Abstract
Background
Pulmonary hypertension and right ventricular (RV) dysfunction are major prognostic determinants in patients with heart failure with preserved ejection fraction (HFpEF). The underlying pathomechanisms remain unknown. In this context, we sought to study the pathogenesis of pulmonary hypertension and RV dysfunction in a rat model of obesity‐associated HFpEF.
Methods and Results
HFpEF was induced in obesity‐prone rats fed a high‐fat diet (n=13) and compared with obesity‐resistant rats fed with standard chow (n=9). After 12 months, the animals underwent echocardiographic and hemodynamic evaluation followed by tissue sampling for pathobiological assessment. HFpEF rats presented mild RV pressure overload (with increased RV systolic pressure and pulmonary vascular resistance). No changes in pulmonary artery medial thickness and ex vivo vasoreactivity (to acetylcholine and endothelin‐1) were observed and RNA sequencing analysis failed to identify gene clustering in HFpEF lungs. However, released nitric oxide levels were decreased in HFpEF pulmonary artery, while lung expression of preproendothelin‐1 was increased. In HFpEF rats, RV structure and function were altered, with RV enlargement, decreased RV fractional area change and free wall longitudinal fractional shortening, together with altered right ventricle–pulmonary artery coupling (estimated by tricuspid annular plane systolic excursion/systolic pulmonary artery pressure). Hypertrophy and apoptosis (evaluated by transferase biotin‐ dUTP nick‐end labeling staining) were increased in right and left ventricles of HFpEF rats. There was an inverse correlation between tricuspid annular plane systolic excursion/systolic pulmonary artery pressure and RV apoptotic rate. Plasma levels of soluble suppression of tumorigenicity‐2, interleukin‐1β, ‐6 and ‐17A were increased in HFpEF rats.
Conclusions
Obesity‐associated HFpEF in rats spontaneously evolves to pulmonary hypertension–HFpEF associated with impaired right ventricle–pulmonary artery coupling that appears disproportionate to a slight increase in RV afterload.
Keywords: apoptosis, group 2 pulmonary hypertension, heart failure with preserved ejection fraction, metabolic syndrome, right ventricular dysfunction
Subject Categories: Animal Models of Human Disease, Basic Science Research, Heart Failure, Metabolic Syndrome, Pulmonary Hypertension
Nonstandard Abbreviations and Acronyms
- HFpEF
heart failure with preserved ejection fraction
- LFS
longitudinal fractional shortening
- mPAP
mean pulmonary artery pressure
- PAP
pulmonary artery pressure
- PH
pulmonary hypertension
- RAP
right atrial pressure
- sPAP
systolic pulmonary artery pressure
- sST2
soluble suppression of tumorigenicity‐2
- TAPSE
tricuspid annular plane systolic excursion
Research Perspective.
What Is New?
Heart failure with preserved ejection fraction (HFpEF) is a growing clinical and public health problem accounting for approximately half of all cases of heart failure and associated hospital admissions; there is an urgent need for therapeutic approaches and strategies that target mechanisms specific for HFpEF.
In an original experimental model of HFpEF, we showed that the alterations in right ventricle–pulmonary artery coupling occur before the development of pulmonary artery remodeling in HFpEF, suggesting load‐independent mechanisms involved in this right ventricular dysfunction.
What Questions Should Be Addressed Next?
Our findings warrant future specific attention to the right ventricle–pulmonary artery uncoupling even before the development of pulmonary hypertension in patients with HFpEF.
The right ventricle–pulmonary artery coupling should be therefore assessed as early as possible in the clinical evaluation of patients with HFpEF.
HFpEF accounts for ≈50% of all cases of heart failure and is expected to become even more prevalent in the coming years due to the demographic shift toward an aging population with an increasing prevalence of comorbidities. 1 , 2 Right ventricular (RV) dysfunction, which is highly prevalent (≈30%–50%) among patients with left‐sided heart failure with preserved ejection fraction (HFpEF), 3 , 4 has been recognized as a major independent factor of prognosis and adverse outcome. 5 , 6 , 7 In HFpEF, increased left‐sided filling pressures lead to pulmonary hypertension (PH) though passive backward transmission. 7 Secondarily, RV dysfunction develops due to load‐dependent mechanisms. Load‐independent factors can also contribute to RV dysfunction, including left‐to‐right ventricular interaction 3 , 8 and atrial fibrillation, 5 as well as systemic inflammation and endothelial dysfunction. 3 , 9
A clinical picture of the so‐called “right heart phenotype” is frequently described in patients with HFpEF presenting an RV rather than a left ventricular (LV) dysfunction. As the LV systolic function is preserved in HFpEF, RV contractility should be maintained by systolic interventricular interaction until advanced stages of the disease. However, accumulating evidence indicates that this is not always the case. 10 Indeed, the right heart phenotype may develop early in HFpEF due to insufficient adaptation of the RV systolic function to a mild increase in pulmonary artery pressure (PAP). 11 This can be assessed by considering the right ventricle and the pulmonary circulation as a sole functional unit, through the evaluation of the RV–pulmonary artery (PA) coupling. 12 However, mechanisms underlying the pathogenesis of altered RV function and PH observed in HFpEF remain poorly understood, which considerably delays therapeutic innovation.
In this context, we developed an experimental rat model of HFpEF associated with multiple comorbidities, as a robust preclinical model of HFpEF mimicking key features observed in patients including aging and comorbidities. 13 In the present study, we evaluated whether these rats developed PH and RV dysfunction as observed in patients with HFpEF and studied underlying pathophysiological mechanisms.
METHODS
The authors declare that all supporting data are available within the article.
Protocol and Hemodynamic Assessment
The present study was approved by the Institutional Animal Care and Use Committee of the Faculty of Medicine of the Université Libre de Bruxelles (Brussels, Belgium; protocol acceptation number: 656N). Applicable guidelines were followed in accordance with the Guide for Care and Use of Laboratory Animals published by the US National Institutes of Health (Eighth Edition, update 2011).
Animals used in the present study were diet‐induced obesity‐prone and obesity‐resistant Sprague Dawley rats raised using selected breeders obtained from Charles River Laboratories Inc. (Wilmington, MA). As previously reported, 13 experimental HFpEF associated with multiple comorbidities (ie, metabolic syndrome) was induced in 4‐week‐old obesity‐prone rats (n=13) fed with a high‐fat diet (473 kcal/100 g; 45% of energy from lipids and 17% from sucrose; D1245; Research Diets, New Brunswick, NJ) for 12 months. Nine 4‐week‐ old obesity‐resistant rats fed with a standard rat chow diet (339 kcal/100 g; 5% of energy from lipids and 2% from sucrose; A03 Safe Diets, Augy, France) for 12 months were used as controls. Along the protocol, the animals were maintained under controlled environmental conditions (21 °C, 60% humidity atmosphere and 12‐hour light/dark cycles) with ad libitum access to water and diet. At the end of the protocol (after 12 months), HFpEF and control rats were evaluated by echocardiography and invasive hemodynamic assessment. Blood samples were drawn from the femoral vein, placed in EDTA‐coated tubes, and centrifuged to collect plasma for biological analyses. Under profound anesthesia (using isoflurane 5%), rats were finally euthanized by exsanguination (by section of the abdominal aorta). Lung tissue was quickly harvested and snap‐frozen in liquid nitrogen and stored at –80 °C for further biological experiments or after 24‐hour fixation in 10% neutral buffered formalin, embedded in paraffin for histopathological evaluation. After dissection of the heart, the right ventricle was weighted and the various parts of the heart were embedded in paraffin after fixation in 10% neutral buffered formalin.
Biochemical analysis including fasting glycemia, glucose tolerance testing obtained from the area under the curve measured from the glycemic values versus time points, plasma levels of triglycerides, total cholesterol, and high‐density lipoprotein cholesterol were determined as previously described. 13 Plasma levels of cardiac biomarkers, including N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) and interleukin‐1 receptor‐like 1 (also called soluble suppression of tumorigenicity‐2 [sST2]), were evaluated using rat NT‐proBNP (MBS012301; MyBioSource, San Diego, CA) and sST2 (MBS9348955; MyBioSource) ELISA kits, respectively, according to manufacturers' instructions.
Echocardiography and Doppler Imaging
Transthoracic 2‐dimensional, M‐mode, and Doppler imaging was performed using a digital ultrasound machine (Vivid‐7, GE Healthcare, Chicago, IL) equipped with a 12‐MHz phased array transducer (Hewlett Packard, Palo Alto, CA). The rats were sedated (ketamine 24 mg/kg−1 and medetomidine 0.32 mg/kg−1 IP), placed on a temperature‐controlled rodent pad, while they were spontaneously breathing during the procedure. ECG was monitored via limb leads throughout the procedure. The rats were continuously observed while anesthetized with monitoring of sedation by the toe‐pinch response and detection of any clinical signs of deterioration, including a dramatic reduction in heart rate and abnormal breathing patterns. All measurements were obtained by the same operator according to methods recommended by the American Society of Echocardiography currently applied to humans. 14
Characterization of HFpEF
As previously described, 13 LV echocardiography was performed in anesthetized rats in the right parasternal (long‐ and short‐axis) and subcostal views. Briefly, diastolic and systolic, septal (SWT) and posterior wall thickness and LV diameters (left ventricular end‐diastolic diameter [LVEDD] and left ventricular end‐systolic diameter) were measured in M‐mode from a LV short‐axis view at the level of the chordae tendinae. LV ejection fraction was derived using the Teicholz formula. To estimate LV hypertrophy, relative wall thickness [(diastolic septal wall thickness + diastolic posterior wall thickness)/LVEDD] and relative wall area [(LV epicardial short‐axis area—LV endocardial short‐axis)/LV epicardial short‐axis area] were calculated. LV mass was calculated using the American Society of Echocardiography recommended formula: LV mass=1.05 (5/6. A. L), where A (LV epicardial area—LV endocardial area) is a planimetered, short‐axis area obtained at the papillary muscle level, and L is the LV length (apex to midmitral annulus plane) obtained from the parasternal long‐axis view normalized for tibial length to assess LV weight independently of body weight. Aortic flow was measured from the subcostal window to calculate forward stroke volume and cardiac output. All parameters were measured in at least 3 heartbeats at end diastole and end systole, and an averaged value was used.
Assessment of PA Hemodynamics and RV Structure and Function
Pulmonary hemodynamics were assessed through images acquired from a modified left parasternal long‐axis view. The PA and the aorta diameter were measured in the 2‐dimensional right parasternal short‐axis view at the level of the aortic valve to calculate the PA/aorta ratio.
