Abstract
Lysozyme is a β-1,4-glycosidase that hydrolyzes the polysaccharide backbone of bacterial cell walls. With an additional bactericidal function mediated by a separate protein domain, lysozyme is considered a uniquely important antimicrobial molecule contributing to the host's innate immune response to infection. Elevated lysozyme production is found in various inflammatory conditions while patients with genetic risks for inflammatory bowel diseases demonstrate abnormal lysozyme expression, granule packaging, and secretion in Paneth cells. However, it remains unclear how a gain- or loss-of-function in host lysozyme may impact the host inflammatory responses to pathogenic infection. We challenged Lyz1−/− and ectopic Lyz1-expressing (Villin-Lyz1TG) mice with S. Typhimurium and then comprehensively assessed the inflammatory disease progression. We conducted proteomics analysis to identify molecules derived from human lysozyme-mediated processing of live Salmonella. We examined the barrier-impairing effects of these identified molecules in human intestinal epithelial cell monolayer and enteroids. Lyz1−/− mice are protected from infection in terms of morbidity, mortality, and barrier integrity, whereas Villin-Lyz1TG mice demonstrate exacerbated infection and inflammation. The growth and invasion of Salmonella in vitro are not affected by human or chicken lysozyme, whereas lysozyme encountering of live Salmonella stimulates the release of barrier-disrupting factors, InvE-sipC and Lpp1, which directly or indirectly impair the tight junctions. The direct engagement of host intestinal lysozyme with an enteric pathogen such as Salmonella promotes the release of virulence factors that are barrier-impairing and pro-inflammatory. Controlling lysozyme function may help alleviate the inflammatory progression.
Keywords: lysozyme, Salmonella Typhimurium, virulence, infection, intestine, barrier function, Lpp 1, sipC, InvE
Lysozyme, also known as muramidase, is a 14-kDa enzyme found in various mucosal tissues and secretions. Through catalytic cleavage of the β-1,4-glycosidic bond between the N-acetylmuramic acid and N-acetylglucosamide that form the peptidoglycan monomers, lysozyme promotes the hydrolysis of polysaccharide backbone of the bacterial cell wall. As a part of the innate immune system, lysozymes are categorized into the c-type (or chicken type), the g-type (goose type), and the i-type (invertebrate type) (1). The lysozyme in chicken egg white was the first extensively studied lysozyme at biochemical and functional levels. Along with the human lysozyme, both are c-type isoforms and share approximately 59% sequence identity. Biochemical analysis of chicken lysozyme revealed a helix-loop-helix bactericidal domain mediating its membrane-permeabilizing action (2, 3). The human lysozyme exhibits a higher antibacterial activity than the chicken isoform, and intriguingly, the bactericidal activity of lysozyme is structurally and functionally separate from its catalytic domain (4). Amino acids responsible for lysozyme’s enzymatic and bactericidal functions are evolutionarily conserved across animal species. These dual mechanistic actions of lysozymes uniquely distinguish them from the other antimicrobial peptides, as the majority of the latter do not possess enzymatic functions.
The human lysozyme is encoded by the LYZ gene on chromosome 12q15 and can be abundantly produced by Paneth cells that reside in the small intestinal epithelia as well as by innate immune cells such as macrophages and neutrophils. Mice have two lysozyme genes, Lyz1 and Lyz2, contributing to the lysozyme proteins predominantly found in the Paneth cells and the macrophages, respectively. Paneth cells are crypt-localized intestinal epithelial cells secreting lysozyme into the gut lumen where the commensal microbiota colonize (5). By genetically disrupting the Lyz1 gene in mice, we have previously reported that the basal Nod-like receptor microbial signaling pathway is weakened and the commensal bacterial landscape is altered (6). In Lyz1−/− mouse intestines, there are increased goblet and tuft cell populations, which are caused by an elevated basal type 2 immune response originating from the innate lymphocyte 2 in the lamina propria (6). Loss or gain of function in intestinal lysozyme in mice dampened or exacerbated dextran sulfate sodium-induced colitis, suggesting that proper control of the abundance of intestinal luminal lysozyme is important for inflammatory disease progression (6).
Elevated fecal lysozyme has been reported in patients with inflammatory bowel diseases (IBD) (7, 8, 9, 10, 11, 12). Interestingly, elevated lysozyme was observed in the colonic epithelia containing metaplastic Paneth-like cells in ulcerative colitis patients (13). Patients with IBD carrying ATG16L1 T300A, or NOD2 disease-associated alleles show abnormal lysozyme expression and packaging in their Paneth cells (14, 15). Mutation in a major IBD risk gene LRRK2 was shown to disrupt lysozyme packaging in the dense core secretory granules in Paneth cells (16). Given the reported bactericidal activity, these reports suggest a potential host response to diminish the adherence or colonization by opportunistic pathobiont or pathogenic bacteria at the intestinal epithelial surface.
In terms of lysozyme interaction with pathogenic bacteria, it was reported in mice that S. Typhimurium infection reduces lysozyme production in Paneth cells (17), and the infection stimulates Paneth cells to release lysozyme via a secretory autophagy pathway (5). However, it was unclear if a gain or loss of function in host lysozyme may impact the host–pathogen interaction thereby affecting the pathogenesis and inflammatory responses caused by infection. In this report, we performed in vivo S. Typhimurium infection studies and comprehensively assessed the inflammatory disease progression in genetically modified Lyz1−/− and Lyz1-overexpressing (Villin-Lyz1TG) mice. We found that increased intestinal lysozyme production exacerbates the morbidity and mortality following infection while diminishing intestinal lysozyme unexpectedly dampens the inflammatory progression triggered by the infection. In vivo, barrier function analysis demonstrated that mice with an elevated lysozyme production had increased gut permeability at steady-state and during infection, whereas Lyz1-deficient mice exhibit significantly elevated barrier functions. Although the growth and colonization of Salmonella are resistant to lysozyme in vitro and in vivo, proteomics and biochemical analysis revealed that engagement of lysozyme with Salmonella promoted the release of several known and poorly characterized virulence factors. We show that these factors impair the epithelial barrier via different mechanisms involving the activation of the Type Three Secretion System (T3SS) that builds translocons on the host cell membrane (18, 19, 20) as well as promoting epithelial cell TNFα production. Blocking lysozyme’s enzymatic action via inhibitor or denaturing temperature abolished its stimulation on the release of Salmonella virulence factor, attenuating the barrier impairment. Single-cell (sc)RNA-Seq analysis demonstrated that Salmonella infection robustly suppressed lysozyme RNA abundances in Paneth cells. We propose that the engagement of host intestinal lysozyme with an enteric pathogen such as Salmonella promotes the release of barrier-impairing factors; therefore, elevated or abnormal lysozyme production is pro-inflammatory during pathogenic bacterial infection.
Results
S. Typhimurium infection reduces lysozyme abundance in Paneth cells
Lysozyme is considered an antimicrobial peptide against bacterial infection (21). We first evaluated lysozyme expression in mice at homeostasis and after 108 CFU S. Typhimurium (SL1344) infection via oral gavage. We found that infection reduced the lysozyme expression in Paneth cells at protein (Fig. 1A) and RNA levels (Fig. 1B). The reduction was not due to loss of Paneth cells as dual RNA base-scope analysis revealed a reduction of Lyz1 (red) with a concomitant increase of Ang4 (blue), a member of the ribonuclease A superfamily having an antimicrobial activity (22), in the infected mouse crypts compared to control mice inoculated by PBS (Fig. 1B).
Figure 1.
S. Typhimurium infection reduces lysozyme in Paneth cells.A, immunohistochemistry for lysozyme in uninfected and Salmonella-infected mouse ileum. B, dual RNA base scope for Lyz1 (red) and Ang4 (blue) in uninfected and Salmonella-infected mouse ileum. C, Seurat UMAP analysis of the single-cell transcriptome of 16,530 Paneth cells flow-sorted from uninfected (red) and Salmonella-infected (blue) Lyz13′UTR-IRES-CreER; Rosa26R-dTomatoLSL mouse ileum. D and E, Seurat UMAP analysis for Lyz1 and Olfm4. F, UMAP shows the clusters. Clusters 1, 2, 4, 9, 10, and 14 are mature Paneth cells in uninfected mice. Clusters 0, 3, 5, and 13 are mature Paneth cells in infected mice. Cluster 12 is mature Paneth cells shared by the two groups. Cluster 6, 7, 8, 11, clusters of Paneth cells progenitors. G, dot plots for Lyz1 (boxed in red) and other antimicrobial genes across the mature Paneth cell clusters. H, violin plot for Lyz1 across the mature Paneth cell clusters. The length of all scale bars in (A and B) are 100 μm.
To test if infection changes Lyz1 transcript abundance in a subpopulation of Paneth cells, we compared the scRNA profiles of ileal Paneth cells (SAL versus PBS) flow-sorted 4-days post-infection (4 dpi) (Fig. 1C) (23). UMAP for Lyz1 demonstrates a reduced transcript abundance in Paneth cells of infected mice (compare SAL to PBS, Fig. 1D). UMAP for Olfm4 distinguishes progenitor from mature Paneth cells (Fig. 1E), and there was a relatively more pronounced Lyz1 reduction in the mature Paneth cells of infected mice (Fig. 1D). Within the 11 mature Paneth cell clusters (Olfm4-negative) (Fig. 1, E and F), differential gene analysis shows that Lyz1 is significantly reduced in SAL specific clusters 1, 2, 4, 9, 10, and 14, when compared to PBS clusters 0, 3, 5, and 13 (red box, Fig. 1G). Cluster 12 is shared by PBS and SAL Paneth cells, where cells with both low and high Lyz1 abundances can be observed in the violin plots (Fig. 1H). In contrast to Lyz1, mature SAL clusters showed elevated abundances of Reg3g, Mptx2, and Mmp7 (Fig. 1G), genes known to enrich in Paneth cells, especially during infection (23). Reg3g is a Paneth cell enriched antimicrobial peptide (24), Mptx2 is a Paneth cell mucosal pentraxin required for activation of lectin complement pathway (23, 25). Mmp7 is matrix metallopeptidase secreted by Paneth cells to process and activate pro-defensins (26). These data demonstrated an overall reduction of lysozyme in Paneth cells in response to infection, a change different from other Paneth cell-enriched antimicrobial genes.
Intestinal epithelial lysozyme overexpression exacerbates Salmonella-induced inflammation
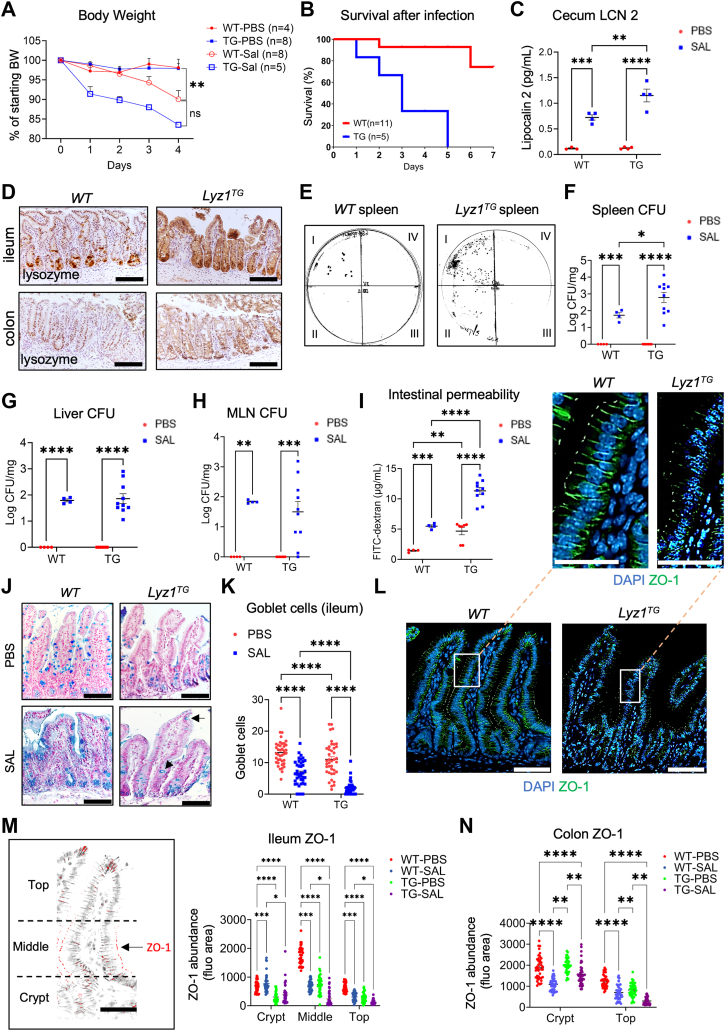
Within the mouse intestinal epithelium, Lyz1 expression is restricted to Paneth cells (27, 28, 29). A transgenic line Villin-Lyz1TG was previously developed by us to ectopically express lysozyme throughout the intestinal epithelial cells (6). To determine the impact of lysozyme overproduction on Salmonella infection, we inoculated S. Typhimurium (SL1344) to WT and Villin-Lyz1TG mice (all on C57BL/6 background). Upon infection, Villin-Lyz1TG mice demonstrated a significantly increased morbidity reflected by severe body weight loss (Fig. 2A), increased mortality (Fig. 2B), and elevated cecum lipocalin 2 (LCN2) (p = 0.0013, Fig. 2C), an indicator of inflammation (30). WT and Villin-Lyz1TG mice that were inoculated with sterile PBS did not show a difference in body weight change (Fig. 2A) or in cecum LCN2 abundance (Fig. 2C).
Figure 2.
