Abstract
BACKGROUND
Although loss of muscle mass may be associated with general weakness, intolerance to physical activity and fatigue, it is underestimated and poorly understood in patients with sarcoidosis.
AIM
To compare the quadriceps femoris muscle (QFM) thickness measured by ultrasonography (US) between the female patients with sarcoidosis and controls, secondly to assess the correlation between the muscle strength, fatigue and QFM thickness.
DESIGN
Observational, case-control study.
SETTING
Physical Medicine and Rehabilitation Department of a University Hospital.
POPULATION
Thirty-one women with sarcoidosis and 27 healthy volunteers were included in the study.
METHODS
The participants were evaluated for the following outcomes: 1) handgrip strength; 2) QFM thickness measured using US; and 3) sonographic thigh adjustment ratio (STAR). The sarcoidosis group was also evaluated with the 30-second chair stand test (30s-CST) and Fatigue Severity Scale (FSS).
RESULTS
The QFM thickness and STAR values of the patients with sarcoidosis were significantly lower than those of the controls (P=0.0001). However, no statistically significant difference was observed between the handgrip strengths of the groups (P=0.581). There was no statistically significant correlation between the STAR values and handgrip strength in the sarcoidosis group; however, there was a significant positive correlation between the STAR values and 30s-CST (r=0.467, P=0.008).
CONCLUSIONS
Loss of muscle mass is one of the musculoskeletal conditions in patients with sarcoidosis that may be associated with nonspecific symptoms, such as general debility, intolerance to physical activity, and fatigue. In the present study, no difference was observed in hand grip strength between the groups, while we found that QFM thickness was affected in patients with sarcoidosis when compared to the controls. The ultrasonographic QFM evaluation seems to be an innovative tool which may be used at all stages of sarcoidosis patient follow-up.
CLINICAL REHABILITATION IMPACT
The grip strength is a commonly used test to detect muscle weakness, but onset of a decrease in muscle mass in the lower extremities may occur earlier. Considering the increased burden of musculoskeletal problems in this population, performing 30s-CST and sonographic QFM thickness is practical methods to identify risky patients.
Key words: Sarcoidosis, Quadriceps muscle, Ultrasonography
Sarcoidosis is a multi-organ disorder described by the existence of noncaseating granulomas in the affected tissues. The clinical course of the disease is variable with a possibility to affect any of the systems.1, 2 The subclinical involvement of the musculoskeletal system is estimated to occur in 50-80% of individuals with sarcoidosis.3 Loss of muscle mass and sarcopenia are often neglected issues, attributed to aging and rarely suspected in individuals with sarcoidosis, which often manifests at a younger age. Moreover, it is unclear how these overlooked conditions are linked to symptoms including general weakness, intolerance to physical activity, and fatigue, which are common in sarcoidosis.3, 4 Fatigue is a common problem affecting quality of life, it is still underestimated and poorly understood in patients with sarcoidosis.5 Fatigue does not correlate with pulmonary function test results.5, 6 Fatigue can be explained by peripheral muscle weakness and exercise intolerance, and both can be caused by multiple factors, such as skeletal muscle sarcoidosis, decreased lung function, impaired physical activity status and corticosteroid-induced myopathy.7 On the other hand, exercise intolerance and reduced muscle strength may occur in both fatigued and non-fatigued sarcoidosis patients.8
Sarcopenia is defined as an accentuated loss of both muscle mass and strength, especially in the elderly population.9 Moreover, it is a treatable precursor of negative adverse outcomes such as functional disability, cognitive impairment, depressive symptoms, and multimorbidity, especially in patients with chronic inflammatory disorders. There have been an increasing number of publications suggesting that, contrary to common belief, loss of muscle mass and strength may begin early in life. But associated factors remains unclear.9, 10 In this study, since our participants were relatively young, we aimed to examine the quadriceps femoris muscle (QFM) thickness measurements rather than diagnosing sarcopenia. Ultrasonography (US) is an easily accessible, affordable, and radiation-free diagnostic tool that has been widely used, particularly in the field of musculoskeletal disorders.11, 12 Sarcopenia guidelines suggest using US to diagnose sarcopenia and probable sarcopenia9 as it supplies data relevant to the evaluation of peripheral muscles. Additionally, it is quick, cost-effective, and simple and these advantages outweigh those of magnetic resonance imaging. Sonographic measurement of the QFM thickness appears to be a practical method for predicting sarcopenia. A systematic review of adult data revealed that US findings are valid and reliable for the measurements of anterior thigh region.13
In this study, first we aimed to compare the handgrip strength, QFM thickness, and sonographic thigh adjustment ratio (STAR) values between the control and study groups. Second, we aimed to determine the relationship between QFM thickness assessed using US with the clinical parameters, including handgrip strength, 30-second chair stand test (30s-CST), and fatigue severity scale (FSS) commonly used in patients with stable sarcoidosis who are referred to rehabilitation clinics.