RV morphology was assessed with RV end‐diastolic diameter and LVEDD to interpret the RV end‐diastolic diameter/LVEDD ratio. The ultrasonic beam was placed across the RV wall perpendicular to the RV long axis at the level of the mitral valve/RV free wall thickness, and end‐diastolic cavity dimension was measured in the parasternal short‐axis view just below the level of the aortic valve. This view was chosen because it offered the most consistent image of the RV free wall in this animal model. Basal RV end‐diastolic diameter was evaluated from the 2‐dimensional apical 4‐chamber view. In this view, RV function was assessed with the endocardial borders from the RV end‐diastolic area and end‐systolic area were traced manually and the fractional area change (in %) was calculated as (RV end‐diastolic area–RV end‐systolic area/RV end‐diastolic area)×100. 15 For the tricuspid annulus plane systolic excursion (TAPSE), the length of the longitudinal systolic excursion of the RV annulus segment was measured at peak systole from a standard 2‐dimensional apical 4‐chamber window. TAPSE was acquired after positioning the M‐mode cursor through the tricuspid annulus, parallel to the longitudinal movement of the RV free wall. Values were measured between end diastole and peak systole. RV free wall longitudinal fractional shortening (LFS, in %) was calculated as [−(TAPSE/RV diastolic length)×100]. 16
Catheterization: Invasive Hemodynamic Data Acquisition and Analysis
RV and LV hemodynamic measurements were performed in spontaneously breathing closed‐chest rats under continuous anesthesia using a rodent anesthesia system (isoflurane: 4% for induction, 2% during surgery, and 1% during hemodynamic measurements; Minerve, Esternay, France), and recorded and analyzed with a ADV500 PV data acquisition system (Transonic, AD Instruments, The Netherlands), as previously reported. 13 Briefly, a microtip pressure catheter (rodent catheter 1.6F; Transonic, The Netherlands) was inserted in the RV flow tract through the right jugular vein cannulation to acquire RV systolic pressure and right atrial pressure. Correct anatomic placement was confirmed by respective pressure contours. Thereafter, the catheter was moved and placed in the LV flow tract through left carotid cannulation to acquire LV end‐diastolic pressure. All measurements were performed after stabilization of the pressure line. Mean PAP (mPAP) was calculated as RV systolic pressure×0.6+2. 17 Pulmonary vascular resistance (PVR) was calculated as the ratio between (calculated mPAP−LV end‐diastolic pressure) and cardiac output (measured noninvasively by Doppler echocardiography). LV end‐diastolic pressure was used as a surrogate to estimate LV filling pressure, as pulmonary artery occlusion pressure (also called pulmonary artery wedge pressure) cannot be measure in rodents. Right atrial pressure was calculated by averaging the maximal and minimal values of the RA pressure recording. The right ventricle–PA coupling was assessed as the ratio between the TAPSE and the systolic PAP. 18 , 19
Pathobiological and Histological Assessments
Experiments of vasoreactivity were performed as previously described. 20 Briefly, 3‐mm‐length PA segments were mounted on 2 stainless steel hooks in 5‐mL organ baths filled with Krebs–Henseleit solution (118 mmol·L−1 NaCl; 4.7 mmol·L−1 KCl; 1.2 mmol·L−1 MgSO4;1.2 mmol.L−1 KH2PO4; 2.5 mmol·L−1 CaCl2; 25 mmol·L−1 NaHCO3; 5.1 mmol·L−1 glucose; Merck, Darmstadt, Germany) bubbled with 95% O2 and 5% CO2 and maintained at 37 °C for continuous isometric tension recording (EMKA Technologies, Paris, France), with a resting tension of 600 mg.
After a 60‐minute equilibration period, contraction capacity was assessed with 8.10−2 mol/L−1 KCl (Merck). After a washout period, endothelial function was evaluated with acetylcholine chloride (10−10 to 10−4 mol/L−1; Sigma‐Aldrich, St. Louis, MO) in phenylephrine hydrochloride (10−6 mol/L−1; Sigma‐Aldrich) precontracted rings. Finally, after a washout period allowing the artery rings to return to their basal vascular tone, increasing concentrations of endothelin‐1 (3−10 to 10−6 mol/L−1; Sigma‐Aldrich) were successively tested in endothelium‐intact PA rings.
Histological Analysis: Pulmonary Artery Morphometry
Three‐micrometer serial sections were taken along the longitudinal axis of the lung lobes and stained with hematoxylin–eosin and orcein for morphological analysis, as previously described. 21 In orcein‐stained lung sections, only small PA with an external diameter <100 μm and a complete muscular coat were measured. Medial thickness was related to arterial size by the formula %medial thickness=(medial thickness/external diameter)×100 and was measured by counting at least 15 to 20 PA per lung lobe from each animal. In hematoxylin–eosin‐stained lung sections, pulmonary vascular density (number of pulmonary vessels/mm2) was evaluated (at a 100‐fold magnification in light microscopy) of pulmonary sections randomly selected. All morphological analyses were performed using a LEICA DFC425C camera and LEICA DM2000 microscope (Leica Microsystems, Heerbrugg, Germany) and ImageJ analysis software by 2 independent investigators in a blinded manner. The mean value was used for analysis.
Pulmonary fibrosis was assessed with Picrosirius Red staining to assess the presence of collagen accumulation and fibrosis within lung sections.
Histological Analysis: Cardiac Morphometry and Capillary Density
Five 3‐μm myocardial sections were taken along the transversal axis of the heart and stained with hematoxylin–eosin for morphological analysis, as previously described. 22 , 23 Briefly, the mean cross‐sectional area of cardiomyocytes was calculated by measuring at least 75 cells for each myocardial sample (at 400‐fold magnification in light microscopy). Each animal's heart is represented by 5 dots corresponding to various myocardial sections.
Capillary density was assessed on myocardial sections stained for the endothelial‐ specific marker CD31. Briefly, myocardial sections were blocked for endogenous peroxidase activity (with 3% hydrogen peroxide) and for nonspecific binding sites (with 5% normal rat serum). After overnight incubation at 4 °C with a polyclonal goat anti‐mouse/rat CD31 primary antibody (1:100 diluted in PBS; R&D Systems, Minneapolis, MN), sections were incubated for 1 hour with a biotinylated rabbit anti‐goat immunoglobulin G secondary antibody (1:200 diluted in PBS; CiteAb, Bath, UK). Colorimetric immunostaining of endothelial layer was performed using the biotin‐streptavidin immunoperoxidase method (Vector Laboratories, Burlingame, CA) with 3,3‐diaminobenzidine (Vector Laboratories) as a chromogen, according to manufacturer's instructions. Nuclei were counterstained with hematoxylin. Images were acquired with a LEICA DFC425C camera and LEICA DM2000 microscope (Leica Microsystems, Wetzlar, Germany). The capillary density was calculated as the number of capillaries (CD31‐ positive stained small vessels) reported to the number of cardiomyocytes in 30 different randomly chosen fields (at 40× magnification) in the right and left ventricles. All counts were performed by 2 independent investigators in a blinded manner. The mean value was used for analysis.
Immunohistochemistry: Terminal Deoxynucleotidyl Transferase dUTP Nick‐End Labeling
Detection of cardiac cells in apoptosis was achieved by transferase biotin‐dUTP nick‐end labeling staining using the ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit (Chemicon, Temecula, CA) according to manufacturer's instructions. Negative control run without terminal deoxynucleotidyl transferase enzyme and rat lung samples were used as positive controls. For each cardiac specimen, 20 different randomly chosen fields (at 40× magnification) were examined. Cardiac apoptotic rate was calculated as the ratio of apoptotic nuclei (transferase biotin‐dUTP nick‐end labeling‐positive or brown nuclei) to total nuclei (brown+blue nuclei) (100× to be expressed in percentage). All counts were performed in a blinded fashion by 2 independent investigators. The mean value was used for analysis.
Multiplex Cytokine Magnetic Bead Panel Assay
Plasma circulating levels of interleukin‐6, ‐1β, ‐17A, and ‐18; monocyte chemoattractant protein 1 and vascular endothelial growth factor were determined using a magnetic bead‐based multiplex assay using the Luminex technology (MILLIPLEXMAP; Merck Laboratories, Germany), as previously reported. 24
Nitric Oxide Assay
PA segments were incubated during 1 hour in a Krebs–Henseleit solution (118 mmol/L−1 NaCl; 4.7 mmol/L−1 KCl; 1.2 mmol/L−1 MgSO4; 1.2 mmol/L−1 KH2PO4; 2.5 mmol/L−1 CaCl2; 25 mmol/L−1 NaHCO3; 5.1 mmol/L−1 glucose; Merck) in a 24‐well cell culture plate. Supernatants were collected and nitric oxide (NO) levels were measured indirectly by the determination the nitrite levels, using the Measure‐iT™ High‐Sensitivity Nitrite Assay kit (Molecular Probes, Eugene, OR) according to the manufacturer's instructions. Briefly, fluorescence was measured with a microplate reader at 365/450 nm. Nitrite concentrations were obtained by referring to a standard curve realized in parallel and expressed in mol/L−1. Results are expressed as the mean concentration values of 3 separate measurements performed for each condition and referred to the weight (in mg) of PA tissue.
RNA Extraction and RNA Sequencing Data Acquisition and Analysis
Total RNA was extracted from snap‐frozen lung tissue sampled in HFpEF and control rats, using TRIzol reagent (Invitrogen, Merelbeke, Belgium) followed by a chloroform/ethanol extraction and a final purification using RNeasy Mini kit (QIAGEN, Hilden, Germany), according to manufacturer's instructions. RNA concentration was determined with a standard spectrophotometer Nanodrop (ND‐1000; Isogen Life Sciences, De Meern, The Netherlands), and RNA integrity was assessed by visual inspection of GelRed (Biotium, Hayward, CA) stained agarose gels. RNA quality was evaluated using a Fragment Analyzer 5200 (Agilent, Santa Clara, CA).
RNA sequencing was performed at the Brussels Interuniversity Genomics High‐Throughput core (www.brightcore.be). Briefly, indexed cDNA libraries were obtained using the TruSeqRNA sample preparation kit (Illumina, San Diego, CA) following manufacturer's instructions. The multiplexed libraries were loaded on a Novaseq 6000 (Illumina) using an S2 flow cell, and sequences were produced using a 200 Cycle Kit. Approximately 25 million paired‐end reads were mapped per sample against the rattus norvegicus (Rnor 6.0) reference genome using STAR software (version STAR_2.5.3a) to generate read alignments for each sample. Annotations Rnor 6.0.103.gtf were obtained from ftp.Ensembl.org.
