Abstract

Platinum nanoparticles are widely used in electrocatalysis and sensors. In this work, a novel modified carbon paste electrode based on PANI-TiO2/Pt nanocomposites was developed electrochemically. To improve the performance of the PANI-TiO2/Pt composite-modified electrode, a Taguchi orthogonal array was used for experimental design, and the best values of factors affecting the performance were determined using the technique for order preference by similarity to ideal solution (TOPSIS) and Taguchi optimization methods. The electrochemical catalytic activities of the PANI-TiO2/Pt composite-modified electrode on the oxidation of nitrite was calculated by electrochemical analysis. The experimental results demonstrate that the PANI-TiO2/Pt electrode has pretty excellent electrocatalytic ability for the oxidation of nitrite. The sensing performances of the proposed electrode were evaluated, including linear detection range, sensitivity, LOD, selectivity, and stability for nitrite sensing.
1. Introduction
In recent years, water and air pollution due to human survival activities has become one of the most serious problems in the world. The main water pollutants such as nitrite and ammonium salts can be produced by human activities and industrial wastewater from chemical fertilizer plants or metal plants.1 Generally, nitrite can be produced by industrial wastes or polluted rainwater due to environmental pollution, which has become one of the major challenges in the world recently due to human activities. Nitrite is one of the toxic substances present in foods, such as fish, meat products, and vegetables, which has a great impact on human life and health. When nitrite ions enter the body, they bind to blood hemoglobin to form metahemoglobin, causing it to lose its oxygen-carrying capacity. It also combines with amines and amides of the digestive argan to produce the carcinogenic nitrosamine compound.2 Therefore, an accurate measurement of the nitrite concentration is very important for protecting human life and health. So far, various methods of detecting nitrite, such as chromatography, spectrophotometry, and electrochemical analysis, have been developed and used.3,4 Among these methods, the detection of nitrite ions by electrochemical sensing electrodes is a major candidate for nitrite detection due to its high sensitivity, selectivity, and fast response time.5
In recent years, there have been many studies on the development and practical application of amperometric sensing electrodes based on the oxidation or reduction of nitrite ions.6−9
Nitrite sensing methods based on the selective reduction of nitrite to ammonia using noble metals and transition metal–based catalysts and their compounds (Cu nanoparticle anchored on a TiO2 nanobelt array on a Ti plate (Cu@TiO2/TP),11 Ag nanoparticle–decorated TiO2 nanoribbon array on a Ti plate (Ag@TiO2/TP),12 CoP nanoparticle–decorated TiO2 nanoribbon array on a Ti plate (CoP@TiO2/TP),13 Ni nanoparticle supported on a molasses-derived carbon sheet)14 have been proposed.10
The synthesis and catalytic properties of many materials with electrocatalytic properties used in electrochemical sensing have been studied. For example, 2,3-diaminophenazine hydrochloride for cyanide detection in water,15 Ag nanoparticles for nitrite detection,16 Cu–Co/rGO nanocomposites17 and CuS-based nanocomposites18 for glucose detection, and rGO/ZnFe2O4 nanocomposite for efficient photocatalytic improvement of methylene blue19 have been proposed.
Among the synthesis methods of these catalytic materials, especially those used for electrochemical sensing, electrochemical synthesis has received much attention. Several catalytic materials, such as polymer-coated Au nanoparticle/black phosphorus nanocomposite for electrochemical sensing of pefloxacin,20 α-zirconium phosphate supported on nitrogen-doped graphene nanosheets (α-ZrP/NG) for the detection of levofloxacin,21 UiO-66/carboxylated multiwalled carbon nanotube composites (UiO-66/MWCNT-COOH) for gatifloxacin,22 and a zirconium-based metal–organic framework UiO-66/nitrogen-doped grapheme nanocomposite for marbofloxacin,23 have been synthesized by electrochemical synthesis.
In particular, modified electrodes based on conducting polymers, including polyaniline, polypyrrole, and polyphenylene, have received great attention in the development of sensing electrodes using them because of their ability to prevent surface contamination and improve the electrochemical properties of the electrodes.24 Among the conducting polymers, polyaniline has been widely used as a modified material for sensors because of its good electrical conductivity, electrochemical catalytic activities, ease of synthesis, and low cost. Synthesis methods for improving the properties of PANI have been reported.24 Recently, several PANI-based composites with metal nanoparticles (Au,25 Pt,26,27 Cu,28 and Pd29), carbon materials (CNT,30,31 GO),32 and metal oxides (WO3)33 have been developed and utilized for the electrochemical oxidation or reduction of nitrite.
Among the synthesis methods of conducting polymer-based composites, the current interest is electrochemical synthesis. The electrochemical synthesis method has the advantages of having less reagent consumption and ease of control of the synthesis process by adjusting the electrical parameters compared to the chemical synthesis method.
The Taguchi optimization method has been applied to determine the reasonable composition and/or process parameters for improving the performance. Rezazadeh et al.34 synthesized GO/PANI and GO/EDA nanomaterials by chemical method and determined the reasonable parameter values, such as the pH of the solution, stirring speed, reaction time, added salt additive amount, and the amount of donor phase, in order to determine the optimal parameters for improving the performance. Wang et al.35 employed PANI for the solid-phase microextraction of determining benzenes in the samples and optimized the parameters using the Taguchi method. Zahed et al.36 fabricated an electrochemical anticancer drug 5-fluorouracil (5-FU) sensor based on the Ag/PANI nanotube (AgNPs@PANINTs), and the AgNPs@PANINT nanocomposite was produced under optimal conditions obtained from the Taguchi method for a considerable electrocatalytic activity of AgNPs@PANINTs.
Although previous works applied the Taguchi method to optimize the material composition and fabrication process, they optimized individual performance response but did not optimize multiple performance responses simultaneously. It resulted in inconsistent optimal compositions and process parameters according to the individual performance responses. Therefore, it is very important to apply an effective method to optimize multiple performance responses simultaneously and not individually.
In this work, a novel composite containing PANI and Pt, TiO2 nanoparticles were synthesized on the surface of the carbon paste electrode by an electrochemical method.
In general, conductive polymer–based nanocomposite is synthesized by preparing nanosized conductive polymers with various metal oxide nanomaterials or by the incorporation of nanometal or carbon nanomaterials into the conductive polymer and used in electrochemical sensors.
The proposed material is a composite that combines the redox catalytic properties of PANI, nanostructured properties of nano-TiO2, and catalytic properties of Pt and exhibits excellent catalytic properties for the electrochemical detection of nitrite.
