Abstract
Background
Hypertrophic cardiomyopathy is a burdensome condition that inflicts both physical and psychological impairment on those with the disease, negatively impacting health‐related quality of life (HRQoL). Given the abundance of evidence suggesting a role of physical activity (PA) in modulating HRQoL in healthy populations of children, we sought to determine the relationship between HRQoL and PA in children diagnosed with hypertrophic cardiomyopathy.
Methods and Results
A multicenter prospective observational cohort study was conducted, with patients with hypertrophic cardiomyopathy aged 10 to 19 years being provided a wrist‐worn activity tracker (Fitbit Charge HR) to wear for 14 days. Patients self‐reported on Pediatric Quality of Life 4.0 quality of life inventory items, which were associated with PA metrics following covariate adjustment using linear regression. A total of 56 participants were recruited to the study. The median age at enrollment was 15.5 years (interquartile range, 13.8–16.8), and 16 out of 56 (29%) of the cohort were girls. The cohort reported decreased metrics of physical, psychosocial, and total summary scores compared with health reference populations, with scores comparable with that of published populations with chronic disease. Increased physical HRQoL scores were significantly associated with increased daily steps taken, distance traveled, and flights of stairs climbed.
Conclusions
These results show that impaired PA correlates with reduced HRQoL in children with hypertrophic cardiomyopathy, suggesting PA may partially mediate HRQoL in this population.
Keywords: exercise, health‐related quality of life, hypertrophic cardiomyopathy, pediatrics
Subject Categories: Exercise, Pediatrics, Cardiovascular Disease, Cardiomyopathy, Hypertrophy
Nonstandard Abbreviations and Acronyms
- HCM
hypertrophic cardiomyopathy
- MD
mean difference
- PA
physical activity
- PedsQL
Pediatric Quality of Life
Clinical Perspective.
What Is New?
Children and adolescents with hypertrophic cardiomyopathy scored significantly lower than healthy reference populations in measures of health‐related quality of life.
Increases in some everyday physical activities, measured by a wrist‐worn activity tracking device, correlated with increases in physical component scores of health‐related quality of life.
What Are the Clinical Implications?
These results suggest that physical activity in children and adolescents with hypertrophic cardiomyopathy could potentially play a role in mediating health‐related quality of life.
Hypertrophic cardiomyopathy (HCM) is the most common inheritable cardiac condition, characterized by disorganization of the myocytes and sarcomeres, myocardial fibrosis, and pathological thickening of the myocardium. 1 , 2 HCM can impact physical activity (PA) because it may cause exercise‐induced arrhythmias, ischemia, and left ventricular outflow tract obstruction. 3 Previous studies have suggested that athletes with HCM are at higher risk of sudden cardiac death compared with those without. 4 , 5 , 6 , 7 These findings have led to historical PA restriction among patients with HCM, particularly from competitive sports. 8 , 9 However, ambiguity around the amount and types of exercise patients should safely perform have resulted in some patients adopting a sedentary lifestyle. 3 The development of unhealthy habits and behaviors during childhood can tack into adulthood and may contribute to an increased prevalence of obesity. 10 , 11 Up to 70% of adults with HCM are overweight or obese, leading to increased risk for cardiovascular complications and a worsening of left ventricular outflow tract obstruction. 12 This may lead to decreased health‐related quality of life (HRQoL), especially in children. 13 , 14 HRQoL is a global measure of disease burden in the individual, which also quantifies perceptions on functional capability. 15 HRQoL scores reflect subjective perceptions about a patient's overall satisfaction with their health and as such have been an effective method to compare among diverse populations. 16 Although HRQoL has been reported on in adult patients with HCM, 17 , 18 , 19 the association between HRQoL and PA in patients, especially children with HCM, is unexplored.
Thus, the objectives of the current study were to (1) evaluate HRQoL in children with HCM and compare it with the general population and (2) determine if HRQoL in children with HCM is related to objective measures of PA. We hypothesized that HRQoL scores in children with HCM are lower compared with healthy populations and that measures of PA are independently associated with overall, physical, and psychosocial HRQoL.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. This is a multicenter prospective observational cohort study (Research Ethics Board registration number Pro00083230, clinical trial registration number NCT05510180) done in collaboration with the Canadian Congenital and Pediatric Cardiology Research Network. 20 Children who met diagnostic criteria for primary HCM (secondary causes for myocardial hypertrophy ruled out), aged 10 to 19 years, were recruited from 10 Canadian pediatric care sites: British Columbia Children's Hospital (Vancouver, BC), Stollery Children's Hospital (Edmonton, AB), Jim Pattison Children's Hospital (Saskatoon, SK), Variety Children's Hospital Centre (Winnipeg, MB), The Hospital for Sick Children (Toronto, ON), CIUSS del'Estrie‐CHUS (Sherbrooke, QC), L'Hopital de Montreal Pour Enfants (Montreal, QC), CHU de Quebec (Quebec City, QC), IWK Heart Centre (Halifax, NS), and CHU Sainte‐Justine (Montreal, QC). Recruitment took place between May 2019 and January 2023. Research ethics board approval for the study was obtained by all participating centers. Patients and their guardians provided written consent.
Demographic data included sex as well as age, height, and weight at enrollment. Body mass index (BMI) was calculated according to World Health Organization standards, with normal weight corresponding to BMI <85th percentile, overweight between 85th and 97th percentile, and obese corresponding to BMI >97th percentile. 21 Clinical data collected included New York Heart Association class, current heart failure symptoms and medication use, cardiac arrest history, family history, genetic diagnosis, and previous surgical interventions (eg, myectomy, implantable cardioverter‐defibrillator placement).
