Abstract
Background
Cardiovascular disease (CVD) is the most important comorbidity in patients with chronic obstructive pulmonary disease (COPD). COPD exacerbations not only contribute to COPD progression but may also elevate the risk of CVD. This study aimed to determine whether COPD exacerbations increase the risk of subsequent CVD events using up to 15 years of prospective longitudinal follow‐up data from the COPDGene (Genetic Epidemiology of Chronic Obstructive Pulmonary Disease) study.
Methods and Results
The COPDGene study is a large, multicenter, longitudinal investigation of COPD, including subjects at enrollment aged 45 to 80 years with a minimum of 10 pack‐years of smoking history. Cox proportional hazards models and Kaplan‐Meier survival curves were used to assess the risk of a composite end point of CVD based on the COPD exacerbation rate. Frequent exacerbators exhibited a higher cumulative incidence of composite CVD end points than infrequent exacerbators, irrespective of the presence of CVD at baseline. After adjusting for covariates, frequent exacerbators still maintained higher hazard ratios (HRs) than the infrequent exacerbator group (without CVD: HR, 1.81 [95% CI, 1.47–2.22]; with CVD: HR, 1.92 [95% CI, 1.51–2.44]). This observation remained consistently significant in moderate to severe COPD subjects and the preserved ratio impaired spirometry population. In the mild COPD population, frequent exacerbators showed a trend toward more CVD events.
Conclusions
COPD exacerbations are associated with an increased risk of subsequent cardiovascular events in subjects with and without preexisting CVD. Patients with COPD experiencing frequent exacerbations may necessitate careful monitoring and additional management for subsequent potential CVD.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT00608764.
Keywords: cardiovascular events, chronic obstructive pulmonary disease, clinical epidemiology, COPD exacerbation, preserved ratio impaired spirometry
Subject Categories: Cardiovascular Disease, Epidemiology
Nonstandard Abbreviations and Acronyms
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- PRISm
preserved ratio impaired spirometry
Clinical Perspective.
What Is New?
Frequent chronic obstructive pulmonary disease exacerbators show increased risk of subsequent cardiovascular events in subjects with and without preexisting cardiovascular disease.
What Are the Clinical Implications?
To reduce the risk of cardiovascular events in this patient population, careful monitoring and optimal management for chronic obstructive pulmonary disease exacerbations should be undertaken.
Cardiovascular disease (CVD) and chronic obstructive pulmonary disease (COPD) are leading global causes of death. 1 CVD represents the most prevalent comorbidity in patients with COPD, affecting up to 70% of patients with COPD. 2 , 3 Patients with COPD exhibit an elevated risk for CVD, independent of smoking status or the Global Initiative for Chronic Obstructive Lung Disease (GOLD) spirometric grade, compared with those without COPD. 4 Additionally, prior studies suggest that patients with COPD face an increased risk not only for CVD but also for cardiovascular risk factors such as diabetes and hypertension. 5 , 6 The presence of CVD in patients with COPD is associated with heightened risk of hospitalization for COPD, extended length of hospital stay, and increased all‐cause and CVD‐related mortality. 5 , 6 , 7 Conversely, in hypertensive patients, COPD has been found to increase the risk of not only myocardial infarction but also cerebrovascular diseases such as stroke. 8 Because the presence of both COPD and CVD leads to worse outcomes, effective management of comorbidities in patients with COPD is crucial, as emphasized in guidelines to improve overall patient survival. 9 , 10 , 11 COPD exacerbations significantly contribute to the COPD burden, with moderate to severe exacerbations linked to declining lung function and substantial health care costs. 3 , 12 Therefore, elucidating the relationship between COPD exacerbations and CVD risk is essential for improved management of these patients.
Although several studies have demonstrated an association between COPD exacerbations and CVD, they possess limitations. 13 , 14 , 15 , 16 For instance, some studies, such as the SUMMIT (Study to Understand Mortality and Morbidity Trial) trial, exclusively included patients with COPD with CVD or high CVD risk. 15 Furthermore, these previous studies focused on CVD events within individuals before and after COPD exacerbations, without specifically investigating the difference in CVD risk between populations with frequent versus infrequent exacerbations. 14 , 15 , 16 To overcome these limitations, we used up to 15 years of prospective longitudinal follow‐up data from the COPDGene (Genetic Epidemiology of Chronic Obstructive Pulmonary Disease) study, a large, longitudinal, epidemiological cohort enriched for COPD subjects. This allowed us to identify the temporal relationship between COPD exacerbations and cardiovascular events by meticulously tracking the period from patient enrollment to the initial cardiovascular event, along with an assessment of COPD exacerbation frequency. We hypothesized that COPD exacerbations increase the risk of subsequent CVD events.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
The COPDGene study is a prospective, multicenter, longitudinal cohort investigation exploring the epidemiology, genetics, and natural history of COPD across 21 centers in the United States (clinicaltrial.gov identifier: NCT00608764, www.copdgene.org). 17 , 18 The study enrolled non‐Hispanic White and Black participants aged 45 to 80 years with a minimum of 10 pack‐years of cigarette smoking history. In the eligibility assessment for the study, participants self‐reported their ethnicity (Hispanic/Latino or non‐Hispanic/Latino) and race (Black/African American, White, American Indian/Alaska Native, Asian, and Pacific Islander). 19 The smoking criterion was chosen because a significant smoking history is closely associated with a higher risk of developing COPD. The COPDGene study aims to uncover genetic, other biological, and environmental factors contributing to COPD susceptibility and progression. The study includes a large number of participants from these 2 racial backgrounds to investigate potential differences in COPD prevalence, heterogeneity, progression, and comorbidities. 20 All participants provided written informed consent, and institutional review board approval was obtained at each study center. Subjects were recruited to represent the full range of spirometric abnormalities as well as normal spirometry, and then were classified by the GOLD spirometric grading system. Normal spirometry was characterized by a postbronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio ≥0.7 and FEV1 ≥80% predicted. GOLD spirometry grades 1 to 4 were defined as follows:
GOLD 1: FEV1/FVC <0.70 and postbronchodilator FEV1 ≥80% predicted.
GOLD 2: FEV1/FVC <0.70 and postbronchodilator FEV1 between 50% and 79% predicted.
GOLD 3: FEV1/FVC <0.70 and postbronchodilator FEV1 between 30% and 49% predicted.
GOLD 4: FEV1/FVC <0.70 and postbronchodilator FEV1 <30% predicted.
In addition to GOLD grades 1 to 4, we analyzed subjects with preserved ratio impaired spirometry (PRISm), defined as a postbronchodilator FEV1/FVC ratio ≥0.70 but with FEV1 <80% predicted. 21 We included COPDGene data from the baseline visit (visit 1) and the longitudinal follow‐up. Enrolled subjects were contacted every 6 months through the COPDGene Longitudinal Follow‐Up program via phone or online survey to prospectively gather data on incident COPD‐related events, comorbidities, and death. COPD‐related events and comorbidities encompassed self‐reported COPD exacerbations (defined as acute worsening of respiratory symptoms necessitating treatment with systemic steroids and/or antibiotics), severe exacerbations (defined as any exacerbation requiring an emergency room visit and/or hospital admission), and CVD events (a composite end point including coronary artery disease, stroke, heart attack indicating myocardial infarction, coronary artery angioplasty, coronary artery bypass graft surgery, and peripheral artery disease). The target population was divided into 2 groups: frequent exacerbators (≥1 exacerbation per year) and infrequent exacerbators (<1 exacerbation per year).
