Abstract
Herpes simplex virus 1 infected cell protein 22 (ICP22) localizes in small, dense nuclear bodies of primate cells early in infection and in the more diffuse replicative complexes after the onset of DNA synthesis. UL4, a γ2 protein, localizes in cytoplasm and in the small nuclear structures containing ICP22 but not in replicative complexes. In rabbit skin cells, both ICP22 and UL4 localize in the dense nuclear bodies late in infection. The results suggest that the small nuclear structures perform a function involving both proteins late in infection.
Of the 84 herpes simplex virus 1 (HSV-1) open reading frames (ORFs), more than half can be deleted without significantly impairing the ability of the virus to replicate in cells grown in culture (20). The UL4 ORF, one of the dispensable ORFs, has no apparent function in infected cells in culture or in experimental animal systems (3, 4, 13). In other studies, Singh and Wagner (22) reported that UL4 is encoded by a 0.8-kb mRNA, and Yamada et al. (25) reported, while this work was in progress, that the product of the HSV-2 UL4 gene is a very late (γ2) protein that accumulates in the cytoplasms of transfected cells but accumulates in punctate nuclear structures late in infection. Homologs of the UL4 gene have also been reported to occur in the genomes of a number of members of the Alphaherpesvirinae subfamily of herpesviruses (7, 8, 10, 17, 23, 24).
We report that the UL4 protein colocalizes with the pre-DNA synthesis isoforms of infected cell protein 22 (ICP22), a 420-amino-acid protein encoded by the α22 gene (11, 12). The domain of the α22 gene also encodes a protein designated US1.5 whose sequence is identical to the 249 carboxyl-terminal amino acids of ICP22 (6). The promoter of US1.5 is located in the 5′ coding sequence of the α22 gene. ICP22 is dispensable for growth in continuous human primate cell lines (18). The deletion mutant is apathogenic when inoculated intracerebrally into mice and replicates poorly in restricted (e.g., rodent or rabbit) cells or in primary human fibroblasts (21). ICP22 localizes in small, dense nuclear structures early in infection. After the onset of viral DNA synthesis, ICP22 localizes in replicative complexes with nascent DNA and RNA polymerase II, ICP4 (the major viral regulatory protein), and other proteins. The transition from the small, dense nuclear structures to the replicative complexes requires the phosphorylation of ICP22 by the viral protein kinase encoded by the UL13 gene (15).
To carry out these studies, we made polyclonal rabbit antibody to the UL4 protein and constructed a virus (R4660) containing a UL4 gene carrying in frame a small sequence encoding an epitope of the glycoprotein B of the human cytomegalovirus (CMV) (16). The monoclonal antibody to this protein, CH28-2, was purchased from the Goodwin Cancer Research Institute (Plantation, Fla.).
The glutathione S-transferase (GST)–UL4 chimeric protein used for rabbit immunization was made as follows. Plasmid pRB5249 was constructed by the in-frame insertion of an EcoRI-digested PCR product containing the entire UL4 ORF cloned into the EcoRI site of the vector pGEX4T-1 (Pharmacia Biotech). The GST-UL4 protein encoded by pRB5249 was expressed in BL21 cells, purified according to the manufacturer’s directions, and used for the immunization of two rabbits according to standard protocols (Josman Laboratories, Napa, Calif.). Serum from rabbit A was used in the experiments described in this report.
The recombinant virus R4660 was constructed as follows. Plasmid pRB4660 contained a CMV tag in the correct orientation and in frame with the UL4 ORF. It was constructed in three steps. First, the oligonucleotide 5′-AAGGGACAGAAG CCCAACCTGCTAGACCGACTGCGACACCGCAAAAA CGGGTACCGACAC-3′, annealed with its complement (not shown), was inserted at the SmaI site of a plasmid containing the BamHI-to-MluI fragment of the UL4 gene in pGEM3Zf+ (Fig. 1, line 3). Next, a DraIII fragment, containing the DraIII-to-EcoRI sequences encoding the N terminus of UL4 plus an EcoRI-to-DraIII fragment from the pGEM3Zf+ vector, was inserted into the DraIII site of the first construct to complete the UL4 gene. Last, a 332-bp XhoI-to-BamHI fragment encoding the C terminus of UL3 was inserted between the SalI and BamHI sites of the polylinker in the construct from the second step. Recombinant virus R4660 was selected and plaque purified from the progeny of cotransfection of R7205 viral DNA (3) and plasmid pRB4660 as described elsewhere (18).