To improve the performance of the PANI-TiO2/Pt composite–modified electrode, a Taguchi orthogonal array–based experimental design was conducted, and the best values of the main factors affecting the performance were determined using the TOPSIS and Taguch optimization methods. The electrochemical performance of the proposed modified electrode was studied using an amperometric sensor with a three-electrode (3E) system.
2. Methods
2.1. Manufacturing Method of PANI-TiO2/Pt CompositeModified Electrode and Its Characterization Method
2.1.1. Reagents and Materials
Aniline (99%), K2PtCl6, nanoTiO2, and PBS (6.86pH) were of analytical grade. These reagents were purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). In all experiments, deionized water was used.
2.1.2. Apparatus
All the electrochemical experiments were conducted with a MEC-16 multifunction electrochemical analyzer. A 3E system with CPE modified with the PANI, PANI-TiO2, PANI-TiO2/Pt composites was used as the working electrode (WE), Pt electrode was used as the counter electrode (CE), and Ag/AgCl electrode was used as the reference electrode (RE). SEM was performed by using a Hitachi S-3000H scanning electron microscope. EIS measurements were performed using an IM6ex ZAHNER. The amperometric experiments were performed using a CHI627A potentiostat.
2.1.3. Preparation Method of PANI-TiO2/Pt Composite–Modified Carbon Paste Electrode
Prior to modification, the CPE electrode was polished with a polishing paper and then washed with deionized water (DW) and ethanol. Nano-TiO2 was added to the aniline monomer solution and then ultrasonically dispersed for 30 min. Then, the monomer solution was placed in the reaction vessel, and nitrogen was blown into the solution prior to electropolymerization (EP).
The EP of aniline was performed by continuous scanning using CV consisting of a 3E system. This electrode was then rinsed with DW and dried at room temperature. Pt nanoparticles (PtNPs) were modified by potential cycling (PC) at potentials of 1.2∼−0.5 V. Then, they were washed with DW and ethanol to remove the loosely attached PtNPs on the electrode surface, followed by drying.
2.2. Experimental Design Method for Improving the Performance of PANI-TiO2/Pt Composite–Modified Electrode
The main factors affecting the performance of the PANI-TiO2/Pt composite–modified electrode are as follows:
concentration of K2PtCl6 solution (mM),
addition amount of nano TiO2 (mg),
concentration of anline solution (M),
concentration of HCl solution (M),
CV scan rate (V/s) for electropolymerization of aniline, and
CV scan number for electropolymerization of aniline.
From among these six factors, the concentration of K2PtCl6 solution, which is an expensive material, was maintained at a constant value (1 mM), and the other five factors were selected as controllable factors. The five factors and their levels are listed in Table 1.
Table 1. Five Factors and Their Levels.
| controllable factors | symbol | levels |
|||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| addition amount of nano TiO2, mg | A | 0.3 | 0.4 | 0.5 | 0.6 |
| concentration of aniline solution, M | B | 0.08 | 0.10 | 0.12 | 0.14 |
| concentration of HCl solution, M | C | 0.8 | 1.0 | 1.2 | 1.4 |
| CV scan rate, V·s–1 | D | 0.06 | 0.08 | 0.1 | 0.12 |
| CV scan number | E | 15 | 20 | 25 | 30 |
Since the number of selected factors is 5 and the number of levels is 4, Taguchi orthogonal array L16(45) (OA L16) was selected for the design of experiment. The five controllable factors, were, respectively, assigned to each column of OA L16. Table 2 summarizes the experimental design using OA L16.
Table 2. Experimental Design Using OA L16.
| trial no. | A (mg) | B (M) | C (M) | D, (V·s–1) | E |
|---|---|---|---|---|---|
| 1 | 0.3 | 0.08 | 0.8 | 0.06 | 15 |
| 2 | 0.3 | 0.1 | 1 | 0.08 | 20 |
| 3 | 0.3 | 0.12 | 1.2 | 0.1 | 25 |
| 4 | 0.3 | 0.14 | 1.4 | 0.12 | 30 |
| 5 | 0.4 | 0.08 | 1 | 0.1 | 30 |
| 6 | 0.4 | 0.1 | 0.8 | 0.12 | 25 |
| 7 | 0.4 | 0.12 | 1.4 | 0.06 | 20 |
| 8 | 0.4 | 0.14 | 1.2 | 0.08 | 15 |
| 9 | 0.5 | 0.08 | 1.2 | 0.12 | 20 |
| 10 | 0.5 | 0.1 | 1.4 | 0.1 | 15 |
| 11 | 0.5 | 0.12 | 0.8 | 0.08 | 30 |
| 12 | 0.5 | 0.14 | 1 | 0.06 | 25 |
| 13 | 0.6 | 0.08 | 1.4 | 0.08 | 25 |
| 14 | 0.6 | 0.1 | 1.2 | 0.06 | 30 |
| 15 | 0.6 | 0.12 | 1 | 0.12 | 15 |
| 16 | 0.6 | 0.14 | 0.8 | 0.1 | 20 |
For evaluating the performance of the PANI-TiO2/Pt composite–modified electrode, we selected three performance responses (PRs): sensitivity (S), current value of the oxidation/reduction peak (CORP), and charge transfer resistance (CTR). Among the three responses, S and CORP are benefit responses (the higher the better), and CTR is a cost one (the lower the better).
2.3. Method to Determine Best Values of Controllable Factors Using TOPSIS and Taguchi Methods
This subsection proposes a method to determine best values of controllable factors for improving the overall performance of the PANI-TiO2/Pt composite–modified electrode using TOPSIS and Taguchi optimization methods.37,38
The proposed method consists of the following five major steps:
Step 1. The PANI-TiO2/Pt composite–modified electrodes were manufactured at every experimental trial, according to the OA L16 in Table 2 and their three PRs, such as S, CORP, and CTR, were measured.
Let yik be the values
of kth PR at the ith trial (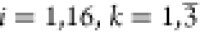 ). Namely, yi1, yi1, and yi1 are the measurement
values of S, CORP, and CTR, respectively, at the ith trial.
). Namely, yi1, yi1, and yi1 are the measurement
values of S, CORP, and CTR, respectively, at the ith trial.
The values of S, CORP, and CTR at every trial construct a decision matrix (DM)Y = (yik)16×3.
Step 2. The overall performance
scores (OPSs) 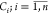 of the PANI-TiO2/Pt composite–modified
electrodes manufactured at every trial using the TOPSIS method were
calculated.
of the PANI-TiO2/Pt composite–modified
electrodes manufactured at every trial using the TOPSIS method were
calculated.
