Abstract
ABSTRACT
Introduction
Contemporary data on the burden of chronic respiratory diseases in sub-Saharan Africa is limited. More so, their economic burden is not well described. This study aims to establish a chronic respiratory disease observatory for Africa. Specific study aims are (1) to describe the prevalence and determinants of asthma with a target to screen up to 4000 children and adolescents across four African cities; (2) to determine the prevalence and determinants of chronic obstructive pulmonary disease (COPD) with a target to screen up to 3000 adults (≥18 years) across five African cities; (3) to describe the disease burden by assessing the frequency and severity of symptoms and exacerbations, medication use, emergency healthcare utilisation and hospitalisation; and (4) to assess the economic burden and affordability of the medicines for these diseases.
Methods and analysis
Surveys will be conducted in schools to identify children and adolescents with asthma using the Global Asthma Network screening questionnaire in Ghana, Nigeria, the Democratic Republic of Congo, and Uganda. Community surveys will be conducted among adults using an adapted version of the Burden of Obstructive Lung Disease Questionnaire to identify persons with COPD symptoms in Nigeria, Burkina Faso, Mozambique, Rwanda, and Sierra Leone. Fractional exhaled nitric oxide and pre-bronchodilator and post-bronchodilator spirometry will be done for children with asthma or asthma symptoms and for all adult participants. Children and adults with respiratory symptoms or diagnoses will complete the health economic questionnaires. Statistical analysis will involve descriptive and analytical statistics to determine outcomes.
Ethics and dissemination
Ethical approval has been obtained from participating institutions. This study’s results will inform deliberations at the United Nations General Assembly high-level meeting on non-communicable diseases in 2025. The results will be shared through academic conferences and journals and communicated to the schools and the communities.
Keywords: Asthma; Pulmonary Disease, Chronic Obstructive
WHAT IS ALREADY KNOWN ON THIS TOPIC
The prevalence of asthma and chronic obstructive pulmonary disease (COPD) is available only for a few African countries and the economic impact of these diseases has not been well described in the continent. There is a need for broad-based data from Africa on these common respiratory diseases.
WHAT THIS STUDY ADDS
This study will provide contemporary and standardised data on the prevalence, determinants and economic impact of asthma and COPD across different age groups from nine African countries. Additionally, it will provide much-needed data on the pattern of airway inflammation from a large African population.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our findings will guide advocacy and inform policy that might improve the current state of disease management and outcomes in Africa.
Introduction
The WHO reports that non-communicable diseases (NCDs) account for up to 70% of global mortality, with three-quarters of NCD-related deaths occurring in low-income and middle-income countries (LMICs), largely due to increased exposure to the risk factors and limited access to health services.1 Chronic respiratory diseases (CRDs), together with diabetes, cardiovascular disease and cancer, are four priority diseases responsible for 80% of NCD-related mortality.
Reduction in the burden of NCDs is a global priority, with the WHO targeting a 25% reduction in mortality by 2025.1 Similarly, the United Nations (UN) Sustainable Development Goal 3.4 (SDG 3.4) targets a one-third decrease in premature deaths due to NCDs by 2030.2 Achieving these goals requires multisectoral interventions, including achievement of SDG 3.8, ‘access to safe, effective, quality and affordable essential medicines and vaccines for all’. A UN high-level meeting on NCDs is planned for 2025 to assess progress towards achieving the 2030 SDG goals. Contemporary data on the burden, determinants and access to diagnostics and medicines for managing NCD, including CRDs, must be available in time for that meeting.
Sub-Saharan Africa (sSA) bears a substantial burden of CRDs, with the prevalence of asthma across all age groups ranging between 10% and 15%,3 4 and the prevalence of chronic obstructive pulmonary disease (COPD) between 1.7% and 24%.5,7 The wide variation in the prevalence of these diseases is partly attributable to differences in case definition and study methodology. Data from many African countries are also limited, making it difficult to estimate the regional disease burden. Contemporaneously, underdiagnosis is a major challenge in the region, contributing to poor disease outcomes. For example, data from Nigeria reports an under-diagnosis rate of about 50% and 99% for asthma and COPD, respectively.8 9 Uncontrolled asthma is associated with absenteeism, poor health-related quality of life and increased mortality.10 11 In Uganda, preventable asthma deaths occur at rates three times higher than in high-income countries.11 12 COPD is projected to be the third leading cause of death by 2030, and currently, 90% of COPD deaths occur in LMIC, where resources for prevention, diagnosis and treatment are scarce.13
In addition to the health burden, CRDs impose an economic burden on patients, their families and society. Inhaled medicines are the cornerstone for treating asthma and COPD; access depends on availability and affordability. Data from a few individual African countries suggest these medicines are not readily available and mostly unaffordable.14,16 Economic costs are categorised as direct costs incurred from using healthcare resources for diagnosis and treatment and indirect costs from lost productivity or absenteeism.17 Indirect costs include income loss due to caregiver absenteeism and reduced productivity borne by co-workers who unexpectedly work without their colleagues. COPD-related lost work days were reported as 16.4 million in the USA in 2010 (averaging 3.2 days lost per worker with treated COPD).18 Due to underdiagnosis and undertreatment of asthma and COPD in sSA, the total societal economic burden of these diseases is expected to be much higher.
