Abstract
Background
Extracellular microRNAs (miRNAs) are a class of noncoding RNAs that remain stable in the extracellular milieu, where they contribute to various physiological and pathological processes by facilitating intercellular signaling. Previous studies have reported associations between miRNAs and cardiovascular diseases (CVDs); however, the plasma miRNA signatures of CVD and its risk factors have not been fully elucidated at the population level.
Methods and Results
Plasma miRNA levels were measured in 4440 FHS (Framingham Heart Study) participants. Linear regression analyses were conducted to test the cross‐sectional associations of each miRNA with 8 CVD risk factors. Prospective analyses of the associations of miRNAs with new‐onset obesity, hypertension, type 2 diabetes, CVD, and all‐cause mortality were conducted using proportional hazards regression. Replication was carried out in 1999 RS (Rotterdam Study) participants. Pathway enrichment analyses were conducted and target genes were predicted for miRNAs associated with ≥5 risk factors in the FHS. In the FHS, 6 miRNAs (miR‐193b‐3p, miR‐122‐5p, miR‐365a‐3p, miR‐194‐5p, miR‐192‐5p, and miR‐193a‐5p) were associated with ≥5 risk factors. This miRNA signature was enriched for pathways associated with CVD and several genes annotated to these pathways were predicted targets of the identified miRNAs. Furthermore, miR‐193b‐3p, miR‐194‐5p, and miR‐193a‐5p were each associated with ≥2 risk factors in the RS. Prospective analysis revealed 8 miRNAs associated with all‐cause mortality in the FHS.
Conclusions
These findings highlight associations between miRNAs and CVD risk factors that may provide valuable insights into the underlying pathogenesis of CVD.
Keywords: cardiovascular disease, extracellular RNA, microRNA, pathways, risk factors
Subject Categories: Cardiovascular Disease, Risk Factors
Nonstandard Abbreviations and Acronyms
- exRNA
extracellular RNA
- FDR
false discovery rate
- FHS
Framingham Heart Study
- GO
gene ontology
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- miRNA
microRNA
- RS
Rotterdam Study
Clinical Perspective.
What Is New?
This study elucidates plasma extracellular microRNA signatures of cardiovascular disease risk factors and identifies 6 microRNAs (miR‐193b‐3p, miR‐122‐5p, miR‐365a‐3p, miR‐194‐5p, miR‐192‐5p, and miR‐193a‐5p) associated with ≥5 risk factors.
The 6‐miRNA signature was predicted to target mRNAs that are enriched for several biological processes and cellular pathways that may contribute to cardiovascular disease risk.
What Are the Clinical Implications?
These findings support a substantive role of extracellular microRNAs as biomarkers of cardiovascular disease risk and may stimulate additional research into the utility of microRNAs as clinically useful diagnostic, prognostic, and therapeutic targets.
RNAs were once thought to regulate only the cells in which they were synthesized. It is now appreciated that extracellular RNAs (exRNAs), a diverse group of noncoding RNAs present in extracellular spaces, have the capacity to facilitate cell‐to‐cell communication and regulate gene expression in distant cells. 1 , 2 Accordingly, exRNAs are synthesized in the host cell, exported out of the cell, and taken up by the recipient cell, initiating intercellular signaling. 3 , 4 , 5 Most exRNAs are packaged in membranous vesicles or are bound to and chaperoned by lipids or lipoproteins, allowing them to resist degradation by RNAse and circulate in the extracellular milieu. For example, exRNAs in extracellular vesicles are enclosed by a lipid bilayer, permitting them to travel outside their “host” cell. Extracellular vesicle‐associated exRNAs can then enter a recipient cell through selective or nonselective mechanisms. 2 , 4 , 5 , 6 , 7
There are a variety of exRNA types, including mRNA, tRNA, ribosomal RNA, microRNA (miRNA), and piwi‐interacting RNA, that differ in composition, abundance, source, location within the cell, and role in cellular communication. The most abundant and well‐characterized exRNAs are miRNAs. 6 , 8 , 9 , 10 These short noncoding RNAs regulate gene expression posttranscriptionally by mediating mRNA degradation or attenuating protein translation to alter protein levels. 3 , 11 , 12 The extent to which miRNAs regulate cell behavior is not well understood, but they are believed to regulate signal transduction within and between cells in a variety of ways, including binding to toll‐like receptors and activating downstream signaling cascades. 13 , 14 Hence, miRNAs may serve as chemical messengers, influencing various biological processes such as cell differentiation and proliferation, immune responses, and apoptosis. 6 , 12 , 15 Recent research has demonstrated associations of miRNAs with several complex diseases such as cancer, diabetes, chronic kidney disease, and neurodegenerative disease. 11 , 16 , 17 , 18 The differential expression of miRNAs has also been linked to differences in lifestyle behaviors, such as exercise, alcohol consumption, cigarette smoking, and diet. 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 Given that miRNAs are stable in various bodily fluids and their expression is specific to tissue or disease stage, they have the potential to be used as both diagnostic and prognostic biomarkers of disease. 3 , 11 , 12 , 18 , 30 , 31 , 32 , 33 Advancing understanding of the roles of miRNAs in health and disease holds the promise of harnessing miRNAs as circulating biomarkers of disease and as a therapeutic tool for its treatment or prevention. 1 , 3 , 11 , 16 , 31 , 33
Numerous studies have reported associations between exRNAs—mostly miRNAs—and a variety of cardiovascular diseases (CVD), including acute myocardial infarction, heart failure, and stroke. 34 , 35 , 36 , 37 , 38 , 39 , 40 Plasma miRNAs have also been shown to be associated with atrial fibrillation across multiple studies, including the FHS (Framingham Heart Study), the RS (Rotterdam Study), and the miRhythm Study. 41 , 42 , 43 Further, previous studies have reported differential expression of vascular and inflammation‐associated miRNAs from plasma in patients with coronary heart disease (CHD). 44 , 45 , 46 Freedman et al. found that expression levels of 8 plasma miRNAs were over 2‐fold higher in participants with significant coronary disease (≥70% stenosis) compared with those with minimal coronary disease (<70% stenosis) or healthy participants. 45 These findings advance understanding of the role of miRNAs in regulating biological processes involved in CHD.
Although previous studies have reported associations of miRNAs with CVD risk factors, many of these studies have examined associations between miRNAs and a single risk factor or a small subset of CVD risk factors. 1 , 21 , 47 , 48 , 49 As the clustering of multiple CVD risk factors is frequently seen clinically, it is prudent to examine associations of miRNAs with multiple CVD risk factors. 50 A study by Streese et al. conducted untargeted sequencing of miRNAs from serum in 158 older participants who were healthy or had disease, revealing 30 circulating miRNAs associated with cardiovascular health and physical activity or disease. 51 Mens et al. identified 22 plasma miRNAs associated with at least 1 cardiometabolic trait and further reported 4 miRNAs to be strongly linked to lipid traits following multiomic analysis. 52 Although miRNAs signatures of cardiometabolic traits support the hypothesis that circulating miRNA patterns can be identified for CVD risk factors, data are largely limited by small sample sizes, cohorts of primarily European ancestry, and lack of longitudinal analysis or independent replication. These findings warrant further investigation into the miRNAs signatures of additional CVD risk factors and outcomes.
In this study, we investigated associations of plasma extracellular miRNAs with CVD and its risk factors in a large middle‐aged population followed by replication in an independent prospective population‐based cohort study of older participants. The CVD risk factors of interest included blood pressure (BP), fasting lipid levels, fasting blood glucose, body mass index (BMI), and cigarette smoking. The clinical outcome events of interest included new onset of hypertension, type 2 diabetes, and obesity, and incidence of CVD events and all‐cause mortality.
METHODS
Discovery Study Population
The FHS is a multigenerational, community‐based observational cohort study. In 1948, the Original FHS cohort was recruited from residents of Framingham, MA, to characterize risk factors for CVD. 53 , 54 , 55 In 1971, children of the Original cohort and the spouses of these children were enrolled in the Offspring cohort. 53 In 2002, the Third Generation cohort was established with the recruitment of children of the FHS Offspring cohort. 53 , 54 To broaden the diversity of the FHS study population and reflect Framingham's changing demographics, the first Omni cohort began recruitment in 1995. This cohort included Black, Hispanic, Asian, Indian, Pacific Islander, and Native American participants. The second Omni cohort started enrolling participants in 2003. 54
The study sample for this investigation included individuals from the FHS Third Generation cohort (n=4095 at Exam 1) and the Omni II cohort (n=410 at Exam 1), in whom plasma miRNA levels were measured. Follow‐up extended through Exam 3, approximately 14 years later for the Third Generation cohort and 13 years later for the Omni II cohort. The study design is displayed in Figure 1. The FHS protocol was approved by the Boston Medical Center institutional review board; all participants provided written informed consent. All miRNA expression data and phenotype data have been deposited in dbGaP and can be accessed at http://www.ncbi.nlm.nih.gov/gap under accession number phs000007.v32.p13.
Figure 1. Flow chart of study design.
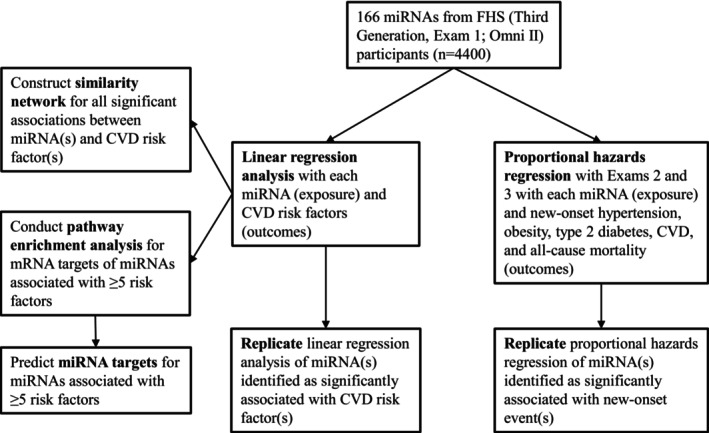
CVD indicates cardiovascular disease; FHS, Framingham Heart Study; and miRNAs, microRNAs.
Evaluation of Plasma miRNA Levels in the FHS
miRNA Isolation
miRNAs from plasma were isolated using the ThermoFisher MagMAX mirVana Total RNA Isolation Kit (Applied Biosystems; catalog #A27828). EDTA was added to the proteinase K digestion buffer and elution buffer at a final concentration of 1 mmol/L. The volume of magnetic beads was increased to 30 μL, the volume of the lysis buffer was increased to 195 μL, and the volume of isopropanol added to the beads/lysis buffer mix was increased to 375 μL. C. elegans miRNA 39.3p was phosphorylated and added as an internal control into the lysis buffer. Samples were processed using the ThermoFisher Flex Magnetic Particle Processor, 96DW. See Data S1 for the list of materials and equipment product numbers and Table S1 for the 282 miRNA targets measured in the current study.
RNA Concentration and Stabilization
Using the Biotage SPE instruments, 25 μL samples of eluted miRNAs were dried under warmed nitrogen (50 °C) and stored at −80 °C until further use.
cDNA Synthesis and Preamplification
Isolated miRNAs were converted to cDNAs using ThermoFisher TaqMan Advanced miRNA cDNA synthesis kits (catalog #A28007). Syntheses began with the addition of 5 μL Poly(A) Reaction Mix to the dried miRNA sample; sample volume in the reaction was made up of RNAse free water added to the reaction mix. All other steps followed vendor instructions. cDNAs derived from reverse transcription underwent 16 cycles of preamplification according to the vendor's recommended thermal‐cycling conditions. Synthesis reactions and preamplification were performed using either an Applied Biosystems ProFlex (96‐well polymerase chain reaction System Catalog #4484075) or Quant Studio 3 polymerase chain reactionsystem (Product A28137).