The TOPSIS converts three PRs into a single OPS as follows:
Step 2–1. A normalized DM Z = (zik)16×3 was
constructed from the DM Y = (yik)16×3 using the following
linear min-max normalization equation: (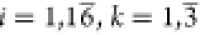 )
)
| 1 |
where ykmin and ykmax are the minimum and maximum values of the kth PR, respectively.
Step 2–2. A weighted normalized DM V = (vik)16×3 was constructed using the following equation:
| 2 |
where w1, w2, and w3 are the importance weights of three PRs (S, CORP, and CTR), respectively.
Step 2–3. Positive and negative ideal solutions 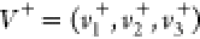 and
and 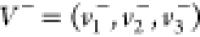 were determined, where
were determined, where
| 3 |
Step 2–4. The Euclidean distances were calculated from every experimental trial to the positive and negative ideal solutions using the following equations:
| 4 |
Step 2–5. The OPSs, namely, relative closeness values, were determined for every experimental trial using the following equation:
| 5 |
Overall, it reflects three PRs at each experimental trial. The larger the value is, the better is the overall performance of the PANI-TiO2/Pt composite–modified electrode.
Step 3. The mean OPSs 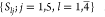 of five factors at four levels were conducted,
where Slj is the mean
OPS of the jth factor at lth level.
of five factors at four levels were conducted,
where Slj is the mean
OPS of the jth factor at lth level.
Step 4. The ranges of mean OPSs of the five factors were determined.
The range of the mean OPSs of jth factor was determined
as the difference between the maximum and minimum OPSs of the jth factor as follows: 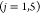
| 6 |
It represents the influence degree of the jth factor.
Step 5. The best values of the controllable factors that maximize the OPS were found.
Step 5–1. The best condition B, was found, which consists of the levels that maximize the mean OPSs.
| 7 |
Step 5–2. The best values of controllable factors x1*, x2*,..., x5* were determined where xj* is the value according to the bjth level of the jth factor.
3. Results and Discussion
3.1. Best Values of Controllable Factors Using TOPSIS and Taguchi Methods
We conducted experiments to determine the best values of the controllable factors for maximizing the overall performance of the PANI-TiO2/Pt composite–modified electrode.
Table 3 summarizes the experimental results according to OA L16.
Table 3. Experimental Results According to OA L16.
| controllable
factors |
PRs |
|||||||
|---|---|---|---|---|---|---|---|---|
| trial no. | A (mg) | B (M) | C (M) | D (V·s-1) | E | S (μA·mM–1) | CORP (μA) | CTR (Ω) |
| 1 | 0.3 | 0.08 | 0.8 | 0.06 | 15 | 20.8 | 36.5 | 454 |
| 2 | 0.3 | 0.1 | 1 | 0.08 | 20 | 24.5 | 39.1 | 482 |
| 3 | 0.3 | 0.12 | 1.2 | 0.1 | 25 | 23.7 | 40.7 | 489 |
| 4 | 0.3 | 0.14 | 1.4 | 0.12 | 30 | 21.6 | 43.9 | 459 |
| 5 | 0.4 | 0.08 | 1 | 0.1 | 30 | 25.2 | 39.3 | 453 |
| 6 | 0.4 | 0.1 | 0.8 | 0.12 | 25 | 24.9 | 40.6 | 454 |
| 7 | 0.4 | 0.12 | 1.4 | 0.06 | 20 | 25.9 | 38.4 | 463 |
| 8 | 0.4 | 0.14 | 1.2 | 0.08 | 15 | 24.7 | 41.2 | 462 |
| 9 | 0.5 | 0.08 | 1.2 | 0.12 | 20 | 26.4 | 44.1 | 441 |
| 10 | 0.5 | 0.1 | 1.4 | 0.1 | 15 | 26.3 | 44.7 | 455 |
| 11 | 0.5 | 0.12 | 0.8 | 0.08 | 30 | 27.9 | 43.2 | 449 |
| 12 | 0.5 | 0.14 | 1 | 0.06 | 25 | 25.7 | 42.4 | 431 |
| 13 | 0.6 | 0.08 | 1.4 | 0.08 | 25 | 26.5 | 44.6 | 430 |
| 14 | 0.6 | 0.1 | 1.2 | 0.06 | 30 | 24.7 | 42.2 | 420 |
| 15 | 0.6 | 0.12 | 1 | 0.12 | 15 | 26.7 | 47.8 | 427 |
| 16 | 0.6 | 0.14 | 0.8 | 0.1 | 20 | 26.1 | 46.5 | 438 |
The best values of controllable factors were determined for improving the overall performance of the PANI-TiO2/Pt composite-modified electrode using TOPSIS and Taguchi methods.
To convert the three PRs into a single OPS, the TOPSIS method, which is a well-known MADM, was applied. Based on the authors’ knowledge and practical experience, the importance weights of S, CORP, and CTR were set as follows:
The DM was constructed with the last three columns corresponding to the three PRs (S, CORP, and CTR), as summarized in Table 3.
Table 4 summarizes the normalized DM, weighted normalized DM, and the OPSs (relative closeness values). Table 5 summarizes the OA L16 and the OPSs (relative closeness values) at 16 trials.
Table 4. Normalized DM, weighted Normalized DM, and OPSs.
| normalized
DM |
weighted
normalized DM |
||||||
|---|---|---|---|---|---|---|---|
| rial no. | S | CORP | CTR | S | CORP | CTR | OPS |
| 1 | 0 | 0 | 0.507 | 0 | 0 | 0.101 | 0.146 |
| 2 | 0.521 | 0.230 | 0.101 | 0.261 | 0.069 | 0.020 | 0.417 |
| 3 | 0.408 | 0.372 | 0 | 0.204 | 0.112 | 0 | 0.366 |
| 4 | 0.113 | 0.655 | 0.435 | 0.056 | 0.196 | 0.087 | 0.321 |
| 5 | 0.620 | 0.248 | 0.522 | 0.310 | 0.074 | 0.104 | 0.519 |
| 6 | 0.577 | 0.363 | 0.507 | 0.289 | 0.109 | 0.101 | 0.519 |
| 7 | 0.718 | 0.168 | 0.377 | 0.359 | 0.050 | 0.075 | 0.542 |
| 8 | 0.549 | 0.416 | 0.391 | 0.275 | 0.125 | 0.078 | 0.501 |
| 9 | 0.789 | 0.673 | 0.696 | 0.394 | 0.202 | 0.139 | 0.748 |
| 10 | 0.775 | 0.726 | 0.493 | 0.387 | 0.218 | 0.099 | 0.725 |
| 11 | 1 | 0.593 | 0.580 | 0.500 | 0.178 | 0.116 | 0.786 |
| 12 | 0.690 | 0.522 | 0.841 | 0.345 | 0.157 | 0.168 | 0.660 |
| 13 | 0.803 | 0.717 | 0.855 | 0.401 | 0.215 | 0.171 | 0.785 |
| 14 | 0.549 | 0.504 | 1 | 0.275 | 0.151 | 0.200 | 0.579 |
| 15 | 0.831 | 1 | 0.899 | 0.415 | 0.300 | 0.180 | 0.862 |
| 16 | 0.746 | 0.885 | 0.739 | 0.373 | 0.265 | 0.148 | 0.773 |
Table 5. OA L16 and OPSs at 16 Trials.
