Abstract
Background
Adverse cardiovascular events during pregnancy (eg, preeclampsia) occur at higher rates among individuals with overweight or obesity (body mass index ≥25 kg/m2) and have been associated with postpartum depression. The present study examined whether changes in cardiovascular health (CVH) during the perinatal period, as defined by the American Heart Association's Life's Essential 8 framework, predicted postpartum psychological functioning among individuals with prepregnancy body mass index ≥25 kg/m2.
Methods and Results
Pregnant individuals (N = 226; mean ± SD age = 28.43 ± 5.4 years; mean body mass index = 34.17 ± 7.15 kg/m2) were recruited at 12 to 20 weeks of gestation (mean, 15.64 ± 2.45 weeks) for a longitudinal study of health and well‐being. Participants completed ratings of depression and perceived stress and reported on CVH behaviors (dietary intake, physical activity, nicotine exposure, and sleep) at baseline and at 6 months postpartum. Body mass index and CVH behaviors were used to calculate a composite CVH score at both time points. Linear regression analyses were performed to examine whether change in CVH related to postpartum symptom scores. Because sleep was measured in only a subset of participants (n = 114), analyses were conducted with and without sleep. Improved CVH was associated with lower postpartum depression (β = −0.18, P<0.01) and perceived stress (β = −0.13, P=0.02) scores. However, when including sleep, these relationships were no longer significant (all P>0.4).
Conclusions
Improvements in CVH from early pregnancy to 6 months postpartum were associated with lower postpartum depressive symptoms and perceived stress but not when including sleep in the CVH metric, potentially due to the large reduction in sample size. These data suggest that intervening during pregnancy to promote CVH may improve postpartum psychological functioning among high‐risk individuals.
Keywords: cardiovascular health, depression, health behaviors, postpartum, pregnancy, stress
Subject Categories: Pregnancy, Mental Health, Obesity, Risk Factors, Lifestyle
Nonstandard Abbreviations and Acronyms
- AHA
American Heart Association
- CES‐D
Center for Epidemiologic Studies–Depression Scale
- CVH
cardiovascular health
- HEI
Healthy Eating Index
- LE8
Life's Essential 8
- LS7
Life's Simple 7
- PA
physical activity
- PSS
Perceived Stress Scale
- PSQI
Pittsburgh Sleep Quality Index
Clinical Perspective.
What Is New?
This is the first study to apply the American Heart Association's Life's Essential 8 framework to understand the longitudinal relationship between cardiovascular health and psychological functioning during the perinatal period.
We found that worsening cardiovascular health over the course of pregnancy and postpartum was associated with higher severity symptoms of depression and perceived stress at 6 months postpartum.
What Are the Clinical Implications?
Our findings suggest that measuring cardiovascular health using a holistic set of indicators rather than just diagnosable disease may be useful for risk detection and prevention during pregnancy.
Interventions targeting cardiovascular health during pregnancy may improve maternal psychological functioning following delivery, an important area for future research.
Pregnancy is a period in which significant physiologic changes occur in the cardiovascular system to meet the additional metabolic demands associated with maintaining fetal growth and development. 1 Difficulty adapting to these demands is associated with adverse pregnancy outcomes, such as gestational hypertension, preeclampsia and eclampsia, and gestational diabetes, 1 which occur in about 33% of individuals. Further, adverse pregnancy outcomes are the primary cause of maternal death in the United States 2 , 3 and predict future cardiovascular disease (CVD) risk, even among individuals for whom these conditions resolve following delivery. 4 , 5 Given that individuals engage with the health care system frequently during pregnancy, pregnancy represents not only a period of heightened vulnerability for CVD but also a window of opportunity to positively influence the health trajectories of birthing individuals.
In addition to being at elevated risk for CVD later in life, individuals who are diagnosed with pregnancy‐related cardiovascular conditions are more likely to experience postpartum psychological distress, particularly symptoms of depression. A recent meta‐analysis of 13 studies demonstrated that hypertensive disorders of pregnancy were associated with higher severity of self‐reported postpartum depressive symptoms. 6 Further, data from a large epidemiological study of nearly 5000 individuals found evidence that those diagnosed with preeclampsia were more than twice as likely to develop postpartum depression compared with those with normotensive pregnancies. 7 Gestational diabetes has also been linked to higher risk of postpartum depression. Two recent meta‐analyses covering data from ≈4 million individuals with limited overlap in study inclusion found evidence that the odds of postpartum depression were 32% to 59% higher among individuals whose pregnancies were affected by gestational diabetes compared with those whose were not. 8 , 9 Comparatively little research has been conducted to examine the effect of cardiovascular conditions in pregnancy on other postpartum psychological outcomes, despite the fact that symptoms such as anxiety, obsessions, compulsions, and mania are relatively common during the postpartum period. 10 , 11 However, 1 study leveraging data from a nationwide health registry in Denmark demonstrated that cardiovascular conditions in pregnancy, such as gestational hypertension and diabetes, were associated with higher risk not only of depression but also symptoms of psychosis and acute stress. 12 It is important to note that psychiatric symptoms during the perinatal period have a detrimental impact on the health and well‐being of the birthing person, as well as infant and child outcomes. 13 , 14 , 15 , 16 , 17 Taken together, these data suggest that interventions targeting cardiovascular risk during pregnancy may improve maternal psychological functioning, which may in turn exert downstream benefits on child health and development.
Although previous research has established a link between diagnosed pregnancy‐related cardiovascular conditions and risk for depression during the postpartum period, few studies have explored how indicators of cardiovascular health (CVH) affect postpartum psychological functioning. Conceptually, CVH, a more holistic concept, emphasizes optimization of health through management of modifiable CVD risk factors. To more effectively identify individuals at risk for CVD before the onset of diagnosable disease, the American Heart Association (AHA) developed the Life's Simple 7 (LS7) CVH framework, yielding a composite indicator of CVH that encompasses the 7 behavioral and physiological factors most closely associated with CVD risk. 18 These factors include nicotine use and exposure, dietary quality, physical activity, body composition, fasting blood glucose, total cholesterol, and blood pressure. Numerous studies conducted in the general population have demonstrated that higher LS7 scores (indicative of better CVH) are robustly associated with reduced risk of CVD‐related morbidity and death. 19 More recently, the LS7 metric was updated to Life's Essential 8 (LE8) and now includes sleep duration on the basis of research indicating that insufficient (<7 hours per night) and excessive (>9 hours per night) sleep duration independently increase risk for CVD. 19 As with LS7, data from large epidemiologic surveys suggest that better CVH as indicated by LE8 scores is associated with lower risk of CVD. 20 , 21 These studies highlight the utility of more broadly and holistically defining CVH for identifying individuals who may benefit from earlier intervention to mitigate CVD risk.
To date, however, there is limited research examining either of these CVH frameworks in the context of pregnancy. Evidence from extant studies employing the LS7 framework indicates that the majority of individuals exhibit poor CVH in pregnancy. 22 Further, poor maternal CVH in pregnancy is associated with elevated risk of adverse obstetric outcomes (eg, unplanned cesarean section), 23 greater postpartum maternal atherosclerosis, 24 as well as worse CVH in their children. 25 However, there have been no studies to explore the relationship between CVH in pregnancy and postpartum maternal psychological functioning. Therefore, it remains unclear whether variation in CVH during pregnancy relates to risk of postpartum psychological distress. Given that individuals who begin pregnancy with overweight or obesity (body mass index [BMI] ≥25 kg/m2) are more likely to experience cardiovascular conditions during pregnancy 26 , 27 as well as postpartum psychological distress 28 , 29 compared with individuals with a BMI <25, the present study aimed to examine the relationship between changes in CVH from the second trimester of pregnancy to 6 months postpartum and postpartum psychological functioning among individuals who began their pregnancies with overweight or obesity. A composite score using the LE8 framework was calculated to index CVH and was composed of body composition and CVH behaviors. CVH scores were computed with and without sleep duration included given that only a subset of participants completed the sleep assessment and that sleep is a recent addition to the AHA's current CVH framework (LE8), which has received minimal research attention in pregnancy. Therefore, a secondary aim of the present study was to evaluate whether the inclusion of sleep changed the relationship between CVH scores and postpartum psychological functioning. It was hypothesized that greater improvements in LS7 CVH scores would be associated with fewer symptoms of psychological distress during the postpartum period and that the inclusion of sleep would strengthen the association between LE8 CVH scores and postpartum psychological functioning.
Methods
Participants and Study Procedures
The present study is a secondary analysis of data collected for a longitudinal study 30 of health and well‐being during the perinatal period among individuals who began their pregnancies with a BMI ≥25 kg/m2. The data, code used to analyze the data, and nonproprietary materials from the current study are available from the corresponding author upon reasonable request. Pregnant individuals (N = 257) were recruited from local obstetrics clinics and were eligible if they had overweight or obesity before becoming pregnant, had a singleton pregnancy, and were ≥14 years of age at enrollment. Exclusion criteria included diagnosis of type I diabetes, taking medications or diagnosed with conditions known to influence weight, participating in a weight management program, or experiencing acute psychiatric symptoms warranting immediate intervention (eg, suicidality). Participants aged ≥18 years provided written informed consent before the initiation of study procedures. Verbal assent was obtained from participants aged <18 years (n = 4), and written informed consent was provided by a parent or legal guardian. Procedures were approved by the University of Pittsburgh Institutional Review Board (PRO11070083). All procedures performed were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Eligible individuals attended up to 7 visits over the course of the perinatal period to complete assessments of demographic (age, racial identity, marital status, educational background, household income, and parity), medical, and psychosocial factors and reported on health behavior engagement. The initial baseline assessment occurred when participants’ pregnancies were at 12 to 20 weeks of gestation (time 0 or T0; n = 257). Subsequent assessments occurred at 18 to 22 weeks of gestation (T1; n = 253), 23 to 26 weeks of gestation (T2; n = 252), 27 to 30 weeks of gestation (T3; n = 245), 31 to 34 weeks of gestation (T4; n = 240), 35 weeks of gestation through delivery (T5; n = 206), and 6 months postpartum (T6; n = 237). Data that were missing due to participant dropout were not addressed statistically; these participants were excluded from analyses. Please see Levine et al 30 for further details about dropout. Data collection for the parent study began in September 2012 and was completed in January 2017.