Statistical Analysis
The primary outcome variables of interest were incidence of a composite end point of CVD and time until the first cardiovascular event. We further analyzed the incidence of individual types of cardiovascular disease events. Each analysis was performed on 2 groups, categorized based on whether they had CVD at baseline. The predictor was the dichotomous indicator variable for exacerbation frequency (<1 versus ≥1 exacerbation per year). Because cardiovascular events may affect incidence of COPD exacerbations, we used prospective longitudinal follow‐up survey data to calculate the annual rate of exacerbations from the phase 1 visit until the occurrence of the first cardiovascular event.
The subject's baseline characteristics and comorbidity prevalence rates were calculated, and we performed χ2 tests for categorical variables and Wilcoxon rank sum tests for continuous variables to analyze differences between the subgroups. We generated survival curves using the Kaplan‐Meier method and used Cox proportional hazards models for data analysis. The Cox models were adjusted for relevant baseline demographic and clinical characteristics. In assessing the risk of cardiovascular events, we adjusted for age, sex, race, body mass index, current smoking status, pack‐years of cigarette smoking, self‐reported diabetes, postbronchodilator FEV1 as a percentage of predicted, self‐reported hypertension, and self‐reported hypercholesterolemia. We checked whether our analysis meets the proportional hazard assumption (Table S1). In addition, we performed a competing risks analysis using the Fine‐Gray model, which models the subdistribution hazard for the cardiovascular event in the presence of the competing death event (n=1793). We adjusted for the same covariates used for the Cox models. The results are presented as subdistribution hazard ratios (HRs) with 95% CIs and P values. Statistical significance was defined as P < 0.05. The data were analyzed with R (version 4.1.0) in R Studio Pro Server.
Results
Study Subjects and Baseline Characteristics
Figure 1 displays the CONSORT (Consolidated Standards of Reporting Trials) diagram of study participants, with a median follow‐up duration of 9.6 years among included subjects. A total of 10 652 eligible subjects were enrolled in COPDGene Phase I. Subjects who underwent lung transplantation or lung volume reduction surgery, or who lacked longitudinal follow‐up data, were excluded. Furthermore, subjects with normal spirometry were excluded. The remaining subject group (n=5083) was divided into subgroups based on spirometric values. Each subgroup was further categorized into groups with or without CVD at baseline (Table 1).
Figure 1. Study flow diagram.
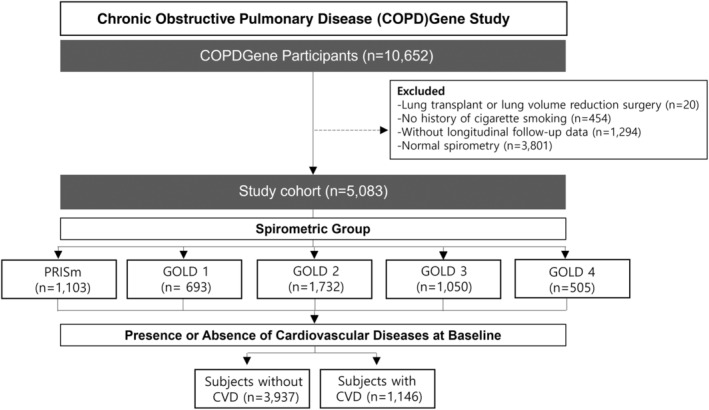
From all eligible participants (n=10 652) enrolled in COPDGene Phase I, those who subsequently underwent lung transplantation or lung volume reduction surgery, who had no smoking history, or who lacked longitudinal follow‐up data, were excluded. Furthermore, subjects with normal baseline spirometry (n=3801) were also excluded. The remaining study population (n=5083) was subdivided based on spirometry and GOLD spirometric classification. Each subgroup was further classified into groups with or without CVD at baseline. CVD includes coronary artery disease, congestive heart failure, stroke, transient ischemic attack, peripheral vascular disease, angina, heart attack, and history of coronary artery angioplasty or coronary artery bypass graft surgery. COPD indicates chronic obstructive pulmonary disease; COPDGene, Genetic Epidemiology of Chronic Obstructive Pulmonary Disease; CVD, cardiovascular disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; and PRISm, preserved ratio impaired spirometry.
Table 1.
Baseline Characteristics of Study Subjects With and Without CVD at Baseline
| Characteristic | Subjects without CVD at baseline (n=3937) | Subjects with CVD at baseline (n=1146) | P value* |
|---|---|---|---|
| Age, y, mean±SD | 61±9 | 65±8 | <0.001 |
| Women, n (%) | 1984 (50%) | 424 (37%) | <0.001 |
| Race, n (%) | <0.001 | ||
| Non‐Hispanic White | 2908 (74%) | 918 (80%) | |
| Black | 1029 (26%) | 228 (20%) | |
| BMI, mean±SD | 29±6 | 30±7 | <0.001 |
| Spirometric group, n (%) | <0.001 | ||
| PRISm | 868 (22%) | 235 (21%) | |
| GOLD 1 | 575 (15%) | 118 (10%) | |
| GOLD 2 | 1336 (34%) | 396 (35%) | |
| GOLD 3 | 776 (20%) | 274 (24%) | |
| GOLD 4 | 382 (9.7%) | 123 (11%) | |
| FEV1% pred, post‐BD | 61±21 | 57±20 | <0.001 |
| FEV1/FVC, post‐BD | 0.58±0.16 | 0.57±0.15 | 0.015 |
| Current smokers, n (%) | 1875 (48%) | 403 (35%) | <0.001 |
| Smoking history, pack‐years, mean±SD | 48±25 | 57±31 | <0.001 |
| Hypertension, n (%) | 1706 (43%) | 781 (68%) | <0.001 |
| Hypercholesterolemia, n (%) | 1440 (37%) | 749 (65%) | <0.001 |
| Diabetes, n (%) | 443 (11%) | 280 (24%) | <0.001 |
BMI indicates body mass index; CVD, cardiovascular disease; FEV1, forced expiratory volume in 1 second; FEV1% pred, percent predicted value of forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; post‐BD, postbronchodilator; and PRISm, preserved ratio impaired spirometry.
Wilcoxon rank sum test for continuous variables or Pearson χ2 test for categorical variables.
In Table S2, which presents baseline characteristics of subjects without a history of cardiovascular disease at study entry, frequent exacerbators were slightly older, exhibited a higher proportion of non‐Hispanic White subjects, and had more severe COPD than infrequent exacerbators. Frequent exacerbators were less likely to be current smokers, probably because more severe COPD subjects had lower rates of current smoking. No significant difference was observed in the proportion of other cardiovascular risk factors, such as hypertension, dyslipidemia, and diabetes between frequent and infrequent exacerbators not only in subjects without preexisting CVD, but also in subjects with CVD at baseline (Table S3).