| OA L16 |
||||||
|---|---|---|---|---|---|---|
| trial no. | A | B | C | D | E | OPS |
| 1 | 1 | 1 | 1 | 1 | 1 | 0.146 |
| 2 | 1 | 2 | 2 | 2 | 2 | 0.417 |
| 3 | 1 | 3 | 3 | 3 | 3 | 0.366 |
| 4 | 1 | 4 | 4 | 4 | 4 | 0.321 |
| 5 | 2 | 1 | 2 | 3 | 4 | 0.519 |
| 6 | 2 | 2 | 1 | 4 | 3 | 0.519 |
| 7 | 2 | 3 | 4 | 1 | 2 | 0.542 |
| 8 | 2 | 4 | 3 | 2 | 1 | 0.501 |
| 9 | 3 | 1 | 3 | 4 | 2 | 0.748 |
| 10 | 3 | 2 | 4 | 3 | 1 | 0.725 |
| 11 | 3 | 3 | 1 | 2 | 4 | 0.786 |
| 12 | 3 | 4 | 2 | 1 | 3 | 0.660 |
| 13 | 4 | 1 | 4 | 2 | 3 | 0.785 |
| 14 | 4 | 2 | 3 | 1 | 4 | 0.579 |
| 15 | 4 | 3 | 2 | 4 | 1 | 0.862 |
| 16 | 4 | 4 | 1 | 3 | 2 | 0.773 |
Table 6 summarizes the mean OPSs (mean relative closeness values) of five factors at four levels and their ranges obtained from TOPSIS. Figure 1 shows the mean relative closeness values of the controllable factors at four levels obtained from TOPSIS.
Table 6. Mean OPSs of Five Factors at Four Levels and Their Ranges were Obtained From TOPSIS.
| controllable
factors |
|||||
|---|---|---|---|---|---|
| levels | A | B | C | D | E |
| 1 | 0.313 | 0.550 | 0.556 | 0.482 | 0.559 |
| 2 | 0.520 | 0.560 | 0.615 | 0.622 | 0.620 |
| 3 | 0.730 | 0.639 | 0.548 | 0.596 | 0.582 |
| 4 | 0.750 | 0.564 | 0.593 | 0.612 | 0.551 |
| ranges | 0.437 | 0.089 | 0.066 | 0.140 | 0.069 |
| influence ranks | 1 | 3 | 5 | 2 | 4 |
| best levels | 4 | 3 | 2 | 2 | 2 |
Figure 1.
Mean relative closeness values of controllable factors at four levels were obtained from TOPSIS.
From Table 6, the influence ranking and scores of the five factors were as follows:
The addition amount of nano TiO2 has the highest influence on the overall performance of the PANI-TiO2/Pt composite–modified electrode, followed by CV scan rate, concentration of aniline solution, CV scan number, and concentration of HCl solution.
From Table 6 and Figure 1, the best condition was A4B3C2D2E2. Therefore, the best values of the five controllable factors to maximize the overall performance of the PANI-TiO2/Pt composite–modified electrode were as follows:
addition amount of nano TiO2: 0.6 mg,
concentration of aniline solution: 0.12 M,
concentration of HCl solution: 1 M,
CV scan rate for electropolymerization of aniline: 0.08 V·s–1, and
CV scan number for electropolymerization of aniline: 20.
3.2. Preparation of PANI-TiO2/Pt Composite–Modified Carbon Paste Electrode and Its Properties
3.2.1. Preparation of PANI-TiO2/Pt Composite–Modified Carbon Paste Electrode
After polishing, the CPE electrode was cleaned and immersed in 0.12 M aniline solution with 1 M HCl containing 0.6 mg of nano-TiO2. The EP of aniline on CPE was performed by continuous PC for 20 cycles in the range of −0.2 to 1.2 V vs the Ag/AgCl reference electrode at a scan speed of 0.08 V·s–1. Then, this electrode was washed with DW and dried at room temperature. Then, this electrode was added to a 1 × 10–3 M K2PtCl6 solution in 0.5 M H2SO4. PtNPs were deposited on the surface of the electrode by potential scanning in the range of −0.5 to 1.2 V for 10 cycles. The electrode was washed with DW in order to remove loosely attached PtNPs and then dried. For comparative study, CPE/PANI and CPE/PANI-TiO2 electrodes were manufactured under the same conditions.
3.2.2. Morphology Characterization of PANI-TiO2/Pt Nanocomposite
The morphology characterization of the PANI-TiO2/Pt nanocomposite was researched by using SEM. Figure 2 shows the SEM images of various films prepared on CPE by an electrochemical method. Figure 2a is an image of the PANI-TiO2, and Figure 2b is one of the PANI-TiO2/Pt nanocomposite. From Figure 2a, it can be seen that the polyaniline film surface is a nanoporous structure with a size of less than 100 nm. Several morphologies of the PANI, such as schistose, porous, sheet, and curling, can be obtained using different preparation methods. In this work, it is shown that the morphology of the prepared composites is porous in terms of the addition of nano-TiO2. The presence of TiO2 affects the process of polymerizing aniline. Therefore, the morphology of the PANI composite would be obtained nanoporous.
Figure 2.
SEM images of PANI-TiO2 (a) and PANI-TiO2/Pt (b) nanocomposite films.
On the contrary, PtNPs with particle sizes ranging from 100 to 200 nm were deposited on the electrode surface (Figure 2b). Since the obtained PANI-TiO2 had a nanoporous structure, the electrode surface was a suitable polymer matrix for the deposition of PtNPs.
3.2.3. Structural Characterization of PANI-TiO2/Pt Composite
Figure 3 shows the FTIR spectra of PANI and PANI-TiO2/Pt composites. The spectra of the PANI composite was observed with the peaks at 1560 cm–1, 1470 cm–1, 1304 cm–1, 1143 cm–1, and 1104 cm–1 corresponding to the vibrations of C=C (for the quinonoid unit), C=C (for the benzenoid unit), C–N, C=N, and C–H, respectively. The wide peak at 500–800 cm–1 in the spectra of the nanocomposite corresponds to the Ti–O bending mode of TiO2.