The purchase of medicines accounts for the highest direct costs incurred in managing CRDs.17 19 In most parts of Africa, where payment is largely out of pocket, management of CRDs leads to catastrophic health costs, with the poorest being the most affected.19 Medication prices vary between countries due to differing subsidy rates, margins and procurement strategies.14 20
Health policies and interventions are underpinned by evidence from reliable data sources that show the burden, determinants and consequences of disease as well as the cost-effectiveness of potential interventions. The wide variation in the global burden of asthma and COPD merits regional surveillance to inform local and international policymaking. It is likely that the limited availability of facilities and medicines for managing asthma and COPD in parts of sSA partly reflects low prioritisation due to the inadequate information on disease burden. Broad-based data from studies that use standardised methods implementable in various settings are well suited to provide the much-needed reliable comparative data to drive policies. A few but not all African countries are included in the largest global epidemiological surveys of asthma and COPD: the Global Asthma Network (GAN) study21 and the Burden of Obstructive Lung Disease (BOLD) study.22 Furthermore, the lack of information on the economic burden of CRD in African countries and insufficient information on medication availability and affordability underscore the need for a broad-based regional study.
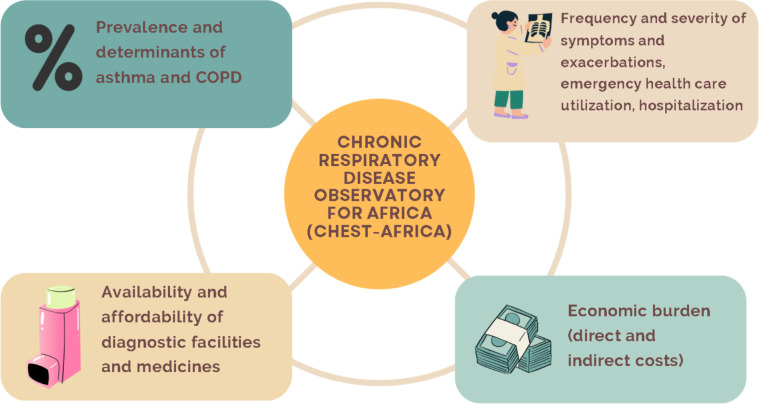
Disease observatories provide high-quality, relevant regional health intelligence that informs public health policies. By synthesising existing data and targeted data collection using strong networks, chronic disease observatories serve as a repository of health data accessible to all who need them. The agenda-setting for disease observatories is time-sensitive and evolves based on epidemiological trends, available resources and policy needs. Hence, the initial focus of this chronic respiratory disease observatory for Africa (CHEST-Africa) is on asthma and COPD, the most common non-communicable respiratory diseases.
We set out to establish CHEST-Africa, with the aim of providing standardised data on the prevalence, burden and determinants of asthma and COPD in sSA, including the economic burden and the current state of availability and affordability of medicines for these diseases. The observatory focuses on collating data across the life course, from children, adolescents and adults by using predominantly digitalised primary data collection, management, analysis and presentation tools. We will draw on the well-established methodologies and experience of the GAN and BOLD consortia in conducting large-scale global surveys for CRD for data collection, processing, analysis and presentation.
Aims and objectives
The overall objective of CHEST-Africa is to provide a cross-sectional view of accessible data on the prevalence, risk factors and economic burden of asthma and COPD in sSA. The primary objectives are:
To describe the prevalence and determinants of asthma with a target to screen up to 2000 children (6–7 years old) and 2000 adolescents (13–14 years old) across four sSA cities.
To determine the prevalence and determinants of COPD with a target to screen up to 3000 adults (>18 years old) in five sSA countries.
