Abstract
Background
This study assesses the diagnostic utility of strain parameters from cardiovascular magnetic resonance feature tracking across all cardiac chambers in patients with acute myocarditis, stratified by ejection fraction.
Methods and Results
Our cohort included 65 patients with acute myocarditis and 25 healthy controls; all underwent cardiac magnetic resonance imaging. Patients were divided into 2 groups based on left ventricular ejection fraction (EF)with a 55% cutoff: acute myocarditis with preserved EF, EF ≥55%, n=48; and acute myocarditis with reduced EF, EF <55%, n=17. The control group matched for age and sex. Cardiovascular magnetic resonance feature tracking evaluated strain parameters across all cardiac chambers. Both acute myocarditis with preserved EF and acute myocarditis with reduced EF groups showed significant decreases in left atrial peak early negative strain rate compared with controls. The acute myocarditis with reduced EF group had significantly reduced left ventricular circumferential strain relative to acute myocarditis with preserved EF and controls. Receiver operating characteristic curve analysis confirmed the diagnostic accuracy in distinguishing patients with acute myocarditis with preserved EF from controls, with left atrial peak early negative strain rate achieving 92.9% specificity, left ventricular circumferential strain demonstrating an area under the curve of 0.832, and similarly effective results for left ventricular longitudinal strain and right ventricular longitudinal strain. Additionally, left atrial peak early negative strain rate and left ventricular circumferential strain showed significant correlations with troponin I levels, indicating myocardial injury.
Conclusions
Cardiovascular magnetic resonance feature‐tracking–derived strain parameters, particularly left atrial peak early negative strain rate and left ventricular circumferential strain, effectively diagnose acute myocarditis across different EFs, enhancing diagnostic accuracy and facilitating early detection, notably in patients with preserved EF.
Keywords: cardiovascular magnetic resonance, feature tracking, myocardial strain, myocarditis
Subject Categories: Magnetic Resonance Imaging (MRI), Machine Learning
Nonstandard Abbreviations and Acronyms
- AMY‐pEF
acute myocarditis with preserved ejection fraction
- AMY‐rEF
acute myocarditis with reduced ejection fraction
- CS
circumferential strain
- FT
feature tracking
- ICC
intraclass correlation coefficient
- LASRe
left atrial peak early negative strain rate
- LAVpreac
left atrial volume preatrial contraction
- LS
longitudinal strain
- LVCS
left ventricular circumferential strain
- LVED
left ventricular end diastolic
- LVLS
left ventricular longitudinal strain
- RS
radial strain
- RVLS
right ventricular longitudinal strain
- RVRS
right ventricular radial strain
Clinical Perspective.
What Is New?
We advance the use of cardiovascular magnetic resonance to discern strain parameters in patients with myocarditis with preserved ejection fraction, an area less explored in the current literature.
By comparing strain metrics between healthy controls and patients with myocarditis (both with preserved and reduced ejection fraction), our study highlights significant differences that offer potential for enhanced diagnostic methods.
Our use of receiver operating characteristic curves underscores the potential of specific strain measures in differentiating patients with myocarditis with preserved ejection fraction from healthy individuals.
What Are the Clinical Implications?
Our insights equip medical professionals with innovative tools, leveraging strain metrics for accurate categorization of myocarditis cases, especially those maintaining a preserved ejection fraction.
Our findings have the potential to reshape the current diagnostic paradigm for myocarditis, paving the way for more targeted therapeutic approaches.
As myocardial imaging techniques advance, our proposed strain parameters have the potential to become cornerstone metrics in future diagnostic protocols, elevating the standards of myocarditis diagnosis and treatment, although it is imperative to acknowledge that their integration into standard care requires rigorous validation and further research.
The diagnosis of acute myocarditis poses significant challenges because of its diverse origins, varied clinical manifestations, and the absence of a singular definitive diagnostic test. 1 Research suggests that the incidence of myocarditis may be frequently underestimated in clinical practice. 2 Early and precise diagnosis is vital for initiating timely and appropriate treatment strategies, thereby improving patient outcomes.
Cardiovascular magnetic resonance (CMR) imaging has proven to be an invaluable noninvasive diagnostic tool for myocarditis assessment, as it provides detailed insights into myocardial tissue characterization and function. 3 Among the numerous functional parameters that CMR assesses, myocardial strain, which measures myocardial deformation, has garnered significant attention in recent years. Myocardial strain has demonstrated its capability to serve as a sensitive marker for myocardial dysfunction, even in patients with preserved ejection fraction, thus offering additional diagnostic information. 4 Moreover, several studies have reported that myocardial strain analysis could potentially provide additional diagnostic and prognostic data, especially in patients with preserved ejection fraction (EF), in whom conventional parameters might not sufficiently capture the extent of myocardial involvement. 5 , 6
In recent years, there has been an increasing emphasis on the role of right ventricular function in cardiovascular diseases, with impaired right ventricular function being closely associated with adverse clinical outcomes. 1 , 7 Against this backdrop, this study seeks to conduct an extensive and systematic evaluation of cardiac morphologic features in patients with varied EF acute myocarditis using CMR feature tracking (FT), by analyzing strain parameters of the left atrium, left ventricle, right atrium, and right ventricle. We propose that these parameters will exhibit high diagnostic value for assessing cardiac function in patients with acute myocarditis, even among those with preserved EF.
In addition to ventricular strain parameters, atrial strain parameters are emerging as crucial indicators of cardiac function, especially in conditions like acute myocarditis. Atrial strain, measured through advanced CMR techniques, offers insights into atrial mechanics and function. 8 This is particularly relevant in myocarditis, where atrial involvement can occur even when ventricular function is preserved. 9 By incorporating atrial strain analysis into our study, we aim to provide a more comprehensive assessment of myocardial dysfunction in acute myocarditis.
The aim of this study is to further explore the role of cardiac functional parameters, including myocardial strain parameters, across different heart chambers in the assessment of patients with acute myocarditis of varied EF. This could potentially unveil new diagnostic markers and provide insights into disease progression. We propose that these parameters may offer additional diagnostic value beyond conventional EF assessment. Our hope is that this study will contribute to a better understanding of acute myocarditis and aid in the development of more effective diagnostic and therapeutic strategies.
Methods
Because of the sensitive nature of the personal health information involved in our study, and adherence to strict privacy regulations, we are unable to provide public access to the original data set. All data analyses were conducted with respect to privacy protection and ethical review compliance. The methods used in our analysis are detailed within the article to ensure the reproducibility of our research. We commit to facilitating legitimate research inquiries with appropriate data protection measures in place, providing necessary data and support where possible without contravening privacy principles.
Study Population and Data Collection
Study Population
This retrospective study included 65 patients diagnosed and treated for acute myocarditis at the Cardiology Department of our institution between January 2020 and December 2022. Acute myocarditis within 2 weeks of onset was diagnosed on the basis of clinical manifestations, laboratory findings, ECG alterations, and cardiovascular magnetic resonance, following the updated Lake Louise Criteria 10 (Figure 1, Table S1).
Figure 1. Patient recruitment and selection flowchart.
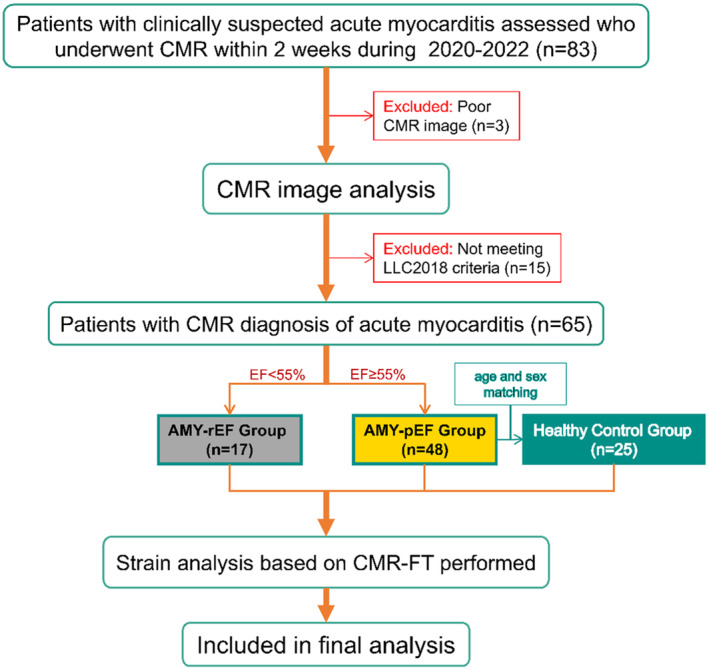
This flowchart details the recruitment, selection, and classification of patients for the study, adhering to the Lake Louise Criteria 2018 for Cardiac Magnetic Resonance in Nonischemic Myocardial Inflammation (LLC2018) Guidelines. AMY‐pEF indicates acute myocarditis with preserved EF; AMY‐rEF, acute myocarditis with reduced EF; CMR, cardiovascular magnetic resonance; EF, ejection fraction; and FT, feature tracking.
The acronym “AMY” will be used to refer to the acute myocarditis group, which encompasses all patients diagnosed with acute myocarditis throughout this study. Additionally, we will use “AMY‐pEF” to indicate patients with acute myocarditis with preserved ejection fraction, and “AMY‐rEF” to represent patients with acute myocarditis with reduced ejection fraction. Patients were further stratified into 2 subgroups based on left ventricular ejection fraction: AMY‐pEF group with an EF of ≥55% (comprising 48 patients), and AMY‐rEF group with an EF of <55% (consisting of 17 patients).