Figure 3.

FTIR spectra of PANI and PANI-TiO2–Pt.
3.2.4. Electrocatalytic Property of PANI-TiO2/Pt Composite–Modified Electrode
The performance of the PANI can be improved by incorporating various materials, such as TiO2.
The CV curves of the CPE, PANI/CPE, PANI-TiO2/CPE, and PANI-TiO2/Pt/CPE electrodes obtained at a potential of −0.2–1.0 V and at a scan rate of 100 mVs–1 in a 0.5 M H2SO4 solution containing 100 μM nitrite are presented in Figure 4.
Figure 4.

Cyclic voltammograms of the bare CPE, PANI/CPE, PANI-TiO2/CPE, and PANI-TiO2/Pt/CPE electrodes in 0.5 M H2SO4 solutions containing 100 μM nitrite with the scan rate of 100 mVs–1.
PtNPs improve the electrochemical properties such as the catalytic activity of the nanocomposite.
Figure 4 shows that the PtNPs in the composite improved the electrical conductivity and redox catalytic properties of the material. Therefore, the highest redox peak was obtained for the composites containing PtNPs.
In general, by adjusting the PC rates, the diffusion process on the surface of the modified electrode can be controlled. Therefore, the CPE/PANI-TiO2/Pt electrode was immersed in 0.5 M H2SO4 (pH 0) solution containing 100 μM nitrite and treated with varying scan rates from 20 to 100 mV·s–1. Figure 5 shows that with the increasing scan rate, the oxidation peak (Oxpeak) current also increased, and the peak potential shifted toward the positive direction.
Figure 5.
Cyclic voltammograms of the CPE/PANI-TiO2/Pt electrode obtained at different scan rates: 20 to 100 mVs–1 in 0.5 M H2SO4 (pH 0) solution containing 100 μM nitrite. Insets: plot of Oxpeak current vs scan rate.
Also, in Figure 5 (inset), the relationship between Oxpeak current and scan rate shows linearity, and the linear correlation coefficient was calculated to be about 0.9976.
3.2.5. Electrochemical Behavior of NO2– at Different Electrodes
From the CV curves of several modified electrodes such as CPE, CPE/PANI, CPE/PANI-TiO2, and CPE/PANI-TiO2/Pt electrodes, the electrochemical catalytic properties of the proposed composite for the oxidation of nitrite were evaluated.
The irreversible peak obtained at the CPE was due to the oxidation of NO2– to NO3–. In the acidic medium, NO2– is oxidized at the electrode surface as follows.
| 8 |
The Oxpeak current (Ipa) obtained at the CPE/PANI-TiO2/Pt electrode for 100 μM nitrite was the highest at 2.14 μA and the Oxpeak potential was lowest at 0.63 V. It means that the CPE/PANI-TiO2/Pt electrode is far superior for electrochemical oxidation of nitrite than other electrodes. On the contrary, the Oxpeak potentials were measured for bare CPE, CPE/PANI, and CPE/PANI-TiO2 of about 1.04, 0.77, and 0.76 V, respectively. At the CPE/PANI-TiO2/Pt electrode, the OxPeak appeared at 0.63 V, which shows a 0.13 V negative shift compared to CPE/PANI-TiO2. This shift of the Oxpeak potential shows that PtNPs have the electrochemical catalytic activity for the oxidation of nitrite. Consequently, the CPE/PANI-TiO2/Pt electrode has excellent electroactive properties for the electrochemical oxidation of nitrite.
Figure 6 shows a schematic diagram of nitrite sensing of the PANI-TiO2/Pt–modified electrode.
Figure 6.

Schematic diagram nitrite sensing of the PANI-TiO2/Pt–modified electrode.
3.2.6. Electrocatalysis of NO2– at PANI-TiO2/Pt Nanocomposite–Modified Electrodes
Figure 7 shows the CV curves of the PANI-TiO2/Pt nanocomposite–modified CPE obtained in a 0.5 M H2SO4 solution with varying nitrite concentrations from 50 μM to 300 μM.
Figure 7.
Cyclic voltammograms of CPE/PANI-TiO2/Pt obtained in 0.5 M H2SO4 solution of different concentrations of nitrite (50 μM to 300 μM) at a scan rate of 50 mV s–1. Insets: the Oxpeak current versus different concentrations of nitrite.
As the concentration of nitrite increased, the Oxpeak current increased, but the Oxpeak potential remained unchanged.
The linear dependence of the Oxpeak current on the concentration of nitrite is shown in Figure 7inset). The Oxpeak current increases linearly with the concentration of nitrite with a slope of 25.816 nAμM –1 and the linear regression coefficient, R2 = 0.9992. This means that the PANI-TiO2/Pt nanocomposite is an excellent electrocatalytic material for the oxidation of nitrite.
3.2.7. pH Effect on the Electrochemical Behavior of CPE/PANI-TiO2/Pt
The pH effect on the electrochemical behavior of CPE/PANI-TiO2/Pt was considered and is shown in Figure 8. The experiments were performed in solutions with three pH values (1.0, 4.01, and 6.86). With increasing pH, the Oxpeak current value of the PANI-TiO2/Pt composite-modified elecrode decreased. The experimental results show that the electrochemical properties of PANI-TiO2/Pt are strongly dependent on the pH of the solution. The inset in Figure 8 shows the current values of Oxpeak, which were obtained in H2SO4 solution with 500 μM nitrite by varying its pH from 0 to 7.
Figure 8.
Cyclic voltammograms of CPE/PANI-TiO2/Pt in H2SO4 solution with different pH values. Insets: anodic OxPeak current was obtained in an N2-saturated H2SO4 solution by varying its pH values from 0 to 7 in the presence of 500 μM nitrite.
3.3. Sensing Performance of PANI-TiO2/Pt Composite-Modified Carbon Paste Electrode for Nitrite
3.3.1. Amperometric Study
The sensitivity, linear range, and LOD of the prepared electrode were determined using the amperometric technique. The response current was recorded in an N2-saturated 0.5 M H2SO4 solution by varying the concentration of nitrite from 0 to 250 μM. Figure 9 shows the response current curve of the proposed electrode with respect to the nitrite concentration.
Figure 9.
Response current curve of the prepared electrode with different nitrite concentrations.
