Abstract
Background
Robust risk assessment is crucial for the growing repaired tetralogy of Fallot population at risk of major adverse clinical outcomes; however, current tools are hindered by lack of validation. This study aims to develop and validate a risk prediction model for death in the repaired tetralogy of Fallot population.
Methods and Results
Patients with repaired tetralogy of Fallot enrolled in the INDICATOR (International Multicenter Tetralogy of Fallot Registry) cohort with clinical, arrhythmia, cardiac magnetic resonance, and outcome data were included. Patients from London, Amsterdam, and Boston sites were placed in the development cohort; patients from the Toronto site were used for external validation. Multivariable Cox regression was used to evaluate factors associated with time from cardiac magnetic resonance until the primary outcome: all‐cause death. Of 1552 eligible patients (n=1221 in development, n=331 in validation; median age at cardiac magnetic resonance 23.4 [interquartile range, 15.6–35.6] years; median follow up 9.5 years), 102 (6.6%) experienced the primary outcome. The multivariable Cox model performed similarly during development (concordance index, 0.83 [95% CI, 0.78–0.88]) and external validation (concordance index, 0.80 [95% CI, 0.71–0.90]) and identified older age at cardiac magnetic resonance, obesity, type of tetralogy of Fallot repair, higher right ventricular end‐systolic volume index, and lower biventricular global function index as independent predictors of death. A risk‐scoring algorithm dividing patients into low‐risk (score ≤4) versus high‐risk (score >4) groups was validated to effectively discriminate risk of death (15‐year survival of 95% versus 74%, respectively; P<0.001).
Conclusions
This externally validated mortality risk prediction algorithm can help identify vulnerable patients with repaired tetralogy of Fallot who may benefit from targeted interventions.
Keywords: cardiovascular magnetic resonance, congenital heart disease, death, outcomes, risk score, tetralogy of Fallot, ventricular function
Subject Categories: Congenital Heart Disease, Magnetic Resonance Imaging (MRI), Imaging
Nonstandard Abbreviations and Acronyms
- BVGFI
biventricular global function index
- INDICATOR
International Multicenter Tetralogy of Fallot Registry
- PVR
pulmonary valve replacement
- rTOF
Repaired tetralogy of Fallot
Clinical Perspective.
What Is New?
Repair type, older age at cardiac magnetic resonance, obesity, lower biventricular global function index, and higher right ventricular end‐systolic volume index were identified as independent predictors of all‐cause death in repaired tetralogy of Fallot.
A clinically practical scoring algorithm was developed and externally validated to allow clinicians to effectively risk stratify patients with tetralogy of Fallot on the basis of demographic and imaging biomarkers.
What Are the Clinical Implications?
Our externally validated risk‐scoring algorithm informs clinicians on risk stratification for patients with repaired tetralogy of Fallot, which may guide clinical decision making as well as the design of future interventional trials in this population.
Advances in the care of children with complex congenital heart disease have led to marked decreases in the mortality rate for patients with repaired tetralogy of Fallot (rTOF). 1 However, as most patients with rTOF experience residual hemodynamic abnormalities subsequent to the complex pathophysiologic sequelae of right ventricular (RV) outflow tract dysfunction, they are subject to increasing rates of morbidity and premature death in adulthood. 2 , 3 , 4 This trend underscores the need for robust, practical, and generalizable risk stratification tools for this growing population.
To circumvent the limitations of small single‐center studies that have attempted to address this issue, 5 , 6 the INDICATOR (International Multicenter Tetralogy of Fallot Registry) was established. Previously, an INDICATOR cohort study identified RV hypertrophy, ventricular dysfunction, and atrial arrhythmias as independent predictors of death or sustained ventricular tachycardia (VT) in patients with rTOF. 7 A subsequent multicenter study confirmed the importance of ventricular dysfunction as a risk predictor in this population. 8 However, both models performed modestly during external validation on a single institution data set, 9 highlighting the need for a larger cohort with longer duration of follow‐up to rigorously develop and validate a robust risk stratification model. Recently, we have updated our INDICATOR cohort to include extended length of follow‐up, which has provided the statistical power to address clinically important topics such as the lower risk of death or sustained VT for propensity score–matched patients with rTOF who received pulmonary valve replacement (PVR). 10
In this study, we leveraged this updated INDICATOR cohort to develop and externally validate a novel risk score model for all‐cause death in patients with rTOF using readily available clinical and imaging biomarkers. Using this model, we then risk stratified patients into low‐ and high‐risk groups to potentially inform the need for closer monitoring or interventions.
METHODS
Patients
Detailed descriptions of the INDICATOR cohort, including recruitment protocol, inclusion and exclusion criteria, and data collection and analysis have been published previously. 7 Briefly, 4 participating congenital heart centers in Toronto, London, Amsterdam, and Boston identified patients fulfilling the following inclusion criteria: (1) rTOF; (2) post‐rTOF cardiovascular magnetic resonance (CMR) performed after January 1, 2002; and (3) clinical follow‐up ≥1 year or occurrence of the primary outcome after the qualifying CMR. Patients with inadequate CMR data (ie, missing RV volume and/or mass measurements) and/or body surface area < 1.0 m2 (given paucity of normative data for this group, as well as the phenomenon of heteroscedasticity preventing extrapolation) were excluded. In our secondary outcome analysis, patients were also excluded if the secondary outcome was experienced before qualifying CMR. Each participating center received institutional review board or ethics committee approval, and requirement for individual patient consent was waived.
Upon application to the corresponding author and with approval of the INDICATOR Cohort Steering Committee, the data supporting the findings of this study will be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Data Collection
Study data were submitted by the participating study sites and analyzed at the coordinating center at Boston Children's Hospital, which included a data repository, CMR core laboratory, and a statistical core. The CMR imaging protocols, analysis methods, and quality assurance procedures were published previously. 2
Clinical, arrhythmia, CMR, and outcome data were collected. Age‐related predictor variables included age at repair, age at CMR, and time from repair to CMR. Age at CMR was considered as a predictor given our objective to risk stratify at a given clinical evaluation time point. Other patient data included demographics (sex), anatomic type of tetralogy of Fallot (TOF; classified as TOF with pulmonary stenosis or pulmonary atresia), related TOF anomalies (major aortopulmonary collateral arteries, absent pulmonary valve), type of TOF repair (classified as transannular patch, right ventricle‐to‐pulmonary artery conduit, or other, inclusive of a variety of pulmonary valve–preserving procedures such as infundibulotomy and valvuloplasty as well as other types of RV outflow reconstructions except transannular patch and right ventricle‐to‐pulmonary artery conduit), associated cardiovascular anomalies, clinical arrhythmia history (including ECG and Holter results, ablation, and pacemaker/implantable cardioverter defibrillator placement), genetic abnormalities, other cardiac procedures (including RV outflow tract/pulmonary valve dilation, RV outflow tract/pulmonary valve stent, branch pulmonary artery dilation or stent, pulmonary artery surgery, and other surgical or catheter‐based interventions), and clinical outcomes.
Diagnostic studies included CMR, ECG, and ambulatory 24‐hour Holter monitor. The qualifying CMR was defined as the earliest postrepair CMR including all required data elements. CMR measurements included RV and left ventricular mass and volumes. Calculations included ventricular stroke volume, ejection fraction, and biventricular global function index (BVGFI). 11 Measurements were normalized to body surface area, which was calculated using the Haycock formula. QRS duration was extracted from ECG tracing. The 24‐hour Holter monitor was reviewed for atrial arrhythmias (defined as atrial fibrillation, atrial flutter, ≥3 consecutive beats of supraventricular tachycardia) and nonsustained (lasting <30 seconds) or sustained (lasting ≥30 seconds) VT.