Additionally, 25 age‐ and sex‐matched healthy volunteers without a history of cardiovascular disease, with normal ECG results, and with unremarkable CMR examination findings were enrolled to form the healthy control group. All participants were Han Chinese ethnicity.
In this retrospective analysis, the adequacy of our sample size was retrospectively validated. A post hoc power analysis was conducted on the basis of the observed effect sizes and the variability in the data, confirming that the sample of 65 patients and 25 healthy controls provided sufficient statistical power to detect significant differences in myocardial strain measurements. This retrospective validation ensures confidence in the findings despite the study's observational nature. The study protocol received approval from the institutional review board, and informed consent was procured from all participants.
Baseline Characteristics and Data Collection
Demographic and anthropometric data, including age, sex, height, weight, and body surface area (BSA), were extracted from medical records for all participants. Clinical characteristics, like heart rate, ECG findings, and laboratory test results, such as levels of troponin I, were gathered from patients' records. Moreover, data on cardiac function parameters, including left ventricular ejection fraction (LVEF), left ventricular end‐systolic volume/BSA, left ventricular end‐diastolic (LVED) wall mass/BSA, left atrial volume preatrial contraction (LAVpreac)/BSA, right ventricular ejection fraction, total left atrial emptying fraction, and passive left atrial emptying fraction, were obtained from CMR reports.
CMR Acquisition Parameters
Our CMR scans were performed using a Siemens Magnetom Aera 1.5‐T magnetic resonance imaging scanner, equipped with a PERU ECG gating board compatible with magnetic resonance imaging and an Antmed high‐pressure injector. Gadopentetate dimeglumine injection (Magnevist) was the contrast agent administered. Before the examination, subjects with regular heart rates underwent breath‐holding training at the end of expiration. The CMR scans were performed while the subjects were in a relaxed state.
For cardiac morphology scanning, a fast low‐angle shot imaging sequence was used. This allowed for the rapid acquisition of axial, sagittal, and coronal images of the heart. Subsequent scanning was done on the heart's sagittal image to obtain short‐axis images from the apex to the root of the aorta. We used the Siemens Syngo platform's automatic positioning method (day optimizing throughput) to place positioning coordinates on the pseudo‐short‐axis image of the heart at the center of the left atrium, the root of the aorta, the corner of the right ventricle, the center of the mitral valve, and the apex of the heart. Breath‐holding scanning allowed us to obtain standard left ventricular long‐axis 2‐, 3‐, and 4‐chamber positioning images.
The TrueFisp sequence was used to scan each layer of the left ventricular long axis (2‐, 3‐, and 4‐chamber views) and the short axis (10 layers were taken with positioning lines perpendicular to the line connecting the apex to the midpoint of the mitral valve), enabling us to acquire cardiac cine images. The scanning parameters included echo time/repetition time/flip angle=1.45 ms/45.37 ms/54°, slice thickness=8 mm, and matrix=205×256.
We obtained edema‐sensitive black‐blood T2‐weighted sequences for short‐axis and 4‐chamber views. Before enhancement scanning, we acquired pre‐T1 mapping and T2 mapping sequence images, including 4‐chamber and short‐axis images. Postenhancement, we captured T1 mapping images and late gadolinium enhancement images. Parameters included echo time/repetition time/flip angle=1.28 ms/688 ms/40°, slice thickness=8 mm, and matrix=156×256.
CMR Image Analysis
Scanned images were transferred to an imaging workstation for postprocessing, which was performed using the Cardiovascular Imaging42 software developed by Circle Cardiovascular Imaging in Canada. Two cardiac imaging radiologists (Xu J and Zhang S), each with >20 years of experience in magnetic resonance imaging, independently performed a double‐blind analysis of all images, adhering to uniform standards.
Left/right ventricular function measurements were performed on left ventricular short‐axis cine sequence images. The largest left ventricular cavity area was manually selected for end diastole, whereas the smallest left ventricular cavity was chosen for end systole. Using a semiautomatic method, the endocardial and epicardial contours of the end‐diastolic and end‐systolic images of the left/right ventricle in the short‐axis position were outlined layer by layer. Papillary muscles and blood pools were excluded for the left ventricle. After outlining, the software was able to calculate the ejection fraction (EF) of the left and right ventricles, standardized end‐diastolic volume, end‐systolic volume, stroke volume, and left ventricular myocardial mass (systolic/diastolic).
The endocardial contours of the left atrium at end diastole and end systole were manually traced on the 2‐ and 4‐chamber levels of the left atrium. The software then automatically averaged the results from the left atrial 2‐ and 4‐chamber views to generate the outcome. Standardized left atrial maximum and minimum volumes were obtained, and the LAVpreac was determined by manually selecting and delineating the left atrial active presystolic endocardium. Using these data, we were able to derive the total left atrial emptying fraction, passive left atrial emptying fraction, and active left atrial emptying fraction. The passive emptying fraction and active emptying fraction represent the conduit function and booster function of the left atrium, respectively.
Strain Analysis
For the assessment of myocardial deformation, we leveraged FT imaging technology for the strain analysis of CMR images. FT imaging is a postprocessing technique that quantifies myocardial strain without the need for extra dedicated imaging sequences, such as tagging or displacement encoding with stimulated echoes imaging. 11 The FT imaging method traces the motion of features or patterns within the myocardium throughout the cardiac cycle, facilitating the calculation of strain parameters.
In this study, our attention was concentrated on 3 specific strain parameters, all ventricular strains covered in the article are global strain: radial strain (RS), circumferential strain (CS), and longitudinal strain (LS). RS reflects the variations in myocardial wall thickness throughout the cardiac cycle, whereas CS represents changes in myocardial circumference that are primarily influenced by the obliquely oriented myocardial fibers extending from the apex to the base of the heart in the subepicardial layer. LS, on the other hand, illustrates alterations in the length of the myocardium along the long axis of the heart. 12 , 13
These strain parameters were selected because of their comprehensive portrayal of myocardial deformation and function, offering us a well‐rounded perspective on cardiac mechanics.
CMR images were processed for strain analysis using the Cardiovascular Imaging42 software package from Circle Cardiovascular Imaging. The software autonomously identifies and tracks myocardial boundaries across the entire cardiac cycle, computing strain values for each segment based on the American Heart Association's 17‐segment model. 14 Global strain values for each strain parameter were then generated by averaging the segmental strain values.
Atrial strain was measured by manually outlining the endocardial borders (excluding pulmonary veins and auricles) at the end of systole. The software subsequently autonomously outlines the endocardial borders throughout the cardiac cycle. Physicians verified the quality of automatic tracking and made manual adjustments if needed, followed by manually drawing the epicardial contours to finally yield the endocardial/epicardial contours for each phase. Once the strain analysis was initiated, the software generates a time versus strain/strain rate graph, from which total strain, passive strain, active strain, peak positive strain rate, peak early negative strain rate, and peak late negative strain rate could be derived. Left atrial strain data are based on results at the 2‐ and 4‐chamber levels, averaged to obtain left atrial strain and strain rate. The average of 2 measurements was taken as the outcome for right atrial strain and strain rate, which were obtained only at the 4‐chamber level.
Total strain represents the reservoir function of the atria, which involves the collection and storage of pulmonary venous blood during ventricular systole. Passive strain denotes the conduit function of the atria, which channels blood from the pulmonary veins through the atria into the ventricles during early ventricular diastole, facilitated by a pressure difference between the ventricles and atria. Active strain, on the other hand, represents the booster pump function of the atria, in which the left atrium actively contracts to enhance ventricular filling during late ventricular diastole. Peak positive strain rate, peak early negative strain rate, and peak late negative strain rate correspond to the atrial strain rate, which is the rate of deformation of the inner atrial myocardium per unit of time.
After outlining the endocardium of the left and right ventricles at the short‐axis level during cardiac function measurements, the software automatically traces the endocardium of the left and right ventricle in the 2‐ and 4‐chamber views, whereas the epicardium of the right ventricle is manually traced at each level. On the basis of these tracings, ventricular CS, RS, and LS can be calculated.
All experimental results were measured twice, with the average taken as the outcome.
Statistical Analysis
The data were processed using the SPSS 25.0 statistical software and were presented as the mean±SD. The Kolmogorov‐Smirnov test was used to determine data normality. The myocardial strain values of the healthy control group and the myocarditis group were compared using the independent sample t‐test, whereas categorical variables were compared using either the χ2 or Fisher exact test, depending on suitability.
Box plots were used to visualize the myocardial strain values within each subgroup of the healthy control and myocarditis groups. One‐way ANOVA was implemented to compare the myocardial strain values among various groups within each subgroup for both healthy controls and myocarditis cases. Pairwise comparisons between groups were conducted using the least significant difference t‐test. If the results showed statistical significance, a receiver operating characteristic (ROC) curve analysis was performed to establish the optimal diagnostic threshold based on the maximum Youden index.
To manage the family‐wise error rate and decrease the probability of type I errors, we applied the Bonferroni correction because of multiple comparisons made in this study. The significance level (α) was adjusted by dividing it by the number of comparisons (m). The resulting adjusted significance level (α/m) was then used as the threshold to determine statistical significance.
