Abstract
Background
The study aimed to describe the patterns and trends of initiation, discontinuation, and adherence of oral anticoagulation (OAC) in patients with new‐onset postoperative atrial fibrillation (POAF), and compare with patients newly diagnosed with non‐POAF.
Methods and Results
This retrospective cohort study identified patients newly diagnosed with atrial fibrillation or flutter between 2012 and 2021 using administrative claims data from OptumLabs Data Warehouse. The POAF cohort included 118 366 patients newly diagnosed with atrial fibrillation or flutter within 30 days after surgery. The non‐POAF cohort included the remaining 315 832 patients who were newly diagnosed with atrial fibrillation or flutter but not within 30 days after a surgery. OAC initiation increased from 28.9% to 44.0% from 2012 to 2021 in POAF, and 37.8% to 59.9% in non‐POAF; 12‐month medication adherence increased from 47.0% to 61.8% in POAF, and 59.7% to 70.4% in non‐POAF. The median time to OAC discontinuation was 177 days for POAF, and 242 days for non‐POAF. Patients who saw a cardiologist within 90 days of the first atrial fibrillation or flutter diagnosis, regardless of POAF or non‐POAF, were more likely to initiate OAC (odds ratio, 2.92 [95% CI, 2.87–2.98]; P <0.0001), adhere to OAC (odds ratio, 1.08 [95% CI, 1.04–1.13]; P <0.0001), and less likely to discontinue (odds ratio, 0.83 [95% CI, 0.82–0.85]; P <0.0001) than patients who saw a surgeon or other specialties.
Conclusions
The use of and adherence to OAC were higher in non‐POAF patients than in POAF patients, but they increased over time in both groups. Patients managed by cardiologists were more likely to use and adhere to OAC, regardless of POAF or non‐POAF.
Keywords: adherence, oral anticoagulation, postoperative atrial fibrillation
Subject Categories: Atrial Fibrillation, Anticoagulants
NONSTANDARD ABBREVIATIONS AND ACRONYMS
- OAC
oral anticoagulation
- POAF
postoperative atrial fibrillation
- sHR
subdistribution hazard ratio
Research Perspective.
What Is New?
The use of and adherence to oral anticoagulation increased over time for both patients with postoperative atrial fibrillation (POAF) and those with non‐POAF, but the use and adherence were higher in non‐POAF in comparison to POAF.
Patients managed by cardiologists were more likely to use and adhere to oral anticoagulation, regardless of POAF or non‐POAF.
What Question Should Be Addressed Next?
Randomized controlled trials are needed to address whether oral anticoagulation should be used in patients with POAF; in the meantime, effective interventions to increase medication initiation and adherence should be developed and implemented to further improve guideline‐recommended care in non‐POAF patients, especially for those who are not routinely managed by cardiologists.
New‐onset postoperative atrial fibrillation (POAF) is associated with increased risk of stroke and mortality, in both short term and long term, in both patients who underwent cardiac surgeries and noncardiac surgeries. 1 , 2 , 3 However, how to prevent stroke in patients with POAF is less certain, especially balancing the reduction of stroke risk and the increase of bleeding risk with long‐term oral anticoagulation (OAC).
Current US guidelines make a modest Class II recommendation for OAC in POAF based on prior nonrandomized studies, but there remains some uncertainty about the usefulness/efficacy of OAC in the context of POAF. 4 , 5 Without a clear consensus and evidence from randomized controlled trials, there have been great variations in how patients are managed in routine clinical practice. However, limited data exist to describe the patterns of the initiation, discontinuation, and adherence of OAC in patients with POAF and the trends over time.
By contrast, in most patients diagnosed with atrial fibrillation (AF) unrelated to a recent surgery (hereafter called “non‐POAF”), there has long been compelling evidence and guideline recommendations for using OAC for stroke prevention. 5 The underuse of OAC and nonadherence in routine practice have been documented in prior studies. 6 , 7 , 8 , 9 In recent years, the use of OAC has become a key quality of care measurement in AF management. 10 Furthermore, many institutions implemented different quality improvement efforts to improve the initiation and adherence to OAC. 11 It would be important to document the OAC use in this population and put the OAC utilization patterns in POAF into the big picture.
Therefore, the current article aims to describe the initiation, adherence, and discontinuation of OAC in patients with POAF between 2012 and 2021 and compare them with the patterns in patients newly diagnosed with non‐POAF.
METHODS
Study Population
This retrospective cohort analysis used de‐identified administrative claims data from the OptumLabs Data Warehouse, which includes medical and pharmacy claims, and enrollment records for commercial and Medicare Advantage enrollees. The database contains longitudinal health information for >200 million enrollees and patients, representing a mixture of ages and geographical regions across the United States. 12 The OptumLabs Data Warehouse has been extensively utilized to investigate population‐level health outcomes related to various health conditions, especially in AF. 13 , 14 , 15 The Mayo Clinic Institutional Review Board determined that this study was exempt from review because it used preexisting, de‐identified data. Because of the nature of the OptumLabs Data Warehouse data, requests to access the dataset from qualified researchers may be sent to the corresponding author.
The study population included adult patients (≥18 years) newly diagnosed with AF, including AF and atrial flutter, between January 1, 2012 and December 31, 2021. The patients were divided into 2 groups for comparison: POAF and non‐POAF. The POAF cohort included those newly diagnosed with AF within 30 days after surgery (excluding obstetric procedures, organ transplantation, and surgeries of the lymphatic system). The non‐POAF cohort included the remaining patients who were newly diagnosed with AF but not within 30 days after surgery. The exclusion criteria were as follows: (1) lack of continuous medical and pharmacy coverage of at least 12 months before and 90 days after surgery, (2) missing age or sex, (3) age <18 years, (4) hospitalized >30 days, (5) patients with established AF diagnosis, and (6) patients with native mitral stenosis, and mechanical or bioprosthetic valve replacement. The diagnosis and procedure codes used to define eligibility are provided in Table S1. The flowchart for cohort creation can be found in Figure S1.
Baseline Characteristics
Baseline characteristics include demographic information, medical history, and surgical categories. Race and ethnicity were provided by OptumLabs, classified as non‐Hispanic White (White), non‐Hispanic Black (Black), Asian, Hispanic, or other/unknown. Self‐report was the primary source, and when it was missing, imputation was made by the data provider based on other available administrative data. 16 Procedure codes, including International Classification of Diseases, Ninth Revision (ICD‐9), International Classification of Diseases, Tenth Revision, (ICD‐10) Healthcare Common Procedure Coding System, and Current Procedural Terminology, were linked to Clinical Classification Software procedure categories that were used to define and categorize surgery (Table S2). 17 Medical history was determined using medical claims before the AF diagnosis date, which was defined as the index date.