Clustering and differential gene expression analysis was performed with 3 randomly chosen lung mRNA samples from HFpEF and from control rat groups, using the integrated website application for analysis of RNA sequencing data iDEP (http://bioinformatics.sdstate.edu/idep/). Genes with a P value <0.1 and an absolute log‐fold change >1 were selected for further evaluation. In parallel, manual selected literature analysis was realized for each differentially expressed gene regarding their molecular function.
Real‐Time Quantitative Polymerase Chain Reaction
Reverse transcription was carried out using SuperScript™ II Reverse Transcriptase (Invitrogen), according to manufacturer's instructions. For real‐time quantitative polymerase chain reaction experiments, sense (5′‐ CAGACCAAGGGAACAGATGC‐3′) and antisense (5′‐ACGCCTTTCTGCATGGTACT‐3′) primer were designed using the Primer3 program for rattus norvegicus preproendothelin‐1 mRNA sequence. To avoid inappropriate amplification of residual genomic DNA, intron‐spanning primers were selected, and an analysis was run to check if primer pairs were only matching the sequence of interest. For each lung tissue sample, the amplification reaction was performed in triplicate using SYBR‐Green PCR Master Mix (Quanta Biosciences, Gaithersburg, MD), specific primers and diluted template cDNA. Result analysis was performed using an iCycler system (Bio‐Rad Laboratories, Hercules, CA). Relative mRNA quantification was achieved with the Pfaffl method 25 by normalization with the housekeeping genes GAPDH (sense: 5′‐AAGATGGTGAAGGTCGGTGT‐3′; antisense: 5′‐ATGAAGGGGTCGTTGATGG‐3′) and hypoxanthine phosphoribosyltransferase 1 (sense: 5′‐ACAGGCCAGACTTTGTTGGA‐3′; antisense: 5′‐TCCACTTTCGCTGATGACCAC‐3′). Results were expressed as relative fold increase over the mean value of mRNA expression of the control rat group arbitrary fixed to 1.
Statistical Analysis
All data were presented as mean±standard error of the mean (SEM). Statistical analysis was performed using Graphpad Prism 9.0 (Graphpad, San Diego, California, USA). A parametric Student's t test was used to assess the statistical significance of the differences between the two tested groups (HFpEF versus control rats). A value of P<0.05 was considered as statistically significant; n represents the number of individual data.
Correlations were analyzed parametrically by the determination of the Pearson correlation coefficient.
RESULTS
Characterization of HFpEF Associated With Multiple Comorbidities
As shown in Table 1, HFpEF was confirmed in rats with diastolic dysfunction (assessed by increased LV end‐diastolic pressure) and concentric LV hypertrophy, characterized by increased LV mass and relative wall thickness. LV ejection fraction was preserved in both groups of rats, with values >60% (Table 1). Left atrial enlargement characterized by increased left atrial diameter was present in HFpEF rats (Table 1). No changes in heart rate (207±7 versus 223±5 beats/min; P=0.10) and stroke volume (0.27±0.02 versus 0.24±0.01 mL/beat; P=0.12) were observed between HFpEF and control rats. Plasma levels of sST2 markedly increased in HFpEF rats, while NT‐proBNP levels decreased (Table 1).
Table 1.
Echocardiographic and Hemodynamic Characterization of HFpEF in Rats
| Control | HFpEF | P value | |
|---|---|---|---|
| LVEDP, mm Hg | 1.6±0.2 | 8.9±1.9 | <0.05 |
| LVEF, % | 63±2 | 69±4 | NS |
| LV mass, g | 0.14±0.01 | 0.18±0.01 | <0.05 |
| LVRWT, mm | 0.41±0.02 | 0.48±0.03 | <0.05 |
| LA diameter, mm | 4.21±0.10 | 5.06±0.21 | <0.05 |
| Plasma NT‐proBNP, pg·mL−1 | 230±7 | 131±10 | <0.001 |
| Plasma sST2, pg·mL−1 | 551±47 | 2803±700 | <0.001 |
Values are expressed as means±SEM. Comparisons are made in HFpEF (n=13) vs control rats (n=9). HFpEF indicates heart failure with preserved ejection fraction; LA, left atrial; LV, left ventricular; LVEDP, left ventricular end‐diastolic pressure; LVEF, left ventricular ejection fraction; LVRWT, left ventricular relative wall thickness; NT‐ proBNP, N‐terminal pro‐B‐type natriuretic peptide; and sST2, soluble suppression of tumorigenicity‐2.
Body weights of 4‐week‐old obesity‐resistant and obesity‐prone rats were similar at baseline (93±2 versus 93±1 g). After 12‐month specific dieting, HFpEF rats presented with dramatically increased body weights compared with control rats (Table 2), together with higher weights of abdominal fat (data not shown). Fasting blood glucose levels (85±3 versus 76±2 mg·dL−1; P=0.056) were not different, while total area under the curve of blood glucose levels (between 0 and 150 minutes after an intraperitoneal glucose tolerance test) were increased in HFpEF rats (Table 2), suggesting glucose intolerance. As illustrated in Table 2, plasma levels of triglycerides were increased in HFpEF rats, as well as the low‐density lipoprotein–to–high‐density lipoprotein ‐cholesterol ratio. LV pressure was also increased, with LV systolic pressure >160 mm Hg (Table 2).
Table 2.
Biochemical and Hemodynamic Characterization of Major Comorbidities Observed in Rats With HFpEF
| Control | HFpEF | P value | |
|---|---|---|---|
| Body weight, g | 554±42 | 719±21 | <0.001 |
| Fasting blood glucose, mg·dL−1 | 76±2 | 85±3 | NS |
| Total blood glucose AUC, mg/dL−1·min | 18 114±865 | 25 063±2802 | <0.05 |
| Triglycerides, mg/dL−1 | 120±13 | 968±123 | <0.001 |
| LDL‐to‐HDL ratio | 0.36±0.03 | 1.61±0.18 | <0.001 |
| LVSP, mm Hg | 120±5 | 164±7 | <0.001 |
Total glycemia AUC corresponds to the geometric mean value to quantify the total increased blood glucose during a glucose tolerance test. Values are expressed as means±SEM. Comparisons are made in HFpEF (n=13) vs control rats (n=9). AUC indicates area under the curve; HDL, high‐density lipoprotein; HFpEF, heart failure with preserved ejection fraction; LDL, low‐density lipoprotein; and LVSP, left ventricular systolic pressure.
Characterization of Pulmonary Hypertensive Disease in Rats With HFpEF
Invasive RV catheterization, using a closed‐chest approach, was performed to measure RV systolic pressure as a surrogate for PAP measurement in rats. As illustrated in RV pressure waveform monitorings (Figure 1A), rats with HFpEF presented with increased RV systolic pressure (Figure 1B) and increased mPAP (Figure 1C), while no change in cardiac output was observed (Figure 1D). There were significant correlations between left atrial enlargement and hemodynamic parameters as RV systolic pressure (r=0.63, P<0.05), and mPAP (r=0.64, P<0.05). PVR was increased in HFpEF rats (Figure 1E), as well as right atrial pressure (Figure 1F). PA diameter (Figure 1G) and PA‐to‐aorta diameter ratio (Figure 1H) were increased in HFpEF rats. PH was thus observed in rats with HFpEF associated to multiple comorbidities.
Figure 1. Hemodynamic and echocardiographic characterization of pulmonary hypertension in HFpEF rats.
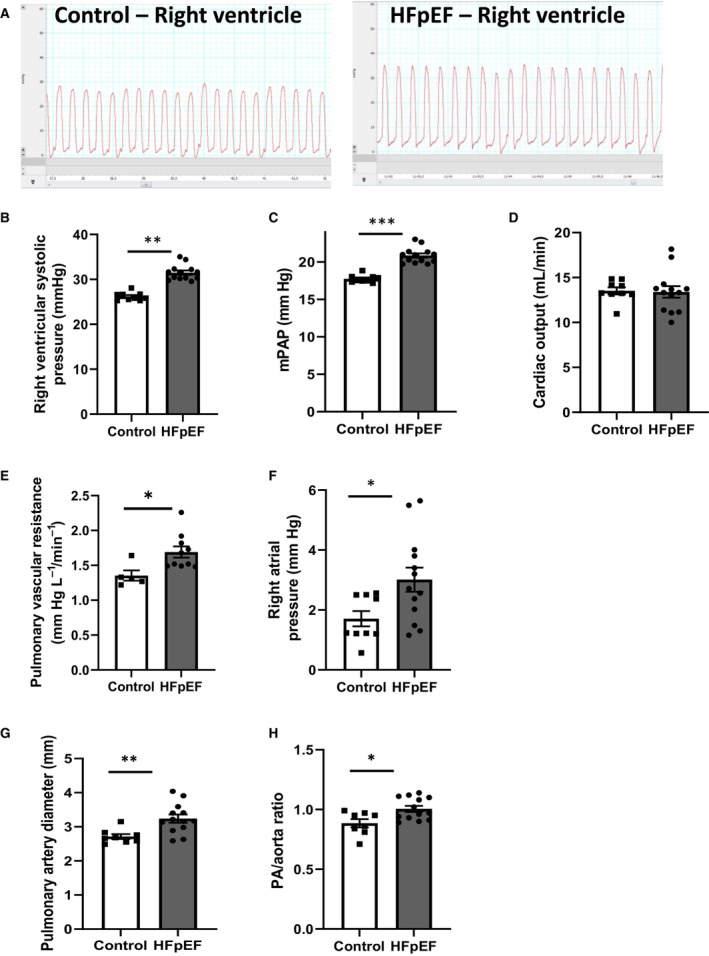
A, Right ventricular pressure waveform monitoring obtained at right heart catheterization in control (left panel) and HFpEF (right panel) rats, (B) right ventricular systolic pressure (in mm Hg), (C) calculated mPAP (in mm Hg), (D) cardiac output (in mL/L−1), (E) pulmonary vascular resistance (in mm Hg/L−1·min−1), (F) right atrial pressure (in mm Hg) in rats with HFpEF (black bars; n=13) and control rats (white bars; n=9). Echocardiographic evaluation of (G) pulmonary artery diameter (in mm) and (H) PA‐to‐aorta diameter ratio in control (white bars; n=9) and HFpEF (black bars; n=13) rats. Values are expressed as mean±SEM. * 0.01<P<0.05, ** 0.001<P<0.01, *** 0.001>P HFpEF vs control rats. HFpEF indicates heart failure with preserved ejection fraction; mPAP, mean pulmonary artery pressure; and PA, pulmonary artery.