Spearman rank correlation analysis was applied to evaluate the relationships between left atrial peak early negative strain rate (LASRe), left ventricular CS (LVCS), LVEF, and myocardial mass (LVED wall mass), separately. The consistency and reproducibility of the measurements were examined using the intraclass correlation coefficient (ICC) and Bland‐Altman analysis. To ensure the integrity and reliability of our intraobserver and interobserver analysis, CMR observers were systematically blinded to all demographic and clinical parameters of the participants.
Results
Study Population and Baseline Characteristics
Study Population
This retrospective study included 65 patients with acute myocarditis and 25 age‐ and sex‐matched healthy volunteers serving as the control group. On the basis of LVEF criteria, patients were categorized into 2 groups: EF preserved (48 patients) and EF reduced (17 patients), as detailed in Table 1.
Table 1.
Comparative Baseline Clinical and Laboratory Profiles of Study Participants Stratified by EF Levels
| Characteristic | AMY‐pEF (n=48) | AMY‐rEF (n=17) |
|---|---|---|
| Age, y | 28±8 | 32±5 |
| Male sex | 38 (79.2) | 14 (82.4) |
| Female sex | 10 (20.8) | 3 (17.6) |
| Height, cm | 172±7 | 170±10 |
| Weight, kg | 71±12 | 76±16 |
| Laboratory markers: TnI, ng/mL | 7.33±4.29 | 28.56±6.90 |
| Acute chest pain | 23 (47.9) | 15 (88.2) |
| Fever | 15 (31.3) | 4 (23.5) |
| Respiratory or gastrointestinal tract symptoms | 20 (41.7) | 5 (29.4) |
| Dyspnea | 25 (52.1) | 16 (94.1) |
| Chest discomfort | 31 (64.6) | 17 (100) |
| Palpitation | 18 (37.5) | 14 (82.4) |
| Presyncope or syncope | 6 (12.5) | 6 (35.3) |
| Unexplained cardiogenic shock | 0 (0) | 2 (11.8) |
| ECG/Holter/stress test features | 32 (66.7) | 17 (100) |
Data are presented as mean±SD or as number (percentage).
AMY‐pEF indicates acute myocarditis with preserved EF; AMY‐rEF, acute myocarditis with reduced EF; EF, ejection fraction; and TnI, troponin I.
In our study cohort, a notable male/female ratio of 4:1 was observed, reflecting well‐documented sex disparities in myocarditis incidence, as seen in both human and murine studies. 15 , 16 , 17 This trend underscores the importance of considering sex differences in myocardial strain and their clinical implications.
Baseline Characteristics and Anthropometric Variables
The groups showed no significant differences in anthropometric variables, including age, sex, height, weight, and BSA.
Clinical Characteristics
Clinical characteristics, including heart rate, ECG manifestations, laboratory test results, and cardiac function parameters, were analyzed for each group (Tables 1, 2, 3).
Table 2.
Comparative Analysis of Cardiac Function Parameters Between the Control Group and the AMY Group
| Parameter | Control group (n=25) | AMY group (n=65) |
|---|---|---|
| Height, cm | 168±8 | 171±7 |
| Weight, kg | 66±12 | 73±13 |
| Heart rate, bpm | 73±7 | 73±8 |
| LVEF, % | 64±5 | 57±6** |
| LVED volume/BSA, mL/m2 | 77±10 | 81±8 |
| LVES volume/BSA, mL/m2 | 29±6 | 34±8* |
| LVED wall mass/BSA, [g/m2 (without papillary muscles) | 43±7 | 51±8** |
| RVEF, % | 63±5 | 55±7** |
| RVED volume/BSA, mL/m2 | 82±10 | 78±17 |
| RVES volume/BSA, mL/m2 | 37±7 | 43±10 |
| RVSV/BSA | 45±6 | 39±9 |
| LAVmax/BSA | 36±5 | 37±7 |
| LAVpreac/BSA | 17±4 | 22±6** |
| LAVmin/BSA | 11±2 | 13±5 |
| LAEFtotal, % | 70±3 | 64±7** |
| LAEFpassive, % | 53±7 | 40±8*** |
| LAEFbooster, % | 35±12 | 40±8 |
Data are presented as mean±SD. AMY indicates acute myocarditis; bpm, beats per minute; BSA, body surface area; LAEFbooster, active left atrial emptying fraction; LAEFpassive, passive left atrial emptying fraction; LAEFtotal, total left atrial emptying fraction; LAVmax, maximum left atrial volume; LAVmin, minimum left atrial volume; LAVpreac, left atrial volume preatrial contraction; LVED, left ventricular end diastolic; LVEF, left ventricular ejection fraction; LVES, left ventricular end systolic; RVED, right ventricular end diastolic; RVEF, right ventricular ejection fraction; and RVES, right ventricular end systolic; RVSV, right ventricle stroke volume.
P<0.05.
P<0.01.
P<0.001.
Table 3.
Comparative Analysis of Cardiac Function Parameters Between the Control Group and the AMY‐pEF Group
| Parameter | Control group (n=25) | AMY‐pEF group (n=48) |
|---|---|---|
| Age, y | 29±4 | 28±8 |
| Height, cm | 168±8 | 172±7 |
| Weight, kg | 66±12 | 71±12 |
| Heart rate, bpm | 73±7 | 73±9 |
| LVEF, % | 64±5 | 61±4 |
| LVED volume/BSA, mL/m2 | 77±10 | 79±8 |
| LVES volume/BSA, mL/m2 | 29±6 | 30±5 |
| LVED wall mass/BSA, g/m2 (without papillary muscles) | 43±7 | 49±5* |
| RVEF, % | 63±5 | 59±7** |
| RVED volume/BSA, mL/m2 | 82±10 | 79±16 |
| RVES volume/BSA, mL/m2 | 37±7 | 42±9 |
| RVSV/BSA | 45±6 | 39±9 |
| LAVmax/BSA | 36±5 | 37±6 |
| LAVpreac/BSA | 17±4 | 22±2* |
| LAVmin/BSA | 11±2 | 12±3 |
| LAEFtotal, % | 70±3 | 67±5 |
| LAEFpassive, % | 53±7 | 42±7** |
| LAEFbooster, % | 35±12 | 42±6 |
Data are presented as mean±SD. AMY‐pEF indicates acute myocarditis with preserved ejection fraction; bpm, beats per minute; BSA, body surface area; LAEFbooster, active left atrial emptying fraction; LAEFpassive, passive left atrial emptying fraction; LAEFtotal, total left atrial emptying fraction; LAVmax, maximum left atrial volume; LAVmin, minimum left atrial volume; LAVpreac, left atrial volume preatrial contraction; LVED, left ventricular end diastolic; LVEF, left ventricular ejection fraction; LVES, left ventricular end systolic; RVED, right ventricular end diastolic; RVEF, right ventricular ejection fraction; and RVES, right ventricular end systolic; RVSV, right ventricle stroke volume.
P<0.05.
P<0.01.
Strain Analysis
Atrial Strain Analysis
The acute myocarditis group displayed notably lower values in left atrial strain parameters, including Left Atrial Total Strain LAEs, LAEe, LASRs, and LASRe, compared with the healthy control group. The preserved EF acute myocarditis subgroup showed reductions in LAEs and LAEe, and a significant decline in LASRe (Table 4, Figure 2). In the EF‐preserved subgroup, we observed a nonsignificant yet notable trend toward reduced right atrial strain and strain rate when compared with controls, suggesting a subtle but discernible impact of myocarditis on atrial function. These observations, although preliminary, warrant further investigation to elucidate the underlying disease mechanisms and may become more pronounced in studies with larger patient cohorts.
Table 4.
Comparative Analysis of Atrial Strain Parameters in AMY
| Variable | Control group | AMY group | P value | AMY‐pEF | AMY‐rEF | Control vs AMY‐pEF | Control vs AMY‐rEF | AMY‐pEF vs AMY‐rEF | |
|---|---|---|---|---|---|---|---|---|---|
| LA longitudinal strain, % | Es | 54.1±8.3 | 42.9±11.2 | 0.005** | 45.3±12.1 | 39.9±10.0 | 0.042* | 0.007** | 0.245 |
| Ee | 39.6±5.9 | 29.5±9.0 | 0.001** | 32.0±8.9 | 24.4±7.6 | 0.017* | 0.000*** | 0.043* | |
| Ea | 18.7±3.8 | 16.9±5.5 | 0.264 | 16.4±5.5 | 17.7±5.9 | 0.258 | 0.678 | 0.587 | |
| LA longitudinal strain SR, s−1 | SRs | 2.9±0.6 | 2.3±0.7 | 0.012* | 2.4±0.7 | 2.0±0.5 | 0.073 | 0.005** | 0.134 |
| SRe | −4.1±0.8 | −2.8±0.9 | 0.000*** | −3.0±0.7 | −2.4±1.0 | 0.002** | 0.000*** | 0.132 | |
| SRa | −2.9±0.4 | −2.7±0.5 | 0.149 | −2.8±0.4 | −2.4±0.5 | 0.523 | 0.034* | 0.094 | |
| RA longitudinal strain, % | Es | 57.2±19.9 | 45.8±15.3 | 0.102 | 46.4±15.0 | 44.6±16.9 | 0.123 | 0.137 | 0.827 |
| Ee | 44.3±15.0 | 35.3±9.4 | 0.059 | 37.8±6.9 | 27.6±9.4 | 0.162 | 0.004** | 0.052 | |
| Ea | 14.7±5.4 | 14.7±8.5 | 0.978 | 12.5±6.8 | 19.0±10.4 | 0.435 | 0.221 | 0.060 | |
| RA longitudinal strain SR, s−1 | SRs | 3.1±1.2 | 2.6±1.0 | 0.230 | 2.5±0.8 | 2.8±1.3 | 0.159 | 0.565 | 0.534 |
| SRe | −3.2±1.4 | −2.5±1.1 | 0.166 | −2.8±1.0 | −2.0±1.0 | 0.379 | 0.044* | 0.170 | |
| SRa | −2.3±0.7 | −2.0±1.0 | 0.418 | −1.9±1.0 | −2.3±1.1 | 0.290 | 0.935 | 0.325 |
Data are presented as mean±SD. AMY indicates acute myocarditis; AMY‐pEF, AMY with preserved ejection fraction; AMY‐rEF, AMY with reduced ejection fraction; Ea, active strain; Ee, passive strain; Es, total strain (sum of Ee and Ea); LA, left atrial; RA, right atrial; SR, strain rate; SRa, peak late negative SR; SRe, peak early negative SR; and SRs, peak positive SR.