Associations Between Moderate/Severe COPD Exacerbations and Subsequent Cardiovascular Events
Initially, we compared the incidence of cardiovascular events between subjects with and without previous CVD, which showed significant difference in the cumulative incidence of cardiovascular events between these groups across various subpopulations stratified by lung function abnormalities (Figure S1). We analyzed data from the full study population with impaired lung function, including GOLD 1 to 4 and PRISm subjects, dividing them into participants with and without CVD at baseline. Regardless of CVD presence at study enrollment, frequent exacerbators demonstrated a higher cumulative incidence of the composite CVD end point than infrequent exacerbators (without CVD: HR, 1.79 [95% CI, 1.47–2.17]; with CVD: HR, 1.84 [95% CI, 1.45–2.32]) (Figures 2A and 2B, Tables 2 and 3, Tables S4 and S5). After adjusting for covariates including age, sex, race, body mass index, current smoking status, FEV1% predicted, pack‐years, hypertension, dyslipidemia, and diabetes, frequent exacerbators still exhibited higher HRs than the infrequent group (without CVD: HR, 1.81 [95% CI, 1.47–2.22]; with CVD: HR, 1.92 [95% CI, 1.51–2.44]). Focusing on moderate to very severe patients with COPD (GOLD 2–4), similar results were observed as in the larger study population (without CVD: HR, 1.77 [95% CI, 1.41–2.21]; with CVD: HR, 1.80 [95% CI, 1.37–2.36]) (Figures 2C and 2D). After adjusting for covariates, HRs remained essentially unchanged (Tables 2 and 3). When severe exacerbation criteria were applied to the same analysis, higher HRs were observed for the moderate to very severe COPD group (GOLD 2–4) (without CVD: HR, 2.30 [95% CI, 1.60–3.31]; with CVD: HR, 1.97 [95% CI, 1.32–2.95]) compared with results using moderate exacerbation criteria (Figures 2E and 2F), and this trend did not change after adjusting for covariates (without CVD: HR, 2.32 [95% CI, 1.59–3.38]; with CVD: HR, 2.02 [95% CI, 1.33–3.06]).
Figure 2. Kaplan‐Meier plots for the cumulative incidence of a composite cardiovascular disease end point in the entire study population (GOLD 1–4 and PRISm) and in GOLD 2 to 4 subjects only.
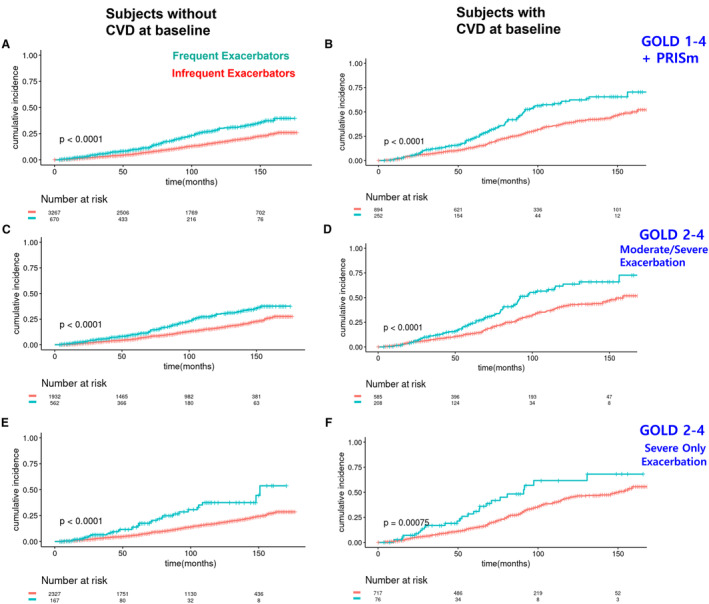
CVD events include coronary artery disease, stroke, heart attack, coronary artery angioplasty, coronary artery bypass graft surgery, and peripheral artery disease. Cumulative incidence in the GOLD 1 to 4 and PRISm populations without CVD at baseline (A) and with CVD at baseline (B). Cumulative incidence in the GOLD 2 to 4 subjects without CVD at baseline (C) and with CVD at baseline (D). Cumulative incidence in the GOLD 2 to 4 subjects without CVD at baseline, using a dichotomous indicator variable for only severe exacerbations (requiring emergency room visit and/or hospitalization) instead of moderate/severe exacerbations (E) and with CVD at baseline (F). The P values in the graphs represent the log‐rank test results. CVD indicates cardiovascular disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; and PRISm, preserved ratio impaired spirometry.
Table 2.
Association of Chronic Obstructive Pulmonary Disease Exacerbation Frequency With a Composite of Cardiovascular Events Among Participants Stratified by Lung Function Abnormality Without CVD at Baseline
| Groups | N | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Without CVD at baseline | |||||||
| GOLD 1–4+PRISm | 3937 | 1.79 | 1.47–2.17 | <0.001 | 1.81 | 1.47–2.22 | <0.001 |
| GOLD 2–4 | 2494 | 1.77 | 1.41–2.21 | <0.001 | 1.87 | 1.48–2.37 | <0.001 |
| GOLD 1 | 575 | 1.86 | 0.86–4.02 | 0.12 | 1.78 | 0.80–3.96 | 0.2 |
| PRISm | 868 | 1.81 | 1.06–3.09 | 0.030 | 1.93 | 1.12–3.35 | 0.018 |
Covariates include age, sex, race, body mass index, current smoking status, pack‐years of cigarette smoking, self‐reported diabetes, baseline postbronchodilator forced expiratory volume in 1 second as a percentage of predicted, self‐reported hypertension, and self‐reported hypercholesterolemia. HRs in the table indicate the ratio of hazard rate of the composite of cardiovascular events comparing frequent to infrequent exacerbators. The reference group was the infrequent exacerbators in each model. CVD indicates cardiovascular disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HR, hazard ratio; and PRISm, preserved ratio impaired spirometry.
Table 3.
Association of Chronic Obstructive Pulmonary Disease Exacerbation Frequency With a Composite of Cardiovascular Events Among Participants Stratified by Lung Function Abnormality With CVD at Baseline
| Groups | Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| N | HR | 95% CI | P value | HR | 95% CI | P value | |
| With CVD at baseline | |||||||
| GOLD 1–4+PRISm | 1146 | 1.84 | 1.45–2.32 | <0.001 | 1.92 | 1.51–2.44 | <0.001 |
| GOLD 2–4 | 793 | 1.80 | 1.37–2.36 | <0.001 | 1.87 | 1.41–2.47 | <0.001 |
| GOLD 1 | 118 | 1.64 | 0.64–4.18 | 0.3 | 2.05 | 0.73–5.79 | 0.2 |
| PRISm | 235 | 2.03 | 1.19–3.47 | 0.010 | 2.73 | 1.54–4.86 | <0.001 |
HRs in the table indicate the ratio of hazard rate of the composite of cardiovascular events comparing frequent to infrequent exacerbators. The reference group was the infrequent exacerbators in each model. CVD indicates cardiovascular disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HR, hazard ratio; and PRISm, preserved ratio impaired spirometry.
Associations Between Moderate/Severe Exacerbations and Subsequent Cardiovascular Events in PRISm and GOLD 1 Subjects
In the PRISm population analysis, a similar trend was observed, showing that frequent exacerbators had a higher incidence of composite CVD events than infrequent exacerbators (without CVD: HR, 1.81 [95% CI, 1.06–3.09]; with CVD: HR, 2.03 [95% CI, 1.19–3.47]) (Figures 3A and 3B, Tables 2 and 3, Tables S4 and S5). After adjusting for covariates, multivariable HRs for the PRISm population were higher than univariable HRs (without CVD: HR, 1.93 [95% CI, 1.12–3.35]; with CVD: HR, 2.73 [95% CI, 1.54–4.86]). In the mild COPD (GOLD 1) population, frequent exacerbators exhibited a trend toward increased cardiovascular events, but the results were not statistically significant (without CVD: HR, 1.86 [95% CI, 0.86–4.02]; with CVD: HR, 1.64 [95% CI, 0.64–4.18]) (Figures 3C and 3D, Tables 2 and 3), and covariate adjustment did not substantially change these results.
Figure 3. Kaplan‐Meier plots for the cumulative incidence of a composite cardiovascular end point separately in the GOLD 1 and the PRISm populations.
