Abstract
Background
Popliteal artery aneurysms (PAAs) are the most common peripheral aneurysm. However, due to its rarity, the cumulative body of evidence regarding patient patterns, treatment strategies, and perioperative outcomes is limited. This analysis aims to investigate distinct phenotypical patient profiles and associated treatment and outcomes in patients with a PAA by performing an unsupervised clustering analysis of the POPART (Practice of Popliteal Artery Aneurysm Repair and Therapy) registry.
Methods and Results
A cluster analysis (using k‐means clustering) was performed on data obtained from the multicenter POPART registry (42 centers from Germany and Luxembourg). Sensitivity analyses were conducted to explore validity and stability. Using 2 clusters, patients were primarily separated by the absence or presence of clinical symptoms. Within the cluster of symptomatic patients, the main difference between patients with acute limb ischemia presentation and nonemergency symptomatic patients was PAA diameter. When using 6 clusters, patients were primarily grouped by comorbidities, with patients with acute limb ischemia forming a separate cluster. Despite markedly different risk profiles, perioperative complication rates appeared to be positively associated with the proportion of emergency patients. However, clusters with a higher proportion of patients having any symptoms before treatment experienced a lower rate of perioperative complications.
Conclusions
The conducted analyses revealed both an insight to the public health reality of PAA care as well as patients with PAA at elevated risk for adverse outcomes. This analysis suggests that the preoperative clinic is a far more crucial adjunct to the patient's preoperative risk assessment than the patient's epidemiological profile by itself.
Keywords: cluster analysis, outcomes, peripheral aneurysm, phenomapping, popliteal artery aneurysm
Subject Categories: Quality and Outcomes, Complications, Cardiovascular Surgery, Cardiovascular Disease, Risk Factors
Nonstandard Abbreviations and Acronyms
- ALI
acute limb ischemia
- PAA
popliteal artery aneurysm
- POPART
Practice of Popliteal Artery Aneurysm Repair and Therapy
Clinical Perspective.
What Is New?
Patients with popliteal artery aneurysms underwent phenomapping, and distinct patient subgroups were identified in a multicenter registry.
Patients with popliteal artery aneurysms are primarily separated by clinical signs and symptoms and secondarily by comorbidities.
Clusters diverge significantly in treatment and outcomes; a complex interplay exists between traditional risk factors, the clinical course, and subsequent outcomes.
What Are the Clinical Implications?
Specific patient clusters might benefit from distinct treatment protocols.
Popliteal artery aneurysms (PAAs) are the most common peripheral aneurysms. 1 , 2 , 3 However, due to their rarity, there are limited data available on PAA treatment practices and outcomes, with the largest patient cohorts typically coming from population‐based data, national insurance claim data sets, or vascular registries. 4 , 5 , 6 , 7 , 8 , 9 While demographic descriptions of larger collectives of patients with PAAs have been conducted before, including subgroup analyses stratified by sex, 5 , 10 symptoms, 6 , 8 or treatment modalities, 11 , 12 the complexity of aneurysm pathophysiology, the heterogeneity, and interactions among patient characteristics make a solely hypothetico‐deductive approach challenging, and many questions remain unanswered. For instance, risk patterns for symptomatic presentation and perioperative complications have not been conclusively clarified yet. 13
As routine clinical data become more accessible for research purposes, new opportunities are emerging for describing rare vascular diseases beyond traditional association studies. 14 , 15 , 16 Clustering algorithms are an established approach to identify and characterize patient clusters that may not have been identified or addressed by clinicians before but can help explain variations in outcomes and heterogeneity of treatment responses. 17 , 18 , 19 , 20 , 21 , 22 , 23
While phenotype clustering is becoming increasingly important to facilitate a transition to precision medicine in cardiovascular diseases in general, it remains underused in vascular surgery. This analysis aims to identify phenotype clusters in patients with PAAs on the basis of 27 different parameters comprising demographics, comorbidities, anatomic features, and clinical symptoms to investigate the diversity of patients with PAAs and associated treatment approaches and outcomes.
Methods
Data can be made available upon reasonable request to the corresponding author.
Study Design
The POPART (Practice of Popliteal Artery Aneurysm Repair and Therapy) registry is a multicenter outcome registry for endovascular and open PAA repair, established in 2014 by the German Institute for Vascular Health Research. Data requests should be directed to the German Institute for Vascular Health Research. The registry was described in detail in previous publications. Until February 2023, 42 centers in Germany and Luxembourg have become part of POPART, of which 38 centers have entered patients into the data set. Participating centers were required to offer both open and endovascular PAA repair. All patients aged >18 years who had undergone PAA treatment since 2010 were eligible for enrollment. Before study inclusion, patients had to provide informed and written consent. As a noninterventional study, the indication for treatment was solely at the discretion of the attending surgeon and unrelated to study participation. No study‐related follow‐up visits or examinations were conducted. Data entry was conducted via an electronic case report form. Study participants were consistently pseudonymized before being entered into the data set. General information regarding treatment and associated outcomes and a descriptive analysis of the registry have been previously reported. 12
Ethics Approval
POPART is approved by the Ethics Committee of the University Hospital Frankfurt (approval no. 218/4). POPART is also listed in the German Registry of Clinical Studies (Identification No. DRKS00017609).
