Abstract
Background
In nonvalvular atrial fibrillation (NVAF), the left atrial appendage (LAA) is the source of thrombus in up to 90% of patients. LAA pseudothrombus (LAAPT), defined as a filling defect on the initial but not the 60‐second delayed acquisition on cardiovascular computed tomography scan (CCT), is a recognized phenomenon in NVAF, with unknown clinical relevance. We aimed to determine the relationship between LAAPT and history of stroke in patients with NVAF.
Methods and Results
The study included 213 consecutive patients with NVAF undergoing CCT who were assessed for LAAPT. LA and LAA dimensions and LAA morphology correlated with clinical demographics including cardiovascular risk factors, history of stroke, thromboembolic stroke, and transient ischemic attack. Mean age (±SD) was 65.1±10.5 years (range 31–89) and 150 of 213 (70.4%) were men. LAAPT was present in 59 of 213 (27.7%) patients. Greater mean LAA ostium area (5.7 versus 4.5, P<0.001), greater mean LAA ostium area:curved length (0.11 versus 0.08, P<0.001), increased LAA volume (14.0 versus 10.2, P<0.001), and lower mean LAA tortuosity index (1.17 versus 1.38, P<0.001) were all associated with the presence of LAAPT. On multivariable analysis, LAAPT on CCT (odds ratio [OR], 3.20 [95% CI, 1.40–7.20]; P<0.006) and higher CHA2DS2‐VASc score (OR, 1.65 [95% CI, 1.16–2.35]; P=0.01) were associated with all strokes, with LAAPT remaining a statistically significant risk factor even after adjustment for CHA2DS2‐VASc score.
Conclusions
LAAPT on CCT is common in patients with NVAF. It has a strong positive association with stroke prevalence, even after adjustment for CHA2DS2‐VASc score. LAAPT on CCT may potentially allow further stratification for stroke risk, additive to the CHA2DS2‐VASc score.
Keywords: atrial fibrillation, computed tomography, left atrial appendage, pseudothrombus, stroke
Subject Categories: Computerized Tomography (CT), Atrial Fibrillation, Cerebrovascular Disease/Stroke
Nonstandard Abbreviations and Acronyms
- AU
Agatston units
- LAA
left atrial appendage
- NVAF
nonvalvular atrial fibrillation
Clinical Perspective.
What Is New?
This paper shows, for the first time, that in patients with nonvalvular atrial fibrillation, left atrial appendage pseudothrombus on cardiovascular computed tomography is associated with increased prior stroke prevalence, even after adjustment for CHA2DS2‐VASc score.
What Are the Clinical Implications?
Left atrial appendage pseudothrombus has the potential to aid identification of patients who may be at higher risk of stroke and who may benefit from targeted anticoagulation.
This includes those patients with CHA2DS2‐VASc scores that currently suggest no anticoagulation is required or those with higher CHA2DS2‐VASc scores, already on anticoagulation, where consideration of a left atrial appendage exclusion device may be appropriate, as stroke risk may persist despite their anticoagulation.
Atrial fibrillation (AF) is associated with significant morbidity and mortality, a 5‐fold increase in ischemic stroke risk, 1 and an annualized absolute risk of stroke of 3% to 4%. 2 However, determination of an individual's stroke risk remains a significant clinical challenge, especially in those without significant comorbidities. 3
The left atrial appendage (LAA) is the source of thrombus in up to 90% of patients with nonvalvular AF (NVAF) and 57% in valvular AF. 4 The role of the LAA in thrombogenesis has been extensively investigated with LAA anatomy, morphology, histology, and function, all implicated in the generation of thrombi. 5 LAA characteristics implicated in increased thrombogenesis, and that have been shown to be independently associated with thromboembolic risk (noting the small sample size of many studies), are increased LAA ostial size, 6 , 7 , 8 increased LAA volume, 9 , 10 , 11 LAA flow velocity, 12 , 13 , 14 increased LAA trabeculation, 6 increased LAA fibrosis, 15 and LAA endothelial dysfunction. 16 LAA morphology is often described using a descriptive classification, namely windsock (long and straight), chicken wing (angulated by 90 degrees or more), cactus (windsock with significant side lobes), and cauliflower (multiple small lobes). The cauliflower morphology is associated with greater likelihood of thrombus formation. 17 Anatomically, this subtype tends to have larger LAA ostial size and lower LAA flow velocity. 18 Conversely, a chicken wing subtype is associated with decrease in prior stroke in patients with low stroke risk (CHADS2 score 0–1). 19 , 20
Stroke risk is usually calculated using the CHA2D2S‐VASc method, which does not consider LAA characteristics. Where CHA2D2S‐VASc score is low, and thus a patient may not need anticoagulation, there may be a clinical role for further risk stratification based on LAA characteristics, with some studies suggesting the potential use of LAA flow 13 , 14 and LAA morphology as part of stroke risk stratification. 20 Additionally, the LAA morphology may also be valuable in the risk stratification of those who do require anticoagulation (as certain LAA morphologies may lead to residual risk).
Cardiovascular computed tomography (CCT) scanning reliably and accurately identifies cardiac anatomy, including the LAA, and is often used for assessment before AF ablation. 21 LAA pseudothrombus (LAAPT) is a filling defect on a standard (first pass) CCT scan that is commonly seen in patients with AF. 22 It is caused by relatively slow mixing of contrast with the blood pool and gives the appearance of an LAA thrombus. A delayed scan (often at 60 seconds) normalizes as the contrast and blood pool more completely mix and the filling defect resolves, excluding a true thrombus 23 (Figure 1).
Figure 1. True thrombus versus pseudothrombus.