Assessment of HRQoL
To assess HRQoL, participants were administered the Pediatric Quality of Life (PedsQL) Inventory Quality of Life Module 4.0. 22 The PedsQL Quality of Life Module is a self‐reporting instrument that consists of 23 items in 4 domains: physical functioning (8 items), emotional functioning (5 items), social functioning (5 items), and school functioning (5 items). It has been designed for multiple age groups, including a parent proxy report if required. It has been shown to be valid and reliable, with an internal consistency for the total scale score of 0.88 for the child self‐report and 0.9 for the parent proxy report 22 , 23 in a large US population. It has been used to measure HRQoL in healthy children and adolescents and those with acute and chronic health conditions. 24 , 25 Scores within individual areas are reverse scored and linearly transformed into a 0 to 100 scale, such that higher score indicates better HRQoL. One SD below the population mean has been identified as a cutoff for those at risk of impaired HRQoL. 22 , 23
Physical Activity
PA was measured using the wrist‐worn Fitbit Charge Activity Tracker (Fitbit, San Francisco, CA). The commercial activity tracker Fitbit has recently been validated in comparison to traditional accelerometers such as Actigraph (GT3x, GT9X; ActiGraph, Pensacola, FL), including in children with congenital heart disease. 26 , 27 The device was chosen because it is a popular device, with the hopes of increasing patient compliance in the study, given previous studies describing poor compliance with the Actigraph accelerometer. 28 , 29 The Fitbit Charge can measure heart rate, steps taken, distance covered, activity intensity, energy expenditure, stair flights climbed, sleep, hourly activity, and active minutes. The Fitbit can quantify activity intensity via metabolic equivalent of tasks (METs), which is the ratio of energy expenditure during the activity to energy expended at rest. An activity with an intensity of <1.5 METs is classified as sedentary time, an intensity of 1.5 to 3 METs is classified as light activity, an intensity of 3 to 6 METs as moderate activity, and >6 METs is classified as very active. We captured data on average daily heart rate, steps taken, distance covered, activity intensity, stair flights climbed, and active minutes.
Subjects were instructed to wear the Fitbit for 2 weeks, with the goal of capturing at a minimum of 5 complete days of activity to a maximum of 14 days of complete data. Based on previous reports, a cutoff point of approximately 12 500 Fitbit steps per day correlates well with adherence to recommended PA guidelines of 60 minutes of moderate to vigorous PA per day. 27 This correlation has been validated with receiver operating characteristic analysis, with an area under the curve of 0.82 (95% CI, 0.74–0.9). 27
All study data were collected using Research Electronic Data Capture. Study data were hosted at the University of Alberta (Edmonton, AB) and the Canadian Congenital Pediatric Cardiology Research Network. Research Electronic Data Capture is a secure, web‐based software platform designed to support data capture for research studies. 30 , 31 Data from Fitbit activity tracking were managed via the Fitabase platform (https://www.fitabase.com).
Statistical Analysis
Continuous variables were reported with mean±SD or as median (interquartile range [IQR] [25th percentile–75th percentile]), depending on the distribution. Categorical variables were reported as frequency and percent. Normality was assessed visually using histograms and Q‐Q plots. No imputation method was used, and proportions of missing data were presented if needed.
HRQoL scores were calculated and reported as mean±SD and compared with a published cohort of healthy children and those with children with chronic conditions. 22 Comparison with population reference was made using a 1‐sample t test. A Spearman correlation matrix was calculated for PA parameters. Simple and multiple linear regression modeling was conducted to assess the strength of association between PA metrics as measured by the Fitbit and physical, psychosocial, and total summary scores obtained from the PedsQL Quality of Life Inventory 4.0. Covariate adjustments were made for age at enrollment, sex, BMI class at enrollment, cardiac arrest history, and implantable cardioverter‐defibrillator implantation, and activity restrictions were made based on covariances of these variables. Results are presented as mean difference (MD) with 95% CI. Results were obtained using R software version 4.2.3. Results are presented according to best practices on reporting results of statistical tests, with a 2‐sided P value <0.05 considered a statistically significant result. 32 , 33
Results
Cohort Characteristics
A total of 56 participants were recruited to the study. The median age at enrollment was 15.5 years (IQR, 13.8–16.8), and 16 out of 56 (29%) of the cohort were girls. More than half of participants were overweight or obese (30/56, 54%), and most were activity restricted (42/56, 75%) by their primary cardiologist. A more detailed outline of cohort parameters can be found in Table 1.
Table 1.
Baseline Characteristics of the Cohort
| Variable | Sample (n=56) |
|---|---|
| Demographic and comorbidities | |
| Age at enrollment, y, median (IQR) | 15.5 (13.8–16.8) |
| Age at diagnosis, y, median (IQR) | 11 (4.3–13.8) |
| Female sex, n (%) | 16 (29) |
| BMI at enrollment, median (IQR) | 23.3 (19.5–27.7) |
| BMI at diagnosis, median (IQR) | 20.3 (17.4–23.4) |
| BMI class | |
| Normal weight | 26 (46) |
| Overweight | 15 (27) |
| Obese | 15 (27) |
| Clinical presentation | |
| New York Heart Association class, n (%) |
Class I: 34 (61.8) Class II: 21 (38.2) Class III and IV: 0 (0) |
| Activity restriction by cardiologist, n (%) | 42 (75) |
| Exercise intolerance, n (%) | 11 (19.6) |
| Cardiac arrest history, n (%) | 6 (11) |
| LVOT gradient, n (%) | 18 (35) |
| LVOT gradient, median [IQR] | 32 [23–57] |
| ICD implant, n (%) | 17 (30.4) |
| Myectomy, n (%) | 7 (12.5) |
| Shortness of breath, n (%) | 11 (19.6) |
| Chest pain, n (%) | 9 (16.1) |
| Dizziness, n (%) | 10 (17.9) |
| Palpitations, n (%) | 12 (21.4) |
| Fainting, n (%) | 1 (1.8) |
| Edema, n (%) | 3 (5.4) |
BMI indicates body mass index; ICD, implantable cardioverter‐defibrillator; IQR, interquartile range; and LVOT, left ventricular outflow tract.
