Abstract
Background
Gestational hypertension (GHTN) and preeclampsia are established risk indicators for chronic hypertension. While recurrence is associated with a greater risk, it is unclear whether there are differences in risk when these gestational complications occur for the first time in an earlier pregnancy versus first occurrence in a subsequent one. We hypothesized that the absence of recurrence reflects a transition toward a lower hypertension risk trajectory, whereas a new occurrence in a later pregnancy indicates a transition toward elevated risk.
Methods and Results
We analyzed linked data in Quebec, Canada, from public health care insurance administrative databases and birth, stillbirth, and death registries. Our retrospective cohort study included mothers with 2 singleton deliveries between April 1990 and December 2012. The primary exposure was patterns of GHTN or preeclampsia across 2 pregnancies (GHTN/preeclampsia in neither, first only, second only, or both). The outcome was incident chronic hypertension. We performed an adjusted multivariable Cox regression analysis. Among 431 980 women with 2 singleton pregnancies, 27 755 developed hypertension during the follow‐up period. Compared with those without GHTN/preeclampsia, those with GHTN/preeclampsia only in the first pregnancy had a 2.7‐fold increase in hazards (95% CI, 2.6–2.8), those with GHTN/preeclampsia only in the second had a 4.9‐fold increase (95% CI, 4.6–5.1), and those with GHTN/preeclampsia in both pregnancies experienced a 7.3‐fold increase (95% CI, 6.9–7.6). Patterns and estimates were similar when we considered GHTN and preeclampsia separately.
Conclusions
The magnitude of hypertension risk is associated with the number and sequence of GHTN/preeclampsia‐affected pregnancies. Considering both allows more personalized risk estimates.
Keywords: chronic hypertension, gestational hypertension, preeclampsia, pregnancy, recurrence
Subject Categories: Preeclampsia, Hypertension, Epidemiology, Pregnancy, Lifestyle
Nonstandard Abbreviations and Acronyms
- CCDSS
Canadian Chronic Disease Surveillance System
- GDM
gestational diabetes
- GHTN
gestational hypertension
- LGA
large for gestational age
- SGA
small for gestational age
Clinical Perspective.
What Is New?
In women with 2 singleton pregnancies, without chronic hypertension before or between pregnancies, those who developed gestational hypertension or preeclampsia for the first time in the second pregnancy were at higher risk for future chronic hypertension, compared with those who had gestational hypertension or preeclampsia in the first pregnancy but not in the second.
Our findings were similar when we considered gestational hypertension alone and when we considered preeclampsia alone.
Gestational diabetes was independently associated with chronic hypertension, with the highest chronic hypertension risk among those with gestational diabetes in both pregnancies.
What Are the Clinical Implications?
In gauging chronic hypertension risks, health care providers should ask not only about previous history of gestational hypertension and preeclampsia but also about the number of pregnancies and specifically in which pregnancies these adverse pregnancy outcomes did or did not occur; the number of gestational diabetes occurrences should also be queried.
Globally, >1 billion people have hypertension. 1 Its prevalence has doubled over the past 3 decades 1 , 2 due to population aging, higher obesity rates, and lower physical activity levels. 3 Chronic hypertension–induced vascular injury contributes to heart disease and stroke in the longer term, 4 as well as the development of renal disease, retinopathy, dementia, and peripheral vascular disease. 5 In the shorter term, hypertensive disorders of pregnancy can lead to organ injury during pregnancy itself, in the form of preeclampsia (new‐onset blood pressure elevation at or after 20 weeks' gestation, accompanied by proteinuria or other maternal organ dysfunction). 6 Beyond its urgent importance during pregnancy, preeclampsia predicts the future development of chronic hypertension. 7 , 8 Gestational hypertension (GHTN) without preeclampsia is also a hypertension risk marker. 8 , 9 Although women average 2 offspring globally, 10 few studies have examined patterns of GHTN/preeclampsia across pregnancies, in relationship to future development of hypertension. Such information could allow further refinement of hypertension risk assessment, with the dual goals of prevention and early detection. We hypothesized that many women with GHTN/preeclampsia in a first pregnancy but not in a second pregnancy had modified their dietary or physical activity behaviors in response to their experiences in the first pregnancy. These behaviors could both reduce their risks for another GHTN/preeclampsia recurrence and their longer‐term hypertension risk.
A 2022 meta‐analysis 8 across 13 studies estimated that preeclampsia confers a 3‐fold risk increase for chronic hypertension; the estimate across 3 studies that specifically evaluated GHTN was similar or higher. 8 , 9 , 11 Some studies have examined risks associated with preeclampsia recurrence 12 , 13 , 14 , 15 , 16 ; a 2018 meta‐analysis across 7 studies reported a doubling of chronic hypertension risk with recurrence, compared with 1 occurrence. 12 A single 2009 Danish study 13 distinguished first‐pregnancy preeclampsia from second‐pregnancy preeclampsia. Compared with absence of preeclampsia, preeclampsia only in the first pregnancy was associated with a 2.7‐fold increase in risk for hypertension, preeclampsia only in the second pregnancy with a 4.3‐fold increase, and preeclampsia in both with a 6‐fold increase. This suggests an upwards risk trajectory in women with a first preeclampsia occurrence in a second pregnancy, and a downward risk trajectory in those with preeclampsia only the first pregnancy.
Now, more than a decade later, we build on these Danish findings 13 using a large Canadian database in women with at least 2 consecutive pregnancies. We evaluated both GHTN and preeclampsia, combined and separately, in relationship to the development of chronic hypertension. In contrast to other studies, we concurrently accounted for patterns of gestational diabetes (GD), 17 , 18 , 19 along with other adverse pregnancy complications associated with hypertension development (preterm delivery 2 , 20 and small [SGA] or large [LGA] for gestational age offspring 21 , 22 ). Pregnancy is a time when younger adults are interested in addressing health issues, to optimize the short‐ and long‐term health of the family. 23 Our overarching goal is to generate precision medicine‐oriented clinical and social measures to refine risk estimates and stimulate action.
Methods
The McGill University Health Centre's Research Ethics Board (2019–5029; 12/11/2018) and Quebec Access to Information Commission (1019371‐S; 11/18/2019) approved the protocol. These bodies waived informed consent because we used deidentified data, performed analyses at the Quebec Statistical Institute's secured research data centers, and rounded frequencies to multiples of 5. We followed the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines.
The data that we analyzed are available only through Quebec's Statistical Institute Centers for Access to Research Data, secure environments available to accredited researchers in Quebec for research purposes. Data requests must be made through the Quebec Statistical Institute (https://statistique.quebec.ca/recherche/) and are subject to ethical and scientific review.
Design and Data Sources
We conducted a retrospective cohort study in Quebec, Canada, where residents are publicly insured for physician services and hospitalization. We examined health administrative databases of the public health insurance plan, linked to birth, stillbirth, and death registries. We obtained mothers' health territory of residence and month and year of birth from the public health insurance registry. The Physician Services Claims (International Classification of Diseases, Ninth Revision [ICD‐9]: Table S1) and Hospitalization Discharge Databases (ICD‐9 codes to April 1, 2006; International Classification of Diseases, Tenth Revision [ICD‐10] codes thereafter) include diagnostic codes, hospitalization dates, medical services, and surgical procedure codes; we used these to define outcomes, exposures, and covariables, alongside data from birth/stillbirth registries. Variables from these registries include offspring birthdates, gestational age at birth, birth weight, fetal sex, parental country of birth and first language, and years of maternal education. Where applicable, we obtained ICD‐coded cause of death from the stillbirth and death registries (ICD‐9 codes until December 31, 1999; ICD‐10 codes thereafter). We had mothers' Institut national de santé publique du Québec material and social deprivation indices, derived from the 6‐digit postal code in the public health registry and corresponding small‐area census data. 24 The Quebec Statistical Institute performed probabilistic database linkage based on multiple identifiers (G‐link software, Statistics Canada).
Study Population
We studied women with ≥2 consecutive singleton deliveries between April 1, 1990, and December 31, 2012, who were alive at 12 weeks after the second delivery (index date). We accessed follow‐up data to April 1, 2019, for these women, their offspring, and, for liveborn offspring, the fathers. We excluded mothers from families with missing gestational age in either offspring (required to distinguish chronic hypertension from GHTN/preeclampsia 25 ), those with preexisting hypertension or diabetes before 20 weeks' gestation of first pregnancy, and those who developed these conditions between pregnancies. To exclude women with these preexisting conditions, we applied the validated Canadian Chronic Disease Surveillance System (CCDSS) hypertension definition 26 of 2 outpatient or 1 hospitalization diagnostic code(s) to (1) the 2‐year period before 20 weeks' gestation in the first pregnancy and (2) the period from 12 weeks after delivery of the first pregnancy to 20 weeks' gestation of the second pregnancy (between pregnancies). We applied a validated parallel diabetes definition 27 , 28 to the same time periods.
