074-7613(00)80458-6. [DOI] [PubMed] [Google Scholar]
- 39.Lovec H, Grzeschiczek A, Kowalski M B, Moroy T. Cyclin D1/bcl-1 cooperates with myc genes in the generation of B-cell lymphoma in transgenic mice. EMBO J. 1994;13:3487–3495. doi: 10.1002/j.1460-2075.1994.tb06655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu M, Shenk T. Human cytomegalovirus infection inhibits cell cycle progression at multiple points, including the transition from G1 to S. J Virol. 1996;70:8850–8857. doi: 10.1128/jvi.70.12.8850-8857.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marrack P, Kappler J. Positive selection of thymocytes bearing αβ T cell receptors. Curr Opin Immunol. 1997;9:250–255. doi: 10.1016/s0952-7915(97)80144-6. [DOI] [PubMed] [Google Scholar]
- 42.Matsushime H, Ewen M E, Strom D K, Kato J Y, Hanks S K, Roussel M F, Sherr C J. Identification and properties of an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D type G1 cyclins. Cell. 1992;71:323–334. doi: 10.1016/0092-8674(92)90360-o. [DOI] [PubMed] [Google Scholar]
- 43.Matsushime H, Roussel M F, Ashmun R A, Sherr C J. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell. 1991;65:701–713. doi: 10.1016/0092-8674(91)90101-4. [DOI] [PubMed] [Google Scholar]
- 44.Meyerson M, Harlow E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol Cell Biol. 1994;14:2077–2086. doi: 10.1128/mcb.14.3.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mittrucker H W, Pfeffer K, Schmits R, Mak T W. T-lymphocyte development and function in gene-targeted mutant mice. Immunol Rev. 1995;148:115–150. doi: 10.1111/j.1600-065x.1995.tb00096.x. [DOI] [PubMed] [Google Scholar]
- 46.Mueller A, Odze R, Jenkins T D, Shahsesfaei A, Nakagawa H, Inomoto T, Rustgi A K. A transgenic mouse model with cyclin D1 overexpression results in cell cycle, epidermal growth factor receptor, and p53 abnormalities. Cancer Res. 1997;57:5542–5549. [PubMed] [Google Scholar]
- 47.Murphy C, Kretschmer C, Biesinger B, Beckers J, Jung J U, Desrosiers R C, Muller-Hermelink H K, Fleckenstein B W, Ruther U. Epithelial tumors induced by a herpesvirus oncogene in transgenic mice. Oncogene. 1994;9:221–226. [PubMed] [Google Scholar]
- 48.Nakagawa H, Wang T C, Zukerberg L, Odze R, Togawa K, May G H, Wilson J, Rustgi A K. The targeting of the cyclin D1 oncogene by an Epstein-Barr virus promoter in transgenic mice causes dysplasia in the tongue, esophagus and forestomach. Oncogene. 1997;14:1185–1190. doi: 10.1038/sj.onc.1200937. [DOI] [PubMed] [Google Scholar]
- 49.Nicholas J, Cameron K R, Honess R W. Herpesvirus saimiri encodes homologs of G protein-coupled receptors and cyclins. Nature. 1992;355:362–365. doi: 10.1038/355362a0. [DOI] [PubMed] [Google Scholar]
- 50.Penit C, Lucas B, Vasseur F. Cell expansion and growth arrest phases during the transition from precursor (CD4−8−) to immature (CD4+8+) thymocytes in normal and genetically modified mice. J Immunol. 1995;154:5103–5113. [PubMed] [Google Scholar]
- 51.Puglielli M T, Woisetschlaeger M, Speck S H. oriP is essential for EBNA gene promoter activity in Epstein-Barr virus-immortalized lymphoblastoid cell lines. J Virol. 1996;70:5758–5768. doi: 10.1128/jvi.70.9.5758-5768.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rainbow L, Platt G M, Simpson G R, Sarid R, Gao S-J, Stoiber H, Herrington C S, Moore P S, Schulz T F. The 222- to 234-kilodalton latent nuclear protein (LNA) of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) is encoded by orf73 and is a component of the latency-associated nuclear antigen. J Virol. 1997;71:5915–5921. doi: 10.1128/jvi.71.8.5915-5921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robles A I, Larcher F, Whalin R B, Murillas R, Richie E, Gimenez-Conti I B, Jorcano J L, Conti C J. Expression of cyclin D1 in epithelial tissues of transgenic mice results in epidermal hyperproliferation and severe thymic hyperplasia. Proc Natl Acad Sci USA. 1996;93:7634–7638. doi: 10.1073/pnas.93.15.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rogers H W, Callery M P, Deck B, Unanue E R. Listeria monocytogenes induces apoptosis of infected hepatocytes. J Immunol. 1996;156:679–684. [PubMed] [Google Scholar]
- 55.Russo J J, Bohenzky R A, Chien M-C, Chen J, Yan M, Maddalena D, Parry J P, Peruzzi D, Edelman I S, Chang Y, Moore P S. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salvant B S, Fortunato E A, Spector D H. Cell cycle dysregulation by human cytomegalovirus: influence of the cell cycle phase at the time of infection and effects on cyclin transcription. J Virol. 1998;72:3729–3741. doi: 10.1128/jvi.72.5.3729-3741.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 58.Sarid R, Flore O, Bohenzky R A, Chang Y, Moore P S. Transcription mapping of the Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) genome in a body cavity-based lymphoma cell line (BC-1) J Virol. 1998;72:1005–1012. doi: 10.1128/jvi.72.2.1005-1012.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schang L M, Hossain A, Jones C. The latency-related gene of bovine herpesvirus 1 encodes a product which inhibits cell cycle progression. J Virol. 