As shown in Figure 9, the linear response range, sensitivity, and LOD of the electrode for nitrite were 0.5–194, μM, 25.6 nA·μM–1, and 0.2 μM, respectively.
3.3.2. Exploring the Sensing Performance of PANI-TiO2/Pt Composite with pH
As mentioned above, since the electrochemical catalytic properties of the PANI-TiO2/Pt vary with the pH of the solution, the sensitivity of the electrochemical nitrite sensors based on the PANI-TiO2/Pt nanocomposite varies greatly with pH. Table 7 summarizes the changes in the sensitivity of the sensor with the pH of the solution.
Table 7. Changes in the Sensitivity of the Sensor with the pH of the Solution.
| pH | sensitivity, nA·μM–1 |
|---|---|
| 0 | 25.7 |
| 1 | 21.6 |
| 2 | 17.9 |
| 3 | 11.7 |
| 4 | 4.2 |
Since the proposed composite has the best electrochemical catalytic performance at pH 0, the sensitivity of the sensor is also the highest.
3.3.3. Comparison of the Catalytic Properties of the Prepared Sensor with PANI-TiO2/Pt–Modified and PANI-TiO2–Modified Electrodes for Detecting Nitrite
To evaluate the catalytic performance of the proposed composite–modified electrode for nitrite sensing, the sensitivity and low detecting limits (LOD) of the sensors based on PANI-TiO2/Pt and PANI-TiO2 were compared.
As summarized in Table 8, the sensitivity of the sensor based on the PANI-TiO2/Pt nanocomposite is greater than that of the sensor based on the PANI-TiO2. It is attributed to the catalytic performance of the PtNPs in the composite.
Table 8. Comparison of Catalytic Performance of the Sensors based on PANI-TiO2/Pt and PANI-TiO2 for Nitrite Sensing.
| modified material | sensitivity, μA·μM–1 | LOD, μM |
|---|---|---|
| PANI-TiO2 | 18.7 | 1.1 |
| PANI-TiO2/Pt | 25.7 | 0.2 |
3.3.4. Selectivity
The selectivity of the electrochemical sensor is very important for its practical application. To evaluate the selectivity, the response current of the PANI-TiO2/Pt composite–modified electrode was measured in an N2-saturated 0.5 M H2SO4 solution with 500 μM nitrite containing various interferences such as NO3–, Cl–, and SO42–. Here, the interference concentrations were 10 mM.
As summarized in Table 9, the response current for nitrite was much larger than that of other interferences except ClO–, and the amount of current response of the interferences could be negligible. In the case of water disinfection using chlorine-based disinfectants, as in swimming pools, ClO– is present in water but is generally not present in natural water. Therefore, the experimental results showed that the PANI-TiO2/Pt composite–modified electrode had excellent selectivity for detecting nitrite in natural water.
Table 9. Response Current in Several Interferences, μA.
| nitrite | NO3– | ClO– | Cl– | SO42– | Na+ | K+ | Cu2+ | Fe3+ |
|---|---|---|---|---|---|---|---|---|
| 12.6 | 0.1 | 1.37 | 0.12 | 0.08 | 0.05 | 0.04 | 0.06 | 0.14 |
3.3.5. Stability, Repeatability, and Reproducibility
The stability of the proposed modified electrode for nitrite sensing was studied in an N2-saturated 0.5 M H2SO4 solution with 100 μM nitrite. The results showed that the response currents of the proposed electrode obtained in the 100 μM nitrite sample were retained at 97.32% of the original response current.
In order to evaluate the reproducibility, five prepared electrodes were selected, and the response currents were measured in 100 μM nitrite sample solution. In result, the relative standard deviation (RSD) was 2.3%.
In order to evaluate repeatability, the experiments of measuring response current in the 100 μM nitrite sample solution were conducted 10 times using the proposed electrode. In result, RSD was 2.1%.
Therefore, the proposed electrode has good stability, repeatability, and reproducibility for nitrite sensing.
3.3.6. Practical Application
To study the practical application of the prepared electrode, the response current was measured in rainwater and river water by using the standard addition method. Table 10 summarizes the experiment results.
Table 10. Response Current of the Prepared Electrode in Various Water Samples.
| water samples | added, μM | found, μM | recovery(%) |
|---|---|---|---|
| rain water | 100 | 101.4 | 101.4 |
| river water | 200 | 199.3 | 99.65 |
As ummarized in Table 10, the PANI-TiO2/Pt composite–modified electrode showed good recovery, and it means that this electrode is suitable for practical applications.
4. Conclusions
In thiswork, the PANI-TiO2/Pt nanocomposite was synthesized on CPE by an electrochemical method.
The experimental design was conducted for improving the performance of the PANI-TiO2/Pt composite–modified electrode, and the best values were determined using TOPSIS and Taguchi methods. The proposed composite showed an excellent electrochemical catalytic property for the oxidation of nitrite. The PANI-TiO2/Pt nanocomposite–modified electrode showed superior linearity for detecing nitrite with a sensitivity of 25.82 nA·μM –1.
The major optimal parameters to manufacture the PANI-TiO2/Pt nanocomposite–modified electrode are as follows:
addition amount of nano TiO2: 0.6 mg,
concentration of aniline solution: 0.12 M,
concentration of HCl solution: 1 M,
CV scan rate for electropolymerization of aniline: 0.08 V·s–1, and
CV scan number for electropolymerization of aniline: 20.
The significance and innovation of this work are as follows:
By determining the optimal parameter values with TOPSIS and Taguchi methods, the performance of modified electrode, such as sensitivity, current value of oxidation/reduction peak, and charge transfer resistance, were improved.
The CPE/PANI-TiO2/Pt electrode can be used as a working electrode of amperometric sensor by using the electrochemical oxidation of nitrite.
This work lacks in constructing the amperometric sensor by using the proposed nanocomposite- modified electrode and in increasing selectivity of sensor. Future work needs to improve the selectivity of sensors by using the conductive polymer nanocomposite–modified electrode.
The proposed method can be applied to manufacture conductive polymer nanocomposite–modified electrodes with an excellent performance.
Acknowledgments
The authors would like to acknowledge the Kim Chaek University of Technology, Pyongyang, Democratic People’s Republic of Korea.
Data Availability Statement
All data that support the findings of this work are included within this article.
This research did not receive the external funding.
The authors declare no competing financial interest.
Notes
Ethics approval. The authors approve to observe the ethics standard of this journal.
