Abstract
Background
Socioeconomic factors may lead to a disproportionate impact on health care usage and death among individuals with congenital heart defects (CHD) by race, ethnicity, and socioeconomic factors. How neighborhood poverty affects racial and ethnic disparities in health care usage and death among individuals with CHD across the life span is not well described.
Methods and Results
Individuals aged 1 to 64 years, with at least 1 CHD‐related International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) code were identified from health care encounters between January 1, 2011, and December 31, 2013, from 4 US sites. Residence was classified into lower‐ or higher‐poverty neighborhoods on the basis of zip code tabulation area from the 2014 American Community Survey 5‐year estimates. Multivariable logistic regression models, adjusting for site, sex, CHD anatomic severity, and insurance‐evaluated associations between race and ethnicity, and health care usage and death, stratified by neighborhood poverty. Of 31 542 individuals, 22.2% were non‐Hispanic Black and 17.0% Hispanic. In high‐poverty neighborhoods, non‐Hispanic Black (44.4%) and Hispanic (47.7%) individuals, respectively, were more likely to be hospitalized (adjusted odds ratio [aOR], 1.2 [95% CI, 1.1–1.3]; and aOR, 1.3 [95% CI, 1.2–1.5]) and have emergency department visits (aOR, 1.3 [95% CI, 1.2–1.5] and aOR, 1.8 [95% CI, 1.5–2.0]) compared with non‐Hispanic White individuals. In high poverty neighborhoods, non‐Hispanic Black individuals with CHD had 1.7 times the odds of death compared with non‐Hispanic White individuals in high‐poverty neighborhoods (95% CI, 1.1–2.7). Racial and ethnic disparities in health care usage were similar in low‐poverty neighborhoods, but disparities in death were attenuated (aOR for non‐Hispanic Black, 1.2 [95% CI=0.9–1.7]).
Conclusions
Racial and ethnic disparities in health care usage were found among individuals with CHD in low‐ and high‐poverty neighborhoods, but mortality disparities were larger in high‐poverty neighborhoods. Understanding individual‐ and community‐level social determinants of health, including access to health care, may help address racial and ethnic inequities in health care usage and death among individuals with CHD.
Keywords: congenital heart defect, death, health care usage, poverty, race
Subject Categories: Epidemiology, Congenital Heart Disease
Nonstandard Abbreviations and Acronyms
- CDC
Centers for Disease Control and Prevention
- nHB
non‐Hispanic Black
- nHW
non‐Hispanic White
Clinical Perspective.
What Is New?
Non‐Hispanic Black individuals with congenital heart defects who reside in higher‐poverty neighborhoods have a higher mortality rate compared with non‐Hispanic White individuals with congenital heart defects residing in high‐poverty neighborhoods, an effect mitigated in lower‐poverty neighborhoods.
Increased emergency department visits and hospitalizations were associated with race and ethnicity in both high‐ and low‐poverty neighborhoods.
What Are the Clinical Implications?
Providers should be aware of the adverse effects on outcomes of race, ethnicity and neighborhood poverty and take steps to mitigate this risk.
Congenital heart defects (CHD) affect ≈1% of all births in the United States. 1 Advances in surgery, technology, and perioperative care for children with CHD have improved survival to adulthood, 2 , 3 , 4 , 5 , 6 , 7 , 8 resulting in more adults than children with CHD in the United States. 9 , 10 Between 1998 and 2005, the number of hospital admissions for adults with CHD more than doubled 11 and death from CHD has decreased, 12 yet racial and ethnic and socioeconomic disparities in health care usage and death exist.
Social determinants of health are associated with racial and ethnic disparities in health care usage and death in the population, and disparities in outcomes have been described among children with CHD. Children with CHD within racial and ethnic minority groups, compared with White children with CHD, have been found to experience greater health care lapses among those who underwent CHD surgery, 13 higher severity of illness scores, 14 increased odds of complications, 15 , 16 greater odds of surgery at lower‐volume CHD surgical centers for those with hypoplastic left heart syndrome, 8 and higher mortality rates. 14 , 15 , 17 , 18 , 19 The National Institute of Minority Health and Health Disparities Research Framework considers multiple domains and levels of influence to conceptualize health disparities, including the role of community environment and resources, and health care policies to better understand the relationship of structural discrimination and health disparities. 20 , 21
Socioeconomic factors and neighborhood poverty are key social determinants of health associated with health care usage and death among individuals with CHD. While previous studies have shown a decrease in death from CHD, 12 , 22 , 23 studies have shown disparities in death by race, ethnicity, and type of health insurance. 12 , 24 Among children undergoing CHD surgery, those with private insurance had improved outcomes, 12 , 15 , 16 , 25 , 26 and those from low‐ compared with high‐income neighborhoods had higher mortality rates. 27 Similarly, among adolescents and adults with CHD in a Colorado cohort, 28 poverty was associated with higher rates of hospitalization, emergency department (ED) visits, and adverse cardiac outcomes.
Few studies have examined how social determinants of health, including neighborhood poverty, mediate the relationship between race and ethnicity and health care usage and death among individuals, including adults, with CHD. One study among individuals with CHD examined the modifying effect of neighborhood household income on the relationship between race and death, finding that Black individuals had a longer length of stay and higher mortality rates compared with their White counterparts, with death potentiated by lower neighborhood income. 29 A review by Richardson et al showed significant racial and ethnic disparity in health care usage among individuals with CHD, and socioeconomic factors mediated the risk. 15 To expand knowledge on this topic, this study aims to examine the association between race and ethnicity and health care usage (outpatient visits, hospitalizations, and ED visits) and death, by neighborhood poverty status, among individuals with CHD aged 1 to 64 years. Study findings may help determine disparities in morbidity and death by race and ethnicity and neighborhood poverty status among people with CHD.
METHODS
Data Availability
The data that support the findings of this study are available from the Centers for Disease Control and Prevention (CDC). Restrictions apply to the availability of these data, which were used under license for this study. Data are available with permission from the CDC. Contact Jill Glidewell at iyp0@cdc.gov.
This retrospective study of children and adults with CHD used data from 4 sites (Georgia, North Carolina, New York, and Utah) participating in a collaborative CHD surveillance project funded by the CDC (CDC‐RFA‐DD15‐1506). The CDC funded 5 sites via a competitive mechanism on the basis of suitability to conduct CHD surveillance activities. One site, Colorado, was unable to provide socioeconomic data necessary for the analysis; thus, data from this site were excluded from this analysis. Individuals with CHD were identified on the basis of at least 1 CHD‐related International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) code within the 745.xx to 747.xx range. 30 These codes were identified from electronic administrative and clinical sources, state Medicaid claims, state vital records, and birth defect registries. Compilation and sharing of deidentified data with the CDC were approved by each site's institutional review board with complete Health Insurance Portability and Accountability Act waiver of consent. A detailed methodology of the parent project has been published. 30
CHD Case Classification
CHD diagnostic codes, which are based on native CHD anatomy, were categorized into 1 of 5 mutually exclusive hierarchical CHD severity groupings similar to the Marelli et al classification scheme, 2 integrating both hemodynamic severity and basic anatomy: (1) severe; (2) shunt (excluding isolated 745.5 secundum atrial septal defect/patent foramen ovale); (3) valve; (4) shunt and valve lesions; and (5) “other” CHDs. 30 Severe CHD includes endocardial cushion defects (745.6/745.60/745.69), interrupted aortic arch (747.11), tetralogy of Fallot (745.2), total anomalous pulmonary venous return (747.41), tricuspid atresia (746.1), transposition complexes (745.1/745.10/745.11/745.12/745.19), truncus arteriosus (745.0), and univentricular hearts (745.3). Individuals with multiple CHD‐related ICD‐9‐CM codes who had at least 1 severe code were classified as having a severe condition regardless of the number of nonsevere codes they had.