Cluster Analysis
Cluster formation was performed applying the k‐means clustering algorithm, an unsupervised learning strategy, on the scaled data set. Cluster analysis involves grouping observations that are more similar to each other on the basis of a set of available variables. In brief, input variables will contribute to the final clustering with decreasing influence to final grouping depending on the level of separation a given variable creates between observations. K was optimized using different methods, including the total within sum of square, the silhouette method, as well as the NbClust function of the NbClust package in R (R Foundation for Statistical Computing, Vienna, Austria), which uses 30 different metrics to optimize k. Clusters were formed including 27 different parameters comprising demographics, comorbidities, anatomic features, and clinical symptoms. In a sensitivity analysis, clusters were formed without clinical symptoms. The list of variables is shown in Table S1. Optimization suggested different values for k depending on the method for within sum of square (6), silhouette (2), and NbClust (2) including symptoms. Differences were again obtained using within sum of square (6), silhouette (8), and NbClust (10) excluding symptoms. Clusters were formed with centers set to 2 and nstart set to 25 (Figure S1). Optimization of k and cluster formation were performed using a set seed. Because clustering requires no missing data points, 13 patients (1.0%) had to be removed from the registry data set. This was deemed to be reasonable without introducing a significant selection bias.
Statistical Analysis
All statistical analyses were conducted using R version 4.1.3. Descriptive statistics were used to display study population and cluster characteristics. Proportions are shown in percentages. Groups were compared by t test or χ2 test depending on the variable. A P value <0.05 was considered statistically significant.
Results
Study Population
The study population included 1223 patients of the POPART registry. In summary, 56 (4.6%) patients were women, and 1167 (95.4%) were men. The median age was 69 years (interquartile range, 61.5–77.0 years). Popliteal artery aneurysms had an average (arithmetic mean) diameter of 29.5 mm. Baseline characteristics are shown in Table 1. Patients were recruited to the register between 2010 and 2022.
Table 1.
Baseline Study Population Characteristics
| Variable | N=1223 |
|---|---|
| Female: Male, n (%) | 56 (4.6): 1167 (95.4) |
| Age, y | 69.0 (61.5–77.0) |
| Aneurysm diameter, mm | 29.5 (+/− 13.0) |
| Unimpeded inflow, % | 12.2 |
| Runoff vessels, % | |
| 0 | 6.0 |
| 1 | 19.3 |
| 2 | 31.6 |
| 3 | 43.0 |
| Hypertension, % | 67.3 |
| Chronic kidney disease, % | 12.1 |
| Diabetes, % | 16.5 |
| Smoking, % | 33.0 |
| AAA, % | 32.2 |
| Contralateral PAA, % | 7.9 |
AAA indicates abdominal aortic aneurysm; and PAA, popliteal artery aneurysms.
Clustering Including Symptoms
Forming 2 clusters resulted in a partition with 922 patients and a second partition with 301 patients. While the clusters appeared similar regarding demographic parameters and the PAA diameter, large differences between cluster 1 and cluster 2 were observed regarding the mean number of runoff vessels (2.3 versus 1.5, P<0.001), proportion of asymptomatic patients in general (66.3% versus 1.3%, P<0.001), and acute limb ischemia in specific (0% versus 45.5%, P<0.001). This was further reflected by the rates of emergency surgeries and perioperative complications such as myocardial infarctions or major amputations. While the main driver of separation between the 2 clusters appeared to be presence or absence of symptoms potentially at least partially mediated by the number of runoff vessels, a subset analysis within cluster 2 was performed. We found no statistically significant differences between acute limb ischemia (ALI) and patients without ALI in cluster 2 regarding age, sex, smoking, obesity, or quality of inflow and number of outflow vessels (all P>0.05). However, patients with ALI had significantly larger maximal PAA diameters compared with patients without ALI in cluster 2 (32.0 versus 26.9 mm, P= 0.002). (Table 2).
Table 2.
Two Clusters Formed Using All Available Demographic and Clinical Features in the Registry
| Cluster 1 | Cluster 2 | |
|---|---|---|
| No. | 922 | 301 |
| Age, y | 68.9 | 68.3 |
| Female sex, % | 4.3 | 5.3 |
| Diameter, mm | 29.4 | 29.2 |
| Runoff vessels | 2.3 | 1.5 |
| Any complication (in surgical cases), % | 11.6 | 33.2 |
| Endovascular treatment, % | 12.9 | 5.0 |
| Vein graft, % | 73.7 | 73.1 |
| Asymptomatic cases, % | 66.3 | 1.3 |
| ALI, % | 0.0 | 45.5 |
| Emergency, % | 2.4 | 55.1 |
| Periprocedural myocardial infarction, % | 0.2 | 2.2 |
| Periprocedural amputation, % | 0.3 | 4.4 |
ALI indicates acute limb ischemia.
Forming 6 clusters as suggested by a different k optimization approach produced a more granular picture of patients within the registry. In summary, the following clusters were found: Cluster 1 is characterized by small PAAs (28.8 mm), a small proportion of female patients (0.3%), the highest proportion of concomitant aortic aneurysms (89.0%), and satisfying outcomes and comparably lower rates of comorbidity. Cluster 2 included the oldest patients on average (mean, 74.3 years) with poor runoff (mean, 2.1 vessels). Cluster 3, the smallest, consisted of younger, healthier patients, with the highest proportion of women (17.2%), the smallest rate of endovascular cases (0%) and the smallest complication rate during open surgical treatment (21.9%). Cluster 4 was the biggest (552 patients) and was in between the other clusters regarding baseline characteristics as well as treatment strategies and outcomes. Cluster 5 was mainly characterized by a high rate of emergency cases (82.1%). Cluster 6 included older patients (mean, 72.3 years), with poor runoff (mean, 2.1 vessels) and large PAAs (34.0 mm). Notably, all patients in Cluster 6 had a documented malignant disease, and all patients with cancer in the registry were exclusively assigned to Cluster 6 (Table 3).
Table 3.