References
- Chan T. Y. K. Vegetable-borne nitrate and nitrite and the risk of methaemoglobinaemia. Toxicol. Lett. 2011, 200 (1–2), 107–108. 10.1016/j.toxlet.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Thompson B. M.; Nokes C. J.; Cressy P. J. Intake and risk assessment of nitrate and nitrite from New Zealand foods and drinking water. Food Addit. Contam. 2007, 24, 113–121. 10.1080/02652030600934206. [DOI] [PubMed] [Google Scholar]
- Erkekoglu P.; Sipahi H.; Baydar T. Evaluation of nitrite in ready-made soups. Food Analytical Methods 2009, 2, 61–65. 10.1007/s12161-008-9045-0. [DOI] [Google Scholar]
- Helaleh M. I. H.; Korenaga T. Ion chromatographic method for simultaneous determination of nitrate and nitrite in human saliva. J. Chromatogr. B: Biomed. Sci. Appl. 2000, 744, 433–437. 10.1016/S0378-4347(00)00264-4. [DOI] [PubMed] [Google Scholar]
- Liu T. S.; Kang T. F.; Lu L. P.; Zhang Y.; Cheng S. Y. Au–Fe(III) nanoparticle modified glassy carbon electrode for electrochemical nitrite sensor. J. Electroanal. Chem. 2009, 632, 197–200. 10.1016/j.jelechem.2009.04.023. [DOI] [Google Scholar]
- Zheng D.; Hu C.; Peng Y.; Hu S. A carbon nanotube/polyvanillin composite film as an electrocatalyst for the electrochemical oxidation of nitrite and its application as a nitrite sensor. Electrochim. Acta 2009, 54, 4910–4915. 10.1016/j.electacta.2009.04.004. [DOI] [Google Scholar]
- Ge X.; Wang L.; Liu Z.; Ding Y. Nanoporous gold leaf for amperometric determination of nitrite. Electroanalysis 2011, 23, 381–386. 10.1002/elan.201000320. [DOI] [Google Scholar]
- Yu C.; Guo J.; Gu H. Electrocatalytical oxidation of nitrite and its determination based on Au@Fe3O4 nanoparticles. Electroanalysis 2010, 22, 1005–1011. 10.1002/elan.200900465. [DOI] [Google Scholar]
- Kozub B. R.; Rees N. V.; Compton R. G. Electrochemical determination of nitrite at a bare glassy carbon electrode; why chemically modify electrodes. Sens. Actuators, B 2010, 143, 539–546. 10.1016/j.snb.2009.09.065. [DOI] [Google Scholar]
- He X.; Li Z.; Yao J.; Dong K.; Li X.; Hu L.; Sun S.; Cai Z. W.; Zheng D.; Luo Y. S.; et al. High-efficiency electrocatalytic nitrite reduction toward ammonia synthesis on CoP@TiO2 nanoribbon array. iScience 2023, 26, 107100. 10.1016/j.isci.2023.107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue L.; Song W.; Zhang L.; Luo Y.; Wang Y.; Li T.; Ying B.; Sun S.; Zheng D.; Liu Q.; et al. Recent Advance in Heterogenous Electrocatalysts for Highly Selective Nitrite Reduction to Ammonia Under Ambient Condition. Small Struct. 2023, 4, 2300168. 10.1002/sstr.202300168. [DOI] [Google Scholar]
- Yang L.; Fan X.; Li Z.; He X.; Sun S.; Cai Z.; Luo Y.; Zheng D.; Ying B.; Zhang J.; Alshehri A. A.; Wang Y.; Ma K.; Sun X. High-efficiency electroreduction of nitrite to ammonia on a Cu@TiO2 nanobelt array. Chem. Commun. 2023, 59, 1625–1628. 10.1039/D2CC06261E. [DOI] [PubMed] [Google Scholar]
- Fan X.; He X.; Ji X.; Zhang L.; Li J.; Hu L.; Li X.; Sun S.; Zheng D.; Luo Y.; et al. High-efficiency electrosynthesis of ammonia with selective reduction of nitrite over an Ag nanoparticle-decorated TiO2 nanoribbon array. Inorg. Chem. Front. 2023, 10, 1431–1435. 10.1039/D2QI02409H. [DOI] [Google Scholar]
- He X.; Li X.; Fan X.; Li J.; Zhao D.; Zhang L.; Sun S.; Luo Y.; Zheng D.; Xie L.; Asiri A. M. Ambient electroreduction of nitrite to ammonia over Ni nanoparticle supported on molasses-derived carbon sheets. ACS Appl. Nano Mater. 2022, 5 (10), 14246–14250. 10.1021/acsanm.2c03720. [DOI] [Google Scholar]
- Yong B. R.; Wei T. B.; Qu W. J.; Lin Q.; Zhang Y. M.; Yao H. Highly selective and sensitive chemosensor based on 2,3-diaminophenazine hydrochloride for the detection of cyanide in pure water and its application in plant seed samples. New J. Chem. 2018, 42, 14766–14771. 10.1039/C8NJ02316F. [DOI] [Google Scholar]
- Ramachandran K.; Kalpana D.; Sathishkumar Y.; Lee Y. S.; Ravichandran K.; Kumar G. G. A facile green synthesis of silver nanoparticles using Piper betle biomass and its catalytic activity toward sensitive and selective nitrite detection. J. Ind. Eng. Chem. 2016, 35, 29–35. 10.1016/j.jiec.2015.10.033. [DOI] [Google Scholar]
- Babu K. J.; Sheet S.; Lee Y. S.; Kumar G. G. Three-Dimensional Dendrite Cu?Co/Reduced Graphene Oxide Architectures on a Disposable Pencil Graphite Electrode as an Electrochemical Sensor for Nonenzymatic Glucose Detection. ACS Sustainable Chem. Eng. 2018, 6, 1909–1918. 10.1021/acssuschemeng.7b03314. [DOI] [Google Scholar]
- Siva G.; Aziz M. A.; Kumar G. G. Engineered tubular nanocomposite electrocatalysts based on CuS for high-performance, durable glucose fuel cells and their stack. ACS Sustainable Chem. Eng. 2018, 6 (5), 5929–5939. 10.1021/acssuschemeng.7b04326. [DOI] [Google Scholar]
- Rani G. J.; Rajan M. A. J.; Kumar G. G. Reduced graphene oxide/ZnFe2O4 nanocomposite as an efficient catalyst for the photocatalytic degradation of methylene blue dye. Res. Chem. Intermed. 2017, 43, 2669–2690. 10.1007/s11164-016-2788-0. [DOI] [Google Scholar]
- Li G.; Qi X.; Wu J.; Wan X.; Wang T.; Liu Y.; Chen Y.; Xia Y. Highly stable electrochemical sensing platform for the selective determination of pefloxacin in food samples based on a molecularly imprinted-polymer-coated gold nanoparticle/black phosphorus nanocomposite. Food Chem. 2024, 436, 137753. 10.1016/j.foodchem.2023.137753. [DOI] [PubMed] [Google Scholar]
