Abstract
The cervical squamocolumnar junction of normal and dysplastic human xenografts was maintained in SCID-beige mice. Dysplastic tissue maintained a dysplastic morphology, irregular pattern of keratin expression, elevated levels of cellular proliferation, and human papillomavirus type 16 and/or type 18 DNA. Hyperplastic changes of normal xenografts occurred via high-dose estrogen exposure, and through recombinant adenovirus infection, the introduction and stable expression of an exogenous gene was accomplished.
Cancer of the uterine cervix is the most common cause of cancer-associated mortality in women worldwide (14). The molecular mechanisms which underly the development of cervical neoplasia are not completely understood and are difficult to assess without an intact and manipulatable biologic model (9, 21). As the premalignant and malignant lesions of the cervix arise from the transformation zone (an area of immature metaplasia between the mature stratified squamous epithelium of the exocervix and the columnar epithelium of the endocervix) (17), an ideal model should maintain this squamocolumnar junction. In addition, since over 90% of cervical malignancies are associated with infection by the high-risk human papillomavirus (HPV) subtypes (e.g., HPV 16 and 18) (2, 6), the ability to assess viral factors would also be a prerequisite for the model. Many biological mechanisms may be evaluated in cell culture (23, 29); however, such systems are usually unable to address important in vivo phenomena such as cytokine signal transduction, angiogenesis, and host immune surveillance.
Previous published experiences with animal models have been promising (1, 3–5, 7, 11, 18, 19, 24, 25, 41, 42). In 1985, Kreider and coworkers reported a nude mouse (i.e., athymic) xenograft model which maintained low-oncogenic-risk human papillomavirus infection (24, 25). However, the absence of the human cervical transformation zone and failure to support HPV cervical cancer-associated viral subtypes limit this model.
Working with transgenic mice expressing the HPV 16 oncoproteins E6 and/or E7, several investigators have elucidated molecular mechanisms associated with the induction of epidermal hyperplasia, angiogenesis, and DNA damage (19, 41, 42). Recently, Arbeit and colleagues used transgenic mice expressing the HPV 16 oncoproteins from a keratin 14 (K14) promoter and demonstrated synergy between the viral oncoproteins and chronic estrogen exposure in the development of squamous carcinogenesis along multiple sites (cervix and vagina) of the murine female reproductive tract (1). Although this is clear evidence of the ability of these viral oncogenes to induce cancer in vivo, these results do not offer sufficient insight that can explain the underlying mechanism which restricts disease to the cervical transformation zone. While HPV is known to infect all areas of the human female genital tract, infection of the transformation zone has deleterious consequences as cervical cancer is a problem of global epidemic proportions; malignant disease very rarely develops following infection of other lower genital tract sites such as the vagina and vulva. Therefore, transgenic models, although important, do not address this crucial biological issue.
Discovered in 1980, severe combined immunodeficiency (SCID) mice lack B and T lymphocytes. The animals have limited adaptive immune responses which precludes the rejection of human xenogenic tissue grafts (40). In this communication, we report the ability to maintain and manipulate the human cervical squamocolumnar junction in a SCID mouse strain which lacks natural killer cell activity and harbors macrophage defects (i.e., SCID-beige).
Animals.
C.B.-17 ICR (ICR background SCID mice are nonleaky and do not produce the low levels of antibody which are often seen with non-ICR SCID mice [12] [data not shown]) SCID-Bg mice (Harlan-Sprague-Dawley, Indianapolis, Ind.) were used at 4 to 6 weeks of age. The animals were housed in microisolator cages and were fed sterilized water and mouse chow. All experimental protocols were approved by the University of California, Irvine Institutional Animal Care and Use Committee.
Human tissue.
