Abstract
About one-third of major depressive disorder (MDD) patients demonstrate unresponsiveness to classic antidepressants, and even the clinical efficacy of fast-acting drugs such as ketamine varies significantly among patients with treatment-resistant depression. Nevertheless, the lack of suitable animal models that mimic a possible ketamine-resistant phenotype challenges the understanding of resistance to drug treatment. In this study, we showed that PI3Kγ knock-out (KO) mice do not respond to classical doses of ketamine and classical antidepressants. PI3Kγ KO mice were unresponsive to both the rapid and sustained antidepressant-like effects of a single dose of ketamine in the forced swimming test. Additionally, they were unresponsive to the antidepressant-like effects induced by the tricyclic antidepressant imipramine and the selective serotonin reuptake inhibitor fluoxetine. However, acute pharmacological inhibition of PI3Kγ did not block the antidepressant-like effect of ketamine, showing that a chronic deficiency of the PI3Kγ-mediated pathway is necessary for the effects of classic doses of ketamine and antidepressants. Therefore, we propose that PI3Kγ participates in the antidepressant activity and is likely implicated in the neurobiology and phenotype observed in patients with MDD who demonstrate treatment resistance.
Keywords: PI3Kγ, Major depressive disorder, Ketamine, Antidepressants, Coping behavior
1. Introduction
According to the World Health Organization (WHO), major depressive disorder (MDD) affects over 280 million people worldwide, making it the leading cause of years lost to disability (WHO). The prevalence of MDD increased by approximately 26 % following the COVID-19 pandemic (Twenge and Joiner, 2020). Moreover, approximately one-third of MDD patients do not respond appropriately to at least two of the available antidepressant therapies (Serafini et al., 2014, Salahudeen et al., 2020).
Ketamine is a dissociative anesthetic that, at subanesthetic doses, induces a rapid and sustained antidepressant action after acute administration. Recently, its S-enantiomer was approved by the FDA for treating severe cases of MDD and patients who experience resistance to treatment with classical antidepressants. In addition, among patients with Treatment-Resistant Depression (TRD), the clinical efficacy of ketamine varies significantly, with an estimated 30–50 % of TRD patients not responding to ketamine (Schwartz et al., 2016, Alnefeesi et al., 2022). An analysis of microRNAs in depressed patients proposed that let-7b and let-7c could be used as biomarkers for TRD (Gururajan et al., 2016). Interestingly, these microRNAs regulate the expression of 27 genes related to the PI3K-Akt-mTOR signaling pathway.
The protein kinase PI3K comprises three classes of proteins classified according to their primary and secondary structure, regulatory mechanisms, and substrate specificity (Foster et al., 2003, Vanhaesebroeck et al., 2010). Class Ia PI3K (isoforms α, β, and δ) consists of a catalytic and a regulatory domain and is typically coupled to tyrosine kinase (class Ia, isoforms α and β) or G-protein receptors (class Ib, γ isoform). The latter, of particular interest for this study, is mainly expressed in the immune system, heart, and brain (Rückle et al., 2006).
Several pieces of evidence suggest that the efficacy of antidepressant treatment is associated with the activation of intracellular pathways that coordinate protein synthesis, cell proliferation, cell survival, and synaptogenesis. Both ketamine and classical monoaminergic antidepressants increase Akt and mTOR activation, an effect significantly blocked by the administration of a non-selective PI3K inhibitor (Park et al., 2014, Fukumoto et al., 2018a) Additionally, the pan pharmacological inhibition of PI3K prevents ketamine-induced behavioral effects (Zhou et al., 2014, Price et al., 2022).
However, little is known about the involvement of class Ib PI3K in the efficacy of fast-acting antidepressant drugs, particularly those observed after long-term clinical follow-ups (Alnefeesi et al., 2022, Price et al., 2022). In the present study, we characterized and demonstrated that PI3Kγ knock-out (KO) mice fail to exhibit positive coping behaviors in the context of preclinical tests commonly used in the field of Psychopharmacology for screening antidepressant activity. We found that male PI3Kγ KO mice do not show the rapid and sustained antidepressant-like effects of ketamine.
2. Methods
2.1. Animals
Adult male C57Bl/6 J (wild-type, W.T.) and PI3Kγ Knock-out (K.O (Takehiko Sasaki et al., 2000).) mice, aged 8–10 weeks, were derived from established colonies in the Animal Care Facility at the Institute of Biological Sciences, Federal University of Minas Gerais (UFMG). These mice were allowed to acclimatize for at least two weeks in our animal facility before the beginning of behavioral experiments. Mice were housed in groups of 4–5 per cage and were kept in a quiet room with controlled temperature and humidity, following a 12:12-hour light/dark cycle (with lights on at 6:30 am), and provided with free access to food and water. They were randomly assigned to experimental groups and received pharmacological treatments. The Experimental Protocols were approved by the Ethical Committee of Animal Experimentation at UFMG, following Brazilian laws and the ARRIVE guidelines (CETEA protocol number: 108/11-UFMG).
2.2. Drugs
The following drugs were used: ketamine chlorinated (10 mg/kg, i.p.; Syntec, Brazil; racemic mixture), imipramine hydrochloride (15 mg/kg, 1.p.; Sigma-Aldrich, Germany), fluoxetine hydrochloride (15 mg/kg, i.p.; EMS, Brazil), and AS605240 (15, 30, 60 mg/Kg, v.o.; Cayman, USA). All drugs were dissolved in sterile saline except AS605240, dissolved in carboxymethylcellulose. Drugs were prepared freshly under sterile conditions and administered intraperitoneally at 10 mL/kg.
2.3. Behavioral tests
Forced Swim Test (FST): The forced swim test (FST) was conducted as previously described (Takehiko Sasaki et al., 2000). It served as a behavioral test for screening the response of rodents to antidepressant drugs, reflecting the ability of antidepressants to reduce immobility and promote active coping strategies (Petit-Demouliere et al., 2005). Each mouse was placed in a cylinder (diameter: 16 cm; height: 31 cm) containing 15 cm of water at a temperature of 24°C ± 2°C. The water was changed, and the cylinders were cleaned between each session. The test lasted for 6 minutes, with immobility time measured during the final 4 minutes, while the first 2 minutes were considered as a pre-test period. Immobility behavior was recorded when mice ceased struggling and moved slowly, maintaining a floating position to keep their heads above water. The test was recorded and analyzed by an experimenter who was blind to treatment and genotype status.