Inclusion and Exclusion Criteria
The 2011 to 2013 population included 72 433 individuals with ≥1 health care encounters during the surveillance period with a CHD‐related ICD‐9‐CM code. Exclusions included those (1) whose age was <1 year or >64 years (n=10 181 excluded, where (10 181/72443=14.1% of total)), leaving 62 252; cases with age<1 year were excluded due to the known high rate of spontaneous closure of ventricular septal defect, atrial septal defect, and patent ductus arteriosus in early infancy, 1 which could lead to inaccurate reporting of outcomes among the younger population in this study; (2) diagnosed with a 745.5 code in isolation (secundum atrial septal defect/patent foramen ovale) or in combination with either 746.89 (other specified anomalies of the heart) or 746.9 (unspecified anomaly of the heart) (n=14 228 excluded, where 14 228/62252=22.8% of the remaining total, leaving 48 024); (3) diagnosed with only “other CHD” code (n=11 885 excluded, where 11 885/47520=25.0% of the remaining total, leaving 36 139), as these codes are known to have poor positive predictive value for CHD 31 , 32 ; (4) with unknown sex (n=1 excluded, where 1/36139 = <1.0% of the remaining total, leaving 36 138); (5) with unknown neighborhood income status (n=249 excluded, where 249/36138=0.7% of the remaining total, leaving 35 889); (6) with unknown neighborhood poverty status (n=1 excluded, where 1/35889=<1.0% of the remaining total, leaving 35 888); and (7) with unknown race and ethnicity (n=4346 excluded, where 4346/35888=12.1% of the remaining total, leaving 31 542) (Figure).
Figure 1. CHD Cohort construction.
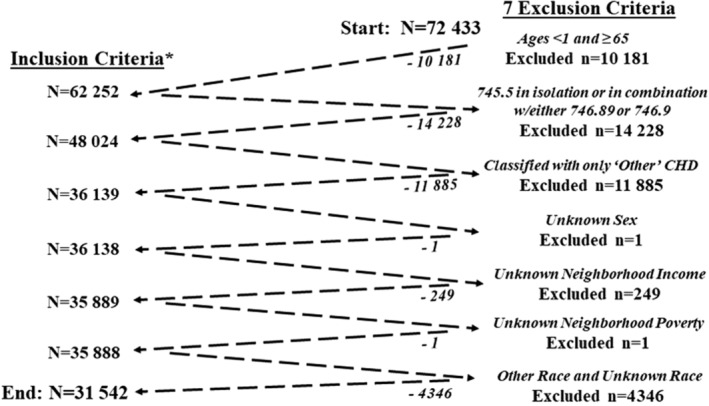
*Case inclusion definition: individuals aged 1 to 64 y from 4 sites (Georgia, North Carolina, New York, and Utah) who (1) had at least 1 health care encounter between January 1, 2011 and December 31, 2013; (2) had at least 1 ICD‐9‐CM CHD‐related code 30 ; and (3) resided in site‐specific catchment areas. CHD indicates congenital heart defects; and ICD‐9‐CM, International Classification of Diseases, Ninth Revision, Clinical Modification.
After implementing the above exclusions, 31 542 individuals with probable CHD aged 1 to 64 years, who (1) had at least 1 health care encounter between January 1, 2011, and December 31, 2013; (2) had at least 1 ICD‐9‐CM CHD‐related code documented 30 ; and (3) resided in the 4 site‐specific catchment areas were eligible and included in the analysis. Site‐specific catchment areas spanned the entire state for individuals who resided in North Carolina and Utah. For Georgia, data were collected from individuals residing within 1 of the 5 metropolitan Atlanta counties (Clayton, Cobb, DeKalb, Fulton, and Gwinnett), and for New York, individuals resided in 1 of 11 New York counties (Allegany, Bronx, Cattaraugus, Chautauqua, Erie, Genesee, Monroe, Niagara, Orleans, Wyoming, and Westchester). The excluded Colorado site had 6511 cases, of whom 1988 were aged between 11 and 18 years, with no children aged <11 years. Of the Colorado cases, race was unknown for 33.5%, 54% were White individuals and 3% were Black individuals. 30 Details of the cohort from each site can be found in Glidewell et al. 30
Study Variables
Health Care Usage (Outcome Variable)
Health care usage was categorized as outpatient visits, hospitalizations, and ED visits. If an ED visit led to a hospitalization, the encounter was captured as a hospitalization. Separately by encounter type, the number of encounters were calculated by combining overlapping date ranges into a single encounter, summing encounters over the surveillance period, and dichotomizing as 0 and ≥ 1. When dates of different encounter types overlapped, a hierarchical scheme was applied in the following order: (1) inpatient hospitalizations; (2) ED visits; and (3) outpatient visits.
Death (Outcome Variable)
Mortality status was determined by deterministically linking individuals to state‐specific death certificates at each site. If the individual did not match to a death record during the surveillance period, the person was considered alive.
Race and Ethnicity
Race and ethnicity were based on data recorded in the electronic health records and categorized as non‐Hispanic White (nHW), non‐Hispanic Black (nHB), Hispanic, and other race (Asian, American Indian/Native American, Native Hawaiian/Pacific Islander, and multiracial [excluding Black multiracial]). Individuals could have >1 race recorded. For this analysis, individuals with nHB race were categorized as nHB, even if they had other races recorded. All other individuals with multiple races recorded were categorized as multiracial (n=161/31542). For purposes of analysis, racial groups comprising a small percentage of the data set were combined into a category called “Other” because the sample sizes were too small.
Neighborhood Economic Status
Two metrics were used to examine neighborhood economic status: (1) neighborhood median income and (2) neighborhood poverty status. Values for each metric were based on individuals' zip code tabulation area from the 2014 American Community Survey 5‐year estimates, estimated across the years 2010 to 2014. 33 Annual neighborhood median income in US dollars was classified into 3 groups: <$40 000, $40 000 to $75 000, and >$75 000. Neighborhood poverty status was defined as the percentage of households in the zip code tabulation area below 100% of the federal poverty level and was classified into low neighborhood poverty (≤25% of households below federal poverty level) and high neighborhood poverty (>25% of households below federal poverty level).
Other Co‐Variables
Individual‐level covariates were determined on the basis of literature review and included site, age, sex, CHD anatomic severity, and health insurance. Age in years was calculated by subtracting the individual's date of birth from the date of the individual's first health care encounter during the 2011 to 2013 surveillance period where an eligible CHD‐related ICD‐9‐CM code appeared. Age was categorized into 4 groups: 1 to 10 years, 11 to 18 years, 19 to 44 years, and 45 to 64 years. Sex was classified as male or female. CHD anatomic severity was categorized as (1) severe, (2) shunt (excluding isolated 745.5 secundum atrial septal defect/patent foramen ovale), (3) valve, and (4) shunt and valve lesions. Health insurance status was classified as (1) “any public” if health insurance was documented at any health care encounter as Medicaid or Medicare; (2) “private” if health insurance was documented at all health care encounters as a private company or other government, which includes military, Veterans Affairs, Tricare, and other federal employee insurance benefits or other health insurance coverage; (3) “none” if all health care encounters indicated self‐pay, uninsured, or “no insurance coverage”; and (4) “unknown” when health insurance status was unavailable.