Outcomes
The primary outcome was OAC use pattern, including initiation, discontinuation, and adherence. The OACs included warfarin and non‐vitamin K antagonist oral anticoagulants (including apixaban, dabigatran, edoxaban, and rivaroxaban). OAC initiation was defined as a prescription fill record within 90 days of the first AF diagnosis; and we performed a sensitivity analysis using 30 days. We also investigated the OAC initiation pattern by the specialty of providers. If a patient had a visit with a cardiologist within 90 days of the first AF diagnosis date, we classified the patient as in the cardiology group; if not and if the patient had a visit with a primary care clinician, we classified the patient as in the primary care group; and if the patient had neither cardiology or primary care visits, we classified the patients as in the “surgery and others” group. We also conducted sensitivity analyses subsetting to patients with an increased risk of stroke (ie, men with CHA2DS2‐VASc ≥2 and women with CHA2DS2‐VASc ≥3). 5
Discontinuation of OAC was defined as having 91 or more days of a gap in OAC. The discontinuation date was calculated based on the last fill date before the gap plus the quantity days' supply. If a patient had multiple discontinuation dates during follow‐up, the first one was counted in the analysis. If a patient was censored within the 91 days after the discontinuation date, either due to death or discontinuation of enrollment, the patient was not counted as discontinuing OAC.
Adherence was measured by the proportion of days covered at the end of 1 year since the medication initiation. Patients with a proportion of days covered ≥80% were considered as being adherent. When considering discontinuation or calculating the proportion of days covered, all OACs were considered regardless of whether the dose was changed, or the patient switched from 1 agent to another. The adherence analysis was limited to patients who started OAC within the first 90 days and had continuous enrollment for the first 12 months after the OAC initiation and did not die within the first 12 months.
Statistical Analysis
Multivariable logistic regression analyses were used to assess factors associated with OAC initiation and adherence, which are binary outcomes. Time to discontinuation was analyzed as a time‐to‐event outcome, and death was considered as a competing risk when plotting the cumulative incidence curve, and the Fine and Gray model was used to obtain subdistribution hazard ratio (sHR). 18 Odds ratios (ORs) or sHRs and the associated 95% CIs for all independent variables included in a multivariable regression model were presented. Such variables included age, sex, race, year of AF diagnosis, medical history, and contraindications of OAC use as listed in Table 1, and provider specialty. When the POAF and non‐POAF patients were pooled, whether a patient had POAF was included as an independent variable in the regression. When analyzing POAF patients alone, an additional variable, whether the surgery was cardiac surgery, was included. Studies using administrative claims data generally do not have the problem of missing data, per se. We defined the presence of a condition, outcome, or drug use by the presence of a claim with eligible diagnosis or procedure codes or prescription fills. Patients were considered to have a comorbidity, outcome, or drug exposure if they had a claim and were considered not to have a comorbidity, outcome, or drug exposure if they did not have a claim. Therefore, we did not have missing data on comorbidities, drug use, or outcomes. However, misclassification may exist. This is a limitation of using claims data, but the algorithms used to define our outcomes of interest and important covariates have been commonly used and have demonstrated good performance in previous studies. 19 , 20 , 21 , 22 , 23 A significance level of P <0.05, determined through 2‐sided testing, was considered statistically significant for all analyses. When performing regression analyses investigating patient, clinician, and procedure characteristics associated with the OAC initiation, adherence, and discontinuation, multiple testing correction was not conducted, and all these analyses should be considered exploratory. All analyses were conducted using SAS Enterprise Guide 7.1 (SAS Institute, Cary NC) and Stata 16.0 (Stata Corp, College Station TX).
Table 1.
Patient Baseline Characteristics
| non‐POAF (N=315 832) | POAF (N=118 366) | Total (N=434 198) | P value | |
|---|---|---|---|---|
| Age, y, mean±SD | 70.7±11.6 | 71.6±10.3 | 71.0±11.3 | <0.0001 |
| 18–64 y | 78 704 (24.9%) | 25 361 (21.4%) | 104 065 (24.0%) | <0.0001 |
| 65–74 y | 95 965 (30.4%) | 39 896 (33.7%) | 135 861 (31.3%) | |
| 75+ y | 141 163 (44.7%) | 53 109 (44.9%) | 194 272 (44.7%) | |
| Female | 147 684 (46.8%) | 54 289 (45.9%) | 201 973 (46.5%) | |
| Race or ethnicity | <0.0001 | |||
| Asian | 6687 (2.1%) | 2458 (2.1%) | 9145 (2.1%) | |
| Black | 38 916 (12.3%) | 14 009 (11.8%) | 52 925 (12.2%) | |
| Hispanic/Latino | 24 117 (7.6%) | 9691 (8.2%) | 33 808 (7.8%) | |
| White | 237 273 (75.1%) | 89 019 (75.2%) | 326 292 (75.1%) | |
| Other/Unknown* | 8839 (2.8%) | 3189 (2.7%) | 12 028 (2.8%) | |
| Medical history | ||||
| Heart failure | 71 835 (22.7%) | 31 469 (26.6%) | 103 304 (23.8%) | <0.0001 |
| Hypertension | 260 892 (82.6%) | 103 458 (87.4%) | 364 350 (83.9%) | <0.0001 |
| Diabetes | 113 302 (35.9%) | 49 719 (42.0%) | 163 021 (37.5%) | <0.0001 |
| Thromboembolism | 52 944 (16.8%) | 22 511 (19.0%) | 75 455 (17.4%) | <0.0001 |