To describe the disease burden among those identified by assessing the frequency and severity of symptoms and exacerbations, medication use, emergency healthcare utilisation and hospitalisation for these CRDs.
To assess the economic burden of asthma and COPD among those identified with either disease.
A secondary objective is to evaluate access to treatment by surveying the availability and affordability of medicines for managing asthma and COPD in sSA.
The data collected from these studies will be used to initiate an online dashboard of the burden of CRD in Africa. Figure 1 shows the thematic framework for CHEST-Africa.
Figure 1. Thematic framework for CHEST-Africa. CHEST-Africa, chronic respiratory disease observatory for Africa.
Study design
This is a multicountry cross-sectional survey involving the assessment of asthma in children and adolescents and COPD in adults across nine African cities (Kumasi, Ghana; Kampala, Uganda; Kano, Nigeria; Lubumbashi, Democratic Republic of Congo (DRC); Lagos, Nigeria; Ouagadougou, Burkina Faso; Freetown, Sierra Leone; Kigali, Rwanda; Maputo, Mozambique). This will include the measurement of lung function using spirometry and the measurement of eosinophilic lung inflammation using the fractional exhaled nitric oxide (FeNO) test. In those identified to have asthma and COPD, a health economic questionnaire has been designed to capture the individual direct and indirect costs of asthma and COPD care. Additionally, surveys of public and private pharmacies will be conducted across these nine cities and other sSA cities to determine the availability and affordability of medicines for asthma and COPD.
Methods
Site investigators
Data collection at all nine urban cities will be led by a local principal investigator with an academic position at an African University or research institute.
Study participants
The study participants will comprise males and females of four age groups: 6–7 years, 13–14 years, 18–39 years and >40 years.
Participant recruitment and data collection
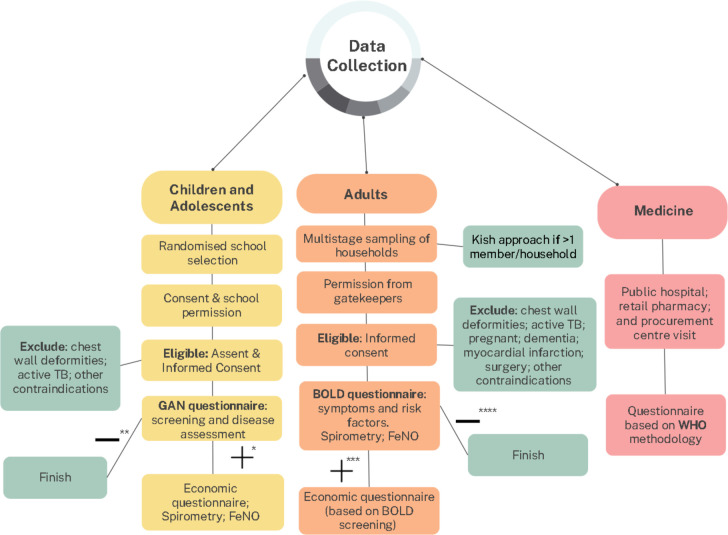
Children and adolescents will be recruited from schools and adults will be recruited from the community (figure 2). Spirometry and FeNO levels will be measured for all children and adolescents who have a diagnosis of asthma or symptoms suggestive of asthma and for all adult participants.
Figure 2. Flow diagram of the recruitment and data collection process. *Screened positive for asthma, **Screened negative for asthma, ***Screened positive for asthma or COPD, ****Screened negative for asthma or COPD.
The economic burden data will be collected only from children, adolescents and adults with symptoms suggestive of asthma or COPD.
Figure 2 shows the flow diagram for recruitment of participants and data collection.
Recruitment of children and adolescents
All children and adolescents will be recruited from schools in four cities (table 1). Schools will be selected at random from a sampling frame provided for each participating site, including private and public primary (6–7 years—children) or secondary (13–14 years—adolescents) schools. Following local requirements for establishing contact, seeking permission from the school management and gaining access to the schools, each site will aim to recruit a minimum sample of 1000 children from the selected schools. All children within the target age group in selected schools who assent and whose parents provide informed consent will be eligible to participate. Children with chest wall deformities or those on active tuberculosis treatment or other contraindications for spirometry will be excluded from testing.