Quantitative Polymerase Chain Reaction
miRNAs were quantitated in multiplex, 96 samples×96 samples, using the Fluidigm BioMark HD instrument (#BMKHD‐BMKHD) and ThermoFisher TaqMan Advanced miRNA assays (#A25576). The integrated fluidic circuit chips, were loaded with samples and assays using the Fluidigm HX (#68000112) instrument. Data are reported as Ct‐values.
Primary methods for miRNA isolation have been previously published and minor adjustments were made for ease of implementation or changes according to product availability or recommendations. 56 , 57
Statistical Analysis in the FHS
We conducted linear regression analyses to test the cross‐sectional associations of each miRNA (exposure) with CVD risk factors (outcomes). The risk factors of interest included systolic BP (SBP), diastolic BP (DBP), triglycerides, high‐density lipoprotein (HDL) cholesterol, total cholesterol, fasting blood glucose, BMI, and cigarette smoking status (current/former/never). Current smokers were participants who reported smoking on average≥1 cigarette per day 1 month before the FHS Research Center examination, former smokers reported smoking on average≥1 cigarette per day but had not smoked 1 month before the exam, and never smokers reported never smoking. Two‐level smoking analyses were conducted for current and former cigarette smoking using never smoker as reference. Triglyceride levels, total cholesterol, and fasting blood glucose were log‐transformed. As miRNAs can be bound to and guided by lipoproteins, the regression models for some risk factors included lipid covariates. SBP and DBP were further adjusted for triglycerides, total cholesterol, and HDL. The lipid risk factors were adjusted for each other (eg, analysis of HDL was adjusted for total cholesterol and triglycerides). As confounding by lipids is likely due to the chaperoning of miRNAs by lipids or lipoproteins, fasting blood glucose and BMI were not adjusted for lipid levels. Other covariates in the model included age, sex, and cohort. We required that a miRNA be measurable in at least 50% of the participants to be included in analyses, which resulted in 166 miRNAs that were carried forward. We applied a false discovery rate (FDR) with a threshold FDR <0.05 for associations, separately for each risk factor.
Prospective analyses were conducted using proportional hazards regression on new‐onset obesity, hypertension, and type 2 diabetes, with discrete follow up time to Exams 2 and 3 (roughly 6 and 13 years, respectively), and on new‐onset CVD and death from all causes, without continuous follow‐up until occurrence of a qualifying event or censoring. Obesity was defined as having a BMI ≥30 kg/m2. Hypertension was defined as having a SBP of ≥130 mm Hg, DBP of ≥80 mm Hg, or the current use of antihypertensive medication. Type 2 diabetes was defined as having a fasting blood glucose level of ≥126 mg/dL or the use of glucose‐lowering medication. New‐onset CVD is a composite of CVD death, heart failure, stroke, CHD, and revascularization. In the prospective analyses, the miRNA was the exposure, and CVD events and time to events were the outcomes. The covariates for the proportional hazards models included SBP, DBP, triglycerides (log‐transformed), total cholesterol (log‐transformed), HDL, fasting blood glucose (log‐transformed), BMI, current smoking, age, sex, and cohort. Using proportional hazards regression models with Exams 2 and 3 as the time metric allowed us to account for censored observations and time to event. Once again, we set thresholds as 50% threshold percentage measurable miRNA and FDR <0.05 separately for each outcome.
All statistical analyses were carried out using SAS 9.4 (SAS Institute Inc. 2020. SAS/STAT® 15.2 User's Guide. SAS Institute Inc., Cary, NC) procedures REG and PHREG.
Bioinformatic Analyses in the FHS
A similarity network was constructed for all discovery associations using the R igraph package. 58 The similarity score was defined as the number of overlapping miRNAs divided by the total number of miRNAs for the 2 CVD risk factors. Each edge indicates that the connected nodes share miRNA(s) at a similarity score greater than 0.1. The size of each node reflects the number of miRNAs associated with the CVD risk factor.
Gene ontology (GO) biological process terms were tested and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis was performed for mRNA targets of miRNAs associated with 5 or more risk factors in the FHS using the R enrichR package. 59 Fisher's exact tests were used to assess whether the set of miRNA targets showed enrichment for genes involved in GO/KEGG pathways. To correct for multiple testing, the Benjamini‐Hochberg method was employed and an FDR threshold of 0.001 was applied. Potential gene targets of miRNAs associated with 5 or more risk factors in the FHS were identified using miRTarBase, a database where miRNA‐target interactions are gathered manually by reviewing relevant literature after natural language processing of the text to screen for research articles related to functional miRNA studies. 60 The miRNA‐target interactions are generally validated by reporter assay, western blot, microarray, and next‐generation sequencing experiments. 60
Replication Study Population
The RS is a large prospective population‐based cohort study among middle‐aged and elderly in the suburb Ommoord in Rotterdam, the Netherlands. In 1990, 7983 inhabitants aged 55 years old and older were recruited to participate in the first cohort (RS‐I). In 2000, the study was extended with a second cohort of 3011 participants (RS‐II) who became 55 years old or moved into the study district since the beginning of the study. In 2006, a further extension of the cohort was initiated with 3932 participants (RS‐III), aged 45 to 54 years. In 2016, the recruitment of another extension with around 3000 new participants (RS‐IV) started that targeted participants aged 40 years and over. A detailed description of the RS can be found elsewhere. 61
In the current study, we used the levels of circulating miRNAs in plasma samples, collected between 2002 and 2005, from a random subset (n=1000) of the fourth visit of the first cohort (RS‐I‐4) and a random subset (n=999) of the second visit of the second cohort (RS‐II‐2). Participants were followed from study entry until death, last health status update when they were known to be alive, or October 20, 2022, whichever came first. The RS has been approved by the Medical Ethics Committee of the Erasmus MC University Medical Center (registration number MEC 02.1015) and the Dutch Ministry of Health, Welfare and Sport (Population Screening Act WBO, license number 1071272‐159521‐PG). The participants included in the current study provided written informed consent.
Evaluation of Plasma miRNA Levels in the RS
Blood samples were collected in EDTA‐treated containers and centrifuged. Plasma was then aliquoted and frozen at −80 °C according to standard procedures. Subsequently, the levels of cell‐free miRNAs in plasma were determined using the HTG EdgeSeq miRNA Whole Transcriptome Assay, which quantitatively detect the expression of 2083 human miRNAs transcripts (HTG Molecular Diagnostics, Tuscon, AZ, USA) by using the Illumina NextSeq 500 sequencer (Illumina, San Diego, CA, USA). Quantification of miRNA expression was based on counts per million. Log2 transformation of counts per million was used as standardization and adjustment for total reads within each sample. MiRNAs with log2 counts per million <1.0 were indicated as not expressed in the samples. The lower limit of quantification was used to select well‐expressed miRNAs. The lower limit of quantification was based on a monotonic decreasing spline curve fit between the means and SDs of all miRNAs in the data set with 1999 subjects. In total, 591 miRNAs with >50% values above the lower limit of quantification were considered to be well expressed in plasma and included in the study. A detailed description of miRNA profiling in the RS can be found elsewhere. 62 , 63
Statistical Analysis in the RS
Covariates information and CVD risk factors data on age, sex, subcohort, and smoking status (never, former, current) were obtained from questionnaires. SBP and DBP were measured (mm Hg) in a seating position after a 5‐minute rest period on the right upper arm of the participant using a random‐zero sphygmomanometer. BMI was calculated based on weight in kilograms divided by the height in meters squared. Blood samples were collected during the visit to the research center. Fasting glucose levels were measured in mmol/L and log‐transformed. Levels of HDL and total serum cholesterol were measured in mmol/L. Total cholesterol was log‐transformed.
Population characteristics were represented as mean±SD for continuous variables, median (interquartile range) for log‐transformed variables, and categorical variables were expressed as numbers and percentages. Participants with missing values on multiple covariates were excluded from the analysis. Multivariable linear regression models were used to investigate the associations between plasma miRNA levels (exposure) and CVD risk factors (outcome), including smoking status, SBP and DBP, BMI, fasting blood glucose levels, and total serum cholesterol. Cox proportional hazard regression was used to estimate hazard ratios (HR) with 95% CIs for the association between plasma miRNA levels and death from all causes, without continuous follow‐up until the occurrence of a qualifying event or until October 20, 2022, whichever came first. Models were adjusted for age, sex, and subcohort. Additional adjustments were based on the outcomeBP was additionally adjusted for log total serum cholesterol and HDL. All‐cause mortality was additionally adjusted for SBP, DBP, log‐transformed cholesterol, HDL, log‐transformed fasting blood glucose, BMI, and smoking status. The Bonferroni correction was applied for the number of miRNAs available for replication analyses in the RS for each risk factor and outcome separately.
Replication was achieved when the P value in the RS was less than the Bonferroni threshold and when regression analysis of miRNAs and CVD risk factors or all‐cause mortality yielded opposite effect size signs in the FHS and the RS. Due to differences in assay technologies (quantitative reverse transcription‐polymerase chain reaction and RNA‐seq), opposite signs indicate that the directions of the estimates are in agreement.
All analyses were performed using SPSS statistical software (SPSS, version 25; IBM Crop) and R software version 4.1.2 (The R Foundation for Statistical Computing).
RESULTS
Characteristics of FHS Participants
The clinical characteristics of FHS participants (n=4440, mean age: 40±9 years) are presented in Table 1. Among eligible participants, 2368 (53%) were women, 2592 (58%) were never smokers, 1195 (27%) were former smokers, and 649 (15%) were current smokers. The analyses for the new‐onset events were performed on participants who had at least 1 follow‐up exam (n=3925). At follow‐up, 243 (7%) met criteria for incident type 2 diabetes, 586 (19%) met criteria for incident obesity, and 874 (36%) met criteria for incident hypertension. There were 108 (3%) participants who developed new‐onset CVD and there were 56 (1%) participants who died during a mean follow‐up of 13 years. Full baseline characteristics of FHS participants are given in Data S2a; follow‐up data for FHS participants are summarized in Data S2b.
Table 1.
Clinical Characteristics of the FHS Study Sample
| Clinical characteristic | Mean±SD |
|---|---|
| Age, y | 40 ±9 |
| Systolic blood pressure, mm Hg | 117 ±14 |
| Diastolic blood pressure, mm Hg | 75 ±10 |
| Triglycerides, mg/dL | 116 ±89 |
| Total cholesterol, mg/dL | 189 ±36 |
| High‐density lipoprotein cholesterol, mg/dL | 54 ±16 |
| Fasting blood glucose, mg/dL | 95 ±18 |
| Body mass index, kg/m2 | 26.9 ±5.5 |
| Dichotomous outcome | Number of participants (%) |
|---|---|
| Never smoker | 2592 (58.4) |
| Former smoker | 1195 (26.9) |
| Current smoker | 649 (14.6) |
| Incident diabetes | 243 (6.5)* |
| Incident hypertension | 874 (36.0)* |
| Incident obesity | 586 (19.3)* |
| Incident cardiovascular disease | 108 (2.8)* |
| All‐cause mortality | 56 (1.4)* |
FHS indicates Framingham Heart Study.
Number of new‐onset events for participants who had at least 1 follow‐up exam (n=3925).
Associations of miRNAs with Continuous Risk Factors in the FHS
At a discovery FDR<0.05, 14 miRNAs were significantly associated with SBP and 1 miRNA (miR‐193b‐3p) was associated with DBP in the FHS. Moreover, 13 miRNAs were associated with triglycerides (log‐transformed), 154 miRNAs were associated with total cholesterol, nine miRNAs were associated with fasting blood glucose, and 57 miRNAs were associated with BMI. No miRNAs were significantly associated with HDL cholesterol. There were 59 miRNAs associated with current cigarette smoking, whereas no miRNAs were associated with former cigarette smoking. The top 5 miRNA associations in the FHS for each risk factor are presented in Table 2. The similarity network (Figure 2) illustrates that total cholesterol was associated with the greatest number of miRNAs, followed by BMI. Additionally, BMI, DBP, total cholesterol, and smoking status were central in the similarity network. These 4 risk factors each had 4 edges, indicating that their associated miRNAs had significant overlap with other risk factors in the similarity network. A full list of cross‐sectional associations between miRNAs and CVD risk factors in the FHS can be found in Data S2c through S2l.