Measures of Psychological Distress
Depressive Symptoms
Depressive symptoms were assessed using the Center for Epidemiologic Studies–Depression Scale (CES‐D), 31 a self‐report measure of the frequency of 20 common depressive symptoms rated along a 0 (“rarely or none of the time”) to 3 (“most or all of the time”) Likert scale. Responses are summed to yield a total score, with higher scores reflecting more severe depressive symptoms. The CES‐D has demonstrated adequate reliability and validity in a number of populations, 32 , 33 , 34 including among individuals who are pregnant. 35
Perceived Stress
The Perceived Stress Scale (PSS) 36 was administered to assess experiences of daily life stress. The PSS is a 14‐item instrument on which respondents use a 0 (“never”) to 4 (“very often”) Likert scale to rate the degree to which daily life events are perceived to be uncontrollable, unpredictable, or unmanageable. Responses are summed to form a total score, with higher scores indicating more perceived stress. This scale has been shown to exhibit satisfactory reliability (Cronbach's α = 0.85) and validity, 37 including during the perinatal period. 38
Measures of Cardiovascular Health
Cardiovascular health was indexed using the AHA LE8 metric, 19 a composite score composed of the 8 health behaviors and biomarkers that are most strongly linked to individual differences in risk for cardiovascular disease. The components included in the LE8 are dietary patterns, physical activity, nicotine exposure, sleep health, BMI, blood lipids, blood glucose, and blood pressure. Of the 8 components included in the LE8 composite score, the following 5 were evaluated as part of the study assessment battery: weight and height for calculating BMI, physical activity, nicotine use and history, dietary intake, and sleep. Data for the present study were drawn from baseline (T0) and 6 months postpartum (T6) visits, as these were the time points at which all available components of the LE8 score were measured. The measure of sleep health (described in more detail below) was added to the assessment battery in February 2015 and was therefore available for only a subset of participants. Thus, we computed a composite score that does not include sleep, reflecting domains originally in the LS7, and computed composite scores that include sleep among the participants with these data. Scoring guidelines for the LE8 were used to compute both composite scores. Complete data were available for BMI, physical activity, nicotine use and history, and dietary intake on 226 (87.9%) participants at the T0 and T6 time points, and of those 226 participants, sleep data were available at both time points on 114 (50.4%). Table 1 depicts the time points that each of the LE8 metrics were collected in the parent study to provide additional clarity.
Table 1.
Collection of the American Heart Association's LE8 Metric Components by Study Time Point
| Component | T0 | T1 | T2 | T3 | T4 | T5 | T6 |
|---|---|---|---|---|---|---|---|
| Diet quality | x | ‐ | ‐ | x | ‐ | ‐ | x |
| Physical activity | x | x | x | x | x | x | x |
| Nicotine use | x | ‐ | ‐ | ‐ | ‐ | x | x |
| Sleep duration (n = 114) | x | x | x | x | x | x | x |
| BMI | x | x | x | x | x | x | x |
| Blood pressure | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Blood lipids | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Blood glucose | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
T0, baseline assessment, 12–20 weeks of gestation; T1, 18–22 weeks of gestation; T2, 23–26 weeks of gestation; T3, 27–30 weeks of gestation; T4, 31–34 weeks of gestation; T5, 35 weeks of gestation through delivery; T6, 6 months postpartum. BMI indicates body mass index; and LE8, Life's Essential 8.
Body Mass Index
To calculate BMI during early pregnancy and 6 months postpartum, weight and height were objectively measured using a digital scale and a calibrated stadiometer during the T0 and T6 assessments. Participants also self‐reported prepregnancy weight to calculate prepregnancy BMI. Lloyd‐Jones et al 19 proposed alternative BMI cut points and scoring guidelines for individuals of Asian and Pacific descent, which we applied to the scoring of BMI for the 1 participant who identified as Asian in the sample. Note that use of the revised scoring guidelines did not change the LE8 score for BMI for this participant.
Physical Activity
Physical activity was assessed using the Paffenbarger Physical Activity Survey, 39 a 7‐day activity recall survey that evaluates the amount of physical activity due to activities of daily living (eg, walking) and leisure activity that involves physical exertion (eg, gardening, jogging). The Paffenbarger is a widely used instrument for assessing habitual physical activity that exhibits good reliability 40 and has been shown to correlate highly with objective measures of body composition 41 and physical activity. 42 Trained staff prompted participants to recall the number of blocks walked, flights of stairs climbed, and any other physical activity for sport, exercise, or recreation within the previous week. When a participant reported engaging in activity that could vary widely in intensity (eg, using an elliptical machine), interviewers conducted follow‐up questioning to obtain a more accurate assessment of intensity level (eg, requesting distance and time). Trained raters categorized exercise and recreation activities according to the 2011 Ainsworth Compendium of Physical Activities, 43 a comprehensive coding system that classifies physical activities by rate of associated energy expenditure in metabolic equivalents of task (METs). Per Ainsworth et al, activities classified as expending 3.0 to 6.0 METs were considered moderate‐intensity physical activity (PA), while activities classified as expending ≥6.0 METs were considered to be in the vigorous‐intensity range. 43 All surveys were rated by 2 individuals, the interviewer who administered the survey and a second independent reviewer. In the case of a discrepant classification, the activity with the lower MET value was chosen, as this was considered to be a more conservative approach. MET values associated with each activity were then used to calculate the number of minutes spent engaging in moderate or vigorous PA. In accordance with the AHA LE8 guidelines for scoring physical activity, minutes of vigorous PA were multiplied by 2. Minutes of moderate or vigorous physical activity were then summed to obtain total minutes per week.
Nicotine Use and History
Participants provided information on their current nicotine use, and if applicable, years of nicotine use, age of onset, number of quit attempts, and quit date, on a health survey. For participants who formerly used nicotine, quit date was subtracted from the date of assessment to determine number of years quit, which was then used to derive the nicotine use score. The health survey was updated shortly after data collection began to include additional questions on other nicotine delivery systems apart from combustible tobacco (eg, e‐cigarettes, vaporizers, hookah). Therefore, while all participants provided information about use of combustible tobacco, 15 participants were missing data on use of other nicotine delivery system due to having completed the survey before the revision. Given evidence suggesting that the prevalence of nicotine delivery system use compared with combustible tobacco use was far lower during the period in which data were collected for the parent study, 44 data for the 15 participants who were missing information on nicotine delivery system use were retained for the calculation of LE8 nicotine use scores.
Dietary Intake and Quality
At T0 and T6 time points, participants completed two 24‐hour dietary recall interviews either over the phone or in person during the baseline assessment. Dietary recall interviews were conducted to obtain a detailed record of all foods and beverages consumed in the previous 24 hours and designed to capture 1 weekday and 1 weekend day given evidence that reports of dietary intake vary significantly depending on what point during a week the recall occurs. 45 Interviews were administered by master's‐level clinicians who received certifications after completing on‐site training in Nutrition Data System for Research software and Nutrition Coordinating Center Food and Nutrient Database, conducting dietary interviews, and dietary recall quality assurance (University of Minnesota, 2023). The Nutrition Data System for Research 46 analysis software was then used to calculate the 2015 version of the Healthy Eating Index (HEI) on the basis of the dietary intake data obtained from the 2 food recall interviews, from which an average HEI score was derived. The HEI is a measure of dietary quality developed to quantify the degree to which an individual's dietary intake patterns conform to the recommendations put forth in the 2015 to 2020 Dietary Guidelines for Americans. 47 The HEI is composed of 13 components, and scores on each component are summed to form a total HEI score ranging from 0 to 100, with higher scores indicating dietary intake more closely aligned with dietary guidelines. In the general population, higher HEI 2015 scores have been associated with lower all‐cause death, and reduced risk of death specifically from cardiovascular disease, type II diabetes, and cancer, 48 , 49 , 50 and the HEI 2015 has been shown to exhibit satisfactory construct and criterion validity. 49 Data on the psychometric properties of the HEI 2015 during the perinatal period are limited. However, HEI 2015 scores have previously been used in the assessment of CVH during pregnancy. 22
Sleep Duration
Participants completed the Pittsburgh Sleep Quality Index (PSQI), 51 a 19‐item self‐report measure on which respondents rate their sleep patterns in the past month using a 0 to 3 Likert Scale. The PSQI has been validated for use in a variety of populations, 52 including among individuals who are pregnant. 53 , 54 For the purposes of the present study, total sleep duration as reported on the PSQI was used to calculate the sleep component of the LE8 score.