1996;70:3807–3814. doi: 10.1128/jvi.70.6.3807-3814.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schang L M, Phillips J, Schaffer P A. Requirement for cellular cyclin-dependent kinases in herpes simplex virus replication and transcription. J Virol. 1998;72:5626–5637. doi: 10.1128/jvi.72.7.5626-5637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shan B, Lee W-H. Deregulated expression of E2F-1 induces S-phase entry and leads to apoptosis. Mol Cell Biol. 1994;14:8166–8173. doi: 10.1128/mcb.14.12.8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shindler K S, Roth K A. Double immunofluorescent staining using two unconjugated primary antisera raised in the same species. J Histochem Cytochem. 1996;44:1331–1335. doi: 10.1177/44.11.8918908. [DOI] [PubMed] [Google Scholar]
- 63.Sinclair A J, Palmero I, Holder A, Peters G, Farrell P J. Expression of cyclin D2 in Epstein-Barr virus-positive Burkitt’s lymphoma cell lines is related to methylation status of the gene. J Virol. 1995;69:1292–1295. doi: 10.1128/jvi.69.2.1292-1295.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sofer-Levi Y, Resnitzky D. Apoptosis induced by ectopic expression of cyclin D1 but not cyclin E. Oncogene. 1996;13:2431–2437. [PubMed] [Google Scholar]
- 65.Soonpaa M H, Koh G Y, Pajak L, Jing S, Wang H, Franklin M T, Kim K K, Field L J. Cyclin D1 overexpression promotes cardiomyocyte DNA synthesis and multinucleation in transgenic mice. J Clin Investig. 1997;99:2644–2654. doi: 10.1172/JCI119453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Surh C D, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372:100–103. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- 67.Swanton C, Mann D J, Fleckenstein B, Neipel F, Peters G, Jones N. Herpes viral cyclin/Cdk6 complexes evade inhibition by CDK inhibitor proteins. Nature. 1997;390:184–187. doi: 10.1038/36606. [DOI] [PubMed] [Google Scholar]
- 68.Tommasino M, Crawford L. Human papillomavirus E6 and E7: proteins which deregulate the cell cycle. Bioessays. 1995;17:509–518. doi: 10.1002/bies.950170607. [DOI] [PubMed] [Google Scholar]
- 69.Tornell J, Farzad S, Espander-Jansson A, Matejka G, Isaksson O, Rymo L. Expression of Epstein-Barr nuclear antigen 2 in kidney tubule cells induce tumors in transgenic mice. Oncogene. 1996;12:1521–1528. [PubMed] [Google Scholar]
- 70.Virgin H W, IV, Latreille P, Wamsley P, Hallsworth K, Weck K E, Dal Canto A J, Speck S H. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J Virol. 1997;71:5894–5904. doi: 10.1128/jvi.71.8.5894-5904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Virgin H W, IV, Presti R M, Li X-Y, Liu C, Speck S H. Three distinct regions of the murine gammaherpesvirus 68 genome are transcriptionally active in latently infected mice. J Virol. 1999;73:2321–2332. doi: 10.1128/jvi.73.3.2321-2332.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang T C, Cardiff R D, Zukerberg L, Lees E, Arnold A, Schmidt E V. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature. 1994;369:669–671. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]
- 73.Weck K E, Barkon M L, Yoo L I, Speck S H, Virgin H W., IV Mature B cells are required for acute splenic infection, but not for establishment of latency, by murine gammaherpesvirus 68. J Virol. 1996;70:6775–6780. doi: 10.1128/jvi.70.10.6775-6780.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weck K E, Dal Canto A J, Gould J D, O’Guin A K, Roth K A, Saffitz J E, Speck S H, Virgin H W., IV Murine gammaherpesvirus 68 causes large vessel arteritis in mice lacking interferon-gamma responsiveness: a new model for virus induced vascular disease. Nat Med. 1997;3:1346–1353. doi: 10.1038/nm1297-1346. [DOI] [PubMed] [Google Scholar]
- 75.White E. Regulation of p53-dependent apoptosis by E1A and E1B. Curr Top Microbiol Immunol. 1995;199:34–58. [PubMed] [Google Scholar]
- 76.Wildin R S, Garvin A M, Pawar S, Lewis D B, Abraham K M, Forbush K A, Ziegler S F, Allen J M, Perlmutter R M. Developmental regulation of lck gene expression in T lymphocytes. J Exp Med. 1991;173:383–393. doi: 10.1084/jem.173.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wilson J B, Bell J L, Levine A J. Expression of Epstein-Barr virus nuclear antigen-1 induces B cell neoplasia in transgenic mice. EMBO J. 1996;15:3117–3126. [PMC free article] [PubMed] [Google Scholar]
- 78.Wilson J B, Weinberg W, Johnson R, Yuspa S, Levine A J. Expression of the BNLF-1 oncogene of Epstein-Barr virus in the skin of transgenic mice induces hyperplasia and aberrant expression of keratin 6. Cell. 1990;61:1315–1327. doi: 10.1016/0092-8674(90)90695-b. [DOI] [PubMed] [Google Scholar]
- 79.Wu X, Levine A J. p53 and E2F-1 cooperate to mediate apoptosis. Proc Natl Acad Sci USA. 1994;91:3602–3606. doi: 10.1073/pnas.91.9.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zuniga-Pflucker J C, Lenardo M J. Regulation of thymocyte development from immature progenitors. Curr Opin Immunol. 1996;8:215–224. doi: 10.1016/s0952-7915(96)80060-4. [DOI] [PubMed] [Google Scholar]