| Other supraventricular arrhythmia | 22 046 (7.0%) | 8012 (6.8%) | 30 058 (6.9%) | 0.0145 |
| Ventricular arrhythmia | 17 057 (5.4%) | 8012 (6.8%) | 25 069 (5.8%) | <0.0001 |
| CAD | 126 311 (40.0%) | 61 612 (52.1%) | 187 923 (43.3%) | <0.0001 |
| PAD | 40 661 (12.9%) | 20 079 (17.0%) | 60 740 (14.0%) | <0.0001 |
| Major bleeding | 66 304 (21.0%) | 30 442 (25.7%) | 96 746 (22.3%) | <0.0001 |
| Stage 3–5 CKD | 50 865 (16.1%) | 22 971 (19.4%) | 73 836 (17.0%) | <0.0001 |
| Liver disease | 44 074 (14.0%) | 20 503 (17.3%) | 64 577 (14.9%) | <0.0001 |
| Non–skin cancer | 57 427 (18.2%) | 27 059 (22.9%) | 84 486 (19.5%) | <0.0001 |
| Fall | 59 737 (18.9%) | 26 380 (22.3%) | 86 117 (19.8%) | <0.0001 |
| Anemia | 130 611 (41.4%) | 58 676 (49.6%) | 189 287 (43.6%) | <0.0001 |
| Alcoholism | 15 754 (5.0%) | 6928 (5.9%) | 22 682 (5.2%) | <0.0001 |
| Concomitant use of antiplatelet | 28 032 (8.9%) | 14 565 (12.3%) | 42 627 (9.8%) | <0.0001 |
| CHA2DS2‐VASc, mean±SD | 3.9±2.0 | 4.2±1.9 | 3.9±2.0 | <0.0001 |
| HAS‐BLED, mean±SD | 2.8±1.4 | 3.1±1.4 | 2.8±1.4 | <0.0001 |
| Contraindications of OAC use | ||||
| None | 234 856 (74.4%) | 80 511 (68.0%) | 315 367 (72.6%) | <0.0001 |
| Intracranial bleeding | 6509 (2.1%) | 3254 (2.7%) | 9763 (2.2%) | <0.0001 |
| Recent bleed event | 4060 (1.3%) | 3381 (2.9%) | 7441 (1.7%) | <0.0001 |
| Cerebral amyloid angiopathy | 90 (0.0%) | 30 (0.0%) | 120 (0.0%) | 0.5780 |
| Cerebral aneurysm | 2025 (0.6%) | 910 (0.8%) | 2935 (0.7%) | 0.0001 |
| Pericarditis/pericardial effusions | 368 (0.1%) | 305 (0.3%) | 673 (0.2%) | <0.0001 |
| Renal failure requiring dialysis | 3485 (1.1%) | 2871 (2.4%) | 6356 (1.5%) | <0.0001 |
| Coagulation defects | 12 108 (3.8%) | 6155 (5.2%) | 18 263 (4.2%) | <0.0001 |
| End‐stage liver disease | 15 395 (4.9%) | 7275 (6.1%) | 22 670 (5.2%) | <0.0001 |
| Gastrointestinal cancer | 16 497 (5.2%) | 8735 (7.4%) | 25 232 (5.8%) | <0.0001 |
| Other gastrointestinal contraindications | 47 633 (15.1%) | 21 133 (17.9%) | 68 766 (15.8%) | <0.0001 |
| Provider specialty | <0.0001 | |||
| Surgery and others | 56 741 (18.0%) | 25 323 (21.4%) | 82 064 (18.9%) | |
| Cardiology | 176 560 (55.9%) | 57 669 (48.7%) | 234 229 (54.0%) | |
| Primary care | 82 531 (26.1%) | 35 374 (29.9%) | 117 905 (27.2%) | |
| CCS category | ||||
| Operation on the cardiovascular system | … | 40 586 (34.3%) | … | … |
| Operation on the digestive system | … | 13 663 (11.5%) | … | … |
| Operation on the ear | … | 2882 (2.4%) | … | … |
| Operation on the endocrine system | … | 2983 (2.5%) | … | … |
| Operation on the eye | … | 9016 (7.6%) | … | … |
| Operation on the genital organs | … | 2064 (1.7%) | … | … |
| Operation on the integumentary system | … | 9131 (7.7%) | … | … |
| Operation on the musculoskeletal system | … | 27 933 (23.6%) | … | … |
| Operation on the nervous system | … | 9068 (7.7%) | … | … |
| Operation on the nose/mouth/pharynx | … | 11 808 (10.0%) | … | … |
| Operation on the respiratory system | … | 2058 (1.7%) | … | … |
| Operation on the urinary system | … | 4232 (3.6%) | … | … |
| Year of AF diagnosis | <0.0001 | |||
| 2012 | 21 602 (6.8%) | 7716 (6.5%) | 29 318 (6.8%) | |
| 2013 | 23 497 (7.4%) | 8612 (7.3%) | 32 109 (7.4%) | |
| 2014 | 21 205 (6.7%) | 8014 (6.8%) | 29 219 (6.7%) | |
| 2015 | 24 523 (7.8%) | 8989 (7.6%) | 33 512 (7.7%) | |
| 2016 | 26 660 (8.4%) | 9723 (8.2%) | 36 383 (8.4%) | |
| 2017 | 38 356 (12.1%) | 13 780 (11.6%) | 52 136 (12.0%) | |
| 2018 | 40 360 (12.8%) | 15 336 (13.0%) | 55 696 (12.8%) | |
| 2019 | 42 267 (13.4%) | 16 051 (13.6%) | 58 318 (13.4%) | |
| 2020 | 37 245 (11.8%) | 14 046 (11.9%) | 51 291 (11.8%) | |
| 2021 | 40 117 (12.7%) | 16 099 (13.6%) | 56 216 (12.9%) | |
AF indicates atrial fibrillation; CAD, coronary artery disease; CCS, clinical classifications software; CKD, chronic kidney disease; OAC, oral anticoagulation; PAD, peripheral artery disease; and POAF, postoperative atrial fibrillation.
Other/unknown includes patients who identified themselves outside any of the prior categories or such information was not available.
RESULTS
Patient Characteristics
We identified 118 366 patients diagnosed with POAF between 2012 and 2021. The mean age was 71.6 (SD 10.3) years, 54 289 (45.9%) were female, and the mean CHA2DS2‐VASc was 4.2 (SD 1.9); 40 586 (34.3%) received a cardiac surgery. We also identified 315 832 newly diagnosed with non‐POAF. The mean age was 70.7 (SD 11.6) years, 147 684 (46.8%) were female, and the mean CHA2DS2‐VASc was 3.9 (SD 2.0; Table 1).
OAC Initiation and Choice
A total of 42 803 (36.2%) of patients with POAF started OAC within 90 days of diagnosis and 149 717 (47.4%) of patients with non‐POAF did so. The OAC initiation rates by surgical categories in POAF can be found in Table S3. Patients with POAF were less likely to initiate OAC than those with non‐POAF (OR, 0.64 [95% CI, 0.63–0.65]; P <0.0001; Table 2). The initiation of OAC was increased over time for both patients with POAF (28.9% in 2012 and 44.0% in 2021; OR, 2.01 [95% CI, 1.88–2.14]; P <0.0001; Figure 1 and Table S4) and non‐POAF (37.8% in 2012 and 59.9% in 2021; OR, 2.64 [95% CI, 2.54–2.75]; P <0.0001; Figure S2 and Table S5). The patterns were similar in patients with an elevated risk of stroke (ie, CHA2DS2‐VASc≥2 for in men and ≥3 in women; Figure S3). Among patients who initiated OAC, regardless of POAF or non‐POAF, most started on a non‐vitamin K antagonist oral anticoagulant (Table S6); in 2021, only 4.6% of non‐POAF patients who started on OAC used warfarin, whereas 80.9% used apixaban; similar trends were found for POAF (Figure 2).