LE8 Scoring
Scoring guidelines for the AHA's LE8 metric have been updated from the LS7 formulation to increase sensitivity for detecting individual differences in CVH. Previously, individual components were rated as “ideal,” “intermediate,” or “poor” on the basis of a set of predefined criteria, which obscured the impact of within‐component variation on outcomes of interest. The revised scoring algorithm for the LE8 now assigns each individual component a score ranging from 0 to 100 points, which are used to create a global CVH metric by calculating the unweighted average of all included component scores. The criteria for scoring each individual component were defined by the AHA LE8 working group 19 and are described in Table 2. The AHA has developed different scoring systems for adults (ie, age ≥20) and children (ie, age ≤ 19) to account for developmental factors that impact CVH metrics. 19 Nevertheless, participants aged <20 years in the current study (n = 15; age range, 15.39–19.92 years) were scored as adults on the basis of evidence that diet quality, physical activity, nicotine use, and BMI measured during pregnancy do not differ significantly by age when comparing adults with late adolescents. 44 , 55 , 56 , 57 , 58 LE8 scores were calculated both with and without sleep. This was done for 2 reasons. First, sleep is a new addition to the AHA's composite CVH score, and there have been no studies exploring how inclusion of sleep affects CVH scores in the context of pregnancy. Second, significantly more participants had complete data on BMI, PA, nicotine use and history, and dietary intake at both time points than on sleep. To evaluate the impact of longitudinal change in CVH from T0 to T6, a CVH change score was calculated by subtracting LE8 scores at T0 from LE8 scores at T6. This was done for LE8 scores with and without sleep included, which permitted comparisons of the effect of CVH scores without sleep to the effect of CVH scores with sleep. Of the 257 individuals who participated in the parent study, 10 were missing data for calculating CVH scores at T0, and 28 were missing data for calculating CVH scores at T6.
Table 2.
American Heart Association Scoring Guidelines for the LE8 Metric Components
| Component | Score units | Component value | Score assigned | n (%) at T0 | n (%) at T6 |
|---|---|---|---|---|---|
| Diet quality | Percentiles | 1st– 24th | 0 | 1 (0.4) | 3 (1.3) |
| 25th–49th | 25 | 136 (60.2) | 148 (65.5) | ||
| 50th–74th | 50 | 86 (38.1) | 74 (32.7) | ||
| 74th–94th | 80 | 3 (1.3) | 1 (0.4) | ||
| ≥95th | 100 | 0 (0.0) | 0 (0.0) | ||
| Physical activity | Minutes per week of MVPA | 0 | 0 | 145 (64.2) | 127 (56.2) |
| 1–29 | 20 | 13 (5.8) | 5 (2.2) | ||
| 30–59 | 40 | 11 (4.9) | 3 (1.3) | ||
| 60–89 | 60 | 16 (7.1) | 11 (4.9) | ||
| 90–119 | 80 | 7 (3.1) | 9 (4.0) | ||
| 120–149 | 90 | 9 (4.0) | 10 (4.4) | ||
| ≥150 | 100 | 25 (11.1) | 61 (13.7) | ||
| Nicotine use | Use of combustible tobacco or other NDS | Current use | 0 | 36 (15.9) | 53 (23.4) |
| Quit <1 y or current use of other NDS | 25 | 40 (17.7) | 20 (8.8) | ||
| Quit 1 to <5 y | 50 | 17 (7.5) | 19 (8.4) | ||
| Quit ≥5 y | 75 | 20 (8.8) | 21 (9.3) | ||
| Never used | 100 | 113 (50.0) | 113 (50.0) | ||
| Sleep duration (n = 114) | Average hours of sleep per night | <4 | 0 | 3 (2.6) | 33 (20.1) |
| 4–<5 | 20 | 6 (5.3) | 17 (10.6) | ||
| 5–<6 or ≥10 | 40 | 9 (7.9) | 20 (12.5) | ||
| 6–<7 | 70 | 22 (19.3) | 22 (13.8) | ||
| 9–<10 | 90 | 11 (9.6) | 43 (26.9) | ||
| 7–<9 | 100 | 63 (55.3) | 25 (15.6) | ||
| BMI | kg/m2 | ≥40.0 | 0 | 47 (20.8) | 49 (21.7) |
| 35.0–39.9 | 15 | 43 (19.0) | 47 (20.8) | ||
| 30.0–34.9 | 30 | 50 (22.1) | 62 (27.4) | ||
| 25.0–29.9 | 70 | 82 (36.3) | 61 (27.0) | ||
| <25.0 | 100 | 4 (1.8) | 7 (3.0) |
Percentages for sleep duration categories were calculated using the number of participants who had data for this variable as the denominator.
Lloyd‐Jones et al 19 proposed alternative BMI cut points and scoring guidelines for individuals of Asian and Pacific descent, which we applied to the scoring of BMI for the 1 participant who identified as Asian in the sample. T0, baseline assessment, 12–20 weeks of gestation; T6, 6 months postpartum assessment.
BMI indicates body mass index; LE8, Life's Essential 8; MVPA, moderate and vigorous intensity physical activity; and NDS, nicotine delivery system.
Statistical Analysis
Before hypothesis testing, all data were examined to assess missingness, identify extreme values, and confirm that the data structure met analytic assumptions (eg, normality). A square root transformation was applied to CES‐D scores to adjust for positive skew. Univariate outlier detection was conducted using Rosner's Test 59 in the R package EnvStats 60 (R Foundation for Statistical Computing, Vienna, Austria). Descriptive analyses were conducted to examine sample characteristics. Multiple linear regression analyses were then performed to evaluate whether changes in LE8 scores from T0 to T6 were associated with T6 CES‐D and PSS scores. Analyses were performed with and without sleep included in the LE8 metric. All models were adjusted for baseline LE8 scores to account for the influence of individual variation on CVH during the second trimester of pregnancy on change in CVH through 6 months postpartum. Prediction performance of each model was evaluated by calculating the root mean square error. Two logistic regression analyses were conducted to evaluate whether change in CVH scores (with and without sleep) were associated with odds of scoring ≥16 on the CES‐D at T6, which is the typical cut of score used to indicate presence of a major depressive episode. Because the Asian and American Indian or Alaska Native racial identity categories only had 1 member each, it was not possible to estimate their effects on odds of scoring ≥16 on the CES‐D at T6. Therefore, we collapsed the racial identity variable to White racial identity, Black racial identity, and other racial identity categories for logistic regression models. In addition, weeks of gestation at the time of enrollment, age, racial identity, household income (dichotomized as ≤$30,000 or > $30,000 per year), and educational attainment were included as covariates in all models. These covariates were chosen on the basis of evidence that health behavior engagement changes over the course of pregnancy 61 , 62 , 63 , 64 and that demographic characteristics and social determinants of health are associated with differences in CVD risk. 19 , 65 Baseline scores on the CES‐D and PSS were also included as covariates in the relevant models. Model fit was evaluated using the overall F‐test and regression diagnostic plots were visually inspected to confirm that the assumptions of linear regression were met. Presence of high‐leverage outliers was evaluated via Cook's distance values using a cutoff of ≥0.5; no values exceeded this threshold for any analysis. Post hoc paired‐sample t tests were performed to evaluate differences between baseline and postpartum LE8 component scores. For all tests, the level of statistical significance was set at P<0.05, and standardized coefficients were selected for reporting significant effects. Analyses were conducted in R Studio 66 using R version 4.2.2. 67
Results
Sample Characteristics
Participants completed their initial baseline assessment visit when their pregnancies were 15.64 (SD, 2.45) weeks of gestation. Mean CVH scores excluding sleep were 40.27 (SD, 17.64) at baseline and 41.97 (SD,19.98) at 6 months postpartum. With the inclusion of sleep in the CVH metric, mean CVH scores were 55.05 (SD, 15.03) at baseline and 46.86 (SD, 17.92) at 6 months postpartum. The intraclass correlation between CVH scores with and without sleep was 0.84 (95% CI, 0.212–0.945) at T0 and 0.89 at T6 (95% CI, 0.838–0.921). Mean postpartum depressive symptoms were in the mild range (mean, 10.75 [SD, 9.58]; range, 0–49), and 51 individuals (22.6%) scored above the clinical cutoff of 16, suggestive of risk for a depressive episode. Ratings of perceived stress were in the moderate range of severity (mean, 20.73 [SD, 8.97]; range, 3–45). Demographic and clinical characteristics of the sample at each time point are presented in more detail in Table 3.
Table 3.
Demographic and Clinical Characteristics of Sample
| T0 | T6 | |
|---|---|---|
| Mean±SD | Mean±SD | |
| Weeks of gestation | 15.64±2.45 | ‐ |
| Prepregnancy BMI, kg/m2 | 32.74±6.55 | ‐ |
| Gestational weight gain, lbs* | ‐ | 29.43±20.41 |
| Postpartum weight retention, lbs† | ‐ | 13.37±18.96 |
| Age, y | 28.43±5.40 | ‐ |
| CES‐D | 12.46±9.88 | 10.75±9.58 |
| PSS | 20.91±8.73 | 20.73±8.97 |
| CVH total (sleep included; n = 114) | 53.05±15.03 | 46.86±17.92 |
| CVH total (sleep excluded) | 40.27±17.64 | 41.97±19.98 |
| BMI, kg/m2 | 34.17±7.15 | 34.94±7.41 |
| 2015 HEI scores‡ | 47.36±10.96 | 45.73±10.78 |
| Minutes of weekly MVPA§ | 120.15±293.96 | 138.01±316.50 |
| Hours of sleep per night (n = 114) | 7.05±1.62 | 6.12±3.22 |
| n (%) | n (%) | |
| CES‐D scores ≥16|| | 65 (28.76) | 51 (22.56) |
| Current combustible tobacco or other NDS use | 52 (23.01) | 72 (31.85) |
| Yearly household income | ‐ | |
| ≤$30,000 | 148 (65.48) | |
| >$30,000 | 78 (34.51) | |
| Education | ‐ | |
| Grade school or some high school | 28 (12.39) | |
| High school graduate or GED | 47 (20.80) | |
| Some college or technical school | 86 (38.05) | |
| 4‐y college graduate | 31 (13.72) | |
| Postgraduate degree | 34 (15.04) | |
| Racial identity | ‐ | |
| Asian | 1 (0.44) | |
| American Indian or Alaska Native | 3 (1.32) | |
| Black | 113 (50.0) | |
| Multiracial | 9 (3.98) | |
| Unknown | 4 (1.77) | |
| White | 96 (42.48) | |
| Hispanic ethnicity | 7 (6.8) | ‐ |
T0, baseline assessment; T6, postpartum assessment. BMI indicates body mass index; CES‐D, Center for Epidemiologic Studies Depression Scale; CVH = cardiovascular health; GED = General Educational Development; HEI, Healthy Eating Index; MVPA = moderate and vigorous intensity physical activity; NDS = nicotine delivery system; and PSS, Perceived Stress Scale.