Salmonella infection caused more pronounced inflammatory disease in Villin-Lyz1TGmice.A, mouse body weight change during 4 days post Salmonella infection. PBS or 108 CFU of Salmonella were orally inoculated into WT or Villin-Lyz1TG mice. Animal numbers for individual groups are specified in the graph. B, survival of WT or Villin-Lyz1TG mice during 4 days post Salmonella infection. All samples analyzed below were collected from the survival mice on day 4. C, cecum lipocalin-2 (LCN-2) abundances were measured by ELISA from uninfected and infected WT and Villin-Lyz1TG mice at sacrifice. D, immunohistochemistry for lysozyme in the infected distal ileum and colon in WT and Villin-Lyz1TG mice. E, Salmonella CFU analysis in spleens from infected WT and Villin-Lyz1TG mice at sacrifice. Every milligram of tissue was homogenized in 10 μl PBS. Region I represents a non-diluted homogenized tissue solution, while regions II to IV represent a 10-fold serial dilution of the previous tissue diluents. Each region had a triplicated 5μl-drop of the indicated dilutant. F–H, Salmonella CFUs in spleen, liver, and MLN of WT and Villin-Lyz1TG mice at sacrifice. Each data point represents a biological replicate. Log10 of the exact CFUs was presented. I, in vivo permeability assay. Serum FITC concentrations (μg/ml) for individual biological replicates are plotted for WT and Villin-Lyz1TG mice at sacrifice. J and K, representative alcian blue staining and quantification of goblet cells in the distal ileum of infected WT and Villin-Lyz1TG mice. Each data point represents a crypt-villus unit scored from 6 to 10 different regions per animal. Five animals per genotype were analyzed. L, representative immunofluorescent staining for ZO-1 in ileum of WT and Villin-Lyz1TG mice. M, quantitative analysis was based on different crypt-villus regions: crypt, middle of the villi, and villus tip, and obtained from uninfected and infected WT and Villin-Lyz1TG mice. N, quantitative of ZO-1 in different colonic regions of uninfected and infected WT and Villin-Lyz1TG mice. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. The length of scale bars in (D, J, L, and M) are 100 μm. The length of scale bars in the L zoom magnification panels is 50 μm.
Immunohistochemistry analysis demonstrated villus blunting in the ileum of infected Villin-Lyz1TG mice (Fig. 2D). Salmonella colony formation unit (CFU) analysis detected a significantly increased Salmonella dissemination to the spleen of infected Villin-Lyz1TG mice when compared to WT mice (p = 0.01, Fig. 2, E and F). Liver and intestinal mesenteric lymph node (MLN) of WT and Villin-Lyz1TG mice showed equivalent Salmonella CFUs in WT and Villin-Lyz1TG mice (Fig. 2, G and H), suggesting a preferential dissemination into the spleen of Villin-Lyz1TG mice.
To determine if Salmonella induced different degrees of intestinal barrier disruption in WT and Villin-Lyz1TG mice, we performed an in vivo gut permeability assay by gavaging FITC-dextran (60 mg/gram of BW) 4 h before euthanizing the mice for serum fluorescent analysis. Infected Villin-Lyz1TG mice demonstrated a significantly higher intestinal permeability than WT mice (Fig. 2I). Interestingly, uninfected Villin-Lyz1TG mice also exhibited a significantly elevated permeability than WT mice (p < 0.01, Fig. 2I), suggesting that the gut permeability in Villin-Lyz1TG mice was already elevated before Salmonella infection whereas the infection further exacerbated the barrier impairment.
Abnormal permeability could be due to goblet cell reduction and epithelial mucin deficiency. Therefore, we examined the goblet cell population in WT and Villin-Lyz1TG mice before and after infection. We found that in steady-state, Villin-Lyz1TG mice have 25% fewer goblet cells per villus than WT mice (Fig. 2, J and K). Salmonella infection further reduced goblet cells in Villin-Lyz1TG mice (Fig. 2, J and K). We noted a particularly diminished goblet cell population in the upper villus regions of infected Villin-Lyz1TG mice (arrowhead, Fig. 2J).
We also examined tight junction (TJ) markers as their integrity is related to barrier function. Importantly, under steady-state conditions, ZO-1 is reduced in the ileum (Fig. 2, L–M), but not in the colon (Fig. 2N), of Villin-Lyz1TG mice when compared to WT animals. After infection, there is a further reduction in ZO-1 in the ileal crypt, mid-villus, and upper villus epithelial regions (Fig. 2, L–M). Colonic epithelial ZO-1 was reduced in infected WT and Villin-Lyz1TG mice, but the difference between the genotypes is insignificant (Fig. 2N). These results suggest a basal reduction in epithelial TJ proteins in Villin-Lyz1TG mice, and this reduction was exacerbated by infection.
Lyz1 ablation protects against infection-induced gut inflammation
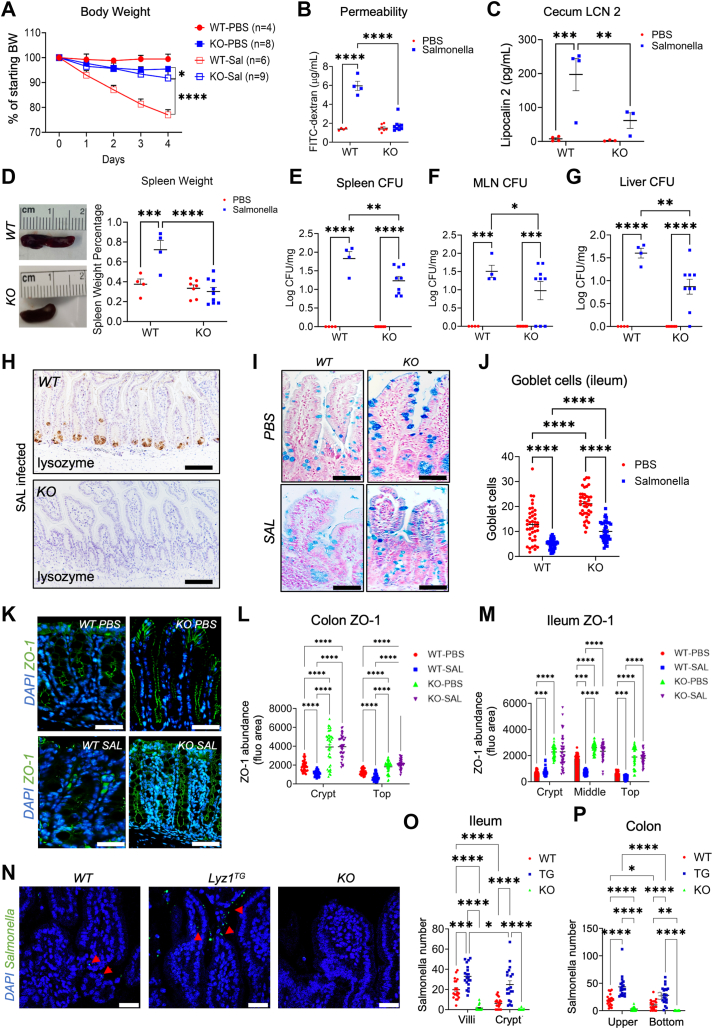
We then investigated how Lyz1−/− mice, which lack the intestinal lysozyme (6), would respond to Salmonella infection. We performed the same infection procedures in WT and Lyz1−/− littermate mice and monitored mouse body weight daily. Surprisingly, Lyz1−/− mice do not exhibit a noticeable body weight loss in response to infection, as shown by WT mice, which had a 20% body weight reduction by day 4 (Fig. 3A). No mice died in these experiments. The protection against body weight loss seen in infected Lyz1−/− mice was significant when compared to WT mice. FITC-dextran gut permeability analysis before sacrifice showed near total protection in infected Lyz1−/− mice (Fig. 3B). Cecum LCN2 abundances in infected Lyz1−/− mice were approximately 30% of WT mice (p = 0.009, Fig. 3C), while uninfected WT and Lyz1−/− mice showed similar cecum LCN2 abundances (Fig. 3C).
Figure 3.
Lyz1-deficient mice show attenuated inflammatory response to Salmonella infection.A, mouse body weight changes during the course of 4 days of Salmonella infection. PBS or 108 CFU of Salmonella were orally inoculated to WT or Lyz1−/− mice. B, intestinal permeability was measured as serum FITC-dextran concentration in uninfected and infected WT and Lyz1−/− mice. All samples analyzed below were collected from the survival mice on day 4. C, cecum LCN-2 was measured in uninfected and infected WT and Lyz1−/− mice at sacrifice. D, gross morphology of spleens and the percentage of spleen weights over body weights in uninfected and infected WT and Lyz1−/− mice. E–G, Salmonella CFUs in the spleen, MLN, and liver of uninfected and infected WT and Lyz1−/− mice. Log10 of the exact CFUs was presented. H, immunohistochemistry for lysozyme in infected WT and Lyz1−/− mouse ileum. I and J, representative alcian blue staining and goblet cell quantification in distal ileum in WT and Lyz1−/− mice. K, immunofluorescent staining for ZO-1 in distal colons of uninfected and infected WT and Lyz1−/− mice. L and M, quantification of ZO-1 in colon and ileum in uninfected and infected WT and Lyz1−/− mice. N, Salmonella staining in WT, Villin-Lyz1TG, and Lyz1−/− mice. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. The length of all scale bars in (H, I, K, and N) are 100 μm.
To assess the progression of the systemic infection, spleen weight, and Salmonella CFU in the spleen were examined in infected WT and Lyz1−/− mice at 4 d.p.i. The spleen-to-body weight ratio was 40% greater in WT mice than Lyz1−/− mice (Fig. 3D). The reduced spleen weights reflect decreased systemic inflammation, consistent with a reduced Salmonella CFU per milligram of spleen tissues in Lyz1−/− mice (Fig. 3E). Similarly, reduced CFUs were observed in Lyz1−/− MLNs and livers (Fig. 3, F and G), but to a less extent.
Histopathology analysis suggested a much normal-appearing Lyz1−/− intestinal epithelial morphology compared to WT mice (Fig. 3H), and a substantially increased number of goblet cells in Lyz1−/− mice before and after infection (Fig. 3, I and J). Colonic epithelial cell ZO-1 abundances in Lyz1−/− mice are also higher than in WT mice, before and after infection (Fig. 3, K–L). Salmonella infection reduced colonic ZO-1 in WT mice, but this reduction was not observed in Lyz1−/− mice (Fig. 3L). ZO-1 abundances were overall higher in Lyz1−/− ileal epithelial cells compared to WT mice (Fig. 3M).
As Villin-Lyz1TG and Lyz1−/− mice displayed contrasting phenotypes in response to Salmonella infection, we scored the visible number of invasive Salmonella in these mice (arrowheads in Fig. 3N). Compared to infected WT mice, Salmonella counts were elevated in Villin-Lyz1TG mice but drastically reduced in Lyz1−/− mice in both the ileum and colon (Fig. 3, O and P).
Gut microbiota accounts in part for the increased permeability in Villin-Lyz1TG mice at the steady state
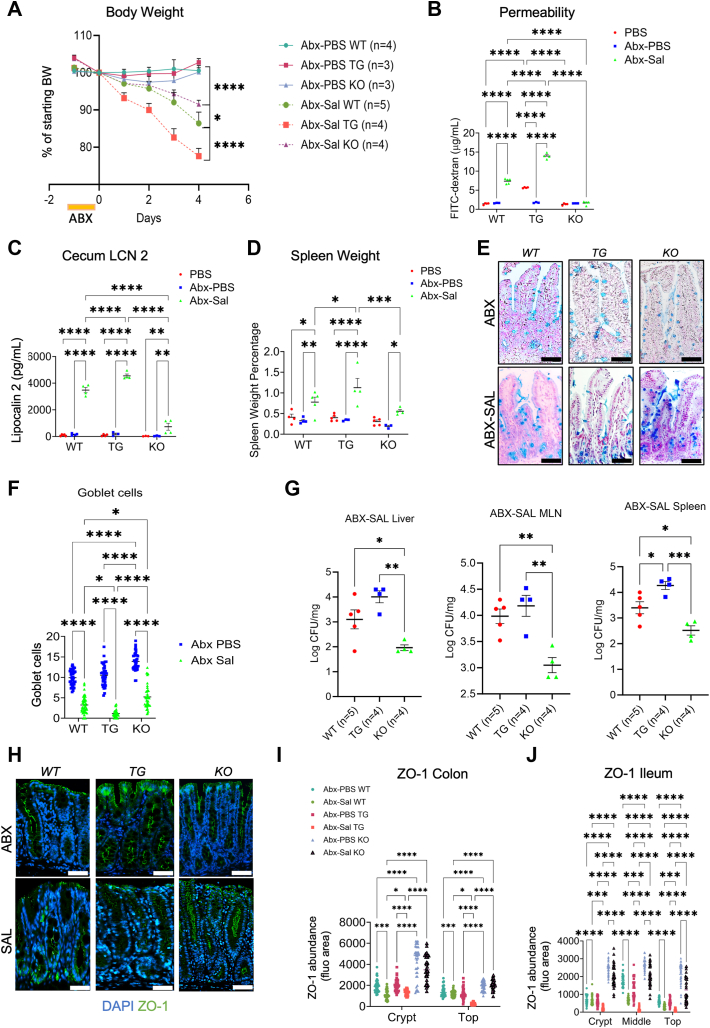
Gut microbiota landscapes are changed in Villin-Lyz1TG and Lyz1−/− mice (6). To assess whether the changed microbiota might have contributed to the observed infection susceptibility in Villin-Lyz1TG and Lyz1−/− mice, we administrated mice with antibiotics (Abx) in drinking water to disrupt the microbiota, then challenged these mice with oral Salmonella inoculation. Overall, Abx-pretreated Villin-Lyz1TG mice remained highly susceptible to infection, whereas Abx-pretreated Lyz1−/− mice remained protected from the infection, based on body weight changes following Salmonella challenge (p = 0.042, WT versus KO, Fig. 4A). However, Abx-pretreatment appeared to partially reduce the protection observed in Lyz1−/− mice, suggesting that the preexisting microbiota in these mice may have partially contributed to the protective role (Fig. 4A). Interestingly, gut permeability assay showed that the increased permeability in Villin-Lyz1TG mice was diminished by Abx pretreatment (compare red and blue for TG, Fig. 4B), suggesting that the preexisting gut microbiota in Villin-Lyz1TG mice to some extent was responsible for the elevated permeability at steady-state in these mice. However, upon challenge by Salmonella, the permeability was drastically elevated in these mice (compare blue to green for TG, Fig. 4B). In contrast, Lyz1−/− mice exhibited minimal changes in gut permeability in response to Abx treatment or to the infection (Fig. 4B). These responses were largely reflected in cecum LNC2 abundances (Fig. 4C), spleen weights (Fig. 4D), goblet cell counts (Fig. 4, E and F), and Salmonella CFU in liver, MLN, and spleen (Fig. 4G). Immunofluorescent analysis for ZO-1 in the colon and ileum showed that Abx-treated WT and Villin-Lyz1TG mice have similar ZO-1 abundances at steady states (Fig. 4, H–J). However, there was a significant infection-induced ZO-1 loss in Abx-pretreated WT and Villin-Lyz1TG mice; and this reduction was not seen in the Lyz1−/− mice (Fig. 4, H–J).