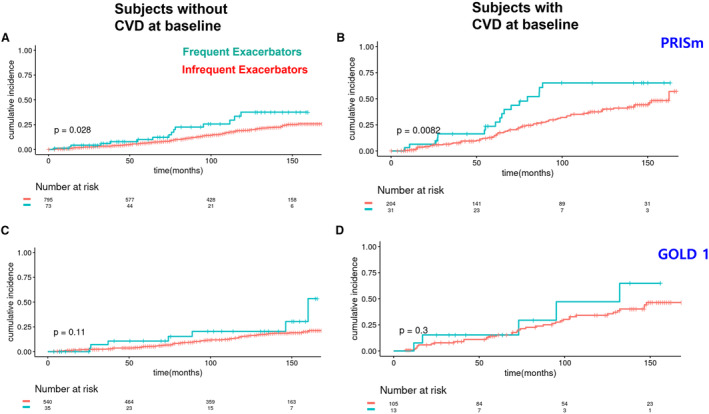
Cumulative incidence in the PRISm subjects without CVD at baseline (A) and with CVD at baseline (B). Cumulative incidence in the GOLD 1 subjects without CVD at baseline (C) and with CVD at baseline (D). The P values in the graphs are for the log‐rank test. Cardiovascular events include coronary artery disease, stroke, heart attack, coronary artery angioplasty, coronary artery bypass graft surgery, and peripheral artery disease. CVD indicates cardiovascular disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; and PRISm, preserved ratio impaired spirometry.
Associations Between Moderate/Severe Exacerbations and Subsequent Cardiovascular Events in Individual Cardiovascular Outcomes
As a sensitivity analysis, we conducted additional analyses using individual cardiovascular outcomes, such as coronary artery disease, heart attack, stroke, peripheral artery disease, coronary angioplasty, and coronary artery bypass graft surgery. The results demonstrate that individual cardiovascular outcomes yielded generally similar results to those observed with the composite cardiovascular event outcome, although statistical significance varied depending on each variable (Tables S6 and S7).
Because the occurrence of competing death events in this cohort precludes the occurrence of cardiovascular events, we performed competing risk analysis as an additional sensitivity analysis. The subdistribution HRs were estimated using a Fine‐Gray competing risks model, with cardiovascular events as the event of interest and death event as the competing event. The frequent exacerbators in the combined population of GOLD 1 to 4 and PRISm still had a significantly higher risk of subsequent CVD than infrequent exacerbators after adjusting for competing risk and other covariates (without CVD: HR, 1.46 [95% CI, 1.20–1.78]; with CVD: HR, 1.64 [95% CI, 1.32–2.05]) (Table S8 and S9).
Discussion
In this study, we examined the relationship between COPD exacerbations and subsequent cardiovascular events in a large prospective cohort of subjects with varying degrees of lung function impairment. We observed a higher HR for CVD events in frequent exacerbators compared with infrequent exacerbators in the entire study population and in subgroups of patients with moderate to very severe COPD (GOLD 2–4). This association persisted after adjusting for potential confounders and was consistent across individual cardiovascular outcomes. Moreover, a higher hazard ratio was observed when analyzing severe exacerbation criteria, suggesting a stronger association between severe exacerbations and cardiovascular events. In the PRISm population, frequent exacerbators had a higher incidence of composite CVD than infrequent exacerbators. After adjusting for covariates, the multivariable hazard ratios increased, indicating the robustness of this association. However, in the GOLD 1 population, the association between frequent exacerbators and cardiovascular events was not statistically significant, which may be due to a smaller sample size or the relatively low severity of COPD in this subgroup. In the analysis for individual cardiovascular outcomes, although we found a significant association between COPD exacerbations and each cardiovascular disease among individuals without CVD at baseline, the analysis for subjects with CVD at baseline resulted in a loss of significance, which may be due to the smaller number of subjects with preexisting CVD.
Previous studies that demonstrated a relationship between COPD exacerbations and CVD events included limited subgroups depending on the purpose of the study, and also analyzed specific CVD outcomes. 13 , 14 , 15 , 16 In contrast, our study not only incorporated subjects with varying severities of COPD, ranging from mild to very severe, but also included PRISm subjects, while examining a broad range of cardiovascular outcomes. In addition, we stratified the subjects based on the presence of CVD at baseline, whereas other studies included subjects with a previous CVD event. 15 , 22
Moreover, many of those earlier clinical trials were designed to observe the impact of COPD medication on outcomes, necessitating the use of prespecified drugs in all instances. 15 , 16 In contrast, our study, reflecting real‐world practices, includes subjects who are treated with standard clinical approaches depending on their condition, suggesting that this study could reflect the natural course of COPD. In addition, as previously described, although the earlier studies compared CVD risk before and after COPD exacerbation within individuals, 23 this study compared the subsequent risk of CVD events between frequent versus infrequent exacerbators. Furthermore, because CVD events could potentially influence COPD exacerbations, our study enhanced its accuracy by restricting the detection period to the time preceding the first CVD event after COPDGene enrollment.
Association Between COPD Exacerbations and Subsequent CVD in the PRISm Population
The term PRISm refers to individuals who have a preserved FEV1/FVC ratio, but their FEV1% predicted is reduced. This physiological abnormality has multiple potential causes, including restrictive lung disease (eg, interstitial lung disease, neuromuscular weakness, chest wall abnormalities, and obesity) or airflow obstruction (eg, COPD). 11 , 21 Although this heterogenous population has respiratory symptoms and a higher risk of developing COPD in the future, a significant portion of these PRISm patients either maintain their current condition or return to normal spirometry. 11 , 24 , 25 The connection between the PRISm population and CVD is not yet understood, but some studies suggest that individuals with PRISm have a higher risk of CVD compared with those with normal lung function. 26 , 27 , 28 , 29 , 30 , 31 This increased risk may be attributable to shared risk factors for both COPD and CVD, such as smoking, aging, and inflammation. 32 , 33 The presence of respiratory symptoms and reduced lung function in the PRISm population could also contribute to the development of CVD by increasing the workload on the heart or through systemic inflammation. 32 To the best of our knowledge, our study is the first study showing the association between COPD exacerbations and subsequent cardiovascular events in the PRISm population, suggesting that the findings in our study could be used to determine the most effective strategies for identifying and managing CVD risk in these individuals. Identifying and treating PRISm when effective therapies become available may help prevent or delay the progression of both COPD and CVD, ultimately improving the health outcomes for these patients.
Potential Underlying Mechanisms Linking COPD Exacerbations and CVD
COPD and CVD have many shared risk factors such as smoking and advanced age, which contribute to endothelial dysfunction. 34 Endothelial dysfunction fundamentally contributes to the development of atherosclerosis that finally leads to ischemic heart disease. 35 The mechanism by which COPD increases CVD risk are not clear, but patients with COPD often display abnormally high concentrations of circulating systemic inflammatory biomarkers such as CRP (C‐reactive protein), IL‐6 (interleukin‐6), and fibrinogen, which may contribute to the development and progression of atherosclerosis. 36 , 37 , 38 During a COPD exacerbation, the release of the above‐mentioned proinflammatory cytokines and other mediators in the lungs can spill over into the bloodstream, leading to a transient deterioration of endothelial function and increased inflammation status throughout the body. 38 This could translate into an increased risk for macrovascular complications such as myocardial infarction and stroke. 39 In addition, hypoxemia derived from COPD exacerbation can stress the heart, increase blood pressure, and promote the formation of blood clots, all of which increase the risk of cardiovascular events. 40 Oxidative stress during COPD exacerbations could not only damage cells, leading to inflammation, but also increase platelet activity, resulting in increased risk of thrombus formation. 41 Drugs used to treat COPD (eg, macrolide antibiotics) can have cardiac side effects, and physicians may avoid prescribing effective cardiac medications (eg, β‐blockers) to patients with COPD due to concerns about adverse events. All of these factors may enable COPD exacerbations to increase the risk of acute cardiovascular outcomes, but further research will be required to define which mechanisms are most important in specific groups of patients with COPD. 42
Clinical Consideration in the Treatment of COPD and CVD
Despite the known strong relationship between COPD and CVD, numerous cases of underdiagnosis and undertreatment of CVD in patients with COPD and vice versa persist. 43 For instance, 1 study showed that many patients undergoing coronary intervention are not diagnosed with concurrent COPD. 44 , 45 Additionally, in 70% of patients experiencing acute COPD exacerbations, electrocardiographic indicators of prior myocardial infarction remain undetected. 4 This challenge primarily impacts early or moderate COPD stages, during which enhanced preventative and therapeutic measures are still feasible. 2 Existing current guidelines largely focus on individual cardiac or respiratory diseases. 11 , 46 , 47 However, an integrated approach is necessary, particularly given the limited long‐term data on patients with both COPD and CVD. As life expectancy increases, a growing number of patients with COPD undergo high‐risk noninvasive procedures like transfemoral aortic valve replacement. Without appropriate management for both COPD and CVD, these procedures could result in fatal cardiovascular events. 48 , 49 By considering the relationship between COPD and CVD, proper patient management could help minimize adverse events associated with both conditions.