Gestational weight gain was calculated as the measured weight before delivery, obtained from medical records, minus self‐reported prepregnancy weight obtained during the initial phone screen. Data were missing for 4 participants.
Postpartum weight retention was calculated by subtracting self‐reported prepregnancy weight in lbs from measured weight in lbs obtained at T6.
HEI scores for the weekend and weekday dietary intake assessments were averaged to create a single HEI score representing overall diet quality during the week of assessment.
Minutes of vigorous physical activity were not doubled when calculating descriptive statistics presented in this table. They were only doubled for the purposes of calculating the Life's Essential 8 metric, per the American Heart Association's scoring guidelines.
Scores of ≥16 on the CES‐D are considered indicative of risk for a major depressive episode.
Comparison of Individuals With and Without Sleep Data
As described above, because the PSQI was added to the assessment battery midway through the study, only 114 participants (50.4%) completed it at the baseline assessment. At baseline, there were significant differences between those with and without PSQI data in terms of household income (X 2[1, N = 226] = 6.56; P=0.01) and weeks of gestation of pregnancy at the time of enrollment (β = −0.18, P<0.01). Regarding household income, 57% of individuals who completed the PSQI reported earning ≤$30,000 compared with 74% of individuals who did not complete the PSQI. Individuals who completed the PSQI also entered the study later in their pregnancies compared with those who did not complete the PSQI (mean weeks of gestation with versus without PSQI: 16.67 ± 2.39 versus 14.65 ± 2.09). There were no other significant differences in baseline demographic characteristics between those with and without PSQI data (all P>0.06). When comparing those with and without PSQI data on postpartum outcomes, individuals who completed the PSQI exhibited significantly higher CVH scores calculated excluding sleep (mean, 44.77 [SD, 20.31]) compared with those who did not complete the PSQI (mean, 39.12 [SD, 19.31]; β = −0.18, P<0.01). There were no significant differences between those with and without PSQI data on postpartum CES‐D or PSS scores (all P>0.36).
Changes in Individual CVH Components From Baseline to Postpartum
Compared with baseline, participants had significantly lower scores for BMI (t[225] = 2.92, P<0.01) and sleep (t[112] = 5.69, P < 0.01) and significantly higher scores for PA (t[225] = −3.91, P<0.01) at the postpartum assessment. There were no significant differences from baseline to postpartum in LE8 component scores for dietary intake or nicotine use (all P>0.06).
Relationship Between CVH Behaviors and Postpartum Psychological Distress
When excluding sleep from the CVH metric, worsening CVH scores from baseline to 6 months postpartum predicted higher postpartum depressive symptoms (model root mean squared error = 1.21; β = −0.18, [95% CI, −0.33 to −0.01]; P < 0.01) and ratings of perceived stress (model root mean squared error = 7.0; β = −0.13 [95% CI, −0.26 to −0.04]; P = 0.02). Overall predictive performance of both models was satisfactory, though it was far stronger for CES‐D scores compared with PSS scores. When examining unadjusted means, compared with those whose CVH improved by >1 SD, individuals whose CVH worsened by >1 SD scored 6.42 points higher on the CES‐D (mean ± SD: mean CES‐D, 15.25 ± 10.92 versus 8.52 ± 6.90) and 6.12 points higher on the PSS (mean PSS, 24.45 ± 8.29 versus 17.83 ± 8.70) at 6 months postpartum. Adjusting for covariates, this difference in mean scores persisted, with individuals whose CVH scores worsened by >1 SD reporting higher severity symptoms compared with those whose scores improved by >1 SD (mean CES‐D, 3.53 ± 0.78 versus 2.59 ± 0.81; mean PSS, 23.34 ± 8.97 versus 17.88 ± 5.69), although the difference was attenuated. The impact of adjustment on mean scores was more pronounced for the CES‐D than the PSS, which is consistent with results from regression models demonstrating that covariates were more strongly associated with CES‐D scores than they were PSS scores. Further, improved CVH was associated with lower odds of having depressive symptom severity above the cutoff score of 16 typically used to identify the presence of a depressive episode (odds ratio, 0.975 [95% CI, −0.049 to −0.002]; P = 0.04). These relationships persisted after adjusting for baseline CVH, baseline symptom scores, as well as demographic factors such as age, racial identity, educational attainment, and household income. The Figure depicts differences between postpartum CES‐D and PSS scores for those whose CVH improved versus worsened from pregnancy to postpartum. Table 4 includes more detailed results from linear regression models using the CVH metric that excluded sleep. Full details of the logistic regression model outputs can be found in Tables S1 and S2.
Figure . Differences in postpartum CES‐D and PSS scores among participants whose CVH worsened from pregnancy to postpartum compared with those whose CVH scores improved.
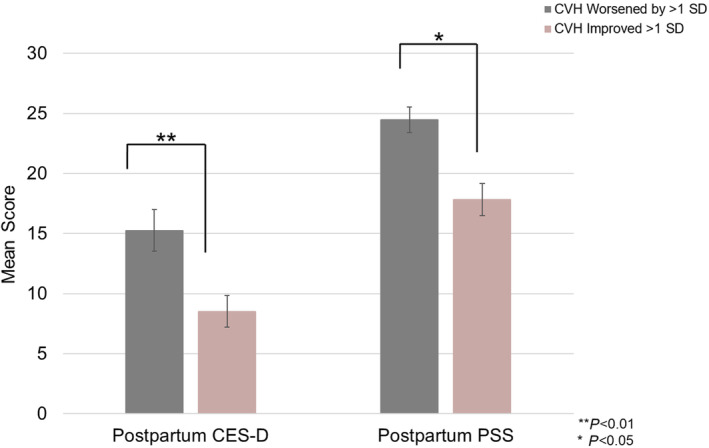
Error bars reflect the standard error of the mean. CES‐D indicates Center for Epidemiologic Studies Depression Scale; CVH, cardiovascular health; and PSS, Perceived Stress Scale.
Table 4.
Associations Between the Changes in CVH (Excluding Sleep) From Baseline to 6 Months Postpartum and Postpartum Psychological Functioning
| Coefficient | Estimate (SE) | P value |
|---|---|---|
| Model: CES‐D; RMSE = 1.21; F(8217) = 12.89, P < 0.01 | ||
| (Intercept) | 2.076 (0.624)* | 0.008* |
| Change in CVH scores from T0 to T6 | −0.017 (0.005)* | 0.002* |
| T0 CVH scores | 0.002 (0.006) | 0.679 |
| T0 CES‐D scores | 0.071 (0.009)* | <0.001* |
| T0 age | −0.002 (0.019) | 0.570 |
| T0 gestational age, wks | −0.020 (0.035) | 0.911 |
| Black racial identity | 0.453 (0.225)* | 0.045* |
| Asian racial identity | 0.043 (1.261) | 0.973 |
| American Indian or Alaska Native racial identity | −0.074 (0.748) | 0.921 |
| Multiracial identity | 0.479 (0.449) | 0.287 |
| Unknown racial identity | 1.104 (0.754) | 0.286 |
| High school graduate/GED | −0.249 (0.296) | 0.399 |
| Some college or technical school | −0.382 (0.282) | 0.177 |
| 4‐y college degree | −0.593 (0.403) | 0.183 |
| Postgraduate degree | −0.906 (0.442)* | 0.006* |
| Annual household income >$30,000 | 0.399 (0.305) | 0.191 |
| Coefficient | Estimate (SE) | P value |
| Model: PSS; RMSE = 7.0; F(8217) = 17.46, P < 0.01 | ||
| (Intercept) | 2.179 (5.138) | 0.672 |
| Change in CVH scores from T0 to T6 | −0.078 (0.032)* | 0.018* |
| T0 CVH scores | 0.047 (0.035) | 0.181 |
| T0 PSS scores | 0.607 (0.059)* | <0.001* |
| T0 age | 0.133 (0.204) | 0.513 |
| T0 gestational age, wks | 0.134 (0.108) | 0.217 |
| Black racial identity | 0.296 (1.293) | 0.819 |
| Asian racial identity | 2.715 (7.209) | 0.707 |
| American Indian or Alaska Native racial identity | −1.787 (4.265) | 0.676 |
| Multiracial identity | −0.739 (2.571) | 0.774 |
| Unknown racial identity | −3.177 (4.329) | 0.464 |
| High school graduate/GED | −1.616 (1.692) | 0.341 |
| Some college or technical school | −2.007 (1.601) | 0.211 |
| 4‐y college degree | −3.096 (2.309) | 0.182 |
| Postgraduate degree | −5.063 (2.521)* | 0.046* |
| Annual household income > $30,000 | 0.789 (1.732) | 0.649 |
Coefficients are unstandardized. Reference groups for categorical variables are as follows: White racial identity; less than a high school education or equivalent; annual household income ≤$30,000. T0, baseline assessment; T6, postpartum assessment. CES‐D indicates Center for Epidemiologic Studies Depression Scale; CVH, cardiovascular health; GED, General Educational Development; PSS, Perceived Stress Scale; and RMSE, root mean squared error.
