Abstract
Background
Extracorporeal cardiopulmonary resuscitation (ECPR) is an option for refractory cardiac arrest, and immediate initiation after indication is recommended. However, the practical goals of ECPR preparation (such as the door‐to‐needle time) remain unclear. This study aimed to elucidate the association between the door‐to‐needle time and neurological outcomes of out‐of‐hospital cardiac arrest.
Methods and Results
This is a post hoc analysis of a nationwide multicenter study on out‐of‐hospital cardiac arrest treated with ECPR at 36 institutions between 2013 and 2018 (SAVE‐J [Study of Advanced Cardiac Life Support for Ventricular Fibrillation with Extracorporeal Circulation in Japan] II study). Adult patients without hypothermia (≥32 °C) in whom circulation was not returned at ECPR initiation were included. The probability of favorable neurological function at 30 days (defined as Cerebral Performance Category ≤2) was estimated using a generalized estimating equations model, in which institutional, patient, and treatment characteristics were adjusted. Estimated probabilities were then calculated according to the door‐to‐needle time with 3‐minute increments, and a clinical threshold was assumed. Among 1298 patients eligible for this study, 136 (10.6%) had favorable neurological function. The estimated probability of favorable outcomes was highest in patients with 1 to 3 minutes of door‐to‐needle time (12.9% [11.4%–14.3%]) and remained at 9% to 10% until 27 to 30 minutes. Then, the probability dropped gradually with each 3‐minute delay. A 30‐minute threshold was assumed, and shorter door‐to‐extracorporeal membrane oxygenation/low‐flow time and fewer adverse events related to cannulation were observed in patients with door‐to‐needle time <30 minutes.
Conclusions
The probability of favorable functions after out‐of‐hospital cardiac arrest decreased as the door‐to‐needle time for ECPR was prolonged, with a rapid decline after 27 to 30 minutes.
Registration
URL: https://center6.umin.ac.jp/cgi‐open‐bin/ctr/ctr_view.cgi?recptno=R000041577; Unique identifier: UMIN000036490.
Keywords: cardiopulmonary resuscitation, Cerebral Performance Category, extracorporeal cardiopulmonary resuscitation
Subject Categories: Cardiopulmonary Resuscitation and Emergency Cardiac Care, Cardiopulmonary Arrest
Nonstandard Abbreviations and Acronyms
- CPC
Cerebral Performance Category
- ECPR
extracorporeal cardiopulmonary resuscitation
- OHCA
out‐of‐hospital cardiac arrest
- ROSC
return of spontaneous circulation
Clinical Perspective.
What Is New?
The probability of favorable outcomes after extracorporeal cardiopulmonary resuscitation for out‐of‐hospital cardiac arrest was highest when extracorporeal membrane oxygenation cannulation was initiated within 1 to 3 minutes after hospital arrival (12.9% [11.4%–14.3%]).
The probability of favorable neurological outcomes remained at 9% to 10% until 27 to 30 minutes of door‐to‐needle time and dropped gradually with each 3‐minute delay.
What Are the Clinical Implications?
A 30‐minute threshold is recommended as door‐to‐needle time of extracorporeal cardiopulmonary resuscitation on patients with out‐of‐hospital cardiac arrest, after which in‐hospital mortality increased and low‐flow time was significantly prolonged.
Out‐of‐hospital cardiac arrest (OHCA) is a major cause of mortality worldwide. 1 Although various treatment options, such as high‐quality chest compression, immediate defibrillation, and targeted temperature management, have maintained a chain of survival, >70% of patients with OHCA still suffer from unfavorable neurologic function. 2 , 3 , 4 In addition, many patients are refractory to standard basic and advanced cardiac life support, in whom even strenuous resuscitative efforts often fail to achieve the return of spontaneous circulation (ROSC). 5
The idea of extracorporeal cardiopulmonary resuscitation (ECPR), which entails applying veno‐arterial extracorporeal membrane oxygenation (VA‐ECMO) during cardiac arrest, is to restore organ perfusion without ROSC. 6 Although ECPR theoretically shortens the low‐flow time and enables the simultaneous treatment of the underlying cause of cardiac arrest, the usefulness and futility of ECPR were both reported in different randomized controlled trials. 7 , 8 , 9 Notably, because the discrepancy of previous studies can be explained by the differences in the candidates, settings, and timing for ECPR, 10 the number of patients treated with ECPR is still increasing without a definitive conclusion on its clinical effects. 6
Clinical consequences of ECPR would be maximally improved by the optimal identification of candidates and the rapid initiation of ECMO after determining an indication. 11 , 12 A certain duration of failed conventional cardiopulmonary resuscitation (CPR) would be considered a criterion for ECPR to avoid overuse, 13 whereas the probability of survival decreases significantly when the low‐flow period is prolonged. A time window is limited for the appropriate ECPR. 14 Therefore, it has been recommended that the ECPR team should be activated immediately after potential candidates present, rather than wait for the failure of conventional CPR. 15 , 16
However, there is a paucity of literature on the clinically feasible goals of ECPR preparation and resuscitation team quality, such as the door‐to‐needle time for ECMO cannulation. Although the association between approximately <30 to 60 minutes of low‐flow time following rapid ECPR initiation and increased survival to discharge has been suggested, 6 , 17 it remains unclear whether short door‐to‐needle time would allow for rapid ECMO induction and shorten the low‐flow time safely with favorable neurological function. Accordingly, to eventually determine the specific target for the ECPR initiation time (if it exists), this study aimed to elucidate the relationship between the door‐to‐needle time for ECPR cannulation and the neurological outcomes of patients with OHCA.
METHODS
Transparency and Openness Promotion Statement
The data of this study are available from the SAVE‐J (Study of Advanced Cardiac Life Support for Ventricular Fibrillation with Extracorporeal Circulation in Japan) II study group; however, restrictions apply to the availability of these data, which were used with permission for the current study and are not publicly available. The data that support the findings of this study are available from the corresponding author upon reasonable request and with the permission of the SAVE‐J II study group.
Study Design and Setting
This was a post hoc analysis of a nationwide multicenter retrospective study conducted by the SAVE‐J II study group to provide real‐world data on ECPR performed on approximately 2000 patients. 17 The SAVE‐J II study included consecutive patients with OHCA aged ≥18 years who were transported to 36 participating hospitals between January 2013 and December 2018 where they received ECPR. Patients with in‐hospital cardiac arrest and those transferred from another nonparticipating institution after receiving treatment for OHCA were not included in the SAVE‐J II study. The SAVE‐J II study was registered in the University Hospital Medical Information Network Clinical Trial Registry on April 15, 2019 (UMIN‐CTR ID, UMIN000036490) before study initiation. The SAVE‐J II study was approved by the institutional review board for research with human participants at the head institute of the SAVE‐J II study (approval number 2018‐110 from Kagawa University). This study was conducted per the principles of the Helsinki Declaration, and written informed consent was waived due to the anonymity of the data.