Table 2.
Factors Associated With OAC Initiation, Adherence, and Discontinuation
| Initiation | Adherence | Discontinuation | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | sHR (95% CI) | P value | |
| Postoperative AF | 0.64 (0.63–0.65) | <0.0001 | 0.66 (0.64– 0.67) | <0.0001 | 1.23 (1.21–1.24) | <0.0001 |
| Age | ||||||
| 18–64 y | Ref | Ref | Ref | Ref | Ref | Ref |
| 65–74 y | 1.13 (1.10–1.15) | <0.0001 | 1.17 (1.12–1.21) | <0.0001 | 0.76 (0.75–0.78) | <0.0001 |
| 75+ y | 1.14 (1.11–1.16) | <0.0001 | 1.30 (1.25–1.35) | <0.0001 | 0.78 (0.76–0.79) | <0.0001 |
| Female | 0.99 (0.97–1.00) | 0.0720 | 1.22 (1.19–1.25) | <0.0001 | 0.87 (0.86–0.88) | <0.0001 |
| Race or ethnicity | ||||||
| White | Ref | Ref | Ref | Ref | Ref | Ref |
| Asian | 0.78 (0.74–0.81) | <0.0001 | 0.84 (0.78–0.92) | 0.0001 | 1.06 (1.01–1.10) | 0.0180 |
| Black | 0.86 (0.84–0.87) | <0.0001 | 0.79 (0.76–0.82) | <0.0001 | 1.14 (1.12–1.16) | <0.0001 |
| Hispanic/Latino | 0.82 (0.80–0.85) | <0.0001 | 0.75 (0.71–0.78) | <0.0001 | 1.16 (1.13–1.19) | <0.0001 |
| Other/Unknown* | 0.91 (0.87–0.95) | <0.0001 | 0.91 (0.85–0.98) | 0.0151 | 1.08 (1.04–1.12) | 0.0001 |
| Year of AF diagnosis | ||||||
| 2012 | Ref | Ref | Ref | Ref | Ref | Ref |
| 2013 | 1.02 (0.98–1.06) | 0.2593 | 1.07 (1.00–1.15) | 0.0465 | 0.96 (0.93–0.99) | 0.0076 |
| 2014 | 1.07 (1.03–1.11) | 0.0007 | 1.12 (1.04–1.20) | 0.0014 | 0.91 (0.88–0.95) | <0.0001 |
| 2015 | 1.07 (1.03–1.11) | 0.0002 | 1.32 (1.24–1.41) | <0.0001 | 0.80 (0.77–0.82) | <0.0001 |
| 2016 | 1.23 (1.18–1.27) | <0.0001 | 1.41 (1.32–1.50) | <0.0001 | 0.76 (0.73–0.78) | <0.0001 |
| 2017 | 1.26 (1.22–1.30) | <0.0001 | 1.56 (1.47–1.65) | <0.0001 | 0.73 (0.71–0.75) | <0.0001 |
| 2018 | 1.61 (1.55–1.66) | <0.0001 | 1.51 (1.43–1.60) | <0.0001 | 0.74 (0.72–0.76) | <0.0001 |
| 2019 | 1.77 (1.71–1.83) | <0.0001 | 1.68 (1.58–1.77) | <0.0001 | 0.72 (0.70–0.74) | <0.0001 |
| 2020 | 2.14 (2.07–2.22) | <0.0001 | 1.68 (1.58–1.78) | <0.0001 | 0.75 (0.73–0.77) | <0.0001 |
| 2021 | 2.44 (2.36–2.52) | <0.0001 | 1.65 (1.56–1.75) | <0.0001 | 0.81 (0.79–0.84) | <0.0001 |
| Medical history | ||||||
| Heart failure | 0.97 (0.95–0.98) | 0.0001 | 0.93 (0.90–0.96) | <0.0001 | 1.10 (1.08–1.12) | <0.0001 |
| Hypertension | 1.14 (1.11–1.18) | <0.0001 | 1.14 (1.09–1.19) | <0.0001 | 0.81 (0.80–0.83) | <0.0001 |
| Diabetes | 1.06 (1.04–1.07) | <0.0001 | 0.98 (0.95–1.00) | 0.0719 | 0.99 (0.98–1.01) | 0.4162 |
| Thromboembolism | 1.08 (1.06–1.10) | <0.0001 | 1.07 (1.04–1.10) | <0.0001 | 0.97 (0.95–0.98) | <0.0001 |
| Other supraventricular arrhythmia | 0.73 (0.71–0.75) | <0.0001 | 1.06 (1.01–1.12) | 0.0193 | 0.99 (0.97–1.02) | 0.4785 |
| Ventricular arrhythmia | 0.82 (0.79–0.84) | <0.0001 | 1.02 (0.97–1.08) | 0.4492 | 0.97 (0.94–1.00) | 0.0281 |
| CAD | 0.80 (0.79–0.81) | <0.0001 | 0.86 (0.84–0.88) | <0.0001 | 1.07 (1.06–1.09) | <0.0001 |
| PAD | 0.96 (0.94–0.98) | 0.0001 | 0.95 (0.92–0.99) | 0.0079 | 1.08 (1.06–1.10) | <0.0001 |
| Major bleeding | 0.96 (0.93–0.99) | 0.0167 | 1.07 (1.01–1.12) | 0.0198 | 1.00 (0.97–1.03) | 0.9786 |
| Stage 3–5 CKD | 0.99 (0.98–1.01) | 0.5109 | 0.98 (0.95–1.01) | 0.2046 | 1.06 (1.05–1.08) | <0.0001 |
| Liver disease | 0.93 (0.91–0.96) | <0.0001 | 0.96 (0.92–1.00) | 0.0527 | 1.03 (1.01–1.06) | 0.0016 |
| Non–skin cancer | 0.96 (0.94–0.98) | <0.0001 | 0.98 (0.95–1.01) | 0.1023 | 1.05 (1.04–1.07) | <0.0001 |