PA Morphometry
To investigate the mechanisms underlying the pathogenesis of PH in these rats, we searched for PA morphological alterations. In the lungs, vascular remodeling assessed by the medial wall thickness (Figure 2D and 2F) and vascular density evaluated as the number of pulmonary vessels/mm2 of lung parenchyma (Figure 2A and 2E) were similar between groups. No differences in terms of inflammatory cell infiltration (evaluated in hematoxylin–eosin‐stained sections; Figure 2A and 2B) and perivascular and interstitial fibrosis (in Picro Sirius Red–stained sections; Figure 2C) were observed in the lungs of HFpEF compared with control rats.
Figure 2. Pulmonary artery morphometry in HFpEF rats.
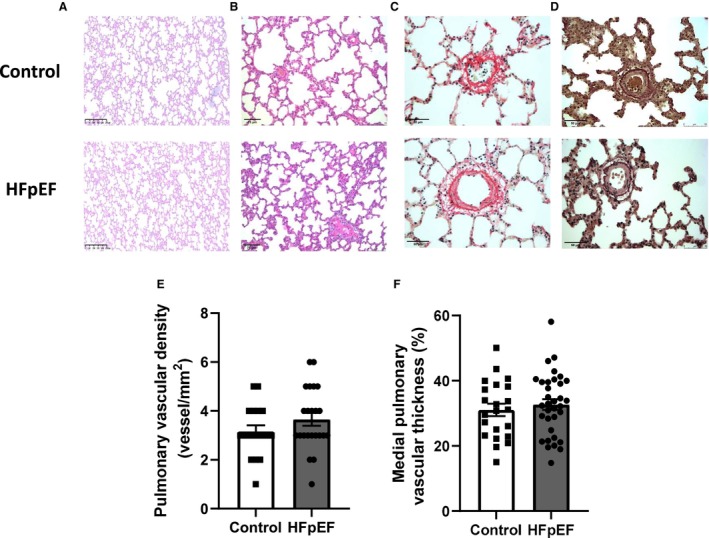
Representative slides of hematoxylin–eosin‐stained pulmonary (A; scale bar: 250 μm) and pulmonary artery (B; scale bar: 75 μm) sections, Picrosirius Red‐ (C; scale bar: 50 μm) and orcein‐ (D; scale bar: 50 μm) stained pulmonary artery sections. PicroSirius Red staining was performed to detect fibrotic areas, with collagen fibers stained in red and orcein staining to detect internal and external elastic lamellae. Vascular density (E; expressed as the number of pulmonary arteries/mm2 lung parenchyma) and morphometry on pulmonary arterioles (with external diameter <150 μm) expressed as medial wall thickness percentage (%MT) vs external diameter (F) in lungs of control (white bars; n=9) and HFpEF (black bars; n=13) rats. Values are expressed as mean±SEM. HFpEF indicates heart failure with preserved ejection fraction.
Ex Vivo PA Reactivity and Expression of Vasoactive Molecules
To further investigate the mechanisms responsible for the increase in PA pressure observed in HFpEF rats, we assessed ex vivo vascular reactivity in freshly dissected PA. After precontraction with phenylephrine (10−6 mol/L−1), acetylcholine (tested from 10−10 to 10−4 mol/L−1) induced a similar endothelium‐dependent relaxation in PA segments from HFpEF and control rats (Figure 3A). In addition, endothelin‐1 (tested from 3.10−10 to 3.10−6 mol/L−1), a well‐known potent PA constrictor, similarly induced contraction in PA segments from HFpEF and control rats (Figure 3B). These results showed preserved endothelial function and contractile properties in PA from HFpEF rats.
Figure 3. Ex vivo pulmonary artery vasoactive assessment and pulmonary pathobiological evaluation, including RNA sequencing in HFpEF rats.
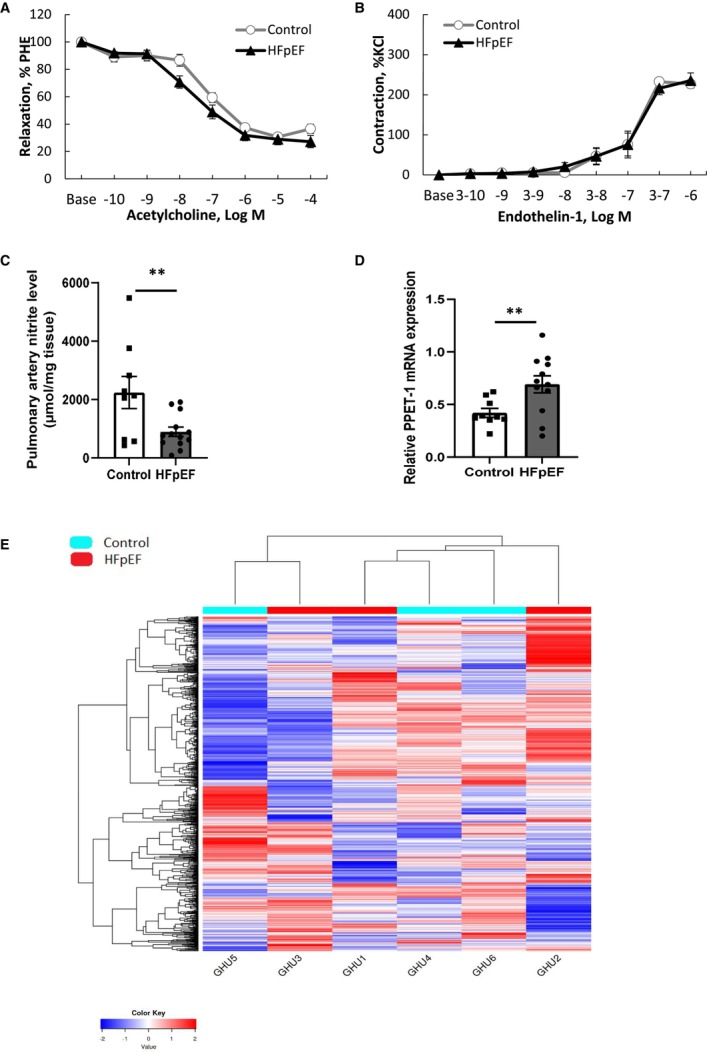
Concentration‐response curves to acetylcholine (A; tested from 10−10 to 10−4 mol/L−1) after phenylephrine (PHE) precontraction and to endothelin‐1 (B; tested from 10−8 to 10−3 mol/L−1) in endothelium‐intact pulmonary artery segments from control (white circles; n=9) and HFpEF (black triangles; n=13) rats. Concentration of nitrites in supernatants of endothelium‐intact pulmonary artery rings from control (white bars; n=9) and HFpEF (black bars; n=13) rats and incubated during 1 hour in Krebs solution (C). Relative mRNA expression of pre‐proendothelin‐1 (PPET‐1) in lungs from control (white circles; n=9) and HFpEF (black triangles; n=13) rats (D), Values are expressed as mean±SEM. ** 0.001<P<0.01, HFpEF vs control rats. Heatmap (E) showing nonclustering of genes from RNA sequencing in 3 randomly chosen control (in blue) and in three randomly chosen HFpEF (in red) rats. Raw Z scores are shown on the heatmap. HFpEF indicates heart failure with preserved ejection fraction.
Thereafter, we compared the release and expression of molecules regulating the pulmonary vasomotion, including endothelium‐derived vasodilator NO and vasoconstrictor endothelin‐1, in HFpEF rats. Basal release of NO, evaluated by the production of its metabolite nitrite, was decreased in PA from HFpEF (Figure 3C), while pulmonary gene expression of endothelin‐1 precursor, the pre‐proendothelin‐1, increased (Figure 3D).
Pulmonary Pathobiological Characterization
As illustrated in Figure 3E, RNA sequencing analysis failed to identify clusters of genes differentially expressed in HFpEF compared with control lungs. The analysis indicated only 7 genes differentially expressed between the 2 groups of rats. The genes were the neurotrophic receptor tyrosine kinase 2 (Ntrk2), the collagen type X alpha 1 chain, the myosin heavy chain 7 (Myh7), the myosin light chain 2, the aggrecan, the adiponectin, and the natriuretic peptide A.
Assessment of RV Structure and Function in Rats With HFpEF
To assess RV mass, dissected right ventricles were weighted. In HFpEF rats, RV mass was increased (217±25 versus 164±6 mg, P=0.03; HFpEF versus control rats).
In the 2 groups of rats, echocardiography was performed to estimate RV structure and function. An RV enlargement, defined by an increased ratio of RV‐to‐LV end‐diastolic size (RV end‐diastolic diameter/LVEDD) in apical 4‐chamber views, was observed in HFpEF rats (Figure 4A). As illustrated in Figure 4B, the RV fractional area change, an index of RV systolic function, was decreased in HFpEF rats. Quantification of TAPSE was also performed to characterize RV longitudinal systolic function in apical 4‐chamber views allowing the visualization of the tricuspid valve (in M‐mode), as illustrated by TAPSE images obtained in the 2 groups of rats (Figure 4C). There were no changes in TASPE values between the two groups (Figure 4D). As recently described, 16 RV LFS can be used as a surrogate of RV strain. Values of LFS in HFpEF rats were slightly above –20.2%, which indicated an abnormal RV function in these rats (Figure 4E).
Figure 4. Echocardiographic evaluation of RV structure and function in HFpEF rats.