Table 1. Study sites, disease focus and age group and of participants.
| Country | City | Age group (years) | Disease focus | Target sample size |
| Ghana | Kumasi | 6–7 | Asthma | 1000 |
| Uganda | Kampala | 6–7 | Asthma | 1000 |
| Nigeria | Kano | 13–14 | Asthma | 1000 |
| DRC | Lubumbashi | 13–14 | Asthma | 1000 |
| Burkina Faso | Ouagadougou | 18–39 | COPD | 600 |
| Nigeria | Lagos | 18–39 | COPD | 600 |
| Sierra Leone | Freetown | >40 | COPD | 600 |
| Rwanda | Kigali | >40 | COPD | 600 |
| Mozambique | Maputo | >40 | COPD | 600 |
COPDchronic obstructive pulmonary disease
Recruitment of adults
Adult participants (18–39 years or >40 years) will be recruited from selected households using multistage cluster sampling of pre-defined government-demarcated areas in five cities (table 1). Clusters (administrative/enumeration areas) will be selected by simple random selection. Within each selected cluster, households will be selected randomly. For each selected household, a maximum of one male and one female belonging to the target age group will be recruited. Where more than one male and/or one female household member is of the target age group, a Kish approach will be used to select one of each sex. The Kish selection method is widely used in epidemiological surveys to reduce bias in selecting household members.23 It uses a number grid to select individuals based on assigned numbers of eligible participants (ranked according to age) juxtaposed with the assigned household number. The aim is to recruit at least 300 males and 300 females per site. The city administrators’ and community gatekeepers’ permission will be obtained before community entry. Adults with chest wall deformity, on active tuberculosis treatment, pregnant, or those with dementia, recent myocardial infarction, surgery, or other contraindications for spirometry will be excluded.
Questionnaire screening for asthma and assessment of disease burden among children and adolescents
Informed consent will be obtained from the parent or guardian and assent will be obtained from the children before data collection. The participant information sheet and consent form will be sent home to the parents through the class teachers and returned before enrolling any participant. With the support of the class teachers, reminders will be sent to parents to return the forms, and the research team will be available in the school to offer clarification to parents or guardians. The adapted GAN asthma questionnaire for 6–7-year-olds or 13–14-year-olds will be used for the respective age groups to screen participants for asthma and assess the disease burden among persons with asthma. This questionnaire asks about a history of asthma symptoms (wheezing) or ‘ever asthma’ to identify persons with asthma. We will define asthma as a ‘history of wheeze in the preceding 12 months’ or/and affirmation to ‘ever asthma’. The questionnaire also obtains information on physician diagnosis of asthma, medication use and healthcare service use for persons with asthma.
For the 6–7-year-old children, the parents will complete a paper version of the questionnaire. The study team will subsequently enter the information into the electronic data management tool.
The 13–14-year-old adolescents will complete a self-administered electronic version of the adapted GAN questionnaire in the school.
Translations to the predominant local languages for the questionnaires will be done at each site with forward and backward translations.
Questionnaire screening for COPD or asthma and assessment of disease burden among adults
A questionnaire adapted from the BOLD study core form will be administered by a trained interviewer and completed on a tablet computer. The BOLD Questionnaire covers information on cough, phlegm, breathlessness and wheezing, and previous doctor diagnoses of asthma, emphysema, bronchitis and COPD, including comorbidities. It also collects information on exposure to COPD risk factors and the participant’s knowledge about the effects of some risk factors. The questionnaire also assesses medication and healthcare service use among persons with respiratory symptoms or a previous respiratory diagnosis. A positive screen for asthma is based on report of ‘current wheeze or ever asthma’ or finding reversibility in pre-bronchodilator airflow obstruction, while COPD is based on previous doctor diagnosed COPD or spirometry finding of post-bronchodilator airflow obstruction with symptoms (cough, phlegm, breathlessness).
Economic burden of asthma and COPD
The parents of all children and adolescents who are identified to have asthma will complete the youth version of the health economic questionnaire, while the adults with asthma or COPD symptoms will complete the adult version of the health economic questionnaire. These questionnaires assess the direct and indirect economic disease burden and were developed following recommended guidelines to suit the African context, as no official validated tool is widely used.24 The questionnaires underwent revision during the investigator meetings to ensure they were context specific.
The questionnaires assess direct costs such as money spent on medicines, hospital stay, at-home care, doctors’ fees, investigations and transportation, as well as indirect costs such as lost time from work for both the patient and caregivers, the number and length of hospitalisations due to exacerbations, the number of outpatient visits and the number of restricted activity days due to asthma or COPD. They also explore catastrophic health expenditure by measuring access to health insurance, whether medicines and diagnostics are covered by health insurance, family income levels, and source of funds for managing asthma or COPD.