In a subgroup of patients, echocardiographic and exercise stress test data were also available within 1 year of the qualifying CMR. Tricuspid regurgitation and RV systolic pressure (based on peak velocities of the tricuspid regurgitation jet and RV outflow) were assessed from Doppler echocardiographic data. Indexed peak oxygen consumption and ventilatory anaerobic threshold were extracted from exercise stress tests.
Outcomes
The primary outcome was defined as time to all‐cause death. All‐cause death was further classified as cardiac, noncardiac, or unknown cause of death. When possible, mortality classification was determined by the US National Death Index underlying cause of death. The composite secondary outcome of major arrhythmias was defined as time to the earliest occurrence of aborted sudden death (defined as resuscitated cardiac arrest or defibrillator shock for ventricular fibrillation), sustained atrial arrhythmia (such as atrial flutter or fibrillation), sustained VT (lasting ≥30 seconds or requiring cardioversion), or nonsustained VT. Outcomes were ascertained through periodic reviews of clinical records and electronic searches of death records in each participating center. The database was closed for analysis on December 31, 2021.
Model Development and External Validation
Patients enrolled in the London, Amsterdam, and Boston sites were placed in the development cohort. Patients from the Toronto site, which comprised 21% of the overall cohort patients, were assigned to the external validation cohort and, therefore, were excluded from the development cohort.
Statistical Analysis
Categorical data were described as number with frequency, and continuous data were described as median with interquartile range or mean with SD, as appropriate. Differences between outcome groups were compared using Student's t test, the Wilcoxon rank‐sum test, Fisher's exact test, or χ2 test, as appropriate.
Cox proportional hazards regression was used to evaluate factors associated with time from CMR until the primary outcome. Patients who did not experience the primary outcome were censored at the time of the last follow‐up. Kaplan–Meier methodology was used to estimate primary outcome event rates. Competing risks (subdistribution hazard) methodology was used to estimate secondary outcome event rates. For univariate modeling, continuous variables had cut points modeled for piecewise linear fits if generalized additive modeling or restricted cubic splines suggested a nonlinear relationship between predictors and the primary outcome. In addition, classification and regression tree modeling was employed to identify potentially important risk subgroups to be included in modeling. Atrial arrhythmia, VT, pacemaker placement, and implantable cardioverter‐defibrillator placement were included in the analysis as time‐dependent covariates due to their potential occurrence after the qualifying CMR. Mean imputation was used for missing values for candidate predictors that had missing data in <2% of patients. Variables with ≥10% missing data were excluded from further analyses.
For Cox multivariable model construction, bidirectional stepwise selection was used; classification and regression tree groupings and variables with a univariate P<0.2 were included as candidates. The criterion for candidate variable entry into the model was P<0.15, and the criterion for remaining in the final model was P<0.05. Similar to others, 12 a continuous risk scoring system for the primary outcome was then constructed from the final multivariable Cox regression model parameter estimates. Each score weight associated with a risk factor is proportional to its log hazard ratio parameter from the final Cox model. The optimal cut point for the risk score to dichotomize patients into low‐ and high‐risk groups was calculated using a log‐rank test statistic‐based approach. 13 The operating characteristics of the binary risk score were evaluated in both cohorts using all available follow‐up, as well as using a follow‐up restricted to specific time points. This study conforms to the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis checklist. 14
A P value <0.05 was considered statistically significant. Harrell's concordance index and hazard ratio are reported with 95% CIs. Analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC) and R 4.03 (R Core Team, Vienna, Austria).
RESULTS
Study Population
Of the 1808 eligible patients enrolled in the INDICATOR database, 1552 fulfilled entry criteria, forming the main study cohort (Figure 1). Birth years ranged from 1932 to 2012. Of these 1552 patients (Table 1), a majority had TOF with pulmonary stenosis (84%), and 16% had TOF with pulmonary atresia. Nearly one third of patients had an initial palliative shunt before TOF repair. Median age at repair was 2.1 (interquartile range, 0.4–6.0) years, with 47% of patients having their TOF repair before 1986. Half of the patients had a transannular patch repair, and 14% had a right ventricle‐to‐pulmonary artery conduit repair. Over half (58%) of patients had a PVR after TOF repair. Qualifying CMR was performed at a median age of 23.4 (interquartile range, 15.6–35.6) years. Atrial arrhythmias and nonsustained VT were experienced by 8% and 12% of patients, respectively.
Figure 1. Strengthening the reporting of observational studies in epidemiology diagram showing patient selection, reasons for exclusion, and primary outcomes.
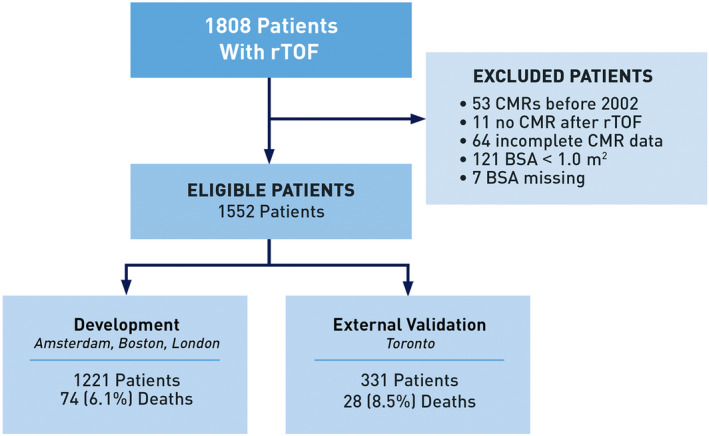
BSA indicates body surface area; CMR, cardiac magnetic resonance imaging; and rTOF repaired tetralogy of Fallot.
Table 1.