Fresh human cervical tissue was obtained from patients treated by members of the Department of Obstetrics and Gynecology under protocols approved by the University of California, Irvine Institutional Review Board. Normal cervical tissue was retrieved from discarded premenopausal hysterectomy specimens. Dysplastic cervical tissue was obtained from diagnostic cervical cold knife conization procedures; tissue lying between two circumferential points with demonstrable dysplasia by frozen section analysis was provided. The tissues were transported in Hanks’ balanced salt solution supplemented with penicillin (500 U/ml), streptomycin (500 μg/ml), and nystatin (200 U/ml) (Life Technologies, Inc., Gaithersburg, Md.). Implants were prepared in 3- by 2- by 2-mm sections containing the squamocolumnar junction and used within 4 h of harvest. One section from each specimen (labeled day 0) was placed in 10% buffered formalin solution and embedded in paraffin.
Implantation procedure.
Mice were transferred to a laminar flow hood and anesthesized by inhalation of methoxyflurane (Metofane; Pittman-Moore, Inc., Mundelein, Ill.). The abdominal surface was prepped with 95% ethanol. A 1-cm incision was made in the lateral wall of the abdomen above the peritoneum. The human cervical implant was inserted into a subcutaneous pocket created by blunt dissection in the adipose tissue. The skin was reapproximated by using 4.0 vicryl (Ethicon, Inc., Somerville, N.J.).
Xenograft excision.
At specific time points, the mice were euthanized by carbon monoxide inhalation. The implants and adjacent mouse tissue were excised en bloc and either fixed in 10% buffered formalin and embedded in paraffin or sucrose saturated and frozen in O.C.T. compound (Sakura Finetek U.S.A., Inc., Torrance, Calif.). Tissue sections of 4 to 6 μm were cut from the paraffin blocks and stained with hematoxylin and eosin.
Vascularization of implanted human tissue.
The xenografts and surrounding tissue were double stained for the human (light blue stain) and mouse (brownish red stain) endothelial cell marker CD31 [36, 37]), using protocols for mouse anti-human CD31 (Becton Dickinson and Company, San Jose, Calif.) and rat anti-mouse CD31 (PharmMingen, San Diego, Calif.), respectively. We observed chimeric vessels (i.e., anastomoses) containing both human and mouse endothelial cells in normal (Fig. 1A) and dysplastic (Fig. 1B) xenografts.
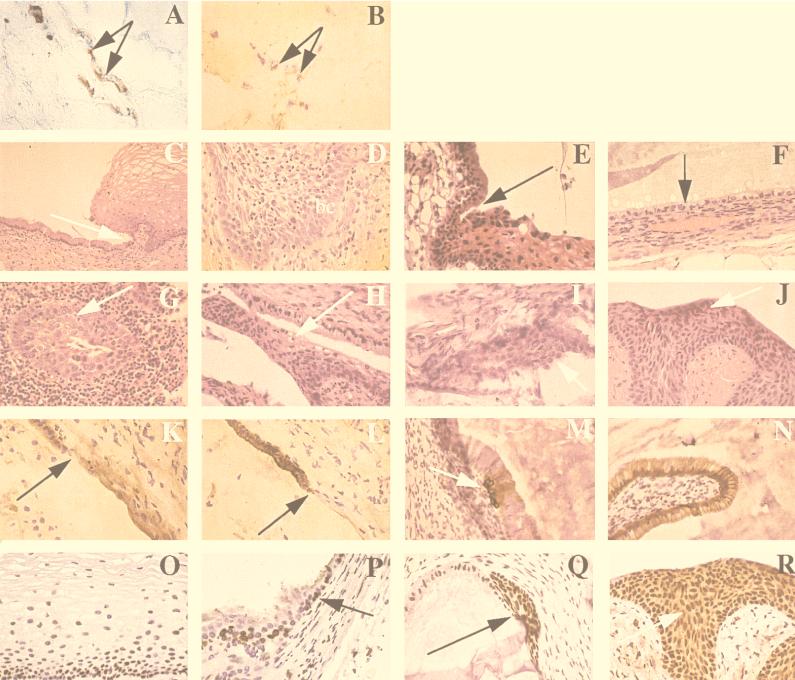
FIG. 1.