FeNO and spirometry measurement
FeNO will be measured using a standardised procedure according to the American Thoracic Society/European Respiratory Society (ATS/ERS) standardised methods using the Spirosure FenomPro point of care breath analyser. It will be measured on only children and adolescents with asthma, as well as for all adult participants. FeNO will be measured prior to spirometry testing. Self-reported history of rhinitis, recent meal and recent tobacco smoking will be documented prior to measurement. FeNO will be interpreted using the ATS clinical practice guideline as high if >50 ppb in adults and >35 ppb in children, intermediate between 25 ppb and 50 ppb in adults and 20–35 ppb in children and low <25 ppb in adults and <20 ppb in children.
Spirometry will be done using the Vyaire Vyintus Pneumo spirometers following the ATS/ERS 2019 standards for only children and adolescent participants with asthma, as well as for all adult participants.25 A calibration verification check will be done daily for all spirometers. Pre-bronchodilator and post-bronchodilator (15–20 min after administration of 400 μg of inhaled salbutamol using a spacer) measurements will be done. All technicians conducting the tests have been trained and certified before the survey.
All spirograms will be assessed for quality using the Artiq.QC quality assurance software and only results that meet all ATS/ERS standards for acceptability will be used. The Artiq.QC software evaluates the quality of the data by checking the numerical quality criteria, as well as the visual shape of the curve with its deep-learning artificial intelligence.26 Doing so mimics the human visual evaluation of the curve, typically done by human over-readers. It can detect artefacts defined by the ATS/ERS standards and evaluate the impact of these artefacts on the acceptability and/or usability of the data. The Artiq.QC agreed with human over-readers in 83% of cases with 93% sensitivity. In addition, there will also be manual over-reading of a small proportion of the tests by a certified spirometry over-reader to compare with Artiq.QC software.
Spirometry measures of interest are the forced vital capacity (FVC) in litres, forced expiratory volume in the first second (FEV1) in litres, the ratio of the FEV1/FVC in percentage and forced expiratory flow 25%–75% in litres.
Availability and cost of medicines for asthma and COPD
Essential asthma and COPD medicine availability and cost will be assessed from cities in countries with a local collaborator. Trained data collectors will collect information using strategic sampling to ensure inclusion of pharmacies representing the public sector, and private sector following an adaptation of the guidance of the WHO/Health Action Initiative method.27 For each city, five public sector hospital pharmacies (main one located in a federal or state-owned tertiary or secondary care hospital and at least four others within the city) and five private sector medicine pharmacies (randomly selected from those closest to each public hospital as identified by data collector). Backup pharmacies for each sector will be identified for each of the pharmacy types, to be surveyed in the event of non-availability of 50% of the listed medicine categories at the primary pharmacy. We will also survey the national procurement centre if available in each participating country.
The presence and cost of the medicines listed on the WHO essential medicine list for the treatment of asthma and COPD (beclomethasone, budesonide, budesonide+formoterol, epinephrine/epinephrine, ipratropium bromide, salbutamol, tiotropium and prednisolone) will be surveyed.28 Information obtained will include the presence (determined by sighting the medicine), the originator and generic brand availability, dosage strengths, quantity and cost per defined pack and per unit tablet/inhalation. Any surveyed medicine will be considered available if present at the time of visit in 80% of all surveyed pharmacies and affordable if the cost is less than a day’s wage of the least-paid government worker or the least-paid unskilled worker in the country.
Study outcome measures
The prevalence of asthma (current wheezing and/or ever asthma) among children, adolescents based on screening questionnaires and the prevalence of COPD among adults based on post-bronchodilator airway obstruction on spirometry with symptoms.
The prevalence of severe asthma defined as ≥4 attacks of wheeze per week, or >1 night per week sleep disturbance from wheeze, or wheeze affecting speech in children and adolescents.
The severity of airflow limitation among children and adolescents with asthma based on pre-bronchodilator airflow obstruction.
The severity of airflow limitation in COPD based on post-bronchodilator airflow obstruction.
The severity of eosinophilic airway inflammation measured by FeNO among children and adolescents with asthma.
The presence of eosinophilic inflammation among adults and its association with COPD.
Direct and indirect economic burden of asthma and COPD based on health economic analysis of responses to health economic questionnaire.
Availability and affordability of asthma and COPD medicines.
Public and patient involvement
All sites will conduct a public involvement programme suitable to their local context to discuss the study protocol before data collection and to share the study findings afterwards. This will include school-based and community-based education programmes on disease prevention and management.