Comparison of Demographic, Anatomic, Surgical, CMR, and Arrhythmia Characteristics Stratified by Study Cohort
| Variable | All patients (N=1552) | Development (n=1221) | Validation (n=331) | P value |
|---|---|---|---|---|
| Demographics | ||||
| Age at repair, y | 2.12 (0.44–6.01) [IQR] | 1.16 (0.32–4.94) [IQR] | 5.22 (3.82–8.48) [IQR] | <0.001 |
| Year of TOF repair ≤1985, n (%) | 734 (47.3) | 497 (40.7) | 237 (71.6) | <0.001 |
| Age at CMR, y | 23.4 (15.6–35.6) [IQR] | 20.7 (13.9–33.0) [IQR] | 31.1 (23.0–40.8) [IQR] | <0.001 |
| Time from repair to CMR, y | 20.2 (13.9–29.4) [IQR] | 18.6 (12.9–28.2) [IQR] | 24.6 (18.7–32.3) [IQR] | <0.001 |
| Follow‐up time after CMR, y | 9.5 (4.1–14.6) [IQR] | 8.2 (3.4–13.4) [IQR] | 13.9 (10.1–15.8) [IQR] | <0.001 |
| Sex, male, n (%) | 853 (55.0) | 660 (54.1) | 193 (58.3) | 0.17 |
| BMI at CMR, kg/m2 | 24.0±5.9 | 23.7±6.2 | 25.3±4.9 | <0.001 |
| Diagnosis | ||||
| TOF diagnosis, n (%) | <0.001 | |||
| TOF/PS | 1296 (83.5) [IQR] | 984 (80.6) [IQR] | 312 (94.3) [IQR] | |
| TOF/PA | 256 (16.5) [IQR] | 237 (19.4) [IQR] | 19 (5.7) [IQR] | |
| Genetic anomaly, n (%) | 192 (12.4) | 159 (13.0) | 33 (10.0) | 0.16 |
| Additional cardiovascular anomaly | 717 (46.2) [IQR] | 540 (44.2) [IQR] | 177 (53.5) [IQR] | <0.01 |
| MAPCAs, n (%) | 58 (3.7) | 53 (4.3) | 5 (1.5) | 0.02 |
| Absent pulmonary valve | 28 (1.8) [IQR] | 22 (1.8) [IQR] | 6 (1.8) [IQR] | 0.99 |
| Prior procedures | ||||
| Pre‐TOF repair palliative shunt, n (%) | 488 (31.4) | 330 (27.0) | 158 (47.7) | <0.001 |
| TOF repair type, n (%) | <0.001 | |||
| Transannular patch | 773 (49.8) [IQR] | 643 (52.7) [IQR] | 130 (39.3) [IQR] | |
| Right ventricle‐to‐pulmonary artery conduit | 221 (14.2) [IQR] | 198 (16.2) [IQR] | 23 (6.9) [IQR] | |
| Other | 558 (36.0) [IQR] | 380 (31.1) [IQR] | 178 (53.8) [IQR] | |
| Postrepair cardiac procedures | 935 (60.2) [IQR] | 712 (58.3) [IQR] | 223 (67.4) [IQR] | <0.01 |
| Postrepair PVR | 893 (57.5) [IQR] | 716 (58.6) [IQR] | 177 (53.5) [IQR] | 0.10 |
| CMR measurement | ||||
| RVEDVi, mL/m2 | 146.1±46.4 | 145.4±46.6 | 148.6±45.6 | 0.27 |
| RVESVi, mL/m2 | 76.2±33.8 | 74.6±34.1 | 81.8±32.3 | <0.001 |
| RVEF, % | 49.1±8.8 | 49.9±8.6 | 45.9±8.6 | <0.001 |
| RV mass index, g/m2 | 32.2±11.5 | 33.3±12.1 | 28.3±8.1 | <0.001 |
| RV mass/volume ratio, g/mL | 0.23±0.07 | 0.24±0.08 | 0.20±0.04 | <0.001 |
| LVEDVi, mL/m2 | 86.9±20.9 | 86.7±20.0 | 87.8±23.7 | 0.42 |
| LVESVi, mL/m2 | 37.6±14.8 | 37.2±13.5 | 39.0±18.7 | 0.11 |
| LVEF, % | 57.5±7.8 | 57.7±7.1 | 56.7±9.9 | 0.07 |
| LV mass index, g/m2 | 51.7±14.3 | 52.4±14.6 | 49.0±13.2 | <0.001 |
| LV mass/volume ratio, g/mL | 0.61±0.15 | 0.62±0.16 | 0.57±0.12 | <0.001 |
| BVGFI | 48.2±8.5 | 48.5±8.3 | 47.2±9.1 | 0.02 |
| RV/LVEDV ratio | 1.73±0.55 | 1.72±0.55 | 1.75±0.57 | 0.42 |
| Rhythm data | ||||
| Atrial arrhythmia, n (%) | 190 (12.2) | 135 (11.1) | 55 (16.6) | <0.01 |
| Nonsustained VT, n (%) | 255 (16.4) | 213 (17.4) | 42 (12.7) | 0.04 |
| Pacemaker, n (%) | 30 (1.9) | 22 (1.8) | 8 (2.4) | 0.50 |
| Implantable cardioverter‐defibrillator, n (%) | 109 (7.0) | 92 (7.5) | 17 (5.1) | 0.15 |
| QRS duration, ms | 144.9±27.2 | 142.6±27.0 | 153.2±26.1 | <0.001 |
| Outcomes | ||||
| All‐cause death, n (%) | 102 (6.6) | 74 (6.1) | 28 (8.5) | 0.13 |
| Cause of death, n (%) | 0.69 | |||
| Cardiac | 47 (46.1) [IQR] | 35 (47.3) [IQR] | 12 (42.9) [IQR] | |
| Noncardiac | 26 (25.5) [IQR] | 17 (23.0) [IQR] | 9 (32.1) [IQR] | |
| Unknown | 29 (28.4) [IQR] | 22 (29.7) [IQR] | 7 (25.0) [IQR] | |
| Nonsustained VT, n (%) | 186 (12.0) | 155 (12.7) | 31 (9.4) | 0.11 |
| Sustained VT, n (%) | 31 (2.0) | 24 (2.0) | 7 (2.1) | 0.83 |
| Aborted sudden death, n (%) | 15 (1.0) | 15 (1.2) | 0 (0) | 0.05 |
| Atrial arrhythmia, n (%) | 126 (8.1) | 91 (7.5) | 35 (10.6) | 0.07 |
BMI indicates body mass index; BVGFI, biventricular global function index; CMR, cardiovascular magnetic resonance imaging; ICD, implantable cardioverter‐defibrillator; LV, left ventricular; LVEDV, left ventricular end‐diastolic volume; LVEDVi, left ventricular end‐diastolic volume index; LVEF, left ventricular ejection fraction; LVESVi, left ventricular end‐systolic volume index; MAPCAs, major aortopulmonary collateral arteries; PA, pulmonary atresia; PS, pulmonary stenosis; PVR, pulmonary valve replacement; RV, right ventricular; RVEDVi, right ventricular end‐diastolic volume index; RVEF, right ventricular ejection fraction; RVESVi, right ventricular end‐systolic volume index; TOF, tetralogy of Fallot; and VT, ventricular tachycardia.
Several differences were found between the development and external validation cohort baseline characteristics (Table 1). Patients in the external validation group had an older age at repair and CMR, earlier repair era, longer follow‐up time, and higher body mass index (BMI) (all P<0.001). In addition, the external validation group had a lower percentage of TOF with pulmonary atresia diagnoses (with corresponding lower major aortopulmonary collateral arteries and lower percentage of transannular patch repair), and differences in several CMR parameters.
Predictors of the Primary Outcome
In the overall cohort, median follow‐up time after qualifying CMR was 9.5 (interquartile range, 4.1–14.6) years. Of these patients, 102 (6.6%) experienced the primary outcome. The mode of death was classified as cardiac in 47 (46.1%), noncardiac in 26 (25.5%), and unknown in 29 (28.4%) patients. Of the 47 cardiac deaths, 15 were sudden cardiac death, 18 were heart failure, and 14 were considered “other” cardiac death (6 related to cardiac interventions, 3 related to endocarditis, 5 unspecified). There were no differences in the mortality rates or the distributions of cause of death for the development and external validation cohorts (Table 1). Kaplan–Meier estimates further highlight the similarity in survival between the cohorts (P=0.55; Figure S1). Survival after repair was also similar across cohorts (P=0.10; Figure S2).
Within the development cohort (n=1221), 74 (6.1%) patients experienced the primary outcome of death. The univariate associations of demographic, clinical, CMR, and arrhythmia variables with the primary outcome in the development data set are shown in Table 2. Among demographic and clinical variables, anatomic TOF type (TOF with pulmonary atresia), major aortopulmonary collateral arteries, type of TOF repair (right ventricle‐to‐pulmonary artery conduit or other versus transannular patch), initial palliative shunt, older age at CMR, earlier year and older age of repair, and higher BMI were associated with a higher risk of death (Table 2). Among CMR variables, RV dysfunction and hypertrophy, as well as left ventricular dysfunction and hypertrophy, were associated with the primary outcome (Table 2). Notably, lower BVGFI, a previously described calculated CMR imaging biomarker, was also associated with earlier time to death (both P<0.001; Table 2). Among arrhythmia variables, atrial arrhythmia, nonsustained VT, implantable cardioverter‐defibrillator implantation, and longer QRS duration were risk factors for death (Table 2). Table S1 and Figure S3 demonstrate similar patient characteristics and survival time estimates when stratifying patients by causes of death.