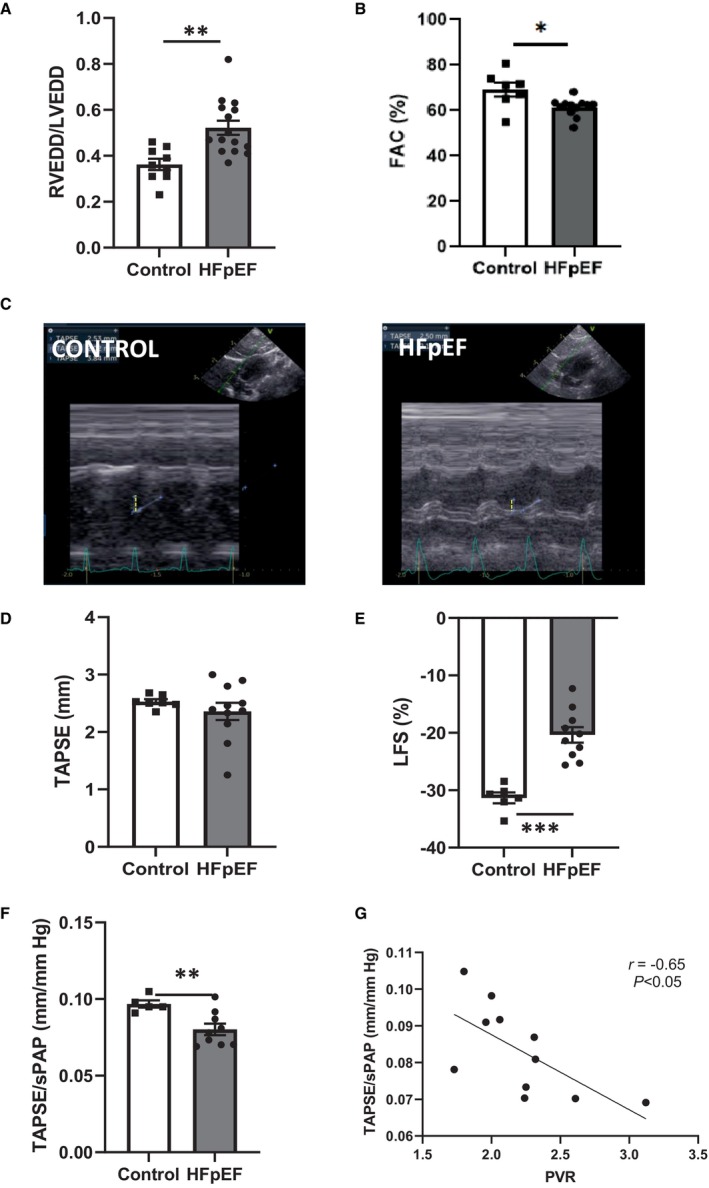
RV enlargement, defined by the ratio of RVEDD and LVEDD A) and RV systolic function, measured as the fractional area change (in %; B), the tricuspid annular plane systolic excursion (TAPSE; in mm; D), as well as representative echocardiographic images (C) derived from control (left panel) and HFpEF rats (right panel) and the RV free wall LFS (in %; E) in control (white bars; n=9) and HFpEF (black bars; n=13) rats. Right ventricule–pulmonary artery coupling, assessed as the TAPSE‐to‐sPAP ratio (F) in control (white bars; n=9) and HFpEF (black bars; n=13) rats. Correlation between TAPSE‐to‐sPAP ratio and PVR (G). Values are expressed as mean±SEM. * 0.01<P<0.05, ** 0.001<P<0.01, *** 0.001>P HFpEF vs control rats. HFpEF indicates heart failure with preserved ejection fraction; LFS, longitudinal fractional shortening; LVEDD, left ventricular end‐diastolic diameter; PVR, pulmonary vascular resistance; RV, right ventricular; RVEDD, right ventricular end‐diastolic diameter; sPAP, systolic pulmonary artery pressure; and TAPSE, tricuspid annular plane systolic excursion.
Assessment of the Right Ventricle–to–PA Coupling
As echocardiography revealed alterations in RV structure and function in HFpEF rats, we evaluated the coupling between the right ventricle and the pulmonary circulation using the TAPSE–to–systolic PAP (sPAP) ratio. This ratio has been validated as an echocardiographic surrogate of right ventricle–to–PA coupling in patients with HFpEF, 19 , 26 , 27 but not yet in rodents. The TAPSE‐to‐sPAP ratio was lower in HFpEF compared with control rats (Figure 4F). This ratio was tightly correlated to PVR (Figure 4G), which suggested impaired adaptation of RV systolic function to its increased afterload.
Heart Morphometry and Assessment of Myocardial Apoptosis
As illustrated in hematoxylin–eosin‐stained LV and RV sections of HFpEF and control rats (Figure 5A), HFpEF rats exhibited LV and RV cardiomyocyte hypertrophy characterized by the increase in cell surface area compared with controls (Figure 5B).
Figure 5. Myocardial structure, including cardiomyocyte hypertrophy and apoptosis in HFpEF rats.
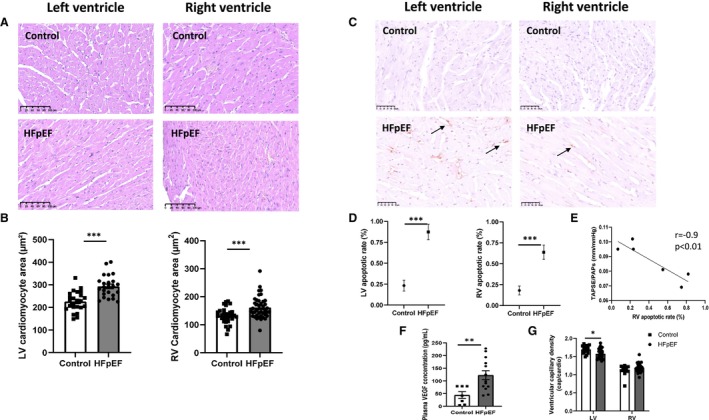
Representative images of hematoxylin–eosin‐stained sections of left and right ventricles in control and HFpEF rats (A; scale bars: 100 μm). Cardiomyocyte hypertrophy in left and in right (B) ventricles (assessed by cardiomyocyte area in μm2 in 5 sections obtained along the transverse myocardial axis of each animal) from control (white bars, n=9) and HFpEF (black bars, n=13) rats. Representative images of terminal deoxynucleotidyl TUNEL‐stained myocardial sections of left and right ventricles in control and HFpEF rats (C; scale bars: 50 μm). Cardiac apoptotic rate (in %; D) was evaluated as the ratio between the number of terminal deoxynucleotidyl TUNEL‐positive cardiomyocytes (brown nuclei mentioned by arrows) and the total number of cardiomyocytes (brown+blue nuclei) in control (while bars; n=9) and HFpEF (black bars; n=13) rats. Correlation between tricuspid annular plane systolic excursion (TAPSE)‐to‐sPAP ratio (TAPSE/sPAP) and RV apoptotic rate (E). Plasma levels of VEGF (F) in control (while bars; n=9) and HFpEF (black bars; n=13) rats. Left and right ventricular capillary density (assessed as the number of small vessels per cardiomyocyte; g) in control (white bars; n = 9) and HFpEF (black bars; n=13) rats. Values are presented as mean ± SEM. *** 0.001>P, HFpEF vs control rats. HFpEF indicates heart failure with preserved ejection fraction; LV, left ventricular; RV, right ventricular; sPAP, systolic pulmonary artery pressure; TUNEL, transferase biotin‐dUTP nick‐end labeling; and VEGF, vascular endothelial growth factor.
Because apoptosis of cardiomyocytes has been implicated in RV dysfunction, 12 we evaluated the activation of apoptotic processes in the heart of HFpEF rats. As illustrated in Figure 5C, transferase biotin‐dUTP nick‐end labeling staining (assessing the termination of apoptotic processes) was performed to evaluate the level of apoptosis in the left and right ventricles. In HFpEF rats, myocardial apoptotic rates were increased in the left and right ventricles (Figure 5D). In the right ventricle, this apoptotic rate was inversely correlated to the TAPSE‐to‐sPAP ratio (Figure 5E).
Circulating plasma levels of vascular endothelial growth factor were significantly elevated in HFpEF rats (Figure 5F). Capillary density was decreased in the left ventricle of HFpEF rats, while it was preserved in the right ventricle (Figure 5G).
Circulating Plasma Levels of Inflammatory Cytokines in HFpEF Rats
Circulating plasma concentrations of interleukin‐6 (Figure 6A), ‐1β (Figure 6B), and ‐17A (Figure 6C) were significantly increased in HFpEF rats. No changes in circulating plasma levels of interleukin‐18 (125±21 versus 88±9 pg/mL−1; P=0.24) and monocyte chemoattractant protein 1 (938±127 versus 842±46 pg/mL−1; P=0.35) were measured between groups.
Figure 6. Circulating plasma levels of proinflammatory cytokines.
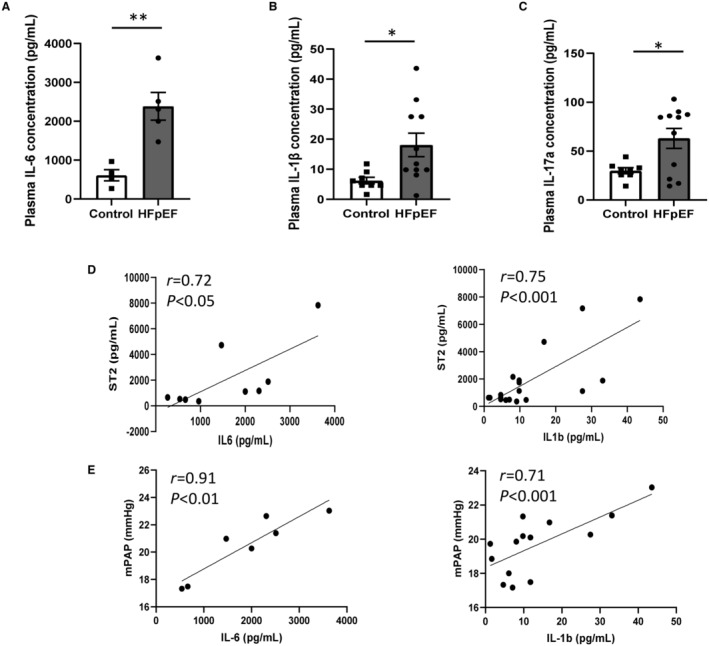
Plasma levels of IL‐6 (A), IL‐1β (B) and IL‐17a (C) in HFpEF (black bars; n=13) vs control (white bars; n=9) rats. Values are presented as mean±SEM. * 0.01<P<0.05, **0.001<P<0.01, HFpEF vs control rats. Correlations between plasma levels of cardiac biomarker sST2 and plasma levels of pro‐inflammatory cytokines, including IL‐6 and IL‐1β (D) and between hemodynamic parameter, mPAP and plasma levels of proinflammatory cytokines, including IL‐6 and IL‐1β (E). Data of HFpEF and control groups were analyzed together using a parametric Pearson's correlation coefficient analysis. HFpEF indicates heart failure with preserved ejection fraction; IL, interleukin; mPAP, mean pulmonary artery pressure.