We removed those with a different partner for each offspring to minimize the heterogeneity of paternal or within‐household factors that could influence health behaviors and subsequent maternal outcomes, 29 , 30 , 31 or baseline preeclampsia risk. 6 , 32 , 33 Using available diagnostic codes, we also excluded those with 2 outpatient visits or 1 hospitalization for cardiovascular disease (CVD) and/or other circulatory system diseases before the index date.
Exposure
Following exclusion of preexisting hypertension as described above, we defined GHTN/preeclampsia as a composite exposure, applying validated codes for both hypertension 26 , 34 and preeclampsia 34 to a pregnancy‐specific period. This period started at 20 weeks' gestation 34 and ended at 12 weeks after delivery, by which point any blood pressure elevation related to GHTN/preeclampsia should have resolved. We required 2 outpatient and/or 1 hospitalization code(s), similar to the validated CCDSS hypertension definition. 26 Our 4 mutually exclusive exposure categories were absence of GHTN/preeclampsia, GHTN/preeclampsia only in the first pregnancy, GHTN/preeclampsia only in the second pregnancy, and GHTN/preeclampsia in both pregnancies. In subgroup analyses, we considered occurrences of GHTN and preeclampsia separately.
Outcome
We examined incident chronic hypertension as the primary outcome, applying the validated CCDSS definition, which requires at least 2 outpatient codes or 1 hospitalization code with a 2‐year period. 26 Follow‐up was until the first of the following: incident chronic hypertension (using the date of the first component of the definition fulfilled), the date coinciding with 120 days before a third delivery (we did not have gestational age data for pregnancies after the second), death, or the end of the study period (April 1, 2019), as applicable.
Covariates
We accounted for other pregnancy‐ and offspring‐related factors previously shown to be associated with hypertension, specifically, GD, preterm delivery (<37 weeks), SGA (<10th percentile) and LGA (>90th percentile) offspring. 35 , 36 We examined these variables for both the first and second pregnancies (4 categories of GDM; 4 categories of preterm delivery; 9 categories of offspring size). To define GD, we applied the CCDSS diabetes definition to the same pregnancy period for which we defined GHTN/preeclampsia, in accordance with a validated GD definition 37 that we used in a previous study. 38
We also accounted for other covariates associated with hypertension development, including time between deliveries (<2, 2–<2.5, 2.5–<3.5, ≥3.5 years), maternal age at the index date (<25, 25–29, 30–34, ≥35 years), material and social deprivation level recorded in the index year (1 [least deprived] to 5 [most deprived]), 24 race or ethnicity based on participant‐reported region of birth and first language (Europid, African/Caribbean, Arabic, Asian, Other [Indigenous language or language unspecified]), presence of comorbid conditions (mood disorders, alcohol/drug dependence; thyroid disorder; arthritis; asthma/chronic obstructive pulmonary disease; defined as ≥1 hospitalization or ≥2 outpatient diagnostic codes occurring within 2 years before the index date) and preexisting paternal diabetes, hypertension, and CVD (validated CCDSS definitions 27 , 28 applied from 2 years before 20 weeks' gestation of the first pregnancy to 12 weeks following the second delivery). We accounted for preexisting spousal diabetes, hypertension, and CVD, given spousal concordance for these conditions, likely related to shared behaviors and environments. 17 , 39 , 40 , 41
We considered other covariates (eg, placental abruption, stillbirth, cancer, offspring sex, offspring congenital anomalies), but these did not meet our variable inclusion criteria (see Statistical Analysis), as described in Data S1.
Statistical Analysis
We computed baseline characteristics (numbers and percentages for categorical variables) and compared them across exposure groups (Pearson χ2 tests for proportions). We constructed Kaplan–Meier curves, calculated the incidence of hypertension by the primary exposure categories, derived crude hazard ratios (HRs; see Tables S2 and S3), and examined for interactions (P<0.05 for interaction terms) and multicollinearity (Cramer's V >0.10) among exposures and covariates. We evaluated applicability of the proportional hazards assumptions (Schoenfeld residuals test and visual inspection of log‐minus‐log survival plots).
We constructed multivariable Cox proportional hazards models to compute HRs for incident chronic hypertension. In the first set of models, the reference group was the absence of GHTN/preeclampsia in either pregnancy. We compared GHTN/preeclampsia in the first pregnancy only, GHTN/preeclampsia in the second pregnancy only, and GHTN/preeclampsia in both pregnancies to this reference category. In other analyses, we compared the GHTN/preeclampsia exposure groups directly to one another. Specifically, we next set GHTN/preeclampsia only in the first pregnancy as the reference group and compared GHTN/preeclampsia in the second pregnancy only and GHTN/preeclampsia in both pregnancies to this group. Then, we set GHTN/preeclampsia only in the second pregnancy as the reference group and compared GHTN/preeclampsia in both pregnancies to this. We considered including covariates in our final statistical models if their univariate associations with hypertension had a P value ≤0.25 and we opted to retain them on the basis of demonstration of a multivariable association with hypertension of P≤0.05 (stepwise selection), and reduced Bayesian information criteria values with inclusion of each additional variable.
The proportional hazards assumptions held when GHTN and preeclampsia were combined as a composite exposure variable and divided into 4 categories (absence of GHTN/preeclampsia, presence in the first pregnancy only, in the second pregnancy only, and in both). These assumptions did not hold within the model when stratified further into 9 categories that distinguished GHTN from preeclampsia (eg, absence of GHTN/preeclampsia in either pregnancy, GHTN in first only, preeclampsia in first only). Therefore, we created 2 subcohorts of women to separately examine GHTN and preeclampsia, in comparison with women without either of these conditions. In 1 subcohort, we removed all women with GHTN in 1 or both pregnancies; in this subcohort, we compared preeclampsia in the first pregnancy only, second pregnancy only, and in both pregnancies, with women who did not have preeclampsia in either pregnancy. In another subcohort, we removed all women with preeclampsia in 1 or both pregnancies; in this subcohort, we compared GHTN in the first pregnancy only, second pregnancy only, and in both pregnancies, with women who did not have GHTN in either pregnancy.
In a sensitivity analysis, we performed indirect adjustments for obesity and smoking status (separately), using established methods of bias analyses. 42 This approach required external estimates for the HRs of obesity and of smoking with incident hypertension in women, which we respectively estimated as 1.85 (obese versus not obese) 43 and 1.02 (smoking versus not smoking). 44 This method also required external cohort data to estimate obesity and smoking prevalence in groups of women with no GHTN/preeclampsia, GHTN/preeclampsia in first pregnancy, GHTN/preeclampsia in second pregnancy, and GHTN/preeclampsia in both pregnancies. We used the Canadian Community Health Survey (cycle 2.2) to estimate these prevalence values, as we had access to these data for another study 45 ; 13% were in the obesity category, and 24% smoked cigarettes. We applied the following formula for the indirect obesity adjustment: (Poe=proportion within specific GHTN/preeclampsia category who have obesity; Pe=proportion of those with specific GHTN/preeclampsia category among all women with 2 consecutive singleton pregnancies; Po=proportion with obesity among all women with 2 consecutive singleton pregnancies; see Data S2, similar formula applied for smoking). All analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC).
Results
Among the 431 980 women with 2 singleton pregnancies following exclusions (Figure 1), 4.9% (n=21 335) had GHTN/preeclampsia only in their first pregnancy, 1.6% had GHTN/preeclampsia in only their second pregnancy (n=6980), and 1.3% had GHTN/preeclampsia in both pregnancies (n=5830; Figure S1). In those with GHTN/preeclampsia in the first, second, and both pregnancies, the following proportions of women had preeclampsia, respectively: 51% (n=10 820), 36% (n=2540), and 64% (n=3750). The distribution of baseline characteristics (numbers and percentages presented) were similar (P>0.05) across each of the exposure groups (Table 1); most percentage differences across these groups were within 1% to 5%.
Figure 1. Cohort construction.