Figure 4.
Antibiotic treatment partially modifies Villin-Lyz1TGand Lyz1−/−mouse susceptibility to Salmonella infection.A, mouse body weight changes during 4 days of Salmonella infection. Mice were orally gavaged with antibiotics (Abx). After 1 day, mice were inoculated with Salmonella or PBS. All samples analyzed below were collected from the survival mice on day 4. B, intestinal permeability was measured based on serum FITC concentration at sacrifice. C, Cecum LCN-2 abundances in WT, Villin-Lyz1TG, and Lyz1−/− mice treated with PBS, Abx, or Abx plus Salmonella. D, the percentage of spleen over total body weights. E and F, alcian blue staining and quantification of goblet cells in the distal ileum of WT, Villin-Lyz1TG, and Lyz1−/− mice treated with Abx or Abx plus Salmonella. G, Salmonella CFUs in the liver, MLN, and spleen of infected WT, Villin-Lyz1TG, and Lyz1−/− mice pretreated with Abx. Log10 of the exact CFUs was presented. H, representative ZO-1 staining of colons of WT, Villin-Lyz1TG, and Lyz1−/− mice treated with Abx or with Abx plus Salmonella. I and J, quantifications of ZO-1 in the colon or ileum of the above mice. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. The length of all scale bars in (E and H) are 100 μm.
Thus, Abx treatment of Villin-Lyz1TG mice improved gut permeability at the steady state, suggesting that the increased lysozyme engagement with gut microbiota in these mice may elicit an enhanced epithelial permeability. However, in terms of Lyz1−/− mice, disrupting microbiota by antibiotics did not drastically alter the protection observed in these mice against disease phenotypes during infection. These data overall point to a pro-inflammatory role of intestinal lysozyme in Salmonella infection.
Direct processing of Salmonella by lysozyme produces barrier-disrupting molecules
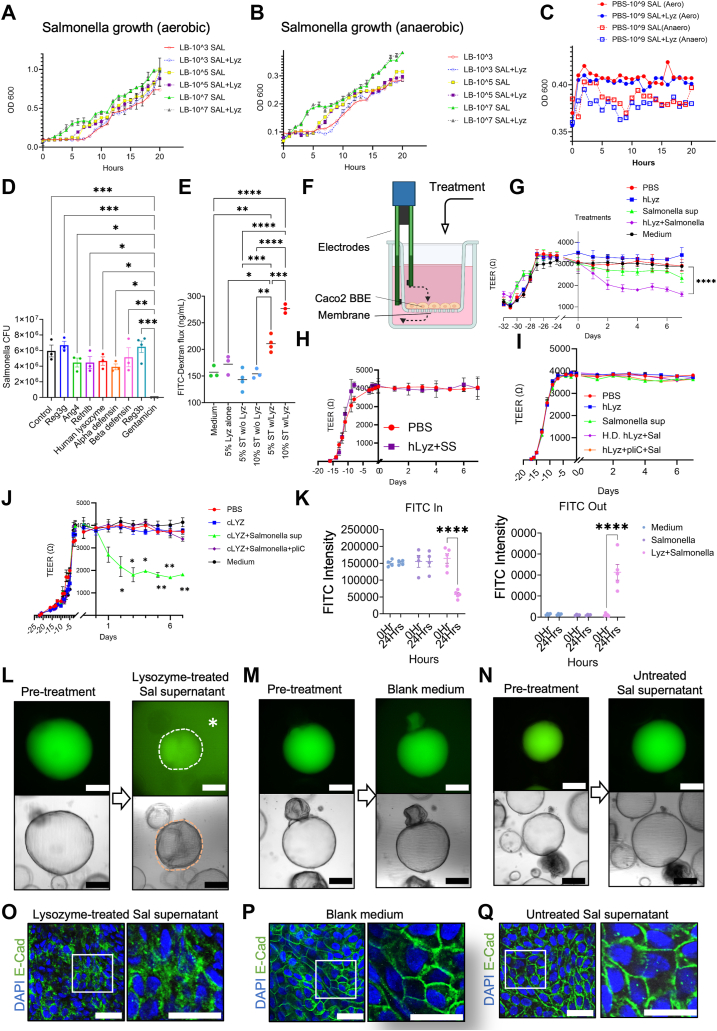
We tested in vitro if lysozyme affects Salmonella’s growth or invasiveness toward enterocytes. We plotted the growth curve of Salmonella under aerobic (Fig. 5A) and anaerobic (Fig. 5B) conditions over 20 h, in the presence or absence of human lysozyme. We used a lysozyme concentration of 20 μg/ml which is approximately 50 to 200 times of lysozyme concentrations in the secretions (31). Regardless of the initial seeding of Salmonella numbers, Salmonella grew at a similar rate in the presence or absence of lysozyme (Fig. 5, A and B). When we incubated 109 CFU Salmonella with the presence or absence of lysozyme in PBS, the Salmonella did not change throughout 20 h (Fig. 5C), suggesting that lysozyme did not kill Salmonella in PBS under aerobic or anaerobic conditions. These data suggest that human lysozyme does not affect Salmonella growth or death.
Figure 5.
Lysozyme-processed Salmonella produces barrier-disrupting molecules.A, Salmonella growth rate curve, reflected as OD600 readings, under aerobic conditions in the presence or absence of 20 μg/ml lysozyme. Cells were seeded at different numbers at initial time points. B, Salmonella growth rate curve, reflected as OD600 readings, under anaerobic conditions in the presence or absence of 20 μg/ml lysozyme. Cells were seeded at different numbers at initial time points. C, Salmonella was resuspended in PBS with or without lysozyme under aerobic or anaerobic conditions. OD600 readings were measured on a time course. D, Caco2 BBE cells were grown to differentiated monolayers and maintained for 2 weeks. Individual antimicrobial proteins were added for 15 min at concentrations described in Methods. Salmonella SL1344 was added to cells at a MOI of 100 and incubated with cells for 30 min to allow invasion at 37 °C, 5% CO2. Cells were then washed with 150 μg/ml gentamicin (Corning, #30-005-CR) for 50 min to remove extracellular bacteria. Cells were lysed and the intracellular Salmonella CFUs were determined from the lysates on XLD agar plates. E, Caco2 monolayers were seeded on trans-wells, and apical medium containing 10 mg/ml 4 kDa FITC-dextran (Sigma, FD4) was supplemented with 5 or 10% of filtered-sterilized LB broth from (i) 16 h Salmonella culture without lysozyme, (ii) LB broth with 4000 U/ml of human lysozyme without bacteria, (iii) conditioned medium from Salmonella culture treated with 4000 U/ml of human lysozyme for 16 h. F, Schematic diagram for TEER measurement. Treatments were applied to the apical side of the Caco2 BBE monolayer. G, TEER readings of monolayer Caco2 BBE in 96-well-plate before and after treatment with cell culture medium, PBS (vehicle), 0.2 μg/ml human lysozyme alone in PBS, 10% untreated Salmonella supernatant or supernatants from Salmonella treated by 0.2 μg/ml human lysozyme in PBS. H, TEER readings of monolayer Caco2 BBE in 96-well-plate before and after treatment with PBS (vehicle), or Salmonella supernatants (SS) preincubated with 0.2 μg/ml human lysozyme. I, TEER readings of monolayer Caco2 BBE in 96-well-plate before and after treatment with PBS, 0.2 μg/ml lysozyme alone in PBS, Salmonella supernatants, supernatant of lysozyme-treated Salmonella in the presence of 1 μg/ml pliC, supernatant from Salmonella treated by heat-denatured (H.D.) 0.2 μg/ml human lysozyme. J, TEER readings of monolayer Caco2 BBE in 96-well-plate before and after treatment with cell culture medium, PBS (vehicle), 0.2 μg/ml chicken lysozyme alone in PBS, 10% untreated Salmonella supernatant or supernatants from Salmonella treated by 0.2 μg/ml chicken lysozyme in PBS, supernatant of lysozyme-treated Salmonella in the presence of 1 μg/ml pliC. K, quantification of FITC fluorescent intensities inside and outside of enteroids with luminally injected FITC-dextran, and subsequently treated 24 h with blank Salmonella culture medium, Salmonella spent medium, and lysozyme-treated Salmonella medium supernatants. L–N, mature enteroids were luminally injected with FITC-dextran, then 3 groups of enteroids were treated with blank Salmonella culture medium, Salmonella spent medium, and lysozyme-treated Salmonella medium supernatants for 24 h. Images were taken pre and after treatments. FITC intensities inside and outside (indicated by an asterisk in K) were quantified and shown in (J). O–Q, Representative immunofluorescent analysis for E-Cadherin and DAPI in the above treated enteroids. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. The length of scale bars in (L–N) is 100 μm, and in (O–Q) is 50 μm.
To test if lysozyme may alter Salmonella’s invasiveness towards human enterocytes, we infected Caco2 BBE monolayers with Salmonella at a MOI of 250 in the presence of human lysozyme preincubated with the cells. After 40 min of infection, we quantified the intracellular Salmonella by CFU assays. Lysozyme preincubation did not significantly reduce Salmonella’s intracellular invasiveness (Fig. 5D). Similar observations were observed with the preincubation of cells with Reg3b, Reg3g, Ang4, Retnlb, α-defensin, or β-defensin, which are antimicrobial peptides enriched in Paneth and goblet cells, and have been previously associated with bactericidal functions and gastrointestinal inflammation (32, 33, 34). However, gentamycin preincubation with the cells completely diminished Salmonella invasiveness (Fig. 5D), serving as a positive control. Thus, lysozyme does not alter Salmonella’s growth capacity or invasiveness in vitro.
To assess the above observation in a 2-D monolayer setting, we performed FITC-dextran permeability assays on Caco2 BBE monolayers using conditioned media from Salmonella culture at 5 or 10% concentration in the apical medium (Fig. 5E). Blank Salmonella culture medium, medium with 4000 U/ml of human lysozyme without bacteria, or conditioned medium from Salmonella culture with 4000 U/ml of human lysozyme. After 24 h of treatment, we found that compared to the control medium group, lysozyme alone or supernatants from untreated Salmonella supernatants did not alter the FITC-dextran permeabilities (Fig. 5E). However, lysozyme-pretreated Salmonella supernatants increased permeability in a concentration-dependent fashion (p5% = 0.0029, p10% < 0.0001, Fig. 5E).
We then directly measured the Trans-Epithelial Electrical Resistance (TEER) of the Caco2 BBE monolayer on a 96 trans-well plate (Fig. 5F). Two days following the addition of human lysozyme-treated Salmonella supernatants TEER was significantly reduced, whereas TEER was not altered in monolayers treated by lysozyme alone or by untreated Salmonella supernatants (Fig. 5G). To distinguish if lysozyme acted on Salmonella directly or on molecules in the Salmonella supernatant (SS) to invoke the barrier disruption, we treated SS with lysozyme, then applied the treated mixture to Caco2 BBE monolayer. No TEER reduction was detected (Fig. 5H). When we added 1 μg/ml periplasmic lysozyme inhibitor of c-type lysozyme (pliC), to the lysozyme and Salmonella mixture, no significant TEER reduction was observed (Fig. 5I). In addition, when we used heat-denatured (HD) lysozyme to treat Salmonella, the resulting supernatants also did not induce TEER change (Fig. 5I), collectively supporting that lysozyme directly engages with live Salmonella potentially via its catalytic function to impair the epithelial barrier. Furthermore, 0.2 μg/ml chicken lysozyme-treated SS also decreased the Caoc2 TEER (Fig. 5J), and pliC also inhibited the decrease (Fig. 5J).