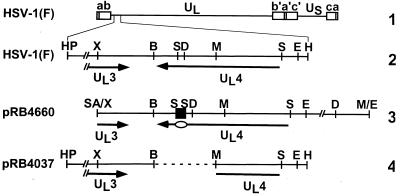
FIG. 1.
Schematic diagram of the sequence arrangement of the HSV-1(F) genome and the sequence arrangement of the region containing the UL4 gene in the plasmids used for the construction of viruses used in this study. Line 1, linear representation of the HSV-1 genome. The thin line represents the unique long (UL) and unique short (US) sequences of the long and short components of the HSV-1 genome, respectively. The open rectangles represent the inverted repeats flanking the unique sequences. Line 2, sequence arrangement of the coding domain for the UL4 protein in HSV-1(F). The arrows labeled UL4 and UL3 represent the coding domains of the genes. Line 3, sequence arrangement of plasmid pRB4660 containing the entire UL4 sequence and the sequence corresponding to the carboxyl-terminal portion of UL3. A 60-bp oligonucleotide (black square) encoding the CMV epitope was inserted into the SmaI site (in the sequence corresponding to the carboxyl terminus) of the UL4 ORF to generate plasmid pRB4660. The oval indicates the location of the CMV tag within the coding domain. Line 4, sequence arrangement of plasmid pRB4037 used in the construction of recombinant virus R7217 as reported elsewhere (3). The dashed line indicates the DNA sequence that had been deleted in R7217. The region between the HpaI site and the XhoI site in lines 2 and 4 is foreshortened in the interest of space. B, BamHI; D, DraIII; E, EcoRI; H, HindIII; HP, HpaI; M, MluI; S, SmaI; SA, SalI; X, XhoI.
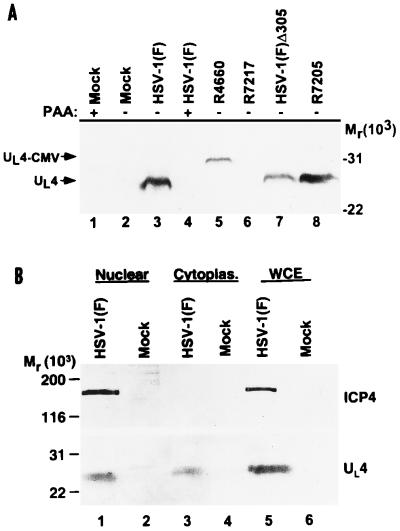
Two series of experiments were done to verify that the rabbit polyclonal antibody generated against the GST-UL4 fusion protein detected the UL4 protein. In the first, an immunoblot of electrophoretically separated lysates of mock infected or infected HEp-2 cells was reacted with the UL4 antiserum. The UL4 antiserum reacted with a protein with an apparent Mr of 26,500 that was present in lysates of cells infected with HSV-1(F) (9), HSV-1(F)Δ305 (18), or R7205 (Fig. 2A, lanes 3, 7, and 8) but not with the lysates of cells either mock infected or infected with the R7217 (ΔUL4 [3]) recombinant virus (Fig. 2A, lanes 2 and 6). As expected inasmuch as the UL4 gene carries an in-frame insert of 20 codons, the anti-UL4 antibody reacted with a more slowly migrating protein band in lysates of cells infected with R4660 (Fig. 2A, lane 5). The results unambiguously verified that the antibody reacted with the UL4 protein. The UL4 gene was assigned to the γ2 kinetic class, based on the absence of the protein from the lysates of HSV-1(F)-infected cells maintained in the presence of the viral DNA synthesis inhibitor phosphonoacetic acid at 300 μg/ml (Fig. 2A, lane 3 versus lane 4).
FIG. 2.
