Abstract
Distance traveled and home range size describe how animals move in space. The seasonal variations of these parameters are important to comprehensively understand animal ecology and its connection with reproductive behavior and energy costs. Researchers usually estimate the distance traveled as the sum of the straight-line displacements between sampled positions, but this approach is sensitive to the sampling frequency and does not account for the tortuosity of the animal’s movements. By means of the continuous-time movement modeling which takes into account autocorrelation and tortuosity of movement data, we estimated the distance traveled and monthly home range size of 28 wild boar Sus scrofa and modeled their inter-sexual seasonal variability. Males traveled longer distances and used larger home ranges than females, particularly during the rut in autumn-winter, consistently with the different biological cycles of males and females. Males enlarged their home ranges during the rut but traveled constant average distances along the year, whereas females traveled shorter distances in correspondence with the peak of food resources and birth periods but exhibited constant home range size across seasons. The differences between the seasonal variation patterns of distance traveled and home range size, observed in both sexes, revealed the complex relationship between these two aspects of spatial behavior and the great opportunity of including both distance traveled and home range size in behavioral ecology investigations. We provided a detailed analysis of wild boar spatial behavior and its relationships with the reproductive cycles of males and females, promoting a deeper comprehension of their behavioral ecology.
Keywords: continuous-time, ctmm, daily range, movements, spatial behavior, Sus scrofa
Investigating the movement patterns of wild animals is crucial for understanding their behavioral ecology, in both theoretical (Wilmers et al. 2015; Calabrese et al. 2016) and applied terms (Ciuti et al. 2012; Podgórski and Śmietanka 2018). For instance, traveling longer distances ensures the acquisition of a higher amount of resources at the cost of an increased energetic expenditure and a higher exposition to risks (Ciuti et al. 2012). Distance traveled is also in a direct relationship with dispersal patterns (Barry et al. 2020), in which inter-sexual differences may influence the gene flow (Peakall et al. 2003) with major implications for the ecological patterns of animal populations.
Remotely recorded spatial location data, such as those provided by satellite telemetry, represent a valid alternative for investigating animal movements and can be profitably applied for measuring the average distance that animals travel over time. Such distance is usually measured as the linear distance between consecutive spatial locations (straight-line displacement, SLD). Even though it is the most used and simple method, SLD tends to underestimate the real distance traveled at coarse sampling frequencies, because animals typically do not move linearly (Rowcliffe et al. 2012). Furthermore, while the SLD method may be modified to overcome biases due to the amount of measurement error (Theuerkauf et al. 2022) it still does not account for autocorrelation, which is the main feature of GPS telemetry with a high sampling rate (i.e., consecutive spatial locations are statistically correlated to each other, Noonan et al. 2019). These limitations have been overcome by the novel continuous-time speed and distance (CTSD) estimation method, which uses autocorrelation as a central and informative feature of the movement process to simulate non-linear trajectories, providing accurate and unbiased estimates of distance traveled (Fleming et al. 2014b, 2016; Noonan et al. 2019). The low sensitivity of this method to the sampling process makes results more robust with missing or irregular samples and enhances the comparability across different case studies (Calabrese et al. 2016; Noonan et al. 2019).
In recent years, the wild boar has become one of the most numerous ungulates in Europe. Its expansion was mainly due to favorable environmental factors and the behavioral plasticity of the species (Apollonio et al. 2010; Vetter et al. 2015). This proliferation led to a negative impact on biodiversity conservation and economic damage to a wide variety of human activities (Massei and Genov 2004; Herrero et al. 2006). Since a science-oriented management of wild boar needs to be based on reliable information about its ecology, understanding the seasonal variation patterns of its spatial behavior can be considered important. Wild boar also represents a valid system to investigate patterns of reproductive investment in the two sexes, which are known to adopt divergent reproductive strategies (e.g., Poteaux et al. 2009; Brogi et al. 2021a). Such inter-sexual differences of life history traits are thus likely to substantially influence the spatial behavior patterns of males and females throughout the year. Males are mostly solitary (Dardaillon 1984) and tend to use larger areas during the rut (Johann et al. 2020). The evidence of males losing body mass and thus experiencing a negative energy balance along the rutting season (Brogi et al. 2021a) suggests an increase of the distances traveled by male wild boar in this season aimed at reaching more mating opportunities, in accordance with the polygynous habits of the species (Dardaillon 1984). Conversely, females form groups together with piglets (Iacolina et al. 2009) and tend to travel shorter distances than males (Spitz and Janeau 1990), especially during the birth and weaning period, when they may use smaller home ranges (Morelle et al. 2015; Joahnn et al. 2020). Nonetheless, studies based on high-resolution spatial data and reliable analytical tools are still missing and most studies on wild boar spatial behavior focused on home range size (Massei et al. 1997; Keuling et al. 2008; Podgórski et al. 2013; Bisi et al. 2018), while less attention was devoted to the distance traveled, typically measured by means of SLD method (Spitz and Janeau 1990; Thurfjell et al. 2014), and thus likely to produce substantial underestimations. In this context, a comprehensive investigation on the inter-sexual seasonal variability of different aspects of spatial behavior such as distance traveled and home range size would provide a deeper understanding of the relationship between reproductive strategies and movement patterns of wild boar.