Six Clusters Formed Using All Available Demographic and Clinical Variables in the Registry
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | Cluster 5 | Cluster 6 | |
|---|---|---|---|---|---|---|
| No. | 328 | 124 | 34 | 552 | 123 | 62 |
| Age, y | 69.6 | 74.3 | 62.4 | 67.0 | 68.7 | 72.3 |
| Female sex, % | 0.3 | 3.3 | 17.2 | 6.0 | 7.9 | 10.7 |
| Diameter, mm | 28.8 | 29.2 | 29.7 | 28.9 | 32.3 | 34.0 |
| Runoff vessels | 2.2 | 2.1 | 2.4 | 2.2 | 2.4 | 2.1 |
| Any complication (surgical cases), % | 15.6 | 15.5 | 15.6 | 13.1 | 40.5 | 24.0 |
| Endovascular treatment, % | 13.4 | 7.3 | 0.0 | 11.8 | 5.7 | 14.5 |
| Vein graft (surgical cases), % | 71.9 | 68.2 | 87.5 | 76.4 | 67.6 | 72.0 |
| Emergency, % | 5.2 | 10.5 | 14.7 | 7.2 | 82.1 | 19.4 |
| Coronary heart disease, % | 37.2 | 54.0 | 0.0 | 33.9 | 34.1 | 45.2 |
| Smoking, % | 15.2 | 32.3 | 2.9 | 19.4 | 19.5 | 16.1 |
| Thrombosed PAA, % | 6.7 | 7.3 | 11.8 | 10.7 | 37.4 | 19.4 |
| Concomitant AAA, % | 89.0 | 40.3 | 0.0 | 0.5 | 23.6 | 32.3 |
| Chronic kidney disease, % | 0.0 | 99.2 | 0.0 | 0.0 | 13.8 | 12.9 |
| Malignant disease, % | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 100 |
AAA indicates abdominal aortic aneurysm; and PAA, popliteal artery aneurysm.
Regardless of the number of clusters, we found no statistically significant intercluster differences regarding complications apart from the ones mentioned above, including differences in vascular versus nonvascular complications, major cardiovascular complications, or reinterventions after endovascular treatment. Impaired wound healing was the most prevalent complication in all clusters.
Clustering Excluding Symptoms
Because symptoms posted a major separator between the clusters, they were excluded, and a sensitivity analysis was conducted to obtain information on patient clusters on the basis of epidemiological profiles. However, Clusters 2 and 4 were identical to Clusters 6 and 3 in the analysis including symptoms. Cluster 1 consisted of the youngest patients (mean, 62.3 years), with the smallest diameters (mean, 27.3 mm), and the best runoff (mean, 2.5 vessels) albeit the largest proportion of smokers (52.0%). Cluster 3 had no female patients, average diameters and runoff, and the lowest proportion of complications after surgical repair. Cluster 5 was characterized by the oldest patients (mean, 75.8 years) with the poorest runoff (mean, 1.7 vessels). Cluster 6 was in between most clusters regarding baseline characteristics; however, it contained the highest proportion of endovascular treatments (23.4%) and simultaneously the highest rate of complications in patients receiving open surgery (50.0%) (Table 4).
Table 4.
Six Clusters Formed After Removing Clinical Signs and Symptoms Before Clustering the Data Set
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | Cluster 5 | Cluster 6 | |
|---|---|---|---|---|---|---|
| No. | 487 | 62 | 129 | 34 | 434 | 77 |
| Age, y | 62.3 | 72.3 | 69.4 | 62.4 | 75.8 | 68.6 |
| Female sex, % | 5.3 | 10.7 | 0.0 | 14.7 | 3.8 | 3.9 |
| Diameter, mm | 27.3 | 34.0 | 30.2 | 29.7 | 31.3 | 29.1 |
| Runoff vessels | 2.5 | 2.1 | 2.1 | 2.4 | 1.7 | 2.0 |
| Any complication (surgical cases), % | 13.4 | 24.0 | 17.0 | 15.6 | 22.4 | 12.5 |
| Endovascular treatment, % | 9.7 | 14.5 | 13.2 | 0.0 | 9.9 | 23.4 |
| Vein graft (surgical cases), % | 66.5 | 72.0 | 68.9 | 87.5 | 69.7 | 80.4 |
| Emergency, % | 16.0 | 19.4 | 10.9 | 14.7 | 17.5 | 3.9 |
| Smoking, % | 52.0 | 25.8 | 34.1 | 0.0 | 15.9 | 27.3 |
| Concomitant AAA, % | 23.4 | 32.3 | 72.1 | 0.0 | 32.7 | 32.4 |
| Chronic kidney disease, % | 4.7 | 12.9 | 14.0 | 0.0 | 20.0 | 15.6 |
| Any symptoms, % | 48.9 | 46.8 | 63.6 | 52.9 | 45.9 | 63.6 |
| Malignant disease, % | 0.0 | 100 | 0.0 | 0.0 | 0.0 | 0.0 |
| Coronary heart disease, % | 12.5 | 45.2 | 39.5 | 0.0 | 62.4 | 45.5 |
AAA indicates abdominal aortic aneurysm.
It was observed that the 6 clusters formed pairs by mean age (1 and 4, 2 and 5, as well as 3 and 6), suggesting that age served as a primary separator between clusters. Major differences between clusters of similar age structure included general rate of comorbidities (1 and 4), specific comorbidities such as cancer (2 and 5), or treatment strategies (6 and 3). A trend for larger diameters and less runoff vessels with increasing mean age among clusters was observed. Clusters 2 and 5 were the ones with the largest PAA diameters, with the highest proportion of patients in need of emergency treatment and the highest rate of perioperative complications of all clusters. However, the rate of emergency procedures might act as a positive confounder regarding perioperative complications instead of reflecting a true association between age and adverse outcomes. Interestingly, the proportion of patients requiring emergency care was inversely related to the proportion of patients who presented with any symptoms due to the PAA before undergoing treatment. Whereas Clusters 1, 3, 4, and 6 differed significantly regarding comorbidities, they showed a similar rate of perioperative complications, not only compared with the one cluster paired by mean age but also to the other clusters. This was particularly apparent in relation to the young and healthy Cluster 4, which did not display a specifically low complication rate.
