Abstract
Histological transformation to small-cell lung cancer (SCLC) is a well-known mechanism of acquired resistance to epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs), and almost all patients receive EGFR-TKIs at the time of transformation. We herein report three cases of EGFR-mutated lung adenocarcinoma that transformed into SCLC long after the cessation of EGFR-TKIs. Rapid tumor progression and elevated SCLC marker levels were observed at the time of transformation. Our case highlights the importance of considering SCLC transformation throughout the clinical course. Careful observation of the tumor behavior and SCLC markers should be performed to avoid diagnostic delays.
Keywords: Small-cell lung cancer transformation, Non-small-cell lung cancer, Acquired resistance, Epidermal growth factor receptor, Tyrosine kinase inhibitors
1. Introduction
The discovery of driver mutations, such as epidermal growth factor receptor (EGFR) activating mutations, anaplastic lymphoma kinase (ALK) gene rearrangement, and Kirsten rat sarcoma (KRAS) mutations, has dramatically transformed the non-small-cell lung cancer (NSCLC) treatment paradigm. Among these, EGFR is the dominant driver mutation, especially in the East Asian population, and tyrosine kinase inhibitors (TKIs) targeting EGFR are the current standard modalities for patients with EGFR-mutated NSCLC in the first-line setting [1]. Although EGFR-TKIs have shown favorable therapeutic efficacy, most patients with EGFR-mutated NSCLC eventually develop acquired resistance to EGFR-TKIs.
Various mechanisms of acquired resistance to EGFR-TKIs have been identified [2]. These mechanisms can be grouped into three categories. The first category is EGFR-dependent ‘on-target’ mechanism mediated by the acquired resistance mutations in EGFR, such as T790M in exon 20. The second category is EGFR-independent ‘off-target’ mechanisms mediated by bypass signaling activation, such as MET proto-oncogene receptor tyrosine kinase (MET) amplification. The third category is phenotypic and histological alterations, including transformation from EGFR-mutated NSCLC to small-cell lung cancer (SCLC) [3].
SCLC transformation accounts for approximately 3–14 % of the mechanisms of acquired resistance to EGFR-TKIs [4]; however, the detailed molecular mechanisms underlying this shift in cellular appearance and biology remain unclear. One possible hypothesis is that the SCLC component co-exists with EGFR-mutated NSCLC in the original tumor and becomes dominant after treatment with EGFR-TKIs [5]. Another hypothesis, which is widely accepted, is that the SCLC component originates from EGFR-mutated NSCLC (de-novo SCLC transformation) because the common founder EGFR mutations persist in the SCLC-transformed tumor specimens [6].
Although SCLC transformation may occur at any point during the clinical course (as early as three months and as late as more than six years after the initial diagnosis of NSCLC), the median time to transformation was reported to be approximately 16–20 months [7,8]. Furthermore, almost all of the patients in these previous studies received EGFR-TKIs at the time of transformation.
We herein report three cases of EGFR-mutated NSCLC that transformed into SCLC long after the cessation of EGFR-TKIs. We also reviewed the relevant literature and summarized cases that followed a similar clinical course.