ECPR was defined as resuscitation from cardiac arrest using VA‐ECMO in the SAVE‐J II study, and the indication for ECPR was determined by a treating physician or an institutional protocol without uniform criteria throughout the study institutions. Management strategies for patients with OHCA before, during, and after ECPR were decided by a treating physician and therefore differ between institutions. Characteristics of the participating institutions (including criteria for ECPR and details of resuscitation) are described in a previous study. 18
Study Population
In this study, we retrieved data of patients with OHCA from the SAVE‐J II study. Our inclusion criteria were as follows: (1) age ≥18 years, (2) the nonobtention of ROSC at VA‐ECMO initiation, and (3) body temperature ≥32 °C on arrival at the hospital. Patients who obtained ROSC but turned into cardiac arrest at VA‐ECMO initiation were therefore included. Patients without available data on the time of hospital arrival and VA‐ECMO initiation were excluded.
Data Collection and Definition
In the SAVE‐J II study, the following data were collected: demographic data such as age, sex, comorbidities, and activities of daily living (performance status); cardiac arrest‐related information such as witness status, bystander CPR, location, and initial cardiac rhythm; prehospital information such as prehospital treatment, physician presence at prehospital, and cardiac arrest on route; and in‐hospital information, including cardiac rhythm on hospital and before ECMO initiation, vital signs on arrival, time course, cause of cardiac arrest, the amount of transfusion administered, and ECMO information. Adverse events related to ECPR, infectious complications during hospital stay, duration of ECMO and ventilator use, length of intensive care unit and hospital stay, cerebral performance category (CPC) at 30 days after admission, and survival status at discharge were also recorded.
The door‐to‐needle time was defined as the time from hospital arrival to the initiation of VA‐ECMO cannulation, whereas the door‐to‐ECMO time was defined as the time from hospital arrival to the establishment of ECMO support. The low‐flow time was defined as the time from cardiac arrest to the establishment of ECMO, in which the time of cardiac arrest was estimated as the time of ambulance activation by calling from the scene if the cardiac arrest was not determined by emergency medical services. The transportation time was defined as the time from ambulance activation to hospital arrival. ROSC was defined as ≥1 minute of continuing spontaneous pulsation. Definitions of adverse events related to ECPR were as follows: cannula malposition was defined as cannulation requiring correct the position or the cannulation of the wrong vessel such as arterial–arterial and veno–veno cannulation; unsuccessful cannulation was defined as failure to complete cannulation; cannulation‐related bleeding was defined as cannulation site bleeding or retroperitoneal hemorrhage that required blood transfusion, angiography, or surgery.
Primary Variable of Interest
The primary variable of interest was door‐to‐needle time for cannulation of ECPR, which was calculated in minutes based on the abovementioned definition. Then, the door‐to‐needle time was classified into 3‐minute increments from 0 to 45 minutes and >45 minutes for the analyses.
Outcome Measures
The primary outcome was favorable neurological function at 30 days after admission, which was defined as a CPC of ≤2, where 1 indicates normal neurological function. CPC is a 1 to 5 scaling system for neurological function, which is widely used to evaluate the neurological outcomes of patients with OHCA and has high interrater reliability. 19 Although a CPC of 5 originally means brain death, it includes cardiac death in this study. Secondary outcomes included the door‐to‐ECMO time, low‐flow time, and the frequency of adverse events related to ECPR. The amount of transfusion until intensive care unit admission, presence of septicemia during hospital stay, days of ECMO and ventilator use, length of intensive care unit and hospital stay, and survival to discharge were also evaluated in post hoc analyses.
Statistical Analysis
Characteristics were compared between patients with and without favorable neurological function. In addition, the numbers of patients with and without favorable neurological function were counted in each minute of door‐to‐needle time.
To investigate the relationship between the door‐to‐needle time and neurological outcomes, the average probability of favorable neurological function at 30 days was calculated with 95% CIs in each door‐to‐needle time category. The probability of neurological function in each patient was estimated using a logistic regression model fitted with generalized estimating equations to develop a population average model, 20 which accounts for within‐institution clustering. Relevant covariates in the model were selected from known or potential predictors for neurological functions of patients treated with ECPR, 1 , 4 , 6 , 11 , 21 , 22 , 23 including age, sex, comorbidity (coronary artery disease, cerebrovascular disease, and chronic kidney disease), performance status, place of cardiac arrest (at home, health care facility, or others), witness status, bystander CPR, initial shockable rhythm, cardiac arrest on route, prehospital airway management (intubation or supraglottic airway device), prehospital physician presence, body temperature on arrival, ROSC on arrival, shockable rhythm on arrival and at ECMO initiation, cause of cardiac arrest (cardiogenic versus noncardiogenic), ECMO cannulation at the emergency department, and surgical cannulation. Before generalized estimating equations model development, missing nonoutcome values were replaced with a set of substituted plausible values by creating 5 filled‐in complete data sets using multiple imputations by a chained equation method. Estimated associations in each of the imputed data sets were averaged together to give overall estimated associations. 24
Moreover, the association between the door‐to‐needle time and the door‐to‐ECMO time, as well as the low‐flow time, was evaluated visually using boxplots and also examined with generalized estimating equations models using the same covariates for the calculation of the probability of favorable neurological function. The door‐to‐ECMO time and low‐flow time were plotted in each door‐to‐needle time category. Furthermore, time between initiation of cannulation and establishment of ECMO support (needle‐to‐ECMO initiation time) was plotted for each door‐to‐needle time category and also evaluated with generalized estimating equation models.
Then, a clinical threshold for the door‐to‐needle time was assumed as the time point after which the probability of a favorable neurological outcome would rapidly decrease. To evaluate this assumption, post hoc analyses were conducted by comparing the primary and several secondary outcomes between patients with shorter versus longer door‐to‐needle time than the threshold. In addition, to ascertain the threshold, a restricted cubic spline curve for estimating 30‐day favorable neurological outcomes by the door‐to‐needle time was created. Furthermore, to adjust the cofounding effects by low‐flow time, the primary outcome was compared between patients with the low‐flow time >60 versus ≤60 minutes among those with the door‐to‐needle time ≤30 minutes.
Subgroup analyses were also conducted considering the possible differences in regional medical systems and quality of the initial response team. Quality of resuscitation was defined as a frequency of rapid cannulation with shorter door‐to‐needle time than the threshold in each institution. Next, favorable neurological outcomes were compared in each subgroup divided by the frequency of rapid cannulation. Moreover, subgroups were generated based on recurrent versus persistent cardiac arrest (ROSC on route or not) and transportation time (>30 versus ≤30 minutes).