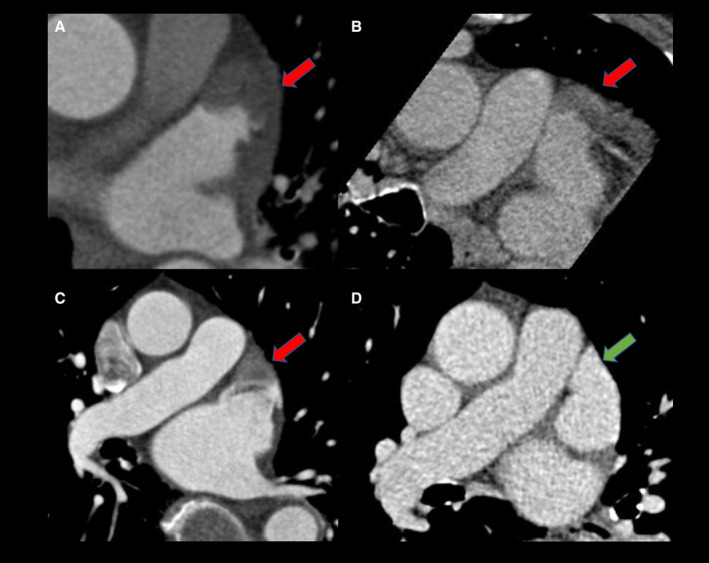
(A) demonstrates initial acquisition with an LAA filling defect (red arrow) that persists in delayed (60s) acquisition (B, red arrow) indicating a true LAA thrombus. C demonstrates initial acquisition with a LAA filling defect (red arrow) that clears in the delayed (60s) acquisition (D, green arrow) indicating an LAA pseudothrombus. LAA indicates left atrial appendage.
Despite representing impaired contrast mixing, likely secondary to LAA dysfunction, the clinical relevance of LAAPT and its association with stroke is unknown. We therefore investigated the relationship between LAAPT on CCT, LAA characteristics, and the prevalence of history of stroke in patients with NVAF.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design
This was a retrospective, single‐center, cross‐sectional study of dual phase (initial and 60‐second late pass acquisition) CCT scans 23 in outpatients with NVAF who underwent CCT as part of preprocedural workup for catheter ablation.
Assessment of Computed Tomography Images
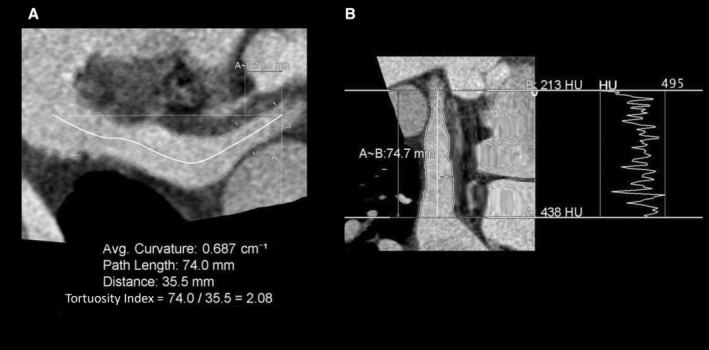
LAAPT was defined as a filling defect on the initial, but not the delayed (60‐second) acquisitions. In our center, a standard protocol is used for venous contrast injection to maintain consistency between patients. In addition, the following LAA characteristics were identified: LA volume, LAA volume and ostial area, LAA morphology (windsock, chicken wing, cauliflower, or cactus), LAA length and tortuosity (Figure 2), and LAA contrast gradient. LAA characteristics were assessed independently, using a Terarecon workstation (Foster City, CA), by 2 Society of Cardiovascular Computed Tomography Level 3 imaging specialists (E.N. and T.S.), both with >10 years CCT experience, blinded to clinical profile. Interobserver analysis was undertaken for LAA morphology and presence of LAA filling defects.
Figure 2. Tortuosity index=curved path length vs straight distance from orifice to apex (A), attenuation gradient (with no gradient) (B).
Clinical Assessment
Clinical assessment was undertaken by reviewing the patient's electronic record. All prior cerebrovascular events were recorded (thromboembolic stroke, hemorrhagic stroke, or transient ischemic attack [TIA]). All events occurred before the CCT scan. To allow for calculation of CHA2DS2‐VASc score, additional clinical assessment included identification of age, sex, atrial arrhythmia (AF, other atrial tachycardia), ventricular arrhythmia, hypertension, heart failure, diabetes, known vascular disease, dyslipidemia, and treatment with anticoagulants or antiarrhythmic drugs at the time of patients attending for their CT scan. Anticoagulation before stroke was also added into the multivariable models, so that an association could be determined.
Clinical Outcomes
The primary clinical end point for this study was the prior occurrence of all strokes. Secondary analysis also included the prior occurrence of any thromboembolic stroke, TIA, and hemorrhagic stroke.
Statistical Analysis
Statistical analysis was performed using Stata (Version 16.2). Normally distributed quantitative variables are presented with mean and SD. Categorical data are presented as frequency (%). Nonnormally distributed data are presented as median and interquartile range. Pearson's chi‐square test, Fisher's exact test, Student t test, and the Mann–Whitney U test were used as appropriate to compare differences between continuous and categorical variables relating to clinical and LAA characteristics between those with LAA pseudothrombus present on CCT and those without. The association of pseudothrombus with cerebrovascular events (ischemic stroke, TIA) was analyzed using logistic regression. Odds ratios (OR) and 95% CI are presented. Oral anticoagulant (OAC) use before CT scan was forced into all multivariable models due to its known association with stroke. Other covariates were adjusted for in multivariable analysis if they were statistically significant on univariable analysis. For univariate and multivariable analysis, if a patient had both a TIA and a stroke, this was counted only once, as a stroke. Thromboembolic stroke and TIA were also analyzed as separate subgroups for univariate and multivariable analysis. To assess the incremental value of LAA pseudothrombus, in addition to CHA2DS2‐VASc score when predicting stroke, the discrimination of CHA2DS2‐VASc with and without the addition of LAA pseudothrombus was compared using the Net Reclassification Index and C‐index. A statistically significant difference was defined as a 2‐sided P value <0.05.