Data management
RedCap (2022 Vanderbilt University) will be used for centralised data collection and management. Only de-identified data will be collected, and no personal information will be shared across countries. A dedicated data management team will continuously monitor the data in real time to address irregularities in a timely manner. The adolescent and adult questionnaires will be completed electronically directly on RedCap. Questionnaires completed on paper (6–7-year-old children and health economic questionnaires for 13–14-year-old adolescents) will be entered on RedCap by the members of the site research team. The FeNO results and the spirometry variables will be recorded on Redcap. The predominant use of electronic case report forms will make data management more practicable, allow real-time validity checks and hence increase internal validity.
Ethics and dissemination
Ethical approval has been obtained from the institutional ethics committees of the following participating sites: Kwame Nkrumah University of Science and Technology, Kumasi, Ghana (CHRPE/AP/334/23); Lagos University Teaching Hospital, Lagos, Nigeria (ADM/DSCST/HREC/APP/5628); Kano State Ministry of Health, Kano, Nigeria (SHREC/2023/3854); University of Lubumbashi, DRC (UNILI/CEM/016/2023); Ministry of Public Health, Burkina Faso (3023-04-081); King Faisal Hospital Rwanda (KFH/2023/063/IRB) and Rwanda National Ethics committee (RNEC 42/2024); Ministry of Health, Sierra Leone (010/02/2023); and Makerere Lung Institute, Kampala, Uganda (MHREC-2023–107).
Only anonymised data will be collected on the questionnaires, and all information will be confidential. Each participant will be identified by a participant ID. Access to identifiable data will only be available within each country and these will not be shared across countries. All study sites will be trained to ensure that all identifiable data are protected and stored securely. Data protection and sharing will adhere to the South African Data protection laws.
Test results will be shared with all participants and any participant who is screened positive for asthma or COPD who did not have a prior diagnosis of disease or who was not receiving treatment for previously diagnosed disease will be referred to the nearest government hospital or other hospital of their choice for further evaluation and treatment.
The study findings will be used to develop and grow a real-time dashboard that will be hosted on the CHEST-Africa observatory as soon as reasonable data becomes available. Findings will be published in peer-reviewed journals and presented at conferences. They will also be shared with the participating schools and communities and shared using several media channels such as the Pan African Thoracic Society website.
Strengths and potential limitations
The strengths of this proposed study include the wide range of African countries and age groups of participants from whom data will be collected. The use of standardised and validated methodologies and questionnaires of the GAN and BOLD consortia is an additional strength. This study will be one of the first to measure airway inflammation using FeNO from a large population-based African sample, including assessing the economic impact of asthma and COPD. We recognise that the selection of children from schools implies that those who do not attend school or those who miss school due to ill health, including asthma exacerbations may be excluded. Also, selecting only urban cities for this study limits the generalisability of our expected findings to rural areas where the situation may be distinct. However, as we intend to grow this observatory, we plan in future studies to widen the scope for participant selection and extend the sites to rural areas.
Acknowledgements
We appreciate the support of the GAN and BOLD consortia and Prof Sonia Buist, as well as all the student and faculty of the Pan African Thoracic Society Methods in Epidemiological Clinical and Operations Research (PATS MECOR) course (2022-2023).
Footnotes
Funding: This publication is supported by Fogarty International Centre of the National Institutes of Health under Grant No. 1R25TW011217-03 African Association for Health Professions Education and Research through the African Forum for Research and Education in AFREhealth (AFREhealth). RM is funded through the NIHR Global Health Research Professorship. This research was funded by the NIHR (NIHR302418) using UK international development funding from the UK Government to support global health research. The views expressed in this publication are those of the author(s) and not necessarily those of the NIHR or the UK government. ML is supported by the Academy of Medical Sciences Professorship (APR7\1005). We thank the following for providing funding and equipment support: African Research Universities Alliance - Imperial Research Seed Fund, Vyaire Medical, Aldama Foundation, Artic.QC®.
Data availability free text: Data are available on reasonable request.
Patient consent for publication: Not applicable.