Physical Activity
Fitbit Charge activity trackers were worn a median time of 14 days (IQR, 13.3–14.0) throughout the study period. Participants took a median of 7586 average steps per day (IQR, 5333–10 324), with 12.5% of individuals reaching an average daily step count of >12 500 steps per day. Participants covered a median daily distance of 5.4 km (IQR, 3.7–7.5 km) and had a median total active time of 246 minutes (IQR, 187–302 minutes) per day. The median daily average flights of stairs climbed was 10.2 (IQR, 5.7–17.0) (Table 2).
Table 2.
Statistics of Physical Activity as Measured by the Fitbit Device
| Variable | Sample (n=54) |
|---|---|
| Time Fitbit worn, d, median [IQR] | 14 [13.25–14] |
| Average daily step count, median [IQR] | 7586 [5333–10 324] |
| Average daily step count ≥12 500, n (%) | 7 (12.5) |
| Average daily distance covered in km, median [IQR] | 5.4 [3.7–7.5] |
| Total of very, moderate, and lightly active, min, median [IQR] | 246 [187–302] |
| Daily average flights of stairs climbed, median [IQR] | 10.2 [5.7–17] |
IQR indicates interquartile range.
PedsQL Quality of Life Module 4.0
Physical, psychosocial, and total PedsQL summary scores for the cohort are summarized in Figure 1 and Table S1. There was evidence to suggest a difference between child and teen PedsQL mean (±SD) scores of our cohort and a reference population of healthy children aged 5 to 16 years for physical summary scores (76.2 [±20.2] versus 87.7 [±13.1], respectively; P<0.0001), psychosocial summary scores (72.5 [±17.5] versus 81.8 [±14.0]; P<0.001), and total scores (73.8 [±16.0] versus 83.9 [±12.5]; P<0.0001). No difference was suggested between child and teen PedsQL scores of our cohort and a reference population of children with chronic conditions for physical, psychosocial, and total summary scores (71.3 [±17.1], 79.5 [±10.1], and 74.2 [±15.4], respectively) for children with chronic disease (P>0.05 for each in comparison with our cohort) (Figure 1 and Table S1). For child and teen reports, the cohort reported mean (±SD) emotional functioning scores of 71.6 (±23.5), social functioning scores of 77.2 (±20.9), and school functioning scores of 68.7 (±22.7). For parent proxy reports, the cohort reported mean physical functioning scores of 66.6 (±20.4), emotional functioning scores of 69.9 (±24.1), social functioning scores of 70.2 (±23.9), school functioning scores of 70.2 (±23.9), psychosocial health summary scores of 72.5 (±20.7), and total scores of 72.9 (±20.3). The proportions (95% CI) of individuals >1 SD below the sample mean for psychosocial, physical, and total HRQoL summary scores are 17.8% (8.9–30.4), 19.6% (10.2–32.2), and 19.6% (10.2–32.2), respectively (Figure 2, Table S2). There was no evidence to suggest these proportions differed from a general pediatric reference population's proportions >1 SD below the mean for psychosocial, physical, and total HRQoL summary scores of 15.8% (14.9–16.7), 14.8% (13.9–15.7), and 16.9 (16.0–17.9), respectively (P>0.05, Figure 2, Table S2). 22
Figure 1. Physical, psychosocial, and total PedsQL 4.0 summary scores in comparison to a published large cohort of healthy children and children with chronic disease. 22 .
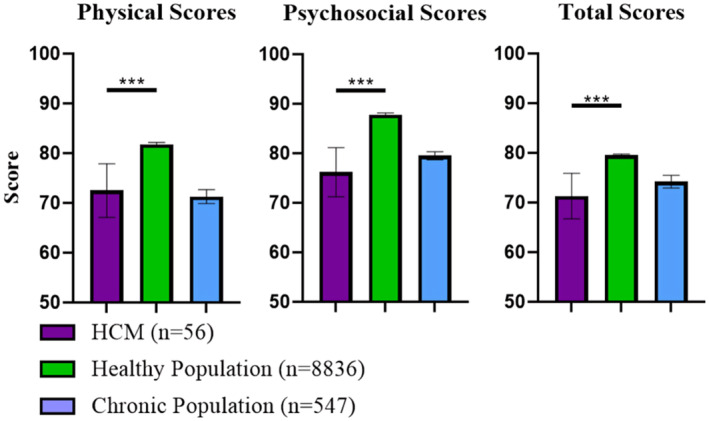
Results are presented as mean with 95% CI error bars. ***P<0.001. HCM indicates hypertrophic cardiomyopathy; and PedsQL, Pediatric Quality of Life.
Figure 2. Proportions (%) of the cohort >1 SD lower than the sample mean with comparison to a reference population 22 with 95% CI error bars.
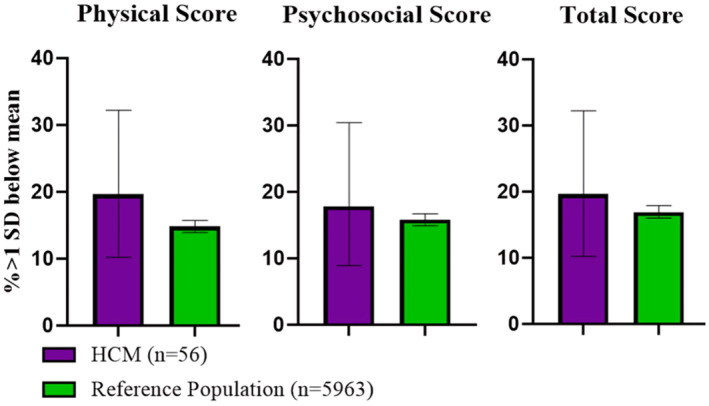
HCM indicates hypertrophic cardiomyopathy.
Relationship of HRQoL by Child and Teen Report and Physical Activity
Unadjusted linear regression models showed a significant association between physical score and both average daily distance covered (MD of 2.22 per km [95% CI, 0.40–4.04]; P=0.018) and daily average flights of stairs climbed (MD of 0.57 [95% CI, 0.04–1.10]; P=0.037). We could not conclude any significant association with any metric of HRQoL with other metrics of PA (P values all >0.05, MDs presented in Table 3).