The Effect of Symptoms on Cluster Formation
We further investigated cluster differences when symptoms were either included or excluded. Two clusters remained identical regardless of the inclusion of symptoms. Apart from this, no major intersections were identified among the different clustering strategies. Clusters including clinical symptoms distributed among several different clusters excluding symptoms. The largest overlap was found between original cluster 4 and cluster 1 after excluding symptoms. (Table 5) The heterogeneous distribution of most patients among different clusters depending on the availability of input variables suggests that epidemiological risk profiles are not necessarily coherent with clinical signs and symptoms of PAAs.
Table 5.
Distribution of Patients Among Clusters Depending on the Inclusion (Rows) or Exclusion (Columns) of Clinical Signs and Symptoms
| Excluding symptoms | ||||||
|---|---|---|---|---|---|---|
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | Cluster 5 | Cluster 6 | |
| Cluster 1 | 95 | 0 | 104 | 0 | 110 | 19 |
| Cluster 2 | 20 | 0 | 17 | 0 | 75 | 12 |
| Cluster 3 | 0 | 0 | 0 | 34 | 0 | 0 |
| Cluster 4 | 319 | 0 | 4 | 0 | 187 | 42 |
| Cluster 5 | 53 | 0 | 4 | 0 | 62 | 4 |
| Cluster 6 | 0 | 62 | 0 | 0 | 0 | 0 |
The cancer cluster (former, 6; new, 2) and the young and healthy cluster (former, 4; new, 3) remained the same.
Discussion
In the phenomapping analysis, we applied several clustering approaches to data of a multicenter registry of patients with PAA, the POPART registry. Using 2 clusters, we observed that the main separator among patients was the presence of symptoms in general and of acute limb ischemia in specific. However, within the cluster that was characterized by a high presence of patients with ALI, only 45.5% were affected by it. The main differentiator in this cluster between patients with ALI and patients without ALI seemed to be the PAA diameter (non‐ALI, 26.9 mm versus ALI, 32.0 mm). Although this is not a matched analysis, these patients are still very similar to each other regarding their risk profiles and clinical appearance. This is a strong indicator that PAA diameter is a relevant predictor for developing ALI in patients with symptomatic PAA. Evidence is scarce regarding the relationship of aneurysm diameter and the probability of acute thromboembolic events. 13 However, data of one of the largest PAA collectives ever reported in literature so far showed similar results with larger PAAs in patients with acute aneurysm thrombosis (mean diameter, 35.5 mm). 6 Further investigations are required, but this might imply that patients with a distinct risk profile as seen with this cluster are at elevated risk for developing ALI and might therefore be suited for accelerated access to surgery.
When using 6 clusters, a picture of higher diversity developed. In summary, we found the following: a cluster with the lowest diameters, almost no female patients, and the lowest rate of emergency procedures (Cluster 1); a cluster with predominantly older patients, with a large proportion of patients with chronic kidney disease (Cluster 2); a small cluster of healthy, young patients (Cluster 3); a middle‐of‐the‐pack cluster (Cluster 4), which was also the largest; cluster with a high rate of emergency cases (Cluster 5); and an exclusive cancer cluster (Cluster 6). In general, these clusters reflect the reality of PAA care from a public health perspective. We argue that Cluster 4 resembles something akin to an average patient with PAA while the other clusters constitute distinct PAA subpopulations with specific profiles regarding both their medical history and risk for adverse outcomes.
While no data regarding prior screenings are available in the POPART registry, Cluster 1 potentially reflects a high proportion of positively screened patients who underwent prophylactic treatment. The high rate of identified abdominal aortic aneurysms (89%), the small aneurysm diameter, and the low proportion of female patients as well as patients in need of emergency treatment suggest that these patients underwent screening for aneurysmatic disease. In Germany, abdominal aortic aneurysm routine screening was established in 2017 for all men aged >65 years. Whereas screening unselected patients for PAA is not recommended, current practice guidelines state that men with larger abdominal aortic aneurysm may benefit from duplex ultrasound examination of their popliteal vessels. 13 , 24 , 25 , 26
The cluster with the highest patient age and highest comorbidity rate with almost all patients requiring hemodialysis was Cluster 2. These polymorbid patients showed poor quality of runoff vessels and in only 68% of open repair cases a reconstruction with a vein bypass. However, in comparison with the other clusters, these patients did not stand out with an excessive overall complication rate, which is surprising considering their polymorbidity, advanced age, and poor runoff.
Cluster 3 was characterized by young and mostly healthy patients. The high rate of open surgical repair in this cluster aligns with the current treatment guidelines for PAAs, with patients having a life expectancy of >5 years, tolerating both kinds of procedures and having an adequate great saphenous vein being considered for primary open surgical treatment. 13 In contrast with general assumptions, these healthy and young patients showed a similar perioperative complication rate as the older multimorbid patients in Cluster 2.
The 2 clusters with the largest PAA diameters, namely, Clusters 5 and 6, exhibited the highest rates of perioperative complications. Notably, there was a stark contrast in the urgency of treatment between these 2 clusters.
Cluster 5, characterized by a predominance of acute thrombosed PAAs alongside generally good overall runoff quality, suggests that these patients were likely asymptomatic before treatment and were not previously identified on the basis of clinical symptoms like claudication. Their initial clinical presentation was prompted by an acute thrombotic event, explaining the high proportion of emergency cases (82%), limited use of vein grafts, and elevated perioperative complication rates typically associated with emergency surgeries. 6 , 12 , 27
In contrast, patients in Cluster 6 exhibited less favorable preoperative anatomic features, including the largest PAA diameters among all clusters and the poorest runoff quality. However, only 19% of cases were emergencies. All patients in this cluster had a malignant disease, which necessitated close medical monitoring and regular diagnostic imaging. Therefore, we hypothesize that PAAs in Cluster 6 were predominantly diagnosed in an asymptomatic state during staging imaging or follow‐up examinations. The underlying malignancy, often associated with limited life expectancy and increased perioperative risk assessment, may also be the reason for the higher proportion of endovascular treatments in this group, as well as the comparatively high complication rate, despite the lower number of emergency procedures.