Indicate statistical significance (P<0.05).
Relationship Between CVH Behaviors, Including Sleep, and Postpartum Psychological Distress
When sleep was included in the CVH metric, the associations between change in CVH scores and postpartum symptom scores were no longer significant (CES‐D model: model root mean squared error, 1.17; β = 0.06 [95% CI, −0.13 to 0.20]; P = 0.4]; CES‐D logistic regression model: odds ratio, 1.002 [95% CI, −0.034 to 0.038]; P = 0.92; PSS model: model root mean squared error, 6.9; β = 0.04 [95% CI, −0.12 to −0.20]; P = 0.6). Overall predictive performance of both models was comparable with that observed for the models excluding sleep. Post hoc assessment of the Pearson's correlation between sleep duration early in pregnancy and postpartum symptom scores indicated that sleep duration at T0 was weakly negatively associated with T6 depressive symptoms (r = −0.09, P = 0.30) and not correlated with T6 perceived stress (r = −0.001, P = 0.99). In contrast, sleep measured at T6 was strongly positively correlated with concurrent depressive symptoms (r = 0.40, P < 0.01) and perceived stress (r = 0.31, P < 0.01), meaning that longer postpartum sleep duration was associated with higher severity symptomatology. Table 5 provides more detailed results from linear regression models using the CVH metric that included sleep.
Table 5.
Associations Between the Changes in CVH Including Seep From Baseline to 6 Months Postpartum and Postpartum Psychological Functioning (N = 114)
| Coefficient | Estimate (SE) | P value |
|---|---|---|
| Model: CES‐D; RMSE = 1.17; F(8104) = 8.031, P < 0.01 | ||
| (Intercept) | 4.363 (1.574)* | 0.007* |
| Change in CVH scores from T0 to T6 | 0.005 (0.008) | 0.493 |
| T0 CVH scores | −0.001 (0.010) | 0.957 |
| T0 CES‐D scores | 0.077 (0.014)* | <0.001* |
| T0 age | −0.051 (0.027) | 0.067 |
| T0 gestational age, wks | −0.041 (0.052) | 0.434 |
| Black racial identity | 0.607 (0.332) | 0.071 |
| Asian racial identity | 0.673 (1.272) | 0.598 |
| American Indian or Alaska Native racial identity | −0.161 (1.328) | 0.903 |
| Multiracial identity | 1.516 (0.961) | 0.118 |
| Unknown racial identity | 1.395 (0.787) | 0.079 |
| High school graduate/GED | −0.886 (0.511) | 0.086 |
| Some college or technical school | −0.668 (0.479) | 0.166 |
| 4‐y college degree | −1.057 (0.604) | 0.083 |
| Postgraduate degree | −1.577 (0.665)* | 0.020* |
| Annual household income > $30,000 | 1.046 (0.423)* | 0.015* |
| Coefficient | Estimate (SE) | P value |
| Model: PSS; RMSE = 6.9; F(8104) = 9.065, P < 0.01 | ||
| (Intercept) | 11.702 (9.599) | 0.226 |
| Change in CVH scores from T0 to T6 | 0.025 (0.048) | 0.606 |
| T0 CVH scores | 0.019 (0.062) | 0.763 |
| T0 PSS scores | 0.604 (0.085)* | <0.001* |
| T0 age | −0.132 (0.167) | 0.427 |
| T0 gestational age, wks | 0.056 (0.318) | 0.860 |
| Black racial identity | 1.103 (2.008) | 0.584 |
| Asian racial identity | 5.634 (7.669) | 0.486 |
| American Indian or Alaska Native racial identity | −0.369 (8.055) | 0.964 |
| Multiracial identity | 1.018 (5.776) | 0.783 |
| Unknown racial identity | −1.315 (4.765) | 0.783 |
| High school graduate/GED | −3.173 (3.050) | 0.301 |
| Some college or technical school | −2.256 (2.856) | 0.432 |
| 4‐y college degree | −3.271 (3.672) | 0.375 |
| Postgraduate degree | −5.816 (4.002) | 0.149 |
| Annual household income > $30,000 | 4.013 (2.533) | 0.116 |
Reference groups for categorical variables are as follows: White racial identity; less than a high school education or equivalent; annual household income ≤ $30,000. T0, baseline assessment; T6, postpartum assessment. CES‐D indicates Center for Epidemiologic Studies Depression Scale; CVH, cardiovascular health; GED, General Educational Development; PSS, Perceived Stress Scale; and RMSE, root mean squared error.
Indicate statistical significance (P<0.05).
Discussion
The present study examined the longitudinal association between change in CVH from early pregnancy to the postpartum period and postpartum psychological distress in a community sample of individuals with BMI ≥25. The AHA's LE8 composite metric was used to index CVH, calculated both with and without sleep, a new addition to the AHA's composite metric, to capitalize on the larger sample of individuals with available data on BMI, nicotine exposure, PA, and diet quality but who did not complete the sleep assessment. Consistent with study hypotheses, improvements in CVH from pregnancy to 6 months postpartum were associated with lower severity of depressive symptoms and perceived stress when excluding sleep from the CVH metric, relationships that persisted after adjusting for the potentially confounding effects of early pregnancy sociodemographic characteristics, CVH, and symptom measures. To our knowledge, this is the first study to examine the relationship between CVH during pregnancy as measured using the AHA's LE8 composite and postpartum psychological outcomes. Prior research exploring how cardiovascular conditions emerging during pregnancy relate to postpartum psychological functioning have predominantly focused on the impact of diagnosed cardiovascular illness. Thus, our findings extend the existing evidence by demonstrating that CVH is associated with postpartum psychological health. These results suggest that measuring cardiovascular health using a holistic construct rather than the presence or absence of a diagnosable disease state may be useful for risk detection and prevention during pregnancy and postpartum. This is consistent with a recent scientific statement from the AHA proposing that use of the LE8 to assess CVH during the perinatal period represents an opportunity to reduce maternal CVD risk. 68
These findings suggest that the AHA's measure of CVH may be useful for identifying individuals before delivery who are at risk for experiencing postpartum depression and elevated stress. Importantly, the factors that are included in the LE8 metric are either routinely collected throughout pregnancy (ie, weight, blood biomarkers) or are relatively convenient to assess using self‐report measures. Therefore, the potential impact on current clinical workflows would be fairly minor, especially when considered in relation to the benefits of monitoring CVH during pregnancy. For example, using this metric for early identification of vulnerable individuals will enable providers to connect patients to prevention and intervention resources to optimize postpartum health and well‐being. Given the pernicious effects of postpartum mental health conditions such as depression on maternal suicide risk 69 and infant development, 70 improving screening and identification of pregnant individuals who are vulnerable to postpartum distress using the CVH metric has the potential to engender benefits to maternal and child health.
Another potential implication of this observed link between change in CVH across pregnancy and postpartum and postpartum psychological distress is that interventions targeting prenatal CVH may improve maternal well‐being following delivery. This idea is consistent with evidence that interventions aimed at increasing engagement in healthy lifestyle behaviors linked to CVH improve postpartum maternal mental health. For example, participating in structured physical activity during pregnancy reduces risk of postpartum depression. 71 An emerging body of research has also demonstrated that brief interventions for insomnia during the perinatal period may reduce risk for postpartum depression, 72 , 73 although this area of research is relatively nascent. It will be important to conduct additional research exploring whether interventions focused on aspects of CVH yield similar benefits to postpartum mental health.
Contrary to our hypotheses, CVH was no longer associated with postpartum psychological distress when sleep was included as a component of CVH. The finding that the addition of sleep to the CVH metric changed the link to psychological distress stands in contrast to previous research demonstrating that poor sleep quality during the perinatal period is associated with increased risk of experiencing postpartum symptoms such as depression and anxiety. 74 , 75 , 76 , 77 However, given that only 50% of the present sample (n = 114) completed the sleep assessment at baseline, the lack of an association between change in CVH and postpartum psychological distress when including sleep may be attributable to the significant sample loss incurred by doing so. Further, there were significant differences in household income, weeks of gestation at the time of enrollment, and postpartum CVH (excluding sleep) between those who did not have sleep data (ie, were recruited earlier in the study period) and those who did, suggesting possible cohort effects. It is also important to note that sleep duration early in pregnancy was only weakly correlated with postpartum depressive symptoms and not correlated with postpartum ratings of perceived stress in the present study. Therefore, it is possible that the lack of an association between change in CVH and postpartum psychological distress when including sleep duration as a component of the CVH metric may be explained by the fact that early‐pregnancy sleep duration was not robustly related to postpartum psychological symptom scores in this sample. In addition, sleep measured at 6 months postpartum was strongly positively correlated with concurrent depressive symptoms and perceived stress, suggesting that individuals whose postpartum sleep duration was in the more ideal range were experiencing higher severity symptoms. This pattern is inconsistent with prior research examining the relationship between sleep duration and postpartum psychological functioning. 74 , 78 , 79 Finally, sleep duration is only 1 of several sleep characteristics that has been linked to health and well‐being outcomes (eg, sleep discontinuity, time spent awake after initiating sleep), and it is unknown whether sleep duration is the characteristic that is most salient during pregnancy and postpartum. Of note, sleep duration recommendations differ by age group for calculating LE8 among children, and it is possible that adjustments to LE8 scoring guidelines for sleep may similarly be warranted during pregnancy. Given that sleep disruption is a common occurrence during the perinatal period, it is possible that the relationship between sleep and postpartum psychological functioning manifests differently during this period. Additional research is needed to better understand how sleep difficulties that are common during the perinatal period impact postpartum health and psychological well‐being.