Table 3.
Strength of Association of Activity Level as Measured by Fitbit on Health‐Related Quality of Life Measures According to Linear Regression Model
| Outcome | Unadjusted mean differences (95% CI) | P value for unadjusted mean differences | Adjusted mean differences (95% CI) | P value for adjusted mean differences |
|---|---|---|---|---|
| Average daily heart rate, per bpm | ||||
| Psychological score | −0.01 (−0.59 to 0.57) | 0.97 | 0.14 (−0.46 to 0.75) | 0.64 |
| Physical score | −0.19 (−0.75 to 0.36) | 0.48 | −0.06 (−0.61 to 0.495) | 0.84 |
| Total score | −0.08 (−0.6 to 0.46) | 0.78 | 0.07 (−0.47 to 0.62) | 0.79 |
| Average daily step count, per 1000 steps | ||||
| Psychological score | 0.27 (−1.1 to 1.6) | 0.70 | 0.48 (−1.06 to 2.04) | 0.53 |
| Physical score | 1.2 (−0.07 to 2.5) | 0.06 | 1.83 (0.53 to 3.13) | 0.007 * |
| Total score | 0.6 (−0.7 to 1.8) | 0.35 | 0.95 (−0.4 to 2.3) | 0.16 |
| Average daily distance covered, per km | ||||
| Psychological score | 1.04 (−0.94 to 3.02) | 0.30 | 1.27 (−0.93 to 3.47) | 0.25 |
| Physical score | 2.22 (0.40 to 4.04) | 0.018 * | 2.88 (1.06 to 4.7) | 0.003 * |
| Total score | 1.45 (−0.35 to 3.25) | 0.12 | 1.83 (−0.07 to 3.74) | 0.06 |
| Total of active times, min, per 100 min | ||||
| Psychological score | −0.06 (−5.4 to 5.3) | 0.98 | −0.4 (−6.03 to 5.16) | 0.88 |
| Physical score | 4.2 (−0.8 to 9.2) | 0.10 | 4.4 (−0.47 to 9.29) | 0.075 |
| Total score | 1.4 (−3.5 to 6.4) | 0.56 | 1.25 (−3.7 to 6.21) | 0.61 |
| Daily average flights of stairs climbed, per flight | ||||
| Psychological score | 0.39 (−0.17 to 0.96) | 0.17 | 0.54 (−0.06 to 1.15) | 0.08 |
| Physical score | 0.57 (0.04 to 1.1) | 0.037 * | 0.76 (0.24 to 1.28) | 0.005 * |
| Total score | 0.45 (−0.06 to 0.97) | 0.08 | 0.62 (0.09 to 1.14) | 0.020 * |
Indicates a P value <0.05.
Adjusted linear regression models found that average daily step count (per 1000 steps, MD of 1.83 [95% CI, 0.53–3.13]; P=0.007) and average daily distance covered per km, MD of 2.88 [95% CI, 1.06–4.7]; P=0.003) were significantly associated with physical HRQoL score. The adjusted daily average flights of stairs climbed was significantly associated with physical (MD of 0.76 [95% CI, 0.24–1.28]; P=0.005) and total summary scores (MD of 0.62 [95% CI, 0.09–1.14]; P=0.020) of HRQoL, but despite a moderate effect size, we could not conclude any significant association for psychological measures (MD of 0.54 [95% CI, 0.06–1.15]; P=0.078).
Average daily heart rate and total active time were not associated with any measure of HRQoL for either adjusted or unadjusted MDs (Table 3).
Discussion
The aim of this study was to investigate HRQoL and determine its relationship with PA in children with HCM. We report that children with HCM have significantly diminished HRQoL dimensions in comparison to healthy populations of children, with HRQoL similar to that of children with chronic conditions. 22 With regard to a reference population of healthy children aged 5 to 18 years, there was a comparable proportion of individuals within our cohort who were at risk for decreased HRQoL (>1 SD lower than the mean). We also found a correlation between PA and dimensions of HRQoL. Generally, these findings support the notion that HCM is a chronic disease with a similar impact on HRQoL to other chronic disorders and suggest there are opportunities for HRQoL to be improved, with PA potentially playing a role.
It is widely acknowledged that children and adolescents with chronic health conditions exhibit lowered HRQoL than their healthy peers. 16 , 34 , 35 Conditions that especially impair physical functioning and the ability to partake in activities with peers are expected to yield larger reductions in HRQoL, given that they indirectly affect all dimensions of HRQoL in addition to the physical dimension. 34 A report comparing HRQoL in a multitude of chronic conditions in children and adolescents found that conditions with high physical impairment, such as cerebral palsy or rheumatoid disorders, had the most impaired HRQoL of a variety of chronic conditions. 16 In children with congenital heart disease, HRQoL scores showed significant correlations with cardiopulmonary exercise test variables, further suggesting that exercise capacity impacts HRQoL. 36 , 37 In the case of HCM, PA restriction or exercise intolerance can similarly prevent individuals from partaking in activities with their peers and place limitations on age‐appropriate functions. Therefore, these limitations may have manifested in lowered HRQoL in the current study, explaining the similarity we find to the reference population for children with chronic conditions. Another contribution to decreased HRQoL may also be obesity. Children and adolescents with severe obesity are shown to have decreased HRQoL in comparison to individuals with normal body weight, and the BMI Z score was correlated with reduced HRQoL. 38 , 39 , 40 Although we adjusted for BMI in our analysis, half of our cohort was overweight or obese, similarly to other cohorts of adults and children with HCM. 12 , 41 Therefore, physical activity may be another potential target for future interventions to improve HRQoL in children with HCM. 36 , 42
Therefore, we explored whether the current activity level of children with HCM in Canada was associated with HRQoL. Using the objective measures of PA obtained through the Fitbit, this study was able to correlate PA with HRQoL. Participants in our study showed high compliance with the Fitbit over the study period, with a median wear time of 14 out of 14 days (Table 2), adding to previous reports of increased compliance with wrist‐worn activity trackers compared with hip‐mounted accelerometers. 28 , 29 The PA parameters of total daily steps, distance covered, and daily flights of stairs were all correlated with physical HRQoL. A meta‐analysis by Wu et al found a dose–response relationship between PA and HRQoL. 13 In agreement, a study by Brudy et al showed that daily steps and minutes of moderate to vigorous physical activity were associated with physical well‐being and everyday functioning in children and adolescents with congenital heart disease via the Kinder Lebensqualitӓt questionnaire. 43 Given that physical HRQoL scores represent an individual's ability to do activities independently or partake in physical activities, the associations made in the current study suggest that regular participation in PA could have a positive effect on HRQoL in the context of HCM. This is supported by studies that have shown that increased PA is associated with improved HRQoL in other pediatric populations with chronic illnesses, such as children with cancer 44 or following kidney transplant. 45 A systematic review and meta‐analysis of the effect of PA on HRQoL in children and adolescents from a number of chronic and healthy populations found a positive effect in both descriptive and intervention studies. 15 Thus, there is ample evidence to suggest the impact of PA on HRQoL.