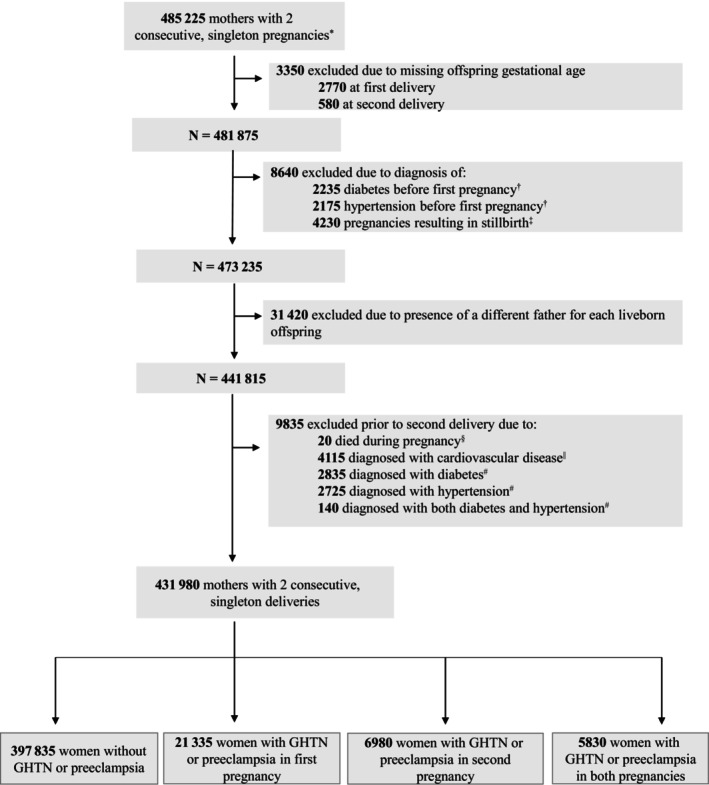
*Values are rounded either up or down to a multiple of ‘5’ (for patient confidentiality purposes). †Preexisting diabetes or hypertension in the mother, defined as ≥1 inpatient and/or ≥2 outpatient ICD codes for any form of diabetes or hypertension in the 2 y before 20 weeks' gestational age of the first pregnancy. ‡Stillbirths were identified from the stillbirth registry. In the case of a stillbirth, it is not possible to identify the father since there is an absence of paternal information in the stillbirth registry (registry is linked only to mothers). §Fatal events occurring at any point between 20 wks' gestation of the second pregnancy and 12 weeks' postpartum. Five deaths were related to a fatal CVD event while the remaining 15 fatalities were related to obstetrical complications related to childbirth, major trauma and suicide. ||The CVD‐related exclusion criteria included codes for myocardial infarction, stroke, angina, and other circulatory system disease conditions (atrial fibrillation, heart failure, other ischemic disease, other cardiac dysrhythmias, peripheral vascular disease, and venous thromboembolism). We required ≥1 inpatient diagnosis, 1 related surgical procedure (angioplasty, endarterectomy, or coronary artery bypass surgery), or ≥2 outpatient diagnoses, occurring 2 y before 12 wks' postpartum of the second pregnancy (index date), to define prior CVD events. #Defined as ≥1 inpatient and/or ≥2 outpatient (within 2 years) diabetes‐related or hypertension‐related ICD codes occurring between 12 wks' postpartum of the first pregnancy and 20 wks' gestation of the second pregnancy. CVD indicates cardiovascular disease; ICD, International Classification of Diseases; and GHTN, gestational hypertension.
Table 1.
Baseline Characteristics, Stratified by GHTN/Preeclampsia Status
| n (%)* | No GHTN/preeclampsia (n=397 835) | GHTN/preeclampsia in first pregnancy (n=21 335) | GHTN/preeclampsia in second pregnancy (n=6980) | GHTN/preeclampsia in both pregnancies (n=5830) |
|---|---|---|---|---|
| Maternal characteristics | ||||
| History of GDM across 2 pregnancies | ||||
| No GDM (n=396 660) | 367 105 (92.3) | 18 640 (87.4) | 5985 (85.7) | 4930 (84.6) |
| GDM in first pregnancy (n=10 915) | 9645 (2.4) | 785 (3.7) | 265 (3.8) | 220 (3.8) |
| GDM in second pregnancy (n=16 150) | 13 990 (3.5) | 1280 (6.0) | 460 (6.6) | 420 (7.2) |
| GDM in both pregnancies (n=8255) | 7095 (1.8) | 630 (2.9) | 270 (3.9) | 260 (4.5) |
| Maternal age at second delivery, y | ||||
| <25 (n=56 460) | 52 135 (13.1) | 2885 (13.5) | 715 (10.2) | 725 (12.4) |
| 25–30 (n=156 305) | 143 700 (36.1) | 8185 (38.4) | 2295 (32.9) | 2125 (36.4) |
| 30–35 (n=157 740) | 145 640 (36.6) | 7480 (35.1) | 2530 (36.2) | 2090 (35.8) |
| >35 (n=61 485) | 56 360 (14.2) | 2785 (13.1) | 1450 (20.8) | 890 (15.3) |
| Time between deliveries, y | ||||
| <2 (n=134 915) | 125 065 (31.4) | 6580 (30.8) | 1565 (22.4) | 1705 (29.2) |
| 2‐ < 2.5 (n=88 105) | 81 120 (20.4) | 4550 (21.3) | 1215 (17.4) | 1220 (20.9) |
| 2.5‐ < 3.5 (n=112 000) | 103 145 (25.9) | 5530 (25.9) | 1790 (25.6) | 1535 (26.3) |
| ≥3.5 (n=96 955) | 88 500 (22.2) | 4675 (21.9) | 2415 (34.6) | 1365 (23.4) |
| Material deprivation index, quintiles† | ||||
| 1=least deprived (n=87 645) | 81 450 (20.5) | 3885 (18.2) | 1260 (18.1) | 1050 (18.0) |
| 2 (n=91 135) | 83 965 (21.1) | 4485 (21.0) | 1440 (20.6) | 1245 (21.4) |
| 3 (n=85 660) | 78 865 (19.8) | 4275 (20.0) | 1365 (19.6) | 1155 (19.8) |
| 4 (n=81 430) | 74 725 (18.8) | 4190 (19.6) | 1385 (19.8) | 1130 (19.4) |
| 5=most deprived (n=78 765) | 72 140 (18.1) | 4105 (19.2) | 1395 (20.0) | 1125 (19.3) |
| Social deprivation index, quintiles† | ||||
| 1=least deprived (n=95 755) | 88 110 (22.1) | 4895 (22.9) | 1450 (20.8) | 1300 (22.2) |
| 2 (n=92 735) | 85 195 (21.4) | 4695 (22.0) | 1525 (21.8) | 1320 (22.6) |
| 3 (n=88 345) | 81 100 (20.4) | 4520 (21.2) | 1525 (21.8) | 1200 (20.6) |
| 4 (n=79 930) | 73 890 (18.6) | 3715 (17.4) | 1260 (18.1) | 1065 (18.3) |
| 5=most deprived (n=67 865) | 62 845 (15.8) | 3115 (14.6) | 1085 (15.5) | 820 (14.1) |
| Ethnicity‡ | ||||
| America, Australia or Europe (n=373 415) | 343 050 (86.2) | 19 175 (89.9) | 5965 (85.5) | 5225 (89.6) |
| Africa or Caribbean (n=8550) | 7680 (1.9) | 430 (2.0) | 285 (4.1) | 155 (2.7) |
| Arab‐speakingregions (n=17 315) | 16 525 (4.2) | 480 (2.3) | 210 (3.0) | 100 (1.7) |
| Asia (n=14 620) | 13 875 (3.5) | 435 (2.0) | 200 (2.9) | 110 (1.9) |
| Other (n=18 080) | 16 705 (4.2) | 815 (3.8) | 325 (4.7) | 235 (4.0) |
| Comorbid conditions | ||||
| Mood disorders, alcohol or drug dependence (n=18 010) | 16 340 (4.1) | 955 (4.5) | 400 (5.7) | 315 (5.4) |
| Thyroid disorder (n=15 015) | 13 525 (3.8) | 900 (4.2) | 330 (4.7) | 260 (4.5) |
| Arthritis (n=9180) | 8305 (2.1) | 505 (2.4) | 220 (3.2) | 150 (2.6) |
| Asthma or COPD (n=8650) | 7695 (2.2) | 545 (2.6) | 225 (3.2) | 185 (3.2) |
| Offspring characteristics | ||||
| Small for gestational age§ | ||||
| Neither pregnancy (n=369 230) | 341 695 (85.9) | 17 390 (81.5) | 5550 (79.5) | 4595 (78.8) |
| First pregnancy only (n=26 110) | 23 375 (5.9) | 1675 (7.9) | 590 (8.5) | 470 (8.1) |
| Second pregnancy only (n=26 145) | 23 415 (5.9) | 1680 (7.9) | 555 (8.0) | 495 (8.5) |
| Both pregnancies (n=10 350) | 9210 (2.3) | 580 (2.7) | 290 (4.2) | 270 (4.6) |
| Large for gestational age§ | ||||
| Neither pregnancy (n=367 635) | 339 380 (85.3) | 17 555 (82.3) | 5845 (83.7) | 4855 (83.3) |
| First pregnancy only (n=26 155) | 23 810 (6.0) | 1475 (6.9) | 475 (6.8) | 395 (6.8) |
|
Second pregnancy only (n=26 070) |
23 725 (6.7) | 1485 (7.0) | 465 (6.7) | 395 (6.8) |
| Both pregnancies (n=11 965) | 10 785 (2.9) | 805 (3.8) | 200 (2.9) | 175 (3.0) |
| Preterm birth | ||||
| Neither pregnancy (n=392 290) | 363 555 (91.4) | 18 510 (86.8) | 5715 (81.9) | 4510 (77.4) |
| First pregnancy only (n=20 330) | 17 315 (4.4) | 1820 (8.5) | 490 (7.0) | 705 (12.1) |
| Second pregnancy only (n=14 630) | 12 890 (3.2) | 750 (3.5) | 635 (9.1) | 355 (6.1) |
| Both pregnancies (n=4730) | 4080 (1.0) | 255 (1.2) | 145 (2.1) | 260 (4.5) |
| Paternal characteristics | ||||
| Prior history of paternal diabetes|| | ||||
| Yes (n=3650) | 3305 (0.8) | 205 (1.0) | 75 (1.1) | 65 (1.1) |
| Prior history of paternal hypertension|| | ||||
| Yes (n=9420) | 8505 (2.1) | 545 (2.6) | 210 (3.0) | 160 (2.7) |
| Prior history of paternal cardiovascular disease# | ||||
| Yes (n=1610) | 1455 (0.4) | 100 (0.5) | 35 (0.5) | 20 (0.3) |
COPD indicates chronic obstructive pulmonary disease; GDM, gestational diabetes; GHTN, gestational hypertension; and ICD, International Classification of Diseases.