Histopathologic features of normal and dysplastic human cervical tissues implanted subcutaneously into SCID-Bg mice and excised at various time points. (A and B) Blood vessel anastomoses between human and mouse vessels within the cervical tissue implants. Sections were stained for human (blue) and mouse (red) CD 31, an endothelial cell marker. Both images were taken at a magnification of ×40. (A) Day 77, normal cervical tissue; (B) day 23, dysplastic tissue. (C to F) Maintenance of cervical histology in implanted normal tissues (hematoxylin and eosin, ×40 unless indicated otherwise). (C) Day 0, prior to implantation; squamocolumnar junction indicated by the arrow (×20); (D) day 8, stratified squamous epithelium with mitotic figures in the basal cell layer (bc); (E) day 77; squamocolumnar junction indicated by the arrow; (F) day 209, only columnar cells present (arrow); a prominent blood vessel is located in the underlying stroma. (G to J) Maintenance of cervical histology in implanted dysplastic tissues (hematoxylin and eosin, all images ×40). (G) Day 0, prior to implantation; a focus of dysplastic cells (arrow) surrounded by inflammatory cells; (H) day 79, koilocytotic changes consistent with HPV infection; (I and J) days 147 and 191, respectively, with foci of dysplastic cells (arrows). (K to N) Maintenance of differential keratin expression (all images at ×40). (K and L) Day 24, normal cervical tissue, with the squamocolumnar junction indicated by the arrow; K5-6 expression present in the stratified squamous epithelium and K8 expression present in the columnar cells, respectively; (M and N) day 147, dysplastic cervical tissue, with K5-6 expression below the columnar layer at a site of squamous metaplasia (arrow) and K8 expression appropriate for columnar cells, respectively. (O to R) Cellular proliferation of epithelium within cervical tissue implants, as determined by staining for proliferating cell nuclear antigen (PCNA) (all images at ×40). (O) Day 0, normal stratified squamous epithelium with proliferating basal layer; (P) day 42, normal cervical tissue, with squamocolumnar junction indicated (arrow) and only mitotically active epithelial cells in the basal layer; (Q and R) days 120 and 191, respectively; dysplastic foci (arrows) remains mitotically active throughout the stratified layers.
Viability of normal human cervical tissue.
Figure 1C to F depicts the tissues at 0, 8, 77, and 209 days postimplantation. Sections from day 0 (Fig. 1C) and day 77 (Fig. 1E) reveal the squamocolumnar junction to be well delineated. A section taken at day 8 (Fig. 1D) demonstrates reparative changes in the presence of mitotic activity near the basal cell layer. After 150 days, primarily columnar epithelium was found; this may indicate that the establishment of new squamous epithelium does not occur beyond certain time points (Fig. 1F, day 209).
Viability of dysplastic human cervical tissue.
A section from day 0 (Fig. 1G) reveals inflammatory cells which were not observed at subsequent time points. Figure 1H (day 79) demonstrates koilocytotic changes consistent with HPV infection. Histologically, these tissues remained viable and maintained their dysplastic state and morphologic evidence of HPV infection for several months (Fig. 1I and J, days 147 and 191).
Differential keratin expression of implanted tissues (normal and dysplastic).
Monoclonal mouse anti-human keratin 5/6 antibody (Boehringer Mannheim Corporation, Indianapolis, Ind.) and monoclonal mouse anti-human keratin 8 antibody (Novocastra Laboratories, Newcastle upon Tyne, United Kingdom) were used to examine tissues for the presence of differential keratin expression (15, 16, 45); in aberrant states, such as metaplasia and dysplasia, keratin expression is often perturbed (13, 33). Consistent with findings at day 0, sections from day 24 (Fig. 1K and L) exhibited the expected pattern of keratin expression at the squamocolumnar junction from K5 positive/K8 negative (squamous epithelium) to K8 positive/K5 negative (columnar epithelium). The keratin expression of dysplastic tissue (Fig. 1M and N), however, was more variable, with a section at day 147 revealing a focus of K5-positive squamous metaplasia below K5-negative columnar cells.
Cellular proliferation of implanted tissues (normal and dysplastic).