Environmental factors are also known to influence the spatial behavior in wild boar. Generally, wild boar move less in cold temperatures and more with increasing temperatures, or with precipitation, especially with warm temperatures (Thurfjell et al. 2014). The spatial distribution of resources may also be expected to substantially influence wild boar spatial behavior, since a higher food availability was shown to provoke a reduction of home range size (Bisi et al. 2018). In addition, the human presence and activities provide suitable habitats and resources to wild boar (Stillfried et al. 2017; Castillo-Contreras et al. 2018) and yet represent its most important source of mortality (Merli et al. 2017). This can cause major changes in the spatial movements of wild boar, as an increase of distance traveled in human-dominated environments (Podgórski et al. 2018).
We investigated the inter-sexual variability of wild boar spatial behavior along the year, to test the hypothesis of the reproductive cycles of the two sexes substantially influencing the seasonal patterns of spatial behavior. We estimated the distance traveled and the monthly home range size from a sample of 28 GPS-tracked wild boars, modeling the variability of these two metrics of spatial behavior along the year. Since moving over wider areas and longer distances would allow males to reach more mating opportunities, we predicted that males increase their home range size and distance traveled during the rut. Conversely, females do not play an active role during the rut, but the presence of piglets may constrain their spatial movements. Accordingly, we predicted that females reduce their home ranges and distance traveled during the birth and weaning seasons.
Material and Methods
Study area
Wild boar spatial data were collected in the Casentino Valley, Northern Apennines (43°48’N, 11°49’E), Tuscany, Italy. Elevation ranges from 330 to 1400 m a.s.l. Forests occupy 74% of the area, and are mainly composed of deciduous species (oaks Quercus spp., beeches Fagus sylvatica, and chestnuts Castanea sativa), with a minor presence of conifers (white fir Abies alba, Douglas fir Pseudotsuga menziesii, and black pine Pinus nigra). Shrubs, natural open areas, and agricultural lands cover 5%, 6%, and 12% of the area, respectively, and human-dominated landscapes occupy the remaining 3%. The area is inhabited by a rich ungulate community including wild boar and three deer species, roe deer Capreolus capreolus, red deer Cervus elaphus, and fallow deer Dama dama. The population density of wild boar was around 14 individuals/km2 during the study period (Guerrasio et al. 2022). In this area, the rutting season of wild boar lasts from November to February, reaching maximum intensity in December, and births occur between March and May (Brogi et al. 2021a, 2021b). Wild boar is the main prey for wolves, Canis lupus (Mattioli et al. 2011; Bassi et al. 2012). The area includes one large (Foreste Casentinesi National Park, 137 km2) and one small (Oasi Alpe di Catenaia, 27 km2) protected areas, in which all forms of hunting were forbidden. Outside the protected areas, hunters annually cull a mean of 6.2 wild boar/km².
Data collection
We captured wild boar using traps baited with maize from June 2013 to July 2020 (n = 28, 17 females and 11 males, Supplementary Table S1). We baited the traps some weeks before the capture period to attract wild boar. During the trapping period, we set the traps at night only in order to minimize the stress due to the high temperatures of the day. We checked the traps in the early morning to reduce the time any captured wild boar would spend in the trap. A veterinarian sedated the captured animals through a mixture of zolazepam and tiletamine (Zoletil 50 + 50 mg ml-1), which was occasionally combined with xylazine (for more details see Brogi et al. 2019).
We estimated the age of captured individuals by teeth eruption and wear. Finally, we equipped them with GPS collars (GPS PRO light collar, Vectronic Aerospace GmbH). All collars recorded wild boar spatial locations every 2 h.
The Regional Hydrological Service provided weather data (temperature and rain precipitation), recorded hourly and daily respectively, at the weather station in Camaldoli (Arezzo province, 43°48’N, 11°48’E).
Data analyses
The distance traveled and the monthly home range size of wild boar was calculated using the CTSD method, based on the continuous-time movement modeling framework (“ctmm” package in R, Calabrese et al. 2016). The main property of this approach is the ability to separate the continuous-time movement process from the discrete-time sampling process, making this method less sensitive to the sampling schedule when estimating parameters and therefore more robust with missing or irregular samples. The CTSD method entails the identification of the best fit continuous-time movement model for the data, based on the level of autocorrelation, and a simulation of possible continuous-time trajectories conditional on the data (Calabrese et al. 2016; Noonan et al. 2019).
To calculate the distance traveled by means of the CTSD method, we had to circumscribe a temporal unit that was large enough to include enough data allowing the model to measure autocorrelation data features, and small enough to inform on the temporal variability of speed. Since the aim of this study was to investigate the variability of the wild boar spatial behavior along the year, we calculated the distance traveled on a monthly base. The spatial locations of each individual were assigned to a specific month and year based on the date of sampling. These sub-datasets have been hereafter called “months/individual,” with each month/individual being composed of, for example, the data collected for the individual x during the month y of the year z. We only used sufficiently precise spatial locations, recorded with dilution of precision smaller than 10 and at least four satellites.