In patients with COPD, exacerbation events are known to contribute to COPD progression. 50 , 51 , 52 Clinically, 2 strategies can help prevent or decrease subsequent CVD events after exacerbation. First, once an exacerbation event occurs in patients with COPD, active monitoring for subsequent cardiovascular events and proper management of exacerbations are recommended. Second, identifying patients at risk of exacerbation could be clinically valuable, because it may enable earlier intervention for COPD exacerbation itself to limit COPD progression and reduce its impact on cardiovascular outcomes.
Study Limitations and Strengths
Our study has several limitations, including the use of self‐reported cardiovascular outcomes, which may lead to biases. Participation in the COPDGene Longitudinal Follow‐Up Program was variable, so comprehensive exacerbation and CVD data were not available in all study participants. In addition, although there are several suggested subtypes of COPD that may have differences in specific inflammatory pathways, we did not analyze these subtypes. Because our cohort lacks the information on causes of hospitalization, more research is needed to fully understand its natural history and the best approaches for management and treatment using these kinds of data in the future. Nonetheless, our study also has several strengths, such as its large, multicenter sample and longitudinal study design that meticulously captured exacerbation data over a 15‐year period. Our study encompassed patients with COPD both with and without CVD, facilitating a comprehensive analysis of the patients with COPD. Our sensitivity analysis using individual cardiovascular outcomes further supported our primary findings, with results consistent with those observed for the composite cardiovascular outcome, indicating that the relationship between COPD exacerbations and cardiovascular events is not driven by a single type of event.
Conclusions
Our study provides robust evidence for an association between COPD exacerbations and subsequent cardiovascular events in subjects with varying degrees of lung function impairment. This underscores the importance of optimal management of COPD exacerbations to reduce the risk of cardiovascular events in this patient population. Future studies should investigate the underlying mechanisms driving this association and evaluate potential strategies for mitigating the risk of cardiovascular events among patients with frequent COPD exacerbations.
Source of Funding
The COPDGene study (NCT00608764) is supported by grants from the National Heart, Lung, and Blood Institute (U01HL089897 and U01HL089856), by National Institutes of Health contract 75N92023D00011, and by the COPD Foundation through contributions made to an Industry Advisory Committee that has included AstraZeneca, Bayer Pharmaceuticals, Boehringer‐Ingelheim, Genentech, GlaxoSmithKline, Novartis, Pfizer, and Sunovion. This study was also supported in part by grants from the National Heart, Lung, and Blood Institute (R01HL133135, R01HL147148, P01HL114501, and R01HL152728). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of data; preparation, review, or approval of this article.
Disclosures
In the past three years, E.K.S. has received grant support from Bayer and Northpond Laboratories. M.T.D. has received grant support from Department of Defense, American Lung Association, and NIH. C.P.H. has received grant support from Alpha‐1 Foundation, Bayer, Boehringer‐Ingelheim, and Vertex. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Supporting information
Tables S1–S9
Figure S1
Appendix A.
COPDGene Investigators
Core Units: Administrative Center: James D. Crapo, MD (Principal Investigator); Edwin K. Silverman, MD, PhD (Principal Investigator); Barry J. Make, MD; Elizabeth A. Regan, MD, PhD. Genetic Analysis Center: Terri H. Beaty, PhD; Peter J. Castaldi, MD, MSc; Michael H. Cho, MD, MPH; Dawn L. DeMeo, MD, MPH; Adel El Boueiz, MD, MMSc; Marilyn G. Foreman, MD, MS; Auyon Ghosh, MD; Lystra P. Hayden, MD, MMSc; Craig P. Hersh, MD, MPH; Jacqueline Hetmanski, MS; Brian D. Hobbs, MD, MMSc; John E. Hokanson, MPH, PhD; Wonji Kim, PhD; Nan Laird, PhD; Christoph Lange, PhD; Sharon M. Lutz, PhD; Merry‐Lynn McDonald, PhD; Dmitry Prokopenko, PhD; Matthew Moll, MD, MPH; Jarrett Morrow, PhD; Dandi Qiao, PhD; Elizabeth A. Regan, MD, PhD; Aabida Saferali, PhD; Phuwanat Sakornsakolpat, MD; Edwin K. Silverman, MD, PhD; Emily S. Wan, MD; Jeong Yun, MD, MPH. Imaging Center: Juan Pablo Centeno; Jean‐Paul Charbonnier, PhD; Harvey O. Coxson, PhD; Craig J. Galban, PhD; MeiLan K. Han, MD, MS; Eric A. Hoffman, Stephen Humphries, PhD; Francine L. Jacobson, MD, MPH; Philip F. Judy, PhD; Ella A. Kazerooni, MD; Alex Kluiber; David A. Lynch, MB; Pietro Nardelli, PhD; John D. Newell Jr., MD; Aleena Notary; Andrea Oh, MD; Elizabeth A. Regan, MD, PhD; James C. Ross, PhD; Raul San Jose Estepar, PhD; Joyce Schroeder, MD; Jered Sieren; Berend C. Stoel, PhD; Juerg Tschirren, PhD; Edwin Van Beek, MD, PhD; Bram van Ginneken, PhD; Eva van Rikxoort, PhD; Gonzalo Vegas Sanchez‐Ferrero, PhD; Lucas Veitel; George R. Washko, MD; Carla G. Wilson, MS; PFT QA Center, Salt Lake City, UT: Robert Jensen, PhD. Data Coordinating Center and Biostatistics, National Jewish Health, Denver, CO: Douglas Everett, PhD; Jim Crooks, PhD; Katherine Pratte, PhD; Matt Strand, PhD; Carla G. Wilson, MS. Epidemiology Core, University of Colorado Anschutz Medical Campus, Aurora, CO: John E. Hokanson, MPH, PhD; Erin Austin, PhD; Gregory Kinney, MPH, PhD; Sharon M. Lutz, PhD; Kendra A. Young, PhD. Mortality Adjudication Core: Surya P. Bhatt, MD; Jessica Bon, MD; Alejandro A. Diaz, MD, MPH; MeiLan K. Han, MD, MS; Barry Make, MD; Susan Murray, ScD; Elizabeth Regan, MD; Xavier Soler, MD; Carla G. Wilson, MS. Biomarker Core: Russell P. Bowler, MD, PhD; Katerina Kechris, PhD; Farnoush Banaei‐Kashani, PhD. COPDGene Investigators–Clinical Centers: Ann Arbor VA: Jeffrey L. Curtis, MD; Perry G. Pernicano, MD. Baylor College of Medicine, Houston, TX: Nicola Hanania, MD, MS; Mustafa Atik, MD; Aladin Boriek, PhD; Kalpatha Guntupalli, MD; Elizabeth Guy, MD; Amit Parulekar, MD; Brigham and Women's Hospital, Boston, MA: Dawn L. DeMeo, MD, MPH; Craig Hersh, MD, MPH; Francine L. Jacobson, MD, MPH; George Washko, MD. Columbia University, New York, NY: R. Graham Barr, MD, DrPH; John Austin, MD; Belinda D'Souza, MD; Byron