Immunoblots of electrophoretically separated proteins. HEp-2 cells were mock infected or exposed to 10 PFU of the indicated virus per cell. Immunoblots were reacted with anti-UL4 polyclonal antiserum (A and B) and ICP4 monoclonal antibody (B). (A) HEp-2 cells harvested 20 h after infection. Lanes 1 and 2, mock infection; lanes 3 and 4, HSV-1(F); lane 5, R4660; lane 6, R7217; lane 7, HSV1(F)Δ305; lane 8, R7205 (the parent virus of R7217 and R4660). PAA, phosphonoacetic acid. (B) Replicate cultures of HEp-2 cells mock infected or infected with HSV-1(F) and harvested 16 h after infection. Nuclear fractions and cytoplasmic (cytoplas.) fractions were prepared with 0.1% Nonidet P-40 as described elsewhere (14). WCE, whole-cell extract.
The second set of experiments was designed to verify the evidence from the immunofluorescence studies (presented below) that UL4 protein was distributed in both the nuclei and the cytoplasms of infected cells. HEp-2 cells were mock infected or infected with HSV-1(F), harvested 16 h after infection, and either solubilized intact or fractionated into nuclear and cytoplasmic fractions as described elsewhere (14). The lysates were then subjected to electrophoresis on denaturing polyacrylamide gels and reacted with the UL4 antiserum and with the monoclonal antibody to ICP4 (H640), which was described elsewhere (1) and was purchased from Goodwin Cancer Research Institute. As expected, ICP4 fractionated with the nuclear extract (Fig. 2B), whereas UL4 protein was detected in both the nuclear and cytoplasmic fractions.
In the first series of immunofluorescence studies (data not shown), we noted that the pattern of UL4 fluorescence was very similar to that of ICP22 (15). To test the hypothesis that ICP22 and UL4 protein colocalize, rabbit skin cells were infected and processed for immunofluorescence analysis as described elsewhere (15) with the monoclonal antibody to the CMV tag (CH28-2) and either the rabbit polyclonal antibody to UL4 or R77 to ICP22 (2). The results were as follows.
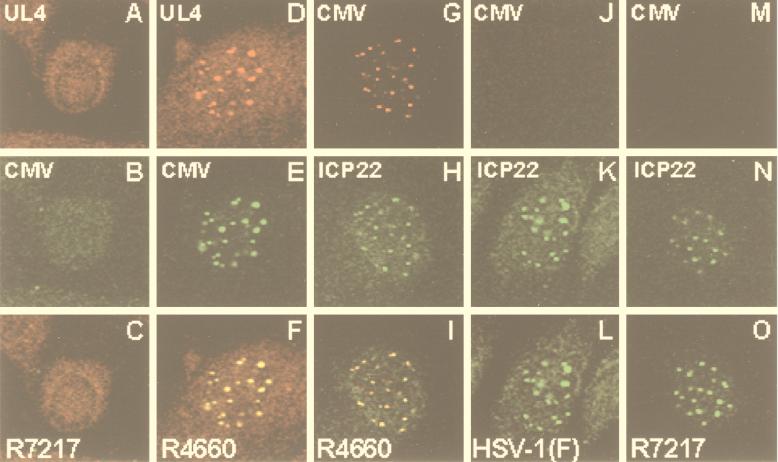
(i) Neither the anti-UL4 nor the anti-CMV antibody reacted with cells infected with R7217 (Fig. 3A to C).
FIG. 3.
Digitized images of rabbit skin cells fixed 17 h after infection and reacted with antibodies either to UL4 and CMV (A to F) or to the CMV epitope and ICP22 (G to O). Rabbit skin cells exposed to approximately 4 PFU of the indicated virus per cell were fixed, reacted with the indicated antibodies, and digitally imaged with a Zeiss confocal fluorescence microscope as described elsewhere (15). The same cell is shown in each vertical triptych. The top and middle panels of each triptych contain images demonstrating the localization of the protein reacting with the indicated antibody. The bottom panel contains an overlay of separately captured images similar to those in the upper panels. The yellow color indicates the colocalization of the proteins shown in the upper panels.
(ii) In cells infected with R4660, the antigens reacting with the UL4 and the CMV antibodies colocalized in the small, dense nuclear bodies (Fig. 3D to F). The UL4 antibody gave a slightly more diffuse pattern of fluorescence than the anti-CMV antibody. This and the results of experiments whose data are not shown indicated that the two antibodies reacted in immunofluorescence assays with identical CMV-tagged proteins and that the native and tagged UL4 proteins localized in identical structures.