Although there was not a specific parameter that was associated with psychological HRQoL, an increase in the number of flights of stairs was associated with overall HRQoL. This finding may suggest that more difficult types of exercise (eg, climbing flights of stairs) may have a more well‐rounded effect on HRQoL, because accomplishing more difficult activities can be a sign of improved self‐efficacy. 46 Studies on the impact of exercise type on HRQoL in various adult and pediatric populations have varied in their conclusions, with some holding that exercise participation matters more than what type of exercise, 42 , 47 and others suggesting only certain types of exercise mediate a benefit to HRQoL. 48 , 49 It is yet to be established which may be true for children with chronic cardiac conditions like HCM. 50 Nonetheless, other studies in healthy children have associated increased physical activity with physical dimensions of HRQoL. 51 , 52 Given the small proportion of patients who completed >12 500 steps per day in this study, further work is needed to determine if prescription of PA can lead to improvements in all scoring metrics of HRQoL and potentially reverse some of the impact of having a chronic disease.
PA could be in the form of exercise programs that in turn have the potential to boost HRQoL. Casey et al found that implementation of an exercise intervention program in adolescent girls without chronic disease resulted in increases in all measures of HRQoL. 53 An additional study targeting an aerobic training program for individuals with metabolic syndrome found that in almost all metrics of HRQoL (via Short Form‐36 scores), motivation and mental health were significantly improved in the intervention group. 54 Although our study demonstrates that walking is associated with physical HRQoL in patients with HCM, longer‐term and intentional aerobic exercise may be more relevant in improving psychosocial HRQoL in this patient population. Studies in adult patients with HCM have trialed mild exercise programs and have concluded that they are safe (neither study reported serious adverse events) and can even boost quality of life. 55 , 56 These studies resulted in small increases in exercise capacity, so it remains to be established what the optimal exercise intensity is in this population and what would be deemed safe. 57 , 58 This current study supports the notion that participation in physical activities measurable by the Fitbit associates with increases in physical HRQoL in patients with HCM.
Limitations
Certain limitations should be considered when interpreting the results of our study. We note a modest sample size, with a low proportion of the cohort being girls (29%), which may skew results given that PA profiles between sexes may be different. However, other studies of HCM in children also report a mostly male cohort. 59 , 60 In our study, the metrics provided by the Fitbit were not supported by objective characterizations of physical fitness (eg, cardiopulmonary exercise testing, the 6‐minute walk test); however, we contend that passive assessment of everyday activities by the Fitbit were more appropriate to associate with HRQoL than measurements of maximal exercise capacity. A time period of 2 weeks to collect Fitbit data may be a relatively short time to evaluate exercise, given that PA can vary seasonally in pediatric populations with cardiac conditions, being highest in the late spring and autumn. 61 This is especially true in northern climates such as Canada, where cold winter temperatures can limit outdoor PA. Weather over the study period may be a confounding variable that prevented some individuals from exercising on certain days, which may dampen the association between the cohort's PA and HRQoL. It should also be emphasized that some study participants were enrolled in the study throughout intermittent COVID‐19 pandemic lockdowns. Although it is uncertain how the PA habits of our cohort were impacted by lockdowns, these circumstances may have influenced the study. We also note that measurement of active minutes by the Fitbit device is only done if the activity at hand reaches a threshold MET; therefore, brief and sporadic bursts of activity may be missed and contributed to sedentary time instead. Finally, in the current study we did not report on the individual correlates with lowered levels of PA. These analyses are the subject of another article on this cohort currently in preparation, with the focus of the current article specifically on the relationship between HRQoL and PA.
Conclusions
Overall, we report low HRQoL in pediatric patients with primary HCM, noting their similarity to individuals with chronic disease. We show that objective measures of PA via wrist‐bound activity tracking were significantly associated with physical measures of HRQoL in children with HCM. Our study further emphasizes the lack of PA in this population, highlighting that exercise interventions in this population of children may have benefits beyond cardiovascular health.
Sources of Funding
This work was supported by funding from the Children's Cardiomyopathy Foundation, Women and Children's Health Research Institute, and the Labbat Family Heart Center.
Disclosures
None.
Supporting information
Data S1
Acknowledgments
Thank you to the Canadian Congenital Pediatric Cardiology Research Network for supporting this study.
This article was sent to Patricia K. Nguyen, MD, Senior Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.033968
For Sources of Funding and Disclosures, see page 8.