Values are randomly rounded up or down to a multiple of 5 (for patient confidentiality purposes). Therefore, column sums for each baseline characteristic may not equal the total number of women in each level of the exposure due to this random rounding process.
Range from 1 (least deprived) to 5 (most deprived). The Institut national de santé publique du Québec material and social deprivation index is computed from small‐area census data. Specifically, the material indices are derived from average income, proportions without high school diploma, and employment to population ratio among those aged ≥15 y. The social indices are derived from the proportion of the population who are single‐parent families; aged ≥15y living alone; and aged ≥15 y who are separated, divorced, or widowed. To assign the Institut national de santé publique du Québec index for each woman, we first checked availability of this variable in the index year (year of second delivery); 7335 women were missing an assigned Institut national de santé publique du Québec index score.
Ethnicity based on the mother's region of birth and reported preferred language. We categorized women as (1) “Europid” if born in North America, South America, Central America, Mexico, East/South/Southern/West Europe or Australia and if their first language was English, French, or other European language; (2) “African or Caribbean” if born in West/South/East/Central Africa or first language is of African or Carribean descent; (3) “Arabic” if born in the Arab league or first language is Arabic or other North African/South‐West Asian language; (4) “Asian” if born in West/East/Central/South/ Southeast/Pacific Asia or if their first language was from this region; or (5) “Other” if their birthplace or first language did not fit into any other category (e.g., if their first language was Indigenous [n=1680]).
150 offspring were missing birthweight required to derive offspring size.
Prior history in the father was defined as ≥1 inpatient and/or ≥2 outpatient ICD codes for any form of diabetes or hypertension, respectively, that occurred during the period from 2 years before 20 wks' gestation of their partner's first pregnancy to 12 wks' postpartum in relationship to the second pregnancy.
Prior history of cardiovascular disease in the father was defined as ≥1 inpatient, ≥1 related surgical procedure (angioplasty, endarterectomy, or coronary artery bypass surgery), and/or ≥2 outpatient ICD codes for any form of myocardial infarction, stroke, and angina that occurred during the period from 2 years before 20 wks' gestation of their partner's first pregnancy to 12 wks' postpartum in relationship to the second pregnancy.
Associations Between GHTN/Preeclampsia and Incident Hypertension
Over a median 11.0 years (interquartile range, 4.96–18.7; 5 147 250 total person years), a total of 27 755 mothers developed chronic hypertension during the follow‐up period. Kaplan–Meier curves indicated significant differences in event‐free survival across exposure groups (P<0.001; Figure 2). The incidence rates per 1000 person‐years rose across the no GHTN/preeclampsia (4.49), GHTN/preeclampsia in the first pregnancy only (12.0), GHTN/preeclampsia in the second pregnancy only (23.6), and GHTN/preeclampsia in both pregnancies (32.1) categories. Schoenfeld residuals test and visual inspection of log‐minus‐log survival plots indicated that the proportional hazards assumptions applied. There was no significant multicollinearity/interaction detected in our models.
Figure 2. Kaplan–Meier curves for hypertension‐free survival, by GHTN or preeclampsia status.
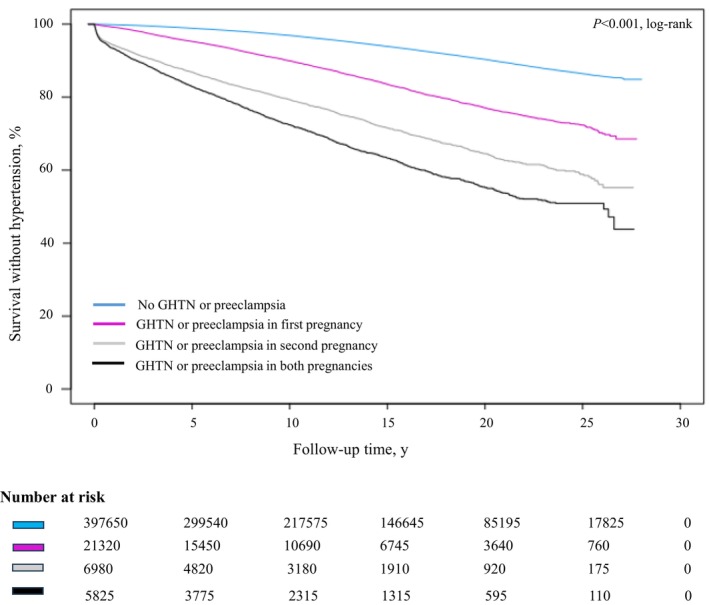
The log‐rank test indicated significant differences in event‐free survival across exposure groups (P<0.001). GHTN indicates gestational hypertension. GHTN indicates gestational hypertension.
In adjusted Cox regression models, compared with absence of GHTN/preeclampsia, those with GHTN/preeclampsia in first pregnancy had 2.67‐fold higher hazards for hypertension (95% CI, 2.57–2.78; Figure 3A), those with GHTN/preeclampsia in the second pregnancy had a 4.85‐fold increase (95% CI, 4.61–5.11), and those with GHTN/preeclampsia affecting both pregnancies demonstrated a 7.25‐fold increase (95% CI, 6.90–7.63). When the reference group was changed to those with GHTN/preeclampsia in the first pregnancy, women with GHTN/preeclampsia in the second pregnancy had an 82% higher hazards (95% CI, 1.71–1.93), and those with GHTN/preeclampsia in both pregnancies had a 2.71‐fold increase (95% CI, 2.55–2.88). Finally, risk for incident hypertension was 1.50‐fold higher among women with GHTN/preeclampsia in both pregnancies (95% CI, 1.37–1.60) compared with those with GHTN/preeclampsia occurring only in the second pregnancy.
Figure 3. Associations of GHTN or preeclampsia in first and second pregnancies with incident chronic hypertension.
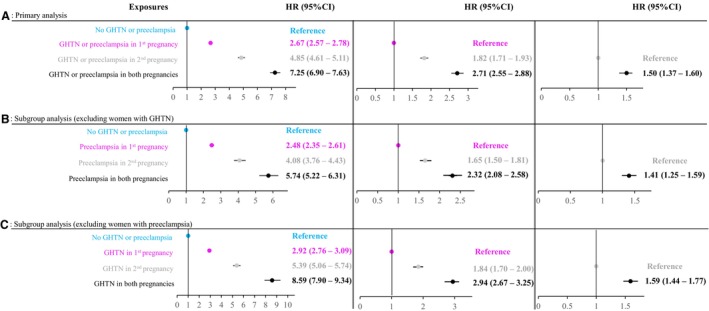
(A) Primary analysis. (B) Subgroup analysis (excluding women with GHTN). (C) Subgroup analysis (excluding women with preeclampsia). CI indicates confidence interval; GHTN, gestational hypertension; and HR, hazard ratio.
Associations Between Preeclampsia and Incident Hypertension
When we removed those with GHTN from the original cohort, 412 735 women remained. Compared with absence of preeclampsia in either pregnancy, those with preeclampsia in the first pregnancy had 2.48‐fold increased hazards for hypertension (95% CI, 2.35–2.61; Figure 3B), those with preeclampsia in the second pregnancy had a 4.08‐fold increase (95% CI, 3.76–4.43), and those with preeclampsia in both pregnancies had a 5.74‐fold increase (95% CI, 5.22–6.31). When women with preeclampsia in the first pregnancy served as the reference group, those with preeclampsia in the second pregnancy had their hazards for hypertension increase by 65% (95% CI, 1.50–1.81), and those with preeclampsia in both pregnancies had a 2.32‐fold increase (95% CI, 2.08–2.58). Finally, compared with those with preeclampsia occurring only in the second pregnancy, risk for incident hypertension was 1.41‐fold higher among women with preeclampsia in both pregnancies (95% CI, 1.25–1.59).
Associations Between GHTN and Incident Hypertension
Removal of women who had preeclampsia in either or both pregnancies (n=17 105) resulted in a sample of 414 875 women. Compared with absence of GHTN in either pregnancy, those with GHTN in the first pregnancy demonstrated 2.92‐fold (95% CI, 2.76–3.09; Figure 3C) increased hazards for subsequent hypertension development, those with GHTN in the second pregnancy had 5.39‐fold (95% CI, 5.06–5.74) increase, and those with GHTN affecting both pregnancies had an 8.59‐fold (95% CI, 7.90–9.34) increase. When the reference group was changed to those with GHTN in the first pregnancy, women with GHTN in the second pregnancy had an 84% higher hazards (95% CI, 1.70–2.00), and those with GHTN in both pregnancies had a 2.94‐fold increase (95% CI, 2.67–3.25). Finally, compared with those with GHTN affecting only the second pregnancy, hazards for incident hypertension was 1.59‐fold higher among women with GHTN in both pregnancies (95% CI, 1.44–1.77).