To further test if lysozyme-treated Salmonella may release barrier-degrading factors, we collected supernatants from human lysozyme-treated and untreated Salmonella and tested their effects on epithelial permeability in human enteroids. FITC-dextran was microinjected into the enteroid lumen, followed by treating the enteroids with lysozyme-treated (Fig. 5L), blank Salmonella culture medium (Fig. 5M), or untreated Salmonella spent medium (Fig. 5N). After 24 h of treatment, FITC-dextran was observed to leak out from the enteroids treated with lysozyme-treated SS (asterisk, Fig. 5L). The FITC-dextran signals were measured inside of the lumen and outside of the enteroid (Fig. 5K). The functional integrity of these treated enteroids was analyzed by immunofluorescent staining for E-Cadherin (Fig. 5, O–Q), and cytosolic localization of E-Cadherin in Lyz+Sal treated enteroids reflected the increased permeabilities (Fig. 5, K–M).
Proteomic identification of lysozyme-induced release of Salmonella virulence factors
We next performed an untargeted proteomic profiling to investigate the identities of molecules that are present in lysozyme-treated Salmonella supernatants. Salmonella can survive in PBS for up to 30 weeks (35). We, therefore, prepared three conditions: (i) live Salmonella were spun down and resuspended in PBS containing 0.2 μg/ml human lysozyme, (ii) live Salmonella were spun down and resuspended in PBS without lysozyme, and (iii) 0.2 μg/ml human lysozyme in PBS. Samples in triplicates were incubated for 24 h at 37 °C, collected, resolved by SDS-PAGE, and subjected to in-gel digestion and proteomic analysis by mass spectrometry (Fig. 6A). The proteomic analysis identified a total of 1961 proteins, however, only 35 proteins were differentially present in lysozyme-processed versus un-processed Salmonella (p-value < 0.05, 20 increased and 15 decreased) (Fig. 6, B and C and Table 1). Compared to untreated Salmonella, 30% of the increased proteins in lysozyme-treated Salmonella supernatants are membrane proteins, 35% are enzymes, while 10% and 5% are chaperones and LPS related enzymes (Fig. 6D). The majority of reduced proteins are enzymes, chaperones, and iron or chlorine related proteins (Fig. 6D).
Figure 6.
Proteomic identification of lysozyme-stimulated release of Salmonella virulent factors.A, supernatants from untreated Salmonella and Salmonella treated with 0.2 μg/ml lysozyme, as well as 0.2 μg/ml lysozyme alone in PBS were resolved on SDS-PAGE. Protein bands were stained and cut for each sample and subjected to proteomic analysis by mass spectrometry. Three biological replicates were used for each condition. B, increased and decreased proteins were plotted on a volcano plot, with the red dots representing targets with more than 1.3-fold change and p-value < 0.05, between Sal versus Lyz+Sal groups. Log10 of the exact fold changes, and -Log10 of p-Value were presented. C, Heatmap of 35 significantly changed proteins. D, functional categorizations of increased and decreased protein targets in Lyz+Sal groups compared to Sal alone. E, the top increased and decreased proteins in the supernatant of lysozyme-treated Salmonella. The abundance of a peptide is measured by the total number of MS/MS peptide spectra matches (PSMs, please also see Table 1). Each point represents the protein abundance based on PSM in each sample. F, sipC ELISA analysis from PBS, lysozyme alone, Salmonella supernatants, supernatants from Salmonella treated with 0.2 ug/ml lysozyme, supernatants from Salmonella treated with heat-denatured lysozyme, and supernatants from Salmonella treated with lysozyme in presence of recombinant pliC. G, sipC ELISA analysis from cecum feces from uninfected and Salmonella-infected WT, Villin-LyzTG, and Lyz−/− mice. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
Table 1.
35 significantly changed proteins
| Protein name | Protein ID | Coverage (%) | Spectrum counts | Unique peptides | Amin acids | Molar weights | SAL abundance | LYZ+SAL Abundance |
Fold changes | p-Value | Function |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Siderophore- interacting protein | yqjH | 10.98 | 4 | 3 | 255 | 28.9 | 184.4333 | 0 | 0 | 0.019824 | Ferric reductase in different iron assimilation pathways |
| N-acetyltransferase | 21.71 | 3 | 3 | 175 | 19.1 | 200 | 0 | 0 | 0.033553 | Transfer actyl group from intercellular into bacteria | |
| Cytoplasmic protein | 22.37 | 3 | 1 | 76 | 8.3 | 200 | 0 | 0 | 0.046521 | Cytoplasmic proteins | |
| Serine protease | 11.72 | 6 | 3 | 273 | 29.1 | 173.9667 | 14.86667 | 0.1 | 0.042902 | Hydrolaysis serine | |
| PadR family transcriptional regulator | yqji | 14.88 | 9 | 3 | 215 | 24.2 | 173.5667 | 26.43333 | 0.2 | 0.005656 | Represses the expression of YqjH which is involved in iron homeostasis under excess nickel conditions. |
| Ethanolamine utilization microcompartment protein EutM | EutM | 37.5 | 11 | 3 | 96 | 9.8 | 173.1333 | 26.86667 | 0.2 | 0.046791 | A transcriptional repressor, is important for preventing expression when other preferred sources of energy are available |
| Ribosomal RNA small subunit methyltransferase I | yraL | 24.74 | 15 | 6 | 287 | 31.5 | 173.3 | 26.7 | 0.2 | 0.042308 | Active on the assembled 30S subunit |
| Conjugal transfer protein, ATP-binding | traJ | 14.4 | 14 | 5 | 382 | 42.5 | 173.1 | 26.9 | 0.2 | 0.038769 | Activates the PY promoter and the transcription of the operon |
| Putative epimerase LsrE | LsrE | 19.29 | 11 | 4 | 254 | 27.6 | 159 | 41 | 0.3 | 0.01799 | Putative epimerase, is the final protein in the A-2 quorum sensing pathway yet to be characterize |
| Copper homeostasis protein CutF (Lipoprotein nlpE) | nlpE | 9.01 | 9 | 3 | 233 | 25.2 | 151.2333 | 48.73333 | 0.4 | 0.002936 | Involved in both copper efflux and the delivery of copper to copper-dependent enzymes |
| D-threonate kinase | 18.68 | 10 | 6 | 423 | 45 | 153.2667 | 46.7 | 0.5 | 0.044597 | A kinase involved in the catabolic pathway of D-threonate | |
| Bacteriophage repressor protein cI | cl | 19.37 | 11 | 3 | 191 | 20.5 | 143.2333 | 56.73333 | 0.5 | 0.018677 | Prevents lytic growth by directly repressing two promoters needed to express lytic functions, PL and PR |
| Type III secretion system effector protein, deubiquitinase | sseL | 16.4 | 18 | 5 | 317 | 35.5 | 140.9 | 59.1 | 0.5 | 0.008821 | Translocated into host cells during intracellular infection |
| Cytosine deaminase | codA | 34.98 | 46 | 11 | 426 | 47.6 | 137.5667 | 62.46667 | 0.6 | 0.025808 | Allows the cell to utilize cytosine for pyrimidine nucleotide synthesis |
| Protease 3 | 15.7 | 56 | 13 | 962 | 107.4 | 133.3 | 66.7 | 0.7 | 0.031043 | Endopeptidase (alkaline protease) of the serine protease family and cleaves proteins in the tissue section | |
| FadR family transcriptional regulator | yieP | 23.58 | 7 | 5 | 229 | 26 | 81.43333 | 118.5667 | 1.8 | 0.027262 | Regulates the expression of genes encoding fatty acid biosynthetic and degrading enzymes |
| Histidine transport ATP-binding protein | hisP | 14.34 | 9 | 3 | 258 | 28.8 | 80.9 | 119.1 | 1.8 | 0.048651 | Part of the ABC transporter complex HisPMQJ involved in histidine transport |
| tRNA/tmRNA (uracil- C(5))-methyltransferase | trmA | 20.49 | 19 | 6 | 366 | 41.9 | 78.23333 | 121.7333 | 2.1 | 0.043658 | Dual-specificity methyltransferase that catalyzes the formation of 5- methyluridine at position 54 (m5U54) in all tRNAs, and that of position 341 in tmRNA |
| Asparagine synthase B | asnB | 25.27 | 52 | 13 | 554 | 62.5 | 71.03333 | 128.9667 | 2.2 | 0.021078 | Catalyzes the ATP- dependent conversion of aspartate into asparagine, using glutamine as a source of nitrogen |
| Hypothetical membrane protein | yijP | 11.36 | 10 | 2 | 132 | 14.8 | 69.63333 | 130.3333 | 2.3 | 0.027148 | Membrane protein |
| Cell invasion protein | invE | 18.82 | 17 | 6 | 372 | 42.4 | 65.36667 | 134.6 | 2.5 | 0.032865 | Triggering of intracellular events that lead to microbial internalization |
| Type I restriction enzyme | hsdS | 8.1 | 10 | 3 | 469 | 51.9 | 63.86667 | 136.1667 | 2.6 | 0.000564 | Recognizes the target DNA sequence |
| Major outer membrane lipoprotein Lpp | Lpp1 | 62.82 | 85 | 6 | 78 | 8.4 | 63.6 | 136.3667 | 2.7 | 0.000564 | Major outer membrane |
| Chaperone protein SicP | slcP | 6.9 | 3 | 1 | 116 | 12.9 | 58.86667 | 141.1667 | 3 | 0.03567 | Binds SptP, which in its absence completely is degraded within tie bacterial cytoplasm |
| Peptidoglycan-binding protein LysM | lysM | 52.35 | 17 | 6 | 149 | 16.1 | 55.73333 | 144.2667 | 3.1 | 0.048491 | Outer membrane protein, activates a common signal transduction pathway response |
| N-acetyl-gamma-glutamyl-phosphate reductase | argC | 7.19 | 4 | 2 | 334 | 35.9 | 55.4 | 144.6 | 3.1 | 0.031262 | Catalyze NADPH- dependent reductive dephosphorylation of N-acetyl-gamma- glutamyl-phosphate to N-acetylglutamate- gamma-semialdehyde |
| UPF0194 membrane protein YbhG | YbhG | 45.32 | 34 | 12 | 331 | 36.3 | 55.96667 | 144.0667 | 3.2 | 0.023133 | Membrane protein |
| Abequosyltransferase RfbV | RfbV | 16.22 | 17 | 6 | 333 | 38.6 | 56.36667 | 143.6333 | 3.3 | 0.033607 | Catalyzes the transfer of CDP-abequose on D-mannosyl-L- rhamnosyl-D- galactose-1- diphospholipid to yield D-abequosyl-D-mannosyl-rhamnosyl-D-galactose-1-diphospholipid |
| Cysteine/glutathione ABC transporter ATP- binding protein/permease CydC | CydC | 15.36 | 12 | 6 | 573 | 63 | 22.33333 | 177.6667 | 9 | 0.032605 | It is involved in the export of glutathione from the cytoplasm to the periplasm and is required for the assembly of both cytochrome c and cytochrome bd |
| ATP-independent periplasmic protein-refolding chaperone | spy | 16.15 | 5 | 3 | 161 | 18.2 | 20.66667 | 179.3333 | 10 | 0.03805 | Folding landscape of the substrate in determining chaperone mechanisms |
| Aspartate--ammonia | asnA | 17.88 | 11 | 5 | 330 | 36.8 | 22.76667 | 177.2667 | 10.6 | 0.033566 | Catalyzes the ATP- dependent conversion of Asn |
| Hypothetical membrane protein (SPI- 6 associated) | sclP | 1.61 | 2 | 1 | 434 | 47.3 | 0 | 200 | 100 | 0.029191 | Membrane protein |
| H(+)/Cl(−) exchange transporter ClcA | clcA | 2.75 | 1 | 1 | 473 | 50.4 | 0 | 200 | 100 | 0.009301 | Proton-coupled chloriode transporter |
| Oligopeptide ABC transporter permease OppC | oppC | 7.95 | 2 | 2 | 302 | 33.1 | 0 | 200 | 100 | 0.020463 | Binds peptides of 3 amino acids to at least 16 amino acids in length independent of amino acid content |
| TDC operon transcriptional activator | tdcA | 6.73 | 7 | 2 | 312 | 34.8 | 0 | 200 | 100 | 0.023254 | Membrane protein |
Spectrum counts (SC) and unique peptides (UP) were utilized to rank the confidence scores (Table 1). Increased membrane proteins with the highest confidence scores and were consistently represented in all replicates include InvE (p = 0.033), Lpp1 (p = 0.036), UPF 0194 (YbhG) (p = 0.034), and LysM (p = 0.048) (Fig. 6F). InvE is a cell invasion protein that recruits sipB and sipC to form a translocon complex (20), and shows 2.6-fold increase in lysozyme-processed Salmonella supernatants (Fig. 6E). Lpp1 is a major outer membrane protein reported to stimulate TNFα production (36), and shows 2.7-fold increase. YbhG is an uncharacterized membrane protein associated with resistant chloroamphenicol and virulence production (36, 37, 38), and shows 3.2-fold increase. LysM, a liposaccharide binding protein linked to virulence by recognizing the enzymatic target, GlcNAc-X-GlcNAc (39), shows 3.1-fold increase.
sipC can be recruited by InvE in the Salmonella T3SS and forms a translocon complex with sipB on the host cell membrane with the help of other effectors (20). As sipC shows borderline significance in our proteomic analysis (Fig. 6E), we performed ELISA analysis for sipC to directly measure its abundances in lysozyme-treated versus untreated Salmonella supernatants. We found that lysozyme treatment significantly stimulated Salmonella to release sipC to the supernatants (Fig. 6F). Such induction of sipC was diminished when Salmonella was treated with heat-denatured lysozyme or in the presence of lysozyme inhibitor, pliC (Fig. 6F). To extent this observation to in vivo analysis, we measured sipC in the cecum feces of WT, Villin-Lyz1TG and Lyz1−/− mice. Interestingly, under the steady-state condition (without infection), Villin-Lyz1TG mice showed slightly elevated sipC in the cecum contents (Fig. 6G). After infection, there was an increased sipC in all groups, but the Villin-Lyz1TG mice demonstrated the most robust elevation of sipC (p < 0.0001, Fig. 6G). These results suggest that lysozyme engagement with commensal bacteria may contribute to basal level of sipC production, and that lysozyme interaction with Salmonella during infection triggers the release of more sipC from the pathogen.