Statistical Analysis
SAS version 9.4 (SAS Institute Inc., Cary, NC) was used for all analyses. Descriptive analyses were conducted to examine the frequency distributions and percentages of individual characteristics by race and ethnicity. Bivariate analyses were conducted to describe and compare the associations between race and ethnicity, the covariables, and health care usage (inpatient hospitalizations, ED visits, and outpatient visits) and death, using 2‐sided χ2 tests. P<0.05 was considered statistically significant. Effect modification was considered a priori by neighborhood poverty status. Adjusted odds ratios (aORs), 95% CIs and P values were estimated using multivariable logistic regression analysis. Potential confounders were selected applying a 10% change‐in‐estimate criterion. Models were stratified by neighborhood poverty status.
RESULTS
There were 31 542 individuals with CHD identified from 4 surveillance sites who were eligible for analysis. Overall, 57.6% were nHW individuals, 22.2% were nHB individuals, 17.0% were Hispanic individuals, and 3.2% were another race and ethnicity (Table 1 ). Distribution of all demographics and health care types (P<0.001) and mortality rate (P<0.001) differed by race and ethnicity. Among nHW individuals with CHD, 32.0% were 1 to 10 years of age as compared with 51.3% among nHB individuals and 51.5% among Hispanic individuals with CHD. The proportion of individuals with CHD aged 45 to 64 years was lower than all other age groups in all racial and ethnic groups. Among nHW individuals with CHD, 18.9% were aged 45 to 64 years as compared with 10.9% and 10.5% for nHB and Hispanic individuals, respectively. Additionally, ≈70% of nHB individuals (70.0%) and Hispanic individuals (69.1%) were aged between 1 and 18 years, compared with slightly >50% of nHW individuals (53.1%). Overall, median age was 14.0 years. Among nHW individuals with CHD, valve lesions were the most common (44.8%), while shunt lesions were most prevalent among nHB (35.1%) and Hispanic (38.7%) individuals. Additionally, 33.9% of nHW individuals with CHD had public health insurance compared with 71.3% of nHB and 82.0% of Hispanic individuals. Similar patterns of neighborhood median income and poverty status emerged, with <20% of nHW and close to half of nHB and Hispanic individuals living in lower‐income/higher‐poverty neighborhoods. For all characteristics, values for individuals with “other” race and ethnicity generally mirrored those of nHB and Hispanic individuals or fell between those of nHW and nHB/Hispanic individuals. Among individuals with CHD, 93.1% had at least 1 outpatient visit, 42.9% had at least 1 inpatient hospitalization, and 34.2% visited the ED at least once (Table 1). All 3 encounter types differed significantly by race and ethnicity (P<0.001 for all). Outpatient care was the most frequent across all racial and ethnic groups. Overall, death of individuals with CHD during the surveillance period was 1.2%, ranging from 0.9% for Hispanic individuals to 1.6% for nHB individuals (P<0.001) (Table 1). Patterns in mortality among nHW, nHB, and Hispanic individuals were similar for younger (1–18 years; nHW, 0.5%; nHB, 0.8%, Hispanic, 0.6%; P<0.05) and older age groups (19–64 years; nHW, 1.8%; nHB, 3.5%, Hispanic, 1.7%;P<0.001), with nHB individuals having higher mortality rates than nHW and Hispanic individuals (data not shown).
Table 1.
Characteristics of Individuals With CHDs, Total and by Racial and Ethnic Group
| Characteristics | Total N=31 542 | Non‐Hispanic White n=18 173 (57.6%) | Non‐Hispanic Black n=6991 (22.2%) | Hispanic n=5365 (17.0%) | Other* n=1013 (3.2%) | P value† |
|---|---|---|---|---|---|---|
| N (%) | n (%)‡ | n (%)‡ | n (%)‡ | n (%)‡ | ||
| Site | ||||||
| Georgia | 6070 (19.2) | 2854 (15.7) | 2415 (34.5) | 522 (9.7) | 279 (27.5) | <0.001§ |
| North Carolina | 11 830 (37.5) | 8033 (44.2) | 2454 (35.1) | 981 (18.3) | 362 (35.7) | |
| New York | 10 240 (32.5) | 4247 (23.4) | 2091 (29.9) | 3534 (65.9) | 368 (36.3) | |
| Utah | 3402 (10.8) | 3039 (16.7) | 31 (0.4) | 328 (6.1) | <10 (−‐) | |
| Age¶, y | ||||||
| 1–10 | 12 700 (40.3) | 5815 (32.0) | 3585 (51.3) | 2765 (51.5) | 535 (52.8) | <0.001§ |
| 11–18 | 6251 (19.8) | 3842 (21.2) | 1311 (18.7) | 943 (17.6) | 155 (15.3) | |
| 19–44 | 7705 (24.4) | 5076 (27.9) | 1333 (19.1) | 1096 (20.4) | 200 (19.8) | |
| 45–64 | 4886 (15.5) | 3440 (18.9) | 762 (10.9) | 561 (10.5) | 123 (12.1) | |
| Age, y | ||||||
| 1–18 | 18 951 (60.1) | 9657 (53.1) | 4896 (70.0) | 3708 (69.1) | 690 (68.1) | <0.001§ |
| 19–64 | 12 591 (39.9) | 8516 (46.9) | 2095 (30.0) | 1657 (30.9) | 323 (31.9) | |
| Sex | ||||||
| Female | 15 376 (48.8) | 8295 (46.6) | 3718 (53.2) | 2834 (52.8) | 529 (52.2) | <0.001§ |
| Male | 16 166 (51.2) | 9878 (54.4) | 3273 (46.8) | 2531 (47.2) | 484 (47.8) | |
| CHD anatomic severity | ||||||
| Severe | 7815 (24.8) | 4488 (24.7) | 1829 (26.2) | 1230 (22.9) | 268 (26.5) | <0.001§ |
| Shunt | 9067 (28.8) | 4163 (22.9) | 2452 (35.1) | 2076 (38.7) | 376 (37.1) | |
| Valve | 12 059 (38.2) | 8145 (44.8) | 2052 (29.3) | 1601 (29.8) | 261 (25.8) | |
| Shunt and valve | 2601 (8.2) | 1377 (7.6) | 658 (9.4) | 458 (8.6) | 108 (10.6) | |
| Health insurance‖ | ||||||
| Any public | 16 071 (51.0) | 6157 (33.9) | 4987 (71.3) | 4399 (82.0) | 528 (52.1) | <0.001§ |
| Private | 12 561 (39.8) | 10 063 (55.4) | 1440 (20.6) | 700 (13.1) | 358 (35.3) | |
| None | 310 (1.0) | 148 (0.8) | 87 (1.2) | 67 (1.3) | <10 (−‐) | |
| Unknown | 2600 (8.2) | 1805 (9.9) | 477 (6.8) | 199 (3.7) | 119 (11.8) | |
| Neighborhood median income, US$ | ||||||
| <$40 000 | 10 003 (31.7) | 3295 (18.1) | 3313 (47.4) | 3039 (56.7) | 356 (35.1) | <0.001§ |
| $40 000–$75 000 | 17 031 (54.0) | 11 142 (61.3) | 3398 (48.6) | 2035 (37.9) | 456 (45.0) | |
| >$75 000 | 4508 (14.3) | 3736 (20.6) | 280 (4.0) | 291 (5.4) | 201 (19.9) | |
| Neighborhood poverty status | ||||||
| Low‐poverty neighborhood (<25% households below FPL) | 21 234 (67.3) | 14 684 (80.8) | 3587 (51.3) | 2328 (43.4) | 635 (62.7) | <0.001§ |
| High‐poverty neighborhood (≥25% households below FPL) | 10 308 (32.7) | 3489 (19.2) | 3404 (48.7) | 3037 (56.6) | 378 (37.3) | |
| Health care usage | ||||||
| ≥1 outpatient visits | 29 352 (93.1) | 16 730 (92.1) | 6532 (93.4) | 5175 (96.5) | 915 (90.3) | <0.001§ |
| ≥1 hospitalizations | 13 523 (42.9) | 7318 (40.3) | 3099 (44.3) | 2599 (48.4) | 507 (50.1) | <0.001§ |
| ≥1 emergency department visits | 10 778 (34.2) | 4827 (26.6) | 2453 (35.1) | 3226 (60.1) | 272 (26.9) | <0.001§ |
| Death | ||||||
| Yes | 374 (1.2) | 197 (1.1) | 115 (1.6) | 50 (0.9) | 12 (1.2) | <0.001§ |
Cell sizes <10 are not displayed directly and are reported as “<10” per site‐specific cell size suppression policy; this policy does not affect the reporting of cell size values of “0,” which are reported directly. CHD indicates congenital heart defect; and FPL, federal poverty level.