Materials and methods
An observational case-control study was conducted on patients with stable sarcoidosis and healthy controls. All patients signed consent form prior to enrollment. The study adhered to the guidelines outlined in the Declaration of Helsinki and received approval from the hospital’s institutional review board (Istanbul University Cerrahpasa, E-83045809-604.01.01-439452).
All patients had a clinical or radiographical finding expected in sarcoidosis, and a biopsy that demonstrated noncaseating granulomas in at least two of the affected organs. All patients were women with normal serum angiotensin-converting enzyme and calcium levels. They were clinically asymptomatic in terms of sarcoidosis organ involvement. After the pulmonologist’s assessment, providing that their disease was stable, the patients were included in the study. Patients with endocrine, renal or metabolic comorbidities, disabling diseases, those who had to be hospitalized for any reason within the previous 3 months, or those undergoing immunosuppresive treatment were excluded. Patients who had received corticosteroids in the last 6 months were also excluded. The healthy volunteers were women in similar age group without chronic respiratory, cardiac, renal or endocrine disorders. Individuals who had neurological/ musculoskeletal conditions which may cause disabilities were not included in the control group.
The main outcome variables were as follows.
Muscle ultrasonography
Transverse images were obtained using a B-mode ultrasound device (ESAOTE My Lab 70) and a linear transducer (7-12 MHz). The anterior thigh muscle thickness of the dominant side was measured (Figure 1).
Figure 1.
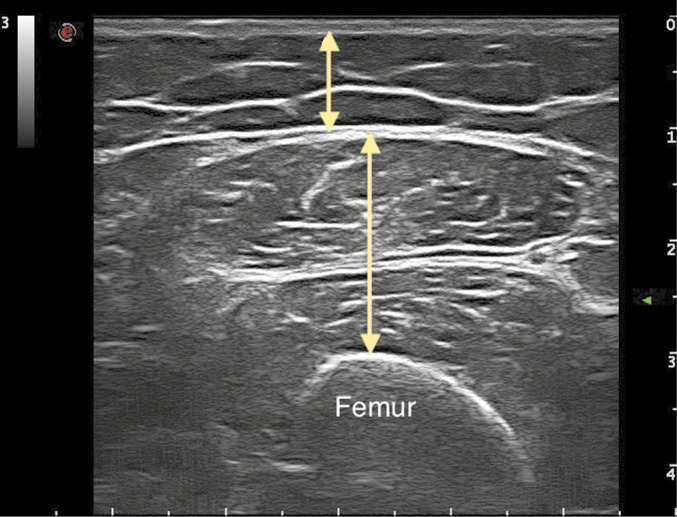
—Sonographic measurement of anterior thigh muscles. Short arrow, subcutaneous fat tissue thickness; long arrow, quadriceps femoris muscle thickness.
The patient was positioned supine with their knees fully extended during measurement. The probe was placed at a 90° angle to the skin at the measuring site, the appropriate amount of transmission gel applied without compression. The thickness of the subcutaneous fat tissue and QFM were measured. The STAR was determined by dividing the muscle thickness values of the patients by their Body Mass Index (BMI).14
Muscle strength
Hand grip strength test was performed in a seated position using a Jamar dynamometer. Three repetitive measurements were taken from the dominant hand in the position elbow flexed at a 90° angle as recommended by Roberts et al.15 Then, the average measurement was noted. In the 30s-CST, after hearing the command to ‘go’ participants were instructed to transition as quickly as they could from a seated position to a standing position with straight legs and a straight back, followed by a return to sitting position. Before the test, the investigator gave each participant instructions, a demonstration, and a 10-second practice. The investigators instructed participant to exert their maximum performance during the test. The participants were allowed a short rest period prior to test.