InvE, Lpp1, and sipC impair the intestinal epithelial barrier
To investigate if any of the above lysozyme-stimulated Salmonella factors may impair barrier integrity, we functionally examined InvE, Lpp1, YbhG, and sipC in TEER assays. Caco2 BBE monolayers were apically treated with individual factors at a concentration gradient of 0.1, 0.5, 1.0, or 5.0 μg/ml, each with 4 replicates. TEER readings were collected every day before and after the treatment.
InvE, at a concentration of 0.5 μg/ml or above significantly decreased TEER 1 day after treatment. While cells treated with 0.5 μg/ml InvE sustained their TEER for up to 7 days, higher InvE concentrations abolished TEER entirely (Fig. 7A). In contrast, cells treated with 0.1 μg/ml did not show any change in the TEER (Fig. 7A). sipC treatment significantly reduced TEER reading even at a concentration of 0.1 μg/ml. Higher concentrations of sipC diminished TEER on the first day of treatment (Fig. 7B). Likewise, cells treated with 1 or 5 μg/ml of Lpp1 demonstrated a reduced TEER, while lower concentrations did not affect TEER (Fig. 7C). Treating cells with YbhG (UPF 0194), an uncharacterized membrane protein, did not elicit any TEER reduction (Fig. 7D). Interestingly, when InvE and Lpp1 were applied from the basolateral side of the monolayers, no significant TEER reduction was detected, whereas 0.5 μg/ml sipC induced a slight TEER reduction on days 2 and 3 from the basolateral side (Fig. 7E).
Figure 7.
InvE, sipC, and Lpp1 impair barrier function.A, TEER readings of Caco2 BBE monolayer in a 96-well plate before and after the treatment with increasing concentrations of InvE, applied apically. B, TEER readings of Caco2 BBE monolayer in a 96-well plate before and after the treatment with increasing concentrations of sipC, applied apically. C, TEER readings of Caco2 BBE monolayer in a 96-well plate before and after the treatment with increasing concentrations of Lpp1, applied apically. D, TEER readings of Caco2 BBE monolayer in 96-well plate before and after the treatment with increasing concentrations of YbhG, applied apically. Note, that the medium control for (A–D) was used as the baseline for all these individual treatments ran in the same experiments. E, TEER readings of Caco2 BBE monolayer in 96-well-plate before and after the treatment with 0.5 μg/ml InvE, or 0.5 μg/ml sipC, or 1.0 μg/ml Lpp1, applied from the basolateral side. F, TEER readings of Caco2 BBE monolayer in 96-well-plate before and after the treatment with different combinations of 0.1 μg/ml InvE, 0.1 μg/ml sipC, or 0.5 μg/ml Lpp1, applied apically. Note, the medium control for (E and F) was used as a baseline for these individual treatments ran in the same experiments. G, TNFα ELISA was performed on Caco2 BBE cells treated with blank medium, PBS, Lpp1 (0.5 or 1 μg/ml), InvE (0.5 μg/ml), sipC (0.1 or 0.5 μg/ml), and YbhG (5 μg/ml). H, cell death was determined by Trypan blue analysis on cells with the above treatments. I, immunofluorescent staining for ZO-1 on Caco2 BBE cells with the above treatments. Arrows point to the perturbed junctional structures by sipC, InvE, and Lpp1. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. The length of scale bars in (I) is 50 μm.
Since 0.1 μg/ml InvE, 0.1 μg/ml sipC, and 0.5 μg/ml Lpp1 did not induce a pronounced TEER reduction, we investigated their potential synergy in perturbing the epithelial barrier. We evaluated all potential combinatory effects of any two or all three of these factors of the above concentrations. A clear synergistic effect was observed on sipC and Lpp1 combination (green, Fig. 7F), and the triple combination elicited the most significant TEER reduction (purple, Fig. 7F).
Lpp1-induced TNFα production as an immune response (36, 40). TNFα is proinflammatory and can impair epithelial and tight junction integrity (41, 42, 43). To test of any of the above proteins may elicit TNFα production from Caco2 BBE monolayers, we treated cells with individual proteins at a non-lethal concentration, for 24 h and measured TNFα by ELISA. We found that 1 μg/ml Lpp1 elicited 8-fold above the baseline, and the highest production of TNF-a (p < 0.0001) (Fig. 7G). With 0.5 μg/ml InvE and 5 μg/ml YbhG treatments, no change was observed (Fig. 7G). However, 0.1 μg/ml of sipC slightly increased TNFα (p = 0.0007), and this induction appeared to be sipC dose-dependent (Fig. 7G). YbhG does not affect TNFα production. Based on trypan blue analysis, we found that low dosages of InvE and sipC treatments caused some cell deaths after 24 h, while Lpp1 treatment had minimal impact on cell death (Fig. 7H). With the same treatment as the Trypan Blue test, we stained ZO-1 on the Caco2 BBE cell. Interestingly, InvE, sipC, and Lpp1 seem to drastically perturb the epithelial cell tight junction structures, morphology, and localization of ZO-1 based on confocal immunofluorescent analysis (Fig. 7I).
Discussion
Lysozyme was reported to have bactericidal activity on both Gram-positive and Gram-negative species (4, 44, 45, 46), while it displays a stronger bactericidal activity towards Gram-positive ones in vitro (4). S. Typhimurium is Gram-negative, and with the experiments performed by us, we failed to detect any bactericidal effects of human or chicken lysozymes on the growth or invasion by Salmonella under aerobic or anaerobic conditions. In our previous study, we found that elevated levels of intestinal lysozyme exacerbate chemically-induced inflammation whereas deleting Lyz1 protects from the inflammatory responses (6). Here, we demonstrated that during Salmonella infection, lysozyme overexpression increased bacterial invasion and the inflammatory response, whereas Lyz1 deficiency significantly protected against pathogen-induced inflammatory response. These findings are in agreement that intestinal lysozyme may play a pro-inflammatory role during disease progression.
Because lysozyme shapes the intestinal bacterial landscape under a steady state. Specifically, Villin-Lyz1TG mice have increased Bacteroidetes and decreased Firmicutes compared to wild-type mice (6). We suspected that this changed microbial composition may contribute to Salmonella susceptibility. Thus, we used antibiotics to pretreat mice before infection and observed a similar barrier disruption in Villin-Lyz1TG mice as well as protection in Lyz1−/− mice following infection. These data suggest that a direct interaction between lysozyme and Salmonella may contribute to disease exacerbation.
Our data suggest that lysozyme does not exhibit a bactericidal effect against Salmonella, reflected as either the growth or invasion in vitro. However, the supernatant from lysozyme-treated Salmonella cultures damaged epithelial barrier function in the human Caco2 cells or in enteroids. These data are consistent with an increased Salmonella invasion in Villin-Lyz1TG.
Salmonella invades epithelial cells by actively secreting virulent factors through the T3SS (18, 47). Through proteomic analysis, we identified significantly increased releases of Salmonella-derived factors that were stimulated by the direct engagement with lysozyme. These factors include components of the T3SS, i.e., InvE and sipC, as well as major outer membrane protein Lpp1 (36), all of which may drive the intracellular invasion by the pathogen via multiple parallel pathways (36, 48, 49).
Interestingly, InvE is a T3SS cell invasion protein also found in other bacteria species, such as Escherichia coli (48, 50, 51, 52, 53), suggesting that the observed lysozyme-stimulated InvE production may also apply to non-Salmonella species. T3SS is a transmembrane apparatus utilized by Salmonella and other invasive pathogens to engage with the host cell membrane, assemble translocon then inject effector proteins, including SopB, SopE, sipB, sipC, InvA, and InvE (18, 19, 20). Without activation of the T3SS, InvE is not typically released. Our data suggest that lysozyme’s engagement with Salmonella may provoke an aberrant activation of the T3SS. As InvE recruits sipB, sipC, sipD and sscA to form a translocon complex on the host cell membrane (19), we indeed also detected an elevated sipC abundance in lysozyme-treated cultures or in infected Villin-Lyz1TG mice with a modified gain-of-function in lysozyme. sipC is known to hijack host cellular F-actin and CDC42 to facilitate bacterial invasion, and previous studies demonstrated an interaction of InvE with F-actin (54, 55). These reports are consistent with our findings that InvE and sipC drastically perturbed the epithelial junctional morphology and TEER in Caco2 monolayers.
Lpp1 is a Salmonella major outer membrane protein, a deficiency of which decreases its cellular invasion (36, 40). Lpp1 has been shown to induce host cells to produce TNFα (40) as also observed in our experiments. However, to our knowledge, we demonstrated for the first time that Lpp1 directly impairs the epithelial barrier and increases gut permeability in Caco2 monolayers. Although a low concentration of TNFα helps epithelial wound healing (56) via the Wnt pathway (57, 58), a high concentration of TNFα can induce cell death, damage the epithelial integrity through MLCK activation (41, 42), perturb barrier function via Occludin endocytosis (43), and drive pathogen invasion (59, 60, 61). It is plausible that enhanced Lpp1 release triggered by lysozyme-Salmonella interaction provokes a feed-forward mechanism, indirectly mediated through TNFα for barrier disruption and Salmonella invasion. This effect could be appreciated from the observed temporal TEER changes elicited by Lpp1 in our time-course experiments. InvE, sipC, and Lpp1 all induced ZO-1 perturbation and TEER reduction. Thus, when the paracellular pores increase to 62 Å radius due to ZO-1 perturbation (43), Salmonella and other Gram-negative bacteria, such as E. coli, can cross the epithelial barriers. These changes may be reflected by the contrasting phenotypes observed in lysozyme-sufficient versus deficient mouse models. Thus, we propose that lysozyme engagement with Salmonella drives the release of virulence factors jointly perturbed epithelial tight junction integrity via T3SS- and TNFα-mediated mechanisms.
Interestingly, Bacteroidetes are anaerobic Gram-negative rods that employ the Type IX Secretion System (T9SS) to release virulence proteins (62, 63, 64, 65). Both T3SS and T9SS play essential roles in transferring virulence factors from the bacterial matrix to host cells or the intercellular space. Bacteroidetes are increased by approximately 20% in Villin-Lyz1TG mice (6), therefore lysozyme may use a similar mechanism on Bacteroidetes to release virulence factors controlled by T9SS. Indeed, we observed an increased intestinal permeability in Villin-Lyz1TG mice under homeostatic conditions compared to WT mice. This line of investigation should be pursued in the future.
Our study is clinically relevant as abnormal production of human c-type lysozyme was reported in various intestinal inflammatory diseases (7, 8, 9, 10, 11, 66, 67, 68, 69). While no causation has been established to link any single agent to chronic IBD, enteric pathogens and opportunistic pathobionts are associated with the development or exacerbation of gut inflammation (70, 71, 72). S. Typhimurium, Campylobacter (73), E. coli (74), Helicobacter pylori (75), and C. difficile (76) among others, have been linked to intestinal inflammation. Population cohort studies showed that individuals with gastrointestinal infection by Salmonella/and Campylobacter before disease onset had an increased IBD risk (73). A portion of later-diagnosed patients with IBD presented serum antibodies against several bacterial and fungal antigens (77), and the disease progression and aggravation were directly correlated with serum toxins of Salmonella and other enteric pathogens (78), anti-Salmonella antibodies (79), and antibodies against Saccharomyces cerevisiae, E. coli, P. fluorescens (80). While some studies suggested that microbial dysbiosis driven by the course of IBD may increase the risk of infection by pathogen (81, 82), others analyzing antibiotics therapy data for IBD standard care attributed the disease causative to pathogenic agents (83). By reporting a potentially deleterious role of host lysozyme in promoting inflammatory progression during infection, our study points to the potentially context-dependent mechanism underlying the host-pathogen interaction. The observed reduction of lysozyme expression in Paneth cells in response to Salmonella infection shown by us and others (17) may reflect an adaptive protection by the host. It would be interesting to delineate the mechanism behind the pathogen-activated circuit responsible for activating this host protection.
We did not observe barrier-regulating effects by YbhG, which is associated with the survivability of bacteria (37, 38). Also, we did not test all the other possible targets such as the SPI-6-associated protein, the hypothetical membrane proteins, or chaperones, primarily due to a lack of available reagents for these proteins. Our data suggest that the lysozyme-induced virulence factor release depends on lysozyme’s enzymatic function, as heat-inactivated lysozyme failed to elicit TEER response. A previous study reported that heat-denatured lysozyme still maintained bactericidal function (84). confirmed that the pliC inhibits the barrier-disrupting effects resulting from lysozyme-mediated Salmonella processing. pliC is known to bind the bactericidal domain H-35 while also blocking lysozyme’s enzymatic function (85). Future work will also determine how lysozyme promotes Salmonella to release these factors at the molecular level.
Experimental procedures
Bacterial strain, culture, and growth analysis
Salmonella enterica Typhimurium strain (ATCC SL1344 or ATCC14028) (86) was cultured in Luria-Bertani (LB) broth (Sigma L3022) with 100 μg/ml ampicillin (Sigma A9518-25G) at 37 °C with shaking.