Table 2.
Univariate Association of Demographic, Anatomic, Surgical, CMR, and Arrhythmia Characteristics With All‐Cause Death in the Development Cohort
| Variable | Survivor (n=1147) | Death (n=74) | Univariate Cox model, HR (95% CI) | P value |
|---|---|---|---|---|
| Demographics | ||||
| Age at repair, y | 1.00 (0.30–3.99) | 8.39 (3.23–14.08) | 1.08 (1.06–1.09) | <0.001 |
| Year of TOF repair ≤1985, n (%) | 444 (38.7) | 53 (71.6) | 2.46 (1.48–4.10) | <0.001 |
| Age at CMR, y | 19.8 (13.6–31.5) | 39.2 (25.3–50.5) | 1.06 (1.05–1.08) | <0.001 |
| Time from repair to CMR | 18.4 (12.6–27.8) | 29.3 (18.5–35.6) | 1.07 (1.04–1.09) | <0.001 |
| Sex, male, n (%) | 619 (54.0) | 41 (55.4) | 1.04 (0.66–1.65) | 0.86 |
| BMI at CMR, kg/m2 | 23.5±6.1 | 26.5±6.0 | 1.07 (1.03–1.10) | <0.001 |
| Obese (BMI ≥30), n (%) | <0.001 | |||
| Yes | 160 (13.9) | 21 (28.4) | 2.48 (1.49–4.12) | |
| No | 987 (86.1) | 53 (71.6) | Reference | |
| Diagnosis | ||||
| TOF diagnosis, n (%) | 0.03 | |||
| TOF/PS | 931 (81.2) | 53 (71.6) | Reference | |
| TOF/PA | 216 (18.8) | 21 (28.4) | 1.74 (1.05–2.88) | |
| Genetic anomaly, n (%) | 153 (13.3) | 6 (8.1) | 0.65 (0.28–1.51) | 0.32 |
| Additional cardiovascular anomaly, n (%) | 514 (44.8) | 26 (35.1) | 0.94 (0.58–1.52) | 0.79 |
| MAPCAs | 46 (4.0) | 7 (9.5) | 3.26 (1.50–7.12) | <0.01 |
| Absent pulmonary valve | 22 (1.9) | 0 (0) | — | — |
| Prior procedures | ||||
| Pre‐TOF repair palliative shunt, n (%) | 288 (25.1) | 42 (56.8) | 2.80 (1.76–4.43) | <0.001 |
| TOF repair type, n (%) | <0.01 | |||
| Transannular patch | 619 (54.0) | 24 (32.4) | Reference | |
| Right ventricle‐to‐pulmonary artery conduit | 179 (15.6) | 19 (25.7) | 2.50 (1.37–4.57) | |
| Other | 349 (30.4) | 31 (41.9) | 2.36 (1.39–4.03) | |
| Postrepair cardiac procedures, n (%) | 661 (57.6) | 51 (68.9) | 1.84 (1.12–3.02) | 0.02 |
| Postrepair PVR, n (%) | 674 (58.8) | 42 (56.8) | 1.06 (0.67–1.70) | 0.79 |
| CMR parameters | ||||
| RVEDVi, mL/m2 | 144.2±45.2 | 164.0±62.5 | 1.08 (1.03–1.12)* | <0.001 |
| RVESVi, mL/m2 | 73.1±32.1 | 98.2±51.4 | 1.14 (1.09–1.19)* | <0.001 |
| RV EF, % | 50.4±8.2 | 42.3±10.9 | 0.41 (0.33–0.51)* | <0.001 |
| RV mass index, g/m2 | 32.7±11.4 | 42.7±16.8 | 1.48 (1.30–1.68)* | <0.001 |
| RV mass/volume ratio, g/mL | 0.24±0.08 | 0.27±0.09 | 1.34 (1.07–1.67)† | 0.011 |
| LVEDVi, mL/m2 | 86.2±19.3 | 94.8±28.0 | 1.16 (1.07–1.27)* | <0.001 |
| LVESVi, mL/m2 | 36.7±12.8 | 44.8±20.2 | 1.38 (1.23–1.54)* | <0.001 |
| LV EF, % | 58.0±6.8 | 54.0±9.5 | 0.45 (0.34–0.60)* | <0.001 |
| LV mass, index, g/m2 | 51.6±13.6 | 64.7±21.6 | 1.46 (1.30–1.63)* | <0.001 |
| LV mass/volume ratio, g/mL | 0.61±0.15 | 0.70±0.22 | 1.25 (1.12–1.40)† | <0.001 |
| BVGFI | 49.0±7.9 | 40.3±9.9 | 0.90 (0.88–0.92) | <0.001 |
| RV/LVEDV ratio | 1.72±0.54 | 1.82±0.71 | 1.03 (1.00–1.07)† | 0.09 |
| Arrhythmia data | ||||
| Atrial arrhythmia, n (%)‡ | 108 (9.4%) | 27 (36.5%) | 5.15 (3.16–8.39) | <0.001 |
| Nonsustained VT, n (%)‡ | 190 (16.6%) | 23 (31.1%) | 2.43 (1.48–4.01) | <0.001 |
| Pacemaker, n (%)‡ | 19 (1.7%) | 3 (4.1%) | 2.09 (0.65–6.74) | 0.22 |
| Implantable cardioverter‐defibrillator, n (%)‡ | 78 (6.8%) | 14 (18.9%) | 2.37 (1.30–4.33) | 0.047 |
| Sustained VT n (%)‡ | 33 (2.9%) | 3 (4.1%) | 1.69 (0.53–5.41) | 0.38 |
| QRS duration, ms | 141.5±26.7 | 160.4±26.6 | 1.24 (1.14–1.36) | <0.001 |
BMI indicates body mass index; BVGFI, biventricular global function index; CMR, cardiovascular magnetic resonance imaging; HR, hazard ratio; ICD, implantable cardioverter‐defibrillator; LV, left ventricular; LVEDV, left ventricular end‐diastolic volume; LVEDVi, left ventricular end‐diastolic volume index; LVEF, left ventricular ejection fraction; LVESVi, left ventricular end‐systolic volume index; MAPCAs, major aortopulmonary collateral arteries; PA, pulmonary atresia; PS, pulmonary stenosis; PVR, pulmonary valve replacement; RV, right ventricular; RVEDVi, right ventricular end‐diastolic volume index; RVEF, right ventricular ejection fraction; RVESVi, right ventricular end‐systolic volume index; TOF, tetralogy of Fallot; and VT, ventricular tachycardia.
HR per 10‐unit increase.
HR per 0.1‐unit increase.
Time‐dependent covariate.
Using the development cohort, a multivariable Cox regression model was constructed (Table 3 and Figure 2), which included 5 independent predictors of shorter survival: older age at CMR (hazard ratio [HR], 1.06 per year [95% CI, 1.04–1.08]; P<0.001), obesity (BMI ≥30 kg/m2) (HR, 1.70 [95% CI, 1.01–2.86]; P=0.046), TOF repair type (right ventricle‐to‐pulmonary artery conduit (HR, 2.97 [95% CI, 1.58–5.57]), or other (HR, 1.95 [95% CI, 1.16–3.38]) versus transannular patch; P=0.002), higher RV end‐systolic volume index (HR, 1.09 per 10 mL/m2 increase [95% CI, 1.04‐1.15]; P<0.001), and lower BVGFI (HR, 1.97 per 10% decrease [95% CI, 1.44–2.70]; P<0.001). The concordance index of the final model was 0.83 (95% CI, 0.78–0.88) and 0.80 (95% CI, 0.71–0.90) in the development and validation cohorts, respectively.