By means of the “ctmm” R package (Calabrese et al. 2016) we imported the original dataset in the R software as a “telemetry” object (Calabrese et al. 2016) and assigned each month/individual to one of the following movement models: independent identically distributed (IID, position and velocities not autocorrelated), Ornstein–Uhlenbeck (OU, autocorrelated positions but not velocities), and Ornstein–Uhlenbeck foraging (OUF, autocorrelated positions and velocities). We discarded the month/individuals in which the best model was represented by IID and OU, because they do not allow to estimate the distance traveled in the absence of velocity autocorrelation, and calculated the distance traveled (km/day) for the month/individuals in which the best model was OUF. In order to prevent biases, we used the methods implemented in the package “ctmm” to identify and filter possible outliers (Fleming et al. 2020). To provide a comparative measure of distance traveled estimated through the classic SLD method, we also measured straight-line distances between consecutive spatial locations and divided it by the time elapsed from the two locations to estimate the SLD distance traveled. To account for the sensitivity of the SLD method to the sampling frequency, we discarded the displacements between pairs of consecutive spatial locations which were separated by more than 2 h (due to temporary failures of the GPS process). Finally, we averaged the SLD distance traveled for each month/individual.
Furthermore, we estimated the average home range size (km2) of the previously selected month/individuals, by means of the autocorrelation kernel density estimator (AKDE, Fleming et al. 2015). After that, we calculated the availability of different habitat types as their proportional coverage (%) within the individual monthly home range. We used GIS software (QGIS 3.4.7) to intersect home range polygons with a freely available CORINE Land Cover database. We then classified the land cover classes into broader habitat types: forest, bush, open areas, agricultural areas, and human-dominated areas (see Supplementary Table S6 for more details).
To investigate the inter-sexual variability of CTSD distance traveled and home range size along the year, we used these two traits of spatial behavior as dependent variables in two sets of Generalized Additive Mixed Models (GAMMs), with the individual identity inserted as a random factor. We considered the following predictive variables: wild boar sex, age, month of sampling, weather parameters (average monthly temperature and monthly total precipitation), and the proportions of different habitats within home ranges. Then, we used the Pearson correlation coefficient (rp) and the variance inflation factor to check for possible collinearity and multicollinearity, respectively, between predictive variables (Zuur et al. 2009). We found non-negligible collinear relationships between agricultural areas and forest proportions within the home range (rp = −0.9). With a random forest calculation (“randomForest” package in R) we then ranked the predictors on the basis of their ability to explain the variability of the dependent variables. Since the proportion of forest performed better than that of agricultural areas for both distance traveled and home range size datasets, the proportion of agricultural was excluded from the subsequent models on both dependent variables (see Supplementary Tables S2 and S3 for a summary of the predictor variables being included in random forest, full models, and best models). In order to properly investigate the effect of the month of the year on the variability of wild boar distance traveled and home range size, we had to consider the temporal order of different months and also the continuity between December and January. Accordingly, the sampling month was inserted as a circular numerical predictor (ranging from 1 to 12) to make the model correctly consider January as contiguous to December. The predictor month of the year was included in interaction with sex, in order to evaluate the effect of the month of the year separately for males and females. We used these predictive variables to build two GAMMs, one for distance traveled and one for home range size, with a full-model structure by means of the “mgcv” R package. We applied the dredge function (“MuMIn” package in R) to fit a set of models with all possible combinations of the variables of each full model. Separately for distance traveled and home range size, we selected the best model by using the Akaike’s information criterion. We assumed models with ΔAIC < 2 to be as good as the minimum AIC model. Finally, among the models with ΔAIC < 2, we chose the one with the least predictors as the best model.
Results
We analyzed a total of 294 months/individual belonging to the 28 wild boar monitored. Out of these, 198 months/individuals were represented by the movement model OUF, allowing the estimation of distance traveled and home range size through the CTSD method. Conversely, the remaining months/individual did not include enough autocorrelation of position and velocity and were discarded from subsequent analyses. Across the set of months/individual represented by the movement model OUF, the average CTSD distance traveled was 5.86 km/day in males (SD = 1.98, n = 91) and 5.13 km/day in females (SD = 1.79, n = 107). Monthly home range size averaged 5.26 km2 (SD = 7.11, n = 91) and 2.61 km2 (SD = 3.36, n = 107) in males and females, respectively. See Table 1 for more details about the inter-sexual variability of raw CTSD distance traveled and home range size along the year and for a descriptive comparison with SLD estimates.
Table 1.