Table 2.
Top 5 miRNAs Associated With Each CVD Risk Factor in the FHS
| CVD risk factor | miRNA | No. full | Effect size | SE | P value | FDR‐adjusted P value |
|---|---|---|---|---|---|---|
| Systolic blood pressure | miR‐365a‐3p | 3341 | −0.775 | 0.169 | 4.68E‐06 | 0.000772 |
| miR‐193b‐3p | 2721 | −0.645 | 0.150 | 1.70E‐05 | 0.00140 | |
| miR‐20b‐5p | 4134 | 0.511 | 0.127 | 5.47E‐05 | 0.00301 | |
| miR‐16‐5p | 4376 | 0.378 | 0.0957 | 7.78E‐05 | 0.00321 | |
| miR‐25‐3p | 4389 | 0.414 | 0.113 | 0.000240 | 0.00790 | |
| Diastolic blood pressure | miR‐193b‐3p | 2721 | −0.428 | 0.103 | 3.18E‐05 | 0.00524 |
| miR‐130a‐3p* | 4144 | 0.228 | 0.0738 | 0.00200 | 0.0825 | |
| miR‐122‐5p* | 4382 | −0.214 | 0.0682 | 0.00170 | 0.0825 | |
| miR‐365a‐3p* | 3341 | −0.372 | 0.118 | 0.00158 | 0.0825 | |
| miR‐34a‐5p* | 3612 | −0.305 | 0.103 | 0.00319 | 0.105 | |
| Triglycerides | miR‐122‐5p | 4382 | −0.0292 | 0.00315 | 2.44E‐20 | 4.02E‐18 |
| miR‐193b‐3p | 2721 | −0.0412 | 0.00479 | 1.35E‐17 | 1.11E‐15 | |
| miR‐193a‐5p | 4261 | −0.0288 | 0.00399 | 6.68E‐13 | 3.68E‐11 | |
| miR‐365a‐3p | 3341 | −0.0378 | 0.00546 | 5.22E‐12 | 2.15E‐10 | |
| miR‐194‐5p | 4047 | −0.0279 | 0.00436 | 1.65E‐10 | 5.43E‐09 | |
| Total cholesterol | miR‐126‐3p | 4265 | −0.0112 | 0.00172 | 7.29E‐11 | 1.20E‐08 |
| miR‐191‐5p | 4259 | −0.00880 | 0.00141 | 4.52E‐10 | 2.14E‐08 | |
| miR‐425‐3p | 4064 | −0.00961 | 0.00155 | 5.77E‐10 | 2.14E‐08 | |
| miR‐425‐5p | 4256 | −0.00883 | 0.00143 | 6.48E‐10 | 2.14E‐08 | |
| miR‐625‐3p | 4266 | −0.00888 | 0.00142 | 4.69E‐10 | 2.14E‐08 | |
| High‐density lipoprotein | miR‐204‐5p* | 4131 | −0.315 | 0.112 | 0.00493 | 0.409 |
| miR‐375‐3p* | 4196 | −0.339 | 0.115 | 0.00324 | 0.409 | |
| miR‐301a‐3p* | 3478 | −0.334 | 0.133 | 0.0121 | 0.622 | |
| miR‐502‐3p* | 2635 | −0.436 | 0.186 | 0.0187 | 0.622 | |
| miR‐532‐3p* | 2718 | −0.364 | 0.153 | 0.0173 | 0.622 | |
| Fasting blood glucose | miR‐192‐5p | 4130 | −0.00647 | 0.00136 | 1.93E‐06 | 0.000114 |
| miR‐193a‐5p | 4263 | −0.00594 | 0.00123 | 2.05E‐06 | 0.000114 | |
| miR‐193b‐3p | 2723 | −0.00789 | 0.00161 | 1.00E‐06 | 0.000114 | |
| miR‐122‐5p | 4384 | −0.00441 | 0.000995 | 9.61E‐06 | 0.000399 | |
| miR‐365a‐3p | 3343 | −0.00684 | 0.00181 | 0.000155 | 0.00515 | |
| Body mass index | miR‐122‐5p | 4384 | −0.357 | 0.0411 | 4.97E‐18 | 8.26E‐16 |
| miR‐193b‐3p | 2723 | −0.462 | 0.0611 | 5.19E‐14 | 4.31E‐12 | |
| miR‐193a‐5p | 4263 | −0.364 | 0.0520 | 3.01E‐12 | 1.67E‐10 | |
| miR‐365a‐3p | 3343 | −0.489 | 0.0702 | 4.02E‐12 | 1.67E‐10 | |
| miR‐101‐3p | 4155 | 0.269 | 0.0512 | 1.61E‐07 | 5.33E‐06 | |
| Current cigarette smoking | miR‐150‐5p | 4382 | −0.334 | 0.0711 | 2.71E‐06 | 0.000339 |
| miR‐152‐3p | 3743 | −0.300 | 0.0631 | 2.03E‐06 | 0.000506 | |
| miR‐125a‐5p | 4360 | −0.307 | 0.0707 | 1.42E‐05 | 0.00118 | |
| miR‐34a‐5p | 3613 | −0.277 | 0.0666 | 3.14E‐05 | 0.00130 | |
| miR‐584‐5p | 4237 | −0.315 | 0.763 | 3.72E‐05 | 0.00132 | |
| Former cigarette smoking | miR‐99a‐3p* | 1200 | −0.254 | 0.0845 | 0.00268 | 0.671 |
| miR‐744‐5p* | 3375 | 0.0182 | 0.0580 | 0.754 | 0.981 | |
| miR‐425‐3p* | 4064 | 0.0184 | 0.0581 | 0.752 | 0.984 | |
| miR‐15a‐5p* | 4356 | −0.0215 | 0.0671 | 0.749 | 0.985 | |
| miR‐29a‐3p* | 4265 | 0.0242 | 0.0543 | 0.657 | 0.989 |
Associations after adjustment for age, sex, cohort, and lipid levels. CVD indicates cardiovascular disease; FDR, false discovery rate; FHS, Framingham Heart Study; and miRNA, microRNA.
Association not statistically significant (FDR>0.05).
Figure 2. Similarity network for continuous risk factors in the Framingham Heart Study.
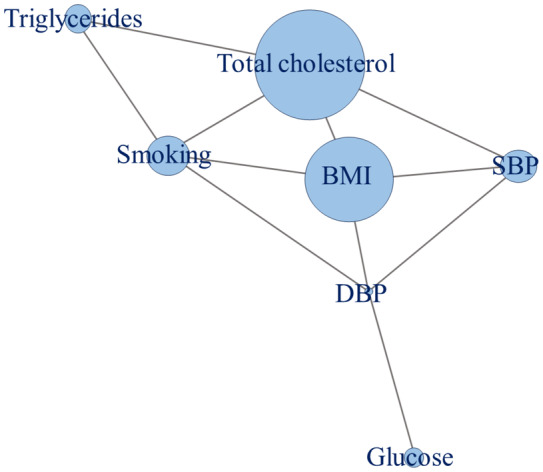
BMI indicates body mass index; DBP, diastolic blood pressure; and SBP, systolic blood pressure.
In the FHS, miR‐193b‐3p and miR‐122‐5p were significantly associated with 6 of the 8 risk factors. Four additional miRNAs (miR‐365a‐3p, miR‐194‐5p, miR‐192‐5p, and miR‐193a‐5p) were each significantly associated with 5 CVD risk factors. The associations of miRNAs with multiple risk factors are presented in Table 3.
Table 3.
miRNAs Associated With ≥5 CVD Risk Factors in the FHS
| miRNA | Risk factors |
|---|---|
| miR‐193b‐3p | SBP, DBP, triglycerides, total cholesterol, fasting blood glucose, BMI |
| miR‐122‐5p | SBP, triglycerides, total cholesterol, fasting blood glucose, BMI, current smoking |
| miR‐365a‐3p | SBP, triglycerides, total cholesterol, fasting blood glucose, BMI |
| miR‐194‐5p | Triglycerides, total cholesterol, fasting blood glucose, BMI, current smoking |
| miR‐192‐5p | Triglycerides, total cholesterol, fasting blood glucose, BMI, current smoking |
| miR‐193a‐5p | Triglycerides, total cholesterol, fasting blood glucose, BMI, current smoking |
Associations after adjustment for age, sex, cohort, and lipid levels. BMI indicates body mass index; CVD, cardiovascular disease; DBP, diastolic blood pressure; FHS, Framingham Heart Study; miRNAs, microRNAs; and SBP, systolic blood pressure.
Associations of miRNAs With New‐Onset Hypertension, Obesity, Type 2 Diabetes, CVD, and All‐Cause Mortality in the FHS
The proportional hazards models showed no significant associations of miRNAs with new‐onset type 2 diabetes, hypertension, or obesity or the composite CVD outcome in our longitudinal analyses in the FHS. Eight miRNAs were associated with all‐cause mortality in the FHS (Table 4). Of these, 4 miRNAs (miR‐193b‐3p, miR‐122‐5p, miR‐192‐5p, and miR‐193a‐5p) were also associated with 5 or more continuous CVD risk factors in the FHS. All prospective associations in the FHS can be found in Data S3a through S3e.
Table 4.
miRNAs Associated With All‐Cause Mortality in the FHS
| miRNA | No. analyzed | Events* | Effect size | SE | P value | FDR‐adjusted P value |
|---|---|---|---|---|---|---|
| miR‐193a‐5p | 3716 | 52 | −0.309 | 0.0649 | 1.84E‐06 | 0.000292 |
| miR‐192‐5p | 3602 | 51 | −0.252 | 0.0797 | 0.00156 | 0.0422 |
| miR‐320e | 3703 | 53 | −0.213 | 0.0670 | 0.00152 | 0.0422 |
| miR‐122‐5p | 3830 | 55 | −0.226 | 0.0679 | 0.000885 | 0.0422 |
| miR‐193b‐3p | 2380 | 37 | −0.277 | 0.0892 | 0.00186 | 0.0422 |
| miR‐210‐3p | 3616 | 52 | −0.227 | 0.0685 | 0.00094 | 0.0422 |
| miR‐34a‐5p | 3162 | 50 | −0.246 | 0.0784 | 0.00170 | 0.0422 |
| miR‐301a‐3p | 3043 | 42 | −0.255 | 0.0831 | 0.00218 | 0.0432 |
Associations after adjustment for age, sex, cohort, and CVD risk factors. CVD indicates cardiovascular disease; FDR, false discovery rate; FHS, Framingham Heart Study; and miRNAs, microRNAs.
Number of events associated with each miRNA out of total 56 new‐onset mortality events.
Enriched Pathways and miRNA Target Genes in the FHS
Pathway enrichment analysis was conducted for predicted mRNA targets of the 6 identified miRNAs associated with 5 or more CVD risk factors in the FHS. At an FDR<0.001, there were 71 enriched GO biological process terms. The top 3 enriched GO biological process terms included DNA replication (P=1.59E‐14), DNA metabolic process (P=4.10E‐13), and cellular response to DNA damage stimulus (P=8.02E‐13). The full list of enriched GO pathways can be found in Data S4a. There were 34 enriched KEGG cellular pathways at an FDR<0.001. The top 3 enriched KEGG cellular pathway terms were cell cycle (P=4.72E‐14), pancreatic cancer (P=1.82E‐07), and colorectal cancer (P=2.33E‐07). The full list of enriched KEGG pathways can be found in Data S4b. The top 3 enriched GO and KEGG terms and the first 10 listed annotated genes associated with CVD are reported in Table 5.