P<0.05.
P<0.01.
P<0.001.
Figure 2. Differential cardiac strain profiles in myocarditis: a comparison across ejection fraction (EF) spectrum.
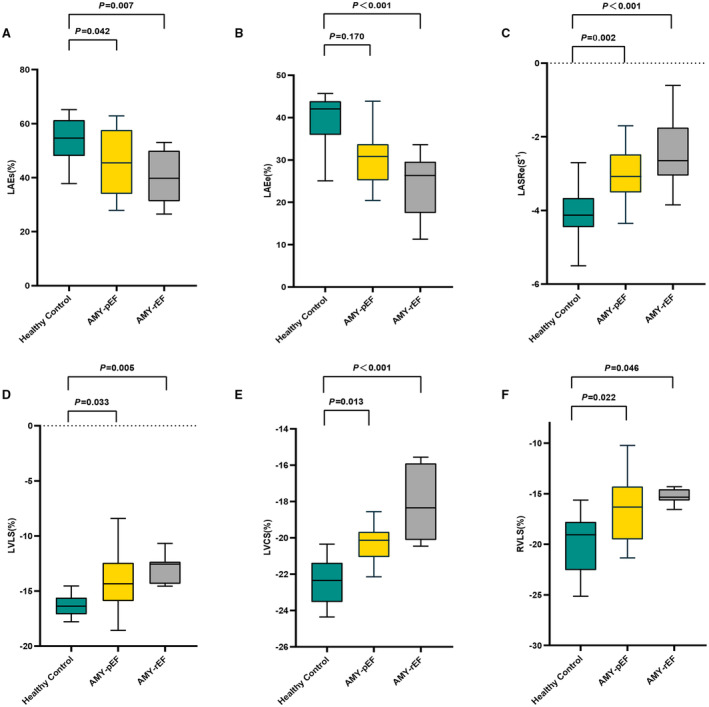
This figure illustrates the disparity in key cardiac strain indicators among healthy individuals and patients with myocarditis with both preserved and reduced EF. Strains, such as left atrial total strain (sum of passive strain and active strain; LAEs) (A), left atrial passive strain (LAEe) (B), and left atrial peak early negative strain rate (LASRe) (C), provide insight into left atrial function, whereas left ventricular longitudinal strain (LVLS) (D), left ventricular circumferential strain (LVCS) (E), and right ventricular longitudinal strain (RVLS) (F) reflect ventricular mechanics. Notably, even when EF is preserved, patients with myocarditis show significant strain alterations, underlying the nuanced impact of myocarditis on cardiac function and emphasizing the value of comprehensive strain assessment in this context. AMY‐pEF indicates acute myocarditis with preserved EF; and AMY‐rEF, acute myocarditis with reduced EF.
Ventricular Strain Analysis
In our exploration of ventricular strain parameters, we observed distinct differences between patients with myocarditis and healthy controls. Specifically, patients with myocarditis exhibited a significant reduction in left ventricular LS (LVLS), averaging −13.9%±2.8%, compared with −16.4%±1.4%, in the control group (P=0.002). Subgroup analysis revealed that both those with preserved EF (LVEF ≥55%, −14.3%±3.3%) and those with reduced EF (LVEF <55%, −13.0%±1.4%) were significant in LVLS when compared with controls (P=0.033 and P=0.005, respectively). However, the difference between the 2 myocarditis subgroups was not statistically significant (P=0.229).
We noted significant differences in LVCS between the patients with myocarditis (−20.0%±2.0%) and the healthy controls (−22.4%±1.3%; P<0.001). The preserved (LVEF ≥55%, −20.7%±1.7%) and reduced EF (LVEF <55%, −18.7%±2.2%) myocarditis subgroups both showed notable decreases in LVCS compared with controls (P=0.013 and P<0.001, respectively). The reduced LVEF group also exhibited a significant decrease compared with the preserved LVEF group (P=0.016) (Figure 2).
Similarly, right ventricular LS (RVLS) and right ventricular radial strain (RVRS) were significantly decreased in patients with myocarditis relative to controls (RVLS: −16.0%±3.4% versus −19.9%±3.5%, P = 0.013; RVRS: 21.2%±7.6% versus 26.3%±5.9%, P=0.039, respectively). Additionally, in subgroup analysis, RVLS was notably reduced in both the preserved and the reduced LVEF subgroups compared with controls (P=0.022 and P=0.046, respectively), but with no significant difference observed between the 2 myocarditis subgroups (P=0.742). For RVRS, a significant decrease was noted in the reduced LVEF subgroup relative to both controls (P<0.001) and the preserved LVEF group (P=0.002), but not between the preserved LVEF group and controls (P=0.410) (Table 5).
Table 5.
Comparative Analysis of Ventricular Strain Parameters in AMY
| Parameters | Control group | AMY group | P value | AMY‐pEF | AMY‐rEF | Control vs AMY‐pEF | Control vs AMY‐rEF | AMY‐pEF vs AMY‐rEF |
|---|---|---|---|---|---|---|---|---|
| LVLS, % | −16.4±1.4 | −13.9±2.8 | 0.002** | −14.3±3.3 | −13.0±1.4 | 0.033* | 0.005** | 0.229 |
| LVRS, % | 37.2±2.9 | 32.4±7.1 | 0.053 | 34.1±7.4 | 29.2±5.9 | 0.231 | 0.013* | 0.105 |
| LVCS, % | −22.4±1.3 | −20.0±2.0 | 0.000*** | −20.7±1.7 | −18.7±2.2 | 0.013* | 0.000*** | 0.016* |
| RVLS, % | −19.9±3.5 | −16.0±3.4 | 0.013* | −16.2±3.6 | −15.5±3.3 | 0.022* | 0.046* | 0.742 |
| RVRS, % | 26.3±5.9 | 21.2±7.6 | 0.039* | 24.3±7.4 | 14.9±2.5 | 0.410 | 0.000*** | 0.002** |
| RVCS, % | −12.9±4.7 | −10.5±4.3 | 0.207 | −10.9±4.6 | −8.6±1.2 | 0.304 | 0.234 | 0.526 |
Data are presented as mean±SD. AMY indicates acute myocarditis; AMY‐pEF, AMY with preserved ejection fraction; AMY‐rEF, AMY with reduced ejection fraction; LVCS, left ventricular circumferential strain; LVLS, left ventricular longitudinal strain; LVRS, left ventricular radial strain; RVCS, right ventricular circumferential strain; RVLS, right ventricular longitudinal strain; and RVRS, right ventricular radial strain.
P<0.05.
P<0.01.
P<0.001.
Sex Differences in Myocardial Strain Analysis
Our cohort displayed a male/female ratio of 4:1, reflecting the sex disparity reported in myocarditis incidence. The sample size determined by G*Power 3.1, for the acute myocarditis group (AMY) and the preserved ejection fraction myocarditis group (AMY‐pEF) was deemed sufficient for our analysis. However, the reduced ejection fraction myocarditis group (AMY‐rEF) did not meet the required sample size for robust statistical evaluation. Independent sample t‐tests within the AMY and AMY‐pEF groups showed no significant differences in myocardial strain between sexes (Table S2).
Diagnostic Performance of Myocardial Strain Parameters in EF‐Preserved Patients With Myocarditis
We assessed the diagnostic capability of myocardial strain parameters in identifying patients with EF‐preserved myocarditis through ROC curve analysis (see Table 6 for details). This analysis highlighted the potential of these parameters to detect myocarditis even when traditional cardiac function measures, like EF, are normal.
Table 6.
ROC Curve Analysis for Discriminating AMY‐pEF Cases
| Parameter | AUC | Cutoff | Sensitivity | Specificity | P value |
|---|---|---|---|---|---|
| LAEe | 0.789 | 34.7 | 0.861 | 0.762 | <0.001 |
| LAEs | 0.722 | 46.5 | 0.833 | 0.548 | 0.001 |
| LASRe | 0.822 | −3.8 | 0.639 | 0.929 | <0.001 |
| LVLS | 0.769 | −15.4 | 0.738 | 0.778 | <0.001 |
| LVCS | 0.832 | −20.2 | 0.667 | 0.889 | <0.001 |
| RVLS | 0.748 | −17.3 | 0.643 | 0.833 | <0.001 |
AMY‐pEF indicates acute myocarditis with preserved ejection fraction; AUC, area under the curve; LAEe, left atrial passive strain; LASRs, left atrial peak positive strain rate; LASRe, left atrial peak early negative strain rate; LVCS, left ventricular circumferential strain; LVLS, left ventricular longitudinal strain; ROC, receiver operating characteristic; and RVLS, right ventricular longitudinal strain.