Circulating plasma levels of interleukin‐6 (Figure 6) were correlated to sST2 levels (Figure 6D) and mPAP (Figure 6E). Similarly, interleukin‐1β levels were correlated to sST2 levels (Figure 6D) and mPAP (Figure 6E). There was no significant correlation between interleukin‐17A level and any biomarker/hemodynamic measurements.
DISCUSSION
The present results show that HFpEF associated with multiple comorbidities in rats induces alterations in RV structure and systolic function, as well as in right ventricle–PA coupling that appears early compared with the slight increase in RV afterload (assessed by mPAP and PVR). The alteration in right ventricle–PA coupling was associated with a hypertrophic response and an activation of apoptotic pathways in cardiomyocytes of the right ventricle (also observed in the left ventricle) in the presence of a systemic inflammatory state characterized by increased circulating plasma levels of proinflammatory cytokines, including interleukin‐6, ‐1β, and ‐17A.
PH, a key clinical feature in left heart disease, is highly prevalent (35%–80%) in patients with HFpEF. 3 , 27 , 28 In our preclinical model of HFpEF, a mild increase in mPAP and PVR was observed, defining a model of PH‐HFpEF. Right atrial pressure was also increased. The mechanisms of PH in HFpEF rely first on increased LV stiffness resulting in elevated filling pressures, with passive backward transmission through the atrium to the pulmonary circulation, leading to increased pulmonary venous and arterial pressures. 29 Pulmonary vascular alterations in HFpEF first occur in venules and capillaries with a venous histopathological pattern typified as arterialization with both medial and intimal thickening, 28 , 30 which was not observed in the present experimental model of early HFpEF. Pulmonary vascular morphometric and ex vivo vasoreactive evaluation did not show any pulmonary vascular structural or functional alterations in HFpEF compared with control rats. However, we found an imbalance between pulmonary vasoconstrictor and dilator levels, with downregulated NO and upregulated endothelin signaling. This is consistent with previous data showing some degree of pulmonary vasoconstriction related to hypoxia and endothelial dysfunction in the early stages of the disease. 31 , 32 Endothelial dysfunction was first described in the distal pulmonary vessels and after transmitted to the proximal arteries. 33 , 34 Moreover, other factors affecting the pulmonary circulation also exist, such as a sustained wall stress due to increased left atrial pressure, shear stress in the pulmonary vessels and endothelial dysfunction, 35 , 36 as well as a systemic proinflammatory state.
LV diastolic dysfunction remains the hallmark of HFpEF 37 ; however, emerging evidence points out the high prevalence of RV dysfunction in (PH‐)HFpEF and its relevance to the poor prognosis of these patients. 3 , 5 , 7 , 38 Passive backward transmission of elevated left‐sided filling pressure in HFpEF results in increased PVR and subsequently RV pressure overload. 28 , 29 , 39 Consequently, alterations in RV and right atrial function are observed and are closely related to death or hospitalization in patients. 38 In addition to increased RV mass, echocardiography revealed enlarged RV in HFpEF rats with altered RV systolic function, as assessed by RV systolic functional echocardiographic metrics, including RV fractional area change and LFS. These data confirmed alterations in RV structure and function associated with early HFpEF in the present experimental model. Surprisingly, the TAPSE was not altered in HFpEF rats but was shown as being not sufficient to counteract PH, using the TAPSE‐to‐sPAP ratio. Although TAPSE is known to reflect RV longitudinal contractility and to give prognostic information in patients, 5 measuring its value should indeed be carried out according to its afterload for a more realistic description of the RV–pulmonary circulation unit. 3 , 40 TAPSE is a regional parameter, measuring tricuspid annular shortening towards the RV apex, and is thus angle dependent, 41 while LFS provides a more global assessment of RV function.
Measurement of RV adaptation to its afterload, the right ventricle–PA coupling, allows monitoring of the transition from compensated to decompensated RV function. 12 The ratio of TAPSE divided by sPAP, which was independently associated with the invasive measure of right ventricle–PA coupling by right heart catheterization, 19 , 26 has emerged as an important prognostic marker in patients. Although echocardiographic assessment of RV function using TAPSE has been widely used and validated in rodents, 42 the use of the TAPSE‐to‐sPAP ratio has not yet been validated in rodents. Here, we found lower TAPSE‐to‐sPAP ratio in HFpEF rats, strongly suggesting altered right ventricle–PA coupling. It is commonly described in HFpEF that RV function remains maintained during the early stages of the disease (usually referred to isolated postcapillary PH), whereas when pulmonary vascular remodeling is present, combined post‐ and precapillary PH develops with a direct impact on RV function. 3 , 43 In the present experimental model of HFpEF associated with multiple comorbidities, RV dysfunction was present before pulmonary vascular remodeling, thus early in the pathological evolution of the disease. Conflicting results about the relationship between PH severity and RV dysfunction have already been observed in patients with HFpEF. 6 Because the TAPSE‐to‐sPAP ratio was described as an important determinant of symptomatology and outcome in patients with HFpEF, 18 , 44 , 45 the present results call for more attention to the right ventricle–PA coupling as early as possible in patients.
The presence of RV dysfunction in HFpEF observed in the present study is consistent with previous findings. 5 , 6 , 46 There could be several explanations for parallel LV and RV dysfunction in patients with HFpEF. The RV–LV interaction is probably largely mediated through septal interdependence, 47 but it can be notably extended beyond the level of hemodynamic interactions. Indeed, the right and left ventricles are encircled by transverse oriented circular myocardial fibers within a basal loop, 48 while the cross‐striation of oblique fibers in the interventricular septum enhances the septal twisting motion and subsequent RV–LV interactions. 49 Histological analysis revealed hypertrophy and apoptosis in cardiomyocytes of the right and left ventricles, while capillary density rarefaction was present only in the left ventricle of HFpEF rats. This is consistent with previous results showing that myocardial RV activation of apoptotic processes in RV dysfunction was tightly correlated to severity of right ventricle–PA uncoupling. 12 , 50
RV dysfunction is an independent determinant of survival in patients with all forms of PH. 51 Interestingly, RV dysfunction in HFpEF was associated with death independently of mPAP level, 52 highlighting the potential prognostic contribution of associated comorbidities. Patients with HFpEF with RV dysfunction present with more coronary disease, endothelial dysfunction, systemic inflammation, and renal dysfunction than those without RV dysfunction. 53 This strongly suggests that PH–HFpEF might be considered as a systemic disease beyond its impact on primary organ disease. Systemic inflammation has been epidemiologically and mechanistically linked to the pathogenesis of HFpEF. 9 HFpEF is a syndrome driven by multiple comorbidities, 54 which are characterized by a status of chronic systemic inflammation potentially impacting the heart. 9 , 55 In the present study, we showed higher circulating levels of canonical inflammatory cytokines, such as interleukin‐1β and ‐6, and confirmed increased sST2 levels in HFpEF rats, 13 which is consistent with previous data showing proinflammatory status in patients with HFpEF. 56 , 57 This increase in circulating inflammatory biomarkers supports the proposed pathophysiological mechanisms of HFpEF in regard to the multiorgan systemic inflammatory status. Comorbidy‐driven systemic inflammation may also contribute to reduce NO bioavailability, 9 as observed in the pulmonary circulation in HFpEF rats. Indeed, inflammation increases the oxidative stress and thus the production of reactive oxygen species converting in part NO into peroxynitrite and other NO‐related reactive species. Consistently, the nitrosative stress in HFpEF has been identified as a major driver of this syndrome. 58 In addition, a systemic inflammatory state is also known to participate in microvascular endothelial inflammation and dysfunction and in fine to cardiomyocyte remodeling implicated in the development of HFpEF. 9
The majority of experimental models of PH–HFpEF in rats were surgically induced, 59 using aortic banding or aortic constriction to induce pressure overload–induced cardiac hypertrophy, which passively causes an increase in LV ejection pressure, leading to increased pulmonary venous pressure and then to PH. 60 , 61 , 62 , 63 Two‐hit experimental models of PH–HFpEF combining metabolic syndrome and surgical intervention 64 or administration of a vascular endothelial growth factor receptor antagonist SU5416 65 have also been developed in rats. However, the main inconvenience of all these models remains that they do not fulfill all the key clinical features observed in patients with HFpEF. Here, we showed that HFpEF associated with multiple comorbidities (related to the metabolic syndrome) in rats spontaneously evolves to PH–HFpEF and to early alterations in right ventricle–PA coupling, faithfully recapitulating the clinical condition of patients with HFpEF. This experimental rat model is therefore unique to learn more about the pathophysiological evolution of the disease over time.
In conclusion, our findings indicate that obesity‐associated HFpEF in rats spontaneously evolves to PH–HFpEF. In these rats, alterations in right ventricle–PA coupling occur before the development of pulmonary vascular remodeling and cannot simply be seen as a sequela of increased RV afterload consequent to LV dysfunction and passive congestion. This involves more complex pathophysiological processes leading to RV dysfunction characterized by cardiomyocyte hypertrophy, activation of apoptotic processes in both the right and left ventricles.
Sources of Funding
This work was supported by research grants from the Belgian Foundation for Cardiac Surgery obtained by Géraldine Hubesch and Laurence Dewachter (Belgium) and by an ARC Consolidated grant obtained by Laurence Dewachter (Belgium). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosures
None.
Acknowledgments
Drs Hubesch, C. Dewachter, McEntee and L. Dewachter conceptualized and designed the study. Drs Hubesch, Doppler, Thiriard, and McEntee and E. Hupkens, P. Jespers, and G. Vegh performed the research. Drs Hubesch, C. Dewachter, Chomette, Doppler, Remmelink, and L. Dewachter and E. Hupkens, P. Jespers, G. Vegh, U.S. Mohammad, and L. Dewachter analyzed the data. Drs Hubesch, C. Dewachter, Chomette, Doppler, Remmelink, Vachiery, and L. Dewachter and P. Jespers and G. Vegh contributed to the interpretation of the results. Drs Hubesch and L. Dewachter wrote the original draft. All authors read and approved the submission of the manuscript.
This manuscript was sent to Sula Mazimba, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 15.