Other racial and ethnic group includes Asian, American Indian/Native American, Native Hawaiian or Other Pacific Islander, and multiracial. Unknown race excluded from analyses.
χ2 tests compare racial and ethnic groups; analysis does not include “Total” column.
Column percentages reported in cells. Cell sizes <10 are not displayed directly and are reported as “<10” per site‐specific cell size suppression policy; cell size values of “0” are reported directly.
Statistically significant adjusted odds ratio values are bolded.
Estimates for 1‐ to 10‐year‐olds are based on 3 sites: 5 counties in metropolitan Atlanta, Georgia; 11 counties in New York state; and statewide in North Carolina; estimates for 11‐ to 18‐, 19‐ to 44‐, and 45‐ to 64‐year‐olds are based on 4 sites: 5 counties in metropolitan Atlanta, Georgia; 11 counties in New York; and statewide in North Carolina and Utah. Overall, median age was 14.0 y.
Insurance categorization: public defined as Medicaid or Medicare; private defined as private, other government, or other insurance; none defined as self‐pay/uninsured; and unknown defined as unavailable, unknown, or no insurance indicated.
Stratified results by neighborhood poverty status were examined in an effort to assess effect modification (Table 2). Within high‐poverty neighborhoods, nHB and Hispanic individuals together (62.5%; 33.0% nHB individuals and 29.5% Hispanic individuals) accounted for the largest proportion of individuals compared with nHW individuals (33.9%) or other races (3.7%), whereas in low‐poverty neighborhoods, nHW individuals (69.2%) comprised the majority compared with the 27.9% of nHB and Hispanic individuals combined (16.9% nHB individuals and 11.0% Hispanic individuals or other races [3.0%]; P<0.0001) (data not shown). Among those in high‐poverty neighborhoods, nHB individuals (44.4%), Hispanic individuals (47.7%), and individuals of other race and ethnicity (55.0%), respectively, had 1.2 (95% CI, 1.1–1.3; P<0.01), 1.3 (95% CI, 1.2–1.5; P<0.001) and 1.7 (95% CI, 1.3–2.1; P<0.001) times higher odds of being hospitalized than nHW individuals (42.3%; referent), respectively, after adjusting for confounders. nHB individuals (38.9%; aOR, 1.3 [95% CI, 1.2–1.5]; P<0.001) and Hispanic individuals (66.0%; aOR, 1.8 [95% CI, 1.5–2.0]; P<0.001) also had higher aOR of ED visits compared with nHW individuals (26.2%; referent). In addition, in high‐poverty neighborhoods, 1.1% of nHW individuals (referent), 1.9% of nHB individuals (aOR, 1.7 [95% CI, 1.1–2.7]; P<0.05), and 0.9% of Hispanic individuals (aOR, 0.7 [95% CI, 0.4–1.3]; P=ns) died during the surveillance period. Among those in low‐poverty neighborhoods, associations between race and ethnicity and health care usage were of similar magnitude and direction as those observed in high‐poverty neighborhoods, with the exceptions that Hispanic individuals (95.8%) had 1.4 (95% CI, 1.1–1.7; P<0.01) times higher aOR of outpatient visits compared with nHW (92.0%; referent), and associations between death and race and ethnicity between nHB (1.5%) and nHW individuals (1.1%) were attenuated (nHB: aOR, 1.2 [95% CI, 0.9–1.7]; P=ns).
Table 2.
Associations Between Race and Ethnicity and Health Care Usage and Death, by Neighborhood Poverty Status Among Individuals With CHDs
| Outpatient visits* | Hospitalizations* | Emergency department visits* | Death | |||||
|---|---|---|---|---|---|---|---|---|
| n (%) | aOR (95% CI) | n (%) | aOR (95% CI) | n (%) | aOR (95% CI) | n (%) | aOR (95% CI) | |
| High‐poverty neighborhood (≥25% households below federal poverty level†) | ||||||||
| Non‐Hispanic White | 3221 (92.3) | 1.0 (referent) | 1475 (42.3) | 1.0 (referent) | 914 (26.2) | 1.0 (referent) | 38 (1.1) | 1.0 (referent) |
| Non‐Hispanic Black | 3207 (94.2) | 0.9 (0.7–1.1) | 1511 (44.4) | 1.2 (1.1–1.3)‡ | 1325 (38.9) | 1.3 (1.2–1.5)‡ | 63 (1.9) | 1.7 (1.1–2.7)‡ |
| Hispanic | 2944 (96.9) | 1.0 (0.7–1.3) | 1449 (47.7) | 1.3 (1.2–1.5)‡ | 2004 (66.0) | 1.8 (1.5–2.0)‡ | 27 (0.9) | 0.7 (0.4–1.3) |
| Other§ | 342 (90.5) | 0.6 (0.4–0.9)‡ | 208 (55.0) | 1.7 (1.3–2.1)‡ | 119 (31.5) | 0.7 (0.5–0.9)‡ | <10 – | 1.0 (0.4–3.0) |
| Low‐poverty neighborhood (<25% households below federal poverty level†) | ||||||||
| Non‐Hispanic White | 13 509 (92.0) | 1.0 (referent) | 5843 (39.8) | 1.0 (referent) | 3913 (26.7) | 1.0 (referent) | 159 (1.1) | 1.0 (referent) |
| Non‐Hispanic Black | 3325 (92.7) | 0.9 (0.8–1.0) | 1588 (44.3) | 1.2 (1.1–1.3)‡ | 1128 (31.5) | 1.6 (1.5–1.8)‡ | 52 (1.5) | 1.2 (0.9–1.7) |
| Hispanic | 2231 (95.8) | 1.4 (1.1–1.7)‡ | 1150 (49.4) | 1.5 (1.3–1.6)‡ | 1222 (52.5) | 2.0 (1.8–2.2)‡ | 23 (1.0) | 0.7 (0.5–1.2) |
| Other§ | 573 (90.2) | 0.9 (0.7–1.2) | 299 (47.1) | 1.4 (1.2–1.7)‡ | 153 (24.1) | 1.0 (0.8–1.2) | <10 – | 1.4 (0.7–2.9) |
Cell sizes <10 are not displayed directly and are reported as “<10” per site‐specific cell size suppression policy; cell size values of “0” are reported directly. aOR indicates adjusted odds ratio; and CHD, congenital heart defect.