Thomashow, MD. Duke University Medical Center, Durham, NC: Neil MacIntyre, Jr., MD; H. Page McAdams, MD; Lacey Washington, MD. HealthPartners Research Institute, Minneapolis, MN: Charlene McEvoy, MD, MPH; Joseph Tashjian, MD. Johns Hopkins University, Baltimore, MD: Robert Wise, MD; Robert Brown, MD; Nadia N. Hansel, MD, MPH; Karen Horton, MD; Allison Lambert, MD, MHS; Nirupama Putcha, MD, MHS. Lundquist Institute for Biomedical Innovationat Harbor UCLA Medical Center, Torrance, CA: Richard Casaburi, PhD, MD; Alessandra Adami, PhD; Matthew Budoff, MD; Hans Fischer, MD; Janos Porszasz, MD, PhD; Harry Rossiter, PhD; William Stringer, MD. Michael E. DeBakey VAMC, Houston, TX: Amir Sharafkhaneh, MD, PhD; Charlie Lan, DO. Minneapolis VA: Christine Wendt, MD; Brian Bell, MD; Ken M. Kunisaki, MD, MS. Morehouse School of Medicine, Atlanta, GA: Eric L. Flenaugh, MD; Hirut Gebrekristos, PhD; Mario Ponce, MD; Silanath Terpenning, MD; Gloria Westney, MD, MS. National Jewish Health, Denver, CO: Russell Bowler, MD, PhD; David A. Lynch, MB. Reliant Medical Group, Worcester, MA: Richard Rosiello, MD; David Pace, MD. Temple University, Philadelphia, PA: Gerard Criner, MD; David Ciccolella, MD; Francis Cordova, MD; Chandra Dass, MD; Gilbert D'Alonzo, DO; Parag Desai, MD; Michael Jacobs, PharmD; Steven Kelsen, MD, PhD; Victor Kim, MD; A. James Mamary, MD; Nathaniel Marchetti, DO; Aditi Satti, MD; Kartik Shenoy, MD; Robert M. Steiner, MD; Alex Swift, MD; Irene Swift, MD; Maria Elena Vega‐Sanchez, MD. University of Alabama, Birmingham, AL: Mark Dransfield, MD; William Bailey, MD; Surya P. Bhatt, MD; Anand Iyer, MD; Hrudaya Nath, MD; J. Michael Wells, MD. University of California, San Diego, CA: Douglas Conrad, MD; Xavier Soler, MD, PhD; Andrew Yen, MD. University of Iowa, Iowa City, IA: Alejandro P. Comellas, MD; Karin F. Hoth, PhD; John Newell, Jr., MD; Brad Thompson, MD. University of Michigan, Ann Arbor, MI: MeiLan K. Han, MD MS; Ella Kazerooni, MD MS; Wassim Labaki, MD MS; Craig Galban, PhD; Dharshan Vummidi, MD. University of Minnesota, Minneapolis, MN: Joanne Billings, MD; Abbie Begnaud, MD; Tadashi Allen, MD. University of Pittsburgh, Pittsburgh, PA: Frank Sciurba, MD; Jessica Bon, MD; Divay Chandra, MD, MSc; Joel Weissfeld, MD, MPH. University of Texas Health, San Antonio, San Antonio, TX: Antonio Anzueto, MD; Sandra Adams, MD; Diego Maselli‐Caceres, MD; Mario E. Ruiz, MD; Harjinder Singh.
This article was sent to Mahasin S. Mujahid, PhD, MS, FAHA, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.033882
For Sources of Funding and Disclosures, see page 11.
Contributor Information
Edwin K. Silverman, Email: ed.silverman@channing.harvard.edu.
COPDGene Investigators:
James D. Crapo, Edwin K. Silverman, Barry J. Make, Elizabeth A. Regan, Terri H. Beaty, Peter J. Castaldi, Michael H. Cho, Dawn L. DeMeo, Adel El Boueiz, Marilyn G. Foreman, Auyon Ghosh, Lystra P. Hayden, Craig P. Hersh, Jacqueline Hetmanski, Brian D. Hobbs, John E. Hokanson, Wonji Kim, Nan Laird, Christoph Lange, Sharon M. Lutz, Merry‐Lynn McDonald, Dmitry Prokopenko, Matthew Moll, Jarrett Morrow, Dandi Qiao, Elizabeth A. Regan, Aabida Saferali, Phuwanat Sakornsakolpat, Edwin K. Silverman, Emily S. Wan, Jeong Yun, Juan Pablo Centeno, Jean‐Paul Charbonnier, Harvey O. Coxson, Craig J. Galban, MeiLan K. Han, Eric A. Hoffman, Stephen Humphries, Francine L. Jacobson, Philip F. Judy, Ella A. Kazerooni, Alex Kluiber, David A. Lynch, Pietro Nardelli, John D. Newell, Aleena Notary, Andrea Oh, Elizabeth A. Regan, James C. Ross, Raul San Jose Estepar, Joyce Schroeder, Jered Sieren, Berend C. Stoel, Juerg Tschirren, Edwin Van Beek, Bram van Ginneken, Eva van Rikxoort, Gonzalo Vegas Sanchez‐Ferrero, Lucas Veitel, George R. Washko, Carla G. Wilson, Robert Jensen, Douglas Everett, Jim Crooks, Katherine Pratte, Matt Strand, Carla G. Wilson, John E. Hokanson, Erin Austin, Gregory Kinney, Sharon M. Lutz, Kendra A. Young, Surya P. Bhatt, Jessica Bon, Alejandro A. Diaz, MeiLan K. Han, Barry Make, Susan Murray, Elizabeth Regan, Xavier Soler, Carla G. Wilson, Russell P. Bowler, Katerina Kechris, Farnoush Banaei‐Kashani, Jeffrey L. Curtis, Perry G. Pernicano, Nicola Hanania, Mustafa Atik, Aladin Boriek, Kalpatha Guntupalli, Elizabeth Guy, Amit Parulekar, Dawn L. DeMeo, Craig Hersh, Francine L. Jacobson, George Washko, R. Graham Barr, John Austin, Belinda D’Souza, Byron Thomashow, Neil MacIntyre, H. Page McAdams, Lacey Washington, Charlene McEvoy, Joseph Tashjian, Robert Wise, Robert Brown, Nadia N. Hansel, Karen Horton, Allison Lambert, Nirupama Putcha, Richard Casaburi, Alessandra Adami, Matthew Budoff, Hans Fischer, Janos Porszasz, Harry Rossiter, William Stringer, Amir Sharafkhaneh, Charlie Lan, Christine Wendt, Brian Bell, Ken M. Kunisaki, Eric L. Flenaugh, Hirut Gebrekristos, Mario Ponce, Silanath Terpenning, Gloria Westney, Russell Bowler, David A. Lynch, Richard Rosiello, David Pace, Gerard Criner, David Ciccolella, Francis Cordova, Chandra Dass, Gilbert D’Alonzo, Parag Desai, Michael Jacobs, Steven Kelsen, Victor Kim, A. James Mamary, Nathaniel Marchetti, Aditi Satti, Kartik Shenoy, Robert M. Steiner, Alex Swift, Irene Swift, Maria Elena Vega‐Sanchez, Mark Dransfield, William Bailey, Surya P. Bhatt, Anand Iyer, Hrudaya Nath, J. Michael Wells, Douglas Conrad, Xavier Soler, Andrew Yen, Alejandro P. Comellas, Karin F. Hoth, John Newell, Brad Thompson, MeiLan K. Han, Ella Kazerooni, Wassim Labaki, Craig Galban, Dharshan Vummidi, Joanne Billings, Abbie Begnaud, Tadashi Allen, Frank Sciurba, Jessica Bon, Divay Chandra, Joel Weissfeld, Antonio Anzueto, Sandra Adams, Mario E. Ruiz, and Harjinder Singh
References
- 1. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, et al. Global burden of cardiovascular diseases and risk factors, 1990‐2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Divo M, Cote C, de Torres JP, Casanova C, Marin JM, Pinto‐Plata V, Zulueta J, Cabrera C, Zagaceta J, Hunninghake G, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:155–161. doi: 10.1164/rccm.201201-0034OC [DOI] [PubMed] [Google Scholar]
- 3. Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1418–1422. doi: 10.1164/ajrccm.157.5.9709032 [DOI] [PubMed] [Google Scholar]
- 4. Curkendall SM, DeLuise C, Jones JK, Lanes S, Stang MR, Goehring E Jr, She D. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol. 2006;16:63–70. doi: 10.1016/j.annepidem.2005.04.008 [DOI] [PubMed] [Google Scholar]