References
- 1. Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med. 2016;375:1868–1877. doi: 10.1056/NEJMcp1511175 [DOI] [PubMed] [Google Scholar]
- 2. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, et al. 2022 AHA/ACC/ HFSA guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2022;145:e895–e1032. doi: 10.1161/CIR.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 3. Gorter TM, van Veldhuisen DJ, Bauersachs J, Borlaug BA, Celutkiene J, Coats AJS, Crespo‐Leiro MG, Guazzi M, Harjola VP, Heymans S, et al. Right heart dysfunction and failure in heart failure with preserved ejection fraction: mechanisms and management. Position statement on behalf of the heart failure Association of the European Society of cardiology. Eur J Heart Fail. 2018;20:16–37. doi: 10.1002/ejhf.1029 [DOI] [PubMed] [Google Scholar]
- 4. Santas E, Palau P, Guazzi M, de la Espriella R, Minana G, Sanchis J, Bayes‐Genis A, Lupon J, Chorro FJ, Nunez J. Usefulness of right ventricular to pulmonary circulation coupling as an indicator of risk for recurrent admissions in heart failure with preserved ejection fraction. Am J Cardiol. 2019;124:567–572. doi: 10.1016/j.amjcard.2019.05.024 [DOI] [PubMed] [Google Scholar]
- 5. Gorter TM, Hoendermis ES, van Veldhuisen DJ, Voors AA, Lam CS, Geelhoed B, Willems TP, van Melle JP. Right ventricular dysfunction in heart failure with preserved ejection fraction: a systematic review and meta‐analysis. Eur J Heart Fail. 2016;18:1472–1487. doi: 10.1002/ejhf.630 [DOI] [PubMed] [Google Scholar]
- 6. Ghio S, Guazzi M, Scardovi AB, Klersy C, Clemenza F, Carluccio E, Temporelli PL, Rossi A, Faggiano P, Traversi E, et al. Different correlates but similar prognostic implications for right ventricular dysfunction in heart failure patients with reduced or preserved ejection fraction. Eur J Heart Fail. 2017;19:873–879. doi: 10.1002/ejhf.664 [DOI] [PubMed] [Google Scholar]
- 7. Obokata M, Reddy YNV, Melenovsky V, Pislaru S, Borlaug BA. Deterioration in right ventricular structure and function over time in patients with heart failure and preserved ejection fraction. Eur Heart J. 2019;40:689–697. doi: 10.1093/eurheartj/ehy809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rommel KP, von Roeder M, Oberueck C, Latuscynski K, Besler C, Blazek S, Stiermaier T, Fengler K, Adams V, Sandri M, et al. Load‐independent systolic and diastolic right ventricular function in heart failure with preserved ejection fraction as assessed by resting and handgrip exercise pressure‐volume loops. Circ Heart Fail. 2018;11:e004121. doi: 10.1161/CIRCHEARTFAILURE.117.004121 [DOI] [PubMed] [Google Scholar]
- 9. Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092 [DOI] [PubMed] [Google Scholar]
- 10. de Man FS, Naeije R. Sex and the right ventricle in heart failure with preserved ejection fraction. Chest. 2021;159:2156–2158. doi: 10.1016/j.chest.2021.02.001 [DOI] [PubMed] [Google Scholar]
- 11. Ghio S, Recusani F, Klersy C, Sebastiani R, Laudisa ML, Campana C, Gavazzi A, Tavazzi L. Prognostic usefulness of the tricuspid annular plane systolic excursion in patients with congestive heart failure secondary to idiopathic or ischemic dilated cardiomyopathy. Am J Cardiol. 2000;85:837–842. doi: 10.1016/s0002-9149(99)00877-2 [DOI] [PubMed] [Google Scholar]
- 12. Naeije R, Brimioulle S, Dewachter L. Biomechanics of the right ventricle in health and disease (2013 Grover conference series). Pulmonary Circ. 2014;4:395–406. doi: 10.1086/677354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hubesch G, Hanthazi A, Acheampong A, Chomette L, Lasolle H, Hupkens E, Jespers P, Vegh G, Wembonyama CWM, Verhoeven C, et al. A preclinical rat model of heart failure with preserved ejection fraction with multiple comorbidities. Front Cardiovasc Med. 2021;8:809885. doi: 10.3389/fcvm.2021.809885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zoghbi WA, Enriquez‐Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two‐dimensional and doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3 [DOI] [PubMed] [Google Scholar]
- 15. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. doi: 10.1093/ehjci/jev014 [DOI] [PubMed] [Google Scholar]
- 16. Unger P, Paesmans M, Vachiery JL, Rietz M, Amzulescu M, David CA. Right ventricular longitudinal fractional shortening: a substitute to right ventricular free wall longitudinal strain? Heart Vessel. 2022;37:426–433. doi: 10.1007/s00380-021-01928-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chemla D, Castelain V, Humbert M, Hebert JL, Simonneau G, Lecarpentier Y, Herve P. New formula for predicting mean pulmonary artery pressure using systolic pulmonary artery pressure. Chest. 2004;126:1313–1317. doi: 10.1378/chest.126.4.1313 [DOI] [PubMed] [Google Scholar]
- 18. Tello K, Axmann J, Ghofrani HA, Naeije R, Narcin N, Rieth A, Seeger W, Gall H, Richter MJ. Relevance of the TAPSE/PASP ratio in pulmonary arterial hypertension. Int J Cardiol. 2018;266:229–235. doi: 10.1016/j.ijcard.2018.01.053 [DOI] [PubMed] [Google Scholar]
- 19. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS, Escribano‐Subias P, Ferrari P, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618–3731. doi: 10.1093/eurheartj/ehac237 [DOI] [PubMed] [Google Scholar]
- 20. Gomart S, Damoiseaux C, Jespers P, Makanga M, Labranche N, Pochet S, Michaux C, Berkenboom G, Naeije R, McEntee K, et al. Pulmonary vasoreactivity in spontaneously hypertensive rats–effects of endothelin‐1 and leptin. Respir Res. 2014;15:12. doi: 10.1186/1465-9921-15-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Makanga M, Maruyama H, Dewachter C, Da Costa AM, Hupkens E, de Medina G, Naeije R, Dewachter L. Prevention of pulmonary hypoplasia and pulmonary vascular remodeling by antenatal simvastatin treatment in nitrofen‐induced congenital diaphragmatic hernia. Am J Physiol Lung Cell Mol Physiol. 2015;308:L672–L682. doi: 10.1152/ajplung.00345.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sahraoui A, Dewachter C, de Medina G, Naeije R, Aouichat Bouguerra S, Dewachter L. Myocardial structural and biological anomalies induced by high fat diet in Psammomys obesus gerbils. PLoS One. 2016;11:e0148117. doi: 10.1371/journal.pone.0148117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iliev AA, Kotov GN, Landzhov BV, Jelev LS, Kirkov VK, Hinova‐Palova DV. A comparative morphometric study of the myocardium during the postnatal development in normotensive and spontaneously hypertensive rats. Folia Morphol (Warsz). 2018;77:253–265. doi: 10.5603/FM.a2017.0094 [DOI] [PubMed] [Google Scholar]
- 24. Belhaj A, Dewachter L, Hupkens E, Remmelink M, Galanti L, Rorive S, Melot C, Naeije R, Rondelet B. Tacrolimus prevents mechanical and humoral alterations in brain death‐induced lung injury in pigs. Am J Respir Crit Care Med. 2022;206:584–595. doi: 10.1164/rccm.202201-0033OC [DOI] [PubMed] [Google Scholar]
- 25. Pfaffl MW. A new mathematical model for relative quantification in realtime RT‐PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guazzi M, Bandera F, Pelissero G, Castelvecchio S, Menicanti L, Ghio S, Temporelli PL, Arena R. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol. 2013;305:H1373–H1381. doi: 10.1152/ajpheart.00157.2013 [DOI] [PubMed] [Google Scholar]
- 27. Vachiery JL, Adir Y, Barbera JA, Champion H, Coghlan JG, Cottin V, De Marco T, Galie N, Ghio S, Gibbs JS, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62:D100–D108. doi: 10.1016/j.jacc.2013.10.033 [DOI] [PubMed] [Google Scholar]
- 28. Guazzi M, Ghio S, Adir Y. Pulmonary hypertension in HFpEF and HFrEF: JACC review topic of the week. J Am Coll Cardiol. 2020;76:1102–1111. doi: 10.1016/j.jacc.2020.06.069 [DOI] [PubMed] [Google Scholar]
- 29. Rosenkranz S, Gibbs JS, Wachter R, De Marco T, Vonk‐Noordegraaf A, Vachiery JL. Left ventricular heart failure and pulmonary hypertension. Eur Heart J. 2016;37:942–954. doi: 10.1093/eurheartj/ehv512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fayyaz AU, Edwards WD, Maleszewski JJ, Konik EA, DuBrock HM, Borlaug BA, Frantz RP, Jenkins SM, Redfield MM. Global pulmonary vascular remodeling in pulmonary hypertension associated with heart failure and preserved or reduced ejection fraction. Circulation. 2018;137:1796–1810. doi: 10.1161/CIRCULATIONAHA.117.031608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Breitling S, Ravindran K, Goldenberg NM, Kuebler WM. The pathophysiology of pulmonary hypertension in left heart disease. Am J Physiol Lung Cell Mol Physiol. 2015;309:L924–L941. doi: 10.1152/ajplung.00146.2015 [DOI] [PubMed] [Google Scholar]
- 32. Hunt JM, Bethea B, Liu X, Gandjeva A, Mammen PP, Stacher E, Gandjeva MR, Parish E, Perez M, Smith L, et al. Pulmonary veins in the normal lung and pulmonary hypertension due to left heart disease. Am J Physiol Lung Cell Mol Physiol. 2013;305:L725–L736. doi: 10.1152/ajplung.00186.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ruocco G, Gavazzi A, Gonnelli S, Palazzuoli A. Pulmonary arterial hypertension and heart failure with preserved ejection fraction: are they so discordant? Cardiovasc Diagn Ther. 2020;10:534–545. doi: 10.21037/cdt-19-405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leopold JA. Pulmonary venous remodeling in pulmonary hypertension: the veins take center stage. Circulation. 2018;137:1811–1813. doi: 10.1161/CIRCULATIONAHA.118.033013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huston JH, Shah SJ. Understanding the pathobiology of pulmonary hypertension due to left heart disease. Circ Res. 2022;130:1382–1403. doi: 10.1161/CIRCRESAHA.122.319967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Elsanhoury A, Nelki V, Kelle S, Van Linthout S, Tschope C. Epicardial fat expansion in diabetic and obese patients with heart failure and preserved ejection fraction‐a specific HFpEF phenotype. Front Cardiovasc Med. 2021;8:720690. doi: 10.3389/fcvm.2021.720690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kovacs A, Papp Z, Nagy L. Causes and pathophysiology of heart failure with preserved ejection fraction. Heart Fail Clin. 2014;10:389–398. doi: 10.1016/j.hfc.2014.04.002 [DOI] [PubMed] [Google Scholar]