Strengths, Limitations, and Directions for Further Research
In addition to being the first study to employ the AHA's LE8 framework in assessing the relationship between CVH and psychological functioning during the perinatal period, there are other notable strengths of the present study to highlight. We focused our investigation on individuals who began their pregnancies with overweight or obesity on the basis of evidence that this is a population at heightened risk for cardiovascular conditions acquired in pregnancy, obstetric complications, and poor postpartum mental health. 27 , 28 As such, it is important to understand the relationship between indicators of CVH and psychological functioning in this vulnerable group to permit more effective prevention and intervention efforts. In addition, a substantial proportion of the sample self‐identified as being Black (50%), and the majority reported a yearly household income of ≤$30,000 (65%). Prior research, in contrast, has primarily been conducted in samples that are predominantly White identifying and higher income, potentially hampering efforts to better understand the significant inequities in perinatal health and well‐being for individuals from disadvantaged and marginalized communities. 80
Despite these strengths, there are a number of important limitations that should be taken into account when interpreting our findings. First, sleep data were only available for half of the sample, restricting statistical power to examine the relationship between CVH and postpartum psychological functioning using the full set of CVH components measured in the parent study. Further, because we did not collect blood samples or measure blood pressure, CVH scores were limited to BMI and health behaviors known to predict CVD risk. Therefore, we were unable to comprehensively assess the impact of CVH as conceptualized by the AHA on postpartum psychological outcomes. Second, given the dearth of research employing the LE8 metric in the perinatal period, it is unclear whether it is necessary to adapt the metric to account for the unique context of pregnancy. For example, pregnancy is associated with normative changes in health behaviors such as diet (eg, dietary restrictions, vitamin supplementation), weight, and blood pressure that may or may not be relevant for estimating disease risk. Indeed, it is not yet established whether the factors currently included in the CVH metric are the strongest predictors of CVH or CVD risk in childbearing individuals, given that the metric was developed on the basis of research conducted in the general population without regard for the impact of pregnancy. Relatedly, it may be important to consider whether other measures of body weight and composition should be added to the metric beyond BMI, such as gestational weight gain and postpartum weight retention. Further, some studies have found evidence that the established BMI cut points may not be appropriate for Black populations. 81 , 82 , 83 , 84 However, there is inconsistency across studies regarding which cut points are most appropriate for identifying individuals at risk for cardiovascular disease, and no guidelines have been developed regarding which cut points to use in this population. Nevertheless, it is possible that the current BMI scoring for the LE8 may not be appropriate for Black individuals. Additional research exploring these questions is necessary to determine whether the LE8 metric as it is currently composed and scored is appropriate for evaluating CVH during the perinatal period. It also important to note that because we did not validate our models in a second test data set, model predictive performance should be interpreted with caution. Finally, sleep and physical activity were assessed using self‐report, which has been shown to correlate only moderately with objective measures of these behaviors. 85 , 86 Future studies evaluating CVH in the perinatal period would benefit from employing actigraphy to obtain more robust, accurate, and nuanced measures of these behaviors.
Conclusions
The present study demonstrated that worsening of CVH behaviors from pregnancy to postpartum is longitudinally associated with more severe depressive symptoms and greater perceived stress at 6 months postpartum among individuals at high risk for future CVD. These findings provide initial evidence that improved management of cardiovascular risk factors during pregnancy may confer specific benefits to maternal mental health in addition to reducing the likelihood of developing pregnancy‐related cardiovascular conditions. Additional research with more robust and complete measurement of the components of CVH across the perinatal period is needed to further validate these associations and to explore whether interventions targeting CVH may promote improved outcomes for maternal mental health.
Sources of Funding
This work is supported by the National Institute of Child Health and Human Development (R01 HD068802 [M.D.L.]); the National Heart, Lung, and Blood Institute (T32 HL007560 [supporting R.J.J.] and R01 HL132578 [M.D.L.]); and the National Institute of Mental Health (T32 MH018269 [supporting C.C.C.]).
Disclosures
None.
Supporting information
Tables S1–S2
Acknowledgments
Dr Donofry had full access to all of the data included in the present study, conducted all study analyses, and takes full responsibility for the integrity of the data and its analysis.
This manuscript was sent to Tiffany M. Powell‐Wiley, MD MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.034153
For Sources of Funding and Disclosures, see page 12.
References
- 1. Sanghavi M, Rutherford JD. Cardiovascular physiology of pregnancy. Circulation. 2014;130:1003–1008. doi: 10.1161/CIRCULATIONAHA.114.009029 [DOI] [PubMed] [Google Scholar]
- 2. Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy‐related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130:366–373. doi: 10.1097/AOG.0000000000002114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shah A, Patel J, Isath A, Virk HU, Jneid H, Zelop CM, Mehta‐Lee S, Economy KE, Gulati M, Krittanawong C. Cardiovascular complications in pregnancy. Curr Treat Options Cardiovasc Med. 2023;25:391–414. doi: 10.1007/s11936-023-01000-8 [DOI] [Google Scholar]
- 4. Fraser A, Nelson SM, Macdonald‐Wallis C, Cherry L, Butler E, Sattar N, Lawlor DA. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age. Circulation. 2012;125:1367–1380. doi: 10.1161/CIRCULATIONAHA.111.044784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown HL, Warner JJ, Gianos E, Gulati M, Hill AJ, Hollier LM, Rosen SE, Rosser ML, Wenger NK. Promoting risk identification and reduction of cardiovascular disease in women through collaboration with obstetricians and gynecologists: a presidential advisory from the American Heart Association and the American College of Obstetricians and Gynecologists. Circulation. 2018;137:e843–e852. doi: 10.1161/CIR.0000000000000582 [DOI] [PubMed] [Google Scholar]
- 6. Caropreso L, de Azevedo CT, Eltayebani M, Frey BN. Preeclampsia as a risk factor for postpartum depression and psychosis: a systematic review and meta‐analysis. Arch Womens Ment Health. 2020;23:493–505. doi: 10.1007/s00737-019-01010-1 [DOI] [PubMed] [Google Scholar]
- 7. Blom EA, Jansen PW, Verhulst FC, Hofman A, Raat H, Jaddoe VW, Coolman M, Steegers EA, Tiemeier H. Perinatal complications increase the risk of postpartum depression. The generation R study. BJOG Int J Obstet Gynaecol. 2010;117:1390–1398. doi: 10.1111/j.1471-0528.2010.02660.x [DOI] [PubMed] [Google Scholar]
- 8. Arafa A, Dong JY. Gestational diabetes and risk of postpartum depressive symptoms: a meta‐analysis of cohort studies. J Affect Disord. 2019;253:312–316. doi: 10.1016/j.jad.2019.05.001 [DOI] [PubMed] [Google Scholar]
- 9. Azami M, Badfar G, Soleymani A, Rahmati S. The association between gestational diabetes and postpartum depression: a systematic review and meta‐analysis. Diabetes Res Clin Pract. 2019;149:147–155. doi: 10.1016/j.diabres.2019.01.034 [DOI] [PubMed] [Google Scholar]
- 10. Howard LM, Molyneaux E, Dennis CL, Rochat T, Stein A, Milgrom J. Non‐psychotic mental disorders in the perinatal period. The Lancet. 2014;384:1775–1788. doi: 10.1016/S0140-6736(14)61276-9 [DOI] [PubMed] [Google Scholar]
- 11. Jones I, Chandra PS, Dazzan P, Howard LM. Bipolar disorder, affective psychosis, and schizophrenia in pregnancy and the post‐partum period. The Lancet. 2014;384:1789–1799. doi: 10.1016/S0140-6736(14)61278-2 [DOI] [PubMed] [Google Scholar]
- 12. Meltzer‐Brody S, Maegbaek ML, Medland SE, Miller WC, Sullivan P, Munk‐Olsen T. Obstetrical, pregnancy and socio‐economic predictors for new‐onset severe postpartum psychiatric disorders in primiparous women. Psychol Med. 2017;47:1427–1441. doi: 10.1017/S0033291716003020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A meta‐analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67:1012–1024. doi: 10.1001/archgenpsychiatry.2010.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Irwin JL, Davis EP, Hobel CJ, Coussons‐Read M, Dunkel SC. Maternal prenatal anxiety trajectories and infant developmental outcomes in one‐year‐old offspring. Infant Behav Dev. 2020;60:101468. doi: 10.1016/j.infbeh.2020.101468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kingston D, Tough S, Whitfield H. Prenatal and postpartum maternal psychological distress and infant development: a systematic review. Child Psychiatry Hum Dev. 2012;43:683–714. doi: 10.1007/s10578-012-0291-4 [DOI] [PubMed] [Google Scholar]