| Fall | 0.89 (0.88–0.91) | <0.0001 | 0.92 (0.90–0.95) | <0.0001 | 1.12 (1.10–1.13) | <0.0001 |
| Anemia | 0.88 (0.87–0.90) | <0.0001 | 0.92 (0.89–0.94) | <0.0001 | 1.08 (1.07–1.10) | <0.0001 |
| Alcoholism | 0.86 (0.83–0.89) | <0.0001 | 0.80 (0.76–0.85) | <0.0001 | 1.12 (1.09–1.16) | <0.0001 |
| Concomitant use of antiplatelet | 0.78 (0.77–0.80) | <0.0001 | 1.14 (1.09–1.18) | <0.0001 | 0.92 (0.90–0.94) | <0.0001 |
| Contraindications of OAC use | ||||||
| Intracranial bleeding | 0.85 (0.80–0.89) | <0.0001 | 0.97 (0.88–1.06) | 0.4948 | 0.99 (0.94–1.04) | 0.5962 |
| Recent bleed event | 0.56 (0.53–0.59) | <0.0001 | 0.85 (0.75–0.95) | 0.0048 | 1.15 (1.08–1.22) | <0.0001 |
| Cerebral amyloid angiopathy | 0.35 (0.23–0.55) | <0.0001 | 1.40 (0.55–3.56) | 0.4839 | 1.28 (0.88–1.86) | 0.1970 |
| Cerebral aneurysm | 0.91 (0.84–0.99) | 0.0267 | 1.01 (0.87–1.17) | 0.8768 | 1.08 (1.01–1.16) | 0.0286 |
| Pericarditis/pericardial effusions | 0.79 (0.66–0.94) | 0.0075 | 0.88 (0.64–1.21) | 0.4236 | 1.18 (1.00–1.40) | 0.0509 |
| Renal failure requiring dialysis | 0.83 (0.78–0.88) | <0.0001 | 0.75 (0.67–,0.84) | <0.0001 | 1.27 (1.20–1.34) | <0.0001 |
| Coagulation defects | 1.78 (1.72–1.85) | <0.0001 | 1.24 (1.17–1.31) | <0.0001 | 0.82 (0.79–0.84) | <0.0001 |
| End‐stage liver disease | 0.99 (0.96–1.03) | 0.6925 | 1.00 (0.94–1.07) | 0.9481 | 1.01 (0.97–1.04) | 0.6897 |
| Gastrointestinal cancer | 0.98 (0.95–1.01) | 0.1620 | 0.95 (0.91–1.01) | 0.0845 | 1.02 (0.99–1.05) | 0.1831 |
| Other gastrointestinal contraindications | 0.97 (0.94–1.00) | 0.0506 | 0.89 (0.84–0.94) | 0.0001 | 1.03 (1.00–1.06) | 0.0790 |
| Provider specialty | ||||||
| Surgery and others | Ref | Ref | Ref | Ref | Ref | Ref |
| Cardiology | 2.92 (2.87–2.98) | <0.0001 | 1.08 (1.04–1.13) | <0.0001 | 0.83 (0.82–0.85) | <0.0001 |
| Primary care | 1.29 (1.27–1.32) | <0.0001 | 0.98 (0.94–1.03) | 0.4692 | 0.90 (0.88–0.92) | <0.0001 |
AF indicates atrial fibrillation; CAD, coronary artery disease; CKD, chronic kidney disease; OAC, oral anticoagulant; OR, odds ratio; PAD, peripheral artery disease;and sHR, subdistribution hazard ratio; OR and 95% CI were obtained from multivariable logistic regression models. sHR and 95% CI were obtained from multivariable time‐to‐event analyses considering mortality as the competing risk using the Fine and Gray method.
Other/unknown includes patients who identified themselves outside any of the prior categories or such information was not available.
Figure 1. Trends of OAC initiation by follow‐up specialty, 2012 to 2021.
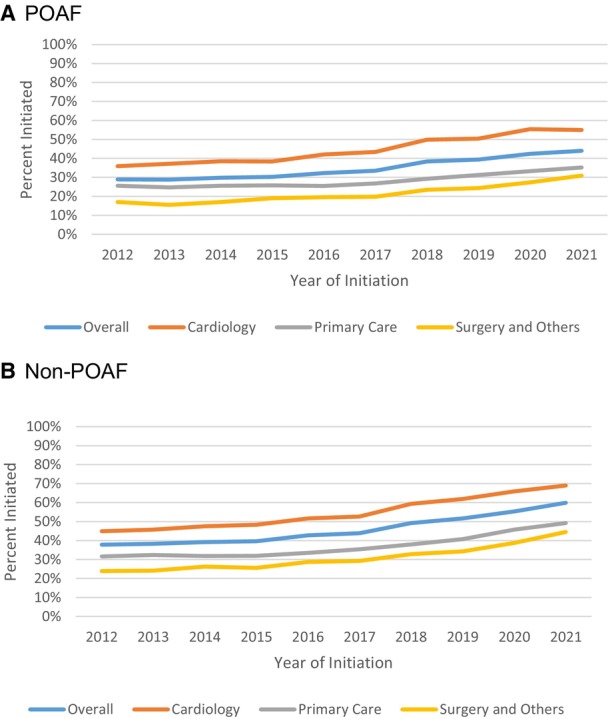
If a patient had a visit with a cardiologist within 90 days of the first AF diagnosis date, we classified the patient as in the cardiology group, if not and if the patient had a visit with a primary care clinician, we classified the patient as in the primary care group, and if the patient had neither cardiology or primary care visits, we classified the patients as in the “surgery and others” group. A, POAF, (B) non‐POAF. AF indicates atrial fibrillation; OAC, oral anticoagulation; and POAF, postoperative atrial fibrillation.
Figure 2. Trends in OAC Choice Among Patients Who Initiated OAC, 2012 to 2021.
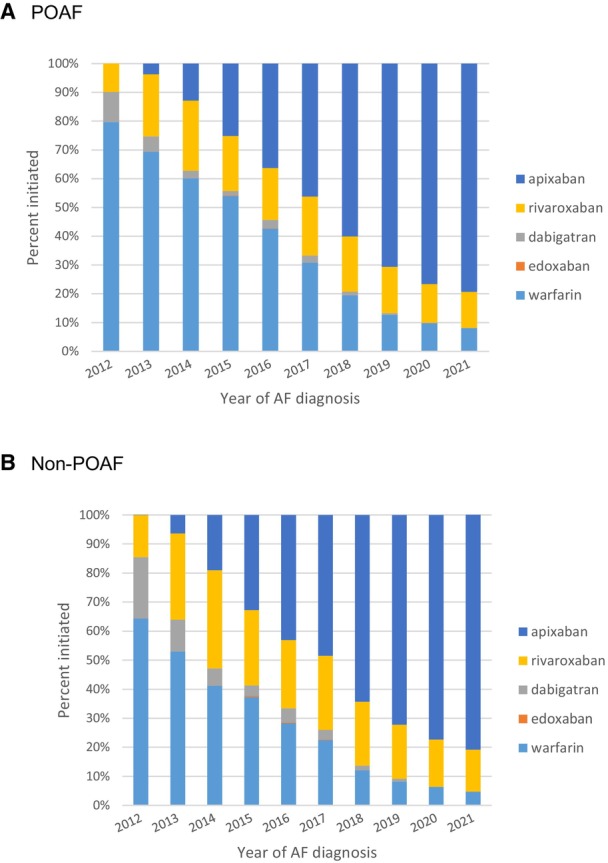
A, POAF, (B) non‐POAF. AF indicates atrial fibrillation or flutter; OAC, oral anticoagulation; and POAF, postoperative atrial fibrillation.