This work was prospectively registered in our institution, as a service evaluation, before being undertaken. The local research and development department advised the requirement for ethical approval was not needed, given this was a retrospective analysis of preexisting data, with no effect of the analysis on the acquisition of the CCT or their subsequent treatment.
Results
Patient Demographics
A total of 355 consecutive patients who had dual‐phase CCT were identified. Patients were excluded if they had congenital heart disease (n=34), arrhythmia other than AF, or AF associated with known valvular disease (n=108). Overall, 213 consecutive patients with NVAF met the inclusion criteria and were included in the analyses. The mean age (±SD) of the cohort was 65.1±10.5 years (range, 31–89 years) and 150 of 213 (70.4%) were men. LAAPT was present in 59 of 213 (27.7%) patients. Full demographics and clinical parameters are shown in Table 1. LAAPT was more prevalent in older patients, those with dyslipidemia, and those with increasing CHA2DS2‐VASc score (Table 1).
Table 1.
Baseline Characteristics
| Variable | Pseudothrombosis | P value* | ||
|---|---|---|---|---|
| Total (n=213) | Yes (n=59) | No (n=154) | ||
| Age, y | 65.1 (10.5) | 68.7 (10.4) | 63.8 (10.3) | 0.002 |
| Female sex | 63 (29.6) | 17 (28.8) | 46 (29.9) | 0.88 |
| Hypertension | 102 (47.9) | 32 (54.2) | 70 (45.5) | 0.25 |
| Diabetes | 35 (16.4) | 10 (16.9) | 25 (16.2) | 0.90 |
| Dyslipidemia | 45 (21.1) | 18 (30.5) | 27 (17.5) | 0.04 |
| Coronary artery disease | 46 (21.6) | 10 (16.9) | 36 (23.4) | 0.31 |
| Heart failure | 39 (18.3) | 13 (22.0) | 26 (16.9) | 0.38 |
| Vascular disease | 20 (9.4) | 4 (6.8) | 16 (10.4) | 0.42 |
| Antiarrhythmic drugs before CT | 95 (44.6) | 22 (37.3) | 73 (47.4) | 0.18 |
| Oral anticoagulants before CT | 96 (45.1) | 22 (37.3) | 74 (48.1) | 0.16 |
| History of bleeding | 32 (15.0) | 12 (20.3) | 20 (13.0) | 0.18 |
| CHA2DS2‐VASc score | ||||
| 0 | 36 (16.9) | 3 (5.1) | 33 (21.4) | <0.001 |
| 1 | 34 (16.0) | 7 (11.9) | 27 (17.5) | |
| 2 | 48 (22.5) | 13 (22.0) | 35 (22.7) | |
| 3 | 35 (16.4) | 12 (20.3) | 23 (14.9) | |
| 4 | 33 (15.5) | 14 (23.7) | 19 (12.3) | |
| 5 | 20 (9.4) | 6 (10.2) | 14 (9.1) | |
| 6 | 6 (2.8) | 4 (6.8) | 2 (1.3) | |
| 7 | 1 (0.5) | 0 (0.0) | 1 (0.6) | |
| CHA2DS2‐VASc score | ||||
| 0 | 36 (16.9) | 3 (5.1) | 33 (21.4) | 0.002 |
| 1 | 34 (16.0) | 7 (11.9) | 27 (17.5) | |
| >1 | 143 (67.1) | 49 (83.1) | 94 (61.0) | |
| LAA characteristics | ||||
| Left atrial volume (mm3) | 135.0 (105.0–165.0) | 150.0 (135.0–274.0) | 131.0 (102.0–163.0) | 0.19 |
| LAA gradient (initial scan) | 1.30 (0.07–3.90) | 9.02 (4.27–45.06) | 0.70 (−0.27–1.92) | <0.001 |
| LAA gradient (delayed scan) | 0.13 (−0.48–0.49) | 0.13 (−0.38–0.73) | 0.12 (−0.52–0.48) | 0.31 |
| Initial: late pass contrast ratio | 0.4 (−3.0–4.5) | 3.7 (−33.6–51.4) | 0.1 (−2.3–2.6) | 0.28 |
| LAA ostium area | 4.8 (2.0) | 5.7 (2.3) | 4.5 (1.8) | <0.001 |
| LAA curved length | 51.1 (13.7) | 50.2 (10.5) | 51.5 (14.7) | 0.55 |
| LAA ostium area:curved length ratio | 0.09 (0.07–0.12) | 0.11 (0.09–0.14) | 0.08 (0.06–0.11) | <0.001 |
| LAA volume (mm3) | 11.1 (8.0–15.3) | 14.0 (10.3–20.5) | 10.2 (7.5–14.0) | <0.001 |
| Tortuosity index | 1.31 (1.16–1.59) | 1.17 (1.10–1.34) | 1.38 (1.19–1.75) | <0.001 |
| LAA morphology | ||||
| Chicken wing | 34 (16.0) | 6 (10.2) | 28 (18.2) | 0.24 |
| Windsock | 148 (69.5) | 51 (86.4) | 97 (63.0) | |
| Cactus | 20 (9.4) | 1 (1.7) | 19 (12.3) | |
| Cauliflower | 11 (5.2) | 1 (1.7) | 10 (6.5) | |
CT indicates computed tomography; and LAA, left atrial appendage.
P values are from t test or Mann–Whitney U test for continuous variables and chi‐square or Fisher's exact test for categorical variables.