Table 3.
Multivariable Cox Regression Model for the Primary Outcome
| Variable | Parameter estimate | HR | 95% CI | P value |
|---|---|---|---|---|
| Age at CMR, y | 0.05785 | 1.06 | 1.04–1.08 | <0.001 |
| Obesity (BMI ≥30 kg/m2) | 0.53076 | 1.70 | 1.01–2.86 | 0.046 |
| TOF repair type | 0.002 | |||
| Transannular patch | 0 | Reference | ||
| Right ventricle‐to‐pulmonary artery conduit | 1.08807 | 2.97 | 1.58–5.57 | |
| Other | 0.66857 | 1.95 | 1.16–3.38 | |
| RVESVi | 0.00883 | 1.09* | 1.04–1.15* | <0.001 |
| BVGFI‡ | −0.06769 | 1.97† | 1.44–2.70† | <0.001 |
Model concordance index 0.83 (95% CI, 0.78–0.88) and 0.80 (95% CI, 0.71–0.90) in the development and validation cohorts, respectively. BMI indicates body mass index; BVGFI, biventricular global function index; CMR, cardiac magnetic resonance; EDV, end‐diastolic volume; ESV, end‐systolic volume; GFI, global function index; HR, hazard ratio; LVGFI, left ventricular global function index; RVESVi, right ventricular end‐systolic volume index; RVGFI, right ventricular global function index; and TOF, tetralogy of Fallot.
Per 10‐unit ↑.
Per 10‐unit ↓.
BVGFI is defined as follows 11 : and BV.
Figure 2. Schematic of mortality risk score model.
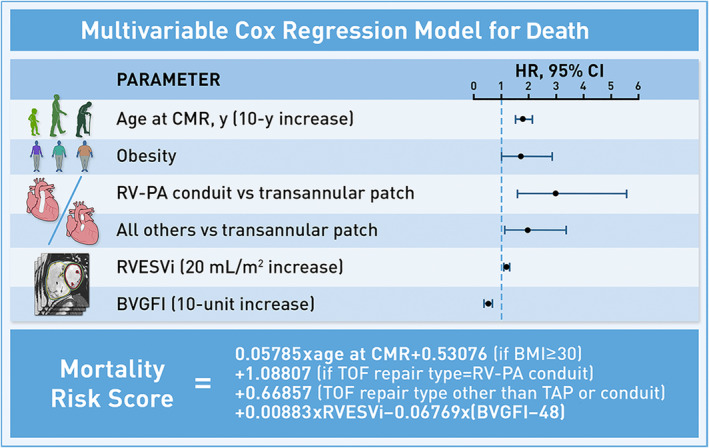
Schematic of the multivariable Cox regression model for death, with mortality risk score equation inset below. BVGFI indicates biventricular global function index; CMR, cardiac magnetic resonance; HR, hazard ratio; RVESVi, right ventricular end‐systolic volume index; RV‐PA, right ventricle‐to‐pulmonary artery; TAP, transannular patch; TOF, tetralogy of Fallot.
Subgroup Analysis
A multivariable Cox regression model was also formulated for a subgroup of patients with exercise stress test and echocardiography data available from a single institution (n=334; 15 primary outcome events). In this cohort, lower indexed peak Vo 2 (P<0.001) was the only exercise or echocardiographic measure identified by multivariable analysis to be included as an independent predictor of shorter time to death (concordance index, 0.93 [95% CI, 0.88–0.98]).
Primary Outcome Risk Stratification Algorithm
The Cox regression model for the primary outcome (Table 3 and Figure 2) was subsequently used to develop a risk‐scoring system defined by the following equation:
 |
The risk scores within the development and validation cohorts had normal distributions with mean values of 2.5 (range, −0.6 to 7.7) and 3.2 (range, 0.5–6.5), respectively (Figure S4). At 5 years, the risk score resulted in areas under the receiver operating curves of 0.86 (95% CI, 0.79–0.94) and 0.95 (95% CI, 0.89–1.00) for the development and validation cohorts, respectively. At 15 years, areas under the receiver operating curves of 0.83 (95% CI, 0.76–0.89) and 0.77 (95% CI, 0.66–0.88) were achieved, respectively.
In the development cohort, there was a higher observed mortality rate for increasing risk score deciles, with a striking increase from the 8th to 9th decile (Figure 3), corresponding to a raw risk score between 3.53 and 4.12. The observed mortality rate was generally similar in the validation cohort, except for the lower observed death rate in the 10th decile (Figure 3). The model was well calibrated in both cohorts through nearly 50% and 25% predicted 10‐year mortality rate in the development and validation cohorts, respectively (Figure S5), which corresponds to the respective ranges of observed 10‐year mortality rate within risk score deciles (Figure 3). Above this, the model began to overestimate mortality risk (Figure S5).
Figure 3. Comparison of observed deaths in development and validation cohorts across risk score deciles.
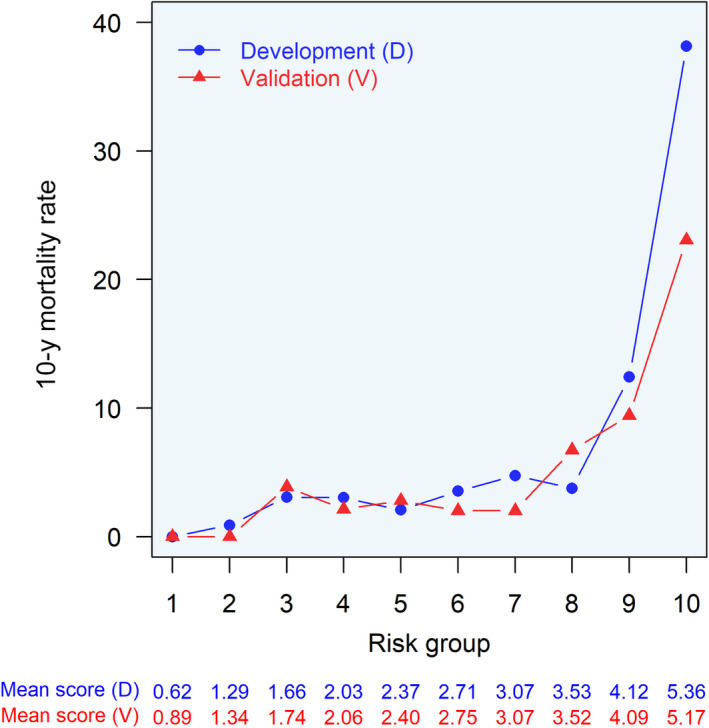
For each risk score decile, observed death at 10 years was compared between the development (blue) and external validation (red) cohorts. Raw mean risk score within each risk score decile is shown below for development (blue text) and external validation (red text) cohorts.
Using a log‐rank test statistic‐based approach on the development cohort, 13 a cutoff score of 4 (≤4 versus >4) was selected to stratify patients into low‐ and high‐risk groups, respectively, in both development and validation cohorts (Figure 4). A cutoff score of 4 effectively discriminated between patients with low and high risk for death at 5, 10, and 15 years in both the development and validation cohorts (both P<0.001; Figure 4 and Table S2). At this cutoff, negative predictive values of 99% and 100% were achieved for 5‐year follow‐up in the development and validation cohorts, respectively (Table 4). At 15‐year follow‐up, positive predictive values were 62% and 43%, respectively (Table 4).
Figure 4. Survival of low‐ and high‐risk groups in development and external validation cohorts.