Table 5.
Top 3 Enriched GO Biological Terms and KEGG Pathways for miRNAs Associated With ≥5 CVD Risk Factors in the FHS
| GO term | Overlap* | Adjusted P value† | Fold change | Annotated genes of interest‡ |
|---|---|---|---|---|
| DNA replication | 51/108 | 1.59E‐14 | 6.143 | FEN1, MCM7, MCM10, BRCA1, PTMS, EXO1, CHEK1, NBN, TOPBP1, RFC2 |
| DNA metabolic process | 88/277 | 4.10E‐13 | 3.219 | TOP2A, FEN1, MCM7, TRRAP, GMNN, MCM10, BRCA1, HMGB1, TRIM28, SUMO1 |
| Cellular response to DNA damage stimulus | 102/350 | 8.02E‐13 | 2.850 | TRRAP, YY1, TRIM28, CCND1, CHEK1, NBN, FOXP1, MSH6, DDIT3, MAPKAPK2 |
| KEGG pathway | Overlap* | Adjusted P value† | Fold change | Annotated genes of interest |
|---|---|---|---|---|
| Cell cycle | 53/124 | 4.72E‐14 | 5.123 | RB1, CDKN1A, CDKN1B, MCM7, YWHAB, BUB1B, CDC20, CCND1, YWHAQ, CHEK1 |
| Pancreatic cancer | 31/76 | 1.82E‐07 | 4.696 | RB1, CDKN1A, PIK3R3, PIK3R2, BRCA2, PLD1, CASP9, MAPK9, MAPK8, BCL2L11 |
| Colorectal cancer | 33/86 | 2.33E‐07 | 4.246 | CDKN1A, LEF1, PIK3R3, PIK3R2, EGFR, CASP9, MAPK9, MAPK8, BCL2L11, CCND1 |
CVD indicates cardiovascular disease; FHS, Framingham Heart Study; GO, gene ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; and miRNAs, microRNAs.
Overlap represents # mRNA that are targets÷total # mRNAs in the pathway.
Adjusted for number of GO terms/KEGG pathways.
The first 10 listed genes that are both annotated to the respective term and are associated with cardiovascular disease. 64
Additionally, each biological process and cellular pathway was annotated to numerous genes, many of which were associated with CVD. 64 To this, there were 51 genes annotated to the top enriched GO term, DNA replication. Of these genes, 49 are implicated in CVD. 64 There were 53 genes annotated to the top enriched KEGG term, cell cycle, and all but 1 of these genes are also implicated in CVD. 64
There were a combined 2741 miRNA‐target interactions generated by miRTarBase for the 6 identified miRNAs associated with 5 or more CVD risk factors. These include 892 gene targets for miR‐193b‐3p (Data S5a), 536 gene targets for miR‐122‐5p (Data S5b), 129 for miR‐365a‐3p (Data S5c), 101 for miR‐194‐5p (Data S5d), 1036 for miR‐192‐5p (Data S5e), and 47 for miR‐193a‐5p (Data S5f). Many of the genes that are both annotated to 1 or more of the top 5 enriched GO biological processes or KEGG cellular pathways and associated with CVD were also found to be targets of the identified miRNAs.
Replication in the RS
The clinical characteristics of RS participants (n=1999, mean age: 72±8 years) are presented in Table 6. Among eligible participants, 1141 (57%) were women, 253 (13%) met criteria for type 2 diabetes, 491 (21%) met criteria for obesity, 1555 (78%) had hypertension, 591 (30%) were never smokers, 1095 (56%) were former smokers, and 285 (15%) were current smokers. There were 300 (15%) participants with new‐onset CVD and 1154 (58%) participants died during a mean follow‐up of 13 years. Full baseline characteristics and follow‐up data for RS participants are given in Data S6a and S6b, respectively.
Table 6.
Clinical Characteristics of the RS Study Sample
| Clinical characteristic | Mean±SD |
|---|---|
| Age, y | 72 ±8 |
| Systolic blood pressure, mm Hg | 148 ±21 |
| Diastolic blood pressure, mm Hg | 79 ±11 |
| Total cholesterol, mmol/L | 5.62 (1.27)* |
| High‐density lipoprotein cholesterol, mmol/L | 1.45 ±0.40 |
| Fasting blood glucose, mmol/L | 5.50 (0.90)* |
| Body mass index, kg/m2 | 27.64 ±4.09 |
| Dichotomous outcome | Number of participants (%) |
|---|---|
| Never smoker | 591 (30.0) |
| Former smoker | 1095 (55.6) |
| Current smoker | 285 (14.5) |
| Incident diabetes | 254 (13.3) |
| Incident hypertension | 1555 (77.8) |
| Incident obesity | 491 (21.0) |
| Incident cardiovascular disease | 300 (15.0) |
| All‐cause mortality | 1154 (57.7) |
RS indicates Rotterdam Study.
Reported values are median and interquartile range.
For each of the CVD risk factors of interest, the miRNAs identified as significantly associated in the FHS (Data S2c through S2l) were tested for replication in the RS. Following Bonferroni correction and comparison of estimate signs in the FHS and the RS, 1 miRNA (miR‐193b‐3p) replicated for SBP (P<0.00357) and DBP (P<0.05). Moreover, 43 miRNAs replicated for total cholesterol (P<0.00038), 5 miRNAs replicated for fasting blood glucose (P<0.00556), 15 miRNAs replicated for BMI (P<0.001), and 10 miRNAs replicated for current cigarette smoking (P<0.00156). The associations of the top miRNAs with each CVD risk factor following replication in the RS are presented in Table 7. The full replication results comparing cross‐sectional associations between miRNAs and CVD risk factors in the FHS and the RS are provided in Data S6c through S6i.
Table 7.
Replication in the RS of the Top miRNAs Associated With Each CVD Risk Factor in the FHS
| CVD risk factor | miRNA | No. full | Effect size | SE | P value |
|---|---|---|---|---|---|
| Systolic blood pressure | miR‐365a‐3p* | 1991 | 0.631 | 0.387 | 0.103 |
| miR‐193b‐3p | 1991 | 3.498 | 0.940 | 0.000204 | |
| miR‐20b‐5p* | 1991 | 2.404 | 1.233 | 0.0513 | |
| miR‐16‐5p* | 1991 | 0.415 | 1.095 | 0.705 | |
| miR‐25‐3p* | 1991 | 0.156 | 1.048 | 0.882 | |
| Diastolic blood pressure | miR‐193b‐3p | 1991 | 2.109 | 0.491 | 1.86E‐05 |
| Total cholesterol | miR‐126‐3p | 1999 | 0.0674 | 0.00921 | 3.80E‐13 |
| miR‐191‐5p* | 1999 | 0.0171 | 0.00856 | 0.0454 | |
| miR‐425‐3p* | 1999 | 0.00474 | 0.00615 | 0.441 | |
| miR‐425‐5p* | 1999 | 0.00503 | 0.00829 | 0.544 | |
| miR‐625‐3p* | 1999 | 0.0115 | 0.00853 | 0.178 | |
| Fasting blood glucose | miR‐192‐5p* | 1999 | 0.0166 | 0.00712 | 0.0200 |
| miR‐193a‐5p | 1999 | 0.0704 | 0.00883 | 2.59E‐15 | |
| miR‐193b‐3p | 1999 | 0.0405 | 0.00864 | 3.03E‐06 | |
| miR‐122‐5p | 1999 | 0.00192 | 0.00483 | 7.26E‐05 | |
| miR‐365a‐3p* | 1999 | 0.00480 | 0.00356 | 0.178 | |
| Body mass index | miR‐122‐5p* | 1958 | 0.246 | 0.108 | 0.0230 |
| miR‐193b‐3p* | 1958 | 0.133 | 0.194 | 0.492 | |
| miR‐193a‐5p | 1958 | 1.626 | 0.195 | 1.59E‐16 | |
| miR‐365a‐3p* | 1958 | 0.0181 | 0.0789 | 0.818 | |
| miR‐101‐3p* | 1958 | −0.349 | 0.226 | 0.123 | |
| Current cigarette smoking | miR‐150‐5p | 1971 | 0.178 | 0.0344 | 2.31E‐07 |
| miR‐152‐3p* | 1971 | 0.0937 | 0.0375 | 0.0126 | |
| miR‐125a‐5p | 1971 | 0.0951 | 0.0245 | 0.000111 | |
| miR‐34a‐5p | 1971 | 0.134 | 0.0424 | 0.00155 | |
| miR‐584‐5p* | 1971 | −0.0372 | 0.0776 | 0.631 |
Associations after adjustment for age, sex, subcohort, smoking status, and lipid levelsCVD indicates cardiovascular disease; FHS, Framingham Heart Study; miRNAs, microRNAs; and RS, Rotterdam Study.
Association does not replicate in the RS.
In the RS, miR‐193b‐3p was significantly associated with 3 CVD risk factors (SBP, DBP, and fasting blood glucose). In addition, 13 miRNAs were significantly associated with 2 CVD risk factors in the RS, including miR‐194‐5p and miR‐193a‐5p. The miRNAs associated with 2 or more CVD risk factors in the RS are displayed in Table 8.
Table 8.
miRNAs Associated With ≥2 CVD Risk Factors Following Replication in the RS
| miRNA | Risk factors |
|---|---|
| miR‐193b‐3p | Systolic blood pressure, diastolic blood pressure, fasting blood glucose |
| miR‐194‐5p | Total cholesterol, fasting blood glucose |
| miR‐99b‐5p | Total cholesterol, fasting blood glucose |
| miR‐193a‐5p | Fasting blood glucose, BMI |
| miR‐16‐5p | Total cholesterol, BMI |
| miR‐17‐5p | Total cholesterol, BMI |
| miR‐29b‐3p | Total cholesterol, current smoking |
| miR‐150‐5p | Total cholesterol, current smoking |
| miR‐29a‐3p | Total cholesterol, current smoking |
| miR‐181b‐5p | Total cholesterol, current smoking |
| miR‐342‐3p | Total cholesterol, current smoking |
| miR‐125a‐5p | Total cholesterol, current smoking |
| miR‐29c‐3p | Total cholesterol, current smoking |
| miR‐155‐5p | Total cholesterol, current smoking |
Associations after adjustment for age, sex, subcohort, smoking status, and lipid levels. BMI indicates body mass index; CVD, cardiovascular disease; miRNAs, microRNAs; and RS, Rotterdam Study.
There were no significant associations of miRNAs with new‐onset type 2 diabetes, hypertension, obesity, or composite CVD outcome in the FHS; therefore, these outcomes were not considered for replication in the RS. Of the 8 miRNAs associated with all‐cause mortality, none of them replicated at the Bonferroni P value threshold (0.00625) in the RS (Data S6j).
DISCUSSION
The principal aim of our study was to explore associations of plasma miRNAs with CVD and its risk factors in a middle‐age sample of adults. Although previous studies have reported associations of the identified miRNAs with impaired glucose metabolism, lipid metabolism, and cardiac dysfunction, the plasma miRNA signatures of CVD risk factors have not been fully elucidated at a population level in middle‐age or older individuals. 17 , 51 , 63 , 65 , 66 , 67 To that end, we elucidated miRNA signatures of CVD risk factors and replicated a subset of these miRNAs in an independent population‐based cohort study. We identified 6 miRNAs (miR‐193b‐3p, miR‐122‐5p, miR‐365a‐3p, miR‐194‐5p, miR‐192‐5p, and miR‐193a‐5p) that were associated with 5 or more CVD risk factors in the FHS. This miRNA signature targets mRNAs that are enriched for several biological processes and cellular pathways associated with CVD and its risk factors. Among the enriched cellular pathways were the AMPK, p53, FoxO, and ErbB signaling pathways (Data 4b). Each of these pathways has been identified as an important signaling pathway related to the pathogenesis of CVD. 68 , 69 , 70 , 71 These findings highlight miRNA biomarkers of CVD and may provide insights into promising targets for its treatment and prevention.