Ethics approval: This study involves human participants and was approved by Lagos University Teaching Hospital, Lagos, Nigeria (ADM/DSCST/HREC/APP/5628); Kano State Ministry of Health, Kano, Nigeria (SHREC/2023/3854); University of Lubumbashi, DRC (UNILI/CEM/016/2023); Ministry of Public Health, Burkina Faso (3023-04-081); King Faisal Hospital Rwanda and Rwanda National Ethics committee (KFH/2023/063/IRB); Ministry of Health, Sierra Leone (010/02/2023); Mulago Hospital Research Ethics Committee, Uganda (MHREC-2023-107). Participants gave informed consent to participate in the study before taking part.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Contributor Information
Obianuju B Ozoh, Email: oozoh@unilag.edu.ng.
Nqobile Ndimande, Email: NdimandeN2@ukzn.ac.za.
Andre F S Amaral, Email: a.amaral@imperial.ac.uk.
Maia Lesosky, Email: m.lesosky@imperial.ac.uk.
Josue Mbonigaba, Email: Mbonigaba@ukzn.ac.za.
Marie Stolbrink, Email: Marie.Stolbrink@lstmed.ac.uk.
Lindsey Zurba, Email: linds@educationforhealth.africa.
Tochukwu Ayo-Olagunju, Email: tochie.ayoolag@gmail.com.
Tony Kayembe-Kitenge, Email: tonykayemb@gmail.com.
Suliaman Lakoh, Email: lakoh2009@gmail.com.
Ana Mocumbi, Email: amocumbi@gmail.com.
Jibril Mohammed, Email: jmohammed.pth@buk.edu.ng.
Rebecca Nantanda, Email: rnantanda@gmail.com.
Elizabete Nunes, Email: dra.elizabete.nunes@gmail.com.
Abdoul Risgou Ouédraogo, Email: oarisgou@yahoo.fr.
Sandra Owusu, Email: abenaboamah18@gmail.com.
Jean Pierre Sibomana, Email: jepisibo@gmail.com.
Refiloe Masekela, Email: masekelar@ukzn.ac.za.
Kevin Mortimer, Email: MortimerK@ukzn.ac.za.
Data availability statement
Data are available upon reasonable request.
References
- 1.Dugani S, Gaziano TA. 25 by 25: achieving global reduction in cardiovascular mortality. Curr Cardiol Rep. 2016;18:10. doi: 10.1007/s11886-015-0679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United Nations Transforming our world: the 2030 agenda for sustainable development.:. sustainable development knowledge platform. 2015. https://sustainabledevelopment.un.org/post2015/transformingourworld/publication Available.
- 3.Musa BM, Aliyu MD. Asthma prevalence in Nigerian adolescents and adults: systematic review and meta-analysis. Afr J Respir Med. 2014;10:4–9. [Google Scholar]
- 4.Mortimer K, Lesosky M, García-Marcos L, et al. The burden of asthma, hay fever and eczema in adults in 17 countries: GAN phase I study. Eur Respir J. 2022;60:2102865. doi: 10.1183/13993003.02865-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Awokola BI, Amusa GA, Jewell CP, et al. Chronic obstructive pulmonary disease in sub-Saharan Africa. Int J Tuberc Lung Dis. 2022;26:232–42. doi: 10.5588/ijtld.21.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ale BM, Ozoh OB, Gadanya MA, et al. Estimating the prevalence of COPD in an African country: evidence from Southern Nigeria. J Glob Health Rep . 2022;6:e2022049. doi: 10.29392/001c.38200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burney P, Patel J, Minelli C, et al. Prevalence and population-attributable risk for chronic airflow obstruction in a large multinational study. Am J Respir Crit Care Med. 2021;203:1353–65. doi: 10.1164/rccm.202005-1990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozoh OB, Aderibigbe SA, Ayuk AC, et al. The prevalence of asthma and allergic rhinitis in Nigeria: a nationwide survey among children, adolescents and adults. PLOS ONE. 2019;14:e0222281. doi: 10.1371/journal.pone.0222281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Obaseki DO, Erhabor GE, Gnatiuc L, et al. Chronic airflow obstruction in a black African population: results of BOLD study, Ile-Ife, Nigeria. COPD J Chronic Obstr Pulm Dis. 2016;13:42–9. doi: 10.3109/15412555.2015.1041102. [DOI] [PubMed] [Google Scholar]
- 10.Ozoh OB, Ayuk AC, Ukwaja KN, et al. Asthma management and control in Nigeria: the asthma insight and reality Nigeria (AIRNIG) study. Expert Rev Respir Med. 2019;13:917–27. doi: 10.1080/17476348.2019.1651201. [DOI] [PubMed] [Google Scholar]
- 11.Kirenga BJ, de Jong C, Mugenyi L, et al. Rates of asthma exacerbations and mortality and associated factors in Uganda: a 2-year prospective cohort study. Thorax. 2018;73:983–5. doi: 10.1136/thoraxjnl-2017-211157. [DOI] [PubMed] [Google Scholar]
- 12.Nantanda R, Tumwine JK, Ndeezi G, et al. Asthma and pneumonia among children less than five years with acute respiratory symptoms in Mulago hospital, Uganda: evidence of under-diagnosis of asthma. PLoS One. 2013;8:e81562. doi: 10.1371/journal.pone.0081562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organisation Chronic obstructive pulmonary disease (COPD) [01-May-2022]. https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) Available. Accessed.