- 5. Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32:962–969. doi: 10.1183/09031936.00012408 [DOI] [PubMed] [Google Scholar]
- 6. Chen W, Thomas J, Sadatsafavi M, FitzGerald JM. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta‐analysis. Lancet Respir Med. 2015;3:631–639. doi: 10.1016/S2213-2600(15)00241-6 [DOI] [PubMed] [Google Scholar]
- 7. Dalal AA, Shah M, D'Souza AO, Rane P. Costs of COPD exacerbations in the emergency department and inpatient setting. Respir Med. 2011;105:454–460. doi: 10.1016/j.rmed.2010.09.003 [DOI] [PubMed] [Google Scholar]
- 8. Perticone M, Maio R, Caroleo B, Suraci E, Corrao S, Sesti G, Perticone F. COPD significantly increases cerebral and cardiovascular events in hypertensives. Sci Rep. 2021;11:7884. doi: 10.1038/s41598-021-86963-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rabe KF, Hurst JR, Suissa S. Cardiovascular disease and COPD: dangerous liaisons? Eur Respir Rev. 2018;27:27. doi: 10.1183/16000617.0057-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andre S, Conde B, Fragoso E, Boleo‐Tome JP, Areias V, Cardoso J; Cronica GD‐GdInDPO . COPD and cardiovascular disease. Pulmonology. 2019;25:168–176. doi: 10.1016/j.pulmoe.2018.09.006 [DOI] [PubMed] [Google Scholar]
- 11. Agusti A, Celli BR, Criner GJ, Halpin D, Anzueto A, Barnes P, Bourbeau J, Han MK, Martinez FJ, Montes de Oca M, et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Eur Respir J. 2023;61:2300239. doi: 10.1183/13993003.00239-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur Respir J. 2017;49:1700214. doi: 10.1183/13993003.00214-2017 [DOI] [PubMed] [Google Scholar]
- 13. Vestbo J, Anderson JA, Brook RD, Calverley PM, Celli BR, Crim C, Martinez F, Yates J, Newby DE, SUMMIT Investigators . Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double‐blind randomised controlled trial. Lancet. 2016;387:1817–1826. doi: 10.1016/S0140-6736(16)30069-1 [DOI] [PubMed] [Google Scholar]
- 14. Rothnie KJ, Connell O, Mullerova H, Smeeth L, Pearce N, Douglas I, Quint JK. Myocardial infarction and ischemic stroke after exacerbations of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2018;15:935–946. doi: 10.1513/AnnalsATS.201710-815OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kunisaki KM, Dransfield MT, Anderson JA, Brook RD, Calverley PMA, Celli BR, Crim C, Hartley BF, Martinez FJ, Newby DE, et al. Exacerbations of chronic obstructive pulmonary disease and cardiac events. A post hoc cohort analysis from the SUMMIT randomized clinical trial. Am J Respir Crit Care Med. 2018;198:51–57. doi: 10.1164/rccm.201711-2239OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dransfield MT, Criner GJ, Halpin DMG, Han MK, Hartley B, Kalhan R, Lange P, Lipson DA, Martinez FJ, Midwinter D, et al. Time‐dependent risk of cardiovascular events following an exacerbation in patients with chronic obstructive pulmonary disease: post hoc analysis from the IMPACT trial. J Am Heart Assoc. 2022;11:e024350. doi: 10.1161/JAHA.121.024350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, Curran‐Everett D, Silverman EK, Crapo JD. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7:32–43. doi: 10.3109/15412550903499522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Washko GR, Lynch DA, Matsuoka S, Ross JC, Umeoka S, Diaz A, Sciurba FC, Hunninghake GM, San Jose Estepar R, Silverman EK, et al. Identification of early interstitial lung disease in smokers from the COPDGene study. Acad Radiol. 2010;17:48–53. doi: 10.1016/j.acra.2009.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Breathett K, Spatz ES, Kramer DB, Essien UR, Wadhera RK, Peterson PN, Ho PM, Nallamothu BK. The groundwater of racial and ethnic disparities research. Circ Cardiovasc Qual Outcomes 2021;1:e007868. doi: 10.1161/circoutcomes.121.007868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mudd‐Martin G, Cirino AL, Barcelona V, Fox K, Hudson M, Sun YV, Taylor JY, Cameron VA. Considerations for cardiovascular genetic and genomic research with marginalized racial and ethnic groups and indigenous peoples: a scientific statement from the American Heart Association. Circ Genom Precis Med. 2021;14:e000084. doi: 10.1161/hcg.0000000000000084 [DOI] [PubMed] [Google Scholar]
- 21. Wan ES, Castaldi PJ, Cho MH, Hokanson JE, Regan EA, Make BJ, Beaty TH, Han MK, Curtis JL, Curran‐Everett D, et al. Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (PRISm) in COPDGene. Respir Res. 2014;15:89. doi: 10.1186/s12931-014-0089-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lokke A, Hilberg O, Lange P, Ibsen R, Telg G, Stratelis G, Lykkegaard J. Exacerbations predict severe cardiovascular events in patients with COPD and stable cardiovascular disease‐a Nationwide, population‐based cohort study. Int J Chron Obstruct Pulmon Dis. 2023;18:419–429. doi: 10.2147/COPD.S396790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mullerova H, Marshall J, de Nigris E, Varghese P, Pooley N, Embleton N, Nordon C, Marjenberg Z. Association of COPD exacerbations and acute cardiovascular events: a systematic review and meta‐analysis. Ther Adv Respir Dis. 2022;16:17534666221113647. doi: 10.1177/17534666221113647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wan ES, Fortis S, Regan EA, Hokanson J, Han MK, Casaburi R, Make BJ, Crapo JD, DeMeo DL, Silverman EK, et al. Longitudinal phenotypes and mortality in preserved ratio impaired spirometry in the COPDGene study. Am J Respir Crit Care Med. 2018;198:1397–1405. doi: 10.1164/rccm.201804-0663OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wijnant SRA, De Roos E, Kavousi M, Stricker BH, Terzikhan N, Lahousse L, Brusselle GG. Trajectory and mortality of preserved ratio impaired spirometry: the Rotterdam study. Eur Respir J. 2020;55:55. doi: 10.1183/13993003.01217-2019 [DOI] [PubMed] [Google Scholar]
- 26. Suissa S, Dell'Aniello S, Ernst P. Long‐acting bronchodilator initiation in COPD and the risk of adverse cardiopulmonary events: a population‐based comparative safety study. Chest. 2017;151:60–67. doi: 10.1016/j.chest.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 27. Lange P, Celli B, Agusti A, Boje Jensen G, Divo M, Faner R, Guerra S, Marott JL, Martinez FD, Martinez‐Camblor P, et al. Lung‐function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373:111–122. doi: 10.1056/NEJMoa1411532 [DOI] [PubMed] [Google Scholar]