(iii) In cultures infected with R4660, the antigens reacting with the monoclonal antibody to the tag in the UL4 protein and to the polyclonal antibody to ICP22 colocalized in the same small, dense nuclear structures (Fig. 3G to I).
(iv) The pattern of accumulation of ICP22 in rabbit skin cells infected with HSV-1(F) (Fig. 3K) could not be differentiated from that observed in cells infected with R4460 (Fig. 3H) or in cells infected with the deletion mutant R7217 (ΔUL4) (Fig. 3N).
(v) Late in infection of human or nonhuman primate cells, ICP22 localizes in replicating structures along with nascent DNA, RNA polymerase II, and other cellular and viral proteins (15). The transition from small, dense nuclear structures to the diffuse intranuclear replication complexes requires the activity of the UL13 protein kinase. Although the rabbit skin cells were examined 17 h after infection, the distribution of ICP22 in these cells resembled that of cells fixed early in infection rather than that of human cells observed late in infection (15).
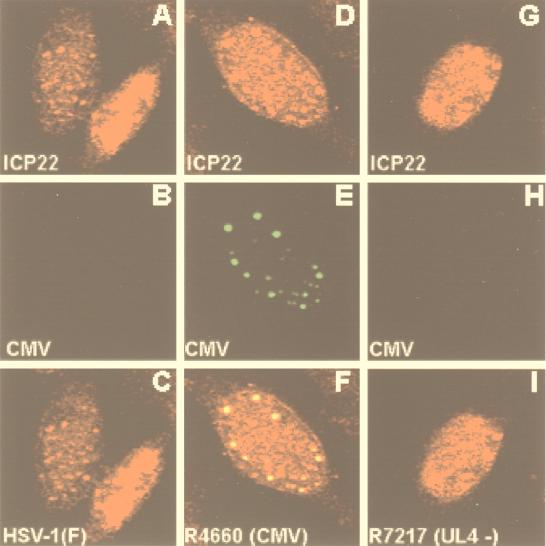
These experiments suggested the possibility that the accumulation of ICP22 in rabbit skin cells may differ from that in HEp-2 cells. To resolve the question of whether UL4 and ICP22 also colocalize in HEp-2 cells fixed late in infection, HEp-2 cells were fixed 17 h after infection with HSV-1(F), R4660, or R7217 and reacted with both the polyclonal antibody to ICP22 and the monoclonal antibody to the CMV tag. The results (Fig. 4) were as follows.
FIG. 4.
Digitized images of HEp-2 cells infected with the indicated virus, fixed 17 h after infection, and doubly stained with primary antibodies to CMV (green fluorescence) and ICP22 (red fluorescence). The organization of each triptych is as described in the legend to Fig. 3.
The antibody to ICP22 reacted with antigens localized in two kinds of structures: small, dense nuclear bodies and more diffuse nuclear materials that in some cells were dispersed and in other cells (e.g., the lower right cell in Fig. 4A) filled a large portion of the nucleus. The monoclonal antibody against the CMV-tagged UL4 protein reacted only with small, dense nuclear structures in cells infected with the R4660 mutant (Fig. 4E). The small, dense nuclear structures illuminated by the ICP22 antibody colocalized with the structures containing the UL4 protein.
We conclude from these studies the following. UL4 protein accumulates in the cytoplasm and in small, dense nuclear bodies. In rabbit skin cells, UL4 protein and ICP22 colocalize and are present predominantly in these structures, apparently throughout the accumulation of UL4. In infected HEp-2 cells, ICP22 and UL4 protein also colocalize in small, dense nuclear bodies. However, as previously reported, by the time UL4 accumulates in sufficient amounts to be detected late in infection, ICP22 is also present in replicative complexes containing nascent DNA, RNA polymerase, and other viral and cellular proteins (15).
The significant points of the findings described in this report are as follows.