Tail suspension Test (TST): The TST evaluates passive and active coping behavior (Ribeiro et al., 2022). On the day of the experiments, all mice were transported from the housing facility to the testing room. Then, they were left there undisturbed for at least 3 h. Animals were individually suspended by the tail to a horizontal ring-stand bar (distance from floor = 35 cm) using adhesive tape (distance from the tip of tail = 2 cm). Typically, mice show several escape-oriented behaviors interspersed with bouts of immobility of increasing length as the session progressed. The test session (6 min) was recorded, and the total immobility time was measured by an experienced experimenter blind to the treatment conditions and genotype. Animals were excluded from the analysis when climbing on their tail.
Open Field Test (OFT): The open field (OF) test was conducted as a control experiment to rule out the possibility of interference from changes in basal locomotion and exploratory behavior in the FST and TST (Seibenhener and Wooten, 2015). The OFT comprises of a square arena (60×60×60cm) made of white acrylic material. Mice were individually removed from their home cage and gently placed immediately in the OF central area. They were allowed to explore the arena for 10 min freely. All trials were recorded, and the total distance traveled, in meters, was analyzed automatically using the AnyMaze software (Stoelting, Germany).
2.4. Experimental design
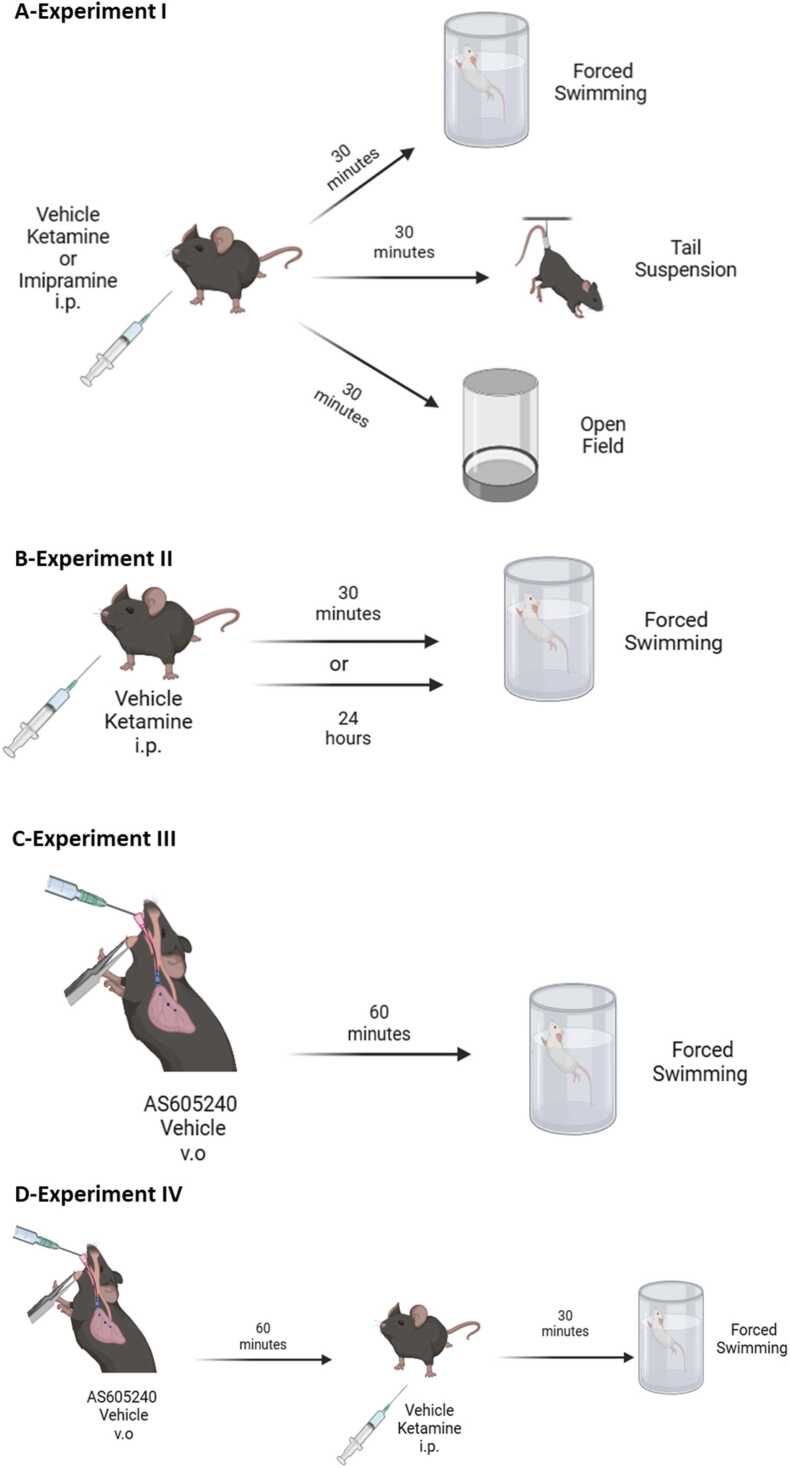
Experiment I: Evaluation of antidepressant-like effects of acute dose of ketamine (10 mg/Kg|) and imipramine (15 mg/Kg) in PI3Kγ KO mice: The experiments were conducted as described in Fig. 1A. Independent groups of WT or PI3Kγ KO mice received i.p. injections of vehicle, ketamine (10 mg/kg) or imipramine (15 mg/kg) and 30 minutes later, were submitted to the FST, TST or OFT. The following groups independent groups were generated: For the FST: WT/Vehicle; WT/ketamine; WT/imipramine, PI3Kγ KO/vehicle, PI3Kγ KO/ketamine, and PI3Kγ KO/imipramine; For the TST: WT/Vehicle; WT/ketamine; WT/imipramine, PI3Kγ KO/vehicle, PI3Kγ KO/ketamine, and PI3Kγ KO/imipramine and the same setting of groups for the OFT. An independent group of WT and PI3Kγ KO mice were submitted to received i.p. treatment with the selective serotonin reuptake inhibitor, fluoxetine (15 mg/kg) and their behavioral response were evaluated in the FST and TST was evaluated 30 min post-treatment (Fig. S1).