Descriptive variables are presented as the median (interquartile range) or frequencies (percentages). Results were presented with 95% CIs. The hypothesis was tested on the primary and secondary outcomes in post hoc analyses, with a 2‐sided α threshold of 0.05 being considered statistically significant. Differences in median were evaluated using the Hodges‐Lehmann estimator. Patient characteristics were compared using Mann‐Whitney U tests, χ2 tests, or Fisher exact tests, as appropriate. All statistical analyses were conducted using the IBM Statistical Package for the Social Sciences Statistics for Windows version 28.0 (IBM, Armonk, NY).
RESULTS
Patient Characteristics
Of the 2157 patients treated with ECPR in the SAVE‐J II study, 1298 adults were not hypothermic on hospital arrival and did not obtain ROSC at the time of ECMO cannulation, which means they were eligible for the study (Figure 1). In total, 136 (10.6%) patients had favorable neurological function (CPC ≤2) 30 days after they were admitted.
Figure 1. Patient flow diagram.
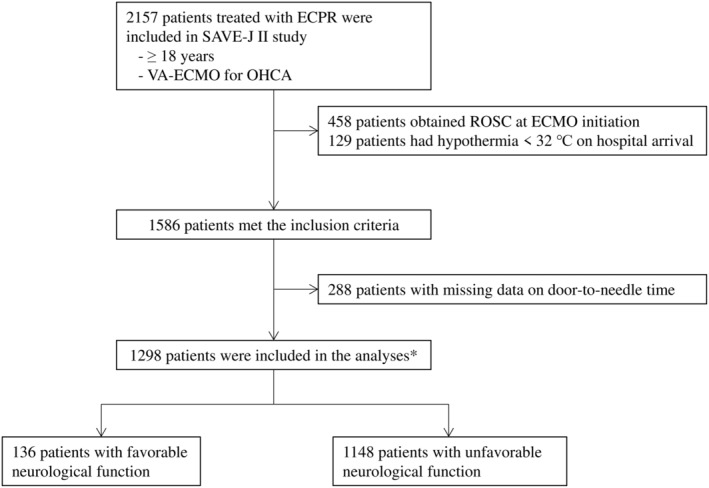
Of the 2157 patients treated with ECPR in the Study of Advanced Cardiac Life Support for Ventricular Fibrillation with Extracorporeal Circulation in Japan (SAVE‐J) II study, 1298 adults were not hypothermic on hospital arrival and did not obtain ROSC at the time of ECMO cannulation; therefore, they were eligible for this study. In total, 136 patients (10.6%) had favorable neurological function (CPC ≤2) 30 days after admission. CPC indicates Cerebral Performance Category; ECMO, extracorporeal membrane oxygenation; ECPR, extracorporeal cardiopulmonary resuscitation; OHCA, out‐of‐hospital cardiac arrest; ROSC, return of spontaneous circulation; and VA‐ECMO, veno‐arterial extracorporeal membrane oxygenation.
Patient characteristics are shown in Table 1. The median door‐to‐needle time was 5 minutes and 7 minutes in patients with and without favorable neurological outcomes, respectively. More patients with favorable function had shockable rhythm at the scene, on hospital arrival, and upon ECMO cannulation (112 [83%], 100 [73.5%], and 102 [76.1%], respectively) than those without. Moreover, witnessed cardiac arrest (116 [85.9%]), bystander CPR (99 [73.9%]), physician presence at prehospital (44 [32.4%]), and cardiogenic cause (114 [83.8%]) were more frequent among patients with favorable neurological outcomes at 30 days.
Table 1.
Characteristics of Patients Treated With ECPR
| Characteristic | Overall | Favorable neurological function (CPC ≤2) | Unfavorable neurological function (CPC ≥3) | P value |
|---|---|---|---|---|
| Cases | 1284 | 136 | 1148 | |
| Door‐to‐needle time, min, median (IQR) | 7 (2–13) | 5 (1–13) | 7 (3–13) | 0.032 |
| Cause for cardiac arrest, cardiogenic, n (%) | 987 (76.9%) | 114 (83.8%) | 873 (76.2%) | 0.045 |
| Age, y, median (IQR) | 59 (48–68) | 51 (43–66) | 60 (49–68) | <0.001 |
| Sex, men, n (%) | 1067 (83.1%) | 99 (72.8%) | 968 (84.3%) | <0.001 |
| Comorbidity, n (%) | ||||
| Coronary artery disease | 308 (24.0%) | 27 (19.9%) | 281 (24.5%) | 0.232 |
| Cerebrovascular disease | 71 (5.5%) | 4 (2.9%) | 67 (5.8%) | 0.110 |
| Chronic kidney disease | 57 (4.4%) | 5 (3.7%) | 52 (4.5%) | 0.648 |
| Performance status, median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.476 |
| Place of cardiac arrest, n (%) | ||||
| At home | 516 (40.2%) | 40 (29.4%) | 476 (41.5%) | 0.007 |
| At health care facility | 5 (0.4%) | 1 (0.7%) | 4 (0.3%) | 0.493 |
| Witness, n (%) | 1001 (78.0%) | 116 (85.9%) | 885 (77.2%) | 0.021 |
| Bystander CPR, n (%) | 718 (55.9%) | 99 (73.9%) | 619 (54.8%) | <0.001 |
| Cardiac rhythm at scene, shockable, n (%) | 854 (66.5%) | 112 (83.0%) | 742 (65.0%) | <0.001 |
| Cardiac arrest on route, n (%) | 137 (10.7%) | 24 (17.6%) | 113 (9.8%) | 0.005 |
| Prehospital treatment, n (%) | ||||
| Intubation | 135 (10.5%) | 16 (11.8%) | 119 (10.8%) | 0.615 |
| Supraglottic airway | 445 (34.7%) | 29 (21.3%) | 416 (36.2%) | <0.001 |
| Epinephrine | 461 (35.9%) | 41 (30.6%) | 420 (36.9%) | 0.151 |
| Mechanical CPR | 116 (9.0%) | 9 (20.5%) | 107 (32.4%) | 0.107 |
| Physician present | 367 (28.6%) | 44 (32.4%) | 323 (28.1%) | 0.303 |
| ROSC on arrival, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | … |
| Body temperature on hospital arrival, °C, median (IQR) | 35.3 (34.3–36.0) | 35.2 (34.2–35.8) | 35.3 (34.3–36.0) | 0.394 |
| Cardiac rhythm on arrival, shockable, n (%) | 618 (48.1%) | 100 (73.5%) | 518 (45.2%) | <0.001 |
| Cardiac rhythm at ECMO cannulation, shockable, n (%) | 646 (50.3%) | 102 (76.1%) | 544 (47.9%) | <0.001 |
| ECMO cannulation at ED, n (%) | 888 (69.2%) | 84 (62.2%) | 804 (70.5%) | 0.049 |
| Surgical cannulation, n (%) | 29 (2.3%) | 0 (0.0%) | 29 (2.6%) | 0.040 |
CPC indicates cerebral performance category; CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation; ECPR, extracorporeal cardiopulmonary resuscitation; ED, emergency department; IQR, interquartile range; and ROSC, return of spontaneous circulation.