(i) The shift from small, dense nuclear structures to replicative complexes after the onset of DNA synthesis in primate cells has led to the suggestion that ICP22 performs different functions before and after the onset of viral DNA synthesis. Entry into replicative complexes requires phosphorylation by the UL13 viral protein kinase and the association with nascent DNA and ICP4 (the major regulatory protein), RNA polymerase II, and other partially defined viral and cellular proteins. In this report, we show that UL4, a γ2 protein, is associated with the small, dense nuclear bodies formed prior to the onset of DNA synthesis but not with the replicative complexes formed after the onset of DNA synthesis. These results suggest that the function of UL4 is associated with that of ICP22 in the context of small, dense nuclear structures and that these structures persist and function after infection inasmuch as they recruit additional viral proteins made late in infection. This observation raises the intriguing possibility that the functions of ICP22 and of UL4 partially overlap, explaining the absence of a phenotype for the UL4 gene.
(ii) Rabbit skin cells are highly permissive for wild-type HSV-1 but are restrictive for mutants lacking the α22 gene. In the restrictive cell lines, the function of ICP22 after the onset of viral DNA synthesis concerns the expression of a subset of late (γ2) genes and the stability and expression of the α0 gene (5, 6, 19). A striking observation to emerge in the studies reported here is that in rabbit skin cells, ICP22 localized only in the small, dense nuclear structures even late in infection and that the UL4 protein colocalized with all of the structures containing ICP22. The significance of this observation is unclear. Hypotheses that remain to be explored further are that in restricted cell lines the compartmentalization of viral functions performed in the nucleus is different from those taking place in permissive cells and that the factors determining the localization of ICP22 play an important role in defining the permissivity of cells.
Acknowledgments
S.J. and N.S.M. contributed equally to this work.
These studies were aided by grants from the National Cancer Institute (CA47451 and CA71933), the U.S. Public Health Service, a Young Investigator Award to N.S.M. from the National Alliance for Research on Schizophrenia and Depression, and a Howard Hughes Medical Institute Summer Research Fellowship for Undergraduates to S.J.
REFERENCES
- 1.Ackermann M, Braun D K, Pereira L, Roizman B. Characterization of herpes simplex virus 1 α proteins 0, 4, and 27 with monoclonal antibodies. J Virol. 1984;52:108–118. doi: 10.1128/jvi.52.1.108-118.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackermann M, Sarmiento M, Roizman B. Application of antibody to synthetic peptides for characterization of the intact and truncated α22 protein specified by herpes simplex virus 1 and the R325 α22− deletion mutant. J Virol. 1985;56:207–215. doi: 10.1128/jvi.56.1.207-215.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baines J D, Roizman B. The open reading frames UL3, UL4, UL10, and UL16 are dispensable for the replication of herpes simplex virus 1 in cell culture. J Virol. 1991;65:938–944. doi: 10.1128/jvi.65.2.938-944.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baines, J. D., and B. Roizman. Unpublished data.
- 5.Carter K L, Roizman B. Alternatively spliced mRNAs predicted to yield frame-shift proteins and stable intron 1 RNAs of the herpes simplex virus 1 regulatory gene α0 accumulate in the cytoplasm of infected cells. Proc Natl Acad Sci USA. 1996;93:12535–12540. doi: 10.1073/pnas.93.22.12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter K L, Roizman B. The promoter and transcriptional unit of a novel herpes simplex virus 1 α gene are contained in, and encode a protein in frame with, the open reading frame of the α22 gene. J Virol. 1996;70:172–178. doi: 10.1128/jvi.70.1.172-178.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson A J, Scott J E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986;67:1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- 8.Dean H J, Cheung A K. Identification of the pseudorabies virus UL4 and UL5 (helicase) genes. Virology. 1994;202:962–967. doi: 10.1006/viro.1994.1419. [DOI] [PubMed] [Google Scholar]