Fig. 1.
Schematic representation of the experimental designed used in the present study. Wild-type (WT) and PI3Kγ K.O. mice were randomly assigned to experimental groups and submitted to behavioral tests used for the screening of antidepressant activity. A- Experiment 1- behavioral tests: forced swimming test (FST), tail suspension and open field test; B- Experiment II- acute and sustained effects of ketamine in the FST was assessed; C-Experiment III- dose response curve of the PI3Kγ inhibitor administered to WT mice and submitted to the FST; D-Experimental design of the Experiment-IV that evaluated the participation of acute pharmacological inhibition of PI3Kγ in the fasting acting effects of ketamine.
Experiment II: Comparison of Acute and sustained antidepressant-like effect of single dose of ketamine in PI3Kγ KO mice: Independent groups of WT and PI3Kγ KO mice received i.p. injections of vehicle or ketamine (10 mg/kg) and their behavioral responses related to antidepressant activity assessed in the FST 30 min or 24 h post-treatment (Fig. 1B). The following groups were analyzed: 30 min- WT/vehicle, WT/ketamine, PI3Kγ KO/vehicle, and PI3Kγ KO/ketamine; and 24 h- WT/vehicle, WT/ketamine, PI3Kγ KO/vehicle, and PI3Kγ KO/ketamine.
Experiment III: Determination of a dose-response curve of AS605240 (acute, v.o.), a selective PI3Kγ inhibitor, in the FST: In this experiment we treated WT mice with acute different doses of AS605240 (15, 30 or 60 mg/kg) or vehicle (v.o.) and, 1 h later, mice were submitted to the FST for antidepressant activity screening. The following groups were evaluated in this experiment: Vehicle, AS605240 15 mg/kg, AS605240 30 mg/kg, and AS605240 60 mg/kg (Fig. 1C).
Experiment IV: Evaluation of antidepressant-like effects under pharmacological PI3Kγ inhibition: Based on the results obtained in Experiment III, we selected the highest ineffective dose of AS605240 to evaluate the role of acute pharmacological inhibition of PI3Kγ on the fast-acting effects of ketamine in the FST. The following groups were tested: vehicle/vehicle, vehicle/ketamine (10 mg/kg), AS605240 (60 mg/kg)/vehicle, and AS605240 (60 mg/kg)/ketamine (10 mg/kg). C57Bl/6 mice were administered the vehicle or AS605240 (60 mg/kg) orally as the first treatment. Then, one hour later, they received the second treatment, either vehicle or ketamine (10 mg/kg) intraperitoneally. Thirty minutes after the last treatment, the mice were subjected to the FST (Fig. 1D).
2.5. Statistical analysis
The data were tested for normality using the Kolmogorov-Smirnov test and for homogeneity of variances using Levene's test before conducting the specific statistical tests. We utilized a two-way ANOVA to assess the effects of individual factors (Factor 1: genotype or treatment 1; Factor 2: treatment or treatment 2) and their possible interactions, with the exception of the AS605240 dose-response curve, which was analyzed using one-way ANOVA. Post hoc analysis was performed using the Duncan post-test to characterize differences between individual groups. P values equal to or less than 0.05 were considered statistically significant. Mice were randomly assigned to each of the experimental groups using block randomization. The calculated statistical power was set at 0.85, with an alpha level of 0.05. Data were expressed as the mean ± standard error of the mean.
3. Results
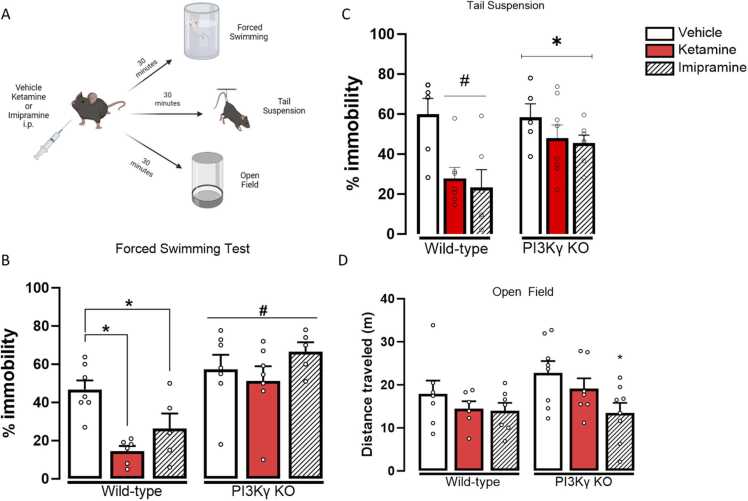
3.1. Acute doses of ketamine (10 mg/Kg) and imipramine (15 mg/Kg) fails to induce antidepressant-like effects in PI3Kγ mice
Two-way ANOVA indicated significant effects of the factors (genotype, F(1,31) = 30.573, p < 0.001; treatment, F(2,31) = 5.068, p = 0.012), and their interaction (F(2,31) = 3.302, p = 0.05). WT animals treated with either ketamine or imipramine showed a decreased % of immobility time compared to those treated with the vehicle, indicating an antidepressant-like effect in the FST (One-way ANOVA followed by Duncan; F(5,31) = 8.704, p < 0.001). In the TST, we observed an effect of the treatment (F(2,35)=3,9, p= 0.018) and an interaction between treatment and genotype (F(2,35)=4,9, p= 0.013). Neither ketamine nor imipramine, at the tested doses, reduced the immobility time of PI3Kγ KO mice in the FST or TST (Fig. 2B-C).
Fig. 2.