Fatigue
Fatigue was assessed using the FSS, a unidimensional, nine-item questionnaire that collects data about the effect and degree of the fatigue. Using response anchors of 1 point for strongly disagreeing and 7 points for strongly agreeing based on the prior week, participants assessed the nine questions on a 7-point Likert-type scale.16 Patients with a score of ≥4 were described as those suffering from significant fatigue. The translation of the FSS into Turkish language as performed according to international standards.17
Statistical analysis
The sample size was calculated to be 26 participants for each group could provide 80% power with an α value of 0.05 using the G*Power version 3.1 software. Statistical analyses were performed using the Number Cruncher Statistical System 2007 Statistical Software (Utah, USA). During the data evaluation, in addition to descriptive statistical methods (mean and standard deviation), the Shapiro-Wilk Normality Test was used to analyze the variables. An independent t-test was used for comparisons of variables with normal distribution. Pearson’s correlation analysis was used to determine the correlations. The results were assessed at a significance level of P<0.05.
Data availability
The data associated with the paper are not publicly available but are available from the corresponding author on reasonable request.
Results
Thirty-one patients with sarcoidosis and 27 healthy controls were included in the study. The mean age was 49.9±10.0 in the sarcoidosis group and 54.5±7.1 in the control group. Both groups consisted of the female gender. No statistically significant difference was observed between the mean age and mean BMI values of the sarcoidosis and control groups (P=0.063 and P=0.161). Additionally, no statistically significant difference was observed between the mean handgrip strength scores of the control and sarcoidosis groups (P=0.581). However, the mean QFM thickness and STAR values of the sarcoidosis group were significantly lower than those of the control group (P=0.0001 and P=0.008) (Table I).
Table I. —The distribution of the demographical and clinical features among groups.
| Characteristics | Control group (N.=27) |
Sarcoidosis group (N.=31) |
P value* |
|---|---|---|---|
| Age | 54.56±7.16 | 49.97±10.04 | 0.063 |
| BMI | 31.14±5.32 | 29.19±5.10 | 0.161 |
| Dominant hand grip strength (kg) | 26.67±4.60 | 27.53±6.87 | 0.581 |
| Dominant QFM thickness (mm) | 38.94±8.19 | 31.08±7.01 | 0.0001 |
| STAR (QFM thickness/BMI) | 1.27±0.24 | 1.08±0.29 | 0.008 |
*Independent t-test. BMI: Body Mass Index; QFM: quadriceps femoris muscle; STAR: sonographic thigh adjustment ratio.
An overview of the sarcoidosis group characteristics is depicted in Figure 2. There were no clinical or demographic differences observed according to the radiological stage of the patients, COVID history, or presence of extrapulmonary involvement in the subgroup analyses of the study group (P>0.05). Dominant handgrip strength values were normal in all patients but the 30s-CST values were impaired in 11 patients.
Figure 2.
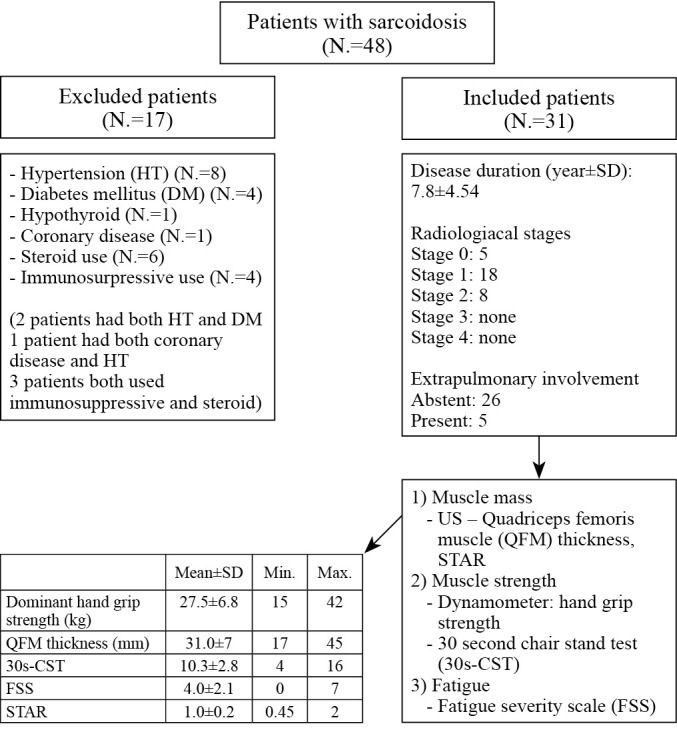
—Flow chart of the sarcoidosis group characteristics and recruitment process.