Proliferating cell nuclear antigen (PCNA) is a cofactor for DNA polymerase delta and is normally present during the late G1 and S phases (8, 28) of the proliferating basal cell layer; in contrast, dysplastic cells may contain high levels of PCNA throughout the epithelium. In analysis using a monoclonal mouse anti-human PCNA antibody (DAKO Corporation, Carpinteria, Calif.), no change in PCNA location or level of expression was observed in normal tissue at day 42 (Fig. 1P) relative to preimplanted day 0 tissue (Fig. 1O). In contrast, implanted dysplastic tissue stained strongly for PCNA in both the columnar cells and the suprabasalary cells of the stratified squamous epithelium for at least 191 days postimplantation (Fig. 1Q and R).
The maintenance of HPV in dysplastic tissue.
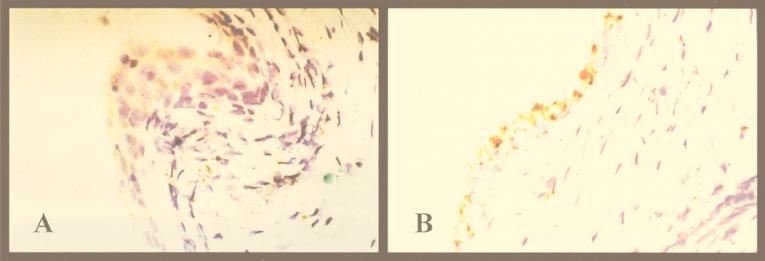
Using the DAKO GenPoint catalyzed signal amplification system and biotinylated HPV wide-spectrum and subtype 16/18-specific DNA probes, we detected high-oncogenic-risk HPV DNA by in situ hybridization in dysplastic tissue retrieved 6 weeks after implantation. Specific signals (brownish stain) were concentrated in the human epithelium of both stratified squamous and columnar cells (Fig. 2). They were not detected in surrounding human stromal tissue or in adjacent murine tissue. These findings were validated by both formalin-fixed cells from a cervical carcinoma cell line carrying one to two chromosomally integrated copies of the HPV 16 genome per cell (positive control) and normal tissue xenografts (negative control).
FIG. 2.
Presence of HPV 16 and/or 18 DNA in dysplastic implants as determined by in situ hybridization studies (both images taken at a magnification of ×40). The signals are concentrated in both stratified squamous (A) and columnar (B) epithelium of the human tissue (day 42). Signals were not detected in human stromal tissue or in murine tissue.
Recombinant viral infection.
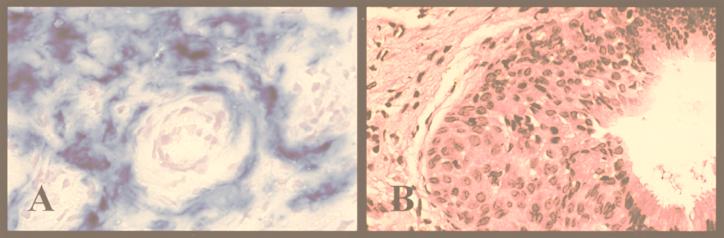
Prior to implantation, normal human cervical tissue was infected ex vivo for 1 h at 109 PFU/ml with the previously published human adenovirus type 5 containing the β-galactosidase gene driven by the human cytomegalovirus immediate-early promoter (20). At day 46, in an assay using 0.1% 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) blue stain (Life Technologies), the excised implant demonstrated expression of the β-galactosidase gene product (Fig. 3A). No evidence of expression was exhibited by neighboring murine tissue or contralateral implants.
FIG. 3.
Biologic and pharmacologic manipulation of normal tissues (magnification, ×40). (A) X-Gal stain of day 46 human cervical tissue infected with human adenovirus containing the β-galactosidase gene. X-Gal substrate turns blue in the presence of β-galactosidase; the diffuse staining pattern is evidence of widespread recombinant viral expression within the implant. (B) Effect on normal cervical tissue following implantation with 90-day-release 17β-estradiol pellet (1.7 mg). At day 24, the tissue exhibited disorganized structure and an increase in number of stratified nucleated epithelium, consistent with hyperplasia and a metaplastic state.