- 16. Slomian J, Honvo G, Emonts P, Reginster JY, Bruyère O. Consequences of maternal postpartum depression: a systematic review of maternal and infant outcomes. Womens Health. 2019;15:1745506519844044. doi: 10.1177/1745506519844044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Van Sieleghem S, Danckaerts M, Rieken R, Okkerse JM, de Jonge E, Bramer WM, Lambregtse‐van den Berg MP. Childbirth related PTSD and its association with infant outcome: a systematic review. Early Hum Dev. 2022;174:105667. doi: 10.1016/j.earlhumdev.2022.105667 [DOI] [PubMed] [Google Scholar]
- 18. Sanchez E. Life's simple 7: vital but not easy. J Am Heart Assoc. 2018;7:e009324. doi: 10.1161/JAHA.118.009324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lloyd‐Jones DM, Allen NB, Anderson CA, Black T, Brewer LC, Foraker RE, Grandner MA, Lavretsky H, Perak AM, Sharma G, et al. Life's essential 8: updating and enhancing the American Heart Association's construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. 2022;146:e18–e43. doi: 10.1161/CIR.0000000000001078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Petermann‐Rocha F, Deo S, Celis‐Morales C, Ho FK, Bahuguna P, McAllister D, Sattar N, Pell JP. An opportunity for prevention: associations between the Life's essential 8 score and cardiovascular incidence using prospective Data from UK biobank. Curr Probl Cardiol. 2023;48:101540. doi: 10.1016/j.cpcardiol.2022.101540 [DOI] [PubMed] [Google Scholar]
- 21. Yi J, Wang L, Guo X, Ren X. Association of Life's essential 8 with all‐cause and cardiovascular mortality among US adults: a prospective cohort study from the NHANES 2005–2014. Nutr Metab Cardiovasc Dis. 2023;33:1134–1143. doi: 10.1016/j.numecd.2023.01.021 [DOI] [PubMed] [Google Scholar]
- 22. Perak AM, Ning H, Khan SS, Van Horn LV, Grobman WA, Lloyd‐Jones DM. Cardiovascular health among pregnant women, aged 20 to 44 years, in the United States. J Am Heart Assoc. 2020;9:e015123. doi: 10.1161/JAHA.119.015123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Perak AM, Lancki N, Kuang A, Labarthe DR, Allen NB, Shah SH, Lowe LP, Grobman WA, Scholtens DM, Lloyd‐Jones DM, et al. Associations of gestational cardiovascular health with pregnancy outcomes: the hyperglycemia and adverse pregnancy outcome study. Am J Obstet Gynecol. 2021;224:210.e1–210.e17. doi: 10.1016/j.ajog.2020.07.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Laura B, Sarah S‐T, Schelling SJC, Steegers Eric AP, Roeters van Lennep Jeanine E. Early pregnancy cardiovascular health and subclinical atherosclerosis. J Am Heart Assoc. 2019;8:e011394. doi: 10.1161/JAHA.118.011394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perak AM, Lancki N, Kuang A, Labarthe DR, Allen NB, Shah SH, Lowe LP, Grobman WA, Lawrence JM, Lloyd‐Jones DM, et al. Associations of maternal cardiovascular health in pregnancy with offspring cardiovascular health in early adolescence. JAMA. 2021;325:658–668. doi: 10.1001/jama.2021.0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Athukorala C, Rumbold AR, Willson KJ, Crowther CA. The risk of adverse pregnancy outcomes in women who are overweight or obese. BMC Pregnancy Childbirth. 2010;10:56. doi: 10.1186/1471-2393-10-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Langley‐Evans SC, Pearce J, Ellis S. Overweight, obesity and excessive weight gain in pregnancy as risk factors for adverse pregnancy outcomes: a narrative review. J Hum Nutr Diet. 2022;35:250–264. doi: 10.1111/jhn.12999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ertel KA, Huang T, Rifas‐Shiman SL, Kleinman K, Rich‐Edwards J, Oken E, James‐Todd T. Perinatal weight and risk of prenatal and postpartum depressive symptoms. Ann Epidemiol. 2017;27:695–700.e1. doi: 10.1016/j.annepidem.2017.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. LaCoursiere D, Barrett‐Connor E, O'Hara M, Hutton A, Varner M. The association between prepregnancy obesity and screening positive for postpartum depression. BJOG Int J Obstet Gynaecol. 2010;117:1011–1018. doi: 10.1111/j.1471-0528.2010.02569.x [DOI] [PubMed] [Google Scholar]
- 30. Levine MD, Tavernier RLE, Conlon RPK, Grace JL, Sweeny GM, Wang B, Cheng Y. Loss of control eating during pregnancy is associated with excessive gestational weight gain among individuals with overweight and obesity. BMC Pregnancy Childbirth. 2023;23:340. doi: 10.1186/s12884-023-05618-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Radloff LS. The CES‐D scale: a self‐report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 32. Cosco TD, Prina M, Stubbs B, Wu YT. Reliability and validity of the Center for Epidemiologic Studies Depression Scale in a population‐based cohort of middle‐aged U.S. adults. J Nurs Meas. 2017;25:476–485. doi: 10.1891/1061-3749.25.3.476 [DOI] [PubMed] [Google Scholar]
- 33. Knight RG, Williams S, McGee R, Olaman S. Psychometric properties of the Centre for Epidemiologic Studies Depression Scale (CES‐D) in a sample of women in middle life. Behav Res Ther. 1997;35:373–380. doi: 10.1016/S0005-7967(96)00107-6 [DOI] [PubMed] [Google Scholar]
- 34. Orme JG, Reis J, Herz EJ. Factorial and discriminant validity of the center for epidemiological studies depression (CES‐D) scale. J Clin Psychol. 1986;42(1):28–33. doi: [DOI] [PubMed] [Google Scholar]
- 35. Maloni JA, Park S, Anthony MK, Musil CM. Measurement of antepartum depressive symptoms during high‐risk pregnancy. Res Nurs Health. 2005;28:16–26. doi: 10.1002/nur.20051 [DOI] [PubMed] [Google Scholar]
- 36. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. doi: 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- 37. Taylor JM. Psychometric analysis of the ten‐item perceived stress scale. Psychol Assess. 2015;27:90–101. doi: 10.1037/a0038100 [DOI] [PubMed] [Google Scholar]
- 38. Solivan AE, Xiong X, Harville EW, Buekens P. Measurement of perceived stress among pregnant women: a comparison of two different instruments. Matern Child Health J. 2015;19:1910–1915. doi: 10.1007/s10995-015-1710-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Paffenbarger RS, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108:161–175. doi: 10.1093/oxfordjournals.aje.a112608 [DOI] [PubMed] [Google Scholar]
- 40. Ainsworth BE, Jacobs DRJ, Leon AS. Validity and reliability of self‐reported physical activity status: the lipid research clinics questionnaire. Med Sci Sports Exerc. 1993;25:92–98. [DOI] [PubMed] [Google Scholar]
- 41. Choo J, Elci OU, Yang K, Turk MW, Styn MA, Sereika SM, Music E, Burke LE. Longitudinal relationship between physical activity and cardiometabolic factors in overweight and obese adults. Eur J Appl Physiol. 2010;108:329–336. doi: 10.1007/s00421-009-1203-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Prince SA, Adamo KB, Hamel ME, Hardt J, Gorber SC, Tremblay M. A comparison of direct versus self‐report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56. doi: 10.1186/1479-5868-5-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr, Tudor‐Locke C, Greer JL, Vezina J, Whitt‐Glover MC, Leon AS. 2011 compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–1581. doi: 10.1249/MSS.0b013e31821ece12 [DOI] [PubMed] [Google Scholar]
- 44. Phillips E, Wang TW, Husten CG, Corey CG, Apelberg BJ, Jamal A, Homa DM, King BA. Tobacco product use among adults — United States, 2015. Morb Mortal Wkly Rep. 2017;66:1209–1215. doi: 10.15585/mmwr.mm6644a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. An R. Weekend‐weekday differences in diet among U.S. adults, 2003–2012. Ann Epidemiol. 2016;26:57–65. doi: 10.1016/j.annepidem.2015.10.010 [DOI] [PubMed] [Google Scholar]
- 46. Miller PE, Mitchell DC, Harala PL, Pettit JM, Smiciklas‐Wright H, Hartman TJ. Development and evaluation of a method for calculating the healthy eating Index‐2005 using the nutrition data system for research. Public Health Nutr. 2011;14:306–313. doi: 10.1017/S1368980010001655 [DOI] [PubMed] [Google Scholar]
- 47. Krebs‐Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, Wilson MM, Reedy J. Update of the healthy eating index: HEI‐2015. J Acad Nutr Diet. 2018;118:1591–1602. doi: 10.1016/j.jand.2018.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Panizza CE, Shvetsov YB, Harmon BE, Wilkens LR, Le Marchand L, Haiman C, Reedy J, Boushey CJ. Testing the predictive validity of the healthy eating Index‐2015 in the multiethnic cohort: is the score associated with a reduced risk of all‐cause and cause‐specific mortality? Nutrients. 2018;10:452. doi: 10.3390/nu10040452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reedy J, Lerman JL, Krebs‐Smith SM, Kirkpatrick SI, Pannucci TE, Wilson MM, Subar AF, Kahle LL, Tooze JA. Evaluation of the healthy eating Index‐2015. J Acad Nutr Diet. 2018;118:1622–1633. doi: 10.1016/j.jand.2018.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schwingshackl L, Hoffmann G. Diet quality as assessed by the healthy eating index, the alternate healthy eating index, the dietary approaches to stop hypertension score, and health outcomes: a systematic review and meta‐analysis of cohort studies. J Acad Nutr Diet. 2015;115:780–800.e5. doi: 10.1016/j.jand.2014.12.009 [DOI] [PubMed] [Google Scholar]