- 9.Ejercito P M, Kieff E D, Roizman B. Characteristics of herpes simplex virus strains differing in their effect on social behavior of infected cells. J Gen Virol. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs W, Mettenleiter T C. DNA sequence and transcriptional analysis of the UL1 to UL5 gene cluster of infectious laryngotracheitis virus. J Gen Virol. 1996;77:2221–2229. doi: 10.1099/0022-1317-77-9-2221. [DOI] [PubMed] [Google Scholar]
- 11.Honess R W, Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974;14:8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honess R W, Roizman B. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc Natl Acad Sci USA. 1975;72:1276–1280. doi: 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jun P Y, Strelow L I, Herman R C, Marsden H S, Eide T, Haarr L, Leib D A. The UL4 gene of herpes simplex virus type 1 is dispensable for latency, reactivation and pathogenesis in mice. J Gen Virol. 1998;79:1603–1611. doi: 10.1099/0022-1317-79-7-1603. [DOI] [PubMed] [Google Scholar]
- 14.Kawaguchi Y, Bruni R, Roizman B. Interaction of herpes simplex virus 1 α regulatory protein ICP0 with elongation factor 1δ: ICP0 affects translational machinery. J Virol. 1997;71:1019–1024. doi: 10.1128/jvi.71.2.1019-1024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leopardi R, Ward P L, Ogle W O, Roizman B. Association of herpes simplex virus regulatory protein ICP22 with transcriptional complexes containing EAP, ICP4, RNA polymerase II, and viral DNA requires posttranslational modification by the UL13 protein kinase. J Virol. 1997;71:1133–1139. doi: 10.1128/jvi.71.2.1133-1139.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu F, Roizman B. The promoter, transcriptional unit, and coding sequence of herpes simplex virus 1 family 35 proteins are contained within and in frame with the UL26 open reading frame. J Virol. 1991;65:206–212. doi: 10.1128/jvi.65.1.206-212.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGeoch D J, Cunningham C, McIntyre G, Dolan A. Comparative sequence analysis of the long repeat regions and adjoining parts of the long unique regions in the genomes of herpes simplex viruses types 1 and 2. J Gen Virol. 1991;72:3057–3075. doi: 10.1099/0022-1317-72-12-3057. [DOI] [PubMed] [Google Scholar]
- 18.Post L E, Roizman B. A generalized technique for deletion of specific genes in large genomes: α gene 22 of herpes simplex virus 1 is not essential for growth. Cell. 1981;25:227–232. doi: 10.1016/0092-8674(81)90247-6. [DOI] [PubMed] [Google Scholar]
- 19.Purves F C, Ogle W O, Roizman B. Processing of the herpes simplex virus regulatory protein α22 mediated by the UL13 protein kinase determines the accumulation of a subset of α and γ mRNAs and proteins in infected cells. Proc Natl Acad Sci USA. 1993;90:6701–6705. doi: 10.1073/pnas.90.14.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roizman B. The function of herpes simplex virus genes: a primer for genetic engineering of novel vectors. Proc Natl Acad Sci USA. 1996;93:11307–11312. doi: 10.1073/pnas.93.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sears A E, Halliburton I W, Meignier B, Silver S, Roizman B. Herpes simplex virus 1 mutant deleted in the α22 gene: growth and gene expression in permissive and restrictive cells and establishment of latency in mice. J Virol. 1985;55:338–346. doi: 10.1128/jvi.55.2.338-346.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh J, Wagner E K. Transcriptional analysis of the herpes simplex virus type 1 region containing the TRL/UL junction. Virology. 1993;196:220–231. doi: 10.1006/viro.1993.1470. [DOI] [PubMed] [Google Scholar]
- 23.Telford E A, Watson M S, McBride K, Davidson A. The DNA sequence of equine herpesvirus-1. Virology. 1992;189:304–316. doi: 10.1016/0042-6822(92)90706-u. [DOI] [PubMed] [Google Scholar]
- 24.Vlcek C, Benes V, Lu Z, Kutish G F, Paces V, Rock D, Letchworth G J, Schwyzer M. Nucleotide sequence analysis of a 30-kb region of the bovine herpesvirus 1 genome which exhibits a colinear gene arrangement with the UL21 to UL4 genes of herpes simplex virus. Virology. 1995;210:100–108. doi: 10.1006/viro.1995.1321. [DOI] [PubMed] [Google Scholar]
- 25.Yamada H, Jiang Y-M, Oshima S, Wada K, Goshima F, Daikoku T, Nishiyama Y. Characterization of the UL4 gene product of herpes simplex virus type 2. Arch Virol. 1998;143:1199–1207. doi: 10.1007/s007050050367. [DOI] [PubMed] [Google Scholar]