Bias Analyses: Indirect Adjustments for Obesity and Smoking
Indirect adjustments for obesity (no GHTN/preeclampsia: reference; GHTN/preeclampsia in first pregnancy: HR, 2.36 [95% CI, 2.27–2.46]; GHTN/preeclampsia in second pregnancy: HR, 4.31 [95% CI, 4.10–4.54]; GHTN/preeclampsia in both pregnancies: HR, 6.44 [95% CI, 6.13–6.77]); and smoking (no GHTN/preeclampsia: reference; GHTN/preeclampsia in first pregnancy: HR, 2.66 [95% CI, 2.56–2.77]; GHTN/preeclampsia in second pregnancy: HR, 4.83 [95% CI, 4.81–5.09]; GHTN/preeclampsia in both pregnancies: HR, 7.21 [95% CI, 6.87–7.60]) slightly attenuated the reported HRs for incident chronic hypertension from our primary analysis.
Associations Between Patterns of Other Pregnancy Complications and Incident Hypertension
GD was also found to be associated with elevated hazards of developing incident hypertension later in life. Compared with those without GDM, this condition conferred an increase of 40% to 45% if occurring in a first or in a second pregnancy alone, and 76% if occurring in both pregnancies (Table 2).
Table 2.
Associations of Covariates With Incident Hypertension
| Covariate* | Adjusted HR (95% CI) |
|---|---|
| Maternal characteristics | |
| History of GD across 2 pregnancies | |
| Absence of GDM | Reference |
| GD in first pregnancy | 1.40 (1.31–1.49) |
| GD in second pregnancy | 1.44 (1.37–1.52) |
| GD in both pregnancies | 1.76 (1.65–1.88) |
| Age of mother at second delivery, y | |
| <25 | Reference |
| 25–29 | 1.24 (1.18–1.30) |
| 30–34 | 1.58 (1.50–1.66) |
| ≥35 | 2.22 (2.11–2.34) |
| Time between deliveries, y | |
| <2 | Reference |
| 2–<2.5 | 0.96 (0.92–0.99) |
| 2.5–<3.5 | 0.97 (0.94–1.00) |
| ≥3.5 | 1.08 (1.05–1.12) |
| Material deprivation index, quintiles† | |
| 1 (least deprived) | Reference |
| 2 | 1.15 (1.11–1.20) |
| 3 | 1.17 (1.13–1.22) |
| 4 | 1.25 (1.20–1.29) |
| 5 (most deprived) | 1.32 (1.27–1.38) |
| Social deprivation index, quintiles† | |
| 1 (least deprived) | Reference |
| 2 | 1.01 (0.97–1.05) |
| 3 | 1.05 (1.01–1.09) |
| 4 | 1.08 (1.04–1.12) |
| 5 (most deprived) | 1.07 (1.03–1.11) |
| Ethnicity‡ | |
| Europid‐descent: America, Australia or Europe | Reference |
| Africa or Caribbean | 2.20 (2.07–2.35) |
| Arab‐speaking regions | 0.82 (0.76–0.89) |
| Asia | 1.14 (1.07–1.22) |
| Other | 0.99 (0.93–1.05) |
| Comorbid conditions§ | |
| Mood disorders, alcohol or drug dependence | 1.21 (1.14–1.28) |
| Thyroid disorder | 1.06 (1.00–1.14) |
| Arthritis | 1.24 (1.15–1.33) |
| Asthma or COPD | 1.38 (1.29–1.49) |
| Offspring characteristics | |
| Offspring size|| | |
| AGA: both offspring | Reference |
| SGA: first offspring only | 1.05 (1.00–1.10) |
| SGA: second offspring only | 1.06 (1.01–1.11) |
| SGA: both offspring | 1.15 (1.07–1.23) |
| LGA: first offspring only | 1.08 (1.03–1.14) |
| LGA: second offspring only | 1.07 (1.02–1.12) |
| LGA: both offspring | 1.10 (1.02–1.18) |
| SGA: first offspring, LGA: second offspring | 1.16 (0.87–1.56) |
| LGA: first offspring, SGA: second offspring | 0.92 (0.68–1.26) |
| Gestational age of offspring at birth | |
| Term birth: both offspring | Reference |
| Preterm birth: first offspring only | 1.06 (1.00–1.11) |
| Preterm birth: second offspring only | 1.15 (1.09–1.22) |
| Preterm birth: both offspring | 1.12 (1.01–1.23) |
| Paternal characteristics | |
| Prior history of paternal diabetes# | 1.25 (1.12–1.41) |
| Prior history of paternal hypertension# | 1.21 (1.13–1.30) |
| Prior history of paternal cardiovascular disease** | 1.21 (1.03–1.42) |
AGA indicates appropriate for gestational age; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; GDM, gestational diabetes; HR, hazard ratio; ICD, International Classification of Diseases; LGA, large for gestational age; and SGA, small for gestational age.
The Cox proportional hazards model adjusted for the gestational hypertension/preeclampsia occurrences across pregnancies, as well as each of the variables listed.
7335 women were missing a value for the Institut national de santé publique du Québec material deprivation index.
Compared with women of Europid descent, those from African or Arabic ethnic origins demonstrated increased risk of developing hypertension, respectively, during the follow‐up period. Other indicates indigenous language or language unspecified.
The reference group are women with the absence of each condition, respectively. The presence of each comorbid condition was associated with higher hypertension hazards. Comorbid conditions were defined in accordance with the Chronic Disease Surveillance System's definition of chronic disease, requiring ≥1 inpatient or ≥2 outpatient ICD codes to be present within 2 years before the index date.
150 offspring were missing birth weight required to derive offspring size.
Prior history in the father was defined as ≥1 inpatient and/or ≥2 outpatient ICD codes for any form of diabetes or hypertension, respectively, that occurred during the period from 2 y before 20 wks' gestation of their partner's first pregnancy to 12 wks' postpartum in relationship to the second pregnancy. The reference group are those without diabetes or hypertension, respectively.
Prior history of CVD in the father was defined as ≥1 inpatient, ≥1 related surgical procedure (angioplasty, endarterectomy, or coronary artery bypass surgery), and/or ≥2 outpatient ICD codes for any form of myocardial infarction, stroke, and angina, that occurred during the period from 2 y before 20 wks' gestation of their partner's first pregnancy to 12 wks' postpartum in relationship to the second pregnancy. The reference group are those without CVD.
SGA and LGA, compared with appropriate for gestational age; and preterm delivery status, compared with term delivery, each elevated hypertension risk by 5% to 15% compared with women without each of these conditions. SGA in a single pregnancy was independently associated with an ≈5% increase in hypertension hazards, which rose to a 15% increase when occurring in both pregnancies, compared with women delivering appropriate for gestational age offspring in both pregnancies. Any occurrence of LGA independently demonstrated 7% to 10% increased hypertension hazards compared with appropriate for gestational age offspring size in both pregnancies. Having SGA in 1 pregnancy and LGA in another (or vice versa) was an infrequent occurrence, with no conclusive associations for such combinations. Preterm delivery status was also independently associated with increased hazards for incident hypertension. Compared with women with 2 term deliveries, those with preterm delivery only in a first pregnancy demonstrated a 6% increase in hypertension hazards, rising to a 12% to 15% increase when occurring only in a second pregnancy or in both pregnancies.
Associations Between Paternal Risk Indicators and Other Maternal Risk Indicators With Incident Hypertension
Paternal diabetes, hypertension, and CVD were each independently associated with roughly a 20 to 25% increase in hazards for incident hypertension in the mother, compared with mothers with partners in which these conditions were absent (Table 2). Maternal age was associated with a stepwise increase in risk of hypertension, as was material deprivation. Ethnicity and comorbid conditions were also conclusively associated with increased hypertension hazards.
Discussion
Our analyses demonstrated that in women with at least 2 singleton pregnancies, the future risk for chronic hypertension is associated with the cumulative number of GHTN/preeclampsia‐affected pregnancies, and the ordinal pregnancy in which it occurs, in the case of a single occurrence. The risk for chronic hypertension doubled with GHTN/preeclampsia in the first pregnancy alone, rose >4‐fold with occurrence only in the second, and >7‐fold higher with GHTN/preeclampsia in both pregnancies, compared with their absence in either pregnancy. We observed similar patterns for preeclampsia and for GHTN when modeled separately. GD was also independently associated with an increase in hazards for hypertension, at 40% to 45% for GD in the first or second pregnancy alone, and 76% with GD in both. SGA, LGA, and preterm status each conferred a 5% to 15% increase in hazards, similar for 1 or both pregnancies. Paternal diabetes, hypertension, and CVD were each associated with a roughly 20% to 25% increase in hazardsfor hypertension in the mother. Age at second delivery, material deprivation, ethnicity, and the presence of comorbidities were also associated with chronic hypertension development.