Interobserver Variability in LAA Morphology and Identification of LAAPT
Interobserver agreement for LAA morphologies was 100% for chicken wing, 98% for windsock, 90% for cactus, and 100% for cauliflower type. There was a 99% agreement for the diagnosis of LAAPT.
Imaging Analysis of LAA Characteristics
Assessment of the LAA characteristics as outlined in the methods was performed on all 213 subjects. LA volume analysis was performed on 187 of 213 (87.8%) subjects, 176 (94.1%) of whom had severely dilated LA. The almost ubiquitous prevalence of severe LA dilation in both subgroups (with/without LAA pseudothrombus) precluded further comparative analysis of this parameter. Individuals with increased LA volume, larger, wider, straighter, and shorter LAA (low tortuosity index), those with larger LAA ostial area, and those with larger LAA ostial area to curved length ratio were statistically more likely to demonstrate LAAPT (all P<0.0001; Table 1). Classical LAA morphology subtype distinctions (cauliflower, cactus, chicken wing, windsock) were not associated with a history of stroke or presence of LAAPT.
Neurological Events
A total of 67 of 213 (31.5%) patients had a stroke or TIA. All events occurred before the CCT scan. Stroke events occurred in 40 of 213 (18.8%); of these patients, 35 of 40 (87.5%) had a thromboembolic event versus 5 of 40 (12.5%) of those who sustained a hemorrhagic stroke. TIA in isolation was recorded in 27 of 213 (12.7%) patients. At the time of their event, OACs were being taken by 14 of 40 (35%) in the group with thromboembolism, 3 of 5 (60%) in the group with hemorrhagic stroke, and 17 of 27 (63.0%) in the group with TIA (Table 2).
Table 2.
Outcomes
| Outcome | Total (n=213) | Pseudothrombosis | P value* | |
|---|---|---|---|---|
| Yes (n=59) | No (n=154) | |||
| All strokes | 40 | 21 (35.6) | 19 (12.3) | <0.001 |
| Thromboembolic stroke | 35 | 19 (32.2) | 16 (10.3) | <0.001 |
| Transient ischemic attack | 27 | 9 (15.3) | 18 (11.7) | 0.48 |
| Hemorrhagic stroke | 5 | 2 (3.4) | 3 (2.0) | 0.62 |
P values are from chi‐square test or Fisher's exact test.
Antecedent TIA occurred in 10 of 35 (28.6%) of the patients who had a thromboembolic stroke. For the purpose of univariate and multivariable analysis, if a patient had both a TIA and a stroke, this was counted only once, as a stroke (Table 3). Thromboembolic stroke and TIA in isolation were analyzed as separate subgroups for univariate and multivariable analysis (Tables S1 and S2, respectively).
Table 3.
Univariable and Multivariable Analyses of All Strokes (Including Hemorrhagic)
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| Pseudothrombosis | 3.93 (1.92–8.05) | <0.001 | 3.03 (1.32–6.95) | 0.009 |
| Age, y | 1.12 (1.07–1.17) | <0.001 | 1.04 (0.98–1.11) | 0.18 |
| Female sex | 0.54 (0.23–1.24) | 0.15 | … | … |
| Hypertension | 1.42 (0.71–2.84) | 0.32 | … | … |
| Diabetes | 0.51 (0.17–1.53) | 0.23 | … | … |
| Dyslipidemia | 1.82 (0.84–3.95) | 0.13 | … | … |
| Coronary artery disease | 1.27 (0.57–2.84) | 0.56 | … | … |
| Heart failure | 0.75 (0.29–1.93) | 0.55 | … | … |
| Vascular disease | 3.35 (1.27–8.86) | 0.01 | 2.26 (0.70–7.35) | 0.18 |
| Antiarrhythmic drugs before CT scan | 0.90 (0.45–1.80) | 0.77 | … | … |
| Oral anticoagulants before CT scan* | 0.60 (0.29–1.22) | 0.16 | 0.57 (0.24–1.32) | 0.19 |
| CHA2DS2‐VASc score | 2.10 (1.61–2.74) | <0.001 | 1.73 (1.20–2.49) | 0.003 |
| LAA characteristics† | Univariable odds ratio (95% CI) | P |
|---|---|---|
| LAA gradient (initial scan) | ||
| <0.07 | 0.99 (0.45, 2.17) | 0.67 |
| 0.07–1.3 | 0.29 (0.09, 0.88) | |
| 1.3+ | Reference group | |
| LAA gradient (delayed scan) | ||
| <−0.5 | 0.46 (0.16, 1.31) | 0.35 |
| −0.5 to 0.12 | 1.85 (0.86, 4.02) | |
| 0.12+ | Reference group | |
| Initial: late pass contrast ratio | ||
| <−3 | 1.81 (0.75, 4.37) | 0.22 |
| −3 to 4 | Reference group | |
| 4+ | 2.98 (1.31, 6.79) | |
| LAA ostium area >3.5 cm | 3.66 (1.24, 10.82) | 0.02 |
| LAA curved length | 1.00 (0.98, 1.03) | 0.72 |
| LAA ostium area:curved length ratio >0.068 | 7.72 (1.79, 33.24) | 0.006 |
| LAA volume >11 mm3 | 1.81 (0.89, 3.66) | 0.10 |
| LAA morphology | ||
| Chicken wing | 0.96 (0.36, 2.55) | 0.25 |
| Windsock | Reference group | |
| Cactus | 0.50 (0.11, 2.28) | |
| Cauliflower | 3.73 (1.06, 13.14) | |
CT indicates computed tomography; and LAA, left atrial appendage.
Forced into multivariable model due to known association with stroke.
LAA characteristics were not considered as potential confounders and thus were included in univariable analysis only.