Zero counts for visit types are not included; health care usage visit categories are not mutually exclusive.
Assessed on the basis of individuals' zip code tabulation area from the 2014 American Community Survey 5‐year estimates, estimated across years 2010 to 2014 (US Census Bureau, 2014).
Statistically significant aOR values are bolded.
Other racial and ethnic group includes Asian, American Indian/Native American, Native Hawaiian or Other Pacific Islander, and multiracial. Unknown race excluded from analyses.
We conducted separate stratified analyses for younger (aged 1–18 years) and older (aged 19–64 years) individuals with CHD (Table S1). Among both younger and older age groups, all 3 encounter types differed significantly by race and ethnicity (P<0.001 for each). Among both the younger and older age groups, nHB and Hispanic individuals had the highest point prevalence estimates for outpatient visits (younger: 93.7% nHB individuals and 96.6% Hispanic individuals; older: 92.9% nHB individuals and 96.1% Hispanic individuals), hospitalizations (younger: 38.3% nHB individuals and 42.5% Hispanic individuals; older: 58.4% nHB individuals and 61.9% Hispanic individuals), and ED visits (younger: 31.9% nHB individuals and 56.7% Hispanic individuals; older: 42.6 nHB individuals and 67.8% Hispanic individuals).
Table S2 presents stratified results by age and neighborhood poverty status in an effort to examine effect modification more closely. Among adults in high‐poverty neighborhoods, nHB (aOR, 1.6 [95% CI, 1.1–2.3]; P<0.05), and Hispanic adults (aOR, 1.8 [95% CI, 1.1–3.0]; P<0.05) had higher prevalence of outpatient visits than nHW adults. However, associations were in the opposite direction for children, where nHB (aOR, 0.7 [95% CI, 0.5–0.9]; P<0.01) and Hispanic children (aOR, 0.8 [95% CI, 0.6–1.1]; P=ns) had lower prevalence of outpatient visits than their nHW counterparts. In higher‐poverty neighborhoods, racial and ethnic differences were revealed for hospitalizations for children with CHD but not for adults with CHD. After adjusting for confounders, the odds of hospitalization among nHB (aOR, 1.2 [95% CI, 1.0–1.3]; P<0.05) and Hispanic children (aOR, 1.4 [95% CI, 1.2–1.6];, P<0.001) was higher compared with nHW children, while the likelihood of hospitalization did not differ for nHB (aOR, 1.0 [95% CI, 0.8–1.2]; P=ns) or Hispanic adults (aOR, 1.1 [95% CI, 0.9–1.4]; P=ns) compared with nHW adults. No substantial racial and ethnic differences were revealed between younger or older individuals with CHD living in either high‐ or low‐poverty neighborhoods for ED visits or death.
DISCUSSION
In this 3‐year multistate health administrative data‐based study of racial and ethnic disparities in health care usage and death among children and adults with CHD aged 1 to 64 years, we found that nHB and Hispanic individuals were significantly more likely to have hospitalizations and ED visits compared with nHW individuals, irrespective of their neighborhood poverty status. However, when examining the mortality rate, nHB individuals in high‐poverty neighborhoods had a nearly 2 times higher odds of death compared with nHW individuals, but this disparity in mortality rate was attenuated for those living in low‐poverty neighborhoods. This study not only adds to the current understanding of the role of neighborhood poverty contributing to racial and ethnic disparities in health care usage among individuals with CHD in the United States but also extends the understanding of how neighborhood poverty status modifies those associations. Findings from the study are representative of the populations that use health care services living in regions with similar socioeconomic and demographic profiles.
We did not find any previous studies that examined the association between race and ethnicity and health care usage or death for adults up to age 64 years with CHD, stratified by neighborhood poverty status. A study using data from the Pediatric Health Information System among individuals aged <26 years 29 found the association between race and ethnicity and mortality rate to be significant among those with low median neighborhood income (a measure often considered equivalent to neighborhoods of higher poverty), 29 similar to our finding showing a significant association between race and ethnicity and mortality rate among people with CHD residing in high‐poverty neighborhoods. In our study among individuals aged 1 to 64 years, the racial and ethnic disparity in mortality rate was attenuated for those living in low‐poverty neighborhoods. While findings are similar, the studies are not directly comparable due to differences in the age of individuals in the 2 studies, where the prior study limited their individuals to age<26 years, 29 while our study sample consisted of individuals aged 1 to 64 years.
Similar to findings from other studies, 12 , 34 the mortality was lower for Hispanic individuals compared to nHW individuals and higher for nHB individuals compared with nHW individuals in our study. Our study was one of the first to adjust for CHD anatomic severity along with age, sex, surveillance site, and insurance status, and stratify by neighborhood poverty status. Our analysis did not look at death before age 1 year. Karamlou et al showed increased death among nHB neonates compared with neonates of other racial and ethnic groups including nHW, Hispanic, Asian, American Indian, and Pacific Islander individuals, and the association was modified by neighborhood household income. 29 Our study found that racial and ethnic differences in mortality rate for people with CHD persist beyond the first year of life, particularly in higher‐poverty neighborhoods.
Our study found that in high‐poverty neighborhoods, both nHB and Hispanic individuals with CHD experienced higher ED use compared with nHW individuals. In a recent study by Benavidez et al, individuals of Hispanic ethnicity with CHD were significantly more likely to experience hospital readmissions compared with individuals who do not identify as Hispanic. 35 Postoperative death by race was not predicted by accessing care alone. In a review of congenital heart surgery outcomes, socioeconomic disparities were noted to be a factor in worse outcomes following congenital surgery in children of nHB and Hispanic families. 15 Barriers impacting health care access or long gaps in care are often associated with complications for selected types of CHDs. 7 , 36 While rates of health insurance have improved for adults with CHD since the Affordable Care Act was implemented, 37 gaps in care usage persist, with about 50% of adolescents with CHD experiencing barriers to transitioning from pediatric to adult CHD specialty care. 5 Future studies could examine the effects of Affordable Care Act on individuals with CHD and their access to care.
While gaps in CHD specialty care have been associated with adverse outcomes, 5 , 38 our data show increased hospitalizations and ED visits among nHB and Hispanic individuals in both low‐ and high‐poverty neighborhoods. In addition, no difference by race and ethnicity in outpatient visits in high‐poverty neighborhoods were revealed, while in low‐poverty neighborhoods, Hispanic individuals had higher outpatient usage. We were not able to determine the provider specialty for outpatient visits; thus, while there was little difference in having ≥1 outpatient encounters by race and ethnicity, there may be differences in the number of outpatient visits and in the number of congenital cardiology outpatient encounters. It is possible that individuals with complications due to CHD may present to the ED in a more advanced disease state that could contribute to an increased mortality rate. Surgical complications are an additional factor associated with high health care usage in terms of hospital readmissions; efforts to reduce such complications have been proposed to decrease health care usage and associated costs. 39 , 40
Ongoing regular care with a congenital cardiologist reduces death 2 , 38 and improves outcomes across the CHD anatomic severity levels. 41 Individuals may be out of routine congenital cardiology care for a variety of socioeconomic reasons, including limited access to transportation to centralized tertiary care centers, lack of insurance access, language barriers, lack of paid sick leave, inability to take time off work, economic constraints, and limited understanding of the purpose and benefits of routine surveillance care for their CHD. 5 Thus, individuals who are out of specialty care may seek care through the ED at a higher rate and in a sicker state than those individuals who have remained in congenital cardiology care. Individuals who identify as nHB or as Hispanic may be more likely to live in higher‐poverty neighborhoods and experience factors that impact access to outpatient CHD specialty care, which may contribute to higher inpatient and ED usage and adverse outcomes. Those living in higher‐poverty neighborhoods may experience additional challenges accessing congenital cardiology care due to school/work environment, availability and quality of health care services, insurance coverage, health literacy, and community resource constraints. 20 , 21 Our study also appears to demonstrate disparities by age and race and ethnicity in individuals who have had any type of health care encounter. It has been reported that children with CHD who identify as Black or Hispanic are less likely to have cardiology follow‐up and remain in cardiology care, 13 , 42 which may be related to a variety of issues impacting access to care such as health insurance, transportation, language barriers, implicit bias in the health care system, and other social determinants.