- 14.Stolbrink M, Thomson H, Hadfield RM, et al. The availability, cost, and affordability of essential medicines for asthma and COPD in low-income and middle-income countries: a systematic review. Lancet Glob Health. 2022;10:e1423–42. doi: 10.1016/S2214-109X(22)00330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozoh OB, Eze JN, Garba BI, et al. Nationwide survey of the availability and affordability of asthma and COPD medicines in Nigeria. Trop Med Int Health. 2021;26:54–65. doi: 10.1111/tmi.13497. [DOI] [PubMed] [Google Scholar]
- 16.Kibirige D, Kampiire L, Atuhe D, et al. Access to affordable medicines and diagnostic tests for asthma and COPD in sub Saharan Africa: the Ugandan perspective. BMC Pulm Med. 2017;17:179. doi: 10.1186/s12890-017-0527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehteshami-Afshar S, FitzGerald JM, Doyle-Waters MM, et al. The global economic burden of asthma and chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2016;20:11–23. doi: 10.5588/ijtld.15.0472. [DOI] [PubMed] [Google Scholar]
- 18.Iheanacho I, Zhang S, King D, et al. Economic burden of chronic obstructive pulmonary disease (COPD): a systematic literature review. Int J Chron Obstruct Pulmon Dis. 2020;15:439–60. doi: 10.2147/COPD.S234942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ughasoro MD, Eze JN, Ayuk AC, et al. Economic burden of childhood asthma in children attending a follow-up clinic in a resource-poor setting of Southeast Nigeria. Paediatr Respir Rev. 2021;37:74–9. doi: 10.1016/j.prrv.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Babar Z-U-D, Lessing C, Mace C, et al. The availability, pricing and affordability of three essential asthma medicines in 52 low- and middle-income countries. Pharmacoeconomics. 2013;31:1063–82. doi: 10.1007/s40273-013-0095-9. [DOI] [PubMed] [Google Scholar]
- 21.García-Marcos L, Asher MI, Pearce N, et al. The burden of asthma, hay fever and eczema in children in 25 countries: GAN phase I study. Eur Respir J. 2022;60:2102866. doi: 10.1183/13993003.02866-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amaral AFS, Potts J, Knox-Brown B, et al. Cohort profile: burden of obstructive lung disease (BOLD) study. Int J Epidemiol. 2023;52:e364–73. doi: 10.1093/ije/dyad146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kish L. A procedure for objective respondent selection within the household. J Am Stat Assoc. 1949;44:380–7. doi: 10.1080/01621459.1949.10483314. [DOI] [Google Scholar]
- 24.Laberge M, Coulibaly LP, Berthelot S, et al. Development and validation of an instrument to measure health-related out-of-pocket costs: the cost for patients questionnaire. Value Health. 2021;24:1172–81. doi: 10.1016/j.jval.2021.03.016. [DOI] [PubMed] [Google Scholar]
- 25.Gaillard EA, Kuehni CE, Turner S, et al. European respiratory society clinical practice guidelines for the diagnosis of asthma in children aged 5-16 years. Eur Respir J. 2021;58:2004173. doi: 10.1183/13993003.04173-2020. [DOI] [PubMed] [Google Scholar]
- 26.Topole E, Biondaro S, Montagna I, et al. Artiq.QC facilitates spirometry quality control in asthma and COPD clinical trials. Eur Respir J. 2021;58:A2505. doi: 10.1183/13993003.congress-2021.PA2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization . WHO Technical Report Series, Vol 20. 2007. Measuring medicine prices, availability, Affordability and price components; pp. 763–5. [Google Scholar]
- 28.World Health Organisation WHO model lists of essential medicines. 2019. [20-Aug-2021]. https://www.who.int/groups/expert-committee-on-selection-and-use-of-essential-medicines/essential-medicines-lists Available. Accessed.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.