Risk of All Strokes
Univariable analysis demonstrated all strokes were more frequent in those with LAAPT on CCT (OR, 3.93 [95% CI, 1.92–8.05]; P<0.001), higher CHA2DS2‐VASc score (OR, 2.10 [95% CI, 1.61–2.74]; <0.001), older age (OR, 1.12 [95% CI, 1.07–1.17]; P<0.001), higher LAA ostium area to curved length ratio (OR, 7.72 [95% CI, 1.79–33.24]; P=0.006), known vascular disease (OR, 3.35 [95% CI, 1.27–8.86], P=0.01), and greater LAA ostial area (>3.5 cm; OR, 3.66 [95% CI, 1.24–10.82]; P=0.02). On multivariable analysis adjusting for age and vascular disease, CHA2DS2‐VASc score and LAAPT remained associated with all strokes (Table 3).
Risk of Thromboembolic Stroke
Univariable analysis demonstrated prior thromboembolic stroke was more frequent in those with LAAPT on CCT (OR, 4.10 [95% CI, 1.93–8.69]; P<0.001), older age (OR, 1.10 [95% CI, 1.05–1.16]; P<0.001), higher CHA2DS2‐VASc score (OR, 2.08 [95% CI, 1.58–2.74]; P<0.001), LAA ostium area to curve length ratio (OR, 6.45 [95% CI, 1.49–27.87]; P=0.01), known vascular disease (OR, 3.17 [95% CI, 1.16–8.65]; P=0.02), and greater LAA ostial area (OR, 3.03 [95% CI, 1.02–9.02]; P=0.05). Multivariable analysis demonstrated that the presence of LAAPT (OR, 3.33 [95% CI, 1.42–7.81]; P=0.006) remained associated with thromboembolic stroke after adjusting for other predictors, including OAC usage (Table S1).
Risk of TIA and Hemorrhagic Stroke
Univariable analysis demonstrated that neither TIA nor hemorrhagic stroke was associated with LAAPT and LAA imaging characteristics (Table S2). Further TIA data are shown in Table S2.
Incremental Value of LAAPT
Among patients with any stroke, 52.5% had higher predictions using the statistical model that includes both LAAPT and CHA2DS2‐VASc score compared with the predictions with the model using only CHA2DS2‐VASc score. Among patients without any stroke, 78.0% had lower predictions with the model of LAAPT and CHA2DS2‐VASc score, leading to an overall Net Reclassification Index of 0.61 (95% CI, 0.27–0.95). In addition, the discrimination of the model with LAAPT and CHA2DS2‐VASc score was higher than that of the model with CHA2DS2‐VASc alone (C‐index 0.83 versus 0.80). Similarly, the addition of LAAPT to CHA2DS2‐VASc score improved the prediction of thromboembolic stroke with an overall Net Reclassification Index of 0.64 (95% CI, 0.27–1.00) and an increase in C‐index from 0.80 to 0.83.
Anticoagulation in the Group With Pseudothrombus and the Control Group
OAC was recorded in 22 of 59 (37.3%) patients in the group with pseudothrombus versus 74 of 154 (48.1%) patients in the group without pseudothrombus (P=0.16). However, in multivariable analysis, OAC usage was a predictor of a reduced incidence of prior thromboembolic stroke (OR, 0.28 [95% CI, 0.11–0.73]; P=0.009) and TIA (OR, 2.39 [95% CI, 0.99–5.79]; P=0.05).
Discussion
The objective of this study was to evaluate the relationship between the incidence of LAAPT on CCT and stroke prevalence. The main finding was that LAAPT detected by CT is associated with a history of stroke even when accounting for CHA2DS2‐VASc score and other LAA characteristics. LAAPT was the only CCT imaging parameter associated with stroke.
Greater LAA volume, larger LAA ostial size, and lower LAA tortuosity are all positively associated with the presence of LAAPT on CCT.
LAAPT and Association With Stroke Risk
LAAPT represents a LAA filling defect, secondary to sluggish flow on CCT scanning, 23 and although common in patients with AF 22 its clinical significance has not been fully established. True LAA thrombus is well known to be responsible for most stroke events in AF and the source of up to 90% of thrombi in NVAF and 57% in valvular AF, respectively. 4 This is why stroke prevention (in addition to anticoagulation) is targeted by either surgical excision 24 or percutaneous LAA ligation. 25 Randomized control trials of LAA occlusion such as PROTECT AF 26 (WATCHMAN Left Atrial Appendage System for Embolic Protection in Patients With Atrial Fibrillation) and PREVAIL 27 (Evaluation of the WATCHMAN Left Atrial Appendage Closure Device in Patients With Atrial Fibrillation Versus Long‐Term Warfarin Therapy) have demonstrated noninferiority of this approach to warfarin for the prevention of stroke, systemic embolism, or death. Our finding that LAAPT remained associated with prevalence of stroke, after adjustment for other variables, is a novel finding.
LAA Attributes on CT Scanning and Their Relationship With Stroke Prevalence
There have been many studies that have evaluated LAA attributes derived from CCT imaging and their association with stroke risk. In a study of 88 patients with AF who had acute cardioembolic stroke, larger LAA orifice and large LAA volumes on CCT were positively associated with cardioembolic stroke, as well as cerebral infarct volume. 28 In those with cryptogenic stroke/TIA, LAA volume on CCT scan during middiastole was 67% larger than in age‐ and sex‐matched controls, 10 whereas transesophageal echocardiogram studies have demonstrated larger LAA orifice size, low LAA flow velocities, and larger LAA volumes are all positively associated with increased stroke prevalence. 7 , 17 , 29 In a large study of 1063 patients with AF who underwent CCT, an increase in LAA trabeculations was associated with a higher likelihood of history of stroke; however, in contradiction with much of the literature, a smaller LAA orifice was associated with increased stroke risk. 6
In our study, LAAPT was the only LAA attribute on CCT that was associated with prior stroke prevalence when accounting for all confounding factors. Univariable analysis did show a positive association between greater LAA ostial area, greater LAA ostium area: lower curved length ratio (ie, comparatively short and wide LAA), and stroke prevalence; however, after adjusting for other variables, these no longer remained significant on multivariable analysis. Most previous literature that assesses the relationship between LAA characteristics on CCT and stroke does not include many clinical factors or an extensive multivariable analysis, and these study limitations are clearly acknowledged in previous research in this field. 6 , 30
LAA Morphology and Stroke Risk
There are conflicting reports in the literature regarding the correlation of LAA morphology and stroke, with some studies demonstrating an association 18 , 20 and others not. 6 , 10 , 28 Our study is consistent with the latter and demonstrates no independent association between LAA subtype and stroke prevalence in detailed and robust multivariable analysis.