2. Case presentation
2.1. Case 1
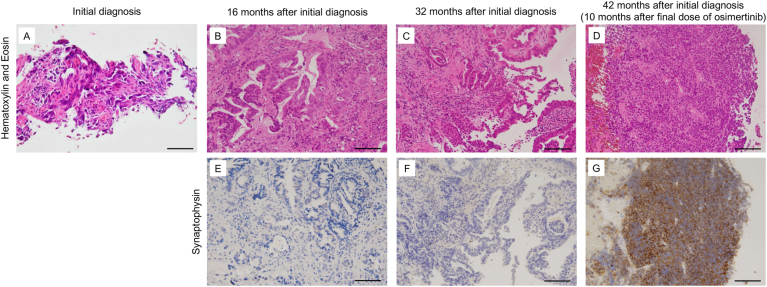
A 26-year-old woman with a primary tumor in the left upper lobe and multiple metastases in the lungs, bones, and kidneys was diagnosed with lung adenocarcinoma (cT3N0M1c, stage IVB) (Fig. 1A) with EGFR exon 19 deletion mutation and referred to our hospital. We initiated treatment with afatinib, a second-generation EGFR-TKI. However, eight months later, re-progression of the primary tumor was observed. We treated her with several cytotoxic chemotherapies (cisplatin + pemetrexed (PEM) + bevacizumab, followed by docetaxel (DTX) + ramucirumab (RAM), tegafur-gimeracil-oteracil potassium (S-1), afatinib rechallenge, and gemcitabine); however, none showed favorable therapeutic efficacy. A re-biopsy of the primary tumor was performed, but no acquired resistance mutations or histological transformation were detected (Fig. 1B). Although afatinib was administered again, we subsequently detected EGFR T790M in the pleural effusion and switched to osimertinib, a third-generation EGFR-TKI. Five months later, her tumor re-progressed and serum neuron-specific enolase (NSE) was slightly elevated, which reminded us of SCLC transformation. Although we performed a re-biopsy of the primary tumor, SCLC transformation could not be confirmed (Fig. 1C). We treated her with nine cycles of nanoparticle albumin-bound paclitaxel followed by amrubicin (AMR). After the fourth cycle of AMR, which corresponded to 10 months after the cessation of osimertinib, rapid tumor progression in the left hilar lymph node was observed on computed tomography (CT). Furthermore, rapid elevation of SCLC markers, such as pro-gastrin-releasing peptide (ProGRP) and NSE, but not carcinoembryonic antigen (CEA), which was elevated at the initial diagnosis, was identified. We then performed a third re-biopsy of the metastatic tumor in the left lower lobe and finally diagnosed the patient with SCLC transformation (Fig. 1D). Although her SCLC-transformed tumor cells were positive for synaptophysin, retrospective evaluation revealed that the previously biopsied tumor cells were negative for synaptophysin (Fig. 1E–G). We then treated the patient with carboplatin (CBDCA) + etoposide (VP-16) + durvalumab. Unfortunately, the chemoimmunotherapy did not induce a favorable anti-tumor effect. Finally, she died four months after the initiation of CBDCA + VP-16 + durvalumab.
Fig. 1.
The histological findings in Case 1.
(A–D) Hematoxylin and Eosin staining of biopsy specimens. The patient was diagnosed with adenocarcinoma based on the evaluation of the initial biopsy specimen (A). Adenocarcinoma was identified in the first re-biopsy performed at 16 months after the initial diagnosis (B). Adenocarcinoma was also identified in the second re-biopsy at 32 months after the initial diagnosis (C). SCLC transformation was identified in the third re-biopsy at 42 months after the initial diagnosis (10 months after the final dose of EGFR-TKI) (D). (E–G) Immunostaining for synaptophysin. While the tumor cells obtained in the first and second re-biopsies were negative for synaptophysin, a representative SCLC marker (E and F), the tumor cells from the third re-biopsy were positive (G). Bar indicates 100 μm.
2.2. Case 2
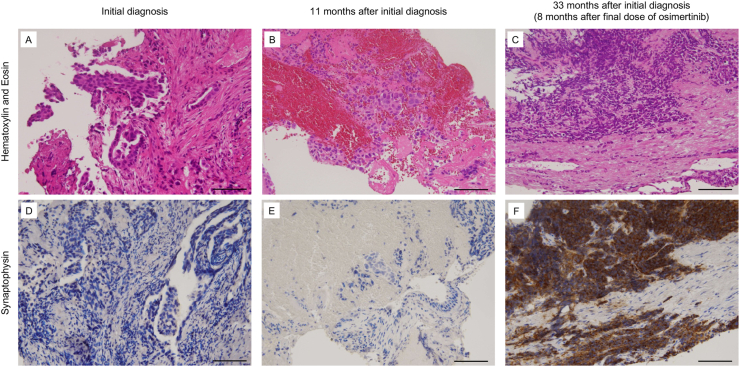
A 67-year-old man with a primary tumor in the right lower lobe and multiple metastases in both lungs was referred to our hospital and diagnosed with lung adenocarcinoma (cT4N2M1a, stage IVA) (Fig. 2A) with EGFR exon 19 deletion mutation. We initially treated the patient with gefitinib, a first-generation EGFR-TKI, which resulted in a partial response. However, re-progression of the primary tumor and the emergence of pleural effusion were observed 10 months later. We performed transbronchial re-biopsy of the primary tumor and identified the EGFR T790M mutation, but not histological transformation (Fig. 2B). We initiated osimertinib treatment; however, tumor progression in the right hilar lymph node was observed 10 months later. We treated the patient with several cytotoxic chemotherapies (CBDCA + PEM followed by DTX); however, none showed favorable therapeutic efficacy. After the fifth cycle of DTX (eight months after the cessation of osimertinib), the rapid progression of liver metastases and elevation of ProGRP were observed, which reminded us of SCLC transformation. The CEA level, which was elevated at the initial diagnosis, was not elevated at this time. Transbronchial re-biopsy of the primary tumor revealed SCLC transformation with the expression of synaptophysin (Fig. 2C and F). A retrospective immunohistochemical analysis revealed that the previously biopsied tumor cells were negative for synaptophysin (Fig. 2D and E). We then treated the patient with several cytotoxic chemotherapies (CBDCA + VP-16, paclitaxel, and AMR); however, these did not induce a favorable anti-tumor effect. Finally, the patient died eight months after the initiation of CBDCA + VP-16.