- 38. Mohammed SF, Hussain I, AbouEzzeddine OF, Takahama H, Kwon SH, Forfia P, Roger VL, Redfield MM. Right ventricular function in heart failure with preserved ejection fraction: a community‐based study. Circulation. 2014;130:2310–2320. doi: 10.1161/CIRCULATIONAHA.113.008461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guazzi M, Naeije R. Pulmonary hypertension in heart failure: pathophysiology, pathobiology, and emerging clinical perspectives. J Am Coll Cardiol. 2017;69:1718–1734. doi: 10.1016/j.jacc.2017.01.051 [DOI] [PubMed] [Google Scholar]
- 40. Harjola VP, Mebazaa A, Celutkiene J, Bettex D, Bueno H, Chioncel O, Crespo‐Leiro MG, Falk V, Filippatos G, Gibbs S, et al. Contemporary management of acute right ventricular failure: a statement from the heart failure association and the working group on pulmonary circulation and right ventricular function of the European Society of Cardiology. Eur J Heart Fail. 2016;18:226–241. doi: 10.1002/ejhf.478 [DOI] [PubMed] [Google Scholar]
- 41. Aloia E, Cameli M, D’Ascenzi F, Sciaccaluga C, Mondillo S. TAPSE: an old but useful tool in different diseases. Int J Cardiol. 2016;225:177–183. doi: 10.1016/j.ijcard.2016.10.009 [DOI] [PubMed] [Google Scholar]
- 42. Zhu Z, Godana D, Li A, Rodriguez B, Gu C, Tang H, Minshall RD, Huang W, Chen J. Echocardiographic assessment of right ventricular function in experimental pulmonary hypertension. Pulmonary Circ. 2019;9:2045894019841987. doi: 10.1177/2045894019841987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rosenkranz S, Lang IM, Blindt R, Bonderman D, Bruch L, Diller GP, Felgendreher R, Gerges C, Hohenforst‐Schmidt W, Holt S, et al. Pulmonary hypertension associated with left heart disease: updated recommendations of the Cologne consensus conference 2018. Int J Cardiol. 2018;272S:53–62. doi: 10.1016/j.ijcard.2018.08.080 [DOI] [PubMed] [Google Scholar]
- 44. Guazzi M, Dixon D, Labate V, Beussink‐Nelson L, Bandera F, Cuttica MJ, Shah SJ. RV contractile function and its coupling to pulmonary circulation in heart failure with preserved ejection fraction: stratification of clinical phenotypes and outcomes. JACC Cardiovasc Imaging. 2017;10:1211–1221. doi: 10.1016/j.jcmg.2016.12.024 [DOI] [PubMed] [Google Scholar]
- 45. Guazzi M, Naeije R, Arena R, Corra U, Ghio S, Forfia P, Rossi A, Cahalin LP, Bandera F, Temporelli P. Echocardiography of right ventriculoarterial coupling combined with cardiopulmonary exercise testing to predict outcome in heart failure. Chest. 2015;148:226–234. doi: 10.1378/chest.14-2065 [DOI] [PubMed] [Google Scholar]
- 46. Damy T, Kallvikbacka‐Bennett A, Goode K, Khaleva O, Lewinter C, Hobkirk J, Nikitin NP, Dubois‐Rande JL, Hittinger L, Clark AL, et al. Prevalence of, associations with, and prognostic value of tricuspid annular plane systolic excursion (TAPSE) among out‐ patients referred for the evaluation of heart failure. J Card Fail. 2012;18:216–225. doi: 10.1016/j.cardfail.2011.12.003 [DOI] [PubMed] [Google Scholar]
- 47. Friedberg MK, Redington AN. Right versus left ventricular failure: differences, similarities, and interactions. Circulation. 2014;129:1033–1044. doi: 10.1161/CIRCULATIONAHA.113.001375 [DOI] [PubMed] [Google Scholar]
- 48. Buckberg GD, Hoffman JI, Coghlan HC, Nanda NC. Ventricular structure‐function relations in health and disease: part II. Clinical considerations. Eur J Cardiothorac Surg. 2015;47:778–787. doi: 10.1093/ejcts/ezu278 [DOI] [PubMed] [Google Scholar]
- 49. Plunkett MD, Buckberg GD. Pathophysiologic implications of the helical ventricular myocardial band: considerations for right ventricular restoration. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2007; 10:68–75. doi: 10.1053/j.pcsu.2007.01.011 [DOI] [PubMed] [Google Scholar]
- 50. Dewachter C, Dewachter L, Rondelet B, Fesler P, Brimioulle S, Kerbaul F, Naeije R. Activation of apoptotic pathways in experimental acute afterload‐induced right ventricular failure. Crit Care Med. 2010;38:1405–1413. doi: 10.1097/CCM.0b013e3181de8bd3 [DOI] [PubMed] [Google Scholar]
- 51. Bernardo RJ, Haddad F, Couture EJ, Hansmann G, de Jesus Perez VA, Denault AY, de Man FS, Amsallem M. Mechanics of right ventricular dysfunction in pulmonary arterial hypertension and heart failure with preserved ejection fraction. Cardiovasc Diagn Ther. 2020;10:1580–1603. doi: 10.21037/cdt-20-479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Raina A, Meeran T. Right ventricular dysfunction and its contribution to morbidity and mortality in left ventricular heart failure. Curr Heart Fail Rep. 2018;15:94–105. doi: 10.1007/s11897-018-0378-8 [DOI] [PubMed] [Google Scholar]
- 53. Aschauer S, Kammerlander AA, Zotter‐Tufaro C, Ristl R, Pfaffenberger S, Bachmann A, Duca F, Marzluf BA, Bonderman D, Mascherbauer J. The right heart in heart failure with preserved ejection fraction: insights from cardiac magnetic resonance imaging and invasive haemodynamics. Eur J Heart Fail. 2016;18:71–80. doi: 10.1002/ejhf.418 [DOI] [PubMed] [Google Scholar]
- 54. Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype‐specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation. 2016;134:73–90. doi: 10.1161/CIRCULATIONAHA.116.021884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rabinovitch M, Guignabert C, Humbert M, Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res. 2014;115:165–175. doi: 10.1161/CIRCRESAHA.113.301141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chirinos JA, Orlenko A, Zhao L, Basso MD, Cvijic ME, Li Z, Spires TE, Yarde M, Wang Z, Seiffert DA, et al. Multiple plasma biomarkers for risk stratification in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2020;75:1281–1295. doi: 10.1016/j.jacc.2019.12.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Collier P, Watson CJ, Voon V, Phelan D, Jan A, Mak G, Martos R, Baugh JA, Ledwidge MT, McDonald KM. Can emerging biomarkers of myocardial remodelling identify asymptomatic hypertensive patients at risk for diastolic dysfunction and diastolic heart failure? Eur J Heart Fail. 2011;13:1087–1095. doi: 10.1093/eurjhf/hfr079 [DOI] [PubMed] [Google Scholar]
- 58. van Heerebeek L, Hamdani N, Falcao‐Pires I, Leite‐Moreira AF, Begieneman MP, Bronzwaer JG, van der Velden J, Stienen GJ, Laarman GJ, Somsen A, et al. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation. 2012;126:830–839. doi: 10.1161/CIRCULATIONAHA.111.076075 [DOI] [PubMed] [Google Scholar]
- 59. Liu SF, Yan Y. Animal models of pulmonary hypertension due to left heart disease. Anim Model Exp Med. 2022;5:197–206. doi: 10.1002/ame2.12214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhuang R, Wu J, Lin F, Han L, Liang X, Meng Q, Jiang Y, Wang Z, Yue A, Gu Y, et al. Fasudil preserves lung endothelial function and reduces pulmonary vascular remodeling in a rat model of end‐stage pulmonary hypertension with left heart disease. Int J Mol Med. 2018;42:1341–1352. doi: 10.3892/ijmm.2018.3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lambert M, Mendes‐Ferreira P, Ghigna MR, LeRibeuz H, Adao R, Boet A, Capuano V, Rucker‐Martin C, Bras‐Silva C, Quarck R, et al. Kcnk3 dysfunction exaggerates the development of pulmonary hypertension induced by left ventricular pressure overload. Cardiovasc Res. 2021;117:2474–2488. doi: 10.1093/cvr/cvab016 [DOI] [PubMed] [Google Scholar]
- 62. Zhang YT, Xue JJ, Wang Q, Cheng SY, Chen ZC, Li HY, Shan JJ, Cheng KL, Zeng WJ. Dehydroepiandrosterone attenuates pulmonary artery and right ventricular remodeling in a rat model of pulmonary hypertension due to left heart failure. Life Sci. 2019;219:82–89. doi: 10.1016/j.lfs.2018.12.056 [DOI] [PubMed] [Google Scholar]
- 63. Chen Y, Guo H, Xu D, Xu X, Wang H, Hu X, Lu Z, Kwak D, Xu Y, Gunther R, et al. Left ventricular failure produces profound lung remodeling and pulmonary hypertension in mice: heart failure causes severe lung disease. Hypertension. 2012;59:1170–1178. doi: 10.1161/HYPERTENSIONAHA.111.186072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ranchoux B, Nadeau V, Bourgeois A, Provencher S, Tremblay E, Omura J, Cote N, Abu‐Alhayja’a R, Dumais V, Nachbar RT, et al. Metabolic syndrome exacerbates pulmonary hypertension due to left heart disease. Circ Res. 2019;125:449–466. doi: 10.1161/CIRCRESAHA.118.314555 [DOI] [PubMed] [Google Scholar]
- 65. Lai YC, Tabima DM, Dube JJ, Hughan KS, Vanderpool RR, Goncharov DA, St Croix CM, Garcia‐Ocana A, Goncharova EA, Tofovic SP, et al. SIRT3‐AMP‐activated protein kinase activation by nitrite and metformin improves hyperglycemia and normalizes pulmonary hypertension associated with heart failure with preserved ejection fraction. Circulation. 2016;133:717–731. doi: 10.1161/CIRCULATIONAHA.115.018935 [DOI] [PMC free article] [PubMed] [Google Scholar]