OAC Discontinuation and Adherence
Among patients with POAF, the median time to discontinuation of OAC was 177 days (interquartile range, 63, 445) within a median of 742 days of follow‐up (interquartile range 383, 1359). Among patients with non‐POAF, the median time to discontinuation was 242 days (interquartile range 91, 569), with a median of 754 days of follow‐up (interquartile range 400, 1359). An estimated 19.2% (95% CI, 18.9%–19.6%) of POAF patients discontinued OAC at 3 months in comparison to 13.7% (95% CI, 13.5%–13.9%) in non‐POAF; and 45.6% (95% CI, 45.2%–46.1%) of POAF patients discontinued OAC at 12 months, versus 36.7% (95% CI, 36.4%–36.9%) in non‐POAF (Figure S4). Patients with POAF were more likely to discontinue OAC than non‐POAF (sHR, 1.23 [95% CI, 1.21–1.24]; P <0.0001); patients in 2021 were less likely to discontinue OAC than patients in 2012 (sHR, 0.81 [95% CI, 0.79–0.84]; P <0.0001; Table 2).
Among patients who initiated OAC and had at least 12 months of follow‐up, patients with POAF were less likely to adhere to OAC, 57.0% of POAF patients were adherent versus 66.2% of non‐POAF patients (Table S7 and Table 2; OR, 0.66 [95% CI, 0.64–0.67]; P <0.0001 comparing POAF versus non‐POAF). The medication adherence increased over time from 47.0% in 2012 to 61.8% in 2021 in patients with POAF, and 59.7% to 70.4% in patients with non‐POAF. Patients in 2021 were more likely to adhere to OAC than patients in 2012 (OR, 1.65 [95% CI, 1.56–1.75]; P <0.0001; Table 2 and Figure S5).
Patient Characteristics Associated With Initiation, Adherence, and Discontinuation
In multivariable regression analyses, regardless of POAF or non‐POAF, the OAC initiation rates appeared to be higher in patients who had a visit with a cardiologist within 90 days of the diagnosis (cardiology OR, 2.92 [95% CI, 2.87–2.98]; P <0.0001, primary care OR, 1.29 [95% CI, 1.27–1.32]; P <0.0001; surgery and other specialties were used as the reference group; Table 2). The results on adherence were less substantial (cardiology OR, 1.08 [95% CI, 1.04, 1.13]; P <0.0001; primary care OR, 0.98 [95% CI, 0.94–1.03]; P=0.47; Table 2 and Figure 1). A similar pattern was found for discontinuation because patients who had a visit with a cardiologist were less likely to discontinue OAC (cardiology sHR, 0.83 [95% CI, 0.82–0.85]; P <0.0001; primary care sHR, 0.90 [95% CI, 0.88–0.92]; P <0.0001).
Older age was associated with an increased likelihood of OAC initiation and adherence and a lower likelihood of discontinuation; women were as likely to initiate OAC as men (OR, 0.99 [95% CI, 0.97–1.00]; P=0.07), but women were more likely to adhere to OAC (OR, 1.22 [95% CI, 1.19–1.25]; P <0.0001) and less likely to discontinue (OR, 0.87 [95% CI, 0.86–0.88]; P <0.0001). Minorities were less likely to initiate or adhere to OAC, and more likely to discontinue. A history of intracranial bleeding was associated with a lower likelihood of initiating OAC (OR, 0.85 [95% CI, 0.80–0.89]; P <0.0001), but once initiated, these patients had a similar likelihood of adhering to OAC (OR, 0.97 [95% CI, 0.88–1.06]; P=0.49) and discontinuing OAC (OR, 0.99 [95% CI, 0.94–1.04]; P=0.60). Table 2 illustrates the regression results in the overall cohort including both POAF and non‐POAF. The model results run in POAF and non‐POAF patients separately can be found in Tables S4 and S5.
DISCUSSION
This is one of the first large studies examining the utilization patterns of OAC in patients with POAF in routine clinical practice and put the findings into the context to compare with patients with non‐POAF. We found regardless of POAF or non‐POAF, patients in 2021 were much more likely to initiate OAC and adhere to OAC, and less likely to discontinue OAC. This trend might be a result of increasing awareness over time as well as the availability of NOACs with the ease of dosing and higher adherence than warfarin, and some of the NOACs also have a lower bleeding risk. 6 , 24 Furthermore, in most patients with non‐POAF, they have a Class IA guideline recommendation for OAC and there have been efforts from numerous learning health systems to improve the initiation and adherence of OAC. 11
All these factors combined have led to increasing use and adherence of OAC over the past decade. However, in those with non‐POAF, substantial gaps remain in the quality of care: over one third of patients with AF did not initiate OAC after the initial diagnosis, and among those who initiated, ≈30% became nonadherent within a year. The current study examined several patients' and clinicians' characteristics associated with the OAC initiation, adherence, and discontinuation.
Interestingly, patients who visited a cardiologist were far more likely to initiate anticoagulation and adhere to it. This could be because cardiologists are more familiar with the evidence and guidelines surrounding OAC, but given that in POAF where the evidence is much weaker, a cardiologist visit was also associated with a large increase in the likelihood of OAC initiation, it is likely that cardiologists in general tend to think about AF stroke prevention and OAC; the lower OAC initiation in other specialties might not be a conscious decision of not to start OAC after weighing the tradeoffs and evidence, but rather, OAC might not be on the list or the top of the list that clinicians in other specialties consider, regardless of whether the patients have POAF or non‐POAF.
Another important finding of the study is that in comparison to the warfarin‐era data that indicated women were less likely to use OAC, 25 the current study found women were as likely as men to initiate OAC; women were in fact more likely to adhere and less likely to discontinue OAC once prescribed. However, in comparison to non‐Hispanic White people, different racial/ethnical minority groups have been consistently less likely to initiate OAC, less likely to adhere, and more likely to discontinue. The findings indicate barriers that persist into the contemporary era despite increasing awareness and efforts on social determinants of health.