Fig. 2.
The histological findings in Case 2.
(A–C) Hematoxylin and Eosin staining of biopsy specimens. The patient was diagnosed with adenocarcinoma based on the evaluation of the initial biopsy specimen (A). Adenocarcinoma was identified in the first re-biopsy at 11 months after the initial diagnosis. An EGFR T790M mutation was detected in this specimen (B). SCLC transformation was identified in the second re-biopsy 33 months after the initial diagnosis (8 months after the final dose of EGFR-TKI) (C). (D–F) Immunostaining for synaptophysin. While the tumor cells obtained at the initial biopsy and first re-biopsy were negative for synaptophysin (D and E), the tumor cells from the second re-biopsy were positive (F). Bar indicates 100 μm.
2.3. Case 3
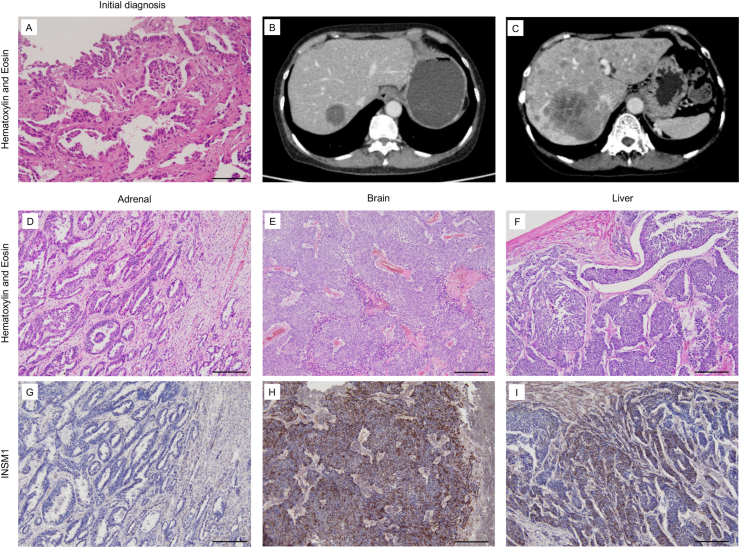
A 70-year-old woman with a primary tumor in the right lower lobe and multiple metastases in the liver, bone, adrenal gland, brain, and pancreas was referred to our hospital and diagnosed with lung adenocarcinoma (cT1bN3M1c, stage IVB) (Fig. 3A) with an EGFR exon 19 deletion mutation. We initiated first-line treatment with osimertinib, which resulted in partial response. We were able to treat the patient with osimertinib for a relatively long duration. However, new liver metastases were observed 24 months later. After treatment with cytotoxic chemotherapy (CBDCA + PEM followed by DTX + RAM), which corresponded to eight months after the cessation of osimertinib, her metastatic tumors in the liver and brain rapidly progressed (Fig. 3B and C), while the metastatic tumor in the adrenal gland was relatively stable. The serum ProGRP and NSE levels were also elevated. Although we suspected SCLC transformation, severe disseminated intravascular coagulation (DIC) due to rapid tumor progression enabled us to perform a re-biopsy and additional chemotherapy. Finally, she died four months after the cessation of DTX + RAM. We performed an autopsy with the patient's family's consent. Severe hemorrhage in multiple organs was observed macroscopically, which is consistent with neoplastic DIC. The direct cause of death was brainstem hemorrhage. Regarding the tumor progression, multiple metastases were observed in the brain, liver, kidneys, adrenal gland, and lungs. A histological examination revealed that the tumor cells in relatively stable areas, such as the adrenal gland, were highly differentiated and formed a glandular structure (Fig. 3D). Furthermore, these tumor cells were negative for insulinoma-associated protein 1 (INSM1), which is a representative immunohistochemical marker of neuroendocrine tumors (Fig. 3G). In contrast, the tumor cells in rapidly progressive areas, such as the liver and brain, were identical to those in well-differentiated adenocarcinoma. The relatively small undifferentiated tumor cells with a high N/C ratio proliferated focally in these areas (Fig. 3E and F), and the tumor cells were positive for INSM1 (Fig. 3H and I), leading to a final diagnosis of SCLC transformation.