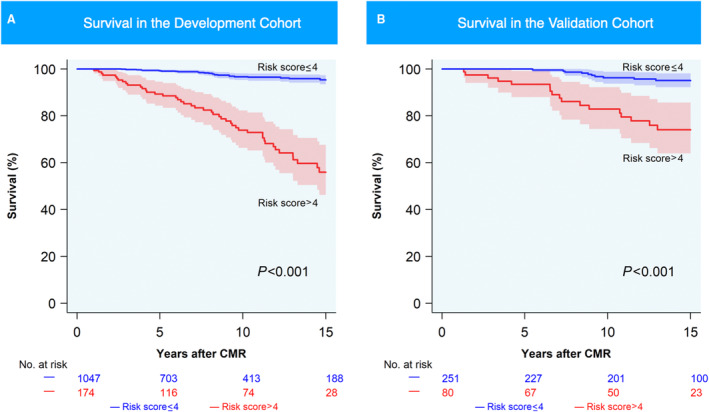
Kaplan–Meier estimates of survival in the (A) development and (B) external validation cohorts for low‐risk (blue; mortality risk score≤4) and high‐risk (red; mortality risk score>4) groups. CMR indicates cardiac magnetic resonance.
Table 4.
Mortality Rates Stratified by Risk Score Groups
| Development | Validation | |||
|---|---|---|---|---|
| High risk score>4 | Low risk score≤4 | High risk score>4 | Low risk score≤4 | |
| 5‐y follow‐up | ||||
| No. | 131 | 709 | 72 | 227 |
| Death, n (%) | 15 (11.5) | 6 (0.9) | 5 (6.9) | 0 (0) |
| Survival, n (%) | 116 (88.6) | 703 (99.2) | 67 (93.1) | 227 (100) |
| PPV, % | 11.5 | 6.9 | ||
| NPV, % | 99.2 | 100 | ||
| Sensitivity, % | 71.4 | 100 | ||
| Specificity, % | 85.8 | 77.2 | ||
| Accuracy, % | 85.5 | 77.6 | ||
| 15‐y follow‐up | ||||
| No. | 73 | 212 | 40 | 110 |
| Death, n (%) | 45 (61.6) | 24 (11.3) | 17 (42.5) | 10 (9.1) |
| Survival, n (%) | 28 (38.4) | 188 (88.7) | 23 (57.5) | 100 (90.9) |
| PPV, % | 61.6 | 42.5 | ||
| NPV, % | 88.7 | 90.9 | ||
| Sensitivity, % | 65.2 | 63.0 | ||
| Specificity, % | 87.0 | 81.3 | ||
| Accuracy, % | 81.8 | 78.0 | ||
| Overall | ||||
| No. | 174 | 1047 | 80 | 251 |
| Death, n (%) | 46 (26.4) | 28 (2.7) | 17 (21.3) | 11 (4.4) |
| Survival, n (%) | 128 (73.6) | 1019 (97.3) | 63 (78.7) | 240 (95.6) |
| PPV, % | 26.4 | 21.3 | ||
| NPV, % | 97.3 | 95.6 | ||
| Sensitivity, % | 62.2 | 60.7 | ||
| Specificity, % | 88.8 | 79.2 | ||
| Accuracy, % | 87.2 | 77.6 | ||
Data sets restricted to those with specified follow‐up (or death prior). NPV indicates negative predictive value; and PPV positive predictive value.
Secondary Outcome Predictors
For the secondary composite outcome analysis, 170 patients were excluded for experiencing the outcome before the qualifying CMR. In the development cohort, there were 1090 qualifying patients, and the composite secondary outcome was experienced in 172 (15.8%) patients. Of those, 19 (11.0%) patients met the primary outcome after the secondary outcome. Figure S6 shows a competing risks analysis for the cumulative incidence of the primary and secondary outcomes. A multivariable Cox regression model (Table S3) identified the following variables as independently associated with the composite secondary outcome: older age at CMR (HR, 1.04 [95% CI, 1.02–1.05]; P<0.001), additional cardiac procedures (HR, 2.22 [95% CI, 1.60–3.08]; P<0.001), RV end‐systolic volume index (HR, 1.08 for 10 mL/m2 increase [95% CI, 1.04–1.12]; P<0.001), and left ventricular mass index (HR, 1.27 for 10 g/m2 increase [95% CI, 1.15–1.40]; P<0.001). The model concordance index was 0.72 (95% CI, 0.67–0.76) in the development cohort and 0.69 (95% CI, 0.61–0.77) in the external validation cohort.
DISCUSSION
The increasing rates of morbidity and premature death in the growing population of adults with rTOF has motivated the development of risk stratification tools using invasive and noninvasive measures. 3 , 7 , 8 , 9 However, these studies are often hindered by small sample size, lack of population diversity, short length of follow‐up, paucity of major outcome events, and lack of model validation, all leading to limited generalizability. The use of a large cohort derived from 4 congenital heart centers in Europe and North America followed for nearly a decade after detailed phenotyping at study entry enabled us, for the first time, to derive and externally validate a mortality risk prediction model for rTOF. In addition, the external validation of low‐ and high‐risk strata to effectively discriminate risk of death provides a practical tool to identify high‐risk patients with rTOF who require frequent surveillance and may benefit from targeted interventions.
Clinical Implications
To be broadly applicable, a risk stratification algorithm should be easily accessible and operational. Indeed, this risk calculator is available online (https://github.com/rTOF‐INDICATOR/Mortality‐Risk‐Score). In addition, a risk stratification algorithm should be derived and externally validated on a sufficiently large and diverse population. As shown in Table 1, there were several notable differences between our development and validation cohorts, even among the predictor variables included in the final multivariable model: older age at CMR, BMI, TOF repair type, RV end‐systolic index, and BVGFI. Despite these notable differences in baseline patient characteristics, model performance remained high in the external validation cohort, suggesting that the model is broadly applicable even when the populations are not identical.
From a translational perspective, our model provides a framework for risk stratification of patients with rTOF, which can inform frequency of evaluations, use of testing resources, and recommendations for therapeutic interventions such as PVR for high‐risk patients. As a thought example, patients with rTOF with low risk scores (ie, ≈80% of patients in our study) would have a negative predictive value of 99% to 100% within 5 years, which may help guide clinical decision making to decrease the frequency of evaluations. For example, recent guidelines 15 recommend CMR surveillance every 2 to 3 years for patients with no‐to‐mild disease progression; in contrast, our high negative predictive values for low‐risk patients may support decreased frequency of routine CMR examinations to every 5 years. In contrast, patients with high risk scores would have 15‐year positive predictive values of 43% to 62%, which may prompt clinicians to consider therapeutic interventions such as PVR.
Consistent with previous work, we found that PVR was not associated with death when not adjusting for pre‐PVR baseline characteristics. 16 In contrast, Bokma et al 10 recently demonstrated in propensity score–matched analysis that individuals receiving PVR had lower risk of a composite end point of death or sustained VT than those not undergoing PVR. In addition, in patients with RV end‐systolic volume index >80 mL/m2, PVR was associated with a lower risk of adverse outcome. Interestingly, our final multivariable model also identified elevated RV end‐systolic volume index as an independent predictor of death. Together, these studies may help inform future work to determine the optimal timing of an intervention such as PVR to prevent the mechanoelectrical cardiomyopathy of rTOF. 3
Age‐Related Risk Factors
Broadly, advancing age likely accounts for the cumulative pathophysiologic burden of the disease process in this population. Therefore, in this study, we considered age at CMR, age at repair, and time from repair to CMR as predictor variables. Age at CMR, unlike age at repair, reflects this cumulating disease burden, as well as (1) age‐related comorbidities that may accumulate in the adult congenital heart disease population over time; and (2) postoperative considerations that may influence timing of surveillance. Accordingly, age at CMR aligns with our objective to risk stratify at a given clinical evaluation time point and was proven to be a superior predictor variable in our multivariable analysis.