Monthly averages of home range size and distance traveled estimated by means of continuous time speed and distance (CTSD) and straight-line displacement (SLD) methods, separately for male and female wild boar GPS tracked in Central Italy
| Month of the year | Sex | n | CTSD distance traveled (km/day) |
CTSD distance traveled SD | SLD distance traveled (km/day) |
SLD distance traveled SD | Home range size (km2) | Home range size SD |
|---|---|---|---|---|---|---|---|---|
| January | f | 9 | 4.95 | 1.03 | 1.65 | 0.51 | 2.60 | 1.57 |
| m | 9 | 6.51 | 2.07 | 2.48 | 1.04 | 11.06 | 6.96 | |
| February | f | 8 | 4.25 | 1.22 | 1.70 | 0.59 | 2.33 | 1.17 |
| m | 7 | 6.35 | 1.99 | 2.46 | 0.93 | 5.82 | 4.89 | |
| March | f | 7 | 4.26 | 0.82 | 1.61 | 0.46 | 2.22 | 0.67 |
| m | 6 | 6.05 | 2.41 | 2.30 | 0.95 | 3.95 | 4.41 | |
| April | f | 6 | 4.13 | 0.68 | 1.46 | 0.70 | 2.15 | 0.60 |
| m | 8 | 5.68 | 2.35 | 2.31 | 0.88 | 2.75 | 2.86 | |
| May | f | 7 | 4.68 | 0.73 | 1.82 | 0.17 | 1.22 | 0.67 |
| m | 8 | 4.84 | 2.86 | 1.95 | 0.89 | 2.04 | 1.34 | |
| June | f | 6 | 4.38 | 1.63 | 1.91 | 0.54 | 1.87 | 1.65 |
| m | 5 | 4.73 | 1.47 | 2.13 | 0.60 | 1.95 | 0.62 | |
| July | f | 8 | 4.91 | 1.34 | 1.91 | 0.77 | 1.39 | 0.59 |
| m | 5 | 5.77 | 1.08 | 2.17 | 0.94 | 2.04 | 0.95 | |
| August | f | 10 | 6.02 | 2.00 | 2.25 | 0.78 | 1.91 | 0.57 |
| m | 6 | 6.17 | 1.81 | 2.52 | 0.75 | 3.19 | 1.25 | |
| September | f | 9 | 5.86 | 2.20 | 2.28 | 0.68 | 5.84 | 8.60 |
| m | 8 | 6.39 | 1.71 | 2.34 | 0.78 | 2.38 | 1.68 | |
| October | f | 14 | 5.33 | 1.41 | 2.42 | 0.65 | 2.38 | 1.03 |
| m | 11 | 5.39 | 1.33 | 2.58 | 0.60 | 4.37 | 3.06 | |
| November | f | 13 | 5.73 | 2.41 | 2.58 | 0.94 | 2.51 | 1.89 |
| m | 9 | 6.50 | 2.48 | 2.33 | 0.83 | 9.79 | 15.87 | |
| December | f | 10 | 5.62 | 2.65 | 1.98 | 1.02 | 4.04 | 6.17 |
| m | 9 | 5.76 | 1.70 | 2.40 | 0.69 | 9.16 | 9.55 |
f = female; m = male; n = number of months/individual used for averaging; CTSD distance traveled = average distance traveled estimated by means of the CTSD method; SD = standard deviation; SLD distance traveled = average distance traveled estimated by means of the SLD method; home range size = average surface occupied by the monthly home range, estimated by means of the AKDE method. See the Methods section for more details about the analytical processes.
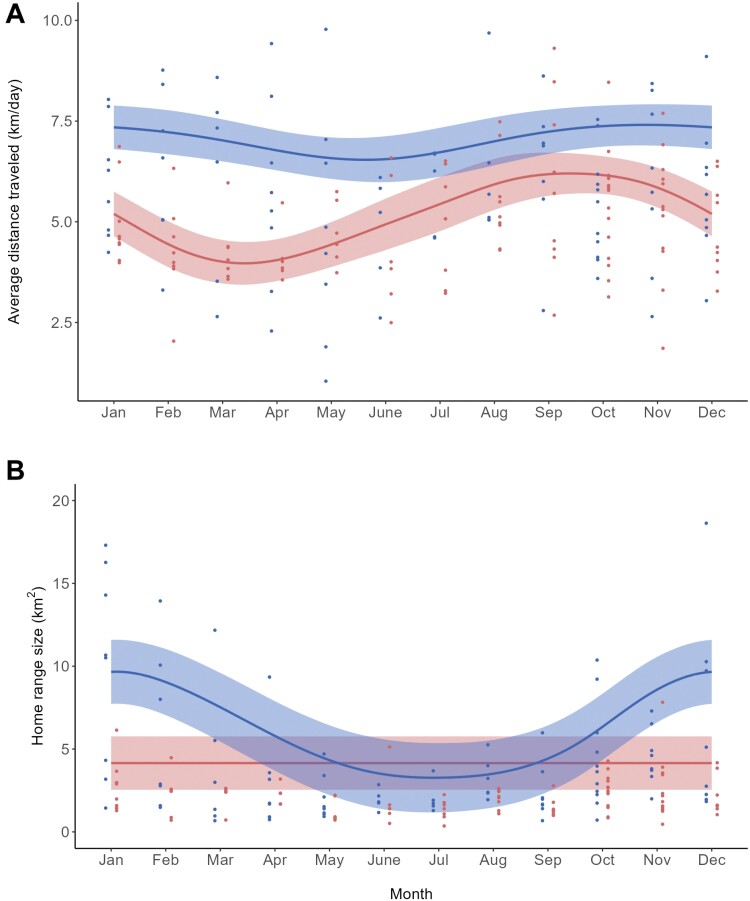
The best predictive model explaining the variability of the CTSD distance traveled (R2 = 0.56) included as predictors the month of the year in interaction with the individual sex, the average daily temperature, and the proportion of human-dominated areas within the monthly home range, despite only the month of the year in interaction with sex had a significant effect (Supplementary Table S2). Males traveled constant distances throughout the year. Conversely, females moved longer distances between August and October, and from November onward they progressively reduced their distance traveled until reaching the minimum between March and April (Figure 1A).