Acute ketamine and imipramine treatment fail to induce antidepressant-like effects in PI3Kγ K.O mice. A-Experimental Design representation; B- Immobility time in the Forced swimming Test (FST)- N= 7,6,5,7,7,5/group; and C- Tail Suspension Test (TST)- N=6,5,7,8,6,7/group. D- Distance traveled in the Open Field of WT and PI3Kℽ-KO mice treated with vehicle, ketamine (10 mg/kg) or imipramine (15 mg/kg- N=7.6.7.8.7.8/group. Data represented as Mean ± SEM. (*) represents p<0.05 in comparison to the group of the same genotype treated with vehicle; (#) represents p<0.05 in comparison to the other genotype (Two-way ANOVA; One-way ANOVA followed by Duncan).
To verify if the genotype or treatments influences in the basal locomotor activity, which could contaminate the results obtained in the FST and TST, WT and K.O. mice treated with ketamine or imipramine were tested in the OFT. We did not observe an effect of the genotype (F(1,37) = 2.235, p = 0.143) or an interaction between the factors (F(2,37) = 0.781, p = 0.466). However, treatment had a significant effect (F(2,37) = 3.791, p = 0.032) on the distance traveled in the OFT. In PI3Kγ KO mice, imipramine significantly reduced the distance traveled in the open field (One-way ANOVA followed by Duncan; F(5,37) = 2.343, p = 0.060) (Fig. 2C).
Given that acute treatments with classic antidepressants might trigger anxiety-like behaviors, and anxiety disorders are common comorbidities in MDD patients, we administered acute doses of imipramine. Thirty minutes later, we subjected both WT and K.O. mice to the Elevated Plus Maze (EPM) (see Fig. S1). Our results demonstrated that acute imipramine decreased the time spent in the open arms of the EPM. Furthermore, PI3Kγ K.O. mice exhibited increased basal levels of anxiety, as indicated by a decrease in the percentage of time spent in the open arms of the EPM. This effect was not potentiated by acute doses of imipramine (F(3,24) = 4.1, p = 0.018; One-way ANOVA followed by Duncan posttest) (see Fig. S1). No effects on the percentage of entries into the open arms or the frequency of entries into the enclosed arms of the EPM were found to be related to treatments or genotype.
We also investigated whether PI3Kγ would influence the behavioral effects of another class of antidepressants: the selective serotonin reuptake inhibitor (SSRI). Therefore, we conducted a similar experiment, but this time, we treated an independent group of mice with fluoxetine (15 mg/Kg) and, 30 minutes later, tested them in the Forced Swim Test (FST) or the Tail Suspension Test (TST) (see Fig. S2A). Our results demonstrated that fluoxetine decreased the percentage of immobility in WT mice tested in the FST and TST. However, PI3Kγ KO mice did not show a reduction in immobility time in the FST or TST when treated with fluoxetine (see Fig. S2B-C).
3.2. Acute and sustained effects of ketamine (10 mg/Kg) are absent in PI3Kℽ KO mice evaluated in the FST
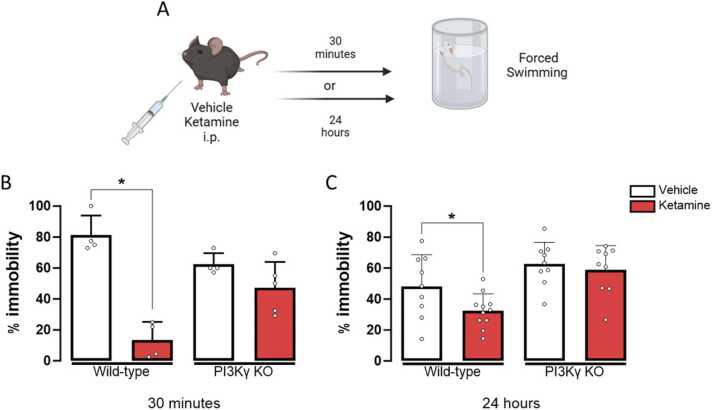
To determine if the sustained effects of ketamine would be preserved in PI3Kγ KO mice, we tested the mice at two different time points after ketamine (10 mg/kg) administration. In the groups tested 30 minutes after ketamine treatment, a two-way ANOVA revealed a significant effect of the treatment factor (F(1,13) = 44.080, p < 0.001) and a significant interaction between genotype and treatment (F(1,13) = 17.652, p = 0.001). WT mice treated with ketamine showed a significantly lower percentage of immobility time than the WT group treated with the vehicle, indicating a fast antidepressant-like effect of ketamine (One-way ANOVA followed by Duncan; F(3,13) = 20.024, p < 0.001). However, in PI3Kγ KO mice, this fast antidepressant-like effect of ketamine was absent (Fig. 3B).
Fig. 3.
Ketamine fails to induce acute or sustained antidepressant-like activity in PI3Kγ K.O mice submitted to the forced swimming test 30 minutes or 24 after the pharmacological treatments. A- schematic representation of the experimental design. Percentage of immobility time in the forced swimming test of WT and PI3Kℽ-KO mice treated with vehicle or ketamine (10 mg/kg) and tested 30 min (B) or 24 h (C) after drug administration(n=4–11). Data represented as Mean ± SEM. (*) represents p<0.05 in comparison to the group of the same genotype treated with vehicle; (#) represents p<0.05 in comparison to the other genotype (Two-way ANOVA; One-way ANOVA followed by Duncan). B-N=4,4,4,5/group respectively; B- N= 9,12,9,9/group respectively.
In the groups tested 24 hours after ketamine treatment, a significant effect of the factor genotype (F(1,34) = 16.594, p < 0.01) was observed, but there was no effect of treatment (F(1,34) = 3.806, p = 0.059) or interaction between factors (F(1,34) = 1.383, p = 0.248). Treatment with ketamine significantly reduced the immobility time of WT mice, indicating a sustained antidepressant-like effect (F(3,34) = 7.799, p < 0.001). However, ketamine treatment did not exert a sustained antidepressant-like activity in PI3Kγ KO mice (Fig. 3C).