A significant correlation was found between the 30s-CST and dominant handgrip strength values in the sarcoidosis group (r=0.56, P=0.001). No statistically significant correlation was observed between the sonographic measurements and handgrip strength values (P>0.05). However, a statistically significant positive correlation was observed between the STAR and 30s-CST values (r=0.46, P=0.008). A statistically significant negative correlation was also observed between the 30s-CST and FSS values (r=-0.48, P=0.006) (Table II).
Table II. —The correlation analyzes of the sarcoidosis group.
| Parameter | 30s-CST | FSS | STAR |
|---|---|---|---|
| Dominant hand grip strength (kg) | |||
| r | 0.567 | -0.565 | 0.314 |
| P value | 0.001 | 0.001 | 0.085 |
| Dominant QFM thickness (mm) | |||
| r | 0.269 | -0.003 | 0.634 |
| P value | 0.143 | 0.989 | 0.0001 |
| 30s-CST | |||
| r | 1 | -0.482 | 0.467 |
| P value | 0.006 | 0.008 | |
| FSS | |||
| r | -0.482 | 1 | -0.276 |
| P value | 0.006 | 0.133 |
Pearson correlation test. 30s-CST: 30-second chair stand test; FSS: Fatigue Severity Scale; QFM: quadriceps femoris muscle; STAR: sonographic thigh adjustment ratio.
A statistically significant negative correlation was observed between the age and 30s-CST values (r=-0.55, P=0.001). No other significant correlation was observed between the demographic and clinical parameters (P>0.05) (Table III). When the patients were divided based on the radiological involvement pattern, no difference was observed in clinical and ultrasonographic findings (P>0.05) (Table IV).
Table III. —The correlation analyzes of the demographic and clinical features.
| Parameter | Age | BMI | Disease duration |
|---|---|---|---|
| Dominant hand grip strength (kg) | |||
| r | -0.324 | -0.117 | -0.269 |
| P value | 0.075 | 0.53 | 0.144 |
| Dominant QFM thickness (mm) | |||
| r | -0.246 | 0.078 | 0.233 |
| P value | 0.182 | 0.677 | 0.206 |
| 30s-CST | |||
| r | -0.559 | -0.151 | -0.209 |
| P value | 0.001 | 0.417 | 0.259 |
| FSS | |||
| r | 0.054 | 0.326 | 0.016 |
| P value | 0.775 | 0.073 | 0.931 |
Pearson correlation test. BMI: Body Mass Index; 30s-CST: 30-second chair stand test; FSS: Fatigue Severity Scale; QFM: quadriceps femoris muscle; STAR: sonographic thigh adjustment ratio.
Table IV. —Comparison of clinical and ultrasonographic measurements of patients according to radiological involvement.
| Measurement | Parenchymal involvement (N.=5) | Mediastinal involvement (N.=18) | Parenchymal and mediastinal involvement (N.=8) | P value* |
|---|---|---|---|---|
| Dominant hand grip strength (kg) | 28.4±6.84 | 26.14±6.3 | 30.13±8.13 | 0.389 |
| Dominant QFM thickness (mm) | 31.74±7.86 | 31.49±6.99 | 29.73±7.27 | 0.826 |
| 30s-CST | 10.2±2.17 | 11±2.66 | 9±3.46 | 0.263 |
| FSS | 3.88±2.47 | 3.81±1.91 | 4.68±2.54 | 0.634 |
| STAR | 1.02±2.25 | 1.09±0.34 | 1.05±0.19 | 0.846 |
QFM: Quadriceps femoris muscle, 30s-CST: 30 second chair stand test, FSS: Fatigue severity scale, STAR: Sonographic thigh adjustment ratio. *One-way analysis of variance.
Discussion
Sarcoidosis is a chronic disease that tends to affect the musculoskeletal system subclinically at the onset of the disease. Although weakness, fatigue, and intolerance to exercise are common symptoms in patients with sarcoidosis, there is limited data available to assess the muscle mass in these patients.3, 5, 7 In this study, we found that the sarcoidosis group’s mean QFM thickness and STAR values were significantly lower than the controls. While, there was no statistically significant difference observed between the mean handgrip strength scores of the sarcoidosis group and controls. Despite being a regularly used diagnostic test, the handgrip strength may not reveal obvious signs of the sarcopenia at early stages. Generally, the onset of a decrease in grip strength and knee extensor strength does not occur simultaneously; grip strength is known to be affected later.18 In our study, the patients were relatively young and active with normal handgrip strength scores that similar to those of the control group. However, QFM thickness and STAR values were impaired. Our results are consistent with the hypothesis loss of muscle mass occurs earlier in the lower extremities.