Figure 1.
Variability of the distance traveled by wild boar (A) and that of their monthly home range size (B) along the year as predicted by the best Generalized Additive Mixed Models (lines), superimposed on the raw datasets of distance traveled and monthly home range size (dots). Blue and red elements represent males and females, respectively. Color-shaded areas represent 95% confidence intervals estimated by the models (see the text for more details).
As for monthly home range size, the best model (R2 = 0.46) included as predictors the month of the year in interaction with the individual sex, the individual age, and the proportions of human-dominated areas, forest, and open areas (Supplementary Table S3 and Figure S1). The two sexes showed home range size variation patterns opposite to those observed for distance traveled. Female home range size did not vary significantly along the year, with the month of the year having a negligible effect on their home range size. Conversely, males exhibited significant variations of home range size along the year, with winter home ranges being about three times larger than those used in summer (Figure 1B).
Discussion
Males traveled longer distances and used larger home ranges than females, especially in autumn and winter. Nonetheless, in both sexes, these two aspects of spatial behavior showed different variation patterns along the year. Males traveled constant distances but exhibited a strongly variable home range size throughout the year, whereas females traveled variable distances and exhibited constant home range size. Unsurprisingly, using the autocorrelation of position and velocity to account for movement tortuosity, the CTSD method provided an estimate of distance traveled (5.47 km/day) much higher than the one estimated through the classic SLD method on the same dataset (2.16 km/day). The CTSD estimates were also higher than the distance traveled previously reported for the species by Morelle et al. (2015, 3–4 km/day). However, Podgórski et al. (2013) estimated even longer daily distances traveled by Polish wild boar in forested areas, averaging 6.8 km/day, despite using the SLD method with a sampling frequency of 30 min. Nevertheless, since the SLD method provides sampling frequency-dependent estimates (Calabrese et al. 2016), direct comparisons across different studies should be taken with caution. Conversely, using the CTSD to estimate the distance traveled and the home range size of different populations of the same species may ensure a more comprehensive understanding of the biological and ecological drivers of spatial behavior.
In partial accordance with our prediction, during the rutting season (autumn-winter in our study area, Brogi et al. 2021a) males increased their home range size but, unexpectedly, they did not travel substantially longer distances. Males thus likely reduced their site fidelity, traveling the same average distance for the rest of the year, but across wider areas to reach more females. The increase of the monthly home range size of male wild boar during the rut confirms the results reported by Johann et al. (2020) on a daily scale. Conversely, this first evidence of no seasonal variations of distance traveled occurring in male wild boar is in contrast with reports of males of other polygynous ungulates traveling longer distances during the rut (e.g., moose, Alces alces, Leblond et al. 2010). This evidence depicts the loss of body condition experienced by male wild boars during the rut (Brogi et al. 2021a) as not directly driven by the higher costs of spatial movements. The rutting season broadly coincides with the local peak of food availability (seeding of beech, chestnut, and turkey oak, Chianucci et al. 2021), which would have potentially allowed wild boar to acquire the same amount of resources with reduced movements (Larter and Gates 1994; Bisi et al. 2018). The evidence of males increasing their home range size and traveling constant distances during this food-abundant period thus indicates that reproductive opportunities overruled the current resources acquisition as driver of spatial behavior of male wild boar, consistently with their life history (Brogi et al. 2021a). Females traveled the least distances between February and April, broadly coinciding with the wild boar birth season (Brogi et al. 2021b) and in accordance with our prediction. This interpretation is consistent with previous studies reporting reduced movements in lactating females of other ungulate species (Bertrand et al. 1996; Grignolio et al. 2007; Bongi et al. 2008). Presumably, this occurred when females started to reduce their movements in preparation for the subsequent phase of weaning, typically occupying hidden places close to foraging sites (Kurz and Marchinton 1972; Frädrich 1974). Nonetheless, females started to travel shorter distances as soon as October, much earlier than the birth season. This was likely accountable to the seasonality of food resources availability rather than directly driven by the reproductive cycle. Indeed, contrary to males, females can be expected to be involved in the rut only passively, and we can speculate that their reduction of distance traveled in autumn-winter may be the outcome of the acorns and chestnut peak availability (Gamelon et al. 2017; Chianucci et al. 2021), allowing them to acquire the same amount of resources with shorter movements. Analogously to what was observed in males and contrary to our prediction, female home range size variation patterns did not follow those of distance traveled, highlighting the great opportunity of investigating both parameters for a comprehensive understanding of animal spatial behavior. Indeed, the average size of the monthly home ranges remained constant across all seasons. We should thus interpret the longer distances traveled by females between August and October as more intense use of the same home ranges, rather than as the result of enhanced large-scale displacements (e.g., from forested to agricultural areas and vice versa).