- Li G.; Wan X.; Xia Y.; Tuo D.; Qi X.; Wang T.; Mehmandoust M.; Erk N.; He Q.; Li Q. Lamellar α-Zirconium Phosphate Nanoparticles Supported on N-Doped Graphene Nanosheets as Electrocatalysts for the Detection of Levofloxacin. ACS Appl. Nano Mater. 2023, 6 (18), 17040–17052. 10.1021/acsanm.3c03162. [DOI] [Google Scholar]
- Wan X.; Du H.; Tuo D.; Qi X.; Wang T.; Wu J.; Li G. UiO-66/Carboxylated Multiwalled Carbon Nanotube Composites for Highly Efficient and Stable Voltammetric Sensors for Gatifloxacin. ACS Appl. Nano Mater. 2023, 6 (20), 19403–19413. 10.1021/acsanm.3c03874. [DOI] [Google Scholar]
- Wang T.; Xia Y.; Wan X.; Zhang Y.; Chen N.; Jin Y.; Li G. A facile and efficient voltammetric sensor for marbofloxacin detection based on zirconium-based metal-organic framework UiO-66/nitrogen-doped grapheme nanocomposite. Microchem. J. 2024, 201, 110673. 10.1016/j.microc.2024.110673. [DOI] [Google Scholar]
- Tanguy N. R.; Thompson M.; Yan N. A review on advances in application of polyaniline for ammonia detection. Sens. Actuators, B 2018, 257, 1044–1064. 10.1016/j.snb.2017.11.008. [DOI] [Google Scholar]
- Xu Q.; Leng J.; Li H. B.; Lu G. J.; Wang Y.; Hu X. Y. The preparation of polyaniline/gold nanocomposites by self-assembly and their electrochemical applications. React. Funct. Polym. 2010, 70, 663–668. 10.1016/j.reactfunctpolym.2010.05.012. [DOI] [Google Scholar]
- Kinyanjui J. M.; Wijeratne N. R.; Hanks J.; Hatchett D. W. Chemical and electrochemical synthesis of polyaniline/platinum composites. Electrochim. Acta 2006, 51 (14), 2825–2835. 10.1016/j.electacta.2005.08.013. [DOI] [Google Scholar]
- Jamal R.; Xu F.; Shao W.; Abdiryim T. The study on the application of solid state method for synthesizing the polyaniline/noble metal (Au or Pt) hybrid matarials. Nanoscale Res. Lett. 2013, 117, 8. 10.1186/1556-276X-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil U. V.; Ramgir N. S.; Karmakar N.; Bhogale A.; Debnath A. K.; Aswal D. K.; Gupta S. K.; Kothari D. C. Room temperature ammonia sensor based on copper nanoparticle intercalated polyaniline nanocomposite thin films. Appl. Surf. Sci. 2015, 339, 69–74. 10.1016/j.apsusc.2015.02.164. [DOI] [Google Scholar]
- Wang Q.; Jing X.; Han J.; Yu L.; Xu Q. Design and fabrication of low-loading palladium nano particles on polyaniline (nano Pd@PANI): An effective catalyst for Suzuki cross-coupling with high TON. Mater. Lett. 2018, 215, 65–67. 10.1016/j.matlet.2017.12.064. [DOI] [Google Scholar]
- Ogurtsov N. A.; Noskov Y. V.; Bliznyuk V. N.; Ilyin V. G.; Wojkiewicz J. L.; Fedorenko E. A.; Pud A. A. Evolution and interdependence of structure and properties of nanocomposites of multiwall carbon nanotubes with polyaniline. J. Phys. Chem. C 2016, 120, 230–242. 10.1021/acs.jpcc.5b08524. [DOI] [Google Scholar]
- Guo M.; Chen J.; Li J.; Tao B.; Yao S. Fabrication of polyaniline/carbon nanotube composite modified electrode and its electrocatalytic property to the reduction of nitrite. Anal. Chim. Acta 2005, 532, 71–77. 10.1016/j.aca.2004.10.045. [DOI] [Google Scholar]
- Sivakumar M.; Sakthivel M.; Chen S. M.; Pandi K.; Chen T. W.; Yu M. C. An electrochemical selective detection of nitrite sensor for polyaniline doped grapheme oxide modified electrode. Int. J. Electrochem. Sci. 2017, 12, 4835–4846. 10.20964/2017.06.24. [DOI] [Google Scholar]
- Zou B. X.; Bian L. J.; Wang Y.; Li X. J.; Liu X. X. Electrocatalytic reduction of bromate, chlorate, nitrite and 4-nitrophenol at WO3/PANI modified electrode. Acta Chim. Sinica 2011, 69, 1575–1581. [Google Scholar]
- Rezazadeh T.; Dalali N.; Sehati N. Investigation of adsorption performance of graphene oxide/polyaniline reinforced hollow fiber membrane for preconcentration of Ivermectin in some environmental samples. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2018, 204, 409–415. 10.1016/j.saa.2018.06.040. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Li Y.; Feng J.; Sun C. Polyaniline-based fiber for headspace solid-phase microextraction of substituted benzenes determination in aqueous samples. Anal. Chim. Acta 2008, 619 (2), 202–208. 10.1016/j.aca.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Zahed F. M.; Hatamluyi B.; Lorestani F.; Zarrin E. Silver nanoparticles decorated polyaniline nanocomposite based electrochemical sensor for the determination of anticancer drug 5-fuorouracil. Int. J. Pharm. Res. Biomed. Anal. 2018, 161, 12–19. 10.1016/j.jpba.2018.08.004. [DOI] [PubMed] [Google Scholar]
- Yang Y. C.; Choe C. M.; Kim J. S.; Om M. S.; Kim U. H. Materials selection method using improved TOPSIS without rank reversal based on linear max-min normalization with absolute maximum and minimum values. Mater. Res. Express 2022, 9 (6), 065503. 10.1088/2053-1591/ac2d6b. [DOI] [Google Scholar]
- Yang Y. C.; Yang J. Y.; Kim R. C.; Om M. S.; Kim U. H.; Ri W. S.; Sok S. H. Multi-attribute optimization and influence assessment methodology of casting process parameters combined with integrated MADM and Taguchi method. Int. J. Adv. Des. Manuf. Technol. 2023, 129, 681. 10.1007/s00170-023-12275-3. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data that support the findings of this work are included within this article.