In our study, we presented myocardial strain imaging from 2 cases. The first case was a healthy control subject, displaying normal myocardial strain measurements with LVCS at −23.6% and LASRe at −4.6 s−1. The second case involved a patient with acute myocarditis and preserved EF, exhibiting reduced myocardial strain, as evidenced by LVCS at −20.3% and LASRe at −3.2 s−1 (as illustrated in Figure 3). This comparison highlights the significant strain differences between healthy individuals and patients with myocarditis, even when EF is preserved.
Figure 3. Comparative analysis of cardiac strain in healthy control and myocarditis with preserved ejection fraction (EF).
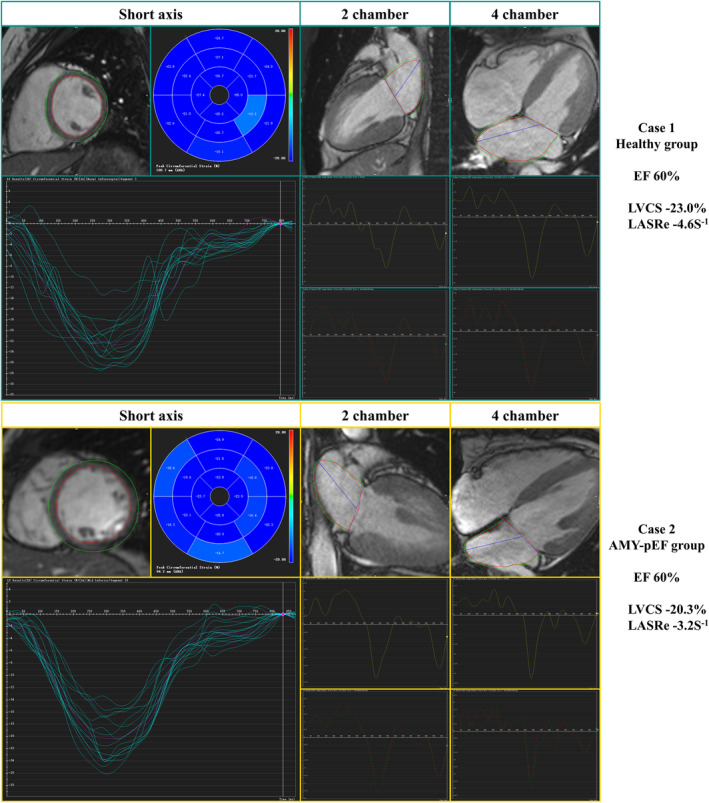
Case 1, representing the healthy control group, demonstrates standard levels of EF, left ventricular circumferential strain (LVCS), and left atrial peak early negative strain rate (LASRe), establishing a baseline for healthy myocardial function. In contrast, case 2, a patient with acute myocarditis with preserved EF (AMY‐pEF), maintains normal EF levels but exhibits reduced LVCS and LASRe. Notably, the LASRe shows a marked decrease. This comparison underlines the significant myocardial strain differences observed in patients with myocarditis with preserved EF when compared with healthy individuals, despite similar EF readings.
These results demonstrated that LAEe showed 86.1% sensitivity and 76.2% specificity at a threshold of 34.7%, with an area under the curve (AUC) of 0.789 (P<0.001). LASRe had a sensitivity of 63.9% and a specificity of 92.9% at a threshold of −3.8 s−1 (AUC of 0.822, P<0.001). LAEs indicated a sensitivity of 83.3% and a specificity of 54.8% at a threshold of 46.5%, with an AUC of 0.722 (P=0.001).
Focusing on the left ventricle, LVLS at −15.4% manifested an AUC of 0.769, with 73.8% sensitivity and 77.8% specificity (P<0.001). LVCS at −20.2% revealed an AUC of 0.832, with 66.7% sensitivity and 88.9% specificity of (P<0.001). Last, RVLS at a cutoff of −17.3%, showed an AUC of 0.748, with 64.3% sensitivity and 83.3% specificity (P<0.001) (Table 6, Figure 4).
Figure 4. Receiver operating characteristic (ROC) curve analysis for strain parameters in myocarditis with preserved ejection fraction (EF).
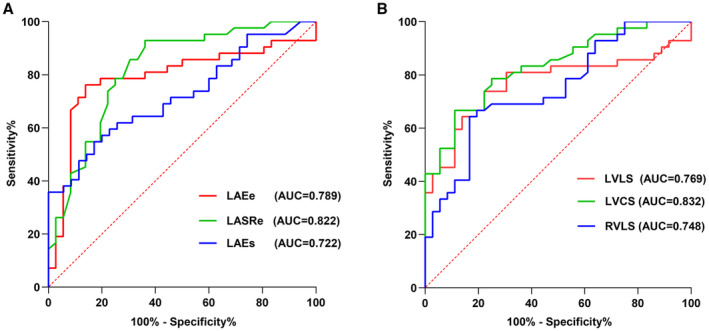
This figure presents the ROC curves assessing the diagnostic performance of atrial and ventricular strain measurements in differentiating patients with preserved EF myocarditis from healthy controls. The area under the curve (AUC) values for each parameter are shown, indicating their respective predictive accuracies. Analysis based on atrial (A) and ventricular (B) strain parameters. LAEe indicates left atrial passive strain; LAEs, left atrial strain (summation of passive and active strain); LASRe, left atrial systolic reservoir strain rate; LVCS, left ventricular circumferential strain; LVLS, left ventricular longitudinal strain; and RVLS, right ventricular longitudinal strain.
Additional Cardiac Parameters in Myocarditis and Controls
In our comprehensive analysis of cardiac function, we observed significant differences in standard cardiac parameters between the acute myocarditis group and the healthy controls. Specifically, noticeable differences (P<0.05) were identified in parameters, including LVEF, left ventricular end‐systolic volume/BSA, LVED wall mass/BSA, LAVpreac/BSA, right ventricular ejection fraction, total left atrial emptying fraction, and passive left atrial emptying fraction, when comparing the acute myocarditis group and the healthy control group (Table 2). When comparing the acute myocarditis preserved EF group with the healthy control group, significant differences (P<0.05) emerged in LVED wall mass/BSA, LAVpreac/BSA, and passive left atrial emptying fraction (Table 3).
Correlation and Reproducibility Analysis
Correlation Analysis
The relationship between LVCS and LASRe and cardiac function parameters (LVEF, LVED wall mass/BSA) and laboratory markers (troponin I) was evaluated.
With Cardiac Function Parameters
Both LVCS and LASRe revealed negative correlations with LVEF: a moderate correlation (R=−0.512, P<0.001) was seen with LVCS, whereas LASRe demonstrated a weak correlation (R=−0.340, P=0.006). Moreover, LVCS and LASRe were positively correlated with LVED wall mass/BSA, displaying weak correlations (R=0.382, P=0.002 for LVCS; R=0.341, P=0.006 for LASRe).
With Laboratory Markers
LVCS and LASRe were positively correlated with troponin I, presenting moderate correlations (R=0.617, P<0.001 for LVCS; R=0.592, P<0.001 for LASRe) (Figure 5).
Figure 5. Correlation between strain parameters and cardiac functional biomarkers.
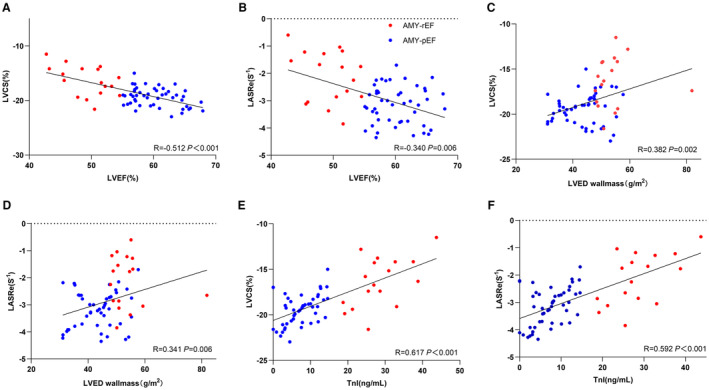
This figure illustrates the relationships between cardiac strain measurements and various indicators of cardiac function. Scatterplots depict the following correlations: left ventricular circumferential strain (LVCS) and left ventricular ejection fraction (LVEF) (A), left atrial systolic reservoir strain rate (LASRe) and LVEF (B), LVCS and left ventricular end‐diastolic (LVED) wall mass (C), LASRe and LVED wall mass (D), LVCS and troponin I (TnI) (E), and LASRe and TnI (F).
Reproducibility Analysis
The strain parameters' reproducibility was assessed using Bland‐Altman analysis by computing the ICC, CI, and coefficient of variation for both intraobserver and interobserver measurements. The results are depicted in Table S3.
In terms of intraobserver reproducibility, ICC values ranged between 0.848 (RVLS) and 0.967 (LAEs), and coefficient of variation values extended from 6.0% Right Atrial Total Strain (RAEs) to 16.1% (right ventricular circumferential strain), indicating solid consistency in repeated measurements by the same observer. As for interobserver reproducibility, ICC values varied from 0.739 (LASRs) to 0.925 Right Atrial Active Strain (RAEa), and coefficient of variation values were between 9.7% (LVCS, LASRe) and 19.3% (right ventricular circumferential strain, Right Atrial Peak Late Negative Strain Rate [RASRa]), suggesting satisfactory agreement between measurements from different observers (Figure 6).