Estrogen administration.
A 90-day time-released 1.7-mg 17β-estradiol pellet (resulting in circulating estrogen levels of 500 to 600 pg/ml, according to the manufacturer [Innovative Research of America, Sarasota, Fla.]) was placed subcutaneously, using the implantation procedure described above. Pellets were placed contralateral to normal tissue xenografts. At day 24, all implants demonstrated regions of hyperplasia and squamous metaplasia (Fig. 3B), which were not present at day 0.
Potential applications of this model.
The presence of HPV infection in the vast majority of cervical neoplasms has been considered evidence of an etiologic role for HPV in the development of cervical dysplasia and eventually carcinoma (10, 26, 27, 31, 32, 39, 43, 44). Because the effect of HPV has generally been limited to immortalization, its precise role in the multistage process of cervical carcinogenesis is difficult to study. Despite evidence from transgenic murine systems which suggests that the viral genes E6 and E7 possess essential activity to effect a fully transformed phenotype, some have argued that HPV infection is not causative but represents an essential cofactor or even an opportunistic pathogen in a host whose immune system is compromised by disease (35). This area of controversy has led to a considerable effort invested into creating a mouse xenograft model that could propagate anogenital HPV. Indeed, several SCID mouse systems have been evolved to permit isolation and propogation of both low- and high-oncogenic-risk HPV subtypes (4, 5, 7, 11, 24, 25). However, these systems have yet to address the causative role of HPV infection.
Although the site of HPV integration appears to be random with respect to the host genome, during the integration process the viral E6 and E7 open reading frames are consistently retained. Our model should permit assessment of interactions of E6 and E7 with cellular proteins specific to the transformation zone. The inactivation of tumor suppressor gene products (e.g., p53 and retinoblastoma) secondary to protein binding or mutation may disrupt control of cellular proliferation and apoptosis, but why this might especially apply to the transformation zone is unknown.
In our model, the induction of cervical hyperlasia and metaplasia in normal tissue xenografts via concomitant high-dose estrogen exposure is noteworthy. The observed metaplastic changes of the cervical transformation zone that occur normally at puberty have been attributed to the effects of high circulating levels of estrogen present during that period. Indeed, the role of estrogen in the development of cervical dysplasia has been debated (38). Contemporary reports have documented synergistic effects between steroid hormones and HPV; specifically, in vitro studies have demonstrated that estrogen can enhance transcription of HPV 16 E6 and E7 oncogenes (22).
In summary, the importance of our model lies in the maintenance of the squamocolumnar junction, the preservation of normal and dysplastic features over extended periods of time, and the opportunity to effect change within the system. These attributes constitute many of the essential characteristics of a biologic model through which one may study HPV-mediated human disease. The interactions between steroid hormones and HPV oncogenes as well as gene therapy and even immune system reconstitution (30, 34, 36) represent areas which may be pursued with this SCID mouse model.
Acknowledgments
C.C.W.H. and L.P.V. contributed equally to this work.
We thank Sharon P. Wilczynski for her expertise in surgical pathology.
This work was supported by a grant awarded to C.C.W.H. from The Chao Family Comprehensive Cancer Center of the University of California, Irvine—Medical Center, a grant awarded to L.P.V. from the Cancer Research Coordinating Committee of the University of California, and The Center for Viral Vector Design of the University of California, Irvine.