The number of patients treated with ECPR decreased as the door‐to‐needle time increased, and it rapidly decreased particularly after 12 to 15 minutes (Figure 2). The number of patients who had favorable functions also decreased over time. There was a paucity of patients with CPC ≤2 at 30 days of admission after 27 to 30 minutes of door‐to‐needle time (Figure 2).
Figure 2. Number of patients treated with ECPR.
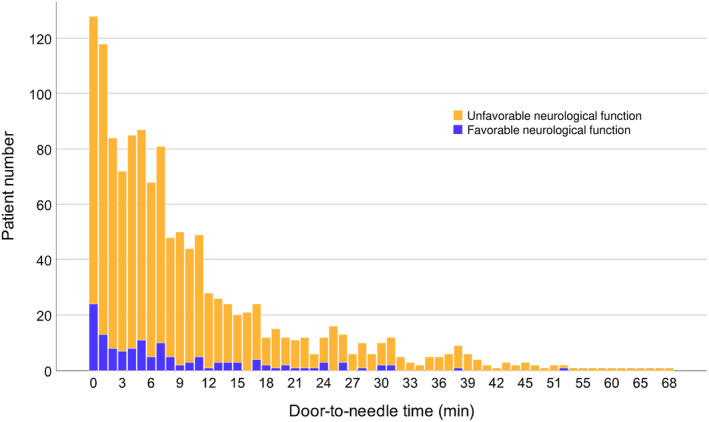
The number of patients treated with ECPR decreased as the door‐to‐needle time increased, and it rapidly decreased particularly after 12 to 15 minutes. The number of patients who had favorable functions was also reduced over time. There was a paucity of patients with CPC ≤2 30 days after admission after 27 to 30 minutes of door‐to‐needle time. CPC indicates Cerebral Performance Category; and ECPR, extracorporeal cardiopulmonary resuscitation.
Probability of Favorable Neurological Outcomes
The crude and estimated probabilities of favorable functions (adjusted probabilities) in each door‐to‐needle time category are shown in Figure 3 and Table S1. The estimated probability was highest in patients with 1 to 3 minutes of door‐to‐needle time (12.9% [95% CI, 11.4%–14.3%]) and remained at 9% to 10% until 27 to 30 minutes. Then, the probability of favorable neurological function sharply decreased to approximately 7.0% and further dropped gradually with each 3‐minute delay in door‐to‐needle time for ECMO cannulation.
Figure 3. Probability of favorable neurological outcomes.
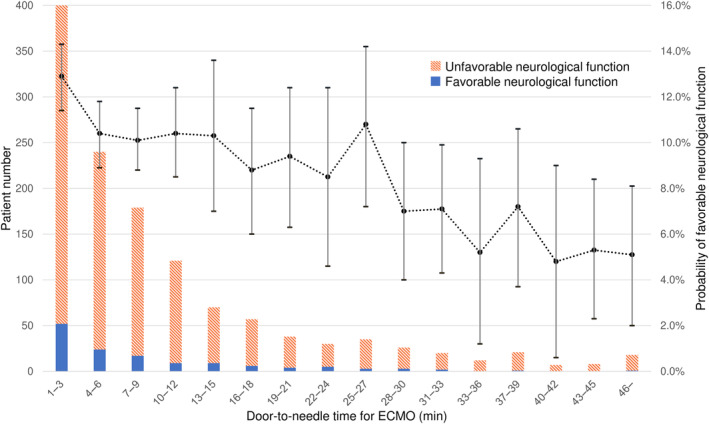
The estimated probabilities of favorable functions in each door‐to‐needle time category are shown (dashed lines) with 95% CIs (error bars) along with the number of patients (stacked bar). The estimated probability was highest in patients with 1 to 3 minutes of door‐to‐needle time (12.9% [95% CI, 11.4%–14.3%]) and remained 9% to 10% until 27 to 30 minutes. Then, the probability of favorable neurological function sharply decreased to approximately 7.0% and further dropped gradually with each 3‐minute delay in door‐to‐needle time for ECMO cannulation. ECMO indicates extracorporeal membrane oxygenation.
Based on the estimated probability and the crude number of patients with favorable function, a clinically relevant threshold for door‐to‐needle time was assumed as 27 to 30 minutes. The cubic spline curve also indicated a similar threshold (Figure S1). Post hoc analyses were performed to assess this assumption (Table 2). Patients with door‐to‐needle time <30 minutes had a higher probability of favorable neurological function, higher in‐hospital survival, and shorter door‐to‐ECMO and low‐flow time than those with door‐to‐needle time ≥30 minutes. Also, adverse events related to ECMO cannulation were fewer when the door‐to‐needle time was <30 minutes than when it was ≥30 minutes (19.2% versus 27.8%; odds ratio, 0.61 [0.39–0.98]). Additional analyses to consider the confounding effects by low‐flow time revealed that comparable probability of favorable neurological outcomes in patients with the low‐flow time >60 versus ≤60 minutes (13.8% versus 10.7%, P = 0.384) among those with the door‐to‐needle time ≤30 minutes.
Table 2.
Door‐to‐Needle Time and Clinical Outcomes in Post Hoc Analysis
| Variable | Door‐to‐needle time <30 min | Door‐to‐needle time ≥30 min | P value | Median difference (95% CI) | OR (95% CI) |
|---|---|---|---|---|---|
| Probability of favorable neurological function, % (95% CI) | 11.0% (10.3% to 11.6%) | 6.6% (5.2% to 7.9%) | <0.001 | 1.7% (0.7% to 2.9%) | |
| Door‐to‐ECMO time, min, median (IQR) | 21 (15 to 28) | 48 (43 to 69) | <0.001 | −29 (−31 to −27) | |
| Low‐flow time, min, median (IQR) | 54 (45 to 63) | 77 (70 to 90) | <0.001 | −24 (−27 to −21) | |
| Adverse events related to ECMO cannulation, n (%) | 230 (19.2%) | 27 (27.8%) | 0.039 | 0.61 (0.39 to 0.98) | |
| Amount of transfusion until ICU admission, mean, median (IQR) | |||||
| Red blood cell, mL | 210, 0 (0 to 0) | 370, 0 (0 to 560) | 0.017 | 0 (0 to 0) | |
| Fresh frozen plasma, mL | 160, 0 (0 to 0) | 360, 0 (0 to 240) | 0.056 | 0 (0 to 0) | |
| Septicemia, n (%) | 61 (6.7%) | 0 (0.0%) | 0.005 | … | |
| ECMO use, d, median (IQR) | 3 (2 to 5) | 2 (2 to 5) | 0.052 | 1 (0 to 1) | |
| Ventilator use, d, median (IQR) | 2 (1 to 8) | 2 (1 to 5) | 0.039 | 0 (0 to 1) | |
| Length of ICU stay, d, median (IQR) | 2 (1 to 9) | 2 (1 to 4) | 0.026 | 1 (0 to 1) | |
| Length of hospital stay, d, median (IQR) | 2 (1 to 14) | 2 (1 to 5) | 0.044 | 0 (0 to 1) | |
| In‐hospital survival, n (%) | 259 (21.6%) | 11 (11.3%) | 0.017 | 2.15 (1.13 to 4.09) | |
ECMO indicates extracorporeal membrane oxygenation; ICU, intensive care unit; IQR, interquartile range; and OR, odds ratio.