Fig. 3.
Histological and imaging findings in Case 3.
(A) Hematoxylin and Eosin staining of biopsy specimens. The patient was diagnosed with adenocarcinoma based on the evaluation of the initial biopsy specimen. Bar indicates 100 μm. (B and C) At 32 months after the initial diagnosis (8 months after the final dose of EGFR-TKI), the patient's liver metastases rapidly progressed in 2 months. (D–I) Autopsy findings from metastatic colonies in the adrenal gland, brain, and liver. The tumor cells in the adrenal gland, which showed relatively stable disease activity, were well differentiated and negative for INSM1. On the other hand, the tumor cells in the brain and liver, which showed aggressive disease activity, were poorly differentiated with a high N/C ratio and were positive for INSM1, leading to a diagnosis of SCLC transformation. Bar indicates 200 μm.
3. Discussion
We encountered three cases of EGFR-mutated NSCLC that transformed into SCLC a relatively long time (8–10 months) after the cessation of EGFR-TKIs. After the first report of SCLC transformation as a mechanism of acquired resistance to EGFR-TKI by Zakowski et al., in 2006 [9], several case reports, case series, and retrospective cohort studies have been published. In a large study of 58 patients by Marcoux et al., the median time from the initiation of TKI therapy to transformation and from the initial diagnosis to transformation was 15.8 months and 17.8 months, respectively. In this cohort, 53 patients (91 %) were receiving EGFR-TKIs at the time of transformation [8]. In other studies, Ferrer et al. and Wang et al. demonstrated that 39 of 48 patients (81 %) and 22 of 25 patients (88 %) were receiving EGFR-TKIs at the time of transformation [7,10], suggesting that some patients with EGFR-mutated NSCLC show SCLC transformation after the cessation of EGFR-TKIs. Therefore, we performed a literature review [3,9,[11], [12], [13], [14], [15]] and summarized these cases (Table 1). In most of these reports, SCLC transformation was observed within two to seven months of cessation of TKI administration. However, Otoshi et al. reported a case of SCLC transformation 19 months after the cessation of osimertinib [14]. Interestingly, all patients harbored an exon 19 deletion mutation. These cases, including ours, suggest that SCLC transformation should be considered at any time, even long after the cessation of TKI therapy. Furthermore, a relatively longer duration of TKI administration in patients with exon 19 deletion mutations would affect the frequency of SCLC transformation after the cessation of TKI therapy.
Table 1.
Summary of cases with EGFR-mutated NSCLC with SCLC transformation after the cessation of EGFR-TKI therapy.