Recent recommendations with policy implications for improving health care delivery for individuals with CHD recognize the role of individuals' socioeconomic factors, in addition to health care delivery and workforce improvements. 43 A framework focusing on access to care, affordability, and accessibility for all populations with CHD has been proposed as a vision for 2030, along with engaged leaders and identification of areas of improvement, training future workforce, and addressing barriers to care. 43 Our findings support the complex interplay between race and ethnicity and neighborhood poverty status in health care usage and death among individuals with CHD. Addressing these would require solutions related to expanded accessibility for lifelong CHD care, improved Medicaid funding or universal health care, and understanding issues related to patients, as recommended by Chowdhury et al. 43 Mainly, our study supports efforts highlighted by Chowdhury et al for addressing and assessing health care disparities, including consistency in care and resources available for minority populations.
Our analysis is strengthened by the large study size spanning multiple geographic regions within the United States and diverse health administration data sources and vital records. We included both pediatric and adult health care systems and a broad age spectrum. Similar to other studies of CHD using administrative data, our data set may contain individuals without CHD and may miss individuals with CHD, although in contrast to other studies using administrative data, we constrained our CHD codes to those with higher positive predictive value. We could examine CHD anatomic grouping as a covariable in understanding racial and ethnic disparities by neighborhood poverty status for different types of health care usage and death. However, there are some key limitations. Overall, 12% of individuals were excluded who met the diagnostic criterion, but who did not have information on their race and ethnicity. We conducted separate analyses by including those individuals with “unknown” race and ethnicity (Table S3), and while the majority of findings did not change remarkably from the current analysis, for individuals with “unknown” race residing in either high‐ or low‐poverty neighborhoods, the odds of having a hospitalization or having an emergency room visit was less compared with White individuals (Table S4). However, heterogeneity of measurement, aggregation of multiple racial groups into the “other” category, and missing data issues on race and ethnicity pose challenges when ascertaining and interpreting our findings on health care usage disparities in select minority populations. 44 Limited availability of racial and ethnic data not only affects the current analysis but also is necessary to the measurement, identification, understanding, and, ultimately, the elimination of disparities in health as well as to the improvement of the quality of health care in a standardized way for all individuals. 45 , 46 , 47 , 48 We used electronic health record data, which misses individuals who did not seek medical care. We did not have data on type of outpatient visit (eg, receipt of congenital cardiology care), limiting our ability to examine variability in the type of outpatient care individuals received. Death among individuals with CHD was based on state‐specific vital records at each site, rather than on National Death Index data, and was limited to the 3‐year surveillance period. Our reliance on state death certificates may have underestimated the mortality rate over the surveillance period, especially if some deaths occurred outside the catchment regions. Additionally, a longer longitudinal analysis of health care usage and survival in this population would enhance our understanding of health care usage and its effect on the risk of death. In addition, there could be multiple reasons for hospitalizations among individuals with CHD, and these reasons can be age specific. 39 Four age groups were examined in the current study, including children (aged 1–10 years), adolescents (aged 11–18 years), and 2 groups of adults: 19 to 44 years and 45 years. While these groupings allow us to examine age‐related effects, more granular analysis of age by race and ethnicity would be possible with larger sample sizes, specifically among older age categories.
Future research opportunities to address gaps in the current study include understanding access to primary care, congenital cardiology practices, and other subspecialty care. Understanding the primary reason for ED visits and hospitalizations would further improve the understanding of racial and ethnic differences in health care usage among people with CHD.
In conclusion, our study showed that health care usage was associated with race and ethnicity among individuals with CHD in both low‐ and high‐poverty neighborhoods, and with death in high‐poverty neighborhoods. Assessing community‐level social determinants of health, along with access to health care, may help close the gaps in racial and ethnic inequities in health care usage and death among individuals with CHD.
Source of Funding
This work was supported by the Centers for Disease Control and Prevention, Grant/Award Number: CDC‐RFA‐DD15‐1506.
Disclosures
None.
Supporting information
Data S1
Acknowledgments
The authors thank all collaborators from each participating site and the CDC: Trenton Hoffman, Carol Hogue, Daphne Hsu, Aida Soim, Alissa Van Zutphen, Kristin Sommerhalter, Sergey Krikov, Matthew Reeder, Kevin Whitehead, Grace Ryan, Lauren Sarno, Timothy M. Hoffman, Karl Welke, Michael Walsh, Tiffany Colarusso, Karrie Downing, and Bobby Lyles. The authors also thank the Intermountain Healthcare's Adult Congenital Disease Program and all institutions that contributed data to the project. DBDID Replication Statement: Analyses have been replicated by Vanessa Villamil, Centers for Disease Control and Prevention |National Center for Birth Defects and Developmental Disabilities|Division of Birth Defects and Infant Disorders|Birth Defects Monitoring and Research Branch (CDC|NCBDDD|DBDID|BDMRB). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
This manuscript was sent to Monik C. Jiménez, SM, ScD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Preprint posted on MedRxiv December 14, 2023. doi: https://doi.org/10.1101/2023.12.12.23299887.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.033937
For Sources of Funding and Disclosures, see page 10.