3.3. The acute pharmacological inhibition of PI3Kγ does not prevent the antidepressant-like effect of ketamine in the FST
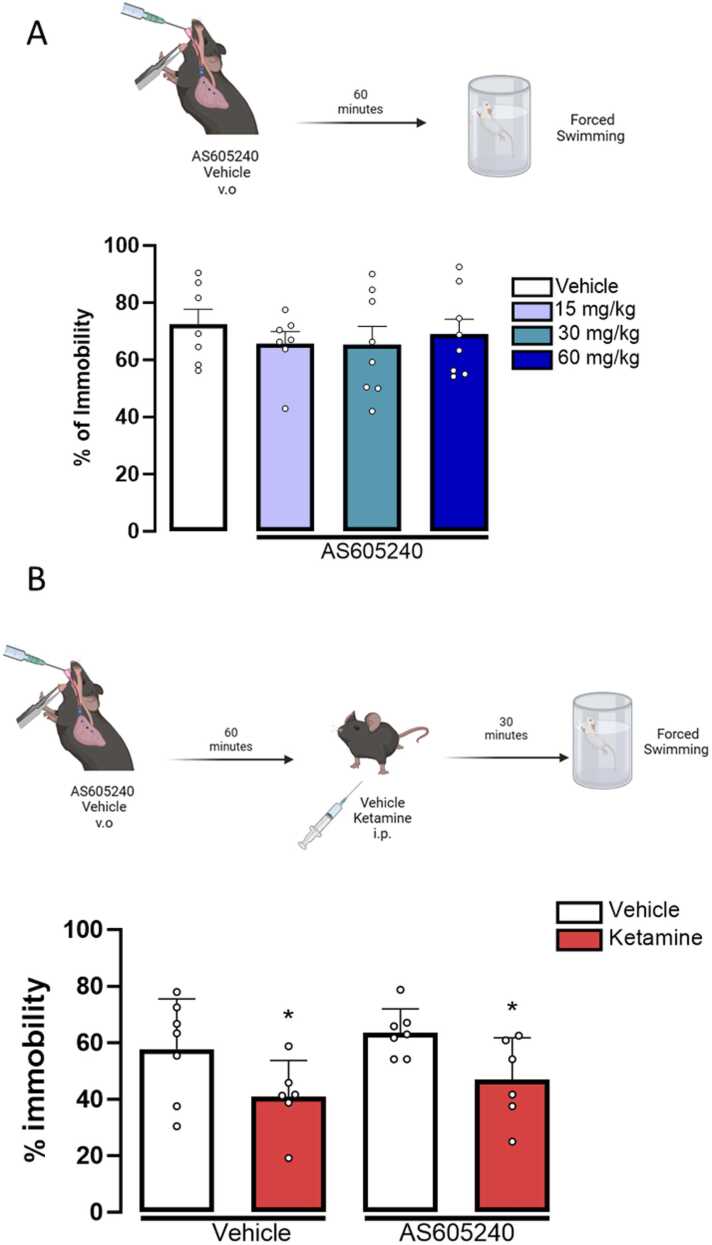
To determine whether the acute inhibition of PI3Kγ would yield similar outcomes as observed in the PI3Kγ KO mice, we pre-treated animals with the PI3Kβ inhibitor AS605240. However, before the antagonism assay, we conducted an experiment to establish the dose-response curve of the PI3Kγ inhibitor, AS605240, in the FST. As shown in Fig. 4B, none of the tested doses significantly altered the immobility percentage (One-way ANOVA, F(3,36) = 0.370, p = 0.775) in the FST. This profile was to what we observed in the PI3Kγ KO mice, indicating that the genetic ablation of the PI3Kγ isoform does not induce a more susceptible behavioral phenotype of the mice in the FST or TST. Therefore, for the next experiment, we selected the highest ineffective dose of AS605240 (60 mg/kg) to investigate whether the acute inhibition of PI3Kγ would affect the effects of ketamine in the FST. Mice pretreated with vehicle or AS605240 (Treatment 1) and then treated with vehicle or ketamine (Treatment 2) showed a significant effect of Treatment 2 (F(1,22) = 9.335, p = 0.006), but no interaction between treatments, indicating that ketamine induced an antidepressant-like effect even after the pre-treatment with the PI3Kγ inhibitor (Fig. 4C)
Fig. 4.
The acute inhibition of PI3Kγ does not prevent the antidepressant-like effect of ketamine in mice tested in the FST. A- Dose-response curve of AS605140 (15 mg/kg, 30 mg/kg, and 60 mg/kg) in the percentage of immobility of mice in the forced swimming test (N=7,7,8,8/group respectively); Percentage of immobility time in the forced swimming test of mice pre-treated with vehicle or AS605240 (60 mg/kg) and treated with vehicle or ketamine (10 mg/kg) (n=7,6,7,6/group respectively). Data represented as Mean ± SEM (Two-way ANOVA; One-way ANOVA followed by Duncan posttest).
4. Discussion
In the present study, we demonstrate that the typical dose of ketamine (10 mg/Kg- after acute administration) often tested in C57Bl6 mice submitted to the Forced Swim Test (FST) failed to induce acute and sustained antidepressant-like effects in PI3Kγ KO mice. PI3Kγ KO mice also showed unresponsiveness to single/acute doses of classical tricyclic and SSRI antidepressants. Although the genetic deletion of this enzyme does not result in increased immobility time in the FST and TST, these findings offer new insights, suggesting the involvement of the γ isoform of class-Ib-PI3K in the behavioral effects of acute treatment with classic antidepressants, imipramine and fluoxetine and acute and sustained effects of the fast-acting drug, ketamine in mice submitted to behavioral tests largely used for the screening of antidepressant compounds (Petit-Demouliere et al., 2005, Armario, 2021). Our results replicate previous results suggesting that PI3Kγ KO mice are also unresponsive to the antidepressant-like effect induced by the anticonvulsant and mood-stabilizer valproic acid in the FST (Lima et al., 2017).