Our results highlight the association of miR‐193b‐3p with 6 CVD risk factors (SBP, DBP, triglycerides, total cholesterol, fasting blood glucose, and BMI) and all‐cause mortality in the FHS. Further, miR‐193b‐3p was associated with 3 CVD risk factors (SBP, DBP, and fasting blood glucose) in the RS. Previous studies reported that higher abundance of miR‐193b‐3p was associated with prediabetes and type 2 diabetes, which are characterized higher glucose levels. 17 , 22 Hu et al. further found that miR‐193b‐3p may affect glucose metabolism by directly targeting YWHAZ/14‐3‐3ζ, and upregulating the expression of transcription factors in the FoxO signaling pathway. 17
Similarly, we found miR‐122‐5p to be positively associated with 6 CVD risk factors (SBP, triglycerides, total cholesterol, fasting blood glucose, BMI, and current cigarette smoking) and with all‐cause mortality in the FHS. In the RS, miR‐122‐5p was significantly associated with fasting blood glucose. Previous studies have also reported associations between circulating miR‐122‐5p levels and acute myocardial infarction, CHD, fatty liver disease, and cardiometabolic disorders. 63 , 65 , 72 , 73 , 74 , 75 , 76 , 77 Further, circulating levels of miR‐122‐5p have also been reported to be implicated in diabetic kidney disease and diabetic cardiomyopathy. 75 , 76 Consistent with this, miR‐122 has been identified as an independent risk factor for insulin resistance and to be involved in cholesterol and lipid regulation. 77 , 78 , 79 , 80
In the FHS, we also found miR‐365a‐3p to be significantly associated with 5 CVD risk factors, including SBP, triglycerides, total cholesterol, fasting blood glucose, and BMI. A study by Satake et al. reported that expression of circulating plasma miR‐365a‐3p is correlated with hyperglycemia in type 1 diabetes. 66 Circulating miR‐365a‐3p levels were also reported to be positively associated with hemoglobin A1C, albumin‐to‐creatinine ratio, and SBP, which serve as markers of CVD risk in individuals with or without in type 1 diabetes. 66 , 81 , 82
Next, we found miR‐194‐5p to be associated with triglycerides, total cholesterol, fasting blood glucose, BMI, and smoking status in the FHS and with total cholesterol and fasting blood glucose in the RS. Previously, Demirsoy et al. reported that circulating miR‐194‐5p levels were downregulated following metformin treatment in patients with type 2 diabetes. 83 Metformin, a widely used biguanide class of antidiabetic drug, reduces blood glucose through inhibiting glucose production in the liver. Thus, circulating miR‐194‐5p appears to be more expressed in plasma in patients with type 2 diabetes with high blood glucose compared with patients receiving treatment that lowers blood glucose. 83
Our results also showed the association of miR‐192‐5p with 5 CVD risk factors (triglycerides, total cholesterol, fasting blood glucose, BMI, and smoking status) and all‐cause mortality in the FHS. Previous studies reported that circulating levels of miR‐192‐5p significantly increase in subjects with prediabetes compared with subjects with normal glucose. 22 , 84 Jaeger et al. further reported that circulating levels of miR‐192 and miR‐194 are elevated in subjects with prevalent in type 1 diabetes compared with healthy subjects. 85
In addition to diabetes, both miR‐194 and miR‐192 have been implicated in cardiac dysfunction. 67 , 86 , 87 , 88 Matsumoto et al. revealed that miR‐192, miR‐194, and miR‐34a levels were significantly upregulated in subjects who developed ischemic heart failure within 1 year of acute myocardial infarction onset. 86 Moreover, the expression of miR‐194 and miR‐34a were correlated with miR‐192, suggesting that the 3 miRNAs were coordinately upregulated in a single cascade, such as the p53 signaling pathway. After p53 activation, both intracellular and extracellular levels of miR‐192, miR‐194, and miR‐34a were elevated. Therefore, these p53‐responsive miRNAs, including miR‐192 and miR‐194, are likely to be involved in the pathogenesis of heart failure after acute myocardial infarction. 86
We also found that miR‐193a‐5p was significantly associated with triglycerides, total cholesterol, fasting blood glucose, BMI, and current cigarette smoking in the FHS and with fasting blood glucose and BMI in the RS. Previous studies have identified miR‐193a‐5p to be positively correlated to BMI and to be upregulated in patients with obesity, diabetes, and nonalcoholic fatty liver disease. 89 , 90 , 91 Additionally, serum levels of miR‐193a‐5p have been shown to decrease in obese subjects following diet‐induced weight loss. 92 , 93 As miR‐193a‐5p is associated with insulin resistance parameters, the reduction of miR‐193a‐5p levels may serve to improve glucose metabolism. 94
Associations of miRNAs with Cholesterol
In mammalian cells, cholesterol modulates cell membrane fluidity and signaling and serves as a chemical precursor of steroid hormones and vitamin D. These are carried in the blood and delivered to cells by lipoproteins. 95 , 96 , 97 Due to its critical function in cells, cholesterol is closely regulated through various mechanisms, including posttranscriptional regulation by miRNAs. 97 We found that most candidate miRNAs were associated with total cholesterol levels, suggesting that statistical associations between miRNAs and total cholesterol may be influenced by lipid chaperoning; however, this finding warrants further investigation into the potential role of miRNAs in regulating cholesterol homeostasis.
Previous studies have shown several plasma miRNAs associated with apolipoprotein A1, a major component of HDL, likely due to the binding and chaperoning of miRNAs by lipoproteins for their delivery and transport to the extracellular matrix. A study by Vickers et al. demonstrated that HDL not only transports miRNAs but these miRNAs mediate some of the biological effects of the lipoproteins. 98 They also found that miRNA delivery to hepatocytes by HDL depends on SR‐B1 (scavenger receptor, class B type 1), a membrane receptor that mediates the uptake of HDL. 98 Vickers et al. also reported that delivery of HDL‐carried miRNAs increased the activity of atherosclerosis‐altered genes (including NDST1, BMPR2, and FLT1), which play a role in lipid metabolism and inflammation. 98 , 99
In contrast, we found no associations between circulating miRNAs with serum HDL cholesterol levels in the FHS. This finding may be due to our adjustment for triglycerides and total cholesterol while analyzing the association of miRNAs with HDL. It is also possible that adjusting for HDL and triglycerides when analyzing the cholesterol‐miRNA associations may not have fully accounted for residual confounding.
The most strongly associated miRNA with total cholesterol in the FHS, miR‐126‐3p, has previously been reported to be positively associated with low‐density lipoprotein cholesterol, very‐low‐density lipoprotein cholesterol, and triglycerides. 100 , 101 Notably, miR‐126‐3p is abundant in endothelial cells and plays a role in maintaining endothelial homeostasis and vascular integrity. 102 Endothelial dysfunction precedes CVD and is often marked by albuminuria, a condition in which there is an increase in urinary albumin excretion. 103 Martinez‐Arroyo et al. found that plasma levels of miR‐126‐3p were higher in patients with hypertension with albuminuria compared with patients with hypertension without albuminuria. 103 The expression of plasma miR‐126‐3p is also positively correlated with presence of urinary albumin excretion in a general population. Furthermore, higher plasma miR‐126‐3p levels were also associated with a higher incidence rate of CVD events, regardless of presence of albuminuria. 103
Associations of miRNAs with Smoking Status
Cigarette smoking poses a significant risk factor for many cardiovascular diseases, including lung cancer, obstructive pulmonary disease, and asthma. 104 Takahashi et al. found that differences in the plasma miRNA profiles between smokers and nonsmokers can be attributed to repeated cigarette smoking. 48 A larger number of miRNAs were detected in smokers than in nonsmokers; furthermore, two thirds of these plasma miRNAs had higher expression levels in smokers than nonsmokers. 48 Importantly, the plasma miRNA profiles of former smokers were altered after smoking cessation, and the profiles largely resembled those of nonsmokers. 21 , 48
We conducted association analyses using never smoker as reference to determine the associations between miRNAs and smoking status. Following replication analyses in the RS, 10 miRNAs were identified as significantly associated with smoking status, including miR‐150‐5p and miR‐29a‐3p. Notably, Dorna et al. found that plasma levels of circulating miR‐150‐5p were higher following tobacco intake via cigarette smoking. 105 Bioinformatic analysis was used to identify oxidative stress, and the ErbB signaling pathway was found to be among the enriched pathways regulated by genes targeted by miRNAs. This pathway has previously been implicated in numerous smoking‐related cardiovascular diseases due. 71 , 106
Silverman et al. identified miR‐150‐5p and miR‐29a‐3p to be significantly associated with elevated risk of sudden cardiac or arrhythmic death in patients with CHD, a disease in which tobacco use and cigarette smoking are risk factors. 107 Additionally, bioinformatic analysis identified pathways related to fibrosis, inflammation, and apoptosis/cell apoptosis to be significantly enriched. 107
miRNAs and New‐Onset of CVD and Mortality
We conducted prospective outcomes analyses using proportional hazards regression to determine the associations between each miRNAs and incident type 2 diabetes, obesity, hypertension, CVD, and all‐cause mortality. Although we observed no significant associations between miRNAs and new‐onset type 2 diabetes, obesity, hypertension, or CVD in the FHS, 8 miRNAs were found to be significantly associated with all‐cause mortality. Of these miRNAs associated with all‐cause mortality, 4 (miR‐193a‐5p, miR‐192‐5p, 122‐5p, and miR‐193b‐3p) were found to be associated with 5 or more CVD risk factors in the FHS. Although previous studies have found miRNAs to be significantly associated with CVD outcomes, including acute coronary syndrome and myocardial infarction, these findings may differ from the results of our study due to differences in study populations. 33
There were 4 miRNAs (miR‐320e, miR‐34a‐5p, miR‐210‐5p, and miR‐301a‐3p) associated with all‐cause mortality that were not robustly associated with CVD risk factors in the FHS. Each of these miRNAs have also been reported to be positively associated with CVD in the literature. 108 , 109 , 110 , 111 A study by Ali et al. found that miR‐320e was predicted to target all 7 members of the 14‐3‐3 gene family comprising YWHAZ, YWHAQ, YWHAH, YWAHE, YWHAB, YWHAG, and SFN. 108 In addition, they found multiple protein–protein interactions connecting all 7 members of the 14‐3‐3 family. 108 Furthermore, the miR‐34 family, which includes miR‐34a‐5p, has been implicated in the regulation of myocardial physiology and pathophysiological processes. 109 Notably, miR‐34a has been reported to be an essential regulator in cardiovascular fibrosis, dysfunction, and related cardiovascular diseases. 109 Furthermore, SIRT1, NOTCH1, SMAD4, PNUTS, FOXO3, FOXM1, and PTEN have been verified as miR‐34a‐5p target genes. 109 Taken together, miR‐34a‐5p can be considered to play a role in controlling cellular apoptosis, autophagy, inflammation, aging, fibrosis, and remodeling through these gene targets. 109 , 112
Limitations
This study has several strengths, in which the main strengths is using a large sample size of 4440 participants in the discovery cohort with available plasma extracellular RNA data in the FHS and successful external replication of cross‐sectional associations of extracellular RNAs with CVD risk factors in 1999 participants in the RS. Also, the availability of various clinical outcomes related to CVD with long‐time follow‐up in these 2 cohort studies provided us the opportunity to conduct a prospective analysis with miRNAs, as well. Yet, there exist some limitations of this study that need to be addressed. The method of RNA isolation and measurement used in the replication study differ from those used in discovery analyses. Further, differences in the mean ages and sizes of the 2 study populations may have contributed to discrepancies between results in the FHS and the RS. These differences may have resulted in inconsistencies across results in the FHS and the RS, such that miRNAs significantly associated to CVD risk factor(s) in the FHS were not successfully replicated in the RS. In addition, the pathway enrichment analysis conducted in this study employs a hypergeometric approach, which does not account for natural intrapathway protein correlations and, therefore, may produce false positives. Moreover, given that the miRNAs were isolated from plasma rather than from tissues or cells, pathway enrichment analysis is unable to provide evidence of miRNA communication with specific tissues or cell types. To further validate the relations of miRNAs to CVD risk factors, replication in a study population of similar age and functional validation of the identified miRNAs and their targets are warranted. In addition, the study population consists largely of participants with European ancestry, limiting the generalizability of the results. Therefore, future studies that include cohorts with greater racial and ethnic diversity are still needed.