Conflicting data exist on the association of increased aneurysm growth in patients with an abdominal aortic aneurysm receiving chemotherapy. 28 , 29 , 30 However, whether the large PAA diameter in this cluster may be related to chemotherapy can only be hypothesized and not answered with this data set.
By applying different clustering strategies and feeding different sets of input variables to the algorithm, we aimed at examining the internal validity and stability of our results as suggested for cluster analyses. 31 Multiple analyses were conducted to validate the results, including varying the number of clusters (relative validation), investigating cluster homogeneity regarding input variables (internal validation), and analyzing clinical outcomes (external validation).
Because symptoms acted as a significant differentiator among the clusters, they were excluded during a sensitivity analysis, to gather insights into patient clusters according to epidemiological risk profiles. When comparing these clusters, it was observed that the rate of perioperative complications increased as the proportion of emergency procedures within a cluster rose. Notably, the proportion of patients who exhibited symptoms before treatment showed an inverse relationship with the proportion of patients requiring emergency care. We hypothesize that patients with chronic symptoms attributed to the PAA, such as claudication due to microembolism or local compression from the aneurysm, are more likely to seek medical attention and subsequently be scheduled for elective treatment than patients who remain asymptomatic until they experience an acute thromboembolic event.
This intriguingly led to the observation that clusters with a higher proportion of patients having any symptoms before treatment experienced a lower rate of perioperative complications. This was strikingly apparent by the findings of the first cluster analysis, showing a similar complication rate for the cluster of young and healthy patients compared with the cluster comprising mostly elderly and multimorbid patients. In the sensitivity analysis, the 2 clusters with the highest patient age, the largest aneurysm diameter, and the highest rate of acutely treated patients displayed the highest complication rate, aligning with the previous observations that PAA diameters, a primary factor for ALI, increase with advancing age. While the presence of symptoms is associated with fewer emergency treatments due to ALI, age is linked to increasing PAA diameters, which in turn appear to increase the proportion of emergency treatments. Emergency treatments are strongly associated with perioperative complications, whereas an association of age and complications is at least partially confounded by diameters and acute surgery.
This analysis suggests that the preoperative clinic is a far more crucial adjunct to the patient's preoperative risk assessment than the patient's epidemiological profile by itself, also supported by the observation that patients were primarily differentiated according to the absence or presence of clinical symptoms when using only 2 clusters. A summary of these findings is shown in the Figure.
Figure Figure. . Interplay of different factors in PAA.
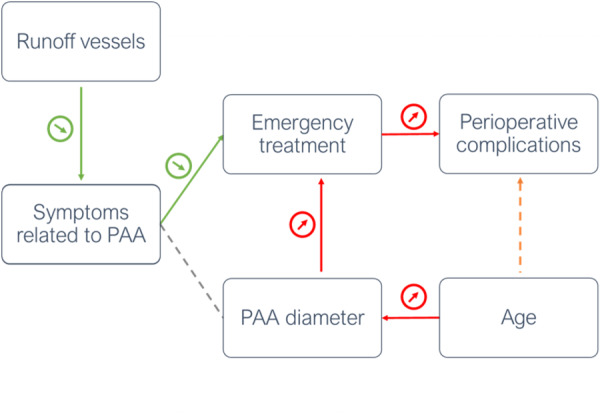
A higher number of patent runoff vessels is associated with fewer preoperative symptoms. However, the presence of symptoms is simultaneously associated with fewer emergency treatments due to acute limb ischemia. Age is linked to increasing PAA diameters, which appears to increase the proportion of emergency treatments. Emergency treatments are the driving factor for perioperative complications, whereas an association of age and complications is at least partially confounded by diameters and acute surgery. PAA indicates popliteal artery aneurysm.
Limitations
This study is limited by the design as an observational registry study. Clinical assessment of the patient's symptoms, the preoperative imaging, and treatment indication were at the sole discretion of the attending surgeon and could not be externally verified by the study team. Furthermore, potential confounding variables, including specific data on aneurysm morphology, intraluminal thrombus configuration, and medication usage before hospital admission, were not considered in the electronic case report form. Due to the limited number of perioperative complications within the study group, it was not feasible to conduct a separate analysis of perioperative outcomes stratified by cardiopulmonary, vascular, and nonvascular complications.
We conducted a distinct validation approach as described above, but it is not conclusively understood whether certain validation strategies are superior to others.
Conclusions
These analyses revealed an insight to the public health reality of PAA care in Germany and Luxembourg as well as adding to the body of evidence regarding patients with PAAs at elevated risk for adverse outcomes. Cluster analysis can identify prognostically distinct phenotypes beyond predefined assumptions or historical classifications, which can help improve surgical decision‐making by developing a more accurate understanding of risk‐modifying factors.
Sources of Funding
The article processing charges are supported by the Gefäßforum Österreich.
Disclosures
None.
Supporting information
Data S1.
Appendix A.