Subgroup analyses according to the frequency of short door‐to‐needle time at each institution are shown in Table S2. Door‐to‐needle time <30 minutes was associated with favorable neurological outcomes regardless of the frequency of rapid cannulation (whether door‐to‐needle time <30 minutes was achieved in >80% of patients). In addition, patients who experienced ROSC on route and those who did not, as well as patients with transportation time >30 and ≤30 minutes, had greater prevalence of favorable neurological outcomes for a door‐to‐needle time <30 minutes compared with door‐to‐needle time ≥30 minutes. The median difference in probability of favorable neurological outcomes between patients with the door‐to‐needle time <30 minutes and ≥30 minutes in each subgroup was shown as a forest plot (Figure S2).
Door‐to‐Needle, Door‐to‐ECMO, and Low‐Flow Time
The door‐to‐ECMO and low‐flow times in each door‐to‐needle time category are shown in Figure 4. The door‐to‐ECMO time was increased linearly with door‐to‐needle time. Conversely, the low‐flow time remained constant or only slightly increased until 30 minutes after hospital arrival (low‐flow time was approximately 60–65 minutes when door‐to‐needle time was <30 minutes), and then rapidly rose with a steep incline (low‐flow time was >75 minutes when door‐to‐needle time ≥30 minutes). The needle‐to‐ECMO initiation time was generally consistent across the door‐to‐needle time categories (Figure S3). Without the consideration of 30‐minute threshold, the door‐to‐ECMO and low‐flow time were correlated to the door‐to‐needle time, whereas the needle‐to‐ECMO initiation time was not.
Figure 4. Boxplots of the door‐to‐ECMO and low‐flow time.
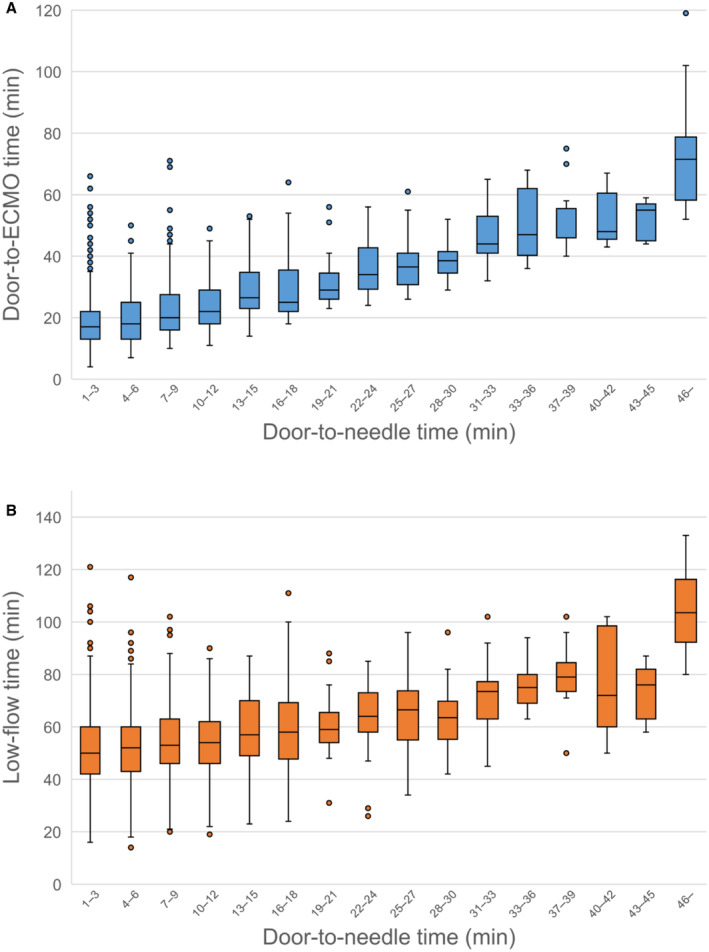
The door‐to‐ECMO time (A) and low‐flow time (B) in each door‐to‐needle time category are shown in boxplots. The door‐to‐ECMO time increased linearly with the door‐to‐needle time. Conversely, the low‐flow time either remained constant or only slightly increased until 30 minutes after hospital arrival (door‐to‐needle time <30 minutes), and then rapidly rose with a steep incline. ECMO indicates extracorporeal membrane oxygenation.
DISCUSSION
This study revealed that the probability of favorable neurological functions of patients treated with ECPR decreased as the door‐to‐needle time for ECMO cannulation increased, with a rapid decline after 27 to 30 minutes. It is noteworthy that the probability of favorable outcomes was approximately 10% and the low‐flow time remained constant at 50 to 60 minutes until 27 to 30 minutes of the door‐to‐needle time.
The findings of this study have several clinically relevant implications. First, the door‐to‐needle time (which is a practical target) can easily be measured. Although several studies discussed the clinical effects of low‐flow time before ECMO initiation, the accurate time of no‐flow and low‐flow time is difficult or impossible to obtain during resuscitation because the exact time of cardiac arrest is often unknown. 25 Second, because there is only a slight change in the estimated probability of favorable neurological function until 27 to 30 minutes of door‐to‐needle time, a door‐to‐needle time of <30 minutes would be a feasible goal for ECPR preparation. Importantly, the probability was estimated after adjusting the place and method for ECMO cannulation, and this management index can be adopted in various institutions with different ECPR practices. Third, the current results indicated that a shorter door‐to‐needle time would predict favorable neurologic function after ECPR, whereas most previous studies of low‐flow time examined only in‐hospital survival. 26 , 27 , 28 , 29 Because a considerable number of patients with OHCA would suffer from neurologic disabilities even after circulation is restored, the door‐to‐needle time would be useful to forecast clinical consequences relevant to a patient's well‐being after OHCA.
It should be emphasized that adverse events related to ECMO cannulation are less frequent among patients with door‐to‐needle time <30 minutes. Although the rapidity of the ECPR procedure is crucial for the early restoration of organ perfusion in patients with OHCA, suboptimal preparation with immediate cannulation has been associated with increased rates of adverse events. 6 , 30 , 31 However, the results of this study suggest that quick ECMO induction could be performed safely, even at the emergency department; ECMO cannulation was conducted at the emergency department in more than two‐thirds of this study's participants.