Conclusions
This population‐based study reveals several individual miRNAs that were significantly associated with CVD risk factors. Moreover, our findings encourage further investigation into the molecular targets of miRNAs and the mechanistic pathways contributing to CVD. Extending miRNA knowledge to young and middle‐aged adults may prove central to better understanding their roles in promoting CVD risk in an age group in which risk factors are emerging and transitioning to overt clinical events. This effort may stimulate additional research into the utility of miRNAs as clinically useful diagnostic, prognostic, and even therapeutic targets.
Sources of Funding
Framingham Heart Study participant exams were funded by National Heart, Lung, and Blood Institute contract 75N92019D00031.
Disclosures
All disclosures are correct. There are no further disclosures to report.
Supporting information
Data S1
Table S1
This article was sent to Julie K. Freed, MD, PhD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.033674
For Sources of Funding and Disclosures, see page 13.
References
- 1. Jones Buie JN, Goodwin AJ, Cook JA, Halushka PV, Fan H. The role of miRNAs in cardiovascular disease risk factors. Atherosclerosis. 2016;254:271–281. doi: 10.1016/j.atherosclerosis.2016.09.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. O'Brien K, Breyne K, Ughetto S, Laurent LC, Breakefield XO. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat Rev Mol Cell Biol. 2020;21:585–606. doi: 10.1038/s41580-020-0251-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Condrat CE, Thompson DC, Barbu MG, Bugnar OL, Boboc A, Cretoiu D, Suciu N, Cretoiu SM, Voinea SC. miRNAs as biomarkers in disease: latest findings regarding their role in diagnosis and prognosis. Cells. 2020;9:9. doi: 10.3390/cells9020276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lu K‐C, Zhang Y, Song E. Extracellular RNA: mechanisms of its transporting into target cells. ExRNA. 2019;1:22. doi: 10.1186/s41544-019-0020-2 [DOI] [Google Scholar]
- 5. Zhao C, Sun X, Li L. Biogenesis and function of extracellular miRNAs. ExRNA. 2019;1:38. doi: 10.1186/s41544-019-0039-4 [DOI] [Google Scholar]
- 6. Kim S, Jeon OH, Jeon Y‐J. Extracellular RNA: emerging roles in cancer cell communication and biomarkers. Cancer Lett. 2020;495:33–40. doi: 10.1016/j.canlet.2020.09.002 [DOI] [PubMed] [Google Scholar]
- 7. Sohel MH. Extracellular/circulating MicroRNAs: release mechanisms, functions and challenges. Achiev Life Sci. 2016;10:175–186. doi: 10.1016/j.als.2016.11.007 [DOI] [Google Scholar]
- 8. Crescitelli R, Lässer C, Szabó TG, Kittel A, Eldh M, Dianzani I, Buzás EI, Lötvall J. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles. 2013;2:20677–20686. doi: 10.3402/jev.v2i0.20677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lasser C, Shelke GV, Yeri A, Kim DK, Crescitelli R, Raimondo S, Sjostrand M, Gho YS, Van Keuren Jensen K, Lotvall J. Two distinct extracellular RNA signatures released by a single cell type identified by microarray and next‐generation sequencing. RNA Biol. 2017;14:58–72. doi: 10.1080/15476286.2016.1249092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Freedman JE, Gerstein M, Mick E, Rozowsky J, Levy D, Kitchen R, Das S, Shah R, Danielson K, Beaulieu L, et al. Diverse human extracellular RNAs are widely detected in human plasma. Nat Commun. 2016;7:11106. doi: 10.1038/ncomms11106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang J, Chen J, Sen S. MicroRNA as biomarkers and diagnostics. J Cell Physiol. 2016;231:25–30. doi: 10.1002/jcp.25056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Etheridge A, Lee I, Hood L, Galas D, Wang K. Extracellular microRNA: a new source of biomarkers. Mutat Res. 2011;717:85–90. doi: 10.1016/j.mrfmmm.2011.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O'Brien J, Hayder H, Zayed Y, Peng C. Overview of MicroRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne). 2018;9:402. doi: 10.3389/fendo.2018.00402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Finn NA, Searles CD. Intracellular and extracellular miRNAs in regulation of angiogenesis signaling. Curr Angiogenes. 2012;4:299–307. doi: 10.2174/2211552811201040299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell‐to‐cell communication. Kidney Int. 2010;78:838–848. doi: 10.1038/ki.2010.278 [DOI] [PubMed] [Google Scholar]
- 16. Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282 [DOI] [PubMed] [Google Scholar]
- 17. Hu H, Zhao M, Li Z, Nie H, He J, Chen Z, Yuan J, Guo H, Zhang X, Yang H, et al. Plasma miR‐193b‐3p is elevated in type 2 diabetes and could impair glucose metabolism. Front Endocrinol (Lausanne). 2022;13:814347. doi: 10.3389/fendo.2022.814347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Quinn JF, Patel T, Wong D, Das S, Freedman JE, Laurent LC, Carter BS, Hochberg F, Van Keuren‐Jensen K, Huentelman M, et al. Extracellular RNAs: development as biomarkers of human disease. J Extracell Vesicles. 2015;4:27495. doi: 10.3402/jev.v4.27495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mori MA, Ludwig RG, Garcia‐Martin R, Brandão BB, Kahn CR. Extracellular miRNAs: from biomarkers to mediators of physiology and disease. Cell Metab. 2019;30:656–673. doi: 10.1016/j.cmet.2019.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karabegović I, Abozaid Y, Maas SCE, Labrecque J, Bos D, De Knegt RJ, Ikram MA, Voortman T, Ghanbari M. Plasma MicroRNA signature of alcohol consumption: the Rotterdam study. J Nutr. 2022;152:2677–2688. doi: 10.1093/jn/nxac216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karabegovic I, Maas SCE, Shuai Y, Ikram MA, Stricker B, Aerts J, Brusselle G, Lahousse L, Voortman T, Ghanbari M. Smoking‐related dysregulation of plasma circulating microRNAs: the Rotterdam study. Hum Genomics. 2023;17:61. doi: 10.1186/s40246-023-00504-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parrizas M, Brugnara L, Esteban Y, Gonzalez‐Franquesa A, Canivell S, Murillo S, Gordillo‐Bastidas E, Cusso R, Cadefau JA, Garcia‐Roves PM, et al. Circulating miR‐192 and miR‐193b are markers of prediabetes and are modulated by an exercise intervention. J Clin Endocrinol Metab. 2015;100:E407–E415. doi: 10.1210/jc.2014-2574 [DOI] [PubMed] [Google Scholar]
- 23. Hernandez‐Alonso P, Giardina S, Salas‐Salvado J, Arcelin P, Bullo M. Chronic pistachio intake modulates circulating microRNAs related to glucose metabolism and insulin resistance in prediabetic subjects. Eur J Nutr. 2017;56:2181–2191. doi: 10.1007/s00394-016-1262-5 [DOI] [PubMed] [Google Scholar]
- 24. Quintanilha BJ, Pinto Ferreira LR, Ferreira FM, Neto EC, Sampaio GR, Rogero MM. Circulating plasma microRNAs dysregulation and metabolic endotoxemia induced by a high‐fat high‐saturated diet. Clin Nutr. 2020;39:554–562. doi: 10.1016/j.clnu.2019.02.042 [DOI] [PubMed] [Google Scholar]
- 25. Assmann TS, Riezu‐Boj JI, Milagro FI, Martinez JA. Circulating adiposity‐related microRNAs as predictors of the response to a low‐fat diet in subjects with obesity. J Cell Mol Med. 2020;24:2956–2967. doi: 10.1111/jcmm.14920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li F, Bai M, Xu J, Zhu L, Liu C, Duan R. Long‐term exercise alters the profiles of circulating micro‐RNAs in the plasma of Young women. Front Physiol. 2020;11:372. doi: 10.3389/fphys.2020.00372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barber JL, Zellars KN, Barringhaus KG, Bouchard C, Spinale FG, Sarzynski MA. The effects of regular exercise on circulating cardiovascular‐related MicroRNAs. Sci Rep. 2019;9:7527. doi: 10.1038/s41598-019-43978-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lew JK, Pearson JT, Saw E, Tsuchimochi H, Wei M, Ghosh N, Du CK, Zhan DY, Jin M, Umetani K, et al. Exercise regulates MicroRNAs to preserve coronary and cardiac function in the diabetic heart. Circ Res. 2020;127:1384–1400. doi: 10.1161/CIRCRESAHA.120.317604 [DOI] [PubMed] [Google Scholar]
- 29. Panico A, Tumolo MR, Leo CG, Donno A, Grassi T, Bagordo F, Serio F, Idolo A, Masi R, Mincarone P, et al. The influence of lifestyle factors on miRNA expression and signal pathways: a review. Epigenomics. 2021;13:145–164. doi: 10.2217/epi-2020-0289 [DOI] [PubMed] [Google Scholar]
- 30. Lawrie CH, Soneji S, Marafioti T, Cooper CD, Palazzo S, Paterson JC, Cattan H, Enver T, Mager R, Boultwood J, et al. MicroRNA expression distinguishes between germinal center B cell‐like and activated B cell‐like subtypes of diffuse large B cell lymphoma. Int J Cancer. 2007;121:1156–1161. doi: 10.1002/ijc.22800 [DOI] [PubMed] [Google Scholar]
- 31. Cakmak HA, Demir M. MicroRNA and cardiovascular diseases. Balkan Med J. 2020;37:60–71. doi: 10.4274/balkanmedj.galenos.2020.2020.1.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peters LJF, Biessen EAL, Hohl M, Weber C, van der Vorst EPC, Santovito D. Small things matter: relevance of MicroRNAs in cardiovascular disease. Front Physiol. 2020;11:793. doi: 10.3389/fphys.2020.00793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou S‐s, Jin J‐P, Wang J‐q, Zhang Z‐g, Freedman JH, Zheng Y, Cai L. miRNAS in cardiovascular diseases: potential biomarkers, therapeutic targets and challenges. Acta Pharmacol Sin. 2018;39:1073–1084. doi: 10.1038/aps.2018.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang R, Li N, Zhang Y, Ran Y, Pu J. Circulating microRNAs are promising novel biomarkers of acute myocardial infarction. Intern Med. 2011;50:1789–1795. doi: 10.2169/internalmedicine.50.5129 [DOI] [PubMed] [Google Scholar]
- 35. De Rosa S, Eposito F, Carella C, Strangio A, Ammirati G, Sabatino J, Abbate FG, Iaconetti C, Liguori V, Pergola V, et al. Transcoronary concentration gradients of circulating microRNAs in heart failure. Eur J Heart Fail. 2018;20:1000–1010. doi: 10.1002/ejhf.1119 [DOI] [PubMed] [Google Scholar]
- 36. Shah RV, Rong J, Larson MG, Yeri A, Ziegler O, Tanriverdi K, Murthy V, Liu X, Xiao C, Pico AR, et al. Associations of circulating extracellular RNAs with myocardial remodeling and heart failure. JAMA Cardiol. 2018;3:871–876. doi: 10.1001/jamacardio.2018.2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tran KV, Tanriverdi K, Aurigemma GP, Lessard D, Sardana M, Parker M, Shaikh A, Gottbrecht M, Milstone Z, Tanriverdi S, et al. Circulating extracellular RNAs, myocardial remodeling, and heart failure in patients with acute coronary syndrome. J Clin Transl Res. 2019;5:33–43. [PMC free article] [PubMed] [Google Scholar]