| Cases | Age | Sex | Primary EGFR mutation | Primary TKI (duration) | Treatment after primary TKI | Duration from final TKI to SCLC transformation (months) |
|---|---|---|---|---|---|---|
| Previously reported cases | ||||||
| Sequist LV et al. [3] | 40 | F | Ex. 19 del | erlotinib (more than 2 years) | NAa | 2 |
| van Riel S et al. [13] | 42 | F | NAa | erlotinib (10 months) | CBDCA + PTX, erlotinib, DTX | 2 |
| Zakowski MF et al. [9] | 45 | F | NAa | erlotinib (18 months) | mitomycin + vinblastine | 2 |
| Morinaga R et al. [12] | 46 | F | Ex. 19 del | gefitinib (10 months) | NAa | 5 |
| Yang MH et al. [15] | 50 | M | Ex. 19 del | erlotinib (6.5 months) | CBDCA + PTX + toripalimab | 6.6 |
| Popat S et al. [11] | 46 | F | Ex. 19 del | erlotinib (12 months) | CDDP + PEM, thoracic radiotherapy | 7 |
| Otoshi R et al. [14] | 68 | M | Ex. 19 del | osimertinib (11 months) | Erlotinib, CBDCA + PTX,DTX, PEM, S-1 | 19 |
| Our cases | ||||||
| Case 1 | 26 | F | Ex. 19 del | afatinib (8 months) | CDDP + PEM + Bev, DTX + RAM,S-1, afatinib, GEM, osimertinib, nab-PTX, AMR | 10 |
| Case 2 | 67 | M | Ex. 19 del | gefitinib (10 months) | osimertinib, CBDCA + PEM,DTX | 8 |
| Case 3 | 70 | F | Ex. 19 del | osimertinib (24 months) | CBDCA + PEM, DTX + RAM | 8 |
NA, not available; EGFR, epidermal growth factor receptor; NSCLC, non-small-cell lung cancer; SCLC, small-cell lung cancer; TKI, tyrosine kinase inhibitor; Ex. 19 del, exon 19 deletion; CBDCA, carboplatin; PTX, paclitaxel; DTX, docetaxel; CDDP, cisplatin; PEM, pemetrexed; S-1, tegafur-gimeracil-oteracil potassium; Bev, bevacizumab; RAM, ramucirumab; GEM, gemcitabine; nab-PTX, nanoparticle albumin bound-paclitaxel; AMR, amrubicin.
It is also known that EGFR-mutated NSCLC can transform to SCLC without the administration of EGFR-TKI [3]. Furthermore, several case series demonstrated that a few patients with NSCLC without EGFR mutation transformed to SCLC [10,16]. Although EGFR-wild-type NSCLC is less likely to transform to SCLC than EGFR-mutated NSCLC [3], these reports suggest that NSCLC can transform to SCLC throughout the entire clinical course independently of EGFR inhibition or EGFR mutation.
SCLC transformation was originally identified as a mechanism of acquired resistance to first- or second-generation EGFR-TKIs. After osimertinib, a third-generation EGFR-TKI, became the current standard care for patients with advanced NSCLC with EGFR mutation, the frequency of first- or second-generation EGFR-TKI administration in these patients has been decreasing in recent years. Some studies have demonstrated that the proportion of SCLC transformation was less with osimertinib than with first- or second-generation EGFR-TKIs among all of the identified mechanisms of acquired resistance in primary therapy [17]. A recent retrospective study reported that 95 out of 327 patients who underwent post-progression re-biopsy showed mechanisms of acquired resistance to first-line osimertinib. Seven of these were identified as SCLC transformation, which was the second most frequent mechanism [18]. All three patients had a history of osimertinib administration, suggesting that SCLC transformation can be caused by any EGFR-TKI, regardless of the generation.
SCLC accounts for approximately 15 % of lung cancers and generally presents a much more aggressive phenotype, characterized by rapid growth and metastatic spread to distant organs, and a poorer prognosis than NSCLC. Among our cases, Case 1 presented with a sudden rapid progression of hilar lymph node metastasis at the time of transformation. Cases 2 and 3 also presented with rapid progression of liver and brain metastases at the time of transformation. The autopsy examination in Case 3 demonstrated that rapidly progressive lesions consisted of SCLC-transformed components, whereas relatively stable lesions consisted of original adenocarcinoma components. These results indicate that SCLC-transformed cells behave like typical SCLC cells, and SCLC transformation should be considered whenever the tumor becomes aggressive. Furthermore, there are reports that transformation can be suspected early by evaluating tumor markers of SCLC, such as NSE and ProGRP [19]. All of our cases showed the elevation of these tumor markers of SCLC, but not of the original adenocarcinoma, when the behavior of their tumors turned aggressive, suggesting that SCLC markers should be evaluated promptly to avoid delaying the re-biopsy.