Notably, for patients with repairs before 1986, the median age at CMR was 35.6 years compared with 16.0 years for patients repaired from 1986 onward. Nearly half of patients were repaired before 1986 (Table 2), highlighting the diversity in our cohort, spanning both older and contemporary procedures and clinical practices.
Other Risk Factors for Adults With rTOF
Our findings also provide insight into other risk factors for adverse clinical outcomes in patients with rTOF. BVGFI, recently shown to be associated with worse clinical outcomes in rTOF, 11 encompasses RV and left ventricular volumes, mass, and function. Each of the core components of the BVGFI equation has been previously shown to be associated with adverse outcomes in rTOF. 3 , 7 , 8 In addition, elevated RV end‐systolic volume was identified as an independent risk factor, which further suggests that RV myopathy has a major impact on clinical decision making in these patients. 10 While not identified in our previous model, in the current study, initial repair with right ventricle‐to‐pulmonary artery conduits and other types of RV outflow reconstruction as compared with a transannular patch were associated with worse outcomes. This is likely related to the underlying pathophysiology of combined pressure and volume overload characterized by mixed pulmonary stenosis and regurgitation, a higher risk for conduit reinterventions, and a higher incidence of underlying TOF/pulmonary atresia with known lower long‐term survival. 17 Obesity, a potentially modifiable risk factor, was also identified as an independent predictor. Previous work showed that elevated BMI was associated with worsened biventricular systolic function and biventricular dilatation (and thus presumably lower BVGFI) late in rTOF. 18 Obesity is closely linked to metabolic syndrome, which is more common in adults with congenital heart disease compared with the general population, 19 and is associated with increased rates of adverse cardiovascular outcomes and all‐cause death. 20 Our findings support a proactive approach to therapeutic interventions in patients with obesity and rTOF, including incorporation of preventive cardiology as part of their routine ambulatory care. In contrast to our previous model, 7 atrial arrhythmia was not identified herein as an independent risk factor of the primary outcome. This may be attributed to differences in primary outcome definitions in comparison with our previous model. 7
Limitations and Future Directions
Our study has several limitations. The cohort is restricted by design to patients with CMR data; therefore, patients with pacemakers or implantable cardioverter‐defibrillators implanted before CMR were excluded. This limitation is mitigated in part by routine use of CMR at participating centers and by including patients receiving such devices after baseline CMR. Although the number of primary outcome events in this cohort is substantial, room for model and threshold refinement remains as the cohort size, follow‐up time, and event rate increase; this may lead to improved model calibration at higher risks of death, which may further improve positive predictive values and facilitate more granular high‐risk stratification. We acknowledge the presence of institutional variability in timing of obtaining CMRs, reflecting real‐life practice variations. However, this limitation is also a strength, as our diverse international data set (spanning older and contemporary procedures and clinical practices) proved robust during external testing. Although our study was performed across 4 large adult congenital heart centers on 2 continents with external validation, the risk stratification algorithm still warrants further validation across a range of care levels to confirm generalizability. While we took a parsimonious approach to grouping repair type, further investigation is warranted to evaluate potential benefits of a valve‐sparing approach during initial repair of TOF. 4 Similarly, further investigation into the role of specific surgical approaches (eg, primary ventriculotomy) on outcomes is warranted. While our traditional statistical model performed well with meaningful interpretability, recent work demonstrated machine learning–based models show promise in risk stratifying patients with rTOF. 21 Other imputation techniques for missing data (eg, multiple imputation) could have also been considered. We limited our primary outcome to all‐cause death, as it has been commonly used in outcome research in adult patients with heart disease. 22 Notably, the distribution of causes of death (cardiac, noncardiac, and unknown) in our study is similar to other reports in patients with congenital heart disease. 9 , 23 , 24 To this end, we acknowledge that the available data are not fully comprehensive, with paucity of information about noncardiac comorbidities/deaths, and lagging information on heart transplants prohibiting such analysis herein. Finally, we acknowledge that despite the predictive value of our scoring systems, patient‐level factors beyond the scoring system should be considered.
Our findings provide several avenues for future investigations. First, future exploration into the predictive utility of machine learning–based models using clinical and imaging biomarker inputs is warranted. In addition, the added value of imaging biomarkers such as myocardial strain, fibrosis by CMR T1 mapping, or late gadolinium enhancement should be evaluated. 9 Other research avenues worth exploring include serum biomarkers or exosomal cargo, 25 the role of social determinants of health in outcomes, 26 and artificial intelligence–enhanced ECG and CMR image analyses to improve outcome prediction. 27 , 28
CONCLUSIONS
In this multicenter cohort study, we developed and externally validated a risk‐scoring algorithm that effectively identified low‐ and high‐risk strata of all‐cause death. These findings represent a step toward improved selection of high‐risk adult patients with rTOF who may benefit from targeted intervention and/or closer monitoring.
Sources of Funding
This work was supported in part by the Thrasher Research Fund Early Career Award (Dr Mayourian), National Institutes of Health/National Heart, Lung, and Blood Institute 2U01HL098147‐12 (Dr Geva), the Lerner Research Award and the Sarah Marie Liamos Fund (Dr Valente), British Heart Foundation EX/18/1/34296 (Dr Babu‐Narayan), the Canadian Institutes of Health Research MOP 119353 (Dr Wald), and by Amsterdam Cardiovascular Sciences (Dr Mulder).
Disclosures
Dr. Valente is an advisor to Elsevier's Advisory Board of Practice.
Supporting information
Tables S1–S3
Figures S1–S6
Acknowledgments
The authors thank Kai‐Ou Tang for assisting with artwork.
This manuscript was sent to John L. Jefferies, MD, MPH, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.034871
For Sources of Funding and Disclosures, see page 12.