- 28. Marott JL, Ingebrigtsen TS, Colak Y, Vestbo J, Lange P. Lung function trajectories leading to chronic obstructive pulmonary disease as predictors of exacerbations and mortality. Am J Respir Crit Care Med. 2020;202:210–218. doi: 10.1164/rccm.201911-2115OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fumagalli G, Fabiani F, Forte S, Napolitano M, Marinelli P, Palange P, Pentassuglia A, Carlone S, Sanguinetti CM. INDACO project: a pilot study on incidence of comorbidities in COPD patients referred to pneumology units. Multidiscip Respir Med. 2013;8:28. doi: 10.1186/2049-6958-8-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Halpin DM, Decramer M, Celli B, Kesten S, Leimer I, Tashkin DP. Risk of nonlower respiratory serious adverse events following COPD exacerbations in the 4‐year UPLIFT(R) trial. Lung. 2011;189:261–268. doi: 10.1007/s00408-011-9301-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wan ES, Balte P, Schwartz JE, Bhatt SP, Cassano PA, Couper D, Daviglus ML, Dransfield MT, Gharib SA, Jacobs DR Jr, et al. Association between preserved ratio impaired spirometry and clinical outcomes in US adults. JAMA. 2021;326:2287–2298. doi: 10.1001/jama.2021.20939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation. 2003;107:1514–1519. doi: 10.1161/01.cir.0000056767.69054.b3 [DOI] [PubMed] [Google Scholar]
- 33. Sidney S, Sorel M, Quesenberry CP Jr, DeLuise C, Lanes S, Eisner MD. COPD and incident cardiovascular disease hospitalizations and mortality: Kaiser Permanente medical care program. Chest. 2005;128:2068–2075. doi: 10.1378/chest.128.4.2068 [DOI] [PubMed] [Google Scholar]
- 34. Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33:1165–1185. doi: 10.1183/09031936.00128008 [DOI] [PubMed] [Google Scholar]
- 35. Gimbrone MA Jr, Garcia‐Cardena G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118:620–636. doi: 10.1161/CIRCRESAHA.115.306301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goedemans L, Bax JJ, Delgado V. COPD and acute myocardial infarction. Eur Respir Rev. 2020;29:29. doi: 10.1183/16000617.0139-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Szucs B, Szucs C, Petrekanits M, Varga JT. Molecular characteristics and treatment of endothelial dysfunction in patients with COPD: a review article. Int J Mol Sci. 2019;20:20. doi: 10.3390/ijms20184329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Clarenbach CF, Senn O, Sievi NA, Camen G, van Gestel AJ, Rossi VA, Puhan MA, Thurnheer R, Russi EW, Kohler M. Determinants of endothelial function in patients with COPD. Eur Respir J. 2013;42:1194–1204. doi: 10.1183/09031936.00144612 [DOI] [PubMed] [Google Scholar]
- 39. Lahousse L, Verhamme KM, Stricker BH, Brusselle GG. Cardiac effects of current treatments of chronic obstructive pulmonary disease. Lancet Respir Med. 2016;4:149–164. doi: 10.1016/S2213-2600(15)00518-4 [DOI] [PubMed] [Google Scholar]
- 40. Chaouat A, Bugnet AS, Kadaoui N, Schott R, Enache I, Ducolone A, Ehrhart M, Kessler R, Weitzenblum E. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:189–194. doi: 10.1164/rccm.200401-006OC [DOI] [PubMed] [Google Scholar]
- 41. Maclay JD, McAllister DA, Macnee W. Cardiovascular risk in chronic obstructive pulmonary disease. Respirology. 2007;12:634–641. doi: 10.1111/j.1440-1843.2007.01136.x [DOI] [PubMed] [Google Scholar]
- 42. Sinden NJ, Stockley RA. Systemic inflammation and comorbidity in COPD: a result of ‘overspill’ of inflammatory mediators from the lungs? Review of the evidence. Thorax. 2010;65:930–936. doi: 10.1136/thx.2009.130260 [DOI] [PubMed] [Google Scholar]
- 43. Soriano JB, Visick GT, Muellerova H, Payvandi N, Hansell AL. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest. 2005;128:2099–2107. doi: 10.1378/chest.128.4.2099 [DOI] [PubMed] [Google Scholar]
- 44. Rutten FH, Moons KG, Cramer MJ, Grobbee DE, Zuithoff NP, Lammers JW, Hoes AW. Recognising heart failure in elderly patients with stable chronic obstructive pulmonary disease in primary care: cross sectional diagnostic study. BMJ. 2005;331:1379. doi: 10.1136/bmj.38664.661181.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Almagro P, Lapuente A, Pareja J, Yun S, Garcia ME, Padilla F, Heredia JL, De la Sierra A, Soriano JB. Underdiagnosis and prognosis of chronic obstructive pulmonary disease after percutaneous coronary intervention: a prospective study. Int J Chron Obstruct Pulmon Dis. 2015;10:1353–1361. doi: 10.2147/COPD.S84482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lawton JS, Tamis‐Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, Bittl JA, Cohen MG, DiMaio JM, Don CW, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: executive summary: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2022;145:e4–e17. doi: 10.1161/CIR.0000000000001039 [DOI] [PubMed] [Google Scholar]
- 47. Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, Blankstein R, Boyd J, Bullock‐Palmer RP, Conejo T, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: executive summary: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2021;144:e368–e454. doi: 10.1161/CIR.0000000000001030 [DOI] [PubMed] [Google Scholar]
- 48. Mok M, Nombela‐Franco L, Dumont E, Urena M, DeLarochelliere R, Doyle D, Villeneuve J, Cote M, Ribeiro HB, Allende R, et al. Chronic obstructive pulmonary disease in patients undergoing transcatheter aortic valve implantation: insights on clinical outcomes, prognostic markers, and functional status changes. JACC Cardiovasc Interv. 2013;6:1072–1084. doi: 10.1016/j.jcin.2013.06.008 [DOI] [PubMed] [Google Scholar]
- 49. Kwak MJ, Bhise V, Warner MT, Balan P, Nguyen TC, Estrera AL, Smalling RW, Dhoble A. National trend of utilization, clinical and economic outcomes of transcatheter aortic valve replacement among patients with chronic obstructive pulmonary disease. Curr Med Res Opin. 2019;35:1321–1329. doi: 10.1080/03007995.2019.1583024 [DOI] [PubMed] [Google Scholar]
- 50. Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–852. doi: 10.1136/thorax.57.10.847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal‐Singer R, Miller B, Lomas DA, Agusti A, Macnee W, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–1138. doi: 10.1056/NEJMoa0909883 [DOI] [PubMed] [Google Scholar]
- 52. Lipson DA, Barnhart F, Brealey N, Brooks J, Criner GJ, Day NC, Dransfield MT, Halpin DMG, Han MK, Jones CE, et al. Once‐daily single‐inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378:1671–1680. doi: 10.1056/NEJMoa1713901 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S9
Figure S1