- 51. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 52. Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non‐clinical samples: a systematic review and meta‐analysis. Sleep Med Rev. 2016;25:52–73. doi: 10.1016/j.smrv.2015.01.009 [DOI] [PubMed] [Google Scholar]
- 53. Qiu C, Gelaye B, Zhong QY, Enquobahrie DA, Frederick IO, Williams MA. Construct validity and factor structure of the Pittsburgh sleep quality index among pregnant women in a Pacific‐northwest cohort. Sleep Breath. 2016;20:293–301. doi: 10.1007/s11325-016-1313-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Skouteris H, Wertheim EH, Germano C, Paxton SJ, Milgrom J. Assessing sleep during pregnancy: a study across two time points examining the Pittsburgh sleep quality index and associations with depressive symptoms. Womens Health Issues. 2009;19:45–51. doi: 10.1016/j.whi.2008.10.004 [DOI] [PubMed] [Google Scholar]
- 55. American Lung Association . Tobacco use among children and teens. Published 2023. Accessed September 18, 2023. https://www.lung.org/quit‐smoking/smoking‐facts/tobacco‐use‐among‐children
- 56. Chang T, Moniz MH, Plegue MA, Sen A, Davis MM, Villamor E, Richardson CR. Characteristics of women age 15‐24 at risk for excess weight gain during pregnancy. PLOS One. 2017;12:e0173790. doi: 10.1371/journal.pone.0173790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Giddens JB, Krug SK, Tsang RC, Guo S, Miodovnik M, Prada JA. Pregnant adolescent and adult women have similarly low intakes of selected nutrients. J Am Diet Assoc. 2000;100:1334–1340. doi: 10.1016/S0002-8223(00)00377-1 [DOI] [PubMed] [Google Scholar]
- 58. Steinl GK, Whisner CM, Pressman EK, Cooper EM, Groth SW, O'Brien KO. Patterns and correlates of self‐reported physical activity in a cohort of racially diverse pregnant adolescents. J Pediatr Adolesc Gynecol. 2019;32:51–56. doi: 10.1016/j.jpag.2018.09.003 [DOI] [PubMed] [Google Scholar]
- 59. Rosner B. On the detection of many outliers. Technometrics. 1975;17:221–227. doi: 10.2307/1268354 [DOI] [Google Scholar]
- 60. Millard SP. EnvStats: An R Package for Environmental Statistics. Springer Science & Business Media; 2013. [Google Scholar]
- 61. Airaksinen V, Ruohomäki A, Hantunen S, Keski‐Nisula L, Luojus MK, Pekkanen J, Tuomainen TP, Heinonen S, Pasanen M, Lehto SM. Longitudinal analyses of diet quality and maternal depressive symptoms during pregnancy: the Kuopio birth cohort study. J Acad Nutr Diet. 2023;123:77–86.e4. doi: 10.1016/j.jand.2022.05.018 [DOI] [PubMed] [Google Scholar]
- 62. Borodulin K, Evenson KR, Wen F, Herring AH, Benson A. Physical activity patterns during pregnancy. Med Sci Sports Exerc. 2008;40:1901–1908. doi: 10.1249/MSS.0b013e31817f1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Donofry SD, Emery RL, Kolko Conlon RP, Germeroth LJ, Wang B, Cheng Y, Levine MD. Documenting the course of loss of control over eating prior to, during and after pregnancy among women with pre‐pregnancy overweight and obesity. Int J Eat Disord. 2021;54:633–638. doi: 10.1002/eat.23454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mindell JA, Cook RA, Nikolovski J. Sleep patterns and sleep disturbances across pregnancy. Sleep Med. 2015;16:483–488. doi: 10.1016/j.sleep.2014.12.006 [DOI] [PubMed] [Google Scholar]
- 65. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart disease and stroke statistics‐2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 66. Racine JS. RStudio: a platform‐independent IDE for R and Sweave. J Appl Econom. 2012;27:167–172. [Google Scholar]
- 67. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2023. URL https://www. R‐project. org [Google Scholar]
- 68. Lewey J, Beckie TM, Brown HL, Brown SD, Garovic VD, Khan SS, Miller EC, Sharma G, Mehta LS. Opportunities in the postpartum period to reduce cardiovascular disease risk after adverse pregnancy outcomes: a scientific statement from the American Heart Association. Circulation. 2024;149:e330–e346. doi: 10.1161/CIR.0000000000001212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lee YL, Tien Y, Bai YS, et al. Association of Postpartum Depression with maternal suicide: a nationwide population‐based study. Int J Environ Res Public Health. 2022;19:5118. doi: 10.3390/ijerph19095118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lubotzky‐Gete S, Ornoy A, Grotto I, Calderon‐Margalit R. Postpartum depression and infant development up to 24 months: a nationwide population‐based study. J Affect Disord. 2021;285:136–143. doi: 10.1016/j.jad.2021.02.042 [DOI] [PubMed] [Google Scholar]
- 71. Poyatos‐León R, García‐Hermoso A, Sanabria‐Martínez G, Álvarez‐Bueno C, Cavero‐Redondo I, Martínez‐Vizcaíno V. Effects of exercise‐based interventions on postpartum depression: a meta‐analysis of randomized controlled trials. Birth. 2017;44:200–208. doi: 10.1111/birt.12294 [DOI] [PubMed] [Google Scholar]
- 72. Felder JN, Epel ES, Neuhaus J, Krystal A, Prather A. Randomized controlled trial of digital cognitive behavior therapy for prenatal insomnia symptoms: effects on postpartum insomnia and mental health. Sleep. 2022;45:zsab280. doi: 10.1093/sleep/zsab280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ladyman C, Sweeney B, Sharkey K, Bei B, Wright T, Mooney H, Huthwaite M, Cunningham C, Firestone R, Signal TL. A scoping review of non‐pharmacological perinatal interventions impacting maternal sleep and maternal mental health. BMC Pregnancy Childbirth. 2022;22:659. doi: 10.1186/s12884-022-04844-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Okun ML. Disturbed sleep and postpartum depression. Curr Psychiatry Rep. 2016;18:66. doi: 10.1007/s11920-016-0705-2 [DOI] [PubMed] [Google Scholar]
- 75. Okun ML, Mancuso RA, Hobel CJ, Schetter CD, Coussons‐Read M. Poor sleep quality increases symptoms of depression and anxiety in postpartum women. J Behav Med. 2018;41:703–710. doi: 10.1007/s10865-018-9950-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Park EM, Meltzer‐Brody S, Stickgold R. Poor sleep maintenance and subjective sleep quality are associated with postpartum maternal depression symptom severity. Arch Womens Ment Health. 2013;16:539–547. doi: 10.1007/s00737-013-0356-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tham EK, Tan J, Chong YS, Kwek K, Saw SM, Teoh OH, Goh DY, Meaney MJ, Broekman BF. Associations between poor subjective prenatal sleep quality and postnatal depression and anxiety symptoms. J Affect Disord. 2016;202:91–94. doi: 10.1016/j.jad.2016.05.028 [DOI] [PubMed] [Google Scholar]
- 78. Lewis BA, Gjerdingen D, Schuver K, Avery M, Marcus BH. The effect of sleep pattern changes on postpartum depressive symptoms. BMC Womens Health. 2018;18:12. doi: 10.1186/s12905-017-0496-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Xiao RS, Kroll‐Desrosiers AR, Goldberg RJ, Pagoto SL, Person SD, Waring ME. The impact of sleep, stress, and depression on postpartum weight retention: a systematic review. J Psychosom Res. 2014;77:351–358. doi: 10.1016/j.jpsychores.2014.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Harris LM, Forson‐Dare Z, Gallagher PG. Critical disparities in perinatal health—understanding risks and changing the outcomes. J Perinatol. 2021;41:181–182. doi: 10.1038/s41372-020-00913-7 [DOI] [PubMed] [Google Scholar]
- 81. Caleyachetty R, Barber TM, Mohammed NI, Cappuccio FP, Hardy R, Mathur R, Banerjee A, Gill P. Ethnicity‐specific BMI cutoffs for obesity based on type 2 diabetes risk in England: a population‐based cohort study. Lancet Diabetes Endocrinol. 2021;9:419–426. doi: 10.1016/S2213-8587(21)00088-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Katzmarzyk PT, Bray GA, Greenway FL, Johnson WD, Newton RL Jr, Ravussin E, Ryan DH, Bouchard C. Ethnic‐specific BMI and waist circumference thresholds. Obesity. 2011;19:1272–1278. doi: 10.1038/oby.2010.319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Javed F, Aziz EF, Sabharwal MS, Nadkarni GN, Khan SA, Cordova JP, Benjo AM, Gallagher D, Herzog E, Messerli FH, et al. Association of BMI and cardiovascular risk stratification in the elderly African‐American females. Obesity. 2011;19:1182–1186. doi: 10.1038/oby.2010.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kruger HS, Schutte AE, Walsh CM, Kruger A, Rennie KL. Body mass index cut‐points to identify cardiometabolic risk in black south Africans. Eur J Nutr. 2017;56:193–202. doi: 10.1007/s00394-015-1069-9 [DOI] [PubMed] [Google Scholar]
- 85. Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Sleep duration: how well do self‐reports reflect objective measures? The CARDIA sleep study. Epidemiol Camb Mass. 2008;19:838–845. doi: 10.1097/EDE.0b013e318187a7b0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Obling KH, Hansen ALS, Overgaard K, Normann K, Sandbaek A, Maindal HT. Association between self‐reported and objectively measured physical fitness level in a middle‐aged population in primary care. Prev Med Rep. 2015;2:462–466. doi: 10.1016/j.pmedr.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2