REFERENCES
- 1. Maron BJ, Desai MY, Nishimura RA, Spirito P, Rakowski H, Towbin JA, Rowin EJ, Maron MS, Sherrid MV. Diagnosis and evaluation of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2022;79:372–389. doi: 10.1016/j.jacc.2021.12.002 [DOI] [PubMed] [Google Scholar]
- 2. Maron BJ, Rowin EJ, Maron MS. Hypertrophic cardiomyopathy: new concepts and therapies. Annu Rev Med. 2022;73:363–375. doi: 10.1146/annurev-med-042220-021539 [DOI] [PubMed] [Google Scholar]
- 3. Basu J, Malhotra A, Papadakis M. Exercise and hypertrophic cardiomyopathy: two incompatible entities? Clin Cardiol. 2020;43:889–896. doi: 10.1002/clc.23343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Corrado D, Basso C, Rizzoli G, Schiavon M, Thiene G. Does sports activity enhance the risk of sudden death in adolescents and young adults? J Am Coll Cardiol. 2003;42:1959–1963. doi: 10.1016/j.jacc.2003.03.002 [DOI] [PubMed] [Google Scholar]
- 5. Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980–2006. Circulation. 2009;119:1085–1092. doi: 10.1161/CIRCULATIONAHA.108.804617 [DOI] [PubMed] [Google Scholar]
- 6. Maron BJ, Haas TS, Murphy CJ, Ahluwalia A, Rutten‐Ramos S. Incidence and causes of sudden death in U.S. college athletes. J Am Coll Cardiol. 2014;63:1636–1643. doi: 10.1016/j.jacc.2014.01.041 [DOI] [PubMed] [Google Scholar]
- 7. Maron BJ, Haas TS, Ahluwalia A, Murphy CJ, Garberich RF. Demographics and epidemiology of sudden deaths in young competitive athletes: from the United States national registry. Am J Med. 2016;129:1170–1177. doi: 10.1016/j.amjmed.2016.02.031 [DOI] [PubMed] [Google Scholar]
- 8. Maron BJ, Udelson JE, Bonow RO, Nishimura RA, Ackerman MJ, Estes NAM, Cooper LT, Link MS, Maron MS. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 3: hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and other cardiomyopathies, and myocarditis: a scientific statement from the American Heart Association and American College of Cardiology. Circulation. 2015;132:273–280. doi: 10.1161/CIR.0000000000000236 [DOI] [PubMed] [Google Scholar]
- 9. Pelliccia A, Fagard R, Bjørnstad HH, Anastassakis A, Arbustini E, Assanelli D, Biffi A, Borjesson M, Carre F, Corrado D, et al. Recommendations for competitive sports participation in athletes with cardiovascular disease: a consensus document from the study Group of Sports Cardiology of the working Group of Cardiac Rehabilitation and Exercise Physiology and the working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005;26:1422–1445. doi: 10.1093/eurheartj/ehi325 [DOI] [PubMed] [Google Scholar]
- 10. Roberts KC, Shields M, de Groh M, Aziz A, Gilbert JA. Overweight and obesity in children and adolescents: results from the 2009 to 2011 Canadian health measures survey. Health Rep. 2012;23:37–41. [PubMed] [Google Scholar]
- 11. Khoury M, Urbina EM. Hypertension in adolescents: diagnosis, treatment, and implications. Lancet Child Adolesc Health. 2021;5:357–366. doi: 10.1016/S2352-4642(20)30344-8 [DOI] [PubMed] [Google Scholar]
- 12. Fumagalli C, Maurizi N, Day SM, Ashley EA, Michels M, Colan SD, Jacoby D, Marchionni N, Vincent‐Tompkins J, Ho CY, et al. Association of obesity with adverse long‐term outcomes in hypertrophic cardiomyopathy. JAMA Cardiol. 2020;5:65–72. doi: 10.1001/jamacardio.2019.4268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu XY, Han LH, Zhang JH, Luo S, Hu JW, Sun K. The influence of physical activity, sedentary behavior on health‐related quality of life among the general population of children and adolescents: a systematic review. PLoS One. 2017;12:e0187668. doi: 10.1371/journal.pone.0187668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ávila‐García M, Esojo‐Rivas M, Villa‐González E, Tercedor P, Huertas‐Delgado FJ. Relationship between sedentary time, physical activity, and health‐related quality of life in Spanish children. Int J Environ Res Public Health. 2021;18:2702. doi: 10.3390/ijerph18052702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marker AM, Steele RG, Noser AE. Physical activity and health‐related quality of life in children and adolescents: a systematic review and meta‐analysis. Health Psychol oOff J Div Health Psychol Am Psychol Assoc. 2018;37:893–903. doi: 10.1037/hea0000653 [DOI] [PubMed] [Google Scholar]
- 16. Varni JW, Limbers CA, Burwinkle TM. Impaired health‐related quality of life in children and adolescents with chronic conditions: a comparative analysis of 10 disease clusters and 33 disease categories/severities utilizing the PedsQL™ 4.0 generic Core scales. Health Qual Life Outcomes. 2007;5:43. doi: 10.1186/1477-7525-5-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Magnusson P, Mörner S, Gadler F, Karlsson J. Health‐related quality of life in hypertrophic cardiomyopathy patients with implantable defibrillators. Health Qual Life Outcomes. 2016;14:62. doi: 10.1186/s12955-016-0467-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Capota R, Militaru S, Ionescu AA, Rosca M, Baicus C, Popescu BA, Jurcut R. Quality of life status determinants in hypertrophic cardiomyopathy as evaluated by the Kansas City cardiomyopathy questionnaire. Health Qual Life Outcomes. 2020;18:351. doi: 10.1186/s12955-020-01604-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zaiser E, Sehnert AJ, Duenas A, Saberi S, Brookes E, Reaney M. Patient experiences with hypertrophic cardiomyopathy: a conceptual model of symptoms and impacts on quality of life. J Patient Rep Outcomes. 2020;4:102. doi: 10.1186/s41687-020-00269-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dallaire F, Battista MC, Greenway SC, Harris K, Jean‐St‐Michel E, Mackie AS. The Canadian pediatric cardiology research network: a model national data‐sharing organization to facilitate the study of pediatric heart diseases. CJC Open. 2021;3:510–515. doi: 10.1016/j.cjco.2020.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Onis M. Development of a WHO growth reference for school‐aged children and adolescents. Bull World Health Organ. 2007;85:660–667. doi: 10.2471/BLT.07.043497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003;3:329–341. doi: [DOI] [PubMed] [Google Scholar]