POPART registry collaborators (in the order of their time of participation): Kyriakos Oikonomou, MD, PhD, Department of Vascular and Endovascular Surgery, University Hospital Frankfurt am Main, Frankfurt am Main, Germany; Martin Storck, MD, PhD, Department of Vascular and Thoracic Surgery, Klinikum Karlsruhe, Karlsruhe, Germany; Kai Balzer, MD, PhD, Department of Vascular and Endovascular Surgery, St. Marien‐Hospital, Bonn, Germany; Ulrich Kugelmann, MD, Department of Vascular and Endovascular Surgery, Kreiskliniken Guenzburg‐Krumbach, Guenzburg, Germany; Christina Schneider, MD, Department of General and Vascular Surgery, Krankenhaus der Barmherzigen Brueder Trier, Trier, Germany; Michael Engelhardt, MD, PhD, Department of Vascular and Endovascular Surgery, Bundeswehrkrankenhaus Ulm, Ulm, Germany; Michael Petzold, MD, Krankenhaus Maerkisch Oderland, Strausberg, Germany; Barbara Weis‐Mueller, MD, PhD, Kliniken Maria Hilf, Department of Vascular surgery and Angiology, Moenchengladbach, Germany; Markus Wortmann, MD, Department of Vascular, Endovascular and Transplantation Surgery, Klinikum Stuttgart, Stuttgart, Germany; Sebastian Popp, MD, Department of Vascular and Endovascular Surgery, Schoen‐Klinik Vogtareuth, Vogtareuth, Germany; Dirk Grotemeyer, MD, PhD, Service de Chirurgie Vasculaire, Hôpital Kirchberg, Hôpitaux Robert Schuman, Luxembourg; Heiner Wenk, MD, PhD, Klinik Lilienthal, Lilienthal, Germany; Roushanak Shayesteh‐Kheslat, MD, Department of General, Visceral, Vascular, and Pediatric Surgery, University Hospital Homburg/Saarland, Homburg/Saar, Germany; Giovanni Torsello, MD, PhD, Institut für vaskulaere Forschung, St. Franziskus‐Hospital GmbH, Muenster, Germany, Katrin Meisenbacher, MD, Department of Vascular and Endovascular Surgery, University Hospital Heidelberg, Heidelberg, Germany; Johannes Hoffmann, MD, PhD, Department of Vascular Surgery and Phlebology, Contilia Herz und Gefaeßzentrum, Essen, Germany; Hubert Schelzig, MD, PhD, Clinic of Vascular and Endovascular Surgery, University Clinic Duesseldorf, Heinrich‐Heine‐University, Duesseldorf, Germany; Yush Roopa, MD, Department of Vascular and Endovascular Surgery, Klinikum am Plattenwald, SLK‐Kliniken Heilbronn GmbH, Bad Friedrichshall, Germany; Thomas Strohschneider, MD, Department of Vascular, Endovascular Surgery and Angiology, Karl‐Olga Krankenhaus, Stuttgart, Germany; Thomas Noppeney, MD, PhD, Department of Vascular Surgery, University Hospital Regensburg, Regensburg, Germany; Viktor Reichert, MD, Department of Vascular and Endovascular Surgery, Klinikum Sindelfingen‐Boeblingen, Sindelfingen, Germany; Uwe Lorenz, MD, Department of Vascular Surgery, Oberhavel Kliniken, Hennigsdorf, Germany; Karin Pfister, MD, PhD, Department of Vascular Surgery, University Hospital Regensburg, Regensburg, Germany; Shoaeddin Damirchi, MD, Department of Vascular Surgery, Evangelische Krankenhaus Herne, Herne, Germany; Tomislav Stojanovic, MD, PhD, Department of Vascular and Endovascular Surgery, Klinikum Wolfsburg, Wolfsburg, Germany; Alexander Oberhuber, MD, PhD, Department of Vascular and Endovascular Surgery, University Hospital Muenster, Muenster, Germany; Bernd Lobenstein, MD, Department of Vascular Surgery, Klinikum Naumburg, Naumburg, Germany; Tolga Atilla Sagban, MD, Department of Vascular Surgery, Sana Klinikum Hameln‐Pyrmont, Hamelin, Germany; Tomas Pfeiffer, MD, PhD, Department of Vascular and Endovascular Surgery, Hegau‐Bodensee‐Klinikum Singen, Singen, Germany; Johann Koller, MD, Department of Vascular and Endovascular Surgery, Kreiskliniken Reutlingen, Reutlingen, Germany; Christian Sprenger, MD, Department of Vascular and Endovascular Surgery, Klinikum Mutterhaus der Borromaerinnen, Trier, Germany; Thomas Kruschwitz, MD, Claus‐Georg Schmedt, MD, PhD, Department of Vascular Surgery, Diakonie‐Klinikum Schwaebisch Hall, Schwaebisch Hall, Germany; Frank Marquardt, MD, Department of Vascular Surgery, Rotes Kreuz Krankenhaus Bremen, Bremen, Germany; Thomas Schmandra, MD, PhD, Department of Vascular and Endovascular Surgery, Rhoen Klinikum, Campus Bad Neustadt, Bad Neustadt, Germany; Dorothee Bail, MD, PhD, Robert‐Bosch‐Krankenhaus Stuttgart, Stuttgart, Germany.
This manuscript was sent to John S. Ikonomidis, MD, PhD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.124.034429
For Sources of Funding and Disclosures, see page 8.
Contributor Information
Amun Georg Hofmann, Email: ah.reply@outlook.com.