Bacterial growth was measured at OD600 by a NanoDrop ONEC (Thermo Scientific), with cell numbers determined based on OD600 of 1.0 = 8 × 108 cells/ml. Aerobic culture condition was used for animal inoculation studies. Anaerobic culture was done in an anaerobic chamber (PLAS LABS 857-OTA) only for testing lysozyme’s effects and was specified in the experimental results. Growth curves were plotted in GraphPad Prism 10, with OD600 values determined hourly after different treatments.
Caco2 BBE cell culture, TEER, and FITC-dextran flux measurement
The Caco2 BBE cell line was cultured in DMEM supplemented with 20% fetal bovine serum (FBS) 1% Pen-strep (Thermo Fisher, 15140-122), and 0.2% Primocin (Thermo Fisher, NC9141815). The cells were grown at 37 °C with 5% CO2. The culture medium was replenished every 2 days. Cells were passaged at 80% confluence. For TEER analysis, 2 × 105 Caco2 BBE cells were initially seeded in 96-well 0.4 μm pore size Transwell plate (STEMCELL, 100-0419). For FITC-dextran permeability cells were seeded in 6-well 0.4 μm pore size Transwell inserts (Sigma Millipore, CLS3450). After a minimum of 16 days, cells were grown into a differentiated monolayer when the TEER reading is stabilized for 3 consecutive days. Treatments were applied to the monolayer from either apical or basolateral side specified in the experimental results. Apical chamber holds 70 μl medium with different concentrations of treatment, while basolateral compartment contains 250 μl medium. Every day, cells were washed with PBS and fresh medium was changed in both chambers. TEER readings were then collected by EVOM (World Precision Instruments, EVM-MT-03-01) at a consistent time of a day. For FITC-dextran flux measurement, medium was changed to FluoroBrite DMEM without phenol red (Thermo Fisher, A1896701). Apical medium containing 10 mg/ml 4 kDa FITC-dextran (Sigma, FD4) was supplemented with 5 or 10% of filtered-sterilized LB broth from (i) 16 h Salmonella culture without lysozyme; (ii) LB broth with 4,000U/ml of human lysozyme without bacteria; (iii) conditioned medium from Salmonella culture treated with 4,000U/ml of human lysozyme for 16 h.
Mice
Lyz1−/−, Villin-Lyz1TG, and Lyz13’UTR-IRES-CreER; Rosa26R-tdTomato mice have been described previously (6, 23, 87). All mice are maintained on C57BL/6 background. Experimental procedures are approved by the Rutgers University Institutional Animal Care and Use Committee. Mice are kept on a 12-h light and dark cycle, given unlimited access to food and drink, and kept in separately ventilated cages free of specific pathogens. All tests were carried out on littermates that were genetically altered or wild type, of both sexes. Power analysis was used to determine the animal numbers based on intra-group experimental variabilities. Mouse numbers were reflected in each data plot.
Salmonella virulence factors
Lpp1 (catalog no. CSB-EP745252SXB) (36), YbhG (CSB-EP856117SXB) (88), and InvE (CSB-EP339573SXB) (48) were purchased from CUSABIO TECHNOLOGY LLC. These proteins were 6xHis-tagged at C-terminal, expressed in E. Coli, purified, and lyophilized in 20 mM TRIS-HCl, 0.5 M NaCl, 6%Trehalose, pH 8.0. The purity of Lpp1 and YbhG is 93%, and InvE is 89%. sipC (catalog. MBS1016763) (89) and pliC (MBS 1177063) (https://www.uniprot.org/uniprotkb/P0AD59/entry) were purchased from MyBioSource LLC. These proteins were 6xHis-tagged at C-terminal, expressed in E. Coli, purified, and lyophilized from a filtered 20 mM Tris-HCl, 0.5 M NaCl, 6% Trehalose, pH 8.0. The purity of pliC is 93% and sipC is 85%. The quality of all these factors was examined by SDS-PAGE with 5% enrichment gel and 15% separation gel. These lyophilized proteins were reconstituted in PBS and filtered through a 0.22 μm filter, stored at −80 °C, used at a concentration described in the experiments.
Salmonella Typhimurium infection
A single colony of S. Typhimurium SL1344 was inoculated in LB-ampicillin broth to allow growth for 16 h at 37 °C with shaking. 100 μl of the culture was inoculated to 5 ml of fresh LB-ampicillin broth, and cultured at 37 °C for 4 h 108 or 109 CFU S. Typhimurium (based on OD600) was inoculated to each mouse. Experiments involving antibiotic pretreatment was specified in the experimental results. Mouse body weight was recorded before and after bacterial inoculation daily. The mice were sacrificed 4 days after infection. Liver, spleen and MLN were used for Salmonella CFU determination as described previously (23). Briefly, tissues were weighed and homogenized by Misonix Sonicator (Qsonica LLC. XL-2000) in PBS at a ratio of 1 mg tissue per 10 μl PBS. Tissue lysates were plated on XLD Agar plate (Sigma Aldrich, 95586) with 10-fold serial dilutions and grown for 24 h in a 37 °C incubator at room temperature in the hood. Numbers of single colonies were counted in each region of different dilutions and averaged to obtain the CFU value per mg tissue. Results were plotted in GraphPad Prism 10 with CFUs transformed on a log10 scale.
In vivo intestinal permeability assay
Four hours prior to sacrifice, food was removed and mice were gavaged with 60 mg/100g body weight fluorescein isothiocyanate–dextran (FITC-Dextran) (Sigma, FD4). Upon sacrifice, blood was removed aseptically by cardiac puncture in 5 mM EDTA and centrifuged at 3000g for 5 min. Plasma was separated from blood cell pellet for each sample. For quantification of FITC-Dextran in plasma samples, a standard curve was created through serial dilutions from 0.1 pg/ml to 1 ng/ml in control plasma. 100 μl of each standard and each experimental sample were transferred to a black-opaque-bottomed 96-well plate. Promega GloMax Plate Reader was used to determine fluorescence in samples with an excitation at 485 nm and emission at 530 nm.
Tissue collection, immunofluorescence, and immunohistochemistry
Mouse intestinal tissues (ileum, colon, and cecum) were collected, fixed in 10% formalin overnight, then transferred into 70% ethanol and stored at 4 °C. Tissues were subjected to paraffin embedding, and 5 μm sections were cut from paraffin blocks, rehydrated, and subjected to H&E staining (Hematoxylin, Sigma-Aldrich, GHS116; Eosin, Sigma-Aldrich, HT110316). Alcian blue staining and procedures of immunofluorescence and immunohistochemistry were described previously (6, 23, 87). For immunostaining analysis, slides were treated in sub-boiling antigen retrieval buffer (1 μM citric acid, pH 6.0 or 1 μM EDTA, pH 8.0) for 10 min and then immediately transferred into running water. Slides were blocked with PBS buffer containing 0.1% Triton X-100, 2% Bovine Serum Albumin (Sigma A3294-100G), and 2% normal goat serum for 2 h at room temperature, and then incubated with indicated primary antibodies at 4 °C overnight. The next day, the slides were washed with PBS, and incubated with biotinylated or immunofluorescent secondary antibodies for 1 h at room temperature. Immunohistochemistry was developed by using the ABC kit (Vector Lab, PK-4000), DAB kit (Vector Lab, SK-4100), and imaged by Nikon TE 2000D with NIS Elements D version 4.4. Fluorescent images were taken by Zeiss LSM 980, and analyzed by NIH Image J software.
RNA BaseScope analysis
The RNA BaseScope in situ hybridization assays were performed following the manufacturer's instructions (Advanced Cell Diagnostics, ACD). Formalin-fixed paraffin-embedded tissue sections (5 μm) were stained by probe BA-Mm-Ang4-1zz-st targeting the mouse Ang4, and probe Ba-Mm-Lyz1-3zz-st targeting the mouse Lyz1. mRNA was detected by BaseScope Duplex Detection Reagent Kit, which was previously described (23).
ELISA assay
Tissues or Caco2 cells were lyzed by 50 mM Tris (pH7.5), 150 mM NaCl, 10 mM EDTA, 0.02%NaN3, 50 mM NaF, 1 mM Na3VO4, 0.5%NP40, 1 mM PMSF, 0.5 mM DTT and protease inhibitors (Sigma), homogenized by sonication, and centrifuged to collect supernatant. Concentrations of protein targets were determined by Protein Assay Dye Reagen Concentrate (Bio-RAD #5000006). 20 μg of total protein was used in each sample. Lipocalin 2 (LCN2) was measured by Quantikine ELISA Mouse Lipocalin-2/NGAL Immunoassay Kit (R&D System, Catlog# MLCN20) following manufacture’s procedures. For TNFα ELISA, mouse TNFα DuoSet (R&D DY410-05) was used. For sipC ELISA, anti-sipC IgG (TGC Biomics, tgc-A203-1) was used as capture antibody and HRP-conjugated anti-Rabbit antibody (Invitrogen, NA934V) was used as detection antibody. The results were read at a wave length of 540 nm by Promega BioSystems Glomax Multi.
FITC-dextran microinjection in enteroids
Human enteroids were passaged and resuspended fragments were mixed with fresh Matrigel at an appropriate ratio to achieve the desired enteroid density. The mixture was spread onto pre-warmed 4-well coverslip chambers. After solidifying at 37 °C for 30 min, the enteroids were overlaid with complete enteroid growth medium. The enteroids were grown for 5 days before injections were performed. Using microinjection hardware, organoids were treated with 59 nl of FITC-dextran diluted in PBS at a concentration of 5 ug/ul. The treated organoids were subsequently imaged using Nikon microscope to assess luminal FITC intensity. The organoids were then treated with the respective media containing microbial toxins or vehicle (PBS). After 24 h luminal intensity was captured using the same settings used on the previous day to quantify loss of luminal FITC intensity.
Computational analysis of Paneth cell scRNA-seq data
The count matrices of scRNA seq are from S. Typhimurium-infected (SAL) and uninfected (PBS) Paneth cell data from previous publications (23). The Paneth cell scRNA dataset is available in Gene Expression Omnibus (GEO) with an accession number: GSE237326. Briefly, Lyz13'UTR-IRES-CreER; Rosa26RtdTomato Paneth cell reporter mice were intraperitoneally injected with tamoxifen to activate the tdTomato in Paneth cells, 24 h before PBS or Salmonella gavage. After 4 days of Salmonella infection, Paneth cells were isolated from ilea of uninfected or infected mice, and were used for single-cell RNA sequencing analysis by 10 × Genomics. The matrices had been loaded into R (version 4.3.1) and were combined and used as the input for the Seurat package (version 4.4.0) (90). Cells with a high percentage of total unique molecular identifier (UMI) counts originating from mitochondrial RNA and cells with low UMI counts were filtered out. Clustering analysis was performed using the FindClusters function with a resolution parameter of 0.8. The clusters were visualized using the Uniform Manifold Approximation and Projection (UMAP) algorithm (91) with principal components as input and dims = 30, n.neighbors = 30. The clusters had been annotated as Paneth cell progenitors and mature Paneth cells based on the expression levels of stem cell markers (Lgr5, Olfm4, and Stmn1) and Paneth cell markers (Reg3g, Mptx2, and Lyz1).
Proteomics for lysozyme-processed Salmonella
S. enterica Typhimurium (ATCC SL1344) was grown on LB agarose (35 g/1L DD water) (Sigma L2897) at 37 °C for 48 h. A single bacteria colony was picked and inoculated in 25 ml autoclaved LB broth (Sigma L3022) with 25 μl ampicillin (Sigma A9518-25G) for 16 h at 37 °C. 100 μl was transferred to fresh LB broth and inoculated for another 4 h. Cells were centrifuged at 3500 rcf for 10 min. The medium was removed, and cells were resuspended in PBS and measured at OD600 by NanoDrop ONEC (Thermo Scientific). 109 cells were suspended in PBS with or without 0.2 μg/ml Human lysozyme (Sigma L1667). Reaction was incubated at 37 °C for 24 h. Samples were centrifuged at 3500 rcf for 10 min. Supernatant was collected, and protein concentrations were determined by Bradford assay. Two mg of total proteins per sample were denatured in LDS and resolved by SDS–PAGE (Invitrogen NP0335BOX). Coomassie Blue staining was performed and protein bands were cut and subjected to in-gel digestion and mass spectrometry.
In vitro Salmonella invasion assay
Caco2 BBE was seeded at 106 cells/well in 6-well plates (35 mm). Cells were grown to differentiated monolayers and then maintained for 2 weeks. Cell culture media was replaced with 20% Fetal Bovine Serum (FBS) in DMEM. Antimicrobial peptides were added 15 min at indicated concentrations prior to addition of bacteria. Antimicrobial peptides we used were 0.175 μg/ml SAP (R&D, 1948-SAB), 0.23 μg/ml CRP (R&D, 1707-CR-200), 0.5 μg/ml Reg3b (R&D, 5110-RG-050), 0.05 μg/ml Retnlb (Abnova, P4630), 0.2 μg/ml α-defensin (Novus Biologicals, H00001670-P01), 0.5 μg/ml β-defesin (Thermofisher, PHC1624), 0.2 μg/ml human lysozyme (Sigma, L1667-1G), 0.05 μg/ml Ang4 (R&D 964-AN-025) and 150 μg/ml. SL1344 was added to cells at a MOI of 100 and incubated with cells for various time points (up to 30 min) at 37 °C, 5% CO2. Cells were then washed with 150 μg/ml gentamicin (Corning, #30-005-CR) for 50 min after removal of bacteria, washed once with PBS, lysed with 0.5% Triton X-100 in PBS, and serial dilutions of lysates were plated on XLD agar plates for quantifying bacterial growth.