Sarcopenia has been considered an advanced form of senility that only strikes the elderly. Despite the common belief, loss of muscle mass and strength may begin in the early stages of life.9 The underlying process of this disorder which becomes clinically overt during the 5th-7th decades of life may start as early as during the third decade as judged by decreases in muscle strength and power. Age-related neurodegeneration, alterations in protein metabolism, and widespread loss of muscle fiber contribute to sarcopenia.19 Also, when there is a clear contributing factor other than aging, such as nutritional deficits, metabolic syndrome, endocrine, cardiopulmonary or inflammatory disorders may lead to loss of muscle mass, strength and power.20 We aimed to investigate reduced muscle mass in sarcoidosis which is a rare, chronic, inflammatory condition and that has not received much attention in the literature.
Strasser et al. showed that the measurement of QFM thickness is a reliable method to assess sarcopenia / probable sarcopenia.21 In a recent study, Eşme et al. analyzed the capacity of muscle thickness measurements to foresee sarcopenia in patients with sarcoidosis. They found that the use of US was more effective than the application of bioelectrical impedance analysis.22 Cruz et al. and Fuentes et al. observed a positive correlation between exercise performance and sonographic muscle thickness in patients with chronic obstructive pulmonary disease.23, 24 The current study is the only controlled study in the literature, that reported muscle mass evaluated sonographically in patients with sarcoidosis.
In our study while we could not find a correlation between QFM thickness and 30s-CST or FSS, there was a correlation between 30s CST and STAR. Body mass adjustment of QFM thickness seems like a useful method in terms of height and weight may have an impact on muscle mass.14 Neuromuscular changes with aging may reduce the number of motor fibers in the lower extremities.25 Moreover, the muscle filament loss starts earlier, particularly in the anterior thigh muscles rather than the upper extremity muscles.26 Our results support the idea that loss of muscle mass may similarly start in the lower extremities in chronic systemic diseases. In rehabilitation clinics, physical performance tests are usually performed on patients with chronic inflammatory diseases. Muscle power is the most quickly deteriorated value and is related more strongly to mobility than to strength, however its assessment needs specialized tools.27 Besides the traditional performance tests, measuring the sonographic muscle mass seems to be a practical method in both screening and follow-up of vulnerable patients.
Patients with sarcoidosis frequently have an intolerance to physical activity, generalized weakness, and fatigue. There are few researches on this subject among patients with sarcoidosis in the literature, and the majority of the studies had small sample sizes or sarcoidosis patients with specific conditions.1, 3, 22, 23 Yet, the main contributors of these physical limitations and their interrelationships remain unclear. Exercise intolerance in chronic cardiac and pulmonary conditions is considerably exacerbated by the dysfunction of the skeletal muscles. Spruit et al. reported the presence of skeletal muscle weakness in patients with sarcoidosis who complain of fatigue. They found some relations in these patients between skeletal muscle weakness, decreased physical capacity, and health status.7 Our study also found a statistically significant negative correlation between the 30s-CST and FSS values and also between the handgrip strength and FSS values. But, no significant correlation was found between STAR and FSS. Although fatigue is a prevalent condition that affects quality of life, it is largely underappreciated and little understood in sarcoidosis patients. The etiology of fatigue in sarcoidosis is most probably multifactorial including cardiopulmonary function status, general inflammation, sleeping disorders, depression and small-fiber neuropathy.5 Additional contribution of such factors, as well as a small sample size, could explain why we were unable to discover a correlation between STAR and FSS.
Limitations of the study
This study has several limitations that various subgroup analyzes could be performed on predictive factors, but our sample size was relatively small for subgroup investigations. Another limitation was that we did not apply the 30s-CST test to healthy controls. Using a nutritional index would also be appropriate for cases. Since the majority of the individuals in the patient group were at an early stage, we are unable to extrapolate our findings to all patients, including patients with severe sarcoidosis. However, the increase in disease severity may accompany comorbidities and increase in steroid use which could also be a separate bias. Randomized controlled trials including more participants are still required for this topic.