- 38. Aldous EK, Toor SM, Parray A, Al‐Sarraj Y, Diboun I, Abdelalim EM, Arredouani A, El‐Agnaf O, Thornalley PJ, Akhtar N, et al. Identification of novel circulating miRNAs in patients with acute ischemic stroke. Int J Mol Sci. 2022;23:3387. doi: 10.3390/ijms23063387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mens MMJ, Heshmatollah A, Fani L, Ikram MA, Ikram MK, Ghanbari M. Circulatory MicroRNAs as potential biomarkers for stroke risk. Stroke. 2021;52:945–953. doi: 10.1161/STROKEAHA.120.031543 [DOI] [PubMed] [Google Scholar]
- 40. Mick E, Shah R, Tanriverdi K, Murthy V, Gerstein M, Rozowsky J, Kitchen R, Larson MG, Levy D, Freedman JE. Stroke and circulating extracellular RNAs. Stroke. 2017;48:828–834. doi: 10.1161/strokeaha.116.015140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McManus DD, Tanriverdi K, Lin H, Esa N, Kinno M, Mandapati D, Tam S, Okike ON, Ellinor PT, Keaney JF Jr, et al. Plasma microRNAs are associated with atrial fibrillation and change after catheter ablation (the miRhythm study). Heart Rhythm. 2015;12:3–10. doi: 10.1016/j.hrthm.2014.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vaze A, Tran K‐V, Tanriverdi K, Sardana M, Lessard D, Donahue JK, Barton B, Aurigemma G, Lubitz SA, Lin H, et al. Relations between plasma microRNAs, echocardiographic markers of atrial remodeling, and atrial fibrillation: data from the Framingham offspring study. PLoS One. 2020;15:e0236960. doi: 10.1371/journal.pone.0236960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Geurts S, Mens MMJ, Bos MM, Ikram MA, Ghanbari M, Kavousi M. Circulatory MicroRNAs in plasma and atrial fibrillation in the general population: the Rotterdam study. Genes (Basel). 2021;13:13. doi: 10.3390/genes13010011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Roxe T, Muller‐Ardogan M, et al. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107:677–684. doi: 10.1161/CIRCRESAHA.109.215566 [DOI] [PubMed] [Google Scholar]
- 45. Freedman JE, Ercan B, Morin KM, Liu CT, Tamer L, Ayaz L, Kanadasi M, Cicek D, Seyhan AI, Akilli RE, et al. The distribution of circulating microRNA and their relation to coronary disease. F1000Res. 2012;1:50. doi: 10.12688/f1000research.1-50.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hinkel R, Ng JK, Kupatt C. Targeting microRNAs for cardiovascular therapeutics in coronary artery disease. Curr Opin Cardiol. 2014;29:586–594. doi: 10.1097/HCO.0000000000000107 [DOI] [PubMed] [Google Scholar]
- 47. Abozaid YJ, Zhang X, Mens MMJ, Ahmadizar F, Limpens M, Ikram MA, Rivadeneira F, Voortman T, Kavousi M, Ghanbari M. Plasma circulating microRNAs associated with obesity, body fat distribution, and fat mass: the Rotterdam study. Int J Obes. 2022;46:2137–2144. doi: 10.1038/s41366-022-01227-8 [DOI] [PubMed] [Google Scholar]
- 48. Takahashi K, Yokota S, Tatsumi N, Fukami T, Yokoi T, Nakajima M. Cigarette smoking substantially alters plasma microRNA profiles in healthy subjects. Toxicol Appl Pharmacol. 2013;272:154–160. doi: 10.1016/j.taap.2013.05.018 [DOI] [PubMed] [Google Scholar]
- 49. Shah R, Murthy V, Pacold M, Danielson K, Tanriverdi K, Larson MG, Hanspers K, Pico A, Mick E, Reis J, et al. Extracellular RNAs are associated with insulin resistance and metabolic phenotypes. Diabetes Care. 2017;40:546–553. doi: 10.2337/dc16-1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McManus DD, Rong J, Huan T, Lacey S, Tanriverdi K, Munson PJ, Larson MG, Joehanes R, Murthy V, Shah R, et al. Messenger RNA and MicroRNA transcriptomic signatures of cardiometabolic risk factors. BMC Genomics. 2017;18:139. doi: 10.1186/s12864-017-3533-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Streese L, Demougin P, Iborra P, Kanitz A, Deiseroth A, Kropfl JM, Schmidt‐Trucksass A, Zavolan M, Hanssen H. Untargeted sequencing of circulating microRNAs in a healthy and diseased older population. Sci Rep. 2022;12:2991. doi: 10.1038/s41598-022-06956-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mens MMJ, Maas SCE, Klap J, Weverling GJ, Klatser P, Brakenhoff JPJ, van Meurs JBJ, Uitterlinden AG, Ikram MA, Kavousi M, et al. Multi‐omics analysis reveals MicroRNAs associated with cardiometabolic traits. Front Genet. 2020;11:110. doi: 10.3389/fgene.2020.00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Oppenheimer GM. Framingham heart study: the first 20 years. Prog Cardiovasc Dis. 2010;53:55–61. doi: 10.1016/j.pcad.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 54. Mahmood SS, Levy D, Vasan RS, Wang TJ. The Framingham heart study and the epidemiology of cardiovascular disease: a historical perspective. Lancet. 2014;383:999–1008. doi: 10.1016/S0140-6736(13)61752-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dawber TR, Meadors GF, Moore FE Jr. Epidemiological approaches to heart disease: the Framingham study. Am J Public Health Nations Health. 1951;41:279–281. doi: 10.2105/ajph.41.3.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Roest HP, IJzermans JN, van der Laan LJ. Evaluation of RNA isolation methods for microRNA quantification in a range of clinical biofluids. BMC Biotechnol. 2021;21:48. doi: 10.1186/s12896-021-00706-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen C, Tan R, Wong L, Fekete R, Halsey J. Quantitation of microRNAs by real‐time RT‐qPCR. Methods Mol Biol. 2011;687:113–134. doi: 10.1007/978-1-60761-944-4_8 [DOI] [PubMed] [Google Scholar]
- 58. Csardi G, Nepusz T. Package ‘igraph’. Network Analysis and Visualization. R package version 1.3.2. 2022. Accessed June 2022. https://CRAN.R‐project.org/package=igraph.
- 59. Jawaid W. Package ‘enrichR’. Provides an R Interface to ‘Enrichr’. R package version 3.1. 2022. Accessed June 2022. https://CRAN.R‐project.org/package=enrichR.
- 60. Huang HY, Lin YC, Cui S, Huang Y, Tang Y, Xu J, Bao J, Li Y, Wen J, Zuo H, et al. miRTarBase update 2022: an informative resource for experimentally validated miRNA‐target interactions. Nucleic Acids Res. 2022;50:D222–D230. doi: 10.1093/nar/gkab1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ikram MA. Objectives, design, and main findings until 2020 from the Rotterdam Study. Eur J Epidemiol. 2020;35:483–517. doi: 10.1007/s10654-020-00640-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yaqub A, Mens MMJ, Klap JM, Weverling GJ, Klatser P, Brakenhoff JPJ, Roshchupkin GV, Ikram MK, Ghanbari M, Ikram MA. Genome‐wide profiling of circulatory microRNAs associated with cognition and dementia. Alzheimers Dement. 2022;19:1194–1203. doi: 10.1002/alz.12752 [DOI] [PubMed] [Google Scholar]
- 63. Zhang X, Mens MMJ, Abozaid YJ, Bos D, Darwish Murad S, de Knegt RJ, Ikram MA, Pan Q, Ghanbari M. Circulatory microRNAs as potential biomarkers for fatty liver disease: the Rotterdam study. Aliment Pharmacol Ther. 2021;53:432–442. doi: 10.1111/apt.16177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rouillard AD, Gundersen GW, Fernandez NF, Wang Z, Monteiro CD, McDermott MG, Ma'ayan A. The harmonizome: a collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database (Oxford). 2016;2016. doi: 10.1093/database/baw100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ling H, Guo Z, Du S, Liao Y, Li Y, Ding C, Song C. Serum exosomal miR‐122‐5p is a new biomarker for both acute coronary syndrome and underlying coronary artery stenosis. Biomarkers. 2020;25:539–547. doi: 10.1080/1354750X.2020.1803963 [DOI] [PubMed] [Google Scholar]
- 66. Satake E, Pezzolesi MG, Md Dom ZI, Smiles AM, Niewczas MA, Krolewski AS. Circulating miRNA profiles associated with hyperglycemia in patients with type 1 diabetes. Diabetes. 2018;67:1013–1023. doi: 10.2337/db17-1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fang L, Ellims AH, Moore XL, White DA, Taylor AJ, Chin‐Dusting J, Dart AM. Circulating microRNAs as biomarkers for diffuse myocardial fibrosis in patients with hypertrophic cardiomyopathy. J Transl Med. 2015;13:314. doi: 10.1186/s12967-015-0672-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Men H, Cai H, Cheng Q, Zhou W, Wang X, Huang S, Zheng Y, Cai L. The regulatory roles of p53 in cardiovascular health and disease. Cell Mol Life Sci. 2021;78:2001–2018. doi: 10.1007/s00018-020-03694-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ronnebaum SM, Patterson C. The FoxO family in cardiac function and dysfunction. Annu Rev Physiol. 2010;72:81–94. doi: 10.1146/annurev-physiol-021909-135931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Salminen A, Kaarniranta K. AMP‐activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev. 2012;11:230–241. doi: 10.1016/j.arr.2011.12.005 [DOI] [PubMed] [Google Scholar]
- 71. Sanchez‐Soria P, Camenisch TD. ErbB signaling in cardiac development and disease. Semin Cell Dev Biol. 2010;21:929–935. doi: 10.1016/j.semcdb.2010.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yao XL, Lu XL, Yan CY, Wan QL, Cheng GC, Li YM. Circulating miR‐122‐5p as a potential novel biomarker for diagnosis of acute myocardial infarction. Int J Clin Exp Pathol. 2015;8:16014–16019. [PMC free article] [PubMed] [Google Scholar]
- 73. Wang S‐S, Wu L‐J, Li J‐J‐H, Xiao H‐B, He Y, Yan Y‐X. A meta‐analysis of dysregulated miRNAs in coronary heart disease. Life Sci. 2018;215:170–181. doi: 10.1016/j.lfs.2018.11.016 [DOI] [PubMed] [Google Scholar]
- 74. Pastukh N, Meerson A, Kalish D, Jabaly H, Blum A. Serum miR‐122 levels correlate with diabetic retinopathy. Clin Exp Med. 2019;19:255–260. doi: 10.1007/s10238-019-00546-x [DOI] [PubMed] [Google Scholar]
- 75. Pofi R, Giannetta E, Galea N, Francone M, Campolo F, Barbagallo F, Gianfrilli D, Venneri MA, Filardi T, Cristini C, et al. Diabetic cardiomiopathy progression is triggered by miR122‐5p and involves extracellular matrix: a 5‐year prospective study. JACC Cardiovasc Imaging. 2021;14:1130–1142. doi: 10.1016/j.jcmg.2020.10.009 [DOI] [PubMed] [Google Scholar]