As previously noted, a 2009 Danish study 13 reported higher hypertension risk for a second pregnancy with preeclampsia alone than for a first pregnancy with preeclampsia alone; specifically, a >2‐fold increase in risk with first pregnancy preeclampsia, a roughly 4‐fold increase with second pregnancy preeclampsia, and a 6‐fold increase with preeclampsia in both. Our findings are consistent with this but demonstrate similar patterns and similar risk increases both for preeclampsia and for GHTN, separately. Specifically, compared with absence of GHTN/preeclampsia in either pregnancy, we determined first pregnancy preeclampsia to be associated with a 2.5‐fold increase in risk for chronic hypertension, second pregnancy preeclampsia with a 4‐fold increase, and preeclampsia in both pregnancies with a 5.7‐fold increase. Compared with absence of GHTN/preeclampsia in either pregnancy, GHTN in the first pregnancy was associated with a nearly 3‐fold increase in risk, in the second pregnancy with a 5.4‐fold increase, and in both with an 8.6‐fold increase. A Nurses' Health Study II analysis 11 examined first‐pregnancy GHTN/preeclampsia and conducted a secondary analysis for GHTN/preeclampsia in “second or later” pregnancies, rather than focusing on the second pregnancy. Their findings were consistent with ours, but their estimates were lower (HR, 1.85 GHTN/preeclampsia in the first pregnancy; HR, 2.40 GHTN/preeclampsia in second or later pregnancy; HR, 3.53 GHTN/preeclampsia in both first pregnancy and in second or later pregnancy). This is likely because they commenced follow‐up at the age of 40 years, rather than soon after the second delivery, and required women to be free of CVD and other risk factors (including hypertension) by the age of 40 years. Additionally, their “second or later” exposure group likely included women with lower risk trajectories (eg, absence of GHTN/preeclampsia in either the first or second pregnancy and presence only in a third pregnancy).
The primary focus of the Nurses' Health Study II analysis 11 was to compare the presence of GHTN or preeclampsia in a first pregnancy versus its absence. In their main analysis, the investigators examined GHTN and preeclampsia, separately, and reported similar associations for both with chronic hypertension development. Consistent with this, in our analyses, we demonstrated that GHTN and preeclampsia have similar patterns of associations with chronic hypertension. Women with severe organ injury related to preeclampsia in a first pregnancy may opt to not have a second pregnancy. Those with a milder course may be similar to women with GHTN, and thus more likely to have a second pregnancy. It is also important to note that while heterogeneity in the pathophysiological processes and clinical phenotypes exist between GHTN and preeclampsia, both conditions share maternal endothelial dysfunction as a central phenomenon. 46 , 47 , 48 , 49
Among women with 2 consecutive singleton pregnancies who have not yet developed chronic hypertension by the second delivery, there thus appears to be a downward shift in risk profile among women with GHTN/preeclampsia occurring only in a first pregnancy. First‐pregnancy GHTN/preeclampsia may motivate some to adopt or enhance health behaviors that lower blood pressure (higher physical activity levels, healthier food intake, optimized weight, smoking cessation). These actions may prevent recurrence in a second pregnancy. The 21% recurrence rate that we observed is similar to that reported in other studies, 12 , 50 , 51 suggesting that a large proportion of women take preventive action, possibly including health behavior change, aspirin therapy before 20 weeks' gestation, and/or calcium supplementation in those at risk for deficiency. 6 Also consistent with some preventive action following a first pregnancy complicated by preeclampsia is a prior study that suggested that even when preeclampsia recurs, it tends to be a milder subtype. 52 Women with GHTN/preeclampsia restricted to the first pregnancy subsequently experience lower risk for chronic hypertension, as we additionally demonstrated for both GHTN and preeclampsia separately, and as reported for preeclampsia in a prior study. 13
While women without recurrence entered a lower risk trajectory, we observed that women with a first GHTN/preeclampsia occurrence in a second pregnancy had entered a higher one. This may be related to difficulty in losing excess gestational weight from the first pregnancy, stress related to motherhood, and time pressures limiting physical activity and healthy eating habits. 53 Additionally, we demonstrated that women with any form of recurrent GHTN/preeclampsia had the highest risk of developing chronic hypertension. As previously discussed, in contrast with our study, no other studies have assessed associations between recurrent GHTN and long‐term hypertension risk, as conducted in our study. However, previous investigators have hypothesized that the potential mechanism underlying associations of recurrent preeclampsia with increased chronic hypertension risk may stem from persistent vascular alterations, dysregulated inflammatory responses, and cumulative metabolic abnormalities. 12 , 14 , 54 Whether these women have a stronger predisposition for chronic hypertension as a result of a more unfavorable cardiovascular risk profile, or if recurrent GHTN/preeclampsia induces direct, cumulative impacts on endothelial dysfunction remains to be elucidated. Understanding these mechanisms is crucial for informing targeted preventive strategies and therapeutic interventions aimed at mitigating long‐term hypertension risk.
We also demonstrated that compared with those without GD, the presence of GD in either a first or second pregnancy conferred 40% to 45% increased risk of developing hypertension, which rose to 76% with GD in both pregnancies. Although GD is a recognized risk marker for chronic hypertension, 17 , 18 , 19 we did not identify any prior study that evaluated GD and GHTN/preeclampsia patterns concurrently across 2 pregnancies, in relationship to hypertension development. Like diabetes and hypertension, both GD and GHTN/preeclampsia emerge from an interplay of genetic factors alongside modifiable household, social, economic, and behavioral factors. These conditions collectively contribute to the emergence of vascular injury and complications. In a previous study, 38 we demonstrated that the risk of CVD increased with the number of GD and GHTN/preeclampsia occurrences across 2 pregnancies, suggesting a cumulative effect over multiple pregnancies.
Preeclampsia results in part from uteroplacental insufficiency that can lead to impaired fetal growth, preterm labor, placental abruption, and stillbirth. Such insufficiency could result in SGA and preterm delivery. Even after accounting for GHTN/preeclampsia, in our analyses, SGA in a single pregnancy was associated with a roughly 5% increased risk for chronic hypertension, and in both pregnancies with a 15% increase, compared with offspring of appropriate size in both pregnancies. Preterm status was also independently associated with a small increase in risk of hypertension, at 6% for the first pregnancy alone and at 12% to 15% for the second pregnancy or both pregnancies.
We also identified a 20% to 25% increase in hypertension hazards for each of hypertension, diabetes, and CVD in the father. Previous studies have demonstrated that spousal concordance exists for type 2 diabetes, hypertension, and CVD, 39 , 40 , 55 , 56 likely as a result of shared behaviors and environments. 31 We previously demonstrated that GD and GHTN/preeclampsia in mothers predict diabetes and CVD development in fathers. 17 , 41 The importance of these associations lies in untapping the potential for couple collaboration in reducing CVD risk, stimulated by their shared risk.
Linkage of Quebec's health administrative and vital statistics databases allowed us to leverage data from nearly half a million women, a population‐based sample with up to 29 years of follow‐up. These databases are designed for administrative purposes and thus have limitations. We could not corroborate ICD‐coded diagnoses of hypertension with direct clinical measurement or medication use, but to mitigate the potential for misclassification, we applied validated definitions. To offset the potential for confounding by obesity and smoking, we performed indirect adjustments for these factors using data from an external cohort in sensitivity analyses, and we observed little impact on our estimates. 42 Further, we accounted for LGA in both pregnancies, a variable that correlates with both maternal prepregnancy obesity and gestational weight gain. 57 , 58 We did not have information on medication use and thus do not know to what degree second pregnancy GHTN recurrence was influenced by implementation of aspirin early in pregnancy or calcium supplementation.
Conclusions
In women with 2 singleton pregnancies, the risk for chronic hypertension associated with new‐onset blood pressure elevation at or after 20 weeks' gestation increases when this elevation occurs in the first pregnancy, is higher when it occurs in the second, and is highest when it complicates both pregnancies. This is true for both GHTN and preeclampsia. The risk for hypertension rises further with GD, preterm delivery, and SGA or LGA offspring. Lack of GHTN/preeclampsia recurrence in a second pregnancy provides some indication that hypertension prevention efforts may be working and should continue. Conversely, GHTN/preeclampsia recurrence or new‐onset GHTN/preeclampsia in a second pregnancy should stimulate preventive action and careful postpartum monitoring (eg, regular blood pressure assessments and comprehensive cardiovascular evaluations) to facilitate early detection and intervention for chronic hypertension. Our findings support a precision medicine‐oriented pathway to hypertension prevention in relationship to GHTN/preeclampsia history in women with at least 2 pregnancies.
Sources of Funding
The Heart and Stroke Foundation of Canada funded this study (grant number: HSF G‐12‐000251). Dr Dasgupta is the recipient of this grant. The Société Québécoise d'Hypertension Artérielle and McGill's Faculty of Medicine provided additional support through doctoral studentships for J. Mussa.