We provided an estimate of the distance traveled by wild boar by means of a large dataset of wild boar spatial positions and the innovative CTSD analytical approach, unbiased by the sampling frequency and the autocorrelation of data. We showed that males and females exhibit different movement patterns throughout the year, consistently with their different reproductive cycles, and that distance traveled and home range size inform on different aspects of animal spatial behavior. It is thus worth considering both to achieve a deeper and more comprehensive understanding of the spatial behavior of wild boar and other species.
The OUF was the most common best movement model in our dataset, allowing the estimation of the distance traveled for most of the considered individual months. However, 31.97% of the months/individual lacked a sufficient autocorrelation of position and/or velocity, preventing a proper application of CTSD. The lack of velocity autocorrelation may be due to lower availability of data in certain months (accountable to temporary collar failures) but it may also reflect the absence of concentrated displacement in a certain time (typically, for foraging, Fleming et al. 2014a, 2014b). In the case of wild boar, this may be related to cases in which resting and feeding sites were adjacent or very close (Thurfjell et al. 2009), and we are aware that our approach may have overlooked these particular cases. Researchers may prevent the lack of velocity autocorrelation by further increasing the number of spatial positions recorded within the time unit, with obvious negative consequences on the collar battery life and consequently on the individual sampling duration.
The CTSD approach required the definition of a temporal unit (one month in this study) long enough to calculate the model parameters used to estimate the average distance traveled and the home range size. While it did not hinder the observation of seasonal inter-sexual differences, this requirement entailed a substantial loss of temporal resolution of the investigated behavioral parameters, preventing the detection of fine-scale temporal variations of distance traveled and home range size.
Supplementary Material
Acknowledgments
The Provincial Administration of Arezzo, the Italian Ministry of Education, University, and Research (PRIN 2010-2011, 20108 TZKHC), and the Foreste Casentinesi National Park (“Studio del comportamento spaziale del cinghiale, con particolare riferimento alle implicazioni gestionali nel Parco Nazionale delle Foreste Casentinesi”) financially and logistically supported the data collection. MA was financed by the “Fondo di Ateneo per la Ricerca 2020.”
Contributor Information
Silvia Cavazza, Department of Veterinary Medicine, University of Sassari, via Vienna 2, I-07100 Sassari, Italy.
Rudy Brogi, Department of Veterinary Medicine, University of Sassari, via Vienna 2, I-07100 Sassari, Italy.
Marco Apollonio, Department of Veterinary Medicine, University of Sassari, via Vienna 2, I-07100 Sassari, Italy.
Conflict of Interest
The authors declare they have no competing interests.
Ethical Statement
This study complies with all national and regional laws dealing with ethics and animal welfare. Protocols for capture and manipulation performed in were approved by Tuscany Regional Administration (no. 103/5936/152—13/03/2002). The research adhered to the ASAB/ABS Guidelines for the Use of Animals in Research (guidelines for the treatment of animals in behavioral research and teaching, 2020).