REFERENCES
- 1.Arbeit J M, Howley P M, Hanahan D. Chronic estrogen-induced cervical and vaginal squamous carcinogenesis in human papillomavirus type 16 transgenic mice. Proc Natl Acad Sci USA. 1996;93:2930–2935. doi: 10.1073/pnas.93.7.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer H M, Ting Y, Greer C E, Chambers J C, Tashiro C J, Chimera J, Reingold A, Manos M M. Genital human papillomavirus infection in female university students as determined by a PCR-based method. JAMA. 1991;265:472–477. [PubMed] [Google Scholar]
- 3.Bonnez W. Murine models of human papillomavirus-infected human xenografts. Papillomavirus Rep. 1998;9:27–38. [Google Scholar]
- 4.Bonnez W, Darin C, Borkhuis C, De Messy Jensen K L, Reichman R C, Rose R C. Isolation and propagation of human papillomavirus type 16 in human xenografts implanted into severe combined immunodeficiency mouse. J Virol. 1998;72:5256–5261. doi: 10.1128/jvi.72.6.5256-5261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnez W, Rose R C, Da Rin C, Borkhuis C, De Mesy Jensen K L, Reichman R C. Propagation of human papillomavirus type 11 in human xenografts using the severe combined immunodeficiency (SCID) mouse and comparison to the nude mouse model. Virology. 1993;197:455–458. doi: 10.1006/viro.1993.1611. [DOI] [PubMed] [Google Scholar]
- 6.Bosch F X, Manos M M, Munos N, Sherman M, Jansen A M, Peto J, Schiffman M H, Moreno V, Kurman R, Shah K V. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 7.Brandsma J L, Brownstein D G, Xiao W, Longley B J. Papilloma formation in human foreskin xenografts after inoculation of human papillomavirus type 16 DNA. J Virol. 1995;69:2716–2721. doi: 10.1128/jvi.69.4.2716-2721.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bravo R, Frank R, Blundell P A, MacDonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987;326:515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- 9.Brisson J, Morin C, Fortier M, Roy M, Bouchard C, Leclerc J, Christen A, Guimont C, Penault F, Meisels A. Risk factors for cervical intraepithelial neoplasia: differences between low- and high-grade lesions. Am J Epidemiol. 1994;140:700–710. doi: 10.1093/oxfordjournals.aje.a117318. [DOI] [PubMed] [Google Scholar]
- 10.Burger R A, Monk B J, Kurosaki T, Anton-Culver H, Vasilev S A, Berman M L, Wilczynski S P. Human papillomavirus type 18: association with poor prognosis in early stage cervical cancer. J Natl Cancer Inst. 1996;88:1361–1368. doi: 10.1093/jnci/88.19.1361. [DOI] [PubMed] [Google Scholar]
- 11.Christensen N D, Koltun W A, Cladel N M, Budgeon L R, Reed C A, Kreider J W, Welsh P A, Patrick S D, Yang H. Coinfection of human foreskin fragments with multiple human papillomavirus types (HPV-11, -40, and -LVX82/MM7) produces regionally separate HPV infections within the same athymic mouse xenograft. J Virol. 1997;71:7337–7344. doi: 10.1128/jvi.71.10.7337-7344.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Custer R P, Bosma G C, Bosma M J. Severe combined immunodeficiency (SCID) in the mouse: pathology, reconstitution, neoplasms. Am J Pathol. 1985;120:464–477. [PMC free article] [PubMed] [Google Scholar]
- 13.Darwiche N, Celli G, G., de Luca L M. Specificity of retinoid receptor gene expression in mouse cervical epithelia. Endocrinology. 1994;134:2018–2025. doi: 10.1210/endo.134.5.8156902. [DOI] [PubMed] [Google Scholar]
- 14.DiSaia P J. Preinvasive disease of the cervix. In: DiSaia P J, Creasman W T, editors. Clinical gynecologic oncology—1998. St. Louis, Mo: Mosby Year Book; 1998. pp. 1–32. [Google Scholar]