Disclosures
None.
Supporting information
Data S1
Acknowledgments
The authors thank the Quebec Statistical Institute (Institut de la statistique du Québec) for data linkage and hosting us at their secured Centers for Access to Research Data.
Author contributions: J. Mussa contributed to the study design, interpreted the data, prepared the first draft of the manuscript and revised on the basis of co‐authors' comments, and approved the final manuscript as submitted. Professor Rahme contributed to the study conception and design, provided oversight of the analysis, critically reviewed and revised the manuscript, and approved the final manuscript as submitted. M. Dahhou contributed to data set cleaning, variable derivation, statistical analyses, and data interpretation; and approved the final manuscript as submitted. Dr Nakhla critically reviewed the manuscript and approved the final version as submitted. Dr Dasgupta conceptualized and designed the study, supervised analyses, interpreted the data, critically reviewed the manuscript and supervised draft revisions, and approved the final manuscript as submitted. Dr Dasgupta is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This manuscript was sent to Tochukwu M. Okwuosa, DO, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.124.034777
For Sources of Funding and Disclosures, see page 13.
References
- 1. Zhou B, Carrillo‐Larco R, Danaei G, Riley L, Paciorek C, Stevens G, et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population‐representative studies with 104 million participants. Lancet. 2021;398:957–980. doi: 10.1016/s0140-6736(21)01330-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Russell MW. JAHA spotlight on pregnancy and its impact on maternal and offspring cardiovascular health. J Am Heart Assoc. 2022;11:e025167. doi: 10.1161/jaha.121.025167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, Chen J, He J. Global disparities of hypertension prevalence and control: a systematic analysis of population‐based studies from 90 countries. Circulation. 2016;134:441–450. doi: 10.1161/circulationaha.115.018912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75:285–292. doi: 10.1161/hypertensionaha.119.14240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tackling G, Borhade MB. Hypertensive heart disease. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC; 2023. [PubMed] [Google Scholar]
- 6. Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, Hall DR, Warren CE, Adoyi G, Ishaku S. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72:24–43. doi: 10.1161/hypertensionaha.117.10803 [DOI] [PubMed] [Google Scholar]
- 7. Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre‐eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta‐analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu J, Li T, Wang Y, Xue L, Miao Z, Long W, Xie K, Hu C, Ding H. The association between hypertensive disorders in pregnancy and the risk of developing chronic hypertension. Front Cardiovasc Med. 2022;9:897771. doi: 10.3389/fcvm.2022.897771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wilson BJ, Watson MS, Prescott GJ, Sunderland S, Campbell DM, Hannaford P, Smith WC. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ. 2003;326:845. doi: 10.1136/bmj.326.7394.845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. The World Bank . Fertility rate, total (births per woman). Accessed February 2, 20202020. https://data.worldbank.org/indicator/SP.DYN.TFRT.IN?locations=CA&name_desc=true.
- 11. Stuart JJ, Tanz LJ, Missmer SA, Rimm EB, Spiegelman D, James‐Todd TM, Rich‐Edwards JW. Hypertensive disorders of pregnancy and maternal cardiovascular disease risk factor development: an observational cohort study. Ann Intern Med. 2018;169:224–232. doi: 10.7326/m17-2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brouwers L, van der Meiden‐van Roest AJ, Savelkoul C, Vogelvang TE, Lely AT, Franx A, van Rijn BB. Recurrence of pre‐eclampsia and the risk of future hypertension and cardiovascular disease: a systematic review and meta‐analysis. BJOG. 2018;125:1642–1654. doi: 10.1111/1471-0528.15394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lykke JA, Langhoff‐Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009;53:944–951. doi: 10.1161/hypertensionaha.109.130765 [DOI] [PubMed] [Google Scholar]
- 14. Magnussen EB, Vatten LJ, Smith GD, Romundstad PR. Hypertensive disorders in pregnancy and subsequently measured cardiovascular risk factors. Obstet Gynecol. 2009;114:961–970. doi: 10.1097/AOG.0b013e3181bb0dfc [DOI] [PubMed] [Google Scholar]
- 15. Spaan JJ, Sep SJ, van Balen VL, Spaanderman ME, Peeters LL. Metabolic syndrome as a risk factor for hypertension after preeclampsia. Obstet Gynecol. 2012;120:311–317. doi: 10.1097/AOG.0b013e31825f21ff [DOI] [PubMed] [Google Scholar]
- 16. Engeland A, Bjørge T, Klungsøyr K, Skjærven R, Skurtveit S, Furu K. Preeclampsia in pregnancy and later use of antihypertensive drugs. Eur J Epidemiol. 2015;30:501–508. doi: 10.1007/s10654-015-0018-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pace R, Brazeau AS, Meltzer S, Rahme E, Dasgupta K. Conjoint associations of gestational diabetes and hypertension with diabetes, hypertension, and cardiovascular disease in parents: a retrospective cohort study. Am J Epidemiol. 2017;186:1115–1124. doi: 10.1093/aje/kwx263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tobias DK, Hu FB, Forman JP, Chavarro J, Zhang C. Increased risk of hypertension after gestational diabetes mellitus: findings from a large prospective cohort study. Diabetes Care. 2011;34:1582–1584. doi: 10.2337/dc11-0268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pirkola J, Pouta A, Bloigu A, Miettola S, Hartikainen AL, Järvelin MR, Vääräsmäki M. Prepregnancy overweight and gestational diabetes as determinants of subsequent diabetes and hypertension after 20‐year follow‐up. J Clin Endocrinol Metab. 2010;95:772–778. doi: 10.1210/jc.2009-1075 [DOI] [PubMed] [Google Scholar]
- 20. Crump C, Sundquist J, Sundquist K. Preterm delivery and Long‐term risk of hypertension in women. JAMA Cardiol. 2022;7:65–74. doi: 10.1001/jamacardio.2021.4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parikh NI, Norberg M, Ingelsson E, Cnattingius S, Vasan RS, Domellöf M, Jansson JH, Edstedt Bonamy AK. Association of Pregnancy Complications and Characteristics with Future Risk of elevated blood pressure: the Västerbotten intervention program. Hypertension. 2017;69:475–483. doi: 10.1161/hypertensionaha.116.08121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cortés YI, Catov JM, Brooks M, Harlow SD, Isasi CR, Jackson EA, Matthews KA, Thurston RC, Barinas‐Mitchell E. History of adverse pregnancy outcomes, blood pressure, and subclinical vascular measures in late midlife: SWAN (study of Women's health across the nation). J Am Heart Assoc. 2017;7:e007138. doi: 10.1161/jaha.117.007138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Edvardsson K, Ivarsson A, Eurenius E, Garvare R, Nyström ME, Small R, Mogren I. Giving offspring a healthy start: parents' experiences of health promotion and lifestyle change during pregnancy and early parenthood. BMC Public Health. 2011;11:936. doi: 10.1186/1471-2458-11-936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pampalon R, Hamel D, Gamache P, Raymond G. A deprivation index for health planning in Canada. Chronic Dis Can. 2009;29:178–191. doi: 10.24095/hpcdp.29.4.05 [DOI] [PubMed] [Google Scholar]
- 25. Feig DS, Berger H, Donovan L, Godbout A, Kader T, Keely E, Sanghera R. Diabetes and pregnancy. Can J Diabetes. 2018;42:S255–S282. doi: 10.1016/j.jcjd.2017.10.038 [DOI] [PubMed] [Google Scholar]
- 26. Pace R, Peters T, Rahme E, Dasgupta K. Validity of health administrative database definitions for hypertension: a systematic review. Can J Cardiol. 2017;33:1052–1059. doi: 10.1016/j.cjca.2017.05.025 [DOI] [PubMed] [Google Scholar]
- 27. Leong A, Dasgupta K, Bernatsky S, Lacaille D, Avina‐Zubieta A, Rahme E. Systematic review and meta‐analysis of validation studies on a diabetes case definition from health administrative records. PLoS One. 2013;8:e75256. doi: 10.1371/journal.pone.0075256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leong A, Dasgupta K, Chiasson JL, Rahme E. Estimating the population prevalence of diagnosed and undiagnosed diabetes. Diabetes Care. 2013;36:3002–3008. doi: 10.2337/dc12-2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jurj AL, Wen W, Li HL, Zheng W, Yang G, Xiang YB, Gao YT, Shu XO. Spousal correlations for lifestyle factors and selected diseases in Chinese couples. Ann Epidemiol. 2006;16:285–291. doi: 10.1016/j.annepidem.2005.07.060 [DOI] [PubMed] [Google Scholar]