Conclusions
In conclusion, sarcoidosis is a heterogeneous, multi-organ disease with numerous nonspecific symptoms that may go beyond typical clinical presentation encountered by physicians. Clinicians must be aware that patients with sarcoidosis may experience muscle weakness, intolerance to activity, and a decline in health status, thus necessitating a multidisciplinary approach including rehabilitation program. In current study, we found that the sarcoidosis group’s mean QFM thickness and STAR values were significantly lower than the age-matched controls. Considering the increased burden of musculoskeletal problems in this population, performing adequate sonographic measurements seem a practical approach to identify patients at risk for sarcopenia or probable sarcopenia. Furthermore, randomized controlled interventional studies are needed for determining the effect of early exercise programs in patients with sarcoidosis.
Footnotes
Conflicts of interest: The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
References
- 1.Nessrine A, Zahra AF, Taoufik H. Musculoskeletal involvement in sarcoidosis. J Bras Pneumol 2014;40:175–82. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24831403&dopt=Abstract 10.1590/S1806-37132014000200012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hutchinson J. Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999;160:736–55. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10430755&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 3.Fayad F, Lioté F, Berenbaum F, Orcel P, Bardin T. Muscle involvement in sarcoidosis: a retrospective and followup studies. J Rheumatol 2006;33:98–103. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16395757&dopt=Abstract [PubMed] [Google Scholar]
- 4.Gea J, Casadevall C, Pascual S, Orozco-Levi M, Barreiro E. Respiratory diseases and muscle dysfunction. Expert Rev Respir Med 2012;6:75–90. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22283581&dopt=Abstract 10.1586/ers.11.81 [DOI] [PubMed] [Google Scholar]
- 5.De Vries J, Rothkrantz-Kos S, van Dieijen-Visser MP, Drent M. The relationship between fatigue and clinical parameters in pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2004;21:127–36. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15281434&dopt=Abstract [PubMed] [Google Scholar]
- 6.Drent M, Wirnsberger RM, de Vries J, van Dieijen-Visser MP, Wouters EF, Schols AM. Association of fatigue with an acute phase response in sarcoidosis. Eur Respir J 1999;13:718–22. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10362029&dopt=Abstract 10.1034/j.1399-3003.1999.13d03.x [DOI] [PubMed] [Google Scholar]
- 7.Spruit MA, Thomeer MJ, Gosselink R, Troosters T, Kasran A, Debrock AJ, et al. Skeletal muscle weakness in patients with sarcoidosis and its relationship with exercise intolerance and reduced health status. Thorax 2005;60:32–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15618580&dopt=Abstract 10.1136/thx.2004.022244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcellis RG, Lenssen AF, Elfferich MD, De Vries J, Kassim S, Foerster K, et al. Exercise capacity, muscle strength and fatigue in sarcoidosis. Eur Respir J 2011;38:628–34. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21436356&dopt=Abstract 10.1183/09031936.00117710 [DOI] [PubMed] [Google Scholar]
- 9.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. ; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30312372&dopt=Abstract 10.1093/ageing/afy169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sayer AA, Syddall H, Martin H, Patel H, Baylis D, Cooper C. The developmental origins of sarcopenia. J Nutr Health Aging 2008;12:427–32. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18615224&dopt=Abstract 10.1007/BF02982703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Correa-de-Araujo R, Harris-Love MO, Miljkovic I, Fragala MS, Anthony BW, Manini TM. The need of standardized assessment of muscle quality in skeletal muscle function deficit and other aging-related muscle dysfunctions: a symposium report. Front Physiol 2017;8:87. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28261109&dopt=Abstract 10.3389/fphys.2017.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ticinesi A, Meschi T, Narici MV, Lauretani F, Maggio M. Muscle ultrasound and sarcopenia in older individuals: a clinical perspective. J Am Med Dir Assoc 2017;18:290–300. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28202349&dopt=Abstract 10.1016/j.jamda.2016.11.013 [DOI] [PubMed] [Google Scholar]