References
- 1. Hoffman JL, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/S0735-1097(02)01886-7 [DOI] [PubMed] [Google Scholar]
- 2. Marelli AJ, Mackie AS, Ionescu‐Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115:163–172. doi: 10.1161/CIRCULATIONAHA.106.627224 [DOI] [PubMed] [Google Scholar]
- 3. Avila P, Mercier LA, Dore A, Marcotte F, Mongeon PF, Ibrahim R, Asgar A, Miro J, Andelfinger G, Mondésert B, et al. Adult congenital heart disease: a growing epidemic. Can J Cardiol. 2014;30:S410–S419. doi: 10.1016/j.cjca.2014.07.749 [DOI] [PubMed] [Google Scholar]
- 4. Gurvitz MZ, Inkelas M, Lee M, Stout K, Escarce J, Chang RK. Changes in hospitalization patterns among individuals with congenital heart disease during the transition from adolescence to adulthood. J Am Coll Cardiol. 2007;49:875–882. doi: 10.1016/j.jacc.2006.09.051 [DOI] [PubMed] [Google Scholar]
- 5. Gurvitz M, Valente AM, Broberg C, Cook S, Stout K, Kay J, Ting J, Kuehl K, Earing M, Webb G, et al. Alliance for adult research in congenital cardiology (AARCC) and adult congenital heart association. Prevalence and predictors of gaps in care among adult congenital heart disease individuals: HEART‐ACHD (the health, education, and access research trial). J Am Coll Cardiol. 2013;61:2180–2184. doi: 10.1016/j.jacc.2013.02.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Connor JA, Gauvreau K, Jenkins KJ. Factors associated with increased resource utilization for congenital heart disease. Pediatrics. 2005;116:689–695. doi: 10.1542/peds.2004-2071 [DOI] [PubMed] [Google Scholar]
- 7. Patel MS, Kogon BE. Care of the adult congenital heart disease individual in the United States: a summary of the current system. Pediatr Cardiol. 2010;31:511–514. doi: 10.1007/s00246-009-9629-5 [DOI] [PubMed] [Google Scholar]
- 8. Williamson CG, Tran Z, Rudasill S, Hadaya J, Verma A, Bridges AW, Satou G, Biniwale RM, Benharash P. Race‐based disparities in access to surgical palliation for hypoplastic left heart syndrome. Surgery. 2022;172:500–505. doi: 10.1016/j.surg.2022.03.017 [DOI] [PubMed] [Google Scholar]
- 9. Gilboa SM, Devine OJ, Kucik JE, Oster ME, Riehle‐Colarruso T, Nembhard WN, Xu P, Correa A, Jenkins K, Marelli AJ. Congenital heart defects in the United States: estimating the magnitude of the affected population in 2010. Circulation. 2016;134:101–109. doi: 10.1161/CIRCULATIONAHA.115.019307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mai CT, Isenburg JL, Canfield MA, Meyer RE, Correa A, Alverson CJ, Lupo PJ, Riehle‐Colarusso T, Cho SJ, Aggarwal D, et al. National Birth Defects Prevention Network. National population‐based estimates for major birth defects, 2010‐2014. Birth Defects Res. 2019;111:1420–1435. doi: 10.1002/bdr2.1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Opotowsky AR, Siddiqi OK, Webb GD. Trends in hospitalizations for adults with congenital heart disease in the U.S. J Am Coll Cardiol. 2009;54:460–467. doi: 10.1016/j.jacc.2009.04.037 [DOI] [PubMed] [Google Scholar]
- 12. Lopez KN, Morris SA, Sexson Tejtel SK, Espaillat A, Salemi JL. US mortality attributable to congenital heart disease across the lifespan from 1999 through 2017 exposes persistent racial and ethnic disparities. Circulation. 2020;142:1132–1147. doi: 10.1161/CIRCULATIONAHA.120.046822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jackson JL, Morack J, Harris M, DeSalvo J, Daniels CJ, Chisolm DJ. Racial disparities in clinic follow‐up early in life among survivors of congenital heart disease. Congenit Heart Dis. 2019;14:305–310. doi: 10.1111/chd.12732 [DOI] [PubMed] [Google Scholar]
- 14. Tjoeng YL, Jenkins K, Deen JF, Chan T. Association between race/ethnicity, illness severity, and mortality in children undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2020;160:1570–1579.e1. doi: 10.1016/j.jtcvs.2020.06.015 [DOI] [PubMed] [Google Scholar]
- 15. Richardson CJ, Itua P, Duong T, Lewars J, Tiesenga F. Racial and socioeconomic disparities in congenital heart surgery: a research article. J Card Surg. 2021;36:2454–2457. doi: 10.1111/jocs.15511 [DOI] [PubMed] [Google Scholar]
- 16. Peyvandi S, Baer RJ, Moon‐Grady AJ, Oltman SP, Chambers CD, Norton ME, Rajagopal S, Ryckman KK, Jelliffe‐Pawlowski LL, Steuer MA. Socioeconomic mediators of racial and ethnic disparities in congenital heart disease outcomes: a population‐based study in California. J Am Heart Assoc. 2018;7:e010342. doi: 10.1161/JAHA.118.010342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oster ME, Strickland MJ, Mahle WT. Racial and ethnic disparities in post‐operative mortality following congenital heart surgery. J Pediatr. 2011;159:222–226. doi: 10.1016/j.jpeds.2011.01.060 [DOI] [PubMed] [Google Scholar]
- 18. Fixler DE, Nembhard WN, Salemi JL, Ethen MK, Canfield MA. Mortality in first 5 years in infants with functional single ventricle born in Texas, 1996 to 2003. Circulation. 2010;121:644–650. doi: 10.1161/CIRCULATIONAHA.109.881904 [DOI] [PubMed] [Google Scholar]
- 19. Benavidez OJ, Gauvreau K, Jenkins KJ. Racial and ethnic disparities in mortality following congenital heart surgery. Pediatr Cardiol. 2006;27:321–328. doi: 10.1007/s00246-005-7121-4 [DOI] [PubMed] [Google Scholar]
- 20. National Institute on Minority Health and Health Disparities NIMHD Research Framework. 2017. Accessed June 6, 2023. https://nimhd.nih.gov/researchFramework.
- 21. Alvidrez J, Castille D, Laude‐Sharp M, Rosario A, Tabor D. The National Institute on minority health and health disparities research framework. Am J Public Health. 2019;109:S16–S20. doi: 10.2105/AJPH.2018.304883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gilboa SM, Salemi JL, Nembhard WN, Fixler DE, Correa A. Mortality resulting from congenital heart disease among children and adults in the United States, 1999 to 2006. Circulation. 2010;122:2254–2263. doi: 10.1161/CIRCULATIONAHA.110.947002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boneva RS, Botto LD, Moore CA, Yang Q, Correa A, Erickson JD. Mortality associated with congenital heart defects in the United States: trends and racial disparities, 1979–1997. Circulation. 2001;103:2376–2381. doi: 10.1161/01.CIR.103.19.2376 [DOI] [PubMed] [Google Scholar]
- 24. Tran R, Forman R, Mossialos E, Nasir K, Kulkarni A. Social determinants of disparities in mortality outcomes in congenital heart disease: a systematic review and meta‐analysis. Front Cardiovasc Med. 2022;9:829902. doi: 10.3389/fcvm.2022.829902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. DeMone JA, Gonzalez PC, Gauvreau K, Piercey GE, Jenkins KJ. Risk of death for Medicaid recipients undergoing congenital heart surgery. Pediatr Cardiol. 2003;24:97–102. doi: 10.1007/s00246-002-0243-z [DOI] [PubMed] [Google Scholar]
- 26. Peterson JK, Catton KG, Setty SP. Healthcare disparities in outcomes of a metropolitan congenital heart surgery center: the effect of clinical and socioeconomic factors. J Racial Ethn Health Disparities. 2018;5:410–421. doi: 10.1007/s40615-017-0384-7 [DOI] [PubMed] [Google Scholar]
- 27. Anderson BR, Fieldston ES, Newburger JW, Bacha EA, Glied SA. Disparities in outcomes and resource use after hospitalization for cardiac surgery by neighborhood income. Pediatrics. 2018;141:e20172432. doi: 10.1542/peds.2017-2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tillman AR, Colborn KL, Scott KA, Davidson AJ, Khanna A, Kao D, McKenzie L, Ong T, Rausch CM, Duca LM, et al. Associations between socioeconomic context and congenital heart disease related outcomes in adolescents and adults. Am J Cardiol. 2021;139:105–115. doi: 10.1016/j.amjcard.2020.10.040 [DOI] [PubMed] [Google Scholar]
- 29. Karamlou T, Hawke JL, Zafar F, Kafle M, Tweddell JS, Najm HK, Frebis JR, Bryant RG. Widening our focus: characterizing socioeconomic and racial disparities in congenital heart disease. Ann Thorac Surg. 2022;113:157–165. doi: 10.1016/j.athoracsur.2021.04.008 [DOI] [PubMed] [Google Scholar]
- 30. Glidewell JM, Farr SL, Book WM, Botto L, Li JS, Soim AS, Downing KF, Riehle‐Colarusso T, D'Ottavio AA, Feldkamp ML, et al. Individuals aged 1–64 years with documented congenital heart defects at healthcare encounters, five U.S. surveillance sites, 2011–2013. Am Heart J. 2021;238:100–108. doi: 10.1016/j.ahj.2021.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rodriguez FH III, Ephrem G, Gerardin JF, Raskind‐Hood C, Hogue C, Book W. The 745.5 issue in code‐based, adult congenital heart disease population studies: relevance to current and future ICD‐9‐CM and ICD‐10‐CM studies. Congenit Heart Dis. 2018;13:59–64. doi: 10.1111/chd.12563 [DOI] [PubMed] [Google Scholar]
- 32. Rodriguez FH III, Raskind‐Hood CL, Hoffman T, Farr SL, Glidewell J, Li JS, D'Ottavio A, Botto L, Reeder MR, Hsu D, et al. How well do ICD‐9‐CM codes predict true congenital heart defects? A Centers for Disease Control and Prevention‐based multisite validation project. J Am Heart Assoc. 2022;11:e024911. doi: 10.1161/JAHA.121.024911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. U.S. Census Bureau . 2014 American Community Survey (ACS) 5‐Year Data Profiles, Extracted by ZCTA and GA Counties. 2014. https://data.census.gov/table?g=040XX00US13&tid=ACSDP5Y2014.DP05.