- 30. Meyler D, Stimpson JP, Peek MK. Health concordance within couples: a systematic review. Soc Sci Med. 2007;64:2297–2310. doi: 10.1016/j.socscimed.2007.02.007 [DOI] [PubMed] [Google Scholar]
- 31. Cobb LK, Godino JG, Selvin E, Kucharska‐Newton A, Coresh J, Koton S. Spousal influence on physical activity in middle‐aged and older adults: the ARIC study. Am J Epidemiol. 2016;183:444–451. doi: 10.1093/aje/kwv104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Verwoerd GR, Hall DR, Grové D, Maritz JS, Odendaal HJ. Primipaternity and duration of exposure to sperm antigens as risk factors for pre‐eclampsia. Int J Gynaecol Obstet. 2002;78:121–126. doi: 10.1016/s0020-7292(02)00130-3 [DOI] [PubMed] [Google Scholar]
- 33. Kho EM, McCowan LM, North RA, Roberts CT, Chan E, Black MA, Taylor RS, Dekker GA. Duration of sexual relationship and its effect on preeclampsia and small for gestational age perinatal outcome. J Reprod Immunol. 2009;82:66–73. doi: 10.1016/j.jri.2009.04.011 [DOI] [PubMed] [Google Scholar]
- 34. Labgold K, Stanhope KK, Joseph NT, Platner M, Jamieson DJ, Boulet SL. Validation of hypertensive disorders during pregnancy: ICD‐10 codes in a high‐burden southeastern United States hospital. Epidemiology. 2021;32:591–597. doi: 10.1097/ede.0000000000001343 [DOI] [PubMed] [Google Scholar]
- 35. Venkatesh KK, Grobman WA, Wu J, Catalano P, Landon M, Scholtens D, Lowe WL, Khan SS. Association of a large‐for‐gestational‐age infant and maternal prediabetes mellitus and diabetes mellitus 10 to 14 years after delivery in the hyperglycemia and adverse pregnancy outcome follow‐up study. Am J Obstet Gynecol. 2023;228:756–758.e753. doi: 10.1016/j.ajog.2023.02.017 [DOI] [PubMed] [Google Scholar]
- 36. Parikh NI, Gonzalez JM, Anderson CAM, Judd SE, Rexrode KM, Hlatky MA, Gunderson EP, Stuart JJ, Vaidya D. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women: a scientific statement from the American Heart Association. Circulation. 2021;143:e902–e916. doi: 10.1161/cir.0000000000000961 [DOI] [PubMed] [Google Scholar]
- 37. Shah BR, Booth GL, Feig DS, Lipscombe LL. Validation of algorithms to identify gestational diabetes from population‐level health‐care administrative data. Can J Diabetes. 2023;47:25–30. doi: 10.1016/j.jcjd.2022.06.010 [DOI] [PubMed] [Google Scholar]
- 38. Mussa J, Rahme E, Dahhou M, Nakhla M, Dasgupta K. Considering gestational diabetes and gestational hypertension history across two pregnancies in relationship to cardiovascular disease development: a retrospective cohort study. Diabetes Res Clin Pract. 2023;206:110998. doi: 10.1016/j.diabres.2023.110998 [DOI] [PubMed] [Google Scholar]
- 39. Sun J, Lu J, Wang W, Mu Y, Zhao J, Liu C, Chen L, Shi L, Li Q, Yang T, et al. Prevalence of diabetes and Cardiometabolic disorders in spouses of diabetic individuals. Am J Epidemiol. 2016;184:400–409. doi: 10.1093/aje/kwv330 [DOI] [PubMed] [Google Scholar]
- 40. Leong A, Rahme E, Dasgupta K. Spousal diabetes as a diabetes risk factor: a systematic review and meta‐analysis. BMC Med. 2014;12:12. doi: 10.1186/1741-7015-12-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dasgupta K, Ross N, Meltzer S, Da Costa D, Nakhla M, Habel Y, Rahme E. Gestational diabetes mellitus in mothers as a diabetes predictor in fathers: a retrospective cohort analysis. Diabetes Care. 2015;38:e130–e131. doi: 10.2337/dc15-0855 [DOI] [PubMed] [Google Scholar]
- 42. Shin HH, Cakmak S, Brion O, Villeneuve P, Turner MC, Goldberg MS, Jerrett M, Chen H, Crouse D, Peters P, et al. Indirect adjustment for multiple missing variables applicable to environmental epidemiology. Environ Res. 2014;134:482–487. doi: 10.1016/j.envres.2014.05.016 [DOI] [PubMed] [Google Scholar]
- 43. Shuger SL, Sui X, Church TS, Meriwether RA, Blair SN. Body mass index as a predictor of hypertension incidence among initially healthy normotensive women. Am J Hypertens. 2008;21:613–619. doi: 10.1038/ajh.2008.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bowman TS, Gaziano JM, Buring JE, Sesso HD. A prospective study of cigarette smoking and risk of incident hypertension in women. J Am Coll Cardiol. 2007;50:2085–2092. doi: 10.1016/j.jacc.2007.08.017 [DOI] [PubMed] [Google Scholar]
- 45. Dasgupta K, Mussa J, Brazeau AS, Dahhou M, Sanmartin C, Ross NA, Rahme E. Associations of free sugars from solid and liquid sources with cardiovascular disease: a retrospective cohort analysis. BMC Public Health. 2023;23:756. doi: 10.1186/s12889-023-15600-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schuermans A, Truong B, Ardissino M, Bhukar R, Slob EAW, Nakao T, Dron JS, Small AM, Cho SMJ, Yu Z, et al. Genetic associations of circulating cardiovascular proteins with gestational hypertension and preeclampsia. JAMA Cardiol. 2024;9:209–220. doi: 10.1001/jamacardio.2023.4994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shen M, Smith GN, Rodger M, White RR, Walker MC, Wen SW. Comparison of risk factors and outcomes of gestational hypertension and pre‐eclampsia. PLoS One. 2017;12:e0175914. doi: 10.1371/journal.pone.0175914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Perišić MM, Vladimir K, Karpov S, Štorga M, Mostashari A, Khanin R. Polygenic risk score and risk factors for preeclampsia and gestational hypertension. J Pers Med. 2022;12:1826. doi: 10.3390/jpm12111826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tyrmi JS, Kaartokallio T, Lokki AI, Jääskeläinen T, Kortelainen E, Ruotsalainen S, Karjalainen J, Ripatti S, Kivioja A, Laisk T, et al. Genetic risk factors associated with preeclampsia and hypertensive disorders of pregnancy. JAMA Cardiol. 2023;8:674–683. doi: 10.1001/jamacardio.2023.1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ebbing C, Rasmussen S, Skjaerven R, Irgens LM. Risk factors for recurrence of hypertensive disorders of pregnancy, a population‐based cohort study. Acta Obstet Gynecol Scand. 2017;96:243–250. doi: 10.1111/aogs.13066 [DOI] [PubMed] [Google Scholar]
- 51. van Oostwaard MF, Langenveld J, Schuit E, Papatsonis DN, Brown MA, Byaruhanga RN, Bhattacharya S, Campbell DM, Chappell LC, Chiaffarino F, et al. Recurrence of hypertensive disorders of pregnancy: an individual patient data metaanalysis. Am J Obstet Gynecol. 2015;212(5):624.e1‐17. doi: 10.1016/j.ajog.2015.01.009 [DOI] [PubMed] [Google Scholar]
- 52. van Rijn BB, Hoeks LB, Bots ML, Franx A, Bruinse HW. Outcomes of subsequent pregnancy after first pregnancy with early‐onset preeclampsia. Am J Obstet Gynecol. 2006;195:723–728. doi: 10.1016/j.ajog.2006.06.044 [DOI] [PubMed] [Google Scholar]
- 53. Rong K, Yu K, Han X, Szeto IM, Qin X, Wang J, Ning Y, Wang P, Ma D. Pre‐pregnancy BMI, gestational weight gain and postpartum weight retention: a meta‐analysis of observational studies. Public Health Nutr. 2015;18:2172–2182. doi: 10.1017/s1368980014002523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Auger N, Fraser WD, Schnitzer M, Leduc L, Healy‐Profitós J, Paradis G. Recurrent pre‐eclampsia and subsequent cardiovascular risk. Heart. 2017;103:235–243. doi: 10.1136/heartjnl-2016-309671 [DOI] [PubMed] [Google Scholar]
- 55. Appiah D, Schreiner PJ, Selvin E, Demerath EW, Pankow JS. Spousal diabetes status as a risk factor for incident type 2 diabetes: a prospective cohort study and meta‐analysis. Acta Diabetol. 2019;56:619–629. doi: 10.1007/s00592-019-01311-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Watanabe T, Sugiyama T, Takahashi H, Noguchi H, Tamiya N. Concordance of hypertension, diabetes and dyslipidaemia in married couples: cross‐sectional study using nationwide survey data in Japan. BMJ Open. 2020;10:e036281. doi: 10.1136/bmjopen-2019-036281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li C, Liu Y, Zhang W. Joint and independent associations of gestational weight gain and pre‐pregnancy body mass index with outcomes of pregnancy in Chinese women: a retrospective cohort study. PLoS One. 2015;10:e0136850. doi: 10.1371/journal.pone.0136850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, Li N, Hu G, Corrado F, Rode L, et al. Association of Gestational Weight Gain with Maternal and Infant Outcomes: a systematic review and meta‐analysis. JAMA. 2017;317:2207–2225. doi: 10.1001/jama.2017.3635 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1