The findings of this study did not refute the usefulness of ECPR after 30 minutes of door‐to‐needle time. Although only a few patients had favorable neurological function when ECMO cannulation was initiated later than 30 minutes after hospital arrival, optimal criteria for candidates of ECPR were not examined, and the importance of each criterion (such as age, cause of cardiac arrest, and CPR duration) was outside the scope of the current study. Moreover, the association between the door‐to‐needle time and clinical consequences would differ with patient characteristics.
The short door‐to‐needle time could be a surrogate marker for short low‐flow time or high quality of resuscitation at an institution, while any measures to reduce low‐flow time must be critically important. Subgroup analyses revealed favorable outcomes with a door‐to‐needle time <30 minutes regardless of transportation time and frequency of rapid cannulation (quality of care at each institution). Therefore, 27 to 30 minutes of door‐to‐needle time could be useful as long as it is appropriately contextualized with other relevant ECPR criteria. Although gathering information and discussing indications for ECPR could hinder rapid cannulation, a concrete goal of ECPR preparation, such as door‐to‐needle time <30 minutes, could improve neurological outcomes in patients with OHCA.
Limitations
This study's findings must be interpreted in the context of its design. We retrospectively retrieved data from medical charts and could not identify the time when ECPR was determined. Therefore, our results may have been different had there been any reasons behind the longer door‐to‐needle time that depended on strong but unrecorded prognostic factors such as the quality of prehospital care, performance on CPR, skills and experience of the physicians and initial response team, postcardiac arrest treatment, institutional ECPR program, and type of institutions. Another limitation was the lack of detailed clinical information on cerebral perfusion or function during or after ECMO. Although the shorter door‐to‐needle time could enhance cerebral oxygenation, it cannot be objectively evaluated. Furthermore, the current study excluded patients who obtained ROSC at ECMO initiation. Although some patients with OHCA may have unstable hemodynamics for a considerable duration after ROSC and would benefit from ECMO, the generalizability of the results for such populations is limited. Finally, this retrospective study did not show any causal effects of shorter door‐to‐needle time on neurological function. Therefore, although the probability of favorable neurological function remained 9% to 10% until 27 to 30 minutes of door‐to‐needle time, early ECMO cannulation would be recommended once the indications for ECPR were determined.
CONCLUSIONS
This study identified the fact that the probability of favorable neurological functions of patients treated with ECPR decreased as door‐to‐needle time for ECMO cannulation was prolonged. A significant decrease in probability was observed after 27 to 30 minutes of door‐to‐needle time. A door‐to‐needle time of <30 minutes as a practical goal for ECPR preparation should be further examined.
APPENDIX
SAVE‐J II Study Group Members
Hirotaka Sawano, MD, PhD (Osaka Saiseikai Senri Hospital, Osaka, Japan); Yuko Egawa, MD, Shunichi Kato, MD (Saitama Red Cross Hospital, Saitama, Japan); Naofumi Bunya, MD, Takehiko Kasai, MD (Sapporo Medical University, Hokkaido, Japan); Shinichi Ijuin, MD, Shinichi Nakayama, MD, PhD (Hyogo Emergency Medical Center, Hyogo, Japan); Jun Kanda, MD, PhD, Seiya Kanou, MD (Teikyo University Hospital, Tokyo, Japan); Toru Takiguchi, MD, Shoji Yokobori, MD, PhD (Nippon Medical School, Tokyo, Japan); Hiroaki Takada, MD, Kazushige Inoue, MD (National Hospital Organization Disaster Medical Center, Tokyo, Japan); Ichiro Takeuchi, MD, PhD, Hiroshi Honzawa, MD (Yokohama City University Medical Center, Kanagawa, Japan); Makoto Kobayashi, MD, PhD, Tomohiro Hamagami, MD (Toyooka Public Hospital, Hyogo, Japan); Wataru Takayama, MD, Yasuhiro Otomo, MD, PhD (Tokyo Medical and Dental University Hospital of Medicine, Tokyo, Japan); Kunihiko Maekawa, MD (Hokkaido University Hospital, Hokkaido, Japan); Takafumi Shimizu, MD, Satoshi Nara, MD (Teine Keijinkai Hospital, Hokkaido, Japan); Michitaka Nasu, MD, Kuniko Takahashi, MD (Urasoe General Hospital, Okinawa, Japan); Yoshihiro Hagiwara, MD, MPH (Imperial Foundation Saiseikai, Utsunomiya Hospital, Tochigi, Japan); Shigeki Kushimoto, MD, PhD (Tohoku University Graduate School of Medicine, Miyagi, Japan); Reo Fukuda, MD (Nippon Medical School Tama Nagayama Hospital, Tokyo, Japan); Takayuki Ogura, MD, PhD (Japan Red Cross Maebashi Hospital, Gunma, Japan); Shin‐ichiro Shiraishi, MD (Aizu Central Hospital, Fukushima, Japan); Ryosuke Zushi, MD (Osaka Mishima Emergency Critical Care Center, Osaka, Japan); Norio Otani, MD (St. Luke's International Hospital, Tokyo, Japan); Migaku Kikuchi, MD, PhD (Dokkyo Medical University, Tochigi, Japan); Kazuhiro Watanabe, MD (Nihon University Hospital, Tokyo, Japan); Takuo Nakagami, MD (Omihachiman Community Medical Center, Shiga, Japan); Tomohisa Shoko, MD, PhD (Tokyo Women's Medical University Medical Center East, Tokyo, Japan); Nobuya Kitamura, MD, PhD (Kimitsu Chuo Hospital, Chiba, Japan); Takayuki Otani, MD (Hiroshima City Hiroshima Citizens Hospital, Hiroshima, Japan); Yoshinori Matsuoka, MD, PhD (Kobe City Medical Center General Hospital, Hyogo, Japan); Makoto Aoki, MD, PhD (Gunma University Graduate School of Medicine, Gunma, Japan); Masaaki Sakuraya, MD, MPH (JA Hiroshima General Hospital Hiroshima, Hiroshima, Japan); Hideki Arimoto, MD (Osaka City General Hospital, Osaka, Japan); Koichiro Homma, MD, PhD (Keio University School of Medicine, Tokyo, Japan); Hiromichi Naito, MD, PhD (Okayama University Hospital, Okayama, Japan); Shunichiro Nakao, MD, PhD (Osaka University Graduate School of Medicine, Osaka, Japan); Tomoya Okazaki, MD, PhD (Kagawa University Hospital, Kagawa, Japan); Yoshio Tahara, MD, PhD (National Cerebral and Cardiovascular Center, Osaka, Japan); Hiroshi Okamoto, MD, MPH (St. Luke's International Hospital, Tokyo, Japan); Jun Kunikata, MD, PhD, Hideto Yokoi, MD, PhD (Kagawa University Hospital, Kagawa, Japan).