the POPART Registry collaborators:
Endovascular Surgery, Martin Storck, Thoracic Surgery, Kai Balzer, Endovascular Surgery, Ulrich Kugelmann, Endovascular Surgery, Christina Schneider, Vascular Surgery, Michael Engelhardt, Endovascular Surgery, Michael Petzold, Barbara Weis‐Mueller, Kliniken Maria Hilf, Markus Wortmann, Transplantation Surgery, Sebastian Popp, Endovascular Surgery, Dirk Grotemeyer, Heiner Wenk, Roushanak Shayesteh‐Kheslat, Pediatric Surgery, Giovanni Torsello, Katrin Meisenbacher, Endovascular Surgery, Johannes Hoffmann, Hubert Schelzig, Endovascular Surgery, Yush Roopa, Endovascular Surgery, Thomas Strohschneider, Endovascular Surgery, Thomas Noppeney, Viktor Reichert, Endovascular Surgery, Uwe Lorenz, Karin Pfister, Shoaeddin Damirchi, Tomislav Stojanovic, Endovascular Surgery, Alexander Oberhuber, Endovascular Surgery, Bernd Lobenstein, Tolga Atilla Sagban, Tomas Pfeiffer, Endovascular Surgery, Johann Koller, Endovascular Surgery, Christian Sprenger, Endovascular Surgery, Thomas Kruschwitz, Frank Marquardt, Thomas Schmandra, Endovascular Surgery, and Dorothee Bail
References
- 1. Trickett JP, Scott RAPP, Tilney HS. Screening and management of asymptomatic popliteal aneurysms. J Med Screen. 2002;9:92–93. doi: 10.1136/jms.9.2.92 [DOI] [PubMed] [Google Scholar]
- 2. Dawson I, Sie RB, Van Bockel JH. Atherosclerotic popliteal aneurysm. Br J Surg. 1997;84:293–299. doi: 10.1002/bjs.1800840304 [DOI] [PubMed] [Google Scholar]
- 3. Szilagyi DE, Schwartz RL, Reddy DJ. Popliteal arterial aneurysms: their natural history and management. JAMA Surg. 1981;116:724–728. doi: 10.1001/archsurg.1981.01380170194034 [DOI] [PubMed] [Google Scholar]
- 4. Ravn H, Wanhainen A, Björck M. Surgical technique and long‐term results after popliteal artery aneurysm repair: results from 717 legs. J Vasc Surg. 2007;46:236–243. doi: 10.1016/j.jvs.2007.04.018 [DOI] [PubMed] [Google Scholar]
- 5. Naazie IN, Arbabi C, Moacdieh MP, Hughes K, Harris L, Malas MB. Female sex portends increased risk of major amputation following surgical repair of symptomatic popliteal artery aneurysms. J Vasc Surg. 2022;76:1030–1036. doi: 10.1016/j.jvs.2022.03.892 [DOI] [PubMed] [Google Scholar]
- 6. Grip O, Mani K, Altreuther M, Bastos Gonçalves F, Beiles B, Cassar K, Davidovic L, Eldrup N, Lattmann T, Laxdal E, et al. Contemporary treatment of popliteal artery aneurysms in 14 countries: a Vascunet report. Eur J Vasc Endovasc Surg. 2020;60:721–729. doi: 10.1016/j.ejvs.2020.07.005 [DOI] [PubMed] [Google Scholar]
- 7. Galiñanes EL, Dombrovskiy VY, Graham AM, Vogel TR. Endovascular versus open repair of popliteal artery aneurysms: outcomes in the US medicare population. Vasc Endovascular Surg. 2013;47:267–273. doi: 10.1177/1538574413475888 [DOI] [PubMed] [Google Scholar]
- 8. Cervin A, Tjärnström J, Ravn H, Acosta S, Hultgren R, Welander M, Björck M. Treatment of popliteal aneurysm by open and endovascular surgery: a contemporary study of 592 procedures in Sweden. Eur J Vasc Endovasc Surg. 2015;50:342–350. doi: 10.1016/j.ejvs.2015.03.026 [DOI] [PubMed] [Google Scholar]
- 9. Beuschel B, Nayfeh T, Kunbaz A, Haddad A, Alzuabi M, Vindhyal S, Farber A, Hassan MH. A systematic review and meta‐analysis of treatment and natural history of popliteal artery aneurysms. J Vasc Surg. 2022;75:121S–125S, e14. doi: 10.1016/j.jvs.2021.05.023 [DOI] [PubMed] [Google Scholar]
- 10. Ravn H, Pansell‐Fawcett K, Björck M. Popliteal artery aneurysm in women. Eur J Vasc Endovasc Surg. 2017;54:738–743. doi: 10.1016/j.ejvs.2017.10.001 [DOI] [PubMed] [Google Scholar]
- 11. Dorigo W, Fargion A, Masciello F, Piffaretti G, Pratesi G, Giacomelli E, Pratesi C. A matched case‐control study on open and endovascular treatment of popliteal artery aneurysms. Scand J Surg. 2018;107:236–243. doi: 10.1177/1457496917748230 [DOI] [PubMed] [Google Scholar]
- 12. Jung G, Leinweber ME, Karl T, Geisbüsch P, Balzer K, Schmandra T, Dietrich T, Derwich W, Gray D, Schmitz‐Rixen T. Real‐world data of popliteal artery aneurysm treatment. Analysis of the POPART registry. J Vasc Surg. 2022;75:1707–1717.e2. doi: 10.1016/j.jvs.2021.12.079 [DOI] [PubMed] [Google Scholar]
- 13. Farber A, Angle N, Avgerinos E, Dubois L, Eslami M, Geraghty P, Haurani M, Jim J, Ketteler E, Pulli R, et al. The Society for Vascular Surgery clinical practice guidelines on popliteal artery aneurysms. J Vasc Surg. 2022;75:109S–120S. doi: 10.1016/j.jvs.2021.04.040 [DOI] [PubMed] [Google Scholar]
- 14. Li B, Feridooni T, Cuen‐Ojeda C, Kishibe T, de Mestral C, Mamdani M, Al‐Omran M. Machine learning in vascular surgery: a systematic review and critical appraisal. Npj Digit Med 2022 51. 2022;5:1–10. doi: 10.1038/s41746-021-00552-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lareyre F, Yeung KK, Guzzi L, Di Lorenzo G, Chaudhuri A, Behrendt CA, Spanos K, Raffort J. Artificial intelligence in vascular surgical decision making. Semin Vasc Surg. 2023;36:448–453. doi: 10.1053/J.SEMVASCSURG.2023.05.004 [DOI] [PubMed] [Google Scholar]