- 15.Eckert R L, Rorke E A. Molecular biology of keratinocyte differentiation. Environ Health Perspect. 1989;80:109–116. doi: 10.1289/ehp.8980109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuchs E, Byrne C. The epidermis: rising to the surface. Curr Opin Genet Dev. 1994;4:725–736. doi: 10.1016/0959-437x(94)90140-x. [DOI] [PubMed] [Google Scholar]
- 17.Gorodeski G I, Romero M F, Hopfer U, Rorke E, Utian W H, Eckert R L. Human uterine cervical epithelial cells grown on permeable support: a new model for the study of differentiation. Differentiation. 1994;56:107–118. doi: 10.1046/j.1432-0436.1994.56120107.x. [DOI] [PubMed] [Google Scholar]
- 18.Griep A E, Lambert P F. Role of papillomavirus oncogenes in human cervical cancer: transgenic animal studies. Proc Soc Exp Biol Med. 1994;206:24–34. doi: 10.3181/00379727-206-43720a. [DOI] [PubMed] [Google Scholar]
- 19.Gulliver G A, Herber R L, Liem A, Lambert P F. Both conserved region 1 (CR1) and CR2 of the human papillomavirus type 16 E7 oncogene are required for induction of epidermal hyperplasia and tumor formation in transgenic mice. J Virol. 1997;71:5905–5914. doi: 10.1128/jvi.71.8.5905-5914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kammesheidt A, Ito K, Kato K, Villarreal L P, Sumikawa K. Transduction of hippocampal CA1 by adenovirus in vivo. Brain Res. 1996;736:297–304. doi: 10.1016/0006-8993(96)00715-9. [DOI] [PubMed] [Google Scholar]
- 21.Katase K, Teshima H, Hirai Y, Hasumi K. Natural history of cervical human papillomavirus lesions. Intervirology. 1995;38:192–194. doi: 10.1159/000150432. [DOI] [PubMed] [Google Scholar]
- 22.Khare S, Pater M M, Tang S C, Pater A. Effect of glucocorticoid hormones on viral gene expression, growth, and dysplastic differentiation in HPV 16-immortalized ectocervical cells. Exp Cell Res. 1997;232:353–360. doi: 10.1006/excr.1997.3529. [DOI] [PubMed] [Google Scholar]
- 23.Kopan R, Traska G, Fuchs E. Retinoids as important regulators of terminal differentiation: examining keratin expression in individual epidermal cells at various stages of keratinization. J Cell Biol. 1987;105:427–440. doi: 10.1083/jcb.105.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kreider J W, Howett M K, Stoler M H, Zaino R J, Welsh P. Susceptibility of various human tissues to transformation in vivo with human papillomavirus type 11. Int J Cancer. 1987;39:459–465. doi: 10.1002/ijc.2910390409. [DOI] [PubMed] [Google Scholar]
- 25.Kreider J W, Howett M K, Wolfe S A, Bartlett G L, Zaino R J, Sedlacek T, Mortel R. Morphological transformation in vivo of human uterine cervix with papillomavirus from condylomata acuminata. Nature. 1985;317:639–641. doi: 10.1038/317639a0. [DOI] [PubMed] [Google Scholar]
- 26.Kurman R J, Schiffman M H, Lancaster W E D, Reid R, Jenson A B, Temple G F, Lorincz A Z. Analysis of individual human papillomavirus types in cervical neoplasia: A possible role for type 18 in rapid progression. Am J Obstet Gynecol. 1988;159:293–296. doi: 10.1016/s0002-9378(88)80070-x. [DOI] [PubMed] [Google Scholar]
- 27.Lombard I, Vincent-Salomon A, Validire P, Zafrani B, De la Rochefordiere A, Clough K, Favre M, Pouillart P, Sastre-Garau X. Human papillomavirus genotype as a major determinant of the course of cervical cancer. J Clin Oncol. 1998;16:2613–2619. doi: 10.1200/JCO.1998.16.8.2613. [DOI] [PubMed] [Google Scholar]
- 28.Matthews M B, Bernstein R M, Franza B R J, Garrels J I. Identity of the proliferating cell nuclear antigen and cyclin. Nature. 1984;309:374–376. doi: 10.1038/309374a0. [DOI] [PubMed] [Google Scholar]