Trypan blue staining
Caco2 BBE was seeded at 104 cells/chamber in 8-chamber slide. Cells were grown to differentiated monolayers and then maintained for 3 days. Cell culture media was replaced with 20% Fetal Bovine Serum (FBS) in DMEM. 0.5 μg/ml of InvE, 0.1 μg/ml of sipC, and 1 μg/ml of Lpp1 were treated in the chambers. Medium and PBS were used as control and vehicle. Within 24 h of treatment, cells were stained with 1:10 diluted Trypan blue (Invitrogen, T10282). Cell viability was determined by counting both stained (non-viable) and unstained (viable) cells. The percentage of viable cells was calculated by dividing the number of unstained cells by the total number of cells counted.
Quantification and statistical analysis
All statistical analysis was done using the GraphPad Prism software unpaired t-tests for 2-group comparison, and two-way ANOVA test for multigroup analysis. Each experiment contained 3 to 11 mice per group. Quantification of immunostaining results was reported from 6 to 10 independent microscopic fields from at least 3 mice for each condition. Mouse numbers per group are reported in individual figure legends. Area and intensity in immunofluorescence staining were determined by NIH Image J. Multi-channel images were split into individual channels using the split channel function. A region of interest was set, then area and intensity functions were determined for channels of interest.
Data availability
Data, analytic methods and study materials will be made available to other researchers upon written request. Mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD049696.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors acknowledge the support from the Advanced Imaging Core Facility of the Department of Biological Sciences and the instrumental grant funding from the NSF-MRI (2117484). The Mass spectrometry data were obtained from an Orbitrap mass spectrometer funded in part by NIH grants, 1S10OD025047-01, for the support of proteomics research at Rutgers Newark Campus. The authors acknowledge the support from Edward M. Bonder for conceptual discussion and support for microscopy. The authors thank Kushabu Patel for technical support.
Author contributions
I. B., R. F., N. G., J. H., R. S., and P. K. conceptualization; I. B., J. A. F., S. B., J. Y., J. H., Y. L., and P. S. data curation; I. B., J. A. F., S. B., J. Y., N. G., J. H., N. G., R. S., P. S., and P. K. methodology; J. Y., J. H., and Y. L. formal analysis; J. Y., R. F., and J. H. software; R. F., N. G., and P. K. funding acquisition; R. F., N. G., and P. K. resources; R. F., N. G., and P. K. supervision, R. F., N. G., J. H., and P. K. validation; N. G. project administration; N. G., J. H. visualization; N. G. and P. K. writing–review & editing; J. H. writing–original draft; Y. L., N. G., and P. K. investigation.
Funding and additional information
This work was supported by NIH grant R01DK119198, R21AI167079, R01DK102934, R01AT010243, R01DK132885, NSF/BIO/IDBR grants (1353890, 1952823), ACS Scholar Award (RSG-15-060-01-TBE), and a Rutgers IMRT award to N. G.; NSF Grant No. IOS 1754783 (RPF).
Reviewed by members of the JBC Editorial Board. Edited by Ursula Jakob
References
- 1.Callewaert L., Michiels C.W. Lysozymes in the animal kingdom. J. Biosci. 2010;35:127–160. doi: 10.1007/s12038-010-0015-5. [DOI] [PubMed] [Google Scholar]
- 2.Canfield R.E., Liu A.K. The disulfide bonds of egg white lysozyme (Muramidase) J. Biol. Chem. 1965;240:1997–2002. [PubMed] [Google Scholar]
- 3.Ibrahim H.R., Thomas U., Pellegrini A. A helix-loop-helix peptide at the upper lip of the active site cleft of lysozyme confers potent antimicrobial activity with membrane permeabilization action. J. Biol. Chem. 2001;276:43767–43774. doi: 10.1074/jbc.M106317200. [DOI] [PubMed] [Google Scholar]
- 4.Ibrahim H.R. On the novel catalytically-independent antimicrobial function of hen egg-white lysozyme: a conformation-dependent activity. Nahrung. 1998;42:187–193. doi: 10.1002/(sici)1521-3803(199808)42:03/04<187::aid-food187>3.3.co;2-6. [DOI] [PubMed] [Google Scholar]
- 5.Bel S., Pendse M., Wang Y., Li Y., Ruhn K.A., Hassell B., et al. Paneth cells secrete lysozyme via secretory autophagy during bacterial infection of the intestine. Science. 2017;357:1047–1052. doi: 10.1126/science.aal4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu S., Balasubramanian I., Laubitz D., Tong K., Bandyopadhyay S., Lin X., et al. Paneth cell-derived lysozyme defines the composition of mucolytic microbiota and the inflammatory tone of the intestine. Immunity. 2020;53:398–416.e8. doi: 10.1016/j.immuni.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Ruscio M., Vernia F., Ciccone A., Frieri G., Latella G. Surrogate fecal biomarkers in inflammatory bowel disease: rivals or complementary tools of fecal calprotectin? Inflamm. Bowel Dis. 2017;24:78–92. doi: 10.1093/ibd/izx011. [DOI] [PubMed] [Google Scholar]
- 8.Klass H.J., Neale G. Serum and faecal lysozyme in inflammatory bowel disease. Gut. 1978;19:233–239. doi: 10.1136/gut.19.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arijs I., De Hertogh G., Lemaire K., Quintens R., Van Lommel L., Van Steen K., et al. Mucosal gene expression of antimicrobial peptides in inflammatory bowel disease before and after first infliximab treatment. PLoS One. 2009;4 doi: 10.1371/journal.pone.0007984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer K., Gellhorn A. Lysozyme in chronic ulcerative colitis. Proc. Soc. Exp. Biol. Med. 1947;65:221. doi: 10.3181/00379727-65-15917p. [DOI] [PubMed] [Google Scholar]
- 11.Meyer K., Gellhorn A. Lysozyme activity in ulcerative alimentary disease; lysozyme activity in chronic ulcerative colitis. Am. J. Med. 1948;5:496–502. doi: 10.1016/0002-9343(48)90100-4. [DOI] [PubMed] [Google Scholar]
- 12.van der Sluys Veer A., Brouwer J., Biemond I., Bohbouth G.E., Verspaget H.W., Lamers C.B. Fecal lysozyme in assessment of disease activity in inflammatory bowel disease. Dig. Dis. Sci. 1998;43:590–595. doi: 10.1023/a:1018823426917. [DOI] [PubMed] [Google Scholar]
- 13.Fahlgren A., Hammarstrom S., Danielsson A., Hammarstrom M.L. Increased expression of antimicrobial peptides and lysozyme in colonic epithelial cells of patients with ulcerative colitis. Clin. Exp. Immunol. 2003;131:90–101. doi: 10.1046/j.1365-2249.2003.02035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cadwell K., Liu J.Y., Brown S.L., Miyoshi H., Loh J., Lennerz J.K., et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456:259–263. doi: 10.1038/nature07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.VanDussen K.L., Liu T.C., Li D., Towfic F., Modiano N., Winter R., et al. Genetic variants synthesize to produce paneth cell phenotypes that define subtypes of Crohn's disease. Gastroenterology. 2014;146:200–209. doi: 10.1053/j.gastro.2013.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Q., Pan Y., Yan R., Zeng B., Wang H., Zhang X., et al. Commensal bacteria direct selective cargo sorting to promote symbiosis. Nat. Immunol. 2015;16:918–926. doi: 10.1038/ni.3233. [DOI] [PubMed] [Google Scholar]
- 17.Salzman N.H., Chou M.M., de Jong H., Liu L., Porter E.M., Paterson Y. Enteric Salmonella infection inhibits Paneth cell antimicrobial peptide expression. Infect Immun. 2003;71:1109–1115. doi: 10.1128/IAI.71.3.1109-1115.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner S., Grin I., Malmsheimer S., Singh N., Torres-Vargas C.E., Westerhausen S. Bacterial type III secretion systems: a complex device for the delivery of bacterial effector proteins into eukaryotic host cells. FEMS Microbiol. Lett. 2018;365 doi: 10.1093/femsle/fny201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myeni S.K., Wang L., Zhou D. SipB-SipC complex is essential for translocon formation. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kubori T., Galan J.E. Salmonella type III secretion-associated protein InvE controls translocation of effector proteins into host cells. J. Bacteriol. 2002;184:4699–4708. doi: 10.1128/JB.184.17.4699-4708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masschalck B., Van Houdt R., Van Haver E.G., Michiels C.W. Inactivation of gram-negative bacteria by lysozyme, denatured lysozyme, and lysozyme-derived peptides under high hydrostatic pressure. Appl. Environ. Microbiol. 2001;67:339–344. doi: 10.1128/AEM.67.1.339-344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abo H., Sultana M.F., Kawashima H. Dual function of angiogenin-4 inducing intestinal stem cells and apoptosis. Front. Cell Dev. Biol. 2023;11 doi: 10.3389/fcell.2023.1181145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balasubramanian I., Bandyopadhyay S., Flores J., Bianchi-Smak J., Lin X., Liu H., et al. Infection and inflammation stimulate expansion of a CD74(+) Paneth cell subset to regulate disease progression. EMBO J. 2023;42 doi: 10.15252/embj.2023113975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin J.H., Bozadjieva-Kramer N., Seeley R.J. Reg3gamma: current understanding and future therapeutic opportunities in metabolic disease. Exp. Mol. Med. 2023;55:1672–1677. doi: 10.1038/s12276-023-01054-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan W., Chen S., Wang Y., You Y., Lu Y., Wang W., et al. Loss of Mptx2 alters bacteria composition and intestinal homeostasis potentially by impairing autophagy. Commun. Biol. 2024;7:94. doi: 10.1038/s42003-024-05785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burke B. The role of matrix metalloproteinase 7 in innate immunity. Immunobiology. 2004;209:51–56. doi: 10.1016/j.imbio.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Hammer M.F., Schilling J.W., Prager E.M., Wilson A.C. Recruitment of lysozyme as a major enzyme in the mouse gut: duplication, divergence, and regulatory evolution. J. Mol. Evol. 1987;24:272–279. doi: 10.1007/BF02111240. [DOI] [PubMed] [Google Scholar]
- 28.Cross M., Mangelsdorf I., Wedel A., Renkawitz R. Mouse lysozyme M gene: isolation, characterization, and expression studies. Proc. Natl. Acad. Sci. U. S. A. 1988;85:6232–6236. doi: 10.1073/pnas.85.17.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cross M., Renkawitz R. Repetitive sequence involvement in the duplication and divergence of mouse lysozyme genes. EMBO J. 1990;9:1283–1288. doi: 10.1002/j.1460-2075.1990.tb08237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu F., Inoue K., Kato J., Minamishima S., Morisaki H. Functions and regulation of lipocalin-2 in gut-origin sepsis: a narrative review. Crit. Care. 2019;23:269. doi: 10.1186/s13054-019-2550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mallo G.V., Kurz C.L., Couillault C., Pujol N., Granjeaud S., Kohara Y., et al. Inducible antibacterial defense system in C. elegans. Curr. Biol. 2002;12:1209–1214. doi: 10.1016/s0960-9822(02)00928-4. [DOI] [PubMed] [Google Scholar]
- 32.Lewin K. The Paneth cell in disease. Gut. 1969;10:804–811. doi: 10.1136/gut.10.10.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barnes S.L., Vidrich A., Wang M.L., Wu G.D., Cominelli F., Rivera-Nieves J., et al. Resistin-like molecule beta (RELMbeta/FIZZ2) is highly expressed in the ileum of SAMP1/YitFc mice and is associated with initiation of ileitis. J. Immunol. 2007;179:7012–7020. doi: 10.4049/jimmunol.179.10.7012. [DOI] [PubMed] [Google Scholar]
- 34.Semple F., Dorin J.R. beta-Defensins: multifunctional modulators of infection, inflammation and more? J. Innate Immun. 2012;4:337–348. doi: 10.1159/000336619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao C.H., Shollenberger L.M. Survivability and long-term preservation of bacteria in water and in phosphate-buffered saline. Lett. Appl. Microbiol. 2003;37:45–50. doi: 10.1046/j.1472-765x.2003.01345.x. [DOI] [PubMed] [Google Scholar]
- 36.Sha J., Fadl A.A., Klimpel G.R., Niesel D.W., Popov V.L., Chopra A.K. The two murein lipoproteins of Salmonella enterica serovar Typhimurium contribute to the virulence of the organism. Infect Immun. 2004;72:3987–4003. doi: 10.1128/IAI.72.7.3987-4003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Branco L.A.C., Souza P.F.N., Neto N.A.S., Aguiar T.K.B., Silva A.F.B., Carneiro R.F., et al. New insights into the mechanism of antibacterial action of synthetic peptide Mo-CBP(3)-PepI against Klebsiella pneumoniae. Antibiotics (Basel) 2022;11 doi: 10.3390/antibiotics11121753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torres-Cabassa A., Gottesman S., Frederick R.D., Dolph P.J., Coplin D.L. Control of extracellular polysaccharide synthesis in Erwinia stewartii and Escherichia coli K-12: a common regulatory function. J. Bacteriol. 1987;169:4525–4531. doi: 10.1128/jb.169.10.4525-4531.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mesnage S., Dellarole M., Baxter N.J., Rouget J.B., Dimitrov J.D., Wang N., et al. Molecular basis for bacterial peptidoglycan recognition by LysM domains. Nat. Commun. 2014;5:4269. doi: 10.1038/ncomms5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fadl A.A., Sha J., Klimpel G.R., Olano J.P., Niesel D.W., Chopra A.K. Murein lipoprotein is a critical outer membrane component involved in Salmonella enterica serovar typhimurium systemic infection. Infect. Immun. 2005;73:1081–1096. doi: 10.1128/IAI.73.2.1081-1096.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang F., Graham W.V., Wang Y., Witkowski E.D., Schwarz B.T., Turner J.R. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am. J. Pathol. 2005;166:409–419. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su L., Nalle S.C., Shen L., Turner E.S., Singh G., Breskin L.A., et al. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology. 2013;145:407–415. doi: 10.1053/j.gastro.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buschmann M.M., Shen L., Rajapakse H., Raleigh D.R., Wang Y., Wang Y., et al. Occludin OCEL-domain interactions are required for maintenance and regulation of the tight junction barrier to macromolecular flux. Mol. Biol. Cell. 2013;24:3056–3068. doi: 10.1091/mbc.E12-09-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ellison R.T., 3rd, Giehl T.J. Killing of gram-negative bacteria by lactoferrin and lysozyme. J. Clin. Invest. 1991;88:1080–1091. doi: 10.1172/JCI115407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ibrahim H.R., Matsuzaki T., Aoki T. Genetic evidence that antibacterial activity of lysozyme is independent of its catalytic function. FEBS Lett. 2001;506:27–32. doi: 10.1016/s0014-5793(01)02872-1. [DOI] [PubMed] [Google Scholar]