This may be in part due to the fact that LAA morphology characterization does not appear to be reliably reproducible, even by experts, 28 and the prevalence of LAA morphologies vary between studies. 6 , 11 Data would suggest that the cauliflower subtype is most likely to be positively correlated with stroke and is considered the most complex LAA type. 20 However, it is thought that this association could be explained by the increased trabeculation and larger ostial size, which are in themselves independent predictors of stroke prevalence. Conversely, the chicken wing subtype is most commonly negatively corelated with stroke prevalence, 20 with some studies suggesting this subtype may also be protective due the usual small ostium and higher LAA flow velocity, 17 again both of which are independently associated with lower stroke prevalence. 17
LAAPT as a Potential Biomarker of LAA Dysfunction, Thrombogenicity, and Stroke Risk
In a cohort of patients who underwent LAA electrical isolation, postisolation LAA flow did not predict stroke or LAA thrombus incidence. 31 This suggests LAA thrombogenicity is multifactorial. In our study, we have shown that there is a relationship between LAAPT and the well‐recognized LA/LAA factors known to be associated with stroke risk. LAAPT could represent an imaging biomarker that encompasses LAA dysfunction and predisposition to thrombogenesis (capturing multiple interrelated factors in a single parameter), which in turn appears to be associated with increased stroke prevalence.
Enhancing Stroke Risk Scoring
The CHA2DS2‐VASc score is effective in identifying patients with low thromboembolic risk 32 ; however, in those who have 1 additional risk factor and thus do not need to be anticoagulated, the annual stroke risk ranges from <1% 32 to 3.5% 31 depending on which stroke‐risk variable is present.
A simple, noninvasive CCT imaging parameter to further refine risk in these patients would be extremely valuable. The binary determination of the presence or absence of LAAPT is also simple and contrasts sharply with the complexity and lack of reproducibility for morphology deduction. 28 , 33 In our study we have shown that by combining LAAPT and CHA2DS2‐VASc score, predictions for all prior stroke prevalence is improved by 52.5% in comparison to CHA2DS2‐VASc score used alone, and among patients without any stroke, 78.0% had lower predictions using the combined model. The use of LAAPT as a potential imaging biomarker warrants further investigation in prospective studies and may add to the stroke‐risk profiling of patients with a single point risk (2 points in women) on their CHA2DS2‐VASc score. Equally, the identification of LAAPT in patients who are anticoagulated may also identify those with residual risk, in whom LAA closure may offer a better prognosis.
Limitations
This is a retrospective study of a relatively small cohort of patients (but consecutive over a 4‐year period) who underwent CCT scanning as part of preprocedural assessment at a tertiary cardiac center and thus the patient cohort is prone to selection bias. The variables are retrospective in relation to the stroke event and thus cannot be considered predictive. Left ventricular function and AF burden at the time of the scan or at the time of stroke event were not available consistently across the cohort and thus are potential confounders. For both cohorts, compliance with OAC and type of OAC were unknown. The exact temporal relationship between oral anticoagulation use and stroke is unknown and the sample size is small, limiting the power for some of the multivariable analyses, with the small number of events driving wide CIs.
Conclusions
LAAPT on CCT is associated with a higher prevalence of previous stroke and potentially represents an important imaging biomarker for LAA dysfunction and elevated stroke risk (Figure 3). Prospective studies are needed to establish its role in predicting who might be at high risk of stroke, especially in patients with low CHA2DS2‐VASc score who do not fulfill anticoagulation criteria or who have pseudothrombus despite therapeutic anticoagulation.
Figure 3. Summarizing image.
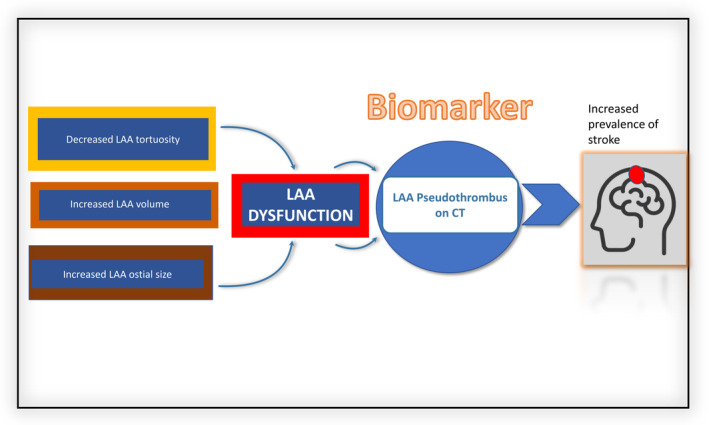
Representation of LAA pseudothrombus as biomarker of LAA dysfunction. Multifactorial LAA variables significantly associated with the presence of pseudothrombus (decreased LAA tortuosity, increased LAA volume and increased LAA ostial size). CT indicates computed tomography; and LAA, left atrial appendage.
Sources of Funding
None.
Disclosures
Dr Nicol is on the Advisory Board of Caristo Diagnostics. The remaining authors have no disclosures to report.