- 23. Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the pediatric quality of life inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39:800–812. doi: 10.1097/00005650-200108000-00006 [DOI] [PubMed] [Google Scholar]
- 24. Lee CT, Lin CY, Strong C, Lin YF, Chou YY, Tsai MC. Metabolic correlates of health‐related quality of life among overweight and obese adolescents. BMC Pediatr. 2018;18:25. doi: 10.1186/s12887-018-1044-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anderson YC, Wynter LE, Treves KF, Grant CC, Stewart JM, Cave TL, Wouldes TA, Derraik JGB, Cutfield WS, Hofman PL. Assessment of health‐related quality of life and psychological well‐being of children and adolescents with obesity enrolled in a New Zealand community‐based intervention programme: an observational study. BMJ Open. 2017;7:e015776. doi: 10.1136/bmjopen-2016-015776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cunningham C, Spence JC, Stearns JA, Carson V, Kantor PF, Urschel S, Conway J. Self‐reported and accelerometer‐measured physical activity in children with cardiomyopathy. J Cardiovasc Nurs. 2020;35:300–3006. doi: 10.1097/JCN.0000000000000629 [DOI] [PubMed] [Google Scholar]
- 27. Voss C, Gardner RF, Dean PH, Harris KC. Validity of commercial activity trackers in children with congenital heart disease. Can J Cardiol. 2017;33:799–805. doi: 10.1016/j.cjca.2016.11.024 [DOI] [PubMed] [Google Scholar]
- 28. Fairclough SJ, Noonan R, Rowlands AV, Van Hees V, Knowles Z, Boddy LM. Wear compliance and activity in children wearing wrist‐ and hip‐mounted accelerometers. Med Sci Sports Exerc. 2016;48:245–253. doi: 10.1249/MSS.0000000000000771 [DOI] [PubMed] [Google Scholar]
- 29. McLellan G, Arthur R, Buchan DS. Wear compliance, sedentary behaviour and activity in free‐living children from hip‐and wrist‐mounted ActiGraph GT3X+ accelerometers. J Sports Sci. 2018;36:2424–2430. doi: 10.1080/02640414.2018.1461322 [DOI] [PubMed] [Google Scholar]
- 30. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wasserstein RL, Lazar NA. The ASA statement on p‐values: context, process, and purpose. Am Stat. 2016;70:129–133. doi: 10.1080/00031305.2016.1154108 [DOI] [Google Scholar]
- 33. Dallaire F. Sounder reporting of study results by systematic screening for erroneous interpretation of p values and statistical tests in cardiology. CJC Pediatr Congenit Heart Dis. 2022;1:245–247. doi: 10.1016/j.cjcpc.2022.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pinquart M. Health‐related quality of life of young people with and without chronic conditions. J Pediatr Psychol. 2020;45:780–792. doi: 10.1093/jpepsy/jsaa052 [DOI] [PubMed] [Google Scholar]
- 35. Bai G, Herten MH, Landgraf JM, Korfage IJ, Raat H. Childhood chronic conditions and health‐related quality of life: findings from a large population‐based study. PLoS One. 2017;12:e0178539. doi: 10.1371/journal.pone.0178539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Amedro P, Basquin A, Gressin V, Clerson P, Jais X, Thambo JB, Guerin P, Cohen S, Bonnet D. Health‐related quality of life of patients with pulmonary arterial hypertension associated with CHD: the multicentre cross‐sectional ACHILLE study. Cardiol Young. 2016;26:1250–1259. doi: 10.1017/S1047951116000056 [DOI] [PubMed] [Google Scholar]
- 37. Abassi H, Gavotto A, Picot MC, Bertet H, Matecki S, Guillaumont S, Moniotee S, Auquier P, Moreau J, Amedro P. Impaired pulmonary function and its association with clinical outcomes, exercise capacity and quality of life in children with congenital heart disease. Int J Cardiol. 2019;285:86–92. doi: 10.1016/j.ijcard.2019.02.069 [DOI] [PubMed] [Google Scholar]
- 38. Schwimmer JB, Burwinkle TM, Varni JW. Health‐related quality of life of severely obese children and adolescents. JAMA. 2003;289:1813–1819. doi: 10.1001/jama.289.14.1813 [DOI] [PubMed] [Google Scholar]
- 39. Wafa SWWBSST, Shahril MRB, Ahmad AB, Zainuddin LRB, Ismail KFB, Aung MMT, Yusoff MBAN. Association between physical activity and health‐related quality of life in children: a cross‐sectional study. Health Qual Life Outcomes. 2016;14:71. doi: 10.1186/s12955-016-0474-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van de Pas KGH, de Krom MAP, Winkens B, van Dielen FMH, Vreugdenhil ACE. Health‐related quality of life in children and adolescents with overweight, obesity, and severe obesity: a cross‐sectional study. Obes Facts. 2023;16:282–292. doi: 10.1159/000529560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Balaji S, DiLorenzo MP, Fish FA, Etheridge SP, Aziz PF, Russell MW, Tisma S, Pflaumer A, Sreeram N, Kubus P, et al. Impact of obesity on left ventricular thickness in children with hypertrophic cardiomyopathy. Pediatr Cardiol. 2019;40:1253–1257. doi: 10.1007/s00246-019-02145-9 [DOI] [PubMed] [Google Scholar]
- 42. Bermejo‐Cantarero A, Sánchez‐López M, Álvarez‐Bueno C, Redondo‐Tébar A, García‐Hermoso A, Martínez‐Vizcaino V. Are physical activity interventions effective in improving health‐related quality of life in children and adolescents? A systematic review and meta‐analysis. Sports Health. 2023;1–9. doi: 10.1177/19417381231190885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brudy L, Meyer M, Oberhoffer R, Ewert P, Müller J. Move more – be happier? Physical activity and health‐related quality of life in children with congenital heart disease. Am Heart J. 2021;241:68–73. doi: 10.1016/j.ahj.2021.07.004 [DOI] [PubMed] [Google Scholar]