- 16. Fischer UM, Shireman PK, Lin JC. Current applications of artificial intelligence in vascular surgery. Semin Vasc Surg. 2021;34:268–271. doi: 10.1053/J.SEMVASCSURG.2021.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cikes M, Sanchez‐Martinez S, Claggett B, Duchateau N, Piella G, Butakoff C, Pouleur AC, Knappe D, Biering‐Sørensen T, Kutyifa V, et al. Machine learning‐based phenogrouping in heart failure to identify responders to cardiac resynchronization therapy. Eur J Heart Fail. 2019;21:74–85. doi: 10.1002/EJHF.1333 [DOI] [PubMed] [Google Scholar]
- 18. Filipow N, Davies G, Main E, Sebire NJ, Wallis C, Ratjen F, Stanojevic S. Unsupervised phenotypic clustering for determining clinical status in children with cystic fibrosis. Eur Respir J. 2021;58:58. doi: 10.1183/13993003.02881-2020 [DOI] [PubMed] [Google Scholar]
- 19. Chao CJ, Barry T, Seri A, El Shaer A, Ponce NC, Chakraborty S, Smith S, Alkhouli M, Thaden J, Fortuin D, et al. Topological data analysis identified prognostically‐distinct phenotypes in transcatheter edge‐to‐edge repair patients. Mayo Clin Proc Digit Heal. 2023;1:381–392. doi: 10.1016/J.MCPDIG.2023.07.002 [DOI] [Google Scholar]
- 20. Ding L, Mane R, Wu Z, Jiang Y, Meng X, Jing J, Ou W, Wang X, Liu Y, Lin J, et al. Data‐driven clustering approach to identify novel phenotypes using multiple biomarkers in acute ischaemic stroke: a retrospective, multicentre cohort study. eClinicalMedicine. 2022;53:101639. doi: 10.1016/j.eclinm.2022.101639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Loftus TJ, Shickel B, Balch JA, Tighe PJ, Abbott KL, Fazzone B, Anderson EM, Rozowsky J, Ozrazgat‐Baslanti T, Ren Y, et al. Phenotype clustering in health care: a narrative review for clinicians. Front Artif Intell. 2022;5. doi: 10.3389/FRAI.2022.842306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kwak S, Lee Y, Ko T, Yang S, Hwang IC, Park JB, Yoon YE, Kim HL, Kim HK, Kim YJ, et al. Unsupervised cluster analysis of patients with aortic stenosis reveals distinct population with different phenotypes and outcomes. Circ Cardiovasc Imaging. 2020;13:9707. doi: 10.1161/CIRCIMAGING.119.009707 [DOI] [PubMed] [Google Scholar]
- 23. Omar AMS, Ramirez R, Haddadin F, Sabharwal B, Khandaker M, Patel Y, Argulian E. Unsupervised clustering for phenotypic stratification of clinical, demographic, and stress attributes of cardiac risk in patients with nonischemic exercise stress echocardiography. Echocardiography. 2020;37:505–519. doi: 10.1111/echo.14638 [DOI] [PubMed] [Google Scholar]
- 24. Diwan A, Sarkar R, Stanley JC, Zelenock GB, Wakefield TW. Incidence of femoral and popliteal artery aneurysms in patients with abdominal aortic aneurysms. J Vasc Surg. 2000;31:863–869. doi: 10.1067/mva.2000.105955 [DOI] [PubMed] [Google Scholar]
- 25. Tuveson V, Löfdahl HE, Hultgren R. Patients with abdominal aortic aneurysm have a high prevalence of popliteal artery aneurysms. Vasc Med. 2016;21:369–375. doi: 10.1177/1358863X16648404 [DOI] [PubMed] [Google Scholar]
- 26. Cervin A, Wanhainen A, Björck M. Popliteal aneurysms are common among men with screening detected abdominal aortic aneurysms, and prevalence correlates with the diameters of the common iliac arteries. Eur J Vasc Endovasc Surg. 2020;59:67–72. doi: 10.1016/j.ejvs.2019.07.042 [DOI] [PubMed] [Google Scholar]
- 27. Kropman RHJ, Schrijver AM, Kelder JC, Moll FL, de Vries JPPM. Clinical outcome of acute leg Ischaemia due to thrombosed popliteal artery aneurysm: systematic review of 895 cases. Eur J Vasc Endovasc Surg. 2010;39:452–457. doi: 10.1016/j.ejvs.2009.11.010 [DOI] [PubMed] [Google Scholar]
- 28. Becker von Rose A, Kobus K, Bohmann B, Lindquist‐Lilljequist M, Eilenberg W, Bassermann F, Reeps C, Eckstein HH, Trenner M, Maegdefessel L, et al. Radiation and chemotherapy are associated with altered aortic aneurysm growth in patients with cancer: impact of synchronous cancer and aortic aneurysm. Eur J Vasc Endovasc Surg. 2022;64:255–264. doi: 10.1016/J.EJVS.2022.07.007 [DOI] [PubMed] [Google Scholar]
- 29. Martin ZL, Mastracci TM, Greenberg RK, Morales JP, Bena J. The effect of chemotherapy for malignancy on the natural history of aortic aneurysm. J Vasc Surg. 2015;61:50–57. doi: 10.1016/J.JVS.2014.06.123 [DOI] [PubMed] [Google Scholar]
- 30. Maxwell DW, Kenney L, Sarmiento JM, Rajani RR. Aortic aneurysm natural progression is not influenced by concomitant malignancy and chemotherapy. Ann Vasc Surg. 2021;71:29–39. doi: 10.1016/J.AVSG.2020.08.137 [DOI] [PubMed] [Google Scholar]
- 31. Ullmann T, Hennig C, Boulesteix AL. Validation of cluster analysis results on validation data: a systematic framework. Wiley Interdiscip Rev Data Min Knowl Discov. 2022;12:e1444. doi: 10.1002/WIDM.1444 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.