Figure 6. Bland‐Altman plots for intraobserver and interobserver reproducibility of left atrial peak early diastolic negative strain rate (LASRe) and left ventricular circumferential strain (LVCS).
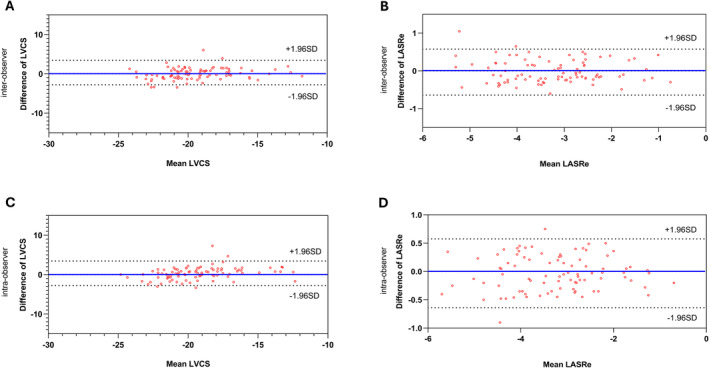
The plots demonstrate high levels of reproducibility for LASRe and LVCS measurements among different observers and the same observer over time.
Discussion
In this study, we validate myocardial strain as a key diagnostic indicator for acute myocarditis, highlighting its reliability across all cardiac chambers. Our data affirm its superiority over traditional functional parameters and reveal nuanced variations in strain between different EF groups. These insights pave the way for more accurate myocarditis diagnosis and underscore the potential for tailored therapeutic strategies based on detailed myocardial strain analysis.
Sex Differences in Myocardial Strain Manifestations
In our study, the observed male predominance in myocarditis aligns with existing literature, 15 suggesting a biological underpinning for sex differences in disease incidence and severity. However, our findings indicate that alterations in myocardial strain, which reflect the mechanical changes within the myocardium attributable to myocarditis, may not be significantly influenced by sex in the AMY and AMY‐pEF groups. This observation contributes to the emerging understanding that myocardial strain metrics are critical indicators of myocardial health and disease, although the small sample size, particularly in the AMY‐rEF group, limits the conclusiveness regarding sex‐specific impacts.
Acknowledging these limitations, we emphasize the need for future studies with larger cohorts to validate our observations and provide a more granular understanding of the potential sex‐specific pathophysiological mechanisms in myocarditis. Expanded research efforts are essential not only for a deeper comprehension of myocarditis and its varied impacts on myocardial function across sexes but also for guiding more personalized treatment approaches and improving overall patient outcomes.
Atrial Strain Parameter Alterations Attributable to Myocarditis Impact
Our findings show that left atrial reservoir function (LAEs), conduit function (LAEe), and LASRe are significantly diminished in patients in the AMY‐pEF group, similar to findings of Dick et al. 18 During early diastole, both active and passive factors synergistically contribute to ventricular dilation facilitating the majority of ventricular blood filling. However, in cases of early left ventricular diastolic dysfunction filling pressure increase, the left atrial–left ventricular pressure gradient decreases, leading to reduced blood entry into the left ventricle and, consequently, left atrial conduit function is diminished. Abnormalities in LAEe and LASRe, which represent conduit function, may thus signal impaired left ventricular diastolic function. Studies have shown that left atrial strain parameters are more sensitive and have more diagnostic efficacy compared with traditional left atrial geometric indexes. 19 , 20 We found no statistical difference in maximum and minimum left atrial volumes compared with controls, as only atrial presystolic volumes could suggest impaired cardiac function, suggesting that impaired left atrial conduit function could serve as a marker of left ventricular diastolic dysfunction. As noted by Kim et al 21 : CMR left atrial strain measurements are linear in diastolic function assessment and vary progressively with diastolic function severity; longitudinal left atrial strain is more diagnostically useful compared with left atrial geometry assessment in diagnosis and grading severity of diastolic dysfunction. In our study, the most effective strain parameter was LASRe, with sensitivity of 63.9% and specificity of 92.9% for diagnosis of cardiac dysfunction in myocarditis at threshold value of −3.8 s−1 (Table 6).
Left Ventricle Strain Parameter Alterations Attributable to Myocarditis Impact
In assessing the changes in left ventricular strain during myocarditis, our study emphasizes LVCS as a crucial diagnostic marker. This finding aligns with existing literature. 4 , 22 , 23 Specifically, LVCS demonstrated exceptional diagnostic efficacy, with an area under the ROC curve of 0.832, and a sensitivity and a specificity of 66.7% and 88.9% at a threshold of −20.2%, indicating the reliability of myocardial strain assessed via CMR for functional assessment.
In our study, we observed a significant reduction in LVLS in patients with acute myocarditis, which aligns with prior research. 23 , 24 This reduction in LVLS can be attributed to pathologic and physiological changes in myocardial cells attributable to inflammation, such as edema, degeneration, necrosis, and exudation. These alterations potentially lead to impaired myocardial cell contractility, manifesting as a decrease in LS. Literature 25 , 26 has reported the association of LVLS with the prognosis of myocarditis, suggesting its importance as a prognostic marker. LVLS could predict cardiac function recovery and might serve as a novel biomarker for enhancing risk stratification in myocarditis. This finding underscores the significance of LVLS as a critical indicator, not only in the diagnosis but also in the prognostic assessment of myocarditis. Given these insights, our future research endeavors will continue to investigate the role of LVLS and other strain parameters in myocarditis, aiming to improve clinical outcomes and provide a deeper understanding of this condition.
Interestingly, we did not observe a statistically significant decrease in radial strain in both the AMY group and the AMY‐pEF subgroup, whereas noteworthy reduction in AMY‐rEF subgroup was observed, suggesting a complex interplay of pathologic processes at different myocardial layers. This indicates that myocarditis has not progressed to a severity level affecting the endocardium. We believe that this observation prompts a deeper exploration of how pathologic changes at different myocardial layers affect myocardial strain in future research.
The findings from Secchi et al 27 and Lee et al 28 provide a contrasting perspective to our study's results. Secchi et al observed a significant reduction in global RS in patients with preserved EF; Lee et al's segmented approach demonstrated a reduction in RS in both the midsection of the left ventricular short axis and the long‐axis view of the bichamber heart. Our study's differing results, with no significant decrease in RS observed in the EF‐preserved group, might reflect the diverse nature of myocarditis and its impact on the myocardium. The results underscore the importance of considering the method used for strain assessment, global versus segmental, and the specific patient population when interpreting strain data. This divergence in findings also emphasizes the need for further research to understand the implications of these differences and to establish a more comprehensive understanding of myocardial strain alterations in myocarditis. Such research could lead to more nuanced diagnostic criteria and tailored treatment strategies based on the specific pattern of myocardial involvement.
Right Ventricle Strain Parameter Alterations Attributable to Myocarditis Impact
Our study delves deep into the nuances of RVLS and RVRS in myocarditis, shedding light on an area that has historically received less attention compared with the left heart. Specifically, the marked reduction in RVRS in the reduced LVEF subgroup signals more extensive myocardial damage. This observation corresponds with previous findings associating reduced right ventricular function with more severe disease in various cardiac conditions. 29 , 30
Furthermore, our observation of diminished RVLS in patients with preserved EF provides an additional perspective compared with some earlier studies. Although Baeßler et al 31 noted an increase in right ventricular basal CS in a similar cohort, it is important to recognize that the differences in our findings might not represent a direct contradiction but rather reflect the distinct methods used in assessing right ventricular strain. Our study focused on a global assessment of right ventricular strain, which may capture a different aspect of myocardial function compared with the localized measurement of basal CS. This difference highlights the necessity for careful consideration of measurement techniques when interpreting and comparing results across studies. Therefore, we advocate for a nuanced understanding of myocardial strain patterns in myocarditis and support a tailored approach that accounts for the unique characteristics of each patient subgroup, as well as the specific methods used in strain assessment.
Strain Parameters as Diagnostic Markers in EF‐Preserved Myocarditis
Our study highlights the significant diagnostic potential of strain parameters, especially for patients with myocarditis with preserved EF. Traditional cardiac function assessments often miss subtle damage in these patients, emphasizing the need for sensitive tools, like strain analysis. Our ROC curve analysis, detailed in Table 6, demonstrates the ability of parameters, like LVCS and LASRe, to differentiate patients with myocarditis from healthy controls, even when EF is normal.
In a detailed case comparison in Figure 3, 1 patient with acute myocarditis and preserved EF exhibited significantly lower strain measurements (LVCS at −20.3% and LASRe at −3.2 s−1) than a healthy control (LVCS at −23.0% and LASRe at −4.6 s−1), potentially leading to earlier intervention and improved outcome. This discrepancy persisted despite the patient's normal EF, emphasizing the additional diagnostic value of myocardial strain parameters. Such individual case analyses underscore the potential of strain analysis to increase the diagnostic sensitivity for myocarditis, particularly in patients with preserved EF, potentially leading to early intervention and improved outcomes.
Our ROC curve analysis further revealed that myocardial strain parameters could serve as reliable diagnostic markers for myocarditis, even in patients with preserved EF. Specifically, the analysis demonstrated high AUC values for LAEe, LAEs, LASRe, LVLS, LVCS, and RVLS, suggesting that these parameters could distinguish patients with myocarditis from healthy controls with reasonable accuracy.