There is no established treatment for patients with NSCLC to SCLC transformation. In general, platinum etoposide regimens are administered to these patients; however, the median overall survival after transformation is reported to be only 9–10 months [7,8,10]. A recent retrospective study of 47 patients harboring EGFR mutations who developed SCLC transformation demonstrated that 11 patients who received atezolizumab-based chemo-immunotherapy had a favorable prognosis compared to the other 36 patients who received conventional chemotherapy [20]. Although two out of three patients in our study failed to receive immune checkpoint inhibitor-containing chemo-immunotherapy and followed an unfavorable clinical course, this regimen should be considered for applicable patients.
In conclusion, we experienced three cases of EGFR-mutated NSCLC in which SCLC transformation developed long after the cessation of EGFR-TKIs. Our case highlights the importance of considering the possibility of SCLC transformation throughout the clinical course. Furthermore, careful observation of tumor behavior and prompt evaluation of SCLC tumor markers can help avoid diagnostic delays.
CRediT authorship contribution statement
Kenya Miyamoto: Investigation, Writing – original draft. Hirokazu Ogino: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. Takumi Kakimoto: Investigation, Writing – review & editing. Yugo Matsumura: Investigation, Writing – review & editing. Keiko Haji: Conceptualization, Investigation, Writing – review & editing. Atsushi Mitsuhashi: Investigation, Writing – review & editing. Yutaka Morita: Investigation, Writing – review & editing. Yuki Tsukazaki: Investigation, Writing – review & editing. Yohei Yabuki: Investigation, Writing – review & editing. Ryohiko Ozaki: Investigation, Writing – review & editing. Hiroto Yoneda: Investigation, Writing – review & editing. Seidai Sato: Investigation, Writing – review & editing. Masaki Hanibuchi: Investigation, Writing – review & editing. Yoshimi Bando: Investigation, Writing – review & editing. Hiroshi Nokihara: Investigation, Writing – review & editing. Yasuhiko Nishioka: Conceptualization, Supervision, Writing – review & editing.
Declaration of competing interest
Hirokazu Ogino reports research grant funding paid to his institution from Taiho Pharmaceutical Co., Ltd. Atsushi Mitsuhashi reports research frees paid to his institution from Taiho Pharmaceutical Co., Ltd. Yasuhiko Nishioka lecture fees as honoraria from Nippon Boehringer lngelheim Co., Ltd., AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., and research grant funding paid to his institution from TMS Co., Ltd., SANSHO Co., Ltd., Taiho Pharmaceutical Co., Ltd., and scholarship donation from Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Asahi Kasei Pharma Co., Eli Lilly Japan K.K. The other authors have declared no conflict of interest to disclose.
Handling Editor: DR AC Amit Chopra
References
- 1.Soria J.C., Ohe Y., Vansteenkiste J., Reungwetwattana T., Chewaskulyong B., Lee K.H., et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 2018;378(2):113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 2.Yu H.A., Arcila M.E., Rekhtman N., Sima C.S., Zakowski M.F., Pao W., et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin. Cancer Res. 2013;19(8):2240–2247. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sequist L.V., Waltman B.A., Dias-Santagata D., Digumarthy S., Turke A.B., Fidias P., et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci. Transl. Med. 2011;3(75) doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper A.J., Sequist L.V., Lin J.J. Third-generation EGFR and ALK inhibitors: mechanisms of resistance and management. Nat. Rev. Clin. Oncol. 2022;19(8):499–514. doi: 10.1038/s41571-022-00639-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oser M.G., Niederst M.J., Sequist L.V., Engelman J.A. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol. 2015;16(4):e165–e172. doi: 10.1016/S1470-2045(14)71180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee J.K., Lee J., Kim S., Kim S., Youk J., Park S., et al. Clonal history and genetic predictors of transformation into small-cell carcinomas from lung adenocarcinomas. J. Clin. Oncol. 2017;35(26):3065–3074. doi: 10.1200/JCO.2016.71.9096. [DOI] [PubMed] [Google Scholar]