The FST and TST have been used as behavioral readout for depressive-like behaviors and acute antidepressant activity for many years since their validation as rodents´ tests (Takehiko Sasaki et al., 2000). While some authors argue that both the TST and FST reflect certain behavioral aspects (such as behavioral despair and learned helplessness) and neurobiological features (e.g., hippocampal involvement) of Major Depressive Disorder (MDD) in humans (Fitzgerald et al., 2019, Cryan and Holmes, 2005), in the last decade, several important reviews and studies have shed light on their significant limitations.
In our study, we chose to employ the FST and TST not as models of MDD but for screening acute and sustained antidepressant activity. The immobility displayed by rodents during these tests is highly sensitive to acute effective doses of classic antidepressants and ketamine (Petit-Demouliere et al., 2005, Armario, 2021, Fitzgerald et al., 2019, Cryan and Holmes, 2005). Furthermore, we use immobility time as an indicator of the effectiveness of antidepressant compounds in enhancing active coping strategies (Armario, 2021), which was notably absent in PI3Kγ KO mice after acute administration of ketamine, imipramine, or fluoxetine at the tested doses.
Another critical aspect regarding the limitations of the TST and FST rely to the acute behavioral responses observed following treatment with classic antidepressants, despite their clinical effects often requiring weeks. Nevertheless, considering that the primary focus of this study was to identify a potential genetic model unresponsive to classic ketamine doses, (typically administered acutely in clinical practice) the use of the TST and FST appears justified.
Regarding the contrast between acute and chronic effects of classic antidepressants, our data suggested that groups receiving acute doses of imipramine and PI3Kγ KO mice exhibited a decreased percentage of time spent exploring the open arms of the elevated plus maze (see Fig. S1), a test commonly employed for screening anxiolytic drugs. However, further studies are needed characterize the role of PI3Kγ in the chronic effects of classic antidepressants drugs, such as fluoxetine and imipramine.
The antidepressant effects of ketamine were initially reported in the 1970s in both animal models (including the FST) and humans. However, it was only after Berman and colleagues' study in 2000, which described a rapid onset of antidepressant effects (within 40 minutes) following ketamine infusion in depressed patients, that the psychopharmacological community became enthusiastic about the potential breakthrough in depression treatment (Cryan and Holmes, 2005). This enthusiasm was particularly significant as it marked a new era from pharmacological interventions not based on the classical Monoaminergic Theory. Our findings include PI3Kγ in the hall of possible druggable target linking classic (monoaminergic) and fasting acting theories of antidepressant action.
The specific mechanism underlying ketamine's rapid and sustained antidepressant effects stays a subject of debate and does not appear to be exclusively related to its role as a non-competitive antagonist of the NMDA receptor. The search for new mechanisms, such as those involving intracellular pathways, as exemplified by the PI3Kγ pathway described here, is particularly significant. This is because ketamine, while effective, is not a definitive cure for MDD, and cases of treatment-resistant depression with ketamine have already been reported. It is estimated that approximately 30–50 % of patients do not respond to standardized dosages and therapeutic regimens of ketamine (Alnefeesi et al., 2022).
On the other hand, classic antidepressants are the first line of treatment for most cases of MDD. A deeper understanding of the pharmacology and mechanisms underlying the effects of classical antidepressants, which extends beyond the monoaminergic theory (including neuroplasticity) may offer valuable insights to the scientific community. These insights could help identify ways to enhance their efficacy (Fukumoto et al., 2018b, Nürnberg and Beer-Hammer, 2019).
Our study revealed that acute intraperitoneal treatment with the selective PI3Kγ inhibitor, AS605240, failed to attenuate the behavioral effects of ketamine in the Forced Swim Test. As a result, we concluded that chronic inhibition of PI3Kγ may be necessary to observe its role in preventing the acute and sustained antidepressant-like effects induced by ketamine. However, it is important to note that the protocol of acute peripheral administrations used in our study may impose limitations on this conclusion (Fukumoto et al., 2018a, Zhou et al., 2014). Zhou and collaborators (2014) demonstrated that ketamine failed to decrease the immobility time of rats in the FST after an intracerebroventricular (icv) administration of LY294002. The icv administration of the PI3K inhibitor also prevented the ketamine-induced increase in mTOR and p7056K phosphorylation in the prefrontal cortex (Fukumoto et al., 2018a). Therefore, further exploration is needed to assess the possible effects of intracerebral administration or chronic pharmacological inhibition of PI3Kγ on antidepressant-like behaviors triggered by psychoactive drugs.
Another point that requires consideration is the relevance of the different biological roles of PI3K isoforms for antidepressant effects. While the class Ia PI3K isoforms (α, ꞵ, and δ) are activated downstream to tyrosine kinase receptors, PI3Kγ activity is modulated by the activation of GPCRs (Neis et al., 2020). The expression pattern of the different isoforms also differs; while the p110β isoform is ubiquitously expressed, p110γ is reliably detected in specific cell types, including CNS cells like microglia and neurons (Neis et al., 2020). Therefore, we cannot rule out that PI3K KO mice might show different basal levels of other isoforms of PI3K, as a compensatory mechanism for the total removal of the Ib isoform (Lim et al., 2009). The relevance of this mechanism for the acute and sustained effects of ketamine requires further investigation.
Another consequence of the genetic deletion of PI3Kγ is the reduction in transforming growth factor-β1 (TGF-β1) levels in tissues like the lung (Zhang et al., 2020). Zhang and co-workers (2020) have shown that ketamine increases TGF-β1 in the prefrontal cortex, and recombinant TGF-β1 treatment induces a ketamine like antidepressant-like effect (Takeda et al., 2019). However, the effect of PI3Kγ KO on brain levels of TGF-β1 still needs investigation. Also, myeloid cells from a human patient with bi-allelic loss of function mutation in PIK3CG encoding the catalytic subunit of PI3Kγ overproduce the interleukins (IL) IL-12 and IL-23 via GSK-3β activation (Zhou et al., 2021). On the other hand, ketamine treatment significantly reduces the levels of IL-12 and IL-23 in severe major depressive disorder and TRD patients (Zhan et al., 2020, Nowak et al., 2019). Besides, GSK-3 inhibition is needed for the rapid antidepressant-like effect induced by ketamine (Beurel et al., 2011).