The pronounced AUC value for LAEe, LAEs, and LASRe highlights the potential importance of left atrial function in myocarditis, which aligns with existing literature emphasizing the prognostic significance of left atrial strain in various cardiac conditions. 32 , 33 In line with previous studies, our findings underscore the diagnostic utility of LVLS and LVCS, suggesting they provide a more intricate assessment of left ventricular function than EF alone. 34 , 35 Moreover, the notable performance of RVLS further emphasizes the importance of right ventricular function in myocarditis, which aligns with research that recognizes the prognostic value of right ventricular strain. 36 , 37
Therefore, the diagnostic potential of strain parameters, as shown in our study, suggest that comprehensive strain analysis could enhance our understanding of myocarditis, even in patients presenting with preserved EF. This approach might facilitate earlier detection and intervention, improving patient outcomes. Nonetheless, broader validation of these insights and a more comprehensive exploration of strain analysis's role in myocarditis with preserved EF are imperative for future research endeavors.
Correlation Analysis of Strain Parameters With Cardiac Function Parameters and Laboratory Markers
In our study, patients with acute myocarditis with preserved EF showed alterations in LVED wall mass/BSA and LAVpreac/BSA, indicating early cardiac function impairment. Notably, a significant yet weak negative correlation was observed between LASRe and LVEF, with a more pronounced negative correlation between LVCS and LVEF. These findings suggest that even with a preserved EF, subtle myocardial impairments are detectable through strain parameters.
Furthermore, the correlations between strain parameters (LVCS, LASRe) and cardiac enzyme marker troponin I underscore their potential in reflecting myocardial damage and their promise for future diagnostic and prognostic applications in myocarditis. Although troponin I's role in prognosis remains to be fully understood, 38 , 39 , 40 its strong correlation with LVCS and LASRe highlights the utility of these strain parameters in complementing traditional cardiac function assessments and laboratory markers.
Reliability of Strain Parameter Measurements
Our reproducibility analysis, detailed in Table S3, confirms the reliability of strain parameter measurements. The intraobserver ICC values exceeded 0.8, demonstrating high consistency in strain measurements by the same observer, which is consistent with prior studies. 41 , 42 Despite slightly lower interobserver ICC values, they remained substantially above 0.7, indicating good agreement between different observers. Both intraobserver and interobserver coefficient of variation values were within acceptable ranges, reinforcing the dependability of these assessments.
These findings underscore the reliability of CMR‐FT–based strain measurements, crucial for their application in clinical practice for diagnosing and monitoring myocarditis.
Limitations
Although our study provides valuable insights into myocardial strain parameters in myocarditis, it is important to consider its limitations in the context of further research opportunities:
Sample size and setting: Our findings are based on a limited sample from a single center. Larger, multicenter studies are needed to confirm these results and enhance their generalizability to different populations.
CMR‐FT software dependence: We used Cardiovascular Imaging42 postprocessing software for CMR‐FT, which, although standard, may yield different results compared with other software. Future studies comparing multiple software platforms could provide broader insights.
Sex‐specific impacts: Although our study suggests myocardial strain alterations may not be significantly influenced by sex in certain myocarditis groups, larger studies are necessary to fully understand the sex‐specific pathophysiological mechanisms in myocarditis.
Conclusions
Our investigation into myocardial strain via CMR‐FT marks a significant step in understanding and diagnosing myocarditis, especially in cases with preserved EF. We have uncovered that despite normal traditional measures, myocarditis can manifest as significant myocardial changes, which CMR‐FT can detect early and accurately. This is particularly critical in patients with preserved EF, in whom traditional diagnostics might miss subtle yet impactful alterations.
Sources of Funding
This work was supported by National Natural Science Foundation of China 82172017 and Sanming Project of Medicine in Shenzhen Nanshan District (No. SZSM202103001).
Disclosures
None.
Supporting information
Tables S1–S3
Acknowledgments
We extend our heartfelt gratitude to Professor Yixiang Wang for his invaluable guidance and expert insights throughout this study. His mentorship was instrumental in shaping the research and significantly contributed to our findings.
This manuscript was sent to Erik B. Schelbert, MD, MS, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.032781
For Sources of Funding and Disclosures, see page 16.
References
- 1. Friedrich MG, Sechtem U, Schulz‐Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel‐Aty H, Gutberlet M, Prasad S, et al. Cardiovascular magnetic resonance in myocarditis: a JACC White paper. J Am Coll Cardiol. 2009;53:1475–1487. doi: 10.1016/j.jacc.2009.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dai H, Lotan D, Much AA, Younis A, Lu Y, Bragazzi NL, Wu J. Global, regional, and National Burden of myocarditis and cardiomyopathy, 1990‐2017. Front Cardiovasc Med. 2021;8:610989. doi: 10.3389/fcvm.2021.610989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eichhorn C, Greulich S, Bucciarelli‐Ducci C, Sznitman R, Kwong RY, Grani C. Multiparametric cardiovascular magnetic resonance approach in diagnosing, monitoring, and prognostication of myocarditis. JACC Cardiovasc Imaging. 2022;15:1325–1338. doi: 10.1016/j.jcmg.2021.11.017 [DOI] [PubMed] [Google Scholar]
- 4. Porcari A, Merlo M, Crosera L, Stolfo D, Barbati G, Biondi F, De Angelis G, Paldino A, Pagnan L, Belgrano M, et al. Strain analysis reveals subtle systolic dysfunction in confirmed and suspected myocarditis with normal LVEF. A cardiac magnetic resonance study. Clin Res Cardiol. 2020;109:869–880. doi: 10.1007/s00392-019-01577-w [DOI] [PubMed] [Google Scholar]
- 5. Park JJ, Hwang IC, Kang SH, Park JB, Park JH, Cho GY. Myocardial strain for heart failure with preserved ejection fraction but without diastolic dysfunction. ESC Heart Fail. 2022;9:3308–3316. doi: 10.1002/ehf2.14078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tanacli R, Hashemi D, Neye M, Motzkus LA, Blum M, Tahirovic E, Dordevic A, Kraft R, Zamani SM, Pieske B, et al. Multilayer myocardial strain improves the diagnosis of heart failure with preserved ejection fraction. ESC Heart Fail. 2020;7:3240–3245. doi: 10.1002/ehf2.12826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park JH, Kusunose K, Motoki H, Kwon DH, Grimm RA, Griffin BP, Marwick TH, Popovic ZB. Assessment of right ventricular longitudinal strain in patients with ischemic cardiomyopathy: head‐to‐head comparison between two‐dimensional speckle‐based strain and velocity vector imaging using volumetric assessment by cardiac magnetic resonance as a "gold standard". Echocardiography. 2015;32:956–965. doi: 10.1111/echo.12740 [DOI] [PubMed] [Google Scholar]
- 8. Cameli M, Mandoli GE, Loiacono F, Dini FL, Henein M, Mondillo S. Left atrial strain: a new parameter for assessment of left ventricular filling pressure. Heart Fail Rev. 2015;21:65–76. doi: 10.1007/s10741-015-9520-9 [DOI] [PubMed] [Google Scholar]
- 9. Doerner J, Bunck AC, Michels G, Maintz D, Baeßler B. Incremental value of cardiovascular magnetic resonance feature tracking derived atrial and ventricular strain parameters in a comprehensive approach for the diagnosis of acute myocarditis. Eur J Radiol. 2018;104:120–128. doi: 10.1016/j.ejrad.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 10. Ferreira VM, Schulz‐Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, Kindermann I, Gutberlet M, Cooper LT, Liu P, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072 [DOI] [PubMed] [Google Scholar]
- 11. Bucius P, Erley J, Tanacli R, Zieschang V, Giusca S, Korosoglou G, Steen H, Stehning C, Pieske B, Pieske‐Kraigher E, et al. Comparison of feature tracking, fast‐SENC, and myocardial tagging for global and segmental left ventricular strain. ESC Heart Fail. 2020;7:523–532. doi: 10.1002/ehf2.12576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sengupta PP, Tajik AJ, Chandrasekaran K, Khandheria BK. Twist mechanics of the left ventricle: principles and application. JACC Cardiovasc Imaging. 2008;1:366–376. doi: 10.1016/j.jcmg.2008.02.006 [DOI] [PubMed] [Google Scholar]