We also investigated other patient characteristics associated with OAC use. The results were similar between POAF and non‐POAF, and consistent across the 3 measurements of OAC use (ie, initiation, adherence, and discontinuation). For example, advanced age was associated with a higher likelihood of OAC initiation, a higher likelihood of adherence, and a lower likelihood of discontinuation; a recent bleed and renal failure requiring dialysis were both associated with a lower likelihood of OAC initiation, a lower likelihood of adherence, and a higher likelihood of discontinuation. An outlier is that a history of intracranial bleeding was associated with a lower likelihood of initiating OAC but once initiated, these patients had a similar likelihood of adhering to OAC and discontinuing OAC.
This study did not examine whether OAC should be used in POAF. In fact, this question has been examined in prior observational studies, 26 , 27 which provided some evidence on the potential benefit of anticoagulation and led to the current guideline recommendations. These observational studies were all subject to residual confounding. Two ongoing randomized controlled trials in cardiac [PACES (Anticoagulation for New‐Onset Post‐Operative Atrial Fibrillation After CABG); NCT04045665] and noncardiac (ASPIRE‐AF; NCT03968393) surgery will inform optimal long‐term OAC use in POAF. Regardless of what the 2 ongoing randomized controlled trials find, the data from the current study will inform the subsequent implementation or de‐implementation strategies following the guideline changes after the trials are concluded.
Limitations
The current study has a few limitations. First, the adherence was calculated based on proportion of days covered over the 12‐month period after initiation. In the POAF setting, it is likely that the clinicians told the patients to stop OAC after a certain time point, so technically such patients did adhere to their clinicians' orders. The administrative claims data cannot distinguish whether patients discontinued OAC due to clinicians' orders or truly became nonadherent. Second, the initiation, adherence, and discontinuation of OAC relied on pharmacy claims. It is likely that a patient filled a prescription, resulting in a claim, but did not take the medication. However, other common methods to measure medication adherence (eg, pill count and patient questionnaire) have their own limitations, and are not feasible when measuring national cohorts over a decade. Third, determining POAF and other comorbidities also depends on administrative claims. Studies using administrative claims data do not have the problem of missing data, per se. We defined the presence of a condition, outcome, or drug use by the presence of claims with eligible diagnosis or procedure codes or prescription fills. Patients were classified as having a comorbidity, outcome, or drug exposure if they had the claims, and not having a comorbidity, outcome, or drug exposure if they did not have claims. Therefore, we did not have missing data, but misclassification may exist. However, misclassification has a limited impact on studies like this looking at population‐level big pictures. Fourth, another important factor affecting the initiation of and adherence to any medication is the cost. Although Affordable Care Act marketplaces and Medicaid expansion have greatly expanded insurance coverage, further efforts might need to investigate and address financial barriers to medication initiation and adherence.
Conclusions
In conclusion, the use of and adherence to OAC improved over the past decade for both patients with POAF and those with non‐POAF. In patients newly diagnosed with non‐POAF where the evidence and guideline recommendations are strong for OAC, there remain substantial gaps in medication initiation and adherence. The similar trends in POAF and non‐POAF and the remaining gaps in care quality indicate that there are many other barriers to initiating and adhering to OAC beyond the strength of evidence and guideline recommendations.
Sources of Funding
This study was funded by the National Institutes of Health (NIH) R01AG062436 and by the Mayo Clinic Robert D and Patricia E Kern Center for the Science of Health Care Delivery. Study contents are the sole responsibility of the authors and do not necessarily represent the official views of the NIH. The study sponsor had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Disclosures
Dr Yao reports in the past 36 months, Dr Yao has received research support through Mayo Clinic from NIA (R01AG062436) and the Food and Drug Administration (FDA, U01FD005938). Dr Noseworthy reports in the past 36 months, that he receives research funding from National Institutes of Health (NIH, including the National Heart, Lung, and Blood Institute [NHLBI] and the National Institute on Aging [NIA]), Agency for Healthcare Research and Quality (AHRQ), Food and Drug Administration (FDA), and the American Heart Association (AHA). Dr Noseworthy is a study investigator in an ablation trial sponsored by Medtronic. Dr Noseworthy and Mayo Clinic are involved in potential equity/royalty relationship with AliveCor. Dr Noseworthy has served on an expert advisory panel for Optum. Dr Noseworthy and Mayo Clinic have filed patents related to the application of AI to the ECG for diagnosis and risk stratification. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S7
Figures S1–S5
Acknowledgments
Holly K. Van Houten has full access to all the data in the study. Dr Xiaoxi Yao and Holly K. Van Houten take responsibility for the integrity of the data and the accuracy of the data analysis.
This manuscript was sent to Luciano A. Sposato, MD, MBA, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.124.035708
For Sources of Funding and Disclosures, see page 9.