Data Availability
All data generated or analyzed during this study are deposited on the Mendeley Data repository and are publicly available at https://data.mendeley.com/datasets/3d2sxjj9g3/1
References
- Apollonio M, Ciuti S, Pedrotti L, Banti P, 2010. European Ungulates and Their Management in the 21th Century. Cambridge: Cambridge University Press. [Google Scholar]
- Barry T, Gurarie E, Cheraghi F, Kojola I, Fagan WF, 2020. Does dispersal make the heart grow bolder? Avoidance of anthropogenic habitat elements across wolf life history. Anim Behav 166:219–231. [Google Scholar]
- Bassi E, Donaggio E, Marcon A, Scandura M, Apollonio M, 2012. Trophic niche overlap and wild ungulate consumption by red fox and wolf in a mountain area in Italy. Mamm Biol 77:369–376. [Google Scholar]
- Bertrand MR, DeNicola AJ, Beissinger SR, Swihart RK, 1996. Effects of parturition on home ranges and social affiliations of female white-tailed Deer. J Wildl Manag 60:899–909. [Google Scholar]
- Bisi F, Chirichella R, Chianucci F, Von Hardenberg J, Cutini A. et al. , 2018. Climate, tree masting and spatial behaviour in wild boar (Sus scrofa L.): Insight from a long-term study. Ann For Sci 75:46. [Google Scholar]
- Bongi P, Ciuti S, Grignolio S, Del Frate M, Simi S. et al. , 2008. Anti-predator behaviour, space use and habitat selection in female roe deer during the fawning season in a wolf area. J Zool 276:242–251. [Google Scholar]
- Brogi R, Brivio F, Bertolucci C, Benazzi M, Luccarini S. et al. , 2019. Capture effects in wild boar: A multifaceted behavioural investigation. Wildl Biol 2019:1–10. [Google Scholar]
- Brogi R, Chirichella R, Brivio F, Merli E, Bottero E, Apollonio M, 2021a. Capital-income breeding in wild boar: A comparison between two sexes. Sci Rep 11:4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogi R, Merli E, Grignolio S, Chirichella R, Bottero E. et al. , 2021b. It is time to mate: Population-level plasticity of wild boar reproductive timing and synchrony in a changing environment. Curr Zool 68:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese JM, Fleming CH, Gurarie E, 2016. ctmm: an r package for analyzing animal relocation data as a continuous-time stochastic process. Methods Ecol Evol 7:1124–1132. [Google Scholar]
- Castillo-Contreras R, Carvalho J, Serrano E, Mentaberre G, Fernández-Aguilar X. et al. , 2018. Urban wild boar prefer fragmented areas with food resources near natural corridors. Sci Total Environ 615:282–288. [DOI] [PubMed] [Google Scholar]
- Chianucci F, Tattoni C, Ferrara C, Ciolli M, Brogi R. et al. , 2021. Evaluating sampling schemes for quantifying seed production in beech Fagus sylvatica forests using ground quadrats. Forest Ecol Manag 493:119294. [Google Scholar]
- Ciuti S, Muhly TB, Paton DG, McDevitt AD, Musiani M, Boyce MS, 2012. Human selection of elk behavioural traits in a landscape of fear. Proc R Soc B 279:4407–4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardaillon M, 1984. Le Sanglier et le MILIEU CAMARGUAis: Dynamique Coadaptative. PhD thesis, Université Paul Sabatier, Toulouse, France.
- Fleming CH, Calabrese JM, Mueller T, Olson KA, Leimgruber P. et al. , 2014a. From fine-scale foraging to home ranges: a semivariance approach to identifying movement modes across spatiotemporal scales. Am Naturalist 183:154–167. [DOI] [PubMed] [Google Scholar]
- Fleming CH, Calabrese JM, Mueller T, Olson KA, Leimgruber P. et al. , 2014b. Non-Markovian maximum likelihood estimation of autocorrelated movement processes. Methods Ecol Evol 5:462–472. [Google Scholar]
- Fleming CH, Drescher-Lehman J, Noonan MJ, Akre TSB, Brown DJ. et al. , 2020. A comprehensive framework for handling location error in animal tracking data. bioRxiv 10.1101/2020.06.12.130195. [DOI] [Google Scholar]
- Fleming CH, Fagan WF, Mueller T, Olson KA, Leimgruber P. et al. , 2015. Rigorous home range estimation with movement data: A new autocorrelated kernel density estimator. Ecology 96:1182–1188. [DOI] [PubMed] [Google Scholar]
- Fleming CH, Fagan WF, Mueller T, Olson KA, Leimgruber P. et al. , 2016. Estimating where and how animals travel: An optimal framework for path reconstruction from autocorrelated tracking data. Ecology 97:576–582. [DOI] [PubMed] [Google Scholar]
- Frädrich H, 1974. A comparison of the behaviour in the Suidae. In: Geist V, Walther F editors. The Behaviour of Ungulates and its Relation to Management. New Series 24. Hearst: IUCN, 133–143. [Google Scholar]
- Gamelon M, Focardi S, Baubet E, Brandt S, Franzetti B. et al. , 2017. Reproductive allocation in pulsed-resource environments: A comparative study in two populations of wild boar. Oecologia 183:1065–1076. [DOI] [PubMed] [Google Scholar]
- Grignolio S, Rossi I, Bertolotto E, Bassano B, Apollonio M, 2007. Influence of the kid on space use and habitat selection of female Alpine ibex. J Wildl Manage 71:713–719. [Google Scholar]
- Guerrasio T, Brogi R, Marcon A, Apollonio M, 2022. Assessing the precision of wild boar density estimations. Wildl Soc Bull 46:e1335. [Google Scholar]