- 7.Wang W., Xu C., Chen H., Jia J., Wang L., Feng H., et al. Genomic alterations and clinical outcomes in patients with lung adenocarcinoma with transformation to small cell lung cancer after treatment with EGFR tyrosine kinase inhibitors: a multicenter retrospective study. Lung Cancer. 2021;155:20–27. doi: 10.1016/j.lungcan.2021.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Marcoux N., Gettinger S.N., O'Kane G., Arbour K.C., Neal J.W., Husain H., et al. EGFR-mutant adenocarcinomas that transform to small-cell lung cancer and other neuroendocrine carcinomas: clinical outcomes. J. Clin. Oncol. 2019;37(4):278–285. doi: 10.1200/JCO.18.01585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zakowski M.F., Ladanyi M., Kris M.G. EGFR mutations in small-cell lung cancers in patients who have never smoked. N. Engl. J. Med. 2006;355(2):213–215. doi: 10.1056/NEJMc053610. [DOI] [PubMed] [Google Scholar]
- 10.Ferrer L., Giaj Levra M., Brevet M., Antoine M., Mazieres J., Rossi G., et al. A brief report of transformation from NSCLC to SCLC: molecular and therapeutic characteristics. J. Thorac. Oncol. 2019;14(1):130–134. doi: 10.1016/j.jtho.2018.08.2028. [DOI] [PubMed] [Google Scholar]
- 11.Popat S., Wotherspoon A., Nutting C.M., Gonzalez D., Nicholson A.G., O'Brien M. Transformation to "high grade" neuroendocrine carcinoma as an acquired drug resistance mechanism in EGFR-mutant lung adenocarcinoma. Lung Cancer. 2013;80(1):1–4. doi: 10.1016/j.lungcan.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 12.Morinaga R., Okamoto I., Furuta K., Kawano Y., Sekijima M., Dote K., et al. Sequential occurrence of non-small cell and small cell lung cancer with the same EGFR mutation. Lung Cancer. 2007;58(3):411–413. doi: 10.1016/j.lungcan.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 13.van Riel S., Thunnissen E., Heideman D., Smit E.F., Biesma B. A patient with simultaneously appearing adenocarcinoma and small-cell lung carcinoma harbouring an identical EGFR exon 19 mutation. Ann. Oncol. 2012;23(12):3188–3189. doi: 10.1093/annonc/mds525. [DOI] [PubMed] [Google Scholar]
- 14.Otoshi R., Sekine A., Okudela K., Asaoka M., Sato Y., Ikeda S., et al. Small-cell lung carcinoma transformation of lung adenocarcinoma diagnosed by pericardial effusion: a case report. Mol. Clin. Oncol. 2020;13(2):129–132. doi: 10.3892/mco.2020.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang M.H., Yu J., Cai C.L., Li W. Small cell lung cancer transformation and tumor heterogeneity after sequential targeted therapy and immunotherapy in EGFR-mutant non-small cell lung cancer: a case report. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.1029282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norkowski E., Ghigna M.R., Lacroix L., Le Chevalier T., Fadel É., Dartevelle P., et al. Small-cell carcinoma in the setting of pulmonary adenocarcinoma: new insights in the era of molecular pathology. J. Thorac. Oncol. 2013;8(10):1265–1271. doi: 10.1097/JTO.0b013e3182a407fa. [DOI] [PubMed] [Google Scholar]
- 17.Johnson M., Garassino M.C., Mok T., Mitsudomi T. Treatment strategies and outcomes for patients with EGFR-mutant non-small cell lung cancer resistant to EGFR tyrosine kinase inhibitors: focus on novel therapies. Lung Cancer. 2022;170:41–51. doi: 10.1016/j.lungcan.2022.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Choudhury N.J., Marra A., Sui J.S.Y., Flynn J., Yang S.R., Falcon C.J., et al. Molecular biomarkers of disease outcomes and mechanisms of acquired resistance to first-line osimertinib in advanced EGFR-mutant lung cancers. J. Thorac. Oncol. 2023;18(4):463–475. doi: 10.1016/j.jtho.2022.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato Y., Tanaka Y., Hino M., Gemma A. ProGRP as early predictive marker of non-small-cell lung cancer to small-cell lung cancer transformation after EGFR-TKI treatment. Respir. Med. Case Rep. 2019;27 doi: 10.1016/j.rmcr.2019.100837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang C.Y., Sun H., Su J.W., Chen Y.Q., Zhang S.L., Zheng M.Y., et al. A potential treatment option for transformed small-cell lung cancer on PD-L1 inhibitor-based combination therapy improved survival. Lung Cancer. 2023;175:68–78. doi: 10.1016/j.lungcan.2022.11.016. [DOI] [PubMed] [Google Scholar]