- 76. Regmi A, Liu G, Zhong X, Hu S, Ma R, Gou L, Zafar MI, Chen L. Evaluation of serum microRNAs in patients with diabetic kidney disease: a nested case‐controlled study and bioinformatics analysis. Med Sci Monit. 2019;25:1699–1708. doi: 10.12659/msm.913265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang R, Hong J, Cao Y, Shi J, Gu W, Ning G, Zhang Y, Wang W. Elevated circulating microRNA‐122 is associated with obesity and insulin resistance in young adults. Eur J Endocrinol. 2015;172:291–300. doi: 10.1530/eje-14-0867 [DOI] [PubMed] [Google Scholar]
- 78. Novák J, Olejníčková V, Tkáčová N, Santulli G. Mechanistic role of MicroRNAs in coupling lipid metabolism and atherosclerosis. In: Santulli G, ed microRNA: Basic Science: from Molecular Biology to Clinical Practice. Springer International Publishing; 2015:79–100. doi: 10.1007/978-3-319-22380-3_5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, Watts L, Booten SL, Graham M, McKay R, et al. miR‐122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87–98. doi: 10.1016/j.cmet.2006.01.005 [DOI] [PubMed] [Google Scholar]
- 80. Raitoharju E, Seppälä I, Lyytikäinen L‐P, Viikari J, Ala‐Korpela M, Soininen P, Kangas AJ, Waldenberger M, Klopp N, Illig T, et al. Blood hsa‐miR‐122‐5p and hsa‐miR‐885‐5p levels associate with fatty liver and related lipoprotein metabolism—the Young Finns study. Sci Rep. 2016;6:38262. doi: 10.1038/srep38262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Hallé JP, Young J, Rashkow A, Joyce C, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421 [DOI] [PubMed] [Google Scholar]
- 82. Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75:285–292. doi: 10.1161/HYPERTENSIONAHA.119.14240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Demirsoy IH, Ertural DY, Balci S, Cinkir U, Sezer K, Tamer L, Aras N. Profiles of circulating MiRNAs following metformin treatment in patients with type 2 diabetes. J Med Biochem. 2018;37:499–506. doi: 10.2478/jomb-2018-0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Misra P, Athira SV, Palaniswamy R, Karthik K, Vashum Y, Ruchira G, Sibin MK. Circulatory levels of multiple microRNA associated with prediabetes. Int J Diabetes Dev Ctries. 2023;43:1043–1051. doi: 10.1007/s13410-023-01208-1 [DOI] [Google Scholar]
- 85. Jaeger A, Zollinger L, Saely CH, Muendlein A, Evangelakos I, Nasias D, Charizopoulou N, Schofield JD, Othman A, Soran H, et al. Circulating microRNAs −192 and −194 are associated with the presence and incidence of diabetes mellitus. Sci Rep. 2018;8:14274. doi: 10.1038/s41598-018-32274-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Matsumoto S, Sakata Y, Suna S, Nakatani D, Usami M, Hara M, Kitamura T, Hamasaki T, Nanto S, Kawahara Y, et al. Circulating p53‐responsive microRNAs are predictive indicators of heart failure after acute myocardial infarction. Circ Res. 2013;113:322–326. doi: 10.1161/CIRCRESAHA.113.301209 [DOI] [PubMed] [Google Scholar]
- 87. Nie H, Pan Y, Zhou Y. Exosomal microRNA‐194 causes cardiac injury and mitochondrial dysfunction in obese mice. Biochem Biophys Res Commun. 2018;503:3174–3179. doi: 10.1016/j.bbrc.2018.08.113 [DOI] [PubMed] [Google Scholar]
- 88. Natsume Y, Oaku K, Takahashi K, Nakamura W, Oono A, Hamada S, Yamazoe M, Ihara K, Sasaki T, Goya M, et al. Combined analysis of human and experimental murine samples identified novel circulating MicroRNAs as biomarkers for atrial fibrillation. Circ J. 2018;82:965–973. doi: 10.1253/circj.CJ-17-1194 [DOI] [PubMed] [Google Scholar]
- 89. Elmoselhi AB, Seif Allah M, Bouzid A, Ibrahim Z, Venkatachalam T, Siddiqui R, Khan NA, Hamoudi RA. Circulating microRNAs as potential biomarkers of early vascular damage in vitamin D deficiency, obese, and diabetic patients. PLoS One. 2023;18:e0283608. doi: 10.1371/journal.pone.0283608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Johnson K, Leary PJ, Govaere O, Barter MJ, Charlton SH, Cockell SJ, Tiniakos D, Zatorska M, Bedossa P, Brosnan MJ, et al. Increased serum miR‐193a‐5p during non‐alcoholic fatty liver disease progression: diagnostic and mechanistic relevance. JHEP Rep. 2022;4:100409. doi: 10.1016/j.jhepr.2021.100409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mens MM, Rima M, Fariba A, Ikram MA, Marina E, Maryam K, Abbas D, Mohsen G. MiR‐139‐5p is a causal biomarker for type 2 diabetes; results from genome‐wide microRNA profiling and Mendelian randomization analysis in a population‐based study. medRxiv . 2021:2021.2005.2013.21257090. doi: 10.1101/2021.05.13.21257090. [DOI]
- 92. Hess AL, Larsen LH, Udesen PB, Sanz Y, Larsen TM, Dalgaard LT. Levels of circulating miR‐122 are associated with weight loss and metabolic syndrome. Obesity. 2020;28:493–501. doi: 10.1002/oby.22704 [DOI] [PubMed] [Google Scholar]
- 93. Ortega FJ, Mercader JM, Catalán V, Moreno‐Navarrete JM, Pueyo N, Sabater M, Gómez‐Ambrosi J, Anglada R, Fernández‐Formoso JA, Ricart W, et al. Targeting the circulating microRNA signature of obesity. Clin Chem. 2013;59:781–792. doi: 10.1373/clinchem.2012.195776 [DOI] [PubMed] [Google Scholar]
- 94. Catanzaro G, Filardi T, Sabato C, Vacca A, Migliaccio S, Morano S, Ferretti E. Tissue and circulating microRNAs as biomarkers of response to obesity treatment strategies. J Endocrinol Investig. 2021;44:1159–1174. doi: 10.1007/s40618-020-01453-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Craig M, Yarrarapu SNS, Dimri M. Biochemistry, cholesterol. In: Craig M, Yarrarapu SNS, Dimri M, eds. StatPearls. Treasure Island; 2022. [Google Scholar]
- 96. Lee KH, Hwang HJ, Cho JY. Long non‐coding RNA associated with cholesterol homeostasis and its involvement in metabolic diseases. Int J Mol Sci. 2020;21:1645. doi: 10.3390/ijms21218337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Jeon TI, Osborne TF. miRNA and cholesterol homeostasis. Biochim Biophys Acta. 2016;1861:2041–2046. doi: 10.1016/j.bbalip.2016.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high‐density lipoproteins. Nat Cell Biol. 2011;13:423–433. doi: 10.1038/ncb2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Linton MRF, Yancey PG, Davies SS, Jerome WG, Linton EF, Song WL, Doran AC, Vickers KC. The role of lipids and lipoproteins in atherosclerosis. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, Dungan K, Hershman JM, Hofland J, Kalra S, et al., eds Endotext. South Dartmouth (MA):Endotext; 2000. [Google Scholar]
- 100. Sun X, Zhang M, Sanagawa A, Mori C, Ito S, Iwaki S, Satoh H, Fujii S. Circulating microRNA‐126 in patients with coronary artery disease: correlation with LDL cholesterol. Thromb J. 2012;10:16. doi: 10.1186/1477-9560-10-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Krause BJ, Carrasco‐Wong I, Dominguez A, Arnaiz P, Farías M, Barja S, Mardones F, Casanello P. Micro‐RNAs Let7e and 126 in plasma as markers of metabolic dysfunction in 10 to 12 years old children. PLoS One. 2015;10:e0128140. doi: 10.1371/journal.pone.0128140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, Mayr A, Weger S, Oberhollenzer F, Bonora E, et al. Plasma microRNA profiling reveals loss of endothelial miR‐126 and other microRNAs in type 2 diabetes. Circ Res. 2010;107:810–817. doi: 10.1161/CIRCRESAHA.110.226357 [DOI] [PubMed] [Google Scholar]
- 103. Martinez‐Arroyo O, Ortega A, Flores‐Chova A, Sanchez‐Garcia B, Garcia‐Garcia AB, Chaves FJ, Martin‐Escudero JC, Forner MJ, Redon J, Cortes R. High miR‐126‐3p levels associated with cardiovascular events in a general population. Eur J Intern Med. 2023;113:49–56. doi: 10.1016/j.ejim.2023.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Willinger CM, Rong J, Tanriverdi K, Courchesne PL, Huan T, Wasserman GA, Lin H, Dupuis J, Joehanes R, Jones MR, et al. MicroRNA signature of cigarette smoking and evidence for a putative causal role of MicroRNAs in smoking‐related inflammation and target organ damage. Circ Cardiovasc Genet. 2017;10:10. doi: 10.1161/CIRCGENETICS.116.001678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Dorna MS, Barbosa EMS, Callegari MA, Tanni SE, Chiuso‐Minicucci F, Felix TF, Seneda AL, Correa CR, Fernandes AAH, Azevedo PS, et al. Orange juice attenuates circulating miR‐150‐5p, miR‐25‐3p, and miR‐451a in healthy smokers: a randomized crossover study. Front Nutr. 2021;8:775515–775524. doi: 10.3389/fnut.2021.775515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zhang L, Wang H, Wang C. Persistence of smoking induced non‐small cell lung carcinogenesis by decreasing ERBB pathway‐related microRNA expression. Thorac Cancer. 2019;10:890–897. doi: 10.1111/1759-7714.13020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Silverman MG, Yeri A, Moorthy MV, Garcia FC, Chatterjee NA, Glinge CSA, Tfelt‐Hansen J, Salvador AM, Pico AR, Shah R, et al. Circulating miRNAs and risk of sudden death in patients with coronary heart disease. JACC: Clin Electrophysiol. 2020;6:70–79. doi: 10.1016/j.jacep.2019.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ali SA, Espin‐Garcia O, Wong AK, Potla P, Pastrello C, McIntyre M, Lively S, Jurisica I, Gandhi R, Kapoor M. Circulating microRNAs differentiate fast‐progressing from slow‐progressing and non‐progressing knee osteoarthritis in the Osteoarthritis Initiative cohort. Ther Adv Musculoskelet Dis. 2022;14:1759720X221082917. doi: 10.1177/1759720X221082917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hua CC, Liu XM, Liang LR, Wang LF, Zhong JC. Targeting the microRNA‐34a as a novel therapeutic strategy for cardiovascular diseases. Front Cardiovasc Med. 2021;8:784044. doi: 10.3389/fcvm.2021.784044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Shen NN, Wang JL, Fu YP. The microRNA expression profiling in heart failure: a systematic review and meta‐analysis. Front Cardiovasc Med. 2022;9:856358. doi: 10.3389/fcvm.2022.856358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sessa F, Salerno M, Esposito M, Cocimano G, Pomara C. miRNA dysregulation in cardiovascular diseases: current opinion and future perspectives. Int J Mol Sci. 2023;24:5192. doi: 10.3390/ijms24065192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zhu JN, Fu YH, Hu ZQ, Li WY, Tang CM, Fei HW, Yang H, Lin QX, Gou DM, Wu SL, et al. Activation of miR‐34a‐5p/Sirt1/p66shc pathway contributes to doxorubicin‐induced cardiotoxicity. Sci Rep. 2017;7:11879. doi: 10.1038/s41598-017-12192-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Table S1