- Herrero J, García-Serrano A, Couto S, Ortuño VM, García-González R, 2006. Diet of wild boar Sus scrofa L. and crop damage in an intensive agroecosystem. Eur J Wildl Res 52:245–250. [Google Scholar]
- Iacolina L, Scandura M, Bongi P, Apollonio M, 2009. Nonkin associations in wild boar social units. J Mammal 90:666–674. [Google Scholar]
- Johann F, Handschuh M, Linderoth P, Heurich M, Dormann CF. et al. , 2020. Variability of daily space use in wild boar Sus scrofa. Wildl Biol 2020:1–12. [Google Scholar]
- Keuling O, Stier N, Roth M, 2008. Annual and seasonal space use of different age classes of female wild boar Sus scrofa L. Eur J Wildl Res 54:403–412. [Google Scholar]
- Kurz JC, Marchinton RL, 1972. Radiotelemetry studies of feral hogs in South Carolina. J Wildl Manage 36:1240. [Google Scholar]
- Larter NC, Gates CC, 1994. Home-range size of wood bison: Effects of age, sex, and forage availability. J Mammal 75:142–149. [Google Scholar]
- Leblond M, Dussault C, Ouellet JP, 2010. What drives fine-scale movements of large herbivores? A case study using moose. Ecography 33:1102–1112. [Google Scholar]
- Massei G, Genov PV, 2004. The environmental impact of wild boar. Galemys 16:135–145. [Google Scholar]
- Massei G, Genov PV, Staines BW, Gorman ML, 1997. Factors influencing home range and activity of wild boar Sus scrofa in a Mediterranean coastal area. J Zool 242:411–423. [Google Scholar]
- Mattioli L, Capitani C, Gazzola A, Scandura M, Apollonio M, 2011. Prey selection and dietary response by wolves in a high-density multi-species ungulate community. Eur J Wildl Res 57:909–922. [Google Scholar]
- Merli E, Grignolio S, Marcon A, Apollonio M, 2017. Wild boar under fire: The effect of spatial behaviour, habitat use and social class on hunting mortality. J Zool 303:155–164. [Google Scholar]
- Morelle K, Podgórski T, Prévot C, Keuling O, Lehaire F. et al. , 2015. Towards understanding wild boar Sus scrofa movement: A synthetic movement ecology approach. Mammal Rev 45:15–29. [Google Scholar]
- Noonan MJ, Fleming CH, Akre TS, Drescher-Lehman J, Gurarie E. et al. , 2019. Scale-insensitive estimation of speed and distance traveled from animal tracking data. Mov Ecol 7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peakall R, Ruibal M, Lindenmayer DB, 2003. Spatial autocorrelation analysis offers new insights into gene flow in the Australian bush rat Rattus Fuscipes. Evolution 57:1182–1195. [DOI] [PubMed] [Google Scholar]
- Podgórski T, Baś G, Jędrzejewska B, Sönnichsen L, Śniezko S. et al. , 2013. Spatiotemporal behavioural plasticity of wild boar Sus scrofa under contrasting conditions of human pressure: Primeval forest and metropolitan area. J Mammal 94:109–119. [Google Scholar]
- Podgórski T, Śmietanka K, 2018. Do wild boar movements drive the spread of African swine fever? Transbound Emerg Dis 65:1588–1596. [DOI] [PubMed] [Google Scholar]
- Poteaux C, Baubet E, Kaminski G, Brandt S, Dobson FS. et al. , 2009. Socio-genetic structure and mating system of a wild boar population. J Zool 278:116–125. [Google Scholar]
- Rowcliffe JM, Carbone C, Kays R, Kranstauber B, Jansen PA, 2012. Bias in estimating animal travel distance: The effect of sampling frequency: Estimating animal travel distance. Methods Ecol Evol 3:653–662. [Google Scholar]
- Spitz F, Janeau G, 1990. Spatial strategies: An attempt to classify daily movements of wild boar. Acta Theriol 35:129–149. [Google Scholar]
- Stillfried M, Gras P, Börner K, Göritz F, Painer J. et al. , 2017. Secrets of success in a landscape of fear: Urban wild boar adjust risk perception and tolerate disturbance. Front Ecol Evol 5:157. [Google Scholar]
- Theuerkauf J, Barrière P, Cadin K, Gula R, 2022. A trial of satellite GPS telemetry on feral pigs in tropical mountain rainforest. Aust Mammal 45:121–124. [Google Scholar]
- Thurfjell H, Ball JP, Åhlén PA, Kornacher P, Dettki H. et al. , 2009. Habitat use and spatial patterns of wild boar Sus scrofa (L.): agricultural fields and edges. Eur J Wildl Res 55:517–523. [Google Scholar]
- Thurfjell H, Spong G, Ericsson G, 2014. Effects of weather, season, and daylight on female wild boar movement. Acta Theriol 59:467–472. [Google Scholar]
- Vetter SG, Ruf T, Bieber C, Arnold W, 2015. What is a mild winter? Regional differences in within-species responses to climate change. PLoS ONE 10:e0132178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmers CC, Nickel B, Bryce CM, Smith JA, Wheat RE. et al. , 2015. The golden age of bio-logging: How animal-borne sensors are advancing the frontiers of ecology. Ecology 96:1741–1753. [DOI] [PubMed] [Google Scholar]
- Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM, 2009. Mixed effects modelling for nested data. In: Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM, editors. Mixed Effects Models and Extensions in Ecology with R. Statistics for Biology and Health. New York: Springer, 101–142. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are deposited on the Mendeley Data repository and are publicly available at https://data.mendeley.com/datasets/3d2sxjj9g3/1