Sources of Funding
None.
Disclosures
None.
Supporting information
Tables S1–S2
Figures S1–S3
Acknowledgments
The authors thank all the members of the SAVE‐J II study group. R.Y. and D.K. designed the study. R.Y., D.K., K.H., A.I., T.H., T.S., and Y.K. collected the relevant data. J.S. managed quality control. R.Y. and D.K. analyzed and interpreted the data. R.Y., D.K., K.H., and J.S. wrote and critically reviewed the article. All authors revised the article.
This article was sent to Kori S. Zachrison, MD, MSc, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.124.034971
For Sources of Funding and Disclosures, see page 11.
Contributor Information
Ryo Yamamoto, Email: ryo.yamamoto@gmail.com.
the SAVE‐J II study group:
Hirotaka Sawano, Yuko Egawa, Shunichi Kato, Naofumi Bunya, Takehiko Kasai, Shinichi Ijuin, Shinichi Nakayama, Jun Kanda, Seiya Kanou, Toru Takiguchi, Shoji Yokobori, Hiroaki Takada, Kazushige Inoue, Ichiro Takeuchi, Hiroshi Honzawa, Makoto Kobayashi, Tomohiro Hamagami, Wataru Takayama, Yasuhiro Otomo, Kunihiko Maekawa, Takafumi Shimizu, Satoshi Nara, Michitaka Nasu, Kuniko Takahashi, Yoshihiro Hagiwara, Shigeki Kushimoto, Reo Fukuda, Takayuki Ogura, Shin‐ichiro Shiraishi, Ryosuke Zushi, Norio Otani, Migaku Kikuchi, Kazuhiro Watanabe, Takuo Nakagami, Tomohisa Shoko, Nobuya Kitamura, Takayuki Otani, Yoshinori Matsuoka, Makoto Aoki, Masaaki Sakuraya, Hideki Arimoto, Hiromichi Naito, Shunichiro Nakao, Tomoya Okazaki, Yoshio Tahara, Hiroshi Okamoto, Jun Kunikata, and Hideto Yokoi
References
- 1. Myat A, Song KJ, Rea T. Out‐of‐hospital cardiac arrest: current concepts. Lancet. 2018;391:970–979. doi: 10.1016/S0140-6736(18)30472-0 [DOI] [PubMed] [Google Scholar]
- 2. Andersen LW, Holmberg MJ, Berg KM, Donnino MW, Granfeldt A. In‐hospital cardiac arrest: a review. JAMA. 2019;321:1200–1210. doi: 10.1001/jama.2019.1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dankiewicz J, Cronberg T, Lilja G, Jakobsen JC, Levin H, Ullén S, Rylander C, Wise MP, Oddo M, Cariou A, et al. Hypothermia versus normothermia after out‐of‐hospital cardiac arrest. N Engl J Med. 2021;384:2283–2294. doi: 10.1056/NEJMoa2100591 [DOI] [PubMed] [Google Scholar]
- 4. Jentzer JC, Clements CM, Wright RS, White RD, Jaffe AS. Improving survival from cardiac arrest: a review of contemporary practice and challenges. Ann Emerg Med. 2016;68:678–689. doi: 10.1016/j.annemergmed.2016.05.022 [DOI] [PubMed] [Google Scholar]
- 5. Goto Y, Funada A, Goto Y. Relationship between the duration of cardiopulmonary resuscitation and favorable neurological outcomes after out‐of‐hospital cardiac arrest: a prospective, Nationwide, population‐based cohort study. J Am Heart Assoc. 2016;5:e002819. doi: 10.1161/JAHA.115.002819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abrams D, MacLaren G, Lorusso R, Price S, Yannopoulos D, Vercaemst L, Bělohlávek J, Taccone FS, Aissaoui N, Shekar K, et al. Extracorporeal cardiopulmonary resuscitation in adults: evidence and implications. Intensive Care Med. 2022;48:1–15. doi: 10.1007/s00134-021-06514-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Belohlavek J, Smalcova J, Rob D, Franek O, Smid O, Pokorna M, Horák J, Mrazek V, Kovarnik T, Zemanek D, et al. Effect of intra‐arrest transport, extracorporeal cardiopulmonary resuscitation, and immediate invasive assessment and treatment on functional neurologic outcome in refractory out‐of‐hospital cardiac arrest: a randomized clinical trial. JAMA. 2022;327:737–747. doi: 10.1001/jama.2022.1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yannopoulos D, Bartos J, Raveendran G, Walser E, Connett J, Murray TA, Collins G, Zhang L, Kalra R, Kosmopoulos M, et al. Advanced reperfusion strategies for patients with out‐of‐hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open‐label, randomised controlled trial. Lancet. 2020;396:1807–1816. doi: 10.1016/S0140-6736(20)32338-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suverein MM, Delnoij TSR, Lorusso R, Brandon Bravo Bruinsma GJ, Otterspoor L, Elzo Kraemer CV, Vlaar APJ, van der Heijden JJ, Scholten E, den Uil C, et al. Early extracorporeal CPR for refractory out‐of‐hospital cardiac arrest. N Engl J Med. 2023;388:299–309. doi: 10.1056/NEJMoa2204511 [DOI] [PubMed] [Google Scholar]
- 10. Holmberg MJ, Granfeldt A, Guerguerian AM, Sandroni C, Hsu CH, Gardner RM, Lind PC, Eggertsen MA, Johannsen CM, Andersen LW. Extracorporeal cardiopulmonary resuscitation for cardiac arrest: an updated systematic review. Resuscitation. 2023;182:109665. doi: 10.1016/j.resuscitation.2022.12.003 [DOI] [PubMed] [Google Scholar]
- 11. Brooks SC, Anderson ML, Bruder E, Daya MR, Gaffney A, Otto CW, Singer AJ, Thiagarajan RR, Travers AH. Part 6: alternative techniques and ancillary devices for cardiopulmonary resuscitation: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132:S436–S443. doi: 10.1161/CIR.0000000000000260 [DOI] [PubMed] [Google Scholar]
- 12. Callaway CW, Soar J, Aibiki M, Böttiger BW, Brooks SC, Deakin CD, Donnino MW, Drajer S, Kloeck W, Morley PT, et al. Part 4: advanced life support: 2015 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 2015;132:S84–S145. doi: 10.1161/CIR.0000000000000273 [DOI] [PubMed] [Google Scholar]
- 13. Soar J, Böttiger BW, Carli P, Couper K, Deakin CD, Djärv T, Lott C, Olasveengen T, Paal P, Pellis T, et al. European resuscitation council guidelines 2021: adult advanced life support. Resuscitation. 2021;161:115–151. doi: 10.1016/j.resuscitation.2021.02.010 [DOI] [PubMed] [Google Scholar]