References
- 1. Lin M‐H, Kamel H, Singer DE, Wu Y‐L, Lee M, Ovbiagele B. Perioperative/postoperative atrial fibrillation and risk of subsequent stroke and/or mortality: a meta‐analysis. Stroke. 2019;50:1364–1371. doi: 10.1161/STROKEAHA.118.023921 [DOI] [PubMed] [Google Scholar]
- 2. Conen D, Alonso‐Coello P, Douketis J, Chan MT, Kurz A, Sigamani A, Parlow JL, Wang CY, Villar JC, Srinathan SK. Risk of stroke and other adverse outcomes in patients with perioperative atrial fibrillation 1 year after non‐cardiac surgery. Eur Heart J. 2020;41:645–651. doi: 10.1093/eurheartj/ehz431 [DOI] [PubMed] [Google Scholar]
- 3. Siontis KC, Gersh BJ, Weston SA, Jiang R, Roger VL, Noseworthy PA, Chamberlain AM. Associations of atrial fibrillation after noncardiac surgery with stroke, subsequent arrhythmia, and death: a cohort study. Ann Intern Med. 2022;175:1065–1072. doi: 10.7326/M22-0434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström‐Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 5. Joglar JA, Chung MK, Armbruster AL, Benjamin EJ, Chyou JY, Cronin EM, Deswal A, Eckhardt LL, Goldberger ZD, Gopinathannair R, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation. Circulation. 2024;149:e1–e156. doi: 10.1161/CIR.0000000000001193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yao X, Abraham NS, Alexander GC, Crown W, Montori VM, Sangaralingham LR, Gersh BJ, Shah ND, Noseworthy PA. Effect of adherence to oral anticoagulants on risk of stroke and major bleeding among patients with atrial fibrillation. J Am Heart Assoc. 2016;5:5. doi: 10.1161/jaha.115.003074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ko D, Lin KJ, Bessette LG, Lee SB, Walkey AJ, Cheng S, Kim E, Glynn RJ, Kim DH. Trends in use of oral anticoagulants in older adults with newly diagnosed atrial fibrillation, 2010‐2020. JAMA Netw Open. 2022;5:e2242964. doi: 10.1001/jamanetworkopen.2022.42964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Navar AM, Kolkailah AA, Overton R, Shah NP, Rousseau JF, Flaker GC, Pignone MP, Peterson ED. Trends in oral anticoagulant use among 436 864 patients with atrial fibrillation in community practice, 2011 to 2020. J Am Heart Assoc. 2022;11:e026723. doi: 10.1161/jaha.122.026723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hsu JC, Maddox TM, Kennedy KF, Katz DF, Marzec LN, Lubitz SA, Gehi AK, Turakhia MP, Marcus GM. Oral anticoagulant therapy prescription in patients with atrial fibrillation across the Spectrum of stroke risk: insights from the NCDR PINNACLE registry. JAMA Cardiol. 2016;1:55–62. doi: 10.1001/jamacardio.2015.0374 [DOI] [PubMed] [Google Scholar]
- 10. Heidenreich PA, Estes NAM 3rd, Fonarow GC, Jurgens CY, Kittleson MM, Marine JE, McManus DD, McNamara RL. 2020 update to the 2016 ACC/AHA clinical performance and quality measures for adults with atrial fibrillation or atrial flutter: a report of the American College of Cardiology/American Heart Association task force on performance measures. J Am Coll Cardiol. 2021;77:326–341. doi: 10.1016/j.jacc.2020.08.037 [DOI] [PubMed] [Google Scholar]
- 11. Gebreyohannes EA, Mill D, Salter S, Chalmers L, Bereznicki L, Lee K. Strategies for improving guideline adherence of anticoagulants for patients with atrial fibrillation in primary healthcare: a systematic review. Thromb Res. 2021;205:128–136. doi: 10.1016/j.thromres.2021.07.014 [DOI] [PubMed] [Google Scholar]
- 12. Wallace PJ, Shah ND, Dennen T, Bleicher PA, Crown WH. Optum labs: building a novel node in the learning health care system. Health Aff. 2014;33:1187–1194. doi: 10.1377/hlthaff.2014.0038 [DOI] [PubMed] [Google Scholar]
- 13. Noseworthy PA, Gersh BJ, Kent DM, Piccini JP, Packer DL, Shah ND, Yao X. Atrial fibrillation ablation in practice: assessing CABANA generalizability. Eur Heart J. 2019;40:1257–1264. doi: 10.1093/eurheartj/ehz085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yao X, Shah ND, Gersh BJ, Lopez‐Jimenez F, Noseworthy PA. Assessment of trends in statin therapy for secondary prevention of atherosclerotic cardiovascular disease in US adults from 2007 to 2016. JAMA Netw Open. 2020;3:e2025505. doi: 10.1001/jamanetworkopen.2020.25505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yao X, Gersh BJ, Holmes DR Jr, Melduni RM, Johnsrud DO, Sangaralingham LR, Shah ND, Noseworthy PA. Association of surgical left atrial appendage occlusion with subsequent stroke and mortality among patients undergoing Cardiac surgery. JAMA. 2018;319:2116–2126. doi: 10.1001/jama.2018.6024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hershman DL, Tsui J, Wright JD, Coromilas EJ, Tsai WY, Neugut AI. Household net worth, racial disparities, and hormonal therapy adherence among women with early‐stage breast cancer. J Clin Oncol. 2015;33:1053–1059. doi: 10.1200/JCO.2014.58.3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elixhauser A, Steiner C, Palmer L. Clinical Classifications Software. US Agency for Healthcare Research and Quality; 2015. http://www.hcup‐us.ahrq.gov/toolssoftware/ccs/ccsjsp [Google Scholar]
- 18. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 19. Tirschwell DL, Longstreth W. Validating administrative data in stroke research. Stroke. 2002;33:2465–2470. doi: 10.1161/01.STR.0000032240.28636.BD [DOI] [PubMed] [Google Scholar]
- 20. Cunningham A, Stein CM, Chung CP, Daugherty JR, Smalley WE, Ray WA. An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiol Drug Saf. 2011;20:560–566. doi: 10.1002/pds.2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arnason T, Wells P, Van Walraven C, Forster A. Accuracy of coding for possible warfarin complications in hospital discharge abstracts. Thromb Res. 2006;118:253–262. doi: 10.1016/j.thromres.2005.06.015 [DOI] [PubMed] [Google Scholar]
- 22. Hwang YJ, Shariff SZ, Gandhi S, Wald R, Clark E, Fleet JL, Garg AX. Validity of the international classification of diseases, tenth revision code for acute kidney injury in elderly patients at presentation to the emergency department and at hospital admission. BMJ Open. 2012;2:e001821. doi: 10.1136/bmjopen-2012-001821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wilchesky M, Tamblyn RM, Huang A. Validation of diagnostic codes within medical services claims. J Clin Epidemiol. 2004;57:131–141. doi: 10.1016/S0895-4356(03)00246-4 [DOI] [PubMed] [Google Scholar]
- 24. Yao X, Abraham NS, Sangaralingham LR, Bellolio MF, McBane RD, Shah ND, Noseworthy PA. Effectiveness and safety of dabigatran, rivaroxaban, and apixaban versus warfarin in nonvalvular atrial fibrillation. J Am Heart Assoc. 2016;5:e003725. doi: 10.1161/jaha.116.003725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baczek VL, Chen WT, Kluger J, Coleman CI. Predictors of warfarin use in atrial fibrillation in the United States: a systematic review and meta‐analysis. BMC Fam Pract. 2012;13:1–14. doi: 10.1186/1471-2296-13-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fragão‐Marques M, Teixeira F, Mancio J, Seixas N, Rocha‐Neves J, Falcão‐Pires I, Leite‐Moreira A. Impact of oral anticoagulation therapy on postoperative atrial fibrillation outcomes: a systematic review and meta‐analysis. Thromb J. 2021;19:89. doi: 10.1186/s12959-021-00342-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Butt JH, Xian Y, Peterson ED, Olsen PS, Rørth R, Gundlund A, Olesen JB, Gislason GH, Torp‐Pedersen C, Køber L, et al. Long‐term thromboembolic risk in patients with postoperative atrial fibrillation after coronary artery bypass graft surgery and patients with nonvalvular atrial fibrillation. JAMA Cardiol. 2018;3:417–424. doi: 10.1001/jamacardio.2018.0405 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S7
Figures S1–S5