- 13. Voigt JU, Cvijic M. 2‐ and 3‐dimensional myocardial strain in cardiac health and disease. JACC Cardiovasc Imaging. 2019;12:1849–1863. doi: 10.1016/j.jcmg.2019.01.044 [DOI] [PubMed] [Google Scholar]
- 14. Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the cardiac imaging Committee of the Council on clinical cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975 [DOI] [PubMed] [Google Scholar]
- 15. Coronado MJ, Bruno KA, Blauwet LA, Tschöpe C, Cunningham MW, Pankuweit S, van Linthout S, Jeon ES, McNamara DM, Krejčí J, et al. Elevated sera sST2 is associated with heart failure in men ≤50 years old with myocarditis. J Am Heart Assoc. 2019;8:8. doi: 10.1161/jaha.118.008968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shah Z, Mohammed M, Vuddanda V, Ansari MW, Masoomi R, Gupta K. National Trends, gender, management, and outcomes of patients hospitalized for myocarditis. Am J Cardiol. 2019;124:131–136. doi: 10.1016/j.amjcard.2019.03.036 [DOI] [PubMed] [Google Scholar]
- 17. Coronado MJ, Brandt JE, Kim E, Bucek A, Bedja D, Abston ED, Shin J, Gabrielson KL, Mitzner W, Fairweather D. Testosterone and interleukin‐1β increase cardiac remodeling during coxsackievirus B3 myocarditis via serpin a 3n. Am J Phys Heart Circ Phys. 2012;302:H1726–H1736. doi: 10.1152/ajpheart.00783.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dick A, Schmidt B, Michels G, Bunck AC, Maintz D, Baessler B. Left and right atrial feature tracking in acute myocarditis: a feasibility study. Eur J Radiol. 2017;89:72–80. doi: 10.1016/j.ejrad.2017.01.028 [DOI] [PubMed] [Google Scholar]
- 19. Morris DA, Takeuchi M, Krisper M, Kohncke C, Bekfani T, Carstensen T, Hassfeld S, Dorenkamp M, Otani K, Takigiku K, et al. Normal values and clinical relevance of left atrial myocardial function analysed by speckle‐tracking echocardiography: multicentre study. Eur Heart J Cardiovasc Imaging. 2015;16:364–372. doi: 10.1093/ehjci/jeu219 [DOI] [PubMed] [Google Scholar]
- 20. Pessoa‐Amorim G, Mancio J, Vouga L, Ribeiro J, Gama V, Bettencourt N, Fontes‐Carvalho R. Impaired left atrial strain as a predictor of new‐onset atrial fibrillation after aortic valve replacement independently of left atrial size. Rev Esp Cardiol (Engl Ed). 2018;71:466–476. doi: 10.1016/j.rec.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 21. Kim J, Yum B, Palumbo MC, Sultana R, Wright N, Das M, You C, Moskowitz CS, Levine RA, Devereux RB, et al. Left atrial strain impairment precedes geometric remodeling as a marker of post‐myocardial infarction diastolic dysfunction. JACC Cardiovasc Imaging. 2020;13:2099–2113. doi: 10.1016/j.jcmg.2020.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Poyraz E, Dinc AL. Dynamic change of left ventricular mechanics in patients with acute myocarditis with preserved left ventricular systolic function: a 2‐year follow‐up study. Turk Kardiyol Dern Ars. 2022;50:485–491. doi: 10.5543/tkda.2022.22358 [DOI] [PubMed] [Google Scholar]
- 23. Isaak A, Kravchenko D, Mesropyan N, Endler C, Bischoff LM, Vollbrecht T, Thomas D, Dabir D, Zimmer S, Attenberger U, et al. Layer‐specific strain analysis with cardiac MRI feature tracking in acute myocarditis. Radiol Cardiothorac Imag. 2022;4:e210318. doi: 10.1148/ryct.210318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luetkens JA, Schlesinger‐Irsch U, Kuetting DL, Dabir D, Homsi R, Doerner J, Schmeel FC, Fimmers R, Sprinkart AM, Naehle CP, et al. Feature‐tracking myocardial strain analysis in acute myocarditis: diagnostic value and association with myocardial oedema. Eur Radiol. 2017;27:4661–4671. doi: 10.1007/s00330-017-4854-4 [DOI] [PubMed] [Google Scholar]
- 25. Fischer K, Obrist SJ, Erne SA, Stark AW, Marggraf M, Kaneko K, Guensch DP, Huber AT, Greulich S, Aghayev A, et al. Feature tracking myocardial strain incrementally improves prognostication in myocarditis beyond traditional CMR imaging features. JACC Cardiovasc Imaging. 2020;13:1891–1901. doi: 10.1016/j.jcmg.2020.04.025 [DOI] [PubMed] [Google Scholar]
- 26. Luetkens JA, Petry P, Kuetting D, Dabir D, Schmeel FC, Homsi R, Schild HH, Thomas D. Left and right ventricular strain in the course of acute myocarditis: a cardiovascular magnetic resonance study. RöFo. 2018;190:722–732. doi: 10.1055/a-0585-0271 [DOI] [PubMed] [Google Scholar]
- 27. Secchi F, Monti CB, Ali M, Carbone FS, Cannao PM, Sardanelli F. Diagnostic value of global cardiac strain in patients with myocarditis. J Comput Assist Tomogr. 2020;44:591–598. doi: 10.1097/RCT.0000000000001062 [DOI] [PubMed] [Google Scholar]
- 28. Lee JW, Jeong YJ, Lee G, Lee NK, Lee HW, Kim JY, Choi B‐S, Choo KS. Predictive value of cardiac magnetic resonance imaging‐derived myocardial strain for poor outcomes in patients with acute myocarditis. Korean J Radiol. 2017;18:18–654. doi: 10.3348/kjr.2017.18.4.643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, et al. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006;114:1883–1891. doi: 10.1161/CIRCULATIONAHA.106.632208 [DOI] [PubMed] [Google Scholar]
- 30. Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease, part I: anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation. 2008;117:1436–1448. doi: 10.1161/CIRCULATIONAHA.107.653576 [DOI] [PubMed] [Google Scholar]
- 31. Baessler B, Schaarschmidt F, Dick A, Michels G, Maintz D, Bunck AC. Diagnostic implications of magnetic resonance feature tracking derived myocardial strain parameters in acute myocarditis. Eur J Radiol. 2016;85:218–227. doi: 10.1016/j.ejrad.2015.11.023 [DOI] [PubMed] [Google Scholar]
- 32. Habibi M, Chahal H, Opdahl A, Gjesdal O, Helle‐Valle TM, Heckbert SR, McClelland R, Wu C, Shea S, Hundley G, et al. Association of CMR‐measured LA function with heart failure development: results from the MESA study. JACC Cardiovasc Imaging. 2014;7:570–579. doi: 10.1016/j.jcmg.2014.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morris DA, Belyavskiy E, Aravind‐Kumar R, Kropf M, Frydas A, Braunauer K, Marquez E, Krisper M, Lindhorst R, Osmanoglou E, et al. Potential usefulness and clinical relevance of adding left atrial strain to left atrial volume index in the detection of left ventricular diastolic dysfunction. JACC Cardiovasc Imaging. 2018;11:1405–1415. doi: 10.1016/j.jcmg.2017.07.029 [DOI] [PubMed] [Google Scholar]
- 34. Ersboll MK. Left ventricular global longitudinal strain in acute myocardial infarction–with special reference to neurohormonal activation, in‐hospital heart failure and prognosis. Dan Med J. 2013;60:B4697. [PubMed] [Google Scholar]
- 35. Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta‐analysis of global longitudinal strain and ejection fraction. Heart. 2014;100:1673–1680. doi: 10.1136/heartjnl-2014-305538 [DOI] [PubMed] [Google Scholar]
- 36. Guendouz S, Rappeneau S, Nahum J, Dubois‐Rande JL, Gueret P, Monin JL, Lim P, Adnot S, Hittinger L, Damy T. Prognostic significance and normal values of 2D strain to assess right ventricular systolic function in chronic heart failure. Circ J. 2012;76:127–136. doi: 10.1253/circj.cj-11-0778 [DOI] [PubMed] [Google Scholar]
- 37. Chen Y, Sun Z, Xu L, Liu J, Li Y, Zhang N, Liu D, Wen Z. Diagnostic and prognostic value of cardiac magnetic resonance strain in suspected myocarditis with preserved LV‐EF: a comparison between patients with negative and positive late gadolinium enhancement findings. J Magn Reson Imaging. 2022;55:1109–1119. doi: 10.1002/jmri.27873 [DOI] [PubMed] [Google Scholar]
- 38. Al‐Biltagi M, Issa M, Hagar HA, Abdel‐Hafez M, Aziz NA. Circulating cardiac troponins levels and cardiac dysfunction in children with acute and fulminant viral myocarditis. Acta Paediatr. 2010;99:1510–1516. doi: 10.1111/j.1651-2227.2010.01882.x [DOI] [PubMed] [Google Scholar]
- 39. Imazio M, Brucato A, Spodick DH, Adler Y. Prognosis of myopericarditis as determined from previously published reports. J Cardiovasc Med (Hagerstown). 2014;15:835–839. doi: 10.2459/JCM.0000000000000082 [DOI] [PubMed] [Google Scholar]
- 40. Miyake CY, Teele SA, Chen L, Motonaga KS, Dubin AM, Balasubramanian S, Balise RR, Rosenthal DN, Alexander ME, Walsh EP, et al. In‐hospital arrhythmia development and outcomes in pediatric patients with acute myocarditis. Am J Cardiol. 2014;113:535–540. doi: 10.1016/j.amjcard.2013.10.021 [DOI] [PubMed] [Google Scholar]
- 41. Taylor RJ, Moody WE, Umar F, Edwards NC, Taylor TJ, Stegemann B, Townend JN, Hor KN, Steeds RP, Mazur W, et al. Myocardial strain measurement with feature‐tracking cardiovascular magnetic resonance: normal values. Eur Heart J Cardiovasc Imaging. 2015;16:871–881. doi: 10.1093/ehjci/jev006 [DOI] [PubMed] [Google Scholar]
- 42. Schmidt B, Dick A, Treutlein M, Schiller P, Bunck AC, Maintz D, Baessler B. Intra‐ and inter‐observer reproducibility of global and regional magnetic resonance feature tracking derived strain parameters of the left and right ventricle. Eur J Radiol. 2017;89:97–105. doi: 10.1016/j.ejrad.2017.01.025 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3