- 14. Yu HY, Wang CH, Chi NH, Huang SC, Chou HW, Chou NK, Chen YS. Effect of interplay between age and low‐flow duration on neurologic outcomes of extracorporeal cardiopulmonary resuscitation. Intensive Care Med. 2019;45:44–54. doi: 10.1007/s00134-018-5496-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abrams D, Garan AR, Abdelbary A, Bacchetta M, Bartlett RH, Beck J, Belohlavek J, Chen YS, Fan E, Ferguson ND, et al. Position paper for the organization of ECMO programs for cardiac failure in adults. Intensive Care Med. 2018;44:717–729. doi: 10.1007/s00134-018-5064-5 [DOI] [PubMed] [Google Scholar]
- 16. Whitmore SP, Gunnerson KJ, Haft JW, Lynch WR, VanDyck T, Hebert C, Waldvogel J, Havey R, Weinberg A, Cranford JA, et al. Simulation training enables emergency medicine providers to rapidly and safely initiate extracorporeal cardiopulmonary resuscitation (ECPR) in a simulated cardiac arrest scenario. Resuscitation. 2019;138:68–73. doi: 10.1016/j.resuscitation.2019.03.002 [DOI] [PubMed] [Google Scholar]
- 17. Inoue A, Hifumi T, Sakamoto T, Okamoto H, Kunikata J, Yokoi H, Sawano H, Egawa Y, Kato S, Sugiyama K, et al. Extracorporeal cardiopulmonary resuscitation in adult patients with out‐of‐hospital cardiac arrest: a retrospective large cohort multicenter study in Japan. Crit Care. 2022;26:129. doi: 10.1186/s13054-022-03998-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hifumi T, Inoue A, Takiguchi T, Watanabe K, Ogura T, Okazaki T, Ijuin S, Zushi R, Arimoto H, Takada H, et al. Variability of extracorporeal cardiopulmonary resuscitation practice in patients with out‐of‐hospital cardiac arrest from the emergency department to intensive care unit in Japan. Acute Med Surg. 2021;8:e647. doi: 10.1002/ams2.647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grossestreuer AV, Abella BS, Sheak KR, Cinousis MJ, Perman SM, Leary M, Wiebe DJ, Gaieski DF. Inter‐rater reliability of post‐arrest cerebral performance category (CPC) scores. Resuscitation. 2016;109:21–24. doi: 10.1016/j.resuscitation.2016.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bible J, Albert PS, Simons‐Morton BG, Liu D. Practical issues in using generalized estimating equations for inference on transitions in longitudinal data: what is being estimated? Stat Med. 2019;38:903–916. doi: 10.1002/sim.8014 [DOI] [PubMed] [Google Scholar]
- 21. Aubin H, Petrov G, Dalyanoglu H, Saeed D, Akhyari P, Paprotny G, Richter M, Westenfeld R, Schelzig H, Kelm M, et al. A suprainstitutional network for remote extracorporeal life support: a retrospective cohort study. JACC Heart Fail. 2016;4:698–708. doi: 10.1016/j.jchf.2016.03.018 [DOI] [PubMed] [Google Scholar]
- 22. Justice CN, Halperin HR, Vanden Hoek TL, Geocadin RG. Extracorporeal cardiopulmonary resuscitation (eCPR) and cerebral perfusion: a narrative review. Resuscitation. 2023;182:109671. doi: 10.1016/j.resuscitation.2022.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shin TG, Jo IJ, Sim MS, Song YB, Yang JH, Hahn JY, Choi SH, Gwon HC, Jeon ES, Sung K, et al. Two‐year survival and neurological outcome of in‐hospital cardiac arrest patients rescued by extracorporeal cardiopulmonary resuscitation. Int J Cardiol. 2013;168:3424–3430. doi: 10.1016/j.ijcard.2013.04.183 [DOI] [PubMed] [Google Scholar]
- 24. Rubin DB, Schenker N. Multiple imputation in health‐are databases: an overview and some applications. Stat Med. 1991;10:585–598. doi: 10.1002/sim.4780100410 [DOI] [PubMed] [Google Scholar]
- 25. Cheskes S, Drennan IR. No flow time, bystander low flow time and EMS system response time: are we looking at two sides of the same coin? Resuscitation. 2021;167:412–413. doi: 10.1016/j.resuscitation.2021.08.033 [DOI] [PubMed] [Google Scholar]
- 26. Chen YS, Lin JW, Yu HY, Ko WJ, Jerng JS, Chang WT, Chen WJ, Huang SC, Chi NH, Wang CH, et al. Cardiopulmonary resuscitation with assisted extracorporeal life‐support versus conventional cardiopulmonary resuscitation in adults with in‐hospital cardiac arrest: an observational study and propensity analysis. Lancet. 2008;372:554–561. doi: 10.1016/S0140-6736(08)60958-7 [DOI] [PubMed] [Google Scholar]
- 27. Chen YS, Yu HY, Huang SC, Lin JW, Chi NH, Wang CH, Wang SS, Lin FY, Ko WJ. Extracorporeal membrane oxygenation support can extend the duration of cardiopulmonary resuscitation. Crit Care Med. 2008;36:2529–2535. doi: 10.1097/CCM.0b013e318183f491 [DOI] [PubMed] [Google Scholar]
- 28. D'Arrigo S, Cacciola S, Dennis M, Jung C, Kagawa E, Antonelli M, Sandroni C. Predictors of favourable outcome after in‐hospital cardiac arrest treated with extracorporeal cardiopulmonary resuscitation: a systematic review and meta‐analysis. Resuscitation. 2017;121:62–70. doi: 10.1016/j.resuscitation.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 29. Wengenmayer T, Rombach S, Ramshorn F, Biever P, Bode C, Duerschmied D, Staudacher DL. Influence of low‐flow time on survival after extracorporeal cardiopulmonary resuscitation (eCPR). Crit Care. 2017;21:157. doi: 10.1186/s13054-017-1744-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bartos JA, Carlson K, Carlson C, Raveendran G, John R, Aufderheide TP, Yannopoulos D. Surviving refractory out‐of‐hospital ventricular fibrillation cardiac arrest: critical care and extracorporeal membrane oxygenation management. Resuscitation. 2018;132:47–55. doi: 10.1016/j.resuscitation.2018.08.030 [DOI] [PubMed] [Google Scholar]
- 31. Danial P, Hajage D, Nguyen LS, Mastroianni C, Demondion P, Schmidt M, Bouglé A, Amour J, Leprince P, Combes A, et al. Percutaneous versus surgical femoro‐femoral veno‐arterial ECMO: a propensity score matched study. Intensive Care Med. 2018;44:2153–2161. doi: 10.1007/s00134-018-5442-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figures S1–S3