Like all preclinical studies, our results have important limitations. The use of immobility behavior in the FST and TST as the primary indicator of antidepressant activity limits the interpretability of our data. Nevertheless, the TST and FST have been widely employed as behavioral tasks predictive of antidepressant-like effects. In our study, only single, acute doses of ketamine, imipramine and fluoxetine were used. This is another limitation of our data, as higher doses of these drugs or even chronic treatments might be necessary to observe a behavioral effect in PI3Kγ KO mice. It is worth noting that we evaluated our mice 30 minutes after ketamine administration, and higher doses of this compound have been shown to induce changes in motor behavior without affecting the FST (in the C57Bl6 strain)[39]. The characterization of the behavioral responses of PI3Kγ KO mice after repeated/chronic treatment with classic antidepressants or ketamine requires further investigation. However, there is no consensus in the literature regarding whether multiple administrations of ketamine are more effective than single doses (Weston et al., 2021).
In summary, our findings suggest that PI3Kγ KO mice are resistant to the antidepressant-like effects induced by ketamine, mirroring a phenotype seen in patients who do not respond to classic dosages of this fast-acting drug.
5. Conclusion
The discovery of the fast and long-lasting antidepressant effects of ketamine marked a groundbreaking revolution in the treatment of this psychiatric disorder. However, a considerable number of patients do not respond as expected to conventional ketamine dosages. To develop more effective treatment strategies, it is crucial to gain a better understanding of the mechanisms associated with ketamine resistance. Our data shows that PI3Kγ KO mice, when tested in the FST and TST, exhibit unresponsiveness to the rapid and sustained antidepressant-like effects of acute ketamine treatment. Further studies may add valuable information in characterizing these mice as a potential genetic model for uncovering the molecular basis of ketamine treatment resistance. This also suggests that PI3Kγ could be a druggable target for the development of new fast-acting antidepressants.
Conflict of interest and compliance with ethical standards
The experimental protocols used in the manuscript, 'Genetic Ablation of the Isoform γ of PI3K Decreases Antidepressant Efficacy of Ketamine in Male Mice,' were approved by the Ethical Committee of Animal Experimentation at UFMG, following Brazilian laws and the ARRIVE guidelines (CETEA protocol number: 108/11-UFMG). Additionally, we, the authors, state that this study was conducted with no financial or personal interest or beliefs that could affect its objectivity and in the absence of any conflict of interest.
CRediT authorship contribution statement
Jose A. Crippa: Writing – original draft, Writing – review & editing. Antonio L. Teixeira: Funding acquisition, Writing – review & editing. Francisco S Guima: Writing – original draft, Writing – review & editing. Jaime E. C. Hallak: Writing – original draft, Writing – review & editing. Antonio C. P. de Oliveira: Conceptualization, Writing – review & editing. Alline Campos: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. Flávia C. Turcato: Data curation, Formal analysis, Investigation, Methodology. Isabel A.V. Lima: Data curation, Formal analysis, Investigation. Gabriela Vaz: Data curation, Formal analysis, Investigation, Methodology. Tamires Amabile Valim Brigante: Data curation, Writing – original draft. Livia C. M. Rodrigues: Data curation, Formal analysis, Writing – review & editing. Franciele F. Scarante: Data curation, Writing – original draft, Writing – review & editing. Melissa R Araujo: Data curation, Writing – original draft.
Acknowledgments
We would like to thank the hard work of lab colleagues and collaborators that made this study possible. We also thank our lab members for keeping such a good and supportive environment and the excellent spirit during our successes and, most importantly, our failures. We apologize to all authors not cited in this manuscript due to space limitations. This work was supported by public funding of the Brazilian Agencies (the FAPESP Young research grant- 2015/05551–0- the thematic grant- 2017/24304–0- and CNPq Universal Grant line A- 400033/2016–0 and CNPq´s productivity 1D scholarship- 304336/2022–0) and Fapemig, process APQ-02559-17. Figures representing experimental designs of the study were made using Biorender (free version).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ibneur.2024.06.002.
Appendix A. Supplementary material
Supplementary material.
References
- WHO, W. H. O. Depressive disorder (depression). 〈https://www.who.int/news-room/fact-sheets/detail/depression〉.