Supporting information
Table S1
Table S2
This article was sent to Luciano A. Sposato, MD, MBA, FRCPC, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.030147
For Sources of Funding and Disclosures, see page 9.
REFERENCES
- 1. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke. 1991;22:983–988. doi: 10.1161/01.STR.22.8.983 [DOI] [PubMed] [Google Scholar]
- 2. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly: the Framingham study. Arch Intern Med. 1987;147:1561–1564. doi: 10.1001/archinte.1987.00370090041008 [DOI] [PubMed] [Google Scholar]
- 3. Alonso A, Norby FL. Predicting atrial fibrillation and its complications. Circ J. 2016;80:1061–1066. doi: 10.1253/circj.CJ-16-0239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61:755–759. doi: 10.1016/0003-4975(95)00887-X [DOI] [PubMed] [Google Scholar]
- 5. Karim N, Ho SY, Nicol E, Li W, Zemrak F, Markides V, Reddy V, Wong T. The left atrial appendage in humans: structure, physiology, and pathogenesis. Europace. 2020;22:5–18. doi: 10.1093/europace/euz212 [DOI] [PubMed] [Google Scholar]
- 6. Khurram IM, Dewire J, Mager M, Maqbool F, Zimmerman SL, Zipunnikov V, Beinart R, Marine JE, Spragg DD, Berger RD, et al. Relationship between left atrial appendage morphology and stroke in patients with atrial fibrillation. Heart Rhythm. 2013;10:1843–1849. doi: 10.1016/j.hrthm.2013.09.065 [DOI] [PubMed] [Google Scholar]
- 7. Lee JM, Shim J, Uhm JS, Kim YJ, Lee HJ, Pak HN, Lee MH, Joung B. Impact of increased orifice size and decreased flow velocity of left atrial appendage on stroke in nonvalvular atrial fibrillation. Am J Cardiol. 2014;15(113):963–969. doi: 10.1016/j.amjcard.2013.11.058 [DOI] [PubMed] [Google Scholar]
- 8. Ernst G, Stöllberger C, Abzieher F, Veit‐Dirscherl W, Bonner E, Bibus B, Schneider B, Slany J. Morphology of the left atrial appendage. Anat Rec. 1995;242:553–561. doi: 10.1002/ar.1092420411 [DOI] [PubMed] [Google Scholar]
- 9. Somerville W, Chambers RJ. Systemic embolism in mitral stenosis relation to the size of the left atrial appendix. Br Med J. 1964;2:1167–1169. doi: 10.1136/bmj.2.5418.1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taina M, Vanninen R, Hedman M, Jäkälä P, Kärkkäinen S, Tapiola T, Sipola P. Left atrial appendage volume increased in more than half of patients with cryptogenic stroke. PloS One. 2013;4(8):e79519. doi: 10.1371/journal.pone.0079519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Korhonen M, Muuronen A, Arponen O, Mustonen P, Hedman M, Jäkälä P, Vanninen R, Taina M. Left atrial appendage morphology in patients with suspected cardiogenic stroke without known atrial fibrillation. PloS One. 2015;10:e0118822. doi: 10.1371/journal.pone.0118822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fatkin D, Kelly RP, Feneley MP. Relations between left atrial appendage blood flow velocity, spontaneous echocardiographic contrast and thromboembolic risk in vivo. J Am Coll Cardiol. 1994;23:961–969. doi: 10.1016/0735-1097(94)90644-0 [DOI] [PubMed] [Google Scholar]
- 13. Handke M, Harloff A, Hetzel A, Olschewski M, Bode C, Geibel A. Left atrial appendage flow velocity as a quantitative surrogate parameter for thromboembolic risk: determinants and relationship to spontaneous echocontrast and thrombus formation–a transesophageal echocardiographic study in 500 patients with cerebral ischemia. J Am Soc Echocardiogr. 2005;18:1366–1372. doi: 10.1016/j.echo.2005.05.006 [DOI] [PubMed] [Google Scholar]
- 14. Uretsky S, Shah A, Bangalore S, Rosenberg L, Sarji R, Cantales DR, Macmillan‐Marotti D, Chaudhry FA, Sherrid MV. Assessment of left atrial appendage function with transthoracic tissue Doppler echocardiography. Eur J Echocardiogr. 2009;10:363–371. doi: 10.1093/ejechocard/jen339 [DOI] [PubMed] [Google Scholar]
- 15. Akoum N, Marrouche N. Assessment and impact of cardiac fibrosis on atrial fibrillation. Curr Cardiol Rep. 2014;16:518. doi: 10.1007/s11886-014-0518-z [DOI] [PubMed] [Google Scholar]
- 16. Fukuchi M, Watanabe J, Kumagai K, Katori Y, Baba S, Fukuda K, Yagi T, Iguchi A, Yokoyama H, Miura M, et al. Increased von Willebrand factor in the endocardium as a local predisposing factor for thrombogenesis in overloaded human atrial appendage. J Am Coll Cardiol. 2001;37:1436–1442. doi: 10.1016/S0735-1097(01)01125-1 [DOI] [PubMed] [Google Scholar]
- 17. Kimura T, Takatsuki S, Inagawa K, Katsumata Y, Nishiyama T, Nishiyama N, Fukumoto K, Aizawa Y, Tanimoto Y, Tanimoto K, et al. Anatomical characteristics of the left atrial appendage in cardiogenic stroke with low CHADS2 scores. Heart Rhythm. 2013;10:921–925. doi: 10.1016/j.hrthm.2013.01.036 [DOI] [PubMed] [Google Scholar]
- 18. Lee Y, Park HC, Lee Y, Kim SG. Comparison of morphologic features and flow velocity of the left atrial appendage among patients with atrial fibrillation alone, transient ischemic attack, and cardioembolic stroke. Am J Cardiol. 2017;119:1596–1604. doi: 10.1016/j.amjcard.2017.02.016 [DOI] [PubMed] [Google Scholar]