References
- 1. Goldstein BH, Petit CJ, Qureshi AM, McCracken CE, Kelleman MS, Nicholson GT, Law MA, Meadows JJ, Zampi JD, Shahanavaz S, et al. Comparison of management strategies for neonates with symptomatic tetralogy of Fallot. J Am Coll Cardiol. 2021;77:1093–1106. doi: 10.1016/j.jacc.2020.12.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Geva T. Repaired tetralogy of Fallot: the roles of cardiovascular magnetic resonance in evaluating pathophysiology and for pulmonary valve replacement decision support. J Cardiovasc Magn Reson. 2011;13:9. doi: 10.1186/1532-429X-13-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Geva T, Mulder B, Gauvreau K, Babu‐Narayan SV, Wald RM, Hickey K, Powell AJ, Gatzoulis MA, Valente AM. Preoperative predictors of death and sustained ventricular tachycardia after pulmonary valve replacement in patients with repaired tetralogy of Fallot enrolled in the INDICATOR cohort. Circulation. 2018;138:2106–2115. doi: 10.1161/CIRCULATIONAHA.118.034740 [DOI] [PubMed] [Google Scholar]
- 4. Smith CA, McCracken C, Thomas AS, Spector LG, St Louis JD, Oster ME, Moller JH, Kochilas L. Long‐term outcomes of tetralogy of Fallot: a study from the pediatric cardiac care consortium. JAMA Cardiol. 2019;4:34–41. doi: 10.1001/jamacardio.2018.4255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Geva T, Sandweiss BM, Gauvreau K, Lock JE, Powell AJ. Factors associated with impaired clinical status in long‐term survivors of tetralogy of Fallot repair evaluated by magnetic resonance imaging. J Am Coll Cardiol. 2004;43:1068–1074. doi: 10.1016/j.jacc.2003.10.045 [DOI] [PubMed] [Google Scholar]
- 6. Knauth AL, Gauvreau K, Powell AJ, Landzberg MJ, Walsh EP, Lock JE, del Nido PJ, Geva T. Ventricular size and function assessed by cardiac MRI predict major adverse clinical outcomes late after tetralogy of Fallot repair. Heart. 2008;94:211–216. doi: 10.1136/hrt.2006.104745 [DOI] [PubMed] [Google Scholar]
- 7. Valente AM, Gauvreau K, Assenza GE, Babu‐Narayan SV, Schreier J, Gatzoulis MA, Groenink M, Inuzuka R, Kilner PJ, Koyak Z, et al. Contemporary predictors of death and sustained ventricular tachycardia in patients with repaired tetralogy of Fallot enrolled in the INDICATOR cohort. Heart. 2014;100:247–253. doi: 10.1136/heartjnl-2013-304958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bokma JP, de Wilde KC, Vliegen HW, van Dijk AP, van Melle JP, Meijboom FJ, Zwinderman AH, Groenink M, Mulder BJM, Bouma BJ. Value of cardiovascular magnetic resonance imaging in noninvasive risk stratification in tetralogy of Fallot. JAMA Cardiol. 2017;2:678–683. doi: 10.1001/jamacardio.2016.5818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ghonim S, Gatzoulis MA, Ernst S, Li W, Moon JC, Smith GC, Heng EL, Keegan J, Ho SY, McCarthy KP, et al. Predicting survival in repaired tetralogy of Fallot: a lesion‐specific and personalized approach. JACC Cardiovasc Imaging. 2022;15:257–268. doi: 10.1016/j.jcmg.2021.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bokma JP, Geva T, Sleeper LA, Lee JH, Lu M, Sompolinsky T, Babu‐Narayan SV, Wald RM, Mulder BJM, Valente AM. Improved outcomes after pulmonary valve replacement in repaired tetralogy of Fallot. J Am Coll Cardiol. 2023;81:2075–2085. doi: 10.1016/j.jacc.2023.02.052 [DOI] [PubMed] [Google Scholar]
- 11. Alsaied T, Geva T, Graf JA, Sleeper LA, Marie VA. Biventricular global function index is associated with adverse outcomes in repaired tetralogy of Fallot. Circ Cardiovasc Imaging. 2021;14:e012519. doi: 10.1161/CIRCIMAGING.121.012519 [DOI] [PubMed] [Google Scholar]
- 12. Mehra MR, Nayak A, Morris AA, Lanfear DE, Nemeh H, Desai S, Bansal A, Guerrero‐Miranda C, Hall S, Cleveland JC Jr, et al. Prediction of survival after implantation of a fully magnetically levitated left ventricular assist device. JACC Heart Fail. 2022;10:948–959. doi: 10.1016/j.jchf.2022.08.002 [DOI] [PubMed] [Google Scholar]
- 13. Contal C, O'Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput Stat Data Anal. 1999;30:253–270. doi: 10.1016/S0167-9473(98)00096-6 [DOI] [Google Scholar]
- 14. Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Circulation. 2015;131:211–219. doi: 10.1161/CIRCULATIONAHA.114.014508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;139:e637–e697. doi: 10.1161/CIR.0000000000000602 [DOI] [PubMed] [Google Scholar]
- 16. Harrild DM, Berul CI, Cecchin F, Geva T, Gauvreau K, Pigula F, Walsh EP. Pulmonary valve replacement in tetralogy of Fallot: impact on survival and ventricular tachycardia. Circulation. 2009;119:445–451. doi: 10.1161/CIRCULATIONAHA.108.775221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blais S, Marelli A, Vanasse A, Dahdah N, Dancea A, Drolet C, Colavincenzo J, Vaugon E, Dallaire F. The 30‐year outcomes of tetralogy of Fallot according to native anatomy and genetic conditions. Can J Cardiol. 2021;37:877–886. doi: 10.1016/j.cjca.2020.10.002 [DOI] [PubMed] [Google Scholar]
- 18. Maskatia SA, Spinner JA, Nutting AC, Slesnick TC, Krishnamurthy R, Morris SA. Impact of obesity on ventricular size and function in children, adolescents and adults with tetralogy of Fallot after initial repair. Am J Cardiol. 2013;112:594–598. doi: 10.1016/j.amjcard.2013.04.030 [DOI] [PubMed] [Google Scholar]
- 19. Deen JF, Krieger EV, Slee AE, Arslan A, Arterburn D, Stout KK, Portman MA. Metabolic syndrome in adults with congenital heart disease. J Am Heart Assoc. 2016;5:5. doi: 10.1161/JAHA.114.001132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The metabolic syndrome and cardiovascular risk a systematic review and meta‐analysis. J Am Coll Cardiol. 2010;56:1113–1132. doi: 10.1016/j.jacc.2010.05.034 [DOI] [PubMed] [Google Scholar]
- 21. Ishikita A, McIntosh C, Hanneman K, Lee MM, Liang T, Karur GR, Roche SL, Hickey E, Geva T, Barron DJ, et al. Machine learning for prediction of adverse cardiovascular events in adults with repaired tetralogy of Fallot using clinical and cardiovascular magnetic resonance imaging variables. Circ Cardiovasc Imaging. 2023;16:e015205. doi: 10.1161/CIRCIMAGING.122.015205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Romano S, Judd RM, Kim RJ, Kim HW, Klem I, Heitner J, Shah DJ, Jue J, White BE, Shenoy C, et al. Association of feature‐tracking cardiac magnetic resonance imaging left ventricular global longitudinal strain with all‐cause mortality in patients with reduced left ventricular ejection fraction. Circulation. 2017;135:2313–2315. doi: 10.1161/CIRCULATIONAHA.117.027740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nieminen HP, Jokinen EV, Sairanen HI. Causes of late deaths after pediatric cardiac surgery: a population‐based study. J Am Coll Cardiol. 2007;50:1263–1271. doi: 10.1016/j.jacc.2007.05.040 [DOI] [PubMed] [Google Scholar]
- 24. Goldstein SA, D'Ottavio A, Spears T, Chiswell K, Hartman RJ, Krasuski RA, Kemper AR, Meyer RE, Hoffman TM, Walsh MJ, et al. Causes of death and cardiovascular comorbidities in adults with congenital heart disease. J Am Heart Assoc. 2020;9:e016400. doi: 10.1161/JAHA.119.016400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jadli AS, Parasor A, Gomes KP, Shandilya R, Patel VB. Exosomes in cardiovascular diseases: pathological potential of Nano‐messenger. Front Cardiovasc Med. 2021;8:767488. doi: 10.3389/fcvm.2021.767488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mayourian J, Brown E, Javalkar K, Bucholz E, Gauvreau K, Beroukhim R, Feins E, Kheir J, Triedman J, Dionne A. Insight into the role of the child opportunity index on surgical outcomes in congenital heart disease. J Pediatr. 2023;259:113464. doi: 10.1016/j.jpeds.2023.113464 [DOI] [PubMed] [Google Scholar]
- 27. Siontis KC, Noseworthy PA, Attia ZI, Friedman PA. Artificial intelligence‐enhanced electrocardiography in cardiovascular disease management. Nat Rev Cardiol. 2021;18:465–478. doi: 10.1038/s41569-020-00503-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mayourian J, La Cava WG, Vaid A, Nadkarni GN, Ghelani SJ, Mannix R, Geva T, Dionne A, Alexander ME, Duong SQ, et al. Pediatric ECG‐based deep learning to predict left ventricular dysfunction and remodeling. Circulation. 2024;149:917–931. doi: 10.1161/CIRCULATIONAHA.123.067750 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figures S1–S6