- Twenge J.M., Joiner T.E. U.S. Census Bureau-assessed prevalence of anxiety and depressive symptoms in 2019 and during the 2020 COVID-19 pandemic. Depress Anxiety. 2020;37:954–956. doi: 10.1002/da.23077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini G., Howland R., Rovedi F., Girardi P., Amore M. The role of ketamine in treatment-resistant depression: a systematic review. Curr. Neuropharmacol. 2014;12:444–461. doi: 10.2174/1570159X12666140619204251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahudeen M.S., Wright C.M., Peterson G.M. Esketamine: new hope for the treatment of treatment-resistant depression? A narrative review. Ther. Adv. Drug Saf. 2020;11 doi: 10.1177/2042098620937899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J., Murrough J.W., Iosifescu D.V. Ketamine for treatment-resistant depression: recent developments and clinical applications: Table 1. Evid. Based Ment. Health. 2016;19:35–38. doi: 10.1136/eb-2016-102355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnefeesi Y., et al. Real-world effectiveness of ketamine in treatment-resistant depression: A systematic review & meta-analysis. J. Psychiatr. Res. 2022;151:693–709. doi: 10.1016/j.jpsychires.2022.04.037. (Jul) [DOI] [PubMed] [Google Scholar]
- Gururajan A., et al. MicroRNAs as biomarkers for major depression: a role for let-7b and let-7c. Transl. Psychiatry. 2016;6 doi: 10.1038/tp.2016.131. e862–e862. e862–e862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster F.M., Traer C.J., Abraham S.M., Fry M.J. The phosphoinositide (PI) 3-kinase family. J. Cell Sci. 2003;116:3037–3040. doi: 10.1242/jcs.00609. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B., Vogt P.K., Rommel C. Springer; Berlin, Heidelberg: 2010. PI3K: From the Bench to the Clinic and Back; pp. 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rückle T., Schwarz M.K., Rommel C. PI3Kγ inhibition: towards an ‘aspirin of the 21st century’? Nat. Rev. Drug Discov. 2006;5:903–918. doi: 10.1038/nrd2145. [DOI] [PubMed] [Google Scholar]
- Park S.W., et al. Differential effects of antidepressant drugs on mTOR signalling in rat hippocampal neurons. Int J. Neuropsychopharmacol. 2014;17:1831–1846. doi: 10.1017/S1461145714000534. [DOI] [PubMed] [Google Scholar]
- Fukumoto K., Iijima M., Funakoshi T., Chaki S. Role of 5-HT1A Receptor Stimulation in the Medial Prefrontal Cortex in the Sustained Antidepressant Effects of Ketamine. Int. J. Neuropsychopharmacol. 2018;21:371–381. doi: 10.1093/ijnp/pyx116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., et al. Akt Mediates GSK-3β Phosphorylation in the Rat Prefrontal Cortex during the Process of Ketamine Exerting Rapid Antidepressant Actions. Neuroimmunomodulation. 2014;21:183–188. doi: 10.1159/000356517. [DOI] [PubMed] [Google Scholar]
- Price R.B., et al. International pooled patient-level meta-analysis of ketamine infusion for depression: In search of clinical moderators. Mol. Psychiatry 2022 27. 2022;12 27:5096–5112. doi: 10.1038/s41380-022-01757-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehiko Sasaki, et al. Function of PI3Kγ in Thymocyte Development, T Cell Activation, and Neutrophil Migration. Science 2000. 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- Petit-Demouliere B., Chenu F., Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacol. (Berl. ) 2005;177(3):245–255. doi: 10.1007/s00213-004-2048-7. (Jan) [DOI] [PubMed] [Google Scholar]
- Ribeiro M.A., Aguiar R.P., Scarante F.F., Fusse E.J., de Oliveira R.M.W., Guimaraes FS F.S., Campos A.C. Spontaneous Activity of CB2 Receptors Attenuates Stress-Induced Behavioral and Neuroplastic Deficits in Male Mice. Front Pharm. 2022 doi: 10.3389/fphar.2021.805758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibenhener M.L., Wooten M.C. Use of the Open Field Maze to Measure Locomotor and Anxiety-like Behavior in Mice. JoVE (J. Vis. Exp. ) 2015;e52434 doi: 10.3791/52434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armario A. The forced swim test: Historical, conceptual and methodological considerations and its relationship with individual behavioral traits. Neurosci. Biobehav Rev. 2021;128:74–86. doi: 10.1016/j.neubiorev.2021.06.014. (Sep) [DOI] [PubMed] [Google Scholar]
- Lima I.V. de A., et al. Antidepressant-like effect of valproic acid—Possible involvement of PI3K/Akt/mTOR pathway. Behav. Brain Res. 2017;329:166–171. doi: 10.1016/j.bbr.2017.04.015. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P.J., Yen J.Y., Watson B.O. Stress-sensitive antidepressant-like effects of ketamine in the mouse forced swim test. PLoS One. 2019;14(4) doi: 10.1371/journal.pone.0215554. Apr 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan J.F., Holmes A. Model organisms: The ascent of mouse: Advances in modelling human depression and anxiety. Nat. Rev. Drug Discov. 2005 doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- Fukumoto K., Iijima M., Funakoshi T., Chaki S. 5-HT1A receptor stimulation in the medial prefrontal cortex mediates the antidepressant effects of mGlu2/3 receptor antagonist in mice. Neuropharmacology. 2018;137:96–103. doi: 10.1016/j.neuropharm.2018.05.001. [DOI] [PubMed] [Google Scholar]
- Nürnberg B., Beer-Hammer S. Function, Regulation and Biological Roles of PI3Kγ Variants. Biomolecules. 2019;9:427. doi: 10.3390/biom9090427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neis V.B., et al. The involvement of PI3K/Akt/mTOR/GSK3β signaling pathways in the antidepressant-like effect of AZD6765. Pharm. Biochem Behav. 2020;198 doi: 10.1016/j.pbb.2020.173020. [DOI] [PubMed] [Google Scholar]
- Lim D.H., et al. PI3K7-deficient mice have reduced levels of allergen-induced eosinophilic inflammation and airway remodeling. Am. J. Physiol. Lung Cell Mol. Physiol. 2009;296:210–219. doi: 10.1152/ajplung.90275.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., et al. Essential role of microglial transforming growth factor-β1 in antidepressant actions of (R)-ketamine and the novel antidepressant TGF-β1. Transl. Psychiatry. 2020;10:32. doi: 10.1038/s41398-020-0733-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A.J., et al. Human PI3Kγ deficiency and its microbiota-dependent mouse model reveal immunodeficiency and tissue immunopathology. Nat. Commun. 2019;10:4364. doi: 10.1038/s41467-019-12311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., et al. Plasma inflammatory cytokines and treatment-resistant depression with comorbid pain: improvement by ketamine. J. Neuroinflamm. 2021;18:200. doi: 10.1186/s12974-021-02245-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y., et al. Alterations of multiple peripheral inflammatory cytokine levels after repeated ketamine infusions in major depressive disorder. Transl. Psychiatry. 2020;10:246. doi: 10.1038/s41398-020-00933-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak W., et al. Pro-inflammatory monocyte profile in patients with major depressive disorder and suicide behaviour and how ketamine induces anti-inflammatory M2 macrophages by NMDAR and mTOR. EBioMedicine. 2019;50:290–305. doi: 10.1016/j.ebiom.2019.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E., Song L., Jope R.S. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol. Psychiatry. 2011;16:1068–1070. doi: 10.1038/mp.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston R.G., Fitzgerald P.J., Watson B.O. Repeated Dosing of Ketamine in the Forced Swim Test: Are Multiple Shots Better Than One? Front Psychiatry. 2021;12 doi: 10.3389/fpsyt.2021.659052. May 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.