- 29.McCance D J, Kopan R, Fuchs E, Laimins L A. Human papillomavirus type 16 alters human epithelial cell differentiation in vitro. Proc Natl Acad Sci USA. 1988;85:7169–7173. doi: 10.1073/pnas.85.19.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCune J M, Namikawa R, Kaneshima H, Shultz L D, Lieberman M, Weissman I L. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 31.Meanwell C A, Cox M F, Blackedge G B, Maitland N J. HPV 16 DNA in normal and malignant cervical epithelium: implications for the aetiology and behaviour of cervical neoplasia. Lancet. 1987;1:703–707. doi: 10.1016/s0140-6736(87)90353-9. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell H, Drake N, Medley G. Prospective evaluation of risks of cervical cancer after cytologic evidence of human papilloma virus infection. Lancet. 1986;1:573–575. doi: 10.1016/s0140-6736(86)92807-2. [DOI] [PubMed] [Google Scholar]
- 33.Moll R, Levy R, Czernobilsky B, Hohlweg-Majert P, Dallenbach-Hellweg G, Franke W W. Cytokeratins of normal epithelia and some neoplasms of the female genital tract. Lab Investig. 1983;49:599–610. [PubMed] [Google Scholar]
- 34.Mosier D E, Gulizia R J, Baird S M, Wilson D B. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988;335:256–259. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- 35.Munoz N, Bosch X, Kaldor J M. Does human papillomavirus cause cervical cancer? The state of the epidemiological evidence. Br J Cancer. 1988;57:1–5. doi: 10.1038/bjc.1988.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murray A G, Petzelbauer P, Hughes C C W, Costa J, Askenase P, Pober J S. Human T-cell-mediated destruction of allogeneic dermal microvessels in a severe combined immunodeficient mouse. Proc Natl Acad Sci USA. 1994;91:9146–9150. doi: 10.1073/pnas.91.19.9146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newman P J. The biology of PECAM-1. J Clin Investig. 1997;99:3–8. doi: 10.1172/JCI119129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piper J M. Oral contraceptives and cervical cancer. Gynecol Oncol. 1985;22:1–14. doi: 10.1016/0090-8258(85)90001-0. [DOI] [PubMed] [Google Scholar]
- 39.Reeves W C, Brinton L A, Garcia M, Brenes M M, Herrero R, Gaitan E, Tenorio F, de Britton R C, Rawls W E. Human papillomavirus infection and cervical cancer in Latin America. N Engl J Med. 1989;320:1437–1441. doi: 10.1056/NEJM198906013202201. [DOI] [PubMed] [Google Scholar]
- 40.Renz J F, Lin Z, de Roos M, Dalal A A, Ascher N L. SCID mouse as a model for transplantation studies. J Surg Res. 1996;65:34–41. doi: 10.1006/jsre.1996.0340. [DOI] [PubMed] [Google Scholar]
- 41.Smith-McCune K, Zhu Y H, Hanahan D, Arbeit J. Cross-species comparison of angiogenesis during the premalignant stages of squamous carcinogenesis in the human cervix and K14-HPV 16 transgenic mice. Cancer Res. 1997;57:1294–1300. [PubMed] [Google Scholar]
- 42.Song S, Gulliver G A, Lambert P F. Human papillomavirus type 16 E6 and E7 oncogenes abrogate radiation-induced DNA damage responses in vivo through p53-dependent and p53-independent pathways. Proc Natl Acad Sci USA. 1998;95:2290–2295. doi: 10.1073/pnas.95.5.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Storey A, Thomas M, Kalita A, Harwood C, Gardiol D, Mantovani F, Breuer J, Leigh I M, Matlashewski G, Banks L. Role of a p53 polymorphism in the development of human papillomavirus-associated cancer. Nature. 1998;393:229–234. doi: 10.1038/30400. [DOI] [PubMed] [Google Scholar]
- 44.Walker J, Bloss J D, Liao S Y, Berman M L, Bergen S, Wilczynski S P. Human papillomavirus genotype as a prognostic indicator in carcinoma of the uterine cervix. Obstet Gynecol. 1989;74:781–785. [PubMed] [Google Scholar]
- 45.Watt F M. Terminal differentiation of epidermal keratinocytes. Curr Opin Cell Biol. 1989;1:1107–1115. doi: 10.1016/s0955-0674(89)80058-4. [DOI] [PubMed] [Google Scholar]