- 34. Nembhard WN, Pathak EB, Schocken DD. Racial and ethnic disparities in mortality related to congenital heart defects among children and adults in the United States. Ethn Dis. 2008;18:442–449. [PubMed] [Google Scholar]
- 35. Benavidez OJ, He W, Lahoud‐Rahme M. Readmissions following congenital heart surgery in surgery admissions in adult hospitals: risk factors and association with death and coinfants and children. Pediatr Cardiol. 2019;40:994–1000. doi: 10.1007/s00246-019-02104-4 [DOI] [PubMed] [Google Scholar]
- 36. Williams RG. Transitioning youth with congenital heart disease from pediatric to adult health care. J Pediatr. 2015;166:15–19. doi: 10.1016/j.jpeds.2014.09.054 [DOI] [PubMed] [Google Scholar]
- 37. Lin CJ, Novak E, Rich MW, Billadello JJ. Insurance access in adults with congenital heart disease in the affordable care act era. Congenit Heart Dis. 2018;13:384–391. doi: 10.1111/chd.12582 [DOI] [PubMed] [Google Scholar]
- 38. Mylotte D, Pilote L, Ionescu‐Ittu R, Abrahamowicz M, Khairy P, Therrien J, Mackie AS, Marelli A. Specialized adult congenital heart disease care: the impact of policy on mortality. Circulation. 2014;129:1804–1812. doi: 10.1161/CIRCULATIONAHA.113.005817 [DOI] [PubMed] [Google Scholar]
- 39. Benavidez OJ, Connor JA, Gauvreau K, Jenkins KJ. The contribution of complications to high resource utilization during congenital heart surgery admissions. Congenit Heart Dis. 2007;2:319–326. doi: 10.1111/j.1747-0803.2007.00119.x [DOI] [PubMed] [Google Scholar]
- 40. Bhatt AB, Rajabali A, He W, Benavidez OJ. High resource use among adult congenital heart morbidities. Congenit Heart Dis. 2015;10:13–20. doi: 10.1111/chd.12169 [DOI] [PubMed] [Google Scholar]
- 41. Willems R, Ombelet F, Goossens E, De Groote K, Budts W, Moniotte S, de Hosson M, Van Bulck L, Marelli A, Moons P, et al. Different levels of care for follow‐up of adults with congenital heart disease: a cost analysis scrutinizing the impact on medical costs, hospitalizations, and emergency department visits. Eur J Health Econ. 2021;22:951–960. doi: 10.1007/s10198-021-01300-5 [DOI] [PubMed] [Google Scholar]
- 42. Serfas JD, Spates T, D'Ottavio A, Spears T, Ciociola E, Chiswell K, Davidson‐Ray L, Ryan G, Forestieri N, Krasuski RA, et al. Disparities in loss to follow‐up among adults with congenital heart disease in North Carolina. World J Pediatr Congenit Heart Surg. 2022;13:707–715. doi: 10.1177/21501351221111998 [DOI] [PubMed] [Google Scholar]
- 43. Chowdhury D, Johnson JN, Baker‐Smith CM, Jaquiss RDB, Mahendran AK, Curren V, Bhat A, Patel A, Marshall AC, Fuller S, et al. Health care policy and congenital heart disease: 2020 focus on our 2030 future. J Am Heart Assoc. 2021;10:e020605. doi: 10.1161/JAHA.120.020605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Viano, S. , & Baker, D. J. How administrative data collection and analysis can better reflect racial and ethnic identities. Rev Res Educ 2020; 44:301–331. doi: 10.3102/0091732X20903321. [DOI] [Google Scholar]
- 45. Institute of Medicine (US) Committee on Quality of Health Care in America . Crossing the Quality Chasm: A New Health System for the 21st Century. Washington (DC): National Academies Press (US); 2001. [PubMed] [Google Scholar]
- 46. Bierman AS, Lurie N, Collins KS, Eisenberg JM. Addressing racial and ethnic barriers to effective health care: the need for better data. Health Aff (Millwood). 2002;21:91–102. doi: 10.1377/hlthaff.21.3.91 [DOI] [PubMed] [Google Scholar]
- 47. Institute of Medicine (US) . In: Ulmer C, McFadden B, Nerenz DR, eds Subcommittee on Standardized Collection of Race/Ethnicity Data for Healthcare Quality Improvement. Race, Ethnicity, and Language Data: Standardization for Health Care Quality Improvement. National Academies Press (US); 2009. [PubMed] [Google Scholar]
- 48. Perez V, Hayden L, Mesa J, Bickell N, Abner P, Richardson LD, Ngai KM. Improving individual race and ethnicity data capture to address health disparities: a case study from a large urban health system. Cureus. 2022;14:e20973. doi: 10.7759/cureus.20973 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Data Availability Statement
The data that support the findings of this study are available from the Centers for Disease Control and Prevention (CDC). Restrictions apply to the availability of these data, which were used under license for this study. Data are available with permission from the CDC. Contact Jill Glidewell at iyp0@cdc.gov.
This retrospective study of children and adults with CHD used data from 4 sites (Georgia, North Carolina, New York, and Utah) participating in a collaborative CHD surveillance project funded by the CDC (CDC‐RFA‐DD15‐1506). The CDC funded 5 sites via a competitive mechanism on the basis of suitability to conduct CHD surveillance activities. One site, Colorado, was unable to provide socioeconomic data necessary for the analysis; thus, data from this site were excluded from this analysis. Individuals with CHD were identified on the basis of at least 1 CHD‐related International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) code within the 745.xx to 747.xx range. 30 These codes were identified from electronic administrative and clinical sources, state Medicaid claims, state vital records, and birth defect registries. Compilation and sharing of deidentified data with the CDC were approved by each site's institutional review board with complete Health Insurance Portability and Accountability Act waiver of consent. A detailed methodology of the parent project has been published. 30