- 44. Speyer E, Herbinet A, Vuillemin A, Briançon S, Chastagner P. Effect of adapted physical activity sessions in the hospital on health‐related quality of life for children with cancer: a cross‐over randomized trial. Pediatr Blood Cancer. 2010;55:1160–1166. doi: 10.1002/pbc.22698 [DOI] [PubMed] [Google Scholar]
- 45. Hamiwka LA, Cantell M, Crawford S, Clark CG. Physical activity and health related quality of life in children following kidney transplantation. Pediatr Transplant. 2009;13:861–867. doi: 10.1111/j.1399-3046.2009.01195.x [DOI] [PubMed] [Google Scholar]
- 46. Han S, Li B, Wang G, Ke Y, Meng S, Li Y, Cui Z, Tong W. Physical fitness, exercise behaviors, and sense of self‐efficacy among college students: a descriptive correlational study. Front Psychol. 2022;13:932014. doi: 10.3389/fpsyg.2022.932014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Choi MJ, Park YG, Kim YH, Cho KH, Nam GE. Association between type of exercise and health‐related quality of life in adults without activity limitations: a nationwide cross‐sectional study. BMC Public Health. 2020;20:599. doi: 10.1186/s12889-020-08699-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim K, Koo KM. Influence of different exercise types on health‐related quality‐of‐life in men with depressive disorder in South Korea. Front Public Health. 2022;10:811168. doi: 10.3389/fpubh.2022.811168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Martínez‐Vizcaíno V, Cavero‐Redondo I, Reina‐Gutiérrez S, Gracia‐Marco L, Gil‐Cosano JJ, Bizzozero‐Peroni B, Rodriguez‐Artalejo F, Guisado‐Ubago E. Comparative effects of different types of exercise on health‐related quality of life during and after active cancer treatment: a systematic review and network meta‐analysis. J Sport Health Sci. 2023;0:1–13. doi: 10.1016/j.jshs.2023.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. West SL, Banks L, Schneiderman JE, Caterini JE, Stephens S, White G, Dogra S, Wells GD. Physical activity for children with chronic disease; a narrative review and practical applications. BMC Pediatr. 2019;19:12. doi: 10.1186/s12887-018-1377-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gu X, Chang M, Solmon MA. Physical activity, physical fitness, and health‐related quality of life in school‐aged children. J Teach Phys Educ. 2016;35:117–126. doi: 10.1123/jtpe.2015-0110 [DOI] [Google Scholar]
- 52. Perry TT, Moore PC, Redwine KM, Robbins JM, Weber JL. Physical activity, screen time and pediatric health‐related quality of life in the Mississippi Delta. Open J Prev Med. 2012;2:105–111. doi: 10.4236/ojpm.2012.21015 [DOI] [Google Scholar]
- 53. Casey MM, Harvey JT, Telford A, Eime RM, Mooney A, Payne WR. Effectiveness of a school‐community linked program on physical activity levels and health‐related quality of life for adolescent girls. BMC Public Health. 2014;14:649. doi: 10.1186/1471-2458-14-649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zupkauskiene J, Lauceviciene I, Navickas P, Ryliskyte L, Puronaite R, Badariene J, Laucevicius A. Changes in health‐related quality of life, motivation for physical activity, and the levels of anxiety and depression after individualized aerobic training in subjects with metabolic syndrome. Hell J Cardiol. 2022;66:41–51. doi: 10.1016/j.hjc.2022.04.003 [DOI] [PubMed] [Google Scholar]
- 55. Klempfner R, Kamerman T, Schwammenthal E, Nahshon A, Hay I, Goldenberg I, Dov F, Arad M. Efficacy of exercise training in symptomatic patients with hypertrophic cardiomyopathy: results of a structured exercise training program in a cardiac rehabilitation center. Eur J Prev Cardiol. 2015;22:13–19. doi: 10.1177/2047487313501277 [DOI] [PubMed] [Google Scholar]
- 56. Saberi S, Wheeler M, Bragg‐Gresham J, Hornsby W, Agarwal PP, Attili A, Concannon M, Dries AM, Shmargad Y, Salisbury H, et al. Effect of moderate‐intensity exercise training on peak oxygen consumption in patients with hypertrophic cardiomyopathy: a randomized clinical trial. JAMA. 2017;317:1349–1357. doi: 10.1001/jama.2017.2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Owens AT, Cappola TP. Recreational exercise in hypertrophic cardiomyopathy. JAMA. 2017;317:1319–1320. doi: 10.1001/jama.2017.2584 [DOI] [PubMed] [Google Scholar]
- 58. Dias KA, Link MS, Levine BD. Exercise training for patients with hypertrophic cardiomyopathy: JACC review topic of the week. J Am Coll Cardiol. 2018;72:1157–1165. doi: 10.1016/j.jacc.2018.06.054 [DOI] [PubMed] [Google Scholar]
- 59. Norrish G, Protonotarios A, Stec M, Boleti O, Field E, Cervi E, Elliott PM, Kaski JP. Performance of the PRIMaCY sudden death risk prediction model for childhood hypertrophic cardiomyopathy: implications for implantable cardioverter‐defibrillator decision‐making. EP Eur. 2023;25:1–8. doi: 10.1093/europace/euad330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Conway J, Min S, Villa C, Weintraub RG, Nakano S, Godown J, Tatangelo M, Armstrong K, Richmond M, Kaufman B, et al. The prevalence and association of exercise test abnormalities with sudden cardiac death and transplant‐free survival in childhood hypertrophic cardiomyopathy. Circulation. 2023;147:718–727. doi: 10.1161/CIRCULATIONAHA.122.062699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kuan MTY, Voss C, Lopez J, Hemphill NM, Harris KC. Children with congenital heart disease exhibit seasonal variation in physical activity. PLoS One. 2020;15:e0241187. doi: 10.1371/journal.pone.0241187 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1