- 46.Laible N.J., Germaine G.R. Bactericidal activity of human lysozyme, muramidase-inactive lysozyme, and cationic polypeptides against Streptococcus sanguis and Streptococcus faecalis: inhibition by chitin oligosaccharides. Infect Immun. 1985;48:720–728. doi: 10.1128/iai.48.3.720-728.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bandyopadhyay S., Zhang X., Ascura A., Edelblum K.L., Bonder E.M., Gao N. Salmonella engages CDC42 effector protein 1 for intracellular invasion. J. Cell Physiol. 2024;239:36–50. doi: 10.1002/jcp.31142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ginocchio C., Pace J., Galan J.E. Identification and molecular characterization of a Salmonella typhimurium gene involved in triggering the internalization of Salmonellae into cultured epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 1992;89:5976–5980. doi: 10.1073/pnas.89.13.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sukhan A., Kubori T., Wilson J., Galan J.E. Genetic analysis of assembly of the Salmonella enterica serovar Typhimurium type III secretion-associated needle complex. J. Bacteriol. 2001;183:1159–1167. doi: 10.1128/JB.183.4.1159-1167.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perna N.T., Plunkett G., 3rd, Burland V., Mau B., Glasner J.D., Rose D.J., et al. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature. 2001;409:529–533. doi: 10.1038/35054089. [DOI] [PubMed] [Google Scholar]
- 51.Allaoui A., Menard R., Sansonetti P.J., Parsot C. Characterization of the Shigella flexneri ipgD and ipgF genes, which are located in the proximal part of the mxi locus. Infect Immun. 1993;61:1707–1714. doi: 10.1128/iai.61.5.1707-1714.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haller J.C., Carlson S., Pederson K.J., Pierson D.E. A chromosomally encoded type III secretion pathway in Yersinia enterocolitica is important in virulence. Mol. Microbiol. 2000;36:1436–1446. doi: 10.1046/j.1365-2958.2000.01964.x. [DOI] [PubMed] [Google Scholar]
- 53.Read T.D., Brunham R.C., Shen C., Gill S.R., Heidelberg J.F., White O., et al. Genome sequences of Chlamydia trachomatis MoPn and Chlamydia pneumoniae AR39. Nucleic Acids Res. 2000;28:1397–1406. doi: 10.1093/nar/28.6.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McGhie E.J., Hayward R.D., Koronakis V. Cooperation between actin-binding proteins of invasive Salmonella: SipA potentiates SipC nucleation and bundling of actin. EMBO J. 2001;20:2131–2139. doi: 10.1093/emboj/20.9.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuan B., Scholz J., Wald J., Thuenauer R., Hennell James R., Ellenberg I., et al. Structural basis for subversion of host cell actin cytoskeleton during Salmonella infection. Sci. Adv. 2023;9 doi: 10.1126/sciadv.adj5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Corredor J., Yan F., Shen C.C., Tong W., John S.K., Wilson G., et al. Tumor necrosis factor regulates intestinal epithelial cell migration by receptor-dependent mechanisms. Am. J. Physiol. Cell Physiol. 2003;284:C953–C961. doi: 10.1152/ajpcell.00309.2002. [DOI] [PubMed] [Google Scholar]
- 57.Mizoguchi E., Mizoguchi A., Takedatsu H., Cario E., de Jong Y.P., Ooi C.J., et al. Role of tumor necrosis factor receptor 2 (TNFR2) in colonic epithelial hyperplasia and chronic intestinal inflammation in mice. Gastroenterology. 2002;122:134–144. doi: 10.1053/gast.2002.30347. [DOI] [PubMed] [Google Scholar]
- 58.Gregorieff A., Clevers H. Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev. 2005;19:877–890. doi: 10.1101/gad.1295405. [DOI] [PubMed] [Google Scholar]
- 59.Kiesslich R., Duckworth C.A., Moussata D., Gloeckner A., Lim L.G., Goetz M., et al. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut. 2012;61:1146–1153. doi: 10.1136/gutjnl-2011-300695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peeters M., Ghoos Y., Maes B., Hiele M., Geboes K., Vantrappen G., et al. Increased permeability of macroscopically normal small bowel in Crohn's disease. Dig. Dis. Sci. 1994;39:2170–2176. doi: 10.1007/BF02090367. [DOI] [PubMed] [Google Scholar]
- 61.Schmitz H., Barmeyer C., Fromm M., Runkel N., Foss H.D., Bentzel C.J., et al. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999;116:301–309. doi: 10.1016/s0016-5085(99)70126-5. [DOI] [PubMed] [Google Scholar]
- 62.Wexler H.M. Bacteroides: the good, the bad, and the nitty-gritty. Clin. Microbiol. Rev. 2007;20:593–621. doi: 10.1128/CMR.00008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lasica A.M., Ksiazek M., Madej M., Potempa J. The type IX secretion system (T9SS): highlights and recent insights into its structure and function. Front. Cell Infect. Microbiol. 2017;7:215. doi: 10.3389/fcimb.2017.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McBride M.J. Bacteroidetes gliding motility and the type IX secretion system. Microbiol. Spectr. 2019;7 doi: 10.1128/microbiolspec.PSIB-0002-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lauber F., Deme J.C., Lea S.M., Berks B.C. Type 9 secretion system structures reveal a new protein transport mechanism. Nature. 2018;564:77–82. doi: 10.1038/s41586-018-0693-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khalil N.A., Walton G.E., Gibson G.R., Tuohy K.M., Andrews S.C. In vitro batch cultures of gut microbiota from healthy and ulcerative colitis (UC) subjects suggest that sulphate-reducing bacteria levels are raised in UC and by a protein-rich diet. Int. J. Food Sci. Nutr. 2014;65:79–88. doi: 10.3109/09637486.2013.825700. [DOI] [PubMed] [Google Scholar]
- 67.Pilarczyk-Zurek M., Chmielarczyk A., Gosiewski T., Tomusiak A., Adamski P., Zwolinska-Wcislo M., et al. Possible role of Escherichia coli in propagation and perpetuation of chronic inflammation in ulcerative colitis. BMC Gastroenterol. 2013;13:61. doi: 10.1186/1471-230X-13-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kabeerdoss J., Sankaran V., Pugazhendhi S., Ramakrishna B.S. Clostridium leptum group bacteria abundance and diversity in the fecal microbiota of patients with inflammatory bowel disease: a case-control study in India. BMC Gastroenterol. 2013;13:20. doi: 10.1186/1471-230X-13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Galecka M., Szachta P., Bartnicka A., Lykowska-Szuber L., Eder P., Schwiertz A. Faecalibacterium prausnitzii and Crohn's disease - is there any connection? Pol. J. Microbiol. 2013;62:91–95. [PubMed] [Google Scholar]
- 70.Schultz B.M., Paduro C.A., Salazar G.A., Salazar-Echegarai F.J., Sebastian V.P., Riedel C.A., et al. A potential role of Salmonella infection in the onset of inflammatory bowel diseases. Front Immunol. 2017;8:191. doi: 10.3389/fimmu.2017.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mann E.A., Saeed S.A. Gastrointestinal infection as a trigger for inflammatory bowel disease. Curr. Opin. Gastroenterol. 2012;28:24–29. doi: 10.1097/MOG.0b013e32834c453e. [DOI] [PubMed] [Google Scholar]
- 72.McPhee J.B., Small C.L., Reid-Yu S.A., Brannon J.R., Le Moual H., Coombes B.K. Host defense peptide resistance contributes to colonization and maximal intestinal pathology by Crohn's disease-associated adherent-invasive Escherichia coli. Infect Immun. 2014;82:3383–3393. doi: 10.1128/IAI.01888-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gradel K.O., Nielsen H.L., Schonheyder H.C., Ejlertsen T., Kristensen B., Nielsen H. Increased short- and long-term risk of inflammatory bowel disease after Salmonella or campylobacter gastroenteritis. Gastroenterology. 2009;137:495–501. doi: 10.1053/j.gastro.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 74.Rahman K., Sasaki M., Nusrat A., Klapproth J.M. Crohn's disease-associated Escherichia coli survive in macrophages by suppressing NFkappaB signaling. Inflamm. Bowel Dis. 2014;20:1419–1425. doi: 10.1097/MIB.0000000000000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Papamichael K.X., Papaioannou G., Karga H., Roussos A., Mantzaris G.J. Helicobacter pylori infection and endocrine disorders: is there a link? World J. Gastroenterol. 2009;15:2701–2707. doi: 10.3748/wjg.15.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bosca-Watts M.M., Tosca J., Anton R., Mora M., Minguez M., Mora F. Pathogenesis of Crohn's disease: bug or no bug. World J. Gastrointest. Pathophysiol. 2015;6:1–12. doi: 10.4291/wjgp.v6.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Israeli E., Grotto I., Gilburd B., Balicer R.D., Goldin E., Wiik A., et al. Anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic antibodies as predictors of inflammatory bowel disease. Gut. 2005;54:1232–1236. doi: 10.1136/gut.2004.060228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qiu H., Sun X., Sun M., He C., Li Z., Liu Z. Serum bacterial toxins are related to the progression of inflammatory bowel disease. Scand. J. Gastroenterol. 2014;49:826–833. doi: 10.3109/00365521.2014.919018. [DOI] [PubMed] [Google Scholar]
- 79.Alvarez-Lobos M., Pizarro D.P., Palavecino C.E., Espinoza A., Sebastian V.P., Alvarado J.C., et al. Role of Salmonella enterica exposure in Chilean Crohn's disease patients. World J. Gastroenterol. 2013;19:5855–5862. doi: 10.3748/wjg.v19.i35.5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bertin D., Grimaud J.C., Lesavre N., Benelmouloud C., Desjeux A., Garcia S., et al. Targeting tissular immune response improves diagnostic performance of anti-Saccharomyces cerevisiae antibodies (ASCA) in Crohn's disease. PLoS One. 2013;8 doi: 10.1371/journal.pone.0080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sartor R.B. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 82.Stecher B., Chaffron S., Kappeli R., Hapfelmeier S., Freedrich S., Weber T.C., et al. Like will to like: abundances of closely related species can predict susceptibility to intestinal colonization by pathogenic and commensal bacteria. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khan K.J., Ullman T.A., Ford A.C., Abreu M.T., Abadir A., Marshall J.K., et al. Antibiotic therapy in inflammatory bowel disease: a systematic review and meta-analysis. Am. J. Gastroenterol. 2011;106:661–673. doi: 10.1038/ajg.2011.72. [DOI] [PubMed] [Google Scholar]
- 84.Hisham R., Ibrahim S.H., Juneja L.R., Kim M., Yamamoto T. A structural phase of heat-denatured lysozyme with novel antimicrobial action. J. Agric. Food Chem. 1996;44:1416–1423. [Google Scholar]
- 85.Um S.H., Kim J.S., Kim K., Kim N., Cho H.S., Ha N.C. Structural basis for the inhibition of human lysozyme by PliC from Brucella abortus. Biochemistry. 2013;52:9385–9393. doi: 10.1021/bi401241c. [DOI] [PubMed] [Google Scholar]
- 86.Clark L., Perrett C.A., Malt L., Harward C., Humphrey S., Jepson K.A., et al. Differences in Salmonella enterica serovar Typhimurium strain invasiveness are associated with heterogeneity in SPI-1 gene expression. Microbiology (Reading) 2011;157:2072–2083. doi: 10.1099/mic.0.048496-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu S., Tong K., Zhao Y., Balasubramanian I., Yap G.S., Ferraris R.P., et al. Paneth cell multipotency induced by notch activation following injury. Cell Stem Cell. 2018;23:46–59.e45. doi: 10.1016/j.stem.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McClelland M., Sanderson K.E., Spieth J., Clifton S.W., Latreille P., Courtney L., et al. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature. 2001;413:852–856. doi: 10.1038/35101614. [DOI] [PubMed] [Google Scholar]
- 89.Kaniga K., Tucker S., Trollinger D., Galan J.E. Homologs of the Shigella IpaB and IpaC invasins are required for Salmonella typhimurium entry into cultured epithelial cells. J. Bacteriol. 1995;177:3965–3971. doi: 10.1128/jb.177.14.3965-3971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stuart T., Butler A., Hoffman P., Hafemeister C., Papalexi E., Mauck W.M., 3rd, et al. Comprehensive integration of single-cell data. Cell. 2019;177:1888–1902.e21. doi: 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leland McInnes J.H., Saul N., Großberger L. UMAP: Uniform Manifold approximation and projection. J. Open Source Softw. 2018;3:861. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data, analytic methods and study materials will be made available to other researchers upon written request. Mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD049696.