- 13.Nijholt W, Scafoglieri A, Jager-Wittenaar H, Hobbelen JS, van der Schans CP. The reliability and validity of ultrasound to quantify muscles in older adults: a systematic review. J Cachexia Sarcopenia Muscle 2017;8:702–12. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28703496&dopt=Abstract 10.1002/jcsm.12210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kara M, Kaymak B, Ata AM, Özkal Ö, Kara Ö, Baki A, et al. STAR—sonographic thigh adjustment ratio: a golden formula for the diagnosis of sarcopenia. Am J Phys Med Rehabil 2020;99:902–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32941253&dopt=Abstract 10.1097/PHM.0000000000001439 [DOI] [PubMed] [Google Scholar]
- 15.Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing 2011;40:423–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21624928&dopt=Abstract 10.1093/ageing/afr051 [DOI] [PubMed] [Google Scholar]
- 16.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989;46:1121–3. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2803071&dopt=Abstract 10.1001/archneur.1989.00520460115022 [DOI] [PubMed] [Google Scholar]
- 17.Gencay-Can A, Can SS. Validation of the Turkish version of the fatigue severity scale in patients with fibromyalgia. Rheumatol Int 2012;32:27–31. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20658235&dopt=Abstract 10.1007/s00296-010-1558-3 [DOI] [PubMed] [Google Scholar]
- 18.Johansson J, Strand BH, Morseth B, Hopstock LA, Grimsgaard S. Differences in sarcopenia prevalence between upper-body and lower-body based EWGSOP2 muscle strength criteria: the Tromsø study 2015-2016. BMC Geriatr 2020;20:461. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33172391&dopt=Abstract 10.1186/s12877-020-01860-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int 2010;21:543–59. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19779761&dopt=Abstract 10.1007/s00198-009-1059-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas DR. Loss of skeletal muscle mass in aging: examining the relationship of starvation, sarcopenia and cachexia. Clin Nutr 2007;26:389–99. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17499396&dopt=Abstract 10.1016/j.clnu.2007.03.008 [DOI] [PubMed] [Google Scholar]
- 21.Strasser EM, Draskovits T, Praschak M, Quittan M, Graf A. Association between ultrasound measurements of muscle thickness, pennation angle, echogenicity and skeletal muscle strength in the elderly. Age (Dordr) 2013;35:2377–88. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23456136&dopt=Abstract 10.1007/s11357-013-9517-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eşme M, Karcıoğlu O, Öncel A, Ayçiçek GŞ, Deniz O, Ulaşlı SS, et al. Ultrasound assessment of sarcopenia in patients with sarcoidosis. J Ultrasound Med 2022;41:951–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34268780&dopt=Abstract 10.1002/jum.15780 [DOI] [PubMed] [Google Scholar]
- 23.Cruz-Montecinos C, Guajardo-Rojas C, Montt E, Contreras-Briceño F, Torres-Castro R, Díaz O, et al. Sonographic measurement of the quadriceps muscle in patients with chronic obstructive pulmonary disease: functional and clinical implications. J Ultrasound Med 2016;35:2405–12. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27698182&dopt=Abstract 10.7863/ultra.15.11032 [DOI] [PubMed] [Google Scholar]
- 24.Ramírez-Fuentes C, Mínguez-Blasco P, Ostiz F, Sánchez-Rodríguez D, Messaggi-Sartor M, Macías R, et al. Ultrasound assessment of rectus femoris muscle in rehabilitation patients with chronic obstructive pulmonary disease screened for sarcopenia: correlation of muscle size with quadriceps strength and fat-free mass. Eur Geriatr Med 2019;10:89–97. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32720275&dopt=Abstract 10.1007/s41999-018-0130-7 [DOI] [PubMed] [Google Scholar]
- 25.Abe T, Loenneke JP, Thiebaud RS, Ogawa M, Mitsukawa N. Age-related site-specific muscle loss in the thigh and zigzag walking performance in older men and women. Acta Physiol Hung 2014;101:488–95. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25201711&dopt=Abstract 10.1556/APhysiol.101.2014.006 [DOI] [PubMed] [Google Scholar]
- 26.Aagaard P, Suetta C, Caserotti P, Magnusson SP, Kjaer M. Role of the nervous system in sarcopenia and muscle atrophy with aging: strength training as a countermeasure. Scand J Med Sci Sports 2010;20:49–64. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20487503&dopt=Abstract 10.1111/j.1600-0838.2009.01084.x [DOI] [PubMed] [Google Scholar]
- 27.Bean RA, Bush KR, McKenry PC, Wilson SM. The impact of parental support, behavioral control, and psychological control on the academic achievement and self-esteem of African American and European American adolescents. J Adolesc Res 2003;18:523–41. 10.1177/0743558403255070 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data associated with the paper are not publicly available but are available from the corresponding author on reasonable request.