- 19. Lupercio F, Carlos Ruiz J, Briceno DF, Romero J, Villablanca PA, Berardi C, Faillace R, Krumerman A, Fisher JD, Ferrick K, et al. Left atrial appendage morphology assessment for risk stratification of embolic stroke in patients with atrial fibrillation: a meta‐analysis. Heart Rhythm. 2016;13:1402–1409. doi: 10.1016/j.hrthm.2016.03.042 [DOI] [PubMed] [Google Scholar]
- 20. Di Biase L, Santangeli P, Anselmino M, Mohanty P, Salvetti I, Gili S, Horton R, Sanchez JE, Bai R, Mohanty S, et al. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J Am Coll Cardiol. 2012;60:531–538. doi: 10.1016/j.jacc.2012.04.032 [DOI] [PubMed] [Google Scholar]
- 21. Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ Jr, Davies DW, DiMarco J, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow‐up, definitions, endpoints, and research trial design. Europace. 2012;14:528–606. doi: 10.1093/europace/eus027 [DOI] [PubMed] [Google Scholar]
- 22. Saremi F, Channual S, Gurudevan SV, Narula J, Abolhoda A. Prevalence of left atrial appendage pseudothrombus filling defects in patients with atrial fibrillation undergoing coronary computed tomography angiography. J Cardiovasc Comput Tomogr. 2008;2:164–171. doi: 10.1016/j.jcct.2008.02.012 [DOI] [PubMed] [Google Scholar]
- 23. Lazoura O, Ismail TF, Pavitt C, Lindsay A, Sriharan M, Rubens M, Padley S, Duncan A, Wong T, Nicol E. A low‐dose, dual‐phase cardiovascular CT protocol to assess left atrial appendage anatomy and exclude thrombus prior to left atrial intervention. Int J Cardiovasc Imaging. 2016;32:347–354. doi: 10.1007/s10554-015-0776-x [DOI] [PubMed] [Google Scholar]
- 24. Healey JS, Crystal E, Lamy A, Teoh K, Semelhago L, Hohnloser SH, Cybulsky I, Abouzahr L, Sawchuck C, Carroll S, et al. Left atrial appendage occlusion study (LAAOS): results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am Heart J. 2005;150:288–293. doi: 10.1016/j.ahj.2004.09.054 [DOI] [PubMed] [Google Scholar]
- 25. Price MJ, Gibson DN, Yakubov SJ, Schultz JC, Di Biase L, Natale A, Burkhardt JD, Pershad A, Byrne TJ, Gidney B, et al. Early safety and efficacy of percutaneous left atrial appendage suture ligation: results from the U.S. transcatheter LAA ligation consortium. J Am Coll Cardiol. 2014;64:565–572. doi: 10.1016/j.jacc.2014.03.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, Mullin CM, Sick P. PROTECT AF investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non‐inferiority trial. Lancet. 2009;374:534–542. [DOI] [PubMed] [Google Scholar]
- 27. Holmes DR Jr, Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, Huber K, Reddy VY. Prospective randomized evaluation of the watchman left atrial appendage closure device in patients with atrial fibrillation versus long‐term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1–12. doi: 10.1016/j.jacc.2014.04.029 [DOI] [PubMed] [Google Scholar]
- 28. Jeong WK, Choi JH, Son JP, Lee S, Lee MJ, Choe YH, Bang OY. Volume and morphology of left atrial appendage as determinants of stroke subtype in patients with atrial fibrillation. Heart Rhythm. 2016;13:820–827. doi: 10.1016/j.hrthm.2015.12.026 [DOI] [PubMed] [Google Scholar]
- 29. Goldman ME, Pearce LA, Hart RG, Zabalgoitia M, Asinger RW, Safford R, Halperin JL. Pathophysiologic correlates of thromboembolism in nonvalvular atrial fibrillation: I. Reduced flow velocity in the left atrial appendage (the stroke prevention in atrial fibrillation [SPAF‐III] study). J Am Soc Echocardiogr. 1999;12:1080–1087. doi: 10.1016/S0894-7317(99)70105-7 [DOI] [PubMed] [Google Scholar]
- 30. Masci A, Barone L, Dedè L, Fedele M, Tomasi C, Quarteroni A, Corsi C. The impact of left atrium appendage morphology on stroke risk assessment in atrial fibrillation: a computational fluid dynamics study. Front Physiol. 2019;9:1938. doi: 10.3389/fphys.2018.01938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim YG, Shim J, Oh SK, Lee KN, Choi JI, Kim YH. Electrical isolation of the left atrial appendage increases the risk of ischemic stroke and transient ischemic attack regardless of postisolation flow velocity. Heart Rhythm. 2018;15:1746–1753. doi: 10.1016/j.hrthm.2018.09.012 [DOI] [PubMed] [Google Scholar]
- 32. Lip GYH, Skjøth F, Rasmussen LH, Larsen TB. Oral anticoagulation, aspirin, or no therapy in patients with nonvalvular AF with 0 or 1 stroke risk factor based on the CHA2DS2‐VASc score. J Am Coll Cardiol. 2015;65:1385–1394. doi: 10.1016/j.jacc.2015.01.044 [DOI] [PubMed] [Google Scholar]
- 33. Taina M, Korhonen M, Haataja M, Muuronen A, Arponen O, Hedman M, Jäkälä P, Sipola P, Mustonen P, Vanninen R. Morphological and volumetric analysis of left atrial appendage and left atrium: cardiac computed tomography‐based reproducibility assessment. PLoS One. 2014;9:e101580. doi: 10.1371/journal.pone.0101580 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2


